Abstract
Neuropathy, typically diagnosed by the presence of either symptoms or signs of peripheral nerve dysfunction, remains a frequently reported complication in the antiretroviral (ART)-treated HIV population. This study was conducted in 109 healthy controls and 57 HIV-infected individuals to investigate CNS regions associated with neuropathy.
An index of objective neuropathy was computed based on 4 measures: deep tendon ankle reflex, vibration sense (great toes), position sense (great toes), and 2-point discrimination (feet). Subjective neuropathy (self-report of pain, aching, or burning; pins and needles; or numbness in legs or feet) was also evaluated. Structural MRI data were available for 126/166 cases.
The HIV relative to the healthy control group was impaired on all 4 signs of neuropathy. Within the HIV group, an objective neuropathy index of 1 (bilateral impairment on 1 measure) or 2 (bilateral impairment on at least 2/4 measures) was associated with older age and a smaller volume of the cerebellar vermis. Moderate to severe symptoms of neuropathy were associated with more depressive symptoms, reduced quality of life, and a smaller volume of the parietal precuneus.
This study is consistent with the recent contention that ART-treated HIV-related neuropathy has a CNS component. Distinguishing subjective symptoms from objective signs of neuropathy allowed for a dissociation between the precuneus, a brain region involved in conscious information processing and the vermis, involved in fine tuning of limb movements.
Keywords: magnetic resonance imaging (MRI), position sense, vibration, reflex, aesthesiometer
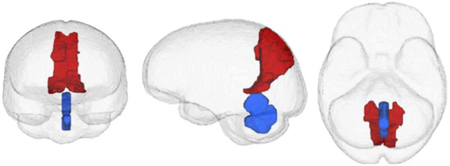
GRAPHICAL ABSTRACT:
In HIV patients, objective signs of neuropathy correlated with smaller cerebellar vermis (red) volumes whereas subjective symptoms of neuropathy were associated with smaller precuneus (blue) volumes.
INTRODUCTION
Peripheral sensory neuropathy is a persisting and prevalent (15-65%)(Ghosh et al. 2012) HIV-associated disturbance in the post-ART era, which may contribute to gait and balance deficits (Ducic et al. 2004), reduced quality of life (Pandya et al. 2005), and increased morbidity and mortality. Contributing factors to HIV-related peripheral neuropathy include older age (e.g., Chen et al. 2013; Saylor et al. 2017), ART toxicity (Adoukonou et al. 2017; Benevides et al. 2017), substance abuse (Morgello et al. 2004), and alcoholism (Chopra and Tiwari 2012).
Excluding the gold standards of invasive nerve biopsy or costly and complex nerve conduction studies (e.g., Asad et al. 2010; Feng et al. 2009; Nelson et al. 2006; Perkins et al. 2001; Ruhdorfer et al. 2015; Sommer 2018), clinical guidelines for the diagnosis of peripheral neuropathies require “the presence of symptoms and/or signs of peripheral nerve dysfunction” (Boulton 1998). Symptoms described can include burning or deep aching pain, stabbing sensations, or numbness, commonly experienced in the lower limbs and feet. Objective neurological signs of neuropathy can include decreased or absent ankle reflexes, decreased distal vibratory sensation, reduced pain or temperature sensation, and muscle weakness (e.g., Cornblath et al. 1999; Kaku and Simpson 2014). Combinations of more than one objective test have an 87% sensitivity for detecting neuropathy in diabetes (Boulton 1998).
Pain is the most distressing symptom of peripheral neuropathy and is often the main reason patients seek medical attention (cf., Tesfaye et al. 2013). Neuropathic pain intensity, however, is not fully explained by the extent of damage to peripheral nerve fibers (Aziz-Donnelly and Harrison 2017; Cherry et al. 2003). Although long-considered a disease of the peripheral nervous system, there is now increasing evidence for Central Nervous System (CNS) involvement in peripheral neuropathy (Keltner et al. 2017; Keltner et al. 2014; Pfefferbaum et al. 2009; Selvarajah et al. 2011; Tesfaye et al. 2016). Several neuroimaging studies in chronic diabetes have incidentally reported CNS correlates of neuropathy (e.g., de Bresser et al. 2010; Frokjaer et al. 2013; Lunetta et al. 1994; Manor et al. 2012). Diabetes with relative to without neuropathy has been associated with gray matter volume loss in somatosensory cortex, supramarginal gyrus, and cingulate gyrus (Selvarajah et al. 2014). Indeed, volume loss of the cingulate cortex, implicated in affective processing of pain (Absinta et al. 2012; Buckalew et al. 2008; Coppieters et al. 2017), is smaller in patients with chronic neuropathic pain (Sugimine et al. 2016) and small-fiber neuropathy (Hsieh et al. 2015). Similarly, the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group reported that more intense symptoms of distal neuropathic pain in HIV are associated with a smaller posterior cingulate volume (Keltner et al. 2017). Together, the extant literature suggests that symptomatic neuropathic pain is associated with compromised integrity of the cingulate cortex.
Involvement of the thalamus in nociception has long been hypothesized based on loss of pain and temperature sensation observed in those with lesions of the thalamus (Dejerine and Roussy 1906; Garcin and Lapresle 1954; Head and Holmes 1911; Schuster 1937). Recently, performance on 2-point discrimination in healthy controls was associated with volume of the thalamus (Schmidt-Wilcke et al. 2018).
The current study aimed to determine CNS regions associated with objective and subjective peripheral neuropathy. A neuropathy index was based on 4 objective signs of bilateral lower limb impairment including perception of vibration (great toes), deep tendon ankle reflexes, position sense (great toes), and 2-point discrimination (feet). Subjective symptoms of neuropathy were considered independently. It was hypothesized that a greater incidence of objective signs would be associated with smaller thalamic volume, whereas greater intensity of subjective symptoms would be associated with a smaller posterior cingulate volume.
MATERIALS AND METHODS
Participants
The Institutional Review Boards of Stanford University and SRI International approved this study. Written informed consent was obtained from all participants in accordance with the Declaration of Helsinki by the signing of consent documents in the presence of staff after staff ensured that each participant understood the information provided and appreciated the reasonably foreseeable consequences of study participation. This study sample included 109 controls (45 women, 64 men, 51.7±14.3 years) and 59 participants with HIV infection (HIV: 17 women, 40 men, 53.3±6.9 years).
HIV patients, with a Karnofsky score (Karnofsky 1949) over 90 at study entry, were referred from local outpatient and treatment centers, or recruited during presentations in clinics by project staff and by distribution of flyers at community events. Comparison participants were recruited from the local community by referrals and flyers. All participants were screened using the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1998), structured health questionnaires, and a semi-structured timeline follow-back interview to quantify lifetime alcohol consumption (Skinner and Sheu 1982). Upon initial assessment, subjects were excluded if they had a significant history of medical (e.g., epilepsy, stroke, multiple sclerosis, uncontrolled diabetes, or loss of consciousness > 30 minutes), psychiatric (i.e., schizophrenia or bipolar I disorder), neurological disorders (e.g., neurodegenerative disease), or recent (i.e., past 3 months) alcohol or substance dependence. Modified from the guidelines of an initial report (Antinori et al. 2007), a standardized score for HIV-related qualify of life was based on responses to the Brief Health and Functioning (Bozzette et al. 1995) and Activities of Daily Living questionnaires (Pfeffer et al. 1982) and Global Assessment of Functioning (i.e., GAF) (Endicott et al. 1976).
