Abstract
Cancer immunotherapy has recently burst onto the center stage of cancer treatment and research. T lymphocyte adoptive cellular transfer (ACT), a form of cancer immunotherapy, has spawned unprecedented complete remissions for terminal patients with certain leukemias and lymphomas. Unfortunately, the successes have been overshadowed by the disappointing clinical results of ACT administered to treat solid tumors, in addition to the toxicities associated with the treatment, a lack of efficacy in a significant proportion of the patient population, and cancer relapse following the treatment. Biomaterials hold the promise of addressing these shortcomings. ACT consists of two main stages – T lymphocyte ex vivo expansion followed by reinfusion into the patient – and biomaterials can improve the efficacy of ACT at both stages. In this review, we highlight recent advances in the use of biomaterials for T lymphocyte adoptive cellular cancer immunotherapy and discuss the challenges at each stage.
Keywords: Artifical APC, T cell, Drug Delivery, Nanoparticle, Scaffold, CAR T Cell
1. Introduction
Malignant neoplasms (cancer) remain the second leading cause of death in the United States [1], with 1 in 2 men and 1 in 3 women developing cancer in his or her lifetime [2]. While the three longstanding pillars of cancer therapy – surgery, radiation, and chemotherapy – are the basis for addressing this threat of cancer, a more recent emerging fourth pillar is becoming the focus of oncology research [3]. That fourth pillar is cancer immunotherapy, in which a patient’s own immune system is manipulated to fight cancer. Thus far, cancer immunotherapy has made its most prominent strides in the form of immune checkpoint blockades, cancer vaccines, and adoptive cellular transfer [4, 5]. Given the recent success of T cell therapies, the scope of this review will be focused on using biomaterials for enhanced T cell treatments.
Adoptive cellular transfer is a treatment in which a cell population is expanded ex vivo and re-infused into the body. ACT for cancer immunotherapy typically utilizes naturally occurring or genetically modified T cells [6], although natural killer (NK) cells have also been studied [7, 8]. Naturally occurring T cells that accumulate in tumors, termed tumor infiltrating lymphocytes (TILs), have been proven to be an effective treatment for metastatic melanoma and have shown promise for treating other cancers as well [9, 10]. After recognizing the potential for the adoptive transfer of T cells, researchers began isolating tumor selective T cells by culture with antigen presenting cells and subsequently progressed to genetically engineering T cells to target specific cancer markers and produce a more durable treatment. Genetically modified T cells used for cancer immunotherapy fall into two categories: T cells constructed to express transgenic T cell receptors (TCRs) or chimeric antigen receptors (CARs) [11]. TCRs bind to peptide-major histocompatibility complexes (MHC), which identify pathogen-derived antigens, and CARs are equipped with a single chain variable fragment (scFv), which is an antibody fragment that can identify and target extracellular biomarkers on a cell in an MHC-independent manner [11]. The year 2017 marked the first FDA approvals of CAR T adoptive cellular therapies, one for treating acute lymphocytic leukemia and a second for large diffuse Non-Hodgkin lymphoma. This followed clinical trials in which CD19 CAR T cells resulted in complete response rates over 80% for patients who had refractory B cell malignancies after previously receiving two other forms of treatment [12-16]. CD19 is a biomarker present solely on B cells [17], which makes it the quintessential target for CAR T cells because other cells in the body are not targeted and B cell aplasia is readily treated by immunoglobulin injections [18].
Despite the outburst of ACT cancer immunotherapy research and clinical trials, the expansion of ACT beyond CAR T cell therapy for liquid cancer treatments remains in question [19-22]. A few of the key challenges that currently prevent cancer immunotherapy ACT from treating solid tumors are the immunosuppressive tumor microenvironment that accompany solid tumors, the production of ample, able T cells during ex vivo expansion, and toxicities associated with ACT. In this review we will discuss how biomaterials can alleviate these problems by producing larger amounts of cytotoxic T cells and providing T cells with additional tools for homing to and fighting cancer and cancer-associated cells. In accordance with the schematic for ACT shown in Fig. 1, we will begin by discussing biomaterials’ impact on T cell expansion methods followed by biomaterials’ potential role in improving T cell in vivo efficacy.
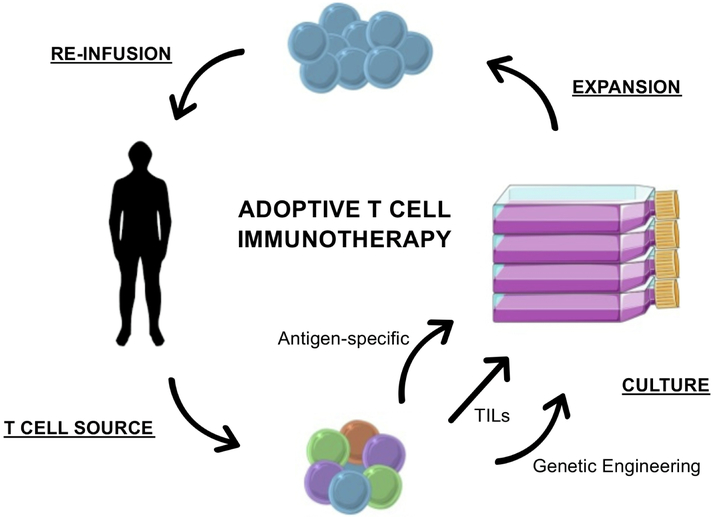
Fig. 1. The Process of Adoptive T Cell Immunotherapy.
T cells are harvested either from tumor (tumor-infiltrating lymphocytes, TILs) or peripheral blood (peripheral blood lymphocytes, PBLs). TILs can be expanded non-specifically since they are preferentially tumor-specific prior to culture. In contrast, tumor specificity must be induced in PBLs, either through antigen-specific expansion or genetic engineering. After several weeks of expansion in culture, tumor-specific T cells can be reinfused into the cancer patient. Fig. 1 is adapted with permission from Adoptive T cell immunotherapy for cancer. Perica K, Varela JC, Oelke M, Schneck J. Rambam Maimonides medical journal. 2015;6:e0004.
2. T cell expansion
Post leukapheresis, T cell expansion is performed to produce a sufficient number of cells for successful treatment of a malignancy. To date, successful ACT for immunotherapy is limited to autologous T cells to prevent graft versus host disease (in which the body rejects the re-infused T cells that are not derived from the host) [23]. T cell activation requires two signals: T cell receptor (TCR) activation and costimulation [24, 25]. A third signal from a pro-survival cytokine such as Interleukin-2 (IL-2) is needed to maintain to the expansion and differentiation of T cells [26, 27]. Antigen presenting cells (APCs), especially dendritic cells (DC), provide these signals to T cells in the body in a specific spatiotemporal manner [28, 29].
Producing large quantities of T cells is not the only concern for ex vivo expansion in cancer immunotherapy. Research has shown that the T cell type and differentiation state upon re-infusion into the body strongly influences the efficacy of the treatment [30-34]. CD8+ T cells, the cytotoxic T cells used to kill cancer cells in ACT, are usually composed of multiple subsets undergoing various degrees of differentiation, including naïve, effector, effector memory, central memory, and stem cell memory T cells, and T cells in less differentiated states produce more durable ACT responses [6, 35-37]. Recently, Fraietta et. al.’s characterization of T cell populations during CAR T cell infusion in patients with chronic lymphocytic leukemia (CLL) revealed that an early memory cytotoxic T cell population resulted in robust therapeutic response [33]. In a simple yet powerful study, Ghassemi et. al. proved that minimally ex vivo expanded CD19 CAR T cells remain less differentiated and exhibit improved effector function in vitro, and the in vivo efficacy of CD19 CAR T cells in a murine xenograft ALL model varied inversely with expansion time [34]. Notably, despite most T cell engineering protocols requiring 9-14 days of ex vivo expansion, CAR T cells administered to the xenograft model after 3 days of expansion produced robust tumor control at a 6-fold lower dosage, in contrast to 9 day cultured CAR T cells which failed to control leukemia at reduced doses. Although longer culture periods increase cell count, “younger,” or less differentiated, T cells possess enhanced anti-tumor capabilities. As discussed below, past expansion methods focus primarily on the production of CD8+ cytotoxic T cells over CD4+ helper T cells, and studies centered on expanding less differentiated CD8+ T cells will be needed to facilitate the administration of these more potent T cells.
2.1. Natural APCs and Cellular aAPCs
Natural APCs, in the form of monocyte originated dendritic cells (moDCs), have been harvested from patients and used to expand T cells ex vivo in a handful clinical trials [38, 39]. Although designed by the body to activate and expand T cells, there are several limitations to using autologous DCs for ex vivo expansion that prevent their widespread usage. The maintenance of DCs for T cell ACT on a clinical scale requires substantial manufacturing and labor costs [36, 40], the functionality of DCs can be compromised in diseased patients [41], and DCs can cause T cell unresponsiveness [42]. In an effort to mitigate these issues, researchers have experimented with various types of cellular artificial antigen presenting cells (aAPCs). Cellular aAPCs, such as insect cells, mouse fibroblasts, and human leukemic cell lines, have been genetically modified to present antigen, adhesion, and costimulatory signals to T cells [43, 44]. While these methods can achieve successful expansion of T cells, and clinical trials relying on human leukemic cell line K562 for ex vivo T cell propagation saw significant clinical benefits of the ACT treatment [45, 46], cellular aAPCs run the risk of tumorigenicity and infection as a result of the genetic modifications and, similar to natural APCs, require additional culturing steps. But above all else, the aforementioned platforms do not allow for the specific control of T cell signal frequency, orientation, and persistence. Engineered biomaterials allow researchers to have full control over the spatio-temporal presentation of signals to maximize the production of more desirable T cells [47, 48]. It is acellular aAPCs that can create an off-the-shelf, simplified T expansion means which eliminate supplementary culture and separation steps - streamlining T cell production.
2.2. Acellular aAPCs
Acellular (synthetic) aAPCs have become the mainstay for T cell expansion in the clinic and laboratory. The key signaling molecules for T cell activation and proliferation can be engineered into biomaterials of different shapes and sizes to maximize the efficacy of T cell production, and thus far have been able to simplify, expedite, and reduce the cost of T cell expansion. TCR agonists targeting CD3, such as anti-CD3 antibodies, can activate T cells in place of the TCR activation that naturally occurs upon the recognition of a peptide-MHC complex [49, 50]. The second and third T cell stimulation signals are most commonly delivered by anti-CD28 antibodies (costimulation) and soluble IL-2 (prosurvival cytokine) [51-53]. The addition of free anti-CD3 and anti-CD28 antibodies for T cell culture fails to provide the simultaneous receptor stimulation necessary for T cell expansion, which has led scientists to bind these signals to various surfaces [54, 55]. The advent of Dynabeads in 1976, magnetizable and superparamagnetic uniform polystyrene spherical beads of five or more microns in diameter, provided such a surface. The signal antibodies are covalently bonded to the spherical beads, and given their magnetic properties, the beads can be easily resuspended or removed from cell culture [56]. The use of magnetic microspheres with conjugated anti-CD3 and anti-CD28 antibodies for ex vivo T cell expansion, with IL-2 culture supplementation, has been utilized in clinical trials [15, 57, 58] as well as the first FDA approved CAR T cell therapy, Novartis’ Kymriah (tisagenlecleucel) [59]. While anti-CD3/anti-CD28 Dynabeads are the standard for translational T cell expansion, there are several limitations to this expansion platform. Bead anti-CD3/anti-CD28 stimulation results in T cells achieving an effector state prior to infusion resulting in diminished anti-tumor efficacy [60], and favors the proliferation of CD4+ helper T cells rather than tumor-killing cytotoxic CD8+ T cells [61]. Furthermore, there is a growing body of work supporting the importance of aAPC shape and surface mobility in optimizing T cell production for ACT [62]. The next section will discuss alterations made to traditional, circular microbeads as well as contemporary T cell expansion platforms like carbon nanotubes and supported lipid bilayers. While each biomaterial platform tries to maximize T cell production in a different way, the underlying principle remains the same – combine the three necessary T cells expansion signals. As the complexity of the acellular aAPCs increases, it is important for researchers to be conscientious of the final goal that is clinical application. Dynabeads are relatively cheap and simple to produce, in addition to being highly reproducible, whereas several of the more recent acellular APCs can be subject to batch to batch inconsistencies, increased materials costs, and prolonged manufacturing procedures. With each new formulation created it is vital that researchers provide scale up and cost analysis, and prove that the platform warrants clinical investigation.
