Abstract
Extracellular vesicles (EVs) are heterogeneous cell-derived membranous vesicles which carry a large diversity of molecules such as proteins and RNA species. They are now considered to be a general mode of intercellular communication by direct transfer of biomolecules. Emerging evidence demonstrates that EVs are involved in multiple pathological processes of brain diseases including neurodegenerative disorders. In this review, we investigate the current knowledge about EV biology. We also provide an overview of the roles of EVs in related brain diseases, particularly in neurodegenerative disorders. Finally, we discuss their potential applications as novel biomarkers as well as the developments of EV-based therapies.
Keywords: Alzheimer’s disease, biomarkers, exosomes, extracellular vesicles, interleukins, microtubule-associated protein tau, neurodegenerative disorders
Introduction
Extracellular vesicles (EVs) are small membranous vesicles bounded by a lipid bilayer and carrying diverse intraluminal cargos of proteins, lipids, and nucleic acids which are secreted into the extracellular milieu [1]. The secretion of EVs was initially described as a consequence of eliminating unneeded compounds from the cell [2]. However, EVs are now known to play vital roles in the intercellular communication that underlies various physiological processes and pathological functions of both recipient and parent cells [3]. The creation of EVs is conserved throughout evolution from bacteria to humans [4]. To our knowledge, EVs are most commonly grouped into three broad types according to their biogenesis: exosomes, microvesicles (MVs) and apoptotic bodies (Table 1) [3]. Most studies have focused on exosomes and microvesicles [5]. They are released from almost all cell types, including the cells of central nervous system (CNS) [6], and their functions in CNS are currently under active investigation [7–9]. Here, we examine the knowledge of EVs biology, focus on their roles in neurodegenerative disorders, and discuss their involvement in pathogenesis as well as in biomarkers for these diseases.
Table 1.
Summary of characteristics of extracellular vesicles subtypes
| EVs subtype |
Alias name | Size | Origin | Known markers |
Biogenesis mechanisms |
Cargos |
|---|---|---|---|---|---|---|
| Exosomes |
|
|
|
|
|
|
| Microvesicles |
|
|
|
|
|
|
| Apoptotic bodies |
|
|
|
|
EVs subtypes
Exosomes
Exosomes, which were first termed in the 1980s [2], are small extracellular nano-size vesicles with typically 30 – 150nm in diameter [10]. Early endosomes undergo inward budding to form multivesicular bodies (MVBs) that contain intraluminal vesicles (ILVs) [11]. By fusion of MVBs with the plasma membrane, ILVs are released into the extracellular environment as exosomes [12]. Alternatively, MVBs can be fused with the lysosomal membrane, resulting in the degradation of the ILVs and their contents [13, 14]. As a consequence of their origin, exosomes contain enriched endosome-associated components, such as Annexins and flotillins [15], the ESCRT (endosomal sorting complex required for transport) component tumour susceptibility gene 101 protein (TSG101), and ALG-2-interacting protein X (ALIX) [16]. Otherwise, membrane proteins that play important roles in biogenesis of endosome or MVBs are also abundant on exosomes. These include tetraspanins such as CD9, CD63, CD81, which are considered as specific markers for exosomes [16, 17].
Exosomes were initially reported in sheep reticulocytes as a mechanism of removing unnecessary proteins and other contents during the maturation of reticulocytes to erythrocytes [2, 18, 19]. In the past decade, secreted exosomes were found not only in cells but the endogenous biofluids (blood, urine, cerebrospinal fluid, etc.) [20–22]. More recently, exosomes have been emerging as long-range messengers, capable of regulating growth and development [23], facilitating inter-cellular communication [24], modulating antigen presentation and inflammation [25, 26], and promoting various stages of tumorigenesis [27].
Microvesicles
Microvesicles, also known as microparticles or ectosomes, are larger than exosomes ranging in size typically from 100 – 1000nm in diameter [11, 23]. Beyond size, microvesicles are also distinguished from exosomes by the fact that they are generated directly by the outward budding of the plasma membrane and are subsequently released into the extracellular space [28]. A recent study identified annexin A1 as a specific marker for microvesicles [29]. However, the components of microvesicle membranes overlap considerably with that of exosomes. For example, although tetraspanins are considered as specific markers for exosomes, these proteins have also recently been observed in microvesicles and other vesicles [30, 31]. Additional experimental data and characterization methods are required to determine whether particular proteins are enriched on microvesicles relative to other specific EV-subgroups.
Apoptotic bodies
Apoptotic bodies are a subpopulation of EVs that are shed by the plasma membrane of apoptotic cells [1]. They are large EVs that range from 100 – 2000 nm in diameter, and contain fragmented subcellular organelles for degradation [26, 32, 33]. The fate of apoptotic bodies is to be taken up by phagocytic cells for digestion, therefore they are not involved in inter-cellular communication as with exosomes and microvesicles [10].
A cautionary note
Although briefly categorized into three subtypes based on the size range, EVs are also found heterogeneous in their origin and molecular constituents, with considerable overlapped in phenotype [34]. Recent studies have applied different EV-isolated techniques to identify diverse subpopulations of EVs [17, 29, 35, 36]. Joanna K et al proposed four subcategories of exosomes (also termed small EVs) based on the expression pattern of tetraspanins, the classic exosome markers, from human dendritic cells by using differential centrifuge and iodixanol gradient floatation: CD63+ CD81+ CD9+ EVs, CD63− CD81− CD9+ EVs, CD63− CD81− CD9− EVs, and EVs enriched in other factors [17]. Moreover, a current study has identified two discernible exosome subpopulations (named Exo-S and Exo-L) and a distinct nanoparticle (the Exomere) which differ in size and contents from mostly reported particles by employing asymmetric flow field-flow fractionation, again highlighting the diversity of EVs and particles secreted by cells [36]. Thus, it’s difficult to make a distinction between the vesicle types simply dependent on protein markers or size alone. To better interpret and replicate the experiment results among EV studies, combined EV extraction methods as well as improved techniques for accurate purification and characterization are recommended. Additionally, a crowdsourcing knowledgebase is now reliable for researchers in EV field to track latest EV biology and methodology [37].
EV Cargos: Nucleic acids and proteins
EVs were initially regarded as “body dust” and a consequence of loading unneeded compounds from the cell [2, 38]. A major breakthrough was the observation of nucleic acids (both mRNA and miRNA) in EVs and their transfer between cells mediated through EVs [39, 40]. Recently, various species of RNA have been detected within EVs. In addition to mRNA and miRNA, a large number of noncoding RNA, circular RNA, ribosomal RNA, transfer RNA fragments, and small interfering RNAs are also contained in EVs [41–45]. RNA profiling showed many enriched RNAs within EVs relative to the originating cells [45–47] and these EVs-derived RNA can be protected by RNaseA treatment [47], indicating the necessity and importance of RNA molecules loaded in EVs. Otherwise, DNA, including mitochondrial DNA and double-stranded DNA, has been found in EVs [48, 49], although there is a debate from a current study that small vesicles are not involved in active DNA release [29]. Nonetheless, increasing evidence indicates nucleic acids within EVs can be delivered and accepted by recipient cells, thus affecting gene expression [50, 51], regulating cell metabolism [52, 53], and facilitating disease progression [54–56].
Apart from nucleic acids, EVs are highly enriched in protein contents. These include endosome associated proteins (e.g., Annexins, TSG101, ALIX and Rab GTPase) and transmembrane proteins or lipids (e.g., tetraspanins, cholesterol, sphingomyelin), which are involved in the biogenesis of EVs [15, 16, 57–59]. They were identified from a variety of cells by proteomic analysis and SDS-PAGE followed by immunoblotting [60]. Recently, the manually curated web-based database Vesiclepedia (http://www.microvesicles.org/) catalogs proteins, RNAs, and lipids identified in different classes of EVs from 41 species which are easily accessed for EV study [61]. In addition, the sub-database ExoCarta (http://www.exocarta.org) lists the corresponding data of both exosomes and microvesicles from independent human studies [62]. According to the current version, 41,860 proteins, >7540 RNA and 1116 lipid molecules have been detected within EVs from human samples (Table 2). It has to be noted that the purification methods of EVs in these reports are not ideal and likely contain contaminated molecules. A further validation is necessary with the EV samples isolated from biospecimens according to the recent recommendations as published in MISEV2018 [63]. Thus, the fact that EVs are loaded with enriched biomolecules which can be targeted to the recipient cells within the nervous system opens an entirely new perspective on cell-cell conversation in the brain.
Table 2.
Overview of EVs cargos based on ExoCarta and Vesiclepedia database (updated on 4/30/2019)
| Studies | Protein entries |
mRNA entries |
miRNA entries |
Lipid molecules |
Species | |
|---|---|---|---|---|---|---|
| ExoCarta | 286 | 41,860 | 4,946 | 2,838 | 1,116 | Human |
| Vesiclepedia | 1,254 | 349,988 | 27,646 | 10,520 | 639 | 41 |
Biogenesis of EVs
Because exosomes and microvesicles differ from their derivation, the cellular machineries involved in their formation and release are likely different in spite of the overlapped mechanistic components (Figure 1). The ESCRT-dependent mechanism was initially interpreted as the biogenesis of ILVs and MVBs, thereafter giving rise to the speculation on its potential role in exosome formation [64, 65]. It’s known that the ESCRT machinery mediates the formation of ILVs in a stepwise manner: ESCRT-0, and ESCRT-I complexes are responsible for recognition and subsequently location of ubiquitinated membrane proteins at the limiting membrane of MVBs, while the ESCRT-II and ESCRT-III as well as their accessories (e.g., TSG101, ALIX and VPS4) perform membrane budding and scission of ILVs [66, 67]. Aside from the ESCRT-dependent mechanism, exosomes can also be generated in an ESCRT-independent manner (Figure 1). Trajkovic et al. first showed biogenesis of proteolipid protein-containing exosomes derived from the oligodendrocyte cell line is dependent on neutral sphingomyelinase 2 (nSMaseII), disruption of which reduced the secretion of exosomes [59, 68]. Sphingomyelinase can hydrolyse the membrane lipid sphingomyelin to ceramide, and ceramide may then induce the aggregation of microdomains, which promotes domain-induced inward budding of ILVs that are secreted as one class of exosomes [59, 69]. Studies in melanocytes also indicate a luminal domain-dependent pathway and CD63 dependent pathway for MVBs formation which are ESCRTs independent [70, 71]. In addition, tetraspanins, the four transmembrane proteins, are directly involved in the formation of ILVs and exosomes [70, 72–75]. The cone-shaped structure of tetraspanins could induce microdomain formation in membrane and then promote the inward budding of exosomes [76].
