Abstract
Emotion regulation dysfunction is characteristic of psychotic disorders, but little is known about how the use of specific types of emotion regulation strategies differs across phases of psychotic illness. This information is vital for understanding factors contributing to psychosis vulnerability states and developing targeted treatments. Three studies were conducted to examine emotion regulation across phases of psychosis, which included 1) adolescent community members with psychotic-like experiences (PLEs; n = 262) and adolescents without PLES (n = 1,226), 2) adolescents who met clinical high-risk (CHR) criteria for a prodromal syndrome (n = 29) and healthy controls (n = 29), and 3) outpatients diagnosed with schizophrenia or schizoaffective disorder (SZ: n = 61) and healthy controls (n = 67). In each study, participants completed the Emotion Regulation Questionnaire and measures of psychiatric symptoms and functional outcome. The three psychosis groups did not differ from each other in reported use of suppression; however, there was evidence for a vulnerability-related dose-dependent decrease in reappraisal. Across each sample, lower use of reappraisal was associated with poorer clinical outcomes. Findings indicate that emotion regulation abnormalities occur across a continuum of psychosis vulnerability and represent important targets for intervention.
Keywords: psychosis, emotion, emotion regulation, prodrome, psychotic-like experiences
Psychosis was traditionally viewed as a bimodal construct, with the majority of the population having no symptoms and a small minority having severe symptoms that reach clinical significance (Johns & van Os, 2001). However, recent evidence suggests that psychotic symptoms vary along a continuum, with a distribution that is continuous, but only half-normal (i.e., the majority of the population has low values, but a significant proportion has non-zero values). At the lowest end of the continuum are individuals with psychotic-like experiences (PLEs). A significant proportion of the general population falls into this category, reporting lifetime psychotic experiences that typically do not occur frequently, do not worsen in severity over time, and do not cause distress (Kelleher & Cannon, 2011). These individuals, by definition, are neither help-seeking nor at immediate risk for transition, and they do not necessitate treatment or contact with the medical system (Linscott & van Os, 2010).
In contrast, in the middle of the severity continuum are those at clinical high-risk (CHR) for developing a psychotic disorder. These individuals meet criteria for a prodromal syndrome or schizotypal personality disorder (Addington et al., 2007). Unlike individuals with PLEs, CHR individuals are considered to be at imminent high risk for psychosis. Inclusion criteria involve recent onset and/or escalating positive symptoms, as evidenced by increasing severity, frequency, distress, and conviction, as well as socio-occupational decline and cognitive impairment (McGlashan, Miller, Woods, Hoffman, & Davidson, 2001; Miller et al., 2003; Rosen, Woods, Miller, & McGlashan, 2002; Yung & McGorry, 1996; Yung, Philips, Yuen, & McGorry, 2004). Reflective of these signs, approximately 15–30% of CHR individuals will transition to a psychotic disorder within 2 years (Cannon et al., 2008; Cannon et al., 2016; Fusar-Poli et al., 2015). As a result, it is possible to make loose inferences about vulnerability and disease related differences between clinical help-seeking samples and those with PLEs, although additional research with a large sample and multiple follow-up time points is necessary before any definitive conclusions can be drawn.
At the highest end of the severity continuum are those with a diagnosable psychotic illness. A key differentiating factor between CHR and a diagnosable illness involves the level of conviction and frequency of psychotic experiences. Full conviction and moderate or greater frequency of psychotic symptoms marks the transition from an at-risk state to formal illness (McGlashan et al., 2001).
Studies examining processes that predict symptoms across the continuum of psychosis vulnerability stages have potential to inform the understanding of pathogenesis and identify targets for novel interventions. One area of focus is affective functioning; however, given that the stages of psychosis vulnerability span many years, such developmental studies face the challenge of distinguishing group differences which are attributable to age and maturation from those which are attributable to psychopathological factors. Despite this challenge, many features of affective disturbance have emerged and seem to supersede developmental factors and predict psychiatric outcomes. Increased stress reactivity is one component of affective disturbance that cuts across the continuum of psychotic experiences and increase vulnerability for illness (Corcoran et al., 2003; Walker, Mittal, & Tessner, 2008, Walker et al., 2013). Emotion regulation, defined as the use of strategies to decrease the frequency, intensity, or duration of emotional response, is a second aspect of emotional functioning that predicts risk for a number of psychiatric diagnoses (Aldao, Nolen-Hoeksema, & Schweizer, 2010). It is unclear, however, whether emotion regulation abnormalities predict vulnerability for psychosis, in particular.
Emotion regulation strategy use is most commonly evaluated using self-report measures, such as the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003), which assesses reported use of reappraisal and suppression to regulate positive and negative emotions. Reappraisal involves the reinterpretation of an event or stimulus to control an emotional response, while expressive suppression involves the intentional reduction of outward emotional expressivity for the purpose of reducing the emotional experience.
Few studies have examined emotion regulation in the schizophrenia-spectrum, and their results have been inconsistent. For example, several studies have found that individuals with schizophrenia (SZ) report significantly greater use of expressive suppression (Horan, Hajcak, Wynn, & Green, 2013; Kimhy et al., 2012, van der Meer, van’t Wout, & Aleman, 2009) and less use of reappraisal (Horan et al., 2013; Kimhy et al., 2012, Livingstone, Harper, & Gillanders, 2009; van der Meer et al., 2009) than controls (CN), while other studies have found no group differences in reported strategy use between SZ and CN groups (Badcock, Paulik, & Maybery, 2011; Henry, Rendell, Green, McDonald, & O’Donnell, 2008; Perry, Henry, & Grisham, 2011). Similar inconsistencies arise in psychosis-risk studies. In a study by Kimhy et al. (2016), CHR youth reported strategy use proportionate to SZ, and both groups reported lower reappraisal than CN. In contrast, van der Meer et al. (2014) found no differences in strategy use between SZ, non-affected siblings of individuals with SZ (i.e., those at CHR due to a genetic predisposition), and CN. The cause for inconsistent findings across studies is unclear, as there is no distinct pattern of similarity among studies with null and significant results in terms of demographic characteristics, symptom severity levels, or neuropsychological impairment among the samples.
At this time, the relationship between emotion regulation strategy use and clinical outcomes is also uncertain. Multiple studies have reported that poor social functioning is related to less habitual use of reappraisal and/or greater use of suppression (Gross & John, 2003; Henry et al., 2008; John & Gross, 2004; Kimhy et al., 2012; Perry et al., 2011; Strauss et al., 2015), even among CHR youth (Kimhy et al., 2016). Likewise, greater self-reported use of suppression has been associated with increased severity of positive and general psychiatric symptoms (e.g., mood, anxiety; Badcock et al., 2011; Horan et al., 2013; John & Gross, 2004), and greater self-reported use of reappraisal has been associated with decreased severity of negative symptoms and depression (Henry et al., 2008; John & Gross, 2004; Perry et al., 2011).
