Abstract
Malignant peripheral nerve sheath tumors (MPNSTs) are highly aggressive soft tissue sarcomas that rarely occur in the general population but have a lifetime incidence of 8% to 13% in those with neurofibromatosis type 1 (NF1). Complete surgical resection is the standard treatment for MPNSTs. Unresectable MPNSTs carry a poor prognosis, and survival appears to be worse in NF1-associated tumors than in sporadic tumors. The response rate of MPNSTs to standard chemotherapeutic agents used to treat pediatric and adult soft tissue sarcomas is unknown and is currently undergoing evaluation in a multi-institutional clinical trial. With an increasing understanding of the molecular pathogenesis of MPNSTs, clinical trials with targeted agents have become available and have established that histology-specific trials in this rare malignancy are feasible. This knowledge, coupled with the availability of preclinical MPNST models, likely will accelerate the development of effective treatments for this malignancy.
Introduction
Malignant peripheral nerve sheath tumors (MPNSTs), also called neurogenic sarcomas, malignant schwannomas, and neurofibrosarcomas, are soft tissue sarcomas that arise from a peripheral nerve or show nerve sheath differentiation and are associated with a high risk of local recurrence and hematogenous metastasis [1]. They account for 10% of all soft tissue sarcomas, and half of these malignancies arise in individuals with neurofibromatosis type 1 (NF1). Early diagnosis of MPNSTs is crucial, because only complete surgical resection has been shown to be curative. For NF1-associated MPNSTs, younger age at diagnosis and worse prognosis have been described [1–3]. This review describes the genetics and clinical manifestations of NF1 and the epidemiology, clinical presentation, diagnosis, and standard treatment of MPNSTs. Advances in the understanding of the molecular pathogenesis of MPNSTs are described, and the resulting histology-specific trials with targeted agents for MPNSTs are discussed.
Epidemiology and Diagnosis of MPNSTs
MPNSTs account for 10% of all soft tissue sarcomas, and half of these malignancies arise in individuals with NF1, with a lifetime risk in NF1 of 8% to 13% [3]. Among individuals with NF1, those with a microdeletion of the NF1 locus may have a higher incidence of MPNSTs [4]. The diagnosis of an MPNST should result in detailed clinical evaluation for NF1, unless this diagnosis is already established.
NF1, previously referred to as von Recklinghausen disease, is a relatively common (1:2500–1:3000) autosomal dominant, progressive tumor predisposition syndrome [5•,6,7]. It is caused by a mutation in the NF1 tumor suppressor gene on chromosome 17q11.2, which comprises 60 exons spanning 350-kb genomic DNA [5•,6]. The gene product neurofibromin (2818 amino acids) contains a domain with significant homology to ras GTPase-activating proteins and thus regulates Ras activity. Neurofibromin accelerates Ras–guanosine triphosphate hydrolysis and thus functions as a potent negative regulator of Ras. Lack of functional neurofibromin in NF1 therefore leads to dysregulated Ras and tumorigenesis [8]. NF1 has 100% penetrance but features variable expressivity. The diagnosis of NF1 is based on clinical criteria including café-au-lait macules; axillary and inguinal freckling; presence of NF1-related tumors such as dermal neurofibromas, the hallmark clinical finding in NF1; and a family history of NF1 and can typically be made by 6 years of age [9]. Many NF1 gene mutations but very few genotype–phenotype correlations have been described. Mutation analysis of the NF1 gene in a Clinical Laboratory Improvement Amendments–certified laboratory has recently become available and allows identification of 95% of mutations, with a wide spectrum of mutations [10]. However, knowledge of the specific NF1 mutation currently does not affect treatment decisions for individuals with NF1.
Importantly, individuals with NF1 have an increased risk of developing tumors of the central and peripheral nervous systems, including plexiform neurofibromas (25%–44%), dermal neurofibromas (> 99%), optic pathway gliomas (15%), brain tumors (2%–3%), MPNSTs (8%–13%), juvenile myelomonocytic leukemia (rarely), pheochromocytomas (2%), and rhabdomyosarcomas (1.5%–6%) [3,11,12].
Plexiform neurofibromas are benign nerve sheath tumors that grow along the length of nerves and involve multiple branches of a nerve [11]. These tumors are usually diagnosed early in life but may develop throughout life. Morbidity from plexiform neurofibromas includes substantial disfigurement, compression of vital structures, progressive neurologic deficit, and often unremitting pain. In addition, plexiform neurofibromas can undergo degeneration to MPNSTs, and most MPNSTs in NF1 develop in preexisting plexiform neurofibromas [1,13]. The only standard treatment for plexiform neurofibromas is surgery. However, given the location, infiltrative nature, high vascularity, and size of plexiform neurofibromas, complete surgical removal usually is not feasible, and up to 44% of tumors progress after the first surgery, most commonly in patients younger than 10 years with head and neck tumors [14]. There is no known effective medical treatment for patients with plexiform neurofibromas, but clinical trials with targeted agents have become available [15].