Evaluation of Peripheral Neuropathy
Four trained and calibrated laboratory personnel (2 PhDs (among them NMZ), 1 RN, and 1 MA with medical training) conducted the examination. Self-report (i.e., subjective) of neuropathy was recorded after questioning participants regarding the bilateral severity (from 1 – 10) of a) pain, aching, or burning; b) pins and needles; or c) numbness in legs or feet and scored as follows: 0 = absence of symptoms, 1 = mild symptoms (1-3), 2= moderate to severe symptoms (4-10).
The four objective signs of lower limb neuropathy evaluated included:
perception of vibration (right or left great toe) normal = 0, impaired = 1: perception of vibration <10 seconds
deep tendon ankle reflexe (right or left ankle) normal = 0, impaired = 1: absent or hypoactive ankle reflex
position sense (right or left great toe) normal = 0, impaired = 1: one or more errors during evaluation
2-point discrimination (right or left soles of feet): a 3-point aesthesiometer was used to determine minimal distance detected between 2 points (e.g., Corkin et al. 1970; Periyasamy et al. 2008). Raw scores were transformed to age-corrected Z-scores. normal = 0, impaired = 1: ≥2 standard deviations from Z-scored control mean.
The peripheral neuropathy (PN) index was tallied as follows: participants were given a score of zero if they were not impaired or had only unilateral impairment on any one of the 4 measures; a score of 1 was assigned for bilateral impairment on any 1 of the 4 measures; and a score of 2 was given for bilateral impairment on at least 2 of the 4 measures.
Quantitative Ataxia Testing
Standing balance was assessed using an ataxia battery (stand heel to toe, walk 10 steps on line, balance on one leg) in eyes open and eyes closed conditions. Age-corrected Z scores were summed for the eyes open and closed conditions separately (Sullivan et al. 2000).
Laboratory Measures
Blood samples (~40cc) were collected and analyzed by Quest Diagnostics for complete blood count with differential, comprehensive metabolic panel including liver enzymes), HIV and hepatitis C virus (HCV) screening.
MRI Acquisition and Analysis
Image Acquisition
MRI data of the brain were acquired on a 3 Tesla GE whole body MR system (General Electric Healthcare, Waukesha, WI) using an 8-channel phased-array head coil. Regional brain volume analyses were based on axial, T1-weighted, Inversion-Recovery Prepared SPGR images (TR=6.55/5.92 ms, TE=1.56/1.93 ms, TI=300ms, matrix = 256×256, thick=1.25 mm, skip=0 mm, 124 slices, field of view (FOV)=24cm). Routine phantom data were used to evaluate spatial fidelity. Drift was corrected by adjusting scanner calibration parameters when necessary to maintain spatial stability within manufacturer guidelines. Imaging data were available for 52 of the 57 HIV subjects.
Image Processing
MRI data were processed using an in-house pipeline (Pfefferbaum et al. 2018). Preprocessing of T1-weighted MRI data involved noise removal (Coupe et al. 2008) and segmentation of brain masks. To generate brain tissue segmentations (gray matter, white matter, and cerebrospinal fluid), average intensity maps were rigidly aligned via ANTS (Avants et al. 2008) before segmentation via Atropos (Avants et al. 2011). Brain tissue was parcellated into regions defined by the SRI24 atlas (Rohlfing et al. 2010) by non-rigidly registering the atlas to the average intensity map via ANTS (Avants et al. 2008). Gray matter volume was quantified for 23 bilateral supratentortial, 5 bilateral infratentorial (including 3 cerebellar regions, vermis, and pons), and 7 bilateral subcortical regions (amygdala, caudate, putamen, pallidum thalamus, parahippocampus, and hippocampus); volumes of corpus callosum, third and lateral ventricles were also quantified (resulting in 38 quantified regions). All brain volumes assessed were corrected for age and supratentorial brain volume corrected (Pfefferbaum et al. 2018).
Statistical Analysis
All statistics were performed using JMP Pro 13.2.0 (SAS Institute Inc. 2016). Two-group differences in the 4 objective measures, the PN index, and subjective neuropathy were assessed using Chi-square tests. Group differences in ataxia measures were evaluated by t-test. Relationships between subjective and objective neuropathy measures with demographic, HIV-related, laboratory, or brain volumes variables were assessed using a 3-group (e.g., NP index score of 0, 1, or 2; subjective symptom score of 0, 1, or 2) analysis of variance (ANOVAs). A nominal logistic regression was conducted to determine the percent of neuropathy (subjective and objective) explained by significantly associated variables. Finally, a data-driven approach including scores on the 4 objective tests, the PN index, subjective neuropathy, and variables associated with the latter two measures were entered into a JMP-based cluster analysis.
RESULTS
Study Participants
Table 1 presents demographic data demonstrating that uninfected-control and HIV-infected participants were matched with respect to age, sex, handedness, body mass index (BMI), systolic and diastolic blood pressure, and heart rate. The HIV relative to the control group had a higher percentage of African-Americans, lower education and socioeconomic status (SES) (Hollingshead 1975), a higher percentage of smokers and individuals with diabetes, more depressive symptoms [as assessed with the Beck Depression Inventory-II (BDI-II) (Beck et al. (1996))], and lower scores on the Dementia Rating Scale (DRS) (Mattis 1998). In addition to traditional indices (length of infection, current and nadir CD4 count, and viral load) and antiretroviral (ART) status, HIV-related variables included evidence for AIDS (i.e., an AIDS-defining illness such as candidiasis or Kaposi’s sarcoma or a CD4 prior nadir <200cells/μL), Global Assessment of Functioning (i.e., GAF) (Endicott et al. 1976), the Veterans Aging Cohort Study (VACS) index, (Tate et al. 2013), and laboratory assessment of HCV infection (Center For Disease Control and Prevention 2017).
Table 1.
