Abstract
Background: Image-guided intensity-modulated radiotherapy (IG-IMRT) is increasingly being used to treat patients with soft-tissue sarcoma (STS) of the head and neck. Although there is no comparison between IMRT and conventional radiation therapy (CRT) concerning their efficacy. In this analysis, we compared CRT and IMRT outcomes for head and neck STS. Patients and Methods: Sixty-seven patients who underwent radiotherapy between 1994 and 2017 were identified. Results: The median follow-up was 31 months. Of the 67 patients, 34% were treated with CRT technique and 66% with IG-IMRT. The locoregional relapse rate following IMRT was 21% versus 70% with CRT (p<0.001) and the 5-year locoregional control was 69% versus 28%, respectively (p=0.01). IG-IMRT was associated with non-significant, less acute, and chronic adverse events. In the multivariate analysis, a significant influence of radiation technique on locoregional control was confirmed (p=0.04). Conclusion: IG-IMRT seems to be associated both with higher locoregional control as well as lower acute and chronic toxicities.
Keywords: Head and neck sarcoma, image-guided radiotherapy, IMRT, local control, prognosis, immunotherapy
Head and neck soft-tissue sarcoma (STS) is a rare tumor arising from soft tissue, and represents ~10% of all sarcomas (1,2). Thus, patient groups presented in such studies are often small and non-homogeneous. STS in the head and neck, specifically, requires special management due to both its location and threat to numerous organs. Prognosis, as well as treatment, of head and neck sarcomas differs from that of other locations, owing to the limited scope for wide local excision due to the presence of important nearby structures and organs. Such localizations bear approximately 10% lower absolute difference in 5-year locoregional control (LRC) and overall survival (OS) as compared to sarcoma of the extremities (1-4). In addition to surgical resection, radiotherapy (RT) represents an important cornerstone of treatment. For instance, the most common RT indications are high tumor grade, large tumor, close resection margins, and locally advanced stage (2,5). Recently, the TNM classification system has been revised to consider tumor size more heavily for better prognostic stratification (6). The role of adjuvant chemotherapy (CTX) is unclear and depends on many factors, such as histological subtype, grade. Therefore, treatment must be individualized and made on a case-by-case basis (5,7). Emerging treatments, such as use of checkpoint inhibitors, is currently under investigation as an adjuvant therapy with the hope of reducing risk of relapse (8).
Image-guided intensity-modulated radiotherapy (IMRT) aims to deliver a homogeneous dose distribution into the tumor bed with maximum protection or sparing of organs at risk (OAR), with optimal positioning of patients (9). In addition, interfractional imaging may allow further adaptive planning in order to escalate the radiation dose, suggesting an improvement of outcome in comparison with conventional radiotherapy (CRT) (10,11).
The purpose of this analysis was to examine the effects of different RT techniques for patients with head and neck STS on survival and locoregional control (LRC) rates. Furthermore, radiation toxicities were investigated in regards to radiotherapy techniques.
Patients and Methods
Patients. In this retrospective study, two closely cooperating German institutions (University Hospital Münster and Paracelsus Clinic Osnabruck) collected data regarding clinical features, treatment concepts, and outcomes of patients who were referred for external beam RT between 1994 and 2017. Inclusion criteria for our study were head and neck STS, completion of treatment course and a minimum follow-up time of three months. RT was delivered as part of a primary management strategy or after exhibiting locoregional relapse (LRR) following other treatment modalities. World Health Organization (WHO) pathological classification and grading systems from the French Federation of Cancer Centers Sarcoma Group (FNCLCC) were utilized at our institutions (12,13). Treatment response, furthermore, was graded in accordance with the response evaluation criteria in solid tumours (14). Imaging data of 64 patients were reviewed for staging according to the recently updated TNM classification of malignant tumor (seventh edition) rubric (6). At the time of final analysis, over 31 patients had died, while 35 were alive, with one patient being lost to follow-up.
Radiation technique. Planning computed tomographic scans (CTs) were performed with intravenous contrast approximately 2 weeks before starting RT. Additional Positron-emission tomography (PET) (N=12) or magnetic resonance imaging (MRI) (N=33) scans were performed on 37 patients for delineation of planning tumor volume (PTV). The median standardized uptake value (SUV) of initial FDG-PET was 8 (range=6-24). Lymphatic irradiation was delivered to nine (14%) patients with involved nodes. Forty-four patients (66%) received image-guided IMRT, 18 (27%) patients received 2D/3D conformal RT, and five (7%) received electron beam. In this study, patients who received CRT were compared with those who received IMRT.
