Abstract
The biophysical mechanisms underlying epileptogenesis and the generation of seizures remain to be better understood. Among many factors triggering epileptogenesis are traumatic brain injury breaking normal synaptic homeostasis and genetic mutations disrupting ionic concentration homeostasis. Impairments in these mechanisms, as seen in various brain diseases, may push the brain network to a pathological state characterized by increased susceptibility to unprovoked seizures. Here, we review recent computational studies exploring the roles of ionic concentration dynamics in the generation, maintenance, and termination of seizures. We further discuss how ionic and synaptic homeostatic mechanisms may give rise to conditions which prime brain networks to exhibit recurrent spontaneous seizures and epilepsy.
Keywords: Ion concentration dynamics, homeostatic synaptic plasticity, epileptic seizures, computational model
I. Introduction
Epilepsy remains one of the most common neurological disorders worldwide (Lukawski et al., 2018). Epilepsy is used to describe a number of neurological disorders characterized by spontaneous, recurrent seizures. These seizures manifest as either convulsive or non-convulsive events with varied effects on the level of consciousness during an attack. Seizures are associated with large increases in neuronal firing, and exhibit periods of synchronous bursting and asynchronous firing (Bazhenov et al., 2004; Frohlich et al., 2008a; Frohlich et al., 2010; González et al., 2015; González et al., 2018; Hamidi and Avoli, 2015; Krishnan and Bazhenov, 2011; Lukawski et al., 2018; Timofeev and Steriade, 2004). Roughly 70% of patients suffering from epilepsy have seizures which are well controlled through pharmacological interventions (Lukawski et al., 2018; Timofeev et al., 2013). For the remaining 30% of patients, their seizures are categorized as pharmaco-resistant or intractable and require more extreme interventions such as resection of epileptic foci to find relief. Additionally, patients who respond well to medications can develop a resistance to chronic use of specific medications and require new cocktails of pharmacological agents or higher doses which can manifest other neurological deficits including memory issues, migraines, and other cognitive impairments (Brodie and French, 2000; Brodie and Kwan, 2001; Ortinski and Meador, 2004; Perucca and Tomson, 2011; Perucca and Gilliam, 2012). In light of the advancements in the treatment of epilepsy and seizure prevention, the underlying mechanisms which give way to epilepsy and seizure generation remain poorly understood.
One difficulty in determining the underlying mechanisms of epileptogenesis and seizure generation stems from the fact that epilepsy has many etiologies (D'Adamo et al., 2013; Lukawski et al., 2018). Epileptogenesis is a set of processes taking place in acquired epilepsies between the initial insult and the onset of epilepsy (Timofeev et al., 2013). Epilepsy can be divided into two main categories: acquired and genetic epilepsies. Many different insults to the brain can lead to the immediate (hours to days) or delayed (months to years) generation of seizures and potential development of epilepsy (Houweling et al., 2005; Nita et al., 2006; Timofeev et al., 2010; Timofeev et al., 2013; Topolnik et al., 2003b). Traumatic brain injury (TBI), stroke, infections, and tumors are commonly associated with the development of acquired epilepsy (Lukawski et al., 2018; Timofeev et al., 2013). TBI remains one of the most common factors leading to epileptogenesis. Penetrating brain wounds commonly result in epileptogenesis with a delay period of months to years following the initial insult (Annegers et al., 1998; Avramescu and Timofeev, 2008; Chauvette et al., 2016; Dinner, 1993; Jin et al., 2006; Kollevold, 1976; Nita et al., 2007; Temkin et al., 1995; Topolnik et al., 2003a). Indeed, 80% of patients suffering severe TBI exhibit paroxysmal activity within 24 hours of injury (Dinner, 1993; Kollevold, 1976). The severity of the trauma has been suggested to play an important role in the susceptibility to the development of paroxysmal activity (Frohlich et al., 2008b; González et al., 2015; Houweling et al., 2005; Volman et al., 2011a; Volman et al., 2011b). Post-traumatic seizures are a risk factor in adults, but it may be less likely for infants (Angeleri et al., 1999). Young children display mainly early seizures, but adolescents and adults are more likely to become epileptic after some latent period (Asikainen et al., 1999). In a recent study (Christensen et al., 2009) assessing the risk of developing epilepsy in children and young adults suffering from TBI, it was found that the risk of epilepsy was dependent on the severity of trauma and the age of a patient at the time of injury with patients who were older at the time of trauma were more likely to develop chronic epilepsy. Though the exact mechanism leading to the development of TBI-induced epilepsy remains poorly understood, it has been suggested that homeostatic synaptic plasticity may be a key culprit in this form of epileptogenesis. Understanding the molecular, cellular and network processes accompanying epileptogenesis will lead to an understanding of the development of epilepsy characterized by unprovoked seizures. Prevention of epileptogenesis will therefore prevent epilepsy.
Idiopathic epilepsies have been associated with various genetic mutations (D'Adamo et al., 2013; Lukawski et al., 2018). Many studies have shown that genetic mutations leading to impaired ion channel expression and regulatory proteins lead to hyperexcitability and epileptic seizures (D'Adamo et al., 2013; Dazzo et al., 2015; Lascano et al., 2016; Nobile et al., 2009; Ottman et al., 2004; Singh et al., 2006). Indeed, studies on excised tissue from epileptic patients have shown impaired Na+/K+ ATPase activity, potassium-chloride co-transporter isoform 2 (KCC2) expression, K+ channel activity, and nicotinic acetylcholine (nACh) channel activity (Buchin et al., 2016; Huberfeld et al., 2007; Lukawski et al., 2018). Specifically, K+-channelopathy has been linked to many forms of intractable epilepsy (D'Adamo et al., 2013). As K+ channels are a key regulator of intrinsic neuronal excitability, disruption or impairment of these channels can lead to hyperexcitability and reduce the threshold for seizure initiation. Interestingly, in addition to mutations causing impairments of specific K+ currents, genetic mutations affecting proteins which regulate or influence K+ channel kinetics have also been shown to cause increased propensity for seizure onset (Dazzo et al., 2015; Nobile et al., 2009; Ottman et al., 2004). Similar to TBI-induced epilepsy, the exact biophysical mechanisms leading to seizure generation and potential development of epilepsy in these forms of genetic epilepsies remains to be fully understood.
In this short review, we will first summarize data related to the role of ionic concentration dynamics in seizure generation. We will then review recent evidence on how ionic and synaptic homeostasis may play a role in epileptogenesis in both trauma- and K+-channelopathy-induced epilepsy. Specifically, we will focus on the roles of the KCC2 co-transporter and homeostatic synaptic scaling in seizure generation and epileptogenesis.
II. Ionic dynamics and seizures
Transmembrane ion currents and neuronal resting potentials are maintained and regulated by ion concentration gradients between the intra- and extracellular space. As such, their relative concentration gradients influence neuronal and network-wide excitability. Because of this crucial role in the regulation of excitability, there exist many mechanisms for maintaining and re-establishing the resting ion concentration gradients (Frohlich et al., 2008a; Somjen, 2002; Wei et al., 2014b). Breakdown in ion concentration homeostasis has been associated with various neurological disorders including epilepsy and seizure generation (Avoli and de Curtis, 2011; Cressman et al., 2009; Filatov et al., 2011; Frohlich et al., 2008a; Frohlich et al., 2010; González et al., 2018; Grisar et al., 1992; Hamidi and Avoli, 2015; Krishnan and Bazhenov, 2011; Krishnan et al., 2015; Ullah et al., 2009; Wei et al., 2014b). Much attention has been focused on the role of K+ concentration regulation in seizure onset due to its involvement in the repolarization phase of the action potential and, therefore, overall excitability (Bean, 2007; Mitterdorfer and Bean, 2002; Pathak et al., 2016). Early hypotheses regarding the generation of seizure activity were centered around the idea that elevated extracellular K+ concentrations ([K+]o) resulted in network hyperexcitability (Fertziger and Ranck, 1970; Frohlich et al., 2008a). This so-called “K+ accumulation hypothesis” suggested that increases in [K+]o could cross a critical concentration threshold initiating a positive feedback loop and thereby increasing neuronal excitability, which in turn further increased [K+]o. An increase in [K+]o to 8–16 mM in vitro (Traynelis and Dingledine, 1988) has been shown to lead to the generation of paroxysmal activity in the hippocampal formation. However, in vivo experiments have shown that seizures develop when perfusion with high K+ medium occurs for at least 10 min (Zuckermann and Glaser, 1968).
This initial hypothesis was met with some skepticism because increased [K+]o by itself was not a sufficient factor in triggering seizures. In fact, there was a lack of evidence for the existence of a [K+]o threshold necessary for seizure generation, and the increase of [K+]o during seizure activity results in a depolarization block and seizure termination (Frohlich et al., 2008a; Seigneur and Timofeev, 2010).