Characteristics of the 2 groups: mean±SD / frequency count
| Control (n=109) | HIV (n=57) | p-value* | |
|---|---|---|---|
| Demographics | |||
| N (men/women) | 64/45 | 40/17 | 0.15 |
| Age (years) | 51.7±14.3 | 53.3±6.9 | 0.44 |
| Handedness (Right/Left or Both) | 97/12 | 52/5 | 0.22 |
| Ethnicity♩ (Caucasian/African American/Asian/Other) | 53/29/23/4 | 24/30/1/2 | 0.0005 |
| Body Mass Index (BMI) | 26.5±4.8 | 25.3±4.4 | 0.13 |
| Systolic Blood Pressure | 128.1±19.6 | 123.8±15.3 | 0.17 |
| Diastolic Blood Pressure | 77.8±12.3 | 75.8±10.8 | 0.32 |
| Heart Rate | 65.8±10.9 | 69.3±14.8 | 0.10 |
| Education (years) | 15.7±2.5 | 13.5±2.6 | <0.0001 |
| Socioeconomic Status# (SES) | 29.1±14.6 | 40.7±15..2 | <0.0001 |
| Smoker (never/past or current) | 93/9 | 34/20 | <0.0001 |
| Nicotine (daily) | 1.0±2.9 | 2.8±5.1 | 0.07 |
| Lifetime Alcohol (kg) | 33.6±56.7 | 76.3±78.8 | <0.0001 |
| Diabetes (yes/no) | 2/59 | 6/34 | 0.03 |
| Beck Depression Index (BDI) | 3.3±4.2 | 10.8±8.7 | <0.0001 |
| Dementia Rating Scale (DRS, total raw score) | 139.5±3.0 | 135.7±5.2 | <0.0001 |
| HIV-Related Variables | |||
| Global Assessment of Functioning (GAF) | 84.3±6.1 | 71.7±10.7 | <0.0001 |
| VACS Index | 14.1±13.4 | 31.1±17.5 | <0.0001 |
| Hepatitis C Virus (HCV, positive/negative) | 3/106 | 21/36 | <0.0001 |
| HIV onset age (years) | - | 36.6±8.6 | na |
| HIV duration (years) | - | 16.7±7.8 | na |
| Viral Load (log copies/mL) | - | 2.1±1.1 | na |
| CD4 cell count (100/mm3) | - | 569.6±267.0 | na |
| CD4 cell count nadir (100/mm3) | - | 195.3±178.8 | na |
| AIDS-defining event (yes/no) | - | 30/27 | na |
| ART (yes/no) | - | 50/7 | na |
| Nucleoside Reverse Transcriptase Inhibitors (NRTI, 0/1/2) | - | 9/38/10 | na |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTI, 0/1 | - | 43/14 | na |
| Protease Inhibitors (PI, 0/1/2) | - | 29/11/17 | na |
| Fusion Inhibitors (FI, 0/1) | - | 49/8 | na |
t-tests used on continueous variables; X2 used on nominal variables (e.g., handedness)
self-defined: Other = Multiracial or Unknown
lower score = higher status
Neuropathy Measures
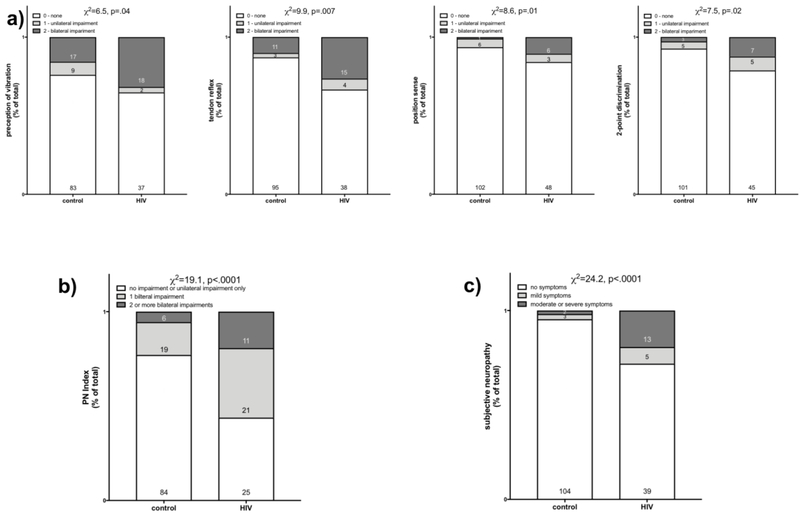
The HIV group had more impairments indicative of objective neuropathy than the control group (Figure 1a): perception of vibration (χ2=6.5, p=.04) [HIV group: normal=37, 6-10s=15, <5s=4; no feeling=1]; deep tendon ankle reflex (χ2=9.9, p=.007) [HIV group: normal=30, absent=1, hyperactive=1, hypoactive=15, could not detect=10]; position sense (χ2=8.6, p=.01) [HIV group: normal=48, 1 error=3, 2+ errors=6] and 2-point discrimination (χ2=7.5, p=.02). The PN index clearly distinguished the 2 groups (χ2=19.1, p<.0001) (Figure 1b).
Fig 1.
a) Each graph presents percent of total group (control or HIV) with no, unilateral, or bilateral impairments on measures of perception of vibration, tendon reflex, position sense, or 2-point discrimination. b) Graph of percent of total group (control or HIV) with a PI Index of 0=no impairment or only unilateral impairment, 1= bilateral impairment on a single measure of the 4, 2=bilateral impairment on 2 or more of the 4 measures. c) Graph of percent of total group (control or HIV) with 0=no symptoms, 1=mild symptoms, 2=moderate to severe symptoms of bilateral neuropathy.
The HIV-group reported a greater frequency of symptoms of subjective neuropathy (χ2=24.2 p<.0001) [HIV group: normal=39, mild pain=5 (all described as numbness), moderate to severe pain=13 (3 described as pain, aching or burning; 10 as numbness] (Figure 1c).
Relationships
Between Subjective Symptoms and Objective Signs
A total of 18 HIV participants reported symptoms of neuropathy (5 mild, 13 moderate to severe). Regardless of intensity, 3 described their symptoms as “pain, aching or burning” and 15 reported “numbness.” All 3 individuals who reported “pain, aching or burning” had at least 1 bilateral sign of objective neuropathy. Of those who reported numbness, 7 had no objective signs of neuropathy and 8 had at least 1 bilateral sign of objective neuropathy. However, there was no relationship between the subjective neuropathy score and the PN index (p=.66) or any of its components (i.e., perception of vibration (p=.53), deep tendon ankle reflex (p=.21), position sense (p=.47), 2-point discrimination (p=.41)).
PN index
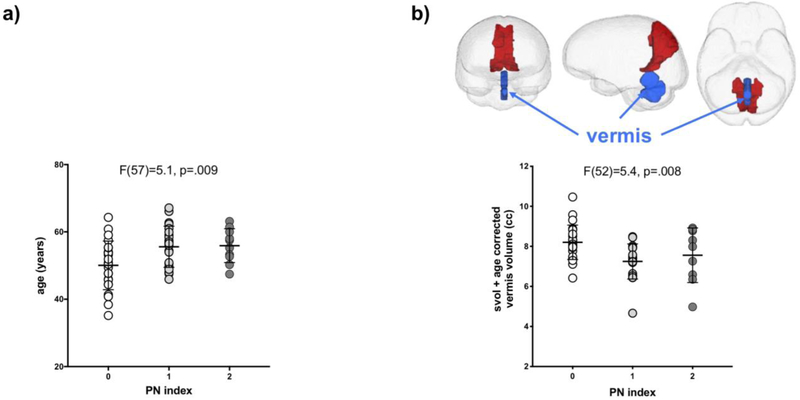
Within the HIV group, a PN index score of 1 or 2 was associated with older age (F(57)=5.1, p=.009; score 0<1=2) (Figure 2a). The index was not related to presence of diabetes, number of depressive symptoms, smoking status, or lifetime quantity of alcohol consumed. Furthermore, the index was not influenced by HIV-related variables including ART medication class (i.e., NRTI, NNRTI, PI, FII) or related to any laboratory blood measures, including presence of HCV.
Fig 2.
Association between the PN Index and a) age (years) and b) volume of the cerebellar vermis (cc). Mean and standard deviation indicated. Inset shows anterior, sagittal, and inferior views of the brain with the cerebellar vermis pictured in blue.
