Abstract
Background: Cancer-induced bone pain remains a serious public health concern, with a need for translational behavioural tests in order to assess nociception in preclinical models of this condition. Burrowing is an innate, ethologically relevant rodent behaviour that has been proven sensitive to chronic pain conditions. Herein, we studied for the first time whether burrowing performance is altered in preclinical models of cancer-induced bone pain. Materials and Methods: Mice and rats were inoculated with syngeneic breast cancer cells. Bone degradation was radiographically evaluated and nociception was assessed in limb-use and burrowing tests. Results: Cancer-bearing rodents showed reduced relative bone density and limb-use scores, confirming disease development. Burrowing performance decreased over time in both rodent models. Conclusion: Burrowing performance was reduced in both rodent models, indicating that the burrowing test is a relevant and reproducible behavioural test for assessing disease development in both mouse and rat models of cancer-induced bone pain.
Keywords: Cancer-induced bone pain, burrowing, animal behaviour, bone degradation
Pain is a severe and debilitating symptom of metastatic bone disease affecting 30-40% of patients with metastatic bone cancer (1,2). As cancer-induced bone pain is poorly controlled with current therapies, preclinical models of this condition have become an important tool for understanding the underlying mechanisms of malignant disease and investigating novel analgesic targets (3).
The first murine model of localized cancer-induced bone pain was established through the direct inoculation of cancer cells into the femoral intramedullary cavity (4). Several mouse and rat models of metastatic bone disease have been developed through inoculation of cancer cells in the intramedullary cavity of long bones, leading to the identification of novel targets for the treatment of cancer-induced bone pain (3,5,6).
Traditionally, pain-related behaviour has been assessed using stimulus-evoked readouts, i.e. tests that measure involuntary responses to a specific stimulus (thermal or mechanical). However, the translatability of stimulus-evoked behavioural tests is currently under debate, as these measure simple spinal reflexes rather than integrating complex pain-like behaviours, where cerebral processing of the nociceptive signal is required (7,8). Additionally, these tests require interaction between the experimenter and the animal, introducing a risk of subjectivity. Therefore, non-stimulus-evoked tests that evaluate changes in ethologically relevant rodent behaviours have received growing interest. While some non-stimulus-evoked tests, such as the limb-use test, are also highly subjective, an example of an objective, ethologically relevant behavioural readout is burrowing (9). Importantly, this innate behaviour can be used to assess the general well-being of rodents (10) and the reduction in the amount of substrate voluntarily burrowed can be used as a surrogate marker of pain-like behaviour (11). As such, the burrowing test exploits an evolutionary-conserved rodent behaviour that is not essential for survival in a laboratory setting; it has thus been hypothesized that this complex behaviour may be comparable to the “activities of daily living” in humans (i.e. housekeeping) (10), suggesting that it might present higher translational validity compared to stimulus-evoked tests (11).
To date, the burrowing test has successfully been used to assess nociception in animal models of inflammatory pain (12-16), nerve injury-induced neuropathy (12,17), post-surgical pain (18), osteoarthritis (19,20), diabetes-associated neuropathy (17), HIV-therapy-induced peripheral neuropathy (21), chemo-therapy-induced mucositis (22,23), chemotherapy-induced neuropathy (24) and complex regional pain syndrome (25).
However, the burrowing test has, to our knowledge, never been used in preclinical models of cancer-induced bone pain. Therefore, the aim of this study was to evaluate the burrowing test in mouse and rat models of cancer-induced bone pain.
Materials and Methods
Mouse experiments
Animals. Experiments in the 4T1-Luc2 mouse model of cancer-induced bone pain were performed at Copenhagen University, Denmark. Five-week-old male BALB/c (21-25 g; Envigo, Venray, the Netherlands) mice were housed in groups of four or five in individually ventilated GM500+ cages (524 cm2) with Tapvei 2HV bedding (Harjumaa, Estonia). The mice were housed in a temperature-controlled room (22±2˚C), on a 12/12 light/dark cycle (lights on at 07:00 AM) and provided water and food (Altromin 1314; Brogaarden, Lynge, Denmark) ad libitum. Environmental enrichment was provided as an S-brick (Tapvei), paper ropes, a red translucent shelter and corn hidden in the bedding. Mice were left to acclimatize to the facility for 1 week prior to initiation of experiments and animal welfare e.g. body weight, breathing, coat condition, stool appearance and abnormal behaviours, was regularly assessed. Mouse experiments were approved by the Danish Animal Experiments Inspectorate (Copenhagen, Denmark) and carried out in accordance with the Danish Act on Animal Experiments (LBK no. 474 of 15/05/2014) and the recommendations and policies of the International Association for the Study of Pain (26). Experiments were conducted between April, 2018 and January, 2019. Baseline behavioural data were obtained for a total of 53 mice used in the experiments as depicted in Figure 1A; 16 were euthanized due to post-surgical complications and four (10%) were excluded from the experimental data due to poor baseline burrowing performance (see below). All behavioural tests were performed by the same researcher, blinded to the experimental groups.