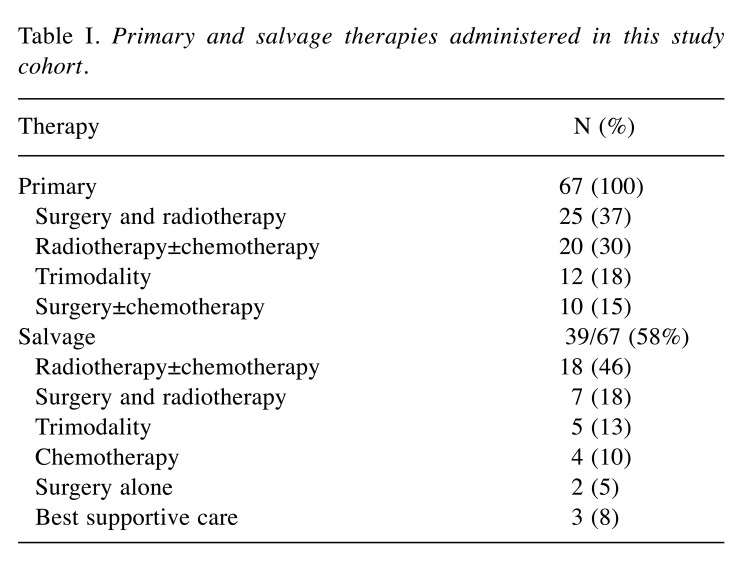
Primary and salvage therapy. Forty-seven (70%) patients underwent surgical resection of primary tumor. Fifty-seven (85%) patients underwent RT of primary tumor (37 postoperative and 20 definitive RT). Thirty-three patients (49%) also received CTX (three concurrently, 23 sequentially to RT, and seven received salvage regimens). In cases of possible relapse, individual salvage therapies were additionally undertaken. Salvage RT was delivered to 12 patients (18%) who developed recurrences following other modalities, and in 13 patients, RT as re-irradiation of local relapse in the head and neck region was utilized. The median interval between the two RT courses was 27 months (Table I).
Table I. Primary and salvage therapies administered in this study cohort.
Statistical analysis. All statistical analyses were conducted with SPSS version 25.0 software (IBM, Armonk, NY, USA). Differences were considered statistically significant at a p<0.05. Chi-squared or Fisher’s exact tests were performed to probe the relationships between two categorical variables. Overall survival (OS) was calculated from the first day of radiation until death, and the progression-free survival (PFS) was calculated from first day of radiation until relapse (locoregional or distant). LRC was calculated from the initiation of RT until the time of documented LRR. Time-dependent event curves were calculated using the Kaplan–Meier method, and were compared using the log-rank test. Independent variables, it must be noted, were initially analysed using univariate analysis. All variables revealed by univariate analysis to be significantly associated with local control or survival were subsequently entered into a Cox proportional hazards regression model for multivariate analysis.
Results
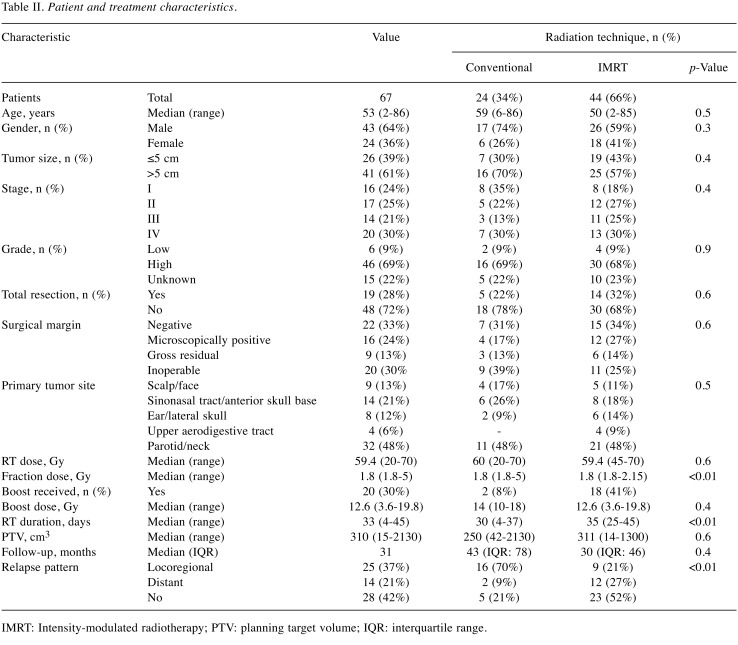
Patient and disease characteristics. There were 90 RT series among the 67 patients. Demographic and key clinical characteristics, including histology of sarcoma, disease stage, tumor location, RT treatment parameters, recurrence, and surgical characteristics, of the study cohort are summarized in Table II. The median tumor size was 5 cm (range=2-20 cm). Twenty-six patients (39%) had T1 and 41 (61%) had T2 disease, according to the older TNM classification systems (seventh edition) (15). Three patients (5%) had T1, six (9%) had T2, 28 (42%) had T3, and 27 (40%) had T4 disease, according to the updated TNM classification system (6). Cervical lymph node metastases were recorded in nine patients (14%). Distant metastases were recorded in 18 patients (27%), most commonly in bone (N=11) and lung (N=7). The overall median age of this cohort at the start of RT was 53 years (range=2-86 years).
