Abstract
During malignant progression cancer cells undergo a series of changes, which promote their survival, invasiveness and metastatic process. One of them is a change in glucose metabolism. Unlike normal cells, which mostly rely on the tricarboxylic acid cycle (TCA), many cancer types rely on glycolysis. Pyruvate dehydrogenase complex (PDC) is the gatekeeper enzyme between these two pathways and is responsible for converting pyruvate to acetyl-CoA, which can then be processed further in the TCA cycle. Its activity is regulated by PDP (pyruvate dehydrogenase phosphatases) and PDHK (pyruvate dehydrogenase kinases). Pyruvate dehydrogenase kinase exists in 4 tissue specific isoforms (PDHK1–4), the activities of which are regulated by different factors, including hormones, hypoxia, and nutrients. PDHK1 and PDHK3 are active in the hypoxic tumor microenvironment and inhibit PDC, resulting in a decrease of mitochondrial function and activation of the glycolytic pathway. High PDHK1/3 expression is associated with worse prognosis in patients, which makes them a promising target for cancer therapy. However, a better understanding of PDC’s enzymatic regulation in vivo and of the mechanisms of PDHK-mediated malignant progression is necessary for the design of better PDHK inhibitors and the selection of patients most likely to benefit from such inhibitors.
Keywords: pyruvate dehydrogenase complex, pyruvate dehydrogenase kinase, hypoxia, glycolytic cancer metabolism
Tumor hypoxia and glucose metabolism
Hypoxia, defined as deficiency in the amount of oxygen reaching a tissue, is a well-known phenomenon found in solid tumors. As the tumor grows, the oxygen that reaches it is limited due to the poor vascularization. In order to overcome the limitations brought on by lack of oxygen, hypoxia inducible factors (HIFs) initiate the primary response of cells to the hypoxic environment1, 2. This response involves gene expression changes facilitating adaptation to hypoxia. Hypoxic metabolic reprogramming is under the control of HIF-1, which is a heterodimer consisting of subunits α and β. HIF-1α’s stability is regulated by oxygen levels, while the expression of the β subunit is constitutive.
In the presence of oxygen, HIF-1α is modified by hydroxylation on prolines 564 and 402. A family of prolyl hydroxylases (PHD; prolyl-4-hydroxylase domain enzymes), which use oxygen as substrate, catalyzes these reactions3. Hydroxylated HIF-1α is recognized by the tumor suppressor pVHL which acts as an E3 ubiquitin ligase and targets HIF-1α for degradation by the proteasome4. Hydroxylated HIF is very unstable in vitro, with a half-life of less than 5 minutes5. Under hypoxia, HIF-1α hydroxylation decreases, it escapes degradation and the protein accumulates.
In general, HIF-target genes mediate adaptation to moderate levels of hypoxia by increasing oxygen delivery (inducing angiogenesis and erythropoiesis), by decreasing oxygen consumption (decreasing mitochondrial function, increasing glucose uptake and glycolysis), and by escaping environments with limited oxygen availability (increasing cell motility and invasion). Under conditions of very severe oxygen deprivation (anoxia) these adaptive responses are unable to sustain survival and growth, and HIF-independent cell death ensues6.
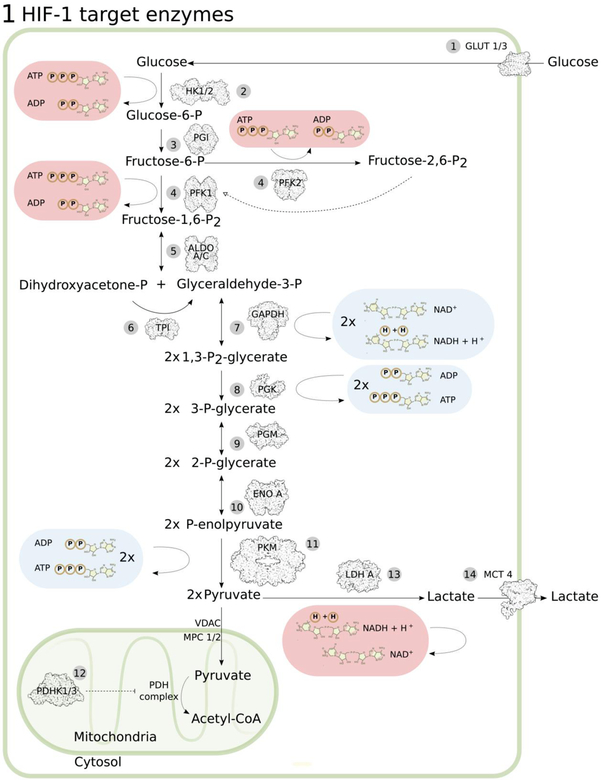
Altered glucose metabolism is a common cancer characteristic. Glucose serves as a main energy source, and under physiologic conditions it is oxidized in mitochondria to generate ATP by oxidative phosphorylation (OXPHOS). Under hypoxic conditions, OXPHOS decreases, and the glycolytic pathway is activated (Figure 1). However, in cancer cells, reliance on glycolysis can occur even in normoxia, a phenomenon known as aerobic glycolysis or the Warburg effect7. Due to the inefficiency of glycolysis in ATP production, there is a higher demand for glucose uptake in cancer cells8, and glucose transporters GLUT1 and GLUT3 are also some of the genes induced by HIF9, 10. Pyruvate is the end-product of cytoplasmic glycolysis and crosses the outer mitochondrial membrane through VDAC11. Two obligatory subunits of the elusive inner mitochondrial membrane pyruvate carrier (MPC1,2) have been recently discovered12, 13, and their expression is downregulated in many cancers14. Moreover, hypoxic tumor cells actively divert pyruvate from mitochondria by inducing pyruvate dehydrogenase kinase (PDHK) 1 and 315–17. The abbreviation PDK(s) is frequently used to describe Pyruvate Dehydrogenase Kinases, however, to avoid confusion with the 3-phosphoinositide-dependent protein kinase-1 (PDK-1/PDPK1), the newer PDHK nomenclature will be used throughout here. These HIF-responsive kinases phosphorylate pyruvate dehydrogenase (PDH) and inactivate the whole pyruvate dehydrogenase complex (PDC). When unphosphorylated, PDC converts pyruvate to acetyl-CoA in the mitochondrial matrix for the TCA cycle. The phosphorylation of serines 293, 300, or 232 (sites 1, 2, 3, respectively) on PDH by PDHKs inhibits this irreversible decarboxylation18 and makes pyruvate available for lactate dehydrogenase (LDH, another HIF-target19) to convert to lactate. This reaction regenerates NAD+ for subsequent glycolysis rounds. Why cancer cells modify their metabolism this way is believed to conserve oxygen20, support lipid and nucleotide synthesis and also redox balance necessary for sustained proliferation21, while maintaining sufficient, albeit lower, energy production. Thus energy-inefficient pathways are selected for in order to sustain the provision of metabolic intermediates and redox equivalents for mass biosynthesis.
Figure 1: Glycolytic HIF-1 target genes.
(1) GLUT1/3, glucose transporter 1 and 39, 10, 119, (2) HK1/2, hexokinase 1 and 2120, 121, (3) PGI, phosphoglucose isomerase121, 122, (4) PFK1/2, phosphofructokinase 1 and 2121, 123, (5) ALDO A/C, aldolase A and C119, 121, 124, (6) TPI, triosephosphate isomerase121, 125, (7) GAPDH, glyceraldehyde-3-phosphate dehydrogenase121, 126, (8) PGK, phosphoglycerate kinase119, 121, 124, (9) PGM, phosphoglycerate mutase121, 127, (10) ENO A, enolase A121, 128, (11) PKM, pyruvate kinase M121, 124, (12) PDHK1/3, pyruvate dehydrogenase kinase 1 and 315–17, (13) LDHA, lactate dehydrogenase A19, 121, 128, (14) MCT4, monocarboxylate transporter 4129. Pyruvate enters mitochondria through VDAC (voltage-dependent anion channel) in the outer and MPC 1/2 (mitochondrial pyruvate carrier 1/2) in the inner mitochondrial membrane, followed by conversion to Acetyl-CoA by the pyruvate dehydrogenase (PDH) complex in the mitochondrial matrix.
Mechanistic regulation of PDC
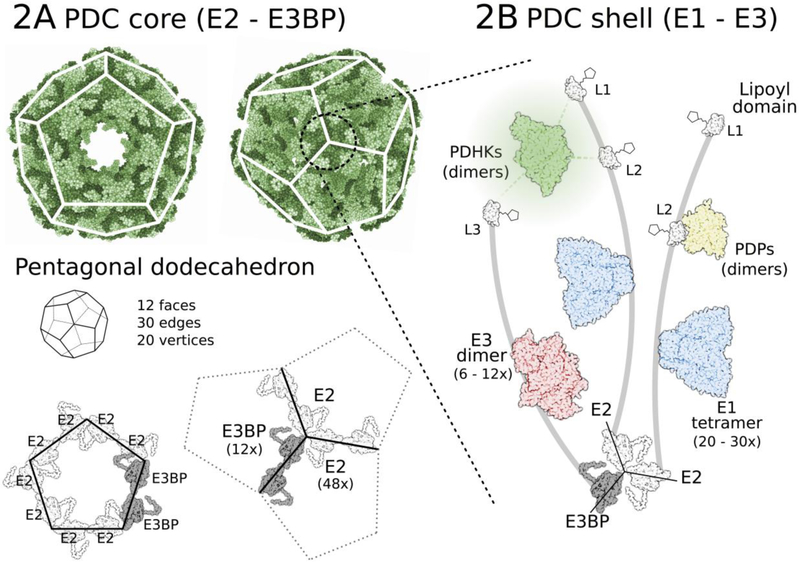
PDC is a 9.5 MDa multi-unit complex. The central core of PDC, forming a pentagonal dodecahedron consists of 48 copies of the E2 subunit and 12 copies of an E2 homolog, the E3 binding protein (E3BP) (Figure 2). The E1 and E3 components of PDC bind non-covalently to the core, as well as the 4 regulatory PDH kinases and 2 phosphatases, creating a flexible shell on top of the complex core. Mobile E2 and E3BP lipoyl domains shuffle substrates and products between active sites of the catalytic E1, E2, and E3 subunits22. Three serine residues of the E1α subunit (293, 300, and 232) are the targets of PDHKs (serine 232 can be phosphorylated only by PDHK123), and phosphorylation of any one of them inactivates the whole PDC complex in vitro, while their dephosphorylation by PDPs restores PDC activity24.
