Abstract
Maduramicin, a polyether ionophore antibiotic used as an anticoccidial agent in poultry industry, has been reported to be toxic to animals and humans if improperly used or by accident, resulting in heart failure, skeletal muscle degeneration and even death. However, the molecular mechanism underlying its cardiotoxicity remains elusive. Mitogen activated protein kinases (MAPKs) and protein phosphatases signaling pathways have been documented to be involved in the cell survival regulation. The present study was set to investigate the role of above pathways in maduramicin-induced myocardial cytotoxicity. Here we observed that maduramicin inhibited cell proliferation and reduced cell viability in H9c2 cells. Furthermore, we found that maduramicin suppressed extracellular signal-regulated kinase 1/2 (ERK1/2) phosphorylation in a concentration-dependent manner. Ectopic expression of constitutively active MKK1 partially prevented the cytotoxicity of maduramicin. Moreover, we showed that maduramicin concentration-dependently activated protein phosphatase 2A (PP2A) by decreasing its phosphorylation and increasing its methylation. Inhibition of PP2A with okadaic acid attenuated maduramicin’s toxicity. Overexpression of dominant negative PP2A partially rescued cells from maduramicin-inhibited ERK1/2 contributing to its cytotoxicity. The results indicate that maduramicin activates PP2A and consequently inhibits ERK1/2, leading to cytotoxicity in H9c2 cells. Our data suggest that manipulation of PP2A-ERK1/2 pathway may be a potential approach to prevent maduramicin-induced cardiotoxicity.
Keywords: maduramicin, cardiotoxicity, extracellular signal-regulated kinase 1/2, protein phosphatase 2A, H9c2
1. Introduction
Coccidiosis, a parasitic disease caused by Eimeria infection, causes great economic loss in the poultry industry (Min et al., 2004; Williams, 1998). Maduramicin is the most potent polyether ionophore antibiotic for coccidiosis prevention (Dorne et al., 2013; Liu et al., 1983). However, clinically maduramicin toxicity has been frequently observed in target animals such as chickens and turkeys because of its using at high dose or for long time (Dorne et al., 2013; Singh and Gupta, 2003). Besides, since maduramicin is excreted rapidly and mainly as unchanged form in broilers (Oehme and Pickrell, 1999), increasing reports have shown that maduramicin is toxic to non-target animals including pigs, cattle and sheep when fed with the broiler litter as a source of protein and minerals (Sanford and McNaughton, 1991; Shimshoni et al., 2014; Shlosberg et al., 1992; Shlosberg et al., 1997). Importantly, there are also some cases of accidental poisoning with maduramicin in humans (Jayashree and Singhi, 2011; Sharma et al., 2005). Pathologically the toxicity of maduramicin is characterized by cardiac and skeletal muscle degeneration (Dorne et al., 2013; Jayashree and Singhi, 2011; Shimshoni et al., 2014).
Mitogen activated protein kinases (MAPKs), including extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 MAPK, play a critical role in the regulation of cell survival (Kim and Choi, 2010; Kyriakis and Avruch, 2012). These serine/threonine (Ser/Thr) kinases are activated through a sequential phosphorylation cascade that amplifies and transduces signals from the cell membrane to the nucleus (Kim and Choi, 2010). MAPKs can also be inactivated by protein phosphatases, which counteract many kinase-driven intracellular signaling pathways through dephosphorylating key proteins (Hinds and Sanchez, 2008; Kalev and Sablina, 2011; Perrotti and Neviani, 2013).
Protein phosphatase 2A (PP2A), a key cellular Ser/Thr phosphatase containing a structural A subunit (PP2A-A), a regulatory B subunit (PP2A-B) and a catalytic C subunit (PP2Ac), can be modulated by phosphorylation of PP2Ac at Tyr307 and Thr304 (Perrotti and Neviani, 2013). Phosphorylation of Tyr307 prevents methylation from occurring (Perrotti and Neviani, 2013). Both phosphorylation events result in inactivation of PP2A (Perrotti and Neviani, 2013). Protein phosphatase 5 (PP5) is also a unique member of Ser/Thr phosphatases based on the presence of tetratricopeptide repeat (TPR) domains within its structure (Hinds and Sanchez, 2008). Since their discovery, PP2A and PP5 has been implicated in wide ranging cellular processes, including MAPK-mediated growth and differentiation (Hinds and Sanchez, 2008). Over all, PP2A negatively regulate the phosphorylation of Erk1/2, JNK and p38, whereas PP5 negatively regulate JNK/p38 pathway (Hinds and Sanchez, 2008; Kalev and Sablina, 2011).
So far the molecular mechanism of maduramicin-induced cardiotoxicity is not well understood. Especially, whether maduramicin affect MAPKs and its phosphatases including PP2A and PP5 in myocardial cells is not clear now. Here we show that maduramicin-induced myocardial cytotoxicity is associated with its intervention of PP2A-ERK1/2 signaling pathway. Mechanistically, maduramicin-activated PP2A results in ERK1/2 inhibition, leading to toxicity in H9c2 cells.