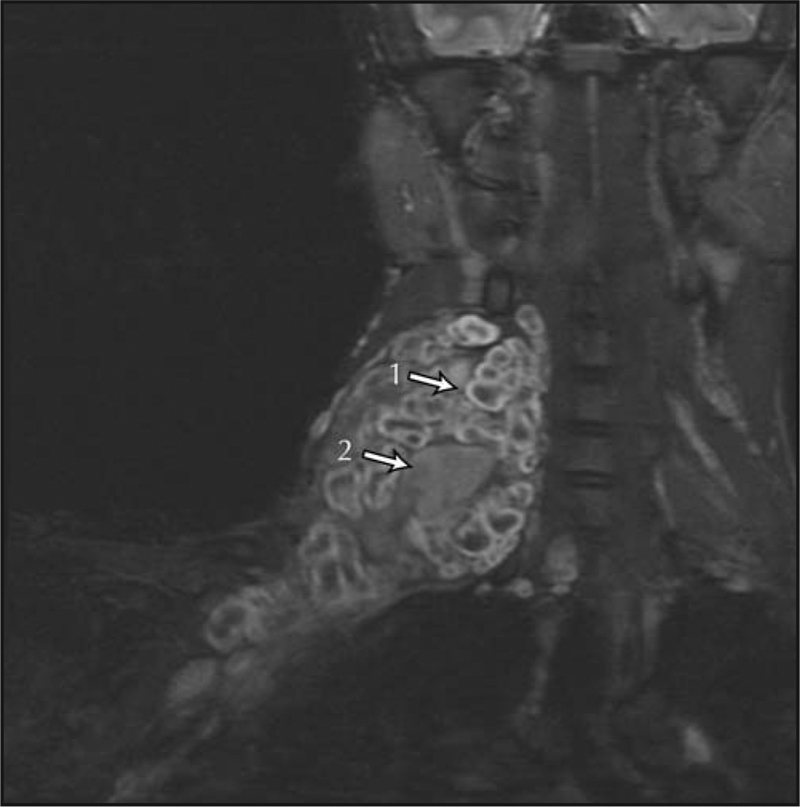
Early diagnosis of MPNSTs is crucial, because only complete surgical resection has been shown to be curative. However, the diagnosis of MPNSTs in NF1 is difficult to establish because clinical indicators of malignancy, such as pain, a growing mass, and neurologic compromise, may also be features of preexisting benign plexiform neurofibromas. The development of an MPNST within a plexiform neurofibroma may require several biopsies to successfully sample the malignant portion of the tumor (Fig. 1). Plexiform neurofibromas demonstrate high signal intensity on fat-suppressed MRI sequences and often show central areas of lower signal intensity (central dot sign) (Fig. 1). This may be lost in areas of malignant degeneration, and MPNSTs appear more heterogeneous. Heterogeneous enhancement on contrast-enhanced MRI may suggest malignancy [16]. 18Fluorodeoxyglucose positron emission tomography (FDG-PET) has been identified as a sensitive and specific tool to assist in the diagnosis of NF1-associated MPNSTs [17••]. FDG-PET also may aid in establishing the diagnosis of MPNSTs by determining the area of greatest FDG uptake for a biopsy [18]. In addition, whole-body MRI is being evaluated as a tool to monitor the neurofibroma burden in NF1 over time with the goal to identify malignant degeneration [19•,20]. Studies suggest that the presence of internal neurofibromas, high whole-body neurofibroma burden [20,21], and the presence of numerous subcutaneous neurofibromas [22] may be associated with a greater risk for MPNST development.
Figure 1.
Malignant peripheral nerve sheath tumor arising in a neck/brachial plexus plexiform neurofibroma, shown on coronal short T1 inversion recovery MRI. The “central dot sign” (1) is not present in the malignant portion of the tumor (2).
The epidemiology and outcome of NF1-associated MPNSTs may be different from those of sporadic MPNSTs in that several studies report a younger age at diagnosis and worse prognosis for NF1-associated MPNSTs (Table 1). Reasons for the potentially worse outcome of NF1-associated MPNSTs are unknown. Two studies indicate that NF1-associated MPNSTs may develop more frequently as central nonextremity lesions, which might affect outcome because central lesions are less amenable to surgery [23,24]. A recently published large retrospective review of 126 individuals with MPNSTs treated in Italian and German studies between 1975 and 1998 found that response to chemotherapy was lower in NF1-associated MPNSTs (3 of 17 individuals [17.6%] responded) than in sporadic MPNSTs (26 of 47 [55.3%] responded). Details regarding the chemotherapeutic agents used were not provided [2]; however, it is not clear that NF1-related MPNSTs have a worse prognosis when adjustments are made for the known prognostic features. For example, one recent study describes excellent survival in patients with MPNSTs, with no difference between sporadic and NF1-associated tumors [25]. Potential reasons for the favorable survival compared with other reports are not discussed in this report, but 70% of the MPNSTs described were extremity lesions, compared with 30% in a central location. Although NF1-associated MPNSTs appear to have a worse outcome than sporadic tumors, gene expression profiling of NF1-associated (n = 25) and sporadic (n = 17) MPNSTs did not identify a molecular signature that could reliably distinguish between the two types [26].
Table 1.
