Abstract
The associations between dietary sodium intake and markers of subclinical cardiovascular disease (CVD), such as high-sensitivity cardiac troponin T (hs-cTnT) and amino terminal pro b-type natriuretic peptide (NT-proBNP), may provide mechanistic insight into the relationship between dietary sodium and cardiovascular events. We studied 6,131 participants of the Multi-Ethnic Study of Atherosclerosis, who were free of clinical CVD at baseline. Food frequency questionnaires were used to assess estimated sodium intake (ESI) at baseline. We tested the associations between 5 quintiles of ESI (quintile 1: 0.2 to 1.3 grams/day, quintile 2: 1.3 to 1.8 grams/day, quintile 3: 1.8 to 2.4 grams/day, quintile 4: 2.4 to 3.2 grams/day and quintile 5: 3.2 to 9.9 grams/day) with cross sectional, and five-year longitudinal, change in hs-cTnT and NT-proBNP concentrations. Restricted cubic-spline plots were utilized to explore the shape of the associations between ESI and biomarker outcomes. A cross-sectional association between baseline sodium intake and hs-cTnT (but not NT-proBNP) was observed, driven predominantly by a strong positive relationship at an intake range of 0.2 – 2.4 g/day. Conversely, a longitudinal association between baseline sodium intake and NT-proBNP (but not hs-cTnT) was observed, driven predominantly by a strong positive relationship at intake levels ≥2.4 g/day. In conclusion, temporal shifts in the association between increased ESI and markers of subclinical cardiovascular disease, hs-cTnT in the short term and NT-proBNP in the longer term, point to the complex pathobiology of the association between salt intake and CVD. There was also no consistent evidence supporting a J-curve (i.e., excess biomarker values at very low ESI)
Keywords: subclinical cardiovascular disease, biomarkers, dietary sodium, risk prediction
INTRODUCTION:
Sodium is a major component of our food supply, and excess dietary intake has an important role in the pathogenesis of hypertension 1. Data from the National Health and Nutrition Examination Survey (NHANES) study estimate that average sodium intake in United States adults remains high, at approximately 3.6 g/day 2,3, which vastly exceeds both the recommended upper limit of 2.3 g/day set by the 2015 United States Dietary Guidelines 4 and the more stringent limit of 1.5 g/day set by the American Heart Association 5. By lowering blood pressure and reducing the risk for hypertension, sodium reduction should theoretically reduce cardiovascular disease.
One approach to further examine the relationships of sodium intake with cardiovascular health is to study the associations between dietary sodium and markers of subclinical cardiovascular disease. However, to our knowledge, the associations between dietary sodium intake and levels of hs-cTnT and NT-proBNP have not yet been thoroughly examined. As such, we tested these associations in the Multi-Ethnic Study of Atherosclerosis (MESA).
METHODS:
Previous reports have described the MESA design in detail 6. The MESA is a prospective observational cohort of 6,814 men and women who, at baseline (2000-2002), were free of clinical cardiovascular disease and were between 45-84 years of age. Information on nutritional intake, predominantly by way of Food Frequency Questionnaires (FFQ), was available for 6,237 MESA participants (92%), consisting of a series of questions pertaining to the frequency and usual serving size for each of 120 food items 7. The FFQ has been validated in relation to other metrics of dietary sodium intake (namely, 24-hour urinary sodium excretion), with an area under the curve (AUC) statistic for an excretion of >100 mmol sodium per day ranging from 0.57 to 0.76 in contemporary studies 8-9. All FFQ responses were processed by the software package (DietSys Nutrient Analysis Program©), which subsequently assigned each participant an average estimated daily intake of sodium (estimated sodium intake, ESI) based on their particular responses. Excluded from the analysis were (a) participants in whom ESI information was missing or those with implausible outlier values above or below the 99.9 and 0.1 percentile marks, respectively), (b) participants with unrealistic total caloric intake (≤500 or ≥5,000 g/day) and (c) participants with missing co-variate data. A flow chart is provide Figure 1.
Figure 1:
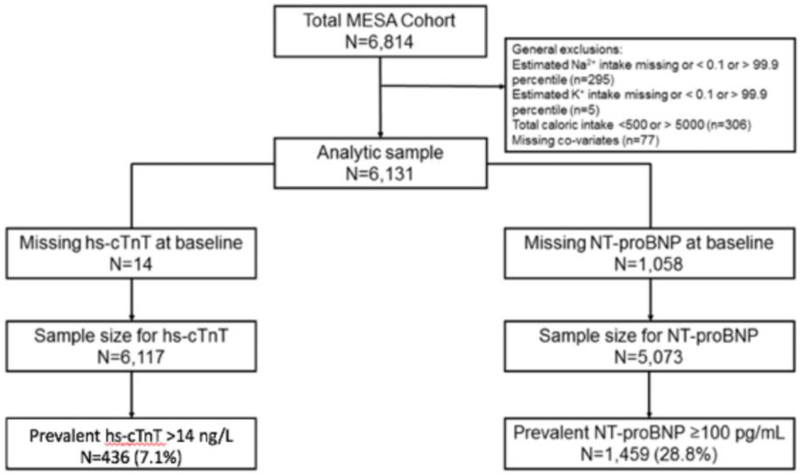
Figure 1 depicts the selection of final analytic cohort following application of aforementioned exclusion criterion.
Hs-cTnT was measured in ethylenediaminetetraacetic acid (EDTA) plasma collected at baseline (examination 1; July 2000 to August 2002) and at examination 3 (March 2004 to September 2005). Hs-cTnT was measured at a MESA collaborative site laboratory (University of Maryland Medical Center; Baltimore, Maryland) using the Cobas e601 Analyzer (Roche Diagnostics; Indianapolis, Indiana©). A previously unthawed 250 μl sample of EDTA plasma was used for analysis. For hs-cTnT, the intraassay coefficients of variation observed for the cohort measurements were 4% at 28 ng/L and 2% at 2154 ng/L. The limit of detection was 3 ng/L.
