Abstract
Plasma medicine comprises the application of physical plasma directly on or in the human body for therapeutic purposes. Three most important basic plasma effects are relevant for medical applications: i) inactivation of a broad spectrum of microorganisms, including multidrug-resistant pathogens, ii) stimulation of cell proliferation and angiogenesis with lower plasma treatment intensity, and iii) inactivation of cells by initialization of cell death with higher plasma treatment intensity, above all in cancer cells. Based on own published results as well as on monitoring of relevant literature the aim of this topical review is to summarize the state of the art in plasma medicine and connect it to redox biology. One of the most important results of basic research in plasma medicine is the insight that biological plasma effects are mainly mediated via reactive oxygen and nitrogen species influencing cellular redox-regulated processes. Plasma medicine can be considered a field of applied redox biology.
Keywords: Plasma medicine, redox biology, cold atmospheric plasma (CAP), review
Plasma medicine is a new field of research combining plasma physics, life science and clinical medicine. Basically, medical application of physical plasma comprises two principal approaches: i) use of plasma-based or plasma-supplemented techniques to treat surfaces, materials or devices to realize specific qualities for subsequent special medical applications, and ii) direct application of physical plasma on or in the human (or animal) body to apply therapeutic effects based on direct interaction of plasma with living tissue. Plasma application for the treatment of medical materials or devices is an important subject of research and has been utilized for several years now (1-8). However, the core area of plasma medicine – as a new field of research – focuses on the use of plasma technology in direct treatment of living cells and tissues. The aim of applied plasma medicine is to exploit a differentiated interaction of specific plasma components with specific structural, as well as functional elements or functionalities of living cells to control, and ideally, normalize therapeutic effects. Besides its antimicrobial activity, exposure of mammalian cells to physical plasma can lead either to stimulation or inhibition of cellular function (9). Consequently, most research and primary medical application of physical plasma is concentrated on wound healing and cancer treatment. During recent years, a broad spectrum of different plasma sources (called by many different names and abbreviations) has been designed and dedicated for biomedical applications (9-12).
Plasma Generation and Plasma Sources for Biomedical Applications
Physical plasma is a special excited gas state, sometimes named “the fourth state of matter” following solid, liquid, and gaseous states. It can be generated by a continuous supply of energy to the atoms or molecules of a neutral gas until an excited state is achieved. The energy required may be provided separately by thermal, chemical, electrical and radiative resources or a combination of all. However, the predominant ionizing mechanism is the collision process that involves inelastic collision, electron impact, radiative interactions and charge exchange. As the typical life span of excited states is about 10 ns stopping the energy supply starts a depletion process rapidly quenching the plasma. The electron impact ionization is the most robust procedure generating a plasma for biomedical purposes. The energy is transferred by inelastic and elastic collisions of high-energy electrons generated by a strong electric field with the atoms or molecules in the gas resulting in its partial ionization. The temperature of such partially ionized gas is always substantially lower than the characteristic ionization temperature. In a well-designed plasma source, ambient temperature of the plasma can be achieved. The physicochemical characteristics of plasma can be complex and they depend on a multitude of parameters, including the type and composition of the gas or gas mixture used for plasma generation, the applied energy and electrode configuration, the pressure, and the environment. Consequentially, a broad range of parameters can be controlled by the plasma source design. With regard to its application, especially in the medical context, useful classifications are thermal versus non-thermal plasmas and low pressure versus atmospheric pressure plasmas (13-15). For a direct application on living tissue as the main aim of plasma medicine, only plasma generated under atmospheric conditions should be used. Medical treatment techniques using such plasmas have been firmly established for a long time in the field of electro surgery, even if they were not explicitly referred to as plasma medicine at the time. Such techniques, like argon plasma coagulation (APC), rely on precisely targeted thermal necrotization of tissue to achieve hemostasis (cauterization), or to cut or remove tissue (16,17). Furthermore, several plasma-based devices in cosmetics, e.g. for wrinkle removal and skin regeneration, also rely on thermal plasma effects (18,19). Since the 1990s, technologies for stable and reproducible plasma generation at low temperature under atmospheric conditions are available on a larger scale, facilitating the generation of so-called cold atmospheric plasmas (CAP). This has led to considerable intensification of research in the field of medical applications of physical plasma at tissue-compatible temperatures. In terms of medical application, “cold” means temperatures lower than 40˚C at the target site during plasma treatment (9).
Simplifying, generation of CAP and its components can be summarized with the following three steps (9,11,14):
i) Ionization and excitation of atoms or molecules of a neutral gas (argon, helium, oxygen, nitrogen, air, or mixtures thereof) via electron impact by supplying electrical energy;
ii) Interaction of electrons and high energy states of atoms or molecules with reaction partners in the plasma phase and its vicinity (ambient air, liquids, surfaces), generating secondary and tertiary reactive species;
iii) Emission of electromagnetic radiation (UV, visible light, IR/heat, electric fields) formed by excitation and depletion processes or charge transport.
It is important to note that the plasma state is maintained as long as the energy supply exists, i.e. it is not possible to store a plasma like a gas.
Because plasma contains highly motile electrons it is conductive and can transfer electrical current to cells and tissue with possible biological consequences (20). Also, the emitted electromagnetic radiation, above all the ultraviolet (UV) light, has the potential to elicit biological effects (21,22). However, according to the current state of knowledge, free electrons, high energy states of atoms and molecules along with ions and radicals in the plasma and those generated in secondary reactions are the main components of the chemical reactivity and biological activity of a plasma (23). The sum of the CAP derived chemical entities is often circumscribed as reactive species.
A large number of plasma sources that are potentially useful for medical applications are described in the literature. They differ in their plasma generation mechanism, source geometry, working gases, and, consequently, vary in their application characteristics (9,12,24-28). During recent years, mainly two fundamental concepts of CAP devices have been tested and are partially applied for medical purposes: i) dielectric barrier discharges (DBD) and ii) plasma jets (9,29-32).
In Figure 1, three technical principles of plasma sources intended for biomedical applications are depicted (11,30).
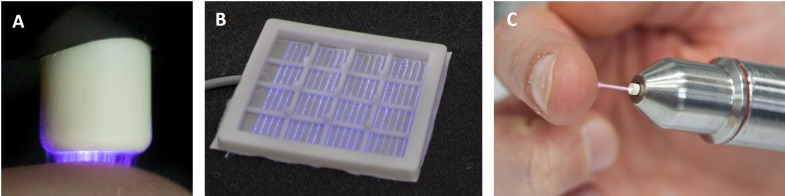
Figure 1. Example photographs of the most common cold atmospheric plasma source principles for biomedical applications: volume DBD (A), surface DBD (B), and plasma jet (C).
The volume DBD (Figure 1A) is characterized by plasma ignition in a gap between an isolated high voltage electrode and the target to be treated. Consequently, cultured cells or living tissues in biomedical application are part of the discharge electrode configuration. Plasma has a direct contact with the target to be treated and the target is directly exposed to the electrical field that is necessary for plasma generation (9,31). In the surface DBD (Figure 1B), plasma is ignited around an individually designed electrode structure (e.g. circular or grid-like), which is isolated from a counter electrode. There is no direct contact of the active plasma with the target to be treated, instead impact is achieved by transport processes bringing the reactive species to the living tissue. With both DBD configurations, atmospheric air usually serves as the working gas for plasma generation. Both volume and surface DBD devices are suitable to generate plasmas over larger areas (9,31). In a plasma jet device (Figure 1C), the electrode setup for plasma generation is located in or around a tube-like arrangement, in most cases inside a pen-like device. Diverse electrode configurations can be used, e.g. pin electrodes, ring electrodes, plate electrodes etc. The plasma is ignited inside the device using a working gas that is flowing through the tube. The so-called plasma effluent (or afterglow) is carried out along the gas flow and can be brought into direct contact with the target to be treated. In order to maintain a low temperature and to achieve excellent controllability of the discharge, most plasma jet devices are using noble gases (helium or argon) as working gas, often doped with small amounts of molecular gases (nitrogen, oxygen). The target to be treated is not part of the electrode configuration. However, because of the conductivity of the plasma and its afterglow, small electrical currents may pass to the target. By choosing an appropriate design of electrode and high voltage waveform these currents can be easily controlled (9,29,32).
One of the best-investigated plasma sources for biomedical application is the argon-driven cold atmospheric pressure plasma jet, kINPen (Figure 1C) (32-34). A needle electrode inside a dielectric capillary is powered with a sinusoidal high voltage (2-6 kVpp) with a frequency of 1.0-1.1 MHz (power <3.5W in the hand-held unit). Argon gas with a flow rate of 3-5 standard liters per minute (slm) is used as the working gas. The plasma is generated at the tip of the needle and is subsequently released with the feed gas flow into the atmospheric environment, thereby generating a typical plasma effluent with a length of 9-12 mm and with 1 mm in diameter. Under these conditions, the electron density in the core plasma region near the high voltage electrode tip is in the order of 1012 cm–1 and one order of magnitude lower in the visible effluent zone. However, electron density depends on several parameters and can be varied by admixture of molecular gases, such as oxygen and nitrogen (32).
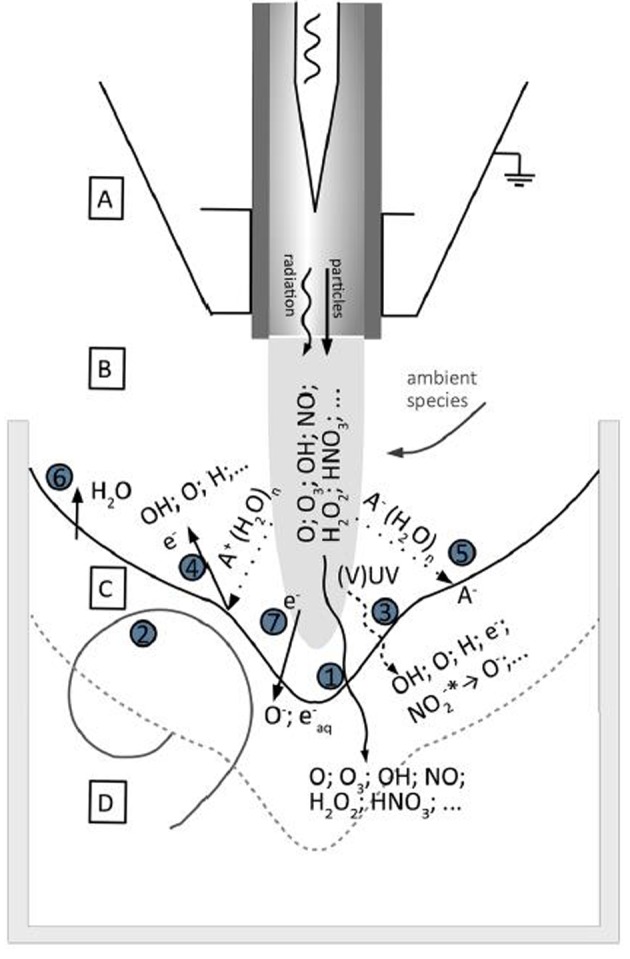
The reactive species generated inside the plasma or as a result of plasma interactions with the surrounding media are considered the most important components responsible for biological plasma effects. In the kINPen, the argon-based plasma effluent is exposed to atmospheric air containing predominantly oxygen, nitrogen, and water. Traces of these, especially the water, are contained in the working gas in low ppm-amounts, too. These atmospheric air compounds are the precursors for secondarily generated non-radical and radical reactive oxygen and nitrogen species (ROS, RNS). Generation of ROS and RNS can also be modulated by controlled admixture of oxygen, nitrogen, water or air to the argon working gas flow, or by gas shielding and modification of the atmosphere around the plasma effluent (32). When the effluent containing the ROS, RNS, and residual high energy states targets a liquid (a tissue), a number of transport processes and tertiary reactions with target molecules occurring is so far not fully understood. Current knowledge assumes that at the interface between gas phase and liquid (or solid) target as well as in the target bulk a considerable rearrangement of the ROS/RNS pattern occurs (Figure 2) (32,35-38). Beside an interaction of the plasma derived species among themselves, the interaction with target biomolecules results in the formation of diverse chemical structures acting as a messenger or a beacon.
