Abstract
Sedatives and anesthetics can injure the developing brain. They cause apoptosis of neurons and oligodendrocytes, impair synaptic plasticity, inhibit neurogenesis and trigger long-term neurocognitive deficits. The projected vulnerable period in humans extends from the third trimester of pregnancy to the third year of life. Despite all concerns, there is no ethically and medically acceptable alternative to the use of sedatives and anesthetics for surgeries and painful interventions. Development of measures that prevent injury while allowing the medications to exert their desired actions has enormous translational value.
Here we investigated protective potential of hypothermia against histological toxicity of the anesthetic sevoflurane in the developing nonhuman primate brain.
Neonatal rhesus monkeys underwent sevoflurane anesthesia over 5hrs. Body temperature was regulated in the normothermic (>36.5°C), mild hypothermic (35–36.5°C) and moderately hypothermic (<35°C) range. Animals were euthanized at 8hrs and brains examined immunohistochemically (activated caspase 3) and stereologically to quantify apoptotic neuronal and oligodendroglial death.
Sevoflurane anesthesia was well tolerated at all temperatures, with oxygen saturations, end tidal CO2 and blood gases remaining at optimal levels. Compared to controls, sevoflurane exposed brains displayed significant apoptosis in gray and white matter affecting neurons and oligodendrocytes. Mild hypothermia (35–36.5°C) conferred significant protection from apoptotic brain injury, whereas moderate hypothermia (<35°C) did not.
Hypothermia ameliorates anesthesia-induced apoptosis in the neonatal primate brain within a narrow temperature window (35–36.5°C). Protection is lost at temperatures below 35°C. Given the mild degree of cooling needed to achieve significant brain protection, application of our findings to humans should be explored further.
Keywords: anesthesia, brain injury, apoptosis, development, neuroprotection
Introduction
Sedative, anesthetic and antiepileptic drugs interact with glutamate and γ-aminobutyric acid A (GABAA) receptors to produce their desired therapeutic effects. Glutamate and GABA are major neurotransmitters in the mammalian brain, which regulate brain development at molecular, cellular and systems level.1–3 Two decades ago, it was reported that antagonists of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors cause widespread apoptotic neurodegeneration in the developing rat brain during the brain growth spurt period.4 This evidence was extended first to GABAA receptor agonists and alcohol5 and later to numerous sedative, antiepileptic drugs (AEDs),5,6 and general anesthetics (ketamine, nitrous oxide, propofol, isoflurane, sevoflurane [SEVO]).7–14 It has now been shown in numerous studies that these classes of drugs can trigger apoptosis of neurons and oligodendroglia, suppress neurogenesis, inhibit normal synapse development and sculpting and cause long-lasting behavioral and cognitive impairments in rodents and non-human primates (NHPs), if exposure occurs during the brain growth spurt period. In humans this period extends from the third trimester of pregnancy to the third year of life.15
Retrospective clinical studies in children exposed to anesthetic or AEDs in utero, during infancy or early childhood have delivered evidence for a correlation between early developmental drug exposure and adverse neurodevelopmental outcomes.16–24 Prospective studies with anesthetics in humans are currently ongoing.25, 26
Millions of human infants are exposed to sedative, anesthetic and antiepileptic agents each year. Despite all concerns, there is no ethically and medically acceptable alternative to the use of these agents in the context of anesthesia for surgical procedures, long-term sedation of critically ill infants and treatment of seizures and status epilepticus in pediatric medicine. Thus, the question arises whether effective and safe protective therapies can be developed to help prevent or minimize iatrogenic injury to the neonatal and infant human brain, while allowing the medications to exert their desired actions. A safe and effective protective treatment would have enormous translational value, as millions of infants would be expected to benefit from it every year worldwide.
Here we investigated the potential of hypothermia (HT) to reduce brain toxicity of sevoflurane (SEVO), a broadly used volatile anesthetic in pediatric medicine. We aimed to determine whether whole body HT may prevent SEVO-induced apoptosis of neurons and oligodendroglia in the short term. Much experience has been gained over the past decade with the use of HT in human neonates in the context of hypoxic ischemic encephalopathy (HIE). HT has been shown to be safe and effective in reducing death and disability from HIE at 22 months and at 6–7 years of age.27–30 Since HT is already an approved treatment and widely applied in neonatal medicine, the translation of the proposed research into clinical practice might be feasible, if a protective effect can be shown.
Materials and Methods
Animals.
All animal procedures were approved by the Wisconsin National Primate Research Center, the Oregon National Primate Research Center (OHSU, Beaverton, Oregon) and the University of Wisconsin Madison Institutional Animal Care and Use Committees (IACUC) and were conducted in full accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Sevoflurane anesthesia was administered to infant rhesus macaque monkeys. Postnatal day 2–9 (P2–P9) infant rhesus macaques (n = 18) were separated from their mothers and exposed for 5hr to SEVO, which was maintained at a concentration that provided a moderate plane of surgical anesthesia as defined by no movement and not more than 10% increase in heart rate or blood pressure in response to a mosquito clamp pinch at hand and foot.
To induce anesthesia, gentle hand-restraint was maintained while a size appropriate face mask was placed over the nose and mouth of the infant and air with 4–5% SEVO was delivered until significant relaxation was noted. Once sufficiently anesthetized, each infant was intubated using a laryngoscope with a zero blade and a 2.0 to 2.5 uncuffed endotracheal tube. Anesthesia was maintained with 2.5–3.5% SEVO. Oxygen saturation and carbon dioxide levels were monitored via pulse oximetry/capnography and maintained at appropriate levels with an appropriate sized ventilator. Blood pressure was recorded using an HDO monitor with software (VetHDO, Vetline LLC), heart rate was monitored via pulse oximeter (Oximax N-65, Nellcor, Mansfield, MA), and cardiac electrical activity was monitored via Lead II ECG. At a minimum, blood samples were collected at 0.5, 2, 4 and 6hr to evaluate blood gases and other metabolic parameters. Anesthetized and recovering animals received supplemental fluid and glucose based on diagnostic values obtained. At 8hr after induction of anesthesia or separation from their mother, animals were injected with an overdose of ketamine and euthanized by transcardial perfusion fixation using phosphate buffered saline followed by 4% paraformaldehyde in phosphate buffer. The brains were subsequently subjected to histopathological analysis.
