Abstract
Native Americans (NAs) have a higher prevalence of chronic pain than any other U.S. racial/ethnic group; however, little is known about the mechanisms for this pain disparity. This study used quantitative sensory testing (QST) to assess pain experience in healthy, pain-free adults (N=137 NAs [87 female], N=145 non-Hispanic whites [NHW; 68 female]) following painful electric, heat, cold, ischemic, and pressure stimuli. Following each stimulus, ratings of pain intensity, sensory pain, affective pain, pain-related anxiety, and situation-specific pain catastrophizing were assessed. Results suggested that NAs reported greater sensory pain in response to suprathreshold electric and heat stimuli, greater pain-related anxiety to heat and ischemic stimuli, and more catastrophic thoughts in response to electric and heat stimuli. Sex differences were also noted; however, with the exception of catastrophic thoughts to cold, these were not moderated by race/ethnicity. Together, findings suggest NAs experience heightened sensory, anxiety, and catastrophizing reactions to painful stimuli. This could place NAs at risk for future chronic pain and could ultimately lead to a vicious cycle that maintains pain (e.g., pain➔anxiety/catastrophizing➔pain).
Keywords: quantitative sensory testing, pain, ethnic differences, Native Americans, pain coping, catastrophizing, anxiety
Perspective:
Native Americans experienced heightened sensory, anxiety, and catastrophizing reactions in response to multiple pain stimuli. Given the potential for anxiety and catastrophic thoughts to amplify pain, this may place them at risk for pain disorders and could lead to a vicious cycle that maintains pain.
Introduction
Native Americans (NAs) have a higher prevalence of chronic pain than any other U.S. racial/ethnic group4,35,83, yet little has been done to understand what contributes to this pain disparity. Access to health care1,46, difficulties assessing/treating pain cross-culturally78, and/or provider biases31,32 may contribute to this disparity; but, it could also stem from differences in the way that pain is processed and experienced37.
Differences in pain experience can be studied in reaction to clinical pain (e.g., fibromyalgia); but, this approach suffers from an internal validity problem because it is unclear whether the source of pain is consistent across persons/groups being studied. As such, experiential differences could simply be due to differences in the disease state. This problem is addressed by assessing reactions to quantitative sensory testing (QST) that involves the delivery of controlled, painful stimuli on healthy, pain-free individuals.
QST studies have been conducted to examine racial/ethnic differences in pain, but most have studied African-Americans (and to a lesser degree Hispanics), because these minority groups also experience high rates of chronic pain52,63. This research suggests they may be at a higher pain risk due, in part, to heightened affective-motivational reactivity to pain, because group differences (when compared to non-Hispanic whites, NHW) are largest in response to suprathreshold stimuli (e.g., pain tolerance) that evoke strong affective reactions52,63. Notably, these suprathreshold measures are believed to have the greatest relationship with, and relevance to, clinical pain19. Given evidence that affective factors (e.g., anxiety) can enhance pain21,44,59,64,66, greater affective-motivational reactions may create a vicious cycle to enhance and promote future pain (e.g., pain➔anxiety➔pain)14,30,66,71. Importantly, it is pain-related affect (e.g., fear/anxiety) that is the primary cause of pain-related suffering38, and clinical studies have shown that racial/ethnic minorities report greater pain-related negative affect in response to clinical pain29,63.
To our knowledge, only 4 published studies have used QST to study pain in NAs41,47,48,65. In contrast to studies of other minorities, 3 noted reduced pain sensitivity and/or a lack of pain facilitation47,48,65, and 1 noted no group differences41. This suggests mechanisms for pain risk may be different for NAs. However, all 4 studies suffered from small sample sizes, or assessed only one pain modality41,65.
To address these gaps in the literature, the present study assessed responses to electric, heat, cold, ischemic, and pressure stimuli in 137 NA and 145 NHW participants. Immediately following each pain task, sensory reactions were assessed from the McGill Pain Questionnaire-Short Form (MPQ-SF) sensory scale and the MPQ-SF visual analog scale (VAS) for pain intensity, whereas affective reactions were assessed from the MPQ-SF affective scale and from a VAS measuring pain-related anxiety. Finally, because pain catastrophizing is a cognitive-affective reaction that is known to promote pain50, the Pain Catastrophizing Scale was administered after each task with instructions to report on catastrophizing that occurred during the painful tasks (i.e., situation-specific pain catastrophizing)11. Given that group differences in the distribution of sex between racial/ethnic groups were found, sex was controlled for in the analyses.
Materials and Methods
Participants
These data were collected as part of the Oklahoma Study of Native American Pain Risk (OK-SNAP). Healthy, pain-free participants were recruited from newspaper ads, tribal newspapers, fliers, personal communications with NA groups, email announcements, and online platforms (e.g., Facebook). Exclusion criteria included: 1) <18 years old, 2) history of cardiovascular, neuroendocrine, musculoskeletal, neurological disorders, 3) chronic pain or current acute pain, 4) BMI≥35 (due to difficulties recording electromyogram for other tasks), 5) use of anti-depressants, anxiolytic, analgesic, stimulant, or anti-hypertensive medication, 6) current psychotic symptoms (assessed by Psychosis Screening Questionnaire (35)) or substance abuse problems, and/or 7) an inability to read and speak English. Data collection occurred between March 2014 and February 2018. The study was approved by Institutional Review Boards of The University of Tulsa, Cherokee Nation, and the Indian Health Service Oklahoma City Area Office. Participants were given an overview of all procedures and told they could withdraw at any time. All participants provided verbal and written informed consent prior to enrollment and received a $100 honorarium for the completion of each testing day (or $10/hour of non-completed days). Table 1 presents the response completion rates by race/ethnicity and sex, whereas Table 2 presents characteristics of the final sample by race/ethnicity and sex. NA participants in the current study represent tribal nations predominately from southern plains and eastern Oklahoma tribes. NA status was verified from Certificate of Degree of Indian Blood (CDIB) or tribal membership cards.
Table 1.
Study completion rate by race and sex
| NHW | NHW | NA | NA | |||||
|---|---|---|---|---|---|---|---|---|
| male | female | male | female | |||||
| N | % | N | % | N | % | N | % | |
| Total N | 77 | 68 | 50 | 87 | ||||
| Quit during day 1 | 3 | 3.9% | 5 | 7.4% | 5 | 10.0% | 16 | 18.4% |
| Completed 1 day (or quit during day 2) | 14 | 18.2% | 10 | 14.7% | 3 | 6.0% | 15 | 17.2% |
| Completed both days | 60 | 77.9% | 53 | 77.9% | 42 | 84.0% | 56 | 64.4% |
Note. NHW=non-Hispanic white. NA=Native American
Table 2.
Participant Characteristics by Race/Ethnicity and Sex
| non-Hispanic white | Native American | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||||||||||
| Continuous Variable | N | M | SD | N | M | SD | N | M | SD | N | M | SD | F | η2 |
| Age (years) | 77 | 28.31 | 13.81 | 68 | 28.69 | 13.19 | 50 | 30.14 | 12.69 | 87 | 31.94 | 13.60 | 1.22 | 0.01 |
| BMI (kg/m2) | 75 | 24.73 | 3.59 | 66 | 23.70 | 3.98 | 49 | 26.20 | 4.21 | 85 | 25.95 | 4.84 | 4.91* | 0.05 |
| Dispositional Pain Catastrophizing (PCS; 0–52) | 77 | 10.65 | 7.98 | 68 | 8.93 | 8.52 | 50 | 11.66 | 8.99 | 86 | 9.29 | 7.80 | 1.43 | 0.02 |
| State Anxiety (STAI; 20–80) | 76 | 32.25 | 6.72 | 68 | 32.00 | 8.01 | 50 | 34.10 | 7.88 | 86 | 33.00 | 7.34 | 0.92 | 0.01 |
| Negative Affect (PANAS; 10–50) | 76 | 2.97 | 3.25 | 68 | 2.79 | 2.92 | 50 | 3.68 | 3.72 | 86 | 3.09 | 2.71 | 0.84 | 0.01 |
| Positive Affect (PANAS; 10–50) | 76 | 18.92 | 7.09 | 68 | 17.87 | 7.35 | 50 | 20.08 | 6.27 | 86 | 18.08 | 8.54 | 1.05 | 0.01 |
| Psychological Distress (SCL-90 GSI; 0–4) | 77 | 0.33 | 0.29 | 65 | 0.33 | 0.37 | 48 | 0.47 | 0.49 | 82 | 0.39 | 0.34 | 2.05 | 0.02 |
| General Health Scale (SF-36; 0–100) | 77 | 64.38 | 10.85 | 65 | 64.34 | 12.48 | 48 | 61.95 | 10.56 | 83 | 62.47 | 12.05 | 0.75 | 0.01 |
| Categorical Variable | N | % | N | % | N | % | N | % | X2 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Marital Status (% single) | 62 | 80.5% | 46 | 67.6% | 32 | 66.7% | 50 | 57.5% | 24.21 | 0.06 |
| Education (% partial college) | 42 | 55.3% | 33 | 48.5% | 24 | 48.0% | 35 | 40.7% | 23.50 | 0.17 |
| Employment (% <40hrs/week) | 35 | 47.3% | 30 | 44.1% | 16 | 33.3% | 34 | 39.1% | 5.17 | 0.82 |
| Income Category | 24.01 | 0.63 | ||||||||
| <$9,999 | 33 | 44.6% | 22 | 32.8% | 11 | 22.9% | 25 | 29.8% | ||
| $10,000-$14,999 | 7 | 9.5% | 9 | 13.4% | 6 | 12.5% | 9 | 10.7% | ||
| $15,000-$24,999 | 8 | 10.8% | 8 | 11.9% | 6 | 12.5% | 13 | 15.5% | ||
| $25,000-$34,999 | 7 | 9.5% | 5 | 7.5% | 8 | 16.7% | 8 | 9.5% | ||
| $35,000-$49,999 | 4 | 5.4% | 9 | 13.4% | 9 | 18.8% | 11 | 13.1% | ||
| ≥$50,000 | 15 | 20.3% | 14 | 20.9% | 8 | 16.7% | 18 | 21.4% | ||
Note: BMI = body mass index. PCS=Pain Catastrophizing Scale. STAI=State Trait Anxiety Inventory. PANAS=Positive and Negative Affect Schedule. SCL-90=Symptom Checklist 90. GSI=Global Severity Index. SF-36=Medical Outcomes Study 36 item Short Form Health Survey.
