Abstract
The ability to detect social signals represents a first step to enter our social world. Behavioral evidence has demonstrated that 6-month-old infants are able to orient their attention towards the position indicated by walking direction, showing faster orienting responses towards stimuli cued by the direction of motion than towards uncued stimuli.
The present study investigated the neural mechanisms underpinning this attentional priming effect by using a spatial cueing paradigm and recording EEG (Geodesic System 128 channels) from 6-month-old infants. Infants were presented with a central point-light walker followed by a single peripheral target. The target appeared randomly at a position either congruent or incongruent with the walking direction of the cue. We examined infants’ target-locked ERP responses and we used cortical source analysis to explore which brain regions gave rise to the ERP responses.
The P1 component and saccade latencies towards the peripheral target were modulated by the congruency between the walking direction of the cue and the position of the target. Infants’ saccade latencies were faster in response to targets appearing at congruent spatial locations. The P1 component was larger in response to congruent than to incongruent targets and a similar congruency effect was found with cortical source analysis in the parahippocampal gyrus and the anterior fusiform gyrus.
Overall, these findings suggest that a type of biological motion like the one of a vertebrate walking on the legs can trigger covert orienting of attention in 6-month-old infants, enabling enhancement of neural activity related to visual processing of potentially relevant information as well as a facilitation of oculomotor responses to stimuli appearing at the attended location.
Keywords: Social stimuli, biological motion, visuo-spatial orienting, ERPs, cortical source analysis
Introduction
Human beings are social creatures whose survival depends on the ability to successfully detect and adequately interact with other social agents. First evidence towards detection and identification of social agents is provided by the kinematics of their movement. Kinematics is effective even when it is conveyed by a small number of point lights attached to the head and major joints of a walking figure (i.e., a point-light walker; Johansson, 1973).
Empirical studies testing adults have demonstrated their ability to retrieve information about biological motion from simple point-light displays (PLDs), ranging from actions (Dittrich, 1993), to emotions (Dittrich, Troscianko, Lea, & Morgan, 1996), to walking direction (Hirai, Saunders & Troje, 2011; Troje & Westhoff, 2006). Importantly, this ability manifests itself only if the stimuli are presented upright. It markedly decreases when the stimuli are presented upside-down (Chang & Troje, 2009; Hirai, Chang, Saunders, & Troje, 2011; Pavlova & Sokolov, 2000; Sumi, 1984; Troje & Westhoff, 2006).
Developmental research has shown that sensitivity to biological motion emerges very early in life, with even two-day-old babies preferentially attending at biological motion displays in comparison to random or mechanical motion displays (Bardi, Regolin & Simion, 2011; Simion, Regolin, & Bulf, 2008). This predisposition is soon associated with the ability to retrieve the articulated shape of a human body, demonstrated by 3-month-old to 5-month-old babies’ ability to extract a configural organization from simple dots in movement (Bertenthal, Proffitt, & Cutting, 1984; Bertenthal, Proffitt, & Kramer, 1987). A neurophysiological study (Reid, Hoel, & Striano 2006), employing a passive viewing condition, demonstrated that the neural correlates elicited by upright and inverted point-light displays depicting a walking human figure differ in 8-month-old infants. Analysis of event-related potential (ERP) showed that the orientation of PLDs elicited a modulation of a positive ERP component peaking between 200–300 ms post-stimulus onset (Reid et al., 2006). This component was greater for upright rather than inverted PLDs and this effect was present only in the right hemisphere, thus indicating that, from early in development, upright and inverted PLD configurations are processed differently. In addition, 6-month-old infants exhibit the ability to detect and discriminate the walking direction of a biological motion PLD representing a human body walking towards either the left or the right (Kuhlmeier, Troje, & Lee, 2010). This evidence suggests that the phenomenon of a preferential attention to biological motion is present since birth and gradually develops during the first months of life.
Recently, research has attempted to investigate the functional meaning accompanying biological motion walking direction processing, contemporarily probing the hypothesis according to which the walking direction of legged vertebrates may trigger mechanisms of visuo-spatial orienting of attention in a way similar to what other directional social cues do (i.e., eye-gaze, pointing and grasping gestures) (Bardi, Di Giorgio, Lunghi & Simion, 2015; Shi, Weng, He & Jiang, 2010). Humans are able to encode the direction of human gestures and to use these as a cue to orient their own visuo-spatial attention. It has been demonstrated that, early in life, humans orient their attention towards the portion of the environment signaled by conspecifics’ gestures and actions (Gredebäck & Daum, 2015; Natale et al., 2016). This evidence indicates that infants can use others’ gestures as relevant cues to detect and orient to potentially salient information in the environment.
Orienting of attention is a mechanism functional since birth and it allows adults as well as infants to process potentially relevant information in the surrounding space. This mechanism can be either overt, when it is accompanied by eye movements, or covert, when attention is deployed to relevant locations without eye movements (Posner, 1980). Moreover, orienting of visual attention can be endogenous, when it is controlled by top-down factors, such as knowledge of the location of the impending target, or it might be exogenous, when it depends on sensory stimulation, such as the sudden appearance of a stimulus. Exogenous orienting is elicited by sensory peripheral stimuli (Posner, 1980). Exogenous orienting produces a processing facilitation indexed by faster and more accurate response to the target priming effect, (i.e., Posner 2016; Muller & Rabbit, 1989). This stronger facilitation is accompanied by a modulation of the P1 and N1 ERP sensory components, supporting the idea that orienting of attention produces sensory consequences (Hillyard, Vogel & Luck, 1998). It appears thus that the process of orienting of visuo-spatial attention toward cued stimuli is coupled with a facilitation of manual and saccadic reaction times and with an enhancement of neural activity related to visual processing.
Traditionally it is believed that only peripheral sensory cues, such as flashes of lights, can elicit a truly automatic, exogenous orienting of attention. However, recently it has been demonstrated that central non-predictive “social” cues can trigger exogenous visuo-spatial orienting of attention producing effects similar to those elicited by peripheral cues. Evidence coming from adults’ studies suggested that when participants are presented with a central non-predictive cue, such as eye gaze, they exhibit faster manual reaction time and a modulation of ERPs sensory components to targets presented in the periphery (Driver et al., 1999; Frischen, Bayliss, & Tipper, 2007). These findings have been explained by proposing the existence of a domain-specific mechanism devoted to eye gaze processing (Baron-Cohen, 1994), which is an important source of social information (Bonato, Priftis, Marenzi, & Zorzi, 2009).