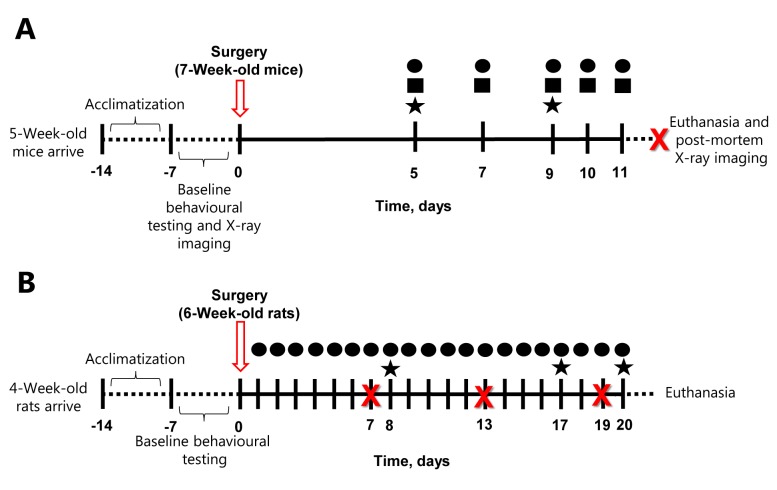
Figure 1. Schematic timeline of the mouse (A) and rat (B) experiments. Mice and rats were left to acclimatize for 1 week upon arrival at the research institution and thereafter baseline measurements were obtained for the behavioral tests within 1 week. Following cancer-induced bone pain surgery, rodents were tested in the limb-use (●), weight-bearing (■) and burrowing test (★) on the days indicated in the figure by a researcher blinded to the experimental groups. When several tests were conducted on the same day, the order was the following: limb-use, followed by weight-bearing and then burrowing. X-Ray images of the hind limbs were captured post-mortem for the mice, and on days 7, 13 and 19 post-surgery for the rats, depicted with an X in the figure.
Cell culture. Mouse mammary gland carcinoma cells (4T1-Luc2; Caliper, Teralfene, Belgium) were cultured as previously described (27). Briefly, the cells were routinely cultured in RPMI 1640 medium (without phenol red) supplemented with 1% penicillin-streptomycin and 10% heat-inactivated foetal bovine serum (FBS) for approximately 10-14 days; cells were split 2 days before surgery. On the day of surgery, cells were harvested with 0.25% trypsin-EDTA, re-suspended in Hank Balanced Salt Solution (HBSS) to a final density of 1.0×106 cells/ml and kept on ice. All reagents were purchased from Thermo Fisher Scientific, Roskilde, Denmark.
Model of cancer-induced bone pain. Seven-week-old mice were briefly anaesthetized with 4% isofluorane (Baxter A/S, 100%; Nomeco, Copenhagen, Denmark) followed by intraperitoneal administration of a ketamine/xylazine cocktail (85.5 mg/kg ketamine from Ketaminol vet; MSD Animal Health, Copenhagen, Denmark; and 12.5 mg/kg xylazine from Nerfasin vet; Virbac, Kolding, Denmark). Eye ointment (Ophtha A/S, Actavis Group, Gentofte, Denmark) was applied to the eyes to avoid dehydration. Thereafter, the inoculation of 4T1-Luc2 cells (or HBSS, sham mice) was performed as previously described (27). Briefly, mice were placed on their dorsal side on a heating pad and a small incision was made on the skin covering the patella ligament of the right hind limb. The retinaculum tendon was loosened and the patella moved aside. Next, a 30-G needle was used to drill a hole into the exposed femoral epiphysis and 1.0×104 4T1-Luc2 cells/10 μl HBSS (n=17) or vehicle alone for the sham group (10 μl HBSS, n=16) was injected intramedullary with a 0.3 ml insulin syringe (BD, Apotek, Copenhagen, Denmark). The bone hole was closed with Ethicon bone wax (Mediq, Brøndby, Denmark) and the wound irrigated with saline. The patella was carefully repositioned on top of the distal femoral epiphyses and two surgical clips (7.5 mm x 1.75 mm, Agnthos, Lidingö, Sweden) were used to close the surgical wound, which was covered with a 2% xylocaine gel (AstraZeneca, Copenhagen, Denmark). Following the surgical procedure, all animals received 500 μl saline and 0.03 mg/kg buprenorphine subcutaneously (Temgesic; Indivior UK Limited, Slough, UK).
Burrowing test. The burrowing test was conducted as previously described with few modifications (16). Each burrowing tube (200 mm length ×72 mm diameter, raised 30 mm from the ground in the frontal, open end) was placed in a standard transparent plastic cage without bedding (125×266×185 mm). Mice underwent a 5-day training program as follows: Day 1, habituation by placing a pair of mice in each burrowing cage with an empty burrowing tube; day 2 and 3, training by placing a pair of mice in each burrowing cage with a burrowing tube filled with 500 g sand (0-3 mm diameter; ScanSand, Herlev, Denmark), and day 4 and 5, baseline testing by placing mice individually in each cage with one burrowing tube filled with 500 g sand. On test days mice were placed individually in a cage with a burrowing tube filled with 500 g sand; the amount of sand left in the tube was weighed at the end of each session. All sessions were conducted for 2 h; cages and burrowing tubes were assigned to each mouse and remained unchanged throughout the entire experiment. Ten percent of the mice (n=4) with the lowest burrowing scores at baseline were excluded from the experiment and the remaining mice were randomized into the sham (n=16) or cancer-bearing (n=17) group according to their baseline burrowing performance; briefly, animals were sorted from highest to lowest in burrowing performance and sequentially assigned into the different experimental groups, ensuring that the average baseline amount of sand burrowed was not significantly different (one-way ANOVA, p>0.05). All burrowing sessions were conducted between 08:00 AM and 12:00 PM, on days 5 and 9 after surgery. When several behavioural tests were conducted on the same day, the order was the following: limb-use, weight-bearing and burrowing.
Limb-use test. Mice were allowed to individually move freely in a transparent plastic cage without bedding (125×266×185 mm). Following 10-min acclimation, the gait of each mouse was observed for 3 min and scored as follows: 4: Normal use of the affected limb, 3: mild or insignificant limping with normal body distribution, 2: pronounced or significant limping accompanied by a shift in body distribution towards the healthy limb, 1: significant limping and partial lack of use of the affected limb and 0: total lack of use of the affected limb. Mice were tested before surgery (baseline) and on days 5, 7, 9, 10 and 11 after surgery, between 07:00 and 10:00 AM. A limb-use score of 0 was defined as the humane endpoint, and it was reached by two cancer-bearing mice on day 9, and seven of the cancer-bearing mice on day 11; all animals were euthanized on day 11.