2. Materials and Methods
2.1. Materials
Maduramicin ammonium (molecular weight = 934.16, purity > 97%, by HPLC) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), dissolved in dimethyl sulfoxide (DMSO) to prepare a stock solution (5 mg/ml), aliquoted and stored at –20°C. Dulbecco’s modified Eagle’s medium (DMEM) and 0.05% trypsin-EDTA were obtained from Mediatech (Manassas, VA, USA). Fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA, USA). Okadaic acid (OA) was from Sigma (Saint Louis, MO, USA). CellTiter 96® AQueous one solution cell proliferation analysis kit was from Promega (Madison, WI, USA). Enhanced chemiluminescence solution was from Perkin-Elmer Life Science (Boston, MA, USA). The following antibodies were used: ERK2, JNK, phosphorylated-JNK (p-JNK) (Thr183/Tyr185), c-Jun, p-c-Jun (Ser63), p38, p-PP2A (Tyr307), demethylated-PP2A (De-PP2A), HA and GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), p-ERK1/2 (Thr202/Tyr204) and p-p38 (Thr180/Tyr182) (Cell signaling technology, Beverly, MA, USA), PP2Ac, PP2A-A, PP2A-B and methylated-PP2A (Me-PP2A) (Upstate biotechnology, Waltham, MA, USA), PP5 (BD transduction laboratories, San Jose, CA, USA), FLAG (Sigma, Saint Louis, MO, USA), goat anti-mouse IgG-horseradish peroxidase and goat anti-rabbit IgG-horseradish peroxidase (Pierce, Rockford, IL, USA).
2.2. Cell line and culture
Rat myocardial myoblasts (H9c2, #CRL-1446, American Type Culture Collection, Manassas, VA, USA) were cultured in antibiotic-free high glucose (4.5 g/L) DMEM supplemented with 10% FBS and 2 mM glutamine, at 37°C and 5% CO2. The cells were split at 1:4 and subcultured every 3 days.
2.3. Recombinant adenoviral constructs and infection of cells
The recombinant adenoviruses expressing FLAG-tagged constitutively active MKK1 (Ad-MKK1-R4F), hemagglutinin (HA)-tagged dominant-negative (dn) PP2A catalytic subunit (PP2Ac) (L199P) (Ad-dn-PP2A), and the control vector expressing green fluorescent protein (GFP) alone (Ad-GFP) were described previously (Liu et al., 2010; Zeng et al., 2015). For experiments, H9c2 cells were grown in the growth medium and infected with the individual adenovirus for 24 h at 5 of multiplicity of infection (MOI = 5). Subsequently, cells were used for experiments. Ad-GFP served as a control. Expression of FLAG-tagged MKK1-R4F and HA-tagged dn-PP2A were determined by Western blot analysis with antibodies to FLAG and HA, respectively.
2.4. Cell proliferation analysis
Cell proliferation analysis was performed by taking pictures as described (Chen et al., 2014). Briefly, H9c2 cells were seeded in DMEM supplemented with 10% FBS in 6-well plates at a density of 2 × 105 cells/well and grown overnight at 37°C in a humidified incubator with 5% CO2. The next day, maduramicin (0–1 μg/ml) was added. In some cases, cells were pretreated with okadaic acid for 1 h or infected with Ad-MKK1-R4F, Ad-dn-PP2A and Ad-GFP for 24 h. Then maduramicin (0.5 and 1 μg/ml) was added. After incubation for 48 h, images were taken with an Olympus inverted phase-contrast microscope equipped with the Quick Imaging system.
2.5. Cell viability assay
Cell viability was evaluated using CellTiter 96® AQueous one solution cell proliferation analysis kit, as described (Chen et al., 2014). Briefly, H9c2 cells suspended in the growth medium were seeded in a 96-well plate at a density of 5 × 103 cells/well and grown overnight at 37°C in a humidified incubator with 5% CO2. The next day, maduramicin (0–1 μg/ml) was added. In some cases, cells were pretreated with okadaic acid for 1 h or infected with Ad-MKK1-R4F, Ad-dn-PP2A and Ad-GFP for 24 h, then exposed to maduramicin (0.5 and 1 μg/ml). After incubation for 48 h, each well was added 20 μl of one solution reagent and incubated for 1 h. Cell viability was determined by measuring the OD at 490 nm using a Wallac 1420 Multilabel Counter (PerkinElmer Life Sciences, Wellesley, MA, USA).
2.6. Western blot analysis
Western blotting was performed, as described previously (Chen et al., 2014). Briefly, equivalent amounts of proteins (whole cell lysates) were separated on 8–12% sodium dodecyl sulfate-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA). Membranes were blocked with 5% non-fat dry milk (dissolved in PBS containing 0.05% Tween 20) for 1 h at room temperature, and then incubated with primary antibodies overnight at 4°C, followed by probing with appropriate secondary antibodies conjugated to horseradish peroxidase overnight at 4°C. Immunoreactive bands were visualized by using enhanced chemiluminescence reagent.