A series of studies of infants (i.e. Gredebäck, Melinder & Daum, 2010; Senju, Johnson & Csibra, 2006; Richards, 2000; Xie & Richards, 2017) have shed light on the possible functional mechanisms and neural correlates associated with this attentional priming effect early in life. Richards (2000) demonstrated that infants are able to shift their attention towards the location indicated by a peripheral cue and the effect is indexed by specific ERP responses. Richards’s study revealed that infants oriented their attention overtly (i.e. gazing) towards the spatial position indicated by peripheral cues showing shorter saccade latencies for congruent rather than incongruent trials. Moreover, infants exhibited distinct ERPs (e.g., P1, N1) responses to spatially cued stimuli compared to non-cued stimuli (Richards, 2000, 2005). The P1 component was found to be larger for congruent than incongruent trials across occipital electrodes in 4.5- and 6-month-old infants whereas no difference was present in 3-month-olds. Similar results were demonstrated for the N1 component, which is the negative deflection following P1 (Richards, 2000, 2005). The modulation of these sensory components (e.g. P1, N1) reveals that infants show covert attentional orienting at very early stages of development.
Different results came from studies employing central directional non-predictive cues (i.e., faces, eye gaze, pointing, hand gesture) in modified versions of the traditional Posner’s cueing paradigm. These studies have indicated that the modulation of ERP components varies as a function of the experimental design and type of stimulus used. For example, a first group of studies investigated the neural correlates of cue-target congruency relations by presenting in one of two possible locations a target stimulus followed by presentation of the cue (a pointing hand or grasping hand). Results indicated that, from 6 months of life, the temporo-parietal component P400 is sensitive to cue-target congruency relations, being higher in amplitude for incongruent relative to congruent conditions (Bakker, Daum, Handl & Gredebäck, 2014; Gredebäck et al., 2010).
A second version of the cueing paradigm requires the initial presentation of a neutral, non-directional cue (a face looking neither right or left) followed by a target appearing either at a left or right spatial location. Disappearance of target stimulus is synchronously coupled with appearance of a directional cue (a face looking right or left). Studies carried out with this paradigm have revealed that the temporo-parietal component N290 is sensitive to congruency relations starting from 9 months of age (Senju et al., 2006). Similar results were found in 6-month-old infants, using a cueing paradigm where the target presentation was preceded by the presentation of a dynamic possible or impossible hand gesture (Natale et al., 2016). Results indicate that the N290 component is modulated by congruency relations between cue and target but only when the cue represents a possible hand gesture and not when the cue represents an impossible hand gesture. This evidence demonstrates that the infant brain can differentiate congruent from incongruent relations in the first few months of life. Nonetheless, since none of these studies made use of the traditional Posner’s cueing paradigm, the potential effect of directional non-predictive cues on attentional orienting and underlying neural correlates still remain to be characterized.
Further, an issue that still needs to be explored relates to the kinematics proprieties of human motion that are crucial in triggering orienting of attention. Farroni and colleagues (2000) demonstrated that perceived motion has a central role in orienting attention in infancy. 4-month-old infants, tested with a cueing paradigm employing gaze as a cue, showed faster responses only when the movement of the eyes was perceived. In contrast, when the motion of the pupils was hidden by a blinking face, infants did not show any facilitation in orienting their attention. Importantly, human motion possesses specific kinematic properties and all human actions (i.e. eye gaze, hand gesture, and walking) obey to specific rules resulting from the physical constraints of the human body (Moore, 2012).
A recent study, demonstrated that 6-month-old infants use information concerning directionality conveyed by human motion to orient their attention towards the spatial hemifield indicated by PLDs of a walking human. Bardi and colleagues (2015) tested infants and adults with a cueing paradigm employing a central PLD of human motion walking on a treadmill followed by a peripheral target. This PLD cue stimulus could be presented upright or upside-down and it was facing either right or left. Adults and infants’ saccadic reaction times were faster in response to targets appearing at congruent rather than incongruent spatial positions when the cue was presented upright but not when it was presented upside-down. This study demonstrated that the walking direction of a human motion PLD can trigger automatic visuo-spatial orienting in both adults and 6-month-old infants. Specifically, both groups of participants exhibited faster saccadic reaction times when the target was presented at the spatial position cued by a PLD of a human walker. Overall, Bardi and colleagues’ results (2015) supports the idea that kinematic information of human motion, as well as motion performed by other stimuli that vehicle social information, can trigger orienting of attention. Moreover, this behavioral evidence points to the idea that human motion can trigger covert orienting of attention preceding and driving eye movements. However, the authors recorded only overt responses. Thus, this study does not clearly demonstrate that human motion can trigger covert orienting of attention in the absence of eye movements.
Rizzolatti and colleagues’ (1987) premotor theory of attention states that eye movement preparation engages covert spatial attention before active stimulation, regardless of the nature (overt or covert) of the attentional shift. Nonetheless, Bardi and colleagues’ results might be argued to be valid only for overt shifts of attention. In particular, these findings (Bardi et al., 2015) do not demonstrate the existence of a sensory gain effect, that is an amplification of the signal in response to targets appearing at cued relative to uncued locations (Hillyard, Vogel & Luck, 1998) when the cue is represented by biological motion walking direction. In order to answer this question, an EEG study is required. If human motion really triggers covert orienting of attention one should observe a specific modulation of the ERP sensory component P1 occurring before eye movements.
The aim of the present study was to investigate the neural correlates of the attentional effect accompanying mechanisms of visuo-spatial orienting triggered by upright biological motion walking direction in 6-month-old infants. A cueing paradigm, similar to that used by Bardi and colleagues (2015) was adopted and coupled with the recording of the EEG signal. As in previous behavioral and EEG infants’ studies (i.e. Bardi et al., 2015; Farroni et al., 2000; Natale et al., 2016), in the following experiment only congruent and incongruent trials were presented. In the absence of neutral trials, facilitatory and inhibitory effects are confounded. Accordingly, we aimed at probing the neural correlates of these attentional effects, regardless of whether they result from facilitation of cued trials, inhibition of uncued trials or both.
From a behavioral perspective, we predicted to observe a modulation of infants’ oculomotor behavior as a function of conguency/incongruency relations between the walking direction of the upright walker and the appearance of a peripheral target stimulus.
We also expected that, due to the social relevance of walking direction, the effects observed might be similar to those found with peripheral cues that elicit exogenous orienting of attention. Thus, from a neural point of view, we predicted that, if human motion orients visuo-spatial attention, a modulation of the sensory ERP component (i.e. P1) should be observed. Accordingly, we hypothesized this early ERP component to exhibit higher amplitude in response to targets appearing at congruent relative to incongruent spatial locations.
In addition, we predicted that the modulation of this sensory component should originate from brain areas involved in visual processing. Therefore, besides analyzing the ERP component P1, we applied cortical source analysis to examine the brain regions that might generate the recorded ERPs involved in infants orienting of attention (Richards, 2005, Xie & Richards 2017). Previous studies have suggested that the cortical regions generating the P1 congruency effect were located in contralateral Brodmann areas (BAs) 18 and 19 (Richards 2005) and in the contralateral ventral temporal areas (Xie & Richards 2017).
Methods
Participants
Twenty-seven healthy, full-term 6-month-old infants (10 females, mean age = 6 months and 7 days, range = 183–224 days) participated in the study. Five infants were tested, but not included in the final sample of participants because of fussiness or excessive movement artifacts. Accordingly, 22 infants were considered behavioral analysis. Eleven out of the 22 infants (7 females, mean age = 6 months and 7 days, range = 183–205 days) were considered for ERP target-locked analysis. The criterion adopted for the inclusion in the sample of the ERP analysis was to have at least 7 good trials in each experimental condition (congruent vs. incongruent).