Hypothermia:
HT was induced with a servo-controlled infant cooling system, which consists of a Blanketrol III hypothermia unit, neonatal cooling blankets and an esophageal/rectal temperature probe (Cincinnati Sub-Zero, OH, USA). Using feedback from the animal’s rectal temperature sensor, the proprietary control algorithm responds by modifying water temperature such that infant target temperature is achieved precisely. This enables management of body temperature in a non-invasive, effective and precise manner. The design of the study was to target four temperature ranges, 33–34°C (n=4; moderate hypothermia), 34–35°C (n=4; moderate hypothermia), 35–36.5°C (n=5; mild hypothermia), 36.5–38°C (normothermia; n=5) and maintain them at that level for 6hr. Subsequently, hypothermic animals were rewarmed by 0.5°C/hr until euthanasia.
Post-anesthesia, all animals were monitored continually to ensure that their vital statistics (e.g., heart rate, respiratory rate), blood gases, and multiple metabolic parameters remained within acceptable limits.
The control group (n=6) consisted of 4–6 day old rhesus macaques who were separated from their mothers but not exposed to anesthesia. These animals were kept in an incubator and their rectal temperature was recorded at 37.3–37.9°C without further intervention. These animals were also euthanized and transcardially perfused at 8hr.
Histopathology Studies.
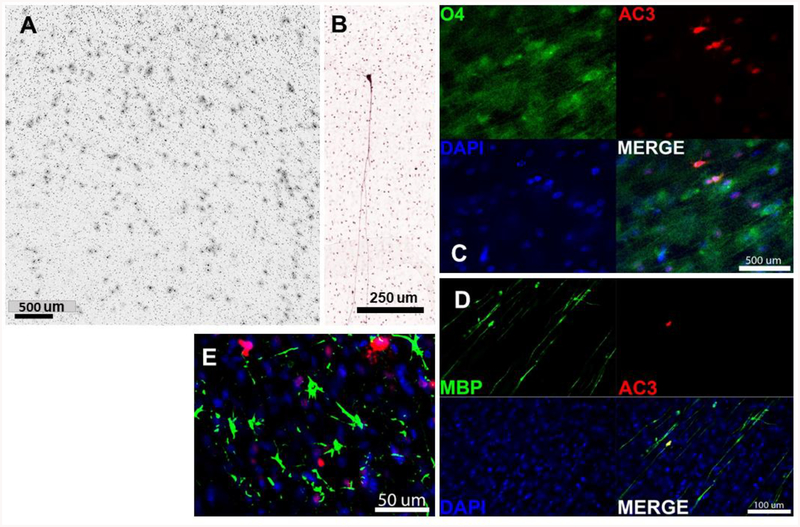
Brains were serially sectioned in the coronal plane using a vibratome at 70uM across the entire rostrocaudal extent and every 64th section immunolabeled for activated caspase-3 (AC3; CAT#9661L; Cell Signaling Technology, Danvers, MA) as a marker of apoptosis. For immunolabeling, sections were first immersed in citrate buffer (pH 6.0) and subjected to heat in a pressure cooker for 10 minutes for antigen retrieval. They were then quenched in 3% hydrogen peroxide in absolute methanol for 10 minutes, immersed for 1 hour in a blocking solution (2% Bovine Serum Abumen, 0.2% Dry Milk, 0.8% TX-100 in PBS), and incubated overnight at 4°C with a 1:1,000 dilution of AC3. The next morning, sections were incubated with a biotinylated secondary antibody (goat anti-rabbit; Vector Labs, Burlingame, CA), reacted with an avidin-biotin conjugate kit (ABC kit), and visualized using the chromogen VIP (Vectastain Elite ABC kit and Vector VIP kits; Vector Labs, Burlingame, CA). The chromogenic AC3 staining method permits comprehensive quantification of cells irreversibly committed to apoptotic death and provides a permanent record of the cell death throughout the brain. This includes both cell bodies and processes that allows one to distinguish early from late stages of degeneration. As previously demonstrated by Brambrink and colleagues7–9, both neurons and oligodendrocytes are sensitive to the proapoptotic effect of anesthetic agents. Neurons and oligodendrocytes were easily distinguished by their location in white or grey matter in addition to each cell’s unique morphological profile. In primate tissue, the AC3 antibody will produce non-specific staining in the nucleus of neurons but, in apoptotic cells, engulfs the entire cell including soma and processes making them easy to distinguish (Fig 2B). Apoptotic oligodendrocytes are distinguished by their immunolabeled soma that is surrounded by a halo of particulate debris (Fig 2A). Histological images were made using a Leica DM 4000B microscope equipped with a Leica DFC310FC camera and Leica Application Suite version 3.7 software.
Figure 2:
Higher magnification views of oligoapoptosis in the corona radiata (A) and an AC3 positive pyramidal neuron in layer II of the superior temporal sulcus in sevoflurane treated animals. Composites C and D show confocal images of O4, MBP, AC3 and DAPI stained sections. There is colocalization of O4 with AC3 and MBP with AC3 indicating oligodendroglial apoptosis. In E, GFAP staining (green) does not colocalize with AC3 (red) indicating that astroglia are not affected by the proapoptotic effect of sevoflurane.
Analysis of Neuronal and Glial Cell Death.
To clarify what cell type was undergoing apoptosis, AC3 was immunofluorescently co-labeled with markers for either neurons (NeuN; 1:100; Chemicon, Pittsburg, PA), oligodendrocytes (myelin basic protein MAB395; 1:200; Millipore, Burlington, MA or O4; 1:50; Sigma Aldrich 07139, St. Louis, MO), or astrocytes (GFAP; 1:400; Sigma Aldrich, St. Louis, MO). After overnight incubation at room temperature, primary antibodies were then labeled with fluorescently tagged secondary antibodies (goat anti-rabbit Alexa 555 and goat anti-mouse Alexa 488; 1:1000; Invitrogen, Waltham, Massachusetts) and counterstained with DAPI nuclear stain.
Quantification of Apoptosis.
Whole Brain Counts:
AC3-positive profiles, visualized by immunoperoxidase staining, were counted by an investigator who was blinded to the treatment using unbiased stereology (optical fractionator method). Tissue was visualized using a Labophot-2 Nikon microscope and QImaging 2000R camera connected to the Stereo Investigator software (version 11.08.1, Microbrightfield, Williston, VT) with an electronically driven motorized stage. For each counted section, Stereo Investigator software was used to mark the location and cell type (red for neurons and green for oligodendrocytes) of each AC3 positive cell along with an outline of each section. From the recorded information, estimates for the density of apoptotic profiles per cubic millimeter and the total number of apoptotic profiles was calculated for the entire brain. In addition, a computer plot showing the regional distribution of stained profiles was generated for comparison between treatment conditions. Apoptotic cells were easily distinguished since AC3-labeling fully engulfs the cell (including processes) and it’s nucleus contains features of typical apoptotic degeneration (condensed or fragmented nucleus). A minimum of 10 fields were counted in each tissue section.