Figure 2. Schematic of interactions between the kINPen plasma effluent with aqueous liquid. There are four reaction regions: (A) core plasma region, (B) effluent and plasma-ambient air interaction zone, (C) plasma/gas/liquid interface, and (D) bulk liquid. Seven processes are dominant for the generation of ROS and RNS, and their transfer: (1), (2) mass transfer from plasma to liquid based on gas/plasma component dissolution and gas and liquid flow, respectively, (3) photolysis based on the plasma UV radiation, (4); positive ions and clusters, which induce sputtering processes releasing water, gases, or electrons from the liquid, (5) negative ions, clusters, and cluster transport, (6) water evaporation, and (7) electron impact or transport. (Adapted from 32).
Because all plasma sources for biomedical applications are working under atmospheric air conditions or use ambient air as working gas, the generation of ROS and RNS from air-based oxygen and nitrogen is a corresponding feature of all these plasma sources. However, the composition and quantity of plasma-generated ROS and RNS, as well as UV irradiance, electrical field and other characteristics, are strongly dependent on specific plasma sources and device parameters as working gas composition, power input and temperature (39).
Biological Plasma Effects and its Medical Use: Focus on Wound Healing
Among the vast number of experimental reports on biological plasma effects (using different plasma sources and devices under varying conditions), three effects most important for a medical application are consistently reported (30):
i) Effective inactivation of a broad spectrum of microorganisms including multidrug-resistant pathogens;
ii) Stimulation of cell proliferation and angiogenesis with lower plasma treatment intensity and time;
iii) Initialization of (programmed) cell death with higher plasma treatment intensity and time, primarily in cancer cells.
With the improved availability of CAP technology in the 1990s, its antimicrobial activity was in the early focus of research with regard to microbial decontamination or sterilization of materials and devices (40,41). Extensive research has been done on the mechanisms of low-pressure cold plasma interaction with microorganisms. This was mainly attributed to UV-based DNA damage in combination with erosion of microorganism structures by UV-based photodesorption and etching processes by reactive plasma species (42-45). However, the knowledge on mechanisms of inactivation of microorganisms by CAP is limited. Microbicidal CAP effects are mainly attributed to the activity of ROS and RNS leading to oxidative damage and modification of cytoplasmatic membrane, proteins, and DNA (46-48). Furthermore, induction of apoptosis-like processes of programmed cell death in bacteria is being discussed as a potential mechanism (49). Other physical mechanisms are currently under investigation, for instance electrostatic disruption by plasma-derived charged particles, or electroporation caused by plasma-related electric fields (49,50).
Regardless of the specific mode of action, early experimental evidence on microbicidal plasma effects on heat or radiation-sensitive material surfaces spurred further efforts to investigate plasma effects on contaminated or infected tissue. Several in vitro studies on effective inactivation of clinically relevant microorganisms and viruses have produced promising results (51-58). The first clinical investigations on antiseptic plasma effects on tissue and wounds that followed have shown rather modest microbial reduction rates (59-63). However, improvement of wound healing in general has been partially seen during these clinical investigations (61,64). Some additional case reports and clinical investigations with different plasma devices support these findings (65,66).
Starting in 2013, the first cold atmospheric pressure plasma devices received CE certification as medical devices for the purpose of treating chronic wounds as well as pathogen-based skin diseases in Germany and Europe. These include the argon-driven, jet-like CAP devices kINPen MED (neoplas tools GmbH Greifswald, Germany) and SteriPlas (ADTEC, Hunslow, UK) as well as the DBD-based devices PlasmaDerm (CINOGY GmbH Duderstadt, Germany) and plasma care (terraplasma medical GmbH Garching, Germany), the latter two using atmospheric air as working gas. Certification of all these devices was based on comprehensive physical and biological characterization of the respective plasma source accompanied with clinical investigations (9,10,25,34,53,55,59-64). However, even if randomized controlled clinical trials are not available as yet (as unfortunately is the case for many wound therapies due to a lack of standardization in wound scoring), positive experiences are consistently reported from the medical practice. Particularly in chronic wounds, e.g. venous leg ulcers where any conventional therapeutic options have been exhausted, a clear benefit of CAP treatment has been found. Some practitioners have reported a re-start or acceleration of wound healing process in more than 80% of cases as a preliminary result, particularly following the use of the kINPen MED (11,30). Now, the most important aim is to validate these results by systematic clinical data.
These clinical experiences confirm a very early hypothesis in plasma medicine research, that plasma effects on wound healing may be a result of a two-step activity: antiseptics on wound surface in combination with stimulation of tissue regeneration (67). Several experimental in vitro studies could demonstrate a direct impact of CAP on cell proliferation and migration as well as on angiogenesis (68-76). The stimulating effect on skin tissue regeneration was confirmed in several in vivo animal experiments (69,74-90) and in human volunteers or patients with reasonably defined wounds (91-93). It has to be pointed out that these last-mentioned wound healing effects in vivo were demonstrated in acute wounds without any interfering microbial contamination. With plasma treatment, the spontaneous wound healing process was not impeded, and there was an acceleration in the early stage of wound healing. With this direct proof of stimulation of wound healing by plasma treatment, it seems that the antiseptic plasma effect can be partially pushed into the background because it may turn out that it is not the dominating process as it was assumed for several years. Consequently, as a next step in clinical research, it should be investigated, if this early stimulation of wound healing may have any beneficial effects also in acute wounds, e.g. with regard to scar formation or prevention of complications in wound healing. Possible fields of application of CAP in acute wound healing could be in patients with co-morbidities leading to a high risk of disturbed wound healing and subsequent chronification, in the case of large-area burns or in the treatment of skin graft donor and acceptor sites (92,94).
Nevertheless, there should be no doubt that plasma-induced antiseptics have an important additional effect in the case of contaminated wounds. Here, a very important question is why plasma is destructive or inactivating for microorganisms while stimulating repair mechanisms on mammalian cells. There is some evidence that the ROS-RNS composition resulting from plasma generation under atmospheric air conditions is more toxic for microorganisms because both oxygen- and nitrogen-containing reactive species are required for antimicrobial effects, whereas mammalian cell toxicity is mostly dependent on oxygen-based reactive species (95). Another very interesting insight is that, because of the role of ROS generated by immune cells to fight wound infection, wounded tissue takes cytoprotective measures to promote some tissue “resilience” for protection against ROS caused damage (96). This physiological mechanism may also be protective against plasma-generated ROS and RNS.
Redox Biology as the Scientific Grounding of Plasma Medicine
The fact that the biological effects of CAPs are mainly based on ROS and RNS, was primarily reasoned from experimental observations in vitro. Plasma effects on mammalian cells were found to be dependent on cell culture media composition, each exhibiting a different antioxidative potential. Additionally, biological plasma effects could be extinguished when antioxidants like N-Acetylcystein (NAC) were added (97-99). A multitude of investigations on plasma-liquid interactions has demonstrated the occurrence of ROS and RNS in liquid phases following plasma treatment (36,37). Moreover, it has been shown several times that liquids, such as water, physiological saline, or cell culture media become biologically effective following plasma treatment (100-105). This underlines a key role of liquid phase composition for biological plasma effects. ROS and RNS like superoxide (O2–•), hydrogen peroxide (H2O2), hydroxyl radical (•OH), singlet oxygen (1O2), ozone (O3), and RNS, such as nitric oxide (•NO), nitrogen dioxide (•NO2) and peroxynitrite (ONOO-), are transferred from plasma into the liquid environment of cells and tissue, or they are generated by a very complex network of secondary liquid reactions (Figure 2) (23,106-111).
This insight of the central role of ROS and RNS has opened up the door to the field of redox biology to explain and interpret biological effects caused by CAP. Redox biology can be taken as the interface between the more or less unspecific impact of external factors and the specific response and adaptation of a cell or an organism via its metabolic and macromolecular structures (112). Meanwhile, it is well known that ROS and RNS are not solely harmful in cells, but also serve as signaling molecules via reversible oxidations and reductions of specific protein structures with cysteine as a major reaction target (113). In a comprehensive in vitro study using different jet-based plasma devices with different working gas mixtures, the cysteine-oriented plasma chemistry could be proven. Furthermore, it could be demonstrated that cysteine is a useful and sensitive tracer compound to discriminate between the chemical potential of different plasma sources or gas mixtures, respectively (114).
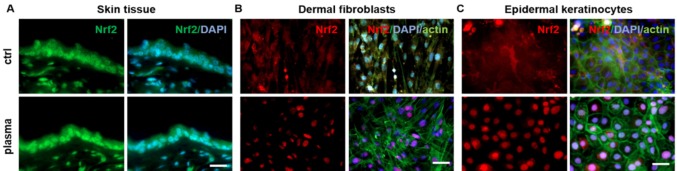
One of the most important players for ROS and RNS-based regulation in cell physiology is the mammalian Kelch-like ECH-associated protein 1 (Keap1)-nuclear factor erythroid 2-related factor (Nrf2) pathway. In regular cell physiology, it uses cysteine oxidation to respond to increased ROS levels. By redox modification of cysteine-residues of Keap1, Nrf2 is released from its complex and the E3 ubiquitin ligase cullin 3 (CUL3). Subsequently, Nrf2 translocates from the cytosol to the nucleus where it binds to antioxidant responsive elements (ARE) on DNA, promoting the upregulation of antioxidant genes (113,115). It has been demonstrated that CAP treatment of human keratinocytes in vitro leads to the stimulation of this Nrf2 pathway, resulting in the translocation of Nrf2 into the nucleus and the subsequent activation of Nrf2-ARE-targets, such as glutathione (GSH), glutathione reductase (GSR), glutathione S-transferase (GST), superoxide dismutase (SOD), heme oxygenase 1 (HMOX-1), and NADPH quinine oxidoreductase 1 (NQO1) (90,116). Moreover, in an acute wound healing study in mice, an early activation of the Nrf2 pathway has also been demonstrated in vivo and ex vivo on skin tissue, dermal fibroblasts and epidermal keratinocytes (Figure 3) (76,117).
Figure 3. CAP treatment induces an early translocation of Nrf2 from the cytoplasm to the nucleus (lower images) in skin tissues (A, 3 s CAP treatment), dermal fibroblasts (B, 60 s CAP treatment) and in epidermal keratinocytes (C, 60 s CAP treatment) in contrast to untreated controls (upper images). Immunofluorescent staining of nuclei (blue: DAPI - 4’,6-Diamidino-2-phenylindole), Nrf2 (green in A, red in B, C), and F-actin cytoskeleton (green in B, C: Phalloidin-FITC). Scale bars=100 μm (See 117 for experimental details).
This Nrf2 pathway stimulation by CAP treatment seems to be one of the most important mechanisms to protect mammalian cells from genotoxic plasma effects. Indeed, a huge number of in vitro studies report on potential genotoxic CAP effects on isolated, naked, or cellular DNA (118). However, several investigations of potential genotoxic effects of CAP by in vitro standard procedures for mutagenicity testing of chemical substances has demonstrated no extended mutation rate of plasma treated cells (119-123). The main conclusion is that CAP treatment causes no enhanced genotoxic risk. This has also been confirmed in an animal study using hairless immunocompetent mice (124), in CAP-treated skin biopsies (125-127), and in clinical follow-up investigations of plasma-treated wounds (128,129).
