Abstract
Background and Purpose
Interest in functionally-guided radiation therapy (RT) planning has been bolstered by the ability to derive lung ventilation maps from 4-Dimensional Computed Tomography. However, this assumes that regional lung ventilation is an accurate surrogate for true regional lung function, i.e., gas exchange between the airspaces and capillary Red Blood Cells (RBCs). This work uses the emerging technology of hyperpolarized (HP)-129Xe Magnetic Resonance Imaging (MRI) to investigate the degree to which lung ventilation and gas exchange are regionally correlated.
Material and Methods
HP-129Xe MRI studies were performed on 17 IRB-approved human subjects, including 13 healthy volunteers, one emphysema patient, and 3 non-small cell lung cancer (NSCLC) patients imaged prior to and ~11 weeks following RT. Subjects inhaled 1 liter of HP-129Xe mixture, followed by the acquisition of interleaved ventilation and gas exchange images, from which maps of relative HP-129Xe distribution were obtained in: 1) the lung airspaces; 2) dissolved interstitially in alveolar barrier tissue; and 3) transferred to the capillary RBCs. The relative spatial distributions of HP-129Xe in airspaces (regional ventilation) and RBCs (regional gas transfer) were compared. Further, we investigated the degree to which ventilation and RBC images identified similar functional regions of interest (ROIs) suitable for functionally-guided RT. For the RT patients, both ventilation and RBC functional images were used to calculate differences in the lung dose-function histogram (DFH) and functional effective uniform dose (fEUD).
Results
The correlation of ventilation and RBC transfer was ρ=0.39±0.15 in healthy volunteers. For the RT patients, this correlation was ρ=0.53±0.02 pre-treatment and ρ=0.39±0.07 post-treatment; for the emphysema patient it was ρ=0.24. Comparing functional ROIs, ventilation and RBC transfer demonstrated poor spatial agreement: DSC=0.50±0.07 and 0.26±0.12 for the highest-33%- and highest10%-function ROIs in healthy volunteers, and in RT patients (pre-treatment) these were 0.54±0.02 and 0.35±0.06. The average magnitude of the differences between RBC- and ventilation-derived fEUD, fV20Gy, fV10Gy, and f5Gy, were 1.5±1.4 Gy, 4.1%±3.8%, 5.0%±3.8%, and 5.3%±3.9%.
Conclusions
Ventilation may not be an effective surrogate for true regional lung function for all patients.
Keywords: Hyperpolarized xenon-129, ventilation imaging, gas exchange, functional-guidance, functional-avoidance, MRgRT, lung radiotherapy
Introduction
Radiation Therapy (RT) to the thorax can be particularly challenging since the lung is particularly sensitive to radiation (1) and therefore limits the ability to deliver effective radiation doses. Incidental radiation to the lung can result in compromised function, which can progress to a clinical presentation of symptomatic radiation pneumonitis (RP) and/or long-term fibrosis (2–4). However, predicting which patients will develop compromised lung function, i.e., radiation-induced lung injury (RILI), is difficult. Historically, this has been pursued using volumetric dose metrics, such as mean lung dose (MLD) (5, 6) and various lung Vx metrics (the volume receiving above a certain radiation dose threshold, x) (7–9), which are used as guidelines for RT planning and optimization. These volume-based metrics are generally known to be somewhat oversimplified and efforts have turned towards including the region of lung being irradiated to better predict lung injury (10). This is bolstered by the fact that, following RT, regional perfusion and ventilation as measured by Single Photon Emission Computed Tomography (SPECT) undergo dose-dependent changes (11, 12). This has led to efforts to evaluate patient-specific spatial distributions of both regional lung function and RT dose to better predict risk for developing injury (13–20). The introduction of dose-function metrics, such as the dose-function-histogram (DFH) (21, 22) and functional Effective Uniform Dose (fEUD) (23), serve to incorporate this regional functional information into the standard DVH and MLD metrics, respectively. Recent studies integrating regional lung function, measured by SPECT perfusion (24, 25) or 4-Dimensional Computed Tomography (4DCT)-derived ventilation (26–28), suggest that dose-function metrics may provide superior predictive power over standard dose-volume metrics.
One approach suggested to reduce the risk of RILI is to orient the treatment beams to avoid the most-functional regions of the lung during 3-Dimensional Conformal RT (29–31). Similarly, one could optimize intensity modulated RT (IMRT) plans to specifically avoid the most-functional lung regions; e.g. optimize based on a ‘dose-function’ parameter, rather than traditional dose-volume metrics. Initial work along these lines used SPECT perfusion as a surrogate for end-to-end regional lung function (21, 29). More recently, interest has spiked in using regional lung ventilation to guide planning, owing to the ability to conveniently derive ventilation maps from 4DCT acquired during the CT simulation study (32–35). Such maps have been shown to correlate with traditional pulmonary function tests (PFTs) (36, 37) and compare reasonably well with regional ventilation as measured by SPECT (37). In light of these findings, several prospective clinical trials have been launched to investigate the safety and feasibility of functionally-guided RT planning based on perfusion images (“FLARE RT,” , https://clinicaltrials.gov/ct2/show/NCT02773238) and ventilation images (38, 39).
Inherent in these studies is the assumption that either ventilation or perfusion images are accurate surrogates for regional end-to-end lung function. Regional function requires that regional airways be ventilated, regional vasculature perfused, and the regional alveolar barrier tissue permits gas exchange (O2 ⇄ CO2) with red blood cells (RBCs) (40). If 4DCT-derived ventilation is to be used for functionally-guided RT planning, it is vital that the correlation between ventilation and gas exchange be understood.