Infants were tested if awake and in an alert state, and after parents gave their informed consent.
The experimental protocol was approved by the Ethical Committee of the University of Padova (protocol number 1956).
Stimuli
Cue stimuli were represented by frame sequences of a walking human figure. The human walker was computed as the average walker from motion-captured data of 50 males and 50 females (Troje, 2002, 2008). A set of 11 markers allocated to the main joints and head of the person was used to represent the figure. The translating component of the walk was removed to display stationary walking. All walkers were presented in profile, either facing leftward or rightward, and were shown with a gait frequency of 0.76 Hz. All stimuli appeared as white dots on a black background and the full point light figure subtended a visual angle of 7.59°x 3.81°. The target stimulus consisted of a colorful ball and it subtended a visual angle of 1.43°x1.43° and the distance between the center of the cue and the center of the target was 12.68° (13.5 cm). At the beginning of each trial, to attract infants’ attention to the center of a screen, a series of silent animated video (e.g. cartoons) was presented as attention getter. The attention getter lasted until the eyes of the infants was aligned with the center of the screen. The experimenter monitored online the eyes of the infants to determine whether the eye gaze was aligned with the center of the screen.
Apparatus and Procedure
Testing took place in a dimly illuminated room. Infants were seated on a parent’s lap approximately 60 cm from screen (24 inches; resolution 1024 × 768 pixels) used for stimulus presentation. A two-machine solution was adopted for experimental control. The sequence and timing of stimulus presentation was controlled using a computer with E-Prime 2.0. This computer was interfaced with Net Station (Electrical Geodesic, Eugene, OR.) via a serial connection. Net Station was used to record the critical sequence of events along with the high-density EEG data. Infants’ eye blinks and saccades were monitored online by an experimenter via a visual inspection of the continuous EEG signal recorded. Additionally, a video camera situated above the screen used for stimulus presentation recorded the infants’ face and gaze behavior.
A cueing paradigm was employed (Natale et al., 2016; Posner, 1980). As shown in Figure 1, each trial began with a visually animated but silent fixation point, (i.e attention getter) randomly selected among 16 different animations, displayed at the center of the screen. As soon as the infant looked at it, this attention getter was replaced by the visuo-spatial cue, namely a PLD of a human body walking toward the right or the left position. The cue was shown for 1200 ms and, after a variable delay (range: 300–500 ms), the target stimulus was displayed for 200 ms at a peripheral spatial location (~10 degrees of visual angle from the center of the screen) either congruent or incongruent with the cue walking direction.
Figure 1: Paradigm demonstration.
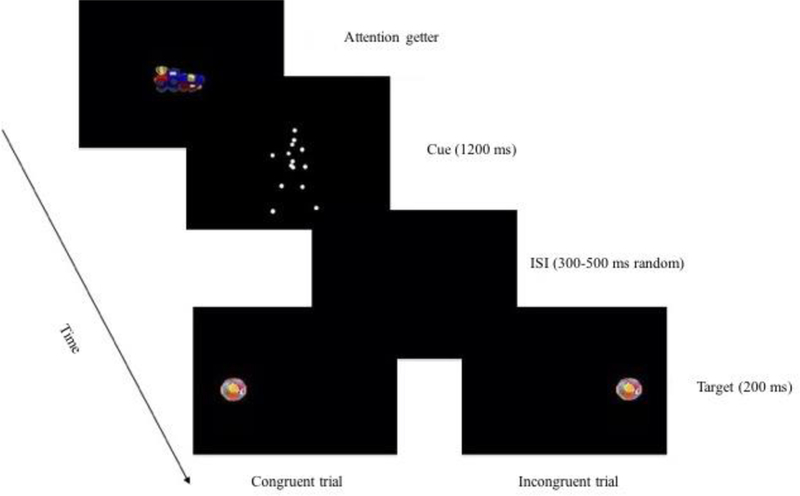
Representation of the sequence of events in the cueing paradigm. Two types of trials were presented: Congruent and Incongruent trials. In congruent trials the target appeared in the spatial position cued by biological motion walking direction; in incongruent trials the target appeared in the spatial position not cued by biological motion walking direction.
Stimuli were presented in blocks of 16 trials, eight congruent (four with left-and four with right-sided targets) and eight incongruent (four with left-and four with right-sided targets) randomly presented within each block. The animated fixation point varied on each trial. Also, the target stimulus varied, being randomly selected among four possible types. In order to obtain as many trials as possible from each infant, there was no restriction in number of blocks or trials shown: they were played as long as the infant was not fussy. The experimental session was terminated when infants looked away from the screen during five consecutive trials. On average, 42 trials (range = 26–53), were presented to each infant, with no difference between number of congruent (N = 20.5) and incongruent (N = 21.4) trials, t (10) = −2, p = .07.
Gaze behavior recording and coding
Infants’ gaze behavior was scored with a computerized frame-by-frame observational coding system (40 ms resolution), enabling two independent coders, blind to the experimental hypothesis, to identify the exact time at which the infant’s pupil began moving horizontally, indicating gaze shift in the direction of the cue and toward/away from the target stimulus. Gaze shifts toward the target were coded to identify correct gaze behavior and to calculate the Saccadic Reaction Time (SRT: the elapsed time between the onset of the target and the onset of the infant’s gaze shift). STRs larger and shorter than 2 standard deviations were excluded. To calculate inter-coders reliability, the second observer coded gaze behavior in a sample of eight participants. Pearson correlation revealed a high degree of agreement between the two coders, r (8) = 0.99, p < .0001.
EEG recording and analysis
Continuous scalp EEG was recorded from a 128-channel HydroCel Geodesic SensorNet (Electrical Geodesic, Eugene, OR) that was connected to a NetAmps 300 amplifier (Electrical Geodesic, Eugene, OR) and referenced on-line to a single vertex electrode (Cz). Electrical signal was recorded from 124 of the 128 channels on the nets with the 4 eye recording electrodes removed to enhance infants’ tolerability of the net. Channel impedance was kept at or below 100 KΩ and signals were sampled at 500 Hz. EEG data were pre-processed off-line using EEGLAB (Delorme & Makeig, 2004). As a first step, data segments were filtered using a 0.3–30 Hz band-pass filter and re-referenced to average reference. The EEG signal was segmented to 350 ms post-stimulus onset, with a baseline period beginning 100 ms prior to target onset and baseline corrected using mean voltage during the 100 ms pre-stimulus period. Automated artifact detection was applied to the segmented data to detect individual epochs that showed >200μV voltage changes within the segment period. A linear interpolation was conducted to correct for any rejected channels using the five closest electrodes if there were less than 12 electrodes that were missing or had bad data. We marked as bad segments belonging to trials in which fast eye movements occurred, and we included in the analysis only trials in which infants’ gaze was aligned with the center of the screen at cue offset, as assessed by off-line coding. Segments in which correct gaze shifts occurred were marked as bad if the gaze shift started before the target off-set. Thus, segments with SRTs faster than 200 ms were marked as bad. Finally, we also marked as bad segments belonging to trials in which distractions occurred. Bad segments identified by either procedure were excluded from further analysis.