Similarly, NT-proBNP was measured in plasma specimens collected at baseline (examination 1) and at exam 2 (September 2002 to February 2004) and exam 3 (March 2004 to September 2005), and were stored at a temperature range −70°C to −80°C prior to thawing for analyasis. NT-proBNP measurements were made on the Elecsys 2010 Analyzers (Roche Diagnostics; Indianapolis, Indiana ©). The analytical measurement range was 5 to 35,000 pg/ml, with a coefficient of variation range of 2 to 5%.
Differences in baseline characteristics among the study participants in various categories of ESI were compared using the chi-square test for categorical variables and analysis of variance for continuous variables. Consistent with previously reported data, ESI was analyzed continuously and as a categorical exposure via quintiles as follows: quintile 1 (ESI 0.2-1.3g/day), quintile 2 (ESI 1.3-1.8 g/day, reference), quintile 3 (ESI 1.8-2.4 g/day), quintile 4 (ESI 2.4-3.2 g/day) and quintile 5 (ESI 3.2-9.9 g/day) 10. Our main outcome measures were average adjusted differences in hs-cTnT and NT-proBNP concentrations at the first examination (cross-sectional linear analysis) as well as the 5-year change in these parameters at subsequent follow up examinations (longitudinal linear analysis). Additional outcomes studied include the odds of prevalent and incident hs-cTnT ≥14 ng/L and NT-proBNP ≥100 pg/mL at examinations 1 and 3, as well the odds of a ≥25% increase in these parameters from baseline. The rationale for utilizing these categorical cut points relates to their association with higher cardiovascular event rates11-13. Individuals meeting these cut points at the time of examination 1 were excluded from this portion of the analysis.
For cross-sectional analyses, we conducted multivariable-adjusted linear regression when assessing biomarker concentrations as continuous outcomes and multivariable-adjusted logistic regression when assessing biomarker concentrations as prevalent categorical outcomes. For prospective longitudinal analyses, we conducted multivariable-adjusted linear regression with generalized estimating equation models in order to determine the continuous association between ESI and temporal change in biomarker level, as well as multivariable-adjusted logistic regression when assessing biomarker levels as incident categorical outcomes. For each of these analyses we used three sequential adjustment models. Model 1 adjusted for age and sex. Model 2 also included race/ethnicity, body mass index (BMI), smoking status (current, former, never), alcohol consumption (current, former, never), total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL), triglycerides (mg/dL), estimated glomerular filtration rate (mL*min −1*1.73 m−2, as estimated by the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation), lipid-lowering medication use (yes or no), anti-hypertensive therapy (yes or no), and history of diagnosed diabetes (yes or no). In addition to the variables included in models 1 and 2, model 3 also adjusted for systolic blood pressure (in mm Hg), a parameter which might mediate any association between ESI and biomarker levels. The measurement of the aforementioned covariates has been previously described 6. In addition, in order to address the potential confounder of sodium reduction among patients with established hypertension, we performed sensitivity analyses excluding individuals with a history of hypertension and those on antihypertensive therapies at the baseline MESA visit.
We also used restricted cubic-spline plots to explore the shape of the association between ESI (as a continuous exposure) and the biomarker outcomes, fitted with four knots (at the 5th, 35th, 65th, and 95th percentiles of the ESI distribution). On the basis of our restricted-cubic-spline plots for the primary outcome and the results of prior analyses, we also added a spline terms to our logistic regressions, evaluating associations between ESI and outcomes below and above a threshold of 2.40 g/day (22). All statistical analyses were performed using the Statistical Analysis Software (SAS) version 9.4
RESULTS:
Baseline characteristics of the study population are outlined in Table 1. The study included 6,814 participants, among whom 683 were excluded according to aforementioned criteria (see Figure 1). In the study sample of 6,131 participants, 14 were missing hs-cTnT and 1,058 were missing NT-proBNP at baseline. Accordingly, the sample sizes were 6,117 participants and 5,073 participants in the hs-cTnT and NT-proBNP outcome groups, respectively. Among all studied participants, mean age was 62 years and 53% were female. Anti-blood pressure was 126.6 mm Hg. There were inverse crude associations observed between ESI quintile and both systolic blood pressure and use of anti-hypertensive medications. Overall, hs-cTnT ≥14 ng/L was present in 436 (7%) while NT-proBNP ≥100 pg/mL was present in 1,459 (29%) of participants. The median baseline values of NT-proBNP and hs-cTnT were 55.2 pg/mL and 14 ng/L, respectively. It is noteworthy that baseline NT-proBNP values were highest in the reference quintile (ESI 1.8 – 2.4 g/day), an observation that was not seen in terms of hs-cTnT.
Table 1.