In this work, we employed a novel Magnetic Resonance Imaging (MRI) technique using hyperpolarized (HP)-129Xe that provides 3D functional maps of ventilation and gas transfer to capillary RBCs (41, 42) to assess, in humans, the degree to which they are spatially correlated. The technology exploits unique frequency shifts of 129Xe that allow it to be independently detected in the airspaces, interstitial barrier tissues and RBCs (43). It has been used to image regional ventilation, barrier uptake and RBC transfer in patients with idiopathic pulmonary fibrosis (IPF) (44), COPD (45, 46), and pulmonary vascular disease (42). Recently, it has also been used to assess RILI in a rat model of injury (47). Importantly, 129Xe gas exchange measures have been shown correlate exceptionally well with the diffusing capacity for carbon monoxide (DLCO), the accepted clinical test of pulmonary gas exchange (44, 48). We therefore suggest that HP-129Xe MRI may represent the most-promising means of imaging true regional lung function (i.e. gas exchange). Therefore, we used this technology to examine the potential effectiveness and/or pitfalls of using ventilation as a surrogate in functionally-guided RT planning.
Materials & Methods
Human Subjects
This IRB approved investigation enrolled 13 healthy volunteers, one patient with emphysema, and three patients with stage-III Non-Small Cell Lung Cancer (NSCLC), who received between 60–66 Gy of radiation therapy in 30–33 fractions (2 Gy/fraction). All subjects were at least 18 years old and had no cardiac arrhythmias; the healthy cohort had no history of smoking, and had never been diagnosed with any pulmonary disorders. The RT patients were imaged both 1-week prior to commencing and between 6–12 weeks after finishing their course of radiation. Subject demographics and pulmonary function values are presented in Table 1.
Table 1.
Human subject details and Pulmonary Function Test (PFT) data, which included: Forced Vital Capacity (FVC), Forced Expiratory Volume in the first second (FEV1), and Diffusing capacity of the Lungs for Carbon Monoxide (DLCO). Average and 1.s.d shown for healthy subjects and RT patients.
| N | Age (years) | FVC (%) | FEV1 (%) | DLCO (%) | |
|---|---|---|---|---|---|
| Healthy subjects | 13 | 34 ± 15 | 92% ± 14% | 92% ± 11% | 91% ± 15% |
| RT patients (pre-RT) | 3 | 68 ± 4 | 72% ± 15% | 71% ± 17% | 73% ± 20% |
| (post-RT) | 85% ± 15% | 87% ± 13% | 78% ± 13% | ||
| Emphysema patient | 1 | 56 | 108% | 58% | not available |
Hyperpolarized Xenon-129 Magnetic Resonance Image Acquisition
Subjects were imaged supine during a series of 15-second breath-hold scans. All were scanned on a 1.5 T GE Healthcare 15M4 EXCITE system, except two RT patients who were imaged on a 3.0 T SIEMENS MAGNETOM Trio. Scans involved an initial calibration, followed by image acquisition as recently detailed by Wang et al. (42). Hyperpolarized xenon was prepared (49) using a commercially available laser-polarizer (Model 9810, Polarean, Inc., Durham, NC), producing 300–750 ml isotopically enriched 129Xe polarized to 18 ± 6%, and augmented with ultra-high-purity helium to fill a 1 L bag. The first dose, consisting of 300 ml HP-129Xe, was inhaled and used for spectroscopic calibration to determine the echo time (TE) at which the RBC and interstitial barrier signals are 90° out of phase (TE90). Subsequently, a 750 ml 129Xe dose was inhaled during which a 3D radial pulse sequence was used to acquire 1000 views each of gaseous and dissolved xenon signals in an interleaved fashion. By acquiring both phases within the same 15 second window, it ensured that the two images were inherently co-registered and subject to the same small variations in radio frequency (RF) coil spatial sensitivity. The gaseous xenon signal was reconstructed directly to create a 3D ventilation image; the dissolved xenon signal underwent further Dixon-based decomposition to separate the barrier and RBC images (41, 42). This process also used an anatomical 1H scan acquired using the same 3D radial sequence after the subject had inhaled 1 L room air from a bag; this provided a thoracic cavity image that could be segmented and registered to the functional images to facilitate quantitative analysis. All images were reconstructed into 6.25-mm isotropic voxels.
Image Registration, Segmentation & SNR
Our analysis of ventilation and gas transfer was restricted to the Region Of Interest (ROI) where the thoracic cavity and ventilated lung overlapped. The former was delineated using semi-automated segmentation of the anatomical 1H image, the latter using a semi-automated k-means approach (41, 50, 51). If needed, slight variations in patient position between anatomical and functional images were corrected by rigid registration. Signal-to-Noise Ratio (SNR) was calculated for each image. Signal was calculated by finding the mean voxel value within the ventilated thoracic cavity, i.e., the intersection of thoracic cavity and ventilated lung ROIs, and then subtracting the mean background. For RBC transfer, signal noise was calculated simply as the standard deviation (s.d.) of the image background. For ventilation, which is derived from a magnitude image, the noise follows a Rician distribution and hence, the signal noise was calculated as the s.d. of the background multiplied by , as explained by Gudbjartsson & Patz (52).
Correlation of Ventilation and Gas Transfer
To test the correlation between the relative xenon signal in the ventilation and RBC phases, images were compared on a per-voxel basis by calculating the Pearson correlation coefficient. To permit analysis by lung region, the thoracic cavity ROI was divided into several partial-lung ROIs, of equal geometric length along superior-inferior and anterior-posterior directions, to examine correlation in various areas of lung:
superior-50% and inferior-50%;
left-50% and right-50%; and
anterior-33%, central(AP)-33%, and posterior-33%.
The finer geometric division along the anterior-posterior axis was implemented to more closely examine the existence of any gravitational functional dependence, given that our subjects were imaged supine.
Functional Avoidance Planning Structures
Further, ROIs were generated to identify the lowest-33%, middle-33%, and highest-33% of each functional volume for ventilation and RBC transfer. These have been suggested for functional-avoidance optimization by Yamamoto et al. (31). Additionally, we identified the top-10% of functional volumes as suggested for the avoidance strategy detailed by Yaremko et al. (30). For each subject, the functional ROIs created from the ventilation images were compared to those created independently from the RBC transfer images by calculating the Dice Similarity Coefficient (DSC), defined as
where |X| and |Y| are the number of voxels within the high-signal ROIs of ventilation and RBC, respectively, and | X ∩ Y| is the number of intersecting voxels. DSC values ~1 represent ROIs that are quantitatively very similar, while those approaching 0 are spatially different.