Though this hypothesis quickly fell out of fashion it has since made a resurgence following the development of new technologies and methods to more precisely probe [K+]o dynamics both in vivo and in vitro, and the development of more detailed biophysical computational models (Kager et al., 2000; Somjen, 2002; Somjen et al., 2008). In vitro experiments in mouse hippocampal brain slices have demonstrated that increases in K+ concentration in bath applied artificial cerebrospinal fluid (ACSF) can result in epileptiform discharges and seizure-like activity resembling interictal spiking and ictal events in epileptic patients (Filatov et al., 2011). The mechanism by which accumulation of [K+]o leads to depolarization and hyperexcitability is most likely through the change in the reversal potential of currents mediated by K+ ions, as predicted by the Nernst equation, which leads to a reduction of hyperpolarizing outward going K+ currents. Therefore, increased [K+]o leads to neuronal depolarization likely through its direct effects on leak current (Pedley et al., 1976; Seigneur and Timofeev, 2010). An increase in [K+]o also produces a depolarizing shift in the reversal potential of the hyperpolarization-activated depolarizing mixed (Na+/K+) current (Ih) that contributes to the generation of seizure activity (Timofeev et al., 2002a). As previously stated, K+ channel activation is associated with the repolarization phase of the action potential thereby regulating neuronal excitability. Increases in [K+]o would depolarize the reversal potential of these channels and render them ineffective. In doing so, the neurons will have prolonged periods of Na+ and Ca2+ channel activation leading to prolonged depolarizations and increased excitability.
Previous computational studies have shown that biophysical models of a cortical network with dynamic K+ concentrations resulted in a “multistability-mediated dynamic repertoire” in which the neuronal network could exist in a number of stable states including physiological /resting activity, asynchronous tonic firing, and synchronous bursting (Frohlich et al., 2010). These results suggest that brain networks could exist in at least two primary states, either a physiological or pathological activity state. Both of these states could be represented as local minima of the network dynamics to which the network activity would converge depending on the initial state of the network (Frohlich et al., 2010).
Under this framework, a stable state (like resting or seizure state) has a region called the “basin of attraction” that represents the values of biophysical parameters (such as ion concentration, synaptic activity, etc.) in which network activity remains within the same state. Thus, a larger basin of attraction for the physiological state compared to the pathological state would represent a more resilient network activity state since a larger perturbation of parameter values (such as ion concentration changes due to external input) would be required to switch from a physiological to a pathological state. In contrast, if the basin of attraction of physiological and pathological conditions are similar, then a smaller perturbation can cause a switch from physiological to pathological or seizure state. As such, in a normal “healthy” brain, this would produce a physiological activity state that would not easily transition to a pathological seizure state. The basin of attraction for the pathological activity state, in a healthy network, would be characterized by a relatively shallow and narrow basin of attraction. It was suggested that in a pathological network, one with mis-regulated K+ concentrations for example, the basin of attraction for the physiological state may be less broad and more shallow, while the pathological domain of attraction may become broader (Frohlich et al., 2010). As a result, the amount of perturbation necessary to transition network activity from the physiological to the pathological domain in the pathological network would be less than needed for the “healthy” network. Therefore, it would facilitate transitions between the two states resulting in the occurrence of recurrent seizures.
Spontaneous transitions between the physiological and pathological network states can also be thought of in terms of intermittency known from dynamical systems theory; that is, the irregular switching between semi-periodic and chaotic dynamics (Velazquez et al., 1999; Zalay et al., 2010). This idea was explored in a hippocampal network model of coupled cognitive rhythm generators (CRGs) and the results were further validated by in vitro experiments on acute mouse hippocampal brain slices under low Mg2+ / high K+ conditions (Zalay et al., 2010). Within a range of network parameters, the system remains in a high-complexity phase-space region characterized by irregular and interictal-like activity. Because of the presence of an unstable saddle-type fixed point, the network could intermittently escape the high-complexity region and approach the periodic-like ictal saddle. The decay constant of the network controls occurrence of the intermittent spontaneous transitions between interictal periods and pathological ictal activity (Zalay et al., 2010). Biologically, reduction of this constant could correspond to increased or prolonged post-synaptic excitability, which can manifest as a result of impaired [K+]o regulation.
In this model, the transitions from an interictal to an ictal orbit is accompanied by a reduction of the Lyapunov exponent, indicative of a reduction in the divergence of nearby phase-space trajectories, a loss of chaotic behavior and an increase in periodicity (Zalay et al., 2010). A similar reduction in high-complexity / chaotic dynamics was also observed at the transition from interictal to ictal periods in the acute brain slices (Zalay et al., 2010). This change in network behavior was suggested to be caused by attractor crisis-induced intermittency in which the boundary of the basin of the chaotic attractor (interictal dynamics) crossed or came into contact with the stable manifold of the saddle (ictal dynamics) (Velazquez et al., 1999; Zalay et al., 2010). The state-space trajectory can, therefore, intermittently enter the area near the low-complexity saddle resulting in transient periodic activity before returning to the higher-complexity attractor. It should be noted that the duration of this transient periodic ictal activity in the model was on the order of a few seconds while biological seizures can vary from a few seconds to minutes (Zalay et al., 2010). The authors suggest that the longer timescales of biological seizures may result from a time constant longer than most ion channel kinetics. It is possible that the infra-slow time scale of resting-state [K+]o fluctuations (Krishnan et al., 2018), which can be exacerbated by mis-regulation of [K+]o, may lead to periods of increased post-synaptic excitability resulting in an intermittency between the different dynamical states, thereby facilitating the transitions between physiological and pathological states of the network.
Because of its role in regulating neuronal excitability, K+ concentration dynamics have been the focus of many seizure related studies. It should be noted that other ionic species have not been as well studied and most likely influence the properties and susceptibility of seizures. Previous work has shown changes to the various ion concentrations prior to and during seizure events (Huberfeld et al., 2007; McCreery and Agnew, 1983; Somjen, 2002; Viitanen et al., 2010). Indeed, extracellular sodium concentrations ([Na+]o) have been shown to reduce during seizures (Somjen, 2002). This is presumably accompanied by increases in intracellular Na+ concentration ([Na+]i). Additionally, intracellular chloride concentration ([Cl−]i) has been shown to change in response to and influence seizure-like activity (Lillis et al., 2012). Extracellular calcium concentration ([Ca2+]o) dramatically drops during seizure activity from about 1.2 mM to 0.6 mM (Heinemann and Lux, 1977; Pumain et al., 1983). At such low levels of [Ca2+]o the synaptic transmission becomes greatly impaired (Crochet et al., 2005; Seigneur and Timofeev, 2010; Somjen, 2002). Therefore, synchronization via chemical synaptic mechanisms may be dramatically reduced or absent within the populations of neurons directly contributing to the seizure activity. At [Ca2+]o below 1.0 mM, hemichannels open (Thimm et al., 2005) creating conditions for paroxysmal synchronization of neuronal activities via electrical synapses (Timofeev et al., 2012).
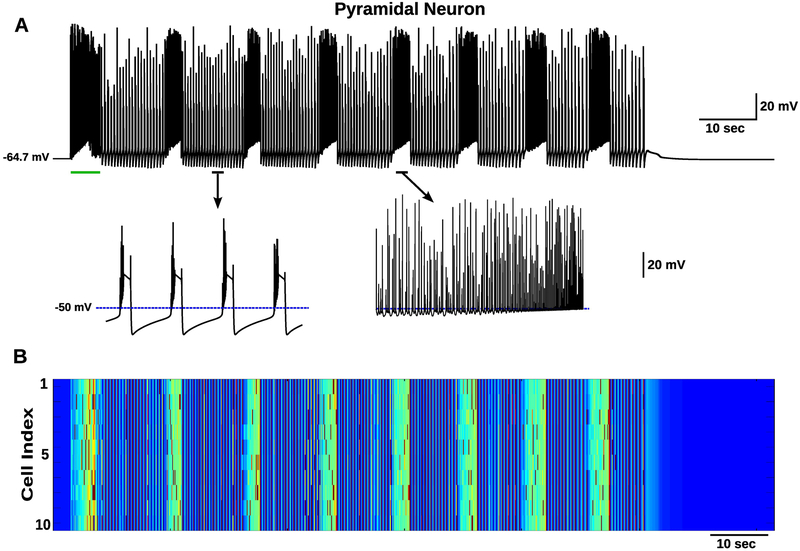
The role of ionic concentration dynamics in seizure termination and postictal depression was explored in computational models in (Krishnan and Bazhenov, 2011). In this cortical network model, in addition to including [K+]o/i dynamics, the dynamics for [Na+]o/i, [Cl−]i, and [Ca2+]i were also represented as dynamic variables controlled by various biophysical mechanisms. A brief depolarizing direct current (DC) input was capable of inducing seizure-like activity which exhibited sustained transitions between periods of tonic firing and synchronized bursting which outlasted the stimulus input but eventually resulted in the termination of the seizure-like activity (figure 1) (Krishnan and Bazhenov, 2011). It was again proposed that these transitions in activity states resulted from a bistability in the network dynamics for a range of [K+]o (Frohlich and Bazhenov, 2006; Frohlich et al., 2010). During tonic firing, the efflux of K+ overwhelmed the K+ regulatory mechanisms allowing [K+]o to reach higher values. Once [K+]o reached a high enough value, the network activity transitioned to synchronized bursting. During the bursting periods, the [K+]o decreased due to lower overall firing (bursting includes higher frequency firing and quiescent periods, figure 1A). The [K+]o reduced until reaching a low enough value at which a return to the tonic spiking regime occurred (figure 1) (Krishnan and Bazhenov, 2011). As a consequence of the repeated tonic and bursting periods, within an episode of seizure, the [K+]o fluctuates as observed in experiments (Heinemann and Lux, 1977).