2.3. Advances in Acellular aAPCs
To more closely resemble natural APCs, researchers have strayed away from Dynabeads towards synthetic aAPCs made of different sizes, shapes, and materials. Although previous experimentation revealed that using microbeads less than 4 micrometers in diameter diminished T cell expansion [63]. Perica et. al. found that magnetic 50-100 nm spherical nanoparticles and 30 nm nanocrystals were effective aAPC platforms [64]. In a subsequent study, Perica et. al. proved that in the presence of magnetic fields, nanoparticles are more advantageous for activating T cells than microparticles [65]. This study highlighted the role of nanoscale TCR clustering in T cell expansion – the nanoparticles allow TCR clusters to form on the T cells much like the clusters that form when natural DCs present antigens and signals to T cells [66, 67], whereas microparticles are less conducive to TCR aggregation. Although the immunological synapse formed between APCs and T cells is on the micrometer scale [68], the more recent discovery that TCRs on a given T cell conglomerate to form nanoclusters for T cell activation [69, 70] can be exploited by nanoparticle aAPCs (naAPCs). Hickey et. al. have since confirmed that in a static magnetic field 50 nm aAPCs produce comparable CD8+ T cell expansion to microparticles without magnetic presence (and showed that a magnetic field had no effect on microparticle T cell expansion), but using a reductionist approach and further analysis concluded that 50 nm aAPCs were less effective than larger aAPCs at stimulating T cells under subsaturated conditions [71]. These results indicate that while nanospheres are useful for the study of TCR clustering, they will not displace microbeads as the standard platform for T cell expansion.
It has also been shown that, while keeping particle volume and antigen content held constant, ellipsoidal poly (lactic-co-glycolic) acid (PLGA) microbeads with high aspect-ratio resulted in significantly enhanced in vitro and in vivo CD8+ T cell activity compared with spherical PLGA beads [72]. Meyer et. al. found that PLGA ellipsoidal naAPC significantly outperformed spherical naAPC for all degrees of stretch, or elongation of a single radius (from 1.5 to 3.5-fold). For example, spherical particles induced a 3-fold expansion of T-cells compared to a 15-fold expansion induced by twofold stretch naAPCs [73]. Biodegradable materials, such as PLGA, hold promise as aAPCs not only due to their structural flexibility, but also given their ability for the paracrine delivery of soluble cues to T cells. The use of polymer aAPCs (paAPCs) for the extended release of IL-2 in addition to activation and costimulation of T cells resulted in enhanced CD8+ T cell proliferation by avoiding the T cell exhaustion that can be instigated by the high concentrations of IL-2 required for polystyrene bead culture methods [74]. paAPCs can result in a high concentration of IL-2 at the T cell activation site despite a low overall culture concentration of IL-2. A distinct disadvantage of biodegradable aAPCs is the loss of surface-bound molecules over time, but conversely this can be a benefit to prevent the over stimulation of T cells – further investigation will be needed to determine the optimal degradation rate of these platforms.
Carbon nanotubes and elongated “nanoworms” have emerged as two other potent T cell expansion structures. Carbon nanotubes and flexible, water-soluble polymer nanoworms provide increased surface area for T cell binding and the clustering of surface receptors. In 2008, Fadel et. al. proved that carbon nanotubes decorated in T cell signaling moieties resulted in more robust T cell stimulation than other high surface area materials [75], and have since matured the platform into a carbon nanotube–polymer composite (CNP) that combines the carbon nanotube surface with magnetite-loaded PLGA nanoparticles that allow for the paracrine delivery of IL-2 [76] and the magnetic extraction of the composite. Traditional Dynabead expansion required a 1000-fold greater concentration of IL-2 than the CNP to produce a comparable level of cytotoxic T cells, and the CNP produced a significantly higher percentage of less differentiated T cells as determined by the presence of the CD27+ marker [59, 77, 78]. The nanoworm platform consists of a poly(isocyano peptide) backbone with functionalized side chains presenting signal molecules, and due to the semi-stiff backbone, was proven to allow TCR clustering to occur readily and cause all the effector molecules on the nanoworm to bind to receptors on the same cell [79]. The elongated structures mark a shift from ellipsoidal and spherical synthetic aAPCs, and indicate an enhanced exploitation of TCR clustering. The nanoworm and nanotube formulations use their pronounced aspect ratio and unique nanoscale topography to promote TCR clustering, which was shown to improve T cell expansion rates. Whereas nanospheres require saturated conditions and the administration of magnetic fields to present T signals in a clustered presentation, the elongated nanostructures can duplicate the T cell activation milieu inherently due to their shape. Further investigation into the use of these nanostructures is warranted, and determining the importance of backbone flexibility in enhancing T cell expansion should be a focus.
The cell membrane, where receptor binding occurs, is composed a lipid bilayer. Thus, to more closely resemble nature, aAPCs composed of lipid bilayers, in the form of liposomes and 2D/3D supported lipid bilayers (SLBs), have been studied. The concentration of MHC molecules on lipid rafts, microdomains on the plasma membrane with high concentrations of cholesterol and glycosphingolipids [80], facilitate antigen presentation [81]. Raft microdomains that contained epitope/MHC complexes were isolated from DCs and reconstituted on liposomes, named RAFTsomes, then successfully expanded CD4+ cells and elicited a strong immune response in an OVA-expressing E.G7 tumor model [82]. In another study, biotinylated monoclonal antibodies preclustered in microdomains held on a liposome scaffold by neutravidin rafts improved polyclonal T cells and MART-1-specific CD8+ T cells expansion compared to traditional microbeads [83]. Liposomes provide fluidity that is conducive for enhanced T cell expansion but lack stability when compared to solid particles [46] and struggle to recreate the immunological synapse, which has led to a focus on SLBs. SLBs are composed of lipid bilayers bonded to a solid flat surface (2D) or a solid scaffold core (3D). 2D SLBs, typically utilizing glass as the solid surface, are powerful tools for studying T cell activation and the immunological synapse [84, 85], but the level, continuous surface fails to recreate the natural T cell – APC interaction resulting in subpar T cell expansion rates. This year Cheung et. al. mimicked APCs by creating a mesoporous silica micro-rod supported fluid lipid bilayer that presented anti-CD3 for TCR stimulation and anti-CD28 for costimulation as membrane bound signals and allowed for the sustained release of soluble signals, namely IL-2 [86]. The novel 3D SLB platform, termed APC-ms for APC-mimetic scaffolds, can be tailored to present the signals in spatiotemporal manner closely resembling natural APCs. The APC-ms promoted up to a tenfold greater polyclonal expansion of mouse and human T cells compared to commercially available Dynabeads, maintained a substantial CD8+ biased skewing, and the CAR T cells expanded by APC-ms showed high in vivo functionality in a disseminated xenograft model of Burkitt’s lymphoma. Furthermore, whereas Dynabeads need to be removed, the evaluation of silicon content in cell culture pellets confirmed the APC-ms degrades prior to cell re-infusion.
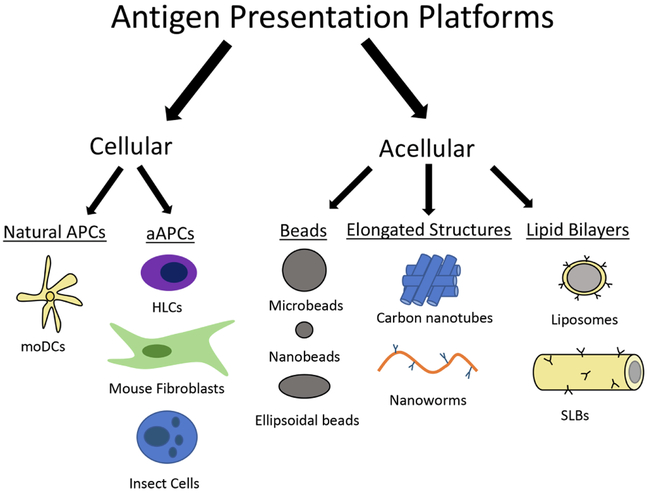
aAPCs are at the interface of T cell immunology and biomaterials, requiring a deep understanding of both fields for the creation of improved T cell expansion platforms. From the advent of Dynabead aAPCs, to naAPCs, elliposoidal aAPCS, paAPCs, and most recently APC-ms (Fig. 2), new discoveries in T cell biology can be incorporated into biomaterials and result in enhanced T cell expansion. Engineered biomaterials provide investigators full autonomy over the presentation of signals to T cells, and thus represent a promising method for achieving the T cell expansion necessary for successful ACT immunotherapy. As research continues, a focus on producing CD8+ T cells of a less differentiated state, rather than a sole focus on producing CD8+ T cells over CD4+ T cells, will likely result in more effective treatments. Research on producing younger T cells for ACT has focalized on preventing T cells differentiation, using molecular inhibitors or cytokines such as IL-7 and IL-15 [87], but these approaches require naïve, non-specific T cells. Emulating Yamanaka and Gurdon’s Nobel Prize for inducing pluripotent cells from mature cells, induced stem cell memory T cells have been generated [88]. The reversion of effector T cells provides a new mechanism for achieving high expansion rates of antigen specific, stem cell memory T cells. The next generation of aAPCs may look to combine the trend towards APC mimicry using biomaterials with the ability to induce stem cell memory T cells. While aAPCs have been investigated for three decades, recent studies involving the recreation organs in vitro are providing additional pathways for expanding T cells, as briefly reviewed next.
Fig. 2. Summary of antigen presentation platforms.
APCs= antigen presenting cells; aAPCs = artificial APCs; moDCs = monocyte originated dendritic cells; HLCs = human leukemic cells; SLBs = supported lipid bilayers
2.4. Organoids Supporting T Cell Expansion
Organoids, or 3D multicellular in vitro organ models, are biomaterials that present a unique avenue for the improved expansion of immune cells, including B cells [89] and T cells. Their three-dimensional nature has facilitated a variety of studies, including the development of neuronal networks among brain cells, the interactions between different types of cells [90], such as the interaction between intraepithelial lymphocytes and intestinal epithelial cells [91], and the gene and pathway alterations associated with the progression of pancreatic cancer [92]. However, in this review, we will focus on the use of organoids to facilitate the generation and expansion of human T cells.
Organoids have also been used to assist in the modeling of the tumor microenvironment and the study of the tumor T cell repertoire in patients. Neal et.al. developed a method to extract patient-derived organoids (PDOs) in a manner that preserved tumor-infiltrating immune cells. Using these PDOs, they were able to expand and activate tumor antigen-specific TILs and demonstrate tumor cytotoxicity [93]. This promising method could potentially facilitate the generation of patient-specific antitumor T cells. Dijkstra et.al. presented an alternative, organoid-based method for the generation of patient-derived TILs. In this report, tumor-reactive T cells were generated from peripheral blood lymphocytes after coculture with autologous tumor organoids. Remarkably, after two weeks of coculture, expanded T cells were shown to react solely with the tumor organoid used in the coculture (mismatch repair-deficient colorectal cancer), and had no reactivity towards organoids derived from another form of colorectal cancer (mismatch repair-proficient colorectal cancer) [94].