Regional Counts:
Regional counts were also performed in the hippocampus (including dentate gyrus, CA regions, and subiculum), thalamus, and temporal lobe using the same tissue used in whole brain counts. The hippocampus and thalamus were quantified by regionally counting all apoptotic cells within each section. For temporal cortex, only sections containing hippocampus were counted. It was demarcated by counting all cells in the temporal lobe ventral to a line drawn through the lateral fissure and continuing medially to the thalamus. No thalamic or hippocampal cells were included in these counts. Counts in each section were used to estimate the total number of cells per region by multiplying by 64 (since every 64th section was immunolabeled).
Statistical Analysis.
Data are presented as means ± standard error of the mean (SEM). One sided ANOVA with post-hoc test for multiple comparisons (for more than 3 groups) or Student’s t-test (for 2 groups) were used. Statistical analysis was performed with Prism (GraphPad Software, La Jolla, CA).
Results
On the day of the experiment, the infant macaques were assigned to receive general anesthesia under a predetermined temperature (33–38°C). Mask induction and maintenance of SEVO anesthesia were well tolerated by all infant animals. At adequate anesthetic depth, the trachea was intubated in all animals. Fluid and glucose application maintained basic homeostasis, documented by continuous monitoring. The measured values for pH, heart rate (HR), pCO2, oxygen saturation (SaO2), end-tidal CO2 (EtCO2), venous lactate, hemoglobin and glucose are shown in Table 1. These remained at physiological levels at all times. The heart rates of moderately hypothermic animals were significantly lower than those of normothermic animals but still within acceptable physiologic range. There were higher lactates values measured at 6 hrs compared to earlier measurements; those did not significantly differ between the 3 groups. EtCO2 was significantly higher in the moderately hypothermic group compared to the normothermic group but still within what is considered an ideal range for this variable. Within 5–10 min after cessation of the SEVO administration, the infant macaques were extubated without complications, and maintained at the desired temperature for another hour, then were slowly rewarmed by increasing the rectal temperature by 0.5°C every hour and euthanized at 8hr.
Table 1:
Physiologic variables in infant rhesus monkeys subjected to SEVO anesthesia during normothermia, mild and moderate hypothermia. Measurements were taken at 0.5, 2, 4 and 6hr after initiation of sevoflurane anesthesia and represent means ± SEM. Statistical comparisons were performed for each variable at each time point between the normothermia, mild and moderate hypothermia groups using one way ANOVA and Dunnet’s post hoc test. *P<0.05; **P<0.01; ****P<0.0001 compared to the normothermia group. HR: heart rate; pCO2: partial CO2 pressure; SaO2: oxygen saturation measured by pulse oximetry; EtCO2: endtidal CO2. Hb: hemoglobin.
| T (°C) (mean ± SEM) |
Weight (kg) (mean ± SEM) |
Age (days) (mean ± SEM) |
Sex distribution |
0.5hr (mean ± SEM) |
2hr (mean ± SEM) |
4hr (mean ± SEM) |
6hr (mean ± SEM) |
|
|---|---|---|---|---|---|---|---|---|
| Normothermia (>36.5°C; n=5) | 37.58 ± 0.19 | 0.55 ± 0.04 | 4.0 ± 1.23 | 2M, 3F | ||||
| pH (venous) | 7.358 ± 0.018 | 7.397 ± 0.019 | 7.425 ± 0.022 | 7.397 ± 0.026 | ||||
| HR (beats/min) | 186.60 ± 8.58 | 175.60 ± 7.67 | 172.20 ± 11.10 | 172.80 ± 3.68 | ||||
| pCO2 mmHg | 43.92 ± 2.30 | 43.86 ± 0.94 | 42.44 ± 1.13 | 35.86 ± 2.98 | ||||
| SaO2 % | 96.20 ± 1.16 | 97.40 ± 0.93 | 98.40 ± 0.81 | 98.20 ± 0.74 | ||||
| EtCO2 % | 22.75 ± 1.44 | 26.60 ± 1.81 | 28.40 ± 1.44 | |||||
| Lactate mM | 2.39 ± 0.12 | 1.70 ± 0.35 | 1.81 ± 0.16 | 3.06 ± 0.51 | ||||
| Hb mg/dl | 15.58 ± 1.66 | 14.62 ± 1.49 | 14.50 ± 1.48 | 14.44 ± 1.74 | ||||
| Glucose mM | 68.5 ± 4.84 | 127.6 ± 49.61 | 57.60 ± 4.55 | 57.60 ± 6.73 | ||||
|
Mild hypothermia (35–36.5°C; n=5) |
36.05 ± 0.24 | 0.55 ± 0.04 | 4.6 ± 1.75 | 5M | ||||
| pH (venous) | 7.388 ± 0.019 | 7.367 ± 0.015 | 7.415 ± 0.021 | 7.412 ± 0.020 | ||||
