Abstract
Background
Individuals with peripheral arterial disease (PAD) have a nearly two-fold increased risk of all-cause and cardiovascular disease mortality compared to those without PAD. This pilot study determined whether metabolomic profiling can accurately identify patients with PAD who are at increased risk of near-term mortality.
Methods
We completed a case-control study using 1H NMR metabolomic profiling of plasma from 20 decedents with PAD, without critical limb ischemia, who had blood drawn within 8 months prior to death (index blood draw) and within 10 to 28 months prior to death (preindex blood draw). Twenty-one PAD participants who survived more than 30 months after their index blood draw served as a control population.
Results
Results showed distinct metabolomic patterns between preindex decedent, index decedent, and survivor samples. The major chemical signals contributing to the differential pattern (between survivors and decedents) arose from the fatty acyl chain protons of lipoproteins and the choline head group protons of phospholipids. Using the top 40 chemical signals for which the intensity was most distinct between survivor and preindex decedent samples, classification models predicted near-term all-cause death with overall accuracy of 78% (32/41), a sensitivity of 85% (17/20), and a specificity of 71% (15/21). When comparing survivor with index decedent samples, the overall classification accuracy was optimal at 83% (34/41) with a sensitivity of 80% (16/20) and a specificity of 86% (18/21), using as few as the top 10 to 20 chemical signals.
Conclusions
Our results suggest that metabolomic profiling of plasma may be useful for identifying PAD patients at increased risk for near-term death. Larger studies using more sensitive metabolomic techniques are needed to identify specific metabolic pathways associated with increased risk of near-term all-cause mortality among PAD patients.
Lower extremity peripheral arterial disease (PAD) affects eight million men and women in the United States and is expected to increase in prevalence as the U.S. population survives longer with chronic disease.1 Men and women with PAD have a higher risk for all-cause and cardiovascular disease (CVD) mortality than men and women without PAD, even after adjusting for CVD risk factors and comorbidities.2, 3 Despite optimal atherosclerotic disease risk factor control, many patients with PAD suffer from cardiovascular events. Conventional cardiovascular risk factors are useful to predict long-term coronary events and mortality, but are less useful for predicting near-term events.4, 5, 6, 7
Many acute coronary events result from plaque rupture and lumen thrombosisat arterial sites with minimally occlusive atherosclerosis.8 Circulating inflammatory and hemostatic biomarkers may contribute to plaque instability and rupture.9, 10, 11 However, these associations are not clearly established. Identifying biomarkers that are elevated immediately prior to an acute coronary event could provide important short-term prognostic information and may elucidate mechanisms of acute coronary events.
Metabolites are the end products of complex interactions between the host genome and environmental stimuli. Thus, metabolic changes are the most proximal reporters of an individual’s physiological status (such as an acute coronary event). Identifying novel metabolic pathways that are activated immediately prior to death may help establish new mechanisms of mortality that may be targeted with novel therapies to prevent adverse outcomes in patients with PAD. Metabolomics,12 a relatively new “omics” system, provides an unbiased means to probe the full spectrum of the metabolic pattern. Metabolomics holds great potential to discover major chemical alterations in response to physiological changes and guide the selection of therapeutic targets.13, 14 Thus, metabolomics represents a potentially powerful tool to capture molecular events that define distinct populations of patients and to attain a high prognostic accuracy for near-term events such as death.
We performed a pilot study to compare the proton nuclear magnetic resonance (1H NMR) metabolic profiles of plasma between PAD patients who died within 8 months of a blood draw and PAD patients who survived for at least 30 months after a blood draw. We hypothesized that metabolomic profiles of plasma can differentiate PAD patients with vs without increased risk of near-term death.
Methods
Study participants and blood sample selections
The study protocol was approved by the institutional review boards of Northwestern University’s Feinberg School of Medicine and participating study sites. Participants gave written informed consent. PAD participants were selected from existing cohorts of outpatients with PAD at Northwestern University including the Walking and Leg Circulation Study (WALCS), II, III cohorts, the Study to Improve Leg Circulation (SILC), and the Reducing Risk Factors in Peripheral Arterial Disease (RRF) study. Detailed descriptions of the WALCS, SILC, and RRF study designs and methods have been published previously.15, 16, 17, 18 Across these studies, PAD was defined as an ankle-brachial index <0.95, documented evidence of lower extremity revascularization, or documented clinical evidence of lower extremity atherosclerosis.