Weight-bearing test. Mice were individually placed in an incapacitance tester (Columbus Instruments, Columbus, OH, USA) with the hind limbs positioned on two separate scales which record the weight load placed on each limb. Triplicates of 3-s readings were collected for each animal and the weight-bearing ratio was calculated as the average weight placed on the right hind limb divided by the total weight placed on both hind limbs. The test was conducted before surgery and on day 5, 7, 9, 10 and 11 after surgery, between 07:00 and 10:00 AM.
X-ray imaging. Upon euthanasia, the right and left hind limbs were dissected and individually placed in a Lumina XR apparatus (Caliper Life Sciences, Teralfene, Belgiun), where X-ray images were obtained. Each image was calibrated to a standard aluminum wedge. Using ImageJ (1.8.0_112; 64-bit 1.8.0_171; 32-bit; National Institute of Health, Bethesda, MD, USA), the average mean grayscale value of two background soft-tissue regions was subtracted from the grayscale value of the femoral area of interest; all analyses were performed by a researcher blinded to the experimental groups. Measurements were then normalized against the standard aluminum wedge so the readout was relative bone density.
Rat Experiments
Animals. Experiments in the MRMT1-Luc2 rat model of cancer-induced bone pain were performed at Grünenthal GmbH, Aachen, Germany. Four-week-old male Sprague Dawley rats (100-124 g; Janvier Laboratories, Le Genest St Isle, France) were housed in groups of four or two in individually ventilated 1500U cages (1,500 cm2, Tecniplast) with Lignocel Flake J. bedding (J. Rettenmaier & Söhne GmbH & Co. KG, Rosenberg, Germany). The rats were housed in a temperature-controlled room (22±2˚C), on a 12/12 light/dark cycle (lights on at 06:00 AM) and provided water and food (Ratte/Maus-Haltung; Ssniff, Soest, Germany) ad libitum. Environmental enrichment was provided as a pure Aspen medium wood block (Ssniff, Soest, Germany). Rats were left to acclimatize to the facility for 1 week prior to initiation of experiments and animal welfare, e.g. body weight, grooming, posture, gait, was assessed on a daily basis. All experiments were performed according to the German Animal Welfare Act and were approved by the local government authority (no. 81-02.05.40.17.087). Experiments were performed in accordance with the recommendations and policies of the International Association for the Study of Pain (26). Experiments were conducted between February and April 2018. Baseline behavioural data were obtained for a total of 70 rats used in the experiments as depicted in Figure 1B; one died in surgery, 12 were excluded from analyses due abnormal/lack of tumour growth, and seven (10%) were excluded due to poor baseline burrowing performance (see below). All behavioural tests were performed by the same researcher, blinded to the experimental groups.
Cell culture. Rat mammary gland carcinoma cells (MRMT1-Luc2; Tohoku University, Japan) were cultured as previously described (28). MRMT1-Luc2 cells were cultured in RPMI 1640 medium (without phenol red) supplemented with 1% penicillin-streptomycin and 10% heat-inactivated FBS for approximately 14 days; cells were split 2 days before surgery. On the day of surgery, cells were harvested with Detachin (Genlantis, San Diego, CA, USA), re-suspended in HBSS to a final density of 1.5×106 cells/ml and kept on ice until use. All reagents were purchased from Thermo Fisher Scientific.
Model of cancer-induced bone pain. The inoculation of the MRMT1-Luc2 cells was performed as previously described by Falk et al. (29). Briefly, approximately 6-week-old animals were anaesthetized with isoflurane (induction 4%; maintenance 2%±0.5%; Baxter Deutschland GmbH, Unterschleissheim, Germany) and placed on their dorsal side. The right hind limb was shaved and disinfected with 70% ethanol prior to making a small (~1 cm) incision on the skin covering the anterior-medial surface of the tibia. A hole was made in the tibia using a 0.7 mm drill bit (Fine Science Tools, Heidelberg, Germany) in order to insert a catheter into the proximal intramedullary cavity. The catheter was connected to a 50-μl Hamilton syringe to inject 1.5×104 MRMT1-Luc2 cells/10-μl HBSS (n=50) or vehicle alone (10 μl HBSS for sham animals, n=20). After removal of the catheter, the hole was closed using bone restorative material (IRM, Dentsply, PSG Procurement services GmbH, Lohmar, Germany) and the wound was sutured (Vicryl sutur 4-0, V292H, FS-2S needle, 45 cm undyed; Johnson & Johnson medical GmbH, Ethicon Deutschland, Norderstedt, Germany). Animals received 5 mg/kg carprofen (ReboPharm GmbH, Bocholt, Germany) subcutaneously 24-h pre-operatively, peri-operatively and for 2 consecutive days post-surgery and xylocaine (xylocaine pump spray; ReboPharm GmbH, Bocholt, Germany) immediately after surgery and on the following 2 days post-surgery.