| HR (beats/min) | 166.40 ± 8.37 | 168.00 ± 11.17 | 177.40 ± 13.79 | 182.80 ± 10.08 | ||||
| pCO2 mmHg | 43.40 ± 2.15 | 41.40 ± 2.57 | 43.76 ± 1.607 | 37.52 ± 21.74 | ||||
| SaO2 % | 94.40 ± 1.99 | 98.80 ± 0.59 | 99.20 ± 0.37 | 97.20 ± 1.12 | ||||
| EtCO2 % | 28.40 ± 1.29 | 27.60 ± 2.37 | 29.4 ± 1.75 | |||||
| Lactate mM | 2.30 ± 0.46 | 1.69 ± 0.57 | 1.63 ± 0.34 | 3.25 ± 0.65 | ||||
| Hb mg/dl | 12.43 ± 2.04 | 12.44 ± 2.04 | 12.44 ± 1.67 | 12.1 ± 2.06 | ||||
| Glucose mM | 114.40 ± 39.24 | 147.00 ± 25.76 | 64.20 ± 5.89 | 71.6 ± 6.013 | ||||
|
Moderate hypothermia (<35°C; n=8) |
33.87 ± 0.26 | 0.53 ± 0.19 | 4.6 ± 0.71 | 6M, 2F | ||||
| pH (venous) | 7.356 ± 0.018 | 7.355 ± 0.026 | 7.385 ± 0.021 | 7.320 ± 0.025 | ||||
| HR (beats/min) | 152.13 ± 2.98** | 139.38 ± 5.48*+ | 131.25 ± 4.57**++ | 153.75 ± 10.75 | ||||
| pCO2 mmHg | 42.56 ± 1.99 | 51.63 ± 1.73 | 44.54 ± 3.41 | 46.38 ± 3.14 | ||||
| SaO2 % | 96.38 ± 1.10 | 95.25 ± 2.92 | 98.50 ± 0.38 | 97.38 ± 1.02 | ||||
| EtCO2 % | 29.29 ± 1.95* | 29.25 ± 2.41 | 28.75 ± 1.67 | |||||
| Lactate mM | 1.41 ± 0.18 | 1.15 ± 0.18 | 1.35 ± 0.12 | 4.17 ± 0.75 | ||||
| Hb mg/dl | 10.70 ± 0.95 | 11.30 ± 1.19 | 12.13 ± 0.84 | 12.09 ± 1.18 | ||||
| Glucose mM | 89.63 ± 11.76 | 84.57 ± 12.39 | 66.38 ± 4.54 | 116.43 ± 14.16 |
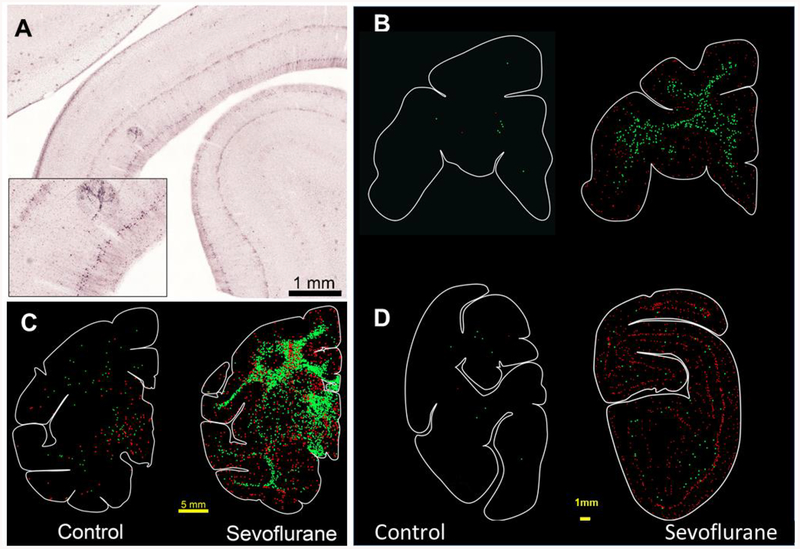
A profound apoptotic response to SEVO was detected in the brains of the exposed infants under normothermic conditions. This was most prominent in several divisions of the neocortex, especially the temporal cortex (layers II and IV) and the primary visual cortex (layers II and V) but also involved the caudate, globus pallidum, hippocampus, thalamus and diffusely the subcortical white matter (Fig. 1, 2). Immunohistochemistry confirmed that both neurons and oligodendrocytes were affected by the apoptosis process (Fig. 2, 3).
Figure 1:
Apoptosis in the neonatal rhesus macaque brain following 5hr SEVO anesthesia. A depicts a histological section of the temporal cortex stained with AC3 immunohistochemistry. There are numerous AC3 positive neurons in layer 2 of the neocortex. B-D show computer generated plots of neuroapoptosis (red dots) and oligoapoptosis (green dots) in the brains of a rhesus control infant macaque and an infant exposed to sevoflurane. There is substantial amount of neuro- and oligoapoptosis in the brain of the sevoflurane treated animal. (B) Frontal cortex neuroapoptosis can be seen in the premotor, dorsolateral, and cingulate cortices along with oligoapoptosis in the corona radiata. (C) At this level there is homogeneous pattern of neuro and oligoapoptosis in the caudate, putamen, extending into the hypothalamus, anterior commissure, corpus callosum and subcortical white matter. A laminar pattern of neuronal apoptosis appears within the cingulate, frontal and temporal neocortices. (D) Degeneration in the visual cortex is more laminar with focused neurodegeneration in layers 2 and 4/5 with more limited oligoapoptosis. Cell plots of control animals show low levels of physiological apoptosis. Scale bar = 5 mm in C and 1mm in B and D.
Figure 3:
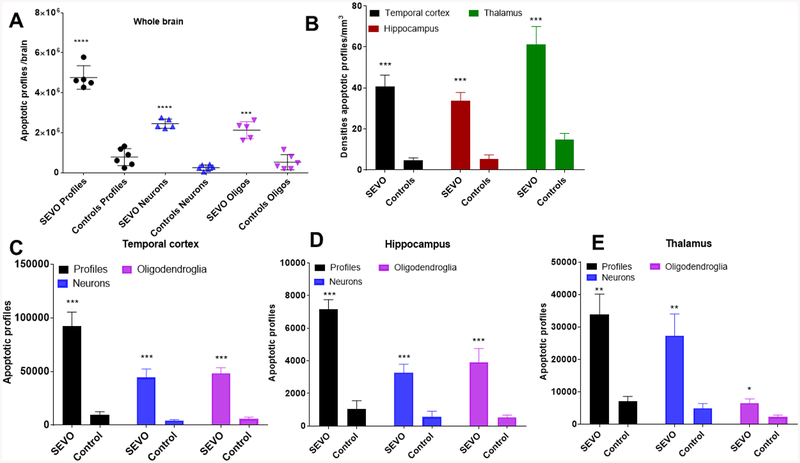
Sevoflurane induces widespread apoptosis in the infant macaque brain affecting neurons and oligodendrocytes. The graphs illustrate significantly higher numbers of apoptotic profiles (black), apoptotic neurons (blue) and apoptotic oligodendrocytes (purple) counted in the brains of control neonatal macaques (n=6) and infants treated with sevoflurane under normothermia (>36.5°C; n=5). Controls are animals not exposed to SEVO, which were kept normothermic. Columns represent means ± SEM of total numbers of apoptotic profiles (black), neurons (blue) and oligodendrocytes (Oligos; purple) counted in the whole brain (A), within the temporal cortex (C), the hippocampus (D) and the thalamus (E). In B the densities of apoptotic profiles (n/mm3) are depicted for the temporal cortex (black), hippocampus (red) and thalamus (green). Statistical comparisons between the control and the SEVO groups were performed by means of Student’s t-test (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001).
