Abstract
Current literature indicates that there is a strong correlation between coronary artery disease (CAD) and type 2 diabetes. The arteriosclerotic progression occurs earlier and in a greater extent in the diabetic than in the non-diabetic population. In diabetic subjects, the detection of arterial disease does not always precede the development of an acute arterial incident. Herein, we reviewed studies published within the last 5 years in order to reveal the risk factors for coronary artery disease in patients with type 2 diabetes. In addition, we aimed to discuss how to diagnose in an early stage or even screen the presence of coronary artery disease in asymptomatic diabetic patients. Possible blood markers as predictors of CAD, which are mostly related to the lipidemic profile of subjects, are included in this review. Less invasive imaging methods than conventional coronary angiography, included in the article, are gradually used more in the diagnosis of CAD and show high effectiveness. Data from 23 articles with 22,350 patients having type 2 diabetes were summarized and presented descriptively.
Keywords: Coronary, artery, disease, diabetes, atherosclerosis, computed, tomography, angiography, review
The rates of diabetes are increasing worldwide. The scientific community estimates that the number of people living with diabetes will rise dramatically the following years and will reach the number of 592 million by 2035 (1). Diabetes mellitus has a wide range of complications which includes both microvascular (renal, retinal, and neuropathic disease) and macrovascular complications [vascular disease and coronary artery disease (CAD)] (2). The main system affected by diabetes, causing death, is the cardiovascular one. As a result, patients suffering from diabetes are prone to more severe cardiovascular diseases and have greater complication rates than non-diabetic patients (3).
Inflammatory elements, vascular smooth muscle cell proliferation and endothelial dysfunction, which characterize atherosclerosis, result in atherosclerotic plaque instability and progression (4-10). Atherosclerosis leading to CAD results in restriction of blood flow to the heart (11). It is common knowledge that the degree of stenosis varies among patients. Therefore, the clinical presentation of patients also varies from asymptomatic to stable angina and acute coronary syndrome (ACS), which includes unstable angina, stemi and non-stemi myocardial infraction (12).
Diabetes is regarded as a CAD risk equivalent. This means that diabetic patients are at risk of having coronary events alike non-diabetic patients, who previously had one (13). Many factors contribute to the appearance of CAD in diabetes type 2 patients and only 25% of these are already known (14). As CAD constitutes a challenging task among practitioners, the aim of our review is to present the correlation between type 2 diabetes mellitus and CAD, according to current scientific reports, and to reveal possible predictive factors that could be used as a screening and risk assessment tool for CAD in the future.
Literature Review
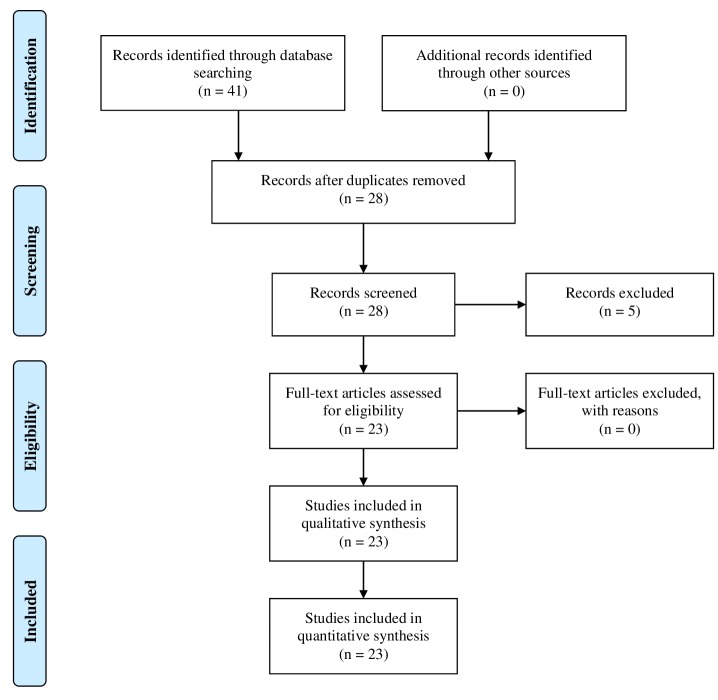
A search was conducted in MEDLINE (via PubMed) in order to retrieve articles from the period of time between 2014-2017. The search strategy was based on the use of keywords such as coronary artery disease, type 2 diabetes, coronary computed tomography, angiography and atherosclerosis. The PRISMA approach was used for the selection of the articles included in the review. A total of 41 records were identified. Following removal of the duplicates 28 records remained. These were screened and five were excluded mainly because they were only abstracts. The full-text articles assessed for eligibility were 23 and none of them was excluded. The inclusion process is demonstrated in Figure 1.