Figure 2: Pyruvate dehydrogenese complex structure (9.5 MDa).
(A) PDC core consisting of 48 E2 and 12 E3BP components is assembled into a pentagonal dodecahedron (3.5 MDa). (B) 20–30 E1 (α2β2 tetramer) components attached to the subunit binding domains of E2 and 6–12 E3 (α2 dimer) units on E3BP make up a plastic shell on the PDC core. Regulatory PDHKs (α2 dimers) and PDPs (αβ dimers) are also part of this shell, binding to lipoyl domains (mostly inner lipoyl L2 domains) on the flexible arm regions of E2 and E3BP. Such formation facilitates easy movement of individual enzymes and reaction intermediates over the complex.
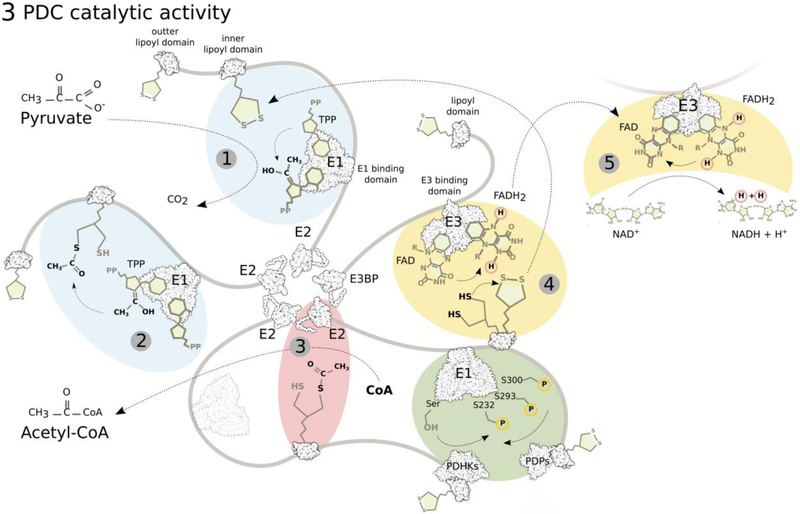
E1 (or PDH) catalyzes the rate-limiting reaction of oxidative decarboxylation of pyruvate and reductive acetylation of the E2 lipoyl moieties, using thiamine pyrophosphate (TPP) as co-enzyme (Figure 3). E2/dihydrolipoyl acetyltransferase (DLT) and E3/dihydrolipoyl dehydrogenase (DLDH) are the two other catalytic subunits of PDC. E3BP is a structural E3 binding subunit, and PDHKs and PDPs are the regulatory components of PDC. PDHKs are tightly associated with mostly the inner E2 lipoyl domain25. To complete PDC reactions, E2 transfers the lipoate-activated acyl residue to CoA, producing acetyl-CoA and reduced dihydrolipoamide. Finally, E3 regenerates oxidized lipoate, while reducing its prosthetic FAD group to FADH2, which is then back-oxidized in a reaction reducing NAD+ to NADH+H+. The structural organization of the complex offers an efficient way of moving reaction intermediates between subunits, executed by “swinging arms” of E2 and E3BP, and a “hand-over-hand” interchange of PDHKs on the surface of the PDC core allowing rapid phosphorylation of E126. A recent simulation of PDC, where layers of the complex were added successively, visualizes the complexity of this efficient system27.
Figure 3: PDC reaction sequence.
E1 (PDH, pyruvate dehydrogenase) first catalyzes thiamine pyrophosphate (TPP)-dependent irreversible decarboxylation of pyruvate (1) and reductive acetylation of lipoate on the lipoyl domains of E2 (and E3BP), the rate-limiting step (2). E2 (DLT, dihydrolipoyl transacetylase) then catalyzes the transfer of the acetyl group from dihydrolipoate to CoA producing Acetyl-CoA (3). E3 (DLDH, dihydrolipoyl dehydrogenase) oxidizes dihydrolipoate back to lipoate by reducing its FAD cofactor to FADH2 (4), which is oxidized back to FAD by NAD+ (5). Movement of the lipoyl domains between active sites of the individual enzymes is executed by the mobile arms of E2 and E3BP.
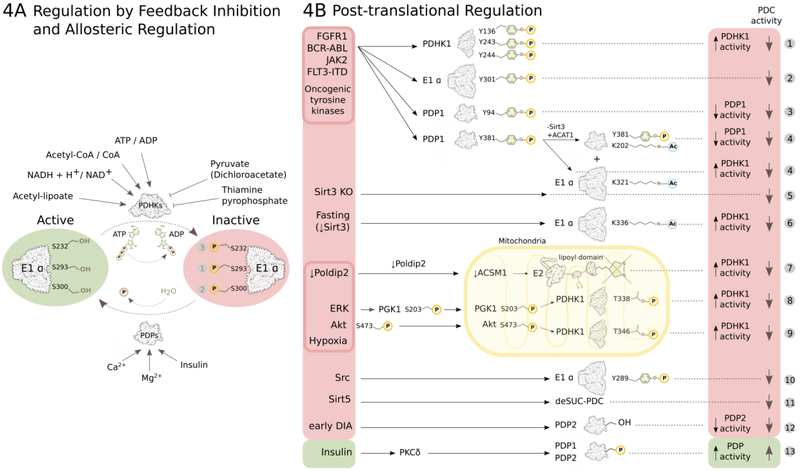
Since E1α phosphorylation-dephosphorylation controls PDC activity28, PDHKs and PDPs play crucial roles in the regulation of carbohydrate flux through the complex. The product-to-substrate ratios of the main PDC reaction greatly affect the activity of PDHKs (Figure 4A). They are activated by high NADH+H+/NAD+ and acetyl-CoA/CoA ratios and inhibited by pyruvate. Pyruvate is an allosteric PDHK inhibitor and binds to PDHKs directly29. NADH and acetyl-CoA on the other hand stimulate PDHKs by reducing and acetylating the E2 lipoyl moieties in reverse E2 and E3 reactions30. Acetyl-lipoamide then induces direct allosteric conformational changes in the lipoyl domain-bound PDHK structure that generate a more active PDHK state, most likely accelerating ADP dissociation. In contrast, full oxidation of the lipoyl groups significantly reduces PDHK activity31.
Figure 4: Regulation of PDC activity.
(A) PDC activity is regulated by reversible phosphorylation and dephosphorylation of its E1α subunit executed by PDHKs and PDPs. PDHKs perform inhibitory phosphorylation of three E1α serine residues (Ser 293 – site 1, Ser 300 – site 2, Ser 232 – site 3) and PDPs activate PDC by dephosphorylating these three sites. High PDC reaction endproduct/substrate ratios activate PDHKs by feedback inhibiton, while pyruvate or its synthetic analogue dichloroacetate inhibit PDHKs allostericaly (details in text). PDPs are activated by Ca2+ and Mg2+ ions, and insulin. (B) Post-translational regulation of PDC activity (details in text). (1)97, (2)100, (3)101, (4)102, (5)103, (6)104, (7)107, (8)98, (9)99, (10)105, (11)106, (12)130, (13)60.
An important feature of PDHKs is that they are active as dimers. The C-terminal tail of each monomer interacts with the lipoyl-binding pocket in the N-terminal domain of the other monomer (summarized in32). The binding to the E2 lipoyl domain not only co-localizes PDHK to E1 but also opens its active site due to a crossover configuration of its C-terminal tail. PDHK dimers interact with two lipoyl domains simultaneously, producing very high binding affinity to PDC and supporting the hand-over-hand migration over the complex. The PDHK active site is at the C-terminus and is folded similarly to the catalytic domains of the ATPase/kinase superfamily (such as histidine protein kinases), unlike other Ser/Thr-specific protein kinases33. PDHKs are thought to form homodimers but a recombinant PDHK1-PDHK2 catalytically active heterodimer has also been reported34.
The three inhibitory serine phosphorylation sites on the E1α subunit were identified 40 years ago35. Since E1 is an α2β2 tetramer with two active sites positioned at the interface of E1α and E1β, there are six serines in total whose phosphorylation could inhibit PDC. Interestingly, phosphorylation of any one site inactivates E1 in vitro24. The reason for the existence of three sites per subunit is probably flexibility in the modulation of PDC inhibition under different conditions. Site 1 (Ser293) is preferentially phosphorylated first, and sites 2 (Ser300) and 3 (Ser232) are sequentially phosphorylated, leading to slower rates of the complex32. However, the dynamics of these post-translational modifications in vivo are not known yet.
Under hypoxia all three regulatory E1α serine residues become phosphorylated, and mitochondrial function decreases. HIF1-dependent transcription of PDHK115, 16 and PDHK317 regulates PDC activity and mitochondrial oxygen consumption, however, the contribution of post-transcriptional and post-translational mechanisms to enzymatic control was not known36. With the development of phospho-serine-specific E1α antibodies37, it became possible to monitor the phosphorylation status of each of the three regulatory serines. We were able to confirm in vivo that phosphorylation of site 3 required PDHK1, as PDHK1 knockout cells completely lost the ability to phosphorylate Ser23238. This incapacity caused a significant decrease in pancreatic model tumor growth38. In vitro studies on purified proteins had shown that phosphorylation of any of the three regulatory serines inactivates PDH24. It is still unknown however, why hypoxia specifically regulates certain PDHK isoforms, and why phosphorylation of sites 1 and 2 is not sufficient for maximum tumor growth, at least in the models tested to date. Additionally, we showed that hypoxic PDH phosphorylation is not entirely dependent on HIF; HIF-1α knockout mouse embryonic fibroblasts were unable to upregulate PDHK1 protein, however, they did maintain some ability to phosphorylate Ser232 in hypoxia, albeit not to the extent of their HIF-expressing counterparts38. Hence hypoxia exerts a dual control over PDHK1, by increasing both the protein levels and its enzymatic activity. Another intriguing, but as of yet untested, possibility is that hypoxia regulates PDHK1 heterodimer formation34 as an additional mechanism of activity control.