Epidemiology and outcome of sporadic versus neurofibromatosis type 1–associated malignant peripheral nerve sheath tumors
| Patients, n | Average age at diagnosis, y | 5-Year survival, % | ||||
|---|---|---|---|---|---|---|
| Study | NF1 | Sporadic | NF1 | Sporadic | NF1 | Sporadic |
| Sordillo et al., 1981 [24] | 65 | 100 | 32 | 48 | 23 | 47 |
| Ducatman et al., 1986 [23] | 62 | 58 | 29 | 40 | 16 | 53 |
| Hruban et al., 1990 [79] | 23 | 20 | 36 | 44 | 38 | 42 |
| deCou et al., 1995 [80] | 11 | 17 | 13 | 16 | 39 | |
| Wong et al., 1998 [39] | 32 | 102 | 37 | 36 | 57 | |
| Evans et al., 2002 [3] | 21 | 37 | 26 | 62 | 21 | 42 |
| Cashen et al., 2004 [25] | 18 | 62 | 31 | 37 | 85* | |
| Carli et al., 2005 [2] | 29 | 138 | — | — | 32 | 55 |
At 11 years, there was no difference between sporadic and NF1-associated malignant peripheral nerve sheath tumors. NF1—neurofibromatosis type 1.
Postirradiation MPNSTs, which have been described with an incidence of 5% to 11% [18], are associated with poor outcome. The potentially greater risk for individuals with NF1 to develop MPNSTs after radiation led to the recommendation that these patients avoid radiation if possible [27].
The diagnosis of MPNSTs can be established only by histopathologic evaluation. Two commonly used grading systems are the US National Cancer Institute system [28] and the Federation National des Centres de Lutte Contre le Cancer, developed by the French Federation of Cancer Centers Sarcoma Group [29]. Both systems evaluate the tumor’s histologic type or subtype and amount of necrosis but also consider cellularity, nuclear pleomorphism, and mitosis. Most MPNSTs are high-grade tumors, and only MPNSTs with perineurial cell differentiation are low grade [30]. MPNSTs are spindle cell neoplasms. MPNSTs with rhabdomyoblastic or other divergent differentiation, called malignant triton tumors [31], may have a more aggressive clinical course than other MPNSTs [32]. Immunohistochemical stains against several antigens, including S-100, Leu7, CD34, p16, p53, p27, MIB-1, and topoisomerase IIα (TOP2A) [13], are used to delineate MPNSTs. A high Ki67 proliferation index (> 25%) was reported to be associated with reduced survival in patients with MPNSTs [33].
Like other soft tissue sarcomas, MPNSTs are most commonly staged using the American Joint Committee on Cancer staging system [34], which includes tumor grade (1–4), tumor size (≤ 5 cm or ≥ 5 cm), location (superficial versus deep), and the presence of distant metastases at the time of diagnosis. Large tumor (> 5 cm), deep location, and the presence of distant metastases are associated with poor outcome. Based on a postoperative nomogram for the 12year sarcoma-specific death risk, the presence of an MPNST carries the highest risk of sarcoma-specific death compared with other histologic types of soft tissue sarcoma [35].
The most frequent sites of MPNST metastasis are lung, liver, brain, soft tissue, bone, regional lymph nodes, and retroperitoneum [23]. Thus the diagnostic evaluation should include MRI of the primary lesion and CT of the chest to evaluate for pulmonary parenchymal disease. In individuals with NF1 and large neurofibroma burden, FDG-PET scanning may be of value as a baseline study before initiating therapy.
Treatment of MPNSTs
Treatment of MPNSTs follows the treatment of other adult soft tissue sarcomas. Only complete surgical resection has been shown to be curative, and it remains the cornerstone of therapy [34,36]. The goal of surgery is to resect the MPNST with wide negative margins. However, the local recurrence rate of MPNSTs is high, ranging from 32% to 65% [18]. Radiotherapy is used in situations in which the sarcoma is not amenable to surgical resection, but when radiotherapy is used as primary treatment, large doses are needed and the control rate is only 30% to 60%. Clinical trials have demonstrated that external beam radiation or brachytherapy in addition to limb-sparing surgery improves local control in patients with soft tissue sarcomas [37,38]. Thus, adjuvant radiotherapy is recommended to improve local control for intermediatet- high-grade lesions greater than 5 cm after a marginal excision [5•,37,39].
The role of chemotherapy for MPNSTs is not yet defined. In adult soft tissue sarcomas, only doxorubicin, dacarbazine, and ifosfamide were consistently associated with response rates of 20% or more in patients with soft tissue sarcomas, and the combination of ifosfamide and doxorubicin produced response rates as high as 46% [40]. However, adjuvant doxorubicinbased chemotherapy did not demonstrate statistically significant improvement in overall survival [40]. In patients with advanced metastatic soft tissue sarcomas, doxorubicin and ifosfamide have the highest activity. The overall response rate for single-agent doxorubicin was 19% (range, 16%–27%), and for ifosfamide, response rates of 36% and 28% were reported [40,41].
Responses to chemotherapy have been described in children and adults with MPNSTs [42–44], but the response rate of MPNSTs to chemotherapy has not been determined prospectively. These tumors are thought to have intermediate chemosensitivity and to be less responsive than synovial sarcoma but more responsive than refractory diseases such as alveolar soft part sarcoma [45].