Figure 1. Prisma flow diagram for the current literature review.
Blood Factors as Predictors for CAD
There is a variety of factors determining the risk of CAD, including blood markers, a common and useful tool for the prediction of CAD. In 2014, Huang et al., reported a study in which they compared sdLDL cholesterol (sdLDL C) levels between healthy individuals and diabetics with CAD in a group of Taiwanese people (Table I). It is known that, sdLDL particles are more atherogenic due to their characteristics, which include lower binding affinity to LDL receptors, higher penetration into subendothelial layer, longer half-life and lower resistance to oxidative stress. According to this study, sdLDL C combined with LDL C leads to better prediction of CAD in diabetic patients than LDL C alone. It is noteworthy that there was an age and sex effect on sdLDL-C as older people and males showed greater levels of sdLDL-C (p< 0.001) (15).
Table I. Clinical trials of blood markers as screening methods for coronary artery disease.
CAD: Coronary artery disease; LDL: low density lipoprotein; SdLDL: small dense low-density lipoprotein; T2DM: type 2 diabetes mellitus; MACE: major adverse cardiovascular event; MI: myocardial infraction; HF: heart failure; CHD: coronary heart disease; ACS: acute coronary syndrome; Apo B: apolipoprotein B.
One year later, a study of 90 participants evaluated the possible association between atherosclerosis and glycated apo B and the later with the glycemic index and sdLDL (Table I) (16). It was shown that hyperglycemia and sdLDL are independently linked to glycation of apo B. They have suggested that the procedure of glycation and its results in the structure of vessels predisposes to atherosclerosis (16).
In addition, another study aimed to link young onset CAD to lipoprotein(a) {Lp(a)} in type 2 diabetic patients (Table I) (17). Based on previous studies, there was the hypothesis that Lp(a)-associated cholesterol can promote coagulation, inflammation, carry proinflammatory oxidized phospholipids and prevent fibrinolysis (18). According to Chen’s study there is significant evidence that higher Lp(a) levels are an independent factor predicting CAD patients with type 2 diabetes, suggesting that Lp(a) level measurement can be beneficial for type 2 diabetic patients in clinical practice (17).
Ozturk et al. have published a study of 158 subjects evaluating the correlation between CAD and blood galectin-3 (Table I) (19). Galectin-3 is defined as a carbohydrate-binding protein with anti-inflammatory and proinflammatory actions, depending on the inflammatory environment and the target cell or tissue. It has also been shown that high galectin-3 levels are positively associated with coronary atherosclerosis (both the extent and the type of plaque), High-sensitivity-C-Reactive Protein (hs-CRP) levels and BMI in diabetic patients (20).
Still in 2015, another study of 76 participants who were angiographically tested for the detection of CAD has estimated the role of insulin resistance and other markers of type 2 diabetes in the development of CAD (Table I) (21). This study has shown that low levels of insulin resistance and microalbuminuria, and female sex constitute negative factors for the development of CAD in patients suffering from diabetes type 2 for more than 10 years. Srinivasan’s study suggested that these factors improve prognosis concerning the CAD profile (21).
A possible relationship between copeptin and cardiovascular mortality has also been tested (Table I) (22). Copeptin, a stable fragment of the vasopressin hormone, has been demonstrated to be an independent predictor for the appearance of diabetes mellitus (23). Several studies have demonstrated that in elderly population CAD and cardiovascular mortality rates increase as the level of blood copentin increases irrespective of diabetes mellitus. As a result, copeptin may be a useful tool for practitioners to assess cardiovascular risk stratification of primary prevention actions (23-25).
In 2016, Chubb et al., presented a study of 1,283 subjects aiming to examine the correlation between the concentration of HCO3 in blood and coronary heart disease CHD), heart failure and mortality (Table I). According to the study, there is an inverse association between serum bicarbonate and CHD events in type 2 diabetic patients (as serum bicarbonate levels decrease, the risk of CHD events increases). Additionally, heart failure and mortality were not demonstrated to be associated to serum bicarbonate levels (26).