Quantitative evaluation of AC3-stained sections from the neonatal brains revealed that the mean (±SEM) number of apoptotic profiles (neurons + oligodendrocytes) per brain in the 6 control neonatal brains was 779,366 ± 175,336 and in the five neonatal brains exposed to SEVO at a mean temperature of 37.58°C (normothermic) it was 4,767,336 ± 262,566, which equals a 6.12-fold increase in the number of apoptotic cells in the SEVO exposed brains compared with the brains from drug-naïve controls (Fig. 3, 4). The total number of neurons undergoing apoptosis in brains of control animals was estimated at 253,159 ± 55,723 and in normothermic animals exposed to SEVO at 2,452,021 ± 106,912, which represents a 9.7 fold increase (Fig. 3). The total number of counted apoptotic oligodendrocytes in control brains was 526,208 ± 155,615 and in the brains of infants exposed to SEVO 2,126,532 ± 191,274, which represents a 4.0 fold increase under normothermic conditions (>36.5°C; Fig. 3).
Figure 4:
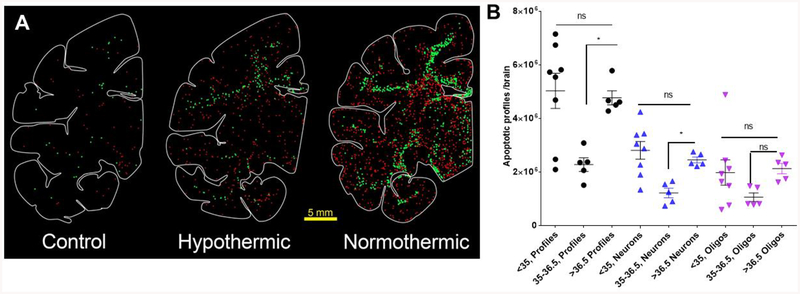
Mild hypothermia ameliorates sevoflurane-induced apoptosis. Computer generated plots of neuroapoptosis (red dots) and oligoapoptosis (green dots) in the brains of a rhesus control monkey, and two infants treated with sevoflurane under mild hypothermic (36.2°C) and normothermic (37.5°) conditions. Mild hypothermia ameliorates sevoflurane-induced neuro- and oligoapoptosis. The graph illustrates mean numbers ± SEM of apoptotic profiles (black), apoptotic neurons (blue) and apoptotic oligodendrocytes (oligos, purple) counted in the whole brains of control neonatal macaques and infants treated with sevoflurane under normothermia (>36.5°C), mild hypothermia (35–36.5°C) or moderate hypothermia (<35°C). Statistical comparisons were performed using one way ANOVA with Dunnet’s multiple comparisons post hoc test. One-way ANOVA revealed that temperature had a significant effect on the numbers of apoptotic profiles [F(2,15)=7.185; P=0.0065] and apoptotic neurons [F(2,15)=8.623; P=0.0032]. Although there is a visible trend towards reduction of apoptotic oligodendrocytes under mild hypothermia, this effect did not reach significance [F(2,14)=1.899; P=0.1863]. Post-hoc analysis using Dunnett’s multiple comparisons test revealed that, compared to normothermia, mild hypothermia significantly reduced apoptotic profiles (P=0.0184) and apoptotic neurons (P=0.0220). The effect of mild hypothermia on oligodendrocyte apoptosis did not reach statistical significance [F(2,15)=1.932; P=0.1793]. *P<0.05; **P<0.01; ***P<0.001.
Animals exposed to SEVO under moderately hypothermic conditions (<35°C; mean 33.87 ± 0.26°C) had an estimated total number of 5,028,812 ± 655.359 apoptotic profiles counted in their brains, a 6.5-fold increase compared to controls (Fig. 4). There was an 11.4 fold increase of neuronal apoptosis (2,809,847 ± 330,871) and a 3.8 fold increase of oligoapoptosis (1,980,061 ± 470,765) attributed to SEVO under moderate hypothermia. Thus, moderate HT did not ameliorate SEVO induced neuro- or oligoapoptosis (Fig. 4).
Interestingly though, mild hypothermia (35–36.5°C: mean 36.05 ± 0.24 °C) conferred a significant degree of protection for total profiles and neurons (Fig. 4 & 5). In these animals, the total number of apoptotic profiles was increased 2.9 fold compared to controls (2,276,256 ± 254,987). This represents 63% less apoptosis attributed to SEVO anesthesia under mild hypothermic conditions.
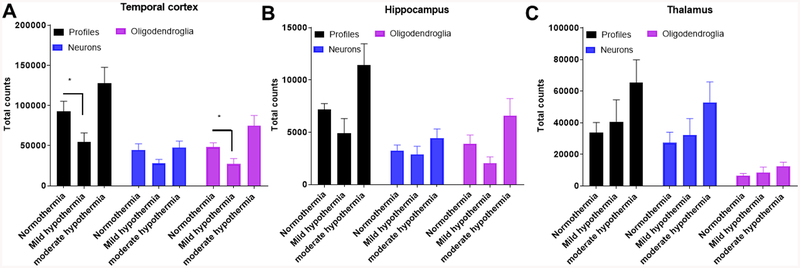
Figure 5:
Effects of mild and moderate hypothermia on sevoflurane-induced apoptosis in the temporal cortex (A), hippocampus (B) and thalamus (C). (A) Within the temporal cortex, body temperature had a significant effect on the numbers of apoptotic profiles [F(2,15)=4.276; P=0.0318] and oligodendrocytes [F(2,15)=5.042; P=0.0212]. The effect on apoptotic neurons did not reach significance but there is a clear trend towards lower neuronal counts under mild hypothermia. Post hoc analysis with Dunnet’s multiple comparisons test revealed that there were significantly fewer apoptotic profiles and apoptotic oligodendrocytes in the temporal cortex of animals exposed to SEVO under mild hypothermia compared to normothermia.
(B) Within the hippocampus, body temperature had a significant effect on the numbers of apoptotic profiles [F(2,15)=3.873; P=0.0441]. Although there is a trend toward lower counts at mild hypothermia, the effect of temperature on apoptotic neurons and oligodendrocytes did not reach statistical significance.
(C) Within the thalamus, body temperature did not have a significant effect on the numbers of apoptotic profiles, apoptotic neurons or oligodendrocytes.
The graphs illustrate mean numbers ± SEM of apoptotic profiles (black), apoptotic neurons (blue) and apoptotic oligodendrocytes (purple) counted in the respective brain regions of neonatal macaque infants treated with sevoflurane under normothermia (>36.5°; n=5), mild hypothermia (35–36.5°C; n=5) or moderate hypothermia (<35°; n=8). Statistical comparisons were performed using one way ANOVA with Dunnet’s multiple comparisons test.