A recent retrospective review of individuals with MPNSTs described better response to ifosfamide-containing regimens and less response to chemotherapy for those with NF1-associated MPNSTs compared with those with sporadic MPNSTs [2].
The primary objective of an ongoing US Department of Defense–sponsored phase 2 clinical trial is to prospectively determine the rate of response of high-grade unresectable chemotherapy-naïve MPNSTs to standard chemotherapy agents (doxorubicin, ifosfamide, and etoposide) used to treat pediatric and adult sarcomas. Because the response to chemotherapy may differ between sporadic and NF1associated tumors, this trial stratifies for the presence of sporadic versus NF1-associated MPNSTs. Depending on its outcome, this trial may serve as a platform for future targeted therapies for MPNSTs.
Molecular Features of Tumorigenesis
With an increasing understanding of the molecular pathogenesis of MPNSTs, targeted treatment trials have become available. Surgical specimens and cell lines from patients with MPNSTs and NF1 demonstrate complete inactivation of NF1 and high levels of Ras activity [46–48]. Benign plexiform neurofibromas also demonstrate complete inactivation of NF1, with evidence suggesting that the Schwann cells represent the neoplastic element [49,50]; therefore, additional genetic alterations and abnormalities of other pathways likely contribute to progression to malignancy. Immunohistochemical analysis and molecular studies have implicated p53, p16, p27, and epidermal growth factor receptor (EGFR) as potential contributors to malignant transformation in peripheral nerve sheath tumors [1,51]. Further highlighting the unique pathogenesis of this tumor type, NF1 and homozygous p16 deletions appear to be relatively restricted to MPNSTs compared with other spindle cell sarcomas with overlapping morphologic features [51].
EGFR has been identified as an upstream activator of Ras in NF1. Although EGFR normally is not expressed by normal Schwann cells, its expression was demonstrated in benign neurofibromas, MPNST cell lines established from NF1 patients [52], and tumor cell lines derived from Nf1: p53 mice. Cell lines responded to epidermal growth factor with activation of the downstream signaling pathways. The growth of these cell lines could be blocked by an EGFR antagonist [53]. EGFR’s potential role in peripheral nerve tumor formation was subsequently demonstrated in transgenic murine Schwann cells expressing EGFR, which elicited features of neurofibromas. In addition, genetic reduction of EGFR in Nf1+/– p53+/– mice that developed sarcomas significantly improved survival [54]. In individuals with MPNSTs, overexpression of EGFR was associated with worse outcome [55].
Angiogenesis also has been implied in MPNST progression. Mutations in ras can upregulate vascular endothelial growth factor (VEGF) expression [56,57], and VEGF and basic fibroblast growth factor also are highly expressed in neurofibromas from patients with NF1 at the mRNA and protein levels [58]. Furthermore, VEGF expression and tumor vascularization are significantly increased in MPNSTs [59]. Use of a specific small molecule inhibitor of VEGF receptor 2 in a mouse explant model of neurogenic sarcomas showed a reduction in tumor growth due to decreased tumor angiogenesis with subsequent reduction in tumor cell proliferation and an increase in apoptosis [59].
Recent studies have demonstrated that NF1 regulates mammalian target of rapamycin (mTOR) pathway activation. NF1 loss in mouse embryonic fibroblasts and primary mouse astrocytes resulted in Ras- and phosphoinositide 3kinase/Akt–dependent mTOR pathway activation, which could be inhibited by sirolimus [60,61]. Increased proliferation associated with loss of neurofibromin expression in human MPNST cell lines also was reduced dramatically with sirolimus treatment [61]. Using a genetic mouse model of NF1-deficient MPNST development, sirolimus completely inhibited the growth of these tumors in vivo [62••]. Similarly, the mTOR inhibitor RAD001 decreased growth in MPNST cell lines and prevented growth of subcutaneously implanted MPNSTs in mice [63]. A negative feedback loop between mTOR and Akt was not found in an NF1 optic glioma and in MPNST preclinical models after treatment with sirolimus [62,64].
Microarray analysis of MPNSTs recently identified TOP2A as the most overexpressed gene in MPNSTs compared with benign neurofibromas, and TOP2A-expressing tumors were associated with poor cancer-specific survival and the presence of metastasis [65]. Topoisomerase II is a primary target for several anticancer agents, including doxorubicin and etoposide. Thus there is a strong rationale for evaluating etoposide (in addition to doxorubicin) in MPNSTs, and the ongoing chemotherapy trial for individuals with chemotherapy-naïve MPNSTs includes both agents.