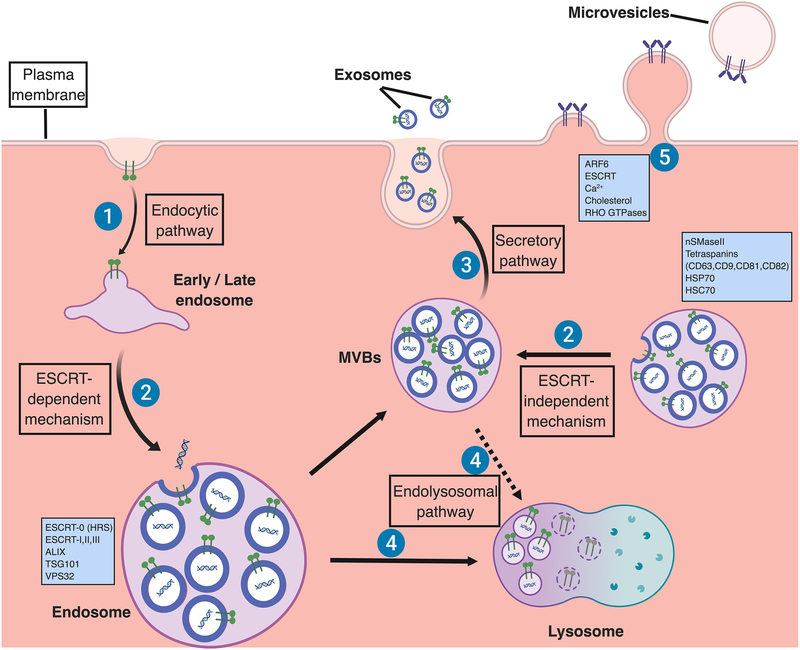
Figure 1. Biogenesis of extracellular vesicles.
Exosomes are secreted from the multivesicular body (MVB), which is formed by invagination of the endosomal membrane. Initially, extracellular cargoes are targeted at the plasma membrane to form early endosomes by endocytic pathway (1). Early endosomes undergo transition to late endosomes and inward budding in which exosomal cargos including specific proteins and nucleic acids are further loaded to form multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) [11]. Endosomal sorting complex required for transport (ESCRT)-dependent mechanism, which are regulated by ESCRT proteins (ESCRT-0, I, II and III) and their accessories (ALIX, TSG101, VPS32) [66, 67], and ESCRT-independent mechanism, which are regulated by neutral sphingomyelinase 2 (nSMaseII), tetraspanins, and the chaperone heat shock proteins (HSP70, HSC70), can develop ILVs (2) [59, 70, 73, 83]. Some of MVBs can be further fused with the lysosomal membrane, resulting in the degradation of the ILVs and their contents for recycling as an endolysosomal pathway (3) [13, 14]. Alternatively, ILVs are released into the extracellular space as exosomes via a secretory pathway (4) [12]. For microvesicles, they are formed by direct outward budding of the plasma membrane, a process which is regulated by the ESCRT components and ADP ribosylation factor 6 (ARF6), some small GTPases, lipids, and Ca2+-dependent enzymatic machineries [1]. (Figure was created with BioRender. com)
Although still largely undefined, the biogenesis of microvesicles requires the involvement of several molecular machineries within the plasma membrane. ESCRT proteins, including ESCRT-I and III, and the generation of ceramide by sphingomyelinase also have an important role in microvesicle biogenesis which is partially common to exosomes [67, 77]. Otherwise, lipids and Ca2+-dependent enzymatic machineries, including aminophospholipid translocases, scramblases and calpain, within the plasma membrane are reported to drive membrane budding and formation of microvesicles [67, 78, 79]. Small GTPases such as RHO family and ARF6, which regulate cytoskeletal rearrangements, have also been implicated in microvesicles formation [44, 80, 81].
As mentioned above, cytosolic proteins and nucleic acids are enriched in EVs and additional mechanisms may be involved in their sorting. The chaperone heat shock proteins such as HSP70 and HSP90, and heat shock cognate protein (HSC70), which are found coimmunoprecipitated with MHC II together with tetraspanins and enriched in exosomes from most cell types, are suggested to be contributing to sorting soluble proteins to ILVs and exosomes [82–84]. Otherwise, some evidence demonstrates that cytosolic proteins can be incorporated into ILVs and exosomes in an ubiquitylation or farnesylation dependent manner [85, 86]. For RNA species, the sorting mechanism is far from clear. Previous studies revealed ESCRT-II as an RNA binding complex or miRNA-induced silencing complex (miRISC) which mediates RNA silencing process and may function to sort RNAs into exosomes [87, 88]. Recent findings described a broad role of YBX1 in sorting not only miRNA but some small noncoding RNA species into exosomes [89, 90]. One study from cancer cells revealed that a zipcode-like 25 nucleotide (nt) sequence in the 3′-untranslated region (3’UTR) of mRNAs appears target mRNAs to microvesicles [91]. In addition, the neuronal gene encoded protein Arc is suggested to package RNA by self-assembling into virus-like capsids that are then loaded in extracellular vesicles [92].
Uptake of EVs by recipient cells
After entering into the extracellular space, EVs can target to recipient cells and deliver their cargos which mediate the physiological processes and pathological progress. EV uptake requires the interactions with surface receptors at the plasma membrane, followed by their fusion with target cells or endocytosis [93]. Several molecules including integrins, tetraspanins, lipids, lectins, proteoglycans, and extracellular matrix (ECM) components, are known to mediate these interactions [67]. For example, previous work from Nazarenko group suggested that exosomal tetraspanin-integrin complexes are involved in target cell binding [94]. In their experiment, they confirmed that Tspan8 can form complexes with integrin alpha4 as well as CD54 to mediate exosome-uptake [95]. Integrins are also shown to interact with cell adhesion molecules such as ICAMs [96] and ECM proteins such as fibronectin [97] at the cell surface, which drive the endocytosis of EVs. In addition, lipids on the surface of EVs can aid in vesicle docking to the cell membrane. Blockade of phosphatidylserine can prevent exosome uptake in microglia [98], and suppression of its binding protein annexin-V is found to disrupt the cellular uptake of tumor-derived microvesicles [99]. Of note, the mode of interactions between EVs and the recipient cells is likely determined by the specificity of proteins at the surface of EVs and cell membrane, which therefore might account for the target cell specificity [67]. In the nervous system, exosomes released upon synaptic activation selectively bind to neurons but not glial cells [100]. CD63-enriched exosomes can bind to both neurons and glia cells while exosomes lacking CD63 specifically bind to neurons [101]. Additionally, microglial vesicles display different dynamics of interaction with the surface of astrocytes compared with microglia [102]. These findings provide us new insights into the intercellular communication in brain, however, the underlying molecular mechanisms that specify the target of EVs still remains elusive.
Following uptake by recipient cells via different mechanisms, extracellular vesicles may undergo various subcellular fates [67]. Current studies combined live-imaging and super-resolution methods to track EVs and found, in most cases, the internalized vesicles are shuttled within endocytic pathway which are ultimately directed to the lysosome for degradation [103– 105]. Otherwise, it is believed that a small portion of internalized EVs may release their contents into the cytoplasm of the recipient cells, instead of being digested, by fusion with plasma membrane and the limiting membrane of the multivesicular bodies [67, 104]. This process is still poorly understood but it is a mechanism of importance for extracellular vesicles to exert regulatory effects on the recipient cells.
EVs in the CNS and neurodegenerative disorders
Both neurons and glia in the nervous system are known to release EVs [106, 107]. These EVs can move from the central nervous system to the systemic circulation by direct transfer into capillaries or through interstitial fluid into the CSF [1, 108]. In several experiments, EVs isolated from body fluids such as blood and cerebrospinal fluid (CSF) are shown to contain neuron- or glia-specific markers. For example, Goetzl et al. found some neuron-associated proteins, such as synaptotagmin and synaptophysin, were present in L1CAM (a neural adhesion protein) immunoprecipitated plasma-derived exosomes [109]. The proteome data from healthy CSF EVs also suggests a high proportion of brain-derived proteins including neuron-specific markers, such as vesicle-associated membrane protein 2 and enolase 2, microglia-specific protein integrin α- M, and oligodendrocyte protein transmembrane protein 132D [110].