While associations between emotion regulation strategy use and symptoms or functional outcome are evident throughout most of the literature, the patterns of association vary, and no conclusions can be made about the nature of these relationships at present. Inconsistent findings in the literature may reflect limited power among prior studies to detect small and medium effect sizes, as well as heterogeneity of demographics, diagnoses (e.g., proportion of schizophrenia vs schizoaffective, see Kimhy et al., 2012), and symptom and developmental profiles.
An important consideration for studies comparing groups of differing clinical profiles (e.g., PLE vs CHR vs SZ) is the influence that age and brain development may have on emotion regulation strategy use apart from illness phase. Specifically, research shows that an individual’s ability to recruit prefrontal regions during emotion regulation improves with age, allowing for more successful regulation through reappraisal in older compared to younger individuals (John & Gross, 2004; Martin & Ochsner, 2016; McRae et al., 2012; Silvers et al., 2012; Theurel & Gentaz, 2018). However, it should be noted that most of the research on emotion regulation and life span development focuses on healthy, non-psychiatric samples, and when evaluating the frequency of strategy use rather than strategy success, studies show no effect of age (Gullone & Taffe, 2011; Theurel & Gentaz, 2018). The latter findings indicate that the success or failure of a strategy does not necessarily predict the frequency in which it is implemented, and therefore, studies evaluating the frequency of strategy use rather than strategy success may not be confounded by age. Additionally, Silvers et al. (2012) reported no effect of age on emotion regulation success after age 18, which suggests that age may be even less of a concern between CHR and SZ samples. Nonetheless, age should be taken into consideration, and a proportion of the inconsistencies between studies may reflect differences in age and development between samples.
In summation, although there is some evidence for aberrant emotion regulation strategy use in youth at CHR for psychosis and adults in the chronic phase of SZ, the current literature is inconsistent and no stable conclusions can be drawn about the nature of these emotion regulation abnormalities or their clinical correlates.
To advance the literature on emotion regulation across the psychosis continuum, three studies were conducted to evaluate differences in emotion regulation across different levels of psychosis vulnerability, including a community sample of youth with psychotic like experiences (PLE), clinical help-seeking CHR youth, and outpatients diagnosed with SZ. Given that the majority of prior studies found group effects (Horan et al., 2013; Kimhy et al., 2016; Kimhy et al., 2012; Livingstone et al., 2009; van der Meer et al., 2009), we first hypothesized that PLE, CHR, and SZ would report less reappraisal and greater suppression than CN. Moreover, based on previous findings that stress reactivity follows a psychosis vulnerability trajectory (Fusar-Poli et al., 2017; Walker et al., 2013), we hypothesized that psychosis vulnerability would influence emotion regulation strategy use, such that PLE > CHR > SZ for reappraisal, and PLE < CHR < SZ for suppression. Given mostly consistent evidence for correlations between strategy use and symptom severity (Badcock et al., 2011; Horan et al., 2013; Perry et al., 2011) as well as social functioning (Gross & John, 2003; Kimhy et al., 2016; Kimhy et al., 2012; Perry et al., 2011; Strauss et al., 2015), we also hypothesized that greater use of suppression and lower use of reappraisal would be associated with greater severity of positive, negative, and general psychiatric symptoms, as well as poorer functional outcome in PLE, CHR, and SZ samples. Further, considering that symptoms, by definition, are more severe in SZ than CHR or PLE, we expected associations between symptoms and strategy use to be strongest in the SZ group. Lastly, based on past findings of sex differences in reported strategy use (Balzarotti, John, & Gross, 2010; Gross & John, 2003; Gullone & Taffe, 2011; Livingstone et al., 2009; McRae, Ochsner, Mauss, Gabrieli, & Gross, 2008), we hypothesized that males would score higher on suppression and reappraisal than females within the psychosis groups.
Method
Study 1: Community School Sample
Participants.
Participants included 1,488 middle and high school students in the northeastern United States (see Table 1 for demographics). The study utilized passive consent from parents and active assent from youth. Parents of all students received a letter detailing the study. Those who did not wish their child to participate were asked to sign the form and return it to the school. Following this, students were asked to give their assent to participate in the study by signing an active assent form. Participation was limited to students whose parents had provided passive consent and who themselves provided active assent.
Table 1.
Demographics for Studies 1–3
| Study 1 | Study 2 | Study 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PLE | NPLE | F/χ2 | CHR | CN | F/χ2 | sz | CN | F/χ2 | |
| Age | 14.25 | 14.14 | F = 0.61 | 19.24 | 19.52 | F=0.29 | 39.98 | 40.57 | F = 0.12 |
| (2.22) | (2.04) | (2.49) | (1.24) | (12.18) | (11.96) | ||||
| Education | 8.04 | 7.88 | F = 1.44 | 12.86 | 13.72 | F=2.96 | 12.56 | 15.18 | F = 45.35** |
| (2.03) | (1.96) | (2.26) | (1.49) | (2.13) | (2.05) | ||||
| % Male | 51.15 | 51.63 | χ2 = 3.71 | 31.03 | 20.70 | χ2=0.81 | 63.9 | 69.12 | χ2 = 0.39 |
| % Ethnicity | χ2 = 24.37** | χ2=4.17 | χ2 = 4.44 | ||||||
| Amer. Ind./ | 4.96 | 2.77 | 0.00 | 0.00 | 1.6% | 0.00 | |||
| Alaskan | |||||||||
| Asian | 5.34 | 2.86 | 17.24 | 6.90 | 3.3% | 4.41 | |||
| Afr. Amer. | 12.60 | 8.32 | 0.00 | 6.90 | 13.1 | 16.18 | |||
| Hispanic | 4.20 | 4.57 | 13.79 | 6.90 | 2.44 | 5.88 | |||
| Biracial | 14.89 | 9.63 | 3.44 | 3.45 | 3.3 | 2.94 | |||
| Caucasian | 56.11 | 70.47 | 65.52 | 75.86 | 70.5 | 69.12 | |||
| Other | 1.91 | 1.39 | 0.00 | 0.00 | 0.0 | 1.47 | |||
Note.
p < 0.001;
p < 0.01;
p < 0.05.
PLE = individuals with psychotic-like experiences; NPLE = individuals without psychotic-like experiences; CHR = individuals at clinical high-risk; CN = controls; SZ = schizophrenia outpatients; Amer. Ind. = American Indian; Afr. Amer. = African American.
Procedures and Measures.