Burrowing test. Each burrowing apparatus (320 mm length ×100 mm diameter, raised 60 mm from the ground in the frontal, open end) was placed in a standard Macrolon type IV cage (595×380×200 mm; floor area 1820 cm2). The burrowing test was performed as previously described (9,30), with small modifications as described by Rutten et al. (17) and Wodarski et al. (15). Rats underwent a 4-day training program prior to the assessment of baseline burrowing performance. The animals were exposed to the burrowing apparatus in pairs on the first 2 days to promote social facilitation. On day 1, rats were placed in pairs with an empty tube for 60 min and on day 2 the same pairs were adapted to the empty test cage for 30 min, followed by 1 h training in presence of the burrowing tube, filled with 2500 g gravel (2-4 mm diameter; ORBIT GmbH, Usingen, Germany). When a pair of rats showed poor burrowing performance (< 500 g), individual animals were exchanged with animals that showed sufficient burrowing performance (>500 g) and an extra social facilitation session was conducted. On days 3 and 4, rats were trained individually by 30-min acclimatization to the empty cage followed by the introduction of the 2,500 g gravel-filled tube for 1 h. On day 5, baseline burrowing performance was conducted similarly to the individual training sessions. Burrowing performance was calculated as 2,500 g of substrate minus the amount of gravel left in the tube in the end of each burrowing session. Ten percent of rats (n=7) with the lowest burrowing scores at baseline were excluded from the experiment and the remaining rats were randomized into the sham (n=17) or cancer-bearing (n=33) group according to baseline burrowing performance. Randomization was performed by sorting animals according to baseline burrowing capacity and sequentially assigning them to the experimental groups, comparing the resulting average baseline amount of gravel burrowed until no statistical differences between groups were observed (one-way ANOVA, p>0.05).
All burrowing sessions were conducted between 08:00 AM and 12:00 PM, and burrowing performance was examined on day 8, 17 and 20 after surgery. During the burrowing test no experimenters were present in the room. If several behavioural tests were conducted on the same day, limb use was assessed before burrowing.
Limb-use test. The limb-use test was performed on a daily basis between 06:00 AM and 10:00 AM, starting on the first day after surgery. In the limb-use test, rats were taken out of their home cages, placed in an open space (60 mm × 120 mm) and allowed to move freely for a maximum of 5 min for behavioural assessment. Scoring was as follows; 3: Normal use of limb, 2: mild or insignificant limping with normal body distribution, 1: significant limping with a shift in body distribution towards the healthy limb, 0: no use of the affected limb. A limb-use score of 0 was defined as a humane endpoint (score 0 was reached in one animal; this animal had extra-tibial tumour growth and was sacrificed 14 days post-surgery).
X-ray imaging. On days 7, 13 and 19 after surgery, relative bone density was measured by X-ray densitometry. Animals were shortly anaesthetized with isoflurane (4% for induction; 2.5% for maintenance; Baxter Deutschland GmbH) and placed on the dorsal side in a Lumina XR apparatus (Caliper Life Sciences), with the operated leg in the capture region. X-ray images of the ipsilateral leg were captured and relative bone density was analysed using ImageJ (ImageJ 1.8.0_112; 64-bit; National Institute of Health). The mean grayscale value of a standard region of interest within the trabecular bone of the proximal tibia was measured and the average of two corresponding background regions in the soft tissue proximal to tibia was subtracted. The relative greyscale value was then normalized to a standard aluminum wedge for each X-ray image to calculate the relative bone density. All X-ray analyses were performed by a researcher blinded to the experimental groups.
Statistical analyses. Data analyses and plots were generated in GraphPad Prism 7.03 (rat experiments) or 7.0 (mice experiments) (Graph Pad Inc, La Jolla, CA, USA); non-parametric data were analysed in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Behavioural data were analysed by 2-way repeated measurements ANOVA followed Bonferroni corrections for multiple comparisons. Limb-use (non-parametric) data was analysed by Friedman’s two-way test, followed by Wilcoxon two-sample test. X-ray images were analysed by 2-way ANOVA repeated measurements followed by the Bonferroni method for correction of multiple comparisons (rat experiments), or by multiple Student’s t-tests followed by the Bonferroni-Dunn correction for multiple comparisons (mice experiments). All data are presented as mean±standard error of the mean (SEM) and the level of significance was in all cases set to p<0.05. Based on post-mortem analysis, animals with tumour growth in the wrong location or without tumours at the end of the experiment were excluded from analysis (n=12); these included: extra-tibial tumour growth (n=1), tumour growth in ankle (n=1) and extinction of the tumour (n=10). One rat died during surgery and 16 mice were euthanized due to post-surgical complications.
Results
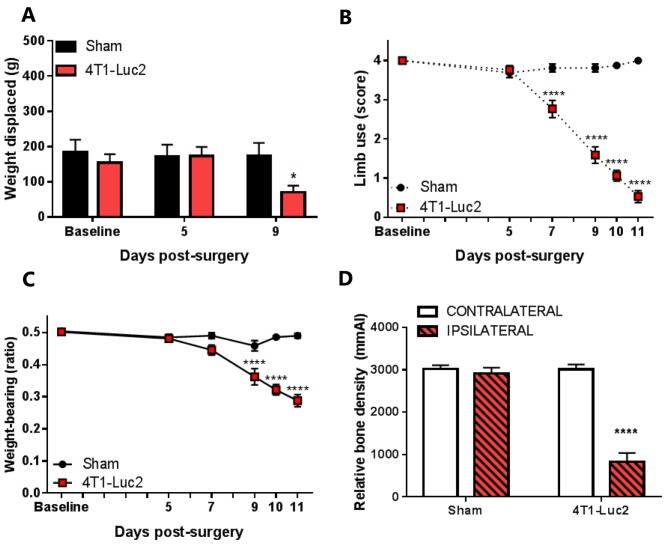
Cancer-bearing mice present nociceptive behaviour and bone degradation. Cancer-bearing mice presented a significant decrease in burrowing performance, compared to sham mice, 9 days after cell inoculation (p<0.05; Figure 2A). The presence of cancer-induced bone pain behaviour in 4T1-inoculated mice was confirmed by the limb-use and weight-bearing tests. Cancer-bearing mice presented a significant decrease in both limb-use scores (p<0.0001; Figure 2B) and weight-bearing ratio (p<0.0001; Figure 2C) compared to sham mice, from day 9 after cell inoculation.