Since the design of the paradigm (i.e. spatial visual cueing with peripheral target) required infants to shift their eyes, saccades occurred during the task. To remove the effect of eye movements, an Independent Component Analysis (ICA) was done using the “extended runica” function in MATLAB. The analysis was performed on the EEG segmented data for each participant. 124 components were plotted based on the decreasing variance explained. All components were visually inspected and those containing eye movements were rejected in accordance with the 2d topographical plots. Those components with a positive/negative distribution suggesting a frontal dipole, i.e. eye movement, and those components with a frontal and posterior opposite polarity, i.e. eye blink, were excluded.
For each participant, average waveforms were generated within each experimental condition (congruent and incongruent separately for left-and right-sided targets) only if at least 7 artifact-free trials (de Klerk, Johnson & Southgate, 2015) collapsed across target side were overall available per condition. On average, the mean number of trials by which each infant contributed to the analysis was N = 10.75 (range: 7–19) for the congruent and the N = 11.5 (range 7–21) for incongruent condition, t (10) = −.949, p = .365.
Cortical Source Localization
Cortical source analysis was performed using the Fieldtrip toolbox (Oostenveld, Fries, Maris, Schoffelen, 2011) and in-house custom MATLAB scripts. The analysis was performed in four steps: 1) selection of MRI template; 2) construction of realistic head models: 3) definition of regions of interests (ROIs); 4) source reconstruction (i.e., current density reconstruction; CDR) (for details, see Xie and Richards, 2017). MRI selection was done referring to the Neurodevelopment MRI Database (Richards and Xie, 2015) by selecting the MRI average template for 6-month-old participants. The infant MRIs were segmented into: scalp, skull, cerebral spinal fluid, white matter, gray matter, nasal cavity and eyes (Richards, 2013). From the segmented head and electrode positions of the average template, the source volume and head volume were computed with Fieldtrip and the inverse filter with the eLORETA constraint (Pascual-Marqui, 2007; Pascual-Marqui, et al. 2011).
Twenty-three brain regions were chosen for ROI analysis based on past identification of dipoles responsible for generating scalp measurements (e.g., P1) in infant studies (i.e. Xie & Richards, 2017). These ROIs included the separate left and right volumes for the anterior fusiform gyrus, middle fusiform gyrus, medial inferior occipital lobe, lateral inferior occipital lobe, middle occipital lobe, superior occipital, parahippocampal gyrus, posterior inferior temporal gyrus, posterior middle-superior temporal gyri, and temporal pole (20 ROIs). A single bilateral ROI was used for the lingual gyrus, central occipital lobe, and parietal lobe (3 ROIs).
ERP data surrounding the P1 peak was used to estimate the current density amplitudes (i.e., CDR values) for every location in the source volume model. The CDR values were then summed over each source location in a ROI and divided by the total volume of the ROI. For statistical analysis, the CDR value was averaged with the time window (±10 ms) around the P1 peak.
Results
Saccadic Reaction Times (SRTs)
Infants included in the ERP target-locked analysis (N = 11) were considered for statistical analysis of behavioral data. On average, the percentage of correct gaze shifts (i.e. saccade toward spatial side where target appeared) was 70% (range = 60–80). No difference between congruent (39%) and incongruent (40%) trials was found, t (10) = −1.5, p = .19. Eighteen per cent of detected gaze shifts were identified as distractions (infants didn’t look at the monitor). Finally, there were a few spatial errors (saccade toward the spatial position where the target didn’t appear) and anticipations/delays (saccade stared before target appearing), i.e. overall 3.2%. A paired t-test between mean SRTs in congruent and incongruent trials was carried out. Infants’ SRTs were significantly faster in response to congruent trials (M = 285.7 ms, SD = 23.5) relative to incongruent trials (M = 313.9 ms, SD = 32.2), t (10) = 2.48, p = .004. In addition, a Wilcoxon non-parametric test between the same variables was performed Congruent vs. Incongruent Z = −2.49 p = .013. This analysis confirms previous parametric analysis. Analogous investigation was carried out for the entire sample of infants tested (N = 22). On average, the percentage of correct gaze shifts was 75.4% (range = 54–90%), with no difference between congruent (36.7%) and incongruent (38.7%) trials, t (21) = −1.5, p = .13. Twenty-one per cent of detected gaze shifts were identified as distractions; 3.2% were identified as spatial errors. A paired t-test was carried out between mean SRTs in congruent and incongruent trials. Infants were faster to orient their gaze in congruent trials (M = 281 ms, SD = 24,5) than in incongruent trials (M = 317ms, SD = 30,4), t (21) = −3.482 p = .002 (Fig. 2). We also performed Wilcoxon non-parametric test that confirms the difference between Congruent vs. Incongruent saccade latencies Z = −3–35 p = .001 in the entire sample.
Figure 2: STRs results.
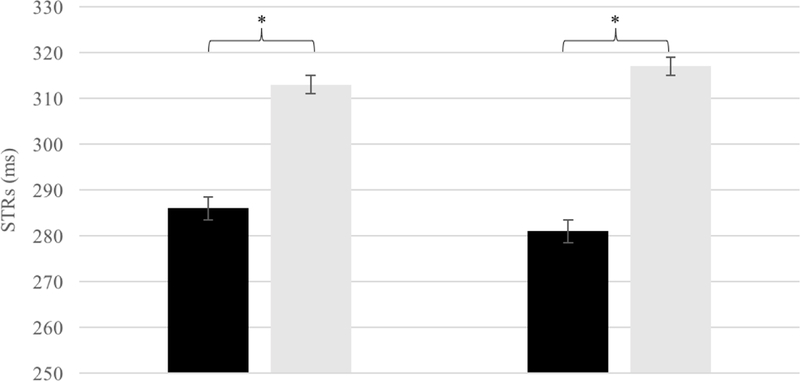
Saccadic reaction times. On the right behavioral data from all participants tested; on the left behavioral data for the sub-sample included in ERPs analysis. * p <.05
Target-locked P1 ERP component
Inspection of the grand-averaged waveforms revealed that the P1 ERP component was reliably elicited at target onset over occipital-parietal scalp sites. Based on visual inspection of both the grand-averaged and individual waveforms, 17 clusters of electrodes were created for the ERP target-locked analysis. According to 10–10 system of electrodes, clusters for O1, Oz, O2, I1, Iz, I2, PO7, PO8, PO9, PO10, P7, P8, P9, P10, TP7, TP8, Cz were created using the average of the closest electrodes (Table 1). Based on the grand-averaged and individual data, peak and mean amplitude of the P1 were extracted within a time window of 100 to 150 ms. This time window was chosen based on previous studies (Richards, 2000 and Xie & Richards, 2017) and on visual inspection of the data.