Baseline characteristics by estimated sodium intake quintile: The Multi-Ethnic Study of Atherosclerosis (MESA; 2000-2002).
| Characteristic | Total | Estimated Sodium Intake (g/day) | p- value** |
||||
|---|---|---|---|---|---|---|---|
| Q1 (0.2 - 1.3 g/d) |
Q2 (1.3 - 1.8 g/d) |
Q3 (1.8 - 2.4 g/d) |
Q4 (2.4 - 3.2 g/d) |
Q5 (3.2 - 9.9 g/d) |
- | ||
| N | 6131 | 1227 | 1226 | 1226 | 1226 | 1226 | |
| Age (years) | 62.3 ± 10.2 | 63.9 ± 10.1 | 63.4 ± 10.3 | 62.0 ± 10.0 | 61.8 ± 10.1 | 60.3 ± 10.3 | <0.001 |
| Women | 3220 (52.5%) | 823 (67.1%) | 762 (62.2%) | 639 (52.1%) | 552 (45.0%) | 444 (36.2%) | <0.001 |
| White | 2427 (39.6%) | 324 (26.4%) | 477 (38.9%) | 564 (46.0%) | 555 (45.3%) | 507 (41.4%) | <0.001 |
| Black | 1614 (26.3%) | 397 (32.4%) | 296 (24.1%) | 290 (23.7%) | 283 (23.1%) | 348 (28.4%) | <0.001 |
| Hispanic | 1361 (22.2%) | 279 (22.7%) | 281 (22.9%) | 229 (18.7%) | 275 (22.4%) | 297 (24.2%) | <0.001 |
| Chinese | 729 (11.9%) | 227 (18.5%) | 172 (14.0%) | 143 (11.7%) | 113 (9.2%) | 74 (6.0%) | <0.001 |
| Body mass index(kg/m2) | 28.3 ± 5.4 | 27.7 ± 5.4 | 27.8 ± 5.3 | 28.2 ± 5.4 | 28.5 ± 5.3 | 29.2 ± 5.5 | <0.001 |
| Current smoker | 770 (12.6%) | 133 (10.8%) | 131 (10.7%) | 152 (12.4%) | 157 (12.8%) | 197 (16.1%) | <0.001 |
| Current alcohol | 3438 (56.1%) | 598 (48.7%) | 650 (53.0%) | 722 (58.9%) | 717 (58.5%) | 751 (61.3%) | <0.001 |
| Total cholesterol (mg/dL) | 194.1 ± 35.4 | 197.3 ± 36.0 | 193.0 ± 33.7 | 194.7 ± 35.2 | 193.5 ± 35.8 | 191.9 ± 35.9 | 0.003 |
| HDL cholesterol (mg/dL) | 51.0 ± 14.9 | 53.7 ± 15.6 | 52.5 ± 15.4 | 51.3 ± 15.5 | 49.8 ± 14.1 | 47.9 ± 13.2 | <0.001 |
| Triglycerides (mg/dL) | 131.5 ± 87.2 | 125.2 ± 77.9 | 130.7 ± 86.1 | 131.6 ± 77.5 | 133.1 ± 99.2 | 136.7 ± 93 | 0.02 |
| eGFR (ml/min per 1.73 m2) | 77.6 ± 16.1 | 76.4 ± 16.1 | 76.2 ± 15.9 | 77.2 ± 16.4 | 78.2 ± 16.1 | 79.9 ± 15.9 | <0.001 |
| Hypertension medications | 2037 (33.2%) | 469 (38.2%) | 440 (35.9%) | 380 (31%) | 380 (31.0%) | 368 (30.0%) | <0.001 |
| Lipid lowering medications | 1005 (16.4%) | 216 (17.6%) | 230 (18.8%) | 185 (15.1%) | 204 (16.6%) | 170 (13.9%) | 0.009 |
| Diabetes mellitus | 755 (12.3%) | 152 (12.4%) | 160 (13.1%) | 128 (10.4%) | 155 (12.6%) | 160 (13.1%) | 0.25 |
| SBP (mmHg) | 126.6 ± 21.3 | 128.0 ± 23.0 | 127.7 ± 21.7 | 125.7 ± 20.7 | 125.8 ± 20.3 | 125.7 ± 20.8 | 0.007 |
| NT-proBNP*, (pg/mL) | 55.2 (24.5 -112.6) | 61.5 (27.3 -118.8) | 64.1 (29.8 -130.4) | 58.7 (26.2 -114.7) | 51.6 (25.1 -109.8) | 42.0 (18.1 -86.9) | <0.001 |
| hs-cTnT*, (ng/L) | 4.4 (3.0 -7.5) | 4.1 (3.0 -6.9) | 4.3 (3.0 -7.0) | 4.4 (3.0 -7.5) | 4.6 (3.0 -7.9) | 4.8 (3.0 -8.2) | <0.001 |
eGFR=estimated glomerular filtration rate
Results are mean ± SD and count (%) unless otherwise specified
Expressed as median (25th - 75th percentile)
Derived for one-way ANOVA for normally distributed continuous variables and Kruskal -Wallis test for skewed continuous variables, chi-square test for categorical variables
NB: P-for-trend was derived by using ESI categories as an ordinal variable and modeling this as a continuous variable in linear regression models using each baseline characteristic as the outcome.
The average adjusted baseline cross-sectional differences in hs-cTnT and NT-proBNP, according to ESI quintile, are shown in Table 2. Compared to the reference quintile (i.e. quintile 2), a statistically significant higher hs-cTnT level was seen in quintile 3 and 5, albeit in a non-graded fashion given the lack of a significant difference in quintile 4. Continuous analysis via linear (Table 2) and restricted-cubic-splines (Figure 2, Panel A) demonstrated a similar cross-sectional trend, with the strongest association between ESI and hs-cTnT seen in an ESI range of 0.2 – 2.4 g/day. In contrast, there was no cross-sectional relationship between ESI and NT-NTproBNP (Table 2 and Figure 2, Panel B).
Table 2.