Figure 2. The effect of 4T1-Luc cell inoculation in the intrafemoral cavity of mice on burrowing (A) limb-use (B), weight bearing (C) and relative bone density (D). Burrowing performance was assessed before surgery and on post-surgical days 5 and 9. The relative bone density of contralateral and ipsilateral femurs was assessed before surgery and post-mortem (after day 11) (D). Data are presented as means±SEM. Significantly different at *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs. sham; n=16-17.
Furthermore, the relative bone density in both ipsi- and contralateral femurs of sham and cancer-bearing animals was analysed by X-ray densitometry upon euthanasia. Ipsilateral cancer-bearing femurs presented a significantly lower relative bone density than their contralateral matches (p<0.0001; Figure 2D), while sham femurs presented similar ipsi- and contralateral relative bone density.
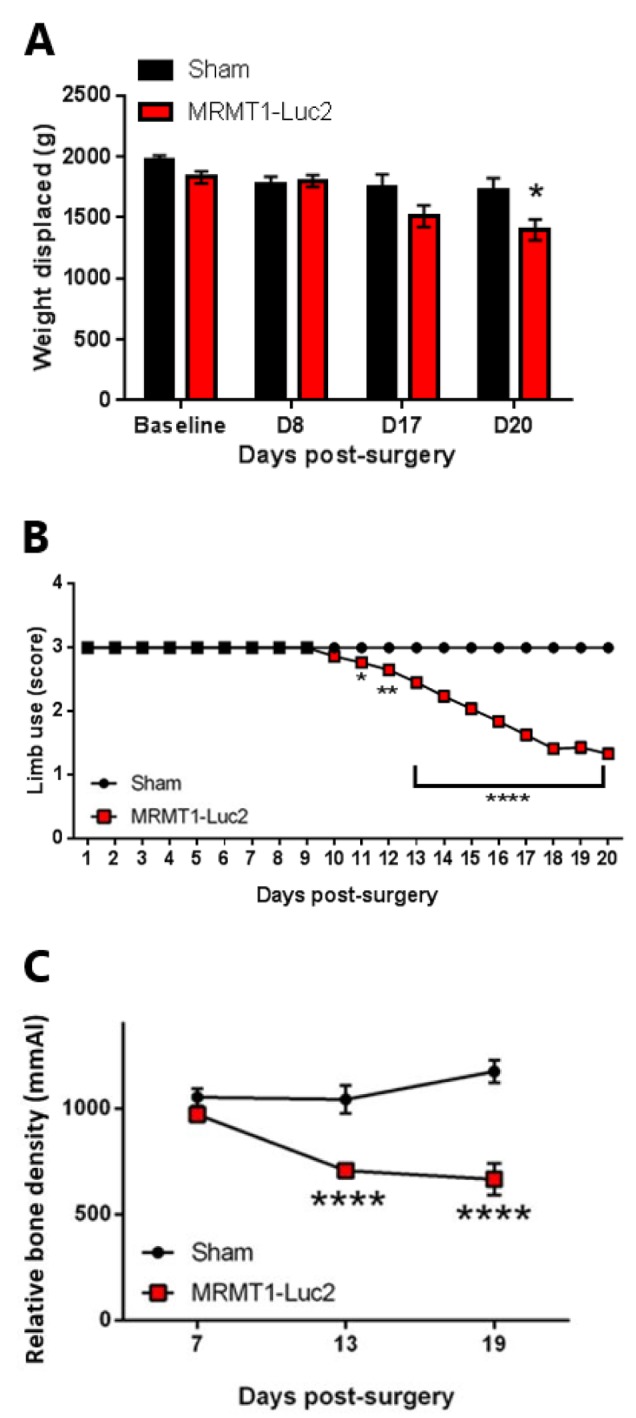
Cancer-bearing rats present nociceptive behaviour and bone degradation. Inoculation of MRMT1-Luc2 cells into the intrafemoral cavity of rat tibia induced a decrease in burrowing performance on post-surgical day 20 (p<0.05; Figure 3A). The presence of cancer-induced bone pain behaviour in the cancer-bearing rats was confirmed in the limb-use test from day 11 after surgery (p<0.05 on post-surgical day 11, p<0.01 on post-surgical day 12, p<0.0001 on post-surgical days 13 to 20; Figure 3B). Furthermore, tibias from cancer-bearing rats showed a significant decrease in relative bone density compared to those from sham mice, from post-surgical day 13 (p<0.0001; Figure 3C).
Figure 3. The effect of MRMT1-Luc2 cell inoculation in the intratibial cavity of rats on burrowing (A), limb use (B) and relative bone density (C). Data are presented as means±SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 vs. sham; n=17-33 per group.
Discussion
The aim of this study was to examine whether burrowing performance is a feasible readout for assessing cancer-induced bone pain in mice and rats. While stimulus-evoked behavioural tests have been traditionally used in preclinical pain research (31-35), it has been argued that they rely on spinal and bulbospinal pathways, which are measures of simple reflexes to nociceptive stimuli as a means to prevent harm, compared with multi-modal and voluntarily altered behavioural traits occurring as a result of induced nociception, e.g. well-being of the animal or nest building (7,8). In this context, the burrowing test is considered more ethologically relevant compared to stimulus-evoked readouts, thereby presenting higher translational validity for the preclinical assessment of pain-related behaviour (11-13,15-20,24,36-38).
The present study is the first to test burrowing performance in rodent cancer-induced bone pain models. For that purpose, syngeneic breast cancer cells were inoculated in the intramedullary cavity of the femur or tibia of immunocompetent mice and rats, respectively; in order to confirm disease progression, the limb-use test and X-ray densitometry were performed in both species and additionally weight bearing was assessed in mice (6,27). In both cancer-bearing mice and rats, the cancer-induced bone pain phenotype developed as expected and was consistent with previous studies (6,39,40).