The current study consists of 20 PAD participants who died within 8 months of their last study blood draws (decedents) and 21 gender- and age-matched (within 3 years) PAD controls (survivors). Decedents died between May 1, 2000 and December 31, 2008. We selected all decedents from the WALCS, SILC, and RRF cohorts who met the following criteria: (a) presence of two blood draws (eg, index and preindex blood draws) prior to death; (b) the final blood draw (index blood draw) was within 1 year before death; and (c) the presence of a matched control survivor PAD participant. The index blood draw is defined as the blood draw that occurred closest to death (2–8 months preceding death). The preindex blood draw is defined as the blood draw at the study visit immediately prior to the index blood draw. The preindex blood draws occurred 7 to 25 months prior to the index blood draws. Each control PAD participant (survivor) was also selected from one of the same cohorts (WALCS I, II, or III, SILC, or RRF) and met the following criteria for inclusion in the study: a) presence of one blood sample that was matched for date of blood draw to within 3 months of the date of the decedent’s index blood draw date; survival to at least 30 months (mean ± standard deviation [sd], 67 ± 28 months) after the date of their blood draws. There were a total of 61 blood samples for the metabolomics experiment, two for each decedent and one for each survivor.
Ascertainment of mortality
Deaths were identified using the Social Security Administration death database and by contact with participants’ family members, proxies, and primary care physicians. Cause of death was determined using death certificates and by a certified nosologist. Cardiovascular deaths were those with International Classification of Disease-10 codes in the ranges I01.0 through I99.9, including deaths due to coronary heart disease, stroke, peripheral vascular disease, and other CVD.
Plasma sample preparation
Blood was collected into Vacutainer (Becton Dickinson, Franklin Lakes, NJ) tubes containing EDTA and sodium citrate and immediately placed on ice. The plasma was separated immediately by centrifugation at 3000 rpm for 20 minutes at 4°C and samples were frozen and stored at −70°C.
Measurement of clinical variables
The clinical variables and blood biomarkers for the current study include ankle-brachial index, total and high density lipoprotein cholesterol levels, D-dimer, CRP, cigarette smoking, body mass index, and medical history. CRP and D-dimer data were only available for 16 survivors and 16 decedents from the WALCS and WALCS II cohorts. The details for calculation and acquisition of all these measures for each study have been reported previously15, 16, 17, 18, 19, 20 and are described briefly in the Appendix (online only).
Plasma 1H NMR spectroscopy protocol and quality control
For each plasma sample, 550 μL of plasma and saline (0.9%) mixture was used for the 1H NMR spectrum measurement at 600.13 MHz on a Bruker DRX-600 spectrometer (Bruker Corporation, Billerica, Mass). The water resonance was suppressed. The experimental temperature was set at 298 k.
1H NMR spectra of plasma were acquired using both Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence and diffusion-edited sequence.21 Diffusion-edited sequence of the 1H NMR spectrum characterized the lipid molecules of lipoproteins, such as the –CH3 group of trigelycerides, cholesterols, phospholipids, and glycophospholipids to generate broad resonances in the spectrum. To characterize the small molecules such as creatinine, lactate, and glucose, we used the spectrometer with CPMG pulse sequence to detect the low molecular weight molecules by suppressing most of the broad resonances. The spectrum from both small molecules and lipoproteins generates an overall chemical structural profile.
Metabolomics data preprocessing, normalization, and statistical analysis
All spectra were phase-corrected and referenced to the chemical shift of alpha-glucose anomeric doublet at 5223 parts per million (ppm). Baseline correction was also applied to each spectrum using a penalized smoothing method.22 Each spectrum was segmented into 1000 chemical shift regions with a binning size 0.01 ppm in the range of chemical shift δ 0–10 for all data analysis. The peak region of water signal between δ 4.5 to 5.2 ppm was removed prior to statistical analysis.
Statistical analysis of plasma 1H NMR spectral data was performed with R 2.10 (R Development Core Team; http://www.R-project.org) using several data analysis strategies, as described below. All binned NMR spectral data were logged and normalized with a quantile normalization procedure. Identification of differential chemical signals (ie, binned variables) was performed using two-sample or paired t-tests as appropriate. Detailed statistical methods for metabolomics data analysis regarding chemical signal selection for sample classification are described in the Appendix (online only). Baseline clinical characteristics between decedents and survivors with PAD were compared using t-tests for continuous variables and χ2 tests for categorical variables.
Self-Organizing Map
We used the Self-Organizing Map (SOM) to create an ordered representation of multi-dimensional metabolomic data to reveal complex correlation structures among samples and metabolite patterns. An algorithm similar to Makinen et al23 was used to perform SOM analyses. Briefly, we produced a map with 5 by 5 hexagonal units using a Gaussian neighborhood function (2.4 samples per unit on average). The SOM algorithm resulted in spectral models of neighboring units that are more similar to each other than those from the remote sites of the SOM. After the map was constructed, each of 61 spectra was allocated to its best-matching spectral model (eg, a hexagonal unit) on the SOM. Samples mapped to the same unit represent a cluster of closely related metabolomic profiles. The interpretation of the SOM is provided in the Appendix (online only). To observe the differences between spectral models, the metabolic spectrum of each hexagonal unit was represented according to the average intensity of the key substructures of lipid or small molecules in the spectra mapped to the certain unit within one unit radius.