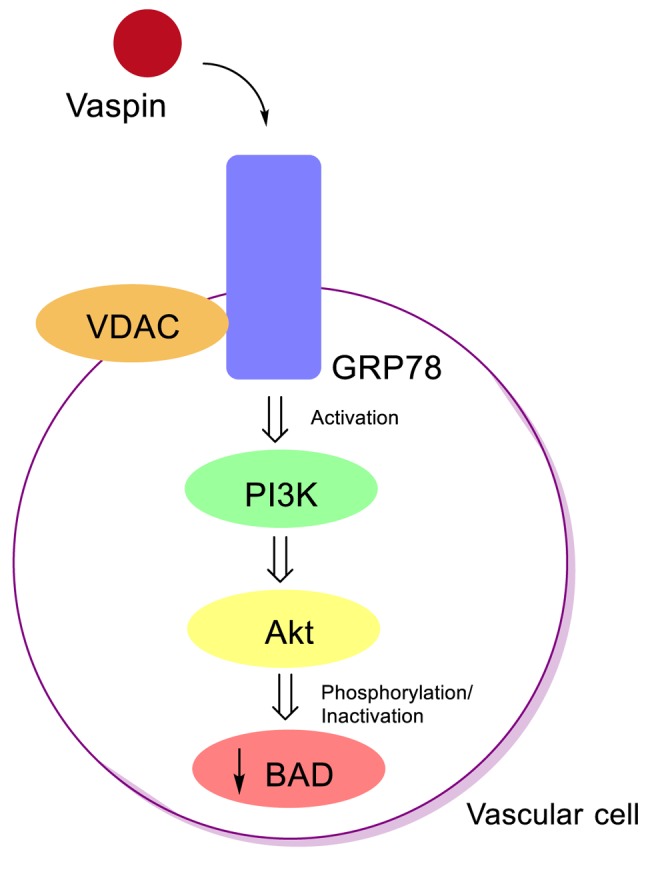
In addition, the relationship between serum vaspin levels and prevalence of CAD has been investigated (Table I) (27). Vaspin is a factor having anti-inflammatory action. Reduced vaspin levels are related to increased CRP and visfatin levels (28). Vaspin, which is a ligand for VDAC/GRP78 complex in the surface of vascular cells, has a protective effect and prevents apoptosis of vascular cells via phosphatidyl-inositide 3 kinase-AKT (PI3K-AKT) signaling pathway (Figure 2) (29). Akt phosphorylates and inactivates BAD, which is a pro-apoptotic factor, inhibiting Bcl-xL (an anti-apoptotic factor) (30). Hao’s et al. study has indicated that diabetic patients with CAD have higher levels of serum vaspin compared to diabetics without CAD and healthy individuals (27).
Figure 2. Anti-apoptotic pathway of vaspin in vascular cells.
Müller et al., have examined whether blood Gremlin-1 and macrophage migration inhibitory factor (MIF) might be related to CAD and the risk of acute coronary syndrome (ACS) (Table I) (31). MIF is a pro-inflammatory factor which regulates monocyte recruitment, leading to atheroprogression and plaque instability (32). Gremlin-1 is an antagonist of MIF preventing foam-cell formation induced by MIF and thus has an atheroprotective action (33). A study has shown that patients with type 2 diabetes and ACS had high levels of both Gremlin-1 and MIF. Additionally, the MIF/gremlin-1 ratio was high, as MIF increased more than gremlin-1. This ratio was demonstrated only in type 2 diabetes with ACS (31).
Valensi et al., have examined the association between asymptomatic myocardial ischemia and asymptomatic CAD with atherogenic dyslipidemia in a study that included 1080 subjects with type 2 diabetes (Table I). Atherogenic dyslipidemia is defined as low levels of high-density lipoprotein cholesterol and high levels of triglycerides. The clinical results confirmed the primary hypothesis, indicating an increased risk of atherogenic dyslipidemia and a possible molecular targeting of atherogenic dyslipidemia could offer improved clinical results (34).
Imaging Methods as Predictors for CAD
Imaging methods are in the forefront of the clinical diagnosis of CAD and the broad range of its complications. In 2014, Gyung-Min Park et al., examined a group of 557 asymptomatic participants who underwent coronary computed tomography angiography (CCTA) evaluation, as a screening method, and found that there is a positive correlation between significant CAD and cardiac events, as well as lower survival rates (Table II) (35). Additionally, approximately 1/3 of asymptomatic type 2 diabetic patients were found to have significant CAD. Thus, this study depicted the potential value of CCTA in the identification of asymptomatic type 2 diabetes being at high risk of cardiovascular complications (35).
Table II. Clinical trials of imaging methods as screening methods for coronary artery disease.
CAD: Coronary artery disease; LDL: low density lipoprotein; SdLDL: small dense low density lipoprotein; T2DM: Type 2 diabetes mellitus; MACE: major adverse cardiovascular event; MI: myocardial infraction; HF: heart failure; CHD: coronary heart disease; ACS: acute coronary syndrome; CCTA: coronary computed tomography angiography; MDCT: multi-detector computed tomography; CAC: coronary artery calcium; ABI: ankle brachial index; CAVI: cardio-ankle vascular index; ICA: invasive coronary angiography; CRF: cardio-respiratory fitness; METs: metabolic equivalents; U/S: ultra-sound; CIMT: carotid intima-media thickness; LA/RA: left atrium/right atrium.