The earliest recognition of PDH site 3 oxygen sensitivity came from the parasitic nematode Ascaris suum39. This species undergoes aerobic/anaerobic transition during development and has two PDH isoforms, specifically an anaerobic adult PDH-I isoform with two phosphorylation sites (serine 232 is changed to alanine) and an aerobic larval PDH-II with all three conserved phosphorylation sites, analogous to mammalian PDH. Anaerobic adult PDH-I lacking site 3 is a better substrate for PDHK but less sensitive to inactivation. This suggested that the expression of the less PDHK-sensitive PDH-I prevents its complete inactivation during transition to anaerobiosis. Similarly, the presence of Ser232 in mammalian PDH may make PDC regulation more flexible during fluctuations in oxygen and fine-tune pyruvate oxidation rates.
Most recently, chronic hypoxia was shown to reduce expression of PDC’s E1β subunit possibly through induction of the mitochondrial matrix LONP protease40. This mechanism maintains glycolytic metabolism even in reoxygenated areas of tumors, further supporting the Warburg effect. Additionally, prolyl hydroxylase PHD3 stabilizes PDC by physically interacting with the E1β subunit, while depleted PHD3 in hypoxia leads to destabilization and reduced PDC activity41.
PDC and clinical implications
The most studied isoform in cancer is PDHK1, due to its involvement in oncogenic transformation15, 16, 36. In addition to PDHK1/3 regulation by tumor hypoxia, other PDHK isoforms are reported to participate in cancer progression42–45. Metabolic control by PDHK extends beyond this hypoxic activation. Oncogene-induced senescence of primary melanocytes by oncogenic BRAFV600E expression requires increased PDC activity, which is mediated by PDHK1 downregulation and PDP2 upregulation46. Forced inhibition of mitochondrial pyruvate metabolism bypassed senescence and permitted tumorigenesis46. Conversely, in established melanomas with acquired resistance to the BRAFV600E inhibitor vemurafenib, combination treatment with PDHK inhibitor dichloroacetate (DCA) and vemurafenib produced more than additive cell killing in vitro47. Additionally, DCA sensitized glycolytic melanoma cell lines to the pro-oxidant elesclomol48.
A growing number of works points to PDH activity downregulation by PDHKs as important for tumor growth (Table 1). Conversely, it has been reported that, for certain cancer models, PDH activity per se is not essential for survival and growth. For example, E1α ablation in cell lines caused significant changes to central carbon metabolism as expected, however, in vitro growth was supported by reliance on extracellular lipids and glutamine-dependent fatty acid synthesis49. In a mouse model of hepatocellular damage and carcinogenesis, liver-specific E1α ablation did not impact tumor growth, despite the decrease in acetyl-CoA levels that inhibition of pyruvate decarboxylation caused50. In contrast to autochthonous liver cancer, liver metastases of breast cancer contain elevated phospho-E1α, and PDHK1 downregulation impaired the ability of mouse breast tumors to specifically colonize the liver but did not impact colonization of the lungs51. Thus, it is becoming evident that alternative pathways exist to coordinate the supply of electron donors to the respiratory chain with TCA metabolite production for biosynthesis.
Table 1:
Summary of published clinical studies evaluating impairments in the pyruvate dehydrogenase complex components and their impact on patient prognosis.
| PDC Impairment | Tumor Type | Patient Prognosis | Predicted PDC Activity |
|---|---|---|---|
| PDHK1 upregulation | Nasopharyngeal carcinoma131–133 | Poor prognosis |  |
| Oropharyngeal carcinoma38 | Poor prognosis |  |
|
| Neuroblastoma134 | Poor prognosis |  |
|
| Retinoblastoma135 |  |
||
| Head&Neck squamous cell carcinoma136 | Poor prognosis |  |
|
| Non-small cell lung carcinoma54, 55 | Poor prognosis |  |
|
| Breast cancer137 | Poor prognosis |  |
|
| Breast cancer liver metastases51 |  |
||
| Gastric carcinoma138 | Poor prognosis |  |
|
| Renal cell carcinoma139 | Lower tumor stage |  |
|
| PDHK1 activation | Glioblastoma98, 99 | Poor prognosis |  |
| PDHK1 downregulation | Renal cell carcinoma139 | Poor prognosis |  |
| PDHK2 upregulation | Gastric carcinoma140 | Poor prognosis |  |
| PDHK3 upregulation | Colon carcinoma43 | Poor prognosis |  |
| PDHK4 upregulation | Bladder carcinoma141 | High grade invasive |  |
| Increased phospho-E1α | Oropharyngeal carcinoma38 | Poor prognosis |  |
| Non-small cell lung carcinoma54 | Poor prognosis |  |
|
| Ovarian carcinoma142 | Poor prognosis |  |
|
| Decreased total E1α | Esophageal squamous cell carcinoma143 | Poor prognosis |  |
| Gastric carcinoma144 | Poor prognosis |  |
|
| Ovarian carcinoma145 | Poor prognosis |  |
|
| Hepatocellular carcinoma146 | Poor prognosis |  |
|
| Increased total E1α | Prostate carcinoma57, 147 | Poor prognosis |  |
| Downregulated E1β | Gastric cardia carcinoma148 | Poor prognosis |  |
| Downregulated E2/E3BP | Non-small cell lung carcinoma56 | Good postoperative outcome |  |
| PDP1 upregulation | Prostate carcinoma57 | Poor prognosis |  |
| Poor prognosis | Epidermal carcinoma149 | Poor prognosis |  |
Conversely, lung and prostate cancers are characterized by high PDC activity. Metabolic profiling of mouse K-ras driven lung tumors and human NSCLC patients showed significant pyruvate flux through PDC in vivo52, 53, and genetic loss of PDHA1 (E1α gene) in KrasG12D-derived cell lines inhibited subsequent tumor growth52. On the other hand, immunohistochemical detection of PDC components on patient biopsies associated their predicted low PDC activity with either poor54, 55 or good56 outcome, for reasons that are not clear yet. In prostate, E1α inactivation inhibited xenograft tumor growth by affecting lipid biosynthesis57. Intriguingly, both mitochondrial and nuclear PDC contributed to lipogenesis by supplying citrate for fatty acid synthesis and acetyl-CoA for epigenetic activation of lipid-biosynthesis genes, respectively57. The mechanism for the nuclear translocation of PDC is not entirely clear but it can be mediated by heat-shock protein 7058.
PDHKs are implicated in other medical conditions in addition to cancer. Therefore, deeper understanding of PDC regulation could find clinical applications in the treatment of cancer and other diseases associated with deregulated pyruvate metabolism. For instance, PDHK2 and PDHK4 are upregulated in diabetes and under fasting conditions, and insulin is a negative regulator of PDHK4 expression, resulting in impairment in glucose oxidation59. Hence, this isoform could be a promising target for therapy of type 2 diabetes. Additionally, insulin stimulates PDC activity through PDP phosphorylation by mitochondrially translocated PKCδ60 (Figure 4B). The highly ubiquitous relevance of PDC function in the organism translates into many medical conditions beyond the scope of this review. Further examples include but are not limited to: acute liver failure61, non-alcoholic steatohepatitis62, obesity63, cardiovascular diseases64–67, viral and bacterial infection68, 69, and even autism70.
PDC defects are the causal factors in pyruvate dehydrogenase complex deficiency, a disease classified as an inborn error of metabolism. The symptoms can range in clinical severity and include congenital lactic acidosis, poor coordination, intellectual disabilities, recurrent ataxia, and delay in development71. PDC inability to convert pyruvate into acetyl-CoA impairs mitochondrial ATP generation by OXPHOS, forcing the cell to rely on glycolysis for energy production. ATP shortage can cause severe damage to the nervous system and other tissues with high-energy demand. The failure to convert pyruvate to acetyl-CoA can result in lactate accumulation, causing lactic acidemia72, 73. In most cases, PDC deficiencies are caused by genetic defects in E1α and impact enzymatic activity and/or E1α protein production. The PDHA1 gene is located on the X chromosome, which accounts for the higher incidence of PDC deficiencies in men74. Mosaicism in the PDHA1 gene due to lyonization of the X chromosome can impeach the diagnosis of PDC deficiency in females75, and also in males76, 77. Defects in other PDC subunits are infrequent78–81. E3 deficient patients suffer additional complications because this PDC subunit is also involved in 2-oxoglutarate dehydrogenase (OGDH) and branched-chain α-ketoacid dehydrogenase (BCKDH) complexes82. Treatment for patients with PDC deficiency is mostly symptomatic. DCA, the common inhibitor of pyruvate dehydrogenase kinases has been used to restore PDC activity in these patients. However, DCA is not a potent and specific PDHK inhibitor83 and can have serious adverse effects84. Therefore, patients with PDC deficiency would benefit from new and more specific approaches to inhibiting PDHKs.
Metabolic regulation of immune function by PDHKs
Another microenvironmental component with important implications in cancer growth is the metabolic reprogramming in innate and adaptive immunity. Cells of the immune system encounter oxygen- and nutrient-deprived conditions in inflamed tissues and must therefore modify their metabolism to cope with the environmental changes. At the same time, metabolic reprogramming can be triggered by pathogens or other activating stimuli, supporting immune cell activation and function85, 86.