Dose-Function Histograms
For the RT patients, the planning CT and anatomical MRI were registered using deformable image registration in RayStation (RaySearch Laboratories, Stockholm, Sweden). The deformation was verified by examining the displacement vector fields for stability and performing a point-by-point check on a range of anatomical features. The CT-to-MRI deformation map permitted the transfer of RT dose into the functional MRI space. This permitted lung DFHs to be computed and compared depending on whether function was defined using ventilation or using RBC transfer.
Modified Functional Effective Uniform Dose (fEUD)
Newer functional optimization techniques for IMRT include the use of the fEUD metric (23, 38), defined as a weighted dose average that considers the voxel’s relative function,
where N is the total number of voxels, and fi and Di are the relative function and dose of voxel i. Given this trend, we also calculated and compared this metric using function derived from both ventilation and RBC-transfer.
Statistical Methods
With fewer than 30 subjects, we do not assume that the subjects’ correlation coefficients for ventilation and RBC transfer are normally distributed. Furthermore, samples are not treated as independent since a given partial-lung ROI may affect the distribution of the other ROI(s) within a given subject. Therefore, to determine whether correlation coefficients between partial-lung ROIs were significant, we used the Wilcoxon signed-rank test. The Wilcoxon z-score was converted into a P-value using a two-tailed approach. Where average numerical values are quoted in the results, cited uncertainties are given as ±1 standard deviation (s.d.). In the healthy cohort, outliers further than 1.5× the Interquartile Range (IQR) above Quartile-3 or below Quartile-1 were removed from statistical analysis but are still shown in figures.
Results
Correlation of Ventilation and RBC
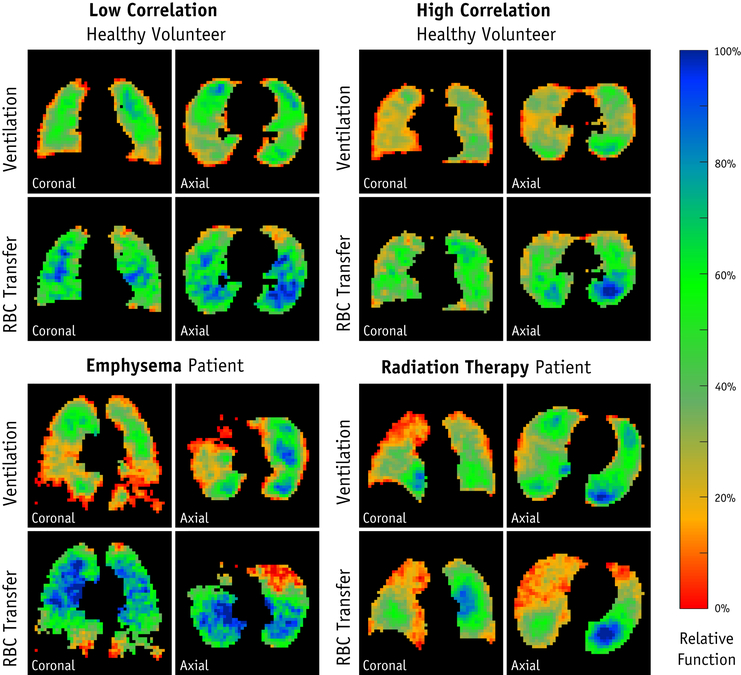
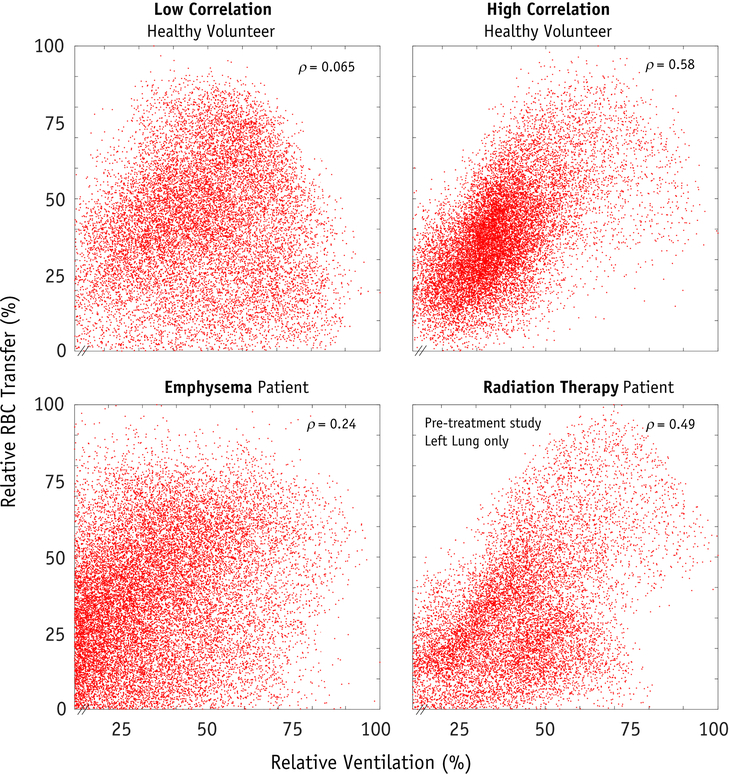
Examples of the ventilation and gas transfer functional images obtained using HP-129Xe MRI are shown in Figure 1. The average SNR for the ventilation and RBC images were 15.2±5.7 and 6.1±2.1, respectively. The average correlation of ventilation and RBC transfer in the healthy volunteer group was weak to moderate (ρ=0.39±0.15). For the RT patients, the average correlation coefficients were ρ=0.53±0.02 and ρ=0.39±0.07 on pre-RT and post-RT imaging, respectively. For one RT patient, the right lung was unventilated during pre-RT imaging, presumably due to a partially obstructing right main stem bronchus tumor (53); thus, only the left lung was analyzed. As for the emphysema patient, RBC transfer signal was weakly correlated with ventilation (ρ = 0.24). The per-voxel comparison of ventilation versus RBC transfer are shown for two healthy subjects, the emphysema patient, and one RT patient (Figure 2).