One year later, a group of 6434 individuals more than 20 years old who underwent CCTA was tested (Table II). Compared to non-diabetics, type 2 diabetic patients presented asymptomatic CAD at higher rates, concerning the low and intermediate risk CAD mostly. Remarkably, diabetic patients with intermediate risk of CAD suffered more cardiac events than the ones with low risk CAD (36).
In 2015, 626 type 2 diabetic patients, not suffering from known cardiovascular disease were examined (Table II). During the study, the coronary artery calcium (CAC) score was compared using multi-detector computed tomography (MDCT), with the cardio-ankle vascular index (CAVI). According to the results, a CAVI ≥9.0 or a CAC score ≥100 were positively correlated with cardiovascular events. Although CAC score has a greater predictive value, CAVI seems also a useful method for the risk stratification of asymptomatic type 2 diabetic patients. Importantly, CAVI presents a range of advantages, such as being a low-cost procedure, is easy to be calculated and is not affected by blood pressure (37).
The same year, investigators tested the usefulness of CCTA in CAD detection in comparison to invasive coronary angiography (ICA) which is regarded as the gold standard method was investigated in 48 type 2 diabetic asymptomatic patients (Table II). The results showed that CCTA has equal sensitivity with ICA. However, CCTA may present many false-positive results reducing its effectiveness as an assessment tool for asymptomatic CAD in type 2 diabetics (38).
In another study, 506 type 2 diabetic patients asymptomatic for CAD were examined via multi-detector computed tomography (MDCT) (Table II). The research group demonstrated that 82% of men and 72% of women were suffering from CAD. Alongside, they assessed other clinical predictors, depending on the gender. Men with high levels of HbA1c, longer duration of diabetes, retinopathy, dyslipidemia and other cardiovascular problems, as well as women with retinopathy and longer duration of diabetes were more susceptible to CAD. As a result, the combination of MDCT with the assessment of risk factors, described above, constitutes a potential screening tool for asymptomatic CAD in type 2 diabetes (39).
Moreover, in another study, cardiorespiratory fitness (CRF) [in peak metabolic equivalents (METs)] and CAC scores of a group of 600 asymptomatic type 2 diabetic individuals were measured via computed tomography (Table II) (40). According to the results, low CRF offers a positive prognostic value for all-cause mortality, stroke and myocardial infraction in asymptomatic diabetics, despite a low CAC. Therefore, lifestyle changes and assessment of other risk factors for cardiovascular problems could be beneficial to patients with low CRF. The study also demonstrated the additive value of CRF along with CAC for the identification of high risk asymptomatic diabetic patients (40).
One year later, a study of 630 diabetic subjects without known CAD underlined that a combination of CCTA with clinical risk stratification methods and coronary artery calcium score is effective in detecting patients with greater risk of primary cardiovascular events (Table II) (41).
In 2016, Srinivasan et al., tested 175 individuals who underwent coronary angiogram for the detection of CAD (Table II). The outcome of the research indicated that there is a correlation between the duration of diabetes and the existence of CAD. Remarkably, patients with more than 5 years with diabetes showed greater vascular structural changes than patients with less than 5 years with diabetes. Therefore, it is of paramount importance to intervene diagnostically and therapeutically in the first 5 years of type 2 diabetes (42).
Next year, Christensen et al., examined a group of 200 patients who underwent echocardiography for the screening of cardiac adipose tissue (CAT) (Table II). According to the results, higher than normal levels of CAT are correlated with greater risk of mortality and cardiovascular incident. In addition, CAT is linked to inflammation, as it is positively associated with IL-8 (43).
In 2017, Nezarat et al., investigated the prevalence and severity of early coronary atherosclerotic disease in 181 patients less than 40 years old (Table II). According to the clinical outcome, type 2 diabetic patients are prone to developing CAD more often and with greater extent, depending on the type of atheromatic plaques, compared to non-diabetic individuals of matched-age. As a result, computed tomography angiography may be used as a detector of subclinical atherosclerosis in this group (44).
In addition, a group of scientists have evaluated the effect of patient’s compliance on the prevention of major adverse cardiovascular event (MACE) in 179 type 2 diabetic patients who underwent coronary angiography before trans-femoral amputation for 1 year (Table II) (45). They proved that non-compliant patients presented more MACEs in comparison with the compliant ones. Additionally, the non-compliant patients with more severe CAD (defined by CCTA), depending on the extent and the position of the lesion, revealed a worse clinical outcome than the non-compliant patients with less severe lesion (45).