Table II. Patient and treatment characteristics.
IMRT: Intensity-modulated radiotherapy; PTV: planning target volume; IQR: interquartile range.
The median initial radiation dose was 59.4 Gy, with 58.8 Gy (range=50-70 Gy) applied for primary RT versus 63 Gy (range=20-70 Gy) applied for postoperative therapy (p=0.007). Thirteen patients (19%) underwent a second RT course with a median RT dose of 50 Gy (range=16-66.6 Gy) and a cumulative dose of 106 Gy (range=70-120 Gy).
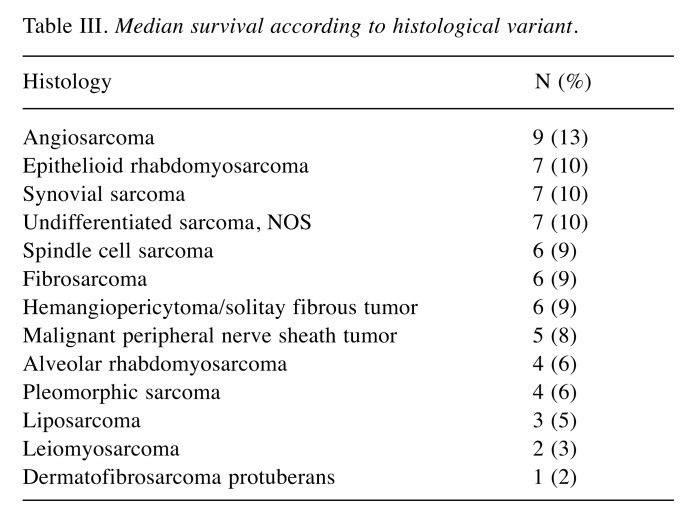
The most common CTX agents were doxorubicin (N=10) and ifosfamide (N=10). The most common histologies were angiosarcoma in nine (13%), rhabdomyosarcomas in 7 (10%), and spindle-cell sarcoma in seven (10%). Median survival according to different histological variants are listed in Table III. In our cohort, there were three (4%) RT-induced sarcomas with previous history of other head and neck malignancies.
Table III. Median survival according to histological variant.
Overall and progression-free survival rates. At end of this analysis, 31 out of 67 patients (46%) had died. The median follow-up time was 31 months. Considering the whole cohort, median OS and median PFS were 55 months [95% confidence intervaI (CI=12-98 months] and 30 months (95%CI=20-40 months), respectively. The 5-year OS and PFS rates were 44% and 33%, respectively.
Patients with low-grade (G1) sarcomas had longer median PFS (p=0.01), and trend towards longer OS (p=0.1) in comparison with those with high-grade (G2-3) sarcomas. There were no significant differences between the CRT and IMRT groups in term of PFS (p=0.4) and OS (p=0.1). Patients who received RT as part of their primary treatment strategy (upfront RT) had a longer PFS in comparison with those given salvage RT (31 versus 21 months, p=0.02), and there was no significant influence on OS (p=0.7). Considering the whole cohort, we did not observe any impact of RT doses on PFS (p=0.8) or OS (p=0.7), although subgroup analysis demonstrated a trend toward better survival outcomes in patients who received >63 Gy using IMRT in comparison with those receiving lower dose (p=0.2). Notably, median PFS was 57 months for patients treated with postoperative RT and 18 months for those treated with definitive RT, with 8 months for those treated with surgery alone (p=0.02). Similarly, median OS following postoperative RT was significantly longer in comparison with definitive RT groups (103 versus 21 months, p<0.0001). Patients with complete remission after primary treatments had significantly longer PFS (84 versus 19, p=0.03) and OS (not reached versus 15 months, p<0.0001) in comparison with those without complete remission. Additionally, patients who received adjuvant CTX after primary therapy displayed a trend for longer PFS (p=0.06), but with no impact on OS (p=0.3). Patients who underwent total resection had a longer PFS (57 versus 19 months, p=0.2) and significantly longer OS (not reached versus 33 months, p=0.01).
Regarding disease stage, patients with early disease (stage 1-2) had a longer PFS (47 versus 18, p=0.1) and significantly longer OS (96 versus 29 months, p=0.01). Patients with an initial tumor size >5 cm had a significantly worse OS in comparison to those with smaller tumors (80 versus 30 months, p=0.05). The site of the sarcoma did not affect the PFS (p=0.2) or OS (p=0.7).