Figure 1.
Functional images of lung obtained using hyperpolarized xenon MRI for four example subjects: healthy volunteers with low and high correlations between ventilation and RBC transfer; the emphysema patient; and an example radiation therapy patient 6-weeks after finishing treatment.
Figure 2.
Four examples of per-voxel scatter plots of relative ventilation versus relative gas transfer, demonstrating the correlation between these two functions. Subject A and B are two example healthy volunteers with low and high correlation coefficients. The emphysema patient and a radiation therapy patient (this time showing the pre-treatment study, left lung only) are shown for comparison. Data is displayed as a percentage of the maximum functioning voxel.
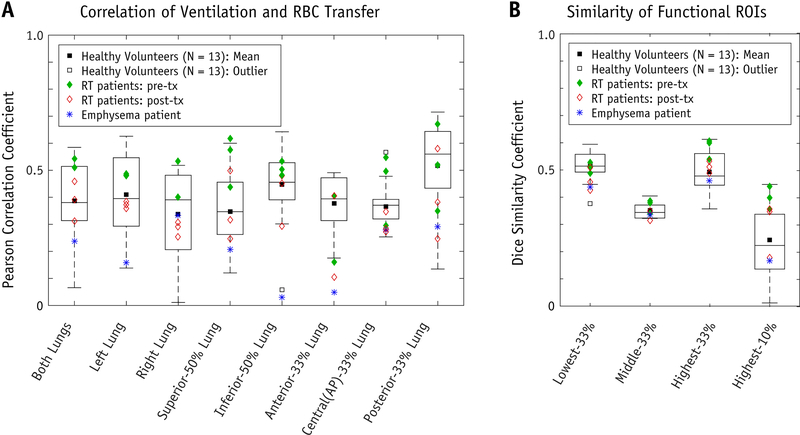
Examining healthy volunteer subjects by ROI, ventilation and gas transfer were significantly more correlated in the inferior-50% (ρ = 0.48 ± 0.10) than in the superior-50% (ρ = 0.35 ± 0.14, P<0.01). Similarly, correlations were significantly higher in the posterior-33% (ρ = 0.52±0.17) than in both the anterior-33% (ρ = 0.38±0.10, P<0.05), and the central(AP)-33% lung (ρ = 0.35±0.06, P<0.01). On average, correlation was stronger in the left lung (ρ = 0.41±0.15) versus the right (ρ = 0.34±0.17), although this difference was not significant (P=0.08). These correlation coefficients, broken down by are R0I, are shown as boxplots for the healthy volunteer cohort in Figure 4; for comparison, we also display these same results for the RT and emphysema patient studies.
Figure 4.
[A] Correlation of 129Xe in ventilation phase and in Red Blood Cells (RBCs). Pearson correlation coefficient, ρ, for “Both Lungs” and for regional ROIs as described along horizontal axis. Distribution of correlation for healthy volunteer cohort (Npatients=13) shown as boxplots (minimum, 1st quartile, median, 3rd quartile, maximum, and mean = ×) and outliers for “Inferior-50% Lung” and “Central(AP)-33% Lung”. The Radiation Therapy (RT) patients’ results (both pre- and post-RT) and emphysema patient’s results are superimposed on the figure as points, as shown in plot legend. [B] Dice Similarity Coefficients comparing functional planning volume ROIs created separately from ventilation and from RBC gas exchange. We examined four potential planning volumes based on what has been used in the literature for functionally-guided RT planning: Lowest-33%-, Middle-33%-, Highest-33%-, and Highest-10%-function.
Similarity of Functional-Guidance Planning ROIs and Metrics
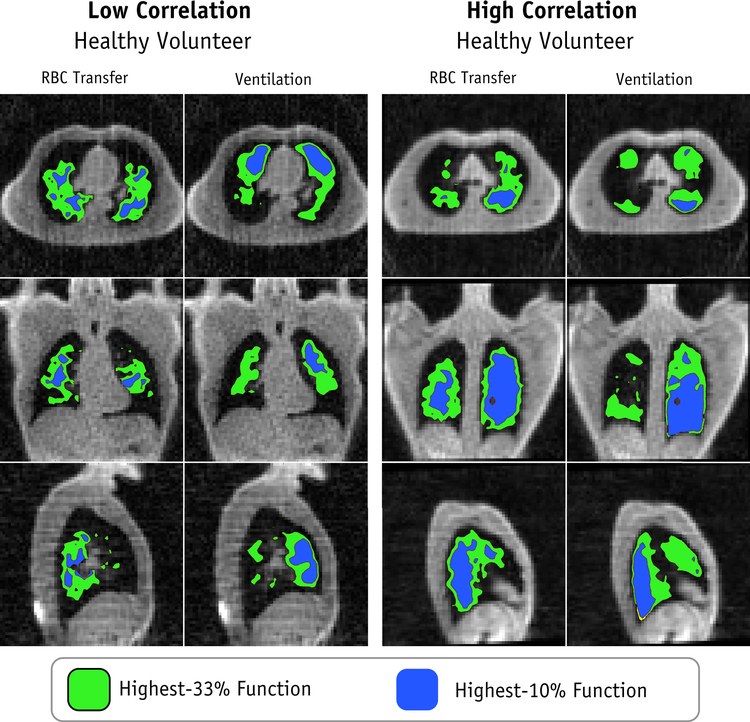
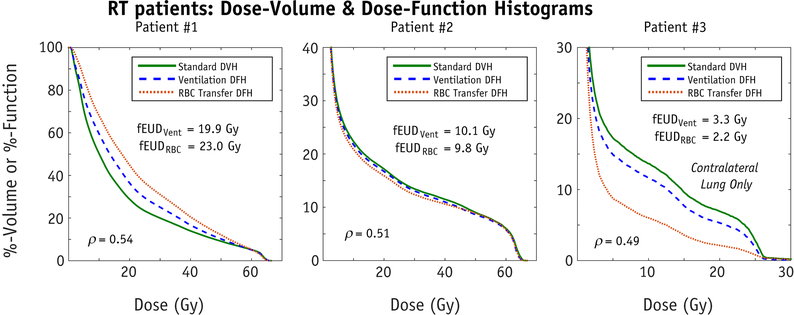
Functional ROIs generated using ventilation and gas exchange are shown in Figure 3. Qualitatively, these ROIs appear significantly different for the least correlated healthy volunteer (Subject A) and appear more similar for the most correlated healthy volunteer (Subject B). Across the entire healthy cohort, the associated DSCs for the lowest-33%, middle-33%, highest-33% and highest-10% functional ROIs were 0.53±0.04, 0.35±0.03, 0.50±0.07, and 0.26±0.12, respectively (Figure 4). Similarly, for the RT patients’ pre-treatment functional images, DSCs were 0.51±0.02, 0.37±0.03, 0.58±0.04, and 0.40±0.04 (Figure 4). Examining the clinical dose distributions of the RT patients, we observed differences in lung dose-function-histograms (DFHs) depending on whether function was defined based on ventilation or RBC transfer (Figure 5). The average magnitude of the differences in fEUD, fV20Gy, fV10Gy, and f5Gy were 1.5±1.4 Gy, 4.1%±3.8%, 5.0%±3.8%, and 5.3%±3.9%.