2.7. Statistical analysis
Results were expressed as mean values ± SE (standard error). Statistical analysis was performed using one-way analysis of variance (ANOVA) followed by Dunnett’s post-test or two-way ANOVA followed by Bonferroni’s post-test for multiple comparisons. A level of P < 0.05 was considered to be significant.
3. Results
3.1. Maduramicin inhibits cell proliferation and reduces cell viability in H9c2 cells
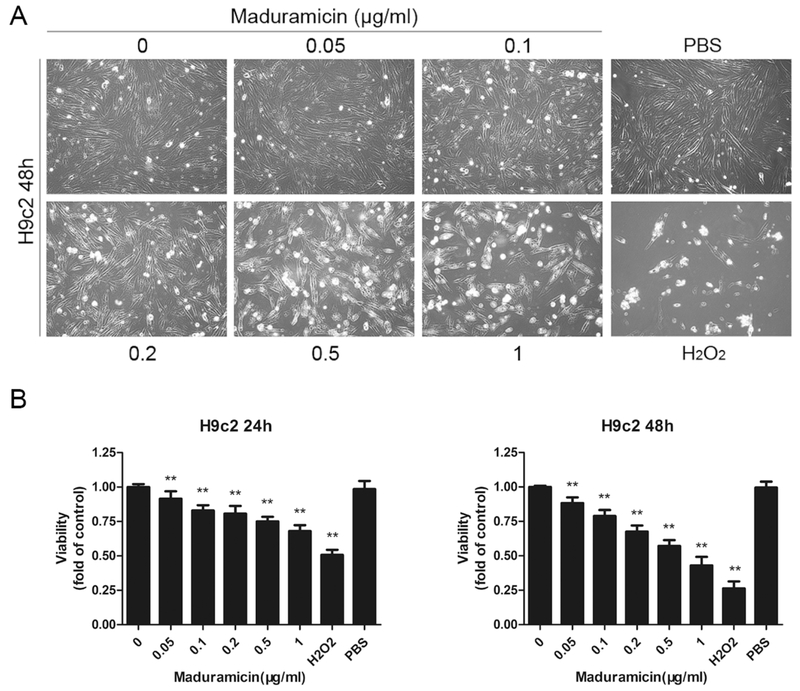
To observe the toxic effect of maduramicin in myocardial cells, H9c2 cells were exposed to 0–1 μg/ml maduramicin for 48 h, followed by taking images under a microscope. We found that maduramicin inhibited cell proliferation in a concentration-dependent manner (Fig. 1A). When cells were exposed to maduramicin (0–1 μg/ml) for 24 or 48 h, one solution assay also revealed concentration- and time-dependent inhibitory effects on cell viability (Fig. 1B). The IC50 value for 48 h treatment was between 0.5–1 μg/ml. Treatment with 1 μg/ml maduramicin for 48 h was able to reduce cell viability to 0.44 fold, compared to the control. The findings suggest that maduramicin exerts its cytotoxicity in H9c2 cells at least by inhibiting cell proliferation and reducing cell viability.
Fig. 1.
Maduramicin inhibits cell proliferation and reduces cell viability in H9c2 cells. H9c2 cells were exposure to maduramicin at indicated concentrations for 24 or 48 h. A. Cell images were taken with an Olympus inverted phase-contrast microscope equipped with the Quick Imaging system. B. Cell viability was detected by one solution assay. For (A), representative photos were shown. Similar results were observed in at least three independent experiments. For (B), all data were expressed as means ± SE (n = 6). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post-test. ** P < 0.01, difference with control group.
3.2. Maduramicin induces cytotoxicity in part by inhibiting ERK1/2 in H9c2 cells
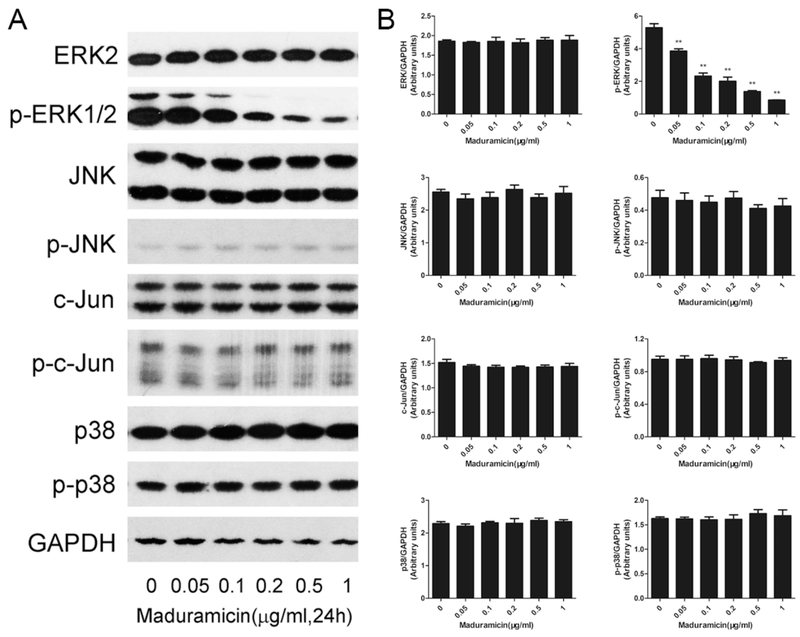
It has been demonstrated that MAPKs play a critical role in cell survival regulation (Kim and Choi, 2010; Kyriakis and Avruch, 2012). To further investigate the toxic mechanism of maduramicin in myocardial cells, Western blotting was used to check the phosphorylation level of MAPK family members (ERK1/2, JNK and p38) after exposed to maduramicin at 0–1 μg/ml for 24 h. As shown in Fig. 2, maduramicin did not change the phosphorylation status of JNK and p38, but obviously decreased the phosphorylation level of ERK1/2 in a concentration-dependent manner. Consistently, exposure to maduramicn also did not alter the phosphorylation status of c-Jun (a downstream substrate of JNK). The results suggest that ERK1/2 was inhibited by maduramicin treatment.