In 2017, Wu et al., tested 1,584 individuals who underwent ultrasound screening for carotid atherosclerosis (CA) (Table II) (46). They demonstrated that the prevalence of carotid atherosclerosis is greater in type 2 diabetics than in non-diabetic patients. Moreover, type 2 diabetic men and elderlies were susceptible to develop CA (gender and age-related). Last but not least, CA was positively related to the appearance of stroke and coronary heart events, setting CA monitoring a necessity (46).
The same year, another study investigated whether cardiovascular death and heart failure are affected by CCTA findings and clinical factors in 735 type 2 diabetic subjects who underwent CCTA and clinical assessment (Table II) (47). The outcome of the research implies that left/right atrial (LA/RA) volume ratio >1 (defined by CCTA), the existence of microvascular disease (retinopathy and nephropathy) and increased systolic blood pressure lead to heart failure and cardiovascular death in asymptomatic patients of low to intermediate risk. Thus, taking these factors under consideration will possibly ameliorate the clinical result (47).
Conclusion
As we have seen in this review, researchers used imaging methods, blood markers and clinical exercises to identify screening tools for CAD. A broad range of factors were investigated, utilizing large groups of subjects suffering from type 2 diabetes. Significant results were obtained from all the studies, described in this review. As CAD reflects one of the most important complications of type 2 diabetes mellitus, it is of paramount importance to find new and more efficient methods to predict CAD both in time and extent and offer clinical benefits to patients. Further research should be performed in order to identify factors predicting high risk diabetic patients for coronary events.
Conflicts of Interest
All Authors declare that there are no conflicts of interest.
Authors’ Contributions
AP and ED designed the study. AP, PF and ED wrote the article. AG, CD, and NG collected the data. AG and DM revised the article.
References
- 1.International Diabetes Federation. International Diabetes Federation. 6th ed. IDF, Brussels. 2013 [Google Scholar]
- 2.Bos M, Agyemang C. Prevalence and complications of diabetes mellitus in Northern Africa, a systematic review. BMC Public Health. 2013;13:387. doi: 10.1186/1471-2458-13-387. PMID: 23617762. DOI: 10.1186/ 1471-2458-13-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albers AR, Krichavsky MZ, Balady GJ. Stress testing in patients with diabetes mellitus. Circulation. 2006;113:583–592. doi: 10.1161/CIRCULATIONAHA.105.584524. PMID: 16449735. DOI: 10.1161/CIRCULATIONAHA.105.584524. [DOI] [PubMed] [Google Scholar]
- 4.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. PMID: 11001066. DOI: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138(5 Pt 2):419–420. doi: 10.1016/s0002-8703(99)70266-8. PMID: 10539839. [DOI] [PubMed] [Google Scholar]
- 6.Hansson GK, Robertson AK, Sӧderberg Nauclér C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. PMID: 18039117. DOI: 10.1146/annurev.pathol.1.110304. 100100. [DOI] [PubMed] [Google Scholar]
- 7.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. PMID: 16322783. DOI: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May AE, Kälsch T, Massberg S, Herouy Y, Schmidt R, Gawaz M. Engagement of glycoprotein IIb/IIIa (α(IIb)β3) on platelets upregulates CD40L and triggers CD40L-dependent matrix degradation by endothelial cells. Circulation. 2002;106:2111–2117. doi: 10.1161/01.cir.0000033597.45947.0f. PMID: 12379582. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. PMID: 21593864. DOI: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 10.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. PMID: 18825131. DOI: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 11.Sanchis Gomar F, Perez Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med. 2016;4:256–256. doi: 10.21037/atm.2016.06.33. PMID: 27500157. DOI: 10.21037/atm.2016.06.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–488. doi: 10.1161/CIRCULATIONAHA.105.537878. PMID: 15983262. DOI: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 13.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. PMID: 9673301. DOI: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 14.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135. PMID: 12087010. [DOI] [PubMed] [Google Scholar]