The neuronal component of this apoptotic response was estimated at 1,217,331 ± 177,729 AC3 positive neurons, which translates to 57% less neuronal apoptosis attributed to SEVO anesthesia under mild hypothermic compared to normothermic conditions (Fig. 4).
There was a non-significant trend (p=0.1661; ANOVA with Dunnett’s multiple comparisons test) towards protection from oligoapoptosis under mild hypothermic conditions. We estimated that 1,058,926 ± 159,442 oligodendrocytes underwent apoptosis in the brains of infants exposed to SEVO at a mean temperature of 36.05 ± 0.24°C. This amounts to 67% less oligoapoptosis triggered by SEVO anesthesia under mild hypothermic compared to normothermic conditions (Fig. 4).
The effect of temperature on the severity of apoptosis in the temporal cortex, hippocampus and thalamus is illustrated in figure 5. Temperature significantly influenced injury severity in the temporal cortex and hippocampus but not in the thalamus.
Discussion
This study was designed to answer the question whether HT has the potential to reduce or even prevent apoptosis induced by an anesthetic drug in the neonatal primate brain. We wanted to mimic the clinical situation of administering anesthesia for a moderately long surgical procedure. SEVO was chosen because it is currently the #1 inhalation volatile anesthetic used in neonatal and pediatric medicine.31 We chose to apply treatment with SEVO for 5hr (as opposed to shorter durations) because, based on experience with isoflurane, we expected that this would be necessary for SEVO to induce robust enough apoptosis of brain cells to allow us to test our hypothesis. This duration is relevant for moderately long surgical cases.
Animals tolerated anesthesia under normothermia, mild and moderate hypothermia well. As table 1 demonstrates, moderate hypothermia resulted in significantly lower heart rates compared to normothermia but no impairments of oxygen saturation, endtidal CO2 or metabolic acidosis. Although endtidal CO2 at 0.5hr was significantly higher than under normothermic conditions, the value of 29.29 itself is within what is considered an ideal range. Somewhat surprising were the higher lactate values at the 6hr time point, which corresponds to 1hr after anesthesia is lifted. These values were higher in all three groups and did not significantly differ between the groups. One explanation for this could be suboptimal hydration, since at 6hr animals were moving around and intravenous fluids had been discontinued. The other possibility is that sevoflurane causes peripheral vasodilation which transitions to vasoconstriction when the anesthetic is discontinued. This peripheral vasoconstriction may be reflected in slightly elevated lactate levels at 6 hr.
We demonstrate that a 5hr exposure of neonatal rhesus monkeys to SEVO causes widespread apoptotic death of neurons and oligodendrocytes in the brain. The response is very similar to the effect of isoflurane reported by Brambrink and colleagues in previous publications.7–9
The study further demonstrates that the amount of apoptosis of both neurons and oligodendrocytes following SEVO anesthesia is temperature dependent, whereby the mode of temperature dependency was rather unexpected. Our histological analysis clearly indicates that, maintaining body temperature at 35–36.5°C significantly reduced the severity of SEVO-induced apoptosis. The protective effect was evident in the significant reduction of apoptotic AC3 positive profiles and AC3 positive neurons in whole brains. We were also able to detect significant regional protective effect of mild hypothermia in the temporal cortex and the hippocampus but not the thalamus, which suggests that not all brain regions benefited equally from the intervention. We attribute inability to reach statistical significance for regional protective effect of mild hypothermia on profiles, neurons and oligodendrocytes (Fig. 5) to the small group sizes, which are generally limiting in nonhuman primate studies.
Surprisingly, hypothermia at 33–35°C, as currently administered in the neonatal intensive care for treatment of perinatal asphyxia, is not protective against SEVO induced apoptosis in the neonatal rhesus monkey brain. This is interesting in view of the fact that numerous in vitro and in vivo studies, including studies in human neurons, have shown that HT to 32–34°C affects every investigated cell death pathway, including pathways leading to excitotoxicity, apoptosis, inflammation and free radical production.32, 33 Admittedly, the apoptosis process triggered by SEVO is pathogenetically different from the excitotoxic and apoptotic cell death response of the brain to perinatal asphyxia. The latter results from reduction of brain perfusion and nutrient delivery, release of excitatory amino acids and initiation of a variety of pathways, which trigger passive and active cell death.33 HT decreases brain oxygen consumption and glucose metabolism by appr. 5% per degree Celsius, preserves high energy phosphate compounds, maintains tissue pH, prevents the release of excitotoxic amino acids and limits calcium influx through AMPA receptors.33 All these mechanisms are highly relevant for hypoxic/ischemic insults but not for anesthesia induced apoptosis.