In terms of diagnostic imaging, an increasing tumor size (continous variable in cm) was associated with a worse PFS (p=0.04) and OS (p=0.036), respectively. According the new TNM classification, we did not detect a significant difference according to the various T-predictors. The initial SUV value had a significant impact on PFS (p=0.04) and a non-significant impact on OS (p=0.4).
There was no significant difference in patients treated outside the Sarcoma Center and patients treated at our Sarcoma Center regarding LRC [hazard ratio (HR)=2, p=0.2], PFS (HR=1.5, p=0.36), and OS (HR=2.2, p=0.06).
Locoregional control. Tumor recurrences were detected in 39 patients (58%), including 25 (37%) LRRs and 14 (21%) distant recurrences. For the whole cohort, median LRC was 120 months (95%CI= 5-234 months). The 5-year LRC was 50±8%. Patients with low-grade sarcomas had a significantly longer LRC (p=0.04) compared with those suffering from a high-grade sarcoma. Combined surgery and RT was associated with LRC improvement as compared to other therapies (p=0.01), while CTX did not affect the rate of LRR or duration of LRC (p=0.3 and p=0.5, respectively). Notably, patients who received upfront RT had a lower relapse rate (50% versus 92%, p=0.005) than those who did not. Local control in patients who had undergone a second RT course was a median of 11 months (range=1-91 months) after salvage RT. There was no significant association between resection margin status and relapse pattern (p=0.8). Use of CTX did not influence the relapse rate. Recurrence rates were similar between patients who received CTX, regardless of whether doxurobucin-based therapies were given (p=0.9).
In terms of RT technique, we found a significant association between the RT technique and the risk of relapse development. Twenty-one out of the 44 patients (48%) treated with IMRT experienced recurrence. In comparison, 18 out of 23 patients (79%) in the CRT group experienced recurrence, while the LRR was 21% and 70%, respectively (p<0.01), which translated to longer 5-year LRC (69±9% versus 28±11% respectively, p=0.01). The initial SUV value did not infleunce the LRC rate significantly (HR=0.8, p=0.5). We did not detect significant differences between the various T-predictors of the eighth edition of the TNM classification.
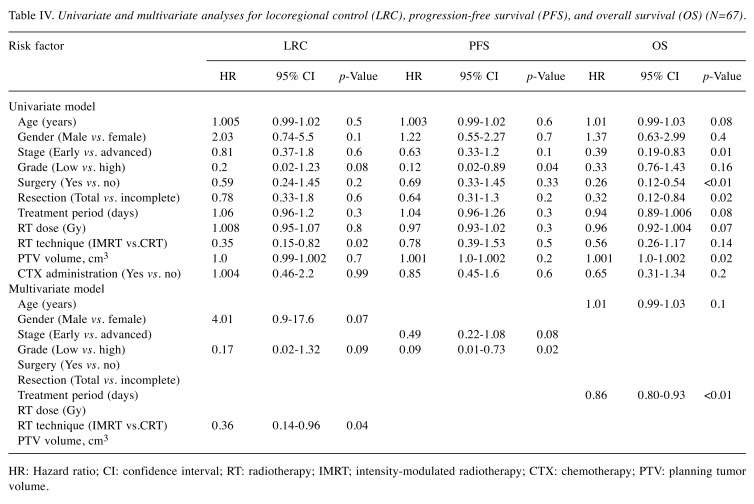
Cox proportional hazards model. Age at the time of RT, gender, stage, histological grade, surgical intervention, resection margin, treatment period, prescribed RT dose, RT technique, PTV, and use of CTX were included in a Cox proportional hazards model (Table IV).
Table IV. Univariate and multivariate analyses for locoregional control (LRC), progression-free survival (PFS), and overall survival (OS) (N=67).
HR: Hazard ratio; CI: confidence interval; RT: radiotherapy; IMRT; intensity-modulated radiotherapy; CTX: chemotherapy; PTV: planning tumor volume.
In the univariate analysis, gender, histological grade, and IMRT usage emerged as potential predictors of LRC, whereas disease stage and histological grade emerged as potential predictors of PFS, while age, disease stage, surgery, total negative resection margin, RT dose, treatment period, and PTV emerged as potential predictor of OS.
In the follow-up multivariate analysis, IMRT technique (p=0.04) on the one hand, remained significantly related for LRC improvement and the histological grade remained significantly related to PFS (p=0.02). On the other hand, duration of RT proved a significant determinate of OS (p<0.001).