Participants completed a series of online questionnaires in their school computer laboratories via Survey Monkey with supervised administration. Questionnaires required approximately one hour to complete and included: 1) the Emotion Regulation Questionnaire for Children and Adolescents (ERQ-CA; Gullone & Taffe, 2011), 2) the Youth Psychosis At-Risk Questionnaire-Brief (YPARQ-B; Ord, Myles-Worsley, Blailes, & Ngiralmau, 2004), and 3) the Strengths and Difficulties Questionnaire (SDQ; Goodman, 1997).
The ERQ-CA is the adapted version of the ERQ (Gross & John, 2003), modified for children and adolescents. Like the ERQ, the ERQ-CA is comprised of 10 items, assessing reported use of reappraisal (6 items) and suppression (4 items); however, the language has been simplified, and self-reports are made on a 1 (strongly disagree) to 5 (strongly agree) scale. The ERQ-CA was developed on a sample of 827 participants, ranging in age from 10 – 18 years. Alpha reliability coefficients for the total sample were .83 for reappraisal and .75 for suppression (Gullone & Taffe, 2011).
The YPARQ-B is a 28-item questionnaire that measures psychotic experiences. Responses are scored as “Yes” (present) or “No/Uncertain” (absent). A cut-off score of ≥11 indicates meeting CHR criteria for developing a psychotic disorder. This cut-off yielded sensitivity of 0.82 and specificity of 0.99 in the original scale development study in 648 high school students (Ord et al., 2004). In a subsequent study by Kline et al. (2012; N = 49), the cutoff ≥ 11 had poorer sensitivity (0.65) and specificity (0.76), and the cut-off ≥13 offered the same sensitivity (0.65) and better specificity (0.90). Using the more conservative cutoff of ≥ 13 (Kline et al., 2012), we identified 262 (17.61%) Psychosis Risk (PLE) and 1,226 (82.39%) comparison control (NPLE) subjects. The PLE and NPLE groups did not differ in age, sex, or grade; however, PLE had a lower proportion of Caucasian participants than NPLE (see Table 1).
The SDQ assesses social and behavioral strengths and difficulties in young children and adolescents, and has adequate reliability and validity (Goodman, 2001). It has subscales for emotional symptoms, conduct problems, hyperactivity, peer problems, and prosocial behavior.
Study 2: CHR Sample
Participants.
This study included 29 CHR and 29 CN participants. CHR participants were recruited from a psychosis risk evaluation program, which received referrals from local clinicians to perform diagnostic assessment and monitor evaluations for youth displaying psychotic experiences. CHR individuals were also recruited via print and online advertisements, in-person presentations to community mental health centers, and calls or in-person meetings with members of the local school system. CHR participants were included if they met criteria for a prodromal syndrome on the Structured Interview for Psychosis-Risk Syndromes (SIPS; Miller et al., 2003; Miller et al., 1999). SIPS criteria included: 1) Attenuated Positive Symptoms (i.e., SIPS score of at least 3–5 on at least one positive symptom item, with worsening symptoms over the past year; n = 27); 2) Genetic Risk and Deterioration Syndrome (i.e., first degree relative with a psychotic disorder and decline in global functioning over the past year; n = 2). CHR participants did not meet lifetime criteria for a DSM-IV-TR psychotic disorder as determined by the Structured Clinical Interview for DSM (SCID; First, Spitzer, Gibbon, & Williams, 2002).
CN participants were recruited from the local community using posted flyers, newspapers advertisements, and electronic advertisements. CN had no current Axis I or Axis II Schizophrenia-Spectrum DSM-IV-TR diagnoses as established by the SCID-I (First et al., 2002) and the Structured Clinical Interview for DSM Axis II disorders (SCID-II; Pfohl, Blum, & Zimmerman, 1997), no family history of psychosis, and were not taking psychotropic medications. All participants were free from lifetime neurological disease. Groups did not significantly differ on age, ethnicity, sex, or personal education (see Table 1). Four of the CHR participants had been prescribed a second-generation antipsychotic.
Procedures and Measures.
Prior to completing the questionnaires, examiners who were trained to reliability standards (ICC > .80) conducted a structured diagnostic interview with all participants to complete the SCID-I, SCID-II and SIPS. CHR participants were also rated on the Prodromal Inventory of Negative Symptoms (PINS; Pelletier-Baldelli, Strauss, Visser, & Mittal, 2017), which is the CHR adapted version of the Brief Negative Symptom Scale (BNSS; Kirkpatrick et al., 2011), and completed the YPARQ-B.
Self-reported habitual emotion regulation strategy use was evaluated using the ERQ (i.e., the adult version; Gross & John, 2003). The ERQ is a 10-item questionnaire that measures the extent to which participants report using reappraisal (6 items) and expressive suppression (4 items) strategies to increase or decrease their positive and negative emotions, respectively. Self-reports are made on a 1 (strongly disagree) to 7 (strongly agree) scale. Higher scores ostensibly reflect greater dispositional tendencies toward using specific strategies across time and different contexts. The ERQ was developed on four undergraduate samples (N = 1,483). Psychometric properties have been considered adequate, with averaged alpha reliability scores of .79 for Reappraisal and .73 for Suppression, and test-retest reliability of .69 (Gross & John, 2003).
Study 3: SZ Sample
Participants.
Participants included 61 individuals diagnosed with SZ and 67 CN. Individuals with SZ were recruited from outpatient community mental health centers. Patients were evaluated during periods of clinical stability marked by no changes in medication type or dosage for at least four weeks. A best-estimate approach was employed for consensus diagnoses based on multiple clinical interviews and psychiatric history. The diagnoses were subsequently confirmed using the SCID-I (First et al., 2002).
CN subjects were recruited by random digit dialing, print and online advertisements and word-of-mouth from recruited participants. CN had no current Axis I or II diagnoses as established by the SCID-I and SCID-II, no family history of psychosis, and were not taking psychotropic medications. All participants denied a history of neurological injury or disease and substance use disorders within the last six months. Written informed consent was obtained for all participants for a protocol approved by the local university Institutional Review Boards.
The CN and SZ groups did not significantly differ in age, gender, or ethnicity; however, SZ had fewer years of personal education than CN. On average, patients displayed moderately severe positive and negative symptoms at the time of testing. Forty-seven of the SZ patients were prescribed a second generation antipsychotic, 3 a first generation antipsychotic, 6 a combination of first and second generation antipsychotics, and 5 were stably unmedicated (see Table 1).
Procedures and Measures.
Participants completed a battery of measures designed to assess emotion regulation, symptoms, and functional outcome. Emotion regulation was assessed using the same version of the ERQ administered in Study 2. A clinical interview was performed to assess symptom severity and functional outcome, after which the BNSS (Kirkpatrick et al., 2011; Strauss et al., 2012), the Brief Psychiatric Rating Scale (BPRS; Overall & Gorham, 1962), and Level of Function Scale (LOF; Hawk, Carpenter, & Strauss, 1975) were rated. Raters were trained to reliability standards (ICC > .80) using gold-standard clinical rating videos.