Figure 1.
Tonic-clonic seizure transitions and spontaneous termination. A, Membrane potential of a single excitatory pyramidal neuron from the network B. A transient DC input (green line) resulted in a depolarization of the neurons and generation of seizure-like activity which continued past the stimulation offset. The resulting seizure-like discharge exhibited several state transitions between tonic (asynchronous firing) and clonic (bursting) periods before spontaneous termination. The bottom plots show activity during bursting (left) and tonic (right) periods. B, Heatmap showing membrane voltage of all pyramidal neurons in the network. Shows highly synchronized firing during bursting episodes. This figure is adapted from (Krishnan and Bazhenov, 2011).
During these seizure-like events, [Na+]i and [Cl−]i increased gradually due to the higher firing rates of excitatory and inhibitory neurons, respectively. [Na+]i progressively increased during the seizure-like event, while [Cl−]i showed a fast increase during seizure initiation and then remained relatively elevated (Krishnan and Bazhenov, 2011), which is in agreement with several experimental paradigms (Lillis et al., 2012; Somjen, 2002). These modeling studies suggested that progressive accumulation of [Na+]i would lead to stronger activation of the Na+/K+ ATPase; this in turn would lead to increase of the Na+/K+ ATPase outward current (at each cycle Na+/K+ ATPase removes 3 Na+ ions and brings in 2 K+ ions, thus creating an outward current). The resulting hyperpolarization of membrane voltage would trigger termination of seizure and initiate postictal depression state (figure 1) (Krishnan and Bazhenov, 2011). Postictal depression following most seizure events was suggested to be a result of the prolonged elevation of [Na+]i and continued Na+/K+ ATPase activity, which would lead to the progressive decrease in [K+]o past its baseline level, a finding that has been previously reported experimentally (Jensen and Yaari, 1997). The role of the Na+/K+ ATPase in the generation of seizure activity has been explored in previous work (Grisar et al., 1992; Krishnan et al., 2015; Wei et al., 2014b). Recent computational and experimental studies have linked oxygen dynamics to the impairments in Na+/K+ ATPase activity leading to the onset of seizures and cortical spreading depression (Ingram et al., 2014; Wei et al., 2014a).
Astrocytes play a major role in the regulation of the extracellular milieu. Indeed, astrocytes have been shown to contribute to the tight regulation of the extracellular ion concentrations and neurotransmitters at the synapse (Coulter and Steinhauser, 2015; de Lanerolle et al., 2010; Kjaerby et al., 2017; Patel et al., 2019; Poskanzer and Yuste, 2016; Tian et al., 2005). Two primary mechanisms by which astrocytes regulate [K+]o include: 1) K+ uptake and 2) spatial buffering through the astrocytic syncytium. [K+]o uptake has been suggested to involve the Na+/K+ ATPase, sodium-potassium-chloride co-transporter isoform 1 (NKCC1), and inwardly rectifying K+ (Kir) channels (Coulter and Steinhauser, 2015; de Lanerolle et al., 2010; Patel et al., 2019; Somjen, 2002). The most predominant mechanism for astrocytic K+ uptake seems to be through Kir channels (Olsen and Sontheimer, 2008; Patel et al., 2019; Steinhauser et al., 2012). As such, many studies have focused on altered expression or impaired Kir channels as a key factor in impaired [K+]o regulation and seizure generation (Olsen and Sontheimer, 2008; Patel et al., 2019; Steinhauser et al., 2012). Kir4.1 is the main Kir subunit expressed in astrocytes and has been shown to be down-regulated in patients suffering mesial temporal lobe epilepsy (mTLE) (Heuser et al., 2012). Additionally, deletion of the astrocytic specific Kir4.1 channel results in spontaneous recurrent seizures in mice (Chever et al., 2010; Djukic et al., 2007; Haj-Yasein et al., 2011). Down-regulation of Kir4.1 channels has also been observed following TBI (D'Ambrosio et al., 2005; D'Ambrosio et al., 1999). TBI commonly presents with increased blood-brain-barrier (BBB) permeability. Dysfunction of the BBB results in extravasation of serum albumin which can directly activate transforming growth factor-β receptor 1 (TGFβR1) leading to the down-regulation of Kir4.1 channels, excitatory amino acid transporter 2 (EAAT2), and gap junction protein connexin 43 (CX43), and alters the expression and trafficking of aquaporin-4 (AQP4) channels all of which have been shown to contribute to the regulation of [K+]o and extracellular glutamate concentrations (Braganza et al., 2012; Coulter and Steinhauser, 2015; Friedman et al., 2009; Seiffert et al., 2004; Stewart et al., 2010; van Vliet et al., 2007).
Altered spatial buffering through the astrocytic syncytium has been suggested to influence seizure initiation (Coulter and Steinhauser, 2015; Giaume et al., 2010; Steinhauser et al., 2012). Once K+enters astrocytes through Kir4.1 channels, K+ is redistributed via gap junctions throughout the astrocytic syncytium (Coulter and Steinhauser, 2015). As previously mentioned, serum albumin activation of TGFβR1 has been shown to result in reduced CX43 expression. CX43, along with CX30, are the two primary connexin protein subunits comprising the gap junctions which form the astrocytic syncytium (Coulter and Steinhauser, 2015; Wallraff et al., 2006). Altered CX43 expression may result in impaired intracellular ionic coupling between astrocytes, thereby affecting K+ spatial buffering, extracellular glutamate uptake, transport of important metabolites, and impair Ca2+wave propagation (Coulter and Steinhauser, 2015; Wallraff et al., 2006). It should be noted that both increases and decreases in CX43/30 expression have been observed in human patients and animal models of epilepsy (Coulter and Steinhauser, 2015; Giaume et al., 2010; Steinhauser et al., 2012). As such, how the differential expression of CXs affects hyperexcitability and seizure generation remains to be fully understood. Taken together these studies suggest that impairments in astrocytic regulation of extracellular ion and transmitter concentrations may result in hyperexcitability and increased propensity for seizure generation. This idea has been explored in a number of computational models showing that proper regulation of the extracellular milieu by astrocytes is important for preventing the transition between physiological and pathological dynamics (Farah et al., 2019; Grigorovsky and Bardakjian, 2018; Ullah et al., 2009; Volman et al., 2012; Volman et al., 2013).
The movements of the charges associated with the high neuronal activity (particularly during seizures) result in the generation of weak electric fields which may play a role in the modulation of neuronal excitability and synchrony (Anastassiou and Koch, 2015; Frohlich and McCormick, 2010; Qiu et al., 2015; Shivacharan et al., 2019; Zhang et al., 2014). It has been shown that these weak endogenous electric fields can cause changes in the resting membrane potential of closely located neurons (Anastassiou and Koch, 2015; Frohlich and McCormick, 2010). Recent studies have demonstrated that the propagation speed (~ 0.1m/s) of pharmacologically-induced seizure-like activity in hippocampal tissue is strongly influenced by the weak endogenous electric field coupling (Qiu et al., 2015; Shivacharan et al., 2019; Zhang et al., 2014). Indeed, the speed of seizure propagation, as measured both in animal models and in epileptic patients, could not be explained by ionic diffusion alone, as diffusion rates are much slower (Shivacharan et al., 2019). Furthermore, propagation speed of seizure-like activity was unaffected in conditions of reduced synaptic transmission suggesting that it is mediated by non-synaptic mechanisms (Qiu et al., 2015; Shivacharan et al., 2019; Zhang et al., 2014). In a recent study, it was shown that cutting the hippocampus and separating the two halves by 400μm prevented the propagation of seizure-like activity between the two halves (Shivacharan et al., 2019). Interestingly, when the two sides were put back together, the spontaneous seizure-like activity was able to propagate between the two cut sections with a propagation speed of (~ 0.09m/s). Application of an external electric field, that was calibrated to negate the endogenous electric field generated during seizure-like activity, prevented the propagation of the seizure-like activity (Shivacharan et al., 2019). These data suggest that weak endogenous electric fields generated during epileptic seizures can aid in the recruitment of the neighboring neuron populations and thereby propagation of the seizure-like activity.