Table 1: Cluster of electrodes list.
On the left column, Cluster of electrodes created for ERP analysis in accordance with 10–10 system. On the right column, electrodes numbers selected from HGSN system to create clusters
| Clusters of Electrodes | Electrodes Number HGSN system |
|---|---|
| O1 | 66 70 71 |
| OZ | 71 75 76 |
| O2 | 76 83 84 |
| I1 | 69 73 74 |
| IZ | 74 81 82 |
| I2 | 82 88 89 |
| PO7 | 59 65 66 |
| PO8 | 84 90 91 |
| PO9 | 64 65 68 |
| PO10 | 90 94 95 |
| P7 | 51 58 59 |
| P8 | 91 96 97 |
| P9 | 57 63 64 |
| P10 | 95 99 100 |
| TP7 | 46 50 51 |
| TP8 | 97 101 102 |
| CZ | 7 31 55 80 106 |
Figure 3 shows the overall ERP responses in the occipital, parietal and parietal-occipital cluster. The peak of the P1 component approximately occurred 100–150 ms following target onset. The P1 component was larger for the congruent condition in the contralateral clusters (right part of Figure 3).
Figure 3: Grand Average plot.

Grand Average plot in occipital and parietal clusters: response to congruent condition is represented in black (solid), whereas response to incongruent condition is represented in red (dotdash)
Figure 4 shows the topographical activation on the scalp for both the congruent and incongruent experimental conditions: a distribution (positive in the back and negative in frontal areas) suggesting the presence of a source dipole that generates the P1 ERP component is evident. However, starting from 50 ms after target onset the activation is greater for the congruent relative to the incongruent condition.
Figure 4: Grand Average topographical potential maps.

Grand Average topoplot. Time labels indicate milliseconds after target stimulus onset. Response to congruent condition is represented in the upper part, whereas response to incongruent condition is represented in the lower part
A repeated measure ANOVA on the peak amplitude with Congruency (Congruent vs. Incongruent) and Electrodes as within subject factors was performed. This analysis revealed a main effect of Congruency, F (1,10) = 4.63, p = .057, η2p = .31, the main effect of Electrodes, F (16,160) = 1.61, p = .071, η2p = .13 and Congruency x Electrodes interaction was not significant, F (16,160) = .878, p = .596, η2p = .08. Overall the amplitude of the P1 component was larger for congruent (4.94 μV) than incongruent trials (3.46 μV). The same analysis was performed on the mean amplitude and no significant effects were found.
To examine whether the trend towards significance (p = .057) in the P1 peak amplitude between congruent and incongruent conditions reflected a real effect, we performed an explorative paired t-test, for each cluster. The analyses revealed a significant effect as a function of congruency both in PO10 and P10 clusters. The P1 component was larger for congruent (6.39 μV) than incongruent trials (1.93 μV) in PO10, t (10) = .3.303 p = .008 and in P10 t (10) = 2.707, p = .022 (congruent trials = 3.48 μV, incongruent trials = - 0.86 μV). Additionally, a Wilcoxon non-parametric test between the same variables for the same clusters was carried out. The analysis confirms the preference of a significant effect both in PO10 Congruent vs. Incongruent Z = −2.401 p = .016 and P10 Congruent vs. Incongruent Z = −1.956 p = .050.
Finally, to further investigate the idea that walking direction can facilitate processing of peripheral information we performed an explorative Pearson correlation on the relation between infants’ SRTs (quantified as difference in SRTs between congruent and incongruent conditions) and the P1 ERP effect (quantified as difference in the P1 peak amplitude between congruent and incongruent conditions). This test was not statistically significant, r = .51, p = .107. Despite the lack of statistical significance, the effect size of this correlation was large (Cohen, 1988). Additionally, 8 out of 11 infants exhibited faster saccadic reaction times and a greater P1 component for congruent relative to incongruent trials.
Cortical Source Analysis
Cortical source analysis focused on the mean current density reconstruction amplitude (CDR value) in the ROIs that were possible cortical sources of the P1. The CDR values around the P1 ERP peak latency (140 ms) were examined in the ROIs for the “congruency effect”. Two ROIs had a significant congruency effect: parahippocampal gyrus and the anterior fusiform gyrus. The congruency effect was significant in the ROIs contralateral to the target position. The congruency effect was not significant in the inferior occipital lobe or in the lateral and medial occipital lobes. Figure 5 shows the CDR values during the time period surrounding the P1 peak (+48 ms/ −48ms) for the congruent and incongruent conditions in the two ROIs showing a significant effect. The congruent condition had larger CDR values (dotted blue line) starting from 16 ms before the P1 peak. Approximately around 30 ms after the P1 peak, the difference between two conditions is greater. Figure 6 shows a bar graph of the CDR values for the two conditions in the two ROI showing a significant effect. The error band indicate that the average CDR values were greater in the congruent than the incongruent condition.
Figure 5:

Line figures of CDR value, Line figures of CDR value from −48 ms to 48 ms around the P1 peak latency. Anterior fusiform gyrus and parahippocampal gyrus are the two ROIs that show significant congruency effect. Both these areas are contralateral to target position.
Figure 6: Bar graph of the CDR value.
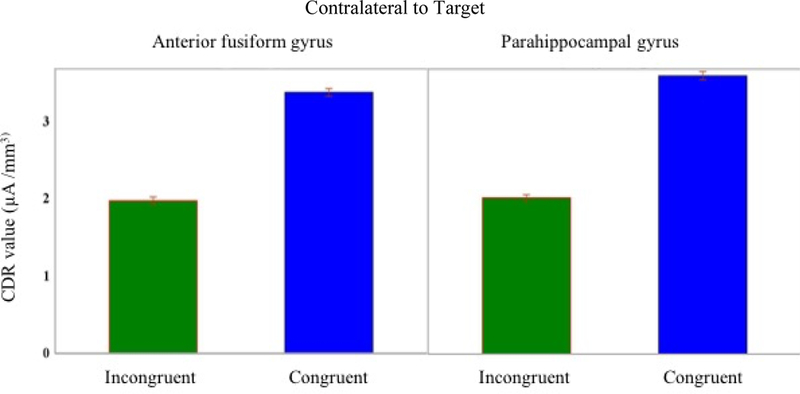
Bar graph of the CDR value in the two areas that show a congruency effect.
Figure 7 shows the brain areas involved as cortical sources of the P1 component. The 2D images depicted in panel a), b), c); show the difference between the congruent and the incongruent conditions. Figure 7, panel d) is a 3D plot depicting the difference between the congruent and incongruent conditions in those brain areas showing a significant effect of the congruency and imply these areas were the cortical sources of the congruency effect in the P1 ERP component.
Figure 7: 2D and 3D plots of brain areas involved in P1 congruency effect.