Within the HIV group, those with a PN index of 1 or 2 had a smaller volume of the cerebellar vermis (F(52)=5.4, p=.008; score 0<1=2) (Figure 2b), but not with the volume of any of the other regions quantified.
A model including age and cerebellar vermis volume was significant (χ2=17.6, p=.002), explained 16% of the variance of the PN index, and was driven by both age (p=.03) and vermis volume (p=.02).
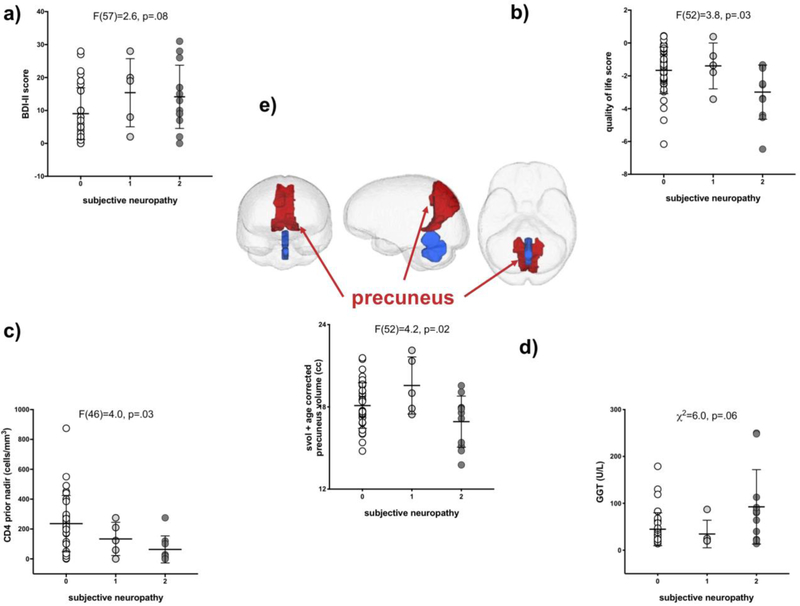
Subjective Neuropathy
Within the HIV group, moderate to severe subjective neuropathy was nominally associated with higher BDI-II scores (F(57)=2.6, p=.08; score 0<2) (Figure 3a) and significantly with poorer quality of life (F(52)=3.8, p=03; score 0=1<2) (Figure 3b) and a lower CD4 prior nadir (F(46)=4.0, p=.03; score 0=1<2)) (Figure 3c). Subjective neuropathy was not associated with lifetime alcohol consumed or days since last drink, but those who reported moderate to severe symptoms had significantly higher γ-glutamyl transferase (GGT) levels (F(55)=4.9, p=.01; score 0=1<2) (Figure 3d).
Fig 3.
Associations between subjective neuropathy scores and a) BDI-II scores, b) quality of life scores, c) CD4 prior nadir (cells/mm3), d) GGT levels (U/L), and e) precuneus volume (cc). Mean and standard deviation indicated. Inset shows anterior, sagittal, and inferior views of the brain with the precuneus pictured in blue.
Those with moderate to severe subjective neuropathy had a smaller volume of parietal precuneus (F(52)=4.2, p=.02; score 0=1>2) (Figure 3e), but not with the volume of any other region quantified, including cingulate (midposterior) volume (F(52)=.54, p=.60) and total cortical volume (F(52)=1.2, p=.30).
A model including BDI-II-derived depressive symptoms, quality of life, CD4 prior nadir, GGT, and parietal precuneus volume was significant (χ2=43.4, p<.0001), explained 65.2% of the variance in subjective neuropathy, and was driven by all 5 associated variables (each p<.0002).
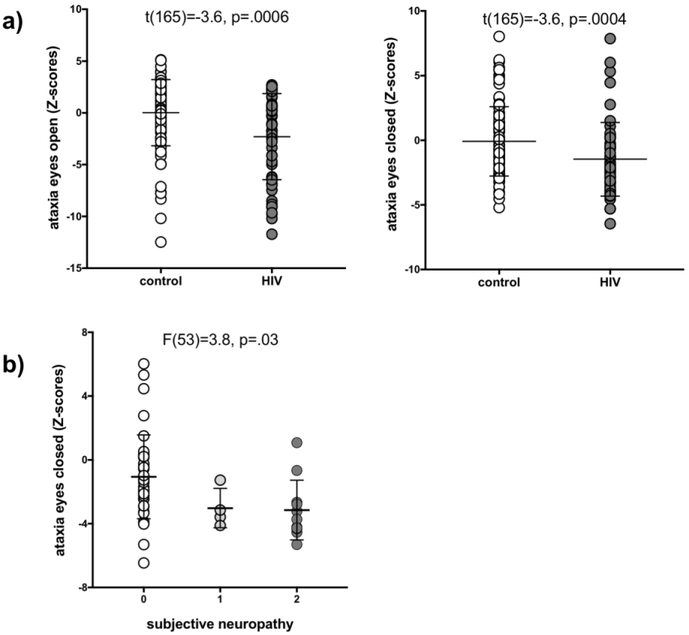
Postural Stability
The HIV group performed worse than the control group on both the eyes open (t(154)=15.1, p=.0001) and closed (t(154)=12.6, p=.0005) quantitative ataxia conditions (Figure 4a). The PN index was not associated with either of the ataxia measures. Mild to severe subjective neuropathy was associated with worse performance on ataxia with eyes closed (F(53)=38, p=.03; score 0>2) (Figure 4b) but not open (p=.22).
Fig 4.
a) Performance on ataxia with eyes open and closed by group (control and HIV). b) relationship between subjective neuropathy scores and ataxia with eyes closed.
Cluster Analysis
Variables including the 4 objective measures, the PN index, subjective neuropathy, and associates of the latter two measures including age, CD4 prior nadir, GGT levels, BDI-II scores, quality of life scores, ataxia with eyes closed, and volumes of the precuneus and vermis were entered into a JMP-based cluster analysis. This data-driven approach assigned the 14 variables into 4 clusters: cluster 1 explained 16% of the overall variance and included the PN index, perception of vibration, tendon reflex, and age; cluster 2 explained 13% of the total variance and included depressive symptoms, quality of life, GGT, and precuneus volume; cluster 3 explained 12% of the variance and included subjective neuropathy, ataxia with eyes closed, and CD4 prior nadir; cluster 4, explaining 10% of the variance included 2-point discrimination, position sense, and vermis volume.
DISCUSSION
As was expected, the HIV relative to the control group had a greater intensity of subjective symptoms of neuropathy, a higher incidence of objective neuropathy, and evidence for postural instability based on quantitative ataxia results. A clear relation between subjective and objective neuropathy scores was not forthcoming (cf., Cherry et al. 2005; McArthur 1998) and therefore supported their independence. A higher incidence of objective neuropathy was associated with older age and volume of the cerebellar vermis. A greater intensity of subjective symptoms of neuropathy was associated with more depressive symptoms, reduced quality of life, a low CD4 prior nadir, GGT levels, and precuneus volume. Moderate to severe subjective neuropathy was also associated with worse performance on ataxia with eyes closed.