Fig. 2.
Maduramicin inactivates ERK1/2 in H9c2 cells. H9c2 cells were exposure to maduramicin at indicated concentrations for 24 h. A. Total cell lysates were subjected to Western blotting using indicated antibodies. B. Blots for indicated proteins were semi-quantified using NIH image J. For (A), representative blots were shown. The blots were probed for GAPDH as a loading control. Similar results were observed in at least three independent experiments. For (B), all data were expressed as means ± SE (n = 3). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post-test. ** P < 0.01, difference with control group.
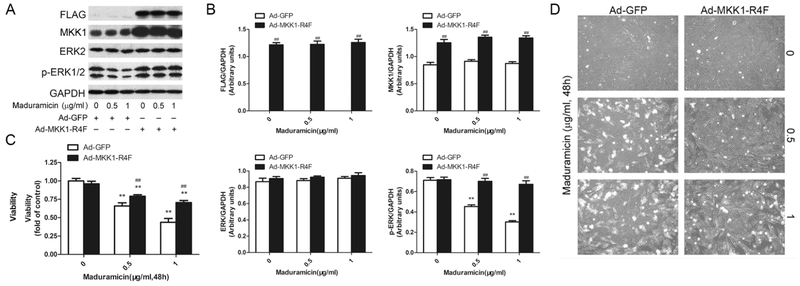
To verify the role of ERK1/2 in maduramicin-induced myocardial cytotoxicity, we employed recombinant adenovirus Ad-MKK1-R4F, encoding FLAG-tagged constitutively active MKK1 (an upstream kinase of Erk1/2). Infection of H9c2 cells with Ad-MKK1-R4F, but not Ad-GFP (control virus), resulted in expression of high levels of FLAG-tagged MKK1 mutant (Fig. 3A and B). Expression of MKK1-R4F led to robust phosphorylation of Erk1/2 even with exposure to Mad (Fig. 3A and B), indicating that the MKK1 mutant function in the cells. Expression of MKK1-R4F significantly attenuated maduramicin-induced cytotoxicity (Fig. 3C and D). The results clearly indicate that maduramicin induces cytotoxic effect in part by inhibiting ERK1/2 in H9c2 cells.
Fig. 3.
Ectopic expression of constitutively active MKK1 intervenes toxicity of maduramicin in H9c2 cells. H9c2 cells, infected with Ad-MKK1-R4F or Ad-GFP (as control), respectively, were exposure to maduramicin at indicated concentrations for 24 h (for Western blotting) or 48 h (for cell viability assay and cell proliferation analysis). A. Total cell lysates were subjected to Western blotting using indicated antibodies. B. Blots for indicated proteins were semi-quantified using NIH image J. C. Cell viability was detected by one solution assay. D. Cell images were taken with an Olympus inverted phase-contrast microscope equipped with the Quick Imaging system. For (A), representative blots were shown. The blots were probed for GAPDH as a loading control. Similar results were observed in at least three independent experiments. For (B), all data were expressed as means ± SE (n = 3). Statistical analysis was performed using two-way ANOVA followed by Bonferroni’s post-test. ** P < 0.01, difference with control group; ## P < 0.01, Ad-MKK1-R4F group versus Ad-GFP group. For (C), all data were expressed as means ± SE (n = 6). Statistical analysis was performed using two-way ANOVA followed by Bonferroni’s post-test. ** P < 0.01, difference with control group; ## P < 0.01, Ad-MKK1-R4F group versus Ad-GFP group. For (D), representative photos were shown. Similar results were observed in at least three independent experiments.