It is generally accepted that activated pro-inflammatory M1 macrophages and inflammatory T helper Th17 cells undergo a switch to glycolytic metabolism similar to cancer cells, and OXPHOS is a hallmark of anti-inflammatory M2 macrophages and anti-inflammatory regulatory T cells (Tregs)87. PDHK1 has been shown to be required for CD4+ Th17 cell function88. Silencing of PDHK1 or DCA treatment inhibited IL-17 production, and through decreased Th17 cell expansion and function, suppressed autoimmunity in vivo88.
The possible role of PDHKs in macrophage metabolic reprogramming and function under different polarization states is an active area of investigation. PDHK1 has been shown to be required for the induction of M1-specific markers in murine bone marrow derived macrophages89. Also, inhibition of PDHK function by DCA was shown to suppress hypoxia-induced migration οf murine peritoneal macrophages in vitro and in vivo90. In contrast to the above studies, prolyl hydroxylase PHD2 knockout macrophages, which cannot hydroxylate HIF-1α and target it for degradation, and thus express HIF-dependent genes like PDHK1 even under normoxia, showed reduced migratory capacity that could be reversed by DCA treatment91. Additionally, stable isotope metabolic flux analyses found glucose flux through PDH to be maintained in activated macrophages, providing carbons for the biosynthesis of the antimicrobial metabolite itaconate and lipids92. The reasons for these discrepancies are not clear yet. The source of cells (bone marrow derived vs elicited peritoneal vs RAW 264.7) and/or differences between genetic and pharmacologic inhibition of PDHKs may be partly responsible. Genetically engineered mouse models of PDHK ablation should help determine their roles in antimicrobial and antitumoral defenses in clinically relevant settings.
PDC regulation by the extracellular matrix
Loss of communication between cells and their extracellular microenvironment has significant ramifications for cell function and physiology, including metabolism. Extracellular matrix (ECM) is the non-cellular scaffolding within tissues and can vary in composition at different sites. During cancer progression, tumor cells must undergo ECM detachment to migrate and then attach again at distant sites. Interestingly, anchorage-independent growth can cause changes in PDC flux93, 94. During ECM-attached growth, ERK activity suppresses the expression of PDHK493. Loss of ECM attachment reduces ERK activity and results in increased PDHK4 levels and reduced PDC activity in normal mammary epithelial MCF-10A cells93. Mechanistically, ERK signaling was shown to control ring-finger protein 126 (RNF126) expression, which acted as an ubiquitin ligase for PDHK1,3,4 (at conserved lysine 258), resulting in their proteasomal degradation and increased PDC activity in MDA-MB-231 breast metastatic cancer cells94. RNF126 depletion profoundly affected cell growth in suspension but not attached growth, indicating its role in anoikis-resistance94. Anoikis is a form of apoptotic cell death caused by loss of ECM-attachment, typical for normal cells, while malignant cancer cells are usually resistant to this stress. ERK phosphorylation and RNF126 are lost under detached conditions in normal cells but are maintained in most cancer cells94, which suggests that oncogenic signaling may determine the balance between aerobic glycolysis and mitochondrial pyruvate oxidation during anchorage-independent growth.
Emerging mechanisms of post-translational control of PDC activity
In recent years, emphasis is being given to post-translational mechanisms of regulation of metabolic enzymes95, 96. PDHK1 is subject to post-translational phosphorylation on several different residues by different kinases, which can alter PDHK1 activity and consequently impact tumor growth (Figure 4B). Among them, the first report showed that PDHK1 could be phosphorylated on at least three different tyrosine residues (136, 243, and 244) by oncogenic tyrosine kinases, such as FGFR1, BCR-ABL, JAK2, and FLT3-ITD97. The authors suggested these Tyr modifications increased ATP binding to the active site of PDHK1, as well as its association with PDC. Nonetheless, Tyr243 phosphorylation of PDHK1 wasn’t altered under hypoxic conditions, so this post-translational modification most likely does not play a role in the induction of PDHK1 activity by hypoxia.
Two reports from 2016 investigated hypoxic PDHK1 activity, although unfortunately none of them measured phosphorylation of the PDHK1-specific target Ser232 of E1α. Li and colleagues proposed an elaborate mechanism, where hypoxia or oncogenic mutations activate ERK, which then phosphorylates the cytosolic glycolytic enzyme PGK1 (phosphoglycerate kinase 1, the first ATP-generating enzyme in the glycolytic pathway)98. Phosphorylated PGK1 subsequently translocates into mitochondria, where it assumes a role of protein kinase and phosphorylates PDHK1 on threonine 338, thereby enhancing its activity98. In addition, they showed that Thr338 phosphorylation promotes tumorigenesis and that high phospho-Thr338 correlates with poor prognosis in glioblastoma patients. On the other hand, Chae and colleagues identified a pool of activated phosphorylated Akt that translocates to the mitochondria during hypoxia, where it phosphorylates PDHK1 on threonine 346 in the ATP lid99. Similar to phospho-Thr338, Thr346 phosphorylation activates PDHK1 and is a negative prognostic factor in glioblastoma patients.
PDHK1 is not the only component modified post-translationally to deactivate the complex (Figure 4B). The same oncogenic tyrosine kinases that phosphorylate PDHK1 on Tyr243 have been shown to phosphorylate E1α at tyrosine 301100. This modification blocks pyruvate binding and hampers tumor growth100. E1α Tyr301 phosphorylation is not subject to hypoxic regulation, so its importance in hypoxic PDH suppression is likely minor. PDP1 is also phosphorylated by FGFR1 on two different residues. Tyrosine 94 phosphorylation inhibits PDP1 by blocking lipoic acid binding on E2101. The second FGFR1-phosphorylated residue on PDP1 is tyrosine 381102, which acts as a primer for a series of post-translational lysine acetylations ultimately leading to downregulated PDC activity. When oncogenic tyrosine kinases phosphorylate PDP1 Tyr381, Sirt3 deacetylase is dissociated and ACAT1 acetyltransferase recruited to PDC. This leads to acetylation of lysine 321 on E1α, which recruits PDHK1 and lysine 202 on PDP1 dissociating it from PDH102. Sirt3 knockout mice have decreased PDC activity103, which is associated with hyper-acetylated and hyper-phosphorylated E1α, and increased fatty acid and aminoacid metabolism in skeletal muscle104.
Src is yet another kinase post-translationally modifying the E1α subunit of PDC on tyrosine 289105. Suppression of Src decreases E1α Tyr289 phosphorylation and increases PDC activity independently of Ser-E1α status. Any potential role of Src-induced phospho-Tyr289 under hypoxia is not known.
A different type of post-translational modification affecting protein function is lysine-succinylation. Multiple sites on PDC have been identified as hypersuccinylated in Sirt5 knockout cells106. Mitochondrial sirtuin Sirt5 removes malonyl and succinyl moieties from target lysines. By desuccinylating PDC, Sirt5 suppresses its activity, however, the physiological significance of PDC (de)succinylation in tumor metabolism is not known.
Furthermore, hypoxia reduces E2 lipoylation and PDC activity via Poldip2 (polymerase-δ interacting protein 2) downregulation107. Poldip2 indirectly protects the lipoic-acid activating enzyme ACSM1 from degradation resulting in lipoylated E2 and active PDC107. Additionally, Poldip2 loss led to decreases in α-ketoglutarate levels and subsequent normoxic stabilization of HIF-1α, PDHK induction and PDC E1α phosphorylation, further contributing to low mitochondrial function.107.
Conclusions
The PDHKs’ involvement in metabolic disorders and cancer warrants the development of specific inhibitors with favorable pharmacokinetic/pharmacodynamic profiles. So far, little success has been made (a new generation of small molecule PDHK inhibitors is far from proving their clinical value108) and only limited early phase clinical data on the chemotherapeutic efficacy of DCA have been published. Most, but not all, trials reported acceptable toxicities but not very compelling improvements in response endpoints109–112. The highly complex regulation of PDC by the microenvironment requires its detailed characterization for the successful design of specific targeting strategies. Tumor hypoxia as an example, regulates PDC by multiple and selective mechanisms, but it is not clear what the adaptive advantage of each additional level of regulation is (Figure 5). It has been known for a long time that HIFs control the expression of metabolic enzymes. Technological advances, such as proteomics and metabolomics, have made it evident that hypoxia can control not only the amount of proteins encoded by HIF-inducible genes but also their activity. This may offer flexibility in responding to acute changes in oxygenation by rapidly modulating the activity of enzymes already present. The array of proteins whose activity is controlled by oxygen concentrations is still emerging, and no single mechanism is apparent from the reports. In addition to PDHK1, it includes lactate dehydrogenase A113, carbonic anhydrase IX114, sphingosine kinase 2115, or ornithine decarboxylase116. It is very likely that hypoxia exerts its acute effects on enzymatic activity not only through post-translational modifications but also through fluctuating metabolite levels. An important consideration for the clinical success of PDHK inhibitors will be the selection of tumors that rely on PDHK activity for growth. In preclinical settings, it should be possible to establish murine models of mutant PDHK and constitutively active or partially regulatable PDH forms to assess tumor physiology, hypoxic metabolism and response to PDHK inhibitors. In clinical trials, it may be necessary to monitor the phosphorylation state of all three E1α serines targeted by PDHKs in parallel with emerging technologies such as in vivo stable isotope metabolic flux analyses53, 117. Although such integrative approaches are not standard clinical practice at present, they are offering great insight into the fate of metabolic substrates, including pyruvate, in human tumors.