Gene expression profiling, immunohistochemistry, and/or Western blot analysis of human MPNSTs showed expression/overexpression of EGFR [26], platelet-derived growth factor receptor (PDGFR)-α and PDGFR-β [66,67], c-Kit [67,68], and matrix metalloproteinase 13 (MMP13) [69]. To identify events contributing to malignant transformation, several studies were performed comparing human Schwann cells with MPNSTs [70••] and plexiform neurofibromas with MPNSTs [65,70••,71,72]. The studies determined that MPNSTs differentially expressed neural crest stem cell markers SOX9 and TWIST1 [70••]; genes involved in cell proliferation (MKI67, TOP2A, CCNE2), apoptosis (BIRC5/Survivin, TP73), and extracellular matrix remodeling (MMP13, MMP9); and genes involved in the Ras (RASF2, HMMR/RHAMM) and Hedgehog-Gli signaling pathways (DHH, Ptch2). In addition, high-resolution array comparative genomic hybridization was used to compare dermal neurofibromas, plexiform neurofibromas, and MPNSTs. This technique revealed amplification of some genes, including PDGFRA, MET, TP73, and HGF, and deletions in NF1, MMP13, p16INK4A, and TP53, demonstrating its potential in identifying MPNST markers [73]. Analysis for somatic mutations in MPNSTs from NF1 patients showed loss of heterozygosity across the TP53 region and TP53 mutation in 14 of 20 tumors [74].
Preclinical and Clinical Trials in MPNSTs
Until recently, few histology-specific clinical trials for MPNSTs were performed. A phase 2 trial of the EGFR inhibitor erlotinib was completed recently in patients with NF1-associated and sporadic MPNSTs [75]. Although erlotinib did not demonstrate activity in MPNSTs, this study demonstrated that timely completion of MPNSTspecific trials is feasible [75]. A phase 2 trial of sorafenib, an inhibitor of Raf kinase and other receptor tyrosine kinases, in adults with several soft tissue sarcoma strata, including an MPNST stratum, was completed recently and reported two minor responses in MPNSTs [76]. These trials required multiple participating sites with sarcoma expertise for completion. A recently established Department of Defense–sponsored NF Consortium has as its primary goal the development of an infrastructure for the timely conduct of NF clinical trials, and it may facilitate trials for individuals with MPNSTs. Likewise, the Children’s Tumor Foundation is funding a preclinical NF consortium to accelerate the identification of effective therapies for NF-associated tumors. The availability of xenograft [63] and transgenic NF1 MPNST mouse models [62••,77] will allow researchers to validate candidate drugs in these models, with the goal of moving the most promising agents to clinical trials [78•].
Conclusions
MPNSTs are highly aggressive soft tissue sarcomas associated with poor outcome, particularly in individuals with the genetic tumor predisposition syndrome NF1. Although complete surgical resection remains the cornerstone of curative treatment, recent advances in the understanding of the molecular pathogenesis of MPNSTs have led to the first targeted treatment trials for MPNSTs. This, coupled with an organized approach toward evaluation of targeted agents in preclinical models of MPNST, will likely accelerate the development of effective treatments for this malignancy.
Acknowledgment
This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research. The views expressed do not necessarily represent views of the National Institutes of Health or the US government.
Footnotes
Disclosure
No potential conflict of interest relevant to this article was reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ferner RE, Gutmann DH: International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res 2002, 62:1573–1577. [PubMed] [Google Scholar]
- 2.Carli M, Ferrari A, Mattke A, et al. : Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol 2005, 23:8422–8430. [DOI] [PubMed] [Google Scholar]
- 3.Evans DG, Baser ME, McGaughran J, et al. : Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 2002, 39:311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leppig KA, Kaplan P, Viskochil D, et al. : Familial neurofibromatosis 1 microdeletions: cosegregation with distinct facial phenotype and early onset of cutaneous neurofibromata. Am J Med Genet 1997, 73:197–204. [DOI] [PubMed] [Google Scholar]
- 5.•.Ferner RE: Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol 2007, 6:340–351. [DOI] [PubMed] [Google Scholar]; This is an excellent, up-to-date review of NF1 and NF2.
- 6.Friedman JM: Neurofibromatosis 1: clinical manifestations and diagnostic criteria. J Child Neurol 2002, 17:548–554; discussion 571–572, 646–651. [DOI] [PubMed] [Google Scholar]
- 7.Korf BR: Clinical features and pathobiology of neurofibromatosis 1. J Child Neurol 2002, 17:573–577; discussion 602–604, 646–651. [DOI] [PubMed] [Google Scholar]
- 8.Cichowski K, Jacks T: NF1 tumor suppressor gene function: narrowing the GAP. Cell 2001, 104:593–604. [DOI] [PubMed] [Google Scholar]
- 9.Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol 1988, 45:575–578. [PubMed] [Google Scholar]
- 10.Messiaen LM, Callens T, Mortier G, et al. : Exhaustive mutation analysis of the NF1 gene allows identification of 95% of mutations and reveals a high frequency of unusual splicing defects. Hum Mutat 2000, 15:541–555. [DOI] [PubMed] [Google Scholar]
- 11.Korf BR: Plexiform neurofibromas. Am J Med Genet 1999, 89:31–37. [DOI] [PubMed] [Google Scholar]
- 12.Korf BR: Malignancy in neurofibromatosis type 1. Oncologist 2000, 5:477–485. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Coffin CM, Perkins SL, et al. : Malignant peripheral nerve sheath tumor: a comparison of grade, immunophenotype, and cell cycle/growth activation marker expression in sporadic and neurofibromatosis 1-related lesions. Am J Surg Pathol 2003, 27:1337–1345. [DOI] [PubMed] [Google Scholar]
- 14.Needle MN, Cnaan A, Dattilo J, et al. : Prognostic signs in the surgical management of plexiform neurofibroma: the Children’s Hospital of Philadelphia experience, 1974–1994. J Pediatr 1997, 131:678–682. [DOI] [PubMed] [Google Scholar]
- 15.Packer R, Gutmann D, Rubenstein A, et al. : Plexiform neurofibromas in NF1: toward biologic-based therapy. Neurology 2002, 58:1461–1470. [DOI] [PubMed] [Google Scholar]
- 16.Mautner VF, Friedrich RE, von Deimling A, et al. : Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma. Neuroradiology 2003, 45:618–625. [DOI] [PubMed] [Google Scholar]
- 17.••.Ferner RE, Golding JF, Smith M, et al. : [18F]2-fluoro-2deoxy-D-glucose positron emission tomography (FDG PET) as a diagnostic tool for neurofibromatosis 1 (NF1) associated malignant peripheral nerve sheath tumours (MPNSTs): a long-term clinical study. Ann Oncol 2008, 19:390–394. [DOI] [PubMed] [Google Scholar]; This is the largest patient series published on the value of FDG-PET in assisting the diagnosis of MPNSTs in NF1. This study confirms the utility of FDG-PET in differentiating neurofibromas from MPNSTs.
