Abstract
Objective:
C-reactive protein (CRP), a marker of systemic inflammation, has been associated with psychiatric disorders including major depressive disorder (MDD) and post-traumatic stress disorder (PTSD). Some research suggests that exposure to trauma can trigger increased activity in the inflammatory system. Dissociation is associated with chronic trauma exposure and may be an important factor in understanding the risk for psychiatric outcomes associated with inflammation. The main objective of the current study was to understand how CRP was related to trauma, dissociation, PTSD and MDD in a sample of 55 traumatized African American women with type 2 diabetes mellitus recruited from an urban hospital.
Method:
High sensitivity CRP (hsCRP) was assayed through blood samples; psychiatric disorders were assessed with structured clinical interviews, dissociation was assessed with the Multiscale Dissociation Inventory, and exposure to trauma in childhood and adulthood was assessed with the Childhood Trauma Questionnaire and the Traumatic Events Inventory, respectively.
Results:
Correlational results showed a significant association between higher concentrations of hsCRP and child abuse (p<.05), overall dissociation severity (p<.001), and PTSD symptoms (p<.01). ANOVA results showed significantly higher levels of hsCRP in those with current MDD, current PTSD, and remitted PTSD. A hierarchical linear regression model demonstrated a significant association between dissociation symptoms and greater hsCRP levels independent of childhood abuse, PTSD, and MDD (R2Δ=0.11, p=.001) and independent of emotion dysregulation (p<.05)
Conclusion:
These findings suggest that dissociation symptoms among those with a history of trauma may be particularly associated with higher levels of inflammation.
Keywords: trauma, PTSD, MDD, dissociation, CRP, inflammation
Introduction
Psychiatric disorders such as major depressive disorder (MDD) and posttraumatic stress disorder (PTSD) have been associated with physical diseases that are characterized by heightened systemic inflammation, including diabetes [1] and cardiovascular disease [2, 3]. A growing body of research suggests that systemic inflammation may be an important contributor to the co-morbidity between these psychiatric and physical disorders, particularly in the context of psychological stress or trauma [4-6]. C-reactive protein (CRP) is a marker of systemic inflammation and many studies have shown positive associations with MDD (e.g., [4, 7-9] and PTSD [10-12]. However, some studies with PTSD and CRP have been equivocal [13, 14]. Increased understanding of psychological factors that relate to changes in inflammatory activity may further clarify the relationship between psychiatric disorders and physical health problems.
One such factor is dissociation, particularly in the context of chronic trauma exposure [15]. Dissociation is characterized by disruptions in memory, identity, and perception of self and the environment [16, 17]; generalized dissociation refers to dissociation not directly associated with trauma-related reminders alone and may represent more severe dissociative tendencies that are pervasive across contexts. Generalized dissociation is associated with both PTSD and MDD and may be predictive of more severe PTSD and MDD symptoms [18, 19]. This tendency toward dissociation could indicate an effort to manage emotional distress [17], and can be seen as a maladaptive emotion regulation strategy often outside the control of the individual. In fact, recent research into dissociative symptoms of PTSD has provided rationale for the addition of a distinct dissociative subtype of PTSD to the diagnostic criteria in the Diagnostic and Statistical Manual of Mental Disorders, Version 5 [20, 21]. Importantly, the DSM-5 dissociative subtype of PTSD only includes depersonalization and derealization, providing a narrow definition of dissociation that may not fully capture the construct of dissociation.
Exposure to severe trauma in childhood is associated with dissociation (e.g., [22, 23]) and evidence suggests that the timing of trauma uniquely impacts symptoms of PTSD, depression, and dissociation, consistent with the notion of stress-sensitive periods in brain development in children [24, 25]. This phenomenon may also affect systemic inflammatory activity. Research indicates that inflammation may mediate the link between early life stress and psychiatric and physical disorders in adulthood [26-28], potentially through dysregulation of the HPA axis which modulates inflammatory activity and is in turn modulated by inflammatory processes [7, 29]. Prior research in our lab has provided initial data indicating that emotion dysregulation is related to higher levels of inflammation among traumatized African American women with type 2 diabetes mellitus (T2DM; [30]). However, the relationship between dissociation and inflammation beyond emotion dysregulation more generally has yet to be tested.
While strong evidence exists for the relationship between child abuse and both dissociation and inflammation, there remains a paucity of literature exploring the relationship directly between dissociation and inflammation. Such investigations could enhance our understanding of how trauma exposure, dissociation, and psychiatric disorders are differentially associated with inflammation, particularly in the context of chronic and early life trauma exposure. Importantly, the few previous studies that examined both current and remitted PTSD in relation to inflammation had equivocal findings [31-33]. Since causal pathways are not yet fully understood, such examination is also essential in our effort to further illuminate these complex associations.
The present study aims to fill these gaps in research by examining the relationship between childhood abuse, other lifetime trauma exposure, dissociative symptoms, MDD, remitted and current PTSD, and CRP levels in a sample of urban dwelling African American women with high rates of trauma exposure and T2DM. This population is particularly relevant for this research question because urban minority women are particularly vulnerable to high levels of interpersonal trauma exposure early in life and show higher rates of PTSD and MDD than the general population (e.g. [34, 35], making them a helpful group for the examination of dissociation. Additionally, since longitudinal studies have shown that elevated circulating concentrations of CRP increases risk for the development of T2DM [36, 37], better understanding of psychological factors associated with increased inflammation levels in individuals with T2DM could be helpful in efforts to improve treatments in this at risk and underserved group of women.
