Abstract
Background/Aim: The aim of the study was to investigate the efficacy of neoadjuvant and chemotherapy (NACT) and adjuvant chemotherapy (ACT) in Hispanic/Latino (H/L) women with TNBC. Patients and Methods: We reviewed the charts of patients with TNBC, stages I–III, treated at TTUHSC from 2006 to 2016. Overall survival (OS) and recurrence-free survival (RFS) were estimated and compared between the treatment groups. Kaplan–Meier curve and Cox proportional hazards regression analyses were conducted to estimate unadjusted and adjusted effects of NACT compared to ACT. Results: A total of 104 patients with TNBC, 30 (29%) received NACT and 74 (71%) ACT. Women undergoing NACT were younger, with a mean age of 50.8 years. Of the 30 patients who received NACT, 12 (40%) had pathologically complete response (pCR). Women who achieved pCR had an excellent RFS (HR=0.5, p=0.001). Women with residual cancer after NACT had worse outcome compared to patients who received ACT (HR=1.7, p=0.005). Conclusion: pCR to NACT is a powerful surrogate for OS in H/L women with TNBC.
Keywords: Triple-negative breast cancer, neoadjuvant chemotherapy, adjuvant chemotherapy, racial disparity, Hispanic/Latino women, locally advancer cancer
Breast cancer is the second leading cause of cancer death after lung cancer. In 2019, the American Cancer Association estimated that there were 252,700 new cases of breast cancer in the United States and 41,000 deaths from the disease (1,2). Breast cancer is a heterogeneous disease, biologically characterized by the expression of one or more steroid hormone receptors such as estrogen receptor (ER) or progesterone receptor (PR) along with the oncogene-epidermal growth factor receptor ErbB2 (Her-2neu). Of those subtypes, triple-negative breast cancer (TNBC) is biologically defined by the absence of expression of ER, PR, and Her-2neu receptors (3). It is a heterogenic subgroup of breast cancer that comprises approximately 15% of all types of breast cancer. TNBC is highly aggressive and has worse disease-specific outcomes than any other subtype of breast cancer (3,4). One possible explanation is the absence of well-defined molecular targets such as ER, PR, or Her2-neu, which limits options for treating this subtype of breast cancer. Systemic chemotherapy has remained the only available option for these patients for almost two decades. Historically, NACT has been used to downstage unresectable breast cancer to allow for better locoregional control and to increase the chance for breast-conserving surgery. It has been recognized that NACT represents an excellent in vivo approach to directly test tumor sensitivity to chemotherapy. Unfortunately, the large National Surgical Adjuvant Breast and Bowel Project (NSABP) randomized clinical trial B-18 did not show any statistically significant difference in the overall survival (OS) between patients who underwent NACT and those who underwent adjuvant chemotherapy (ACT) (5). There was, however, a trend in favor of NACT in women younger than 50 years of age. The major limitation of the B-18 trial was the lack of subgroup analysis by hormonal status. Furthermore, multiple published retrospective clinical studies comparing the efficacy of NACT and ACT showed conflicting results, particularly among TNBC patients (6-8). Recently, Clifton et al. retrospectively analyzed the efficacy of NACT in TNBC patients. They concluded that NACT and ACT lead to similar disease-free survival and OS. However, all patients with stage III breast cancer were excluded from the study, which limits its generalizability (9). Another study by Fisher et al., showed significantly worse survival for patients with residual disease after NACT compared with patients who received ACT (HR=0.51, p=0.007) in a retrospective analysis of 385 patients with TNBC (stages I-III) (8). In another retrospective observation study, Kennedy et al. reported that women who received ACT were less likely to die over the observation period compared with women who received NACT (6). Chavez-Mac Gregor M et al. evaluated NACT and pathologic complete response (pCR) in patients with stage II and III hormonal receptor-positive and negative breast cancer. This large study comprised 2,074 patients with 15% Hispanic/Latino (H/L), 64% Non-Hispanic White (NHW), 15% African American (AA), and 6% others. There was no statistically significant difference in pCR among racial/ethnic groups. Furthermore, while prior studies examined the efficacy of NACT in TNBC, there have been very limited data comparing NACT with ACT in a cohort of H/L women with TNBC. In general, H/L women represent less than 19% of patients in most currently published studies (5,6,8-11).
There are notable differences in breast cancer incidence and mortality between various racial and ethnic groups in the USA. The age-adjusted incidence of breast cancer per 100,000 is 128 for non-Hispanic White (NHW) women, 125 for African American (AA) women, 92 for H/L women, and about 80 for Native American women (1,2,7). Some studies suggest that the prevalence of TNBC among H/L women is 23.1% and that the onset of the disease is approximately 11 years younger than the average age reported for NHW and AA women (10,12-14). Despite the relatively low incidence of breast cancer among H/L women, their risk of mortality is higher than that of NHW women (15). The reasons for this remain unclear, but may include multiple factors such as socioeconomic status, culture, limited health-care and access to health-care, and the biology of the breast cancer, which could reflect differences in gene expression profile between NHW, AA, and H/L women (7,14).