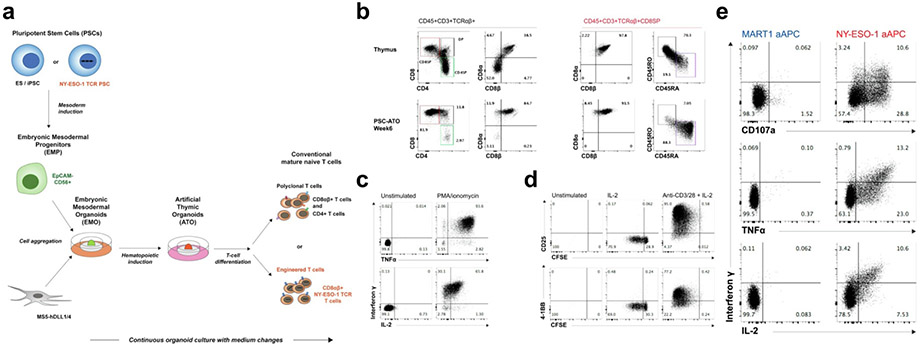
Recently, Montel-Hagen et.al. described a continuous 3D organoid system that induced the differentiation of pluripotent stem cells (PSCs) into terminally differentiated CD3+CD8αβ+ and CD3+CD4+ T cells bearing a diverse TCR repertoire (Fig. 3a) [95]. As shown in Fig. 3b, T cells that were generated from PSCs in the presence of an artificial thymic organoid (ATO) were shown to have a conventional T cell phenotype (CD8αβ+), and were shown to be mature, naive T cells (CD45RA+CD45RO−). These newly generated T cells were able to secrete TNFα, IFN-γ and IL-2 upon stimulation, as well as proliferate in the presence of anti-CD3, anti-CD28 and IL-2 - demonstrating a functional similarity to traditionally expanded T cells (Fig. 3c, 3d). The authors also demonstrated that PSCs transduced with engineered TCR transgenes (specific for NY-ESO-1 ligand) were also able to be expanded with the use of the athymic organoid, while maintaining their functionality as well as antigen specificity (Fig. 3e).
Fig. 3. Artificial thymic organoids for the differentiation of naive or engineered T cells from PSCs.
a) Schematic of workflow for the differentiation of polyclonal or engineered T cells using artificial thymic organoids. b) Flow cytometry plots showing the differences between T cells from the thymus and T cells differentiated from PSCs after six weeks of culture in ATOs. c) Flow cytometry plots demonstrating the newly generated T cells’ ability to produce interferon γ, TNFα, and IL-2 upon stimulation. d) Flow cytometry plots demonstrating the newly generated T cells’ ability to expand upon stimulation with anti-CD3 and anti-CD28 in the presence of IL-2. e) Flow cytometry plots showing antigen-specific activation of the newly generated T cells. a)-e) are reprinted with permission from Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells, Montel-Hagen, A., Seet, C.S., Li, S., Chick, B., Zhu, Y., Chang, P., Tsai, S., Sun, V., Lopez, S., Chen, H-C., He, C., Chin, C.J., Casero, D., Crooks, G.M., Cell stem cell. 2019;24:376-89 e8 [95].
Although further studies must be conducted to assess the feasibility of widespread usage of organoids to generate patient-derived TILs, tumor organoids have the potential to revolutionize the field of TIL therapy by providing the means to dramatically improve TIL expansion ex vivo. The work of Montel-Hagen et.al. lays the foundation for the use of organoids in the large scale generation of engineered, antigen-specific T cells from PSCs – providing an alternative to traditional production methods for T cell therapies. Whether its using organoids to expand TILs using tumor samples or PSCs, there is a clear role for the use of organoids in the expansion of T cells for ACT. In concordance with the use of biomaterials in T cell expansion, biomaterials have been studied for enhancing T cell therapy in vivo. As discussed next, in the form of nanoparticles, small molecular compounds, and scaffolds, biomaterials have the potential to improve the treatment efficacy of T cell therapy through several avenues.
3. Enhancing T Cell Therapy Efficacy
In addition to improving the rate of T cell expansion for ACT in vitro, biomaterials can also play an important role in enhancing the functionality of T cell therapies in vivo. As shown in Table 1, there are several approaches in which biomaterials augment T cell therapy. Biomaterials’ ability to protect drug payloads prior to release and prevent premature drug activity has motivated further research into their use as drug delivery vehicles. As we discuss in this section, the in vivo payload protection and targeting capabilities of current biomaterials are frequently leveraged to enhance drug delivery in vivo. One method in which biomaterials can improve T cell therapy is by allowing for the targeted delivery of immune-modulating pharmaceuticals. Due to the often toxic nature of cancer treatments, it is necessary to ensure the targeted delivery of therapeutic compounds to tumor cells and avoid systemic toxicity. Furthermore, the therapeutic must be protected from degradation, clearance, and off-target delivery prior to reaching its intended destination. The study of nanoparticles has resulted in the development of many safe and effective drug delivery vehicles. Their design [96] and successes [97] are discussed in other reviews. Previous research has exploited the enhanced permeability and retention (EPR) effect to deliver untargeted, drug-loaded nanoparticles to tumor cells [98, 99]. The EPR effect is a mechanism by which nanoparticles are able to accumulate in tumor tissue due to the tumor's abundant vasculature and exclusion of immune cells. Numerous groups have employed the preferential tumor targeting of the EPR effect to deliver nanoparticles, including Wei et.al., who used nanomicelles to delivery doxorubicin to breast cancer cells [100], and Liu et.al., who developed crosslinked, multilamellar liposomal vesicles (cMLVs) for the co-delivery of doxorubicin and paclitaxel [101]. However, successful delivery of a single therapeutic is often insufficient due to the generation of drug-resistant cancer cells [102] and antigen-loss variants [103, 104]. Additional steps must be taken to ensure a lasting antitumor effect by recruiting and facilitating the help of immune cells, and the recent advances in nanoparticle research provides a promising route for this to occur.
Table 1.
Methods for Enhancing T Cell Therapy Efficacy
| Method | Biomaterial | Reference |
|---|---|---|
| Targeted nanoparticle delivery | Antigen-encapsulating nanoparticles presenting IL-15:IL-15Rα | [116] |
| PEGylated liposomes with anti-CD137 and/or IL-2Fc anchored to their surfaces | [117, 118] | |
| Nanolipogels delivering IL-2 and a TGF- β receptor inhibitor | [121] | |
| Liposomes targeted to CD90, an internalizing receptor on T cells, to deliver a TGF-β receptor inhibitor | [124] | |
| PLGA/PEG nanoparticles to deliver a TGF-β receptor inhibitor (SD-208) to exhausted T cells expressing PD-1 | [125] | |
| PEG-histidine-modified alginate to encapsulate CpG oligodeoxynucleotides (ODN), anti-IL-10 ODN, and anti-IL-10 receptor ODN targeting TAMs | [130] | |
| Imatinib-loaded nanoparticles decorated with a peptide targeting Treg cells | [133] | |
| Backpacking nanoparticles | cMLVs containing paclitaxel (chemotherapeutic) or SCH (an A2a adenosine receptor antagonist) conjugated to NK cells | [139, 140] |
| SN-38 (chemotherapeutic) loaded nanocapsules conjugated to T cells | [141] | |
| Nanoparticles loaded with an inhibitor of Shp1 and Shp2 conjugated to T cells | [143] | |
| Protein nanogels containing IL-15 super-agonist (IL-15 Sa) complex conjugated to T cells | [144] | |
| Biomaterials delivering molecular immunotherapies | Alginate matrices to deliver IL-15Sa | [147] |
| Chitosan coformulated with IL-12 for IL-12 delivery | [148] | |
| Hydrogels to deliver either R848, a Toll-like receptor 7/8 agonist, or STING-RR | [149] | |
| Gelatin cryogel delivering granulocyte-macrophage colony-stimulating factor | [150] | |
| Polyglyconate/gelatin scaffold to deliver CCL17 | [151] | |
| PEG-heparin cryogels to deliver engineered mesenchymal stromal cells (MSCs) that secrete an anti-CD33-anti-CD3 bispecific antibody | [154] | |
| Biomaterial scaffolds delivering T cells | Thermoreversible PEG-g-chitosan hydrogel | [159] |
| Chitosan, sodium hydrogen carbonate, and phosphate buffer thermogel | [160] | |
| Polymerized alginate macroporous scaffold with synthetic collagen-mimetic peptide and porous silica microparticles | [161] | |
| Hyaluronic acid-based low viscosity hydrogel | [163] | |
| CAR T Cell Therapy | Synthetic DNA nanocarriers with a CD3ε-targeting antibody fragment to deliver CAR DNA to circulating T cells | [170] |
| Protease activated probody to mask CAR T cells and prevent on-target off-tumor effects | [176] | |
| CAR T cells engineered to secrete cytokines | [177-180] | |
| Liposomes to make the tumor microenvironment more hospitable to CAR T cells | [182] |
Although immune cells have a vital role in killing tumor cells, additional barriers due to the immunosuppressive tumor microenvironment (TME) can prevent immune cells from functioning at full capacity. The TME facilitates many different avenues for the inhibition of T cell activation including maintaining a hypoxic environment [99, 105], and producing excessive lactate, which can hinder export of intracellular lactate by T cells [106, 107]. Tumor cells can also employ other modes of immune suppression by recruiting suppressive immune cells that secrete anti-inflammatory cytokines such as IL-10 and TGF-β, and further deplete the microenvironment of metabolites necessary for T cell function [108]. The immunosuppressive TME presents a unique obstacle that must be considered when developing cancer immunotherapies. This section discusses the research done to deliver small molecules, cytokines, and other immune cell function-supporting molecules to T cells in order to circumvent the immunosuppressive TME and promote antitumor effects. This section also reviews biomaterials that aid in the direct, local delivery of T cells and biomaterials that work in conjunction with CAR T cells.
3.1. Targeted Nanoparticle Delivery
Cytokines play a vital role in T cell function. For instance, IL-2 is responsible for T cell proliferation [20, 109] and IL-15 regulates T cell activation, proliferation, and survival [110, 111]. Although previous attempts at systemically administering IL-2 to cancer patients resulted in the expansion of lymphoid cells, it also demonstrated rapid clearance of the cytokine and significant side effects [112]. A later study involving the high dose administration of IL-2 to metastatic melanoma patients achieved an objective response rate of 16%, but still reported side effects in over half of the patients [113]. IL-2 treatment has also been shown to increase the frequency of immunosuppressive regulatory T cells in patients with melanoma or renal cell carcinoma [114]. Similarly, Berger et.al. showed that IL-15 therapy, though able to expand memory CD8+ and CD4+ T cells, was still subject to a high clearance rate and resulted in transient toxicity in nonhuman primates [115]. These results indicate that cytokine therapies must be administered in a targeted manner in order to limit side effects and have a higher antitumor efficacy.
The high clearance rate and complications with untargeted delivery make cytokines ideal candidates for nanoparticle-based delivery. A study of antigen-encapsulating nanoparticles presenting IL-15:IL-15Rα demonstrated a marked improvement in the life spans of mice bearing OVA-expressing B16 tumors compared to mice receiving antigen-encapsulating nanoparticles and free IL-15:IL-15Rα [116]. In a similar manner, intratumoral injections of PEGylated liposomes with anti-CD137 and IL-2Fc anchored to their surfaces were shown to slow the growth of B16-F10 tumors in vivo while mitigating the toxicity associated with systemic administration of anti-CD137 and IL-2 [117]. In a related study, Zheng et.al. reported that intravenous injections of liposomes with surface IL-2Fc molecules after adoptive cell transfer resulted in a fourfold improvement in the expansion of adoptively transferred T cells [118].