The main objectives of this study were to 1) identify how current MDD and both current and remitted PTSD symptoms and diagnoses were related to inflammation and 2) determine whether dissociation was related to inflammation independent of the effects of trauma and current psychiatric symptoms (depression and PTSD symptom severity). Based on previous findings in this sample [30], we hypothesized that current depression but not current PTSD would be associated with higher CRP levels; note that remitted PTSD diagnosis was also included for exploratory purposes due to the equivocal nature of research on PTSD in relation to inflammation. We also hypothesized that dissociation would be associated with higher concentrations of CRP independent of the effects of trauma exposure and current psychiatric diagnoses. Because prior research in this sample has shown a strong effect of emotion dysregulation on inflammation levels [30], we also included emotion dysregulation as a variable of interest in analyses to evaluate whether the relationship between dissociation and inflammation may hold independent of general emotion dysregulation. Dissociation can, at least in part, be understood as a maladaptive form of emotion regulation [30], and so although they are distinct constructs, there may be important overlap in how they relate to concentrations of CRP. In addition, we also felt it was important to establish the relationship between dissociation and both trauma and psychiatric disorders in this specialized sample. We hypothesized that dissociation severity would be elevated in those with current MDD and PTSD and would be associated with child abuse exposure.
Methods
Procedure
Participants were drawn from a study of risk factors for the development of PTSD in a low socioeconomic, urban minority population. Participants were recruited from waiting rooms in the diabetic, gynecology, and primary care medical clinics at a publically funded hospital in Atlanta, Georgia. We did not narrow recruitment to specific criteria, but approached any individual in the waiting room. To be eligible for participation, participants had to be between the ages of 18 and 65 and able to give informed consent (see [34] for full details regarding study procedures). The investigation was carried out in accordance with the latest version of the Declaration of Helsinki and informed consent of the participants was obtained after the nature of the procedures had been fully explained. After signing the informed consent approved by the Emory Institutional Review Board and the Research Oversight Committee of Grady Memorial Hospital, an interview was administered with questionnaires regarding trauma history and psychological variables. Trained research assistants administered this interview (approximately 45-75 minutes). A subgroup of female participants with T2DM was chosen for a separate associated study. These women returned to participate in structured clinical interviews and phlebotomy. Exclusion criteria included current bipolar or psychotic disorder, autoimmune disorder, systemic treatment with a non-steroidal anti-inflammatory drug, glucocorticoid, or anticonvulsant drug (other than gabapentin), and current treatment with an antipsychotic or benzodiazepine. On the morning of the interview, height and weight were measured for calculation of body mass index (BMI) and fasting blood samples were collected for later batch assessment of CRP concentrations (approximately 2 weeks post-initial assessment). A subsample of the women recruited for this study (n=40) were included in a previous study examining the relationship between emotion dysregulation and hsCRP levels [30].
Participants
The current study included 55 type 2 diabetic African American women with a mean age of 50.89 years (SD = 8.86 years, Range = 28-65). Education levels were as follows: 16.4% reported less than high school education, 31.0% reported having a high school diploma or GED, 36.4% reported having some college or technical school education, and 16.2% reported graduating from technical school or college. Only 27.3% of participants were employed and 77.8% had a household monthly income of $1999 or less.
Psychological Measures
Traumatic Events Inventory (TEI).
The TEI is a 14-item screening instrument for lifetime history of traumatic events [34]; total level of trauma exposure was measured by a sum score reflecting the total number of different types of events that an individual experienced or witnessed in their lifetime (excluding child abuse).
Childhood Trauma Questionnaire (CTQ).
The CTQ [38] is a 25-item, reliable and valid self-report instrument assessing sexual, physical, emotional abuse, and neglect in childhood (α = 0.92 in current study). A continuous measure of overall severity of childhood abuse exposure was calculated and used as the measure of child abuse severity.
Multiscale Dissociation Inventory (MDI).
The MDI is a 30-item validated self-report measure of dissociative symptomatology in the previous month [39] and measures six different types of dissociative response: disengagement, depersonalization, derealization, emotional constriction, memory disturbance, and identity dissociation. The internal consistency of the MDI total scale was high (α=0.91). For the present study, we used an overall measure of dissociative symptoms as well as sums of the six dimensions of dissociation.
Clinician-Administered PTSD Scale (CAPS).
The CAPS is an interviewer-administered psychometrically validated diagnostic instrument measuring PTSD [40, 41]. Interrater reliability (IRR) within this sample has been examined previously and showed good IRR for current diagnosis of PTSD (k = 0.83; [42]). The CAPS assesses current PTSD and was used to determine presence/absence of a PTSD diagnosis based on DSM criteria. Both CAPS for DSM-IV (CAPS-IV) and DSM-5 (CAPS-5) were used in the present study due to switching when CAPS-5 was released; Thirty-four percent (n=19) received the CAPS for DSM-IV and 66% received the CAPS-5. All DSM-IV diagnostic criteria were still asked to participants using the CAPS-5 and thus we could identify whether participants would meet for DSM-IV-TR PTSD criteria1. A sum of PTSD symptoms based on the CAPS items was used as the measure of PTSD symptom severity. In order to combine CAPS-IV and CAPS-5 severity scores together, all scores were weighted to balance the number of items (17 for CAPS-IV and 20 for CAPS-5) and factor in that scoring of frequency and intensity was separate in the CAPS-IV but combined in the CAPS-5.
MINI International Neuropsychiatric Interview (MINI).
The MINI [43] is a reliable and well-validated structured diagnostic interview that assesses mood, anxiety, substance use, and psychotic disorders based on DSM-IV-TR criteria. For the present study, only the current MDD and lifetime PTSD sections were used to assess presence/absence of current MDD and presence of lifetime PTSD to determine participants with remitted PTSD.
Beck Depression Inventory-II (BDI-II).
The BDI-II [44] is a widely used, 21-item self-report measurement of current depressive symptoms. Multiple studies have shown good reliability and validity for the BDI-II (Beck et al., 1996; Dozois et al., 1998). Internal consistency of the BDI scale was high (α=0.93). The BDI was summed to indicate current depression symptom severity in study analyses.
Biological Measures
Body Mass Index (BMI).
Calculated as: BMI=body mass (kg) / (height (m))2 based on measurements obtained during the history and physical conducted by a physician or physician in training prior to blood draw on day of clinical interview.