In summary, the efficacy of NACT compared with ACT is still debatable in many aspects, but in general, multiple studies have demonstrated the similar efficacy for recurrence free survival (RFS) and OS, the only exception being patients with pCR after NACT (6,8,11,16,17). There is a lack of studies evaluating the efficacy of NACT and ACT on RFS or OS among H/L TNBC patients, particularly Mexican Americans. Therefore, we evaluated OS and RFS between H/L TNBC patients who received NACT and ACT at our institution.
The aim of this study was to analyze the outcomes, including OS and RFS, in H/L women with stage I-III TNBC.
Patients and Methods
Patient population. Institutional review board approval was obtained prior to starting this research project. Due to the retrospective nature of the study, written informed consent was not required. We retrospectively reviewed the clinical databases of the Texas Tech Breast Care Center and the University Medical Center in El Paso, Texas, from January 2006 to December 2016 to identify all patients with the diagnosis of stage I-III invasive TNBC who received either NACT or ACT. Only newly registered cases were extracted from the databases. Any patients with incomplete data on outcomes (OS or RFS) were excluded from the study.
Pathologic assessment. Pathological diagnosis, ER/PR status, and Her-2/neu status were determined by core biopsy during initial evaluation, before NACT or surgery. Standard immunohisto-chemical (IHC) staining was used to determine hormonal receptor status. All tumors with less than 1% stained cells were considered to have a negative hormonal receptor status. The oncogene-epidermal growth factor receptor Her-2neu status was evaluated by IHC staining only if it scored 0 or 1+.
For specimens that scored 2+, fluorescence in situ hybridization was used for confirmation.
Outcome measures and statistical analysis. The primary outcome for this study was OS, which was defined as the time from the initiation of systemic chemotherapy to the date of death or date of last follow-up. The secondary endpoint was RFS, defined as the time from date of breast cancer diagnosis to the date of local recurrence or metastasis, whichever occurred first. The date of death or last follow-up was considered as censored time. The OS and RFS between different groups were estimated using the Kaplan–Meier method, summarized with 95% confidence interval (CI), and compared with the log-rank test.