In rodent models of cancer-induced bone pain, development of a pain-related phenotype in the limb-use test has been shown to coincide with decreases in stimulus-evoked tests, e.g. von Frey and Randall Selitto, and non-stimulus-evoked tests, e.g. weight bearing and grid climbing (28,29,41,42). In accordance with this, our data showed a decrease in burrowing performance in cancer-bearing mice parallel to the development of limb-use impairment and a shift in weight distribution. Furthermore, burrowing performance also decreased in cancer-bearing rats over time compared with sham rats. However, the burrowing performance of the cancer-bearing rats was not impaired to the same extent as previously reported in inflammatory and neuropathic pain models (11,12,16,20), despite having similar baseline levels. Moreover, compared to the mouse experiment, rats developed a more subtle pain-like phenotype in burrowing compared with the limb-use test; it remains unclear whether this difference is species-specific or related to the site of cancer cell inoculation (tibia vs. femur). It is important to note, however, that only male adolescent rodents were included in this study, thus hindering general conclusions on the extent and efficacy of burrowing behaviour as a surrogate marker of cancer-induced bone pain in older and in female rodents.
Recently, a prospective multicentre study showed that is possible to use burrowing behaviour as a robust and reliable readout to assess pain-related behaviour in a Complete Freund’s Adjuvant rat model of inflammatory pain, across laboratories (15). This demonstrates that burrowing is robust and reproducible when standardized by the same protocol, model and species. Herein, we examined for the first time whether burrowing is an appropriate behavioural test for the assessment of well-being in two different species at two different laboratories. Our study shows that cancer-induced bone pain induces a decrease in burrowing behaviour, and we suggest that burrowing may be a useful addition to the battery of available behavioural tests for the detection of well-being in rodent models of cancer-induced bone pain.
Conflicts of Interest
Sonny H.J. Sliepen, Johanna Korioth, Thomas Christoph and Kris Rutten are employees of Grünenthal GmbH. Marta Diaz-delCastillo, Rikke Brix Olsen, Camilla Kristine Appel and Anne-Marie Heegaard have no conflict of interest.
Authors’ Contributions
S.H.J.S., M.D., A.M.H., K.R.: Conceptualization, article writing and editing. S.H.J.S., M.D., T.C., J.K., R.B.O., C.K.A.: data collection. S.H.J.S., M.D.: data analysis. A.M.H., K.R.: supervision.
Acknowledgements
This project received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement no. 642720.
References
- 1.Grond S, Zech D, Diefenbach C, Radbruch L, Lehmann KA. Assessment of cancer pain: A prospective evaluation in 2266 cancer patients referred to a pain service. Pain. 1996;64(1):107–114. doi: 10.1016/0304-3959(95)00076-3. PMID: 8867252. DOI: 10.1016/0304-3959(95)00076-3. [DOI] [PubMed] [Google Scholar]
- 2.Caraceni A, Portenoy RK. An international survey of cancer pain characteristics and syndromes. IASP task force on cancer pain. International Association for the Study of Pain. Pain. 1999;82(3):263–274. doi: 10.1016/S0304-3959(99)00073-1. PMID: 10488677. DOI: 10.1016/S0304-3959 (99)00073-1. [DOI] [PubMed] [Google Scholar]
- 3.Slosky LM, Largent-Milnes T, Vanderah TW. Use of animal models in understanding cancer-induced bone pain. Cancer Growth Metastasis. 2015;8:47–62. doi: 10.4137/CGM.S21215. PMID: 26339191. DOI: 10.4137/CGM.S21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwei MJ, Honore P, Rogers SD, Salak Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19(24):10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. PMID: 10594070. DOI: 10.1523/jneurosci.19-24-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falk S, Dickenson AH. Pain and nociception: Mechanisms of cancer-induced bone pain. J Clin Oncol. 2014;32(16):1647–1654. doi: 10.1200/JCO.2013.51.7219. PMID: 24799469. DOI: 10.1200/JCO.2013.51.7219. [DOI] [PubMed] [Google Scholar]
- 6.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain. 2002;96(1-2):129–140. doi: 10.1016/s0304-3959(01)00437-7. PMID: 11932069. DOI: 10.1016/ S0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 7.Mogil JS. Animal models of pain: Progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. PMID: 19259101. DOI: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 8.Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18(4):325–343. doi: 10.1016/0304-3959(84)90045-9. PMID: 6728499. DOI: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]
- 9.Deacon RM. Burrowing in rodents: A sensitive method for detecting behavioral dysfunction. Nat Protoc. 2006;1(1):118–121. doi: 10.1038/nprot.2006.19. PMID: 17406222. DOI: 10.1038/nprot.2006.19. [DOI] [PubMed] [Google Scholar]