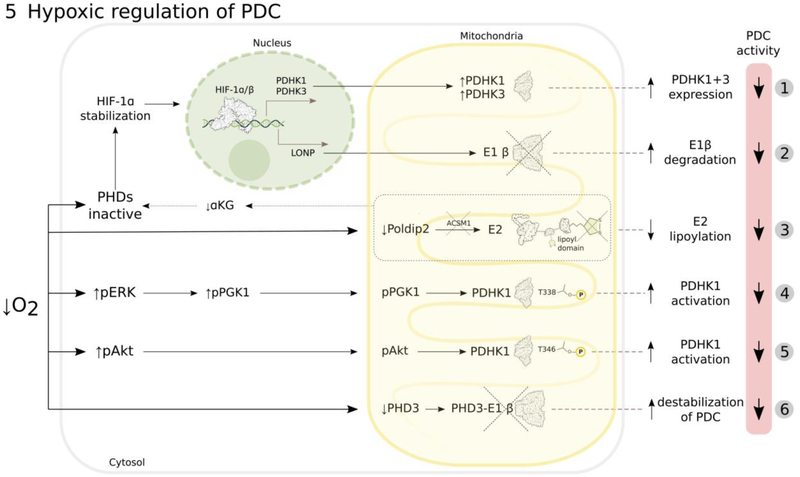
Figure 5: Mechanisms involved in regulation of PDC activity by tumor hypoxia.
Hypoxia decreases PDC activity (more details in text) by: HIF-1-mediated transcription of PDHK1 and PDHK3, which inhibit PDC by phosphorylation of E1α (1)15–17; HIF-1-induced transcription of LONP protease that degrades E1β (2)40; downregulation of Poldip2, resulting in increased degradation of the lipoic-acid activating enzyme ACSM1, which leads to decreased E2 lipoylation and PDC activity, accompanied by decreased α-ketoglutarate (αKG) levels that additionally inhibit prolyl-hydroxylases (PHDs) and stabilize HIF-1α (3)107; activating PDHK1 by post-translational phosphorylation of its threonine residues either by ERK-phosphorylated and mitochondrially-translocated phosphoglycerate kinase 1 (PGK1) (4)98 or by mitochondrially-translocated phosphorylated Akt (5)99; prolyl-hydroxylase PHD3 depletion that leads to PDC destabilization due to decreased PHD3-E1β interaction (6)41.
Acknowledgments
Protein structures used in all illustrations were derived from the Protein Data Bank database (PDB; http://www.rcsb.org/pdb/)118
This work was co-financed by the EU and SAS (SASPRO Program grant 0035/01/02), Slovak Scientific Grant Agency VEGA (2/0155/15, 2/0122/16), and the NIH (CA191653).
Abbreviations:
- Akt
protein kinase B
- ACSM1
lipoate-activating enzyme acetyl-CoA synthetase medium-chain family member 1
- ACAT1
acetyl-CoA acetyltransferase 1
- ADP
adenosine diphosphate
- ATP
adenosine triphosphate
- BCKDH
branched-chain α-ketoacid dehydrognease
- CoA
Coenzyme A
- DCA
dichloroacetate
- DLDH
dihydrolipoyl dehydrogenase, E3 subunit of PDC
- DLT
dihydrolipoyl acetyltransferase, E2 subunit of PDC
- E3BP
E3 binding protein
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FAD
flavin adenine dinucleotide
- GLUT
glucose transporter
- HIF
hypoxia-inducible factor
- LDH
lactate dehydrogenase
- MPC
mitochondrial pyruvate carrier
- NAD
nicotinamide adenine dinucleotide
- OGDH
2-oxoglutarate dehydrogenase
- OXPHOS
oxidative phosphorylation
- PGK1
phosphoglycerate kinase 1
- PDC
pyruvate dehydrogenase complex
- PDH
pyruvate dehydrogenase
- PDHA1
pyruvate dehydrogenase E1α subunit gene
- PDHK, PDK
pyruvate dehydrogenase kinase
- PDP
pyruvate dehydrogenase phosphatase
- PHD
prolyl-4-hydroxylase domain enzymes
- Poldip2
polymerase-δ interacting protein 2
- pVHL
Von Hippel Lindau tumor suppressor protein
- RNF126
ring-finger protein 126
- Sirt
sirtuin, NAD+-dependent deacetylase
- TCA
tricarboxylic acid cycle
- TPP
thiamine pyrophosphate
- VDAC
voltage-dependent anion channel
Footnotes
Publisher's Disclaimer: This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record.
Declarations of interest: none
References
- 1.Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol 1992;12: 5447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 1995;92: 5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science 2001;294: 1337–40. [DOI] [PubMed] [Google Scholar]
- 4.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999;399: 271–5. [DOI] [PubMed] [Google Scholar]
- 5.Berra E, Richard DE, Gothie E, Pouyssegur J. HIF-1-dependent transcriptional activity is required for oxygen-mediated HIF-1alpha degradation. FEBS Lett 2001;491: 85–90. [DOI] [PubMed] [Google Scholar]
- 6.Papandreou I, Krishna C, Kaper F, Cai D, Giaccia AJ, Denko NC. Anoxia is necessary for tumor cell toxicity caused by a low-oxygen environment. Cancer Res 2005;65: 3171–8. [DOI] [PubMed] [Google Scholar]
- 7.Warburg O On respiratory impairment in cancer cells. Science 1956;124: 269–70. [PubMed] [Google Scholar]
- 8.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 2011;27: 441–64. [DOI] [PubMed] [Google Scholar]
- 9.Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem 1995;270: 29083–9. [DOI] [PubMed] [Google Scholar]
- 10.O’Rourke JF, Pugh CW, Bartlett SM, Ratcliffe PJ. Identification of hypoxically inducible mRNAs in HeLa cells using differential-display PCR. Role of hypoxia-inducible factor-1. Eur J Biochem 1996;241: 403–10. [DOI] [PubMed] [Google Scholar]
- 11.Huizing M, Ruitenbeek W, Thinnes FP, DePinto V, Wendel U, Trijbels FJ, Smit LM, ter Laak HJ, van den Heuvel LP. Deficiency of the voltage-dependent anion channel: a novel cause of mitochondriopathy. Pediatr Res 1996;39: 760–5. [DOI] [PubMed] [Google Scholar]
- 12.Bricker DK, Taylor EB, Schell JC, Orsak T, Boutron A, Chen YC, Cox JE, Cardon CM, Van Vranken JG, Dephoure N, Redin C, Boudina S, et al. A mitochondrial pyruvate carrier required for pyruvate uptake in yeast, Drosophila, and humans. Science 2012;337: 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herzig S, Raemy E, Montessuit S, Veuthey JL, Zamboni N, Westermann B, Kunji ER, Martinou JC. Identification and functional expression of the mitochondrial pyruvate carrier. Science 2012;337: 93–6. [DOI] [PubMed] [Google Scholar]
- 14.Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell 2014;56: 400–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006;3: 187–97. [DOI] [PubMed] [Google Scholar]
- 16.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006;3: 177–85. [DOI] [PubMed] [Google Scholar]
- 17.Lu CW, Lin SC, Chen KF, Lai YY, Tsai SJ. Induction of pyruvate dehydrogenase kinase-3 by hypoxia-inducible factor-1 promotes metabolic switch and drug resistance. J Biol Chem 2008;283: 28106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holness MJ, Sugden MC. Regulation of pyruvate dehydrogenase complex activity by reversible phosphorylation. Biochem Soc Trans 2003;31: 1143–51. [DOI] [PubMed] [Google Scholar]
- 19.Firth JD, Ebert BL, Pugh CW, Ratcliffe PJ. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3’ enhancer. Proc Natl Acad Sci U S A 1994;91: 6496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer 2008;8: 705–13. [DOI] [PubMed] [Google Scholar]
- 21.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324: 1029–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans 2006;34: 217–22. [DOI] [PubMed] [Google Scholar]
- 23.Korotchkina LG, Patel MS. Site specificity of four pyruvate dehydrogenase kinase isoenzymes toward the three phosphorylation sites of human pyruvate dehydrogenase. J Biol Chem 2001;276: 37223–9. [DOI] [PubMed] [Google Scholar]
- 24.Korotchkina LG, Patel MS. Mutagenesis studies of the phosphorylation sites of recombinant human pyruvate dehydrogenase. Site-specific regulation. J Biol Chem 1995;270: 14297–304. [DOI] [PubMed] [Google Scholar]
- 25.Yang D, Gong X, Yakhnin A, Roche TE. Requirements for the adaptor protein role of dihydrolipoyl acetyltransferase in the up-regulated function of the pyruvate dehydrogenase kinase and pyruvate dehydrogenase phosphatase. J Biol Chem 1998;273: 14130–7. [DOI] [PubMed] [Google Scholar]
- 26.Liu S, Baker JC, Roche TE. Binding of the pyruvate dehydrogenase kinase to recombinant constructs containing the inner lipoyl domain of the dihydrolipoyl acetyltransferase component. J Biol Chem 1995;270: 793–800. [DOI] [PubMed] [Google Scholar]
- 27.Hezaveh S, Zeng AP, Jandt U. Full Enzyme Complex Simulation: Interactions in Human Pyruvate Dehydrogenase Complex. J Chem Inf Model 2018. [DOI] [PubMed] [Google Scholar]
- 28.Linn TC, Pettit FH, Reed LJ. Alpha-keto acid dehydrogenase complexes. X. Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A 1969;62: 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowker-Kinley MM, Davis WI, Wu P, Harris RA, Popov KM. Evidence for existence of tissue-specific regulation of the mammalian pyruvate dehydrogenase complex. Biochem J 1998;329 (Pt 1): 191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ravindran S, Radke GA, Guest JR, Roche TE. Lipoyl domain-based mechanism for the integrated feedback control of the pyruvate dehydrogenase complex by enhancement of pyruvate dehydrogenase kinase activity. J Biol Chem 1996;271: 653–62. [DOI] [PubMed] [Google Scholar]
- 31.Bao H, Kasten SA, Yan X, Hiromasa Y, Roche TE. Pyruvate dehydrogenase kinase isoform 2 activity stimulated by speeding up the rate of dissociation of ADP. Biochemistry 2004;43: 13442–51. [DOI] [PubMed] [Google Scholar]
- 32.Patel MS, Nemeria NS, Furey W, Jordan F. The pyruvate dehydrogenase complexes: structure-based function and regulation. J Biol Chem 2014;289: 16615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowker-Kinley M, Popov KM. Evidence that pyruvate dehydrogenase kinase belongs to the ATPase/kinase superfamily. Biochem J 1999;344 Pt 1: 47–53. [PMC free article] [PubMed] [Google Scholar]
- 34.Boulatnikov I, Popov KM. Formation of functional heterodimers by isozymes 1 and 2 of pyruvate dehydrogenase kinase. Biochim Biophys Acta 2003;1645: 183–92. [DOI] [PubMed] [Google Scholar]
- 35.Yeaman SJ, Hutcheson ET, Roche TE, Pettit FH, Brown JR, Reed LJ, Watson DC, Dixon GH. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry 1978;17: 2364–70. [DOI] [PubMed] [Google Scholar]