2D and 3D maps of the Congruency effect (different plot: activation for congruent condition minus activation for the incongruent condition) surrounding the P1 peak in brain source. Panel a, b, c, are 2D coronal, sagittal and axial plots. Panel d), is the 3D plot of the brain areas mainly involved to originate P1 congruency effect. All these data are plotted in an average 6-months-old brain template.
Discussion
The goal of the present study was to explore the neural mechanism underlying the orienting of attention cued by a type of biological motion like the one of a vertebrate walking on the legs. To this aim we made use of a spatial cueing paradigm and recorded EEG in 6-month-old infants. Infants were presented with a spatially non-predictive PLDs of a human walker, followed by a peripheral target. Behavioral and ERPs data were analyzed as a function of the congruency relation between cue and target. As in the previous behavioral and EEG infants’ studies (i.e. Bardi et al., 2015; Farroni et al., 2000; Natale et al., 2016), in the present experiment only congruent and incongruent trials were presented. The absence of neutral trials renders it impossible to differentiate facilitatory effects produced by congruent trials from inhibitory effects produced by incongruent trials. However, this distinction is scarcely relevant in the context of the present research, since our goal was to investigate the neural correlates underpinning visuo-spatial orienting triggered by biological motion. All comparisons were thus carried out between congruent and incongruent trials.
Behavioral results from this experiment replicated eye-tracking evidence coming from Bardi and colleagues (2015), confirming the role of upright biological motion walking direction in triggering visuo-spatial orienting. More specifically, data on infants’ behavior indicate that upright walking direction can facilitate SRTs in response to peripherally presented targets appearing at congruent relative to incongruent spatial locations. This advantage in SRTs might be explained by hypothesizing that the PLD walker triggers covert orienting of attention towards walking direction, yielding a facilitation of oculomotor responses to stimuli appearing at the attended location (Posner, 1980). Accordingly, behavioral results from this study confirm the hypothesis that upright biological motion walking direction of a human agent can influence mechanisms of visuo-spatial orienting, at the same time suggesting that such mechanisms may be associated to a functional processing advantage.
Electrophysiological evidence coming from our study demonstrates for the first time that the apparent motion of a legged vertebrate determines a sensory gain effect, yielding a facilitation in processing stimuli coming from the side of the space congruently cued. Importantly, our findings demonstrate that walking direction triggers a covert orienting of attention which, in turn, produces facilitation in cortical areas involved in processing visual information. This functional processing advantage precedes the oculomotor response. These effects resemble those previously found with pure peripheral cues. Differently from previous studies on infants’ covert attention that employed social cues (Natale et al., 2016), here we found a modulation of an early ERP sensory component, namely the P1. Therefore, this study provides the first evidence that sensory facilitation in processing peripheral information is present even when a central non-predictive cue, such as the walking direction of a legged vertebrate, is employed.
Our ERP evidence supports behavioral data and demonstrates that the attentional effect occurs during the initial stages of visual processing. Indeed, target-locked ERP analyses pointed to the existence of an attentional effect indexed by a greater P1 peak amplitude in response to congruent rather than incongruent trials. The P1 attentional effect indicates that the information provided by upright biological motion walking direction can yield a gain control or selective amplification of sensory information in the extra-cortical visual pathways, improving the signal-to-noise ratio so that more information is extracted from relevant portions of the visual field. The P1 attentional effect, obtained in the present study by employing a PLD walker as a central cue, converges with evidence from developmental studies investigating the ERP correlates of covert shifts of attention triggered by pure peripheral cues (Richards, 2000, 2005). These studies have reported an analogous enhancement of the P1 component for congruent relative to incongruent trials in the absence of overt shifts of fixation. As in these studies, also in our study infants’ fixation remained at central location until the target was presented.
Even though in the literature, as reported by Richards (2000) and Natale et al. (2017), the attentional effect is also associated to the N1 component, we decided to analyze only the P1 component for a methodological reason. Since the peak of the N1 in our sample occurs around 250–300 ms, this sensory component in this case might be affected by the offset of the target (target duration =200 ms). An offset of a visual stimulus may have an effect on the sensory components. Therefore, the N1 component was not included in the analysis.
Results, coming from cortical source analysis, confirm that infants manifest a facilitation in processing visual information when this appears at the cued spatial position. In the present study the brain areas that are involved in generating the P1 congruency effect i.e., parahippocampal gyrus and the anterior fusiform gyrus, are located in regions different from those found in previous studies (Richards 2005; Xie & Richards 2017). The brain areas involved are visual areas, therefore demonstrating that the facilitation effect has direct consequences on visual processing. The differences between this and previous studies might be explained by the nature of the cue. Whereas in previous studies (Richards 2005; Xie & Richards 2017) a peripheral cue was used, in this study a central salient cue was used. Altogether, data from both previous studies and the present study suggest that, when the ERPs converge at the scalp level, the nature of the cue affects the brain areas originating the ERP components. In accordance with the adult literature, social stimuli (i.e. eye gaze) seem to rely on specific brain areas compared to non-social stimuli (i.e. arrows) when they act as a cue in spatial cueing paradigms. For example, Hietanen and colleagues (2006), demonstrated that eye gaze activates a more specific area (e.g. temporal and parietal areas, i.e. STS) as compared to arrows, which activate instead broader brain areas. Additionally, evidence has indicated that biological motion information is processed in the superior temporal sulcus (STS, Shiffrar, 1994).
Overall, behavioral and neural evidence coming from this study converge in indicating that biological motion PLDs walking direction triggers automatic visuo-spatial orienting in 6-month-old infants and that effects in oculomotor behavior are associated with functionally significant brain effects. Indeed, the ability to extract such information appears very early in development (Bardi et al., 2015; Kuhlmeier et al., 2010). This ability is presumably based on a predisposition to some general properties characterizing biological motion (Simion et al., 2008). Consequently, it is sensible to hypothesize that this ability may gradually cascade into a wide corpus of skills, from visuo-spatial orienting, to action processing and intention understanding.
The evidence presented in this paper supports the “Directed Attention model” proposed by Reid and Striano (2007) according to which biological motion is one of the key aspects of infants’ detection and identification of socially relevant organisms in the environment. Our results are in line with the hypothesis that, since birth, our species might be endowed with a subcortical mechanism to detect the spatiotemporal parameters of biological motion, (Troje & Westhoff, 2006). Based on the information available through this subcortical mechanism, a second cortical global processing system would become specialized for the recognition of agents’ identity (Chang e Troje 2009).
One possibility is that the sensitivity to spatiotemporal parameters results from newborns’ experience with their own body. Since birth, humans possess a stepping reflex, thus suggesting that the walking schema is present in the motor repertoire very early in life. In light of this evidence, we hypothesize that the motor schema of walking might be the base on which the mechanism to detect biological motion is built during the prenatal life. According to the embodied simulation theory, even if at 6 months of age infants cannot walk independently, they nonetheless possess a schema of walking in their motor repertoire. This schema might act as bias scaffolding infants’ ability to extract the information of directionality conveyed by a PLD of biological motion. Further, it might affect infants’ ability to orient attention in accordance to walking direction (Gallese, Rochat, Cossu, & Sinigaglia, 2009; Frankenhuis & Barret, 2013). Future studies should empirically test this hypothesis.