Figure 3.
High lung function ROIs for the two healthy volunteers with low and high correlation between ventilation and RBC transfer. Lung “function” has been defined as both ventilation and as RBC transfer and the resulting planning ROIs compared.
Figure 5.
Dose-volume histograms (DVH) and dose-function histograms (DFH) using both ventilation and RBC transfer for the three RT patients. These were created by fusing the patient’s CT simulation and treatment planning dose with the pre-treatment HP-129Xe MRI.
Discussion
In our healthy volunteer cohort (N=13), relative lung ventilation demonstrated a weak-to-moderate positive correlation with relative gas exchange, as quantified by RBC transfer. Similar correlation was also observed in the RT patients, and for the emphysema patient it fell >1 s.d. below the healthy average. The functional planning volume ROIs from ventilation demonstrated modest overlap with those from RBC transfer, as shown by DSC scores. Additionally, DSC exhibited significant variability, raising concerns about the degree to which ventilation is a suitable surrogate for RBC transfer for functional RT planning. One could move towards using larger functional volumes for planning, for example the “highest-50%”, which gave DSC = 0.64±0.05 for healthy volunteers, 0.66±0.03 and 0.62±0.02 for RT patients pre- and post-treatment, and 0.60 for the emphysema patient. However, larger percentile functional ROIs trend towards the same metric as simple mean lung dose (MLD). The associated DFHs and fEUDs differed by an average of 5% and 1.5Gy when defining function by either ventilation or RBC transfer. Importantly, these differences are similar in magnitude to the potentially achievable reductions in functional lung doses when using functional-avoidance planning. Recent modelling by Faught et al. (28) showed average achievable reductions of 4.0%, 6.2%, 3.3%, and 1.2Gy in fV5, fV10, fV20, and fEUD. This suggests that the way in which “lung function” is defined in functional-avoidance planning could have as significant an effect on toxicity reduction as the choice to use functional guidance at all.
The weak to moderate correlation between ventilation and RBC transfer, even the healthy subjects (ρ=0.39±0.15), may initially appear counter-intuitive given the widely held notion that ventilation (V) and perfusion (Q) are matched (54, 55). However, it should be noted that while the highest ventilation and perfusion values typically occur in lung units where V/Q ≈1, the associated distributions are, in fact, quite broad. This heterogeneity is caused by both gravitational gradients (56) and lung architectural factors within a given gravitational plane (57). In fact, our understanding of V/Q heterogeneity continues to be frustratingly imperfect as recently detailed (58). Furthermore, to our knowledge, this is one of the few studies that have examined to quantify regional function both on the per voxel level (6.25 mm isotropic), which may be exposing spatial variations in function that were previously unresolved. Ventilation is dictated, to some extent, by the mechanical stretching of lungs, whereas gas-exchange depends on alveolar ventilation as well as physiological parameters such as alveolar wall permeability and capillary vessel presence surrounding the alveolar wall. Hence, ventilation and gas-exchange need not necessarily be correlated. Thus, the modest correlations between ventilation and RBC transfer reported here may simply be a different way to represent the functional heterogeneity known to physiologists.
There are several shortcomings of this current analysis. First, when analyzing image differences on a per-voxel basis, the signal-to-noise ratio (SNR) of the images must be considered. Elevated imaging noise can artificially reduce the measured correlation. Our images demonstrated good SNR, with average for ventilation and RBC at 15.2±5.7 and 6.1±2.1, respectively. The effects of SNR on correlation can be appreciated by considering two identical normal distributions (μS = 1; σS = 0.3), which have ρ=1.0, and then adding independent gaussian noise to each of those distributions (μN=0; σN=μS/13 and σN=μS/7 to simulate our SNR of 13 and 7, respectively). In this scenario, on average, the correlation coefficient will decrease by ~0.1 to a value of 0.9, which is likely the degree to which noise affected the correlation coefficients reported here. In addition, we did not detect a relationship between SNR and correlation. Our least correlated healthy subject had image SNRs of 12.8 and 8.1 for ventilation and RBC transfer, respectively, where the most correlated healthy subject had image SNRs of 12.3 and 5.6; this suggests that our correlation results were not limited by SNR.
Second, most of our subjects were healthy volunteers. It is possible that the relationship between ventilation and RBC transfer in our healthy cohort is not representative of patients with lung cancer presenting for thoracic RT. Our single lung cancer patient had a correlation coefficient within 1.s.d. of the healthy average, and the emphysema patient’s correlation coefficient was > 1.s.d below this cohort average. Further, there is no inherent reason to believe that the correlation coefficients would be better in patients with lung cancer compared to healthy volunteers. Thus, we believe that the current data is a reasonable estimation of the correlation coefficients that can be expected in patients with lung cancer.