Average adjusted* cross-sectional differences in hs-cTnT andNT-proBNP (both log transformed) according to estimated sodium intake quintile at MESA Exam 1.
| CATEGORICAL ANALYSIS | CONTINUOUS ANALYSIS |
||||||
|---|---|---|---|---|---|---|---|
| Q1 (0.2-1.3 g/d) |
Q2 (1.3-1.8 g/d) |
Q3 (1.8-2.4 g/d) |
Q4 (2.4-3.2 g/d) |
Q5 (3.2-9.9 g/d) |
Per 1g/day increment in ESI (≤ 2.4g/day) |
Per 1g/day increment in ESI (> > 2.4g/day) |
|
| ln hs-cTnT, ng/L | |||||||
| N | 1224 | 1222 | 1224 | 1223 | 1224 | 3,687 | 2,430 |
| Model 1 | −0.018 (−0.059, 0.022) | Ref=0 | 0.044 (0.004, 0.085) | 0.033 (−0.007, 0.074) | 0.071 (0.029, 0.112) | 0.043 (0.016, 0.069) | 0.015 (−0.003, 0.032) |
| Model 2 | −0.011 (−0.049, 0.027) | Ref=0 | 0.042 (0.004, 0.080) | 0.029 (−0.009, 0.067) | 0.049 (0.010, 0.088) | 0.034 (0.009, 0.060) | 0.007 (−0.009, 0.024) |
| Model 3 | −0.009 (−0.047, 0.029) | Ref=0 | 0.043 (0.005, 0.081) | 0.030 (−0.008, 0.068 | 0.049 (0.011, 0.088) | 0.034 (0.009, 0.060) | 0.006 (−0.010, 0.023) |
| ln NT-proBNP, pg/mL | |||||||
| N | 1,000 | 1,022 | 1,000 | 1,018 | 1,033 | 3,038 | 2,035 |
| Model 1 | −0.117 (−0.199, −0.036) | Ref=0 | 0.041 (−0.040, 0.123) | 0.016 (−0.065, 0.098) | −0.069 (−0.151, 0.013) | 0.093 (−0.038, 0.147) | −0.044 (−0.079, −0.008) |
| Model 2 | −0.065 (−0.143, 0.013) | Ref=0 | 0.030 (−0.048, 0.108) | 0.005 (−0.073, 0.083) | −0.064 (−0.143, 0.015) | 0.049 (−0.004, 0.102) | −0.028 (−0.062, 0.006) |
| Model 3 | −0.057 (−0.134, 0.02) | Ref=0 | 0.032 (−0.045, 0.109) | 0.009 (−0.068, 0.086) | −0.064 (−0.142, 0.014) | 0.047 (−0.006, 0.099) | −0.030 (−0.064, 0.004) |
Estimates are β coetticients
Model 1: age and sex.
Model 2: Model 1 plus race/ ethnicity, body mass index, smoking status, alcohol consumption, total cholesterol, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, lipid-lowering medication use, blood pressure-lowering medication, and history of diagnosed diabetes.
Model 3: Model 2 plus systolic BP
NB: Linear spline 1 measures the slope for ESI ≤ 2.4g/day and Linear spline 2 measures the slope for ESI> 2.4g/day
Figure 2 (A-B):
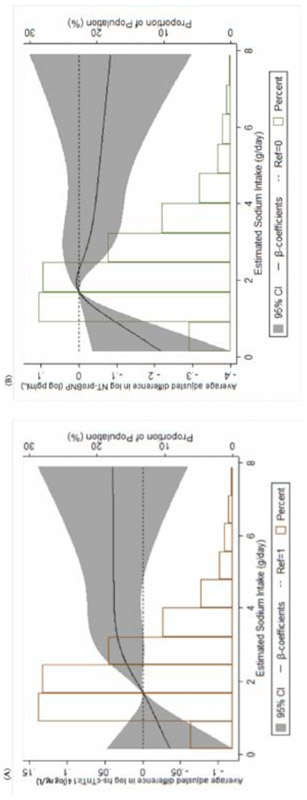
Panel A shows a restricted-cubic-spline plot of the association Between estimated sodium intake and hs-cTnT (log transformed) at MESA Exam 1. Panel B shows a restricted-cubic-spline plot of the association between estimated sodium intake and NT-proBNP (log-transformed) at MESA Exam 1. 95% confidence intervals are depicted within the span of the gray bars. All results are presented following multivariable adjustment.
The average adjusted 5-year differences in hs-cTnT and NT-proBNP, according to ESI, between MESA examinations 1 and 3 are shown in Table 3 and Figure 3. There was no consistent association between baseline ESI quintile and temporal change in hs-cTnT, though there appeared to be excess troponin increases at very low ESI values (quintile 1). Compared to the reference quintile, a statistically significant increase in NT-proBNP was evident between quintile 4 and 5, a trend which was further manifest in both the linear (Table 3) and restricted cubic (Figure 3, Panel B) spline analyses. Unlike the cross-sectional relationship with hs-cTnT, the longitudinal association of ESI with NT-proBNP was driven predominantly by a strong relationship at ESI levels ≥2.4 g/day.
Table 3.
Estimated sodium intake and average adjusted* 5-year change in hs-cTnT andNT-proBNP (both log transformed) at MESA Exam 3.