Within the nervous system, EVs could be secreted from one cell type and targeted to the other (Figure 2) [107, 111, 112], and are suggested to involve multiple functions. EVs transfer not only membrane components but also nucleic acids between different cells, emphasizing their role in intercellular communication [112]. Microglia-derived EVs have been proposed to contain the cytokine interleukin-1β (IL-1β) and regulate inflammatory response [113]. There is also evidence that microvesicles from microglia can stimulate synaptic activity [114]. Similar to microglia, astrocytes release Nef in EVs to mediate neurotoxicity and synapsin 1 to promote neurite outgrowth [115–117]. Oligodendrocyte-derived exosome-like vesicles are reported to participate in myelin formation and maintenance [118], as well as in trophic support of neurons [8]. Taken together, these findings described above indicate that EVs have crucial physiological roles in the CNS. In addition, EVs are considered to contribute to the pathogenesis of many primary CNS disorders such as neurodegenerative diseases. Pathogenic protein aggregates are widely considered to be contributing factors to neurodegeneration. These aggregates would cause toxicity in cells and involve multiple mechanisms including neuronal death [119], synaptic dysfunction [120], and immune activation [121]. Several pathology-associated proteins including prions [122], amyloid peptide [123], Tau [10], and α-synuclein [124] are found present within EVs. These molecules released from EVs are thought to spread between neural cells contributing to disseminating pathogenesis, as well as enter into circulatory system which can be detected by non-invasive tools (Figure 2) [107]. Below, we will outline the roles of EVs in brain diseases particularly the aspects that are relevant to neurodegenerative diseases, as well as their potential biomarker development (Table 3).
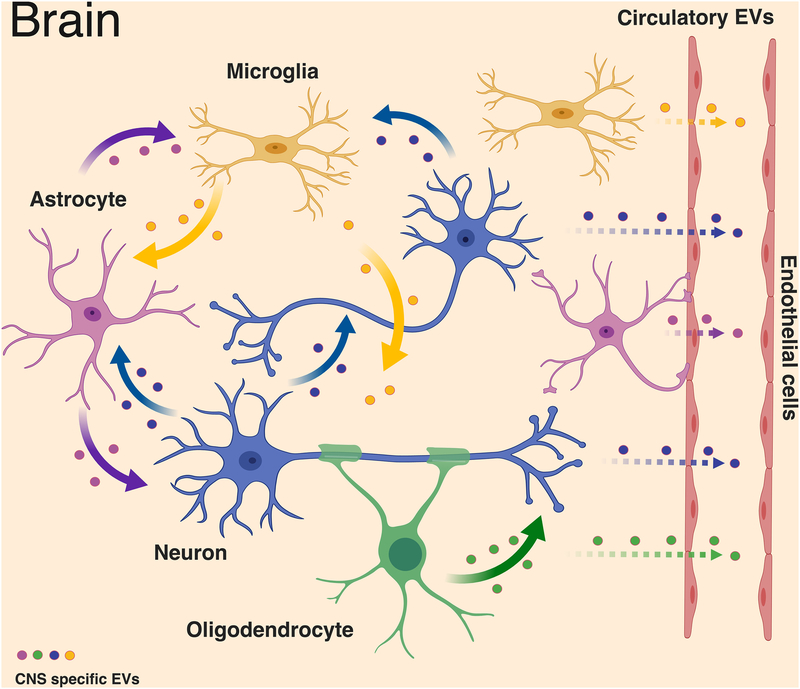
Figure 2. Intercellular communication of neural cells derived extracellular vesicles in brain.
In the central nervous system, EVs could be secreted from one cell type and targeted to the others to engage multiple functions. Microglia have been proposed to secrete EVs containing the pro-inflammatory cytokine interleukin-1 β (IL-1 β), which mediates inflammasome response and the aminopeptidase CD13, which provides metabolic support [113, 198]. Similar to microglia, astrocyte-derived EVs play both neuroprotective and neurotoxic roles such as containing synapsin 1 to promote neurite outgrowth and Nef to mediate neurotoxicity [115–117]. Oligodendrocytes secrete myelin molecules and stress-protective proteins in EVs, which are reported to participate in myelin formation and maintenance [118] as well as trophic support of neurons [8]. Neurodegenerative disorder-associated proteins such as prions [122], amyloid-β peptide [123], Tau [10], and α-synuclein [124] can also be released from EVs of the neural cells, leading to the spread of protein aggregate seeds and disease progression. In addition, these EVs could be exported through blood-brain barrier as circulatory EVs, which can be used for disease-specific biomarkers [1]. (Figure was created with BioRender. com)
Table 3.
Current knowledge of EV involvement in neurodegenerative diseases
| Disease | EVs subtype | Evidence | Biomarker development |
|---|---|---|---|
| Alzheimer’s disease |
|
|
|
|
|
||
| Amyotrophic lateral sclerosis |
|
|
|
|
|
||
| Hungtington’s disease |
|
|
|
|
|||
| Parkinson’s disease |
|
|
|
| Prion disease |
|
|
Alzheimer’s Disease
Alzheimer’s Disease (AD) is a degenerative brain disease and the most common cause of dementia which affects appropriately 30 million patients worldwide [6]. AD is characterized by deposits of amyloid‑ β (Aβ) protein called Aβ plaques, and abnormally folded hyper-phosphorylated tau protein known as neurofibrillary tangles (NFTs) in patients’ brains [125, 126]. Either the Aβ plaques or tau tangles are thought to spread in AD in the manner of a prion disease, where oligomeric proteins act as ‘seeds’ to undergo nucleated polymerization to form eventual pathogenic aggregates [127]. Although the neuropathology of AD has been clearly studied in the past 30 years, the mechanism of how these pathogenic proteins act in the neurodegenerative cascade remains elusive.
The role of EVs in the pathology of AD begins to uncover this cascade since β-cleavage of the amyloid precursor protein (APP) was found in early endosomes followed by delivery of Aβ to multivesicular bodies [123]. Some exosomal proteins such as Alix and Flotilin-1 were also enriched in the Aβ plaques in AD patient brains, suggesting that exosomes may contribute to Aβ deposits and the pathogenesis of AD. Furthermore, direct evidence of the role of extracellular vesicles in AD comes from isolating neuron-derived exosomes (NDEs) or astrocyte-derived exosomes (ADEs) from plasma in AD patients using neuron-specific antibodies against L1 cell adhesion molecule (L1CAM) or neural cell adhesion molecule 1 (NCAM1), and astrocyte-specific antibodies against glial fibrillary acidic protein (GFAP). Fiandaca et al. found levels of amyloid β 1–42 (Aβ1–42) in NDEs from AD were significantly higher than those from case-controls 1 to 10 years before diagnosis, which might be developed as a prediction of AD [128]. In addition, levels of cellular survival factors (e.g. low−density lipoprotein receptor−related protein 6, heat−shock factor−1, and repressor element 1−silencing transcription factor) in NDEs were significantly lower in Alzheimer’s disease patients than controls, possibly explaining the decreased neuronal resistance to neurotoxic proteins in AD [129]. Recently, Goetzl and their colleagues, isolating ADEs from AD patients, noticed significantly raised levels of complement including C1q, C4b, C3d, and pro-inflammatory factors including IL-1, TNF- α , IL-1 β compared to matched controls [130]. Further analysis revealed levels of complement proteins and some complement regulatory proteins in ADEs were associated with the staging of disease, for example, higher complement proteins and lower regulatory proteins in patients at AD2 stage than that at AD1 preclinical stage.
Consistent studies revealed a prominent role of EVs in the AD pathology, however, the relationship is not clear. Numerous evidence indicates that EVs play a neurotoxic role in AD. Higher levels of myeloid MVs were found in the CSF of AD and MCI patients than controls, suggesting the role of EVs in propagation of disease [131]. Besides, the increased myeloid EVs was thought to be released by activated microglia and contain neurotoxic Aβ forms which could drive AD degeneration [132]. Moreover, AD patients’ brains derived exosomes contain elevated toxic Aβ oligomers that can promote the neuron-to-neuron transfer of oligomers which cause toxicity in culture [133]. Therefore, EVs might be a potential therapeutic target for AD treatment. For example, inhibition of nSMase2, a key regulatory enzyme involved in ESCRT-independent exosome biogenesis with a small molecule GW4869, can reduce Aβ deposits both in vitro and in vivo [134]. Additionally, genetic depletion of nSMase2 can ameliorate AD pathology and improve cognitive deficits in 5×FAD mouse model of Alzheimer’s disease [135]. There are also some studies suggesting EVs may play a neuroprotective role in AD such as aiding in the degradation of amyloid plaques. Neuron-derived exosomes assist in conformational changes of A β to nontoxic amyloid fibrils and promote their uptake by microglia [98]. These Aβ were further transported to lysosomes for degradation. Additionally, exosomes derived from N2a cells or human CSF may ameliorate the synaptic-plasticity-disrupting activity of both synthetic and AD brain-derived Aβ in vivo [136]. These controversial studies demonstrate EVs would act as a ‘doubled-edged sword’ in the amyloid accumulations. In the future, more details of EVs, for example cell of origin, in vivo and vitro, and stage of disease, are needed for functional analysis.
Hyper-phosphorylated tau-based NFTs have also been proposed to contribute to the diffusion of neurodegeneration including AD, and this tangle pathology proceeds in a characteristic and predictable spatiotemporal manner [137, 138]. What is the underlying mechanism accounting for the sequential dissemination of tau? Apart from direct cell to cell contact for tau propagation, accumulating evidence recently supports that EVs are engaged in tau spread particularly as a form of long distance communication. Sudad et al. identified exosome-associated tau which is phosphorylated at Thr-181 (AT270), an established phosphotau biomarker for Alzheimer disease (AD), in human CSF samples from early AD [139]. Phosphorylated tau was also present in exosomes derived from plasma in patients with AD or frontotemporal dementia (FTD) [128]. The exosomal levels of P-T181-tau and P-S396-tau in AD were significantly higher than that in healthy controls even 1 to 10 years before AD diagnosis. Currently, induced pluripotent stem cells (iPSCs) have become ideal tools for the postnatal generation of specific diseased cell types from patients [140]. Researchers differentiated AD patient-derived iPSCs into functional neurons and detected more aggregation-competent Tau from neuron-derived EVs compared to healthy controls [141], which provides important insights of EV in the pathology of AD. Furthermore, in our previous study, more details of the EVs-associated tau propagation were provided by developing a mouse model with rapid tau spread. We demonstrated that microglia can spread tau via exosome secretion, and inhibiting exosome synthesis with GW4869 significantly reduced tau propagation in vitro and in vivo [7].