P<.05. Bolded tests are statistically significant.
Sample Size Determination
The parent study was powered to detect racial/ethnic differences in pain tolerance, temporal summation of the nociceptive flexion reflex, and conditioned pain modulation. That power analyses suggested 120 per group would provide power=.80 at alpha=.05, so that was the targeted sample. Based on this sample size, a sensitivity analysis was conducted in G*Power (version 3.1.9.2) to determine what size group difference (i.e., effect size) could be detected (by rejecting the null hypothesis) in the present study. That analysis indicated that, with alpha level set to .05, group differences with d≥.36 would be detected 80% of the time (i.e., power=.80), whereas with an alpha level set to .01, group differences with d≥.44 would be detected 80% of the time (i.e., power=.80). Thus, our targeted sample size provided adequate power to detect a small-to-medium effect size.
General Overview of Procedures/Testing
Testing was conducted over a 2-day period, each lasting 4–6 hours. Informed consent and inclusion/exclusion screening were conducted on the first day. If found to be eligible, participants filled out several questionnaires to assess background characteristics in order to ensure that groups did not differ on important variables (e.g., dispositional pain catastrophizing, state affect, general health perceptions, psychological functioning). On one of the testing days, electric tolerance, heat tolerance, mechanical pain threshold, and the Pain45 heat series were administered in a testing block (with a minimum of a 2-min break between tasks). There was a 10-min break after the first testing block and then cold tolerance and ischemia tolerance were administered in a second testing block (with 20-min break between tasks). The mandatory breaks were employed between tasks to minimize carryover effects. On the other day, suprathreshold electric pain, suprathreshold electric pain series, and the 52°C heat series were administered in a block of tests (with a minimum of a 2-min break between tasks). On both testing days, pain tasks were randomized within each block.
Immediately following each pain task, the McGill Pain Questionnaire-Short Form, pain-related anxiety questionnaire, and the Pain Catastrophizing Scale (using situation-specific instructions) were administered with instructions to rate their experience of the preceding pain task. The order of the two testing days was counterbalanced across participants, blocking for sex and race.
Apparatus
Questionnaire presentation was controlled by a computer with dual monitor capacity. Custom built LabVIEW software (National Instruments, Austin, TX) was used to control timing and order of the experimental protocol. One computer monitor was used by the experimenter to monitor experimental timing, whereas the second monitor was used by the participant to complete electronic questionnaires and to make ratings of stimuli. In order to reduce experimenter influences on testing, all reported experimental outcomes (except pressure pain) were conducted with participants in a sound attenuated and electrically shielded testing room and experimenters monitored the testing from an adjacent control room via a video camera (with a microphone) connected to a flat panel monitor. Participants wore a pair of sound attenuating headphones that allowed them to hear the experimenter and the computer-recorded instructions for each task.
Background Questionnaires
These questionnaires were administered to assess eligibility (e.g., Demographic and Health Status) and to examine whether groups differed on important background characteristics (e.g., mood, anxiety, psychological functioning, health, pain catastrophizing).
Demographics and Health Exclusion.
A custom-built demographic and health status questionnaire was used to obtain standard background information, as well as information regarding health problems. It was administered immediately following informed consent. The questionnaire asked about demographic information such as: age, sex, marital status, education level, employment, and income level, as well as potential exclusionary criteria such as cardiovascular problems, neurological problems, chronic pain, and medication use. Weight and height were assessed from a medical scale to calculate body mass index (BMI).
Current Affect.
Positive and negative affect prior to testing were assessed from the Positive and Negative Affect Schedule (PANAS)88. Each positive and negative affect subscale consists of 10 items that measure positive and negative emotions, with subscales ranging from 10 to 50. Higher scores on each scale indicate greater positive or negative affect. In the current study, Chronbach’s alpha (reliability) for the negative affect scale was .76 for NHWs and .73 for NAs, and for the positive affect scale it was .88 for NHWs and .88 for NAs.
State Anxiety.
State anxiety prior to testing was assessed by the 20-item state anxiety subscale of the State Trait Anxiety Inventory (STAI)69. The subscale ranges from 20–80, with higher scores indicating greater current anxiety. In the current study, Chronbach’s alpha (reliability) was .89 for NHWs and .88 for NAs.
Psychological Functioning.
The Symptom Checklist-90-Revised (SCL-90-R) was used to assess general psychological functioning16. The scale consists of 90 items that assess various psychological symptoms (e.g., somatization, obsessive-compulsive, depression, phobic anxiety, paranoia). The Global Severity Index (GSI) of the SCL-90-R was used to assess overall psychological distress. Total GSI scores range from 0 to 4 with higher scores indicating greater distress. In the current study, Chronbach’s alpha (reliability) was .97 for NHWs and .97 for NAs.
Health Perceptions.
The 5-item General Health subscale of the Medical Outcomes Study 36-item Short Form Health Survey (SF-36)87 was used to determine whether groups differed in their perceptions of their health. Example items from this subscale are: “I seem to get sick a little easier than other people,” “I expect my health to get worse,” and “My health is excellent.” Scores are standardized to range from 0 to 100, with higher scores indicating better health. In the current study, Chronbach’s alpha (reliability) of the General Health subscale was .57 for NHWs and .56 for NAs.
Traditional (Dispositional) Pain Catastrophizing.
The 13-item Pain Catastrophizing Scale (PCS)76 was used to assess dispositional catastrophizing at the beginning of the testing session to assess how each participant generally reacts to painful events. This was done to establish whether groups generally differed in pain catastrophizing. Scores ranged from 0 to 52, with higher scores indicating greater catastrophic thoughts. In the current study, Chronbach’s alpha (reliability) was .93 for NHWs and .93 for NAs.
Sensory, Affective, and Catastrophizing Reactions
The current study administered questionnaires to determine group differences in sensory (MPQ – Sensory), affective (MPQ – Affective, and Pain-Related Anxiety), and cognitive-emotional (Situation Specific Catastrophizing,) responses to pain tasks.
McGill Pain Questionnaire – Short Form (MPQ-SF).
Immediately following each painful task, the MPQ-SF42 was administered to assess sensory and affective reactions. The MPQ-SF consists of 15 descriptors (11 sensory, 4 affective) that describe the quality of pain. The sensory subscale includes throbbing, shooting, stabbing, sharp, cramping, gnawing, hot-burning, aching, heavy, tender, and splitting. The affective subscale includes tiring-exhausting, sickening, fearful, and punishing-cruel. Subscales are summed creating total scores that range from 0–33 (sensory) and 0–12 (affective). The MPQ visual analog scale (VAS) was also used to assess pain intensity (a sensory reaction). In the current study, Chronbach’s alpha (reliability) for the MPQ-SF sensory subscale was .76 for NHWs and .84 for NAs and for the MPQ-SF affective subscale it was .73 for NHWs and .81 for NAs.
Pain-related Anxiety.
Immediately following each painful task, a computer-presented visual analog scale (VAS) was administered to assess pain-related anxiety57. Instructions asked the participant to rate the level of anxiety they experienced as a result of the task. Anchors of the VAS ranged from “not at all anxious” to “extremely anxious.” The computer converted the scores so that they ranged from 0 to 100, with higher scores indicating greater pain-related anxiety. These scores were used as a measure of pain-related affect.
Situation-Specific Pain Catastrophizing.
The Pain Catastrophizing Scale (PCS)76 was also used to assess situation-specific (SS) pain catastrophizing. To assess SS pain catastrophizing, the SS-PCS was administered immediately following each painful task with altered instructions asking the participant to report the degree of catastrophizing they engaged in during the task. Scores ranged from 0 to 52, with higher scores indicating greater catastrophic thoughts. SS pain catastrophizing was used as a cognitive-emotional reaction to pain. In the current study, Chronbach’s alpha (reliability) was .95 for NHWs and .96 for NAs.
Exposure to Noxious Stimuli
To assess reactions to pain, participants underwent several suprathreshold pain tasks that involved electric, heat, cold, ischemic, and mechanical pressure stimuli. Following each pain task, participants were administered the MPQ-SF, PCS (situation-specific instructions), and the pain-related anxiety VAS (order randomized).
Electric Pain Tasks
Three tasks were used to assess responses to suprathreshold electric pain. To assess these responses, a bipolar electrode (Nicolet; 30 mm inter-electrode distance) filled with a conductive gel (EC60, Grass Technologies) was placed over the retromalleolar surface of the left ankle after the skin had been cleaned with alcohol and abraded with an exfoliating cream (NuPrep, Weaver and Company, Aurora, CO). Stimulations were delivered by an isolated, constant current stimulator (Digitimer DS7A; Hertfordshire, England). Each stimulus consisted of a train of five 1 ms rectangular wave pulses with a 3 ms inter-pulse interval (250 Hz); however, the train was always experienced as a single stimulation. The maximum stimulation intensity was set at 50-mA to ensure safety.