High Sensitivity CRP.
Serum samples were stored at −80°C until the time of high sensitivity CRP (hsCRP) assay. Serum hsCRP concentrations were determined using an immunoturbidometric assay from Sekisui Diagnostics (Lexington, Mass.) on the Beckman AU480 chemistry analyzer, with an interassay coefficient of variation (CV) of 5.2% and an intraassay CV of 3.1%. Individuals with circulating concentrations of hsCRP >20 mg/L were excluded from analysis because hsCRP > 20 mg/L suggests the presence of an active infection or other illness that could seriously confound study findings2.
Glucose.
Plasma samples of glucose were stored at −80°C until the time of assay. Glucose is measured by enzymatic methods on the Beckman AU480 using reagents from Beckman Coulter (Fullerton, CA). Average glucose level for this sample was 151.35 mg/dl (SD=72.61, range=63-518.00 mg/dl).
Hemoglobin A1c (HbA1c).
Whole blood samples were stored at −80°C until the time of assay. HbA1c was measured using high performance liquid chromatography by ARUP laboratories (Salt Lake City, Utah). Average HbA1c for this sample was 7.83 (SD=2.04, range=4.90-16.00).
Data Analysis
The overall analytic approach was to examine the associations between trauma exposure, PTSD and MDD symptoms and diagnoses, and dissociative symptoms in this sample, as well as the predictive utility of dissociative symptoms on hsCRP concentrations independent of trauma, PTSD, and MDD. Because emotion dysregulation is a construct closely aligned with dissociation and has previously been found to be associated with concentrations of hsCRP in a subsample of these women [30], we also included emotion dysregulation as a variable of interest in all analyses. We first examined the distributions of key predictor variables. Concentrations of hsCRP and dissociation variables were positively skewed. However, the level of skewness (Range: 0.79 - 1.37) and kurtosis (Range: 0.63 – 1.61) in this sample fell within acceptable parameters for the sample size on these variables [45]3. Descriptive statistics of the variables of interest were computed (Table 1). Differences in dissociation and CRP were examined by both current MDD and PTSD diagnosis (remitted and current) using analysis of variance. For continuous variables of interest, bivariate correlations were also computed to determine associations with dissociation and with hsCRP in this sample. Then, based on the results of the correlational analyses and analysis of variance results, a hierarchical linear regression model was fit to examine the unique predictive value of dissociation on hsCRP independent of other variables of interest. Potential covariates for regression analysis were first identified based on previous research suggesting associations with circulating concentrations of hsCRP: age, income, BMI, hemoglobin A1c, and baseline blood glucose level [8, 9, 46, 47]. Due to the small sample size and risk for low power in detecting significant effects, associations between hsCRP and these potential covariates were assessed to determine if their inclusion was warranted. No significant associations emerged between any of the variables and hsCRP except for BMI (r=0.46, p<0.001) and therefore only BMI was included as a covariate in the regression analysis. All analyses were conducted with SPSS 23.0 software package.
Table 1.
Descriptive characteristics of variables of interest
| Mean (SD, range) | |
|---|---|
| Dissociation Symptoms | 44.83 (11.61; 30-82) |
| Disengagement | 9.54 (3.28; 5-17) |
| Depersonalization | 5.94 (1.63; 5-11) |
| Derealization | 8.01 (3.14; 5-15) |
| Emotional constriction | 7.96 (2.91; 5-15) |
| Memory disturbance | 7.54 (2.77; 5-19) |
| Identity dissociation | 5.81 (1.90; 5-13) |
| Child Abuse Severity | 37.69 (15.59; 25-100) |
| Overall Trauma Load (excluding child abuse) | 4.74 (2.34; 1-12) |
| Emotion Dysregulation Total | 70.1 (19.49; 38-120) |
| PTSD Symptom Severity | 16.32 (11.35; 0-41) |
| Depression Symptom Severity | 15.13 (10.38; 0-38) |
| hsCRP | 4.49 (4.06; 0.15-18.06) |
| Body Mass Index | 36.43 (6.80; 20.95-54.15) |
| % (N) | |
| Current MDD | 27.3 (15) |
| Current PTSD | 32.7 (18)5 |
| Remitted PTSD | 29.0 (16) |
N = 55; Note: trauma load was measured using the TEI; child abuse severity was measured using the CTQ; emotion dysregulation was measured using the DERS; current PTSD diagnosis and symptom severity were measured using the CAPS; current depressive episode was measured using the MINI; current depression symptom severity was measured using the BDI.