Although transforming growth factor β (TGF-β) is a cytokine that regulates cell proliferation in healthy cells, mutations in the TGF-β pathway in cancer cells result in uncontrolled proliferation and immune evasion [119, 120]. Intratumoral injections of nanolipogels (nLG) delivering IL-2 and a TGF- β receptor inhibitor (SB505124), were shown to significantly inhibit tumor growth and prolong survival in a mouse metastatic melanoma model. Furthermore, intratumoral injections of nLG SB505124, IL-2, or both compounds increased the amount of tumor infiltrating CD8+ T cells and natural killer (NK) cells compared to injections with an empty nLG [121]. However, there have also been reports of systemic administration of TGF-β receptor inhibitors resulting in cardiac toxicity [122, 123], suggesting that TGF-β receptor inhibitors are a promising form of cancer therapy that demonstrate a need for targeted delivery to mitigate any potential off-tumor side effects. Zheng et.al. used liposomes targeted to CD90, an internalizing receptor on T cells, to deliver a TGF-β receptor inhibitor (SB525334) to adoptively transferred T cells. The targeted liposomes were shown to inhibit tumor growth compared to nontargeted liposomes. Interestingly, liposomes targeted to CD45, a noninternalizing T cell receptor, did not inhibit tumor growth as well as those liposomes targeted to CD90 - suggesting that targeting an internalizing receptor can result in the enhanced uptake of delivered drugs [124]. Schmid and coworkers employed a similar strategy, using PLGA and polyethylene glycol (PLGA/PEG) nanoparticles to deliver a TGF-β receptor inhibitor (SD-208) to exhausted T cells expressing programmed cell death protein 1 (PD-1). Treatment of C57BL/6 mice bearing MC38 tumors with PD-1 targeted nanoparticles containing SD-208 was shown to significantly inhibit tumor growth and prolong overall survival compared to treatment with untargeted SD-208-loaded nanoparticles [125].
The use of nanoparticles to support T cell function is not limited to therapies directed towards cytokine signaling. Targeting cells that are responsible for maintaining the immunosuppressive tumor microenvironment has also been an active area of research. For instance, tumor associated macrophages (TAMs) are known to inhibit T cell function [126] as well as contribute to angiogenesis and tumor invasion [127-129]. To combat TAMs, Huang et.al. used PEG-hisitidine-modified alginate (PHA), a pH-sensitive material, to encapsulate CpG oligodeoxynucleotides (ODN), anti-IL-10 ODN, and anti-IL-10 receptor ODN. TAM targeting was achieved by associating ODNs with galactosylated cationic dextran, which is known to bind to Mg1 - a lectin that is highly expressed on TAMs. This strategy proved to be successful by reducing the expression of IL-10 receptor threefold, changing the TAM phenotype, and achieving a twofold inhibition of tumor growth in vivo [130]. Regulatory T cells (Treg) are vital to the prevention of autoimmune disease, but are also another type of immune cell that inhibits T cell function and facilitates tumor evasion [131, 132]. In a recent study, Ou and coworkers targeted Treg cells with imatinib-loaded nanoparticles decorated with a peptide targeting motif. They observed that delivering imatinib to Treg cells resulted in enhanced tumor inhibition and the increased presence of intratumoral CD8+ T cells [133].
An alternative strategy to elicit T cell-mediated antitumor responses centers on the activation of T cells in a cytokine-free manner. T cell activation requires antigen binding to the T cell receptor (TCR), and a simultaneous costimulatory signal, typically from binding to the CD28 or 4-1BB (CD137) receptors on the T cell. However, activated T cells can become exhausted upon its PD-1 receptor binding to programmed death-ligand 1 (PD-L1) [134, 135]. Although PD-L1 expression on healthy cells prevents autoimmune diseases, its expression on cancer cells results in the evasion of tumor specific T cells. A recent study described the use of nanoparticles coated with two different antibodies that are specific for 4-1BB and PD-L1 (immunoswitches), resulting in T cell stimulation while simultaneously preventing PD-1-mediated T cell exhaustion. Intratumoral injection of immunoswitches were also found to inhibit the growth of B16-SIY tumors in C57BL/6 mice, both in the presence and absence of adoptively transferred CD8+ T cells [136]. Another approach involved redirecting CTLs towards tumors cells with antigen-specific T cell redirectors (ATR) - nanoparticles decorated with moieties binding to antigen-specific T cells and tumor cells. In this study, Schutz et.al. used either peptide-loaded MHC complexes or a clonotypic anti-T cell receptor (TCR) antibody as the T cell binding moiety, and anti-human CD19 antibody as the tumor binding moiety and showed that ATRs could effectively facilitate the killing of CD19+ Raji tumor cells by T cells lacking anti-CD19 TCRs, both in vitro and in vivo [137].
Targeted delivery of therapeutics to tumors has been shown to facilitate the "reawakening" of T cells within the tumor microenvironment. Nanoparticles can help increase the presence of pro-immune cytokines and molecules within the tumor by protecting them from degradation and clearance during the delivery process. Currently, nanoparticle research has shifted its sights from creating protective nanoparticles to developing innovative methods to direct nanoparticles and their payloads to specific cells within the tumor microenvironment in a targeted fashion.
3.2. Backpacking Nanoparticles
The concept of backpacking involves conjugating nanoparticles to immune cells in order to promote the successful delivery of a therapeutic to the "backpacked" immune cell in a pseudoautocrine manner. This strategy utilizes the abundance of free thiol groups on the cell surface as a means to conjugate nanoparticles bearing surface maleimide groups. It was first developed by the Irvine group to deliver adjuvants to cells commonly used in cell therapy, including CD8+ T cells and hematopoietic stem cells [138], but this method has since been adapted to attaching differing payloads to immune cells.
For instance, NK cells with a chimeric antigen receptor (CAR) targeting CD19 have been used to deliver cMLVs containing paclitaxel (cMLV PTX), a chemotherapeutic agent, to SKOV3 CD19 tumors in NSG mice. In this study, Siegler et.al. demonstrated the importance of conjugating cMLV (PTX) to the CAR NK cells by showing that mice receiving coadministered cMLV (PTX) and CAR NK cells had significantly larger tumor volumes than those receiving cMLV (PTX) directly conjugated to CAR NK cells. Furthermore, they observed that the targeted nature of PTX delivery allowed for a decreased dose of PTX and by extension, minimal side effects associated with PTX therapy [139]. Similarly, Siriwon and coworkers conjugated cMLV containing SCH (cMLV SCH), an A2a adenosine receptor antagonist, to CD19-targeting CAR T cells. Again, the antitumor efficacy of cMLV SCH conjugated to CAR T cells was shown to be substantially higher than coadministration of cMLV SCH and CAR T cells in NSG mice with SKOV3 CD19 tumors. Additionally, conjugation of cMLV SCH to CAR T cells was shown to significantly reduce intratumoral CAR T cell hypofunction and nearly double the amount of tumor-infiltrating CAR T cells compared to the unconjugated combination therapy [140].
Similar to paclitaxel, SN-38 is a chemotherapeutic agent that is known to have poor pharmacokinetic properties. The conjugation of SN-38 - loaded nanocapsules to T cells was shown to promote SN-38 mediated killing of lymphoma cells in vitro and in vivo. This delivery strategy exploited the innate ability of T cells to traffic to lymphoid organs to target disseminated lymphoma tumors. Compared to systemically administered SN-38, cell-mediated delivery of SN-38-loaded nanocapsules was reported to increase the concentration of SN-38 in lymph nodes by 90-fold, and could successfully prolong the survival of C57BL/6 mice with disseminated lymphoma tumors [141]. Jones et. al. showed that conjugating drug-loaded lipid nanoparticles to cytotoxic T lymphocytes (CTL) allowed the contents to be delivered to cells targeted by the CTLs in vivo. Drug release was triggered by the release of perforins from CTL activation, which was shown to result in the killing of targeted cells [142].
Researchers have also used nanoparticles to backpack molecules that can promote T cell function. For example, Stephan et. al. showed that the conjugation of nanoparticles loaded with an inhibitor of Shp1 and Shp2 to T cells prior to adoptive transfer resulted in a fourfold enhancement of T cell expansion at the tumor site [143]. In another study, Tang et.al. employed this backpacking strategy to conjugate and deliver protein nanogels containing IL-15 super-agonist (IL-15 Sa) complex to T cells. These nanogels relied upon the increase in T cell surface reduction potential following antigen binding to release their cargo. This novel release mechanism allowed for the transport of protein therapeutics to the tumor microenvironment and for antigen recognition to trigger cargo release, resulting in a 16-fold higher expansion of T cells in tumors compared to treatment with soluble IL-15Sa, and the ability to deliver eightfold higher doses of cytokines, while avoiding toxicity associated with systemic administration. Additionally, compared to CAR T cell therapy alone, IL-15 Sa nanogels were able to substantially improve the efficacy of EGFR-targeting CAR T cells and improve the survival of NSG mice with adoptively transferred human T cells and U-87 MG tumors [144].
Backpacking has the potential to provide even more control over the release of the drug payload by the inclusion of stimuli-responsive nanoparticles, such as light-responsive or temperature-responsive nanoparticles. However, additional research must be conducted in order to evaluate their efficacy. Nonetheless, backpacking is a unique workaround that bypasses the need to provide a targeting moiety to nanoparticles, while still maintaining their ability to protect the drug payload. This promising delivery method has the potential to enhance the efficacy of adoptive cellular therapies by providing the stimuli needed to promote immune cell activation and proliferation, while counteracting immune suppressive signals in the tumor microenvironment.
3.3. Biomaterials delivering molecular immunotherapies
Biocompatible scaffolds have also been investigated as a drug delivery vehicle. Current T cell therapies require the large bolus systemic administration of cytokine IL-2, which can cause cytokine release syndrome (CRS) and high toxicity in patients [145, 146]. As a result, many groups have developed biocompatible scaffolds for the sustained release of molecules such as IL-2 to enhance the effects of immunotherapy.
Injectable hydrogels have been used to deliver cytokines to tumor sites. A study used alginate matrices to deliver IL-15Sa to B16-OVA tumors and showed that peritumoral injections of IL-15Sa-carrying gels achieved about 40-fold higher concentrations of IL-15Sa in the tumor site than systemically administered IL-15Sa and suppressed tumor growth [147]. In an orthotopic bladder cancer mouse model, intravesical treatment with chitosan/IL-12 (IL-12 coformulated with chitosan) cured 88% to 100% of mice, compared to 38% to 60% of mice treated with IL-12 alone. Mice treated with chitosan/IL-12 were also shown to have durable protection upon intravesical tumor rechallenge [148]. Hydrogels have also been used to prevent tumor recurrence at tumor resection sites. In a recent report, Park et.al. used hydrogels to deliver either R848, a Toll-like receptor 7/8 agonist, or STING-RR, a stimulator of interferon genes (STING) agonist, to tumor resection sites and demonstrated the importance of hydrogel delivery. A majority of the mice receiving hydrogel-delivered R848 or STING-RR (60% - 70%) survived beyond 90 days, while mice receiving local delivery of either agonist had a median survival of under 60 days [149].
Biomaterials have also been used to deliver cytokines for the purpose of attracting host immune cells. The sustained release of granulocyte-macrophage colony-stimulating factor (GM-CSF) from gelatin cryogels implanted into C57BL/6J mice was shown to attract about 20 times more immune cells than a blank cryogel [150]. Another study reported using a polyglyconate/gelatin scaffold to deliver CCL17, a chemoattractant for CCR4+ CD8+ T cells, to pancreatic cancer cells in vivo. The CCL17-eluting scaffold inhibited tumor growth and prevented the metastasis of pancreatic cancer cells to the liver, while the non-eluting CCL17 scaffold was shown to be nonfunctional in both of these regards [151].