Electric Pain Tolerance.
Similar to previous studies56, electric pain tolerance was assessed using a single ascending staircase of stimulations that started at 0-mA and increased in 2-mA steps. After each stimulus, the participant rated their pain intensity on a VAS that ranged from “no pain” to “maximum tolerable pain.” The stimulation intensity was increased until the participant rated the stimulus as the maximum tolerable pain. Afterwards, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Suprathreshold Electric Stimuli.
In the parent study, single suprathreshold electric stimuli were delivered to assess the size of individual nociceptive flexion reflexes (NFR), but the current analyses focus only on subjective reactions to these stimuli. First, validated procedures were used to determine the stimulus intensity that reliably elicits NFRs79 (for detailed procedures,80). In brief, stimulation intensity was set at 1.2X the highest of stimulation intensity resulting from: (1) NFR threshold25,55 or (2) 3-stimulation threshold79. NFR threshold assessment involved the delivery of stimulations starting at 0-mA and increased in 2-mA steps (8–12 s interstimulus interval, ISI) until an NFR was evoked. The intensity was decreased in 1-mA steps until an NFR was no longer present. This ascending-descending staircase procedure was repeated 2 more times in 1-mA steps until the NFR appeared and disappeared 2 more times. 3-stimulation threshold involved the delivery of a train of 3 electric stimulations (0.5 s ISI). The intensity of the train was increased in 2-mA steps (8–12 s ISI between trains) until the third stimulus in the train elicited an NFR.
Once the suprathreshold stimulus intensity was determined by the procedures above, participants received 5 individual suprathreshold electric stimuli (8–12 s ISI) and then rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Suprathreshold Electric Series.
The current analyses focused on the subjective reactions to this task. However, in the parent study, these stimuli were used to assess temporal summation of the nociceptive flexion reflex. Stimulation intensity for this task was the same as the suprathreshold electric stimuli noted above. This task involved the delivery of 5 series (trains) of 3 suprathreshold stimulations with an ISI = 0.5 s. There was an 8–12 s break between trains. After all 5 trains were delivered, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Heat Tasks
Heat stimuli were generated using a Medoc (Haifa, Israel) Pathway device with a Contact Heat Evoked Potential Stimulator (CHEPS) thermode. The maximum intensity of any heat stimulus was set to 52°C (51°C for heat tolerance). Three different tasks were used to assess responsivity to painful heat. For all 3 tasks, the thermode was attached to the participants’ left volar forearm using a Velcro strap.
Heat Pain Tolerance.
Similar to prior studies9, heat pain tolerance was assessed 4 times after an initial practice trial. Each trial started from a baseline of 32°C and heated at a rate of 0.5°C/s. Participants were told to terminate the stimulus by pushing a button as soon as the heat became intolerable. In between trials, the thermode was moved slightly to avoid sensitization and then there was a 25–35 s randomly determined inter-trial interval. Heat pain tolerance was defined as the average of the 4 trials. Immediately after the 4th trial, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Pain45 Heat Series.
In the parent study, 5 series of suprathreshold heat stimuli were delivered to assess temporal summation of heat, but the current analyses focus only on the global sensory, affective, and catastrophizing reactions to this task. This task involved 5 series of 10 heat stimuli delivered with a 2.5 s ISI70. Each pulse peaked at an individually calibrated temperature corresponding to a rating of 45 on a numerical rating scale with the following labels70: 10=warm; 20=a barely painful sensation; 30=very weak pain; 40=weak pain; 50=moderate pain; 60=slightly strong pain; 70=strong pain; 80=very strong pain; 90=nearly intolerable pain; 100=intolerable pain. The interval between each 10-pulse series was always 2 min. After the delivery of the 5th series, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
52°C Heat Series.
In the parent study, 6 blocks of 5 heat pulses (8–12 s ISI) were delivered to assess contact heat evoked potentials from EEG28. However, the current analyses focused only on the subjective reactions to this task. Each pulse started at a baseline of 32°C and increased to 52°C at a rate of 70°C/s and then returned to baseline at a rate of 40°C/s28. Each 65 s block of 5 pulses was separated by a short (~1-min) break. After the 5th block of pulses, participants rated their reactions using the MPQSF, pain-related anxiety VAS, and SS-PCS.
Cold Pressor Tolerance
A cold pressor procedure9,40,54 was used to assess responses to cold pain. Participants were asked to submerge their hand and forearm into a circulating water bath (Thermo Fisher Scientific, Pittsburgh, PA) held at 6±0.1°C. Participants were instructed to keep their fingers spread apart and to place their hand on the bottom of the water tank and keep it there for as long as they could tolerate it. The water level was kept constant (6” deep) across all participants to keep procedures standardized and maintain similar cold exposure to participants’ hands/forearms. During the task, a computer timed the length of the hand/arm immersion. The maximum cold water exposure was set to 5 min, but the participant was not informed of the limit. Immediately after tolerance was reached, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Ischemia Pain Tolerance
To measure responses to ischemia pain, a standard forearm tourniquet test was employed24. First, participants used their non-dominant hand to conduct hand exercises with a dynamometer (Lafayette Hand Dynamometer, Lafayette Instrument Company, IN) at 50% grip strength for 2 min (1x/sec). Immediately after the last exercise, the non-dominant arm was raised for 15 s to allow the blood to drain from the forearm, then a blood pressure cuff was inflated to 220 mm/Hg around the non-dominant biceps to occlude blood flow to the forearm. Participants were instructed to keep the non-dominant arm still during the task and indicate by computer when they could no longer tolerate the ischemic pain. During the task, the computer timed the length of the arm occlusion until tolerance was reached. The maximum exposure to ischemic pain was set to 25 min, but the participant was not made aware of this limit. Immediately after tolerance was reached, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Pressure Pain Threshold
To measure responses to mechanical pressure, a Medoc-Wagner computerized algometer was used (Haifa, Israel). Similar to previous studies53, pressure pain threshold was assessed at 3 body sites (masseter muscle, trapezius muscle, thumbnail) in random order. Pressure was applied at a rate of 98 kPa/s until the participant reached pain threshold by pressing a button. Pressure pain threshold was defined as the average of the 3 trials. For parsimony, only responses to the masseter muscle were used because this site elicited greater pain (and lower thresholds) than the other two sites. Immediately after the last pressure pain trial, participants rated their reactions using the MPQ-SF, pain-related anxiety VAS, and SS-PCS.
Data Analysis
Prior to analyses, variable distributions were screened using boxplots, histograms, and normality statistics. Those that were skewed were log or square transformed to reduce positive and negative skew, respectively. If a variable was transformed, this is noted in the units next to the variable name in the tables. Reverse transformed variables are presented and labeled when appropriate. Next, outliers were identified using Wilcox’s MAD-median procedure (using the recommended 2.24 cutoff) and then winsorized by replacing the outlier value with the next nearest non-outlier value90. The following variables were windsorized as a result of outliers: 1) heat tolerance (17 cases) and pressure threshold (20 cases), 2) MPQ-SF sensory ratings for electric tolerance (13 cases), suprathreshold electric stimuli (19 cases), suprathreshold electric series (9 cases), heat tolerance (17 cases), Pain45 heat series (14 cases), 52°C series (5 cases), cold tolerance (14 cases), ischemia tolerance (22 cases), pressure threshold (22 cases), and electric tolerance (13 cases), 3) MPQ-SF VAS for 52°C heat series (6 cases), pressure threshold (19 cases), suprathreshold electric stimuli (4 cases), heat tolerance (12 cases), and ischemia tolerance (6 cases), 4) Pain-related anxiety for heat tolerance (20 cases), Pain45 heat series (21 cases), and ischemia tolerance (39 cases), and 5) SS pain catastrophizing for suprathreshold electric series (7 cases), heat tolerance (9 cases), and cold tolerance (26 cases).
Background Characteristics.
Preliminary analyses determined that there were significant group differences in sex (i.e., there were more women in the NA group), therefore to examine group differences on background characteristics a new independent variable (IV) was created that coded for race and sex in the same variable (i.e., a grouping variable that coded for: NHW men, NHW women, NA men, NA women). This IV was then used in 1-way ANOVAs (for continuous dependent variables [DV]) and chi-square analyses (for categorical DVs) to explore group differences. Significance level for group differences in background characteristics were set to α<.05 (2-tailed).
Primary Analyses.
Analyses for stimulus intensity (e.g., pain tolerance) outcomes were conducted using 2-way factorial ANOVAs with race (NHW vs. NA) and sex (men vs. women) as IVs. Significance level for group differences in stimulus intensity were set to α<.05 (2-tailed). For analyses of sensory, affective, and SS catastrophizing outcomes, ANCOVAs were used to control for the fact that stimulus intensity was individually calibrated to the participant (except for 52°C heat series where the stimulus was constant, so ANOVAs were used). Significance levels for group differences for primary analyses used a Bonferroni adjusted alpha of .01 that accounted for the 5 outcomes (intensity, sensory, affect, anxiety, SS catastrophizing) measured in response to each stimulus (.05 / 5 = .01). However, because this was the first study of its kind we also note when results would have been significant at an alpha level of .05.
Results
Final Sample
338 persons consented to participate, but 56 did not meet inclusion criteria; thus, 282 persons were enrolled (Table 1). Of those, 75% (N=211) completed both days of testing. 15% (N=42) completed only one day and 10% (N=29) quit during the first testing day. Given this, Ns across analyses differed and will be reported. Non-completers were more likely to be NA women (P=.045) and have a lower education level (P=.015); however, there were no differences on age, BMI, marital status, positive affect, negative affect, state anxiety, psychological functioning, or pain catastrophizing (all Ps>.18).