Results
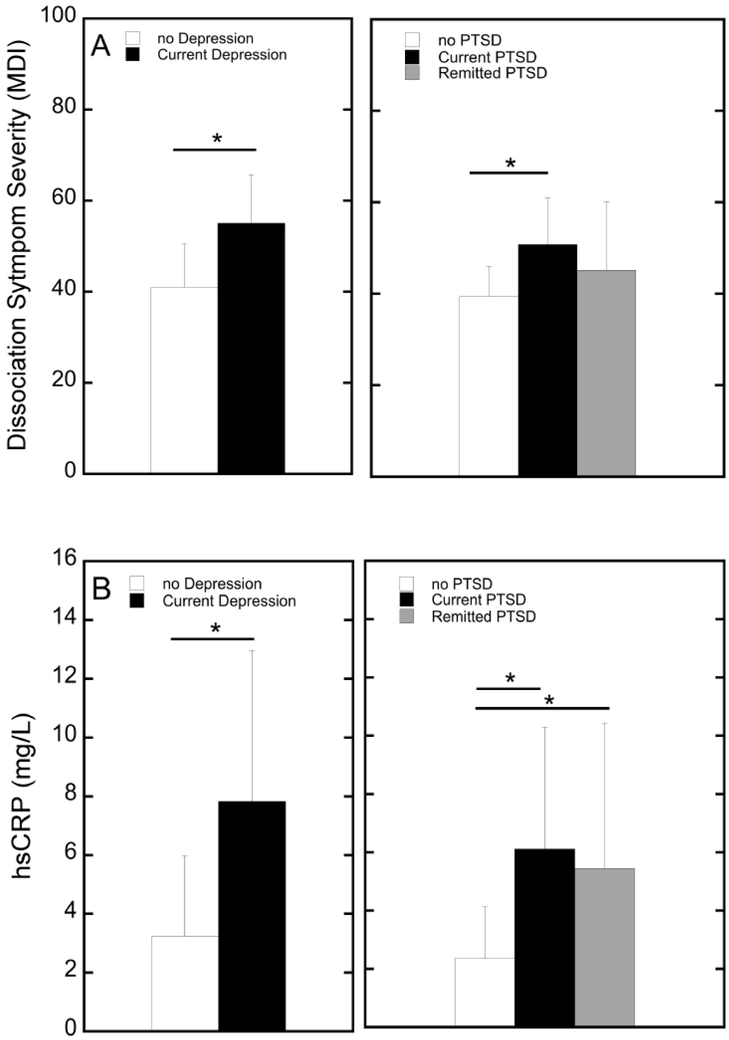
To determine the extent of association between dissociation with our other variables of interest (trauma exposure, emotion dysregulation, depression symptom severity, and PTSD symptom severity), we first calculated Pearson correlation coefficients. Dissociation symptom severity was positively correlated with emotion dysregulation (r=0.63, p<0.001), depression symptom severity (r=0.66, p <0.001), and PTSD symptom severity (r=0.56, p <0.001) all surviving Bonferroni correction for multiple comparisons4; the association between dissociation and child abuse severity trended toward significance (r=0.26, p=0.060), but dissociation was not associated with other trauma exposure (r=0.14). Next, one-way ANOVA tests were run examining mean differences in dissociation symptoms with current MDD and PTSD diagnosis (none, current, and remitted). As shown in Figure 1A, results showed a significant main effect of current MDD on higher dissociation symptoms (F=22.33, p<0.001) as well as a main effect of PTSD diagnosis on dissociation symptoms (F=5.47, p=0.007). Planned pair-wise comparison tests showed that participants with no history of PTSD reported significantly lower dissociation symptoms than current PTSD (p=0.002); no other group differences were significant (Figure 1A).
Figure 1.
Mean differences in dissociation symptom severity (A) and hsCRP concentrations (B) between groups based on Depression and PTSD diagnoses. Asterisks denote significant differnces between groups (p<0.05).
Associations between hsCRP concentrations and independent variables of interest
Next, associations between independent variables of interest and hsCRP were examined using Pearson correlation analyses. As shown in Table 2, hsCRP was significantly positive correlated with dissociation symptoms (total score and all six dimensions measured; all p’s < 0.05; all survived Bonferroni correction except for emotional constriction), child abuse severity (p<0.05), emotion dysregulation (p<0.01), depression symptom severity (p<0.001), and PTSD symptom severity (p<0.01).
Table 2.
Bivariate Pearson’s correlations (r) between variables of interest and hsCRP
| hsCRP | |
|---|---|
| Dissociation Total | 0.63*** |
| Disengagement | 0.53*** |
| Depersonalization | 0.40** |
| Derealization | 0.54*** |
| Emotional constriction | 0.30* |
| Memory disturbance | 0.54*** |
| Identity dissociation | 0.47*** |
| Child abuse severity | 0.33* |
| Other trauma exposure | 0.20 |
| Emotion dysregulation total | 0.56** |
| PTSD symptom severity | 0.38** |
| Depression symptom severity | 0.55*** |
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Note: Dissociation symptom severity was measured using the MDI, child abuse severity was measured using the CTQ, and trauma exposure was measured using the TEI, Emotion dysregulation was measured using the DERS, PTSD symptom severity was measured using the CAPS, and depression symptom severity was measured using the BDI.
One-way ANOVA tests were then used to examine mean differences in hsCRP by current MDD and PTSD diagnosis. As shown in Figure 1B, results showed a significant main effect of current MDD on hsCRP concentrations (F=18.40, p<0.001). For PTSD diagnosis, ANOVA results also showed a significant main effect of PTSD diagnosis on hsCRP concentrations (F=5.66, p=0.006). Planned pair-wise comparison tests showed that participants with no history of PTSD had significantly lower levels of hsCRP concentrations than either current or remitted PTSD groups (p=0.003 and p=0.016, respectively); there were no significant differences found between current PTSD and remitted PTSD groups (see Figure 1B).
Next, a hierarchical linear regression model was run to test the differential associations of current PTSD symptom severity, current depression severity, child abuse severity, emotion dysregulation, and dissociation with hsCRP concentrations. Step 1 included BMI, child abuse severity, depression symptom severity, and PTSD symptom severity. Step 2 included BMI, child abuse severity, depression symptom severity, PTSD symptom severity, and dissociation symptoms. Finally, Step 3 was run including BMI, child abuse severity, depression symptom severity, PTSD symptom severity, dissociation symptoms, and emotion dysregulation. As shown in Table 3, Step 1 was significant (p<0.001), showing that higher BMI (p<0.001) and higher levels of current depression symptoms (p<.01) were significantly related to higher hsCRP concentrations, explaining 45% of unique variance in hsCRP concentrations. In Step 2, when dissociation symptoms were entered into the regression model, dissociation symptoms were significantly predictive of hsCRP (p=0.001), surviving Bonferroni correction for multiple comparisons and accounting for 11% of unique variance in predicting higher hsCRP concentrations independent of BMI, child abuse severity, and current psychiatric symptoms. Depression symptoms were no longer significantly associated with hsCRP concentrations in this step. When emotion dysregulation was included in Step 3, dissociation remained significantly predictive of hsCRP (p=0.03), although the association was attenuated to some extent. Emotion dysregulation was not significantly predictive of hsCRP but trended toward significance (p=0.06), suggesting that high levels of emotion dysregulation may help to explain some, but not all, of the association between dissociation and hsCRP concentrations in the current sample.