Results
Characteristics of PAD participants
The baseline clinical characteristics of the participants are shown in the Table. There were no significant differences in the majority of the clinical variables between survivors and decedents. However, the prevalence of several comorbidities (eg, angina, stroke, myocardial infarction, and pulmonary disease) was higher among the decedents, and there is a significant difference between the decedents and survivors (74% vs 33%; P = .03) in active atherosclerotic vascular diseases (ie, a combination of stroke, angina, and myocardial infarction). Additionally, the number of stair flights walked was significantly higher in the survivor group (6.2 vs 17.7; P = .03). For the 20 decedents, seven died of coronary heart disease or CVD, six died of cancer, five died of non-coronary heart disease/CVD or noncancer causes, and two died of an unknown cause.
Table.
Baseline clinical characteristics of peripheral arterial disease (PAD) participants, according to whether they were decedents vs survivors
| Variables | Decedent (n = 120) | Survivor (n = 21) | P valuea |
|---|---|---|---|
| Age, years | 75.9 ± 7.8 | 73.9 ± 8.0 | .42 |
| Male, % | 80 (16/20) | 81 (17/21) | .99 |
| Ankle-brachial index | 0.74 ± 0.24 | 0.73 ± 0.20 | .94 |
| Body mass index, kg/m2 | 26.5 ± 5.3 | 25.3 ± 3.5 | .38 |
| Total cholesterol, mg/dL | 168.8 ± 38.1 | 173.0 ± 22.9 | .70 |
| High-density lipoproteins, mg/dL | 41.1 ± 11.0 | 43.2 ± 17.6 | .67 |
| CRP,b mg/L (range) | 6.11 ± 6.75 (0.2–23.9) | 5.32 ± 7.98 (0.2–33.4) | .75 |
| D-dimer,b μg/L (range) | 0.80 ± 0.47 (0.23–1.71) | 0.77 ± 0.45 (0.32–1.92) | .81 |
| Current smoker, % | 30 (6/20) | 14 (3/21) | .64 |
| Hypertension, % | 95 (19/20) | 75 (15/20) | .18 |
| Statin use, % | 40 (8/20) | 45 (9/20) | .99 |
| Diabetes, % | 55 (1½0) | 41 (7/17) | .61 |
| Stroke, % | 17 (3/18) | 0 (0/19) | .21 |
| Angina, % | 37 (7/19) | 19 (4/21) | .37 |
| Cancer, % | 26 (5/19) | 29 (6/21) | .99 |
| Congestive heart failure, % | 16 (3/19) | 0 (0/21) | .20 |
| Myocardial infarction, % | 42 (8/19) | 25 (5/20) | .43 |
| Pulmonary disease, % | 21 (4/19) | 10 (2/21) | .56 |
| Atherosclerotic vascular disease,c % | 74 (14/19) | 33 (7/21) | .03 |
| Classic claudication symptoms, % | 15 (3/20) | 24 (5/21) | .69 |
| Antiplatelet use, % | 50 (10/20) | 71 (15/21) | .27 |
| Physical activity | |||
| Number of blocks walked last week | 28.3 ± 38.8 | 33.6 ± 46.7 | .70 |
| Number of stair flights climbed last week | 6.2 ± 8.1 | 17.7 ± 19.1 | .03 |
CRP, C-reactive protein.
P values were obtained from two-sample t-tests or χ2 tests.
CRP and D-dimer data were only available for 16 survivors and 16 decedents from the WALCS and WALCS II cohorts. WALCS, Walking and Leg Circulation Study.
Comprising stroke, angina, or myocardial infarction.
Metabolic patterns of decedents vs survivors
We observed a large number of spectrum signals with distinct intensity levels between the decedent index, decedent preindex, and survivor blood samples from the diffusion-edited spectra. Specifically, 26% of signals for index decedent samples vs survivor samples, 23% of signals for preindex decedent samples vs survivor samples, and 12% of signals for index decedent samples vs preindex decedent samples were significantly different at P values <.05. In contrast, no distinct CPMG 1H NMR metabolomic patterns were observed between these three groups (data not shown). Thus, we focused on the diffusion-edited spectra data for the remaining analyses.
The SOM analysis used 222 signals (false discovery rate <10%) of the diffusion-edited spectral data from the comparison between the decedent preindex samples and the survivor samples. As shown in Fig 1, most survivor samples shared metabolomic patterns on the upper-right hexagonal units of the SOM while most decedent pre-index and decedent index samples had similar metabolomic patterns on the left and lower hexagonal units. For example, most survivor samples (86%; Fig 2) had metabolomic patterns characterizing metabolites from signals of the following chemical groups: –CH3 (0.7 ppm), (–CH2–)n (1.3 ppm), βCH2 (1.45 ppm), CH–CH2CH2– (1.95 ppm), αCH2 (2.2 ppm), and CH–CH2–CH (2.82 ppm) of fatty acyl chain protons of various lipid types as part of lipoproteins as well as –N(CH3)3 (3.26 ppm) and NCH2 (3.6 ppm) from the choline head group protons of phospholipids. The intensity levels of these chemical signals are significantly different between survivors and decedents (Fig 3). Relative to survivor samples, most of these chemical signals that have higher (or lower) mean intensity levels in the decedent preindex samples have even higher (or lower) mean intensity levels in the decedent index samples, although none of them reaches statistical significance (P < .05).