- 36.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S, Califano JA, et al. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem 2008;283: 22700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rardin MJ, Wiley SE, Naviaux RK, Murphy AN, Dixon JE. Monitoring phosphorylation of the pyruvate dehydrogenase complex. Anal Biochem 2009;389: 157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golias T, Papandreou I, Sun R, Kumar B, Brown NV, Swanson BJ, Pai R, Jaitin D, Le QT, Teknos TN, Denko NC. Hypoxic repression of pyruvate dehydrogenase activity is necessary for metabolic reprogramming and growth of model tumours. Sci Rep 2016;6: 31146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang YJ, Walker D, Chen W, Klingbeil M, Komuniecki R. Expression of pyruvate dehydrogenase isoforms during the aerobic/anaerobic transition in the development of the parasitic nematode Ascaris suum: altered stoichiometry of phosphorylation/inactivation. Arch Biochem Biophys 1998;352: 263–70. [DOI] [PubMed] [Google Scholar]
- 40.Yonashiro R, Eguchi K, Wake M, Takeda N, Nakayama K. Pyruvate Dehydrogenase PDH-E1beta Controls Tumor Progression by Altering the Metabolic Status of Cancer Cells. Cancer Res 2018;78: 1592–603. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi D, Minamishima YA, Nakayama K. Prolyl-hydroxylase PHD3 interacts with pyruvate dehydrogenase (PDH)-E1beta and regulates the cellular PDH activity. Biochem Biophys Res Commun 2014;451: 288–94. [DOI] [PubMed] [Google Scholar]
- 42.Contractor T, Harris CR. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res 2012;72: 560–7. [DOI] [PubMed] [Google Scholar]
- 43.Lu CW, Lin SC, Chien CW, Lee CT, Lin BW, Lee JC, Tsai SJ. Overexpression of pyruvate dehydrogenase kinase 3 increases drug resistance and early recurrence in colon cancer. Am J Pathol 2011;179: 1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinidad AG, Whalley N, Rowlinson R, Delpuech O, Dudley P, Rooney C, Critchlow SE. Pyruvate dehydrogenase kinase 4 exhibits a novel role in the activation of mutant KRAS, regulating cell growth in lung and colorectal tumour cells. Oncogene 2017;36: 6164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Geng L, Yi H, Huo W, Talmon G, Kim YC, Wang SM, Wang J. Transforming Growth Factor beta Mediates Drug Resistance by Regulating the Expression of Pyruvate Dehydrogenase Kinase 4 in Colorectal Cancer. J Biol Chem 2016;291: 17405–16. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M, Shlomi T, Gottlieb E, Peeper DS. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 2013;498: 109–12. [DOI] [PubMed] [Google Scholar]
- 47.Parmenter TJ, Kleinschmidt M, Kinross KM, Bond ST, Li J, Kaadige MR, Rao A, Sheppard KE, Hugo W, Pupo GM, Pearson RB, McGee SL, et al. Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov 2014;4: 423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kluza J, Corazao-Rozas P, Touil Y, Jendoubi M, Maire C, Guerreschi P, Jonneaux A, Ballot C, Balayssac S, Valable S, Corroyer-Dulmont A, Bernaudin M, et al. Inactivation of the HIF-1alpha/PDK3 signaling axis drives melanoma toward mitochondrial oxidative metabolism and potentiates the therapeutic activity of pro-oxidants. Cancer Res 2012;72: 5035–47. [DOI] [PubMed] [Google Scholar]
- 49.Rajagopalan KN, Egnatchik RA, Calvaruso MA, Wasti AT, Padanad MS, Boroughs LK, Ko B, Hensley CT, Acar M, Hu Z, Jiang L, Pascual JM, et al. Metabolic plasticity maintains proliferation in pyruvate dehydrogenase deficient cells. Cancer Metab 2015;3: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jackson LE, Kulkarni S, Wang H, Lu J, Dolezal JM, Bharathi SS, Ranganathan S, Patel MS, Deshpande R, Alencastro F, Wendell SG, Goetzman ES, et al. Genetic Dissociation of Glycolysis and the TCA Cycle Affects Neither Normal nor Neoplastic Proliferation. Cancer Res 2017;77: 5795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupuy F, Tabaries S, Andrzejewski S, Dong Z, Blagih J, Annis MG, Omeroglu A, Gao D, Leung S, Amir E, Clemons M, Aguilar-Mahecha A, et al. PDK1-Dependent Metabolic Reprogramming Dictates Metastatic Potential in Breast Cancer. Cell Metab 2015;22: 577–89. [DOI] [PubMed] [Google Scholar]
- 52.Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O’Brien JP, Pierce KA, Gui DY, Sullivan LB, et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab 2016;23: 517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, Wodzak M, Klimko C, et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016;164: 681–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giatromanolaki A, Sivridis E, Arelaki S, Koukourakis MI. Expression of enzymes related to glucose metabolism in non-small cell lung cancer and prognosis. Exp Lung Res 2017;43: 167–74. [DOI] [PubMed] [Google Scholar]
- 55.Liu T, Yin H. PDK1 promotes tumor cell proliferation and migration by enhancing the Warburg effect in non-small cell lung cancer. Oncol Rep 2017;37: 193–200. [DOI] [PubMed] [Google Scholar]
- 56.Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Harris AL, Tumor, Angiogenesis Research G. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 2005;7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Guccini I, Mitri DD, Brina D, Revandkar A, Sarti M, Pasquini E, Alajati A, Pinton S, Losa M, Civenni G, Catapano CV, et al. Compartmentalized activities of the pyruvate dehydrogenase complex sustain lipogenesis in prostate cancer. Nat Genet 2018;50: 219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, Flaim E, Michelakis ED. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell 2014;158: 84–97. [DOI] [PubMed] [Google Scholar]
- 59.Roche TE, Hiromasa Y, Turkan A, Gong X, Peng T, Yan X, Kasten SA, Bao H, Dong J. Essential roles of lipoyl domains in the activated function and control of pyruvate dehydrogenase kinases and phosphatase isoform 1. Eur J Biochem 2003;270: 1050–6. [DOI] [PubMed] [Google Scholar]
- 60.Caruso M, Maitan MA, Bifulco G, Miele C, Vigliotta G, Oriente F, Formisano P, Beguinot F. Activation and mitochondrial translocation of protein kinase Cdelta are necessary for insulin stimulation of pyruvate dehydrogenase complex activity in muscle and liver cells. J Biol Chem 2001;276: 45088–97. [DOI] [PubMed] [Google Scholar]
- 61.Ferriero R, Nusco E, De Cegli R, Carissimo A, Manco G, Brunetti-Pierri N. Pyruvate dehydrogenase complex and lactate dehydrogenase are targets for therapy of acute liver failure. J Hepatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang M, Zhao Y, Li Z, Wang C. Pyruvate dehydrogenase kinase 4 mediates lipogenesis and contributes to the pathogenesis of nonalcoholic steatohepatitis. Biochem Biophys Res Commun 2018;495: 582–6. [DOI] [PubMed] [Google Scholar]
- 63.Wu CY, Tso SC, Chuang JL, Gui WJ, Lou M, Sharma G, Khemtong C, Qi X, Wynn RM, Chuang DT. Targeting hepatic pyruvate dehydrogenase kinases restores insulin signaling and mitigates ChREBP-mediated lipogenesis in diet-induced obese mice. Mol Metab 2018;12: 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Crewe C, Kinter M, Szweda LI. Rapid inhibition of pyruvate dehydrogenase: an initiating event in high dietary fat-induced loss of metabolic flexibility in the heart. PLoS One 2013;8: e77280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Handzlik MK, Constantin-Teodosiu D, Greenhaff PL, Cole MA. Increasing cardiac pyruvate dehydrogenase flux during chronic hypoxia improves acute hypoxic tolerance. J Physiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schafer C, Young ZT, Makarewich CA, Elnwasany A, Kinter C, Kinter M, Szweda LI. Coenzyme A-mediated degradation of pyruvate dehydrogenase kinase 4 promotes cardiac metabolic flexibility after high-fat feeding in mice. J Biol Chem 2018;293: 6915–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sun W, Liu Q, Leng J, Zheng Y, Li J. The role of Pyruvate Dehydrogenase Complex in cardiovascular diseases. Life Sci 2015;121: 97–103. [DOI] [PubMed] [Google Scholar]
- 68.Grayczyk JP, Harvey CJ, Laczkovich I, Alonzo F 3rd., A Lipoylated Metabolic Protein Released by Staphylococcus aureus Suppresses Macrophage Activation. Cell Host Microbe 2017;22: 678–87 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jung GS, Jeon JH, Choi YK, Jang SY, Park SY, Kim SW, Byun JK, Kim MK, Lee S, Shin EC, Lee IK, Kang YN, et al. Pyruvate dehydrogenase kinase regulates hepatitis C virus replication. Sci Rep 2016;6: 30846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vallee A, Vallee JN. Warburg effect hypothesis in autism Spectrum disorders. Mol Brain 2018;11: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnerias C, Saudubray JM, Touati G, De Lonlay P, Dulac O, Ponsot G, Marsac C, Brivet M, Desguerre I. Pyruvate dehydrogenase complex deficiency: four neurological phenotypes with differing pathogenesis. Dev Med Child Neurol 2010;52: e1–9. [DOI] [PubMed] [Google Scholar]