- 18.Gupta G, Mammis A, Maniker A: Malignant peripheral nerve sheath tumors. Neurosurg Clin N Am 2008, 19:533–543, v. [DOI] [PubMed] [Google Scholar]
- 19.•.Cai W, Kassarjian A, Bredella MA, et al. : Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: determination on whole-body MR images. Radiology 2009, 250:665–673. [DOI] [PubMed] [Google Scholar]; The authors describe a novel method of volumetric MRI analysis of NF1and NF2-related tumors. This method may have applicability in prospective studies monitoring NF1and NF2-related tumors.
- 20.Mautner VF, Asuagbor FA, Dombi E, et al. : Assessment of benign tumor burden by whole-body MRI in patients with neurofibromatosis 1. Neuro Oncol 2008, 10:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker T, Wolkenstein P, Revuz J, et al. : Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology 2005, 65:205–211. [DOI] [PubMed] [Google Scholar]
- 22.Khosrotehrani K, Bastuji-Garin S, Riccardi VM, et al. : Subcutaneous neurofibromas are associated with mortality in neurofibromatosis 1: a cohort study of 703 patients. Am J Med Genet A 2005, 132A:49–53. [DOI] [PubMed] [Google Scholar]
- 23.Ducatman BS, Scheithauer BW, Piepgras DG, et al. : Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer 1986, 57:2006–2021. [DOI] [PubMed] [Google Scholar]
- 24.Sordillo PP, Helson L, Hajdu SI, et al. : Malignant schwannoma—clinical characteristics, survival, and response to therapy. Cancer 1981, 47:2503–2509. [DOI] [PubMed] [Google Scholar]
- 25.Cashen DV, Parisien RC, Raskin K, et al. : Survival data for patients with malignant schwannoma. Clin Orthop Relat Res 2004, 69–73. [DOI] [PubMed] [Google Scholar]
- 26.Watson MA, Perry A, Tihan T, et al. : Gene expression profiling reveals unique molecular subtypes of Neurofibromatosis Type I-associated and sporadic malignant peripheral nerve sheath tumors. Brain Pathol 2004, 14:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducatman BS, Scheithauer BW: Postirradiation neurofibrosarcoma. Cancer 1983, 51:1028–1033. [DOI] [PubMed] [Google Scholar]
- 28.Costa J, Wesley RA, Glatstein E, et al. : The grading of soft tissue sarcomas. Results of a clinicohistopathologic correlation in a series of 163 cases. Cancer 1984, 53:530–541. [DOI] [PubMed] [Google Scholar]
- 29.Trojani M, Contesso G, Coindre JM, et al. : Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int J Cancer 1984, 33:37–42. [DOI] [PubMed] [Google Scholar]
- 30.Kourea HP, Cordon-Cardo C, Dudas M, et al. : Expression of p27(kip) and other cell cycle regulators in malignant peripheral nerve sheath tumors and neurofibromas: the emerging role of p27(kip) in malignant transformation of neurofibromas. Am J Pathol 1999, 155:1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woodruff JM, Chernik NL, Smith MC, et al. : Peripheral nerve tumors with rhabdomyosarcomatous differentiation (malignant “Triton” tumors). Cancer 1973, 32:426–439. [DOI] [PubMed] [Google Scholar]
- 32.Brooks JS, Freeman M, Enterline HT: Malignant “Triton” tumors. Natural history and immunohistochemistry of nine new cases with literature review. Cancer 1985, 55:2543–2549. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Oda Y, Tamiya S, et al. : Malignant peripheral nerve sheath tumours: high Ki67 labelling index is the significant prognostic indicator. Histopathology 2001, 39:187–197. [DOI] [PubMed] [Google Scholar]
- 34.Scaife CL, Pisters PW: Combined-modality treatment of localized soft tissue sarcomas of the extremities. Surg Oncol Clin N Am 2003, 12:355–368. [DOI] [PubMed] [Google Scholar]
- 35.Kattan MW, Leung DH, Brennan MF: Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol 2002, 20:791–796. [DOI] [PubMed] [Google Scholar]
- 36.Abbas JS, Holyoke ED, Moore R, et al. : The surgical treatment and outcome of soft-tissue sarcoma. Arch Surg 1981, 116:765–769. [DOI] [PubMed] [Google Scholar]
- 37.Yang JC, Chang AE, Baker AR, et al. : Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 1998, 16:197–203. [DOI] [PubMed] [Google Scholar]
- 38.Pisters PW, Leung DH, Woodruff J, et al. : Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol 1996, 14:1679–1689. [DOI] [PubMed] [Google Scholar]
- 39.Wong WW, Hirose T, Scheithauer BW, et al. : Malignant peripheral nerve sheath tumor: analysis of treatment outcome. Int J Radiat Oncol Biol Phys 1998, 42:351–360. [DOI] [PubMed] [Google Scholar]
- 40.Verma S, Bramwell V: Dose-intensive chemotherapy in advanced adult soft tissue sarcoma. Expert Rev Anticancer Ther 2002, 2:201–215. [DOI] [PubMed] [Google Scholar]