Background Characteristics
Chi-square analysis indicated that groups differed by sex, χ2(df=1)=7.85, P=.005. 64% of the NA group were women, whereas only 47% of the NHW group were women. Thus, all background characteristic analyses used a 4 level IV that coded for race/ethnicity and sex. Inferential statistics, as well as means and SDs (or N’s and %’s), for these analyses are reported in Table 2. As shown, groups were similar on most variables except for BMI. NHW women had a significantly lower BMI than NA men and NA women (Ps<.005).
Ethnic/Racial Differences in Stimulus Intensity/Stimulus Exposure
Prior to examining group differences in sensory, affective, and catastrophizing reactions, stimulus intensity/duration was analyzed. As shown in Table 3, there were significant group differences in suprathreshold electric stimulus intensity (P=.010) and cold pressor pain tolerance (P=.015). The average intensity of the electric stimuli was higher for the NA group than the NHW group. Cold pressor tolerances were lower in the NA group than the NHW group (P=.015), but this was qualified by a Group X Sex interaction (P=.032) that indicated NA women had lower pain tolerances than NHW women (M=36.84 sec, SD=1.74 vs. M=62.29 sec, SD=2.76; P=.001; means/SD reversed transformed), whereas NA men and NHW men did not differ (M=63.73 sec, SD=2.46 vs. M=65.98 sec, SD=2.56; P=.834; means/SD reversed transformed).
Table 3.
Group differences in painful stimulus intensity/duration
| non-Hispanic white |
Native American |
Cohen’s | Group | Sex | GxS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stimulus Variable | N | M | SD | N | M | SD | d | F | F | F |
| Electric Tolerance Stimulus (mA) | 126 | 31.01 | 13.10 | 111 | 30.82 | 13.17 | 0.01 | 0.01 | 0.68 | 0.05 |
| Suprathreshold Electric Intensity (mA) | 131 | 21.70 | 10.02 | 124 | 25.02 | 10.05 | −0.33 | 6.73* | 0.01 | 0.03 |
| Heat Pain Tolerance Stimulus (°C) | 126 | 45.89 | 2.07 | 111 | 45.52 | 1.66 | 0.19 | 2.44 | 30.98* | 0.03 |
| Heat Pain45 Series Intensity (°C) | 126 | 47.34 | 1.79 | 112 | 47.30 | 1.85 | 0.02 | 0.03 | 0.59 | 1.05 |
| Cold Pressor Pain Tolerance Stimulus (Log[sec+1]) | 127 | 1.81 | 0.42 | 110 | 1.69 | 0.33 | 0.32 | 6.04* | 7.07* | 4.64* |
| Ischemia Pain Tolerance Stimulus (Log[sec+1]) | 126 | 2.21 | 0.44 | 111 | 2.11 | 0.43 | 0.23 | 3.21 | 5.56* | 1.16 |
| Pressure Pain Threshold (kPa) | 127 | 162.24 | 61.32 | 111 | 155.31 | 57.49 | 0.12 | 0.79 | 2.60 | 1.14 |
Note. G × S = Group x Sex interaction.
P<.05. Bolded F-tests are statistically significant. Cohen’s d is reported for the effect size for the group mean comparisons.
Sex Differences in Stimulus Intensity/Stimulus Exposure
Sex differences were also found for heat, ischemia, and cold pain tolerance. Compared to men, women had lower heat tolerances (M=45.05°C, SD=1.62 vs. M=46.36°C, SD=1.95; P<.001; d=0.77), lower ischemia tolerances (M= 125.20 sec, SD= 2.77 vs, M= 170.15 sec, SD= 2.63; P=.019; d=0.33; means/SD reversed transformed), and lower cold pressor tolerances (M=47.90 sec, SD=2.33 vs. M=64.84 sec, SD=2.51; P=.008; d=0.36; means/SD reversed transformed). This last effect was moderated by race/ethnicity as described in the previous paragraph. There were no group or sex differences in electric pain tolerance, heat Pain45 intensity, or pressure pain threshold.
Ethnic/Racial Differences in Subjective Reactions to Painful Stimuli
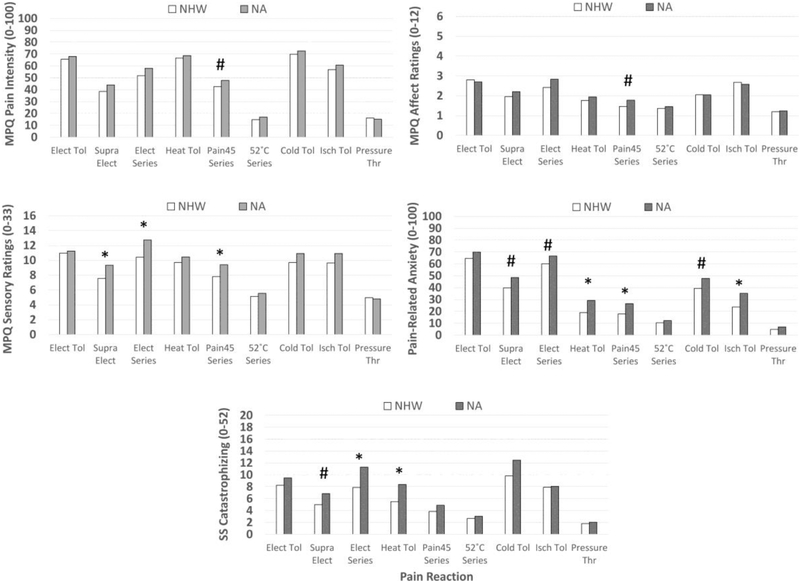
Table 4 reports Ms, SDs, Cohen’s d, and inferential statistics for pain intensity ratings, sensory ratings, affective ratings, pain-related anxiety, and situation-specific catastrophizing in response to the various painful stimulus modalities. Figure 1 depicts these group differences after using reverse operations (e.g., 10X, square root) to put transformed variables back into their original units. Results indicated that, compared to NHW participants, NAs reported: 1) higher pain intensity in response to Pain45 stimuli (P=.041), 2) higher MPQ-SF sensory ratings in response to suprathreshold electric stimulations (P=.008), suprathreshold electric series (P=.004), and Pain45 stimuli (P=.009); 3) higher MPQ-SF affective ratings to the Pain45 heat series (P=.017); 4) higher pain-related anxiety in response to suprathreshold electric stimuli (P=.025), suprathreshold electric series (P=.047), heat pain tolerance stimuli (P=.003), Pain45 heat series (P=.004), cold pressor tolerance stimuli (P=.030), and ischemia tolerance stimuli (P=.002); and 5) greater situation-specific catastrophizing in response to suprathreshold electric stimuli (P=.033), suprathreshold electric series (P=.008), and heat tolerance stimuli (P=.004).
Table 4.
Sensory, affective, and catastrophizing reactions to pain tasks
| ANCOVA F-tests | non-Hispanic white | Native American | Cohen’s | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulus Type | Reactivity Variable | Group | Sex | G x S | M | SD | N | M | SD | N | d |
| Electric Tolerance | Pain Intensity (squared MPQ VAS) |
0.74 | 0.21 | 2.27 | 4312.0 4 |
2672.82 | 12 6 |
4616.2 7 |
2731.7 3 |
11 1 |
–0.11 |
| Sensory (MPQ; 0–33) | 0.15 | 0.11 | 0.27 | 10.97 | 4.70 | 12 6 |
11.24 | 5.73 | 11 1 |
–0.05 | |
| Affect (Log[MPQ+1]) | 0.15 | 2.99 | 0.17 | 0.45 | 0.32 | 12 6 |
0.43 | 0.33 | 11 1 |
0.05 | |
| Anxiety (squared VAS) | 3.04 | 0.52 | 0.16 | 4185.7 7 |
2967.82 | 12 6 |
4892.8 3 |
3254.7 2 |
11 1 |
–0.23 | |
| SS Catastrophizing (Log[PCS+1]) |
0.96 | <.01 | 0.21 | 0.92 | 0.48 | 12 6 |
0.98 | 0.47 | 11 1 |
–0.13 | |
| Suprathr Electric | Pain Intensity (MPQ VAS; 0– 100) |