Thus, the intricate interactions between various ionic species in the brain may underlie the transitions and maintenance of seizure onset, progression, and termination. As the maintenance of the relative ionic concentration gradients is a critical component in the resilience of a network against transitions to pathological seizure states, it is not surprising that there are exist many homeostatic mechanisms implemented in the brain to maintain ion concentrations within a physiological range. Therefore, impairments in these homeostatic mechanisms, such as the Na+/K+ ATPase and the KCC2 co-transporter, would increase seizure susceptibility.
III. K-channelopathy-related epilepsy and KCC2 co-transporter
Various channelopathies have been associated with increased seizure susceptibility and epileptogenesis (D'Adamo et al., 2013; Lascano et al., 2016). K+-channelopathies are characterized by mutated or misregulated K+ channels resulting in network hyperexcitability. The term K+-channelepsy has been coined to describe a number of neurological disorders which exhibit increased propensity for epileptogenesis due to underlying K+ channel impairments (D'Adamo et al., 2013). K+ channels represent one of the most diverse and largest family of ion channels, and as such, we will focus here on impairment of the K+ channels which mediate the outward going A-current (IA). For a more extensive review of K+-channelepsy and the various mutations which give rise to them, we direct the reader to the following review (D'Adamo et al., 2013).
The outward going IA has been shown to influence action potential firing by modulating the inter-spike interval in response to prolonged subthreshold current injections (Bean, 2007; Mitterdorfer and Bean, 2002; Pathak et al., 2016). The IA, along with the dendrotoxin sensitive K+-type D-current (ID), have been shown to account for much of the hyperpolarizing K+-currents present during the repolarization phase of mammalian action potentials within regions of the cortex and hippocampus (Bean, 2007). IA is mediated by multimeric channels, which are comprised of voltage-gated K+ (KV) channel KV1 and KV4 α-subunits in combination with modulatory β-subunits (D'Adamo etal., 2013; Singh et al., 2006). Mutations in these α-subunit families have been shown to exist in a population of patients exhibiting pharmaco-resistant epilepsy (D'Adamo et al., 2013; Singh et al., 2006). Attenuation of IA, as a result of these mutations, has been shown to increase the likelihood for seizure generation (Singh et al., 2006). Furthermore, mutations resulting in impaired expression of IA regulating proteins, such as the leucine-rich glioma-inactivated 1 (LGI1) gene, have been shown to result in the rapid inactivation of IA and the development of paroxysmal activity (Dazzo et al., 2015; Nobile et al., 2009; Ottman et al., 2004). The robust convulsive compound 4-aminopryidine (4AP) is a strong IA antagonist (Avoli and de Curtis, 2011). Indeed, application of 4AP leads to the transition from physiological/resting activity to pathological seizure-like discharges both in vitro and in vivo (Avoli et al., 1996; Fragoso-Veloz et al., 1990; Levesque et al., 2013; Lopantsev and Avoli, 1998). It should be noted that the direct knockout of the KV4.2 α-subunit was unable to generate spontaneous recurrent seizures, though it did increase the susceptibility for seizure onset in response to additional convulsive pharmacological compounds (Barnwell et al., 2009). In light of these findings, the previously proposed mechanism for 4AP-induced ictogenesis in which reduction of IA promotes seizure by directly increasing neuronal excitability has come into question (Galvan et al., 1982; Gustafsson et al., 1982; Yamaguchi and Rogawski, 1992). As such, the exact mechanism by which altered IA activity leads to the development of spontaneous seizures and epileptogenesis remains to be fully understood.
The balance between excitatory and inhibitory activity is required for the maintenance of stable physiological network activity. Seizures have been traditionally believed to be caused by a breakdown of this balance favoring the reduction of inhibition and subsequent run-away excitability (Ben-Ari et al., 1979; Dingledine and Gjerstad, 1980; Schwartzkroin and Prince, 1980). Interestingly, recent work has shown that increase in inhibitory γ-aminobutyric acid class-A (GABAA) receptor signaling may underlie seizure generation (Sessolo et al., 2015; Shiri et al., 2016; Yekhlef et al., 2015). Typically, during seizures most pyramidal neurons are depolarized and, due to depolarization block, their firing is dramatically reduced, but the interneuron firing and therefore overall GABAA-dependent Cl− influx is increased which may result in Cl− currents becoming depolarizing (Cohen et al., 2002; Timofeev et al., 2002b; Timofeev and Steriade, 2004). However, following 4AP treatment, increases in [Cl−]i in excitatory neurons, and increases in inhibitory interneuron firing at the onset of seizure-like discharges have been reported both in vivo (Grasse et al., 2013; Toyoda et al., 2015) and in vitro (Levesque et al., 2016; Lillis et al., 2012; Uva et al., 2015). Additionally, intense stimulation of GABAergic interneurons has been demonstrated to increase [K+]o and generate long-lasting depolarizations (Rivera et al., 2005; Viitanen et al., 2010). These transient GABAergic excitatory [K+]o signals elicit prolonged depolarizations in rat temporal lobe, and may contribute to seizure generation (Lopantsev and Avoli, 1998; Viitanen et al., 2010).
The potassium-chloride co-transporter isoform 2 (KCC2) has recently been suggested to play a crucial role in the development of 4AP-induced seizures (González et al., 2018; Hamidi and Avoli, 2015; Levesque et al., 2016). The KCC2 co-transporter is one of the primary membrane-bound proteins responsible for maintaining the Cl− concentration gradient (Payne et al., 2003). Unlike the Na+/K+ ATPase, the KCC2 is a passive ion transporter, relying on the K+ concentration gradient to transport both Cl− and K+ out of the cell. Increases in [Cl−]i lead to KCC2 activation and the efflux of one Cl− and one K+ ion, while elevations of [K+]o halt its activity (Payne et al., 2003). Due to this activation paradigm, it has been proposed that the synchronized activation of GABAergic interneurons may cause a gradual accumulation of [Cl−]i, activating the KCC2 co-transporter. This activation would initiate the efflux of both Cl− and K+, elevating the [K+]o and triggering the positive feedback loop between increases in [K+]o and neuronal excitability described above. 4AP-induced ictal events and high-frequency stimulation-induced increases in [K+]o could be prevented through the reduction of KCC2 activity (Hamidi and Avoli, 2015).
One recent study explored the role of the KCC2 co-transporter in the generation of seizure-like activity in a biophysically realistic network model of K+-channelopathy dependent epilepsy (González et al., 2018). In vitro data showed that the selective activation of either parvalbumin (PV)-positive or somatostatin (SOM)-expressing inhibitory interneurons, in the presence of 4AP, could induced ictal-like discharges in mouse entorhinal cortex suggesting that the mechanism of seizure generation was dependent on synchronized GABAergic signaling rather than dependent on the target of the inhibition (peri-somatic vs. dendritic) (González et al., 2018). Modeling the application of 4AP as a network-wide reduction of IA, the model developed to explain these experiments was able to generate seizure-like events in response to synchronized GABAergic activation. This synchronized GABAergic activity led to progressive increases in [Cl−]i in excitatory principal neurons (figure 2A left, and B). Increased [Cl−]i activated the KCC2 co-transporter generating a gradual accumulation of [K+]o, which eventually reached a value high enough to trigger the positive feedback loop between [K+]o and neuronal depolarization necessary for seizure onset (figure 2A right, and B). Figure 2D shows the progression of these events for a single principal neuron. Transient increases in inhibitory neuron activation caused a brief silencing and hyperpolarization of principal neurons (figure 2D black), which was accompanied by an increase in [Cl−]i (figure 2D green). The accumulation of [K+]o (figure 2D purple) resulted from the Cl−-dependent activation of the KCC2 co-transporter. Similar to previous experimental work, reduction of KCC2 activity or impairment of the [Cl−]i dependent activation of KCC2 prevented seizure generation (González et al., 2018). It is important to note that the GABAA reversal potential did not depolarize in response to the accumulation of [Cl−]i in the model. Therefore, the development of seizure activity in the model was not a result of ineffective GABAA receptor activation as inhibitory synapses were still functional.
Figure 2.
Cl−-dependent activation of KCC2 leads to gradual accumulation of [K+]o and ictogenesis. A, Mean [Cl−]i (left) and [K+]o (right) for excitatory neurons in a “healthy” network, and a “pathological” reduced A-current network (red and black respectively). The blue trace indicates the pattern of synchronized interneuron stimulation. B and C, network-wide [Cl−]i and [K+]o for excitatory neurons. D, Overlay of the spiking of a single excitatory neuron (black) from the “pathological” network, and the corresponding [Cl−]i (green), [K+]o (purple), and interneuron stimulation (blue). This figure is adapted from (González et al., 2018).
The brain has developed a number of homeostatic mechanisms by which it is capable of maintaining and re-establishing ionic concentration gradients. In the healthy brain, these mechanisms serve the purpose of maintaining normal physiological activity. However, in the pathological brain, such as brains afflicted by K+-channelopathies, these homeostatic mechanisms may actually exacerbate existing network hyperexcitability shifting the network to pathological seizure activity states.