3.3. Maduramicin inhibits ERK1/2 and induces cytotoxicity partially by activating PP2A in H9c2 cells
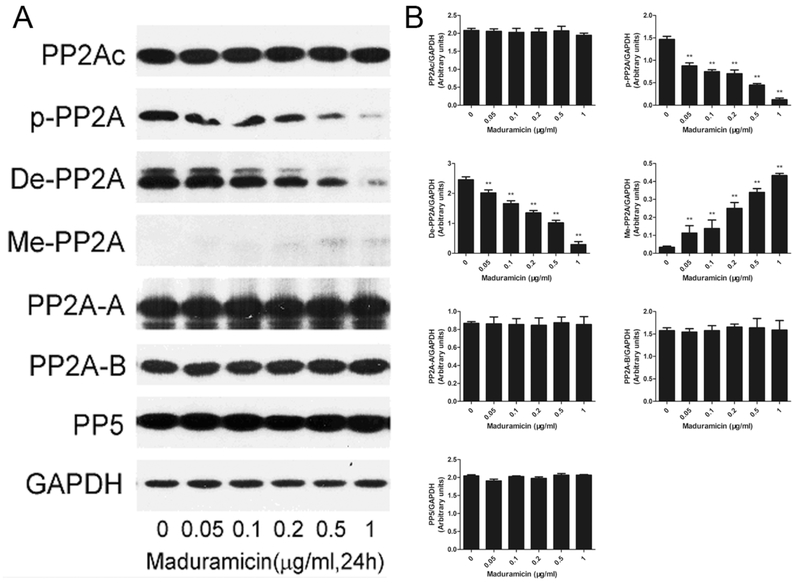
Since MAPKs can be negatively regulated by PP2A and PP5 (Chen et al., 2009), we reasoned that maduramicin might inhibit ERK1/2 by activating these protein phosphatases. To this end, H9c2 cells were exposed to 0–1 μg/ml maduramicin for 24 h, followed by Western blotting. As shown in Fig. 4, maduramicin did not apparently alter the cellular protein level of the structural subunit PP2A-A, the regulatory subunit PP2A-B and the catalytic subunit PP2Ac as well as the expression of PP5, but reduced the expression of phosphorylated- and demethylated-PP2A and increased the protein level of methylated-PP2A, which related to PP2A activity (Perrotti and Neviani, 2013), in a concentration dependent manner. The results suggest that maduramicin activates PP2A in myocardial cells.
Fig. 4.
Maduramicin activates PP2A in H9c2 cells. H9c2 cells were exposure to maduramicin at indicated concentrations for 24 h. A. Total cell lysates were subjected to Western blotting using indicated antibodies. B. Blots for indicated proteins were semi-quantified using NIH image J. For (A), representative blots were shown. The blots were probed for GAPDH as a loading control. Similar results were observed in at least three independent experiments. For (B), all data were expressed as means ± SE (n = 3). Statistical analysis was performed using one-way ANOVA followed by Dunnett’s post-test. ** P < 0.01, difference with control group.
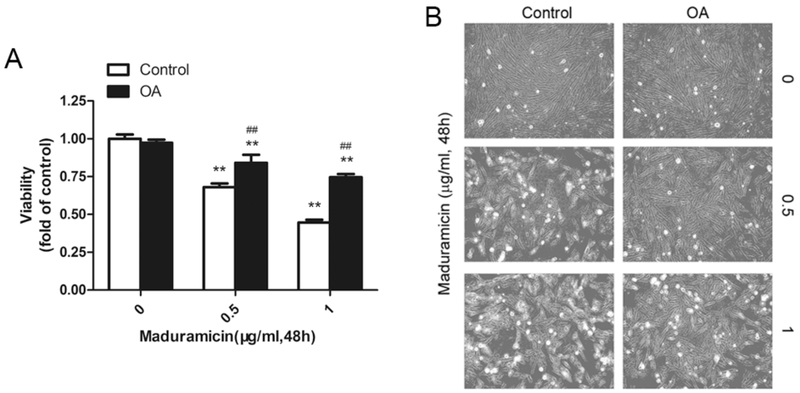
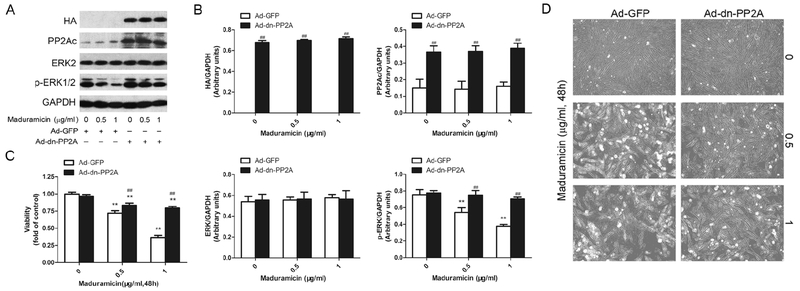
To pinpoint the role and significance of PP2A in maduramicin-induced cytotoxicity in myocardial cells, PP2A inhibitor okadaic acid (OA) was utilized. We found that pre-incubation with OA attenuated toxicity of maduramicin in H9c2 cells (Fig. 5). To further confirm the effect of PP2A on maduramicin-induced ERK1/2 inhibition and cytotoxicity in myocardial cells, we infected H9c2 cells with recombinant adenovirus Ad-dn-PP2A, encoding HA-tagged dominant negative PP2A. Infection of cells with Ad-dn-PP2A, but not Ad-GFP (control virus), resulted in expression of high levels of HA-tagged dn-PP2A mutant (Fig. 6A and B). Over-expression of dn-PP2A substantially relieved maduramicin-reduced phosphorylation of ERK1/2 in the cells (Fig. 6A and B). Consistently, overexpression of dn-PP2A attenuated maduramicin-induced cell toxicity (Fig. 6C and D). It suggests that maduramicin induces cytotoxicity by intervening PP2A-ERK1/2 signaling pathway in H9c2 cells.