Data Analysis
A similar analytic approach was used to evaluate hypothesis 1 in relation to each study. First, a 2 Group × 2 Emotion Regulation Strategy repeated measures ANOVA was used to determine whether PLE/CHR/SZ and NPLE/CN groups reported different patterns of reappraisal and expressive suppression strategy use. One-way ANOVAs were performed to follow-up all significant interaction effects. To evaluate hypothesis 2 regarding changes in emotion regulation strategy use across the continuum of psychosis vulnerability, Z-scores were calculated separately for the psychosis group of each study using the mean and standard deviation of their respective control/comparison group and a 3 Group × 2 Strategy repeated measures ANOVA was conducted. The Z-score approach was necessary to account for use of two different versions of the ERQ (the Child/Adolescent version in Study 1 and the Adult version in Studies 2 and 3), which utilize different scales (0–5 versus 0–7, respectively). These analyses were then repeated using sex as an additional between-subjects factor. To evaluate hypothesis 3, Spearman correlations were calculated to determine associations between emotion regulation strategy use and clinical outcomes. Lastly, one-way ANOVAs were used to compare mean strategy use between males and females within and between psychosis groups. ERQ data have not previously been published on any of these samples, although data on other measures have been published from these samples for studies on SZ, CHR, and PLE (Strauss & Chapman, 2018; Strauss, Raugh, Mittal, Gibb, & Coles, 2018; Strauss, Ruiz, Visser, Crespo, & Dickinson, 2018; Sullivan & Strauss, 2017).
Results
Control Versus Psychosis Group Differences in Strategy Use
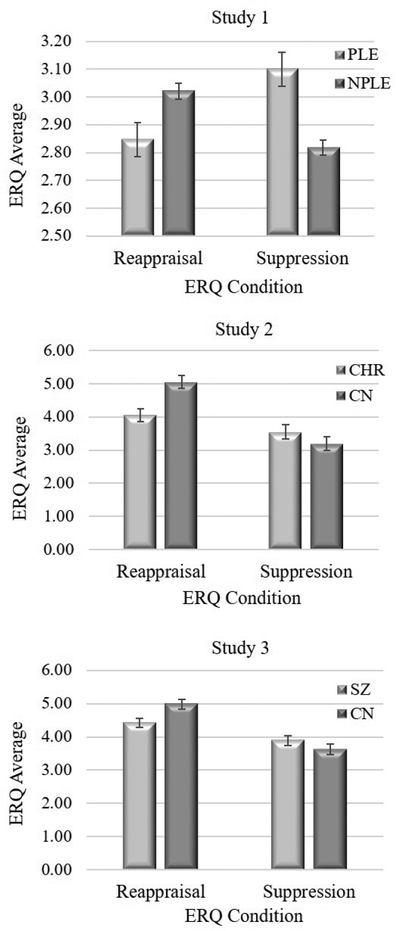
Table 2 presents ANOVA results for each study. Figure 1 presents group means and standard errors for ERQ variables in each study. In Study 1, there was a significant Group × Strategy interaction; however, the main effects of Group and Strategy were nonsignificant. Post hoc one-way ANOVAs indicated that PLE reported significantly less reappraisal and more suppression than NPLE. In Studies 2 and 3, the main effect of Strategy and the Group × Strategy interaction were significant, but the main effect of Group was not. In both studies, post hoc oneway ANOVAs indicated that SZ and CHR reported significantly less reappraisal than CN, but there was no group difference for suppression.
Table 2.
ANOVA Results for Studies 1–3
| Study 1 | Study 2 | Study 3 | |
|---|---|---|---|
| PLE: n = 262 | CHR: n = 29 | SZ: n = 61 | |
| NPLE: n= 1226 | CN: n = 29 | CN: n = 67 | |
| Group | F = 0.93, d = 0.06 | F = 2.16, d=0.41 | F = 1.39, d = 0.20 |
| Strategy | F = 0.44, d = 0.03 | F = 38.60***, d= 1.67 | F = 29.58***, d = 0.97 |
| Group × Strategy | F= 38.22***, d= 0.47 | F= 12.34**, d= 1.26 | F= 6.49*, d= 0.62 |
| Post Hoc Oneway ANOVAs | |||
| Reappraisal | F = 6.65*, d = 0.13 | F = 12.73**, d = 0.95 | F = 7.84**, d= 0.50 |
| Suppression | F= 17.62***, d= 0.22 | F = 1.28, d= 0.30 | F= 1.09, d = 0.19 |
Note.
p < 0.001;
p < 0.01;
p < 0.05.
PLE = individuals with psychotic-like experiences; NPLE = individuals without psychotic-like experiences; CHR = individuals at clinical high-risk; CN = controls; SZ = schizophrenia outpatients.
Figure 1.
Mean Strategy Use for Studies 1–3 96×210mm (150 × 150 DPI)
Effect of Psychosis Vulnerability
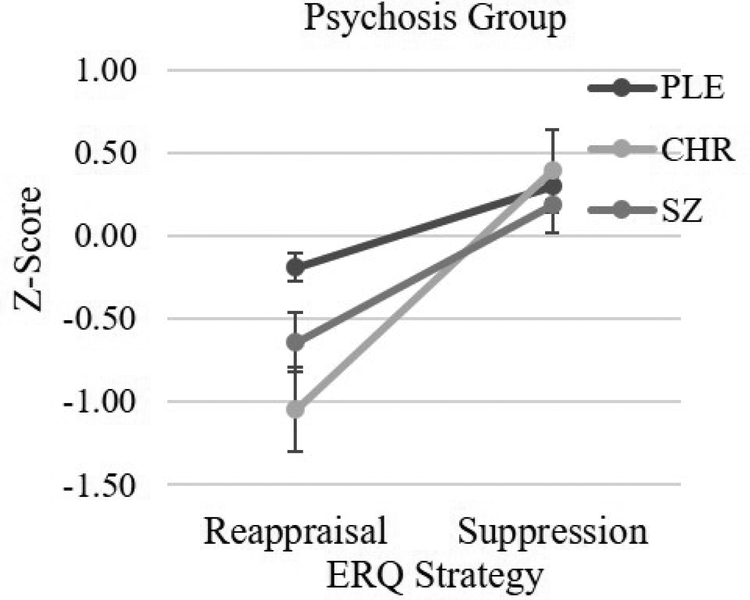
Figure 2 presents mean reappraisal and suppression Z-scores for each psychosis group relative to their respective control sample. There was a significant Psychosis Group (PLE, CHR, SZ) × Strategy (Reappraisal, Suppression) interaction. Additionally, the main effect of Strategy was significant; however, the main effect of Group was nonsignificant. Follow-up one-way ANOVAs indicated that the three groups differed on reappraisal, but not suppression. Post hoc LSD contrasts conducted on reappraisal indicated that PLE reported more use of reappraisal than CHR (p < 0.01) or SZ (p < 0.03); however, CHR and SZ did not differ (p = 0.20).1 Thus, there was evidence that reappraisal was specifically associated with vulnerability for psychosis, with scores declining as risk vulnerability increased from PLE to CHR states. Reappraisal did not differ between CHR and SZ, suggesting that use of reappraisal does not decrease with illness onset.2
Figure 2.