IV. Homeostatic synaptic scaling and TBI-induced epilepsy
Patients suffering from penetrating brain wounds after returning from the Vietnam war showed increased likelihood for epileptogenesis up to 15 years after the initial trauma (Salazar et al., 1985). As mentioned earlier, severe brain trauma, including penetrating brain wounds, are commonly associated with the development of epilepsy (Frohlich et al., 2008b; Timofeev et al., 2010; Timofeev et al., 2013). Experimentally, partial cortical deafferentation in cats has been used as a model of penetrating brain wounds to study posttraumatic epilepsy (PTE) (Avramescu and Timofeev, 2008; Nita et al., 2006; Nita et al., 2007; Topolnik et al., 2003a; Topolnik et al., 2003b). A similar type of paroxysmal activity was also found in an undercut model of epileptogenesis in mice (Chauvette et al., 2016; Ping and Jin, 2016). Similarly, slices from isolated cortical slabs show increased sensitivity to convulsive pharmacological compounds, and readily exhibit epileptiform discharges (Prince and Tseng, 1993). Partial cortical deafferentation presents with reduced neuronal excitability and firing rates immediately following trauma (Avramescu and Timofeev, 2008; Nita et al., 2006; Topolnik et al., 2003a; Topolnik et al., 2003b). This perturbation of network activity has been shown to result in the modification of synaptic strengths through homeostatic synaptic plasticity (HSP) (Avramescu and Timofeev, 2008). Though severe penetrating brain wounds remain a common factor leading to acquired epilepsy, the exact underlying mechanism by which such wounds lead to the transition of the healthy brain to a pathological state over a prolonged delay period remains to be understood but may involve HSP regulation of synaptic strength.
HSP is a slow negative feedback bidirectional process which aims to maintain a target network firing rate through the activity-dependent modulation of post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor densities (Burrone and Murthy, 2003; Pozo and Goda, 2010; Turrigiano, 2008). Network-wide reduction of activity, through cortical deafferentation or the application of tetrodotoxin (TTX), has been demonstrated to result in increased synaptic strength and neuronal excitability in order to recover a baseline firing rate through HSP (Avramescu and Timofeev, 2008; Echegoyen et al., 2007; Ibata et al., 2008; Jin et al., 2006; Lemieux et al., 2014; Nita et al., 2006; Topolnik et al., 2003a; Topolnik et al., 2003b; Trasande and Ramirez, 2007; Wierenga et al., 2005). Activation of this bidirectional mechanism has been suggested to involve activity-dependent [Ca2+]i sensors influencing relative tumor necrosis factor-α (TNFα) and brain derived neurotrophic factor (BDNF) expression levels (Burrone and Murthy, 2003; Pozo and Goda, 2010; Turrigiano, 2008). Additionally, the bidirectionality of HSP appears to be differentially regulated, with TNFα and BDNF levels influencing synaptic up-scaling and changes in post-synaptic density protein-95 (PSD-95) affecting down-scaling of synaptic strengths in response to prolonged increases in network activity (Pozo and Goda, 2010; Sun and Turrigiano, 2011; Turrigiano, 2008). Therefore, impairments in the bidirectionality of this mechanism could mediate transitions to an epileptic state following TBI.
In healthy tissue, HSP works to counteract the intrinsically unstable Hebbian plasticity, prevent run-away excitability, and maintain overall network stability (Pozo and Goda, 2010). However, in the case of chronic reduction of activity, as observed in response to cortical deafferentation, HSP may fail to precisely compensate for the loss of network-wide activity, thereby promoting hyperexcitability and the generation of paroxysmal epileptiform discharges (Frohlich et al., 2008b; Houweling et al., 2005; Volman et al., 2011a; Volman et al., 2011b). Indeed, previous studies have shown that, in a computational model of the cortical network, deafferentation of afferent inputs to a subpopulation of neurons within the network initiated homeostatic up-regulation of AMPA receptor conductance (González et al., 2015; Houweling et al., 2005; Volman et al., 2011a). Though the network was able to recover its baseline firing rate, the synaptic strengths increased substantially, putting the network in a pathological hyperexcitable state in which seizures could be easily generated.
Recent work also suggested that the susceptibility to PTE may be age-dependent (González et al., 2015; Timofeev et al., 2013). Partial cortical deafferentation in young and old cats resulted in the generation of spontaneous recurring electrographic seizures in older animals. Though acute seizures were observed in young animals within hours of the initial insult, sustained epileptogenesis was not observed in these younger cats. It was hypothesized that age-dependent impairment of the down-scaling mechanism of HSP may underlie the increased seizure susceptibility for older animals and patients (Timofeev et al., 2013). Recent experimental work has provided evidence for age-dependent differences in HSP down-scaling. It was demonstrated that the differential expression of the scaffolding protein PSD-95 in older neurons rendered homeostatic down-scaling ineffective (Sun and Turrigiano, 2011). Endogenous levels of PSD-95 increase with age, and it was shown that in old neurons, upregulation of PSD-95 impaired the HSP down-scaling without impacting HSP up-scaling (Sun and Turrigiano, 2011). As such it is possible that the initial compensation for decreased network activity following TBI through HSP up-scaling can drive an older network to a higher AMPA conductance pathological state. In a younger network, HSP down-scaling would correct for this initial overcompensation by reducing AMPA receptor conductances and re-establishing physiological activity state. The reduced or lack of bidirectionality of HSP, due to increased levels of PSD-95 in older animals, may keep the older network in a higher AMPA conductance state rendering the network more prone to seizure onset. This hypothesis was tested in (González et al., 2015).
In this model of a cortical network, excitatory synapses between pyramidal neurons were under HSP regulation such that in an intact non-deafferented network, a brief increase in network firing rate, through direct current (DC) current injection, would result in a transient decrease in synaptic strength (González et al., 2015). After termination of the stimulus pulse, the network firing rate and synaptic strengths returned to baseline levels through HSP regulation of AMPA receptor conductances. Reduction of afferent input to the network, modeling partial cortical deafferentation, caused a global reduction of network firing rates and subsequent up-scaling of synaptic strengths. The sustained increase in synaptic strength following “trauma” in the network ensured its easy transition from physiological spontaneous firing to a pathologic seizure-like state in response to a brief depolarizing DC input (González et al., 2015).
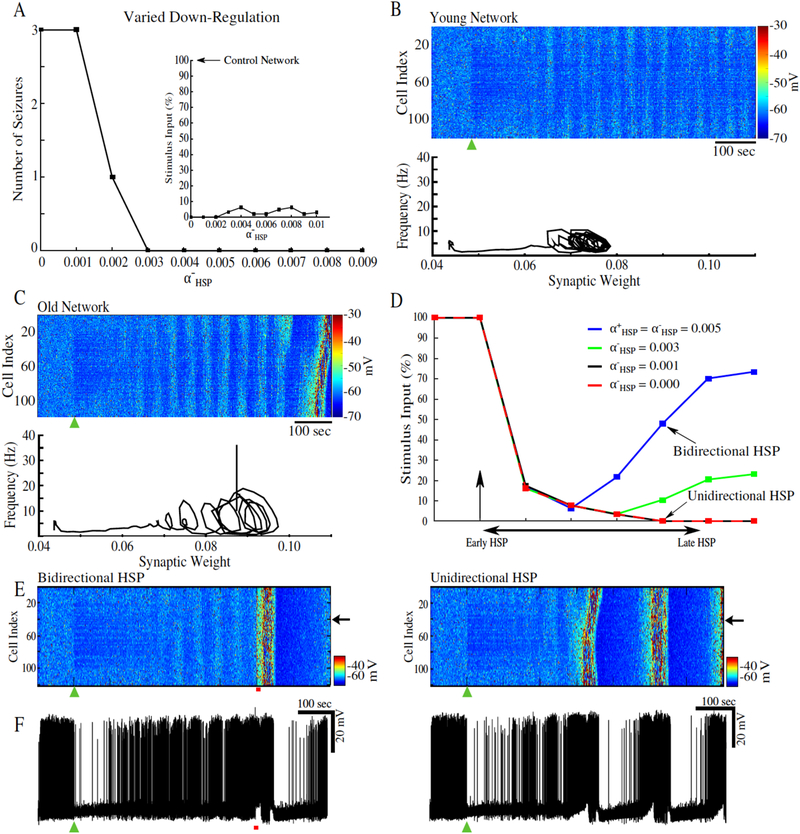
Axonal sprouting is initiated follow cortical trauma (Jin et al., 2011; Jin et al., 2006; Kusmierczak et al., 2015; Salin et al., 1995), and this process is likely age-dependent (Timofeev et al., 2013). The hypothesis that a combination of age-dependent differences in axonal sprouting rate, and age-dependent impairment of the bidirectionality of HSP may underlie the propensity for older animals to develop PTE was explored in computer models (González et al., 2015). Starting with the condition of no axonal sprouting and a reduced rate of HSP down-scaling, it was shown that impaired down-scaling allowed the deafferented cortical network model to generate spontaneous seizure-like activity (figure 3A). Differences were observed in the frequency – synaptic strength phase space trajectories for a “young” network (bidirectional HSP) as compared to an “old” network (impaired down-scaling rate of action) (figure 3B and C, respectively). The phase space trajectories show a transition from a fixed-point like attractor prior to deafferentation to a sustained limit cycle following recovery from deafferentation (figure 3B and C, inset). The radii of the limit cycles reflect the fact that “young” networks were capable of tightly and precisely regulating synaptic strength and network firing rate, while “old” network showed larger deviations from baseline firing rates. These deviations allowed for the progressive accumulation of [K+]o triggering the positive feedback loop necessary for seizure initiation (González et al., 2015). Implementing axonal sprouting allowed “young” networks (those with bidirectional HSP) to recover their initial seizure thresholds over time (figure 3D). This suggests that the combination of bidirectional HSP and axonal sprouting may prevent epileptogenesis in younger animals and patients. On the other hand, “old” network (unidirectional HSP) was unable to recover its pre-trauma seizure threshold and exhibited spontaneous recurrent seizure-like events (figure 3D and E). Together these data predict that age-dependent impairments in the bidirectional nature of homeostatic regulation of excitatory synapses may be responsible to the observed age-dependent differences in PTE.