- 10.Jirkof P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods. 2014;234:139–146. doi: 10.1016/j.jneumeth.2014.02.001. PMID: 24525328. DOI: 10.1016/j.jneumeth.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Andrews N, Harper S, Issop Y, Rice AS. Novel, nonreflex tests detect analgesic action in rodents at clinically relevant concentrations. Ann N Y Acad Sci. 2011;1245:11–13. doi: 10.1111/j.1749-6632.2011.06342.x. PMID: 22211966. DOI: 10.1111/j.1749-6632.2011.06342.x. [DOI] [PubMed] [Google Scholar]
- 12.Andrews N, Legg E, Lisak D, Issop Y, Richardson D, Harper S, Pheby T, Huang W, Burgess G, Machin I, Rice AS. Spontaneous burrowing behaviour in the rat is reduced by peripheral nerve injury or inflammation associated pain. Eur J Pain. 2012;16(4):485–495. doi: 10.1016/j.ejpain.2011.07.012. PMID: 22396078. DOI: 10.1016/ j.ejpain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Gould SA, Doods H, Lamla T, Pekcec A. Pharmacological characterization of intraplantar complete Freund’s adjuvant-induced burrowing deficits. Behav Brain Res. 2016;301:142–151. doi: 10.1016/j.bbr.2015.12.019. PMID: 26704218. DOI: 10.1016/j.bbr.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Jirkof P, Leucht K, Cesarovic N, Caj M, Nicholls F, Rogler G, Arras M, Hausmann M. Burrowing is a sensitive behavioural assay for monitoring general wellbeing during dextran sulfate sodium colitis in laboratory mice. Lab Anim. 2013;47(4):274–283. doi: 10.1177/0023677213493409. PMID: 23828853. DOI: 10.1177/0023677213493409. [DOI] [PubMed] [Google Scholar]
- 15.Wodarski R, Delaney A, Ultenius C, Morland R, Andrews N, Baastrup C, Bryden LA, Caspani O, Christoph T, Gardiner NJ, Huang W, Kennedy JD, Koyama S, Li D, Ligocki M, Lindsten A, Machin I, Pekcec A, Robens A, Rotariu SM, Voß S, Segerdahl M, Stenfors C, Svensson CI, Treede R-D, Uto K, Yamamoto K, Rutten K, Rice ASC. Cross-centre replication of suppressed burrowing behaviour as an ethologically relevant pain outcome measure in the rat: A prospective multicentre study. Pain. 2016;157(10):2350–2365. doi: 10.1097/j.pain.0000000000000657. PMID: 27820160. DOI: 10.1097/j.pain.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutten K, Schiene K, Robens A, Leipelt A, Pasqualon T, Read SJ, Christoph T. Burrowing as a non-reflex behavioural readout for analgesic action in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18(2):204–212. doi: 10.1002/j.1532-2149.2013.00358.x. PMID: 23853119. DOI: 10.1002/j.1532-2149.2013.00358.x. [DOI] [PubMed] [Google Scholar]
- 17.Rutten K, Gould SA, Bryden L, Doods H, Christoph T, Pekcec A. Standard analgesics reverse burrowing deficits in a rat cci model of neuropathic pain, but not in models of type 1 and type 2 diabetes-induced neuropathic pain. Behav Brain Res. 2018;350:129–138. doi: 10.1016/j.bbr.2018.04.049. PMID: 29738803. DOI: 10.1016/ j.bbr.2018.04.049. [DOI] [PubMed] [Google Scholar]
- 18.Jirkof P, Cesarovic N, Rettich A, Nicholls F, Seifert B, Arras M. Burrowing behavior as an indicator of post-laparotomy pain in mice. Front Behav Neurosci. 2010;4:165–165. doi: 10.3389/fnbeh.2010.00165. PMID: 21031028. DOI: 10.3389/fnbeh.2010.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaney A, Su J, Gao T, Agalave N, Svensson CI. Spontaneous burrowing behavior as an outcome measure of the global impact of chronic pain in preclinical models of arthritis. Scand J Pain. 2017;8(1):48–48. DOI: 10.1016/j.sjpain.2015.04.007. [Google Scholar]
- 20.Bryden LA, Nicholson JR, Doods H, Pekcec A. Deficits in spontaneous burrowing behavior in the rat bilateral monosodium iodoacetate model of osteoarthritis: An objective measure of pain-related behavior and analgesic efficacy. Osteoarthr Cartilage. 2015;23(9):1605–1612. doi: 10.1016/j.joca.2015.05.001. PMID: 25966657. DOI: 10.1016/j.joca.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Calvo M, Karu K, Olausen HR, Bathgate G, Okuse K, Bennett DL, Rice AS. A clinically relevant rodent model of the HIV antiretroviral drug stavudine induced painful peripheral neuropathy. Pain. 2013;154(4):560–575. doi: 10.1016/j.pain.2012.12.023. PMID: 23415009. DOI: 10.1016/j.pain.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker AL, Lymn KA, Nicholson A, Howarth GS. The assessment of general well-being using spontaneous burrowing behaviour in a short-term model of chemotherapy-induced mucositis in the rat. Lab Anim. 2015;49(1):30–39. doi: 10.1177/0023677214546913. PMID: 25112495. DOI: 10.1177/0023677214546913. [DOI] [PubMed] [Google Scholar]
- 23.Whittaker AL, Zhu Y, Howarth GS, Loung CS, Bastian SEP, Wirthensohn MG. Effects of commercially produced almond by-products on chemotherapy-induced mucositis in rats. World J Gastrointest Pathophysiol. 2017;8(4):176–187. doi: 10.4291/wjgp.v8.i4.176. PMID: 291847 03. DOI: 10.4291/wjgp.v8.i4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffiths LA, Duggett NA, Pitcher AL, Flatters SJL. Evoked and ongoing pain-like behaviours in a rat model of paclitaxel-induced peripheral neuropathy. Pain Res Manag. 2018;2018:8217613–8217613. doi: 10.1155/2018/8217613. PMID: 29973969. DOI: 10.1155/2018/8217613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das V, Kroin JS, Moric M, Buvanendran A. Biochemical and pharmacological characterization of a mice model of complex regional pain syndrome. Reg Anesth Pain Med. 2017;42(4):507–516. doi: 10.1097/AAP.0000000000000622. PMID: 28609318. DOI: 10.1097/aap.0000000000000622. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. PMID: 6877845. DOI: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 27.Falk S, Uldall M, Appel C, Ding M, Heegaard AM. Influence of sex differences on the progression of cancer-induced bone pain. Anticancer Res. 2013;33(5):1963–1969. PMID: 23645744. [PubMed] [Google Scholar]