Mean Reappraisal and Suppression Z-Scores by Psychosis Group 84×69mm (150 × 150 DPI)
Sex Differences in Emotion Regulation Strategy Use
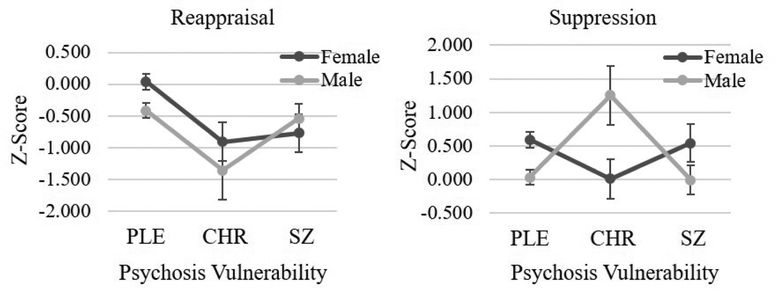
One-way ANOVAs indicated that PLE males scored significantly lower on reappraisal and suppression than PLE females. Among CHR, males scored significantly higher on suppression than females, but sex differences for reappraisal were nonsignificant. Similarly, the SZ group revealed no differences between males and females for either strategy. The Psychosis Group (PLE, CHR, SZ) × Strategy (Reappraisal, Suppression) × Sex (female, male) interaction was significant. Follow-up one-way ANOVAs were conducted separately per sex. For females, there was a significant effect of Psychosis Group for reappraisal, but not suppression. Post hoc LSD contrasts indicated that females with PLEs reported using reappraisal more than CHR or SZ females (ps < 0.01); however, female CHR and female SZ did not differ on reappraisal (p = 0.73). For males, the psychosis groups did not differ on reappraisal, but did differ on suppression. Post hoc LSD contrasts calculated for suppression indicated that male CHR reported more suppression than male PLE or SZ (ps < 0.02). However, male PLE and SZ did not differ on suppression (p = 0.87, see Table 3 and Figure 3).
Table 3.
Strategy Use by Psychosis Vulnerability and Sex
| Studies 1–3 | ||
|---|---|---|
| Psychosis Group | F = 1.82 | d = 0.26 |
| Sex | F = 0.24 | d = 0.06 |
| Strategy | F = 79.04*** | d = 0.91 |
| Psychosis Group × Sex | F = 2.14 | d = 0.31 |
| Psychosis Group × Strategy | F = 977*** | d = 0.68 |
| Sex × Strategy | F = 1.28 | d = 0.17 |
| Psychosis Group × Sex × Strategy | F = 6.42** | d = 0.59 |
| One-Way ANOVAs Across Groups | ||
| Reappraisal - Female | F = 759*** | d = 0.61 |
| Suppression - Female | F = 1.82 | d = 0.30 |
| Reappraisal - Male | F = 1.81 | d = 0.29 |
| Suppression - Male | F = 3.55* | d = 0.40 |
| One-Way ANOVAs Within Groups Comparing Sexes | ||
| Reappraisal | ||
| PLE (M vs F) | F = 7.68** | d = 0.35 |
| CHR (M vs F) | F = 0.67 | d = 0.32 |
| SZ (M vs F) | F = 0.31 | d = 0.14 |
| Suppression | ||
| PLE (M vs F) | F = 11.02*** | d = 0.42 |
| CHR (M vs F) | F = 4.29* | d = 0.81 |
| SZ (M vs F) | F = 3.11 | d = 0.51 |
Note.
p < 0.001;
p < 0.01;
p < 0.05.
PLE = individuals with psychotic-like experiences; NPLE = individuals without psychotic-like experiences; CHR = individuals at clinical high-risk; CN = controls; SZ = schizophrenia outpatients; M = males; F = females.
Figure 3.
Strategy Use by Sex 150×58mm (150 × 150 DPI)
Correlations with Clinical Outcomes
Table 4 presents correlations between ERQ and symptom variables for each study. When examining the effect of vulnerability in PLE and CHR through the YPARQ-B, greater severity of psychosis was associated with less use of reappraisal (r = −.26, p < .001) and suppression (r = −.19, p = .001). Interestingly, this effect was driven by the PLE group (reappraisal: r = −.19, p < .01; suppression: r = −.21, p < .01), as the correlations were nonsignificant in CHR (reappraisal: r = .16, p = .46; suppression: r = .03, p = .88). In Study 1, lower use of reappraisal was also associated with more severe emotional symptoms and less prosocial behavior on the SDQ, and lower use of suppression was associated with more severe emotional symptoms, conduct problems, hyperactivity, peer problems, and less prosocial behavior on the SDQ.
Table 4.
Correlations with Clinical Outcomes for Studies 1–3
| Reappraisal | Suppression | |
|---|---|---|
| Study 1 (PLE: n = 262) | ||
| SDQ Emotional Symptoms | .26*** | .43*** |
| SDQ Conduct Problems | .08 | .22*** |
| SDQ Hyperactivity | −.03 | .20** |
| SDQ Peer Problems | −.10 | .16* |
| SDQ Prosocial | .51*** | .34*** |
| YPARQ-B Total | −.19** | −.21** |
| Study 2 (CHR: n = 29) | ||
| SIPS Positive | .11 | −.02 |
| SIPS Negative | −.11 | .32 |
| SIPS Disorganized | .17 | −.22 |
| SIPS General | −.40* | −.30 |
| BNSS Anhedonia | −.16 | .16 |
| BNSS Avolition | .03 | .17 |
| BNSS Asociality | −.45* | .05 |
| BNSS Blunted Affect | −.15 | .37 |
| BNSS Alogia | −.12 | .32 |
| Study 3 (SZ: rc = 61) | ||
| BPRS Positive | −.14 | .22 |
| BPRS Negative | −.09 | −.05 |
| BPRS Disorganized | .19 | .07 |
| BPRS Total | −.14 | .13 |
| BNSS Anhedonia | −.33** | −.10 |
| BNSS A volition | −.08 | −.13 |
| BNSS Asociality | −.33** | .07 |
| BNSS Blunted Affect | −.17 | .04 |
| BNSS Alogia | −.19 | −.08 |
| LOF Social | .17 | −.09 |
| LOF Work | .06 | .11 |
Note.
p < 0.001;
p < 0.01;
p < 0.05.