- 41.Antman KH, Montella D, Rosenbaum C, et al. : Phase II trial of ifosfamide with mesna in previously treated metastatic sarcoma. Cancer Treat Rep 1985, 69:499–504. [PubMed] [Google Scholar]
- 42.Edmonson JH, Buckner JC, Long HJ, et al. : Phase II study of ifosfamide-etoposide-mesna in adults with advanced nonosseous sarcomas. J Natl Cancer Inst 1989, 81:863–866. [DOI] [PubMed] [Google Scholar]
- 43.Raney B, Schnaufer L, Ziegler M, et al. : Treatment of children with neurogenic sarcoma. Experience at the Children’s Hospital of Philadelphia, 1958–1984. Cancer 1987, 59:1–5. [DOI] [PubMed] [Google Scholar]
- 44.Valdes OS, Maurer HM: Combination therapy with vincristine sulfate (NSC-67574) and cyclophosphamide (NSC-26271) for generalized malignant schwannoma—a case report. Cancer Chemother Rep 1970, 54:65–68. [PubMed] [Google Scholar]
- 45.Santoro A, Tursz T, Mouridsen H, et al. : Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol 1995, 13:1537–1545. [DOI] [PubMed] [Google Scholar]
- 46.Basu TN, Gutmann DH, Fletcher JA, et al. : Aberrant regulation of ras proteins in malignant tumour cells from type 1 neurofibromatosis patients. Nature 1992, 356:713–715. [DOI] [PubMed] [Google Scholar]
- 47.DeClue JE, Papageorge AG, Fletcher JA, et al. : Abnormal regulation of mammalian p21ras contributes to malignant tumor growth in von Recklinghausen (type 1) neurofibromatosis. Cell 1992, 69:265–273. [DOI] [PubMed] [Google Scholar]
- 48.Guha A, Lau N, Huvar I, et al. : Ras-GTP levels are elevated in human NF1 peripheral nerve tumors. Oncogene 1996, 12:507–513. [PubMed] [Google Scholar]
- 49.Kluwe L, Friedrich RE, Mautner VF: Allelic loss of the NF1 gene in NF1-associated plexiform neurofibromas. Cancer Genet Cytogenet 1999, 113:65–69. [DOI] [PubMed] [Google Scholar]
- 50.Perry A, Roth KA, Banerjee R, et al. : NF1 deletions in S-100 protein-positive and negative cells of sporadic and neurofibromatosis 1 (NF1)-associated plexiform neurofibromas and malignant peripheral nerve sheath tumors. Am J Pathol 2001, 159:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry A, Kunz SN, Fuller CE, et al. : Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J Neuropathol Exp Neurol 2002, 61:702–709. [DOI] [PubMed] [Google Scholar]
- 52.DeClue JE, Heffelfinger S, Benvenuto G, et al. : Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest 2000, 105:1233–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li H, Velasco-Miguel S, Vass WC, et al. : Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Res 2002, 62:4507–4513. [PubMed] [Google Scholar]
- 54.Ling BC, Wu J, Miller SJ, et al. : Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell 2005, 7:65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keizman D, Issakov J, Meller I, et al. : Expression and significance of EGFR in malignant peripheral nerve sheath tumor. J Neurooncol 2009. March 28 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 56.Kranenburg O, Gebbink MF, Voest EE: Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta 2004, 1654:23–37. [DOI] [PubMed] [Google Scholar]
- 57.Rak J, Mitsuhashi Y, Bayko L, et al. : Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res 1995, 55:4575–4580. [PubMed] [Google Scholar]
- 58.Kawachi Y, Xu X, Ichikawa E, et al. : Expression of angiogenic factors in neurofibromas. Exp Dermatol 2003, 12:412–417. [DOI] [PubMed] [Google Scholar]
- 59.Angelov L, Salhia B, Roncari L, et al. : Inhibition of angiogenesis by blocking activation of the vascular endothelial growth factor receptor 2 leads to decreased growth of neurogenic sarcomas. Cancer Res 1999, 59:5536–5541. [PubMed] [Google Scholar]
- 60.Dasgupta B, Yi Y, Chen DY, et al. : Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1-associated human and mouse brain tumors. Cancer Res 2005, 65:2755–2760. [DOI] [PubMed] [Google Scholar]
- 61.Johannessen CM, Reczek EE, James MF, et al. : The NF1 tumor suppressor critically regulates TSC2 and mTOR. Proc Natl Acad Sci U S A 2005, 102:8573–8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.••.Johannessen CM, Johnson BW, Williams SM, et al. : TORC1 is essential for NF1-associated malignancies. Curr Biol 2008, 18:56–62. [DOI] [PubMed] [Google Scholar]; This article confirms the mTOR pathway as a target for MPNST therapy. Administration of sirolimus to mice with MPNSTs resulted in substantial improvement in survival compared with control animals.