Researchers are constantly striving to develop novel biomaterials with expanded drug delivery capabilities. Wen et. al. describe the use of EAK16-II peptides to generate an in situ-forming system capable of enhancing the local retention of antibodies and delaying antibody clearance. Structure assembly begins when the EAK16-II and EAKH6 peptides self-assemble into beta sheet bilayers. Upon beta sheet formation, the histidine tags on the EAKH6 peptides are exposed and recognized by an anti-histidine tag antibody, which is also recognized by Protein A/G. The avidity of Protein A/G allows for the binding and retention of any nearby antibody that the structure encounters. The self-assembled system was shown to improve the in vivo retention of intratumorally injected, dye-labeled antibodies by at least three days - demonstrating the potential benefits of this system when used in conjunction with checkpoint inhibitor therapies like anti-PD-1 and anti-CTLA-4 [152]. A recent study described the use of βTail tags as a potential material for the delivery of therapeutic proteins at precise ratios. Fusion proteins containing βtails were shown to coassemble into fibrils when incubated with beta sheet fibrillizing peptides. Using fluorescent proteins, Hudalla et. al. showed that this preparation method allows for the precise control of the ratio of βTail-tagged therapeutic proteins delivered by each fibril, which could potentially mitigate the side effects associated with high dosages of molecular immunotherapies [153].
As discussed below, biomaterials can also be used to deliver engineered cells to support cancer immunotherapy. Aliperta et.al. used star-shaped PEG-heparin cryogels to deliver engineered mesenchymal stromal cells (MSCs) that secrete an anti-CD33/anti-CD3 bispecific antibody for the treatment for acute myeloid leukemia (AML). This strategy was shown to effectively activate T cell-mediated antitumor responses by activating T cells and bringing them into contact with CD33+ AML blasts [154].
3.4. Biomaterial scaffolds delivering T cells
Biomaterials in the form of scaffolds can be used to deliver not only T cell enhancing drugs but also adoptive T cells themselves. Scaffold can provide a means for increasing the efficacy and longevity of T cell therapies while diminishing toxicity associated with the large intravenous dosage of T cells as well as off-tumor reactivity. Similar to synthetic aAPCS, biomaterial scaffolds for enhanced T cell therapy allow the controlled delivery of soluble cues to a target area.
A broad range of materials can be used to create ACT enhancing scaffolds, and each formulation has its own biocompatibility, biodegradability, structural integrity, porosity, and rigidity – factors that play essential roles in the activation and structural support of T cells [155]. Thus far the investigated T cell depots for cancer immunotherapy are hydrogels composed of chitosan, polymerized alginate, or hyaluronic acid. Hydrogels are three-dimensional, cross-linked structures of hydrophilic polymers swollen in water or biological fluids [156], and have received considerable attention over the past 50 years for application in a wide range of fields [157]. The past two decades have seen a rise in the use of hydrogels for immunomodulation, with a focus on enhancing cancer vaccines [158], and are only more recently under investigation for the improvement of T cell therapies. In 2014, Tsao et. al. created a biodegradable, thermal reversible hydrogel made of poly(ethylene glycol)-g-chitosan (PCgel), which was proven to be a suitable reservoir for the harbor and release of T lymphocytes for brain tumor immunotherapy [159]. PCgel is a liquid at low temperatures and forms a gel at body temperature, allowing for the steady release of viable T lymphocytes to a brain tumor without surgical intervention. Anti-EGFR (epidermal growth factor receptor) CAR T cells released from the PCgel retained increased antiglioblastoma activity compared those delivered in Matrigel, and PCgel is clinically preferable over Matrigel and other animal-sourced gels because of its biocompatibility, biodegradability, low immunogenicity, and low cost. Despite the promising preliminary data, PCgel for T cell immunotherapy has yet to be verified in a preclinical model. Two years following the work done by Tsao et. al., a similar chitosan-based thermogel was investigated for the in situ encapsulation and gradual release of cytotoxic T lymphocytes [160] (Fig 4a). The chitosan, sodium hydrogen carbonate, and phosphate buffer thermogel has highly desirable mechanical and biocompatibility properties for the local administration of T lymphocytes through a catheter or needle. The optimized gel solution solidifies rapidly upon injection into mice, and in vitro studies confirmed the sustained migration of cancer fighting T cells from the thermogel.
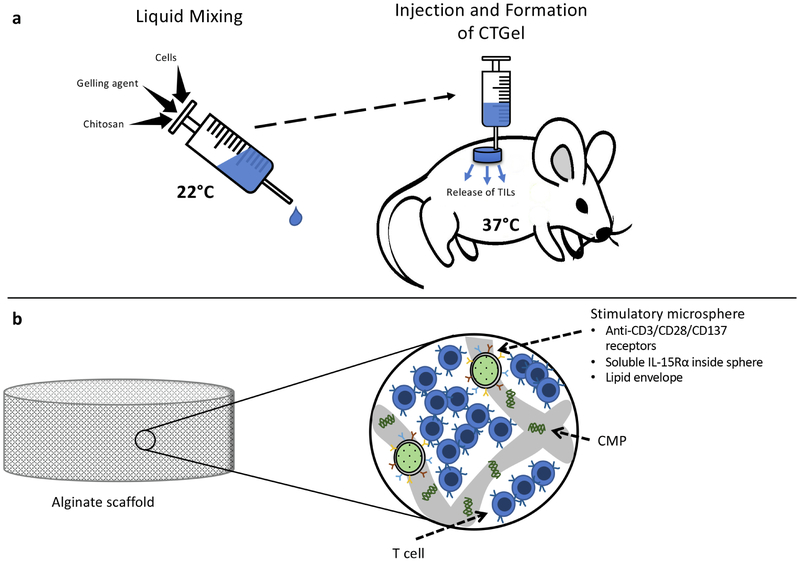
Fig. 4. Scaffolds delivering T cells for enhanced ACT therapy.
a) Monette et. al. combined chitosan, gelling agent (sodium hydrogen carbonate in phosphate buffer), and cell culture medium to create an injectable chitosan-based thermogel (CTGel) that maintains the extended release of cytotoxic T lymphocytes. At room temperature the components mix in solution, and upon injection into the thermogel solidifies, creating a readily administered T cell reservoir. In vitro studies verified the gradual discharge of desirable T cells from the CTGel. b) T cells are seeded onto Stephen et. al.’s polymerized alginate macroporous scaffold, which are surgically implanted into a tumor resection bed or near an inoperable tumor site. A synthetic collagen-mimetic peptide (CMP) is used to bind T cells to the scaffold, and porous silica microparticles presenting membrane-bound ligands anti-CD3, anti-CD28 and anti-CD137 and loaded with soluble interleukin-15 superagonist (IL-15Rα) are added into the scaffold void space. Degradation of the scaffold results in the sustained release and enhanced proliferation of robust antitumor T cells [160].
Stephan et. al. created a macroporous scaffold from polymerized alginate that binds T cells via a synthetic collagen-mimetic peptide and contains porous silica microparticles in the scaffold void spaces [161] (Fig 4b). The microparticles, similar to the liposomes used as aAPCs, provide for the encapsulation and sustained release of soluble biomolecules, and can provide membrane-bound signals mimicking nature when coated with lipid bilayers. In their study, interleukin 15 superagonist was used as the soluble factor and the membrane-bound ligands were anti-CD3, anti-CD28 and anti-CD137 antibodies coupled to the microsphere bilayer. Integrating the microparticles into the modified alginate scaffold resulted in a 22-fold jump in T-cell proliferation and a 8.3-fold increase in T cell migration into the surrounding collagen gel, and additional testing proved the scaffold-released T cells have improved antitumor cytotoxicity and reduced apoptotic susceptibility. The efficacy of the T cell infused scaffolds were tested in a 4T1 mouse breast tumor resection model and an ovarian carcinoma unresectable tumor model. The staggering results revealed that in the 4T1 model, when compared to T cells injected intravenously or directly into the resection bed as well T cells expanded ex vivo with IL-15 superagonist and anti-CD3, anti-CD28 and anti-CD137 antibodies before local injection, none of the mice receiving the T cell bearing scaffolds experienced relapse whereas relapse and subsequent death occurred in all other mice. Scaffold-delivered T cells proliferated to numbers 167-fold higher than locally injected, prestimulated T cells in the tumor resection bed, and despite the high expansion rates maintained a nonexhausted phenotype. Furthermore, the scaffold-delivered T cells migrated to tumor-draining lymph nodes (TDLNs) in significantly higher levels and took on a central memory phenotype in the TDLNs. In the unresectable tumor model, the scaffold-delivered T cells significantly increased survival time, with the disseminated cancer eradicated in 6 of the 10 mice and the other 4 mice averaging 27 days longer survival. Further studies proved the CAR T cells migrate from the scaffold and eliminate tumors more efficiently than systemic delivery of the same cells in pancreatic and melanoma models, and the codelivery of additional agonists from the scaffold improves immune response [162].
Recently, a hyaluronic acid-based low viscosity hydrogel (LVHydrogel) was developed as a novel carrier for convection enhanced delivery (CED) of CAR T cells [163]. Although the blood-brain-barrier limits the accessibility of pharmacological agents to the central nervous system from the bloodstream [164], CED uses a needle/catheter coupled with a positive pressure pump to provide the steady flow of infusates directly into the brain parenchyma [165] and results in significantly (up to 8 times) greater distribution of an agent than delivery from a single direct intracranial injection [166]. Numerous studies and clinical trials have been performed using CED and a variety of therapeutics to treat central nervous diseases [167], but when tasked with dispatching cells, CED has a low efficacy, largely due to the sedimentation of the cells in traditional CED carrier (phosphate buffer saline) [163]. LVHydrogel was demonstrated to be a viable option for the CED of CAR T cells for glioblastoma immunotherapy, as it did not cause acute toxicity in preclinical mouse models, prevented cellular sedimentation during CED, and enhanced post-infusion migration and cytotoxicity profiles of the CAR T cells.
These studies have shown that scaffolds possess the unique ability to store, deliver, and stimulate cancer killing T cells. The scaffolds are made with FDA-approved materials, exhibiting minimal immunogenicity and high biodegradability. The injectable thermosensitive hydrogels are attractive due to their ease of administration but research into the preclinical efficacy of the T lymphocytes in tumor models is necessary to move forward. Furthermore, the addition of T cell stimulators to the gels should be investigated to provide a platform that not only delivers T cells but ensures their proliferation. Stephan et. al.’s surgically implanted scaffold has shown outstanding preclinical success, and can now be further modified to obtain the optimal scaffold formulation. It is possible that including the paracrine delivery of anti-PD-1 and anti-CTLA-4 antibodies can improve the therapy, and a deeper understanding of T cell migration can dictate the placement of the adhesion molecules. The scaffolds will need to be tested on a larger scale for toxicity and potency as humans typically require a 1000-fold increase in the number of T cells administered – although the scaffolds demonstrate no toxicity in mice, the increase in size for human application may invoke serious side effects.
3.5. CAR T Cell Therapy
T cell-mediated killing of tumor cells requires several external stimuli that are often hindered by cancer cells and the tumor microenvironment: recognition of a tumor antigen, activation of costimulatory receptors, and cytokine signals promoting the expansion and continued antitumor effects of the T cell. CAR T cells express an engineered receptor that is activated upon antigen binding - allowing for T cell activation in the absence of a second signal from costimulatory receptors.
Although CAR T therapy has proven to be remarkably successful at treating hematological malignancies [13, 168, 169], there are several logistical considerations that must be addressed in order to successfully treat patients. CAR T cells must be generated from a patient's own immune cells in order to avoid graft-versus-host disease. Currently, CAR T cell production requires harvesting T cells from patients, shipping them to a facility to transduce the patient's T cells with the CAR gene, expanding the newly-generated CAR T cells, and shipping the newly-generated, patient-specific CAR T cells back to the hospital for reinfusion into the patient. The need to generate personalized CAR T cells significantly increases the cost of therapy, which is subsequently passed on to patients. Recently, Smith et.al. developed a method to generate leukemia-targeting T cells in situ by using synthetic DNA nanocarriers with a CD3ε-targeting antibody fragment to deliver CAR DNA to circulating T cells - potentially consolidating the steps of CAR T cell generation into a single injection [170]. The in situ generated CAR T cells were found to prolong the survival of C56BL/6 mice with systemically injected Eμ-ALL01 leukaemia cells as much as an infusion of CAR T cells that were generated ex vivo.