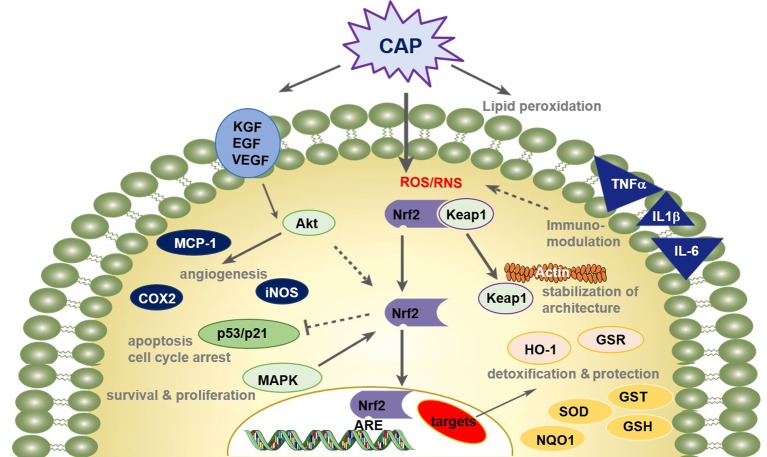
Meanwhile, it is well known that ROS and RNS also play an important role as secondary messengers in the orchestration of wound healing processes (130,131). This idea of redox-based repair of destroyed tissue becomes important in connection with acute and chronic wound healing supported by CAP. There is some evidence that the Nrf2 pathway does not only function in cellular defense against increased ROS levels but it also has central regulatory effects in wound healing (Figure 4) (117,132).
Figure 4. CAP treatment improves wound healing processes via redox-regulated pathways. Enhanced wound healing by CAP involves a distinct temporal pattern of inflammation, a promoting of pro-angiogenetic factors, and a balance of cellular proliferation and apoptotic events. The cellular redox homeostasis is maintained and cells are defended from damage by a strong modulation of the nuclear E2-related factor (Nrf2) pathway.
The schematic overview in Figure 4 summarizes the state of knowledge on molecular patterns in wound healing in response to CAP treatment and aligns these results with insights from redox biology and research on molecular biology of healing processes in acute wounds (90,117,124,132). Briefly, it is assumed that CAP treatment leads to a transient and reversible modification of proteins and the lipid bilayer, which contribute to normal or pathologic stages of wound healing (117,140,141). This is crucially mediated by the Nrf2 pathway (90,116,117). Its key role in up-regulation of detoxifying and antioxidant genes is mentioned above (113,115). Moreover, via activation of Keap1, which does not only act as sensing element in the redox stress reaction of the Nrf2 pathway, CAP stabilizes the architecture of F-actin cytoskeleton and focal adhesions, and increases granulation tissue formation and matrix deposition (117,132). As a key regulator in macrophages, Nrf2 mediates the infiltration of macrophages and neutrophils and the upregulation and secretion of pro- and anti-inflammatory ligands (e.g. TNFα, TGFβ, IL-1β etc.), which activate signaling and intracellular generation of reactive oxygen and nitrogen species (117).
Furthermore, CAP supports angiogenesis by recruiting endothelial cells, stimulates growth factor expression like keratinocyte growth factor (KGF), epidermal growth factor (EGF), or vascular epidermal growth factor (VEGF), and activates protein kinase B (Akt), which induces Nrf2 expression (70,71,138,139). Nrf2-regulated inflammation and angiogenesis is also shown in numerous studies (133-137). Appropriate effects of CAP are also attributed to the regulation of Nrf2 (90,116,117).
Moreover, the activity of the transcription factor p53 depends on the stage of wound healing and reactive species concentration (90,117). The tumor suppressor protein p53 influences cell proliferation and apoptosis and has a central role in angiogenesis and cell cycle regulation and DNA repair. When the expression of p53 is relatively low, p53 enhances the protein level of Nrf2 and its target genes promote cell survival depending on the cyclin-dependent kinase inhibitor 1 (p21). When p53 expression is high, the Nrf2-mediated survival response is inhibited by p53 (142,143).
Taken together, CAP treatment leads to an accelerated repair in acute wounds. All these findings are mainly based on numerous in vitro and in vivo studies using the argon-driven cold atmospheric plasma jet kINPen (32,34).
The long-term and systematic investigation of molecular biological processes of plasma-supplemented wound healing processes has opened several interconnections of plasma medicine and redox biology. In wound healing, insights from redox biology, indicating that redox-sensitive processes are driving factors in tissue repair (130,131), can serve as a sound scientific basis to confirm CAP applications in this field. There is no doubt that this may be true also for other fields of biomedical application of cold atmospheric plasma.
Medical Application of Physical Plasma – Present and Future
Besides wound healing, several other indications for plasma application in dermatology are being taken into consideration, mainly in the treatment of pathogen-based and/or inflammatory skin irritations and diseases (128,144-146). Also, anti-infective plasma applications have been tested in ophthalmology (147-149), while plasma in dentistry is under research for several years, too. Possible dental applications include antimicrobial plasma activity, inactivation and removal of biofilm on teeth and on dental implants, disinfection of tooth root canal, plasma-assisted cleaning and optimization of tooth and implant surfaces to improve bone integration. Additionally, in-growth or bonding of dental fillings and prostheses, decontamination and coating of dental prosthesis, antimicrobial treatment of the oral mucosa, oral wound healing and tooth whitening are under investigation (150). For more details on promising clinical applications of CAP, see a recent review by Metelmann et al. (151).
As it was mentioned above, depending on plasma treatment intensity and time, it is possible to inactivate mammalian cells by initializing programmed cell death in them. This is true particularly for cancer cells. After several reports on apoptosis induction in cancer cells in vitro (152-155), animal studies on transcutaneous plasma treatment of subcutaneously induced solid tumors could prove the general concept of plasma-supported tumor treatment (156,157). However, there are several open questions about the mechanisms of plasma attack on cancer cells, a possible selectivity with regard to healthy tissue or on possible secondary effects distant from the region of local plasma treatment. Most current hypotheses are based on a predominant role of plasma-generated redox active species (158). Briefly, it is assumed that CAP treatment causes apoptosis of cancer cells through a selective rise of intracellular ROS and corresponding ROS-based death pathways. In that regard, enhanced sensitivity of cancer cells may be caused by enhanced ROS levels in the cancer resulting from its unique metabolic activities (113,159). Other hypotheses attribute differences in cell sensitivity to significant variations of aquaporins (AQPs) among different cell lines. Enhanced generation of long-lived species, such as H2O2 via extracellular superoxide dismutase (Ex-SOD, SOD3) on the cytoplasmatic membrane of cancer cells and a subsequent trigger of immune attack on tumoral tissues via H2O2-mediated (second messenger) lymphocyte activation is also discussed (160,161). Another interesting hypothesis is based on the specific action of CAP via singlet oxygen (1O2) generation and the subsequent induction of intercellular ROS-RNS-dependent apoptosis-inducing signaling (162). Finally, a plasma-induced stimulation of immunogenic cell death via damage associated molecular patterns (DAMPs) is under discussion (163,164).
There are several promising experimental results leading to the situation that plasma application in cancer therapy is now one of the most attractive research fields in plasma medicine (155). Based on the experimental proof of inactivation of single layers of cancer cells by local plasma treatment (165), first of all a supportive plasma application in combination with surgical tumor resections in cases where large-scale tumor removal is impossible, seems to be realistic (166,167). Moreover, first CAP applications in palliative care in patients with advanced squamous cell carcinoma of head and neck have not only resulted in the intended reduction of microbial load and resulting reduction of typical fetid odor, but in some particular cases also in transient tumor remission (168-170). Further research will tell us if any direct plasma application is useful for the reduction or complete removal of solid tumors and will lead to a “paradigm shift in cancer therapy” as it was predicted some years ago (156).
Conclusion and Outlook
It is a very interesting perspective to attribute vital processes to electron flow, i.e. energetic electrons are processed by biochemical and molecular biological pathways to transfer their energy into chemical energy to realize metabolic and signaling processes. From this point of view, redox gradients are eventually the driving forces of life (171). Because the primary process of generation of cold atmospheric plasma is the acceleration of electrons by electrical fields, plasma application may be considered as an initial part of a complex electron-transfer process transferring electrical energy via complex chemical reaction cascades into biological effects.
One of the main advantages of biomedical application of CAP is that the active components, such as ROS and RNS, are generated locally and only for the required duration of the application primarily by a physical process. By means of variations of several plasma parameters, the essential ROS and RNS-based energy transfer from plasma to cells and tissue can be easily controlled. This is the main reason why one can describe the biomedical plasma application as a field of applied redox biology.
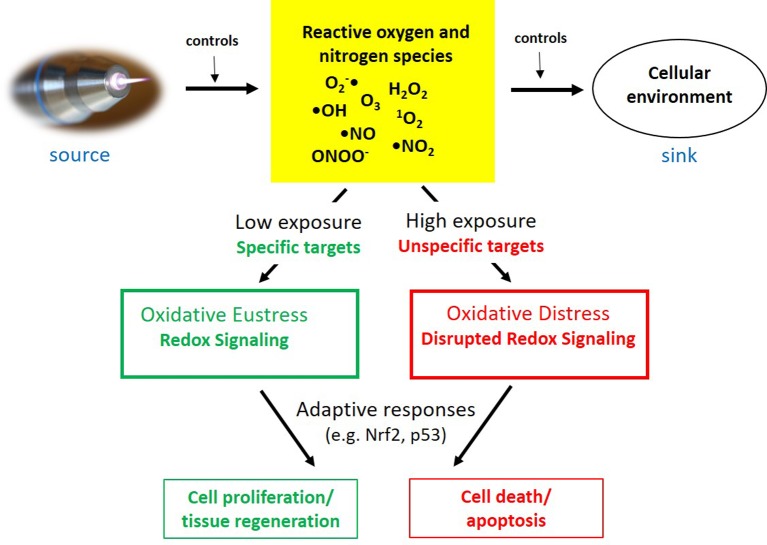
The insight that CAP impact may result either in cell and tissue stimulation or in cell death, depending on exposure conditions (e.g. treatment time), fits very well with the theory of oxidative stress mainly introduced and developed by Sies (172-176). According to that, oxidative stress can be differentiated between oxidative eustress and oxidative distress depending on low or high oxidant exposure. These processes are strongly controlled by adaptive cellular responses, including the Nrf2 pathway that plays a central role above all in cell defence (115,176,177).
While CAP serves as a controllable source of redox-active species, the cellular environment and its antioxidative capacity may act as a “ROS-RNS sink”. The interaction of both components will define the degree of exposure of the living system to redox active species. Depending on the cellular ability of adaptive response as a third component in this process, either oxidative eustress or distress is resulting. In the case of CAP treatment this may result in stimulation of tissue regeneration or cell death (Figure 5).
Figure 5. Different aspects of CAP-triggered oxidative stress, its control, adaptive response and physiological consequences. (Adapted from176).
Based on these insights, redox biology will not only serve as a scientific basis to further explain biological plasma effects. Conversely, CAP may become a useful tool for specific research in redox biology. It is obvious that both plasma medicine and redox biology are interested in similar questions:
i) Are there single and specific ROS and RNS responsible for distinct biological effects or is it only a matter of redox potential at the cellular target sites?
ii) How to identify and analyze specific ROS and RNS at their site of action?
iii) Which cell biological mechanisms are responsible for different sensitivity of several cell types to the impact of CAP as well as redox-active species?
iv) Is it possible to find a measure for biological plasma effects that can serve as a kind of “treatment dose”?
This preliminary small catalogue of questions can be easily extended considering the findings and future prospects of redox biology, both in view of basic research and with respect to specific pathologies (178). Finally, yet importantly, the concept of oxidative eustress and distress and its possible controllability by CAP treatment can also serve for further basic research on the “hormesis” phenomenon, which has received increased attention in recent years (176,179-182).