The baseline characteristics between treatment groups (NACT versus ACT) were compared using Fisher’s exact tests. Multivariable Cox proportional-hazards regression models were used to determine the adjusted effect of treatment and to identify factors that were independently associated with OS and RFS. The results of Cox analyses were presented with Hazards Ratio (HR), 95%CI, and p-value. All analyses were two-sided, and significance was set at a p-value of 0.05. Statistical analyses were carried out using STATA 15.
Results
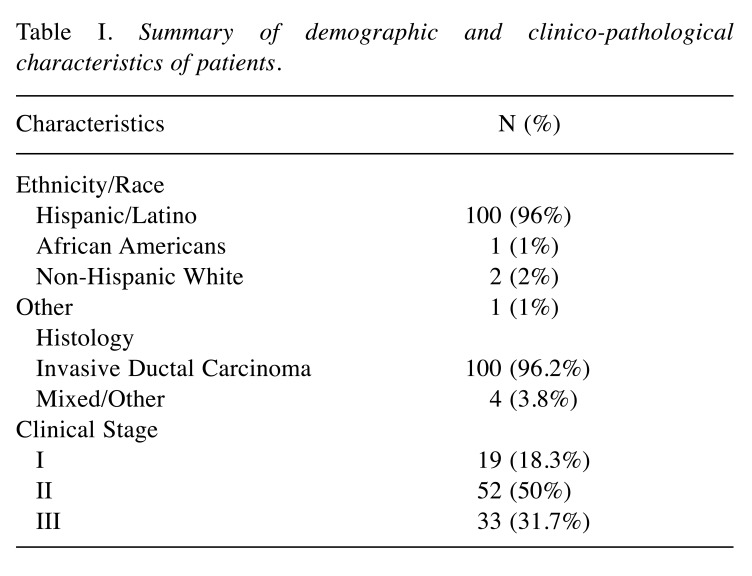
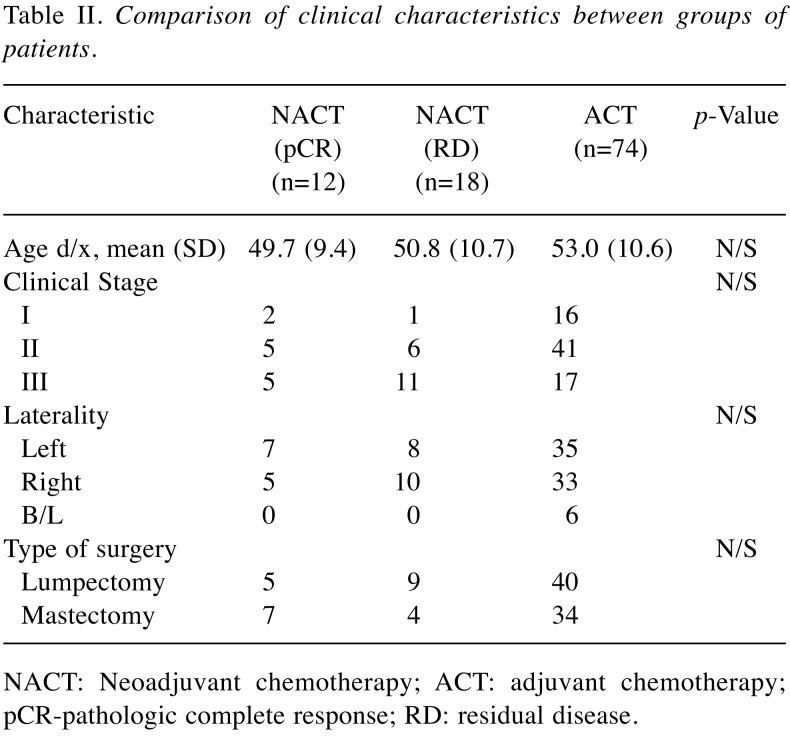
During the study period from 2006-2016, we identified 104 patients with a pathologically confirmed diagnosis of TNBC, stages I-III, who were treated at our institution and met the eligibility criteria. Patients demographic and tumor clinical characteristics are shown in Table I. The majority of patients were postmenopausal at diagnosis, 50 years of age or older (n=69, 60%). The majority of patients with TNBC were H/L (n=104, 90%). Of 104 patients, (n=30, 27.8%) were treated with NACT and (n=74, 72.2%) received ACT postoperatively. The relationship between clinicopathological characteristics of patients with TNBC treated with NACT versus ACT are summarized in Table II. Women undergoing NACT had a more advanced clinical stage at the time of diagnosis (53.3% with stage III in the NACT group compare with 22.9% in the ACT group). The groups did not differ significantly in terms of tumor histology or laterality or primary tumor. The chemotherapy regimens varied overall but did not differ between the groups. The most common regimen was Adriamycin and Taxane based in both groups (Table III).
Table I. Summary of demographic and clinico-pathological characteristics of patients.
Table II. Comparison of clinical characteristics between groups of patients.
NACT: Neoadjuvant chemotherapy; ACT: adjuvant chemotherapy; pCR-pathologic complete response; RD: residual disease.
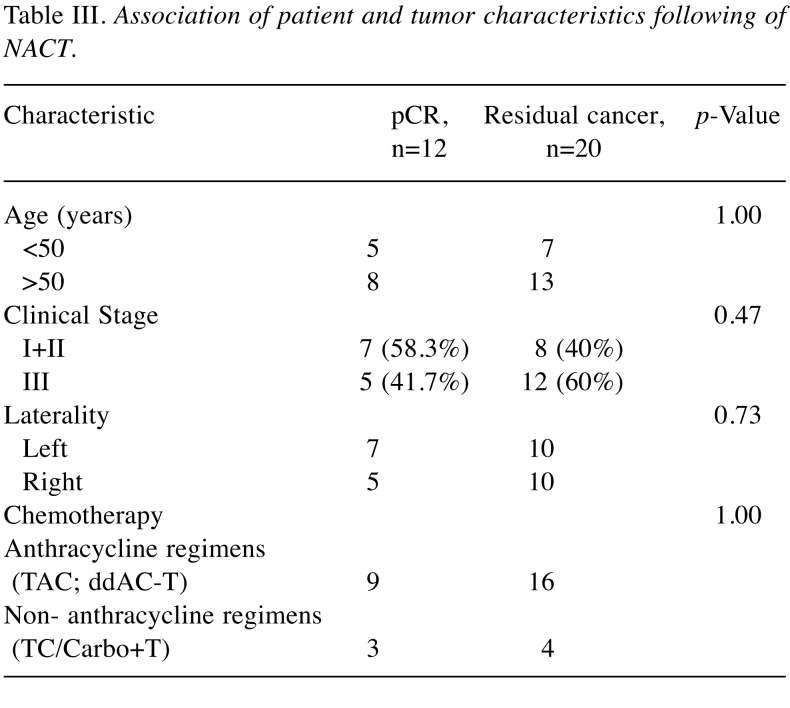
Table III. Association of patient and tumor characteristics following of NACT.
The overall pCR rate in this study was 40%. The median follow-up time was six years (range=1-11 years). OS rate at five years among patients that received ACT was 82% (95%CI=0.71-0.89). In NACT group, OS was 54% (95%CI=0.29-0.74) among patients with residual disease after NACT.