CAR T cell therapy comes with several significant limitations that could potentially be addressed by biomaterials, including on-target, off-tumor effects, patient toxicity, and decreased functionality due to the tumor microenvironment. In recent years, the profound success of CAR T cells has inspired further research into improving the safety and efficacy of CAR T cells. Previous research has shown that proteases such as cathepsin B [171], urokinase-type plasminogen activator [172], legumain [173], and different matrix metalloproteinases are prevalent in the tumor microenvironment [174] - providing a potential avenue for the activation of CAR T cells. To minimize on-target, off-tumor effects, Desnoyers et.al. created a protease-activated "probody" by modifying Cetuximab, an EGFR-binding antibody, to include a peptide linker susceptible to tumor-associated proteases and a peptide "mask" to prevent off-tumor binding to EGFR. The probody was shown to be relatively inactive in nonhuman primates, but could effectively target EGFR+ tumor cells in a mouse xenograft model [175]. Han. et. al. adapted this discovery and created a masked CAR T cell targeting EGFR, which was shown to be inactive among tumor-associated protease negative cells and active among cells expressing tumor-associated proteases. These masked CAR T cells also demonstrated a level of in vivo antitumor efficacy similar to unmasked CAR T cells [176]. Other studies have focused on modifying CAR T cells to secrete various agents that can improve their function within the tumor microenvironment, including cytokines, such as IL-2 [177], IL-7 [178], IL-15 [179], or IL-18 [180], and checkpoint inhibitors such as single chain antibodies targeting PD-1 [181].
Researchers have also reported using liposomes to make the tumor microenvironment more hospitable to CAR T cells. Zhang et.al. used liposomes with the tumor-targeting iRGD peptide to encapsulate PI-3065, a P110δ PI3K kinase inhibitor, and DW8-5, an immunostimulant-invariant natural killer T (iNKT) cell agonist, and demonstrate the importance of preconditioning the tumor microenvironment prior to CAR T cell therapy. Treating mice bearing 4T1 tumors with liposomes encapsulating PI-3065 and 7DW8-5 was shown to decrease the presence of monocytic myeloid derived suppressor cells and TAMs threefold and sevenfold respectively, while increasing the amount of tumor-infiltrating CD8+ T cells and iNKT cells fivefold and 20-fold respectively. Additionally, the study reported that treatment with drug-loaded liposomes prior to anti-ROR1 CAR T cell infusion effectively doubled the mediansurvival of BALB/cJ mice bearing 4T1-ROR1 tumors when compared to mice receiving drug-loaded liposomes after anti-ROR1 CAR T cell infusion [182].
Although the remarkable success of CAR T cell therapy is promising, there are still several challenges that must be addressed. Their high cost, potential for on target, off tumor effects, and susceptibility to inhibition by the tumor microenvironment are all obstacles that may be overcome by additional research. As we continue to make new discoveries and develop novel biomaterials, the field of CAR T cell therapy continues to advance towards unlocking its true potential.
4. Conclusion
Immuno-oncology, in particular T cell therapies, has undergone tremendous advancements in the past decade. Although significant process has been made, remissions are only obtained in certain patients and are often short-lived. Tisagenlecleucel, originally FDA approved to treat ALL, underwent further clinical trials and has received approval for relapsed or refractory Diffuse Large B-Cell Lymphoma treatment (DLBCL) [183]. This followed a tisagenlecleucel DLBCL study generating an objective response rate (ORR) above 54%. Despite achieving an ORR of more than double other treatments [184, 185], the median overall survival (OS) was 11.1 months and the OS probability at 18 months was 43%. T cell immunotherapy is altering the oncological landscape, but we are far from our goal of curing cancer. Researchers around the world are working to engineer an improved T cell – one that can successfully home to cancer cells, proliferate inside the patient’s body, overcome the immunosuppressive tumor microenvironment, and minimize side effects. T cells have progressed through 1st, 2nd, 3rd, and now 4th generations, and a few of the advancements can be seen through the introductory of suicide genes [186], tandem targeting moieties [187], masking peptides [176], secretory abilities [188], and altered receptor/gene expression [189]. While each of these developments holds promise in enhancing T cell therapy, it is vital to explore solutions from a different angle. A shift from a focus on T cells themselves has resulted in a wave of research on engineered biomaterials for improved T cell therapy. Biomaterials were first used for the ex vivo expansion of T cells, providing an off-the-shelf, simplified method for rapidly producing large quantities of tumor fighting lymphocytes. While spherical microbeads coated with anti-CD3 and anti-CD28 antibodies are the gold standard for clinical T cell expansion, recent works have proven that manipulating synthetic aAPCs to mimic nature have resulted in a higher quantity of higher quality T cells. As knowledge of T cell biology, activation, and differentiation expands, the design of improved aAPCs will follow shortly after. The discovery of TCR clustering has paved the path for the optimal placement of signal antigens, and research into T cell development will allow less differentiated T cells to be produced and maintained. The carbon nanotubes, nanoworms, and APC-ms platforms have been proven to enhance T cell production, and warrant further investigation, but it is also important to explore the reversion of differentiated T cells to earlier states as an approach for achieving maximum cell expansion of younger, antigen-specific T cells, and to see if this approach can be used in conjugation with these novel platforms. In parallel to the immunotherapy buzz, research on biomaterials and nanotechnology in medicine has experienced explosive growth [190, 191]. Despite their proven effectiveness, the efficacy and accessibility of T cell immunotherapies still has the opportunity for improvement. There is a constant need for improved in vitro T cell expansion - especially given the recent interest in the generation of allogeneic, off-the-shelf CAR T cells. Once infused into patients, the T cells must be able to efficiently traffic to their intended destination, and proliferate and thrive in a hostile environment. Researchers have recognized the potential of using biomaterials to address these challenges, as shown by the wide array of biomaterials investigated for enhancing the in vivo efficacy of T cell therapies (Table 1). Nanoparticles and scaffolds have been proven to enhance T cell homing to cancer cells, cytotoxic capacities, and T cell persistence in preclinical models – the next step is to diminish the disparity between biomaterial publications and clinical impact [191]. Although the biomaterials discussed in this review need to be tested in additional preclinical models and scaled up to confirm their viability as therapeutics, they show great promise in improving the efficacy of immunotherapy.
Acknowledgements and Disclosure
This work was supported by grants from the National Institutes of Health (R01AI068978, R01CA170820, R01EB017206 and P01CA132681) and a grant from the Ming Hsieh Institute for Research on Engineering-Medicine for Cancer. The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Heron M Deaths: Leading Causes for 2016. National Vital Statistics Reports. 2018;67:1–76. [PubMed] [Google Scholar]
- [2].Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA: a cancer journal for clinicians. 2012;62:220–41. [DOI] [PubMed] [Google Scholar]
- [3].McCune JS. Rapid Advances in Immunotherapy to Treat Cancer. Clinical pharmacology and therapeutics. 2018;103:540–4. [DOI] [PubMed] [Google Scholar]
- [4].Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature reviews Cancer. 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nature reviews Immunology. 2012;12:269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Knorr DA, Bachanova V, Verneris MR, Miller JS. Clinical utility of natural killer cells in cancer therapy and transplantation. Seminars in immunology. 2014;26:161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. [DOI] [PubMed] [Google Scholar]
- [9].Rosenberg SA, Packard BS, Topalian SL, Toy ST, Simon P, Lotze MT, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. N Engl J Med. 1988;319:1676–80. [DOI] [PubMed] [Google Scholar]
- [10].Geukes Foppen MH, Donia M, Svane IM, Haanen JB. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Molecular oncology. 2015;9:1918–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].June Carl H., O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361–5. [DOI] [PubMed] [Google Scholar]
- [12].Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and Toxicity Management of 19–28z CAR T Cell Therapy in B Cell Acute Lymphoblastic Leukemia. Sci Transl Med. 2014;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. The Lancet. 2015;385:517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Maude SL, Pulsipher MA, Boyer MW, Grupp SA, Davies SM, Phillips CL, et al. Efficacy and Safety of CTL019 in the First US Phase II Multicenter Trial in Pediatric Relapsed/Refractory Acute Lymphoblastic Leukemia: Results of an Interim Analysis. Blood. 2016;128:2801. [Google Scholar]
- [17].Uckun FM, Jaszcz W, Ambrus JL, Fauci AS, Gajl-Peczalska K, Song CW, et al. Detailed studies on expression and function of CD19 surface determinant by using B43 monoclonal antibody and the clinical potential of anti-CD19 immunotoxins. Blood. 1988;71:13–29. [PubMed] [Google Scholar]
- [18].Tasian SK, Gardner RA. CD19-redirected chimeric antigen receptor-modified T cells: a promising immunotherapy for children and adults with B-cell acute lymphoblastic leukemia (ALL). Therapeutic Advances in Hematology. 2015;6:228–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gilham DE, Debets R, Pule M, Hawkins RE, Abken H. CAR-T cells and solid tumors: tuning T cells to challenge an inveterate foe. Trends in molecular medicine. 2012;18:377–84. [DOI] [PubMed] [Google Scholar]
- [20].Kakarla S, Gottschalk S. CAR T Cells for Solid Tumors: Armed and Ready to Go? Cancer journal. 2014;20:151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Newick K, O'Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annual review of medicine. 2017;68:139–52. [DOI] [PubMed] [Google Scholar]
- [22].Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nature reviews Cancer. 2016;16:566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Perica K, Varela JC, Oelke M, Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides medical journal. 2015;6:e0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annual review of immunology. 2009;27:591–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nature reviews Immunology. 2013;13:227–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schwartz RH. Costimulation of T lymphocytes: The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–8. [DOI] [PubMed] [Google Scholar]
- [27].Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nature reviews Immunology. 2012;12:180–90. [DOI] [PubMed] [Google Scholar]
- [28].Hamilos DL. Antigen presenting cells. Immunologic Research. 1989;8:980117. [DOI] [PubMed] [Google Scholar]
- [29].Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annual review of immunology. 2002;20:621–67. [DOI] [PubMed] [Google Scholar]
- [30].Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. The Journal of clinical investigation. 2008;118:294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17469–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Oliveira G, Ruggiero E, Stanghellini MTL, Cieri N, D’Agostino M, Fronza R, et al. Tracking genetically engineered lymphocytes long-term reveals the dynamics of T cell immunological memory. Sci Transl Med. 2015;7:1–14. [DOI] [PubMed] [Google Scholar]
- [33].Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nature medicine. 2018;24:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ghassemi S, Nunez-Cruz S, O'Connor RS, Fraietta JA, Patel PR, Scholler J, et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer immunology research. 2018;6:1100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hinrichs CS, Borman ZA, Gattinoni L, Yu Z, Burns WR, Huang J, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117:808–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].June CH. Principles of adoptive T cell cancer therapy. The Journal of clinical investigation. 2007;117:1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Golubovskaya V, Wu L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers. 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:16168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:5060–9. [DOI] [PubMed] [Google Scholar]
- [40].Levine BL. Performance-enhancing drugs: design and production of redirected chimeric antigen receptor (CAR) T cells. Cancer gene therapy. 2015;22:79–84. [DOI] [PubMed] [Google Scholar]
- [41].Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, et al. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–7. [DOI] [PubMed] [Google Scholar]