Unlike previous reports, the current study did not find significant contributions of HCV (but see Cherry et al. 2010; Mapoure et al. 2018), ART (Cherry et al. 2009; but see Lee et al. 2015), or diabetes (Evans et al. 2011) to either objective signs or self-report of symptoms of neuropathy. An association of the PN index with older age, however, was notable (Chen et al. 2013; Saylor et al. 2017) and expected as the incidence of neuropathy is higher, even in healthy aging (Brouwer et al. 2015). This finding highlights challenges facing an aging HIV population (Nookala et al. 2017).
Although no studies to date have implicated the vermis in the pathophysiology of frequently studied neuropathies (e.g., alcoholic, diabetic, HIV- associated), several disorders with symptoms and signs of neuropathy are associated with smaller volume of the cerebellar vermis. A syndrome referred to as CANVAS features ataxia, vestibular areflexia, and neuropathy and includes cerebellar atrophy involving anterior and dorsal vermis (Szmulewicz et al. 2011a; Szmulewicz et al. 2011b; Taki et al. 2018). Similarly, ARCAS, an autosomal recessive ataxia, includes signs of distal sensorimotor neuropathy and atrophy of the superior vermis (Prodi et al. 2013; Vermeer et al. 2008; Vermeer et al. 1993). A case study of 3 incidents of infection with the human T-lymphocyte virus reported peripheral neuropathy of the lower limbs and atrophy of the superior vermis (Castillo et al. 2000). Whether the vermis is principally involved in HIV-related peripheral neuropathy will require further investigation. Loss of pain and temperature sensation following thalamic lesions has led to the hypothesis that the thalamus is involved in nociception (Dejerine and Roussy 1906; Garcin and Lapresle 1954; Head and Holmes 1911; Schuster 1937). Furthermore, performance on 2-point discrimination of the index fingers in healthy controls has recently been found to be associated with volume of the thalamus (Schmidt-Wilcke et al. 2018). The current study did not find a relationship between the PN index and thalamic volume; the volume of the total thalamus, however, was smaller in the combined group (i.e., unilateral+bilateral) of those with position sense impairment (χ2=4.3 p=.04). Future studies focused on high-resolution imaging and segmentation of thalamic subregions (cf., Tourdias et al. 2014) may provide evidence for involvement of specific thalamic nuclei in objective neuropathy.
Comporting with the literature, this study identified relationships between HIV-related neuropathic pain and depressive symptoms (Ellis et al. 2010; Robinson-Papp et al. 2010) and poor quality of life (Keltner et al. 2012). A relation between low CD4 prior nadir and neuropathic pain symptoms was not expected in the post-ART era (cf., Childs et al. 1999), but suggests that low CD4 counts increase the risk for HIV-associated neuropathy. While direct measures of heavy alcohol use (i.e., total lifetime alcohol consumed and recency of drinking) were not related (Bauer et al. 2005; Benevides et al. 2017), GGT levels, often used as an indirect biomarker of recent alcohol consumption (Andresen-Streichert et al. 2018), were higher in those with moderate to severe subjective neuropathy relative to those without neuropathy. The relationship between GGT and neuropathy, however, may not be mediated by alcohol: GGT has been associated with limited joint mobility and neuropathic pain symptoms in diabetics, independent of alcohol consumption, BMI, and metabolic control of diabetes (Arkkila et al. 2001). The relation between GGT and symptomatic neuropathy has also been shown in diabetic She Chinese (Lin et al. 2011) and Korean (Cho 2010) populations. Rather than as a marker for recent alcohol consumption, GGT has been hypothesized to serve as an index of oxidative stress (Lee et al. 2004; Lim et al. 2004) in the etiology of neuropathy (El Boghdady and Badr 2012) and thus may reflect an underlying mechanisms in HIV-related peripheral neuropathy.
This study reports a nominal association between subjective symptoms of neuropathy and postural instability. Although a review of studies focused on the association between peripheral neuropathy and gait and balance deficits in people living with HIV found no significant associations (Berner et al. 2017), peripheral neuropathy is related to joint kinematic impairment in diabetics (Mustapa et al. 2016).
Here although specifically tested, volume of the posterior cingulate was not associated with symptoms of neuropathy (cf., Hellmuth et al. 2016; Keltner et al. 2017; Keltner et al. 2014; Pfefferbaum et al. 2009). Instead, a relation was identified between subjective neuropathy and volume of the precuneus, which participates in reflective self-awareness (Kjaer et al. 2002; Lou et al. 2004) as has been suggested by a number of imaging studies (Cavanna and Trimble 2006; Fiset et al. 1999; Lou et al. 2005). Although distinct regions, the posterior cingulate and the precuneus have the highest level of metabolism in the brain (Gusnard et al. 2001; Laureys 2004), and are together considered pivotal for conscious information processing (Vogt and Laureys 2005).
One limitation of the current study is that despite a reasonable sample size, many relations were evaluated. We would argue, however, that these relations were considered in the context of nominal regressions, which demonstrated, after accounting for significant associations, persisting relations between neuropathy measures and brain volumes. Furthermore, the results of the cluster analysis provide data-driven support for dissociable functions of the vermis and precuneus: whereas the vermis clustered with performance on position sense and 2point discrimination, precuneus was associated with more depressive symptoms, poor quality of life, and high levels of GGT.
A more general limitation of this study is a lack of consensus regarding diagnosis of HIV-related neuropathy. For example, although the CHARTER studies describe objective measures to diagnose HIV-associated sensory neuropathy, their studies on CNS associations appear to rely on self-report of subjective symptoms (Keltner et al. 2017; Keltner et al. 2014).
In conclusion, this study provides support for a CNS substrate of peripheral neuropathy. Specifically, objective signs of neuropathy were associated with smaller volumes of cerebellar vermis, whereas subjective symptoms of neuropathy were associated with smaller volumes of the precuneus. Although these central substrates of neuropathy remain to be confirmed, the current study contributes to a growing body of literature showing CNS changes relevant to both signs and symptoms of neuropathy.
Acknowledgements
The authors would like to thank Priya Asok, Karen Jackson, and Anne-Lise Pitel for their help with data collection. The authors would also like to thank Ehsan Adeli for creating the regional brain atlas figure.
Funding: This study was supported with grant funding from the National Institute of Alcohol Abuse and Alcoholism (NIAAA): AA017347, AA010723, AA017168; and from the National Institute of Mental Health MH113406.
Footnotes
Conflict of Interest: The authors report no competing interests.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Please also sees statement under Participant header in Material and Methods.