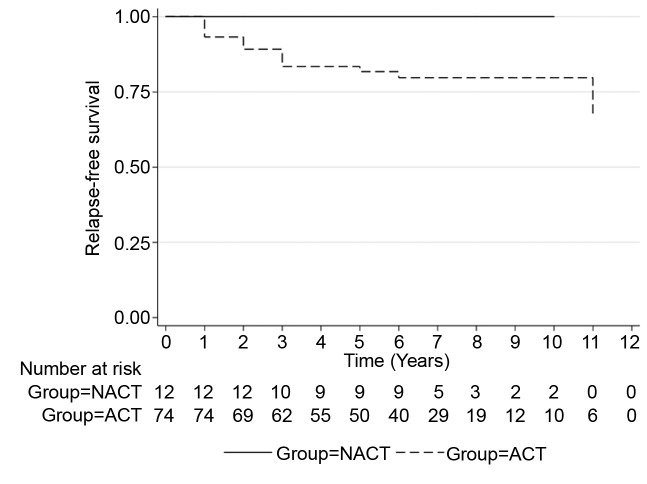
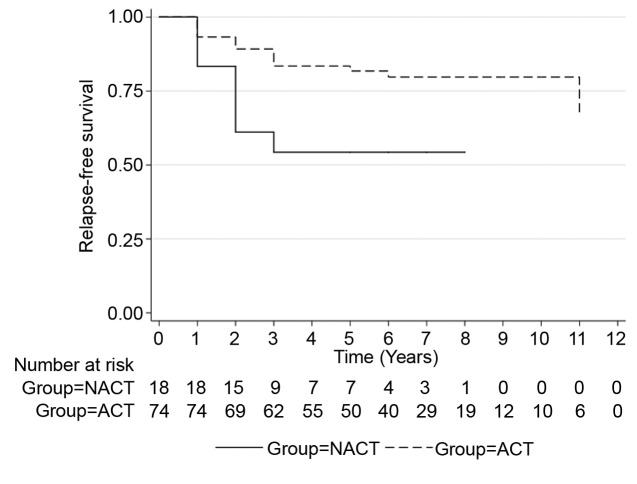
During the observation period, a total of 20% of patients had a recurrence in the ACT group whereas 44% in the NACT group with residual disease. There were no recurrences in the NACT group with pCR. Of 30 patients who underwent NACT, (n=12, 40%) achieved pCR. Figure 1 and Figure 2 illustrate the RFS between treatment groups. Unadjusted analyses showed significantly worse RFS for patients with residual disease after NACT compared with the ACT group (HR=0.35, 95%CI=0.14-0.83, p=0.017). Table III summarizes the patient and tumor characteristics of the two groups as per pathological response status. In general, patients with an early stage (I and II) of cancer had a better chance of achieving pCR (n=8, 66.7%) compared with patients with stage III disease (n=4, 33.3%).
Figure 1. PFS NACT (RD) vs. ACT.
Figure 2. PFS NACT (cPR) vs. ACT.
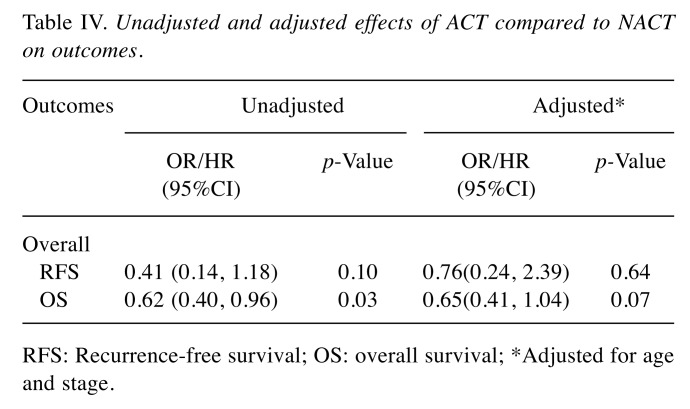
Table IV displays the unadjusted and adjusted effects of ACT compared to NACT on OS and RFS outcomes. In the unadjusted analysis, the ACT group had a significantly lower risk of death (HR=0.62, 95%CI=0.40-0.96, p=0.03) compared with patients in the NACT group. After controlling for all covariates associated with survival, a significantly higher OS rate (HR=0.65, 95%CI=0.41-1.04, p=0.07) was found in the ACT group compared with the NACT group. Although not statistically significant, a reduced risk of recurrence was also observed in the ACT group (HR=0.41, 95%CI=0.14-1.18, p=0.10) compared to the NACT group. After adjusting for potential confounders, no difference in the risk of recurrence was observed between the groups (HR=0.76, 95%CI=0.24-2.39, p=0.64). Among all considered covariates, only the advanced stage patients were independently associated with increased risk of RFS (HR=4.53, 95%CI=1.40-14.7, p=0.012). Patients undergoing ACT were less likely to die during the follow-up period compared to patients undergoing NACT (HR=0.62, 95%CI=0.40-0.96). Compared to patients who underwent NACT and had residual disease, patients who received ACT showed significantly better OS (HR=0.5, 95%CI=0.020.88), based on univariate analysis. On the other hand, patients with stage I and II TNBC who received NACT and had residual disease had a 90% greater chance of recurrence compared with patients in the ACT group (HR=0.10, 95%CI=0.03-0.37, p=0.001). Five-year survival was 100% for patients who received NACT and achieved the pCR, 82% for patients who received ACT, and 54% for patients who received NACT and had residual disease.