Figure 3.
Impaired bidirectionality of HSP may underlie age-dependent differences in seizure susceptibility. Partial deafferentation was applied to the computational model at 100 sec (black arrow or green triangle). A, Number of spontaneous seizures as a function of the HSP down-regulation rate, α−HSP. Inset shows the seizure thresholds for networks with different α−HSP. B, Top, Heatmap of activity of a network with α−HSP = 0.009. Bottom, firing rate – synaptic strength phase space projection showing effects of network deafferentation. Inset, amplitude of the steady-state oscillation in the phase space projections for values of α−HSP = 0.003 – 0.01; note these HSP rates did not produce spontaneous seizures. C, Top, Heatmap of the network activity for α−HSP = 0.002. Bottom, firing rate – synaptic strength phase space projection leading to seizure. D, Time evolution of seizure threshold for networks with varying α−HSP All networks implemented synaptic sprouting; vertical arrow indicates the time of partial deafferentation. E, Heatmap of the network activity with bidirectional HSP (left), and unidirectional HSP (right). F, Corresponding single neuron activity from the networks in E. This figure is adapted from (González etal., 2015).
V. Conclusion
Though it has been extensively studied, epilepsy remains one of the most common neurological disorders. Many potent pharmacological compounds have been identified for their anti-convulsive properties but are ineffective in nearly 30% of patients with epilepsy. Additionally, these medications target the treatment of the symptoms of epilepsy, mainly seizure generation, rather than the underlying cause of the disease. The difficulty in developing appropriate treatments to prevent epileptogenesis comes from the many etiologies which can give rise to hyperexcitability and seizures. Here, we reviewed research exploring the roles of ionic concentration dynamics in seizure generation, and how ionic and synaptic homeostatic mechanisms, which are meant to prevent hyperexcitability, may contribute to network-wide pathological activity in an “unhealthy” brain suggesting that the tight regulation of [K+]o is necessary for maintaining physiological activity states, and that in a brain plagued by K+-channelopathy, KCC2 co-transporter activity may play a critical role in the onset of seizure-like activity. Experiments and computational modeling also predict that the age-dependent differences in the susceptibility to PTE observed in patients may be related to age-related impairments in the bidirectional regulation of excitatory synapses through homeostatic synaptic scaling. It should be noted that this work is not an exhaustive review into the ionic and synaptic mechanisms of epileptic seizures. Additional topics of interest which are omitted here include the roles of oxygen, energetic stress, changes in neuromodulatory tone, sleep disturbances, microglia, and volume dynamics in seizure generation, just to name a few.
Acknowledgements:
This was supported by NIH grant R01 NS104368.
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anastassiou CA, Koch C, 2015. Ephaptic coupling to endogenous electric field activity: why bother? Curr Opin Neurobiol. 31, 95–103. [DOI] [PubMed] [Google Scholar]
- Angeleri F, et al. , 1999. Posttraumatic epilepsy risk factors: one-year prospective study after head injury. Epilepsia. 40, 1222–30. [DOI] [PubMed] [Google Scholar]
- Annegers JF, et al. , 1998. A population-based study of seizures after traumatic brain injuries. N Engl J Med. 338, 20–4. [DOI] [PubMed] [Google Scholar]
- Asikainen I, et al. , 1999. Early and late posttraumatic seizures in traumatic brain injury rehabilitation patients: brain injury factors causing late seizures and influence of seizures on long-term outcome. Epilepsia. 40, 584–9. [DOI] [PubMed] [Google Scholar]
- Avoli M, et al. , 1996. Synchronous GABA-mediated potentials and epileptiform discharges in the rat limbic system in vitro. J Neurosci. 16, 3912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avoli M, de Curtis M, 2011. GABAergic synchronization in the limbic system and its role in the generation of epileptiform activity. Prog Neurobiol. 95, 104–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avramescu S, Timofeev I, 2008. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J Neurosci. 28, 6760–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell LF, et al. , 2009. Kv4.2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia. 50, 1741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazhenov M, et al. , 2004. Potassium model for slow (2-3 Hz) in vivo neocortical paroxysmal oscillations. J Neurophysiol. 92, 1116–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP, 2007. The action potential in mammalian central neurons. Nat Rev Neurosci. 8, 451–65. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, et al. , 1979. Hippocampal seizures and failure of inhibition. Canadian Journal of Physiology and Pharmacology. 57, 1462–1466. [Google Scholar]
- Braganza O, et al. , 2012. Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Epilepsia. 53, 1898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MJ, French JA, 2000. Management of epilepsy in adolescents and adults. Lancet. 356, 323–9. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Kwan P, 2001. The star systems: overview and use in determining antiepileptic drug choice. CNS Drugs. 15, 1–12 discussion 13-5. [DOI] [PubMed] [Google Scholar]
- Buchin A, et al. , 2016. Reduced Efficacy of the KCC2 Cotransporter Promotes Epileptic Oscillations in a Subiculum Network Model. J Neurosci. 36, 11619–11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, Murthy VN, 2003. Synaptic gain control and homeostasis. Curr Opin Neurobiol. 13, 560–7. [DOI] [PubMed] [Google Scholar]
- Chauvette S, et al. , 2016. In vivo models of cortical acquired epilepsy. J Neurosci Methods. 260, 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chever O, et al. , 2010. Implication of Kir4.1 channel in excess potassium clearance: an in vivo study on anesthetized glial-conditional Kir4.1 knock-out mice. J Neurosci. 30, 15769–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, et al. , 2009. Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet. 373, 1105–10. [DOI] [PubMed] [Google Scholar]
- Cohen I, et al. , 2002. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 298, 1418–21. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Steinhauser C, 2015. Role of astrocytes in epilepsy. Cold Spring Harb Perspect Med. 5, a022434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cressman JR Jr., et al. , 2009. The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states: I. Single neuron dynamics. J Comput Neurosci. 26, 159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, et al. , 2005. Modulation of synaptic transmission in neocortex by network activities. Eur J Neurosci. 21, 1030–44. [DOI] [PubMed] [Google Scholar]
- D'Adamo MC, et al. , 2013. K(+) channelepsy: progress in the neurobiology of potassium channels and epilepsy. Front Cell Neurosci. 7, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, et al. , 2005. Progression from frontal-parietal to mesial-temporal epilepsy after fluid percussion injury in the rat. Brain. 128, 174–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrosio R, et al. , 1999. Impaired K(+) homeostasis and altered electrophysiological properties of post-traumatic hippocampal glia. J Neurosci. 19, 8152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo E, et al. , 2015. Autosomal dominant lateral temporal epilepsy (ADLTE): novel structural and single-nucleotide LGI1 mutations in families with predominant visual auras. Epilepsy Res. 110, 132–8. [DOI] [PubMed] [Google Scholar]
- de Lanerolle NC, et al. , 2010. Astrocytes and epilepsy. Neurotherapeutics. 7, 424–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Gjerstad L, 1980. Reduced inhibition during epileptiform activity in the in vitro hippocampal slice. J Physiol. 305, 297–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinner D, 1993. Posttraumatic epilepsy. The Treatment of Epilepsy: Principles. 654–658. [Google Scholar]
- Djukic B, et al. , 2007. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 27, 11354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echegoyen J, et al. , 2007. Homeostatic plasticity studied using in vivo hippocampal activity -blockade: synaptic scaling, intrinsic plasticity and age-dependence. PLoS One. 2, e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah FH, et al. , 2019. Coupled Oscillators Model of Hyperexcitable Neuroglial Networks. Int J Neural Syst. 29, 1850041. [DOI] [PubMed] [Google Scholar]
- Fertziger AP, Ranck JB Jr., 1970. Potassium accumulation in interstitial space during epileptiform seizures. Exp Neurol. 26, 571–85. [DOI] [PubMed] [Google Scholar]
- Filatov G, et al. , 2011. Dynamics of epileptiform activity in mouse hippocampal slices. J Biol Phys. 37, 347–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso-Veloz J, et al. , 1990. Seizures and wet-dog shakes induced by 4-aminopyridine, and their potentiation by nifedipine. Eur J Pharmacol. 178, 275–84. [DOI] [PubMed] [Google Scholar]