Conflicts of Interest
The majority of research work that served as a scientific basis for further development and CE certification of the atmospheric pressure plasma jet kINPen MED as a medical device by neoplas tools GmbH Greifswald, Germany has been realized by INP Greifswald. INP Greifswald is a minority shareholder of neoplas tools GmbH Greifswald, Germany.
Authors’ Contributions
ThvW was responsible for the concept of the manuscript, review of literature and writing of the text in general. AS, SB and KW contributed detailed content on mechanisms on CAP-supported wound healing, cellular redox mechanisms, and plasma chemistry, respectively. AS compiled and designed Figure 4. All authors reviewed the manuscript.
Acknowledgements
The authors acknowledge stimulating discussions on redox biology and their significance for plasma medicine with David Graves, UC Berkeley, USA, and Georg Bauer, Medical Center – University of Freiburg, Germany. The German Federal Ministry of Education and Research financially supports basic research on plasma medicine at INP Greifswald (grant numbers 03Z22DN11&12). Plasma medicine research at INP Greifswald is part of the Research Alliance “Leibniz Health Technologies”.
References
- 1.Favia P, d’Agostino R. Plasma treatments and plasma deposition of polymers for biomedical application. Surf Coat Technol. 1998;98:1102–1106. DOI: 10.1016/S0257-8972(97) 00285-5. [Google Scholar]
- 2.Ohl A, Schröder K. Plasma-induced chemical micropatterning for cell culturing applications: a brief review. Surf Coat Technol. 1999;116-119:820–830. DOI: 10.1016/S0257-8972(99)00150-4. [Google Scholar]
- 3.d’Agostino R, Favia P, Oehr C, Wertheimer MR. Low-Temperature Plasma Processing of Materials: Past, Present, and Future. Plasma Process Polym. 2005;2:7–15. DOI: 10.1002/ ppap.200400074. [Google Scholar]
- 4.Desmet T, Morent R, De Geyter N, Leys C, Schacht E, Dubruel P. Nonthermal Plasma Technology as a Versatile Strategy for Polymeric Biomaterials Surface Modification: A Review. Biomacromolecules. 2009;10:2351–2378. doi: 10.1021/bm900186s. PMID: 19655722. DOI: 10.1021/bm900186s. [DOI] [PubMed] [Google Scholar]
- 5.Bazaka K, Jacob MV, Crawford RJ, Ivanova EP. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011;7:2015–2028. doi: 10.1016/j.actbio.2010.12.024. PMID: 21194574. DOI: 10.1016/j.actbio.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Nikiforov A, Deng X, Xiong Q, Cvelbar U, DeGeyter N, Morent R, Leys C. Non-thermal plasma technology for the development of antimicrobial surfaces: a review. J Phys D Appl Phys. 2016;49:204002–204002. DOI: 10.1088/0022-3727/49/20/204002. [Google Scholar]
- 7.Fiebrandt M, Lackmann J-W, Stapelmann K. From patent to product? 50 years of low-pressure plasma sterilization. Plasma Process Polym. 2018;15:e1800139–e1800139. DOI: 10.1002/ ppap.201800139. [Google Scholar]
- 8.Bekeschus S, Favia P, Robert E, von Woedtke Th. White paper on plasma for medicine and hygiene: Future in plasma health sciences. Plasma Process Polym. 2019;16:e1800033–e1800033. DOI: 10.1002/ppap.201800033. [Google Scholar]
- 9.von Woedtke Th, Reuter S, Masur K, Weltmann K-D. Plasmas for medicine. Phys Rep. 2013;530:291–320. DOI: 10.1016/j.physrep.2013.05.005. [Google Scholar]
- 10.Isbary G, Shimizu T, Li Y-F, Stolz W, Thomas HM, Morfill GE, Zimmermann JL. Cold atmospheric plasma devices for medical issues. Expert Rev Med Devic. 2013;10:367–377. doi: 10.1586/erd.13.4. PMID: 23668708. DOI: 10.1586/erd.13.4. [DOI] [PubMed] [Google Scholar]
- 11.Weltmann K-D, von Woedtke Th. Plasma Medicine - current state of research and medical application. Plasma Phys Control Fusion. 2017;59:014031–014031. DOI: 10.1088/0741-3335/59/ 1/014031. [Google Scholar]
- 12.Tanaka H, Ishikawa K, Mizuno M, Toyokuni S, Kajiyama H, Kikkawa F, Metelmann H-R, Hori M. State of the art in medical applications using non-thermal atmospheric pressure plasma. Rev Mod Plasma Phys. 2017;1:3–3. DOI: 10.1007/s4161 4-017-0004-3. [Google Scholar]
- 13.Braithwaite NStJ. Introduction to gas discharges. Plasma Sources Sci Technol. 2000;9:517–527. DOI: 10.1088/0963-0252/9/4/307. [Google Scholar]
- 14.Conrads H, Schmidt M. Plasma generation and plasma sources. Plasma Sources Sci Technol. 2000;9:441–454. DOI: 10.1088/0963-0252/9/4/301. [Google Scholar]
- 15.Bogaerts A, Neyts E, Gijbels R, van der Mullen J. Gas discharge plasmas and their applications. Spectrochim Acta B. 2002;57:609–658. DOI: 10.1016/S0584-8547(01)00406-2. [Google Scholar]
- 16.Canady J, Wiley K, Ravo B. Argon Plasma Coagulation and the Future Applications for Dual-Mode Endoscopic Probes. Rev Gastroenterol Disord. 2006;6:1–12. PMID: 16520707. [PubMed] [Google Scholar]
- 17.Raiser J, Zenker M. Argon plasma coagulation for open surgical and endoscopic applications: state of the art. J Phys D Appl Phys. 2006;39:3520–3523. DOI: 10.1088/0022-3727/39/ 16/S10. [Google Scholar]
- 18.Foster KW, Moy RL, Fincher EF. Advances in plasma skin regeneration. J Cosmet Dermatol. 2008;7:169–179. doi: 10.1111/j.1473-2165.2008.00385.x. PMID: 18789051. DOI: 10.1111/j.1473-2165.2008.00385.x. [DOI] [PubMed] [Google Scholar]
- 19.Bentkover SH. Plasma Skin Resurfacing: Personal Experience and Long-Term Results. Facial Plast Surg Clin North America. 2012;20:145–162. doi: 10.1016/j.fsc.2012.02.010. PMID: 22537783. DOI: 10.1016/j.fsc. 2012.02.010. [DOI] [PubMed] [Google Scholar]
- 20.McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling Cell Behavior Electrically: Current Views and Future Potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. PMID: 15987799. DOI: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 21.Sinha RP, Häder D-P. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. PMID: 12661961. [DOI] [PubMed] [Google Scholar]
- 22.Sklar LR, Almutawa F, Lim HW, Hamzavi I. Effects of ultraviolet radiation, visible light, and infrared radiation on erythema and pigmentation: A review. Photochem Photobiol Sci. 2013;12:54–64. doi: 10.1039/c2pp25152c. PMID: 23111621. DOI: 10.1039/c2pp25152c. [DOI] [PubMed] [Google Scholar]
- 23.Graves DB. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys. 2012;45:263001–263001. DOI: 10.1088/0022-3727/45/26/263001. [Google Scholar]
- 24.Weltmann KD, Kindel E, von Woedtke Th, Hähnel M, Stieber M, Brandenburg R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl Chem. 2010;82:1223–1237. DOI: 10.1351/PAC-CON-09-10-35. [Google Scholar]
- 25.Weltmann KD, von Woedtke Th. Basic requirements for plasma sources in medicine. Eur Phys J-Appl Phys. 2011;55:13807–13807. DOI: 10.1051/epjap/2011100452. [Google Scholar]
- 26.Park GY, Park SJ, Choi MY, Koo IG, Byun JH, Hong JW, Sim JY, Collins GJ, Lee JK. Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci Technol. 2012;21:043001–043001. DOI: 10.1088/0963-0252/21/4/ 043001. [Google Scholar]
- 27.Setsuhara Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch Biochem Biophys. 2016;605:3–10. doi: 10.1016/j.abb.2016.04.009. PMID: 27109191. DOI: 10.1016/j.abb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Laroussi M, Lu X, Keidar M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J Appl Phys. 2017;122:020901–020901. DOI: 10.1063/1.4993710. [Google Scholar]
- 29.Winter J, Brandenburg R, Weltmann K-D. Atmospheric pressure plasma jets: an overview of devices and new directions. Plasma Sources Sci Technol. 2015;24:064001–064001. DOI: 10.1088/0963-0252/24/6/064001. [Google Scholar]
- 30.Weltmann K-D, Metelmann H-R, von Woedtke Th. Low temperature plasma applications in medicine. Europhysics News (EPN) 2016;47(5-6):39–42. DOI: 10.1051/epn/2016507. [Google Scholar]
- 31.Brandenburg R. Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci Technol. 2017;26:053001–053001. DOI: 10.1088/1361-6595/aaced9. [Google Scholar]
- 32.Reuter S, von Woedtke Th, Weltmann K-D. The kINPen – a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J Phys D Appl Phys. 2018;51:233001–233001. DOI: 10.1088/1361-6463/aab3ad. [Google Scholar]
- 33.Weltmann K-D, Kindel E, Brandenburg R, Meyer C, Bussiahn R, Wilke C, von Woedtke Th. Atmospheric Pressure Plasma Jet for Medical Therapy: Plasma Parameters and Risk Estimation. Contrib Plasma Phys. 2009;49:631–640. DOI: 10.1002/ctpp.200910067. [Google Scholar]
- 34.Bekeschus S, Schmidt A, Weltmann K-D, von Woedtke Th. The plasma jet kINPen – A powerful tool for wound healing. Clin Plasma Med. 2016;4:19–28. DOI: 10.1016/j.cpme.2016.01.001. [Google Scholar]
- 35.Bruggeman P, Leys C. Non-thermal plasmas in and in contact with liquids. J Phys D Appl Phys. 2009;42:53001–53001. DOI: 10.1088/0022-3727/42/5/053001. [Google Scholar]
- 36.Jablonowski H, von Woedtke Th. Research on plasma medicine-relevant plasma–liquid interaction: What happened in the past five years. Clin Plasma Med. 2015;3:42–52. DOI: 10.1016/j.cpme.2015.11.003. [Google Scholar]
- 37.Bruggeman PJ, Kushner MJ, Locke BR, Gardeniers JGE, Graham WG et al. Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci Technol. 2016;25:053002–053002. DOI: 10.1088/0963-0252/25/5/053002. [Google Scholar]
- 38.Vanraes P, Bogaerts A. Plasma physics of liquids – A focused review. Appl Phys Rev. 2018;5:031103–031103. DOI: 10.1063/1.5020511. [Google Scholar]
- 39.Lu X, Naidis GV, Laroussi M, Reuter S, Graves DB, Ostrikov K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys Rep. 2016;630:1–84. DOI: 10.1016/j.physrep.2016.03.003. [Google Scholar]
- 40.Laroussi M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci. 1996;24:1188–1191. DOI: 10.1109/27.533129. [Google Scholar]
- 41.Ehlbeck J, Schnabel U, Polak M, Winter J, von Woedtke Th, Brandenburg R, von dem Hagen T, Weltmann K-D. Low Temperature Atmospheric Pressure Plasma Sources for Microbial Decontamination. J Phys D Appl Phys. 2011;44:013002–013002. DOI: 10.1088/0022-3727/44/1/013002. [Google Scholar]