Table 3.
Hierarchical linear regression model predicting current hsCRP levels from current depression symptom severity, current PTSD symptoms, dissociation symptoms, and overall emotion dysregulation symptoms independent of body mass index (BMI) and child abuse severity.
| β | t | p | R | R2Δ | F change | p change | |
|---|---|---|---|---|---|---|---|
| Step 1 | 0.67 | 0.45 | 10.11 | <.001*** | |||
| BMI | 0.40 | 3.61 | 0.001** | ||||
| Child abuse severity | 0.02 | 0.17 | 0.87 | ||||
| Current Depression symptom severity | 0.42 | 2.74 | 0.008* | ||||
| Current PTSD symptom severity | 0.08 | 0.47 | 0.64 | ||||
| Step 2 | 0.75 | 0.11 | 12.05 | .001** | |||
| BMI | 0.34 | 3.35 | 0.002** | ||||
| Child abuse severity | 0.13 | 1.01 | 0.32 | ||||
| Current Depression symptom severity | 0.18 | 1.13 | 0.26 | ||||
| Current PTSD symptom severity | −0.07 | −0.47 | 0.64 | ||||
| Dissociation symptoms | 0.47 | 3.47 | .001** | ||||
| Step 3 | 0.77 | 0.03 | 3.75 | 0.059 | |||
| BMI | 0.36 | 3.64 | .001** | ||||
| Child abuse severity | 0.07 | 0.58 | 0.57 | ||||
| Current Depression symptom severity | 0.10 | 0.60 | 0.51 | ||||
| Current PTSD symptom severity | −0.01 | −0.04 | 0.97 | ||||
| Dissociation symptoms | 0.33 | 2.23 | 0.03* | ||||
| Emotion dysregulation | 0.25 | 1.94 | 0.06 |
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001;
Note: Child abuse severity was measured using the CTQ; emotion dysregulation was measured using the DERS; current depressive symptoms were measured using the BDI and PTSD symptoms were measured using the CAPS.
Discussion
To our knowledge, this is the first study examining the relationship between childhood abuse, lifetime exposure to trauma, dissociative symptoms, trauma-related psychopathology, and hsCRP concentrations. Prior studies have shown an association between childhood trauma exposure and both dissociative symptoms [15, 23] and inflammation [26, 27]. The current study expanded these findings by demonstrating that general dissociation is also associated with higher concentrations of CRP among African American women with T2DM and high rates of previous trauma exposure. Examining these associations in a sample of women with T2DM is particularly critical since inflammation may present an independent risk of disability among individuals with diabetes [48] and serves as a risk factor in the development of other health conditions, such as cardiovascular disease [49, 50]. This highlights the need to identify and address factors that may influence inflammation among those with T2DM to reduce the long term consequences of the disease.
As hypothesized, we found a significant association between higher levels of circulating concentrations of hsCRP and overall dissociation severity, with dissociation accounting for 11% of unique variance in hsCRP independent of child abuse severity, current PTSD and depressive symptoms, and BMI. Evidence supports dissociation as a multidimensional construct, with various dimensions that are distinct, but overlapping [16]. When examining across the different dimensions of dissociation, after correction for multiple testing, this association between higher concentrations of hsCRP and dissociation was present for five of the six dissociation dimensions assessed, including disengagement, depersonalization, derealization, memory disturbance, and identity dissociation. The only dimension not significantly associated with hsCRP was emotional constriction, a dimension with significant overlap to emotional numbing symptoms in PTSD. In support of previous findings in the same sample (Powers et al., 2016), emotion dysregulation was also predictive of hsCRP when included in the regression model, but dissociation still remained significant. These findings suggest that although emotion dysregulation helps to explain the relationship between dissociation and hsCRP to some extent, there is something unique about the link between dissociation and inflammation.
Dissociation may reflect a neural process whereby high activation in brain regions involved in arousal modulation and emotional regulation (prefrontal regions) inhibit activation in limbic regions [21], and there is evidence that it is related to an inhibited autonomic response and reduced cortisol stress reactivity to stressful stimuli [51]. One explanation for this association between dissociation and inflammation may be through the impact of chronic trauma on the HPA axis and its regulation of inflammatory processes. Chronic activation of the HPA axis may result in elevated glucocorticoid resistance that in turn impairs reduction of stress-induced inflammation [52, 53]. Exactly how the dysfunction develops in the context of dissociation remains unclear, although a study of peritraumatic dissociation in children suggests that polymorphisms within FKBP5, the gene which codes for the glucocorticoid receptor-regulating co-chaperone, may affect HPA axis reactivity in response to stress associated with dissociation [54]. Glucocorticoid resistance may lead to greater inflammation and both could be associated with dissociation. Interestingly, although individuals with a dissociative response to stress may show reduced physiological reactivity, subjectively they often report higher levels of subjective distress than individuals with low dissociation [55, 56]. It is possible that disconnection between the body’s physiological response and subjective experience may also represent a problem in naturally allowing an emotional response and recovery to equilibrium following a stressor, thus leading to a more long term detrimental impact on the body.
In examining the relationship among dissociation, trauma exposure, and psychiatric symptoms in this specialized sample, our results support previous findings that dissociation symptom severity was significantly positively correlated with emotion dysregulation, depression symptoms, and PTSD symptom severity [17]. When examined across current MDD and current and remitted PTSD diagnoses, significant difference in mean levels of dissociation symptoms was present for current MDD and current PTSD (higher dissociation in those with psychiatric diagnoses). Contradictory to what we hypothesized, we did not see a strong association between higher levels of dissociation and child abuse severity or overall trauma load. It is unclear why this is the case, but it suggests that at least within this population of African American women with T2DM, factors beyond child abuse severity or trauma load itself may be important in the presence of dissociative symptoms in adulthood. Prior research suggests that other factors beyond child abuse exposure also contribute to the development of posttraumatic dissociation, such as a history of interpersonal violence, insecure parent-child attachment, and problematic emotion regulation [15]. Our measure of child abuse also focused on any abuse prior to the age of 18, and it is possible that timing of trauma exposure could play a role in the development of dissociation as an emotion regulation strategy, as prior work in our lab has shown that developmental timing of trauma exposure does impact risk for depression and PTSD [57]. A key conclusion from these findings is that dissociation may be an important trans-diagnostic factor that cuts across trauma-related disorders in chronically traumatized populations and that focusing on dissociation only in the context of a current PTSD diagnosis may result in a lack of identification of an important treatment target.