Third, MRI-based imaging is imperfect. For example, geometric distortions can exist due to non-uniform B0 field or encoding gradients within the imaging field of view (FOV). However, most commercial scanners do apply a correction, which can reduce the magnitude to < 2 mm. For this study, any remaining geometric distortion was identically present in both the ventilation and RBC transfer images, and therefore affected neither the correlation nor the DSC calculations. In addition to geometric imperfections, the flexible B1 coil used for this study does not transmit/receive a perfectly uniform RF signal and therefore bias field correction would be needed to derive absolute function. However, given the focus on correlating relative function, any transmit/receive inhomogeneity would, again, affect each image equally. We must also acknowledge that much work remains to be done to establish the accuracy and repeatability of xenon gas transfer MRI. Certainly, 129Xe ventilation MRI has been proven to correlate well with pulmonary function testing while being highly repeatable (59). Moreover, 129Xe gas transfer MRI is exceptionally well correlated with DLCO, the accepted clinical metric for gas exchange of O2 and CO2 (44). Thus, despite differences in density, viscosity, and membrane diffusion coefficients, 129Xe transfer to RBCs is currently the most promising way to approximate regional O2 transfer.
Fourth, the calculated metrics presented in this work (e.g. DFH, fEUD) are based on imperfect models, i.e., both assume a linear relationship between imaged function and true function. While the authors acknowledge this, the calculations are shown simply to illustrate the magnitude of the potential effects resulting from the discordance between ventilation- and RBC transfer-based metrics. Differences of similar magnitude would be expected with other models as well.
Finally, we acknowledge that there may be differences between 4D-CT ventilation and HP-129Xe MR ventilation. While we have not directly compared the two modalities here, a recent study has compared 3He ventilation MRI, which is closely related to 129Xe MRI, with CT-based ventilation (60) and showed acceptable agreement. Therefore, in this work we have assumed that ventilation imaged using 129Xe MRI is representative of what would be derived from 4DCT. Additionally, we did not acquire MR-based perfusion for comparison with RBC transfer, due to the requirement of Gd administration, which has become increasingly challenging in the research environment given the recent emergence of concerns about Gd deposition in the brains of patients. It is also important to note, that MR perfusion has not yet been validated against accepted measures of perfusion, e.g. Tc-99m microaggregated albumin (MAA); MR perfusion results from other subjects tend to show more of the larger vasculature, compared to SPECT Tc-99m, which measures the accumulation of MAA in the capillaries where gas exchange takes place.
Functionally-guided RT planning is a likely useful approach to improve the therapeutic ratio of thoracic RT (24, 28). However, it is not clear if ventilation-based imaging is the ideal surrogate for regional function. In practice, one needs to strike a balance between “ease of access to functional imaging” and the accuracy of the functional information. Within many RT clinics, 4DCT are readily available and thus might be ‘good enough’ for patients whose ventilation and RBC transfer are strongly correlated; the challenge is identifying these patients. There is reason to believe that perfusion might be a better surrogate for RBC transfer than ventilation, since blood vessels constrict in response to poor aeration (e.g. hypoxia) but small airways are unable to constrict in response to poor perfusion (57). However, neither perfusion (61) nor ventilation (26, 27) have been able to strongly predict RP (i.e. AUC > 0.7), even when combined with other prognostic factors; there is still a missing piece to the puzzle. Thus, while functional imaging of RBC transfer with HP-129Xe MRI does require an additional imaging procedure, it presents a potentially unique advantage in being able to measure true regional lung function in a single non-invasive breath-hold scan. Given that existing approaches to functional planning have acknowledged prognostic limitations, this emerging technology provides hope that functional planning can be improved, and RP toxicity better predicted.
Conclusion
Ventilation demonstrated only weak-to-moderate correlation with regional RBC transfer, as measured by xenon MRI. Dose-function histogram and fEUDs were markedly different when defining lung “function” as either ventilation or RBC transfer. The clinical impact of these findings should be explored in future work by studying outcomes of a cohort of RT patients with pre- and post-treatment xenon images.
Functionally-guided radiation therapy (RT) planning using 4-Dimensional Computed Tomography (4DCT)-derived ventilation is gaining momentum. However, an important question remains: is regional lung ventilation a good surrogate for end-to-end lung function, i.e., gas transfer to Red Blood Cells (RBCs). We acquired functional image data for N=17 human subjects, using state-of-the-art hyperpolarized (HP)-129Xe Magnetic Resonance Imaging (MRI). We analyzed the correlation of ventilation and RBC transfer, and calculated the similarity of planning optimization structures created from each.
Acknowledgements
This work was supported in part by grants R01HL105643 and P41 EB015897.
Footnotes
Conflicts of Interest Statement:
Dr. Driehuys is a founder of and shareholder in Polarean Imaging, outside the submitted work; In addition, Dr. Driehuys has a patent US 9625550 B2, receiving royalties paid by Polarean Imaging.