| CATEGORICAL ANALYSIS | CONTINUOUS ANALYSIS | ||||||
|---|---|---|---|---|---|---|---|
| Q1 (0.2- 1.3 g/d) |
Q2 (1.3- 1.8 g/d) |
Q3 (1.8- 2.4 g/d) |
Q4 (2.4- 3.2 g/d) |
Q5 (3.2-9.9 g/d) |
Per 1g/day increment in ESI (≤ 2.4g/day) |
Per 1g/day increment in ESI (> > 2.4g/day) |
|
| ln hs-cTnT, ng/L | |||||||
| N | 663 | 692 | 667 | 706 | 686 | 3,687 | 2,430 |
| Model 1 | 0.069 (0.005, 0.133) | Ref=0 | 0.018 (−0.046, 0.082) | 0.041 (−0.022, 0.104) | 0.031 (−0.033, 0.094) | −0.020 (−0.063, 0.022) | 0.011 (−0.019, 0.041) |
| Model 2 | 0.067 (0.004, 0.131) | Ref=0 | 0.018 (−0.046, 0.082) | 0.043 (−0.020, 0.106) | 0.034 (−0.029, 0.097) | −0.018 (−0.060, 0.025) | 0.011 (−0.018, 0.041) |
| Model 3 | 0.067 (0.003, 0.131) | Ref=0 | 0.018 (−0.045, 0.082) | 0.043 (−0.020, 0.106) | 0.034 (−0.029, 0.0 97) | −0.017 (−0.060, 0.025) | 0.011 (−0.019, 0.041) |
| ln NT-proBNP, pg/mL | |||||||
| N | 849 | 890 | 883 | 896 | 914 | 3,038 | 2,035 |
| Model 1 | 0.025 (−0.023, 0.073) | Ref=0 | 0.007 (−0.040, 0.054) | 0.058 (0.011, 0.105) | 0.113 (0.066, 0.160) | 0.021 (−0.011, 0.052) | 0.030 (0.009, 0.051) |
| Model 2 | 0.023 (−0.025, 0.071) | Ref=0 | 0.005 (−0.042, 0.052) | 0.056 (0.009, 0.103) | 0.110 (0.063, 0.157) | 0.021 (−0.011, 0.053) | 0.029 (0.008, 0.051) |
| Model 3 | 0.023 (−0.025, 0.071) | Ref=0 | 0.006 (−0.042, 0.053) | 0.055 (0.008, 0.103) | 0.110 (0.063, 0.157) | 0.020 (−0.011, 0.052) | 0.029 (0.008, 0.050) |
Estimates are β coefficients
Model 1: age and sex.
Model 2: Model 1 plus race/ ethnicity, body mass index, smoking status, alcohol consumption, total cholesterol, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, lipid-lowering medication use, blood pressure–lowering medication, and history of diagnosed diabetes.
Model 3: Model 2 plus systolic BP
NB: Linear spline 1 measures the slope for ESI ≤ 2.4g/day and Linear spline 2 measures the slope for ESI > 2.4g/day
Figure 3 (A-B):
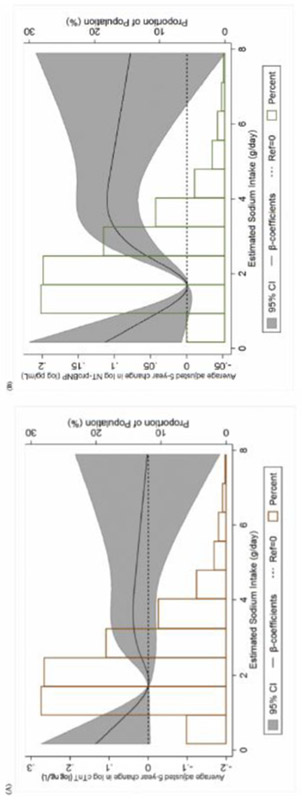
Panel A shows a restricted-cubic-spline plot of the association between baseline estimated sodium intake and 5-year change in hs-cTnT (log-transformed) at MESA Exam 3. Panel B shows a restricted-cubic-spline plot of the association between baseline estimated sodium intake and 5-year change in NT-proBNP (log-transformed) at MESA Exam 3. 95% confidence intervals are depicted within the span of the gray bars. All results are presented following multivariable adjustment.
The odds of prevalent and incident hs-cTnT ≥14 ng/L and NT-proBNP ≥100 pg/mL at MESA examinations 1 and 3, along with their associated linear and cubic restricted splines, are shown in Tables 4 and 5. While point estimates for the odds ratios in these tables were generally consistent with the results in Tables 2 and 3 (i.e., evidence of higher odds for prevalent hs-cTnT ≥14 ng/L and for incident NT-proBNP ≥100 pg/mL according to higher baseline ESI quintile), these categorical results were mostly not statistically significant. Precision was, however, limited. Also consistent with the above results, analysis of linear (Supplemental Table 1) and cubic (Supplemental Figure 2) restricted splines demonstrate a strong association between an ESI ≥2.4 g/day and the odds of a ≥25% temporal increase in NT-proBNP, but not hs-cTnT. Supplemental Table 1 demonstrates the odds of prevalent and incident hs-cTnT ≥14 ng/L and NT-proBNP ≥100 pg/mL at examinations 1 and 3, as well the odds of a ≥25% increase in these parameters from baseline. Sensitivity analyses performed following the exclusion of individuals with either baseline hypertension or on anti-hypertensive therapy corroborated similar cross-sectional (Supplemental Table 2) and longitudinal (Supplemental Table 3) associations with hs-cTnT and NT-proBNP, respectively.
Table 4.
Odds Ratios (95% Cl) of prevalent hs-cTnT≥14 ng/L and NT-proBNP≥100 pg/mL at MESA Exam 1 according to estimated sodium intake quintile.