Apoptosis induced by anesthetics is not triggered by impaired tissue energy supply, acidosis or release of excitotoxic amino acids. It is the result of disturbances in physiologic excitatory neurotransmission which, at early developmental stages of ongoing active synaptogenesis and physiological cell death in the brain, critically controls survival pathways in neurons and immature oligodendrocytes. Yet, it utilizes pathways that are reportedly influenced by HT in ways that let us anticipate a robust protective effect. Mechanisms implicated include reduction in synthesis of neurotrophins, reduced levels of the active phosphorylated forms of extracellular signal regulated kinase (ERK1/2) and protein kinase B (AKT) which suppress the intrinsic apoptotic pathway.6, 34 The cell death process is Bax-dependent35 and involves down regulation of BclxL 36, 37 mitochondrial injury and extra-mitochondrial leakage of cytochrome c.35, 37 This is followed by a sequence of changes culminating in activation of caspase 3.38, 39 Involvement of reactive oxygen species generation in the pathogenesis of this phenomenon has been suggested.41,42 Studies using microarray analysis of rat brains exposed to anesthetics revealed differential expression of multiple genes involved in 45 pathways directly related to brain function, the significance of which remains to be explored.43 Furthermore, anesthetics lead to a persistent decrease of synapses in several brain regions in rodents and suppress neurogenesis.44–46 More recent work in nonhuman primates also implicates involvement of glial activation which may serve as a surrogate marker for neurotoxicity.47
HT has been associated with suppression of AC3, suppression of protein synthesis, the mitochondrial permeability transition, and components of the intrinsic and extrinsic apoptotic pathways.32, 33 In vitro, mild HT directly suppressed neuronal apoptosis induced by serum deprivation, with reduced activation of caspases-3, −8, and, −9 after 24 hours, and reduced cytochrome c translocation, consistent with suppression of the intrinsic and extrinsic pathways of apoptosis. HT has also been shown to slow the extrinsic and intrinsic apoptotic pathways by decreasing BAX expression and increasing BCL-2 expression, blocking of pro-apoptotic PKCδ activation and anti-apoptotic PKCε degradation, reduce production of free radicals, and decrease activation of immune transcription factors.32, 33 There is in fact one brief study by Creeley and Olney11 aimed at determining whether HT can protect against apoptotic injury induced in the developing rodent brain by pediatric drugs. Creeley and Olney first evaluated how changes in ambient temperature influence brain temperature in 4 day-old (P4) infant mice. They conducted a study in which infant mice were exposed to no anesthesia or to anesthesia maintained by isoflurane or ketamine for 4hr under either normothermic (34.7°C) or hypothermic (29.7°C) brain temperature conditions. At 5hr following initiation of drug exposure brains were evaluated for apoptotic injury. Either isoflurane or ketamine, under the normothermic condition, induced a robust apoptotic reaction compared to the no anesthesia controls; under the hypothermic condition there was no apoptotic response to either of these anesthetic drugs.11
Our results in a primate species differ substantially from those of Creeley and Olney and demonstrate that findings in rodents may not be transferrable to primates. In an attempt to mimic the conditions applied in mice and, given that the mean rectal temperature of naïve neonatal macaques is 37.58°C ± 0.19°C (Table 1), we initially aimed for a temperature reduction by 4°C to 33°C which is the degree of HT used in the treatment of perinatal asphyxia in human infants. It soon became evident that this degree of HT was not protective. Subsequently, we performed experiments to explore the impact of milder degrees of HT on SEVO induced apoptosis in the brain and were surprised to see that reduction of body temperature by only 1.5°C (from mean 37.58°C to mean 36.05°C) conferred significant protection. The amount of apoptosis was reduced by 63% (57% for neurons and 67% for oligodendrocytes) compared to normothermic conditions.
Overall, the hypothesis that HT would protect the neonatal primate brain from anesthesia-induced neuro and oligoapoptosis was confirmed in our study, but the finding that this protective effect was abolished at moderate HT levels was surprising. We consider it unlikely that a protective effect of HT below 35°C was abolished during rewarming. We maintained HT at a set temperature for 6hr, rewarmed slowly (0.5°C/hr) and euthanized the infants at 8hr. For those animals cooled at or below 36°C, normothermia was not achieved prior to euthanasia.
We rather suspect that at body temperatures below a threshold level, the protective effect of HT is abolished, possibly because physiological excitatory neurotransmission is impaired to levels that critically compromise its trophic and survival promoting effects on neurons and oligodendrocytes. In the NHP this threshold lies approximately 2.5° below normothermia.
A series of studies has addressed the effects of cooling and rewarming on neuronal activity and EPSP thresholds. Aihara and colleagues investigated effects of cooling on pyramidal neurons in guinea pig hippocampal slices and reported reduction of the excitatory postsynaptic potential (EPSP) slope in a temperature dependent manner between 37° and 30°C.48 Others49 studied influence of cooling rate on activity of ionotropic glutamate receptors in rat olfactory cortex slices and described that amplitudes of NMDA and AMPA mediated EPSPs decreased in a temperature dependent manner in the 37–33°C temperature window, regardless of cooling rate. No comparable studies exploring impact of HT on synaptic activity in the primate brain exist to allow for comparisons between species.
We conducted this study with translation into the clinical setting in mind. We wanted to determine whether HT, when administered in the context of anesthesia, would confer a mode of brain protection, given the fact that HT is a recognized effective treatment for perinatal asphyxia, is well tolerated and applied in neonatal medicine worldwide. It is a fact that, moderate HT, as administered in the clinical setting, has side effects, which include coagulopathy, thrombocytopenia, cardiac arrhythmias and increased susceptibility to infections.50 These potential complications need to be taken into serious consideration when anesthesia is administered for surgical procedures. The fact that significant brain protection from anesthesia toxicity can be accomplished with mild HT is very encouraging, as this degree of HT (1–1.5°C below normal) is easier to achieve and bears fewer complication risks for the patient.
It remains to be shown whether mild hypothermia can protect from apoptotic degeneration induced by other sedative and antiepileptic drugs, especially when exposures are longer than 6hr. Also, the question of whether histological protection translates into neurobehavioral benefits will require neurocognitive and behavioral studies in primate species which are currently being conducted by our group. Although more research on this topic is necessary, translation of our findings to neonatal and pediatric medicine in the future may be feasible.
Highlights.
Sevoflurane triggers apoptosis of neurons and oligodendrocytes in neonatal primates
Mild hypothermia conferred significant protection from apoptosis
Moderate hypothermia did not protect from sevoflurane-induced apoptosis
The protective temperature window in the neonatal primate brain is very narrow
Application of our findings to humans should be explored further.