When mean differences in hsCRP were examined by current MDD and current and remitted PTSD diagnoses, we found a significant main effect of current MDD; there were significantly higher concentrations of mean hsCRP levels in individuals with current MDD compared to those without current MDD and this effect survived multiple test correction. This supports previous research showing a strong relationship between MDD and inflammation [4, 8, 9]. Interestingly, our results also showed a main effect of PTSD diagnosis; levels of hsCRP were higher in women with both current PTSD and remitted PTSD compared to those women without any PTSD diagnosis. We did not find a significant difference in hsCRP concentrations between those with current and remitted PTSD. Previous research examining levels of inflammatory markers in individuals with and without PTSD have varied. While multiple other studies have found increased hsCRP levels in individuals with current PTSD [9, 11, 14, 30, 58], studies examining inflammation in individuals with remitted PTSD report varying results. One study reports lower inflammation in individuals with remitted PTSD compared to individuals with no history of PTSD [32] whereas other studies have found that individuals with remitted PTSD had inflammation levels similar to controls and much lower than those of individuals with current PTSD [31, 33]. Methodological differences in subject selection and diagnostic classification between studies may be the driver of these inconsistent results. Some data suggests that inflammation is increased in individuals exposed to chronic traumatic stress [27, 59], and our sample includes individuals with high levels of trauma exposure, often chronic in nature, and so that may be reflected in observations of systemic inflammation in the individuals with a lifetime, or current, PTSD diagnosis. Additionally, group distribution is affected by the use of categorical groups rather than continuous measures of PTSD symptoms. It is important to note that many individuals do not achieve full remission of symptoms even though they might not fulfill all diagnostic criteria for current PTSD and so even our “remitted” women may exhibit symptoms. Interestingly, when examining current symptom severity and hsCRP concentrations, it was depression symptoms that remained an important predictor of hsCRP concentrations, not PTSD symptoms. The clear and strong relationship between depression and inflammation was reinforced by these results.
The current study has some limitations that should be kept in mind when interpreting the results. First, the study was cross-sectional in nature, which does not allow for the determination of causality. It is certainly possible that chronic inflammation leads to higher levels of dissociation. However, our results showed an association between lifetime PTSD and current hsCRP levels and there is strong evidence that dissociation patterns tend to develop early in life [22, 23], thus supporting the notion that dissociation may lead to chronic inflammation or that they may have an important bidirectional relationship. However, examining the relationship between lifetime PTSD and current hsCRP in this cross-sectional study also makes it difficult to determine causality. Future longitudinal research is needed to better understand pathways of risk and how PTSD, dissociation, and inflammation may influence each other over development within an environment where chronic trauma exposure is common. In addition, dissociation was measured using a self-report questionnaire; participants needed to have insight into their dissociative symptoms in order to report them. A lack of insight could have underestimated the severity of dissociation within this group. We also did not measure current smoking status in our participants and therefore were unable to control for the potential effects of smoking on hsCRP levels within this sample despite prior evidence that smoking is related to elevated concentrations of CRP [60]. The small sample size of this study means that we may not have had enough power to detect small, yet clinically important associations between the variables examined. Additionally, due to the specificity of our sample, it is possible that the findings observed here may be most generalizable to populations with similarly high rates of trauma and comparable medical and demographic characteristics. However, the potential limitation of generalizability is counterbalanced by the public health importance of studying these variables in an often under-researched and under-served population with such high rates of trauma exposure and mental and physical health problems. There are very limited mental health resources available for individuals in this population, despite the strong need for treatment options given the high rates of trauma, trauma-related psychopathology, and medical comorbidities, thus making it critical to understand underlying mechanisms of risk for the development or maintenance of psychiatric and medical conditions in the context of trauma exposure. Finally, it is also critical to acknowledge that this was an all-female sample and therefore it is possible that different outcomes would be observed in men. In fact, there is evidence to suggest important sex differences in mood and socioemotional response to inflammatory challenges [61, 62], highlighting the value of understanding associations between these variables across sex.
Conclusions
Our findings indicate a significant association between generalized dissociative symptoms, current MDD, and both current and remitted PTSD with higher levels of hsCRP in a sample of traumatized, African American women with T2DM. Dissociation appears important in predicting inflammation levels independent of trauma, current psychiatric symptoms, or emotion dysregulation more generally, suggesting that dissociation may be particularly detrimental on individual’s health and may represent an underlying mechanism that increases risk for physical health problems in the context of significant trauma exposure. These data indicate the need for more extensive research on dissociation in the context of inflammation and HPA dysfunction across varied populations. Further, these findings highlight the potential need to address dissociation more directly in the context of behavioral health treatment, particularly among at risk populations with significant trauma exposure and comorbid physical health problems.
Highlights.
This was the first study examining the relationship between trauma exposure, dissociative symptoms, trauma-related psychopathology, and CRP concentrations
Mean levels of CRP were significantly higher in individuals with current MDD than those without MDD
Mean levels of CRP were significantly higher in individuals with current or remitted PTSD diagnoses than those with no PTSD diagnosis
General dissociation was associated with higher concentrations of CRP among traumatized African American women with T2DM independent of trauma exposure or current psychiatric symptoms
Dissociation may represent an underlying mechanism that increases risk for physical health problems in the context of significant trauma exposure
Acknowledgments
Conflicts of Interest and Sources of Funding: This work was primarily supported by the National Institute of Mental Health (MH071537 for Ressler; MH102890 for Powers, MH099211 for Gillespie), the National Institute of Child Health and Human Development (HD071982 for Bradley), and the National Center for Complementary & Integrative Health (K23AT009713 for Powers). Support also included Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01 RR00039). There are no conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLAIMER: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additionally, the contents of this report do not represent the views of the Department of Veterans Affairs or the United States Government.