Overall, our work demonstrates for the first time that the information cued by the apparent motion of a legged vertebrate triggers a covert orienting of visual spatial attention in 6-month-old infants. This covert orienting of attention yields faster saccadic reaction times to targets appearing at congruent relative to incongruent spatial locations. More intriguingly, our ERP evidence shows that facilitation in processing sensory information (i.e. priming effects) presented at the cued spatial location occurs before the onset of the oculomotor response. In line with the adult literature making use of eye gaze as an attentional cue (e.g., Schuller & Rossion, 2001; Schuller & Rossion, 2005), our study provides the first developmental evidence that a modulation of the P1 component occurs in a Posner’s cueing paradigm even when a central rather than peripheral cue is employed (Richards, 2000). Cortical source analysis further supports this evidence, indicating that the congruency effect of the P1 component is generated in anterior fusiform gyrus and parahippocampal gyrus.
Research highlights.
This study investigated the neural correlates of the attentional effect accompanying mechanisms of visuo-spatial orienting triggered by upright biological motion walking direction in 6-month-old infants.
Behaviorally, infants’ saccade latencies are shorter for congruent targets that appeared in the position signaled by walking direction rather than for incongruent targets.
ERPs show that biological motion can trigger infants’ covert orienting of attention, enabling enhancement of the peak amplitude of the P1 component related to visual processing of potentially relevant information.
Visual brain areas are mainly involved as cortical source of the P1 component suggesting that infants’ orienting of attention have direct consequences on visual processing.
Acknowledgments
The authors would like to thank the parents of the infants who took part in the study. We also like to thank Paola Sessa to the help during data processing and Carlo Arrigo Umiltà for the comments during the writing-up phase of the manuscript. This work was supported by PRIN grant, founded by the Ministero dell’Istruzione dell’Università e della Ricerca (MIUR) awarded to Francesca Simion (20152M5A5J).
References
- Bakker M, Daum MM, Handl A, & Gredebäck G (2014). Grasping the difference through experience! Neurophysiological correlates of action perception during the onset of grasping. Social Cognitive and Affective Neuroscience, 10, 769–776. 10.1093/scan/nsu119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardi L, Di Giorgio E, Lunghi M, Troje NF, & Simion F (2015). Walking direction triggers visuo-spatial orienting in 6-month-old infants and adults: An eye tracking study. Cognition, 141, 112–120. 10.1016/j.cognition.2015.04.014 [DOI] [PubMed] [Google Scholar]
- Bardi L, Regolin L, & Simion F (2011). Biological motion preference in humans at birth: role of dynamic and configural properties. Developmental Science, 14(2), 353–359. doi: 10.1111/j.1467-7687.2010.00985.x [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S (1994). How to build a baby that can read minds: Cognitive mechanisms in mindreading. Cahiers de Psychologie/Current Psychology of Cognition, 13, 513–552. [Google Scholar]
- Bertenthal BI, Proffitt DR, & Cutting JE (1984). Infant sensitivity to figural coherence in biomechanical motions. Journal of experimental child psychology, 37(2), 213–230. 10.1016/0022-0965(84)90001-8 [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Proffitt DR, & Kramer SJ (1987). Perception of biomechanical motions by infants: implementation of various processing constraints. Journal of Experimental Psychology: Human Perception and Performance, 13(4), 577 10.1037/0096-1523.13.4.577 [DOI] [PubMed] [Google Scholar]
- Bonato M, Priftis K, Marenzi R, & Zorzi M (2009). Normal and impaired reflexive orienting of attention after central nonpredictive cues. Journal of Cognitive Neuroscience, 21(4), 745–759. 10.1162/jocn.2009.21054 [DOI] [PubMed] [Google Scholar]
- Chang DH, & Troje NF (2009). Characterizing global and local mechanisms in biological motion perception. Journal of Vision, 9(5), 8–8. doi: 10.1167/9.5.8 [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of neuroscience methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dittrich WH (1993). Action categories and the perception of biological motion. Perception, 22(1), 15–22. 10.1068/p220015 [DOI] [PubMed] [Google Scholar]
- Dittrich WH, Troscianko T, Lea SE, & Morgan D (1996). Perception of emotion from dynamic point-light displays represented in dance. Perception, 25(6), 727–738. 10.1068/p250727 [DOI] [PubMed] [Google Scholar]
- de Klerk CC, Johnson MH, & Southgate V (2015). An EEG study on the somatotopic organisation of sensorimotor cortex activation during action execution and observation in infancy. Developmental cognitive neuroscience, 15, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J IV, Davis G, Ricciardelli P, Kidd P, Maxwell E, & Baron-Cohen S (1999). Gaze perception triggers reflexive visuospatial orienting. Visual cognition, 6(5), 509–540. 10.1080/135062899394920 [DOI] [Google Scholar]
- Farroni T, Johnson MH, Brockbank M, & Simion F (2000). Infants’ use of gaze direction to cue attention: The importance of perceived motion. Visual Cognition, 7(6), 705–718. 10.1080/13506280050144399 [DOI] [Google Scholar]
- Frankenhuis WE, & Barrett HC (2013) Design for Learning: The Case of Chasing In Rutherford MD & Kuhlmeier VA(Eds.), Social perception: Detection and interpretation of animacy, agency, and intention (pp. 171–195). MIT Press. [Google Scholar]
- Frischen A, Bayliss AP, & Tipper SP (2007). Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin, 133, 694–724. doi: 10.1037/0033-2909.133.4.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V, Rochat M, Cossu G, & Sinigaglia C (2009). Motor cognition and its role in the phylogeny and ontogeny of action understanding. Developmental psychology, 45(1), 103–113. doi: 10.1037/a0014436 [DOI] [PubMed] [Google Scholar]
- Gredebäck G, & Daum MM (2015). The microstructure of action perception in infancy: Decomposing the temporal structure of social information processing. Child Development Perspectives, 9, 79–83. doi: 10.1111/cdep.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredebäck G, Melinder AMD, & Daum MM (2010). The development and neural basis of pointing comprehension. Social Neuroscience, 5, 441–450. 10.1080/17470910903523327 [DOI] [PubMed] [Google Scholar]
- Hietanen JK, Nummenmaa L, Nyman MJ, Parkkola R, & Hämäläinen H (2006). Automatic attention orienting by social and symbolic cues activates different neural networks: An fMRI study. Neuroimage, 33(1), 406–413. 10.1016/j.neuroimage.2006.06.048 [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Vogel EK, & Luck SJ (1998). Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philosophical Transactions of the Royal Society B: Biological Sciences, 353(1373), 1257–1270. doi: 10.1098/rstb.1998.0281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Chang DH, Saunders DR, & Troje NF (2011). Body configuration modulates the usage of local cues to direction in biological-motion perception. Psychological Science, 22(12), 1543–1549. 10.1177/0956797611417257 [DOI] [PubMed] [Google Scholar]