| Q1 (0.2-1.3 g/d) |
Q2 (1.3-1.8 g/d) |
Q3 (1.8-2.4 g/d) |
Q4 (2.4-3.2 g/d) |
Q5 (3.2-9.9 g/d) |
|
|---|---|---|---|---|---|
| hs-cTnT≥14 ng/L | 68 (5.6%) | 72 (5.9%) | 101 (8.3%) | 93 (7.6%) | 102 (8.3%) |
| Model 1 | 1.03 (0.72, 1.46) | Ref=1 | 1.47 (1.06, 2.05) | 1.22 (0.87, 1.70) | 1.40 (1.00, 1.95) |
| Model 2 | 1.06 (0.72, 1.54) | Ref=1 | 1.52 (1.07, 2.16) | 1.26 (0.89, 1.80) | 1.37 (0.96, 195) |
| Model 3 | 1.07 (0.73, 1.56) | Ref=1 | 1.54 (1.08, 2.19) | 1.27 (0.89, 1.82) | 1.38 (0.97, 1.96) |
| NT -proBNP≥100 pg/mL | 315 (31.5%) | 351 (34.3%) | 290 (29%) | 279 (27.4%) | 224 (21.7%) |
| Model 1 | 0.76 (0.62, 0.93) | Ref=1 | 0.95 (0.77, 1.17) | 0.95 (0.77, 1.17) | 0.83 (0.66, 1.03) |
| Model 2 | 0.81 (0.66, 1.01) | Ref=1 | 0.93 (0.75, 1.15) | 0.93 (0.75, 1.15) | 0.83 (0.66, 1.05) |
| Model 3 | 0.83 (0.67, 1.03) | Ref=1 | 0.93 (0.75, 1.15) | 0.94 (0.75, 1.16) | 0.82 (0.66, 1.04) |
Model 1: age and sex.
Model 2: Model 1 plus race/ ethnicity, body mass index, smoking status, alcohol consumption, total cholesterol, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, lipid-lowering medication use, blood pressure–lowering medication, and history of diagnosed diabetes.
Model 3: Model 2 plus systolic BP
Table 5.
Odds Ratios (95% Cl) of incident hs-cTnT≥14 ng/L and NT-proBNP≥100 pg/mL at MESA Exam 3 according to estimated sodium intake quintile.
| Q1 (0.2-1.3 g/d) |
Q2 (1.3-1.8 g/d) |
Q3 (1.8-2.4 g/d) |
Q4 (2.4-3.2 g/d) |
Q5 (3.2-9.9 g/d) |
|
|---|---|---|---|---|---|
| hs-cTnT≥14 ng/L | 43 (7%) | 44 (7%) | 40 (6.8%) | 56 (8.9%) | 54 (8.9%) |
| N | 611 | 630 | 593 | 631 | 607 |
| Model 1 | 1.11 (0.71, 1.73) | Ref=1 | 0.96 (0.61, 1.52) | 1.20 (0.79, 1.84) | 1.31 (0.85, 2.02) |
| Model 2 | 1.14 (0.72, 1.80) | Ref=1 | 0.98 (0.61, 1.55) | 1.17 (0.76, 1.80) | 1.25 (0.81, 1.95) |
| Model 3 | 1.15 (0.73, 1.81) | Ref=1 | 0.98 (0.62, 1.57) | 1.16 (0.75, 1.80) | 1.24 (0.79, 1.93) |
| NT -proBNP≥100 pg/mL | 103 (18.3%) | 112 (19.7%) | 119 (19.8%) | 112 (17.9%) | 105 (15.6%) |
| N | 563 | 569 | 601 | 627 | 675 |
| Model 1 | 0.86 (0.63, 1.17) | Ref=1 | 1.20 (0.89, 1.63) | 1.01 (0.75, 1.38) | 1.02 (0.75, 1.39) |
| Model 2 | 0.89 (0.65, 1.23) | Ref=1 | 1.23 (0.90, 1.67) | 1.05 (0.77, 1.44) | 1.10 (0.80, 1.51) |
| Model 3 | 0.90 (0.65, 1.23) | Ref=1 | 1.22 (0.89, 1.66) | 1.04 (0.76, 1.42) | 1.10 (0.80, 1.52) |
Model 1: age and sex.
Model 2: Model 1 plus race/ ethnicity, body mass index, smoking status, alcohol consumption, total cholesterol, high-density lipoprotein cholesterol, triglycerides, estimated glomerular filtration rate, lipid-lowering medication use, blood pressure–lowering medication, and history of diagnosed diabetes.
Model 3: Model 2 plus systolic BP
DISCUSSION:
Our study demonstrates that ESI is associated with hs-cTnT predominantly in a cross-sectional manner. In particular, ESI appears positively associated with hs-cTnT when ESI was within a range of 0.2 – 2.4 g/day, while beyond ESI 2.4 g/day the association plateaued. In contrast, ESI is only associated with NT-proBNP in a longitudinal manner, with significant increases in NT-proBNP occurring over the 5 years of follow up. Unlike the association with hs-cTnT, this association appears to be driven primarily by a strong correlation at ESI ≥2.4 g/day. Indeed, ESI in this range was also linked to an increased odds of ≥25% increase in NT-proBNP from baseline at the 5-year examination. Furthermore, in longitudinal (but not cross-sectional analyses) there appeared to be a possible J-curve phenomenon for elevated hs-cTnT and NT-proBNP at low ESI, though this may have been due to chance given there were no consistent, significant findings for a J-curve with either hs-cTnT or NT-proBNP in any of the other analyses performed. To the best of our knowledge, the temporal differences in the impact of sodium excess on these important markers of cardiovascular disease has not been previously described.
The pathobiological basis upon which incremental increases in ESI result in elevations in hs-cTnT in the short-term and NT-proBNP in the long-term is not clear, but are likely to be multifactorial. Clearly established as the gold standard diagnostic and prognostic instrument in acute coronary syndrome patients, by enabling the detection of significantly lower concentrations of troponin than conventional assays, the advent of hs-cTnT has extended the application of this test to prognostication in otherwise stable disease states 15. In addition to detecting minute levels of myocyte necrosis, other proposed pathophysiologic phenomena unrelated to necrosis –including increased cell wall permeability, production of troponin-containing membranous blebs, cellular release of proteolytic degradation products and apoptosis – may now be detectable with the increasingly sensitive assays 16,17. In animal models, salt-loading even in the short term has been linked to several such derangements, and as such, could explain our observed cross-sectional relationship between ESI and hs-cTnT in the short term 18-20.