Toxicities. During the initial RT courses, almost all patients (85%) experienced grade 1 AEx s and 52% patients experienced grade 2 AEs. Grade 3 and 4 toxicities were observed in 12% and 3% of patients, respectively. No radiation-related breaks or deaths occurred. The most common acute AEs were erythema and mucositis. The incidence of grade 3 and 4 toxicities proved lower in patients treated with IMRT (12% vs. 18%, respectively; p=0.7). In terms of chronic AEs, 48% of patients experienced grade 1, 17% grade 2, and 9% grade 3 AEs. There were no incidences of grade 4 chronic AEs. Following IMRT, the incidence of grade 1 (45% vs. 55%, p=0.6) and grade 2 (14% vs. 23%, p=0.5) toxicities were lower compared to those following CRT. However, this advantage did not reach statistical significance. Grade 3 toxicity with IMRT was 12% versus 5% with CRT (p=0.6).
Regarding radiation dose, patients receivedwho had a high dose (>63 Gy) significantly more frequently had grade 2 toxicities (84% vs. 39%, p=0.001) and non-significant more grade 3 AEs (21% vs. 9%, p=0.2). Chronic AEs also proved more frequent in the high-dose RT group: Grade 1: 63% versus 42%, p=0.17; 2: 26% versus 13%, p=0.3; and 3: 10% versus 9%, p>0.99.
Discussion
The aim of this study was to compare the impact of two different RT techniques on LRC, PFS, OS, and radiation-related toxicity. The following findings emerged from this work: i) Radiotherapy technique significantly influenced the 5-year LRC, with a noticeable benefit for those treated with IG-IMRT (69% vs. 28%, p=0.01), and in the multivariate analysis, this benefit remained a significant predictor for LRC (p=0.04). ii) Radiotherapy technique did not Iead to any significant difference regarding PFS (p=0.4). However, a trend towards improved OS in favor of IMRT was detectable (p=0.1). iii) Regardless of RT technique, minimal grade 4 toxicities were noted, while IMRT was associated with non-significant lower incidence of acute and chronic AEs. iv) Upfront RT conferred an advantage in LRC and PFS over delayed RT for treatment of head and neck STS.
In accordance with previous studies, better outcomes were observed in patients treated with a combined modality (16,17). In this analysis, the LRR rate was lower in patients receiving IMRT compared to those receiving CRT (21% vs. 70% with CRT, p<0.01) with a higher 5-year LRC rate. In contrast to our findings, Vitzthum et al. (18) did not detect significant survival differences between results of IMRT and CRT techniques in 48 patients with head and neck STS. In addition, we noted that patients treated with IMRT developed fewer acute and chronic AEs, however, this advantage was not significant, and probably resulted from a small sample size.
In accordance with previous studies (19-21), lymph node involvement was 14%, and distant metastasis rate 27%. Prognostic factors for STS of the head and neck include tumor size over 5 cm, histological grade, and resection margin (2,21). The 5-year LRC was found to range from 41 to 81% with a 5-year window between 50% and 80% (2,19,22). In a modern radiation series, including 26 patients with non-metastatic head and neck STS, Andrä et al. reported 5-year LRC, PFS, and OS of 86%, 82%, and 82%, respectively (23).
Interestingly, complete remission after primary therapies improved PFS (p=0.03) and OS (p<0.0001). Patients who received RT as part of primary treatment strategy had a longer PFS in comparison with salvage RT (31 vs. 21 months, p=0.02). This finding may support the initiation of RT as part of multimodal approach at initial diagnosis.
In a recent review, Crompton et al. reported that surgery and RT are the most important factors for local disease control (8), although other therapeutic options may also have local influence. Gustafson et al. indicated that patients should be treated in a specialized Sarcoma Center, and further reported a 2.4-fold higher risk of LRR in patients who were not treated at such a facility (24). In our patients, there was no significant difference between the treatment outcome at the two insititutions, although we did observe a trend for better OS in patients treated at our Sarcoma Center (HR=2.2, p=0.06), most likely due to the small number of patients not treated at our Sarcoma Center (N=7).
Higher radiation doses result in better local control but also in higher toxicity rates (23,25). In accordance with other studies, we observed an increased incidence of AEs and improved local control at doses >63 Gy compared to lower doses (18,23,26-29). However, the local control did not differ between the two groups. In terms of survival, Kepka et al. reported improved OS and PFS with doses >63 Gy, with even an improvement of 3% per Gy in local control and OS (26). According to Aljabab et al., the recommended doses for low-grade soft tissue sarcomas are 60 Gy, for high-grade sarcomas 65 Gy and for a positive resection margin the dose may be increased by 5-10 Gy (1).