Table IV. Unadjusted and adjusted effects of ACT compared to NACT on outcomes.
RFS: Recurrence-free survival; OS: overall survival; *Adjusted for age and stage.
Discussion
Triple-negative breast cancer represents the most aggressive subtype of breast cancer without any available targeted therapy. For more than two decades, systemic chemotherapy has been the only option for these patients. The role of NACT has evolved over past decades. For unknown reasons, this particular type of breast cancer is highly sensitive to systemic chemotherapy, with a reported pCR in the neoadjuvant setting approaching 45% (18-20). This is in contrast to findings for other subtypes of breast cancer, for which NACT demonstrates very modest benefits, with reported pCR approaching 20% (5,17).
In addition to the highest pathological response rate, NACT presents many advantages for patients. First, it helps to reduce the size of the primary tumor, thus allowing patients to undergo breast-conserving surgery, which would be impossible otherwise. The second advantage is that NACT can eradicate tumor cells from axillary lymph nodes, which helps downstage the cancer and avoid the morbid procedure of axillary lymph node dissection. The third benefit is that it provides an important in vivo observation of the chemosensitivity of the tumor (21).
Despite these advantages, it is still debatable if the use of NACT translates into survival benefits compared with ACT (5,6,8,17,21). Multiple clinical trials in the past have reported conflicting results regarding survival benefits of NACT versus ACT (5,6,8). In the vast majority of previously reported studies using NACT, the distribution of patients was not equal in terms of racial/ethnic subgroups because there was a predominant representation of NHW and AA and significant under-representation of the H/L ethnic group (7,15,22). More importantly, no previous NACT clinical trials have demonstrated survival benefits compared with ACT for H/L patients with TNBC.
A number of studies have examined the differential responses and survival outcomes among women of different racial groups in response to chemotherapy and have shown conflicting results (23). Dawood S. et al. demonstrated that NACT and ACT have comparable efficacy among patients with breast cancer, at least when comparing black patients with white patients (23). Another study evaluated NACT and pCR with anthracycline- and taxane-based NACT in patients with stage II and III breast cancer. Of 2,074 total patients, 6% were AA, 15.2% H/L, 64.3% NHW, and 5.9% other. There were almost no differences in pCR among racial/ ethnic groups (12.5% in AA, 14.24% in H/L, 12.3% in NHW, and 11.5% in others, p=0.788). Although the authors concluded that race/ethnicity was not a predictor of survival outcome, there appeared to be a trend toward improvement in relapse-free survival and OS in H/L women (24).
It has been well documented that H/L and AA women have a slightly lower incidence of breast cancer compared with NHW women, but in contrast they have generally higher mortality rates and a much higher incidence of TNBC (25,26). The reason for the difference in mortality rates is still not fully understood, although some investigators suggest that differences in socioeconomic and cultural factors that limit treatment access contribute to increased mortality rates in these patients (7,14). Other investigators report that the difference in mortality remained significant even after the adjustment for access to health-care, treatment, and sociodemographic factors. They therefore suggested that the reason for the difference is more likely to be found in the biology of breast cancer among different racial/ethnic subgroups (11,25). Few studies in the past have evaluated the association between race/ethnicity and BRCA1 mutation.
Interestingly, among the nonwhite minorities, Hispanics with breast cancer have the highest incidence of pathogenic BRCA1 mutations (27).
In recent decades significant progress has been made in understanding the biology of breast cancer (3,28). We have learnt that TNBC is a heterogeneous disease that can be classified into distinct molecular subtypes by gene-expression profiling. Many research groups have proposed different molecular classifications of TNBC (28-31). Currently, there are at least four stable tumor-specific subtypes of TNBC characterized by the expression of distinct molecular profiles that have a distinct prognosis. Among them, there are two basal-like subtypes (BL-1 and BL-2), a mesenchymal (M) subtype, and a luminal androgen receptor (LAR) subtype (29). According to a study by Lehmann et al., these four subtypes have not only different gene expression profiles but also different biological and clinicopathological characteristics. For example, tumors of the BL-1 subtype are usually characterized by a higher grade, the highest proliferation rates of Ki-67 expression level (>85%), and upregulation of genes involved in cell proliferation and DNA damage response (such as ATR/BRCA pathway, CHEK1, MSH2, RAD21, and MDC1). Importantly, this subtype is highly sensitive to chemotherapy, with reported pCR up to 50% (32,33). The next is the BL-2 subtype, which has all the above-mentioned features and in addition it shows overexpression of growth factor receptors such as EGFR, NGFR, IGF1R, MET pathway, myoepithelial markers, and so on. Clinical studies have shown that the BL-2 subtype is highly resistant to chemotherapy (28,32). The M subtype is associated with the activation of epithelial-mesenchymal transition and cell motility pathways. The last subtype is LAR, which is characterized by overexpression of hormonally regulated pathways (particularly steroid synthesis and androgen/estrogen metabolism). Both subtypes demonstrate a very modest response to chemotherapy, approaching 15% overall response (34). There are no data on the frequency and distribution of the above-mentioned subclasses of TNBC in the general population, nor in different ethnic/racial subgroups of women with TNBC.