Informed consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES
- Absinta M, Rocca MA, Colombo B, Falini A, Comi G, Filippi M (2012) Selective decreased grey matter volume of the pain-matrix network in cluster headache Cephalalgia : an international journal of headache 32:109–115 doi: 10.1177/0333102411431334 [DOI] [PubMed] [Google Scholar]
- Adoukonou TA, Kouna-Ndouongo P, Kpangon A, Gnonlonfoun D, Kpacha B, Dovonou A, Houinato D (2017) Distal sensory polyneuropathy among HIV-infected patients at Parakou University Hospital, Benin, 2011 Medecine et sante tropicales 27:190–194 doi: 10.1684/mst.2017.0685 [DOI] [PubMed] [Google Scholar]
- Andresen-Streichert H, Muller A, Glahn A, Skopp G, Sterneck M (2018) Alcohol Biomarkers in Clinical and Forensic Contexts Deutsches Arzteblatt international 115:309–315 doi: 10.3238/arztebl.2018.0309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A et al. (2007) Updated research nosology for HIV-associated neurocognitive disorders Neurology 69:1789–1799 doi:01.WNL.0000287431.88658.8b [pii] 10.1212/01.WNL.0000287431.88658.8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkkila PE, Koskinen PJ, Kantola IM, Ronnemaa T, Seppanen E, Viikari JS (2001) Diabetic complications are associated with liver enzyme activities in people with type 1 diabetes Diabetes Res Clin Pract 52:113–118 [DOI] [PubMed] [Google Scholar]
- Asad A, Hameed MA, Khan UA, Ahmed N, Butt MU (2010) Reliability of the neurological scores for assessment of sensorimotor neuropathy in type 2 diabetics JPMA The Journal of the Pakistan Medical Association 60:166–170 [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain Medical image analysis 12:26–41 doi: 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration NeuroImage 54:2033–2044 doi: 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Donnelly A, Harrison TB (2017) Update of HIV-Associated Sensory Neuropathies Current treatment options in neurology 19:36 doi: 10.1007/s11940-017-0472-3 [DOI] [PubMed] [Google Scholar]
- Bauer LO, Ceballos NA, Shanley JD, Wolfson LI (2005) Sensorimotor dysfunction in HIV/AIDS: effects of antiretroviral treatment and comorbid psychiatric disorders AIDS 19:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, S RA, B GK ((1996) ) Manual for the Beck Depression Inventory-II. Psychological Corporation, San Antonio, TX [Google Scholar]
- Benevides ML, Filho SB, Debona R, Bergamaschi EN, Nunes JC (2017) Prevalence of Peripheral Neuropathy and associated factors in HIV-infected patients Journal of the neurological sciences 375:316–320 doi: 10.1016/j.jns.2017.02.011 [DOI] [PubMed] [Google Scholar]
- Berner K, Morris L, Baumeister J, Louw Q (2017) Objective impairments of gait and balance in adults living with HIV-1 infection: a systematic review and meta-analysis of observational studies BMC musculoskeletal disorders 18:325 doi: 10.1186/s12891-017-1682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton AJ (1998) Guidelines for diagnosis and outpatient management of diabetic peripheral neuropathy. European Association for the Study of Diabetes, Neurodiab Diabetes Metab 24 Suppl 3:55–65 [PubMed] [Google Scholar]
- Bozzette SA, Hays RD, Berry SH, Kanouse DE, Wu AW (1995) Derivation and properties of a brief health status assessment instrument for use in HIV disease Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association 8:253–265 [DOI] [PubMed] [Google Scholar]
- Brouwer BA, de Greef BT, Hoeijmakers JG, Geerts M, van Kleef M, Merkies IS, Faber CG (2015) Neuropathic Pain due to Small Fiber Neuropathy in Aging: Current Management and Future Prospects Drugs & aging 32:611–621 doi: 10.1007/s40266-015-0283-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckalew N, Haut MW, Morrow L, Weiner D (2008) Chronic pain is associated with brain volume loss in older adults: preliminary evidence Pain medicine 9:240–248 doi: 10.1111/j.1526-4637.2008.00412.x [DOI] [PubMed] [Google Scholar]
- Castillo LC, Gracia F, Roman GC, Levine P, Reeves WC, Kaplan J (2000) Spinocerebellar syndrome in patients infected with human T-lymphotropic virus types I and II (HTLV-I/HTLV-II): report of 3 cases from Panama Acta Neurol Scand 101:405–412 [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates Brain 129:564–583 doi: 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Center For Disease Control and Prevention (2017) Hepatitis C FAQs for Health Professionals. CDC. [Google Scholar]
- Chen H et al. (2013) Peripheral neuropathy in ART-experienced patients: prevalence and risk factors Journal of neurovirology 19:557–564 doi: 10.1007/s13365-013-0216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry CL et al. (2010) Hepatitis C seropositivity is not a risk factor for sensory neuropathy among patients with HIV Neurology 74:1538–1542 doi: 10.1212/WNL.0b013e3181dd436d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry CL et al. (2009) Age and height predict neuropathy risk in patients with HIV prescribed stavudine Neurology 73:315–320 doi: 10.1212/WNL.0b013e3181af7a22 [DOI] [PubMed] [Google Scholar]
- Cherry CL, McArthur JC, Hoy JF, Wesselingh SL (2003) Nucleoside analogues and neuropathy in the era of HAART Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology 26:195–207 [DOI] [PubMed] [Google Scholar]
- Cherry CL, Wesselingh SL, Lal L, McArthur JC (2005) Evaluation of a clinical screening tool for HIV-associated sensory neuropathies Neurology 65:1778–1781 doi: 10.1212/01.wnl.0000187119.33075.41 [DOI] [PubMed] [Google Scholar]
- Childs EA et al. (1999) Plasma viral load and CD4 lymphocytes predict HIV-associated dementia and sensory neuropathy Neurology 52:607–613 [DOI] [PubMed] [Google Scholar]
- Cho HC (2010) The Association between Serum GGT Concentration and Diabetic Peripheral Polyneuropathy in Type 2 Diabetic Patients Korean diabetes journal 34:111–118 doi: 10.4093/kdj.2010.34.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, Tiwari V (2012) Alcoholic neuropathy: possible mechanisms and future treatment possibilities British journal of clinical pharmacology 73:348–362 doi: 10.1111/j.1365-2125.2011.04111.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters I et al. (2017) Decreased Regional Grey Matter Volume in Women with Chronic Whiplash-Associated Disorders: Relationships with Cognitive Deficits and Disturbed Pain Processing Pain physician 20:E1025–E1051 [PubMed] [Google Scholar]
- Corkin S, Milner B, Rasmussen T (1970) Somatosensory thresholds--contrasting effects of postcentral-gyrus and posterior parietal-lobe excisions Archives of neurology 23:41–58 [DOI] [PubMed] [Google Scholar]
- Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T (1999) Total neuropathy score: validation and reliability study Neurology 53:1660–1664 [DOI] [PubMed] [Google Scholar]
- Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C (2008) An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images IEEE Trans Med Imaging 27:425–441 doi: 10.1109/TMI.2007.906087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bresser J, Reijmer YD, van den Berg E, Breedijk MA, Kappelle LJ, Viergever MA, Biessels GJ (2010) Microvascular determinants of cognitive decline and brain volume change in elderly patients with type 2 diabetes Dement Geriatr Cogn Disord 30:381–386 doi: 10.1159/000321354 [DOI] [PubMed] [Google Scholar]