- 15.Huang YC, Chang PY, Hwang JS, Ning HC. Association of small dense low density lipoprotein cholesterol in type 2 diabetics with coronary artery disease. Biomed J. 2014;37:375–379. doi: 10.4103/2319-4170.132883. PMID: 25179702. DOI: 10.4103/2319-4170.132883. [DOI] [PubMed] [Google Scholar]
- 16.Dev K, Sharma SB, Garg S, Aggarwal A, Madhu SV. Glycated apolipoprotein B-A surrogate marker of subclinical atherosclerosis. Diabetes Metab Syndr. 2016;10:78–81. doi: 10.1016/j.dsx.2015.09.012. PMID: 26614298. DOI: 10.1016/j.dsx.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Zhang Y, Liu J, Chen MH, Guo YL, Zhu CG, Xu RX, Dong Q, Li JJ. Role of lipoprotein(a) in predicting the severity of new on-set coronary artery disease in type 2 diabetics: A Gensini score evaluation. Diabetes Vasc Dis Res. 2015;12:258–264. doi: 10.1177/1479164115579004. PMID: 25861813. DOI: 10.1177/147916411 5579004. [DOI] [PubMed] [Google Scholar]
- 18.Gouni-Berthold I, Berthold HK. Lipoprotein(a): current perspectives. Curr Vasc Pharmacol. 2011;9:682–692. doi: 10.2174/157016111797484071. PMID: 21529331. [DOI] [PubMed] [Google Scholar]
- 19.Ozturk D, Celik O, Satilmis S, Aslan S, Erturk M, Cakmak HA, Kalkan AK, Ozyilmaz S, Diker V, Gul M. Association between serum galectin-3 levels and coronary atherosclerosis and plaque burden/structure in patients with type 2 diabetes mellitus. Coron Artery Dis. 2015;26:396–401. doi: 10.1097/MCA.0000000000000252. PMID: 25887000. DOI: 10.1097/MCA.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 20.Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64:1–12. doi: 10.1111/j.0001-2815.2004.00278.x. PMID: 15191517. DOI: 10.1111/j.0001-2815.2004.002 78.x. [DOI] [PubMed] [Google Scholar]
- 21.Srinivasan MP, Kamath PK, Bhat NM, Pai ND, Manjrekar PA, Mahabala C. Factors associated with no apparent coronary artery disease in patients with type 2 diabetes mellitus for more than 10 years of duration: A case control study. Cardiovasc Diabetol. 2015;14:146–152. doi: 10.1186/s12933-015-0307-z. PMID: 26521236. DOI: 10.1186/ s12933-015-0307-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasevska I, Enhörning S, Persson M, Nilsson PM, Melander O. Copeptin predicts coronary artery disease cardiovascular and total mortality. Heart. 2016;102:127–132. doi: 10.1136/heartjnl-2015-308183. PMID: 26661323. DOI: 10.1136/heartjnl-2015-308183. [DOI] [PubMed] [Google Scholar]
- 23.Enhorning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm E, Groop L, Lyssenko V, Orho Melander M, Newton Cheh C, Melander O. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121:2102–2108. doi: 10.1161/CIRCULATIONAHA.109.909663. PMID: 20439785. DOI: 10.1161/CIRCULATIONAHA.109.909663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. PMID: 16269513. DOI: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 25.Abbasi A, Corpeleijn E, Meijer E, Postmus D, Gansevoort RT, Gans RO, Struck J, Hillege HL, Stolk RP, Navis G, Bakker SJ. Sex differences in the association between plasma copeptin and incident type 2 diabetes: The Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Diabetologia. 2012;55:1963–1970. doi: 10.1007/s00125-012-2545-x. PMID: 22526609. DOI: 10.1007/s00125-012-2545-x. [DOI] [PubMed] [Google Scholar]