No previous NACT trials have demonstrated survival benefit or harm compared with ACT. Our study was first to analyze outcomes including OS and disease-free survival for H/L women with local or locally advanced TNBC treated with NACT versus ACT. We demonstrated a statistically significant difference in OS in favor of ACT. In the past, the large NSABP B-18 study reported no statistically significant survival benefits between NACT and ACT; however, the study did not report the hormonal status and ethnic/racial distribution of the breast cancer (5). More recent retrospective studies from Washington University and UT MD Anderson Cancer Center demonstrated no statistically significant difference in survival between patients who underwent NACT versus ACT (6,8,9). The major limitation of these studies was the racial/ethnic distribution of women, with a predominant population of NHW women of about 60%-62%, 15%-39% AA, and only a small proportion of H/L women, around 0%-18% (6-9). This is in contrast to our study, in which the majority of women were H/L, at 90%.
In our study, we demonstrated a statistically significant survival advantage in patients in the ACT group compared with the NACT group (HR=0.62, 95%CI=0.40-0.96). When we analyzed the possible reason for such results, we noticed worse outcomes in the group of patients with residual disease after completion of NACT. Subsequently, we demonstrated a statistically significant survival benefit from NACT in H/L women who achieved pCR after completion of NACT (HR=1.11).
Based on our and others’ results, we suggest that there are potential benefits of NACT for selected groups of patients, which at this point might be determined only by genetic profiling. Therefore, more studies need to be conducted to identify appropriate patient populations that will respond favorably to NACT.
We identified several limitations in our study. The most significant was that it was a retrospective analysis, and therefore, patients were not randomly selected to receive NACT versus ACT. As reported before, younger patients with more aggressive or advanced cancers were more likely to receive neoadjuvant treatment, which could be considered as selection bias in our study. Furthermore, the retrospective nature of this study limited the ability to directly compare “chemosensitive” tumors with “chemoresistant” tumors in the ACT group of patients. Another limitation of our study was the lack of consistency in the chemotherapy regimens. The most common regimens were anthracycline-based, such as Adriamycin/Cytoxan, followed by Taxol (AC-T) or Taxotere, Adriamycin, and Cytoxan (TAC). Even with the above-mentioned limitations, it is clear that patients who receive NACT and show a pCR have a significant survival advantage. For the general TNBC population, ACT seems to have a better overall survival outcome compared with NACT.
To the best of our knowledge, this is the first study to include a larger cohort of Hispanics, particularly Mexican American women, with TNBC treated with NACT versus ACT.
In conclusion, NACT versus ACT resulted in similar PFS among H/L patients with local or locally advanced TNBC. In conjunction with previous research in the neoadjuvant setting, we have shown a benefit to patients with TNBC who achieve pCR. In addition, our data demonstrate that patients with residual disease at the time of surgery have much worse outcomes, especially with stage I and II cancer.
Further investigation of new and promising immunotherapy or chemotherapy, as well as chemosensitivity tumor markers for TNBC, is vital so that patients can be offered NACT alone or in combination with immunotherapy, based on predictive chemosensitivity tumor markers. In addition, we believe that our retrospective data analysis provides justification for the development of a well-designed prospective randomized controlled trial that will demonstrate the benefit of NACT versus ACT in patients with TNBC.
Conflicts of Interest
Philipovskiy Alexander declares that he has no conflict of interest, Corral Javier declares that he has no conflict of interest, Dwivedi Kumar Alok declares that he has no conflict of interest, Heydarian Rosalinda, declares that she has no conflict of interest, Sumit Guar declares that he has no conflict of interest.