2.89 | 0.02 | 0.26 | 38.54 | 24.68 | 12 9 |
44.024 | 26.41 | 11 4 |
–0.22 |
| Sensory (MPQ; 0–33) | 7.11* | 1.17 | 2.83 | 7.57 | 4.83 | 12 9 |
9.33 | 5.67 | 11 4 |
–0.34 | |
| Affect (Log[MPQ+1]) | 1.37 | 0.77 | 3.53 | 0.29 | 0.33 | 12 9 |
0.34 | 0.36 | 11 4 |
–0.15 | |
| Anxiety (0–100) | 5.06# | 0.20 | 1.07 | 39.74 | 29.24 | 12 9 |
48.56 | 31.62 | 11 4 |
–0.29 | |
| SS Catastrophizing (Log[PCS+1]) |
4.61# | 0.00 | 1.61 | 0.70 | 0.50 | 12 9 |
0.84 | 0.50 | 11 4 |
–0.28 | |
|
Suprathr Electric Series |
Pain Intensity (MPQ VAS; 0– 100) |
3.29 | 0.04 | <.01 | 51.78 | 26.90 | 12 8 |
58.02 | 27.02 | 11 2 |
–0.23 |
| Sensory (MPQ; 0–33) | 8.34* | 0.12 | 1.30 | 10.41 | 5.81 | 12 7 |
12.74 | 6.92 | 11 2 |
–0.37 | |
| Affect (Log[MPQ+1]) | 2.12 | 2.09 | 2.72 | 0.38 | 0.35 | 12 8 |
0.45 | 0.38 | 11 2 |
–0.19 | |
| Anxiety (squared) | 3.98# | 1.49 | 2.09 | 3600.4 8 |
3088.85 | 12 8 |
4447.6 8 |
3569.5 5 |
11 2 |
–0.26 | |
| SS Catastrophizing (Log[PCS+1]) |
7.23* | 0.97 | 0.89 | 0.90 | 0.49 | 12 7 |
1.05 | 0.42 | 11 1 |
–0.34 | |
| Heat Tolerance | Pain Intensity (squared MPQ VAS) |
0.67 | 8.63* | 1.33 | 4436.9 3 |
2404.65 | 12 5 |
4718.1 8 |
2859.8 5 |
11 0 |
–0.11 |
| Sensory (MPQ; 0–33) | 1.15 | 4.87* | 2.21 | 9.70 | 4.89 | 12 5 |
10.45 | 5.76 | 11 0 |
–0.14 | |
| Affect (Log[MPQ+1]) | 1.11 | 4.60* | 0.20 | 0.25 | 0.29 | 12 5 |
0.29 | 0.31 | 11 0 |
–0.14 | |
| Anxiety (Log[Anx+1]) | 8.98* | 6.56* | 3.41 | 1.28 | 0.49 | 12 5 |
1.47 | 0.46 | 11 0 |
–0.40 | |
| SS Catastrophizing | 8.66* | 9.62* | 0.57 | 0.74 | 0.50 | 12 | 0.92 | 0.43 | 11 | –0.38 | |
| (Log[PCS+1]) | 5 | 0 | |||||||||
| Pain45 Heat Series | Pain Intensity (MPQ VAS; 0–100) | 4.22# | 4.12* | 0.07 | 42.58 | 19.07 | 12 6 |
47.88 | 20.66 | 11 2 |
–0.27 |
| Sensory (MPQ; 0–33) | 6.76* | 0.77 | 3.11 | 7.81 | 3.97 | 12 6 |
9.41 | 5.42 | 11 2 |
–0.34 | |
| Affect (Log[MPQ+1]) | 5.77# | 0.39 | 0.54 | 0.17 | 0.23 | 12 6 |
0.25 | 0.31 | 11 2 |
–0.31 | |
| Anxiety (Log[Anx+1]) | 8.61* | 0.01 | 0.82 | 1.25 | 0.50 | 12 6 |
1.43 | 0.42 | 11 2 |
–0.37 | |
| SS Catastrophizing (Log[PCS+1]) |
3.01 | 1.62 | 0.16 | 0.59 | 0.44 | 12 6 |
0.69 | 0.48 | 11 2 |
–0.23 | |
| 52°C Heat Series§ | Pain Intensity (Log[MPQ VAS+1]) |
1.13 | 1.39 | 0.07 | 1.17 | 0.42 | 13 0 |
1.23 | 0.45 | 11 2 |
–0.14 |
| Sensory (Log[MPQ+1]) | 0.75 | 1.56 | <.01 | 0.71 | 0.27 | 13 0 |
0.74 | 0.30 | 11 2 |
–0.11 | |
| Affect (Log[MPQ+1]) | 0.75 | 3.02 | 0.25 | 0.13 | 0.23 | 13 0 |
0.16 | 0.26 | 11 2 |
–0.11 | |
| Anxiety (Log[Anx+1]) | 0.89 | 0.17 | 0.11 | 1.02 | 0.58 | 13 0 |
1.09 | 0.58 | 11 3 |
–0.12 | |
| SS Catastrophizing (Log[PCS+1]) |
0.89 | 0.29 | 0.02 | 0.43 | 0.43 | 13 0 |
0.48 | 0.48 | 11 2 |
–0.12 | |
| Cold Pressor Tol | Pain Intensity (squared MPQ VAS) |
1.38 | 7.00* | 0.70 | 4875.1 1 |
2653.93 | 12 6 |
5272.3 2 |
2936.1 2 |
10 9 |
–0.14 |
| Sensory (MPQ; 0–33) | 2.75 | 1.83 | 0.89 | 9.70 | 5.58 | 12 6 |
10.92 | 5.70 | 10 9 |
–0.22 | |
| Affect (Log[MPQ+1]) | <.01 | 2.90 | 1.10 | 0.31 | 0.32 | 12 6 |
0.31 | 0.32 | 10 9 |
0.00 | |
| Anxiety (0–100) | 4.75# | 1.60 | 0.89 | 39.34 | 29.29 | 12 6 |
47.83 | 33.31 | 10 9 |
–0.27 | |
| SS Catastrophizing (Log[PCS+1]) | 3.76 | 6.73* | 4.07* | 0.99 | 0.46 | 12 6 |
1.10 | 0.40 | 10 9 |
–0.24 | |
| Ischemia Tolerance | Pain Intensity (MPQ VAS; 0– 100) |
1.71 | 13.34* | <.01 | 56.89 | 22.75 | 12 6 |
60.70 | 23.14 | 11 0 |
–0.17 |
| Sensory (MPQ; 0–33) | 2.96 | 6.04* | 0.73 | 9.65 | 4.91 | 12 6 |
10.92 | 6.59 | 11 0 |
–0.22 | |
| Affect (Log[MPQ+1]) | 0.17 | 7.36* | 0.10 | 0.43 | 0.29 | 12 6 |
0.41 | 0.32 | 11 0 |
0.05 | |
| Anxiety (Log[Anx+1]) | 9.72* | 6.23* | 0.25 | 1.38 | 0.51 | 12 6 |
1.55 | 0.34 | 11 0 |
–0.39 | |
| SS Catastrophizing (Log[PCS+1]) | 0.02 | 4.09* | 0.38 | 0.90 | 0.51 | 12 6 |
0.91 | 0.50 | 11 0 |
–0.02 | |
| Pressure Threshold | Pain Intensity (Log[MPQ VAS+1]) |
0.28 | 0.33 | 0.52 | 1.21 | 0.31 | 12 5 |
1.18 | 0.39 | 11 1 |
0.09 |
| Sensory (MPQ; 0–33) | 0.19 | 3.43 | 0.05 | 4.98 | 3.05 | 12 5 |
4.80 | 3.21 | 11 1 |
0.06 | |
| Affect (Log[MPQ+1]) | 0.34 | 2.09 | 0.11 | 0.08 | 0.17 | 12 5 |
0.09 | 0.20 | 11 1 |
–0.08 | |
| Anxiety (Log[Anx+1]) | 3.80 | 0.04 | 0.22 | 0.69 | 0.57 | 12 5 |
0.84 | 0.63 | 11 1 |
–0.26 | |
| SS Catastrophizing (Log[PCS+1]) | 0.82 | 2.27 | 0.52 | 0.26 | 0.37 | 12 5 |
0.31 | 0.38 | 11 1 |
–0.12 | |
Note. MPQ=McGill Pain Questionnaire – Short Form. SS=situation-specific. PCS=Pain Catastrophizing Scale. Cohen’s d is reported for the effect size for the group mean comparisons.
P<.05
P<.01. Bolded F-tests are statistically significant.
analysis of this variable did not include stimulus intensity as a covariate because the intensity was the same for all participants.
Figure 1.
Racial/ethnic differences in pain intensity ratings, sensory ratings, affective ratings, pain-related anxiety, and situation-specific (SS) catastrophizing in response to electric tolerance testing (Elect Tol), suprathreshold electric stimulations (Supra Elect), 3 stimulation series of suprathreshold electric stimulations (Elect Series), heat tolerance testing (Heat Tol), 10-pulses series of suprathreshold heat stimuli (Pain45 series), series of 52°C heat stimuli (52°C series), cold pressor tolerance testing (Cold Tol), ischemia tolerance testing (Isch Tol), and pressure threshold testing (Pressure Thr). For variables that were transformed (e.g., log, square) to address non-normality, the reverse operation was conducted on the means (e.g., 10X, square root) in order to display them in the original units. MPQ=McGill Pain Questionnaire-Short Form. #Racial/ethnic group differences at P<.05. *Racial/ethnic group differences at P<.01.