- 42.Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia L’H. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1–21. doi: 10.1016/s0378-5173(01)00752-9. PMID: 11532565. DOI: 10.1016/S0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- 43.Moisan M, Barbeau J, Crevier M-C, Pelletier J, Philip N, Saoudi B. Plasma sterilization. Methods and mechanisms. Pure Appl Chem. 2002;74:349–358. DOI: 10.1351/pac200274 030349. [Google Scholar]
- 44.Laroussi M. Low temperature plasma-based sterilization: overview and state-of-the-art. Plasma Process Polym. 2005;2:391–400. DOI: 10.1002/ppap.200400078. [Google Scholar]
- 45.von Keudell A, Awakowicz P, Benedikt J, Raballand V, Yanguas-Gil A, Opretzka J, Flötgen C, Reuter R, Byelykh L, Halfmann H, Stapelmann K, Denis B, Wunderlich J, Muranyi P, Rossi F, Kylián O, Hasiwa N, Ruiz A, Rauscher H, Sirghi L, Comoy E, Dehen C, Challier L, Deslys JP. Inactivation of Bacteria and Biomolecules by Low-Pressure Plasma Discharges. Plasma Process Polym. 2010;7:327–352. DOI: 10.1002/ppap.200900121. [Google Scholar]
- 46.Winter T, Winter J, Polak M, Kusch K, Mäder U, Sietmann R, Ehlbeck J, van Hijum S, Weltmann K-D, Hecker M, Kusch H. Characterization of the global impact of low temperature gas plasma on vegetative microorganisms. Proteomics. 2011;11:3518–3530. doi: 10.1002/pmic.201000637. PMID: 21751354. DOI: 10.1002/pmic.201000637. [DOI] [PubMed] [Google Scholar]
- 47.Winter T, Bernhardt J, Winter J, Mäder U, Schlüter R, Weltmann K-D, Hecker M, Kusch H. Common versus noble Bacillus subtilis differentially responds to air and argon gas plasma. Proteomics. 2013;13:2608–2621. doi: 10.1002/pmic.201200343. PMID: 23794223. DOI: 10.1002/pmic.201200343. [DOI] [PubMed] [Google Scholar]
- 48.Bourke P, Ziuzina D, Han L, Cullen PJ, Gilmore BF. Microbiological interactions with cold plasma. J Appl Microbiol. 2017;123:308–324. doi: 10.1111/jam.13429. PMID: 28245092. DOI: 10.1111/jam.13429. [DOI] [PubMed] [Google Scholar]
- 49.Liao X, Liu D, Xiang Q, Ahn J, Chen S, Ye X, Ding T. Inactivation mechanisms of non-thermal plasma on microbes: a review. Food Control. 2017;75:83–91. DOI: 10.1016/ j.foodcont.2016.12.021. [Google Scholar]
- 50.Brun P, Bernabè G, Marchiori C, Scarpa M, Zuin M, Cavazzana R, Zaniol B, Martines E. Antibacterial efficacy and mechanisms of action of low power atmospheric pressure cold plasma: membrane permeability, biofilm penetration and antimicrobial sensitization. J Appl Microbiol. 2018;125:398–408. doi: 10.1111/jam.13780. PMID: 29655267. DOI: 10.1111/jam.13780. [DOI] [PubMed] [Google Scholar]
- 51.Daeschlein G, von Woedtke Th, Kindel E, Brandenburg R, Weltmann K-D, Jünger M. Antibacterial Activity of an Atmospheric Pressure Plasma Jet Against Relevant Wound Pathogens in vitro on a Simulated Wound Environment. Plasma Process Polym. 2010;7:224–230. DOI: 10.1002/ppap.2009 00059. [Google Scholar]
- 52.Zimmermann JL, Dumler K, Shimizu T, Morfill GE, Wolf A, Boxhammer V, Schlegel J, Gansbacher B, Anton M. Effects of cold atmospheric plasmas on adenoviruses in solution. J Phys D Appl Phys. 2011;44:505201. DOI: 10.1088/0022-3727/44/50/505201. [Google Scholar]
- 53.Daeschlein G, Scholz S, Arnold A, von Podewils S, Haase H, Emmert S, von Woedtke Th, Weltmann K-D, Jünger M. In vitro susceptibility of important skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Process Polym. 2012;9:380–389. DOI: 10.1002/ppap.20110 0160. [Google Scholar]
- 54.Maisch T, Shimizu T, Li Y-F, Heinlin J, Karrer S, Morfill G, Zimmermann JL. Decolonisation of MRSA, S. aureus and E. coli by Cold-Atmospheric Plasma Using a Porcine Skin Model In Vitro. PLoS ONE. 2012;7:e34610. doi: 10.1371/journal.pone.0034610. PMID: 22558091. DOI: 10.1371/journal.pone.0034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daeschlein G, Napp M, von Podewils S, Lutze S, Emmert S, Lange A, Klare I, Haase H, Gümbel D, von Woedtke Th, Jünger M. In vitro susceptibility of multidrug resistant skin and wound pathogens against low temperature atmospheric pressure plasma jet (APPJ) and dielectric barrier discharge plasma (DBD) Plasma Process Polym. 2014;11:175–183. DOI: 10.1002/ppap.201300070. [Google Scholar]
- 56.Klämpfl TG, Shimizu T, Koch S, Balden M, Gemein S, Li Y-F, Mitra A, Zimmermann JL, Gebel J, Morfill GE, Schmidt H-U. Decontamination of nosocomial bacteria including clostridium difficile spores on dry inanimate surface by cold atmospheric plasma. Plasma Process Polym. 2014;11:974–984. DOI: 10.1002/ppap.201400080. [Google Scholar]
- 57.Gilmore BF, Flynn PB, O’Brien S, Hickok N, Freeman T, Bourke P. Cold plasmas for biofilm control: opportunities and challenges. Trends Biotechnol. 2018;36:627–638. doi: 10.1016/j.tibtech.2018.03.007. PMID: 29729997. DOI: 10.1016/j.tibtech.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo L, Xu R, Gou L, Liu Z, Zhao Y, Liu D, Zhang L, Chen H, Kong MG. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl Environ Microbiol. 2018;84:e00726–e18. doi: 10.1128/AEM.00726-18. PMID: 29915117. DOI: 10.1128/AEM.00726-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Isbary G, Morfill G, Schmidt HU, Georgi M, Ramrath K, Heinlin J, Karrer S, Landthaler M, Shimizu T, Steffes B, Bunk W, Monetti R, Zimmermann JL, Pompl R, Stolz W. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Brit J Dermatol. 2010;163:78–82. doi: 10.1111/j.1365-2133.2010.09744.x. PMID: 20222930. DOI: 10.1111/j.1365-2133.2010.09744.x. [DOI] [PubMed] [Google Scholar]
- 60.Isbary G, Heinlin J, Shimizu T, Zimmermann JL, Morfill G, Schmidt HU, Monetti R, Steffes B, Bunk W, Li Y, Klaempfl T, Karrer S, Landthaler M, Stolz W. Successful and safe use of 2 min cold atmospheric argon plasma in chronic wounds: Results of a randomized controlled trial. Brit J Dermatol. 2012;167:404–410. doi: 10.1111/j.1365-2133.2012.10923.x. PMID: 22385038. DOI: 10.1111/j.1365-2133.2012.10923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brehmer F, Haenssle HA, Daeschlein G, Ahmed R, Pfeiffer S, Görlitz A, Simon D, Schön MP, Wandke D, Emmert S. Alleviation of chronic venous leg ulcers with a hand-held dielectric barrier discharge plasma generator (PlasmaDerm® VU-2010): Results of a monocentric, two-armed, open, prospective, randomized and controlled trial ( NCT01415622) J Eur Acad Dermatol Venereol. 2015;29:148–155. doi: 10.1111/jdv.12490. PMID: 24666170. DOI: 10.1111/jdv.12490. [DOI] [PubMed] [Google Scholar]
- 62.Ulrich C, Kluschke F, Patzelt A, Vandersee S, Czaika VA, Richter H, Bob A, von Hutten J, Painsi C, Hügel R, Kramer A, Assadian O, Lange Asschenfeldt B. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J Wound Care. 2015;24:196–203. doi: 10.12968/jowc.2015.24.5.196. PMID: 25970756. DOI: 10.12968/jowc.2015.24.5.196. [DOI] [PubMed] [Google Scholar]
- 63.Daeschlein G, Napp M, Lutze S, Arnold A, von Podewils S, Guembel D, Jünger M. Skin and wound decontamination of multidrug-resistant bacteria by cold atmospheric plasma coagulation. J Dtsch Dermatol Ges (JDDG) 2015;13:143–149. doi: 10.1111/ddg.12559. PMID: 25597338. DOI: 10.1111/ddg.12559. [DOI] [PubMed] [Google Scholar]
- 64.Isbary G, Stolz W, Shimizu T, Monetti R, Bunk W, Schmidt H-U, Morfill GE, Klämpfl TG, Steffes B, Thomas HM, Heinlin J, Karrer S, Landthaler M, Zimmermann JL. Cold atmospheric argon plasma treatment may accelerate wound healing in chronic wounds: Results of an open retrospective randomized controlled study in vivo. Clin Plasma Med. 2013;1(2):25–30. DOI: 10.1016/j.cpme.2013.06.001. [Google Scholar]
- 65.Chuangsuwanich A, Assadamongkol T, Boonyawan D. The healing effect of low-temperature atmospheric-pressure plasma in pressure ulcer: A randomized controlled trial. Int J Low Extr Wound. 2016;15:313–319. doi: 10.1177/1534734616665046. PMID: 27581113. DOI: 10.1177/ 1534734616665046. [DOI] [PubMed] [Google Scholar]
- 66.López Callejas R, Peña Eguiluza R, Valencia Alvarado R, Mercado Cabrera A, Rodríguez Méndez BG, Serment Guerrero JH, Cabral Prieto A, González Garduño AC, Domínguez Cadena NA, Muñoz Infante J, Betancourt Ángeles M. Alternative method for healing the diabetic foot by means of a plasma needle. Clin Plasma Med. 2018;9:19–23. DOI: 10.1016/ j.cpme.2018.01.001. [Google Scholar]
- 67.Kramer A, Hübner N-O, Weltmann K-D, Lademann J, Ekkernkamp A, Hinz P, Assadian O. Polypragmasia in the therapy of infected wounds – conclusions drawn from the perspectives of low temperature plasma technology for plasma wound therapy. GMS Krankenhaushyg Interdiszip. 2008;3:Doc13. PMID: 20204115. [PMC free article] [PubMed] [Google Scholar]
- 68.Kalghatgi S, Friedman G, Fridman A, Morss Clyne A. Endothelial Cell Proliferation is Enhanced by Low Dose Non-Thermal Plasma Through Fibroblast Growth Factor-2 Release. Ann Biomed Eng. 2010;38:748–757. doi: 10.1007/s10439-009-9868-x. PMID: 20013154. DOI: 10.1007/s10439-009-9868-x. [DOI] [PubMed] [Google Scholar]
- 69.Arndt S, Unger P, Wacker E, Shimizu T, Heinlin J, Li Y-F, Thomas HM, Morfill GE, Zimmermann JL, Bosserhoff A-K, Karrer S. Cold Atmospheric Plasma (CAP) changes gene expression of key molecules of the wound healing machinery and improves wound Healing in vitro and in vivo. PLoS ONE. 2013;8:e79325. doi: 10.1371/journal.pone.0079325. PMID: 24265766. DOI: 10.1371/journal.pone. 0079325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haertel B, Eiden K, Deuter A, Wende K, von Woedtke Th, Lindequist U. Differential effect of non-thermal atmospheric-pressure plasma on angiogenesis. Lett Appl NanoBioScience. 2014;3:159–166. [Google Scholar]
- 71.Haertel B, von Woedtke Th, Weltmann KD, Lindequist U. Non-thermal atmospheric-pressure plasma possible application in wound healing. Biomol Ther. 2014;22:477–490. doi: 10.4062/biomolther.2014.105. PMID: 25489414. DOI: 10.4062/biomolther.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thi Ngo M-H, Liao J-D, Shao P-L, Weng C-C, Chang C-Y. Increased Fibroblast Cell Proliferation and Migration Using Atmospheric N2/Ar Micro-Plasma for the Stimulated Release of Fibroblast Growth Factor-7. Plasma Process Polym. 2014;11:80–88. DOI: 10.1002/ppap.201300098. [Google Scholar]