In contrast, BNP (as well as its n-terminal fragment) is a natriuretic hormone released from myocardial cells in a graded fashion following transcriptional activation in response to chronic increases in either atrial or ventricular wall tension 21,22. Given that the predominant downstream consequence of prolonged excessive dietary sodium intake is systemic hypertension, it is possible that in the short-term, the accrued burden of left ventricular remodeling is insufficient to yield significant increases in plasma NT-proBNP levels. Indeed, various animal models have shown that left ventricular hypertrophy and fibrosis only manifest following prolonged exposure to elevated systemic blood pressures23-25.
Complicating the aforementioned association between excess dietary sodium and cardiovascular events is controversial evidence supporting a “J-curve” relationship between dietary sodium and cardiovascular events 14,26-29. Indeed, in the largest study of its kind, the Prospective Urban Rural Epidemiology (PURE) research team reported that an ESI between 3 and 6 g/day was associated with a lower risk of cardiovascular events and mortality than was either a higher or lower estimated level of intake 14. However, our findings from MESA do not appear to corroborate the PURE findings in that we only found inconsistent evidence for elevated biomarkers at ESI levels well below those reported as harmful in PURE (ESI 0.2-1.3 g/day) in the longitudinal hs-cTnT analysis and not in any other analysis (a finding which could therefore have been due to chance). Thus, while we cannot definitely rule out a J-curve, our findings are more consistent with those reported by Cook and others, who analyzed both phases of the Trials of Hypertension Prevention (TOHP) program and demonstrated a direct linear relation of association of average sodium excretion with cardiovascular disease down to the lowest intake with mortality (6). Mechanistically, the subclinical myocardial damage rendered by hs-cTnT in the short term and by NT-proBNP in the longer term, may account for the linearity of this relationship.
There were several limitations to our study. First, although validated in a variety of nutritional intake studies, the inherent biases of FFQs (e.g. recall bias) as well as their other limitations (e.g. inability to account for within-person variability in intake when a single FFQ is used) must be acknowledged. Second, only baseline ESI data was available, and as such, change in ESI patterns over time was not studied. Third, we recognize that despite consistency between the point estimates for the odds ratios among both the categorical and continuous analyses, the former are inherently underpowered relative to continuous analyses and as such, may be more likely to miss true associations. Furthermore, we recognize the limitations of contextualizing the impact of incremental increases in ESI on biomarkers in absolute terms. Finally, as with any observational study, residual confounding may persist even beyond multivariate adjustment.
In summary, our results demonstrate a relationship between dietary sodium intake and hs-cTnT and NT-proBNP in cross-sectional and longitudinal fashions, respectively. Further study is needed to better define this relationship, and in particular, to further examine the possibility of a salt intake “J-curve” phenomenon from the perspective of these and other novel biomarkers. Doing so will enhance understanding of the mechanisms through which sodium intake influences cardiovascular risk.
Supplementary Material
Supplemental Figure 1 (A-D): Panel A shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent hs-cTnT ≥14 ng/L at MESA Exam 1. Panel B shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent NT-proBNP ≥100 pg/mL at MESA Exam 1. Panel C shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent hs-cTnT ≥14 ng/L at MESA Exam 3. Panel D shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent NT-proBNP ≥100 pg/mL at MESA Exam 3. 95% confidence intervals are depicted within the span of the gray bars. All results are presented following multivariable adjustment.
Supplemental Figure 2 (A-B): Panel A shows a restricted-cubic-spline plot of the association between estimated sodium intake and the odds ratio of ≥25% increase in hs-cTnT at MESA Exam 3. Panel B shows a restricted-cubic-spline plot of the association between estimated sodium intake and the odds ratio of ≥25% increase in NT-proBNP at MESA Exam 3. 95% confidence intervals are depicted within the span of the gray bars. All results are presented following multivariable adjustment.
Acknowledgments
MESA Funding Statement:
This research was supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. Additionally, Roche Diagnostics supported in-part the measure of biomarkers through an investigator initiated grant to the University of Maryland (PI: Christopher deFilippi). The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Disclosures: KK (nothing to disclose); OF (nothing to disclose); WSP (nothing to disclose); PLL (nothing to disclose); EDM (nothing to disclose); CRD (Roche Diagnostics, Inova Consulting Group); JWM (nothing to disclose)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 2.Cogswell ME, Loria CM, Terry AL, Zhao L, Wang C-Y, Chen T-C, Wright JD, Pfeiffer CM, Merritt R, Moy CS, Appel LJ. Estimated 24-Hour Urinary Sodium and Potassium Excretion in US Adults. JAMA 2018;319:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elijovich F, Weinberger MH, Anderson CAM, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL. Salt Sensitivity of Blood Pressure: A Scientific Statement From the American Heart Association. Hypertension 2016;68:e7–e46. [DOI] [PubMed] [Google Scholar]
- 4.McGuire S Scientific Report of the 2015 Dietary Guidelines Advisory Committee Washington, DC: US Departments of Agriculture and Health and Human Services, 2015; Adv Nutr 2016;7:202–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010; 121:586–613. [DOI] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DRJ, Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am JEpidemiol 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 7.Steinemann N, Grize L, Ziesemer K, Kauf P, Probst-Hensch N, Brombach C. Relative validation of a food frequency questionnaire to estimate food intake in an adult population. Food Nutr Res 2017;61:1305193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly C, Geaney F, Fitzgerald AP, Browne GM, Perry IJ. Validation of diet and urinary excretion derived estimates of sodium excretion against 24-h urine excretion in a worksite sample. NutrMetab Cardiovasc Dis 2015;25:771–779. [DOI] [PubMed] [Google Scholar]