Fig 1.

Self-Organizing Maps (SOMs) of 61 proton nuclear magnetic resonance (1H NMR) spectra of plasma from peripheral arterial disease (PAD) patients with and without experiencing near-term death. Each hexagonal unit of the SOM represents a specific metabolic model spectrum and the color coding represents the percentage estimate of samples from a specific category (decedent index, decedent preindex, and survivor) mapped to the corresponding model spectrum. A-C represent the SOMs of 21 survivor samples, 20 decedent preindex samples, and 20 decedent samples, respectively.
Fig 2.
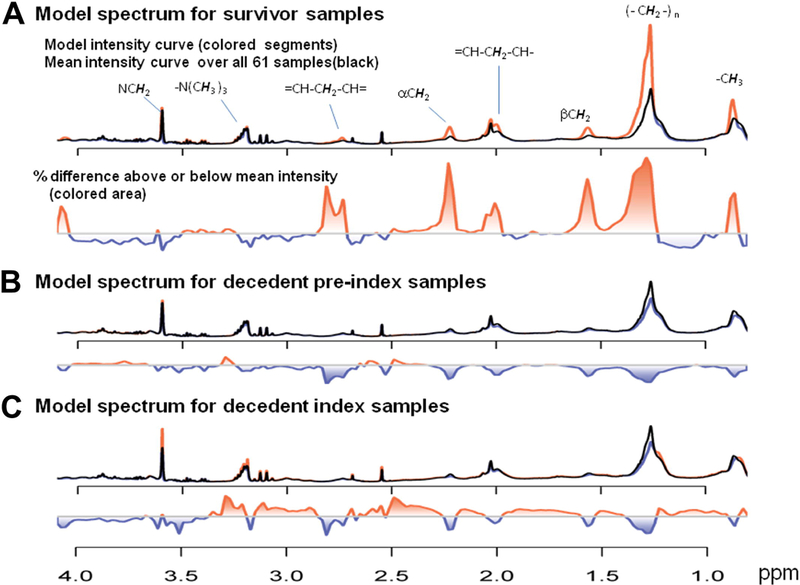
Three key metabolic model spectra of the Self-Organizing Map (SOM). A, The survivor model spectrum mapped to the grid (1,5) in Fig 3, A. B, The decedent preindex model spectrum mapped to the grid (3,1) in Fig 3, B. C, The decedent index model spectrum mapped to the grid (5,3) in Fig 3, C. The red curve indicates the current metabolic model; the black curve indicates the mean spectrum over all 61 spectra, thus serving as a constant reference. The proportional differences of each metabolic model spectrum and the mean spectrum are shown by the colored area below each model spectrum.
Fig 3.
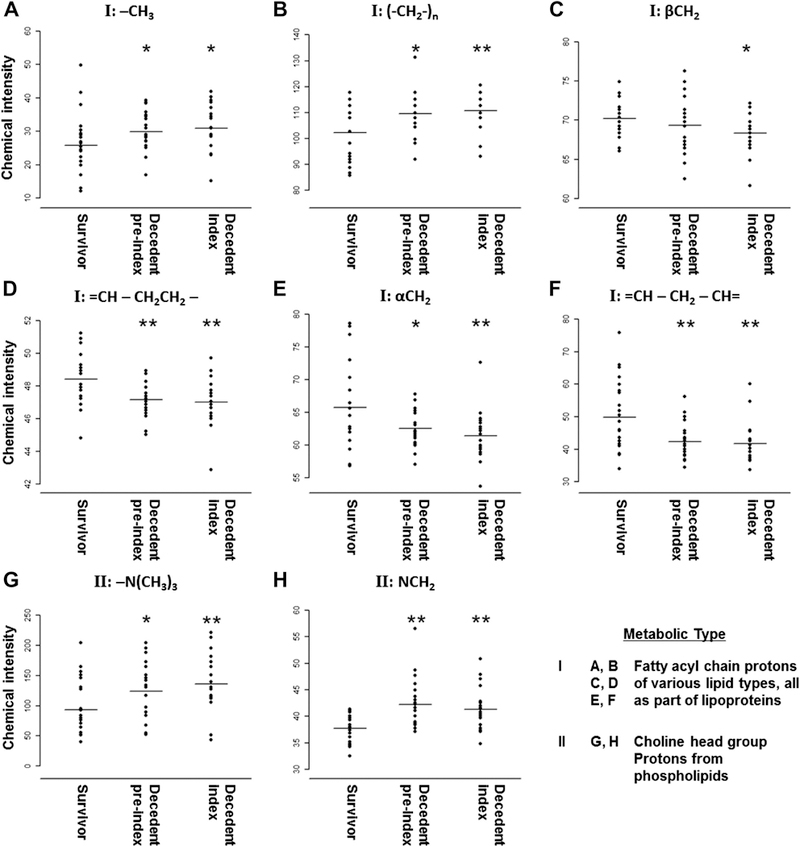
Concentration levels of substructures of lipid molecules from plasma samples of survivors and decedents. Quantified were: A, –CH3 signal at 0.7 parts per million (ppm). B, (–CH2–)n signal at 1.3 ppm. C, βCH2 signal at 1.45 ppm. D, CH–CH2CH2– signal at 1.95 ppm. E, αCH2 signal at 2.2 ppm. F, CH–CH2–CH signal at 2.82 ppm. G, –(NH3)3 signal at 3.26 ppm. H, NCH2 signal at 3.6 ppm. Statistically significant levels for comparison between survivor and preindex decedent and comparison between survivor and index decedent samples are indicated by red star: *P < .05; **P < .01. Intensity levels are not significantly different between preindex and index decedent samples for all eight chemical signals.