- 73.Lendeckel D, Eymann C, Emicke P, Daeschlein G, Darm K, O’Neil S, Beule AG, von Woedtke Th, Völker U, Weltmann K-D, Jünger M, Hosemann W, Scharf C. Proteomic changes of tissue-tolerable plasma treated airway epithelial cells and their relation to wound healing. Biomed Res Int. 2015;2015:506059. doi: 10.1155/2015/506059. PMID: 26539504. DOI: 10.1155/2015/506059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi JH, Song YS, Song K, Lee HJ, Hong JW, Kim GC. Skin renewal activity of nonthermal plasma through the activation of β-catenin in keratinocytes. Sci Rep. 2017;7:6146. doi: 10.1038/s41598-017-06661-7. PMID: 28733577. DOI: 10.1038/s41598-017-06661-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kang SU, Choi JW, Chang JW, Kim KI, Kim YS, Park JK, Kim YE, Lee YS, Yang SS, Kim C-H. N2 non-thermal atmospheric pressure plasma promotes wound healing in vitro and in vivo: Potential modulation of adhesion molecules and matrix metalloproteinase-9. Exp Dermatol. 2017;26:163–170. doi: 10.1111/exd.13229. PMID: 27673439. DOI: 10.1111/exd.13229. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt A, Bekeschus S, Wende K, Vollmar B, von Woedtke Th. A cold plasma jet accelerates wound healing in a murine model of full-thickness skin wounds. Exp Dermatol. 2017;26:156–162. doi: 10.1111/exd.13156. PMID: 27492871. DOI: 10.1111/exd.13156. [DOI] [PubMed] [Google Scholar]
- 77.Nastuta AV, Topala I, Grigoras C, Pohoata V, Popa G. Stimulation of wound healing by helium atmospheric pressure plasma treatment. J Phys D Appl Phys. 2011;44:105204. DOI: 10.1088/0022-3727/44/10/105204. [Google Scholar]
- 78.García Alcantara E, López Callejas R, Morales Ramírez PR, Peña Eguiluz R, Fajardo Muñoz R, Mercado Cabrera A, Barocio SR, Valencia Alvarado R, Rodríguez Méndez BG, Muñoz Castro AE, de la Piedad-Beneitez A, Rojas-Olmedoa IA. Accelerated mice skin acute wound healing in vivo by combined treatment of argon and helium plasma needle. Arch Med Res. 2013;44:169–177. doi: 10.1016/j.arcmed.2013.02.001. PMID: 23506720. DOI: 10.1016/ j.arcmed.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 79.Hirata T, Kishimoto T, Tsutsui C, Kanai T, Mori A. Healing burns using atmospheric pressure plasma irradiation. Jpn J Appl Phys. 2014;53:010302. DOI: 10.7567/JJAP.53.010302. [Google Scholar]
- 80.Ngo Thi M-H, Shao P-L, Liao J-D, Lin C-CK, Yip H-K. Enhancement of Angiogenesis and Epithelialization Processes in Mice with Burn Wounds through ROS/RNS Signals Generated by Non-Thermal N2/Ar Micro-Plasma. Plasma Process Polym. 2014;11:1076–1088. DOI: 10.1002/ppap. 201400072. [Google Scholar]
- 81.Nasruddin, Nakajima Y, Mukai K, Rahayu HSE, Nur M, Ishijima T, Enomoto H, Uesugi Y, Sugama J, Nakatani T. Cold plasma on full-thickness cutaneous wound accelerates healing through promoting inflammation, re-epithelialization and wound contraction. Clin Plasma Med. 2014;2:28–35. DOI: 10.1016/j.cpme.2014.01.001. [Google Scholar]
- 82.Lee OJ, Ju HW, Khang G, Sun PP, Rivera J, Cho JH, Park S-J, Eden JG, Park CH. An experimental burn wound-healing study of non-thermal atmospheric pressure microplasma jet arrays. J Tissue Eng Regen Med. 2016;10:348–357. doi: 10.1002/term.2074. PMID: 26227832. DOI: 10.1002/term.2074. [DOI] [PubMed] [Google Scholar]
- 83.Kang SK, Kim HY, Yun GS, Lee JK. Portable microwave air plasma device for wound healing. Plasma Sources Sci Technol. 2015;24:035020. DOI: 10.1088/0963-0252/24/3/035020. [Google Scholar]
- 84.Kim HY, Kang SK, Park SM, Jung HY, Choi BH, Sim JY, Lee JK. Characterization and Effects of Ar/Air Microwave Plasma on Wound Healing. Plasma Process Polym. 2015;12:1423–1434. DOI: 10.1002/ppap.201500017|. [Google Scholar]
- 85.Hung Y-W, Lee L-T, Peng Y-C, Chang C-T, Wong Y-K, Tung K-C. Effect of a nonthermal-atmospheric pressure plasma jet on wound healing: An animal study. J Chin Med Assoc. 2016;79:320–328. doi: 10.1016/j.jcma.2015.06.024. PMID: 27036493. DOI: 10.1016/j.jcma. 2015.06.024. [DOI] [PubMed] [Google Scholar]
- 86.Fathollah S, Mirpour S, Mansouri P, Dehpour AR, Ghoranneviss M, Rahimi N, Naraghi ZS, Chalangari R, Chalangari KM. Investigation on the effects of the atmospheric pressure plasma on wound healing in diabetic rats. Sci Rep. 2016;6:19144–19144. doi: 10.1038/srep19144. DOI: 10.1038/srep19144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rad ZS, Davani FA. Non-thermal atmospheric pressure dielectric barrier discharge plasma source construction and investigation on the effect of grid on wound healing application. Clin Plasma Med. 2016;4:56–64. DOI: 10.1016/j.cpme. 2016.11.002. [Google Scholar]
- 88.Kubinova S, Zaviskova K, Uherkova L, Zablotskii V, Churpita O, Lunov O, Dejneka A. Non-thermal air plasma promotes the healing of acute skin wounds in rats. Sci Rep. 2017;7:45183. doi: 10.1038/srep45183. DOI: 10.1038/srep45183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chatraie M, Torkaman G, Khani M, Salehi Hand Shokri B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci Rep. 2018;8:5621. doi: 10.1038/s41598-018-24049-z. DOI: 10.1038/ s41598-018-24049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arndt S, Schmidt A, Karrer S, von Woedtke Th. Comparing two different plasma devices kINPen and Adtec SteriPlas regarding their molecular and cellular effects on wound healing. Clin Plasma Med. 2018;9:24–33. DOI: 10.1016/j.cpme. 2018.01.002. [Google Scholar]
- 91.Metelmann H-R, von Woedtke Th, Bussiahn R, Weltmann K-D, Rieck M, Khalili R, Podmelle F, Waite PD. Experimental Recovery of CO2-Laser Skin Lesions by Plasma Stimulation. Am J Cosmetic Surg. 2012;29:52–56. DOI: 10.5992/AJCS-D-11-00042.1. [Google Scholar]
- 92.Heinlin J, Zimmermann JL, Zeman F, Bunk W, Isbary G, Landthaler M, Maisch T, Monetti R, Morfill G, Shimizu T, Steinbauer J, Stolz W, Karrer S. Randomized placebo-controlled human pilot study of cold atmospheric argon plasma on skin graft donor sites. Wound Rep Regen. 2013;21:800–807. doi: 10.1111/wrr.12078. PMID: 23937657. DOI: 10.1111/wrr.12078. [DOI] [PubMed] [Google Scholar]
- 93.Vandersee S, Richter H, Lademann J, Beyer M, Kramer A, Knorr F, Lange Asschenfeldt B. Laser scanning microscopy as a means to assess the augmentation of tissue repair by exposition of wounds to tissue tolerable plasma. Laser Phys Lett. 2014;11:115701. DOI: 10.1088/1612-2011/11/11/115701. [Google Scholar]
- 94.Hartwig S, Doll C, Voss JO, Hertel M, Preissner S, Raguse JD. Treatment of Wound Healing Disorders of Radial ForearmFree Flap Donor Sites Using Cold Atmospheric Plasma: A Proof of Concept. J Oral Maxillofac Surg. 2017;75:429–435. doi: 10.1016/j.joms.2016.08.011. PMID: 27637776. DOI: 10.1016/j.joms.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Jablonowski H, Hänsch MACh, Dünnbier M, Wende K, Hammer MU, Weltmann K-D, Reuter S, von Woedtke Th. Plasma jet’s shielding gas impact on bacterial inactivation. Biointerphases. 2015;10:029506. doi: 10.1116/1.4916533. PMID: 25832438. DOI: 10.1116/1.4916533. [DOI] [PubMed] [Google Scholar]
- 96.Weavers H, Wood W, Martin P. Understanding the inflammatory response to tissue damage in Drosophila: a complex interplay of pro-inflammatory attractant signals, developmental priming and cytoprotection. 8th International Conference on Oxidative Stress in Skin Medicine and Biology, Andros, Greece. Book of Abstracts. 6-9 September 2018:73–73. [Google Scholar]
- 97.Kalghatgi S, Kelly CM, Cerchar E, Torabi B, Alekseev O, Fridman A, Friedman G, Azizkhan Clifford J. Effects of Non-Thermal Plasma on Mammalian Cells. PLoS ONE. 2011;6:e16270. doi: 10.1371/journal.pone.0016270. PMID: 21283714. DOI: 10.1371/journal.pone. 0016270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blackert S, Haertel B, Wende K, von Woedtke Th, Lindequist U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (HaCaT) J Dermatol Sci. 2013;70:173–181. doi: 10.1016/j.jdermsci.2013.01.012. PMID: 23619096. DOI: 10.1016/j.jdermsci.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 99.Wende K, Straßenburg S, Haertel B, Harms M, Holtz S, Barton A, Masur K, von Woedtke Th, Lindequist U. Atmospheric pressure plasma jet treatment evokes transient oxidative stress in HaCaT keratinocytes and influences cell physiology. Cell Biol Int. 2014;38:412–425. doi: 10.1002/cbin.10200. PMID: 24155089. DOI: 10.1002/ cbin.10200. [DOI] [PubMed] [Google Scholar]
- 100.Oehmigen K, Winter J, Hähnel M, Wilke C, Brandenburg R, Weltmann K-D, von Woedtke Th. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process Polym. 2011;8:904–913. DOI: 10.1002/ppap.201000099. [Google Scholar]
- 101.von Woedtke Th, Haertel B, Weltmann K-D, Lindequist U. Plasma pharmacy-physical plasma in pharmaceutical applications. Pharmazie. 2013;68:492–498. PMID: 23923628. DOI: 10.1691/ph.2013.6521. [PubMed] [Google Scholar]
- 102.Joslin JM, McCall JR, Bzdek JP, Johnson DC, Hybertson BM. Aqueous Plasma Pharmacy: Preparation Methods, Chemistry, and Therapeutic Applications. Plasma Med. 2016;6:135–177. doi: 10.1615/PlasmaMed.2016018618. PMID: 28428835. DOI: 10.1615/PlasmaMed. 2016018618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yan D, Talbot A, Nourmohammadi N, Cheng X, Canady J, Sherman J, Keidar M. Principles of using Cold Atmospheric Plasma Stimulated Media for Cancer Treatment. Sci Rep. 2015;5:18339. doi: 10.1038/srep18339. DOI: 10.1038/srep18339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanaka H, Nakamura K, Mizuno M, Ishikawa K, Takeda K, Kajiyama H, Utsumi F, Kikkawa F, Hori M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci Rep. 2016;6:36282. doi: 10.1038/srep36282. PMID: 27824103. DOI: 10.1038/srep36282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bekeschus S, Käding A, Schröder T, Wende K, Hackbarth C, Liedtke KR, van der Linde J, von Woedtke Th, Heidecke C-D, Partecke L-I. Cold Physical Plasma-Treated Buffered Saline Solution as Effective Agent Against Pancreatic Cancer Cells. Anticancer Agents Med Chem. 2018;18:824–831. doi: 10.2174/1871520618666180507130243. PMID: 29732979. DOI: 10.2174/1871520618666180507130243. [DOI] [PubMed] [Google Scholar]