ACKNOWLEDGEMENTS
This research was supported by NIH/NICHD R01HD083001-01A1 grant to C. Ikonomidou and pilot grant award, Office of the Director, NIH P51OD011106 to WNPRC; NIH grants HD052664 and U54-HD087011 the Intellectual and Developmental Disabilities Research Center at Washington University to K. Noguchi.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Seeburg PH: The TINS/TIPS lecture: The molecular biology of mammalian glutamate receptor channels. Trends Neurol Sci 1993;16:359–365. [DOI] [PubMed] [Google Scholar]
- 2.Nicholls D, Attwell D: The release and uptake of excitatory amino acids. Trends Pharmacol Sci 1990;11:462–468. [DOI] [PubMed] [Google Scholar]
- 3.Daniel H, Levenes C, Crepel F: Cellular mechanisms of cerebellar LTD. Trends Neurosci 1998;21:401–407. [DOI] [PubMed] [Google Scholar]
- 4.Ikonomidou C, Bosch F, Miksa M, et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999;283:70–74. [DOI] [PubMed] [Google Scholar]
- 5.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science 2000;287:1056–1060. [DOI] [PubMed] [Google Scholar]
- 6.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci 2002;99:15089–15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brambrink AM, Back SA, Riddle A, et al. Isoflurane-induced apoptosis of oligodendrocytes in the neonatal primate brain. Ann Neurol 2012;2:525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brambrink AM, Evers AS, Avidan MS, et al. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology 2012;116:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brambrink AM, Evers AS, Avidan MS, et al. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology 2010;112:834–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Creeley CE, Dikranian KT, Dissen GA, et al. Propofol-induced apoptosis of neurons and oligodendrocytes in the fetal and neonatal rhesus macaque brain. Br J Anaesth 2010;10;i29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Creeley CE, Olney JW. The young: neuroapoptosis induced by anesthetics and what to do about it. Anesth Analg 2010;110:442–448. [DOI] [PubMed] [Google Scholar]
- 12.Fredriksson A, Ponten E, Gordh T, Eriksson P: Neonatal exposure to a combination of N-methyl-d-aspartate and γ-aminobutyric acid type A receptor anesthetic agents potentiates apoptotic neurodegeneration and persistent behavioral deficits. Anesthesiology 2007;107:427–436. [DOI] [PubMed] [Google Scholar]
- 13.Jevtovic-Todorovic V, Hartman RE, Izumi Y, et al. Early exposure to common anesthetics causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003;23:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slikker W Jr, Zou X, Hotchkiss CE, et al. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci 2007; 98:145–158. [DOI] [PubMed] [Google Scholar]
- 15.Dobbing J, Sands J. The brain growth spurt in various mammalian species. Early Hum Dev 1979;3:79–84. [DOI] [PubMed] [Google Scholar]
- 16.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol 2009;21:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMaggio CJ, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg 2011;113: 1143–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farwell JR, Lee YJ, Hirtz DG, et al. Phenobarbital for febrile seizures - effects on intelligence and on seizure recurrence. N Engl J Med 1990; 322:364–369. [DOI] [PubMed] [Google Scholar]
- 19.Flick RP, Katusic SK, Colligan RC, et al. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics 2011;128:e1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikonomidou C, Scheer I, Wilhelm T, et al. Brain morphology alterations in the basal ganglia and the hypothalamus following prenatal exposure to antiepileptic drugs. Eur J Paediatr Neurol 2007;11:297–301. [DOI] [PubMed] [Google Scholar]
- 21.Meador KJ and NEAD Study Group. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009;360:1597–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meador KJ and NEAD Study Group. Effects of fetal antiepileptic drug exposure: Outcomes at age 4.5 years. Neurology 2012;78:1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sprung J, Flick RP, Katusic SK, et al. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc 2012; 87:120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology 2009;110:796–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davidson AJ, Disma N, de Graaff JC, et al. Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicenter, randomized controlled trial. Lancet 2016;387:239–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun LS, Li G, Miller TL, et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA 2016; 315:2312–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Edwards AD, Brocklehurst P, Gunn AJ, et al. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: synthesis and meta-analysis of trial data. BMJ 2010; 340:C363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guillet R, Edwards AD, Thoresen M, et al. Seven- to eight-year follow-up of the CoolCap trial of head cooling for neonatal encephalopathy. Pediatr Res 2012;71:205–209. [DOI] [PubMed] [Google Scholar]
- 29.Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N Engl J Med 2012;366:2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holzki J: Recent advances in pediatric anesthesia. Korean J Anesthesiol 2011;60:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drury PP, Gunn ER, Bennet L, Gunn AJ: Mechanisms of hypothermic neuroprotection. Clin Perinatol Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nature Rev Neurosci 2012;13:267–278. [DOI] [PubMed] [Google Scholar]
- 33.Yenari MA, Han HS: Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012;13:267–278. [DOI] [PubMed] [Google Scholar]
- 34.Hansen HH, Briem T, Dzietko M, et al. Mechanisms leading to disseminated apoptosis following NMDA receptor blockade in the developing rat brain. Neurobiol Dis 2004;16:440–453. [DOI] [PubMed] [Google Scholar]
- 35.Young C, Klocke BJ, Tenkova T, et al. Ethanol induced neuronal apoptosis in the in vivo developing mouse brain is BAX dependent. Cell Death Differ 2003;10:1148–1155. [DOI] [PubMed] [Google Scholar]
- 36.Sanders RD, Sun P, Patel S, et al. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand 2010;54:710–716. [DOI] [PubMed] [Google Scholar]
- 37.Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V: Anesthesia induces suicide in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 2005;135: 815–827. [DOI] [PubMed] [Google Scholar]
- 38.Olney JW, Tenkova T, Dikranian K, et al. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Dev Brain Res 2001;133:115–126. [DOI] [PubMed] [Google Scholar]
- 39.Olney JW, Tenkova T, Dikranian K, et al. Ethanol-induced caspase-3 activation in the in vivo developing mouse brain. Neurobiol Dis 2002;9:205–219. [DOI] [PubMed] [Google Scholar]
- 40.Papadia S, Soriano FX, Léveillé F, et al. Synaptic NMDA receptor activity boosts intrinsic antioxidant defences. Nat Neurosci 2008;11:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Léveillé F, Papadia S, Fricker M, et al. Suppression of the intrinsic apoptosis pathway by synaptic activity. J Neurosci 2010;30:2623–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu F, Patterson TA, Sadovova N, et al. Ketamine-induced neuronal damage and altered N-methyl-D-aspartate receptor function in rat primary forebrain culture. Toxicol Sci 2012;131:548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu F, Guo L, Zhang J, et al. Inhalation anesthesia-induced neuronal damage and gene expression changes in developing rat brain. Sys Pharmacol 2013;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stefovska V, Czuczwar M, Smitka M, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Ann Neurol 2008;64:434–45. [DOI] [PubMed] [Google Scholar]
- 45.Forcelli PA, Janssen MJ, Vicini S, Gale K. Neonatal exposure to antiepileptic drugs disrupts striatal synaptic development. Ann Neurol 2012;72:363–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jevtovic-Todorovic V, Absalom AR, Blomgren K, et al. Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br J Anaesthesia 2013;111:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang X, Liu S, Newport GD, et al. In vivo monitoring of sevoflurane-induced adverse effects in neonatal nonhuman primates using small-animal positron emission tomography. Anesthesiology 2016;125:133–146. [DOI] [PubMed] [Google Scholar]
- 48.Aihara H, Okada Y, Tamaki N: The effects of cooling and rewarming on the neuronal activity of pyramidal neurons in guinea pig hippocampal slices. Brain Res 2001;893:36–45. [DOI] [PubMed] [Google Scholar]
- 49.Mokrushin AA, Pavlinova LI, Borovikov SE: Influence of cooling rate on activity of ionotropic glutamate receptors in brain slices at hypothermia. J Thermal Biol 2014;44:5–13. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Ma J, Danzeng Q, et al. Safety of moderate hypothermia for perinatal hypoxic-ischemic encephalopathy: a meta-analysis. Ped Neurol 2017;74:51–61. [DOI] [PubMed] [Google Scholar]