Sensitivity and specificity of metabolic profile for predicting near-term mortality
1H NMR metabolomic profiles provide a high predictive accuracy for the near-term all-cause deaths in these PAD participants. For the classification between decedent preindex samples and survivor samples, the overall classification performance was optimal at 78% (32 out of 41 participants), with a specificity of 71% (15 out of 21 survivors) and a sensitivity of 85% (17 out of 20 decedents) using the top 40 to 150 chemical signals of the diffusion-edited data. The predictive accuracy was similar when decedent index and survivor samples were compared. Specifically, the overall classification success was optimal at 83% (34 out of 41 participants) with a specificity of 86% (18 out of 21 survivors) and a sensitivity of 80% (16 out of 20 decedents) using the top 10 to 20 chemical signals. The four decedents who were misclassified included two who died of cancer, one who died of CVD, and one who died of an unknown cause. Logistic regression shows that the metabolomic signature remained significantly associated with near-term all-cause death (P < 10−4) after adjustment for active atherosclerotic diseases status.
Discussion
Our results demonstrate that the global metabolomic profiling of plasma differed between 20 PAD participants who died within 8 months after their blood samples were obtained and 21 PAD participants who survived for at least 30 months after their blood samples were obtained. Importantly, the metabolomics profiling of decedent preindex samples shows that these differences are already detectable more than 1 year before death. Since death involves a global breakdown of the homeostatic regulation of multiple metabolic pathways that fails to counter the overwhelming destructive metabolic reactions,24 the dramatically altered metabolic composition in decedents may reflect underlying physiological changes leading to death. These findings suggest that metabolomic profiling of plasma may be a useful tool to delineate the pathophysiological and biologic mechanisms immediately preceding death.
Our results show that the major chemical signals differentiating survivors from decedents were derived from the diffusion-edited profiling but were not from the CPMG profiling, suggesting lipid metabolism may have a stronger association with near-term death than other small molecules such as carbohydrates or amino acids. Although specific molecular mechanisms may exist in different diseases, the lipid signature identified in the current study may represent a common pathway associated with mortality in different diseases. Evidence is emerging to support this assertion. For example, large epidemiological studies have found an increased risk of developing coronary heart disease and a variety of cancers in individuals with diabetes,25, 26 suggesting the existence of common molecular mechanisms that are associated with the development of various chronic diseases. Additionally, our metabolomics finding is in line with a recent transcriptomic finding by Hirsch et al,27 who identified an unexpected molecular link between lipid metabolism and diverse human diseases including cancer, atherosclerosis, diabetes, and autoimmune diseases. Structural analysis of our metabolomic data showed that the most significant chemical signals arose from lipoproteins and phospholipids. These two lipid groups can be converted through arachidonic acid metabolism to prostaglandins, which play a central role in platelet aggregation, vascular smooth muscle tone, and inflammation.28 This suggests that these two lipid groups have a fundamental role in plaque homeostasis or pathophysiological changes prior to death.29 Experimental studies also demonstrated that inhibition of cyclooxygenase, the rate-limiting enzyme for conversion of arachidonic acid to important signal molecules (ie, prostaglandins), has a profound effect on cellular functions in many tissues, including tumor growth suppression and reduction of atherosclerosis.30, 31, 32, 33 Since the cyclooxygenase pathway plays a critical role in inflammation, future investigation is needed to determine whether these two groups of lipid species (ie, those with fatty acyl chain protons of lipoproteins and the choline head group protons of phospholipids) are involved in this pathway and induce further inflammation or changes in other biological processes related to the pathophysiology of all-cause near-term death. These two groups of lipid species may also be associated with physical activity. The survivor group of our study’s participants had better walking performance than the decedent group. A recent metabolomics analysis34 of the Framingham Heart Study participants showed that baseline plasma concentration of metabolites in the lipolysis pathway that were altered in response to exercise was associated with cardiovascular fitness. Furthermore, several cohort and twin studies show that long-term leisure-time physical activity affects serum concentrations of a wide spectrum of lipoprotein subclasses, independent of body mass index and age.35 Therefore, future studies will be needed to examine whether our observation in the alteration of lipid profiling is linked to physical activity – thereby identifying an enhanced secondary prevention strategy for the high-risk patients with PAD. The intensity levels of the majority of these chemical signals show linear increases or decreases from survivor to decedent preindex to decedent index samples (Fig 3). Intensity levels