Ethics Disclosure:
All human subjects in this work were enrolled on an approved IRB protocol # Pro00060259
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int. J. Radiat. Oncol 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 2.Movsas B, Raffin TA, Epstein AH, et al. Pulmonary Radiation Injury. Chest. 1997;111:1061–1076. [DOI] [PubMed] [Google Scholar]
- 3.GROSS NJ. Pulmonary Effects of Radiation Therapy. Ann. Intern. Med 1977;86:81. [DOI] [PubMed] [Google Scholar]
- 4.Palma DA, Senan S, Tsujino K, et al. Predicting Radiation Pneumonitis After Chemoradiation Therapy for Lung Cancer: An International Individual Patient Data Meta-analysis. Int. J. Radiat. Oncol 2013;85:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwa SLS, Lebesque JV, Theuws JCM, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int. J. Radiat. Oncol 1998;42:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Rodrigues G, Lock M, D’Souza D, et al. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer - a systematic review. Radiother. Oncol 2004;71:127–138. [DOI] [PubMed] [Google Scholar]
- 7.Marks LB, Bentzen SM, Deasy JO, et al. Radiation Dose–Volume Effects in the Lung. Int. J. Radiat. Oncol 2010;76:S70–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int. J. Radiat. Oncol 1999;45:323–329. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues G, Lock M, D’Souza D, et al. Prediction of radiation pneumonitis by dose-volume histogram parameters in lung cancer - a systematic review. Radiother. Oncol 2004;71:127–138. [DOI] [PubMed] [Google Scholar]
- 10.Bradley JD, Hope A, El Naqa I, et al. A Nomogram to Predict Radiation Pneumonitis, Derived From a Combined Analysis of RTOG 9311 and Institutional Data. Int. J. Radiat. Oncol 2007;69:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boersma LJ, Damen EMF, de Boer RW, et al. Dose-effect relations for local functional and structural changes of the lung after irradiation for malignant lymphoma. Radiother. Oncol 1994;32:201–209. [DOI] [PubMed] [Google Scholar]
- 12.Boersma LJ, Damen EMF, de Boer RW, et al. A new method to determine dose-effect relations for local lung-function changes using correlated SPECT and CT data. Radiother. Oncol 1993;29:110–116. [DOI] [PubMed] [Google Scholar]
- 13.Fan M, Marks LB, Hollis D, et al. Can We Predict Radiation-Induced Changes in Pulmonary Function Based on the Sum of Predicted Regional Dysfunction? J. Clin. Oncol 2001;19:543–550. [DOI] [PubMed] [Google Scholar]
- 14.Evans ES, Hahn CA, Kocak Z, et al. The Role of Functional Imaging in the Diagnosis and Management of Late Normal Tissue Injury. Semin. Radiat. Oncol 2007;17:72–80. [DOI] [PubMed] [Google Scholar]
- 15.Fan M, Marks LB, Lind P, et al. Relating radiation-induced regional lung injury to changes in pulmonary function tests. Int. J. Radiat. Oncol. Biol. Phys 2001;51:311–317. [DOI] [PubMed] [Google Scholar]
- 16.Marks LB, Munley MT, Spencer DP, et al. Quantification of radiation-induced regional lung injury with perfusion imaging. Int. J. Radiat. Oncol 1997;38:399–409. [DOI] [PubMed] [Google Scholar]
- 17.Abratt RP, Willcox PA, Smith JA. Lung cancer in patients with borderline lung functions--zonal lung perfusion scans at presentation and lung function after high dose irradiation. Radiother. Oncol 1990;19:317–22. [DOI] [PubMed] [Google Scholar]
- 18.Nioutsikou E, Partridge M, Bedford JL, et al. Prediction of radiation-induced normal tissue complications in radiotherapy using functional image data. Phys. Med. Biol 2005;50:1035–1046. [DOI] [PubMed] [Google Scholar]
- 19.Boersma LJ, Damen EMF, de Boer RW, et al. Estimation of overall pulmonary function after irradiation using dose-effect relations for local functional injury. Radiother. Oncol 1995;36:15–23. [DOI] [PubMed] [Google Scholar]
- 20.Seppenwoolde Y, De Jaeger K, Boersma LJ, et al. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys 2004;60:748–758. [DOI] [PubMed] [Google Scholar]
- 21.Marks LB, Spencer DP, Sherouse GW, et al. The role of three dimensional functional lung imaging in radiation treatment planning: The functional dose-volume histogram. Int. J. Radiat. Oncol 1995;33:65–75. [DOI] [PubMed] [Google Scholar]
- 22.Marks LB, Sherouse GW, Munley MT, et al. Incorporation of functional status into dose-volume analysis. Med. Phys 1999;26:196–199. [DOI] [PubMed] [Google Scholar]
- 23.Miften MM, Das SK, Su M, et al. Incorporation of functional imaging data in the evaluation of dose distributions using the generalized concept of equivalent uniform dose. Phys. Med. Biol 2004;49:1711–1721. [DOI] [PubMed] [Google Scholar]
- 24.Farr KP, Kallehauge JF, Møller DS, et al. Inclusion of functional information from perfusion SPECT improves predictive value of dose–volume parameters in lung toxicity outcome after radiotherapy for non-small cell lung cancer: A prospective study. Radiother. Oncol 2015;117:9–16. [DOI] [PubMed] [Google Scholar]
- 25.Dhami G, Zeng J, Vesselle HJ, et al. Framework for radiation pneumonitis risk stratification based on anatomic and perfused lung dosimetry. Strahlentherapie und Onkol. 2017;193:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinogradskiy Y, Castillo R, Castillo E, et al. Use of 4-dimensional computed tomography-based ventilation imaging to correlate lung dose and function with clinical outcomes. Int. J. Radiat. Oncol. Biol. Phys 2013;86:366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faught AM, Yamamoto T, Castillo R, et al. Evaluating Which Dose-Function Metrics Are Most Critical for Functional-Guided Radiation Therapy. Int. J. Radiat. Oncol 2017;99:202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faught AM, Miyasaka Y, Kadoya N, et al. Evaluating the Toxicity Reduction With Computed Tomographic Ventilation Functional Avoidance Radiation Therapy. Int. J. Radiat. Oncol 2017;99:325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGuire SM, Zhou S, Marks LB, et al. A methodology for using SPECT to reduce intensity-modulated radiation therapy (IMRT) dose to functioning lung. Int. J. Radiat. Oncol 2006;66:1543–1552. [DOI] [PubMed] [Google Scholar]