For the application of EVs to the potential biomarker in disease, It was mentioned that levels of Aβ1–42 and cellular survival factors from NDEs [128, 129], complements from ADEs [130], and phosphorylated tau isoforms from exosomes in CSF and plasma [128, 139] are associated with development of AD. In addition, several studies have identified some dysregulated miRNAs as potential biomarker for AD. A MicroRNA profiling study reported an opposite pattern of the microRNA expression in the exosome-enriched CSF AD samples and control samples; miR-9–5p and miR-598 are present in most of control CSF samples but not in AD samples [142]. Deep sequencing of plasma exosomal miRNAs from control and AD patients screened a panel of seven miRNAs to predict AD status with 83–89% accuracy [143]. Of these miRNAs, miR-342–3p is the most significant and brain-enriched in tissue expression. Notably, it’s common with two previous studies which reported circulating mir-342–3p is down-regulated in AD [144, 145]. Though promising, the literature of EV-associated miRNAs as biomarkers in AD is still limited and these findings require validation in different cohorts.
Amyotrophic lateral sclerosis (ALS)
The major pathological proteins of ALS include TAR DNA-binding protein 43 (TDP- 43), Cu/Zn superoxide dismutase 1 (SOD1) and fused in sarcoma (FUS), abnormal accumulations of which would result in motor neuron death in spinal cord, brain stem, and cortex [146–148]. These aggregates are also thought to propagate in a manner similar to prion proteins by which misfolded proteins seed at a focal site before spreading to contiguous neuroanatomical regions [149, 150]. Familial ALS (FALS) accounts for 5–10% of all cases, while the others are sporadic (SALS). No evidence shows difference in clinical characterization between FALS and SALS patients, suggesting that they may share common pathogenic mechanisms [151].
EVs, as new-discovered intercellular messengers, are raising more interests in the study of neurodegenerative diseases including ALS. The propagation of ALS has been proposed to be mediated through release and uptake of protein aggregates (SOD1, TDP-43, FUS) in EV-dependent pathways [150, 152, 153]. It was observed that wild type and mutant SOD1 were respectively present in exosomes within the supernatant medium from wild type and mutant SOD1 stably expressed NSC-34, a motor neuron-like cell line [154]. Moreover, astrocyte-derived exosomes were found to efficiently transport mutant SOD1 to spinal neurons and induce motor neuron death in primary astrocyte cultures overexpressing mutant SOD1 [155]. The authors believed that these exosomes might help limit the formation of intracellular aggregates and prevent from toxicity. But on the other side, exosome release is also considered to be a pathway for spreading disease [155].
Similar to SOD1 protein, TDP-43 was detected in secreted exosomes from Neuro2a cells and primary neurons, as well as from CSF of ALS patients [156, 157]. Application of exosomes isolated from brains of ALS patients, but not from healthy controls, to Neuro2a cells causes pathological TDP-43 aggregates, suggesting that secreted exosomes might contribute to propagation of TDP-43 proteinopathy [156]. This was supported also by another study that oligomeric TDP- 43 is packaged into exosomes, and is preferentially taken up by recipient cells, thereby leading to greater toxicity than free TDP-43 [158]. More recently, the researchers observed increased size of MVs and exosomes isolated from plasma of sporadic ALS patients compared to healthy controls [150].
Plasma-MVs and-exosomes of ALS patients are enriched in ALS associated proteins including SOD1, TDP-43, phospho-TDP-43, and FUS, suggesting that EVs can be a reliable biomarker [150]. In addition, Xu Q et al. reported the down-regulation of serum-derived exosomal miR-27a-3p which targets the genes promoting osteoblast mineralization in ALS patients compared to healthy subjects [159]. The author suggested it may be involved in the development of ALS, and therefore could be a candidate indicator for the diagnosis of ALS in the clinic. Several studies demonstrated exosomes from adipose-derived stem cells exert a neuroprotective effect in an in vitro model of ALS including increasing motoneuron survival and restoring mitochondrial protein function, demonstrating that EVs could be potential as a novel therapeutic target to halt the course of ALS [160–162].
Huntington’s Disease
The neuropathological feature of Huntington’s disease (HD) is intracellular inclusions consisting of mutant HTT protein, which is encoded by mutant HTT gene with a trinucleotide repeat CAG in the first coding exon [163]. The mutant HTT protein contains the expansion of polyglutamine (polyQ) repeat which can form protein aggregates and fibrils and induce neurotoxicity in brain [164].
EVs are recently considered as an important regulator in HD propagation. Both polyQ protein and the repeat RNA were found incorporation into EVs in a model of human 293T cells with the overexpression of mutant HTT gene [164]. A higher level of the repeat RNA was loaded into EVs than the level of the normal mRNA, indicating the potential role of EVs in transferring toxic repeat RNAs. This is supported by further experiment that these EVs can be taken up by striatal mouse neural cells though no apparent toxicity was observed probably due to a short incubation period [164]. Otherwise, a more recent study determined a neuroprotective role of cell-type specific EVs in HD [165]. The researchers observed the defective exosome release from astrocytes in a mutant huntingtin HD140Q knock-in (KI) mouse model. Further, injection of normal astrocytic exosomes into the striatum of HD140Q KI mice could reduce the density of pathogenic aggregates [165]. Exosomes from adipose-derived stem cells, which are known to secrete various neurotrophic factors and also studied in ALS, are shown to decrease protein aggregates and ameliorate mitochondrial function in an in vitro HD model [166]. All these findings define a progressive role of EVs in HD propagation but also reveal a potential therapeutic effect of EVs on disease.
Parkinson’s Disease
Parkinson’s disease (PD) is characterized by the pathologic accumulation and aggregation of α- synuclein (α-syn) in the neuronal soma (Lewy bodies) and neurites (Lewy neurites) [167, 168]. α-syn aggregates are formed in a step-wise manner that misfolded monomers lead to the formation of oligomers and protofibrils, which eventually deposit as fibril and insoluble aggregates [169]. In PD, Lewy bodies are initially resident in brain stem as well as the substantia nigra at early stages, which become more widespread to cerebral cortex and other brain regions as the disease progresses.
Recent evidence suggests that EVs may play a vital role in the hierarchical spreading of toxic α-syn within brain. α-syn was found elevated in serum-derived EVs from PD patients [170]. It has been proposed that exosomes can participate in the inter-neuronal transmission of α-syn by using neuronal-like cells [124]. Oligomeric α-syn has been detected half within the exosomes and half on the extracellular space by fusion of α-syn to humanized Ganussia Luciferase [171], indicating the presence of EVs-associated α-syn. Additionally, these exosomal α-syn oligomers are more efficient to be taken up by recipient cells and induce toxicity compared with free α-syn oligomers. Further evidence indicates that exosomes can trigger the aggregation of α-syn, probably due to the optimal catalytic environments for nucleation they provide [172]. The authors also concluded that this reaction is more likely to be mediated by phospholipids which are enriched in vesicular membranes. Another study supports this hypothesis. The lipid peroxidation product 4-hydroxynonenal (HNE) was found to increase not only the aggregation of endogenous α-syn but the secretion of EVs containing toxic α-syn as a consequence of degeneration in primary neurons [173]. Moreover, stereotaxic injection of these EVs into the striatum of wild-type mice leads to the spread of synuclein pathology to anatomically connected brain regions including the cerebral cortex, substantia nigra, and hippocampus [173]. Notably, a current study demonstrated a new mechanism based on EV-associated α-syn for PD progression: α-syn-rich EVs produced by periphery erythrocytes could cross the blood-brain barrier and provoke microglial inflammatory responses to initiate CNS α-syn-related pathology [174]. All these findings support a role of EVs in the transcellular propagation of α-syn.
In addition to α-syn, other PD-related proteins such as PARK9 and LRRK2 may be involved in EVs trafficking pathway thereby regulating α-syn transport [169]. For example, overexpression of PARK9 results in increased release and loss of function mutations in PARK9 results in decreased secretion of α-syn into extracellular space via exosomes [175]. Similar functions have been implicated in LRRK2, too. LRRK2 is known to colocalize with MVBs, and the R1441C LRRK2 mutant has been reported to increase the number and size of MVBs; LRRK2 dysfunction would disrupt the dynamics of vesicular release [1, 176]. Another study demonstrated that LRRK2 interacting with Rab5b regulates endocytosis of synaptic proteins, suggesting LRRK2 might also have a role in EVs-associated uptake [177]. Moreover, LRRK2 is released in exosomes from cells [178].
Levels of autophosphorylated LRRK2 (P-S1292) were found to be elvated in urinary exosomes and are correlated with the severity of cognitive impairment and difficulty in accomplishing activities of daily living, which is a promising candidate biomarker for PD [179]. Also, EV-associated miRNAs are useful for diagnosis of PD though the studies are limited [180]. Gui et al. reported that miR-1 and miR-19b-3p are significantly reduced in PD CSF exosome, while miR-153, miR-409–3p, miR-10a-5p, and let-7g-3p are elevated [181]. Further pathway analysis demonstrated that these molecules are targeted to Neurotrophin signaling and Dopaminergic synapse. Another group collected serum-derived exosome-like microvesicles from PD patients and validated the downregulation of miR-19b, the upregulation of miR-195 and miR-24 in PD compared to healthy controls [182].
Prion Diseases
Prion diseases such as Creutzfeldt–Jakob disease are associated with the aggregates of misfolded prion protein (PrPC) [183]. PrPC misfolding results in the generation of pathological prion protein (PrPSc), a key factor in the pathophysiology of prion diseases [184]. Different mechanisms of how prion proteins move and progressively spread between cells have been proposed, including direct release and uptake by nearby cells, and intercellular transfer through tunneling nanotubes [24, 185].