- 9.McLean RM, Farmer VL, Nettleton A, Cameron CM, Cook NR, Campbell NRC. Assessment of dietary sodium intake using a food frequency questionnaire and. J Clin Hypertens (Greenwich) 2017; 19:1214–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvaraj S, Djousse L, Aguilar FG, Martinez EE, Polsinelli VB, Irvin MR, Arnett DK, Shah SJ. Association of Estimated Sodium Intake With Adverse Cardiac Structure and Function: From the HyperGEN Study. J Am Coll Cardiol 2017;70:715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McEvoy JW, Chen Y, Ndumele CE, Solomon SD, Nambi V, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E. Six-Year Change in High-Sensitivity Cardiac Troponin T and Risk of Subsequent Coronary Heart Disease, Heart Failure, and Death. JAMA Cardiol 2016;1:519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniels LB, Clopton P, deFilippi CR, Sanchez OA, Bahrami H, Lima JAC, Tracy RP, Siscovick D, Bertoni AG, Greenland P, Cushman M, Maisel AS, Criqui MH. Serial measurement of N-terminal pro-B-type natriuretic peptide and cardiac troponin T for cardiovascular disease risk assessment in the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 2015;170:1170–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blaha MJ, Cainzos-Achirica M, Greenland P, McEvoy JW, Blankstein R, Budoff MJ, Dardari Z, Sibley CT, Burke GL, Kronmal RA, Szklo M, Blumenthal RS, Nasir K. Role of Coronary Artery Calcium Score of Zero and Other Negative Risk Markers for Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016;133:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014;371:612–623. [DOI] [PubMed] [Google Scholar]
- 15.Sherwood MW, Kristin Newby L. High-sensitivity troponin assays: evidence, indications, and reasonable use. J Am Heart Assoc 2014;3:e000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol 2011; 57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 17.Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AHB, Roberts MS. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta 2010;411:318–323. [DOI] [PubMed] [Google Scholar]
- 18.Tsukamoto Y, Mano T, Sakata Y, Ohtani T, Takeda Y, Tamaki S, Omori Y, Ikeya Y, Saito Y, Ishii R, Higashimori M, Kaneko M, Miwa T, Yamamoto K, Komuro I. A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am J Physiol Heart Circ Physiol 2013;305:H1658–1667. [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto M, Fujita T. Renal mechanisms of salt-sensitive hypertension: contribution of two steroid receptor-associated pathways. Am J Physiol Renal Physiol 2015;308:F377–387. [DOI] [PubMed] [Google Scholar]
- 20.Weil BR, Suzuki G, Young RF, Iyer V, Canty JMJ. Troponin Release and Reversible Left Ventricular Dysfunction After Transient Pressure Overload. J Am Coll Cardiol 2018;71:2906–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Globits S, Frank H, Pacher B, Huelsmann M, Ogris E, Pacher R. Atrial natriuretic peptide release is more dependent on atrial filling volume than on filling pressure in chronic congestive heart failure. Am Heart J 1998;135:592–597. [DOI] [PubMed] [Google Scholar]
- 22.Iwanaga Y, Nishi I, Furuichi S, Noguchi T, Sase K, Kihara Y, Goto Y, Nonogi H. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol 2006;47:742–748. [DOI] [PubMed] [Google Scholar]
- 23.Santos M, Shah AM. Alterations in cardiac structure and function in hypertension. Carr Hypertens Rep 2014; 16:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomek J, Bub G. Hypertension-induced remodelling: on the interactions of cardiac risk factors. J Physiol 2017;595:4027–4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petriz BA, Franco OL. Effects of hypertension and exercise on cardiac proteome remodelling. BiomedRes Int 2014;2014:634132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mente A, O’Donnell MJ, Yusuf S. How Robust Is the Evidence for Recommending Very Low Salt Intake in Entire Populations? J Am Coll Cardiol 2016;68:1618–1621. [DOI] [PubMed] [Google Scholar]
- 27.Cook NR, Appel LJ, Whelton PK. Sodium Intake and All-Cause Mortality Over 20 Years in the Trials of Hypertension Prevention. J Am Coll Cardiol 2016;68:1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mancia G, Oparil S, Whelton PK, McKee M, Dominiczak A, Luft FC, AlHabib K, Lanas F, Damasceno A, Prabhakaran D, La Torre G, Weber M, O’Donnell M, Smith SC, Narula J. The technical report on sodium intake and cardiovascular disease in low- and middle-income countries by the joint working group of the World Heart Federation, the European Society of Hypertension and the European Public Health Association. Ear Heart J 2017;38:712–719. [DOI] [PubMed] [Google Scholar]
- 29.Graudal N, Jurgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens 2014;27:1129–1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 (A-D): Panel A shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent hs-cTnT ≥14 ng/L at MESA Exam 1. Panel B shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent NT-proBNP ≥100 pg/mL at MESA Exam 1. Panel C shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent hs-cTnT ≥14 ng/L at MESA Exam 3. Panel D shows a restricted-cubic-spline plot of the association between estimated sodium intake with prevalent NT-proBNP ≥100 pg/mL at MESA Exam 3. 95% confidence intervals are depicted within the span of the gray bars. All results are presented following multivariable adjustment.
Supplemental Figure 2 (A-B): Panel A shows a restricted-cubic-spline plot of the association between estimated sodium intake and the odds ratio of ≥25% increase in hs-cTnT at MESA Exam 3. Panel B shows a restricted-cubic-spline plot of the association between estimated sodium intake and the odds ratio of ≥25% increase in NT-proBNP at MESA Exam 3. 95% confidence intervals are depicted within the span of the gray bars. All results are presented following multivariable adjustment.