- 72.Robinson BH, Sherwood WG. Lactic acidaemia. J Inherit Metab Dis 1984;7 Suppl 1: 69–73. [DOI] [PubMed] [Google Scholar]
- 73.Robinson BH. Lactic acidemia: disorders of pyruvate carboxylase and pyruvate dehydrogenase In: Scriver CR The metabolic and molecular bases of inherited diseaseed. New York: McGraw-Hill, 2001: 2275–96. [Google Scholar]
- 74.Lissens W, De Meirleir L, Seneca S, Liebaers I, Brown GK, Brown RM, Ito M, Naito E, Kuroda Y, Kerr DS, Wexler ID, Patel MS, et al. Mutations in the X-linked pyruvate dehydrogenase (E1) alpha subunit gene (PDHA1) in patients with a pyruvate dehydrogenase complex deficiency. Hum Mutat 2000;15: 209–19. [DOI] [PubMed] [Google Scholar]
- 75.Ridout CK, Brown RM, Walter JH, Brown GK. Somatic mosaicism for a PDHA1 mutation in a female with pyruvate dehydrogenase deficiency. Hum Genet 2008;124: 187–93. [DOI] [PubMed] [Google Scholar]
- 76.Okajima K, Warman ML, Byrne LC, Kerr DS. Somatic mosaicism in a male with an exon skipping mutation in PDHA1 of the pyruvate dehydrogenase complex results in a milder phenotype. Mol Genet Metab 2006;87: 162–8. [DOI] [PubMed] [Google Scholar]
- 77.Coughlin CR 2nd, Krantz ID, Schmitt ES, Zhang S, Wong LJ, Kerr DS, Ganesh J Somatic mosaicism for PDHA1 mutation in a male with pyruvate dehydrogenase complex deficiency. Mol Genet Metab 2010;100: 296–9. [DOI] [PubMed] [Google Scholar]
- 78.Brown RM, Head RA, Morris AA, Raiman JA, Walter JH, Whitehouse WP, Brown GK. Pyruvate dehydrogenase E3 binding protein (protein X) deficiency. Dev Med Child Neurol 2006;48: 756–60. [DOI] [PubMed] [Google Scholar]
- 79.Head RA, Brown RM, Zolkipli Z, Shahdadpuri R, King MD, Clayton PT, Brown GK. Clinical and genetic spectrum of pyruvate dehydrogenase deficiency: dihydrolipoamide acetyltransferase (E2) deficiency. Ann Neurol 2005;58: 234–41. [DOI] [PubMed] [Google Scholar]
- 80.Maj MC, MacKay N, Levandovskiy V, Addis J, Baumgartner ER, Baumgartner MR, Robinson BH, Cameron JM. Pyruvate dehydrogenase phosphatase deficiency: identification of the first mutation in two brothers and restoration of activity by protein complementation. J Clin Endocrinol Metab 2005;90: 4101–7. [DOI] [PubMed] [Google Scholar]
- 81.Okajima K, Korotchkina LG, Prasad C, Rupar T, Phillips JA 3rd, Ficicioglu C, Hertecant J, Patel MS, Kerr DS Mutations of the E1beta subunit gene (PDHB) in four families with pyruvate dehydrogenase deficiency. Mol Genet Metab 2008;93: 371–80. [DOI] [PubMed] [Google Scholar]
- 82.Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci 2014;71: 2577–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Papandreou I, Goliasova T, Denko NC. Anticancer drugs that target metabolism: Is dichloroacetate the new paradigm? Int J Cancer 2011;128: 1001–8. [DOI] [PubMed] [Google Scholar]
- 84.Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology 2006;66: 324–30. [DOI] [PubMed] [Google Scholar]
- 85.Pearce EL, Poffenberger MC, Chang CH, Jones RG. Fueling immunity: insights into metabolism and lymphocyte function. Science 2013;342: 1242454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity 2013;38: 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013;493: 346–55. [DOI] [PubMed] [Google Scholar]
- 88.Gerriets VA, Kishton RJ, Nichols AG, Macintyre AN, Inoue M, Ilkayeva O, Winter PS, Liu X, Priyadharshini B, Slawinska ME, Haeberli L, Huck C, et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J Clin Invest 2015;125: 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, Liu G. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol 2015;194: 6082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semba H, Takeda N, Isagawa T, Sugiura Y, Honda K, Wake M, Miyazawa H, Yamaguchi Y, Miura M, Jenkins DM, Choi H, Kim JW, et al. HIF-1alpha-PDK1 axis-induced active glycolysis plays an essential role in macrophage migratory capacity. Nat Commun 2016;7: 11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guentsch A, Beneke A, Swain L, Farhat K, Nagarajan S, Wielockx B, Raithatha K, Dudek J, Rehling P, Zieseniss A, Jatho A, Chong M, et al. PHD2 Is a Regulator for Glycolytic Reprogramming in Macrophages. Mol Cell Biol 2017;37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meiser J, Kramer L, Sapcariu SC, Battello N, Ghelfi J, D’Herouel AF, Skupin A, Hiller K. Pro-inflammatory Macrophages Sustain Pyruvate Oxidation through Pyruvate Dehydrogenase for the Synthesis of Itaconate and to Enable Cytokine Expression. J Biol Chem 2016;291: 3932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grassian AR, Metallo CM, Coloff JL, Stephanopoulos G, Brugge JS. Erk regulation of pyruvate dehydrogenase flux through PDK4 modulates cell proliferation. Genes Dev 2011;25: 1716–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yoshino S, Hara T, Nakaoka HJ, Kanamori A, Murakami Y, Seiki M, Sakamoto T. The ERK signaling target RNF126 regulates anoikis resistance in cancer cells by changing the mitochondrial metabolic flux. Cell Discov 2016;2: 16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirschey MD, Zhao Y. Metabolic Regulation by Lysine Malonylation, Succinylation, and Glutarylation. Mol Cell Proteomics 2015;14: 2308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Oliveira AP, Sauer U. The importance of post-translational modifications in regulating Saccharomyces cerevisiae metabolism. FEMS Yeast Res 2012;12: 104–17. [DOI] [PubMed] [Google Scholar]
- 97.Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, Chen GZ, Boggon TJ, et al. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell 2011;44: 864–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, Xia Y, Aldape K, He J, Hunter T, Wang L, Lu Z. Mitochondria-Translocated PGK1 Functions as a Protein Kinase to Coordinate Glycolysis and the TCA Cycle in Tumorigenesis. Mol Cell 2016;61: 705–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chae YC, Vaira V, Caino MC, Tang HY, Seo JH, Kossenkov AV, Ottobrini L, Martelli C, Lucignani G, Bertolini I, Locatelli M, Bryant KG, et al. Mitochondrial Akt Regulation of Hypoxic Tumor Reprogramming. Cancer Cell 2016;30: 257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fan J, Kang HB, Shan C, Elf S, Lin R, Xie J, Gu TL, Aguiar M, Lonning S, Chung TW, Arellano M, Khoury HJ, et al. Tyr-301 phosphorylation inhibits pyruvate dehydrogenase by blocking substrate binding and promotes the Warburg effect. J Biol Chem 2014;289: 26533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shan C, Kang HB, Elf S, Xie J, Gu TL, Aguiar M, Lonning S, Hitosugi T, Chung TW, Arellano M, Khoury HJ, Shin DM, et al. Tyr-94 phosphorylation inhibits pyruvate dehydrogenase phosphatase 1 and promotes tumor growth. J Biol Chem 2014;289: 21413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, Gu TL, Aguiar M, Lonning S, Chen H, Mohammadi M, Britton LM, et al. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell 2014;53: 534–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ozden O, Park SH, Wagner BA, Song HY, Zhu Y, Vassilopoulos A, Jung B, Buettner GR, Gius D. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med 2014;76: 163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jing E, O’Neill BT, Rardin MJ, Kleinridders A, Ilkeyeva OR, Ussar S, Bain JR, Lee KY, Verdin EM, Newgard CB, Gibson BW, Kahn CR. Sirt3 regulates metabolic flexibility of skeletal muscle through reversible enzymatic deacetylation. Diabetes 2013;62: 3404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jin Y, Cai Q, Shenoy AK, Lim S, Zhang Y, Charles S, Tarrash M, Fu X, Kamarajugadda S, Trevino JG, Tan M, Lu J. Src drives the Warburg effect and therapy resistance by inactivating pyruvate dehydrogenase through tyrosine-289 phosphorylation. Oncotarget 2016;7: 25113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, Zhao Y. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell 2013;50: 919–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paredes F, Sheldon K, Lassegue B, Williams HC, Faidley EA, Benavides GA, Torres G, Sanhueza-Olivares F, Yeligar SM, Griendling KK, Darley-Usmar V, San Martin A. Poldip2 is an oxygen-sensitive protein that controls PDH and alphaKGDH lipoylation and activation to support metabolic adaptation in hypoxia and cancer. Proc Natl Acad Sci U S A 2018;115: 1789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stacpoole PW. Therapeutic Targeting of the Pyruvate Dehydrogenase Complex/Pyruvate Dehydrogenase Kinase (PDC/PDK) Axis in Cancer. J Natl Cancer Inst 2017;109. [DOI] [PubMed] [Google Scholar]
- 109.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, et al. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2010;2: 31ra4. [DOI] [PubMed] [Google Scholar]