Sex Differences in Subjective Reactions to Painful Stimuli
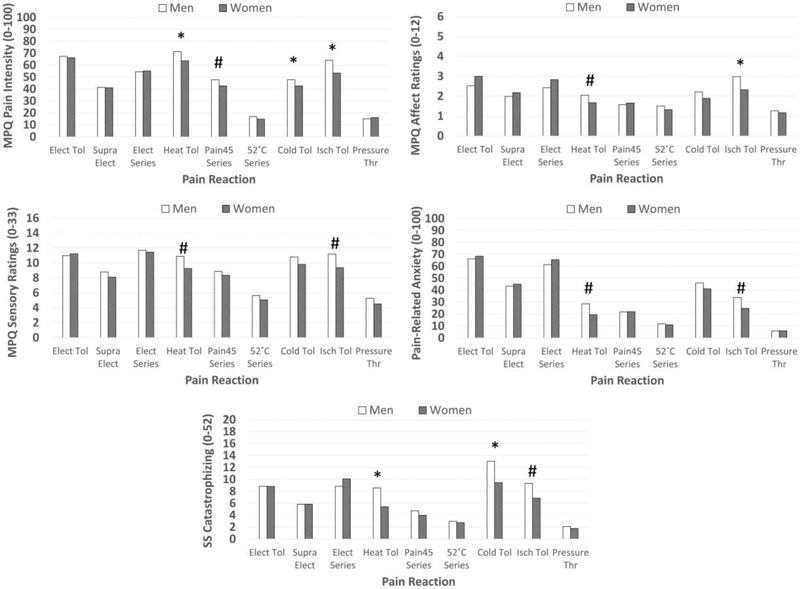
Also, as noted in Table 4, sex differences were also found. Figure 2 depicts these results after using reverse operations (e.g., 10X, square root) to put transformed variables back into their original units. Results indicated that, compare to women, men reported: 1) more pain intensity in response to heat tolerance stimuli (P=.004, d=.41), Pain45 series (P=.044, d=.26), cold tolerance stimuli (P=.009, d=.32), and ischemia tolerance stimuli (P=.0003, d=.47), 2) higher MPQ-SF sensory ratings in response to heat tolerance stimuli (P=.028; d=0.31) and ischemia tolerance stimuli (P=.015; d=0.32), 3) higher MPQSF affective ratings in response to heat tolerance stimuli (P=.033; d=0.30) and ischemia tolerance stimuli (P=.007; d=0.36), 4) higher pain related anxiety in response to heat tolerance stimuli (P=.011; d=0.35) and ischemia tolerance stimuli (P=.013; d=0.30); and 5) higher situation-specific catastrophizing in response to heat tolerance stimuli (P=.002; d=0.43), cold tolerance stimuli (P=.010, d=.23), and ischemia tolerance stimuli (P=.044; d=0.26). The only significant Group X Sex interaction was noted for log situation-specific catastrophizing during cold pressor (P=.045). NA women reported greater situation-specific catastrophizing than NHW women (M= 12.02, SD= 2.34 vs. M= 7.40, SD= 3.21; P=.005; d=0.63, means reverse transformed to ease interpretation), whereas there was no difference in situation-specific catastrophizing between NA men and NHW men (M= 12.94, SD= 2.79 vs. M= 13.06, SD= 2.45; P=.956; d=.01, means reverse transformed to ease interpretation).
Figure 2.
Sex differences in pain intensity ratings, sensory ratings, affective ratings, pain-related anxiety, and situation-specific (SS) catastrophizing in response to electric tolerance testing (Elect Tol), suprathreshold electric stimulations (Supra Elect), 3 stimulation series of suprathreshold electric stimulations (Elect Series), heat tolerance testing (Heat Tol), 10-pulse series of suprathreshold heat stimuli (Pain45 series), series of 52°C heat stimuli (52°C series), cold pressor tolerance testing (Cold Tol), ischemia tolerance testing (Isch Tol), and pressure threshold testing (Pressure Thr). For variables that were transformed (e.g., log, square) to address non-normality, the reverse operation was conducted on the means (e.g., 10X, square root) in order to display them in the original units. MPQ=McGill Pain Questionnaire-Short Form. #Sex differences at P<.05. *Sex differences at P<.01.
Discussion
This study used QST to examine racial/ethnic group differences in reactions to painful stimuli that might contribute to chronic pain risk in NAs4,35,83. To achieve this, we measured pain intensity ratings, sensory ratings, affective ratings, pain-related anxiety, and situation-specific catastrophizing (SS-catastrophizing) following suprathreshold exposure to electric, heat, cold, ischemic, and pressure stimuli.
Race/Ethnic Differences in Pain Reactions
Sensory differences were noted between NAs and NHWs, but were restricted to electric stimuli and Pain45 (heat) series. In all cases, NAs reported higher pain intensity (although non-significant at the P<.01 level) and higher MPQ-SF sensory ratings than NHWs. Thus, these electric and heat stimuli evoked a stronger sensory experience in NAs. Interestingly, all of these stimuli were delivered in a series. 2 of the 3 stimuli (electric series, Pain45 series) were designed to evoke temporal summation of pain (i.e., had inter-stimulus intervals ≤3 s), a phenomenon associated with temporary sensitization of spinal neurons2,22,72. Therefore, these racial/ethnic differences could reflect a heightened sensory experience in NAs resulting from amplification of spinal nociception. However, this cannot fully explain the group differences because NAs also had higher sensory ratings in response to the 5 suprathreshold electric stimuli whose inter-stimulus intervals were too long to evoke temporal summation. Moreover, we have not observed racial/ethnic differences in temporal summation of pain itself (74; data to be reported elsewhere). Because sensitized spinal pathways assessed by temporal summation are not likely to explain the results, it would be interesting to determine whether these heightened sensory experiences correspond to pain signaling differences within the primary or secondary somatosensory cortices43,86, or whether they reflect differences in the interpretation of the pain signal. Whatever the mechanism, these data suggest differences in the sensory experience of NAs that occurs during suprathreshold electric and heat stimuli.
There was only one group difference in MPQ-SF affective ratings and it occurred in response to the Pain45 heat series, but it did not survive after adjusting for familywise Type I error. Some of the most consistent differences were noted for pain-related anxiety. A close examination of Fig 1 finds that NAs reported more anxiety to all stimuli, but only heat tolerance, Pain45, and cold tolerance survived familywise type I error adjustment. Some of the non-significant group differences may be due to ceiling and floor effects because stimuli evoking moderate anxiety showed the strongest group differences. Given that anxiety is known to enhance pain3,39,49,59, this could be a significant risk factor for NAs.
NAs also reported greater SS-catastrophizing in response to suprathreshold electric stimuli, electric series, and heat tolerance stimuli (although suprathreshold electric stimuli did not survive familywise Type I error adjustment). This is interesting given that there were no group differences in traditionally-measured (dispositional) pain catastrophizing (Table 2) and suggests that NAs experienced greater catastrophic thoughts during specific pain tasks. This implies that SS-catastrophizing may be more sensitive in detecting racial/ethnic differences. Given that prior research has found SS-catastrophizing to be a stronger predictor of pain outcomes than dispositional catastrophizing10,12,18,58, greater SS-catastrophizing could put NAs at greater risk of pain.
Implications for Racial/Ethnic Group Differences in Pain Reactions
These differences in pain experience have several implications. First, compared to NHWs, NAs reported heightened sensory, anxiety, and catastrophizing reactions to multiple pain stimuli, suggesting amplification of the pain experience across different stimulus modalities. Notably, these experiential differences were more consistent than differences in pain thresholds/tolerances (Table 3). NAs had lower cold pain tolerances, but there were no group differences for electric/heat tolerance or pressure thresholds. Thus, pain risk in NAs may not stem from a general reduction in pain tolerance, but rather the resulting psychological impact of the noxious event (e.g., anxiety, catastrophizing). These observations are in contrast to prior (small N) studies of NAs that reported dampened pain sensitivity or reduced pain facilitation47,48,65. So, the present study underscores the importance of replicating findings from small studies using larger, more diverse samples and indicates that pain risk in NAs may be similar to other minorities. Specifically, others have noted that ethnic/racial differences are often attributed to psychosocial variables like hypervigilance, anxiety, and/or depression.9,19,20,29,36,52,63,91 It is noteworthy though that we did not find group differences in general psychological status (Table 2; e.g., state anxiety, distress). Thus, future research is needed to explore cultural factors (e.g., historical trauma) that could contribute to heightened pain reactivity in NAs.
Second, the heightened pain reactivity in NAs cannot be attributed to differences in nociceptive input. Even though most stimuli (except 52°C heat series) were individually-calibrated to participants, individual differences in stimulus intensity/exposure were controlled in the analyses. Moreover, group differences cannot be explained by the unequal number of men/women across racial/ethnic groups, because sex was also controlled.
Third, the most consistent group differences across stimuli were noted for pain-related anxiety, sensory ratings, and to a lesser extent SS-catastrophizing. Both anxiety3,13,44,45,49,59,68,85 and catastrophizing10,12,50,57,73 are strong pain enhancers. Anxiety can activate descending, brain-to-spinal cord facilitation circuits33,81 and catastrophizing can facilitate pain via supraspinal circuits17,26,27,57,80,81. Given this, these factors may play a significant role in pain risk for NAs due to their sensitizing effects, perhaps actually promoting the eventual onset of chronic pain. Moreover, anxiety and catastrophizing are related to greater pain-related disability and suffering in those with pain39,50,75; thus, they may also help maintain pain in NAs. This could ultimately form a vicious cycle, in which pain leads to anxiety/catastrophizing, that then enhances pain8,12,39,51,84. Hence, treatments that target pain-related anxiety and catastrophizing may be particularly helpful for NA patients15,34,67,82.
Sex Differences in Pain Reactions
Sex differences in pain are often observed with women showing greater experimental pain sensitivity and a higher likelihood of developing chronic pain5–7,23,60–62,77,89 (although effect sizes can be quite variable62). Consistent with this, we found that women had lower heat pain tolerances, ischemia pain tolerances, and cold pain tolerances, but cold tolerance was moderated by race/ethnicity such that NA women were less tolerant than NA men (P=.001). Interestingly, sex differences in pain experience did not mirror these differences. Rather, men reported greater pain intensity (heat, cold, & ischemia tolerance), MPQ-SF affective pain (ischemia tolerance), and SS-catastrophizing (heat & cold tolerance). Although SS-catastrophizing during cold pain was moderated by race/ethnicity, men in both racial/ethnic groups reported greater SS-catastrophizing than women (Ps<.05).
It is unclear why men reported greater pain reactivity in our study, but it is interesting to note that higher reactivity was only found in response to stimuli that men tolerated longer. By contrast, NAs reported greater pain reactivity in response to stimuli in which their pain tolerances were lower or similar to NHWs. Together, this suggests increased pain reactivity in men may reflect a different mechanism than those observed in NAs. Indeed, the racial/ethnic differences are more likely to reflect pain amplification processes that could promote pain in NAs (i.e., a risk factor), whereas the sex differences seem to reflect that men were able to tolerate pain longer despite experiencing greater pain. Taken together, one could argue this latter observation reflects a resiliency factor for men. However, this hypothesis requires further testing.