All but 3 participants who met criteria for PTSD based on DSM-5 criteria also met for DSM-IV criteria. Main analyses were re-run with those 3 participants classified as remitted PTSD and no changes to the results were found. Therefore, we included them in the group under current PTSD.
Four individuals were excluded from analyses (hsCRP ranging from 21.97 – 61.69).
MDI total had one outlier which affected the level of kurtosis and was >3 SDs above the mean and therefore data from that participant was removed from analyses (MDI score = 100).
Twenty primary analyses were conducted resulting in a Bonferroni correction value of p<.003.
The majority of women with current PTSD had comorbid MDD (18.2% of overall sample, n = 10).
References
- [1].Goodwin RD, Davidson JR. Self-reported diabetes and posttraumatic stress disorder among adults in the community. Preventive medicine. 2005;40:570–4. [DOI] [PubMed] [Google Scholar]
- [2].Kubzansky LD, Koenen KC, Spiro A, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Archives of general psychiatry. 2007;64:109–16. [DOI] [PubMed] [Google Scholar]
- [3].Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosomatic medicine. 2004;66:802–13. [DOI] [PubMed] [Google Scholar]
- [4].Kuo H-K, Yen C-J, Chang C-H, Kuo C-K, Chen J-H, Sorond F. Relation of C-reactive protein to stroke, cognitive disorders, and depression in the general population: systematic review and meta-analysis. The Lancet Neurology. 2005;4:371–80. [DOI] [PubMed] [Google Scholar]
- [5].Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological bulletin. 2014;140:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Speer K, Upton D, Semple S, McKune A. Systemic low-grade inflammation in post-traumatic stress disorder: a systematic review. Journal of inflammation research. 2018;11:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews neuroscience. 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Archives of internal medicine. 2004;164:1010–4. [DOI] [PubMed] [Google Scholar]
- [9].Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosomatic medicine. 2009;71:171–86. [DOI] [PubMed] [Google Scholar]
- [10].Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A, et al. Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA psychiatry. 2014;71:423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, et al. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. American Journal of Psychiatry. 2015;172:353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Miller R, Sutherland A, Hutchison J, Alexander D. C-reactive protein and interleukin-6 receptor in posttraumatic stress disorder: A pilot study Cytokine. 2001;13:253–5. [DOI] [PubMed] [Google Scholar]
- [13].McCanlies EC, Araia SK, Joseph PN, Mnatsakanova A, Andrew ME, Burchfiel CM, et al. C-reactive protein, interleukin-6, and posttraumatic stress disorder symptomology in urban police officers. Cytokine. 2011;55:74–8. [DOI] [PubMed] [Google Scholar]
- [14].von Känel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L, et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. Journal of psychiatric research. 2007;41:744–52. [DOI] [PubMed] [Google Scholar]
- [15].Briere J Dissociative symptoms and trauma exposure: Specificity, affect dysregulation, and posttraumatic stress. The Journal of nervous and mental disease. 2006;194:78–82. [DOI] [PubMed] [Google Scholar]
- [16].Briere J, Weathers FW, Runtz M. Is dissociation a multidimensional construct? Data from the Multiscale Dissociation Inventory. Journal of Traumatic Stress. 2005;18:221–31. [DOI] [PubMed] [Google Scholar]
- [17].Powers A, Cross D, Fani N, Bradley B. PTSD, emotion dysregulation, and dissociative symptoms in a highly traumatized sample. Journal of psychiatric research. 2015;61:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Johnson DM, Pike JL, Chard KM. Factors predicting PTSD, depression, and dissociative severity in female treatment-seeking childhood sexual abuse survivors. Child Abuse & Neglect. 2001;25:179–98. [DOI] [PubMed] [Google Scholar]
- [19].Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological bulletin. 2003;129:52. [DOI] [PubMed] [Google Scholar]
- [20].American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5): American Psychiatric Pub; 2013. [Google Scholar]
- [21].Lanius RA, Brand B, Vermetten E, Frewen PA, Spiegel D. The dissociative subtype of posttraumatic stress disorder: Rationale, clinical and neurobiological evidence, and implications. Depression and anxiety. 2012;29:701–8. [DOI] [PubMed] [Google Scholar]
- [22].D'andrea W, Ford J, Stolbach B, Spinazzola J, van der Kolk BA. Understanding interpersonal trauma in children: Why we need a developmentally appropriate trauma diagnosis. American Journal of Orthopsychiatry. 2012;82:187. [DOI] [PubMed] [Google Scholar]
- [23].Terock J, Van der Auwera S, Janowitz D, Spitzer C, Barnow S, Miertsch M, et al. From childhood trauma to adult dissociation: the role of PTSD and alexithymia. Psychopathology. 2016;49:374–82. [DOI] [PubMed] [Google Scholar]
- [24].Carlson EB, Dalenberg C, McDade-Montez E. Dissociation in posttraumatic stress disorder part I: Definitions and review of research. Psychological Trauma: Theory, Research, Practice, and Policy. 2012;4:479. [Google Scholar]
- [25].Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baumeister D, Akhtar R, Ciufolini S, Pariante C, Mondelli V. Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular psychiatry. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences. 2007;104:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lu S, Peng H, Wang L, Vasish S, Zhang Y, Gao W, et al. Elevated specific peripheral cytokines found in major depressive disorder patients with childhood trauma exposure: a cytokine antibody array analysis. Comprehensive psychiatry. 2013;54:953–61. [DOI] [PubMed] [Google Scholar]