Fig. 5.
Pretreatment with okadaic acid (OA) attenuates toxicity of maduramicin in H9c2 cells. H9c2 cells, pretreated with/without OA (100 nM) for 1 h, were exposure to maduramicin at indicated concentrations for 48 h. A. Cell viability was detected by one solution assay. B. Cell images were taken with an Olympus inverted phase-contrast microscope equipped with the Quick Imaging system. For (A), all data were expressed as means ± SE (n = 6). Statistical analysis was performed using two-way ANOVA followed by Bonferroni’s post-test. ** P < 0.01, difference with control group; ## P < 0.01, difference with OA group. For (B), representative photos were shown. Similar results were observed in at least three independent experiments.
Fig. 6.
Expression of dominant negative PP2A dramatically attenuates maduramicin-inactivated ERK1/2 contributing to its toxicity in H9c2 cells. H9c2 cells, infected with Ad-dn-PP2A or Ad-GFP (as control), respectively, were exposure to maduramicin at indicated concentrations for 24 h (for Western blotting) or 48 h (for cell viability assay and cell proliferation analysis). A. Total cell lysates were subjected to Western blotting using indicated antibodies. B. Blots for indicated proteins were semi-quantified using NIH image J. C. Cell viability was detected by one solution assay. D. Cell images were taken with an Olympus inverted phase-contrast microscope equipped with the Quick Imaging system. For (A), representative blots were shown. The blots were probed for GAPDH as a loading control. Similar results were observed in at least three independent experiments. For (B), all data were expressed as means ± SE (n = 3). Statistical analysis was performed using two-way ANOVA followed by Bonferroni’s post-test. ** P < 0.01, difference with control group; ## P < 0.01, Ad-dn-PP2A group versus Ad-GFP group. For (C), all data were expressed as means ± SE (n = 6). Statistical analysis was performed using two-way ANOVA followed by Bonferroni’s post-test. ** P < 0.01, difference with control group; ## P < 0.01, Ad-dn-PP2A group versus Ad-GFP group. For (D), representative photos were shown. Similar results were observed in at least three independent experiments.
4. Discussion and Conclusions
Maduramicin, a polyether ionophore antibiotic used for coccidiosis prevention in the poultry, has been shown to be toxic to animals and humans because of improperly used or by accident (Dorne et al., 2013; Jayashree and Singhi, 2011; Sharma et al., 2005; Shimshoni et al., 2014; Singh and Gupta, 2003). Importantly, Shimshoni et al. described an acute maduramicin toxicosis affecting 22 pregnant gilts, 2 pregnant sows and 2 boars, resulting in a total mortality of 65% within 2 days (Shimshoni et al., 2014). It suggests that prenatal maduramicin exposure is more harmful and might to cause congenital birth defects in animals. Histopathologically, the toxicity of maduramicin is characterized by severe cardiac and skeletal muscle lesions (Dorne et al., 2013; Jayashree and Singhi, 2011; Shimshoni et al., 2014). However, the toxic mechanism of maduramicin in myocardial cells is not well known. Here we found that maduramicin inhibited cell proliferation and reduced cell viability in H9c2 cells (Fig. 1), suggesting it is toxic to myocardial cells. This is consistent with previous studies which showed that salinomycin and monensin, two other polyether ionophores, also executes its toxic effects by inhibiting cell proliferation and reducing cell viability in a spectrum of human cancer cells (Boehmerle and Endres, 2011; Park et al., 2002).
ERK1/2 has been reported to be a key protein in regulation of cell survival (Baines and Molkentin, 2005; Xia et al., 2016). We found that maduramicin dramatically decreased the expression of phosphorylated ERK1/2 (Fig. 2), which suggested that maduramicin inhibited ERK1/2 pathway in H9c2 cells. To further confirm whether ERK1/2 inhibition contributes to the toxicity of maduramicin in myocardial cells, recombinant adenovirus Ad-MKK1-R4F, encoding FLAG-tagged constitutively active MKK1, was utilized. Expression of MKK1-R4F attenuated cytotoxicity of maduramicin in H9c2 cells (Fig. 3). It suggests that maduramicin inactivates ERK1/2, leading to toxicity in myocardial cells. Many studies have shown similar results in cardiomyocytes and cancer cells. For instance, urotensin II protects cardiomyocytes from doxorubicin-induced apoptosis partly through upregulating the phosphorylation of ERK (Chen et al., 2012). Isobavachalcone induces the apoptosis of gastric cancer cells via inhibition ERK pathway (Jin and Shi, 2016). Monensin increases ERK activity and induces apoptosis in NCI-H929 myeloma cells (Park et al., 2003). Ghrelin protection of dexamethasone-induced INS-1 cell apoptosis is mediated by inducing ERK activation (Zhang et al., 2016).