Authors’ Contributions
AP conceived of the study, prepared the draft of the manuscript. JC participated in the design of the study and approved the manuscript. KAD performed statistical analysis and participated in the drafting the manuscript. RH participated in the drafting the manuscript and approved the manuscript. SG participated in the design of the study and approved the manuscript. All Authors read and approved the final manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. PMID: 29313949. DOI: 10.3322/ caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Goding Sauer A, Ortiz AP, Fedewa SA, Pinheiro PS, Tortolero Luna G, Martinez Tyson D, Jemal A, Siegel RL. Cancer statistics for Hispanics/Latinos, 2018. CA Cancer J Clin. 2018;68:425–445. doi: 10.3322/caac.21494. PMID: 30285281. DOI: 10.3322/caac.21494. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. PMID: 17671126. DOI: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. PMID: 19574486. DOI: 10.3121/cmr. 2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001;30:96–102. doi: 10.1093/oxfordjournals.jncimonographs.a003469. PMID: 11773300. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy CR, Gao F, Margenthaler JA. Neoadjuvant versus adjuvant chemotherapy for triple negative breast cancer. J Surg Res. 2010;163:52–57. doi: 10.1016/j.jss.2010.04.015. PMID: 20599225. DOI: 10.1016/j.jss. 2010.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Patel KR, Martinez A, Stahl JM, Logan SJ, Perricone AJ, Ferris MJ, Buchwald ZS, Chowdhary M, Delman KA, Monson DK, Oskouei SV, Reimer NB, Cardona K, Edgar MA, Godette KD. Breast cancer in Latinas: gene expression, differential response to treatments, and differential toxicities in Latinas compared with other population groups. Oncologist. 2010;15:466–475. doi: 10.1634/theoncologist.2010-0004. PMID: 29900051. DOI: 10.1080/2162402X.2018.1442168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher CS, Ma CX, Gillanders WE, Aft RL, Eberlein TJ, Margenthaler JA. Neoadjuvant chemotherapy is associated with improved survival compared with adjuvant chemotherapy in patients with triple-negative breast cancer only after complete pathologic response. Ann Surg Oncol. 2012;19:253–258. doi: 10.1245/s10434-011-1877-y. PMID: 21725686. DOI: 10.1245/s10434-011-1877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clifton K, Gutierrez Barrera A, Ma J, Bassett R Jr, Litton J, Kuerer H, Moulder S, Albarracin C, Hortobagyi G, Arun B. Adjuvant versus neoadjuvant chemotherapy in triple-negative breast cancer patients with BRCA mutations. Breast Cancer Res Treat. 2018;170:101–109. doi: 10.1007/s10549-018-4727-9. PMID: 29470805. DOI: 10.1007/ s10549-018-4727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin NU, Vanderplas A, Hughes ME, Edge SB, Winer EP, Weeks JC. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118:5463–5472. doi: 10.1002/cncr.27581. PMID: 22544643. DOI: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez Macgregor M, Liu S, De Melo Gagliato D, Chen H, Do KA, Pusztai L, Fraser Symmans W, Nair L, Hortobagyi GN, Mills GB, Meric Bernstam F, Gonzalez Angulo AM. Differences in gene and protein expression and the effects of race/ethnicity on breast cancer subtypes. Cancer Epidemiol Biomarkers Prev. 2014;23:316–323. doi: 10.1158/1055-9965.EPI-13-0929. PMID: 24296856. DOI: 10.1158/1055-9965.EPI-13-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Y, Sarkissyan M, Elshimali Y, Vadgama JV. Triple negative breast tumors in African-American and Hispanic/Latina women are high in CD44+, low in CD24+, and have loss of PTEN. PLoS One. 2013;8:e78259. doi: 10.1371/journal.pone.0078259. PMID: 24167614. DOI: 10.1371/journal.pone.0078259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano SJ Gomez, Fejerman L, Zabaleta J. Breast cancer in Latinas: A focus on intrinsic subtypes distribution. Cancer Epidemiol Biomarkers Prev. 2018;27:3–10. doi: 10.1158/1055-9965.EPI-17-0420. PMID: 29054978. DOI: 10.1158/1055-9965.EPI-17-0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lara Medina F, Pérez-Sánchez V, Saavedra-Pérez D, Blake Cerda M, Arce C, Motola Kuba D, Villarreal Garza C, González Angulo AM, Bargalló E, Aguilar JL, Mohar A, Arrieta Ó. Triple-negative breast cancer in Hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117:3658–3669. doi: 10.1002/cncr.25961. PMID: 21387260. DOI: 10.1002/cncr.25961. [DOI] [PubMed] [Google Scholar]
- 15.Banegas MP, Tao L, Altekruse S, Anderson WF, John EM, Clarke CA, Gomez SL. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat. 2014;144:625–634. doi: 10.1007/s10549-014-2882-1. PMID: 24658879. DOI: 10.1007/s10549-014-2882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. PMID: 24529560. DOI: 10.1016/ S0140-6736(13) 62422-8. [DOI] [PubMed] [Google Scholar]