- 26.Paul Chubb SA, Davis WA, Peters KE, Davis TM. Serum bicarbonate concentration and the risk of cardiovascular disease and death in type 2 diabetes: the Fremantle Diabetes Study. Cardiovasc Diabetol. 2016;15:143–143. doi: 10.1186/s12933-016-0462-x. PMID: 27716263. DOI: 10.1186/s12933-016-0462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hao F, Zhang HJ, Zhu J, Kuang H, Yu Q, Bai M, Mu J. Association between vaspin level and coronary artery disease in patients with type 2 diabetes. Diabetes Res Clin Pract. 2016;113:26–32. doi: 10.1016/j.diabres.2015.12.001. PMID: 26972957. DOI: 10.1016/j.diabres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Aust G, Richter O, Rohm S, Kerner C, Hauss J, Klöting N, Ruschke K, Kovacs P, Youn BS, Blüher M. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis. 2009;204:262–266. doi: 10.1016/j.atherosclerosis.2008.08.028. PMID: 18848328. DOI: 10.1016/j.athero sclerosis.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 29.Jung CH, Lee WJ, Hwang JY, Seol SM, Kim YM, Lee YL, Park JY. Vaspin protects vascular endothelial cells against free fatty acid-induced apoptosis through a phosphatidylinositol 3- kinase/Akt pathway. Biochem Biophys Res Commun. 2011;413:264–269. doi: 10.1016/j.bbrc.2011.08.083. PMID: 21893030. DOI: 10.1016/j.bbrc.2011.08.083. [DOI] [PubMed] [Google Scholar]
- 30.Nakatsuka A, Wada J, Iseda I, Teshigawara S, Higashio K, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Hida K, Eguchi J, Ogawa D, Matsuki Y, Hiramatsu R, Yagita H, Kakuta S, Iwakura Y, Makino H. Vaspin inhibits apoptosis of endothelial cells as a ligand for cell-surface GRP78/VDAC complex. Circ Res. 2013;112:771–780. doi: 10.1161/CIRCRESAHA.111.300049. PMID: 23307819. DOI: 10.1161/CIRCRESAHA.111.300049. [DOI] [PubMed] [Google Scholar]
- 31.Müller KA, Rath D, Schmid M, Schoenleber H, Gawaz M, Geisler T, Müller II. High plasma levels of gremlin-1 and macrophage migration inhibitory factor, but not their ratio, indicate an increased risk for acute coronary syndrome in patients with type 2 diabetes mellitus. Clin Cardiol. 2016;39:201–206. doi: 10.1002/clc.22509. PMID: 27101443. DOI: 10.1002/clc.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Müller II, Müller KA, Karathanos A, Schönleber H, Rath D, Vogel S, Chatterjee M, Schmid M, Haas M, Seizer P, Langer H, Schaeffeler E, Schwab M, Gawaz M, Geisler T. Impact of counterbalance between macrophage migration inhibitory factor and its inhibitor Gremlin-1 in patients with coronary artery disease. Atherosclerosis. 2014;237:426–432. doi: 10.1016/j.atherosclerosis.2014.09.010. PMID: 25463068. DOI: 10.1016/j.atherosclerosis.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Müller II, Schonberger T, Schneider M, Borst O, Ziegler M, Seizer P, Leder C, Müller K, Lang M, Appenzeller F, Lunov O, Büchele B, Fahrleitner M, Olbrich M, Langer H, Geisler T, Lang F, Chatterjee M, de Boer JF, Tietge UJ, Bernhagen J, Simmet T, Gawaz M. Gremlin-1 is an inhibitor of macrophage migration inhibitory factor and attenuates atherosclerotic plaque growth in ApoE−/− Mice. J Biol Chem. 2013;288:31635–31645. doi: 10.1074/jbc.M113.477745. PMID: 24003215. DOI: 10.1074/jbc.M113.477745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Valensi P, Avignon A, Sultan A, Chanu B, Nguyen MT, Cosson E. Atherogenic dyslipidemia and risk of silent coronary artery disease in asymptomatic patients with type 2 diabetes: a cross sectional study. Cardiovasc Diabetol. 2016;15:104–113. doi: 10.1186/s12933-016-0415-4. PMID: 27450534. DOI: 1186/s12933-016-0415-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park GM, Lee SW, Cho YR, Kim CJ, Cho JS, Park MW, Her SH, Ahn JM, Lee JY, Park DW, Kang SJ, Kim YH, Lee CW, Koh EH, Lee WJ, Kim MS, Lee KU, Kang JW, Lim TH, Park SW, Park SJ, Park JY. Coronary computed tomographic angiographic findings in asymptomatic patients with type 2 diabetes mellitus. Am J Cardiol. 2014;113:765–771. doi: 10.1016/j.amjcard.2013.11.028. PMID: 24528613. DOI: 10.1016/j.amjcard.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 36.Park GM, Lee JH, Lee SW, Yun SC, Kim YH, Cho YR, Gil EH, Kim TS, Kim CJ, Cho JS, Park MW, Her SH, Yang DH, Kang JW, Lim TH, Koh EH, Lee WJ, Kim MS, Lee KU, Kim HK, Choe J, Park JY. Comparison of coronary computed tomographic angiographic findings in asymptomatic subjects with versus without diabetes mellitus. Am J Cardiol. 2015;116:372–378. doi: 10.1016/j.amjcard.2015.04.046. PMID: 26037293. DOI: 10.1016/j.amjcard. 2015.04.046. [DOI] [PubMed] [Google Scholar]
- 37.Chung SL, Yang CC, Chen CC, Hsu YC, Lei MH. Coronary artery calcium score compared with cardio-ankle vascular index in the prediction of cardiovascular events in asymptomatic patients with type 2 diabetes. J Atheroscler Thromb. 2015;22:1255–1265. doi: 10.5551/jat.29926. PMID: 26269147. DOI: 10.5551/jat.29926. [DOI] [PubMed] [Google Scholar]