are significantly different between survivors and decedent index samples and between survivor and decedent preindex samples (except for βCH2), but are not significantly different between decedent preindex and index samples. Since both decedent metabolomics profiles are near-term mortality measurements (18.4 vs 3.6 months), the relatively stable concentration of these metabolites over the 1 year or so before death among the decedents (but significantly different from survivors) suggests that these lipid signals are a robust metabolite signature to discriminate those at risk for imminent acute events including death. However, further prospective study is needed, including confirmation of these findings in a larger cohort of individuals with PAD. Identifying these specific metabolites using high-sensitive technologies such as mass spectrometry may help elucidate metabolic mechanisms of mortality and may lead to new therapies to improve longevity.
Some limitations of the current study should be acknowledged. First, the small sample size of our pilot study does not allow adjustment for other risk factors and clinical variables in our classification model. Similarly, our study has a low statistical power to evaluate the correlation between metabolites and known risk factors such as cholesterol levels, thereby limiting the clinical utility of the metabolomic profile in modifying the clinical course. Future studies with a larger sample size are needed to confirm the findings reported here. Second, our pilot study did not have all the clinical variables necessary to construct the 10-year Charlson comorbidity score.36 Therefore, we are not able to assess the incremental predictive value of our metabolomics profiling for near-term mortality in addition to the Charlson comorbidity score. Third, 1H NMR profiling is advantageous because it is capable of screening a large number of metabolites and revealing a global metabolomic pattern. However, the molecular species giving rise to the peaks are generally unknown, and this method tends to have a low sensitivity that can detect only highly abundant metabolites.37 Further studies using technology with high precision and accuracy such as liquid chromatography mass spectrometry may identify unambiguously specific lipid metabolites and other nonlipid metabolites that may complement current existing biomarkers for near-term mortality. Fourth, study participants were outpatients with PAD. Our findings may not be generalizable to individuals at risk for PAD or those with critical limb ischemia. Fifth, we did not have data on diabetes mellitus severity. However, we are unaware of evidence demonstrating that more severe diabetes mellitus is associated with higher mortality in PAD patients without critical limb ischemia.
In summary, our result, as a proof-of-principle, shows that a specific metabolite signature from the lipid species correlated well with near-term death. To our knowledge, this is the first study to examine the utility of metabolomics profiling for near-term mortality prediction in any patient population. Our results suggest that metabolomic profiling may be a useful prognostic tool to identify individuals at high risk for near-term mortality. However, confirmation of these findings in a larger cohort is needed. In addition, further in-depth investigation of these metabolites is required to understand how the alteration of these metabolites’ concentration influences the metabolic pathways that are activated during the days and weeks leading up to death.
Acknowledgments
This research was supported by the National Institutes of Health R01HL58099, R01HL64739, R01-HL073912, R01-HL083064, R01-HL071223, R01-HL073551 (MM) and Taiwan National Science Council, grants number 99–2627-B-002–018 and 99–2321-B-002–042 (JT).
Appendix (online version only).
Measurement of clinical variables
Ankle-brachial index measurement
A hand-held Doppler probe (Pocket-Dop II, Nicolet Vascular, Golden, Colo) was used to measure systolic pressures in the right brachial artery, right dorsalis pedis and posterior tibial arteries, left dorsalis pedis and posterior tibial arteries, and left brachial artery.
Total and high-density lipoprotein cholesterol levels
Total cholesterol levels were measured by using enzymatic reaction with peroxidase-phenol-4-aminophenazone indicator reaction. High-density lipoprotein cholesterol levels were measured by using a direct enzymatic colorimetric assay.
Cigarette smoking, body mass index, and medical history
We determined smoking status by patient report using a structured interview. Body mass index was calculated as weight in kilograms dividing by height in meters squared. Diabetes status was determined based on patient self-report in the SILC and the RRF clinical trials. In the WALCS II, III cohorts, diabetes was considered present if the participant was taking insulin or an oral hypoglycemic agent or if a patient report of diabetes mellitus was confirmed by medical record review or a primary care physician questionnaire. Hypertension was defined as either a patient report of physician-diagnosed hypertension or a physician report of a history of hypertension on the primary care physician questionnaire. The patient report of a physician diagnosis of each comorbid condition was used in the SILC and RRF clinical trials. In WALCS cohorts, we obtained patient reports of physician-diagnosed comorbidities, but the data were also adjudicated from medical record review and a primary care physician questionnaire.