With the implementation of functional imaging, FDG-PET is highly accurate in detecting both primary and metastatic lesions of STS (2,8,30). In our cohort, PET was used in 12 patients and the initial SUV value seemed to predict the PFS (p=0.04), supporting the development of PET-adaptive treatment strategies. Salvage RT was a feasible option in the case of localized recurrences even after extensive pre-treatment. We were able to apply a second RT course in 13 patients without an increase in severe acute or chronic toxicity grades, except in one case of osteoradionecrosis and only after a cumulative dose of 106 Gy.
Nevertheless, our study has several limitations such as its retrospective nature, as well as the low patient number and very heterogenous population of cases and treatments, which is attributable to the low frequency of head and neck STS. Unfortunately, some patient data were lacking, while furthermore one patient was lost during follow-up. Despite our data demonstrating significantly longer local tumor control using IMRT for patients with head and neck STS, some questions remain unsettled which should be addressed by a multi-institutional prospective study. In the era of targeted therapies, further research in term of radiogenomic and personalized medicine is warranted to optimize treatment decisions (31,32). Our understanding of STS is evolving as current investigations continue to improve our comprehension of such molecular mechanisms, especially those concerning this rare entity (8). Regarding targeted therapies, immunotherapy has been demonstrated to have efficacy in patients with metastatic synovial cell sarcoma and other advanced stages (5,33). At present, there are ongoing clinical studies evaluating the toxicity and efficacy of checkpoint inhibitors (durvalumab and tremelimumab) combined with RT and surgery (phase I/II trial, ClinicalTrials.gov NCT03116529); moreover, such data will be enriched further through analyzing the safety and efficacy of neoadjuvant and adjuvant pembrolizumab in patients with high-risk extremity STS (phase II trial, ClinicalTrials.gov NCT03092323). Results of the ongoing trials and future recommendations are expected within a few years.
Conclusion
Upfront RT remains an integral component for head and neck STS management after first diagnosis. IG-IMRT might be associated with higher locoregional control and less acute and chronic toxicity.
Compliance with Ethical Standards
All procedures performed were in accordance with the ethical standards of the University Hospital Münster and National Research Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This research received no specific grant from any funding agency.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Conflicts of Interest
On behalf of all the Authors, the corresponding Author states that there are no conflicts of interest to report.
Authors’ Contributions
KE and DS were involved in formal analysis, and research methodology. All co-authors were involved in conceptualization of article, and article drafting and editing. HTE was the senior author who oversaw the project. All co-authors read and approved the final article.
References
- 1.Aljabab AS, Nason RW, Kazi R, Pathak KA. Head and neck soft-tissue sarcoma. Indian J Surg Oncol. 2011;2:286–290. doi: 10.1007/s13193-012-0127-5. PMID: 23204783. DOI: 10.1007/s13193-012-0127-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bree R de, van der Waal I, de Bree E, Leemans CR. Management of adult soft-tissue sarcomas of the head and neck. Oral Oncol. 2010;46:786–790. doi: 10.1016/j.oraloncology.2010.09.001. PMID: 20947413. DOI: 10.1016/j.oraloncology.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Levay J, O'sullivan B, Catton C, Bell R, Fornasier V, Cummings B, Hao D, Warr D, Quirt I. Outcome and prognostic factors in soft-tissue sarcoma in the adult. Int J Radiat Oncol Biol Phys. 1993;27:1091–1099. doi: 10.1016/0360-3016(93)90529-5. PMID: 8262833. DOI: 10.1016/0360-3016(93)90529-5. [DOI] [PubMed] [Google Scholar]
- 4.Wolden SL, Wexler LH, Kraus DH, Laquaglia MP, Lis E, Meyers PA. Intensity-modulated radiotherapy for head-and-neck rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2005;61:1432–1438. doi: 10.1016/j.ijrobp.2004.08.005. PMID: 15817347. DOI: 10.1016/j.ijrobp.2004. 08.005. [DOI] [PubMed] [Google Scholar]