PLE = individuals with psychotic-like experiences; CHR = individuals at clinical high-risk; SZ = schizophrenia outpatients; SDQ = Strengths and Difficulties Questionnaire; SIPS = Structured Interview for Psychosis-Risk Syndromes; BNSS = Brief Negative Symptom Scale; BPRS = Brief Psychiatric Rating Scale; LOF = Level of Function Scale.
In Study 2, lower use of reappraisal was significantly associated with asociality on the BNSS and general psychiatric symptoms on the SIPS. Follow-up item-level correlations indicated that the correlation with the SIPS general subscale reflected an association between lower reappraisal and more severely impaired tolerance to normal stress. There were no significant correlations with suppression.
In Study 3, reappraisal was significantly associated with greater severity of BNSS asociality and anhedonia. There were no significant correlations with suppression.
Discussion
Across three experiments, we examined self-reported emotion regulation strategy use in groups differing in level of vulnerability for psychosis. Groups included a community sample of youth with PLEs, youth at CHR for psychosis, and adults diagnosed with SZ. As predicted, each of the psychosis-spectrum groups reported using reappraisal significantly less than their comparison control group. Additionally, there was evidence for lower reappraisal in CHR than PLE, consistent with a vulnerability-related dose-dependent decrease. In contrast, mean reappraisal levels were similarly severe in CHR and SZ, suggesting that once individuals reach a clinical help-seeking prodromal stage, reappraisal abnormalities are firmly in place and do not worsen with illness onset. Age was not a significant covariate in the analyses comparing groups within each study. Prior research suggests that reappraisal increases with age in healthy samples (John & Gross, 2004; Martin & Ochsner, 2016; McRae et al., 2012; Silvers et al., 2012); however, we found higher reported use of reappraisal in the youngest group of participants (i.e., PLE). Thus, although there is strong evidence for effects of age on emotion regulation in adolescence, these effects did not supersede the impact of psychosis. Sex, however, may moderate reappraisal differently across phases of illness, with a more pronounced effect on males at the lower end of the psychosis continuum that dissipates in those with diagnosable illness.
With regard to suppression, we hypothesized that all three psychosis groups would report more suppression than CN. Findings were inconsistent with this hypothesis across studies. Youth at CHR for psychosis and adults with SZ did not differ from CN in suppression, whereas youth with PLEs reported using suppression more than NPLE youth. There was also no evidence for a vulnerability-related dose-dependent effect of suppression, which may be due to insufficient power. However, sex had a moderating effect on vulnerability, with female PLEs and SZ reporting more suppression than males, and the opposite pattern in CHR. This effect of sex on vulnerability should be interpreted with caution given the low number of male participants in the CHR group.
These findings help to clarify inconsistencies in group effects among prior studies, which may reflect demographics (e.g., sex, age), symptom profiles, and power. In particular, the proportion of males vs females in a sample may influence whether group differences are observed on reappraisal vs suppression subscales. Additionally, our effect sizes were small to moderate, indicating that prior studies with insufficient sample sizes may not have been adequate to detect potential effects.
Consistent with several past studies, significant associations between emotion regulation strategy use and clinical outcomes were observed (Badcock et al., 2011; Horan et al., 2013; Kimhy et al., 2016; Kimhy et al., 2012; Perry et al., 2011). In all three studies, lower use of reappraisal was associated with asociality. These findings are consistent with some prior studies examining adults with SZ and CHR youth. In the PLE and CHR groups, lower use of reappraisal was associated with greater severity of general psychiatric symptoms. In adults with SZ, lower use of reappraisal was associated with greater severity of anhedonia. These findings indicate that across the continuum of psychotic experiences, lower use of reappraisal is associated with negative and general symptoms. In contrast, suppression only showed significant correlations with clinical variables in the community sample of youth with PLEs, where associations were found with psychosis and general psychiatric symptoms. This suggests that greater severity of psychosis may be driving associations with emotion regulation, regardless of vulnerability status. These findings are consistent with some prior literature indicating that poor clinical outcomes are more strongly associated with abnormalities in reappraisal than suppression (Kimhy et al., 2012; Perry et al., 2011; Strauss et al., 2015), although other studies have found stronger associations with suppression (Aldao et al., 2010; Badcock et al., 2011; Horan et al., 2013; Kimhy et al., 2016; Perry et al., 2011).
Despite the strengths of the current studies, certain limitations should be considered. First, the differences in age and psychosis vulnerability between samples necessitated using common but different measures across studies. The child version of the ERQ (i.e., the ERQ-CA) was used in Study 1, and the original ERQ was used in Studies 2 and 3. These versions have slightly different scale characteristics and psychometric properties. Direct statistical comparisons that were made among the psychosis vulnerability states should be interpreted with this limitation in mind. Additionally, clinical scales used across studies varied, complicating interpretation of symptom associations. Second, although the SZ and PLE samples were relatively large, the CHR sample was not, particularly for analyses examining sex differences in relation to psychosis vulnerability. Given that there were only nine males in the CHR group, sex difference findings should be considered preliminary until replicated. Third, Z-scores were used to compare the clinical samples on reappraisal and suppression. These are influenced by sample size, which was much larger in the community sample than the CHR or SZ samples. Larger samples are more representative and less prone to influences of extreme scores, and therefore, sample size may have influenced group comparisons using Z-scores, making the CHR sample, in particular, more influenced by variations in individual differences. CHR findings should be interpreted with this limitation in mind. Fourth, we did not administer a measure of emotional awareness along with the ERQ. Prior studies have demonstrated that emotional awareness is a significant mediator of the association between emotion regulation and clinical outcomes (Kimhy et al., 2012). Future studies should explore the role of emotional awareness in emotion regulation abnormalities across the psychosis continuum. Fifth, each of the studies used a cross-sectional design. In future studies, it will be important to follow community and CHR groups over time using a prospective longitudinal design to determine whether emotion regulation abnormalities worsen with illness onset. Longitudinal studies are necessary to inform etiological models and highlight novel biomarkers. Sixth, the effects of antipsychotic medication were not systematically evaluated across studies, and it is currently unknown whether antipsychotics contribute to the emotion regulation abnormalities observed in the schizophrenia spectrum. Finally, emotion regulation was evaluated through self-report alone, which can reflect biases regarding gender stereotypes and fails to observe underlying components of emotion regulation (e.g., cognitive demand) that may contribute to emotion regulation abnormalities (McRae et al., 2012; McRae et al., 2008). Future studies should implement neurophysiological and psychophysiological assessments to explore potential mechanisms underlying self-reported abnormalities.