- Dejerine J, Roussy G (1906) Le syndrome thalamique Revue Neurologique 14:521–532 [Google Scholar]
- Ducic I, Short KW, Dellon AL (2004) Relationship between loss of pedal sensibility, balance, and falls in patients with peripheral neuropathy Annals of plastic surgery 52:535–540 [DOI] [PubMed] [Google Scholar]
- El Boghdady NA, Badr GA (2012) Evaluation of oxidative stress markers and vascular risk factors in patients with diabetic peripheral neuropathy Cell Biochem Funct 30:328–334 doi: 10.1002/cbf.2808 [DOI] [PubMed] [Google Scholar]
- Ellis RJ et al. (2010) Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study Archives of neurology 67:552–558 doi: 10.1001/archneurol.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Spitzer RL, Fleiss JL, Cohen J (1976) The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance Archives of general psychiatry 33:766–771 [DOI] [PubMed] [Google Scholar]
- Evans SR et al. (2011) Peripheral neuropathy in HIV: prevalence and risk factors AIDS 25:919–928 doi: 10.1097/QAD.0b013e328345889d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Schlosser FJ, Sumpio BE (2009) The Semmes Weinstein monofilament examination as a screening tool for diabetic peripheral neuropathy Journal of vascular surgery 50:675–682, 682 e671 doi: 10.1016/j.jvs.2009.05.017 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW (1998) Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) Version 2.0 Biometrics Research Department, New York State Psychiatric Institute, New York, NY. [Google Scholar]
- Fiset P et al. (1999) Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study J Neurosci 19:5506–5513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frokjaer JB, Brock C, Softeland E, Dimcevski G, Gregersen H, Simren M, A MD (2013) Macrostructural brain changes in patients with longstanding type 1 diabetes mellitus - a cortical thickness analysis study Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association 121:354–360 doi: 10.1055/s-0033-1345120 [DOI] [PubMed] [Google Scholar]
- Garcin R, Lapresle J (1954) [Sensory syndrome of the thalamic type and with hand-mouth topography due to localized lesions of the thalamus] Rev Neurol (Paris) 90:124–129 [PubMed] [Google Scholar]
- Ghosh S, Chandran A, Jansen JP (2012) Epidemiology of HIV-related neuropathy: a systematic literature review AIDS Res Hum Retroviruses 28:36–48 doi: 10.1089/AID.2011.0116 [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME, Raichle ME (2001) Searching for a baseline: functional imaging and the resting human brain Nat Rev Neurosci 2:685–694 doi: 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Head H, Holmes G (1911) Sensory Disturbances from Cerebral Lesions Brain 34:102–254 [Google Scholar]
- Hellmuth J et al. (2016) Neurologic signs and symptoms frequently manifest in acute HIV infection Neurology 87:148–154 doi: 10.1212/WNL.0000000000002837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A (1975) Four-factor index of social status Department of Sociology, Yale University, New Haven, CT [Google Scholar]
- Hsieh PC et al. (2015) Imaging signatures of altered brain responses in small-fiber neuropathy: reduced functional connectivity of the limbic system after peripheral nerve degeneration Pain 156:904–916 doi: 10.1097/j.pain.0000000000000128 [DOI] [PubMed] [Google Scholar]
- Kaku M, Simpson DM (2014) HIV neuropathy Current opinion in HIV and AIDS 9:521–526 doi: 10.1097/COH.0000000000000103 [DOI] [PubMed] [Google Scholar]
- Karnofsky DA (1949) The clinical evaluation of chemotherapeutic agents in cancer In: MacLeod CM (ed) Evaluation of Chemotherapeutic Agents. Columbia University Press, New York, NY, pp 191–205 [Google Scholar]
- Keltner JR et al. (2017) HIV Distal Neuropathic Pain Is Associated with Smaller Ventral Posterior Cingulate Cortex Pain medicine 18:428–440 doi: 10.1093/pm/pnw180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR et al. (2014) HIV-associated distal neuropathic pain is associated with smaller total cerebral cortical gray matter Journal of neurovirology 20:209–218 doi: 10.1007/s13365-014-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner JR et al. (2012) Health-related quality of life 'well-being' in HIV distal neuropathic pain is more strongly associated with depression severity than with pain intensity Psychosomatics 53:380–386 doi: 10.1016/j.psym.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC (2002) Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core NeuroImage 17:1080–1086 [PubMed] [Google Scholar]
- Laureys S (2004) Functional neuroimaging in the vegetative state NeuroRehabilitation 19:335–341 [PubMed] [Google Scholar]
- Lee AJ, Bosch RJ, Evans SR, Wu K, Harrison T, Grant P, Clifford DB (2015) Patterns of peripheral neuropathy in ART-naive patients initiating modern ART regimen Journal of neurovirology 21:210–218 doi: 10.1007/s13365-015-0327-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DH, Blomhoff R, Jacobs DR Jr. (2004) Is serum gamma glutamyltransferase a marker of oxidative stress? Free radical research 38:535–539 [DOI] [PubMed] [Google Scholar]
- Lim JS, Yang JH, Chun BY, Kam S, Jacobs DR Jr., Lee DH (2004) Is serum gamma-glutamyltransferase inversely associated with serum antioxidants as a marker of oxidative stress? Free Radic Biol Med 37:1018–1023 doi: 10.1016/j.freeradbiomed.2004.06.032 [DOI] [PubMed] [Google Scholar]
- Lin Y et al. (2011) Serum gamma-glutamyltransferase and associated damage among a She Chinese population Diabetic medicine : a journal of the British Diabetic Association 28:924–931 doi: 10.1111/j.1464-5491.2011.03270.x [DOI] [PubMed] [Google Scholar]
- Lou HC et al. (2004) Parietal cortex and representation of the mental Self Proceedings of the National Academy of Sciences of the United States of America 101:6827–6832 doi: 10.1073/pnas.0400049101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou HC, Nowak M, Kjaer TW (2005) The mental self Progress in brain research 150:197–204 doi: 10.1016/s0079-6123(05)50014-1 [DOI] [PubMed] [Google Scholar]
- Lunetta M, Damanti AR, Fabbri G, Lombardo M, Di Mauro M, Mughini L (1994) Evidence by magnetic resonance imaging of cerebral alterations of atrophy type in young insulin-dependent diabetic patients J Endocrinol Invest 17:241–245 doi: 10.1007/BF03348967 [DOI] [PubMed] [Google Scholar]
- Manor B, Newton E, Abduljalil A, Novak V (2012) The relationship between brain volume and walking outcomes in older adults with and without diabetic peripheral neuropathy Diabetes care 35:1907–1912 doi: 10.2337/dc11-2463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapoure NY, Budzi MN, Eloumou S, Malongue A, Okalla C, Luma HN (2018) Neurological manifestations in chronic hepatitis C patients receiving care in a reference hospital in sub-Saharan Africa: A cross-sectional study PLoS One 13:e0192406 doi: 10.1371/journal.pone.0192406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S (1998) Dementia rating scale (DRS) professional manual. Psychological Assessment Resources, Inc, Odessa, FL [Google Scholar]
- McArthur JH (1998) The reliability and validity of the subjective peripheral neuropathy screen J Assoc Nurses AIDS Care 9:84–94 doi: 10.1016/S1055-3290(98)80048-4 [DOI] [PubMed] [Google Scholar]