Strengths and Limitations
This study had a number of strengths. It is currently the largest study of experimental pain in a NA sample. It also used numerous pain stimulus modalities that were pseudorandomly ordered to minimize order effects. We also assessed several measures of pain experience. Analyses also controlled for stimulus intensity and sex. Despite these strengths, a few limitations should be noted.
First, this study was conducted in healthy, pain-free individuals in order to determine whether group differences in pain processing exist that could contribute to chronic pain risk. This helps ensure that observed group differences are not due to differences in disease status or access to health care. As a result, we cannot know whether these findings will generalize to NAs experiencing chronic pain. Moreover, it meant that many of our participants were young adults; thus, results may not generalize to older individuals. Second, NA women were more likely to quit before completing all tasks. Thus, it is possible that our results will not generalize to all NA women. That said, non-completers were not systematically different than completers on other background variables. Related, our NA sample was recruited mostly from northeastern Oklahoma where NAs are not reservation-dwelling. Future studies should determine if our results generalize to NAs from other geographical regions. Third, although we varied the order of pain tasks and scheduled mandatory breaks in between most tasks, several tasks occurred each day. Therefore, it is possible that some carryover occurred, perhaps masking other racial/ethnic differences. And finally, although we adjusted our alpha level, it is possible that the numerous hypothesis tests resulted in inflation of Type I error rate.
Summary
In sum, NAs experienced greater sensory, pain-related anxiety, and catastrophizing reactions to laboratory pain. Given the potential for anxiety and catastrophic thoughts to amplify pain, these enhanced reactions in NAs may, at least partly, explain the greater prevalence of pain in this population.
Highlights.
Native Americans (NAs) experienced greater pain reactivity than non-Hispanic whites
Reactivity differences were noted for sensory ratings, anxiety, and catastrophizing
This might place them at risk for pain disorders
Conflicts of Interest and Source of Funding:
This research was supported by the National Institute on Minority Health and Health Disparities of the National Institute of Health under Award Number R01MD007807. Edward Lannon, Shreela Palit, and Yvette Güereca were supported by a National Science Foundation Graduate Research Fellowship Program. The content is solely the responsibility of the authors and does not necessarily reflect the views of the National Institutes of Health, National Science Foundation, Indian Health Service, or the Cherokee Nation. The authors report no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anderson KO, Green CR, Payne R: Racial and ethnic disparities in pain: causes and consequences of unequal care. The Journal of Pain 10:1187–1204, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Arendt-Nielsen L, Petersen-Felix S: Wind-up and neuroplasticity: is there a correlation to clinical pain? Eur J Anaesthesiol Suppl 10:1–7, 1995 [PubMed] [Google Scholar]
- 3.Bair MJ, Poleshuck EL, Wu J, Krebs EK, Damush TM, Tu W, Kroenke K: Anxiety but not social stressors predict 12-month depression and pain severity. The Clinical Journal of Pain 29:95–101, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes PM, Adams PF, Powell-Griner E: Health characteristics of the American Indian or Alaska Native adult population: United States, 2004–2008 In: Services USDoHaH, ed. Vol no. 20 Hyattesville, MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 5.Bartley EJ, Fillingim RB: Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 111:52–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkley K: Sex differences in pain. Behav Brain Sci 20:371, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Berkley KJ: Sex differences in pain. Behav Brain Sci 20:371–380, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Ze, Katz J: Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. Journal of pain research 8:21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell CM, Edwards RR, Fillingim RB: Ethnic differences in responses to multiple experimental pain stimuli. Pain 113:20–26, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Campbell CM, Kronfli T, Buenaver LF, Haythornthwaite J, Smith MI, Edwards RR: In-vivo vs. standard catastrophizing in multiple pain measures among healthy, TMD and arthritis patients American Pain Society. Tampa, FL: American Pain Society; 2008. [Google Scholar]
- 11.Campbell CM, Kronfli T, Buenaver LF, Smith MT, Berna C, Haythornthwaite JA, Edwards RR: Situational versus dispositional measurement of catastrophizing: Associations with pain responses in multiple samples. The Journal of Pain 11:443–453, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell CM, Quartana PJ, Buenaver LF, Haythornthwaite JA, Edwards RR: Changes in Situation-Specific Pain Catastrophizing Precede Changes in Pain Report During Capsaicin Pain: A Cross-Lagged Panel Analysis Among Healthy, Pain-Free Participants. J Pain doi: 10.1016/j.jpain.2009.1012.1007, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Colloca L, Benedetti F: Nocebo hyperalgesia: how anxiety is turned into pain. Current Opinion In Anaesthesiology 20:435–439, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Crombez G, Vlaeyen JW, Heuts PH, Lysens R: Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain 80:329–339, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Darnall BD, Sturgeon JA, Kao M-C, Hah JM, Mackey SC: From catastrophizing to recovery: a pilot study of a single-session treatment for pain catastrophizing. Journal of pain research 7:219, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derogatis LR: SCL-90-R : symptom checklist-90-R : administration, scoring & procedures manual. Vol. [Minneapolis, Minn.]: [National Computer Systems, Inc.]; 1994. [Google Scholar]
- 17.Edwards RR: The Neural Correlates of Catastrophizing: an fMRI Study. American Pain Society; Tampa, FL; 2008. [Google Scholar]
- 18.Edwards RR, Campbell CM, Fillingim RB: Catastrophizing and Experimental Pain Sensitivity: Only In Vivo Reports of Catastrophic Cognitions Correlate With Pain Responses. J Pain 6:338–339, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Edwards RR, Doleys DM, Fillingim RB, Lowery D: Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med 63:316–323, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Fillingim RB: Ethnic differences in thermal pain responses. Psychosom Med 61:346–354, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O: Ethnic Similarities and Differences in the Chronic Pain Experience: A Comparison of African American, Hispanic, and White Patients. Pain Medicine 6:88–98, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Eide PK: Wind-up and the NMDA receptor complex from a clinical perspective. European Journal of Pain 4:5–15, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Fillingim RB, Fillingim RB: Sex, gender, and pain: A biopsychosocial framework. Vol. Seattle, WA, US: IASP Press; 2000. [Google Scholar]
- 24.Fillingim RB, Maixner W, Girdler SS, Light KC, Sheps DS, Mason GA: Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom Med 559:512, 1997 [DOI] [PubMed] [Google Scholar]
- 25.France CR, Rhudy JL, McGlone S: Using normalized EMG to define the nociceptive flexion reflex (NFR) threshold: further evaluation of standardized NFR scoring criteria. Pain 145:211–218, 2009 [DOI] [PubMed] [Google Scholar]
- 26.Geisser ME, Casey KL, Brucksch CB, Ribbens CM, Appleton BB, Crofford LJ: Perception of noxious and innocuous heat stimulation among healthy women and women with fibromyalgia: Association with mood, somatic focus, and catastrophizing. Pain 102:243–250, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Gracely RH, Geisser ME, Giesecke T, Grant MAB, Petzke F, Williams DA, Clauw DJ: Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain: A Journal Of Neurology 127:835–843, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Granovsky Y, Anand P, Nakae A, Nascimento O, Smith B, Sprecher E, Valls-Solé J: Normative data for Aδ contact heat evoked potentials in adult population: a multicenter study. Pain 157:1156–1163, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kaloukalani DA, Lasch KE, Myers C, Tait RC, Todd KH, Vallerand AH: The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain medicine : the official journal of the American Academy of Pain Medicine 4:277–294, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Hinrichs‐Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EA: Psychosocial predictors and correlates for chronic post‐surgical pain (CPSP)–a systematic review. European journal of pain 13:719–730, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Hirsh AT, Hollingshead NA, Ashburn-Nardo L, Kroenke K: The interaction of patient race, provider bias, and clinical ambiguity on pain management decisions. The Journal of Pain 16:558–568, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman KM, Trawalter S, Axt JR, Oliver MN: Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proceedings of the National Academy of Sciences 113:4296–4301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hubbard CS, Ornitz EM, Gaspar JX, Smith S, Amin J, Labus JS, Kilpatrick LA, Rhudy JL, Mayer EA, Naliboff B: Modulation of nociceptive and acoustic startle responses to an unpredictable threat in men and women. Pain 152:1632–1634, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen MP, Turner JA, Romano JM: Changes in beliefs, catastrophizing, and coping are associated with improvement in multidisciplinary pain treatment. J Consult Clin Psychol 69:655–662, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Jimenez N, Garroutte E, Kundu A, Morales L, Buchwald D: A Review of the Experience, Epidemiology, and Management of Pain among American Indian, Alaska Native, and Aboriginal Canadian Peoples. J Pain 12:511–522, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jordan MS, Lumley MA, Leisen JCC: The relationships of cognitive coping and pain control beliefs to pain and adjustment among African-American and Caucasian women with rheumatoid arthritis. Arthritis Care Res 11:80, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Yang GS, Greenspan JD, Downton KD, Griffith KA, Renn CL, Johantgen M, Dorsey SG: Racial and ethnic differences in experimental pain sensitivity: systematic review and meta-analysis. Pain 158:194–211, 2017 [DOI] [PubMed] [Google Scholar]