- 106.Graves DB. Oxy-nitroso shielding burst model of cold atmospheric plasma therapeutics. Clin Plasma Med. 2014;2:38–49. DOI: 10.1016/j.cpme.2014.11.001. [Google Scholar]
- 107.Hamaguchi S, Ikuse K, Kanazawa T. Generation of free radicals in liquid by atmospheric-pressure plasmas and its application to biology and medicine. JPS Conf Proc. 2014;1:015055. DOI: 10.7566/JPSCP.1.015055. [Google Scholar]
- 108.Wende K, Williams P, Dalluge J, van Gaens W, Aboubakr H, Bischop J, von Woedtke Th, Goyal SM, Bogaerts A, Masur K, Bruggeman PJ. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases. 2015;10:029518. doi: 10.1116/1.4919710. PMID: 25947392. DOI: 10.1116/1.4919710. [DOI] [PubMed] [Google Scholar]
- 109.Gorbanev Y, O’Connell D, Chechik V. Non-Thermal Plasma in Contact with Water: The Origin of Species. Chem Eur J. 2016;22:1–11. doi: 10.1002/chem.201503771. PMID: 26833560. DOI: 10.1002/chem.201503771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lietz AM, Kushner MJ. Air plasma treatment of liquid covered tissue: long timescale chemistry. J Phys D Appl Phys. 2016;49:425204. DOI: 10.1088/0022-3727/49/42/425204. [Google Scholar]
- 111.Verlackt CCW, Van Boxem W, Bogaerts A. Transport and accumulation of plasma generated species in aqueous solution. Phys Chem Chem Phys. 2018;20:6845–6859. doi: 10.1039/c7cp07593f. PMID: 2946 0930. DOI: 10.1039/c7cp07593f. [DOI] [PubMed] [Google Scholar]
- 112.Go Y-M, Jones DP. Redox biology: Interface of the exposome with the proteome, epigenome and genome. Redox Biol. 2014;2:358–360. doi: 10.1016/j.redox.2013.12.032. PMID: 24563853. DOI: 10.1016/j.redox. 2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature Rev Mol Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. PMID: 24854789. DOI: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 114.Lackmann J-W, Wende K, Verlackt C, Golda J, Volzke J, Kogelheide F, Held J, Bekeschus S, Bogaerts A, Schulz-von der Gathen V, Stapelmann K. Chemical fingerprints of cold physical plasmas – an experimental and computational study using cysteine as tracer compound. Sci Rep. 2018;8:7736. doi: 10.1038/s41598-018-25937-0. DOI: 10.1038/s41598-018-25937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bryan HK, Olayanju A, Goldring CE, Park BK. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol. 2013;85:705–717. doi: 10.1016/j.bcp.2012.11.016. PMID: 23219527. DOI: 10.1016/j.bcp.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 116.Schmidt A, Dietrich S, Steuer A, Weltmann K-D, von Woedtke Th, Masur K, Wende K. Non-thermal plasma activates human keratinocytes by stimulation of antioxidant and phase II pathways. J Biol Chem. 2015;290:6731–6750. doi: 10.1074/jbc.M114.603555. PMID: 25589789. DOI: 10.1074/jbc.M114.603555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmidt A, von Woedtke Th, Vollmar B, Hasse S, Bekeschus S. Nrf2 signaling and inflammation are key events in physical plasma-spurred wound healing. Theranostics. 2019;9:1066–1084. doi: 10.7150/thno.29754. PMID: 30867816. DOI: 10.7150/thno.29754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arjunan KP, Sharma VK, Ptasinska S. Effects of Atmospheric Pressure Plasmas on Isolated and Cellular DNA – A Review. Int J Mol Sci. 2015;16:2971–3016. doi: 10.3390/ijms16022971. PMID: 25642755. DOI: 10.3390/ijms16022971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boxhammer V, Li YF, Köritzer J, Shimizu T, Maisch T, Thomas HM, Schlegel J, Morfill GE, Zimmermann JL. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat Res-Gen Tox En. 2013;753:23–28. doi: 10.1016/j.mrgentox.2012.12.015. PMID: 23416235. DOI: 10.1016/j.mrgentox.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 120.Kluge S, Bekeschus S, Bender C, Benkhai H, Sckell A, Below H, Stope MB, Kramer A. Investigating the Mutagenicity of a Cold Argon-Plasma Jet in an HET-MN Model. PLoS ONE. 2016;11:e0160667. doi: 10.1371/journal.pone.0160667. PMID: 27584003. DOI: 10.1371/journal. pone.0160667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wende K, Bekeschus S, Schmidt A, Jatsch L, Hasse S, Weltmann K-D, Masur K, von Woedtke Th. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat Res-Gen Tox En. 2016;798:48–54. doi: 10.1016/j.mrgentox.2016.02.003. DOI: 10.1016/j.mrgentox.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 122.Maisch T, Bosserhoff K, Unger P, Heider J, Shimizu T, Zimmermann JL, Morfill GE, Landthaler M, Karrer S. Investigation of Toxicity and Mutagenicity of Cold Atmospheric Argon Plasma. Environ Mol Mutagen. 2017;58:172–177. doi: 10.1002/em.22086. PMID: 28370324. DOI: 10.1002/em.22086. [DOI] [PubMed] [Google Scholar]
- 123.Bekeschus S, Schmidt A, Kramer A, Metelmann H-R, Adler F, von Woedtke Th, Niessner F, Weltmann K-D, Wende K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ Mol Mutagen. 2018;59:268–277. doi: 10.1002/em.22172. PMID: 29417643. DOI: 10.1002/em.22172. [DOI] [PubMed] [Google Scholar]
- 124.Schmidt A, von Woedtke Th, Stenzel J, Lindner T, Polei S, Vollmar B, Bekeschus S. One year follow up risk assessment in SKH-1 mice and wounds treated with an argon plasma jet. Int J Mol Sci. 2017;18:868. doi: 10.3390/ijms18040868. PMID: 28422070. DOI: 10.3390/ijms18040868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.von Woedtke Th, Metelmann H-R, Weltmann K-D. Editorial. Clin Plasma Med. 2013;1:1–2. DOI: 10.1016/j.cpme. 2013.03.001. [Google Scholar]
- 126.Hasse S, Hahn O, Kindler S, von Woedtke Th, Metelmann H-R, Masur K. Atmospheric pressure plasma jet application on human oral mucosa modulates tissue regeneration. Plasma Med. 2014;4:117–129. DOI: 10.1615/PlasmaMed.2014011978. [Google Scholar]
- 127.Hasse S, Tran T, Hahn O, Kindler S, Metelmann H-R, von Woedtke Th, Masur K. Induction of proliferation of basal epidermal keratinocytes by cold atmospheric pressure plasma. Clin Exp Dermatol. 2015;41:202–209. doi: 10.1111/ced.12735. PMID: 26175125. DOI: 10.1111/ced.12735. [DOI] [PubMed] [Google Scholar]
- 128.Heinlin J, Isbary G, Stolz W, Morfill G, Landthaler M, Shimizu T, Steffes B, Nosenko T, Zimmermann JL, Karrer S. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol. 2011;25:1–11. doi: 10.1111/j.1468-3083.2010.03702.x. PMID: 20497290. DOI: 10.1111/j.1468-3083.2010.03702.x. [DOI] [PubMed] [Google Scholar]
- 129.Metelmann H-R, Vu TT, Do HT, Le TNB, Hoang THA, et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (CAP) treatment: A clinical long term observation. Clin Plasma Med. 2013;1:30–35. DOI: 10.1016/ j.cpme.2012.12.001. [Google Scholar]
- 130.Sen CK, Roy S. Redox signals in wound healing. Biochim Biophys Acta. 2008;1780:1348–1361. doi: 10.1016/j.bbagen.2008.01.006. PMID: 18249195. DOI: 10.1016/j.bbagen.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dunnill C, Patton T, Brennan J, Barrett J, Dryden M, Cooke J, Leaper D, Georgopoulos NT. Reactive oxygen species (ROS) and wound healing: the functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int Wound J. 2017;14:89–96. doi: 10.1111/iwj.12557. PMID: 26688157. DOI: 10.1111/iwj.12557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schmidt A, Bekeschus S. Redox for Repair: Cold Physical Plasmas and Nrf2 Signaling Promoting Wound Healing. Antioxidants. 2018;7:146–146. doi: 10.3390/antiox7100146. PMID: 30347767. DOI: 10.3390/ antiox7100146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Itoh K, Mochizuki M, Ishii Y, Ishii T, Shibata T, Kawamoto Y, Kelly V, Sekizawa K, Uchida K, Yamamoto M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2) Mol Cell Biol. 2004;24:36–45. doi: 10.1128/MCB.24.1.36-45.2004. DOI: 10.1128/MCB.24.1.36-45.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. J Gerontol A Biol Sci Med Sci. 2012;67:821–829. doi: 10.1093/gerona/glr229. PMID: 22219515. DOI: 10.1093/gerona/glr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang Y, Li W, Su Z, Kong A-N T. The complexity of the Nrf2 pathway: beyond the antioxidant response. J Nutr Biochem. 2015;26:1401–1413. doi: 10.1016/j.jnutbio.2015.08.001. PMID: 26419687. DOI: 10.1016/j.jnutbio.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nature Comm. 2016;7:11624–11624. doi: 10.1038/ncomms11624. PMID: 27211851. DOI: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li L, Pan H, Wang H, Li X, Bu X, Wang Q, Gao Y, Wen G, Zhou Y, Cong Z, Yang Y, Tang C, Liu Z. Interplay between VEGF and Nrf2 regulates angiogenesis due to intracranial venous hypertension. Sci Rep. 2016;6:37338–37338. doi: 10.1038/srep37338. PMID: 278691 47. DOI: 10.1038/srep37338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Schmidt A, von Woedtke Th, Weltmann K-D, Masur K. Identification of the molecular basis of non-thermal plasma-induced changes in human keratinocytes. Plasma Med. 2013;3:15–25. DOI: 10.1615/PlasmaMed.2014008535. [Google Scholar]
- 139.Barton A, Wende K, Bundscherer L, Hasse S, Schmidt A, Bekeschus S, Weltmann K-D, Lindequist U, Masur K. Nonthermal Plasma Increases Expression of Wound Healing Related Genes in a Keratinocyte Cell Line. Plasma Med. 2013;3:125–136. DOI: 10.1615/PlasmaMed.2014008540. [Google Scholar]
- 140.Hammer MU, Forbrig E, Kupsch S, Weltmann K-D, Reuter S. Influence of Plasma Treatment on the Structure and Function of Lipids. Plasma Med. 2013;3:97–114. DOI: 10.1615/ PlasmaMed.2014009708. [Google Scholar]
- 141.Yusupov M, Wende K, Kupsch S, Neyts EC, Reuter S, Bogaerts A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci Rep. 2017;7:5761. doi: 10.1038/s41598-017-06412-8. DOI:10.1038/s41598-017-06412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmidt A, Bekeschus S, Jablonowski H, Barton A, Weltmann K-D, Wende K. Role of ambient gas composition on cold physical plasma-elicited cell signaling in keratinocytes. Biophys J. 2017;112:2397–2407. doi: 10.1016/j.bpj.2017.04.030. PMID: 28591612. DOI: 10.1016/ j.bpj.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmidt A, Bekeschus S, Jarick K, Hasse S, von Woedtke Th, Wende K. Cold physical plasma modulates p53 and mitogen-activated protein kinase signaling in keratinocytes. Oxid Med Cell Longev. 2019;2019:7017363. doi: 10.1155/2019/7017363. DOI: 10.1155/2019/7017363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Emmert S, Brehmer F, Hänßle H, Helmke A, Mertens N, Ahmed R, Simon D, Wandke D, Maus-Friedrichs W, Daeschlein G, Schön MP, Viöl W. Atmospheric pressure plasma in dermatology: Ulcus treatment and much more. Clin Plasma Med. 2013;1:24–29. DOI: 10.1016/j.cpme.2012.11.002. [Google Scholar]