- Friedman A, et al. , 2009. Blood-brain barrier breakdown-inducing astrocytic transformation: novel targets for the prevention of epilepsy. Epilepsy Res. 85, 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, Bazhenov M, 2006. Coexistence of tonic firing and bursting in cortical neurons. Phys Rev E Stat Nonlin Soft Matter Phys. 74, 031922. [DOI] [PubMed] [Google Scholar]
- Frohlich F, et al. , 2008a. Potassium dynamics in the epileptic cortex: new insights on an old topic. Neuroscientist. 14, 422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, et al. , 2008b. Pathological effect of homeostatic synaptic scaling on network dynamics in diseases of the cortex. J Neurosci. 28, 1709–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, McCormick DA, 2010. Endogenous electric fields may guide neocortical network activity. Neuron. 67, 129–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich F, et al. , 2010. Network bistability mediates spontaneous transitions between normal and pathological brain states. J Neurosci. 30, 10734–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M, et al. , 1982. Convulsant actions of 4-aminopyridine on the guinea-pig olfactory cortex slice. Brain Res. 241, 75–86. [DOI] [PubMed] [Google Scholar]
- Giaume C, et al. , 2010. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 11, 87–99. [DOI] [PubMed] [Google Scholar]
- González OC, et al. , 2015. Modeling of Age-Dependent Epileptogenesis by Differential Homeostatic Synaptic Scaling. The Journal of Neuroscience. 35, 13448–13462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González OC, et al. , 2018. Role of KCC2-dependent potassium efflux in 4-Aminopyridine-induced Epileptiform synchronization. Neurobiol Dis. 109, 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasse DW, et al. , 2013. Neuronal synchrony and the transition to spontaneous seizures. Experimental neurology. 248, 72–84. [DOI] [PubMed] [Google Scholar]
- Grigorovsky V, Bardakjian BL, 2018. Low-to-High Cross-Frequency Coupling in the Electrical Rhythms as Biomarker for Hyperexcitable Neuroglial Networks of the Brain. IEEE Trans Biomed Eng. 65, 1504–1515. [DOI] [PubMed] [Google Scholar]
- Grisar T, et al. , 1992. Contribution of Na+,K(+)-ATPase to focal epilepsy: a brief review. Epilepsy Res. 12, 141–9. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, et al. , 1982. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 299, 252–4. [DOI] [PubMed] [Google Scholar]
- Haj-Yasein NN, et al. , 2011. Evidence that compromised K+ spatial buffering contributes to the epileptogenic effect of mutations in the human Kir4.1 gene (KCNJ10). Glia. 59, 1635–42. [DOI] [PubMed] [Google Scholar]
- Hamidi S, Avoli M, 2015. KCC2 function modulates in vitro ictogenesis. Neurobiol Dis. 79, 51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann U, Lux HD, 1977. Ceiling of stimulus induced rises in extracellular potassium concentration in the cerebral cortex of cat. Brain Res. 120, 231–49. [DOI] [PubMed] [Google Scholar]
- Heuser K, et al. , 2012. Loss of perivascular Kir4.1 potassium channels in the sclerotic hippocampus of patients with mesial temporal lobe epilepsy. J Neuropathol Exp Neurol. 71, 814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houweling AR, et al. , 2005. Homeostatic synaptic plasticity can explain post-traumatic epileptogenesis in chronically isolated neocortex. Cereb Cortex. 15, 834–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberfeld G, et al. , 2007. Perturbed chloride homeostasis and GABAergic signaling in human temporal lobe epilepsy. J Neurosci. 27, 9866–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata K, et al. , 2008. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 57, 819–26. [DOI] [PubMed] [Google Scholar]
- Ingram J, et al. , 2014. Oxygen and seizure dynamics: I. Experiments. J Neurophysiol. 112, 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Yaari Y, 1997. Role of intrinsic burst firing, potassium accumulation, and electrical coupling in the elevated potassium model of hippocampal epilepsy. J Neurophysiol. 77, 1224–33. [DOI] [PubMed] [Google Scholar]
- Jin X, et al. , 2011. Reorganization of inhibitory synaptic circuits in rodent chronically injured epileptogenic neocortex. Cereb Cortex. 21, 1094–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, et al. , 2006. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 26, 4891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kager H, et al. , 2000. Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. J Neurophysiol. 84, 495–512. [DOI] [PubMed] [Google Scholar]
- Kjaerby C, et al. , 2017. Does Global Astrocytic Calcium Signaling Participate in Awake Brain State Transitions and Neuronal Circuit Function? Neurochem Res. 42, 1810–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollevold T, 1976. Immediate and early cerebral seizures after head injuries. Part I. J Oslo City Hosp. 26, 99–114. [PubMed] [Google Scholar]
- Krishnan GP, Bazhenov M, 2011. Ionic dynamics mediate spontaneous termination of seizures and postictal depression state. J Neurosci. 31, 8870–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, et al. , 2015. Electrogenic properties of the Na(+)/K(+) ATPase control transitions between normal and pathological brain states. J Neurophysiol. 113, 3356–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan GP, et al. , 2018. Origin of slow spontaneous resting-state neuronal fluctuations in brain networks. Proc Natl Acad Sci U S A. 115, 6858–6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmierczak M, et al. , 2015. Changes in long-range connectivity and neuronal reorganization in partial cortical deafferentation model of epileptogenesis. Neuroscience. 284, 153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lascano AM, et al. , 2016. Seizures and Epilepsies due to Channelopathies and Neurotransmitter Receptor Dysfunction: A Parallel between Genetic and Immune Aspects. Mol Syndromol. 7, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux M, et al. , 2014. The impact of cortical deafferentation on the neocortical slow oscillation. J Neurosci. 34, 5689–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, et al. , 2016. Interneurons spark seizure-like activity in the entorhinal cortex. Neurobiol Dis. 87, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, et al. , 2013. Temporal lobe epileptiform activity following systemic administration of 4-aminopyridine in rats. Epilepsia. 54, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis KP, et al. , 2012. Pyramidal cells accumulate chloride at seizure onset. Neurobiol Dis. 47, 358–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopantsev V, Avoli M, 1998. Participation of GABAA-mediated inhibition in ictallike discharges in the rat entorhinal cortex. J Neurophysiol. 79, 352–60. [DOI] [PubMed] [Google Scholar]
- Lukawski K, et al. , 2018. Mechanisms of epileptogenesis and preclinical approach to antiepileptogenic therapies. Pharmacol Rep. 70, 284–293. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Agnew WF, 1983. Changes in extracellular potassium and calcium concentration and neural activity during prolonged electrical stimulation of the cat cerebral cortex at defined charge densities. Exp Neurol. 79, 371–96. [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J, Bean BP, 2002. Potassium currents during the action potential of hippocampal CA3 neurons. J Neurosci. 22, 10106–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nita DA, et al. , 2006. Increased propensity to seizures after chronic cortical deafferentation in vivo. J Neurophysiol. 95, 902–13. [DOI] [PubMed] [Google Scholar]
- Nita DA, et al. , 2007. Waking-sleep modulation of paroxysmal activities induced by partial cortical deafferentation. Cereb Cortex. 17, 272–83. [DOI] [PubMed] [Google Scholar]
- Nobile C, et al. , 2009. LGI1 mutations in autosomal dominant and sporadic lateral temporal epilepsy. Hum Mutat. 30, 530–6. [DOI] [PubMed] [Google Scholar]
- Olsen ML, Sontheimer H, 2008. Functional implications for Kir4.1 channels in glial biology: from K+ buffering to cell differentiation. J Neurochem. 107, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski P, Meador KJ, 2004. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 5 Suppl 1, S60–5. [DOI] [PubMed] [Google Scholar]
- Ottman R, et al. , 2004. LGI1 mutations in autosomal dominant partial epilepsy with auditory features. Neurology. 62, 1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DC, et al. , 2019. Neuron-glia interactions in the pathophysiology of epilepsy. Nat Rev Neurosci. 20, 282–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D, et al. , 2016. Roles of specific Kv channel types in repolarization of the action potential in genetically identified subclasses of pyramidal neurons in mouse neocortex. J Neurophysiol. 115, 2317–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, et al. , 2003. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 26, 199–206. [DOI] [PubMed] [Google Scholar]
- Pedley TA, et al. , 1976. Regulation of extracellular potassium concentration in epileptogenesis. Fed Proc. 35, 1254–9. [PubMed] [Google Scholar]
- Perucca E, Tomson T, 2011. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 10, 446–56. [DOI] [PubMed] [Google Scholar]
- Perucca P, Gilliam FG, 2012. Adverse effects of antiepileptic drugs. Lancet Neurol. 11, 792–802. [DOI] [PubMed] [Google Scholar]