Increasing evidence indicates that exosomes represent a novel and efficient way for prion transmission. Saá et al. first reported the presence of PrPSc in exosomes isolated from plasma in a prion disease mouse model [186]. After, they injected these EVs to PrPC transgenic mice and found the transmission of prion occurs, suggesting exosomes are the most likely carriers of intercellular PrPSc transmission [187]. Further evidence identified the mechanisms by which PrPC or PrPSc is packaged into exosomes. Guo et al. proves that the neutral sphingomyelinase pathway regulates exosome biogenesis and packaging of PrPC into these vesicles by inhibition of the pathway using GW4869 [188]. Moreover, packaging of PrPC into exosomes is dependent on nSMase2 whereas PrPSc packaging occurs independently of nSMase2, providing new insights into prion transmission and identify a pathway which might be targeted to avoid the infection [189].
In addition, some miRNAs were found dysregulated in exosomes from prion infected samples. A small RNA deep sequencing of exosomes released by prion-infected neuronal cells demonstrated a distinct miRNA signature compared to non-infected exosomes (increased let-7b, let-7i, miR-128a, miR-21, miR-222, miR-29b, miR-342–3p, and miR-424 levels with decreased miR-146a levels) [43]. These results might not only be utilized for diagnostic biomarker but provide better understanding of the pathogenic mechanisms in prion disease.
Discussion
In the past decade, the major progress on EVs was finding their active roles in cell-to-cell communication, both under healthy and pathological conditions. The properties that EVs can be accessibly isolated from biofluids and carry cell-specific cargoes, including proteins and nucleotides, give them the potential to harbor disease-specific molecular signatures [1]. Therefore, vesicles have been proposed as useful candidate biomarkers as well as promising therapeutic targets in neurological diseases. For example, the levels of Aβ1–42, P-T181-tau, and P-S396-tau in plasmal exosomes predict the development of AD 1 to 10 years before clinical diagnosis [128]. High complement proteins including C1q, C4b, C3d were found in exosomes from AD patients [130]. For PD, levels of α-syn and autophosphorylated LRRK2 were elevated and found correlation with the severity of disease [170, 179]. In addition, a distinct miRNA signature in exosomes has also been revealed in other neurodegenerations [43].
Development of EV biomarkers show promise; however, reproducibility across studies is poor. One reason is that the pathology of neurodegenerative disorders is associated with distinct subsets of CNS cells, and the heterogeneity of EV populations and technical limitations of current EV extraction methods in terms of EV yield and purity make the identification of reliable biomarkers a challenge [1]. In order to solve these problems, researchers applied immunoaffinity enrichment to immunoprecipitate CNS-specific exosomes from AD samples via cell-specific antibodies, (e.g., neuron-specific L1CAM and astrocyte-specific EAAT1), and indeed obtained some promising results [128, 130, 190]. However, no study shows that these proteins are present in EVs isolated from the different cell types. Therefore, identification of cell-type specific EVs markers is necessary for comprehensive understanding of the sources of pathological EVs and the potential therapeutic targets for halting propagation of diseases. Future research will focus on enabling high-yield capture of EVs and shed light on the nature of the different extracellular vesicle subpopulations that could be associated with distinct pathological stages in a given disease.
Aside from developing new biomarkers and therapeutic targets for neurodegenerative diseases, EVs themselves could be used as non-invasive vectors to deliver defined compounds for therapeutics. The best advantages is that EVs are biocompatible and self-derived, and therefore they are immunologically inert and less likely to trigger innate and adaptive immune responses [191]. Mesenchymal stem cell-derived EVs has been tested to treat acute kidney injury [192], stroke [193] and ischemic brain injury without immunogenicity [194]. A previous study successfully designed the engineered exosomes which were loaded with short interfering RNAs (siRNAs) to target the CNS cells and silence expression of BACE1, a therapeutic target in AD, in wide-type mice [195]. Genetically engineered microvesicles expressing suicide gene can effectively inhibit schwannoma tumor growth [196]. Moreover, application of EV-based drug delivery has been registered in serval clinical trials [197]. Undoubtedly, these aforementioned use of EVs have addressed the potential of EVs as novel therapeutics in various diseases.
Despite the enormous therapeutic potential, there are still many questions of EVs remaining to be answered [107]. It is unclear how EVs are sorted to disparate targets and how recipient cells discriminate among EVs. The mechanisms of EVs uptake by recipient cells also remains unknown, as well as the decision of EV cargoes’ destination of lysosome for degradation or to be utilized by recipient cells. More new in vivo models combined with powerful imaging methods to track the biogenesis, secretion, uptake, and fates of EVs will help us further understand the basic functions of EVs and promote the translation of researches into the clinical applications.
Acknowledgements
We would like to thank Samuel Hersh for editing the manuscript. This work is funded in part by Nancy Lurie Marks Family Foundation (TI), Robert E. Landreth and Dona Landreth Family Foundation (TI), BrightFocus Foundation (A2016551S), Cure Alzheimer’s Fund, NIH R01AG054672 (TI), RF1AG054199 (TI), R56AG057469 (TI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson AG, Gray E, Heman-Ackah SM, Mager I, Talbot K, Andaloussi SE, et al. Extracellular vesicles in neurodegenerative disease - pathogenesis to biomarkers. Nat Rev Neurol. 2016;12(6):346–57. doi: 10.1038/nrneurol.2016.68. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20. [PubMed] [Google Scholar]
- 3.Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16(1):24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450. doi: 10.1371/journal.pbio.1001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciregia F, Urbani A, Palmisano G. Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front Mol Neurosci. 2017;10:276. doi: 10.3389/fnmol.2017.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asai H, Ikezu S, Tsunoda S, Medalla M, Luebke J, Haydar T, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat Neurosci. 2015;18(11):1584–93. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C, Mobius W, Berger H, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: Trophic support for axons? Proteomics Clin Appl. 2007;1(11):1446–61. doi: 10.1002/prca.200700522. [DOI] [PubMed] [Google Scholar]
- 9.Chiarini A, Armato U, Gardenal E, Gui L, Dal Pra I. Amyloid beta-Exposed Human Astrocytes Overproduce Phospho-Tau and Overrelease It within Exosomes, Effects Suppressed by Calcilytic NPS 2143-Further Implications for Alzheimer’s Therapy. Front Neurosci. 2017;11:217. doi: 10.3389/fnins.2017.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLeo AM, Ikezu T. Extracellular Vesicle Biology in Alzheimer’s Disease and Related Tauopathy . J Neuroimmune Pharmacol. 2018;13(3):292–308. doi: 10.1007/s11481-017-9768-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 12.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94(11):3791–9. [PubMed] [Google Scholar]
- 13.Buschow SI, Nolte-’t Hoen EN, van Niel G, Pols MS, ten Broeke T, Lauwen M, et al. MHC II in dendritic cells is targeted to lysosomes or T cell-induced exosomes via distinct multivesicular body pathways. Traffic. 2009;10(10):1528–42. doi: 10.1111/j.1600-0854.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 14.Klumperman J, Raposo G. The complex ultrastructure of the endolysosomal system. Cold Spring Harb Perspect Biol. 2014;6(10):a016857. doi: 10.1101/cshperspect.a016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: a common pathway for a specialized function. J Biochem. 2006;140(1):13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- 16.Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113(8):E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33(3):967–78. [DOI] [PubMed] [Google Scholar]
- 19.Harding C, Stahl P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem Biophys Res Commun. 1983;113(2):650–8. [DOI] [PubMed] [Google Scholar]
- 20.Vella LJ, Greenwood DL, Cappai R, Scheerlinck JP, Hill AF. Enrichment of prion protein in exosomes derived from ovine cerebral spinal fluid. Vet Immunol Immunopathol. 2008;124(3–4):385–93. doi: 10.1016/j.vetimm.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17(7):879–87. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 22.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101(36):13368–73. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janas AM, Sapon K, Janas T, Stowell MH, Janas T. Exosomes and other extracellular vesicles in neural cells and neurodegenerative diseases. Biochim Biophys Acta. 2016;1858(6):1139–51. doi: 10.1016/j.bbamem.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Gousset K, Schiff E, Langevin C, Marijanovic Z, Caputo A, Browman DT, et al. Prions hijack tunnelling nanotubes for intercellular spread. Nat Cell Biol. 2009;11(3):328–36. doi: 10.1038/ncb1841. [DOI] [PubMed] [Google Scholar]
- 25.Lindenbergh MFS, Stoorvogel W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu Rev Immunol. 2018;36:435–59. doi: 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 26.Buzas EI, Gyorgy B, Nagy G, Falus A, Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10(6):356–64. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 27.Ge R, Tan E, Sharghi-Namini S, Asada HH. Exosomes in Cancer Microenvironment and Beyond: have we Overlooked these Extracellular Messengers? Cancer Microenviron. 2012;5(3):323–32. doi: 10.1007/s12307-012-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tricarico C, Clancy J, D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8(4):220–32. doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of Exosome Composition. Cell. 2019;177(2):428–45 e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol Cell Proteomics. 2013;12(3):587–98. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crescitelli R, Lasser C, Szabo TG, Kittel A, Eldh M, Dianzani I, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2. doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momen-Heravi F, Getting SJ, Moschos SA. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol Ther. 2018;192:170–87. doi: 10.1016/j.pharmthera.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardiner C, Di Vizio D, Sahoo S, Thery C, Witwer KW, Wauben M, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobrie A, Colombo M, Krumeich S, Raposo G, Thery C. Diverse subpopulations of vesicles secreted by different intracellular mechanisms are present in exosome preparations obtained by differential ultracentrifugation. J Extracell Vesicles. 2012;1. doi: 10.3402/jev.v1i0.18397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–43. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, et al. EV- TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14(3):228–32. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 38.Wolf P The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–88. [DOI] [PubMed] [Google Scholar]