- [29].Baumeister D, Russel A, Pariante C, Modelli V. Inflammatory biomarker profiles of mental disorders and their relation to clinical, social, and lifestyle factors. Social Psychiatry and Psychiatric Epidemiology. 2014;49:841–9. [DOI] [PubMed] [Google Scholar]
- [30].Powers A, Michopoulos V, Conneely K, Gluck R, Dixon H, Wilson J, et al. Emotion dysregulation and inflammation in African-American women with type 2 diabetes. Neural plasticity. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gill JM, Saligan L, Lee H, Rotolo S, Szanton S. Women in recovery from PTSD have similar inflammation and quality of life as non-traumatized controls. Journal of psychosomatic research. 2013;74:301–6. [DOI] [PubMed] [Google Scholar]
- [32].Kawamura N, Kim Y, Asukai N. Suppression of cellular immunity in men with a past history of posttraumatic stress disorder. American Journal of Psychiatry. 2001;158:484–6. [DOI] [PubMed] [Google Scholar]
- [33].O’Donovan A, Ahmadian AJ, Neylan TC, Pacult MA, Edmondson D, Cohen BE. Current posttraumatic stress disorder and exaggerated threat sensitivity associated with elevated inflammation in the Mind Your Heart Study. Brain, behavior, and immunity. 2017;60:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gillespie C, Bradley B, Mercer K, Smith A, Conneely K, Gapen M, et al. Trauma exposure and stress-related disorders in inner city primary care patients. General Hospital Psychiatry. 2009;31:505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychological bulletin. 2007;133:183. [DOI] [PubMed] [Google Scholar]
- [36].Freeman DJ, Norrie J, Caslake MJ, Gaw A, Ford I, Lowe GD, et al. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes. 2002;51:1596–600. [DOI] [PubMed] [Google Scholar]
- [37].Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. Jama. 2001;286:327–34. [DOI] [PubMed] [Google Scholar]
- [38].Bernstein D, Fink L. Manual for the childhood trauma questionnaire. New York: The Psychological Corporation; 1998. [Google Scholar]
- [39].Brière J MDI, Multiscale dissociation inventory: Professional manual: Psychological Assessment Resources, Incorporated; 2002. [Google Scholar]
- [40].Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Charney DS, Keane TM. Clinician-administered PTSD scale for DSM-IV. Boston: National Center for Posttraumatic Stress Disorder; 1998. [Google Scholar]
- [41].Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. Journal of traumatic stress. 1995;8:75–90. [DOI] [PubMed] [Google Scholar]
- [42].Powers A, Fani N, Carter S, Cross D, Bradley B. Differential predictors of DSM-5 PTSD and ICD-11 complex PTSD among African American women. European journal of psychotraumatology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of clinical psychiatry. 1998. [PubMed] [Google Scholar]
- [44].Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- [45].Tabachnick BG, Fidell LS. Using multivariate statistics. 2001.
- [46].Alley DE, Seeman TE, Kim JK, Karlamangla A, Hu P, Crimmins EM. Socioeconomic status and C-reactive protein levels in the US population: NHANES IV. Brain, behavior, and immunity. 2006;20:498–504. [DOI] [PubMed] [Google Scholar]
- [47].Kaefer M, Piva SJ, De Carvalho JA, Da Silva DB, Becker AM, Coelho AC, et al. Association between ischemia modified albumin, inflammation and hyperglycemia in type 2 diabetes mellitus. Clinical Biochemistry. 2010;43:450–4. [DOI] [PubMed] [Google Scholar]
- [48].Figaro MK, Kritchevsky SB, Resnick HE, Shorr RI, Butler J, Shintani A, et al. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes care. 2006;29:2039–45. [DOI] [PubMed] [Google Scholar]
- [49].Myers GL, Rifai N, Tracy RP, Roberts WL, Alexander RW, Biasucci LM, et al. CDC/AHA workshop on markers of inflammation and cardiovascular disease: application to clinical and public health practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–e9. [DOI] [PubMed] [Google Scholar]
- [50].Ridker PM, Wilson PW, Grundy SM. Should C-reactive protein be added to metabolic syndrome and to assessment of global cardiovascular risk? Circulation. 2004;109:2818–25. [DOI] [PubMed] [Google Scholar]
- [51].Simeon D, Knutelska M, Yehuda R, Putnam F, Schmeidler J, Smith LM. Hypothalamic-pituitary-adrenal axis function in dissociative disorders, post-traumatic stress disorder, and healthy volunteers. Biological psychiatry. 2007;61:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health psychology. 2002;21:531. [DOI] [PubMed] [Google Scholar]
- [53].Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological bulletin. 2007;133:25. [DOI] [PubMed] [Google Scholar]
- [54].Koenen K, Saxe G, Purcell S, Smoller J, Bartholomew D, Miller A, et al. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Molecular psychiatry. 2005;10:1058. [DOI] [PubMed] [Google Scholar]
- [55].Kaufman ML, Kimble MO, Kaloupek DG, McTeague LM, Bachrach P, Forti AM, et al. Peritraumatic dissociation and physiological response to trauma-relevant stimuli in Vietnam combat veterans with posttraumatic stress disorder. The Journal of nervous and mental disease. 2002;190:167–74. [DOI] [PubMed] [Google Scholar]
- [56].McTeague LM, Lang PJ, Laplante M-C, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biological psychiatry. 2010;67:346–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Dunn EC, Nishimi K, Powers A, Bradley B. Is developmental timing of trauma exposure associated with depressive and post-traumatic stress disorder symptoms in adulthood? Journal of psychiatric research. 2017;84:119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. Journal of psychiatric research. 2010;44:15–21. [DOI] [PubMed] [Google Scholar]
- [59].Black PH. Stress and the inflammatory response: a review of neurogenic inflammation. Brain, behavior, and immunity. 2002;16:622–53. [DOI] [PubMed] [Google Scholar]
- [60].Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:2167–76. [DOI] [PubMed] [Google Scholar]
- [61].Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: the role of sex differences. Neuroimage. 2009;47:881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]