- 28.Falk S, Al-Dihaissy T, Mezzanotte L, Heegaard AM. Effect of sex in the MRMT-1 model of cancer-induced bone pain. F1000Res. 2015;4:445–445. doi: 10.12688/f1000research.6827.1. PMID: 26834983. DOI: 10.12688/f1000 research.6827.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falk S, Ipsen DH, Appel CK, Ugarak A, Durup D, Dickenson AH, Heegaard AM. Randall Selitto pressure algometry for assessment of bone-related pain in rats. Eur J Pain. 2015;19(3):305–312. doi: 10.1002/ejp.547. PMID: 25057115. DOI: 10.1002/ejp.547. [DOI] [PubMed] [Google Scholar]
- 30.Contet C, Rawlins JN, Deacon RM. A comparison of 129s2/svhsd and c57bl/6jolahsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: Implications for the study of genetically modified mice. Behav Brain Res. 2001;124(1):33–46. doi: 10.1016/s0166-4328(01)00231-5. PMID: 11423164. DOI: 10.1016/S0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- 31.El Mouedden M, Meert TF. Pharmacological evaluation of opioid and non-opioid analgesics in a murine bone cancer model of pain. Pharmacol Biochem Behav. 2007;86(3):458–467. doi: 10.1016/j.pbb.2007.01.003. PMID: 17306872. DOI: 10.1016/j.pbb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 32.El Mouedden M, Meert TF. Evaluation of pain-related behavior, bone destruction and effectiveness of fentanyl, sufentanil, and morphine in a murine model of cancer pain. Pharmacol Biochem Behav. 2005;82(1):109–119. doi: 10.1016/j.pbb.2005.07.016. PMID: 16125759. DOI: 10.1016/j.pbb.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Saito O, Aoe T, Yamamoto T. Analgesic effects of nonsteroidal antiinflammatory drugs, acetaminophen, and morphine in a mouse model of bone cancer pain. J Anesth. 2005;19(3):218–224. doi: 10.1007/s00540-005-0323-3. PMID: 16032450. DOI: 10.1007/s00540-005-0323-3. [DOI] [PubMed] [Google Scholar]
- 34.Mao QL Ying, Zhao J, Dong ZQ, Wang J, Yu J, Yan MF, Zhang YQ, Wu GC, Wang YQ. A rat model of bone cancer pain induced by intra-tibia inoculation of Walker 256 mammary gland carcinoma cells. Biochem Biophys Res Commun. 2006;345(4):1292–1298. doi: 10.1016/j.bbrc.2006.04.186. PMID: 16725112. DOI: 10.1016/j.bbrc.2006.04.186. [DOI] [PubMed] [Google Scholar]
- 35.Fox A, Medhurst S, Courade JP, Glatt M, Dawson J, Urban L, Bevan S, Gonzalez I. Anti-hyperalgesic activity of the COX-2 Inhibitor lumiracoxib in a model of bone cancer pain in the rat. Pain. 2004;107(1-2):33–40. doi: 10.1016/j.pain.2003.09.003. PMID: 14715386. DOI: 10.1016/j.pain.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Deseure K, Hans G. Orofacial neuropathic pain reduces spontaneous burrowing behavior in rats. Physiol Behav. 2018;191:91–94. doi: 10.1016/j.physbeh.2018.04.020. PMID: 29673859. DOI: 10.1016/j.physbeh. 2018. 04.020. [DOI] [PubMed] [Google Scholar]
- 37.Muralidharan A, Kuo A, Jacob M, Lourdesamy JS, Carvalho LM, Nicholson JR, Corradini L, Smith MT. Comparison of burrowing and stimuli-evoked pain behaviors as end-points in rat models of inflammatory pain and peripheral neuropathic pain. Front Behav Neurosci. 2016;10:88–88. doi: 10.3389/fnbeh.2016.00088. PMID: 27242458. DOI: 10.3389/fnbeh.2016.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutten K, Robens A, Read SJ, Christoph T. Pharmacological validation of a refined burrowing paradigm for prediction of analgesic efficacy in a rat model of sub-chronic knee joint inflammation. Eur J Pain. 2014;18(2):213–222. doi: 10.1002/j.1532-2149.2013.00359.x. PMID: 2385 2581. DOI: 10.1002/j.1532-2149.2013.00359.x. [DOI] [PubMed] [Google Scholar]
- 39.Clohisy DR, Mantyh PW. Bone cancer pain. Clin Orthop Relat Res. 2003;415(Suppl):S279–288. doi: 10.1097/01.blo.0000093059.96273.56. PMID: 14600620. DOI: 10.1097/01.blo.0000093059.96273.56. [DOI] [PubMed] [Google Scholar]
- 40.Jimenez JM Andrade, Mantyh WG, Bloom AP, Ferng AS, Geffre CP, Mantyh PW. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181. doi: 10.1111/j.1749-6632.2009.05429.x. PMID: 20536932. DOI: 10.1111/j.1749-6632. 2009.05429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Minett MS, Falk S, Santana S Varela, Bogdanov YD, Nassar MA, Heegaard AM, Wood JN. Pain without nociceptors? NAV1.7-independent pain mechanisms. Cell Rep. 2014;6(2):301–312. doi: 10.1016/j.celrep.2013.12.033. PMID: 24440715. DOI: 10.1016/j.celrep.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Appel CK, Gallego S Pedersen, Andersen L, Blancheflor S Kristensen, Ding M, Falk S, Sayilekshmy M, Gabel C Jensen, Heegaard AM. The SRC family kinase inhibitor dasatinib delays pain-related behaviour and conserves bone in a rat model of cancer-induced bone pain. Sci Rep. 2017;7(1):4792–4792. doi: 10.1038/s41598-017-05029-1. PMID: 28684771. DOI: 10.1038/s41598-017-05029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]