- 39.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20(9):1487–95. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 40.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Li X. Recent advances in extracellular vesicles enriched with non-coding RNAs related to cancers. Genes Dis. 2018;5(1):36–42. doi: 10.1016/j.gendis.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanale D, Taverna S, Russo A, Bazan V. Circular RNA in Exosomes. Adv Exp Med Biol. 2018;1087:109–17. doi: 10.1007/978-981-13-1426-1_9. [DOI] [PubMed] [Google Scholar]
- 43.Bellingham SA, Coleman BM, Hill AF. Small RNA deep sequencing reveals a distinct miRNA signature released in exosomes from prion-infected neuronal cells. Nucleic Acids Res. 2012;40(21):10937–49. doi: 10.1093/nar/gks832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10): 1603–11. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nolte-’t Hoen EN, Buermans HP, Waasdorp M, Stoorvogel W, Wauben MH, t Hoen PA. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–85. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, et al. Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genomics. 2013;14:319. doi: 10.1186/1471-2164-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3. doi: 10.3402/jev.v3.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J Neural Transm (Vienna) 2010;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- 49.Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–9. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2013;32(22):2747–55. doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 51.Zhou CF, Ma J, Huang L, Yi HY, Zhang YM, Wu XG, et al. Cervical squamous cell carcinoma-secreted exosomal miR-221–3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1. Oncogene. 2019;38(8):1256–68. doi: 10.1038/s41388-018-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci U S A. 2012;109(31):E2110–6. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lafourcade C, Ramirez JP, Luarte A, Fernandez A, Wyneken U. MiRNAs in Astrocyte-Derived Exosomes as Possible Mediators of Neuronal Plasticity. J Exp Neurosci. 2016;10(Suppl 1):1–9. doi: 10.4137/JEN.S39916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herrera M, Llorens C, Rodriguez M, Herrera A, Ramos R, Gil B, et al. Differential distribution and enrichment of non-coding RNAs in exosomes from normal and Cancer-associated fibroblasts in colorectal cancer. Mol Cancer. 2018;17(1):114. doi: 10.1186/s12943-018-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ashley J, Cordy B, Lucia D, Fradkin LG, Budnik V, Thomson T. Retrovirus-like Gag Protein Arc1 Binds RNA and Traffics across Synaptic Boutons. Cell. 2018;172(1–2):262–74 e11. doi: 10.1016/j.cell.2017.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem. 2003;278(13):10963–72. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 58.Brouwers JF, Aalberts M, Jansen JW, van Niel G, Wauben MH, Stout TA, et al. Distinct lipid compositions of two types of human prostasomes. Proteomics. 2013;13(10–11):1660–6. doi: 10.1002/pmic.201200348. [DOI] [PubMed] [Google Scholar]
- 59.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 60.Greening DW, Xu R, Gopal SK, Rai A, Simpson RJ. Proteomic insights into extracellular vesicle biology - defining exosomes and shed microvesicles. Expert Rev Proteomics. 2017;14(1):69–95. doi: 10.1080/14789450.2017.1260450. [DOI] [PubMed] [Google Scholar]
- 61.Pathan M, Fonseka P, Chitti SV, Kang T, Sanwlani R, Van Deun J, et al. Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–D9. doi: 10.1093/nar/gky1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keerthikumar S, Chisanga D, Ariyaratne D, Al Saffar H, Anand S, Zhao K, et al. ExoCarta: A Web-Based Compendium of Exosomal Cargo. J Mol Biol. 2016;428(4):688–92. doi: 10.1016/j.jmb.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7(1):1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20(1):4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Juan T, Furthauer M. Biogenesis and function of ESCRT-dependent extracellular vesicles. Semin Cell Dev Biol. 2018;74:66–77. doi: 10.1016/j.semcdb.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 66.Hurley JH. The ESCRT complexes. Crit Rev Biochem Mol Biol. 2010;45(6):463–87. doi: 10.3109/10409238.2010.502516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 68.Marsh M, van Meer G. Cell biology. No ESCRTs for exosomes. Science. 2008;319(5867):1191–2. doi: 10.1126/science.1155750. [DOI] [PubMed] [Google Scholar]
- 69.Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22(45):7070–7. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- 70.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–21. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Theos AC, Truschel ST, Tenza D, Hurbain I, Harper DC, Berson JF, et al. A lumenal domain-dependent pathway for sorting to intralumenal vesicles of multivesicular endosomes involved in organelle morphogenesis. Dev Cell. 2006;10(3):343–54. doi: 10.1016/j.devcel.2006.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van Niel G, Bergam P, Di Cicco A, Hurbain I, Lo Cicero A, Dingli F, et al. Apolipoprotein E Regulates Amyloid Formation within Endosomes of Pigment Cells. Cell Rep. 2015;13(1):43–51. doi: 10.1016/j.celrep.2015.08.057. [DOI] [PubMed] [Google Scholar]
- 73.Malla RR, Pandrangi S, Kumari S, Gavara MM, Badana AK. Exosomal tetraspanins as regulators of cancer progression and metastasis and novel diagnostic markers. Asia Pac J Clin Oncol. 2018;14(6):383–91. doi: 10.1111/ajco.12869. [DOI] [PubMed] [Google Scholar]
- 74.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190(6):1079–91. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zimmerman B, Kelly B, McMillan BJ, Seegar TCM, Dror RO, Kruse AC, et al. Crystal Structure of a Full-Length Human Tetraspanin Reveals a Cholesterol-Binding Pocket. Cell. 2016;167(4):1041–51 e11. doi: 10.1016/j.cell.2016.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Andreu Z, Yanez-Mo M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:442. doi: 10.3389/fimmu.2014.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nabhan JF, Hu R, Oh RS, Cohen SN, Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109(11):4146–51. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Del Conde I, Shrimpton CN, Thiagarajan P, Lopez JA. Tissue-factor-bearing microvesicles arise from lipid rafts and fuse with activated platelets to initiate coagulation. Blood. 2005;106(5):1604–11. doi: 10.1182/blood-2004-03-1095. [DOI] [PubMed] [Google Scholar]
- 79.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–24. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 80.Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, et al. ARF6- regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19(22):1875–85. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31(45):4740–9. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, et al. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166(12):7309–18. [DOI] [PubMed] [Google Scholar]
- 83.Geminard C, De Gassart A, Blanc L, Vidal M. Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes. Traffic. 2004;5(3):181–93. doi: 10.1111/j.1600-0854.2004.0167.x. [DOI] [PubMed] [Google Scholar]
- 84.Buschow SI, van Balkom BW, Aalberts M, Heck AJ, Wauben M, Stoorvogel W. MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88(8):851–6. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- 85.Buschow SI, Liefhebber JM, Wubbolts R, Stoorvogel W. Exosomes contain ubiquitinated proteins. Blood Cells Mol Dis. 2005;35(3):398–403. doi: 10.1016/j.bcmd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 86.Luhtala N, Aslanian A, Yates JR 3rd, Hunter T Secreted Glioblastoma Nanovesicles Contain Intracellular Signaling Proteins and Active Ras Incorporated in a Farnesylation-dependent Manner. J Biol Chem. 2017;292(2):611–28. doi: 10.1074/jbc.M116.747618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Irion U, St Johnston D. bicoid RNA localization requires specific binding of an endosomal sorting complex. Nature. 2007;445(7127):554–8. doi: 10.1038/nature05503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11(9):1143–9. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 89.Shurtleff MJ, Temoche-Diaz MM, Karfilis KV, Ri S, Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5. doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shurtleff MJ, Yao J, Qin Y, Nottingham RM, Temoche-Diaz MM, Schekman R, et al. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A. 2017;114(43):E8987–E95. doi: 10.1073/pnas.1712108114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bolukbasi MF, Mizrak A, Ozdener GB, Madlener S, Strobel T, Erkan EP, et al. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol Ther Nucleic Acids. 2012;1:e10. doi: 10.1038/mtna.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pastuzyn ED, Day CE, Kearns RB, Kyrke-Smith M, Taibi AV, McCormick J, et al. The Neuronal Gene Arc Encodes a Repurposed Retrotransposon Gag Protein that Mediates Intercellular RNA Transfer. Cell. 2018;172(1–2):275–88 e18. doi: 10.1016/j.cell.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, et al. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70(4):1668–78. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- 95.Rana S, Yue S, Stadel D, Zoller M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int J Biochem Cell Biol. 2012;44(9):1574–84. doi: 10.1016/j.biocel.2012.06.018. [DOI] [PubMed] [Google Scholar]