- Morgello S et al. (2004) HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: the Manhattan HIV Brain Bank Archives of neurology 61:546–551 doi: 10.1001/archneur.61.4.546 [DOI] [PubMed] [Google Scholar]
- Mustapa A, Justine M, Mohd Mustafah N, Jamil N, Manaf H (2016) Postural Control and Gait Performance in the Diabetic Peripheral Neuropathy: A Systematic Review Biomed Res Int 2016:9305025 doi: 10.1155/2016/9305025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson D et al. (2006) Comparison of conventional and non-invasive techniques for the early identification of diabetic neuropathy in children and adolescents with type 1 diabetes Pediatric diabetes 7:305–310 doi: 10.1111/j.1399-5448.2006.00208.x [DOI] [PubMed] [Google Scholar]
- Nookala AR, Mitra J, Chaudhari NS, Hegde ML, Kumar A (2017) An Overview of Human Immunodeficiency Virus Type 1-Associated Common Neurological Complications: Does Aging Pose a Challenge? J Alzheimers Dis 60:S169–S193 doi: 10.3233/JAD-170473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya R, Krentz HB, Gill MJ, Power C (2005) HIV-related neurological syndromes reduce health-related quality of life The Canadian journal of neurological sciences 32:201–204 [DOI] [PubMed] [Google Scholar]
- Periyasamy R, Manivannan M, Narayanamurthy VB (2008) Changes in two point discrimination and the law of mobility in diabetes mellitus patients Journal of brachial plexus and peripheral nerve injury 3:3 doi: 10.1186/1749-7221-3-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins BA, Olaleye D, Zinman B, Bril V (2001) Simple screening tests for peripheral neuropathy in the diabetes clinic Diabetes care 24:250–256 [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S (1982) Measurement of functional activities in older adults in the community Journal of gerontology 37:323–329 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, Sullivan EV (2009) Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking Biological psychiatry 65:680–690 doi:S0006-3223(08)01385-1 [pii] 10.1016/j.biopsych.2008.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, Sullivan EV (2018) Accelerated and Premature Aging Characterizing Regional Cortical Volume Loss in Human Immunodeficiency Virus Infection: Contributions From Alcohol, Substance Use, and Hepatitis C Coinfection Biological psychiatry Cognitive neuroscience and neuroimaging 3:844–859 doi: 10.1016/j.bpsc.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodi E et al. (2013) Supratentorial and pontine MRI abnormalities characterize recessive spastic ataxia of Charlevoix-Saguenay. A comprehensive study of an Italian series Eur J Neurol 20:138–146 doi: 10.1111/j.1468-1331.2012.03815.x [DOI] [PubMed] [Google Scholar]
- Robinson-Papp J et al. (2010) Association of self-reported painful symptoms with clinical and neurophysiologic signs in HIV-associated sensory neuropathy Pain 151:732–736 doi: 10.1016/j.pain.2010.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A (2010) The SRI24 multichannel atlas of normal adult human brain structure Human brain mapping 31:798–819 doi: 10.1002/hbm.20906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhdorfer AS et al. (2015) Selecting a prospective test for early detection of diabetic polyneuropathy Microsurgery 35:512–517 doi: 10.1002/micr.22409 [DOI] [PubMed] [Google Scholar]
- Saylor D, Nakigozi G, Nakasujja N, Robertson K, Gray RH, Wawer MJ, Sacktor N (2017) Peripheral neuropathy in HIV-infected and uninfected patients in Rakai, Uganda Neurology doi: 10.1212/WNL.0000000000004136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Wilcke T et al. (2018) Structural changes in brain morphology induced by brief periods of repetitive sensory stimulation NeuroImage 165:148–157 doi: 10.1016/j.neuroimage.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Schuster P (1937) Beiträge zun Pathologie des Thalamus opticus. Mitteilung Archiv für Psychiatrie und Nervenkrakheiten 106:13–53 [Google Scholar]
- Selvarajah D, Wilkinson ID, Davies J, Gandhi R, Tesfaye S (2011) Central nervous system involvement in diabetic neuropathy Curr Diab Rep 11:310–322 doi: 10.1007/s11892-011-0205-z [DOI] [PubMed] [Google Scholar]
- Selvarajah D et al. (2014) Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy Diabetes care 37:1681–1688 doi: 10.2337/dc13-2610 [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ (1982) Reliability of alcohol use indices. The Lifetime Drinking History and the MAST Journal of studies on alcohol 43:1157–1170 [DOI] [PubMed] [Google Scholar]
- Sommer C (2018) Nerve and skin biopsy in neuropathies Current opinion in neurology 31:534–540 doi: 10.1097/WCO.0000000000000601 [DOI] [PubMed] [Google Scholar]
- Sugimine S, Ogino Y, Kawamichi H, Obata H, Saito S (2016) Brain morphological alternation in chronic pain patients with neuropathic characteristics Molecular pain 12 doi: 10.1177/1744806916652408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A (2000) Pattern of motor and cognitive deficits in detoxified alcoholic men Alcoholism, clinical and experimental research 24:611–621 [PubMed] [Google Scholar]
- Szmulewicz DJ, Waterston JA, Halmagyi GM, Mossman S, Chancellor AM, McLean CA, Storey E (2011a) Sensory neuropathy as part of the cerebellar ataxia neuropathy vestibular areflexia syndrome Neurology 76:1903–1910 doi: 10.1212/WNL.0b013e31821d746e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szmulewicz DJ et al. (2011b) Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis Annals of the New York Academy of Sciences 1233:139–147 doi: 10.1111/j.1749-6632.2011.06158.x [DOI] [PubMed] [Google Scholar]
- Taki M et al. (2018) Cerebellar ataxia with neuropathy and vestibular areflexia syndrome (CANVAS) Auris, nasus, larynx 45:866–870 doi: 10.1016/j.anl.2017.10.008 [DOI] [PubMed] [Google Scholar]
- Tate JP et al. (2013) An internationally generalizable risk index for mortality after one year of antiretroviral therapy AIDS 27:563–572 doi: 10.1097/QAD.0b013e32835b8c7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dickenson AH (2013) Mechanisms and management of diabetic painful distal symmetrical polyneuropathy Diabetes care 36:2456–2465 doi: 10.2337/dc12-1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S, Selvarajah D, Gandhi R, Greig M, Shillo P, Fang F, Wilkinson ID (2016) Diabetic peripheral neuropathy may not be as its name suggests: evidence from magnetic resonance imaging Pain 157 Suppl 1:S72–80 doi: 10.1097/j.pain.0000000000000465 [DOI] [PubMed] [Google Scholar]
- Tourdias T, Saranathan M, Levesque IR, Su J, Rutt BK (2014) Visualization of intra-thalamic nuclei with optimized white-matter-nulled MPRAGE at 7T NeuroImage 84:534–545 doi:S1053-8119(13)00934-8 [pii] 10.1016/j.neuroimage.2013.08.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer S et al. (2008) ARSACS in the Dutch population: a frequent cause of early-onset cerebellar ataxia Neurogenetics 9:207–214 doi: 10.1007/s10048-008-0131-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer S, van de Warrenburg BP, Kamsteeg EJ (1993) Arsacs In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A (eds) GeneReviews((R)). Seattle (WA) [PubMed] [Google Scholar]
- Vogt BA, Laureys S (2005) Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness Progress in brain research 150:205–217 doi: 10.1016/s0079-6123(05)50015-3 [DOI] [PMC free article] [PubMed] [Google Scholar]