- [42].Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, et al. Dendritic Cells Induce Peripheral T Cell Unresponsiveness under Steady State Conditions in Vivo. The Journal of experimental medicine. 2001;194:769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Maus MV, K.Thomas A, Leonard DGB, Allman D, Addya K, Schlienger K, et al. Ex vivo expansion of polyclonal and antigenspecific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4–1BB. Nature biotechnology. 2002;20:143–8. [DOI] [PubMed] [Google Scholar]
- [44].Kim JV, Latouche JB, Riviere I, Sadelain M. The ABCs of artificial antigen presentation. Nature biotechnology. 2004;22:403–10. [DOI] [PubMed] [Google Scholar]
- [45].Butler MO, Hirano N. Human cell-based artificial antigen-presenting cells for cancer immunotherapy. Immunol Rev. 2014;257:191–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hasan A, Selvakumar A, O’Reilly R. Artificial Antigen Presenting Cells: An Off the Shelf Approach for Generation of Desirable T-Cell Populations for Broad Application of Adoptive Immunotherapy. Adv Genet Eng. 2015;4. [PMC free article] [PubMed] [Google Scholar]
- [47].Turtle CJ, Riddell SR. Artificial Antigen-Presenting Cells for Use in Adoptive Immunotherapy. Cancer journal. 2010;16:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Neal LR, Bailey SR, Wyatt MM, Bowers JS, Majchrzak K, Nelson MH, et al. The Basics of Artificial Antigen Presenting Cells in T Cell-Based Cancer Immunotherapies. Journal of Immunology Research and Therapy. 2017;2:68–79. [PMC free article] [PubMed] [Google Scholar]
- [49].Van Wauwe JP, De Mey JR, Goossens JG. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. Journal of immunology. 1980;124:2708–13. [PubMed] [Google Scholar]
- [50].Tsoukas CD, Landgraf B, Bentin J, Valentine M, Lotz M, Vaughan JH, et al. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. Journal of immunology. 1985;135:1719–23. [PubMed] [Google Scholar]
- [51].Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. Journal of Immunological Methods. 1990;128:189–201. [DOI] [PubMed] [Google Scholar]
- [52].Acuto O, Michel F. CD28-mediated co-stimulation: a quantitative support for TCR signalling. Nature reviews Immunology. 2003;3:939–51. [DOI] [PubMed] [Google Scholar]
- [53].Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C, et al. Manufacturing Validation of Biologically Functional T Cells Targeted to CD19 Antigen for Autologous Adoptive Cell Therapy. Journal of Immunotherapy. 2009;32:169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ledbetter JA, Gentry LE, June CH, Rabinovitch PS, Purchio Af. Stimulation of T Cells through the CD3/T-Cell Receptor Complex: Role of Cytoplasmic Calcium, Protein Kinase C Translocation, and Phosphorylation of pp60c-src in the Activation Pathway. Mol Cell Biol. 1987;7:650–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Trickett A, Kwan YL. T cell stimulation and expansion using anti-CD3/CD28 beads. Journal of Immunological Methods. 2003;275:251–5. [DOI] [PubMed] [Google Scholar]
- [56].Neurauter AA, Bonyhadi M, Lien E, Nøkleby L, Ruud E, Camacho S, et al. Cell Isolation and Expansion Using Dynabeads ® In: Kumar A, Galaev IY, Mattiasson B, editors. Cell Separation: Fundamentals, Analytical and Preparative Methods. Berlin, Heidelberg: Springer Berlin Heidelberg; 2007. p. 41–73. [Google Scholar]
- [57].Thompson JA, Figlin RA, Sifri-Steele C, Berenson RJ, Frohlich MW. A Phase I Trial of CD3/CD28-activated T Cells (Xcellerated T Cells) and Interleukin-2 in Patients with Metastatic Renal Cell Carcinoma1. Clinical cancer research : an official journal of the American Association for Cancer Research. 2003;9:3562–70. [PubMed] [Google Scholar]
- [58].Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Molecular therapy oncolytics. 2016;3:16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Tisagenlecleucel (CTL019) for the treatment of pediatric and young adult patients with relapsed/refractory B-cell acute lymphoblastic leukemia. In: FDA, editor.2017. [Google Scholar]
- [60].Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. The Journal of clinical investigation. 2005;115:1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Deeths MJ, Mescher MF. B7–1-dependent co-stimulation results in qualitatively and quantitatively different responses by CD4+ and CDS+ T cells. European journal of immunology. 1997;27:598–608. [DOI] [PubMed] [Google Scholar]
- [62].Eggermont LJ, Paulis LE, Tel J, Figdor CG. Towards efficient cancer immunotherapy: advances in developing artificial antigen-presenting cells. Trends in biotechnology. 2014;32:456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mescher MF. Surface contact requirements for activation of cytotoxic T lymphocytes. J Immunology. 1992;149:2402–5. [PubMed] [Google Scholar]
- [64].Perica K, De Leon Medero A, Durai M, Chiu YL, Bieler JG, Sibener L, et al. Nanoscale artificial antigen presenting cells for T cell immunotherapy. Nanomedicine : nanotechnology, biology, and medicine. 2014;10:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Perica K, Tu A, Richter A, Bieler JG, Edidin M, Schneck JP. Magnetic Field-Induced T Cell Receptor Clustering by Nanoparticles Enhances T Cell Activation and Stimulates Antitumor Activity. ACS nano. 2014;8:2252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Boyle S, Kolin DL, Bieler JG, Schneck JP, Wiseman PW, Edidin M. Quantum dot fluorescence characterizes the nanoscale organization of T cell receptors for antigen. Biophysical journal. 2011;101:L57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Pageon SV, Tabarin T, Yamamoto Y, Yuanqing Ma, Nicovich PR, Bridgeman JS, et al. Functional role of T-cell receptor nanoclusters in signal initiation and antigen discrimination. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5454–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Purtic B, Pitcher LA, van Oers NS, Wulfing C. T cell receptor (TCR) clustering in the immunological synapse integrates TCR and costimulatory signaling in selected T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Molnar E, Swamy M, Holzer M, Beck-Garcia K, Worch R, Thiele C, et al. Cholesterol and sphingomyelin drive ligand-independent T-cell antigen receptor nanoclustering. The Journal of biological chemistry. 2012;287:42664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Matic J, Deeg J, Scheffold A, Goldstein I, Spatz JP. Fine tuning and efficient T cell activation with stimulatory aCD3 nanoarrays. Nano letters. 2013;13:5090–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Hickey JW, Vicente FP, Howard GP, Mao HQ, Schneck JP. Biologically Inspired Design of Nanoparticle Artificial Antigen-Presenting Cells for Immunomodulation. Nano letters. 2017;17:7045–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sunshine JC, Perica K, Schneck JP, Green JJ. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials. 2014;35:269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, et al. Biodegradable nanoellipsoidal artificial antigen presenting cells for antigen specific T-cell activation. Small. 2015;11:1519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Steenblock ER, Fadel T, Labowsky M, Pober JS, Fahmy TM. An artificial antigen-presenting cell with paracrine delivery of IL-2 impacts the magnitude and direction of the T cell response. The Journal of biological chemistry. 2011;286:34883–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Fadel TR, Steenblock ER, Stern E, Li N, Wang X, Haller GL, et al. Enhanced Cellular Activation with Single Walled Carbon Nanotube Bundles Presenting Antibody Stimuli. Nano letters. 2008;8:2070–6. [DOI] [PubMed] [Google Scholar]
- [76].Fadel TR, Sharp FA, Vudattu N, Ragheb R, Garyu J, Kim D, et al. A carbon nanotube-polymer composite for T-cell therapy. Nature nanotechnology. 2014;9:639–47. [DOI] [PubMed] [Google Scholar]
- [77].Cheung AS, Mooney DJ. Engineered Materials for Cancer Immunotherapy. Nano today. 2015;10:511–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Ahlers JD, Belyakov IM. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115:1678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mandal S, Eksteen-Akeroyd ZH, Jacobs MJ, Hammink R, Koepf M, Lambeck AJA, et al. Therapeutic nanoworms: towards novel synthetic dendritic cells for immunotherapy. Chemical Science. 2013;4:4168. [Google Scholar]
- [80].Pike LJ. Lipid rafts: bringing order to chaos. Journal of lipid research. 2003;44:655–67. [DOI] [PubMed] [Google Scholar]
- [81].Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nature Immunology. 2000;1:156–62. [DOI] [PubMed] [Google Scholar]
- [82].Ding Q, Chen J, Wei X, Sun W, Mai J, Yang Y, et al. RAFTsomes containing epitope-MHC-II complexes mediated CD4+ T cell activation and antigen-specific immune responses. Pharmaceutical research. 2013;30:60–9. [DOI] [PubMed] [Google Scholar]
- [83].Zappasodi R, Di Nicola M, Carlo-Stella C, Mortarini R, Molla A, Vegetti C, et al. The effect of artificial antigen-presenting cells with preclustered anti-CD28/-CD3/-LFA-1 monoclonal antibodies on the induction of ex vivo expansion of functional human antitumor T cells. Haematologica. 2008;93:1523–34. [DOI] [PubMed] [Google Scholar]
- [84].Irvine DJ, Doh J. Synthetic surfaces as artificial antigen presenting cells in the study of T cell receptor triggering and immunological synapse formation. Seminars in immunology. 2007;19:245–54. [DOI] [PubMed] [Google Scholar]
- [85].Su X, Ditlev JA, Rosen MK, Vale RD. Reconstitution of TCR Signaling Using Supported Lipid Bilayers In: Baldari CT, Dustin ML, editors. The Immune Synapse: Methods and Protocols. New York, NY: Springer New York; 2017. p. 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Cheung AS, Zhang DKY, Koshy ST, Mooney DJ. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nature biotechnology. 2018;36:160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, et al. IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121:573–84. [DOI] [PubMed] [Google Scholar]
- [88].Kondo T, Imura Y, Chikuma S, Hibino S, Omata-Mise S, Ando M, et al. Generation and application of human induced-stem cell memory T cells for adoptive immunotherapy. Cancer science. 2018;109:2130–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Purwada A, Jaiswal MK, Ahn H, Nojima T, Kitamura D, Gaharwar AK, et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials. 2015;63:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Nozaki K, Mochizuki W, Matsumoto Y, Matsumoto T, Fukuda M, Mizutani T, et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. Journal of gastroenterology. 2016;51:206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J, et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell. 2018;175:1972–88 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF, et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell. 2018;174:1586–98 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Montel-Hagen A, Seet CS, Li S, Chick B, Zhu Y, Chang P, et al. Organoid-Induced Differentiation of Conventional T Cells from Human Pluripotent Stem Cells. Cell stem cell. 2019;24:376–89 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Blanco E, Shen H, Ferrari M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nature biotechnology. 2015;33:941–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nature reviews Cancer. 2017;17:20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Maeda H Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Advanced drug delivery reviews. 2015;91:3–6. [DOI] [PubMed] [Google Scholar]
- [99].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D. Progress and challenges towards targeted delivery of cancer therapeutics. Nature communications. 2018;9:1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Wei T, Chen C, Liu J, Liu C, Posocco P, Liu X, et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:2978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Liu Y, Fang J, Joo KI, Wong MK, Wang P. Codelivery of chemotherapeutics via crosslinked multilamellar liposomal vesicles to overcome multidrug resistance in tumor. PLoS One. 2014;9:e110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nature medicine. 2004;10:294–8. [DOI] [PubMed] [Google Scholar]
- [104].Vyas M, Muller R, Pogge von Strandmann E. Antigen Loss Variants: Catching Hold of Escaping Foes. Frontiers in immunology. 2017;8:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Tannock IF, Rotin D. Acid pH in Tumors and Its Potential for Therapeutic Exploitation. Cancer Res. 1989;49:4373–84. [PubMed] [Google Scholar]
- [106].Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res. 2011;71:6921–5. [DOI] [PubMed] [Google Scholar]
- [107].Fischer K, Voelkl S, Meidenbauer N, Ammer J, Gottfried E, Schwarz S, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109:3812–9. [DOI] [PubMed] [Google Scholar]
- [108].Anderson KG, Stromnes IM, Greenberg PD. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer cell. 2017;31:311–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nature reviews Immunology. 2018;18:648–59. [DOI] [PubMed] [Google Scholar]
- [110].Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and Selective Stimulation of Memory-Phenotype CD81 T Cells In Vivo by IL-15. Immunity. 1998;8:591–9. [DOI] [PubMed] [Google Scholar]