- 96.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104(10):3257–66. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 97.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the Surface of Myeloma Cell-derived Exosomes Mediates Exosome-Cell Interactions. J Biol Chem. 2016;291(4):1652–63. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012;287(14):10977–89. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lima LG, Leal AC, Vargas G, Porto-Carreiro I, Monteiro RQ. Intercellular transfer of tissue factor via the uptake of tumor-derived microvesicles. Thromb Res. 2013;132(4):450–6. doi: 10.1016/j.thromres.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 100.Chivet M, Javalet C, Laulagnier K, Blot B, Hemming FJ, Sadoul R. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722. doi: 10.3402/jev.v3.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laulagnier K, Javalet C, Hemming FJ, Chivet M, Lachenal G, Blot B, et al. Amyloid precursor protein products concentrate in a subset of exosomes specifically endocytosed by neurons. Cell Mol Life Sci. 2018;75(4):757–73. doi: 10.1007/s00018-017-2664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prada I, Amin L, Furlan R, Legname G, Verderio C, Cojoc D. A new approach to follow a single extracellular vesicle-cell interaction using optical tweezers. Biotechniques. 2016;60(1):35–41. doi: 10.2144/000114371. [DOI] [PubMed] [Google Scholar]
- 103.Tian T, Wang Y, Wang H, Zhu Z, Xiao Z. Visualizing of the cellular uptake and intracellular trafficking of exosomes by live-cell microscopy. J Cell Biochem. 2010;111(2):488–96. doi: 10.1002/jcb.22733. [DOI] [PubMed] [Google Scholar]
- 104.Heusermann W, Hean J, Trojer D, Steib E, von Bueren S, Graff-Meyer A, et al. Exosomes surf on filopodia to enter cells at endocytic hot spots, traffic within endosomes, and are targeted to the ER. J Cell Biol. 2016;213(2):173–84. doi: 10.1083/jcb.201506084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, et al. Studying the Fate of Tumor Extracellular Vesicles at High Spatiotemporal Resolution Using the Zebrafish Embryo. Dev Cell. 2019;48(4):554–72 e7. doi: 10.1016/j.devcel.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 106.Perez-Gonzalez R, Gauthier SA, Kumar A, Levy E. The exosome secretory pathway transports amyloid precursor protein carboxyl-terminal fragments from the cell into the brain extracellular space. J Biol Chem. 2012;287(51):43108–15. doi: 10.1074/jbc.M112.404467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17(3):160–72. doi: 10.1038/nrn.2015.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zakharov A, Papaiconomou C, Djenic J, Midha R, Johnston M. Lymphatic cerebrospinal fluid absorption pathways in neonatal sheep revealed by subarachnoid injection of Microfil. Neuropathol Appl Neurobiol. 2003;29(6):563–73. [DOI] [PubMed] [Google Scholar]
- 109.Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30(12):4141–8. doi: 10.1096/fj.201600816R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chiasserini D, van Weering JR, Piersma SR, Pham TV, Malekzadeh A, Teunissen CE, et al. Proteomic analysis of cerebrospinal fluid extracellular vesicles: a comprehensive dataset. J Proteomics. 2014;106:191–204. doi: 10.1016/j.jprot.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 111.Frohlich D, Kuo WP, Fruhbeis C, Sun JJ, Zehendner CM, Luhmann HJ, et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652). doi: 10.1098/rstb.2013.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fitzner D, Schnaars M, van Rossum D, Krishnamoorthy G, Dibaj P, Bakhti M, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(Pt 3):447–58. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 113.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174(11):7268–77. [DOI] [PubMed] [Google Scholar]
- 114.Antonucci F, Turola E, Riganti L, Caleo M, Gabrielli M, Perrotta C, et al. Microvesicles released from microglia stimulate synaptic activity via enhanced sphingolipid metabolism. EMBO J. 2012;31(5):1231–40. doi: 10.1038/emboj.2011.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bianco F, Perrotta C, Novellino L, Francolini M, Riganti L, Menna E, et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009;28(8):1043–54. doi: 10.1038/emboj.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang S, Cesca F, Loers G, Schweizer M, Buck F, Benfenati F, et al. Synapsin I is an oligomannose-carrying glycoprotein, acts as an oligomannose-binding lectin, and promotes neurite outgrowth and neuronal survival when released via glia-derived exosomes. J Neurosci. 2011;31(20):7275–90. doi: 10.1523/JNEUROSCI.6476-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sami Saribas A, Cicalese S, Ahooyi TM, Khalili K, Amini S, Sariyer IK. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2017;8(1):e2542. doi: 10.1038/cddis.2016.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem. 2011;286(1):787–96. doi: 10.1074/jbc.M110.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer’s disease. J Chem Neuroanat. 2004;28(1–2):67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 120.Marttinen M, Kurkinen KM, Soininen H, Haapasalo A, Hiltunen M. Synaptic dysfunction and septin protein family members in neurodegenerative diseases. Mol Neurodegener. 2015;10:16. doi: 10.1186/s13024-015-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat Rev Immunol. 2014;14(7):463–77. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 122.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. Proc Natl Acad Sci U S A. 2004;101(26):9683–8. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, et al. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci U S A. 2006;103(30):11172–7. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, et al. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–51. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamaguchi H, Hirai S, Shoji M, Harigaya Y, Okamoto Y, Nakazato Y. Alzheimer type dementia: diffuse type of senile plaques demonstrated by beta protein immunostaining. Prog Clin Biol Res. 1989;317:467–74. [PubMed] [Google Scholar]
- 126.Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci U S A. 1988;85(11):4051–5. doi: 10.1073/pnas.85.11.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med. 2014;20(2):130–8. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015;11(6):600–7 e1. doi: 10.1016/j.jalz.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goetzl EJ, Boxer A, Schwartz JB, Abner EL, Petersen RC, Miller BL, et al. Low neural exosomal levels of cellular survival factors in Alzheimer’s disease. Ann Clin Transl Neurol. 2015;2(7):769–73. doi: 10.1002/acn3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goetzl EJ, Schwartz JB, Abner EL, Jicha GA, Kapogiannis D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann Neurol. 2018;83(3):544–52. doi: 10.1002/ana.25172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Agosta F, Dalla Libera D, Spinelli EG, Finardi A, Canu E, Bergami A, et al. Myeloid microvesicles in cerebrospinal fluid are associated with myelin damage and neuronal loss in mild cognitive impairment and Alzheimer disease. Ann Neurol. 2014;76(6):813–25. doi: 10.1002/ana.24235. [DOI] [PubMed] [Google Scholar]
- 132.Joshi P, Turola E, Ruiz A, Bergami A, Libera DD, Benussi L, et al. Microglia convert aggregated amyloid-beta into neurotoxic forms through the shedding of microvesicles. Cell Death Differ. 2014;21(4):582–93. doi: 10.1038/cdd.2013.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, et al. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136(1):41–56. doi: 10.1007/s00401-018-1868-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dinkins MB, Dasgupta S, Wang G, Zhu G, Bieberich E. Exosome reduction in vivo is associated with lower amyloid plaque load in the 5XFAD mouse model of Alzheimer’s disease. Neurobiol Aging. 2014;35(8):1792–800. doi: 10.1016/j.neurobiolaging.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dinkins MB, Enasko J, Hernandez C, Wang G, Kong J, Helwa I, et al. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5 XFAD Mouse. J Neurosci. 2016;36(33):8653–67. doi: 10.1523/JNEUROSCI.1429-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O’Dowd ST, et al. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol Brain. 2013;6:47. doi: 10.1186/1756-6606-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–59. [DOI] [PubMed] [Google Scholar]
- 138.Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64(1):4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- 139.Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, et al. Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem. 2012;287(6):3842–9. doi: 10.1074/jbc.M111.277061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tang BL. Patient-Derived iPSCs and iNs-Shedding New Light on the Cellular Etiology of Neurodegenerative Diseases. Cells. 2018;7(5). doi: 10.3390/cells7050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guix FX, Corbett GT, Cha DJ, Mustapic M, Liu W, Mengel D, et al. Detection of Aggregation-Competent Tau in Neuron-Derived Extracellular Vesicles. Int J Mol Sci. 2018;19(3). doi: 10.3390/ijms19030663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Riancho J, Vazquez-Higuera JL, Pozueta A, Lage C, Kazimierczak M, Bravo M, et al. MicroRNA Profile in Patients with Alzheimer’s Disease: Analysis of miR-9–5p and miR-598 in Raw and Exosome Enriched Cerebrospinal Fluid Samples. J Alzheimers Dis. 2017;57(2):483–91. doi: 10.3233/JAD-161179. [DOI] [PubMed] [Google Scholar]
- 143.Lugli G, Cohen AM, Bennett DA, Shah RC, Fields CJ, Hernandez AG, et al. Plasma Exosomal miRNAs in Persons with and without Alzheimer Disease: Altered Expression and Prospects for Biomarkers. PLoS One. 2015;10(10):e0139233. doi: 10.1371/journal.pone.0139233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cheng L, Doecke JD, Sharples RA, Villemagne VL, Fowler CJ, Rembach A, et al. Prognostic serum miRNA biomarkers associated with Alzheimer’s disease shows concordance with neuropsychological and neuroimaging assessment. Mol Psychiatry. 2015;20(10):1188–96. doi: 10.1038/mp.2014.127. [DOI] [PubMed] [Google Scholar]
- 145.Tan L, Yu JT, Tan MS, Liu QY, Wang HF, Zhang W, et al. Genome-wide serum microRNA expression profiling identifies serum biomarkers for Alzheimer’s disease. J Alzheimers Dis. 2014;40(4):1017–27. doi: 10.3233/JAD-132144. [DOI] [PubMed] [Google Scholar]
- 146.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 147.Kaur SJ, McKeown SR, Rashid S. Mutant SOD1 mediated pathogenesis of Amyotrophic Lateral Sclerosis. Gene. 2016;577(2):109–18. doi: 10.1016/j.gene.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 148.Tan RH, Ke YD, Ittner LM, Halliday GM. ALS/FTLD: experimental models and reality. Acta Neuropathol. 2017;133(2):177–96. doi: 10.1007/s00401-016-1666-6. [DOI] [PubMed] [Google Scholar]