- Hirai M, Saunders DR, & Troje NF (2011). Allocation of attention to biological motion: Local motion dominates global shape. Journal of Vision, 11(3), 4–4. doi: 10.1167/11.3.4 [DOI] [PubMed] [Google Scholar]
- Johansson G (1973). Visual perception of biological motion and a model for its analysis. Perception & psychophysics, 14(2), 201–211. [Google Scholar]
- Johnson MH (2006). Biological motion: a perceptual life detector?. Current Biology, 16(10), R376–R377. 10.1016/j.cub.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Kuhlmeier VA, Troje NF, & Lee V (2010). Young infants detect the direction of biological motion in point‐light displays. Infancy, 15(1), 83–93. doi: 10.1111/j.1532-7078.2009.00003.x [DOI] [PubMed] [Google Scholar]
- Moore DG (2012). Understanding of human motion, form and levels of meaning: evidence from the perception of human point light displays by infants and people with autism. In Slaughter V, Brownell CA, Early development of body representations; (122–145), Cambridge. [Google Scholar]
- Müller HJ, & Rabbit PMA (1989). Reflexive and voluntary orienting of visual attention: Time course of activation and resistance to interruption. Journal of Experimental Psychology: Human Perception and Performance, 15, 315–330. 10.1037/0096-1523.15.2.315 [DOI] [PubMed] [Google Scholar]
- Natale E, Addabbo M, Marchis IC, Bolognini N, Macchi Cassia V., & Turati C (2016). Action priming with biomechanically possible and impossible grasps: ERP evidence from 6-month-old infants. Social neuroscience, 1–10. 10.1080/17470919.2016.1197853 [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM (2011). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience, 2011, 1. doi: 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui RD (2007). Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv:0710.3341 [Google Scholar]
- Pascual-Marqui RD, Lehmann D, Koukkou M, Kochi K, Anderer P, Saletu B, Tanaka H, Hirata K, John ER, Prichep L, Biscay-Lirio R, & Kinoshita T (2011). Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences, 369(1952), 3768–3784. doi: 10.1098/rsta.2011.0081 [DOI] [PubMed] [Google Scholar]
- Pavlova M, & Sokolov A (2000). Orientation specificity in biological motion perception. Attention, Perception, & Psychophysics., 62(5), 889–899. 10.3758/BF03212075 [DOI] [PubMed] [Google Scholar]
- Posner MI (1980). Orienting of attention. Quarterly journal of experimental psychology, 32(1), 3–25. 10.1080/00335558008248231 [DOI] [PubMed] [Google Scholar]
- Posner MI (2016). Orienting of attention: then and now. Quarterly journal of experimental psychology, 69(10), 1864–1875. 10.1080/17470218.2014.937446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid VM, & Striano T (2007). The directed attention model of infant social cognition. European Journal of Developmental Psychology, 4(1), 100–110. 10.1080/17405620601005648 [DOI] [Google Scholar]
- Reid VM, Hoehl S, & Striano T (2006). The perception of biological motion by infants: An event-related potential study. Neuroscience letters, 395(3), 211–214. 10.1016/j.neulet.2005.10.080 [DOI] [PubMed] [Google Scholar]
- Richards JE (2000). Localizing the development of covert attention in infants with scalp event-related potentials. Developmental psychology, 36(1):91–108. 10.1037/0012-1649.36.1.91 [DOI] [PubMed] [Google Scholar]
- Richards JE (2005). Localizing cortical sources of event-related potentials in infants’ covert orienting. Developmental Science, 8(3):255–278. doi: 10.1111/j.1467-7687.2005.00414.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE (2013). Cortical sources of ERP in prosaccade and antisaccade eye movements using realistic source models. Frontiers in systems neuroscience, 7:27. doi: 10.3389/fnsys.2013.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE, & Xie W (2015). Brains for all the ages: Structural neurodevelopment in infants and children from a life-span perspective In: Benson J (ed) Advances in child development and behavior, vol 48 Elsevier, Philadephia, pp 1–52 [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, & Umiltá C (1987). Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia, 25(1), 31–40. 10.1016/0028-3932(87)90041-8 [DOI] [PubMed] [Google Scholar]
- Schuller AM, & Rossion B (2001). Spatial attention triggered by eye gaze increases and speeds up early visual activity. Neuroreport, 12(11), 2381–2386. [DOI] [PubMed] [Google Scholar]
- Schuller AM, & Rossion B (2005). Spatial attention triggered by eye gaze enhances and speeds up visual processing in upper and lower visual fields beyond early striate visual processing. Clinical neurophysiology, 116(11), 2565–2576. 10.1016/j.clinph.2005.07.021 [DOI] [PubMed] [Google Scholar]
- Senju A, Johnson MH, & Csibra G (2006). The development and neural basis of referential gaze perception. Social neuroscience, 1(3–4), 220–234. 10.1080/17470910600989797 [DOI] [PubMed] [Google Scholar]
- Shi J, Weng X, He S, & Jiang Y (2010). Biological motion cues trigger reflexive attentional orienting. Cognition, 117(3), 348–354. 10.1016/j.cognition.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrar M (1994). When what meets where. Current Directions in Psychological Science, 3(3), 96–101. 10.1111/1467-8721.ep10770450 [DOI] [Google Scholar]
- Simion F, Regolin L, & Bulf H (2008). A predisposition for biological motion in the newborn baby. Proceedings of the National Academy of Sciences, 105(2), 809–813. doi: 10.1073/pnas.0707021105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi S (1984). Upside-down presentation of the Johansson moving light-spot pattern. Perception, 13(3), 283–286. 10.1068/p130283 [DOI] [PubMed] [Google Scholar]
- Troje NF (2002). Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. Journal of Vision, 2, 371–387. doi: 10.1167/2.5.2 [DOI] [PubMed] [Google Scholar]
- Troje NF (2008). Retrieving information from human movement patterns In Shipley TF & Zacks JM (Eds.), Understanding events: How humans see, represent, and act on events (pp. 308–334). Oxford, England: Oxford University Press. [Google Scholar]
- Troje NF, & Westhoff C (2006). The inversion effect in biological motion perception: Evidence for a “life detector”?. Current Biology, 16(8), 821–824. 10.1016/j.cub.2006.03.022 [DOI] [PubMed] [Google Scholar]
- Vogel EK, & Luck SJ (2000). The visual N1 component as an index of a discrimination process. Psychophysiology, 37(2), 190–203. [PubMed] [Google Scholar]
- Wang L, Yang X, Shi J, & Jiang Y (2014). The feet have it: Local biological motion cues trigger reflexive attentional orienting in the brain. NeuroImage, 84, 217–224. 10.1016/j.neuroimage.2013.08.041 [DOI] [PubMed] [Google Scholar]
- Xie W, & Richards JE (2017). The relation between infant covert orienting, sustained attention and brain activity. Brain Topography, 30(2), 198–219. 10.1007/s10548-016-0505-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