- [111].Waldmann TA. Cytokines in Cancer Immunotherapy. Cold Spring Harbor perspectives in biology. 2018; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Lotze MT, Matory YL, Ettinghausen SE, Rayner AA, Sharrow SO, Seipp CA, et al. In vivo adminsitration of purified human interleukin 2. Journal of immunology. 1985;135:2865–75. [PubMed] [Google Scholar]
- [113].Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-Dose Recombinant Interleukin 2 Therapy for Patients With Metastatic Melanoma: Analysis of 270 Patients Treated Between 1985 and 1993. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17:2105–16. [DOI] [PubMed] [Google Scholar]
- [114].Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Hong E, Usiskin IM, Bergamaschi C, Hanlon DJ, Edelson RL, Justesen S, et al. Configuration-dependent Presentation of Multivalent IL-15:IL-15Ralpha Enhances the Antigen-specific T Cell Response and Anti-tumor Immunity. The Journal of biological chemistry. 2016;291:8931–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Kwong B, Gai SA, Elkhader J, Wittrup KD, Irvine DJ. Localized immunotherapy via liposome-anchored Anti-CD137 + IL-2 prevents lethal toxicity and elicits local and systemic antitumor immunity. Cancer Res. 2013;73:1547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. Journal of controlled release : official journal of the Controlled Release Society. 2013;172:426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–8. [DOI] [PubMed] [Google Scholar]
- [120].Colak S, Ten Dijke P. Targeting TGF-beta Signaling in Cancer. Trends in cancer. 2017;3:56–71. [DOI] [PubMed] [Google Scholar]
- [121].Park J, Wrzesinski SH, Stern E, Look M, Criscione J, Ragheb R, et al. Combination delivery of TGF-beta inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature materials. 2012;11:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-beta signalling inhibitors for cancer therapy. Nature reviews Drug discovery. 2004;3:1011–22. [DOI] [PubMed] [Google Scholar]
- [123].Gueorguieva I, Cleverly AL, Stauber A, Pillay NS, Rodon JA, Miles CP, et al. Defining a therapeutic window for the novel TGF-β inhibitor LY2157299 monohydrate based on a pharmacokinetic/pharmacodynamic model. British Journal of Clinical Pharmacology. 2014;77:796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ. Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors. ACS nano. 2017;11:3089–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, et al. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nature communications. 2017;8:1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Dannenmann SR, Thielicke J, Stockli M, Matter C, von Boehmer L, Cecconi V, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology. 2013;2:e23562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. [DOI] [PubMed] [Google Scholar]
- [128].Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nature reviews Clinical oncology. 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Huang Z, Zhang Z, Jiang Y, Zhang D, Chen J, Dong L, et al. Targeted delivery of oligonucleotides into tumor-associated macrophages for cancer immunotherapy. Journal of controlled release : official journal of the Controlled Release Society. 2012;158:286–92. [DOI] [PubMed] [Google Scholar]
- [131].Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews Immunology. 2008;8:523–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell research. 2017;27:109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Ou W, Thapa RK, Jiang L, Soe ZC, Gautam M, Chang JH, et al. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. Journal of controlled release : official journal of the Controlled Release Society. 2018;281:84–96. [DOI] [PubMed] [Google Scholar]
- [134].Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nature reviews Immunology. 2018;18:153–67. [DOI] [PubMed] [Google Scholar]
- [135].Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Kosmides AK, Sidhom JW, Fraser A, Bessell CA, Schneck JP. Dual Targeting Nanoparticle Stimulates the Immune System To Inhibit Tumor Growth. ACS nano. 2017; 11:5417–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Schütz C, Varela JC, Perica K, Haupt C, Oelke M, Schneck JP. Antigen-specific T cell Redirectors: a nanoparticle based approach for redirecting T cells. Oncotarget. 2016;7:68503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature medicine. 2010;16:1035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Siegler EL, Kim YJ, Chen X, Siriwon N, Mac J, Rohrs JA, et al. Combination Cancer Therapy Using Chimeric Antigen Receptor-Engineered Natural Killer Cells as Drug Carriers. Molecular therapy : the journal of the American Society of Gene Therapy. 2017;25:2607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Siriwon N, Kim YJ, Siegler E, Chen X, Rohrs JA, Liu Y, et al. CAR-T Cells Surface-Engineered with Drug-Encapsulated Nanoparticles Can Ameliorate Intratumoral T-cell Hypofunction. Cancer immunology research. 2018;6:812–24. [DOI] [PubMed] [Google Scholar]
- [141].Huang B, Abraham WD, Zheng Y, López SCB, Luo SS, Irvine DJ. Active targeting of chemotherapy to disseminated tumors using nanoparticle-carrying T cells. Sci Transl Med. 2015;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Jones RB, Mueller S, Kumari S, Vrbanac V, Genel S, Tager AM, et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33:5776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Tang L, Zheng Y, Melo MB, Mabardi L, Castano AP, Xie YQ, et al. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nature biotechnology. 2018;36:707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:3127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Hershkovitz L, Schachter J, Treves AJ, Besser MJ. Focus on adoptive T cell transfer trials in melanoma. Clinical & developmental immunology. 2010;2010:260267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Hori Y, Stern PJ, Hynes RO, Irvine DJ. Engulfing tumors with synthetic extracellular matrices for cancer immunotherapy. Biomaterials. 2009;30:6757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Zaharoff DA, Hoffman BS, Hooper HB, Benjamin CJ Jr., Khurana KK, Hance KW, et al. Intravesical immunotherapy of superficial bladder cancer with chitosan/interleukin-12. Cancer Res. 2009;69:6192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Park CG, Hartl CA, Schmid D, Carmona EM, Kim H-j, Goldberg MS. Extended release of perioperative immunotherapy prevents tumor recurrence and eliminates metastases. Sci Transl Med. 2018;10:1–14. [DOI] [PubMed] [Google Scholar]
- [150].Koshy ST, Ferrante TC, Lewin SA, Mooney DJ. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35:2477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Zhan Q, Shen B, Fang Y, Deng X, Chen H, Jin J, et al. Drug-eluting scaffold inhibited in vivo pancreatic tumorigenesis by engaging murine CCR4(+)CD8(+) T cells. Colloids and surfaces B, Biointerfaces. 2017;158:469–73. [DOI] [PubMed] [Google Scholar]
- [152].Wen Y, Kolonich HR, Kruszewski KM, Giannoukakis N, Gawalt ES, Meng WS. Retaining antibodies in tumors with a self-assembling injectable system. Molecular pharmaceutics. 2013;10:1035–44. [DOI] [PubMed] [Google Scholar]
- [153].Hudalla GA, Sun T, Gasiorowski JZ, Han H, Tian YF, Chong AS, et al. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nature materials. 2014;13:829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Aliperta R, Welzel PB, Bergmann R, Freudenberg U, Berndt N, Feldmann A, et al. Cryogel-supported stem cell factory for customized sustained release of bispecific antibodies for cancer immunotherapy. Scientific reports. 2017;7:42855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Weiden J, Tel J, Figdor CG. Synthetic immune niches for cancer immunotherapy. Nature reviews Immunology. 2018;18:212–9. [DOI] [PubMed] [Google Scholar]
- [156].Peppas NA, Mikos AG. Preparation methods and structure of hydrogels. Boca Raton, FL, USA: CRC Press Inc; 1986. [Google Scholar]
- [157].Ahmed EM. Hydrogel: Preparation, characterization, and applications: A review. Journal of advanced research. 2015;6:105–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Singh A, Peppas NA. Hydrogels and scaffolds for immunomodulation. Advanced materials. 2014;26:6530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Tsao CT, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, et al. Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy. Biomacromolecules. 2014;15:2656–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Monette A, Ceccaldi C, Assaad E, Lerouge S, Lapointe R. Chitosan thermogels for local expansion and delivery of tumor-specific T lymphocytes towards enhanced cancer immunotherapies. Biomaterials. 2016;75:237–49. [DOI] [PubMed] [Google Scholar]
- [161].Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nature biotechnology. 2015;33:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. The Journal of clinical investigation. 2017;127:2176–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Atik AF, Suryadevara CM, Schweller RM, West JL, Healy P, Herndon Ii JE, et al. Hyaluronic acid based low viscosity hydrogel as a novel carrier for Convection Enhanced Delivery of CAR T cells. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2018;56:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Pardridge WM. Blood-brain barrier delivery. Drug discovery today. 2007;12:54–61. [DOI] [PubMed] [Google Scholar]
- [165].Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chaichana KL, Pinheiro L, Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Therapeutic Delivery. 2015;6:353–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Debinski W, Tatter SB. Convection enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci Transl Med. 2011;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, et al. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nature nanotechnology. 2017;12:813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, et al. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes & development. 2006;20:543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Duffy MJ, McGowan PM, Harbeck N, Thomssen C, Schmitt M. UPA and PAI-1 as biomarkers in breast cancer: validated for clinical use in level-of-evidence-1 studies. Breast Cancer Research. 2014;16:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhen Y, Chunlei G, Wenzhi S, Shuangtao Z, Na L, Rongrong W, et al. Clinicopathologic significance of legumain overexpression in cancer: a systematic review and meta-analysis. Scientific reports. 2015;5:16599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Mason SD, Joyce JA. Proteolytic networks in cancer. Trends in cell biology. 2011;21:228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Desnoyers LR, Vasiljeva O, Richardson JH, Yang A, Menendez EEM, Liang TW, et al. Tumor-Specific Activation of an EGFR-Targeting Probody Enhances Therapeutic Index. Sci Transl Med. 2013;5:1–10. [DOI] [PubMed] [Google Scholar]
- [176].Han X, Bryson PD, Zhao Y, Cinay GE, Li S, Guo Y, et al. Masked Chimeric Antigen Receptor for Tumor-Specific Activation. Molecular therapy : the journal of the American Society of Gene Therapy. 2017;25:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Adachi K, Kano Y, Nagai T, Okuyama N, Sakoda Y, Tamada K. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nature biotechnology. 2018;36:346–51. [DOI] [PubMed] [Google Scholar]
- [179].Hoyos V, Savoldo B, Quintarelli C, Mahendravada A, Zhang M, Vera J, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia. 2010;24:1160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Avanzi MP, Yeku O, Li X, Wijewarnasuriya DP, van Leeuwen DG, Cheung K, et al. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell reports. 2018;23:2130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Li S, Siriwon N, Zhang X, Yang S, Jin T, He F, et al. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23:6982–92. [DOI] [PubMed] [Google Scholar]
- [182].Zhang F, Stephan SB, Ene CI, Smith TT, Holland EC, Stephan MT. Nanoparticles That Reshape the Tumor Milieu Create a Therapeutic Window for Effective T-cell Therapy in Solid Malignancies. Cancer Res. 2018;78:3718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- [184].Crump M, Neelapu SS, Farooq U, Neste EVD, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Hitz F, Connors JM, Gascoyne RD, Hoskins P, Moccia A, Savage KJ, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R-CHOP treatment. Annals of hematology. 2015;94:1839–43. [DOI] [PubMed] [Google Scholar]
- [186].Gargett T, Brown MP. The inducible caspase-9 suicide gene system as a "safety switch" to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Frontiers in pharmacology. 2014;5:235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Schneider D, Xiong Y, Wu D, Nlle V, Schmitz S, Haso W, et al. A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines. Journal for immunotherapy of cancer. 2017;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev. 2014;257:83–90. [DOI] [PubMed] [Google Scholar]
- [189].Rupp LJ, Schumann K, Roybal KT, Gate RE, Ye CJ, Lim WA, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Scientific reports. 2017;7:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Emerich DF, Thanos CG. Nanotechnology and medicine. Expert opinion on biological therapy. 2003;3:655–63. [DOI] [PubMed] [Google Scholar]
- [191].Gammon JM, Dold NM, Jewell CM. Improving the clinical impact of biomaterials in cancer immunotherapy. Oncotarget. 2016;7:15421–43. [DOI] [PMC free article] [PubMed] [Google Scholar]