- 5.Casali PG, Abecassis N, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A1, Haas RL, Hannu A, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno S Neumann, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, Van der Graaf W, Whelan J, Wardelmann E, Zaikova O, Blay JY, ESMO Guidelines Committee EURACAN Soft-tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:268–269. doi: 10.1093/annonc/mdy096. PMID: 29846498. DOI: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 6.Brierley J, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. John Wiley & Sons Inc, Chichester, West Sussex, UK. 2017 [Google Scholar]
- 7.Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer. 2008;113:573–581. doi: 10.1002/cncr.23592. PMID: 18521899. DOI: 10.1002/cncr.23592. [DOI] [PubMed] [Google Scholar]
- 8.Crompton JG, Ogura K, Bernthal NM, Kawai A, Eilber FC. Local control of soft tissue and bone sarcomas. J Clin Oncol. 2018;36:111–117. doi: 10.1200/JCO.2017.75.2717. PMID: 29220297. DOI: 10.1200/JCO.2017. 75.2717. [DOI] [PubMed] [Google Scholar]
- 9.Leitzen C, Wilhelm Buchstab T, Müdder T, Heimann M, Koch D, Schmeel C, Simon B, Stumpf S, Vornholt S, Garbe S, Röhner F, Schoroth F, Schild HH, Schüller H. Patientenlagerung bei Kopf-Hals-Tumoren Lageungenauigkeiten und Sicherheitsabstände bei helikaler Tomotherapie. Strahlenther Onkol. 2018;194:386–391. doi: 10.1007/s00066-018-1265-7. PMID: 29372290. DOI: 10.1007/s00066-018-1265-7. [DOI] [PubMed] [Google Scholar]
- 10.Elsayad K, Scobioala S, Kriz J, Haverkamp U, Eich HT. Advances in image-guided radiation therapy for primary cardiac angiosarcoma: The role of PET-CT and MRI. Oncol Res Treat. 2016;39:290–294. doi: 10.1159/000445864. PMID: 27173652. DOI: 10.1159/000445864. [DOI] [PubMed] [Google Scholar]
- 11.Surucu M, Shah KK, Roeske JC, Choi M, Small W, Emami B. Adaptive radiotherapy for head and neck cancer. Technol Cancer Res Treat. 2017;16:218–223. doi: 10.1177/1533034616662165. PMID: 27502958. DOI: 10.1177/1533034616662165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillou L, Coindre JM, Bonichon F, Nguyen BB, Terrier P, Collin F, Vilain MO, Mandard AM, Le Doussal V, Leroux A, Jacquemier J, Duplay H, Sastre-Garau X, Costa J. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft-tissue sarcoma. J Clin Oncol. 1997;15:350–362. doi: 10.1200/JCO.1997.15.1.350. PMID: 8996162. DOI: 10.1200/JCO.1997. 15.1.350. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CDM. WHO classification of tumours of soft tissue and bone: [this book reflects the views of a working group that convened for a consensus and editorial meeting at the University of Zurich, Switzerland, 18-20 April 2012]. Internat. Agency for Research on Cancer, Lyon. 2013 [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. PMID: 19097774. DOI: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. Wiley, Somerset. 2011 [Google Scholar]
- 16.Eeles RA, Fisher C, A'Hern RP, Robinson M, Rhys Evans P, Henk JM, Archer D, Harmer CL. Head and neck sarcomas: prognostic factors and implications for treatment. Br J Cancer. 1993;68:201–207. doi: 10.1038/bjc.1993.314. PMID: 8318414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Q-TX, Fu KK, Kroll S, Fitts L, Massullo V, Ferrell L, Kaplan MJ, Phillips TL. Prognostic factors in adult soft-tissue sarcomas of the head and neck. Int J Radiat Oncol Biol Phys. 1997;37:975–984. doi: 10.1016/s0360-3016(97)00103-x. PMID: 9169803. DOI: 10.1016/S0360-3016(97) 00103-X. [DOI] [PubMed] [Google Scholar]
- 18.Vitzthum LK, Brown LC, Rooney JW, Foote RL. Head and neck soft-tissue sarcomas treated with radiation therapy. Rare Tumors. 2016;8:6165–6165. doi: 10.4081/rt.2016.6165. PMID: 27441072. DOI: 10.4081/rt.2016. 6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tajudeen BA, Fuller J, Lai C, Grogan T, Elashoff D, Abemayor E, St John M. Head and neck sarcomas: The UCLA experience. Am J Otolaryngol. 2014;35:476–481. doi: 10.1016/j.amjoto.2014.02.003. PMID: 24721744. DOI: 10.1016/j.amjoto.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salcedo Hernández RA, Lino Silva LS, Mosqueda Taylor A, Luna Ortiz K. Soft-tissue sarcomas of the head and neck. Clinical and pathological evaluation of 108 cases in Mexico. J Craniomaxillofac Surg. 2014;42:1566–1571. doi: 10.1016/j.jcms.2014.01.033. PMID: 24704280. DOI: 10.1016/j.jcms.