- 110.Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar F, Patel C, von Euw E, et al. Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol 2014;140: 443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs 2014;32: 452–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs 2015;33: 603–10. [DOI] [PubMed] [Google Scholar]
- 113.Marti HH, Jung HH, Pfeilschifter J, Bauer C. Hypoxia and cobalt stimulate lactate dehydrogenase (LDH) activity in vascular smooth muscle cells. Pflugers Arch 1994;429: 216–22. [DOI] [PubMed] [Google Scholar]
- 114.Ditte P, Dequiedt F, Svastova E, Hulikova A, Ohradanova-Repic A, Zatovicova M, Csaderova L, Kopacek J, Supuran CT, Pastorekova S, Pastorek J. Phosphorylation of carbonic anhydrase IX controls its ability to mediate extracellular acidification in hypoxic tumors. Cancer Res 2011;71: 7558–67. [DOI] [PubMed] [Google Scholar]
- 115.Schnitzer SE, Weigert A, Zhou J, Brune B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in A549 lung cancer cells. Mol Cancer Res 2009;7: 393–401. [DOI] [PubMed] [Google Scholar]
- 116.Longo LD, Packianathan S, McQueary JA, Stagg RB, Byus CV, Cain CD. Acute hypoxia increases ornithine decarboxylase activity and polyamine concentrations in fetal rat brain. Proc Natl Acad Sci U S A 1993;90: 692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fan TW, Lane AN, Higashi RM, Farag MA, Gao H, Bousamra M, Miller DM. Altered regulation of metabolic pathways in human lung cancer discerned by (13)C stable isotope-resolved metabolomics (SIRM). Mol Cancer 2009;8: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res 2000;28: 235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maltepe E, Schmidt JV, Baunoch D, Bradfield CA, Simon MC. Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature 1997;386: 403–7. [DOI] [PubMed] [Google Scholar]
- 120.Mathupala SP, Rempel A, Pedersen PL. Glucose catabolism in cancer cells: identification and characterization of a marked activation response of the type II hexokinase gene to hypoxic conditions. J Biol Chem 2001;276: 43407–12. [DOI] [PubMed] [Google Scholar]
- 121.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998;12: 149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoon DY, Buchler P, Saarikoski ST, Hines OJ, Reber HA, Hankinson O. Identification of genes differentially induced by hypoxia in pancreatic cancer cells. Biochem Biophys Res Commun 2001;288: 882–6. [DOI] [PubMed] [Google Scholar]
- 123.Minchenko O, Opentanova I, Caro J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1–4) expression in vivo. FEBS Lett 2003;554: 264–70. [DOI] [PubMed] [Google Scholar]
- 124.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem 1994;269: 23757–63. [PubMed] [Google Scholar]
- 125.Gess B, Hofbauer KH, Deutzmann R, Kurtz A. Hypoxia up-regulates triosephosphate isomerase expression via a HIF-dependent pathway. Pflugers Arch 2004;448: 175–80. [DOI] [PubMed] [Google Scholar]
- 126.Graven KK, Troxler RF, Kornfeld H, Panchenko MV, Farber HW. Regulation of endothelial cell glyceraldehyde-3-phosphate dehydrogenase expression by hypoxia. J Biol Chem 1994;269: 24446–53. [PubMed] [Google Scholar]
- 127.Takahashi Y, Takahashi S, Yoshimi T, Miura T. Hypoxia-induced expression of phosphoglycerate mutase B in fibroblasts. Eur J Biochem 1998;254: 497–504. [DOI] [PubMed] [Google Scholar]
- 128.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 1996;271: 32529–37. [DOI] [PubMed] [Google Scholar]
- 129.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 2006;281: 9030–7. [DOI] [PubMed] [Google Scholar]
- 130.Deng WJ, Nie S, Dai J, Wu JR, Zeng R. Proteome, phosphoproteome, and hydroxyproteome of liver mitochondria in diabetic rats at early pathogenic stages. Mol Cell Proteomics 2010;9: 100–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cai CF, Ye GD, Shen DY, Zhang W, Chen ML, Chen XX, Han DX, Mi YJ, Luo QC, Cai WY, Yang SY. Chibby suppresses aerobic glycolysis and proliferation of nasopharyngeal carcinoma via the Wnt/beta-catenin-Lin28/let7-PDK1 cascade. J Exp Clin Cancer Res 2018;37: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dai Z, Pan S, Chen C, Cao L, Li X, Chen X, Su X, Lin S. Down-regulation of succinate dehydrogenase subunit B and up-regulation of pyruvate dehydrogenase kinase 1 predicts poor prognosis in recurrent nasopharyngeal carcinoma. Tumour Biol 2016;37: 5145–52. [DOI] [PubMed] [Google Scholar]
- 133.Xiang G, Li X, Cao L, Zhu C, Dai Z, Pan S, Lin S. Frequent overexpression of PDK1 in primary nasopharyngeal carcinoma is associated with poor prognosis. Pathol Res Pract 2016;212: 1102–7. [DOI] [PubMed] [Google Scholar]
- 134.Ognibene M, Cangelosi D, Morini M, Segalerba D, Bosco MC, Sementa AR, Eva A, Varesio L. Immunohistochemical analysis of PDK1, PHD3 and HIF-1alpha expression defines the hypoxic status of neuroblastoma tumors. PLoS One 2017;12: e0187206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sradhanjali S, Tripathy D, Rath S, Mittal R, Reddy MM. Overexpression of pyruvate dehydrogenase kinase 1 in retinoblastoma: A potential therapeutic opportunity for targeting vitreous seeds and hypoxic regions. PLoS One 2017;12: e0177744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wigfield SM, Winter SC, Giatromanolaki A, Taylor J, Koukourakis ML, Harris AL. PDK-1 regulates lactate production in hypoxia and is associated with poor prognosis in head and neck squamous cancer. Br J Cancer 2008;98: 1975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Peng F, Wang JH, Fan WJ, Meng YT, Li MM, Li TT, Cui B, Wang HF, Zhao Y, An F, Guo T, Liu XF, et al. Glycolysis gatekeeper PDK1 reprograms breast cancer stem cells under hypoxia. Oncogene 2018;37: 1062–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hur H, Xuan Y, Kim YB, Lee G, Shim W, Yun J, Ham IH, Han SU. Expression of pyruvate dehydrogenase kinase-1 in gastric cancer as a potential therapeutic target. Int J Oncol 2013;42: 44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Baumunk D, Reichelt U, Hildebrandt J, Krause H, Ebbing J, Cash H, Miller K, Schostak M, Weikert S. Expression parameters of the metabolic pathway genes pyruvate dehydrogenase kinase-1 (PDK-1) and DJ-1/PARK7 in renal cell carcinoma (RCC). World J Urol 2013;31: 1191–6. [DOI] [PubMed] [Google Scholar]
- 140.He Z, Li Z, Zhang X, Yin K, Wang W, Xu Z, Li B, Zhang L, Xu J, Sun G, Wang L, Li Q, et al. MiR-422a regulates cellular metabolism and malignancy by targeting pyruvate dehydrogenase kinase 2 in gastric cancer. Cell Death Dis 2018;9: 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woolbright BL, Choudhary D, Mikhalyuk A, Trammel C, Shanmugam S, Abbott E, Pilbeam CC, Taylor JA. The Role of Pyruvate Dehydrogenase Kinase-4 (PDK4) in Bladder Cancer and Chemoresistance. Mol Cancer Ther 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chakraborty PK, Mustafi SB, Xiong X, Dwivedi SKD, Nesin V, Saha S, Zhang M, Dhanasekaran D, Jayaraman M, Mannel R, Moore K, McMeekin S, et al. MICU1 drives glycolysis and chemoresistance in ovarian cancer. Nat Commun 2017;8: 14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhong Y, Huang R, Li X, Xu R, Zhou F, Wang J, Fan H, Goscinski M, Zhang M, Wen JG, Nesland JM, Suo Z. Decreased Expression of PDHE1alpha Predicts Worse Clinical Outcome in Esophageal Squamous Cell Carcinoma. Anticancer Res 2015;35: 5533–8. [PubMed] [Google Scholar]
- 144.Sun XR, Sun Z, Zhu Z, Guan HX, Li CY, Zhang JY, Zhang YN, Zhou H, Zhang HJ, Xu HM, Sun MJ. Expression of pyruvate dehydrogenase is an independent prognostic marker in gastric cancer. World J Gastroenterol 2015;21: 5336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y, Huang R, Li X, Li X, Yu D, Zhang M, Wen J, Goscinski MA, Trope CG, Nesland JM, Suo Z. Decreased expression of pyruvate dehydrogenase A1 predicts an unfavorable prognosis in ovarian carcinoma. Am J Cancer Res 2016;6: 2076–87. [PMC free article] [PubMed] [Google Scholar]
- 146.Sun J, Li J, Guo Z, Sun L, Juan C, Zhou Y, Gu H, Yu Y, Hu Q, Kan Q, Yu Z. Overexpression of Pyruvate dehydrogenase E1a subunit Inhibits Warburg effect and Induces Cell Apoptosis through Mitochondria-mediated Pathway in Hepatocellular Carcinoma. Oncol Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhong Y, Li X, Ji Y, Li X, Li Y, Yu D, Yuan Y, Liu J, Li H, Zhang M, Ji Z, Fan D, et al. Pyruvate dehydrogenase expression is negatively associated with cell stemness and worse clinical outcome in prostate cancers. Oncotarget 2017;8: 13344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cai Z, Zhao JS, Li JJ, Peng DN, Wang XY, Chen TL, Qiu YP, Chen PP, Li WJ, Xu LY, Li EM, Tam JP, et al. A combined proteomics and metabolomics profiling of gastric cardia cancer reveals characteristic dysregulations in glucose metabolism. Mol Cell Proteomics 2010;9: 2617–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Eboli ML, Pasquini A. Transformation linked decrease of pyruvate dehydrogenase complex in human epidermis. Cancer Lett 1994;85: 239–43. [DOI] [PubMed] [Google Scholar]