PP2A has been documented to dephosphorylating Erk1/2 (Hinds and Sanchez, 2008; Kalev and Sablina, 2011; Perrotti and Neviani, 2013). Here, we found that maduramicin concentration-dependently activated PP2A, which was evidenced by the finding that maduramicin decreased the protein expression level of phosphorylated-PP2A and demethylated-PP2A, and increased the level of methylated-PP2A in H9c2 cells (Fig. 4). Next, we used okadaic acid (OA), a pharmacological inhibitor of PP2A, to pinpoint the role and significance of PP2A in maduramicin`s cytotoxicity. We found that inhibition of PP2A by OA attenuated toxicity of maduramicin in H9c2 cells (Fig. 5). Further, to verify the hypothesis that maduramicin-induced ERK1/2 inhibition and cytotoxicity through PP2A-dependent mechanism in myocardial cells, genetic inhibition experiments for PP2A were carried out. It was shown that expression of dominant negative PP2A resulted in high resistance to maduramicin-induced dephosphorylation of ERK1/2 and cytotoxicity in H9c2 cells (Fig. 6). Collectively, maduramicin causes cytotoxicity through inhibition of ERK1/2, which is partially attributed to activation of PP2A in myocardial cells. Similar effects have been reported in different cell lines. For example, PP2A mediates p38 MAPK-induced ERK dephosphorylation contributing to apoptosis in cardiac myocytes (Liu and Hofmann, 2004). Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway (Chen et al., 2009). Rapamycin inhibits hsBAFF-stimulated cell proliferation and survival by activation of PP2A, thereby resulting in inhibition of ERK1/2 pathway in normal and neoplastic B-lymphoid cells (Zeng et al., 2015).
In summary, here we have verified that maduramicin activated PP2A, which resulted in inhibition of ERK1/2, thereby leading to cytotoxicity in H9c2 cells. The results indicate that maduramicin executed its myocardial cytotoxicity at least partially through PP2A-ERK1/2 pathway. Our findings suggest that manipulation of PP2A-ERK1/2 signaling pathway may be a potential approach to prevent maduramicin-induced cardiotoxicity.
Acknowledgments
This work was supported in part by the grants from National Natural Science Foundation of China (No. 31672612, S.J.), the National Institutes of Health (NCI CA115414, S.H.), American Cancer Society (RSG-08–135-01-CNE, S.H.), Nanjing Agricultural University, and Feist-Weiller Cancer Center, Louisiana State University Health Sciences Center.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
dimethyl sulfoxide
- ERK1/2
extracellular signal-regulated kinase 1/2
- FBS
fetal bovine serum
- GFP
green fluorescence protein
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MKK
mitogen-activated protein kinase kinase
- OA
okadaic acid
- PBS
phosphate buffered saline
- PP2A
protein phosphatase 2A
- PP5
protein phosphatase 5
- Ser/Thr
serine/threonine
Footnotes
Competing Interests
The authors have declared that no competing interests exist.
References
- Baines CP, Molkentin JD, 2005. STRESS signaling pathways that modulate cardiac myocyte apoptosis. Journal of molecular and cellular cardiology 38, 47–62. [DOI] [PubMed] [Google Scholar]
- Boehmerle W, Endres M, 2011. Salinomycin induces calpain and cytochrome c-mediated neuronal cell death. Cell death & disease 2, e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu L, Yin J, Luo Y, Huang S, 2009. Hydrogen peroxide-induced neuronal apoptosis is associated with inhibition of protein phosphatase 2A and 5, leading to activation of MAPK pathway. The international journal of biochemistry & cell biology 41, 1284–1295. [DOI] [PubMed] [Google Scholar]
- Chen X, Gu Y, Singh K, Shang C, Barzegar M, Jiang S, Huang S, 2014. Maduramicin inhibits proliferation and induces apoptosis in myoblast cells. PloS one 9, e115652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Loh SH, Chen JJ, Tsai CS, 2012. Urotensin II prevents cardiomyocyte apoptosis induced by doxorubicin via Akt and ERK. European journal of pharmacology 680, 88–94. [DOI] [PubMed] [Google Scholar]
- Dorne JL, Fernandez-Cruz ML, Bertelsen U, Renshaw DW, Peltonen K, Anadon A, Feil A, Sanders P, Wester P, Fink-Gremmels J, 2013. Risk assessment of coccidostatics during feed cross-contamination: animal and human health aspects. Toxicology and applied pharmacology 270, 196–208. [DOI] [PubMed] [Google Scholar]
- Hinds TD Jr., Sanchez ER, 2008. Protein phosphatase 5. The international journal of biochemistry & cell biology 40, 2358–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayashree M, Singhi S, 2011. Changing trends and predictors of outcome in patients with acute poisoning admitted to the intensive care. Journal of tropical pediatrics 57, 340–346. [DOI] [PubMed] [Google Scholar]