D-dimer and CRP levels
D-dimer levels were measured using an Asserachrom D-Di it (Diagnostica Stago, Asniè res-sur-Seine, France) with an enzyme-linked immunosorbent assayprocedure. CRP levels were measured using an immunotechnique on the Behring BN II analyzer (Dade Behring, Wilmington, Del). CRP and D-dimer data were only available for 16 survivors and 16 decedents from the WALCS and WALCS II cohorts.
Physical activity
Patient-reported physical activity was measured with a questionnaire derived from the Harvard Alumni Activity Survey.1 Participants were asked, “During the last week, how many city blocks or their equivalent did you walk? Let 12 city blocks equal 1 mile.” and “In the last week, about how many flights of stairs did you climb up? A flight is 10 steps.”
Statistical methods for chemical signal selection and sample classification
To assess the probability of false positive findings, we used the positive False Discovery Rate method proposed by Storey.2 For predicting all-cause deaths, the leave-one-out cross validation procedure with support vector machine classifiers was used to evaluate the classification accuracy. For each binned variable, a c statistic to evaluate its discriminative power for near-term death was calculated. Binned variables with the largest c statistics were sequentially added to support vector machine classifiers.
Interpretation of SOM
After the locations of the metabolomic spectra on the SOM were obtained, color coding was used for each hexagonal unit to represent the percentage of a specific category of spectra within one unit radius of a certain hexagonal unit. For example, if two spectra of survivor samples are mapped to a certain hexagonal unit, and eight spectra of survivor samples are mapped to the nearby units, then there were 10 (2 + 8) spectra of survivor samples mapped to the certain unit within one unit radius. If a total of 16 experimental spectra were mapped to the certain unit within one unit radius, the color value of the certain hexagon unit for survivor category is 63% (10/16).
Footnotes
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Author conflict of interest: none.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statisticse2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25–146. [DOI] [PubMed] [Google Scholar]
- 2.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA 2008;300:197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heald CL, Fowkes FG, Murray GD, Price JF. Risk of mortality and cardiovascular disease associated with the ankle-brachial index: systematic review. Atherosclerosis 2006;189:61–9. [DOI] [PubMed] [Google Scholar]
- 4.Desai MY, Nasir K, Braunstein JB, Rumberger JA, Post WS, Budoff MJ, et al. Underlying risk factors incrementally add to the standard risk estimate in detecting subclinical atherosclerosis in low- and intermediate-risk middle-aged asymptomatic individuals. Am Heart J 2004;148:871–7. [DOI] [PubMed] [Google Scholar]
- 5.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA 2003;290:891–7. [DOI] [PubMed] [Google Scholar]
- 6.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA 2003;290:898–904. [DOI] [PubMed] [Google Scholar]
- 7.Magnus P, Beaglehole R. The real contribution of the major risk factors to the coronary epidemics: time to end the “only-50%” myth. Arch Intern Med 2001;161:2657–60. [DOI] [PubMed] [Google Scholar]
- 8.Rioufol G, Finet G, Ginon I, Andre-Fouet X, Rossi R, Vialle E, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation 2002;106: 804–8. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, et al. Thrombogenic factors and recurrent coronary events. Circulation 1999;99:2517–22. [DOI] [PubMed] [Google Scholar]
- 10.Libby P Current concepts of the pathogenesis of the acute coronary syndromes. Circulation 2001;104:365–72. [DOI] [PubMed] [Google Scholar]
- 11.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation 2002;105:1135–43. [DOI] [PubMed] [Google Scholar]
- 12.Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: moving from information to knowledge. Diabetes 2009;58:2429–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bathen TF, Sitter B, Sjobakk TE, Tessem MB, Gribbestad IS. Magnetic resonance metabolomics of intact tissue: a biotechnological tool in cancer diagnostics and treatment evaluation. Cancer Res 2010;70:6692–6. [DOI] [PubMed] [Google Scholar]
- 14.Barba I, de Leon G, Martin E, Cuevas A, Aguade S, Candell-Riera J, et al. Nuclear magnetic resonance-based metabolomics predicts exercise-induced ischemia in patients with suspected coronary artery disease. Magn Reson Med 2008;60:27–32. [DOI] [PubMed] [Google Scholar]