- Ping X, Jin X, 2016. Chronic Posttraumatic Epilepsy following Neocortical Undercut Lesion in Mice. PLoS One. 11, e0158231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Yuste R, 2016. Astrocytes regulate cortical state switching in vivo. Proc Natl Acad Sci U S A. 113, E2675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo K, Goda Y, 2010. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 66, 337–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince DA, Tseng GF, 1993. Epileptogenesis in chronically injured cortex: in vitro studies. J Neurophysiol. 69, 1276–91. [DOI] [PubMed] [Google Scholar]
- Pumain R, et al. , 1983. Fast extracellular calcium transients: involvement in epileptic processes. Science. 222, 177–9. [DOI] [PubMed] [Google Scholar]
- Qiu C, et al. , 2015. Can Neural Activity Propagate by Endogenous Electrical Field? J Neurosci. 35, 15800–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, et al. , 2005. Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J Physiol. 562, 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar AM, et al. , 1985. Epilepsy after penetrating head injury. I. Clinical correlates: a report of the Vietnam Head Injury Study. Neurology. 35, 1406–14. [DOI] [PubMed] [Google Scholar]
- Salin P, et al. , 1995. Axonal sprouting in layer V pyramidal neurons of chronically injured cerebral cortex. J Neurosci. 15, 8234–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA, 1980. Changes in excitatory and inhibitory synaptic potentials leading to epileptogenic activity. Brain Res. 183, 61–76. [DOI] [PubMed] [Google Scholar]
- Seiffert E, et al. , 2004. Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 24, 7829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneur J, Timofeev I, 2010. Synaptic impairment induced by paroxysmal ionic conditions in neocortex. Epilepsia. 52, 132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessolo M, et al. , 2015. Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity. J Neurosci. 35, 9544–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri Z, et al. , 2016. Activation of specific neuronal networks leads to different seizure onset types. Ann Neurol. 79, 354–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivacharan RS, et al. , 2019. Self-propagating, non-synaptic epileptiform activity recruits neurons by endogenous electric fields. Exp Neurol. 317, 119–128. [DOI] [PubMed] [Google Scholar]
- Singh B, et al. , 2006. A Kv4.2 truncation mutation in a patient with temporal lobe epilepsy. Neurobiol Dis. 24, 245–53. [DOI] [PubMed] [Google Scholar]
- Somjen GG, 2002. Ion regulation in the brain: implications for pathophysiology. Neuroscientist. 8, 254–67. [DOI] [PubMed] [Google Scholar]
- Somjen GG, et al. , 2008. Computer simulations of neuron-glia interactions mediated by ion flux. J Comput Neurosci. 25, 349–65. [DOI] [PubMed] [Google Scholar]
- Steinhauser C, et al. , 2012. Astrocyte dysfunction in temporal lobe epilepsy: K+ channels and gap junction coupling. Glia. 60, 1192–202. [DOI] [PubMed] [Google Scholar]
- Stewart TH, et al. , 2010. Chronic dysfunction of astrocytic inwardly rectifying K+ channels specific to the neocortical epileptic focus after fluid percussion injury in the rat. J Neurophysiol. 104, 3345–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Turrigiano GG, 2011. PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J Neurosci. 31, 6800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR, et al. , 1995. Causes, prevention, and treatment of post-traumatic epilepsy. New Horiz. 3, 518–22. [PubMed] [Google Scholar]
- Thimm J, et al. , 2005. Calcium-dependent open/closed conformations and interfacial energy maps of reconstituted hemichannels. J Biol Chem. 280, 10646–54. [DOI] [PubMed] [Google Scholar]
- Tian GF, et al. , 2005. An astrocytic basis of epilepsy. Nat Med. 11, 973–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, et al. , 2010. Posttraumatic epilepsy: the roles of synaptic plasticity. Neuroscientist. 16, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, et al. , Neuronal Synchronization and Thalamocortical Rhythms in Sleep, Wake and Epilepsy. In: th, et al., (Eds.), Jasper's Basic Mechanisms of the Epilepsies, Bethesda (MD), 2012. [PubMed] [Google Scholar]
- Timofeev I, et al. , 2002a. Cortical hyperpolarization-activated depolarizing current takes part in the generation of focal paroxysmal activities. Proc Natl Acad Sci U S A. 99, 9533–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, et al. , 2002b. The role of chloride-dependent inhibition and the activity of fast-spiking neurons during cortical spike-wave electrographic seizures. Neuroscience. 114, 1115–32. [DOI] [PubMed] [Google Scholar]
- Timofeev I, et al. , 2013. Age dependency of trauma-induced neocortical epileptogenesis. Front Cell Neurosci. 7, 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev I, Steriade M, 2004. Neocortical seizures: initiation, development and cessation. Neuroscience. 123, 299–336. [DOI] [PubMed] [Google Scholar]
- Topolnik L, et al. , 2003a. Hyperexcitability of intact neurons underlies acute development of trauma-related electrographic seizures in cats in vivo. Eur J Neurosci. 18, 486–96. [DOI] [PubMed] [Google Scholar]
- Topolnik L, et al. , 2003b. Partial cortical deafferentation promotes development of paroxysmal activity. Cereb Cortex. 13, 883–93. [DOI] [PubMed] [Google Scholar]
- Toyoda I, et al. , 2015. Unit activity of hippocampal interneurons before spontaneous seizures in an animal model of temporal lobe epilepsy. Journal of Neuroscience. 35, 6600–6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande CA, Ramirez JM, 2007. Activity deprivation leads to seizures in hippocampal slice cultures: is epilepsy the consequence of homeostatic plasticity? J Clin Neurophysiol. 24, 154–64. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Dingledine R, 1988. Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol. 59, 259–76. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, 2008. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 135, 422–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah G, et al. , 2009. The influence of sodium and potassium dynamics on excitability, seizures, and the stability of persistent states. II. Network and glial dynamics. J Comput Neurosci. 26, 171–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uva L, et al. , 2015. Synchronous inhibitory potentials precede seizure-like events in acute models of focal limbic seizures. J Neurosci. 35, 3048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet EA, et al. , 2007. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 130, 521–34. [DOI] [PubMed] [Google Scholar]
- Velazquez JLP, et al. , 1999. Type III intermittency in human partial epilepsy. European Journal of Neuroscience. 11, 2571–2576. [DOI] [PubMed] [Google Scholar]
- Viitanen T, et al. , 2010. The K+-Cl cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol. 588, 1527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, et al. , 2011a. Pattern of trauma determines the threshold for epileptic activity in a model of cortical deafferentation. Proc Natl Acad Sci U S A. 108, 15402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, et al. , 2012. Computational models of neuron-astrocyte interaction in epilepsy. Front Comput Neurosci. 6, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, et al. , 2013. Divide and conquer: functional segregation of synaptic inputs by astrocytic microdomains could alleviate paroxysmal activity following brain trauma. PLoS Comput Biol. 9, e1002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volman V, et al. , 2011b. Topological basis of epileptogenesis in a model of severe cortical trauma. J Neurophysiol. 106, 1933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallraff A, et al. , 2006. The impact of astrocytic gap junctional coupling on potassium buffering in the hippocampus. J Neurosci. 26, 5438–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, et al. , 2014a. Oxygen and seizure dynamics: II. Computational modeling. J Neurophysiol. 112, 213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, et al. , 2014b. Unification of neuronal spikes, seizures, and spreading depression. J Neurosci. 34, 11733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, et al. , 2005. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 25, 2895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Rogawski MA, 1992. Effects of anticonvulsant drugs on 4-aminopyridine-induced seizures in mice. Epilepsy Res. 11, 9–16. [DOI] [PubMed] [Google Scholar]
- Yekhlef L, et al. , 2015. Selective activation of parvalbumin- or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J Neurophysiol. 113, 1616–30. [DOI] [PubMed] [Google Scholar]
- Zalay OC, et al. , 2010. System characterization of neuronal excitability in the hippocampus and its relevance to observed dynamics of spontaneous seizure-like transitions. J Neural Eng. 7, 036002. [DOI] [PubMed] [Google Scholar]
- Zhang M, et al. , 2014. Propagation of epileptiform activity can be independent of synaptic transmission, gap junctions, or diffusion and is consistent with electrical field transmission. J Neurosci. 34, 1409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckermann EC, Glaser GH, 1968. Hippocampal epileptic activity induced by localized ventricular perfusion with high-potassium cerebrospinal fluid. Exp Neurol. 20, 87–110. [DOI] [PubMed] [Google Scholar]