- 30.Yaremko BP, Guerrero TM, Noyola-Martinez J, et al. Reduction of Normal Lung Irradiation in Locally Advanced Non–Small-Cell Lung Cancer Patients, Using Ventilation Images for Functional Avoidance. Int. J. Radiat. Oncol 2007;68:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto T, Kabus S, von Berg J, et al. Impact of Four-Dimensional Computed Tomography Pulmonary Ventilation Imaging-Based Functional Avoidance for Lung Cancer Radiotherapy. Int. J. Radiat. Oncol 2011;79:279–288. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto T, Kabus S, Klinder T, et al. Four-dimensional computed tomography pulmonary ventilation images vary with deformable image registration algorithms and metrics. Med. Phys 2011;38:1348–1358. [DOI] [PubMed] [Google Scholar]
- 33.Castillo R, Castillo E, Martinez J, et al. Ventilation from four-dimensional computed tomography: density versus Jacobian methods. Phys. Med. Biol 2010;55:4661–4685. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Kabus S, von Berg J, et al. Reproducibility of Four-dimensional Computed Tomography-based Lung Ventilation Imaging. Acad. Radiol 2012;19:1554–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerrero T, Sanders K, Castillo E, et al. Dynamic ventilation imaging from four-dimensional computed tomography. Phys. Med. Biol 2006;51:777–791. [DOI] [PubMed] [Google Scholar]
- 36.Brennan D, Schubert L, Diot Q, et al. Clinical Validation of 4-Dimensional Computed Tomography Ventilation With Pulmonary Function Test Data. Int. J. Radiat. Oncol 2015;92:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto T, Kabus S, Lorenz C, et al. Pulmonary Ventilation Imaging Based on 4-Dimensional Computed Tomography: Comparison With Pulmonary Function Tests and?SPECT Ventilation Images. Int. J. Radiat. Oncol 2014;90:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto T, Kabus S, Bal M, et al. The first patient treatment of computed tomography ventilation functional image-guided radiotherapy for lung cancer. Radiother. Oncol 2016;118:227–231. [DOI] [PubMed] [Google Scholar]
- 39.Hoover D a, Capaldi DP, Sheikh K, et al. Functional lung avoidance for individualized radiotherapy (FLAIR): study protocol for a randomized, double-blind clinical trial. BMC Cancer. 2014;14:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleveland ZI, Virgincar RS, Qi Y, et al. 3D MRI of impaired hyperpolarized 129 Xe uptake in a rat model of pulmonary fibrosis. NMR Biomed. 2014;27:1502–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaushik SS, Robertson SH, Freeman MS, et al. Single-breath clinical imaging of hyperpolarized 129 xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn. Reson. Med 2016;75:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Z, Robertson SH, Wang J, et al. Quantitative analysis of hyperpolarized 129 Xe gas transfer MRI. Med. Phys 2017;44:2415–2428. [DOI] [PubMed] [Google Scholar]
- 43.Norquay G, Leung G, Stewart NJ, et al. 129 Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129 Xe NMR. Magn. Reson. Med 2017;77:1399–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang JM, Robertson SH, Wang Z, et al. Using hyperpolarized 129 Xe MRI to quantify regional gas transfer in idiopathic pulmonary fibrosis. Thorax. 2017;In Press:thoraxjnl-2017–210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qing K, Ruppert K, Jiang Y, et al. Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI. J. Magn. Reson. Imaging 2014;39:346–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qing K, Mugler JP, Altes TA, et al. Assessment of lung function in asthma and COPD using hyperpolarized 129 Xe chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR Biomed 2014;27:1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fox MS, Ouriadov A, Thind K, et al. Detection of radiation induced lung injury in rats using dynamic hyperpolarized 129 Xe magnetic resonance spectroscopy. Med. Phys 2014;41:72302. [DOI] [PubMed] [Google Scholar]
- 48.Kaushik SS, Freeman MS, Yoon SW, et al. Measuring diffusion limitation with a perfusion-limited gas-Hyperpolarized 129Xe gas-transfer spectroscopy in patients with idiopathic pulmonary fibrosis. J. Appl. Physiol 2014;117:577–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Driehuys B, Cates GD, Miron E, et al. High-volume production of laser-polarized 129 Xe. Appl. Phys. Lett 1996;69:1668–1670. [Google Scholar]
- 50.He M, Kaushik SS, Robertson SH, et al. Extending Semiautomatic Ventilation Defect Analysis for Hyperpolarized 129Xe Ventilation MRI. Acad. Radiol 2014;21:1530–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He M, Driehuys B, Que LG, et al. Using Hyperpolarized 129Xe MRI to Quantify the Pulmonary Ventilation Distribution. Acad. Radiol 2016;23:1521–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gudbjartsson H, Patz S. The Rician Distribution of Noisy MRI Data. Magn. Reson. Med 1995;34:910–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Song EJ, Kelsey CR, Driehuys B, et al. Functional airway obstruction observed with hyperpolarized 129 Xenon-MRI. J. Med. Imaging Radiat. Oncol 2017:In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersson J, Glenny RW. Gas exchange and ventilation-perfusion relationships in the lung. Eur. Respir. J 2014;44:1023–1041. [DOI] [PubMed] [Google Scholar]
- 55.West JB. Respiratory Physiology: The Essentials. 9th ed. Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 56.Wagner PD, Saltzman HA, West JB. Measurement of continuous distributions of ventilation-perfusion ratios: theory. J. Appl. Physiol 1974;36:588–599. [DOI] [PubMed] [Google Scholar]
- 57.Glenny RW. Determinants of regional ventilation and blood flow in the lung In: Applied Physiology in Intensive Care Medicine 2. Berlin, Heidelberg: Springer Berlin Heidelberg; 2012:227–236. [DOI] [PubMed] [Google Scholar]
- 58.Robertson HT, Buxton RB. Imaging for lung physiology: What do we wish we could measure? J. Appl. Physiol 2012;113:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ebner L, He M, Virgincar RS, et al. Hyperpolarized 129Xenon Magnetic Resonance Imaging to Quantify Regional Ventilation Differences in Mild to Moderate Asthma: A Prospective Comparison between Semiautomated Ventilation Defect Percentage Calculation and Pulmonary Function Tests. Invest. Radiol 2017;52:120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Capaldi DPI, Zha N, Guo F, et al. Pulmonary Imaging Biomarkers of Gas Trapping and Emphysema in COPD: 3 He MR Imaging and CT Parametric Response Maps. Radiology. 2016;279:597–608. [DOI] [PubMed] [Google Scholar]
- 61.Lind PA, Marks LB, Hollis D, et al. Receiver operating characteristic curves to assess predictors of radiation-induced symptomatic lung injury. Int. J. Radiat. Oncol. Biol. Phys 2002;54:340–347. [DOI] [PubMed] [Google Scholar]