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Fayda M, Aksu G, Yaman Agaoglu F, Karadeniz A, Darendeliler E, Altun M, Hafiz G. The role of surgery and radiotherapy in treatment of soft-tissue sarcomas of the head and neck region: review of 30 cases. J Craniomaxillofac Surg. 2009;37:42–48. doi: 10.1016/j.jcms.2008.07.007. PMID: 18804382. DOI: 10.1016/j.jcms.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Bentz BG, Singh B, Woodruff J, Brennan M, Shah JP, Kraus D. Head and neck soft-tissue sarcomas: A multivariate analysis of outcomes. Ann Surg Oncol. 2004;11:619–628. doi: 10.1245/ASO.2004.03.006. PMID: 15172935. DOI: 10.1245/ASO.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Andrä C, Rauch J, Li M, Ganswindt U, Belka C, Saleh Ebrahimi L, Ballhausen H, Nachbichler SB, Roeder F. Excellent local control and survival after postoperative or definitive radiation therapy for sarcomas of the head and neck. Radiat Oncol. 2015;10:140–140. doi: 10.1186/s13014-015-0449-x. PMID: 26156022. DOI: 10.1186/s13014-015-0449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gustafson P, Dreinhöfer KE, Rydholm A. Soft-tissue sarcoma should be treated at a tumor center. A comparison of quality of surgery in 375 patients. Acta Orthop Scand. 1994;65:47–50. doi: 10.3109/17453679408993717. PMID: 8154283. [DOI] [PubMed] [Google Scholar]
- 25.Jensen AD, Uhl M, Chaudhri N, Herfarth KK, Debus J, Roeder F. Carbon Ion irradiation in the treatment of grossly incomplete or unresectable malignant peripheral nerve sheaths tumors: Acute toxicity and preliminary outcome. Radiat Oncol. 2015;10 doi: 10.1186/s13014-015-0414-8. PMID: 25943106. DOI: 10.1186/s13014-015-0414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kepka L, DeLaney TF, Suit HD, Goldberg SI. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63:852–859. doi: 10.1016/j.ijrobp.2005.03.004. PMID: 16199316. DOI: 10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Tepper JE, Suit HD. Radiation therapy alone for sarcoma of soft tissue. Cancer. 1985;56:475–479. doi: 10.1002/1097-0142(19850801)56:3<475::aid-cncr2820560311>3.0.co;2-s. PMID: 4005809. [DOI] [PubMed] [Google Scholar]
- 28.Zagars GK, Ballo MT. Significance of dose in postoperative radiotherapy for soft-tissue sarcoma. Int J Radiat Oncol Biol Phys. 2003;56:473–481. doi: 10.1016/s0360-3016(02)04573-x. PMID: 12738323. DOI: 10.1016/ S0360-3016(02)04573-X. [DOI] [PubMed] [Google Scholar]
- 29.Hata M, Wada H, Ogino I, Omura M, Koike I, Tayama Y, Odagiri K, Kasuya T, Inoue T. Radiation therapy for angiosarcoma of the scalp: Treatment outcomes of total scalp irradiation with X-rays and electrons. Strahlenther Onkol. 2014;190:899–904. doi: 10.1007/s00066-014-0627-z. PMID: 24622678. DOI: 10.1007/s00066-014-0627-z. [DOI] [PubMed] [Google Scholar]
- 30.Zwirner K, Thorwarth D, Winter RM, Welz S, Weiss J, Schwenzer NF, Schmidt H, La Fougère C, Nikolaou K, Zips D, Gatidis S. Voxelweise Korrelation funktioneller PET/MRT-Parameter in Bestrahlungslagerung bei Patienten mit Kopf-Hals-Tumoren. Strahlenther Onkol. 2018;194:719–726. doi: 10.1007/s00066-018-1292-4. PMID: 29564483. DOI: 10.1007/s00066-018-1292-4. [DOI] [PubMed] [Google Scholar]
- 31.Peeken JC, Goldberg T, Knie C, Komboz B, Bernhofer M, Pasa F, Kessel KA, Tafti PD, Rost B, Nüsslin F, Braun AE, Combs SE. Therapieinformationen verbessern auf maschinellem Lernen basierende prognostische Einschätzungen für Patienten mit Weichteilsarkomen. Strahlenther Onkol. 2018;194:824–834. doi: 10.1007/s00066-018-1294-2. PMID: 29557486. DOI: 10.1007/s00066-018-1294-2. [DOI] [PubMed] [Google Scholar]
- 32.Peeken JC, Nüsslin F, Combs SE. “Radio-oncomics” Das Potenzial von Radiomics in der Strahlenonkologie. Strahlenther Onkol. 2017;193:767–779. doi: 10.1007/s00066-017-1175-0. PMID: 28687979. DOI: 10.1007/ s00066-017-1175-0. [DOI] [PubMed] [Google Scholar]
- 33.Robbins PF, Kassim SH, Tran TLN, Crystal JS, Morgan RA, Feldman SA, Yang JC, Dudley ME, Wunderlich JR, Sherry RM, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Li YF, El-Gamil M, Rosenberg SA. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: long-term follow-up and correlates with response. Clin Cancer Res. 2015;21:1019–1027. doi: 10.1158/1078-0432.CCR-14-2708. PMID: 25538264. DOI: 10.1158/1078-0432.CCR-14-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]