- 145.Hilker L, von Woedtke Th, Weltmann K-D, Wollert H-G. Cold atmospheric plasma: A new tool for the treatment of superficial driveline infections. Eur J Cardiothorac Surg. 2017;51:186–187. doi: 10.1093/ejcts/ezw212. PMID: 27354253. DOI: 10.1093/ejcts/ezw212. [DOI] [PubMed] [Google Scholar]
- 146.Wirtz M, Stoffels I, Dissemond J, Schadendorf D, Roesch A. Actinic keratoses treated with cold atmospheric plasma. J Eur Acad Dermatol. 2018;32:e37–e39. doi: 10.1111/jdv.14465. PMID: 28695987. DOI: 10.1111/jdv.14465. [DOI] [PubMed] [Google Scholar]
- 147.Martines E, Brun P, Brun P, Cavazzana R, Deligianni V, Leonardi A, Tarricone E, Zuin M. Towards a plasma treatment of corneal infections. Clin Plasma Med. 2013;1(2):17–24. DOI: 10.1016/j.cpme.2013.10.001. [Google Scholar]
- 148.Alekseev O, Donovan K, Limonnik V, Azizkhan Clifford J. Nonthermal dielectric barrier discharge (DBD) plasma suppresses herpes simplex virus type 1 (HSV-1) replication in corneal epithelium. Trans Vis Sci Tech. 2014;3:2. doi: 10.1167/tvst.3.2.2. PMID: 24757592. DOI: 10.1167/tvst.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reitberger HH, Czugala M, Chow C, Mohr A, Burkovski A, Gruenert AK, Schoenebeck R, Fuchsluger TA. Argon cold plasma – A novel tool to treat therapy-resistant corneal infections. Am J Ophthalmol. 2018;190:150–163. doi: 10.1016/j.ajo.2018.03.025. PMID: 29580975. DOI: 10.1016/j.ajo.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 150.Cha S, Park Y-S. Plasma in dentistry. Clin Plasma Med. 2014;2:4–10. doi: 10.1016/j.cpme.2014.04.002. PMID: 27030818. DOI: 10.1016/j.cpme.2014. 04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Metelmann H-R, von Woedtke Th, Weltmann K-D (eds) Comprehensive Clinical Plasma Medicine. Cold Physical Plasma for Medical Application. Springer International Publishing AG, part of Springer Nature. 2018:526. DOI: 10.1007/978-3-319-67627-2. [Google Scholar]
- 152.Schlegel J, Köritzer J, Boxhammer V. Plasma in cancer treatment. Clin Plasma Med. 2013;1(2):2–7. DOI: 10.1016/ j.cpme.2013.08.001. [Google Scholar]
- 153.Ratovitski EA, Cheng X, Yan D, Sherman JH, Canady J, Trink B, Keidar M. Anti-cancer therapies of 21st century: Novel approach to treat human cancers using cold atmospheric plasma. Plasma Process Polym. 2014;11:1128–1137. DOI: 10.1002/ppap.201400071. [Google Scholar]
- 154.Hirst AM, Frame FM, Arya M, Maitland NJ, O’Connell D. Low temperature plasmas as emerging cancer therapeutics: the state of play and thoughts for the future. Tumor Biol. 2016;37:7021–7031. doi: 10.1007/s13277-016-4911-7. PMID: 26888782. DOI: 10.1007/s13277-016-4911-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dubuc A, Monsarrat P, Virard F, Merbahi N, Sarrette J-P, Laurencin Dalicieux S, Cousty S. Use of cold-atmospheric plasma in oncology: a concise systematic review. Ther Adv Med Oncol. 2018;10:1–12. doi: 10.1177/1758835918786475. PMID: 30046358. DOI: 10.1177/ 1758835918786475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Keidar M, Walk R, Shashurin A, Srinivasan P, Sandler A, Dasgupta S, Ravi R, Guerrero Preston R, Trink B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Brit J Cancer. 2011;105:1295–1301. doi: 10.1038/bjc.2011.386. PMID: 21979421. DOI: 10.1038/bjc.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vandamme M, Robert E, Lerondel S, Sarron V, Ries D, Dozias S, Sobilo J, Gosset D, Kieda C, Legrain B, Pouvesle JM, Le Pape A. ROS implication in a new antitumor strategy based on non-thermal plasma. Int J Cancer. 2012;130:2185–2194. doi: 10.1002/ijc.26252. PMID: 21702038. DOI: 10.1002/ijc.26252. [DOI] [PubMed] [Google Scholar]
- 158.Graves DB. Reactive species from cold atmospheric plasma: Implications for cancer therapy. Plasma Process Polym. 2014;11:1120–1127. DOI: 10.1002/ppap.201400068. [Google Scholar]
- 159.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach. Nature Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. PMID: 19478820. DOI: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 160.Yan D, Xiao H, Zhu W, Nourmohammadi N, Zhang LG, Bian K, Keidar M. The role of aquaporins in the anti-glioblastoma capacity of the cold plasma-stimulated medium. J Phys D Appl Phys. 2017;50:055401. DOI: 10.1088/1361-6463/aa53d6. [Google Scholar]
- 161.Yan D, Cui H, Zhu W, Talbot A, Zhang LG, Sherman JH, Keidar M. The Strong Cell-based Hydrogen Peroxide Generation Triggered by Cold Atmospheric Plasma. Sci Rep. 2017;7:10831. doi: 10.1038/s41598-017-11480-x. DOI: 10.1038/s41598-017-11480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bauer G, Graves D. Mechanisms of Selective Antitumor Action of Cold Atmospheric Plasma-Derived Reactive Oxygen and Nitrogen Species. Plasma Process Polym. 2016;13:1157–1178. DOI: 10.1002/ppap.201600089. [Google Scholar]
- 163.Miller V, Lin A, Fridman A. Why target immune cells for plasma treatment of cancer. Plasma Chem Plasma Process. 2016;36:259–268. DOI: 10.1007/s11090-015-9676-z. [Google Scholar]
- 164.Bekeschus S, Mueller A, Miller V, Gaipl U, Weltmann K-D. Physical plasma elicits immunogenic cancer cell death and mitochondrial singlet oxygen. IEEE Trans Radiation Plasma Med Sci. 2018;2:138–146. DOI: 10.1109/TRPMS.2017. 2766027. [Google Scholar]
- 165.Partecke LI, Evert K, Haugk J, Doering F, Normann L, Diedrich S, Weiss FU, Evert M, Hübner NO, Guenther C, Heidecke CD, Kramer A, Bussiahn R, Weltmann K-D, Pati O, Bender C, von Bernstorff W. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer. 2012;12:473. doi: 10.1186/1471-2407-12-473. PMID: 23066891. DOI: 10.1186/1471-2407-12-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.von Woedtke Th, Metelmann H-R. Editorial. Clin Plasma Med. 2014;2:37. DOI: 10.1016/j.cpme.2014.11.002. [Google Scholar]
- 167.Yoon YJ, Suh MJ, Lee HY, Lee HJ, Choi EH, Moon IS, Song K. Anti-tumor effects of cold atmospheric pressure plasma on vestibular schwannoma demonstrate its feasibility as an intra-operative adjuvant treatment. Free Radical Bio Med. 2018;115:43–56. doi: 10.1016/j.freeradbiomed.2017.11.011. PMID: 29138018. DOI: 10.1016/j.freerad biomed.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 168.Metelmann H-R, Nedrelow DS, Seebauer C, Schuster M, von Woedtke Th, Weltmann K-D, Kindler S, Metelmann PH, Finkelstein SE, Von Hoff DD, Podmelle F. Head and neck cancer treatment and physical plasma. Clin Plasma Med. 2015;3:17–23. DOI: 10.1016/j.cpme.2015.02.001. [Google Scholar]
- 169.Schuster M, Seebauer C, Rutkowski R, Hauschild A, Podmelle F, Metelmann C, Metelmann B, von Woedtke Th, Hasse S, Weltmann K-D, Metelmann H-R. Visible Tumor Surface Response to Physical Plasma and Apoptotic Cell Kill in Head and Neck Cancer. J Cranio Maxill Surg. 2016;44:1445–1452. doi: 10.1016/j.jcms.2016.07.001. PMID: 27499516. DOI: 10.1016/j.jcms.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 170.Metelmann H-R, Seebauer C, Miller V, Fridman A, Bauer G, Graves DB, Pouvesle J-M, Rutkowski R, Schuster M, Bekeschus S, Wende K, Masur K, Hasse S, Gerling T, Hori M, Tanaka H, Choi EH, Weltmann K-D, Metelmann PH, Von Hoff DD, von Woedtke Th. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin Plasma Med. 2018;9:6–13. DOI: 10.1016/j.cpme.2017.09.001. [Google Scholar]
- 171.Herrmann JM, Dick TP. Redox Biology on the rise. Biol Chem. 2012;393:999–1004. doi: 10.1515/hsz-2012-0111. PMID: 22944698. DOI: 10.1515/ hsz-2012-0111. [DOI] [PubMed] [Google Scholar]
- 172.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–219. doi: 10.1111/j.1432-1033.1993.tb18025.x. PMID: 7688300. DOI: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 173.Jones DP, Radi R. Redox Pioneer: Professor Helmut Sies. Antioxid Redox Signal. 2014;21:2459–2468. doi: 10.1089/ars.2014.6037. PMID: 25178739. DOI: 10.1089/ars.2014.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. PMID: 25588755. DOI: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. PMID: 28441057. DOI: 10.1146/ annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 176.Sies H. On the history of oxidative stress: Concept and some aspects of current development. Curr Opin Toxicol. 2018;7:122–126. DOI: 10.1016/j.cotox.2018.01.002. [Google Scholar]
- 177.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. PMID: 28110218. DOI: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Egea J, Fabregat I, Frapart YM, Ghezzi P, Görlach A. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS) Redox Biol. 2017;13:94–162. doi: 10.1016/j.redox.2017.05.007. PMID: 28577489. DOI: 10.1016/j.redox.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kaiser J. Hormesis. Sipping From a Poisoned Chalice. Science. 2003;302:376–379. doi: 10.1126/science.302.5644.376. PMID: 14563981. DOI: 10.1126/science. 302.5644.376. [DOI] [PubMed] [Google Scholar]
- 180.Calabrese EJ, Baldwin LA. Hormesis: The dose-response revolution. Annu Rev Pharmacol Toxicol. 2003;43:175–197. doi: 10.1146/annurev.pharmtox.43.100901.140223. PMID: 12195028. DOI: 10.1146/annurev.pharmtox.43.100901. 140223. [DOI] [PubMed] [Google Scholar]
- 181.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J . Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hermetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. DOI: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 182.Szili EJ, Harding FJ, Hong S-H, Herrmann F, Voelcker NH, Short RD. The hormesis effect of plasma-elevated intracellular ROS on HaCaT cells. J Phys D Appl Phys. 2015;48:495401. DOI: 10.1088/0022-3727/48/49/495401. [Google Scholar]