- 15.McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301:165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDermott MM, Greenland P, Liu K, Guralnik JM, Celic L, Criqui MH, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study. Ann Intern Med 2002;136:873–83. [DOI] [PubMed] [Google Scholar]
- 17.McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001;286: 1599–606. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Mazor KM, Reed G, Pagoto S, Graff R, Merriam P, et al. Attitudes and behavior of peripheral arterial disease patients toward influencing their physician’s prescription of cholesterol-lowering medication. Vasc Med 2010;15:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDermott MM, Ferrucci L, Guralnik J, Tian L, Liu K, Hoff F, et al. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation 2009;120:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDermott MM, Liu K, Carr J, Criqui MH, Tian L, Li D, et al. Superficial femoral artery plaque, the ankle brachial index, and leg symptoms in peripheral arterial disease: the Walking and Leg Circulation Study (WALCS) III. Circ Cardiovasc Imaging 2011;4: 246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckonert O, Keun HC, Ebbels TM, Bundy J, Holmes E, Lindon JC, et al. Metabolic profiling, metabolomic and metabonomic procedures for NMR spectroscopy of urine, plasma, serum and tissue extracts. Nat Protoc 2007;2:2692–703. [DOI] [PubMed] [Google Scholar]
- 22.Xi Y, Rocke DM. Baseline correction for NMR spectroscopic metabolomics data analysis. BMC Bioinformatics 2008;9:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makinen VP, Soininen P, Forsblom C, Parkkonen M, Ingman P, Kaski K, et al. 1H NMR metabonomics approach to the disease continuum of diabetic complications and premature death. Mol Syst Biol 2008;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marieb EN, Hoehn K. Human Anatomy & Physiology. 7th ed San Francisco, CA: Pearson Benjamin Cummings; 2007. [Google Scholar]
- 25.Adami HO, McLaughlin J, Ekbom A, Berne C, Silverman D, Hacker D, et al. Cancer risk in patients with diabetes mellitus. Cancer Causes Control 1991;2:307–14. [DOI] [PubMed] [Google Scholar]
- 26.Coughlin SS, Calle EE, Teras LR, Petrelli J, Thun MJ. Diabetes mellitus as a predictor of cancer mortality in a large cohort of US adults. Am J Epidemiol 2004;159:1160–7. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch HA, Iliopoulos D, Joshi A, Zhang Y, Jaeger SA, Bulyk M, et al. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 2010;17:348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipollone F, Cicolini G, Bucci M. Cyclooxygenase and prostaglandin synthases in atherosclerosis: recent insights and future perspectives. Pharmacol Ther 2008;118:161–80. [DOI] [PubMed] [Google Scholar]
- 29.Santovito D, Mezzetti A, Cipollone F. Cyclooxygenase and prostaglandin synthases: roles in plaque stability and instability in humans. Curr Opin Lipidol 2009;20:402–8. [DOI] [PubMed] [Google Scholar]
- 30.Earnest DL, Hixson LJ, Alberts DS. Piroxicam and other cyclooxygenase inhibitors: potential for cancer chemoprevention. J Cell Biochem Suppl 1992;16I:156–66. [DOI] [PubMed] [Google Scholar]
- 31.Scioscia KA, Snyderman CH, Rueger R, Reddy J, D’Amico F, Comsa S, et al. Role of arachidonic acid metabolites in tumor growth inhibition by nonsteroidal antiinflammatory drugs. Am J Otolaryngol 1997;18:1–8. [DOI] [PubMed] [Google Scholar]
- 32.Lin DW, Nelson PS. The role of cyclooxygenase-2 inhibition for the prevention and treatment of prostate carcinoma. Clin Prostate Cancer 2003;2:119–26. [DOI] [PubMed] [Google Scholar]
- 33.Burleigh ME, Babaev VR, Oates JA, Harris RC, Gautam S, Riendeau D, et al. Cyclooxygenase-2 promotes early atherosclerotic lesion formation in LDL receptor-deficient mice. Circulation 2002;105:1816–23. [DOI] [PubMed] [Google Scholar]
- 34.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Sci Transl Med 2010;2:33ra37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kujala UM, Makinen VP, Heinonen I, Soininen P, Kangas AJ, Leskinen TH, et al. Long-term leisure-time physical activity and serum metabolome. Circulation 2013;127:340–8. [DOI] [PubMed] [Google Scholar]
- 36.Froehner M, Koch R, Litz RJ, Oehlschlaeger S, Twelker L, Hakenberg OW, et al. Detailed analysis of Charlson comorbidity score as predictor of mortality after radical prostatectomy. Urology 2008;72: 1252–7. [DOI] [PubMed] [Google Scholar]
- 37.Mayr M Metabolomics: ready for the prime time? Circ Cardiovasc Genet 2008;1:58–65. [DOI] [PubMed] [Google Scholar]
Appendix References
- 1.Lee IM, Paffenbarger RS Jr, Hsieh CC. Time trends in physical activity among college alumni, 1962–1988. Am J Epidemiol 1992;135: 915–25. [DOI] [PubMed] [Google Scholar]
- 2.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A 2003;100:9440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