Despite these limitations, findings have important treatment implications. There are now psychosocial interventions developed for emotion regulation, which have proven effective in several psychiatric disorders (Fresco, Mennin, Heimberg, & Ritter, 2013; Renna, Quintero, Fresco, & Mennin, 2017); however, these interventions have yet to be evaluated in the schizophrenia-spectrum to determine their utility for prevention or symptom reduction. The current findings suggest that interventions targeting reappraisal may be particularly beneficial.
Acknowledgments
This work was supported by the National Institute of Mental Health, Grant K23-MH092530 and the Transdisciplinary Areas of Excellence grant from the State University of New York, Binghamton.
Footnotes
When the Tukey-Kramer post hoc test for unequal sample sizes was applied, the group difference between PLE and SZ was at a trend level (p = .058).
After accounting for age as a covariate, the overall omnibus repeated measures ANOVA remained significant (F(1, 349) = 6.34, p = .002, η p2 = .04). Given that the cross-group analyses were potentially confounded, we also ran analyses examining age as a covariate within each group. The Group × Strategy interaction remained significant when controlling for age for the PLE group (F(1, 1478) = 37.8, p <.001, η p2 = .03), the CHR group (F(1, 55) = 14.19, p < .001, η p2 = .21), and the SZ group (F(1, 145) = 7.25, p = .008, η p2 = .05). After accounting for education as a covariate, the overall omnibus repeated measures ANOVA remained significant (F(1, 360) = 4.5, p < .02, η p2 = .03).
References
- Addington J, Cadenhead KS, Cannon TD, Cornblatt B, McGlashan TH, Perkins DO, … Heinssen R. (2007). North American Prodrome Longitudinal Study: A collaborative multisite approach to prodromal schizophrenia research. Schizophrenia Bulletin, 33(3), 665–672. doi: 10.1093/schbul/sbl075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldao A, Nolen-Hoeksema S, & Schweizer S (2010). Emotion-regulation strategies across psychopathology: A meta-analytic review. Clinical Psychology Review, 30(2), 217–237. doi: 10.1016/j.cpr.2009.11.004 [DOI] [PubMed] [Google Scholar]
- Badcock JC, Paulik G, & Maybery MT (2011). The role of emotion regulation in auditory hallucinations. Psychiatry Research, 185(3), 303–308. doi: 10.1016/j.psychres.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Balzarotti S, John OP, & Gross JJ (2010). An Italian adaptation of the Emotion Regulation Questionnaire. European Journal of Psychological Assessment, 26(1), 61–67. doi: 10.1027/1015-5759/a000009 [DOI] [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, … Heinssen R. (2008). Prediction of psychosis in youth at high clinical risk: A multisite longitudinal study in North America. Archives of General Psychiatry, 65(1), 28–37. doi: 10.1001/archgenpsychiatry.2007.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, Yu C, Addington J, Bearden CE, Cadenhead KS, Cornblatt BA, … Kattan MW. (2016). An individualized risk calculator for research in prodromal psychosis. American Journal of Psychiatry, 173(10), 980–988. doi: 10.1176/appi.ajp.2016.15070890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L, & Malaspina D (2003). The stress cascade and schizophrenia: Etiology and onset. Schizophrenia Bulletin, 29(4), 671–692. doi: 10.1093/oxfordjournals.schbul.a007038 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured Clinical Interview for DSM-IV-TR Axis 1 Disorders—Research Version—Patient Edition (SCIDIV-P). New York: New York State Psychiatric Institute, Biometrics Research Department. [Google Scholar]
- Fresco DM, Mennin DS, Heimberg RG, & Ritter M (2013). Emotion regulation therapy for generalized anxiety disorder. Cognitive and Behavioral Practice, 20(3), 282–300. doi: 10.1016/j.cbpra.2013.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Cappucciati M, Rutigliano G, Schultze-Lutter F, Bonoldi I, Borgwardt S, … McGuire P. (2015). At risk or not at risk? A meta-analysis of the prognostic accuracy of psychometric interviews for psychosis prediction. World Psychiatry, 14(3), 322–332. doi: 10.1002/wps.20250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P, Tantardini M, De Simone S, Ramella-Cravaro V, Oliver D, Kingdon J, … & Galderisi S. (2017). Deconstructing vulnerability for psychosis: Meta-analysis of environmental risk factors for psychosis in subjects at ultra high-risk. European Psychiatry, 40, 65–75. doi: 10.1016/j.eurpsy.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Goodman R (1997). The Strengths and Difficulties Questionnaire: A research note. Journal of Child Psychology and Psychiatry, 38(5), 581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x [DOI] [PubMed] [Google Scholar]
- Goodman R (2001). Psychometric properties of the Strengths and Difficulties Questionnaire. Journal of the American Academy of Child and Adolescent Psychiatry, 40(11), 1337–1345. doi: 10.1097/00004583-200111000-00015 [DOI] [PubMed] [Google Scholar]
- Gross JJ, & John OP (2003). Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology, 85(2), 348–362. doi: 10.1037/0022-3514.85.2.348 [DOI] [PubMed] [Google Scholar]
- Gullone E, & Taffe J (2011). The Emotion Regulation Questionnaire for Children and Adolescents (ERQ-CA): A psychometric evaluation. Psychological Assessment, 24(2), 409–417. doi: 10.1037/a0025777 [DOI] [PubMed] [Google Scholar]
- Hawk AB, Carpenter WJ, Strauss JS (1975). Diagnostic criteria and five-year outcome in schizophrenia: A report from the International Pilot Study of Schizophrenia. Archives of General Psychiatry, 32, 343–347. doi: 10.1001/archpsyc.1975.01760210077005 [DOI] [PubMed] [Google Scholar]
- Henry JD, Rendell PG, Green MJ, McDonald S, & O’Donnell M (2008). Emotion regulation in schizophrenia: Affective, social, and clinical correlates of suppression and reappraisal. Journal of Abnormal Psychology, 117(2), 473–478. doi: 10.1037/0021-843x.117.2.473 [DOI] [PubMed] [Google Scholar]
- Horan WP, Hajcak G, Wynn JK, & Green MF (2013). Impaired emotion regulation in schizophrenia: Evidence from event-related potentials. Psychological Medicine, 43(11), 2377–2391. doi: 10.1017/s0033291713000019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, & Gross JJ (2004). Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality, 72(6), 1301–1334. doi: 10.1111/j.1467-6494.2004.00298.x [DOI] [PubMed] [Google Scholar]
- Johns LC, & van Os J (2001). The continuity of psychotic experiences in the general population. Clinical Psychology Review, 21(8), 1125–1141. doi: 10.1016/S0272-7358(01)00103-9 [DOI] [PubMed] [Google Scholar]