- 38.Loeser JD, Melzack R: Pain: an overview. The Lancet 353:1607–1609, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Lumley MA, Cohen JL, Borszcz GS, Cano A, Radcliffe AM, Porter LS, Schubiner H, Keefe FJ: Pain and emotion: a biopsychosocial review of recent research. J Clin Psychol 67:942–968, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meagher MW, Arnau RC, Rhudy JL: Pain and emotion: effects of affective picture modulation. Psychosom Med 63:79–90, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Meehan JP, Stoll AM, Hardy JD: Cutaneous pain threshold in the Native Alaskan Indian and Eskimo. J Appl Physiol 6:397–400, 1954 [DOI] [PubMed] [Google Scholar]
- 42.Melzack R: The short-form McGill pain questionnaire. Pain 30:191–197, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Melzack R: From the gate to the neuromatrix. Pain Suppl 6:S121–126, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Naliboff B, Rhudy JL: Anxiety and functional pain disorders In: Mayer EA, Bushnell MC, (eds). Functional pain syndromes: Presentation and pathophysiology. Seattle: IASP Press; 2009:pp 185–214 [Google Scholar]
- 45.Nash JM, Williams DM, Nicholson R, Trask PC: The contribution of pain-related anxiety to disability from headache. J Behav Med 29:61–67, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Nguyen M, Ugarte C, Fuller I, Haas G, Portenoy RK: Access to care for chronic pain: racial and ethnic differences. The Journal of Pain 6:301–314, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Palit S, Kerr KL, Kuhn BL, DelVentura JL, Terry EL, Bartley EJ, Shadlow JO, Rhudy JL: Examining emotional modulation of pain and spinal nociception in Native Americans: A preliminary investigation. Int J Psychophysiol 90:272–281, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Palit S, Kerr KL, Kuhn BL, Terry EL, DelVentura JL, Bartley EJ, Shadlow JO, Rhudy JL: Exploring pain processing differences in Native Americans. Health Psychol 32:1127–1136, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, Matthews PM, Rawlins JN, Tracey I: Exacerbation of pain by anxiety is associated with activity in a hippocampal network. The Journal Of Neuroscience: The Official Journal Of The Society For Neuroscience 21:9896, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quartana PJ, Campbell CM, Edwards RR: Pain catastrophizing: a critical review. Expert Review Of Neurotherapeutics 9:745–758, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Racine M, Moulin DE, Nielson WR, Morley-Forster PK, Lynch M, Clark AJ, Stitt L, Gordon A, Nathan H, Smyth C: The reciprocal associations between catastrophizing and pain outcomes in patients being treated for neuropathic pain: a cross-lagged panel analysis study. Pain 157:1946–1953, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Rahim-Williams B, Riley JL III, Williams AK, Fillingim RB: A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Medicine 13:522–540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rahim-Williams FB, Riley JL 3rd, Herrera D, Campbell CM, Hastie BA, Fillingim RB : Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain 129:177–184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rhudy JL, Dubbert PM, Parker JD, Burke RS, Williams AE: Affective modulation of pain in substance dependent veterans. Pain Medicine 7:483–500, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Rhudy JL, France CR: Defining the nociceptive flexion reflex (NFR) threshold in human participants: A comparison of different scoring criteria. Pain 128:244–253, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rhudy JL, France CR, Bartley EJ, McCabe KM, Williams AE: Psychophysiological responses to pain: Further validation of the nociceptive flexion reflex (NFR) as a measure of nociception using multilevel modeling. Psychophysiol 46:939–948, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Rhudy JL, Martin SL, Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL: Pain catastrophizing is related to temporal summation of pain, but not temporal summation of the nociceptive flexion reflex. Pain 152:794–801, 2011 [DOI] [PubMed] [Google Scholar]
- 58.Rhudy JL, Maynard LJ, Russell JL: Does in-vivo catastrophizing engage descending modulation of spinal nociception? J Pain 8:325–333, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Rhudy JL, Meagher MW: Fear and anxiety: divergent effects on human pain thresholds. Pain 84:65–75, 2000 [DOI] [PubMed] [Google Scholar]
- 60.Rhudy JL, Williams AE: Gender differences in pain: Do emotions play a role? Gender Med 2:208–226, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Riley J III, Robinson ME, Wade JB, Myers CD, Price DD: Sex differences in negative emotional responses to chronic pain. J Pain 2:354, 2001 [DOI] [PubMed] [Google Scholar]
- 62.Riley JL 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB: Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain 74:181–187, 1998 [DOI] [PubMed] [Google Scholar]
- 63.Riley JL III, Wade JB, Myers CD, Sheffield D, Papas RK, Price DD: Racial/ethnic differences in the experience of chronic pain. Pain 100:291, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Ruehlman LS, Karoly P, Newton C: Comparing the experiential and psychosocial dimensions of chronic pain in African Americans and Caucasians: Findings from a national community sample. Pain Medicine 6:49–60, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherman ED: Sensitivity to Pain:(With an Analysis of 450 Cases). Can Med Assoc J 48:437, 1943 [PMC free article] [PubMed] [Google Scholar]
- 66.Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, Max MB, Goldman D, Maixner W: Influence of psychological factors on risk of temporomandibular disorders. J Dent Res 86:1120–1125, 2007 [DOI] [PubMed] [Google Scholar]
- 67.Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA: Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. The Journal of Pain 7:261–271, 2006 [DOI] [PubMed] [Google Scholar]
- 68.Smith BW, Zautra AJ: The effects of anxiety and depression on weekly pain in women with arthritis. Pain 138:354–361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spielberger CD: State-Trait Anxiety Inventory. Vol. Palo-Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 70.Staud R, Craggs JG, Robinson ME, Perlstein WM, Price DD: Brain activity related to temporal summation of C-fiber evoked pain. Pain 129:130–142, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Staud R, Price DD, Robinson ME, Vierck CJ Jr.: Body pain area and pain-related negative affect predict clinical pain intensity in patients with fibromyalgia. The journal of pain : official journal of the American Pain Society. 5:338–343, 2004 [DOI] [PubMed] [Google Scholar]
- 72.Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD: Abnormal sensitization and temporal summation of second pain (wind-up) in patients with fibromyalgia syndrome. Pain. 91:165–175, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Sturgeon JA, Zautra AJ: State and trait pain catastrophizing and emotional health in rheumatoid arthritis. Ann Behav Med 45:69–77, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sturycz CA, Kuhn BL, Lannon EW, Palit S, Güereca YM, Payne MF, Hellman N, Toledo TA, Hahn BJ, Shadlow JO, Rhudy JL: Does Spinal Sensitization Contribute to Pain Risk in Native Americans?: Preliminary Findings from Oklahoma Study of Native American Pain Risk (OK-SNAP).International Association for the Study of Pain (IASP) World Congress on Pain; Boston, MA; 2018. [Google Scholar]
- 75.Sullivan MJ, Thorn B, Haythornthwaite JA, Keefe F, Martin M, Bradley LA, Lefebvre JC: Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 17:52–64, 2001 [DOI] [PubMed] [Google Scholar]
- 76.Sullivan MJL, Bishop SR, Pivik J: The Pain Catastrophizing Scale: Development and validation. Psychological Assessment 7:524–532, 1995 [Google Scholar]
- 77.Sullivan MJL, Tripp DA, Santor D: Gender differences in pain and pain behavior: The role of catastrophizing. Cognitive Therapy and Research 24:121–134, 2000 [Google Scholar]
- 78.Tait RC, Chibnall JT: Racial/ethnic disparities in the assessment and treatment of pain: psychosocial perspectives. Am Psychol 69:131, 2014 [DOI] [PubMed] [Google Scholar]
- 79.Terry EL, France CR, Bartley EJ, DelVentura JL, Kerr KL, Vincent AL, Rhudy JL: Standardizing procedures to study sensitization of human spinal nociceptive processes: Comparing parameters for temporal summation of the nociceptive flexion reflex (TS-NFR). Int J Psychophysiol 81:263–274, 2011 [DOI] [PubMed] [Google Scholar]
- 80.Terry EL, Thompson KA, Rhudy JL: Experimental reduction of pain catastrophizing modulates pain report but not spinal nociception as verified by mediation analyses. Pain 156:1477–1488, 2015 [DOI] [PubMed] [Google Scholar]
- 81.Terry EL, Thompson KA, Rhudy JL: Does pain catastrophizing contribute to threat-evoked amplification of pain and spinal nociception? Pain 157:456–465, 2016 [DOI] [PubMed] [Google Scholar]
- 82.Thorn BE, Boothby JL, Sullivan MJL: Targeted treatment of catastrophizing for the management of chronic pain. Cognitive and Behavioral Practice 9:127–138, 2002 [Google Scholar]
- 83.USDHHS: Summary of health statistics for U.S. Adults: National health interview survey, 2009 In: Services HaH, ed. Hyattsville, Maryland: DHHS; 2010. [Google Scholar]
- 84.Vlaeyen JW, Linton SJ: Fear-avoidance model of chronic musculoskeletal pain: 12 years on. Pain 153:1144–1147, 2012 [DOI] [PubMed] [Google Scholar]
- 85.Vowles KE, McNeil DW, Sorrell JT, Lawrence SM: Fear and pain: Investigating the interaction between aversive states. J Abnorm Psychol 115:821–833, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo C-W, Kross E: An fMRI-based neurologic signature of physical pain. N Engl J Med 368:1388–1397, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ware JE, Snow KK, Kosinski M, Gandek B: SF-36 Health Survey manual and interpretation guide. Vol. Boston: The Health Institute New England Medical Center; 1993. [Google Scholar]
- 88.Watson D, Clark LA, Tellegen A: Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol 54:1063, 1988 [DOI] [PubMed] [Google Scholar]
- 89.Wiesenfeld-Hallin Z: Sex differences in pain perception. Gender Med 2:137–145, 2005 [DOI] [PubMed] [Google Scholar]
- 90.Wilcox RR: Understanding and applying basic statistical methods using R. Vol John Wiley & Sons; 2016. [Google Scholar]
- 91.Zatzick DF, Dimsdale JE: Cultural variations in response to painful stimuli. Psychosom Med 52:544, 1990 [DOI] [PubMed] [Google Scholar]