- 17.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a metaanalysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. PMID: 15687361. DOI: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 18.Gerber B, Loibl S, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, Schrader I, Kittel K, Hanusch C, Kreienberg R, Solbach C, Jackisch C, Kunz G, Blohmer JU, Huober J, Hauschild M, Nekljudova V, Untch M, von Minckwitz G. German Breast Group Investigators: Neoadjuvant bevacizumab and anthracycline-taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44) Ann Oncol. 2013;24:2978–2984. doi: 10.1093/annonc/mdt361. PMID: 15687361. DOI: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 19.Golshan M, Cirrincione CT, Sikov WM, Berry DA, Jasinski S, Weisberg TF, Somlo G, Hudis C, Winer E, Ollila DW. Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg. 2015;262:434–439. doi: 10.1097/SLA.0000000000001417. PMID: 26222764. DOI: 10.1097/SLA.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, Golshan M, Bellon JR, Collyar D, Hahn OM, Carey LA, Hudis CA, Winer EP. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. PMID: 25092775. DOI: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omarini C, Guaitoli G, Pipitone S, Moscetti L, Cortesi L, Cascinu S, Piacentini F. Neoadjuvant treatments in triplenegative breast cancer patients: where we are now and where we are going. Cancer Manag Res. 2018;10:91–103. doi: 10.2147/CMAR.S146658. PMID: 2939 1830. DOI: 10.2147/CMAR.S146658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Killelea BK, Yang VQ, Wang SY, Hayse B, Mougalian S, Horowitz NR, Chagpar AB, Pusztai L, Lannin DR. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: Results From the National Cancer Data Base. J Clin Oncol. 2015;33:4267–4276. doi: 10.1200/JCO.2015.63.7801. PMID: 26598753. DOI: 10.1200/JCO.2015.63.7801. [DOI] [PubMed] [Google Scholar]
- 23.Dawood S, Broglio K, Kau SW, Green MC, Giordano SH, Meric Bernstam F, Buchholz TA, Albarracin C, Yang WT, Hennessy BT, Hortobagyi GN, Gonzalez Angulo AM. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. J Clin Oncol. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. PMID: 19047281. DOI: 10.1200/JCO. 2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boddie AW, Warso M, Briele H, Sweeney P, Low N, Bork J, Rattan N, Wild L. Multimodal-therapy breast salvage in the urban poor with locally advanced cancer. Arch Surg. 1996;131:424–429. doi: 10.1001/archsurg.1996.01430160082017. PMID: 8615730. [DOI] [PubMed] [Google Scholar]
- 25.Parise CA, Caggiano V. Disparities in race/ethnicity and socioeconomic status: risk of mortality of breast cancer patients in the California Cancer Registry, 2000-2010. BMC Cancer. 2013;13:449–449. doi: 10.1186/1471-2407-13-449. PMID: 24083624. DOI: 10.1186/1471-2407-13-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. PMID: 17387718. DOI: 10.1002/ cncr.22618. [DOI] [PubMed] [Google Scholar]
- 27.John EM, Miron A, Gong G, Phipps AI, Felberg A, Li FP, West DW, Whittemore AS. Prevalence of pathogenic BRCA1 mutation carriers in 5 US racial/ethnic groups. JAMA. 2007;298:2869–2876. doi: 10.1001/jama.298.24.2869. PMID: 18159056. DOI: 10.1001/jama.298.24.2869. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, Pietenpol JA. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. PMID: 21633166. DOI: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y, Moses HL, Sanders ME, Pietenpol JA. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS One. 2016;11:e0157368–e0157368. doi: 10.1371/journal.pone.0157368. PMID: 27310713. DOI: 10.1371/journal.pone. 0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SA, Savage MI, Osborne CK, Hilsenbeck SG, Chang JC, Mills GB, Lau CC, Brown PH. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21:1688–1698. doi: 10.1158/1078-0432.CCR-14-0432. PMID: 25208879. DOI: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, Gräf S, Ha G, Haffari G, Bashashati A, Russell R, McKinney S, METABRIC Group, Langerød A, Green A, Provenzano E, Wishart G, Pinder S, Watson P, Markowetz F, Murphy L, Ellis I, Purushotham A, Børresen Dale AL, Brenton JD, Tavaré S, Caldas C, Aparicio S. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. PMID: 25208879. DOI: 10.1158/1078-0432.CCR-14-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santonja A, Sánchez Muñoz A, Lluch A, Chica Parrado MR, Albanell J, Chacón JI, Antolín S, Jerez JM, de la Haba J, de Luque V, Fernández De Sousa CE, Vicioso L, Plata Y, Ramírez Tortosa CL, Álvarez M, Llácer C, Zarcos Pedrinaci I, Carrasco E, Caballero R, Martín M, Alba E. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget. 2018;9:26406–26416. doi: 10.18632/oncotarget.25413. PMID: 29899867. DOI: 10.18632/oncotarget.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez Angulo AM, Meric Bernstam F, Valero V, Lehmann BD, Pietenpol JA, Hortobagyi GN, Symmans WF, Ueno NT. Differential response to neoadjuvant chemotherapy among 7 triplenegative breast cancer molecular subtypes. Clin Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. PMID: 23948975. DOI: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramson VG, Lehmann BD, Ballinger TJ, Pietenpol JA. Subtyping of triple-negative breast cancer: implications for therapy. Cancer. 2015;121:8–16. doi: 10.1002/cncr.28914. PMID: 25043972. DOI: 10.1002/cncr.28914. [DOI] [PMC free article] [PubMed] [Google Scholar]