- 63.Johansson G, Mahller YY, Collins MH, et al. : Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther 2008, 7:1237–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegedus B, Banerjee D, Yeh TH, et al. : Preclinical cancer therapy in a mouse model of neurofibromatosis-1 optic glioma. Cancer Res 2008, 68:1520–1528. [DOI] [PubMed] [Google Scholar]
- 65.Skotheim RI, Kallioniemi A, Bjerkhagen B, et al. : Topoisomerase-II alpha is upregulated in malignant peripheral nerve sheath tumors and associated with clinical outcome. J Clin Oncol 2003, 21:4586–4591. [DOI] [PubMed] [Google Scholar]
- 66.Badache A, De Vries GH: Neurofibrosarcoma-derived Schwann cells overexpress platelet-derived growth factor (PDGF) receptors and are induced to proliferate by PDGF BB. J Cell Physiol 1998, 177:334–342. [DOI] [PubMed] [Google Scholar]
- 67.Holtkamp N, Okuducu AF, Mucha J, et al. : Mutation and expression of PDGFRA and KIT in malignant peripheral nerve sheath tumors, and its implications for imatinib sensitivity. Carcinogenesis 2006, 27:664–671. [DOI] [PubMed] [Google Scholar]
- 68.Dang I, Nelson J, DeVries G: C-kit receptor expression in normal human Schwann cells and Schwann cell lines derived from neurofibromatosis type 1 tumors. J Neurosci Res 2005, 82:465–471. [DOI] [PubMed] [Google Scholar]
- 69.Holtkamp N, Atallah I, Okuducu AF, et al. : MMP-13 and p53 in the progression of malignant peripheral nerve sheath tumors. Neoplasia 2007, 9:671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.••.Miller SJ, Rangwala F, Williams J, et al. : Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res 2006, 66:2584–2591. [DOI] [PubMed] [Google Scholar]; The authors performed a molecular analysis that identified several potential targets for MPNST treatment.
- 71.Holtkamp N, Mautner VF, Friedrich RE, et al. : Differentially expressed genes in neurofibromatosis 1-associated neurofibromas and malignant peripheral nerve sheath tumors. Acta Neuropathol 2004, 107:159–168. [DOI] [PubMed] [Google Scholar]
- 72.Levy P, Vidaud D, Leroy K, et al. : Molecular profiling of malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1, based on large-scale real-time RT-PCR. Mol Cancer 2004, 3:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantripragada KK, Spurlock G, Kluwe L, et al. : High-resolution DNA copy number profiling of malignant peripheral nerve sheath tumors using targeted microarray-based comparative genomic hybridization. Clin Cancer Res 2008, 14:1015–1024. [DOI] [PubMed] [Google Scholar]
- 74.Upadhyaya M, Kluwe L, Spurlock G, et al. : Germline and somatic NF1 gene mutation spectrum in NF1-associated malignant peripheral nerve sheath tumors (MPNSTs). Hum Mutat 2008, 29:74–82. [DOI] [PubMed] [Google Scholar]
- 75.Albritton KH, Rankin C, Coffin CM: Phase II study of erlotinib in metastatic or unresectable malignant peripheral nerve sheath tumors (MPNST) [abstract]. J Clin Oncol 2006, 24(June 20 Suppl):9518. [Google Scholar]
- 76.Maki RG, Keohan ML, Undevia SD: Updated results of a phase II study of oral multi-kinase inhibitor sorafenib in sarcomas, CTEP study #7060 [abstract]. J Clin Oncol 2008, 24(May 20 Suppl):10531. [Google Scholar]
- 77.Cichowski K, Shih TS, Schmitt E, et al. : Mouse models of tumor development in neurofibromatosis type 1. Science 1999, 286:2172–2176. [DOI] [PubMed] [Google Scholar]
- 78.•.Gutmann DH, Hunter-Schaedle K, Shannon KM: Harnessing preclinical mouse models to inform human clinical cancer trials. J Clin Invest 2006, 116:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article describes the current status of mouse models of cancers and how these models can be used to assist in drug development for human cancers.
- 79.Hruban RH, Shiu MH, Senie RT, et al. : Malignant peripheral nerve sheath tumors of the buttock and lower extremity. A study of 43 cases. Cancer 1990, 66:1253–1265. [DOI] [PubMed] [Google Scholar]
- 80.deCou JM, Rao BN, Parham DM, et al. : Malignant peripheral nerve sheath tumors: the St. Jude Children’s Research Hospital experience. Ann Surg Oncol 1995, 2:524–529. [DOI] [PubMed] [Google Scholar]