- 38.Ulimoen GR, Ofstad AP, Endresen K, Gullestad L, Johansen OE, Borthne A. Low-dose CT coronary angiography for assessment of coronary artery disease in patients with type 2 diabetes – a cross-sectional study. BMC Cardiovasc Disord. 2015;15:147–153. doi: 10.1186/s12872-015-0143-9. PMID: 26573616. DOI: 10.1186/s12872-015-0143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimabukuro M, Saito T, Higa T, Nakamura K, Masuzaki H, Sata M, Fukuoka diabetologists group Risk stratification of coronary artery disease in asymptomatic diabetic subjects using multidetector computed tomography. Circ J. 2015;79:2422–2429. doi: 10.1253/circj.CJ-15-0325. PMID: 26399764. DOI: 10.1253/circj.CJ-15-0325. [DOI] [PubMed] [Google Scholar]
- 40.Zafrir B, Azaiza M, Gaspar T, Mery ID, Azencot M, Lewis BS, Rubinshtein R, Halon DA. Low cardiorespiratory fitness and coronary artery calcification: Complementary cardiovascular risk predictors in asymptomatic type 2 diabetics. Atherosclerosis. 2015;241:634–640. doi: 10.1016/j.atherosclerosis.2015.06.020. PMID: 26117400. DOI: 10.1016/j.atherosclerosis.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Halon DA, Azencot M, Rubinshtein R, Zafrir B, Flugelman MY, Lewis BS. Coronary computed tomography (CT) angiography as a predictor of cardiac and noncardiac vascular events in asymptomatic type 2 diabetics: A 7-year population-based cohort study. J Am Heart Assoc. 2016;5:e003226–e003226. doi: 10.1161/JAHA.116.003226. PMID: 27412899. DOI: 10.1161/JAHA.116.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasan MP, Kamath PK, Bhat NM, Pai ND, Bhat RU, Shah TD, Singhal A, Mahabala C. Severity of coronary artery disease in type 2 diabetes mellitus: Does the timing matter. Indian Heart J. 2016;68:158–163. doi: 10.1016/j.ihj.2015.08.004. PMID: 27133324. DOI: 10.1016/j.ihj.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christensen RH, Scholten BJV, Hansen CS, Heywood SE, Rosenmeier JB, Andersen UB, Hovind P, Reinhard H, Parving HH, Pedersen BK, Jørgensen ME, Jacobsen PK, Rossing P. Epicardial, pericardial and total cardiac fat and cardiovascular disease in type 2 diabetic patients with elevated urinary albumin excretion rate. Eur J Prev Cardiol. 2017;24:1517–1524. doi: 10.1177/2047487317717820. PMID: 28650207. DOI: 10.1177/2047487317717820. [DOI] [PubMed] [Google Scholar]
- 44.Nezarat N, Budoff MJ, Luo Y, Darabian S, Nakanishi R, Li D, MD, Sheidaee N, Kim M, BSa, Alani A, Matsumoto S, Rahmani S, Kanisawa M, Ceponiene I, Osawa K, Qi H, Hamal S, Kitslaar P, Broersen A, Flores F, Ipp E, Khazai B. Presence, characteristics, and volumes of coronary plaque determined by computed tomography angiography in young type 2 diabetes mellitus. Am J Cardiol. 2017;119:1566–1571. doi: 10.1016/j.amjcard.2017.02.023. PMID: 28343599. DOI: 10.1016/j.amjcard.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 45.Shalaeva EV, Saner H, Janabaev BB, Shalaeva AV. Tenfold risk increase of major cardiovascular events after high limb amputation with non-compliance for secondary prevention measures. Eur J Prev Cardiol. 2017;24:708–716. doi: 10.1177/2047487316687103. PMID: 28071959. DOI: 10.1177/2047487316687103. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, He J, Sun X, Zhao YM, Lou HY, Ji XL, Pang XH, Shan LZ, Kang YX, Xu J, Zhang SZ, Wang YJ, Ren YZ, Shan PF. Carotid atherosclerosis and its relationship to coronary heart disease and stroke risk in patients with type 2 diabetes mellitus. Medicine (Baltimore) 2017;96:e8151. doi: 10.1097/MD.0000000000008151. PMID: 28953658. DOI: 10.1097/MD.0000000000008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Halon DA, Ayman J, Rubinshtein R, Zafrir B, Azencot M, Lewis BS. Cardiac computed tomography angiographic findings as predictors of late heart failure in an asymptomatic diabetic cohort: An 8-year prospective follow-up study. Cardiology. 2017;138:218–227. doi: 10.1159/000478995. PMID: 28817814. DOI: 10.1159/000478995. [DOI] [PubMed] [Google Scholar]