- 149.Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68(19):1571–5. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- 150.Sproviero D, La Salvia S, Giannini M, Crippa V, Gagliardi S, Bernuzzi S, et al. Pathological Proteins Are Transported by Extracellular Vesicles of Sporadic Amyotrophic Lateral Sclerosis Patients. Front Neurosci. 2018;12:487. doi: 10.3389/fnins.2018.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hanspal MA, Dobson CM, Yerbury JJ, Kumita JR. The relevance of contact-independent cell-to-cell transfer of TDP-43 and SOD1 in amyotrophic lateral sclerosis. Biochim Biophys Acta Mol Basis Dis. 2017;1863(11):2762–71. doi: 10.1016/j.bbadis.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grad LI, Yerbury JJ, Turner BJ, Guest WC, Pokrishevsky E, O’Neill MA, et al. Intercellular propagated misfolding of wild-type Cu/Zn superoxide dismutase occurs via exosome-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2014;111(9):3620–5. doi: 10.1073/pnas.1312245111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Silverman JM, Fernando SM, Grad LI, Hill AF, Turner BJ, Yerbury JJ, et al. Disease Mechanisms in ALS: Misfolded SOD1 Transferred Through Exosome-Dependent and Exosome- Independent Pathways. Cell Mol Neurobiol. 2016;36(3):377–81. doi: 10.1007/s10571-015-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gomes C, Keller S, Altevogt P, Costa J. Evidence for secretion of Cu,Zn superoxide dismutase via exosomes from a cell model of amyotrophic lateral sclerosis. Neurosci Lett. 2007;428(1):43–6. doi: 10.1016/j.neulet.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 155.Basso M, Pozzi S, Tortarolo M, Fiordaliso F, Bisighini C, Pasetto L, et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem. 2013;288(22):15699–711. doi: 10.1074/jbc.M112.425066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Iguchi Y, Eid L, Parent M, Soucy G, Bareil C, Riku Y, et al. Exosome secretion is a key pathway for clearance of pathological TDP-43. Brain. 2016;139(Pt 12):3187–201. doi: 10.1093/brain/aww237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feneberg E, Steinacker P, Lehnert S, Schneider A, Walther P, Thal DR, et al. Limited role of free TDP-43 as a diagnostic tool in neurodegenerative diseases. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5–6):351–6. doi: 10.3109/21678421.2014.905606. [DOI] [PubMed] [Google Scholar]
- 158.Feiler MS, Strobel B, Freischmidt A, Helferich AM, Kappel J, Brewer BM, et al. TDP-43 is intercellularly transmitted across axon terminals. J Cell Biol. 2015;211(4):897–911. doi: 10.1083/jcb.201504057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu Q, Zhao Y, Zhou X, Luan J, Cui Y, Han J. Comparison of the extraction and determination of serum exosome and miRNA in serum and the detection of miR-27a-3p in serum exosome of ALS patients. Intractable Rare Dis Res. 2018;7(1):13–8. doi: 10.5582/irdr.2017.01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bonafede R, Mariotti R. ALS Pathogenesis and Therapeutic Approaches: The Role of Mesenchymal Stem Cells and Extracellular Vesicles. Front Cell Neurosci. 2017;11:80. doi: 10.3389/fncel.2017.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D, et al. Exosome derived from murine adipose-derived stromal cells: Neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 2016;340(1):150–8. doi: 10.1016/j.yexcr.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 162.Lee M, Ban JJ, Kim KY, Jeon GS, Im W, Sung JJ, et al. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem Biophys Res Commun. 2016;479(3):434–9. doi: 10.1016/j.bbrc.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 163.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72(6):971–83. [DOI] [PubMed] [Google Scholar]
- 164.Zhang X, Abels ER, Redzic JS, Margulis J, Finkbeiner S, Breakefield XO. Potential Transfer of Polyglutamine and CAG-Repeat RNA in Extracellular Vesicles in Huntington’s Disease: Background and Evaluation in Cell Culture. Cell Mol Neurobiol. 2016;36(3):459–70. doi: 10.1007/s10571-016-0350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hong Y, Zhao T, Li XJ, Li S. Mutant Huntingtin Inhibits alphaB-Crystallin Expression and Impairs Exosome Secretion from Astrocytes. J Neurosci. 2017;37(39):9550–63. doi: 10.1523/JNEUROSCI.1418-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lee M, Liu T, Im W, Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur J Neurosci. 2016;44(4):2114–9. doi: 10.1111/ejn.13275. [DOI] [PubMed] [Google Scholar]
- 167.Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, et al. Poly(ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson’s disease. Science. 2018;362(6414). doi: 10.1126/science.aat8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, et al. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152(4):879–84. [PMC free article] [PubMed] [Google Scholar]
- 169.Loov C, Scherzer CR, Hyman BT, Breakefield XO, Ingelsson M. alpha-Synuclein in Extracellular Vesicles: Functional Implications and Diagnostic Opportunities. Cell Mol Neurobiol. 2016;36(3):437–48. doi: 10.1007/s10571-015-0317-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shi M, Liu C, Cook TJ, Bullock KM, Zhao Y, Ginghina C, et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014;128(5):639–50. doi: 10.1007/s00401-014-1314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, et al. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grey M, Dunning CJ, Gaspar R, Grey C, Brundin P, Sparr E, et al. Acceleration of alpha-synuclein aggregation by exosomes. J Biol Chem. 2015;290(5):2969–82. doi: 10.1074/jbc.M114.585703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang S, Eitan E, Wu TY, Mattson MP. Intercellular transfer of pathogenic alpha-synuclein by extracellular vesicles is induced by the lipid peroxidation product 4-hydroxynonenal. Neurobiol Aging. 2018;61:52–65. doi: 10.1016/j.neurobiolaging.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Matsumoto J, Stewart T, Sheng L, Li N, Bullock K, Song N, et al. Transmission of alpha-synuclein-containing erythrocyte-derived extracellular vesicles across the blood-brain barrier via adsorptive mediated transcytosis: another mechanism for initiation and progression of Parkinson’s disease? Acta Neuropathol Commun. 2017;5(1):71. doi: 10.1186/s40478-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsunemi T, Hamada K, Krainc D. ATP13A2/PARK9 regulates secretion of exosomes and alpha-synuclein. J Neurosci. 2014;34(46):15281–7. doi: 10.1523/JNEUROSCI.1629-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, et al. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009;18(21):4022–34. doi: 10.1093/hmg/ddp346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shin N, Jeong H, Kwon J, Heo HY, Kwon JJ, Yun HJ, et al. LRRK2 regulates synaptic vesicle endocytosis. Exp Cell Res. 2008;314(10):2055–65. doi: 10.1016/j.yexcr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 178.Fraser KB, Moehle MS, Daher JP, Webber PJ, Williams JY, Stewart CA, et al. LRRK2 secretion in exosomes is regulated by 14–3-3. Hum Mol Genet. 2013;22(24):4988–5000. doi: 10.1093/hmg/ddt346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Fraser KB, Rawlins AB, Clark RG, Alcalay RN, Standaert DG, Liu N, et al. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov Disord. 2016;31(10):1543–50. doi: 10.1002/mds.26686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Leggio L, Vivarelli S, L’Episcopo F, Tirolo C, Caniglia S, Testa N, et al. microRNAs in Parkinson’s Disease: From Pathogenesis to Novel Diagnostic and Therapeutic Approaches. Int J Mol Sci. 2017;18(12). doi: 10.3390/ijms18122698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gui Y, Liu H, Zhang L, Lv W, Hu X. Altered microRNA profiles in cerebrospinal fluid exosome in Parkinson disease and Alzheimer disease. Oncotarget. 2015;6(35):37043–53. doi: 10.18632/oncotarget.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cao XY, Lu JM, Zhao ZQ, Li MC, Lu T, An XS, et al. MicroRNA biomarkers of Parkinson’s disease in serum exosome-like microvesicles. Neurosci Lett. 2017;644:94–9. doi: 10.1016/j.neulet.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 183.Guest WC, Plotkin SS, Cashman NR. Toward a mechanism of prion misfolding and structural models of PrP(Sc): current knowledge and future directions. J Toxicol Environ Health A. 2011;74(2–4):154–60. doi: 10.1080/15287394.2011.529065. [DOI] [PubMed] [Google Scholar]
- 184.Hartmann A, Muth C, Dabrowski O, Krasemann S, Glatzel M. Exosomes and the Prion Protein: More than One Truth. Front Neurosci. 2017;11:194. doi: 10.3389/fnins.2017.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vilette D, Courte J, Peyrin JM, Coudert L, Schaeffer L, Andreoletti O, et al. Cellular mechanisms responsible for cell-to-cell spreading of prions. Cell Mol Life Sci. 2018;75(14):2557–74. doi: 10.1007/s00018-018-2823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Saa P, Yakovleva O, de Castro J, Vasilyeva I, De Paoli SH, Simak J, et al. First demonstration of transmissible spongiform encephalopathy-associated prion protein (PrPTSE) in extracellular vesicles from plasma of mice infected with mouse-adapted variant Creutzfeldt-Jakob disease by in vitro amplification. J Biol Chem. 2014;289(42):29247–60. doi: 10.1074/jbc.M114.589564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cervenakova L, Saa P, Yakovleva O, Vasilyeva I, de Castro J, Brown P, et al. Are prions transported by plasma exosomes? Transfus Apher Sci. 2016;55(1):70–83. doi: 10.1016/j.transci.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 188.Guo BB, Bellingham SA, Hill AF. Stimulating the Release of Exosomes Increases the Intercellular Transfer of Prions. J Biol Chem. 2016;291(10):5128–37. doi: 10.1074/jbc.M115.684258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Guo BB, Bellingham SA, Hill AF. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem. 2015;290(6):3455–67. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kapogiannis D, Boxer A, Schwartz JB, Abner EL, Biragyn A, Masharani U, et al. Dysfunctionally phosphorylated type 1 insulin receptor substrate in neural-derived blood exosomes of preclinical Alzheimer’s disease. FASEB J. 2015;29(2):589–96. doi: 10.1096/fj.14-262048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.El Andaloussi S, Lakhal S, Mager I, Wood MJ. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65(3):391–7. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 192.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33(11):1711–5. doi: 10.1038/jcbfm.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Donega V, Nijboer CH, van Tilborg G, Dijkhuizen RM, Kavelaars A, Heijnen CJ. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol. 2014;261:53–64. doi: 10.1016/j.expneurol.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 195.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 196.Mizrak A, Bolukbasi MF, Ozdener GB, Brenner GJ, Madlener S, Erkan EP, et al. Genetically engineered microvesicles carrying suicide mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 2013;21(1):101–8. doi: 10.1038/mt.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lener T, Gimona M, Aigner L, Borger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol. 2005;175(4):2237–43. PubMed PMID: 16081791. [DOI] [PubMed] [Google Scholar]