- Jin X, Shi YI, 2016. Isobavachalcone induces the apoptosis of gastric cancer cells via inhibition of the Akt and Erk pathways. Experimental and therapeutic medicine 11, 403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalev P, Sablina AA, 2011. Protein phosphatase 2A as a potential target for anticancer therapy. Anti-cancer agents in medicinal chemistry 11, 38–46. [DOI] [PubMed] [Google Scholar]
- Kim EK, Choi EJ, 2010. Pathological roles of MAPK signaling pathways in human diseases. Biochimica et biophysica acta 1802, 396–405. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J, 2012. Mammalian MAPK signal transduction pathways activated by stress and inflammation: a 10-year update. Physiological reviews 92, 689–737. [DOI] [PubMed] [Google Scholar]
- Liu CM, Hermann TE, Downey A, Prosser BL, Schildknecht E, Palleroni NJ, Westley JW, Miller PA, 1983. Novel polyether antibiotics X-14868A, B, C, and D produced by a Nocardia. Discovery, fermentation, biological as well as ionophore properties and taxonomy of the producing culture. The Journal of antibiotics 36, 343–350. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, Han X, Shen T, Huang S, 2010. Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PloS one 5, e10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Hofmann PA, 2004. Protein phosphatase 2A-mediated cross-talk between p38 MAPK and ERK in apoptosis of cardiac myocytes. American journal of physiology Heart and circulatory physiology 286, H2204–2212. [DOI] [PubMed] [Google Scholar]
- Min W, Dalloul RA, Lillehoj HS, 2004. Application of biotechnological tools for coccidia vaccine development. Journal of veterinary science 5, 279–288. [PubMed] [Google Scholar]
- Oehme FW, Pickrell JA, 1999. An analysis of the chronic oral toxicity of polyether ionophore antibiotics in animals. Veterinary and human toxicology 41, 251–257. [PubMed] [Google Scholar]
- Park WH, Kim ES, Kim BK, Lee YY, 2003. Monensin-mediated growth inhibition in NCI-H929 myeloma cells via cell cycle arrest and apoptosis. International journal of oncology 23, 197–204. [PubMed] [Google Scholar]
- Park WH, Lee MS, Park K, Kim ES, Kim BK, Lee YY, 2002. Monensin-mediated growth inhibition in acute myelogenous leukemia cells via cell cycle arrest and apoptosis. International journal of cancer 101, 235–242. [DOI] [PubMed] [Google Scholar]
- Perrotti D, Neviani P, 2013. Protein phosphatase 2A: a target for anticancer therapy. The Lancet Oncology 14, e229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford SE, McNaughton C, 1991. Ontario. Inonophore (maduramicin) toxicity in pigs. The Canadian veterinary journal = La revue veterinaire canadienne 32, 567. [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Bhalla A, Varma S, Jain S, Singh S, 2005. Toxicity of maduramicin. Emergency medicine journal : EMJ 22, 880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimshoni JA, Britzi M, Pozzi PS, Edery N, Berkowitz A, Bouznach A, Cuneah O, Soback S, Bellaiche M, Younis A, Blech E, Oren P, Galon N, Shlosberg A, Perl S, 2014. Acute maduramicin toxicosis in pregnant gilts. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 68, 283–289. [DOI] [PubMed] [Google Scholar]
- Shlosberg A, Harmelin A, Perl S, Pano G, Davidson M, Orgad U, Kali U, Bor A, Van Ham M, Hoida G, et al. , 1992. Cardiomyopathy in cattle induced by residues of the coccidiostat maduramicin in poultry litter given as a feedstuff. Veterinary research communications 16, 45–58. [DOI] [PubMed] [Google Scholar]
- Shlosberg A, Perl S, Harmelin A, Hanji V, Bellaiche M, Bogin E, Cohen R, Markusfeld-Nir O, Shpigel N, Eisenberg Z, Furman M, Brosh A, Holzer Z, Aharoni Y, 1997. Acute maduramicin toxicity in calves. The Veterinary record 140, 643–646. [DOI] [PubMed] [Google Scholar]
- Singh T, Gupta RP, 2003. Clinico-haematological and mineral studies on experimental maduramicin toxicity in chickens. Veterinary parasitology 116, 345–353. [DOI] [PubMed] [Google Scholar]
- Williams RB, 1998. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. International journal for parasitology 28, 1089–1098. [DOI] [PubMed] [Google Scholar]
- Xia P, Liu Y, Cheng Z, 2016. Signaling Pathways in Cardiac Myocyte Apoptosis. BioMed research international 2016, 9583268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Q, Zhang H, Qin J, Xu Z, Gui L, Liu B, Liu C, Xu C, Liu W, Zhang S, Huang S, Chen L, 2015. Rapamycin inhibits BAFF-stimulated cell proliferation and survival by suppressing mTOR-mediated PP2A-Erk1/2 signaling pathway in normal and neoplastic B-lymphoid cells. Cellular and molecular life sciences : CMLS 72, 4867–4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li L, Zhao B, Jiao A, Li X, Sun N, Zhang J, 2016. Ghrelin Protects against Dexamethasone-Induced INS-1 Cell Apoptosis via ERK and p38MAPK Signaling. International journal of endocrinology 2016, 4513051. [DOI] [PMC free article] [PubMed] [Google Scholar]