- Kelleher I, & Cannon M (2011). Psychotic-like experiences in the general population: Characterizing a high-risk group for psychosis. Psychological Medicine, 41, 1–6. doi: 10.1017/S0033291710001005 [DOI] [PubMed] [Google Scholar]
- Kimhy D, Gill KE, Brucato G, Vakhrusheva J, Arndt L, Gross JJ, & Girgis RR (2016). The impact of emotion awareness and regulation on social functioning in individuals at clinical high risk for psychosis. Psychological Medicine, 46(14), 2907–2918. doi: 10.1017/s0033291716000490 [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Jobson-Ahmed L, Tarrier N, Malaspina D, & Gross JJ (2012). Emotion awareness and regulation in individuals with schizophrenia: Implications for social functioning. Psychiatry Research, 200(2–3), 193–201. doi: 10.1016/j.psychres.2012.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, & Marder SR (2011). The Brief Negative Symptom Scale: Psychometric properties. Schizophrenia Bulletin, 37(2), 300–305. doi: 10.1093/schbul/sbq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E, Wilson C, Ereshefsky S, Denenny D, Thompson E, Pitts SC, … Schiffman J. (2012). Psychosis risk screening in youth: A validation study of three self-report measures of attenuated psychosis symptoms. Schizophrenia Research, 141(1), 72–77. doi: 10.1016/j.schres.2012.07.022 [DOI] [PubMed] [Google Scholar]
- Linscott RJ, & van Os J (2010). Systematic reviews of categorical versus continuum models in psychosis: Evidence for discontinuous subpopulations underlying a psychometric continuum. Implications for DSM-V, DSM-VI, and DSM-VII. Annual Review of Clinical Psychology, 6, 391–419. doi: 10.1146/annurev.clinpsy.032408.153506 [DOI] [PubMed] [Google Scholar]
- Livingstone K, Harper S, & Gillanders D (2009). An exploration of emotion regulation in psychosis. Clinical Psychology and Psychotherapy, 16(5), 418–430. doi: 10.1002/cpp.635 [DOI] [PubMed] [Google Scholar]
- Martin RE, & Ochsner KN (2016). The neuroscience of emotion regulation development: Implications for education. Current Opinion in Behavioral Sciences, 10, 142–148. doi: 10.1016/j.cobeha.2016.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlashan T, Miller TJ, Woods SW, Hoffman RE, Davidson L (2001). Instrument for the assessment of prodromal symptoms and states. In Miller T, Mednick SA, McGlashan TH, Libiger J, Johannessen JO (Series Ed.), NATO Science Series: Vol. 91. Early Intervention in Psychotic Disorders (pp. 135–149). doi: 10.1007/978-94-010-0892-1_7 [DOI] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, … & Ochsner KN. (2012). The development of emotion regulation: An fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7(1), 11–22. doi: 10.1093/scan/nsr093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Ochsner KN, Mauss IB, Gabrieli JJD, Gross JJ (2008). Gender differences in emotion regulation: An fMRI study of cognitive reappraisal. Group Processes and Intergroup Relations, 11(2), 143–162. doi: 10.1177/1368430207088035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, … & Woods SW. (2003). Prodromal assessment with the Structured Interview for Prodromal Syndromes and the Scale of Prodromal Symptoms: Predictive validity, interrater reliability, and training to reliability. Schizophrenia Bulletin, 29(4), 703–715. doi: 10.1093/oxfordjournals.schbul.a007040 [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Woods SW, Stein K, Driesen N, Corcoran CM, … Davidson L. (1999). Symptom assessment in schizophrenic prodromal states. Psychiatric Quarterly, 70(4), 273–287. doi: 10.1023/A:1022034115078 [DOI] [PubMed] [Google Scholar]
- Ord LM, Myles-Worsley M, Blailes F, & Ngiralmau H (2004). Screening for prodromal adolescents in an isolated high-risk population. Schizophrenia Research, 71(2–3), 507–508. doi: 10.1016/j.schres.2004.03.014 [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR (1962). The Brief Psychiatric Scale (BPRS). Psychological Reports, 10, 799–812. doi: 10.2466/pr0.1962.10.3.799 [DOI] [Google Scholar]
- Pelletier-Baldelli A, Strauss GP, Visser KH, & Mittal VA (2017). Initial development and preliminary psychometric properties of the Prodromal Inventory of Negative Symptoms (PINS). Schizophrenia Research, 189, 43–49. doi: 10.1016/j.schres.2017.01.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry Y, Henry JD, & Grisham JR (2011). The habitual use of emotion regulation strategies in schizophrenia. British Journal of Clinical Psychology, 50(2), 217–222. doi: 10.1111/j.2044-8260.2010.02001.x [DOI] [PubMed] [Google Scholar]
- Pfohl BM, Blum N, & Zimmerman M (1997). Structured Interview for DSM-IV Personality (SIDP-IV). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Renna ME, Quintero JM, Fresco DM, & Mennin DS (2017). Emotion regulation therapy: A mechanism-targeted treatment for disorders of distress. Frontiers in Psychology, 8, 98. doi: 10.3389/fpsyg.2017.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen JL, Woods SW, Miller TJ, & McGlashan TH (2002). Prospective observations of emerging psychosis. Journal of Nervous and Mental Disease, 190(3), 133–141. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, & Ochsner KN (2012). Age-related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12(6), 1235–1247. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, & Chapman HC (2018). Preliminary psychometric properties of the brief Negative Symptom Scale in youth at clinical high-risk for psychosis. Schizophrenia Research, 193, 435–437. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Kappenman ES, Culbreth AJ, Catalano LT, Ossenfort KL, Lee BG, & Gold JM (2015). Emotion regulation abnormalities in schizophrenia: Directed attention strategies fail to decrease the neurophysiological response to unpleasant stimuli. Journal of Abnormal Psychology, 124(2), 288–301. doi: 10.1037/abn0000017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Keller WR, Buchanan RW, Gold JM, Fischer BA, McMahon RP, … Kirkpatrick B. (2012). Next-generation negative symptom assessment for clinical trials: Validation of the Brief Negative Symptom Scale. Schizophrenia Research, 142(1–3), 88–92. doi: 10.1016/j.schres.2012.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Raugh IM, Mittal VA, Gibb BE, & Coles ME (2018). Bullying victimization and perpetration in a community sample of youth with psychotic-like experiences. Schizophrenia Research, 195, 534–536. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Ruiz I, Visser KH, Crespo LP, & Dickinson EK (2018). Diminished hedonic response in neuroleptic-free youth at ultra high-risk for psychosis. Schizophrenia Research: Cognition, 12, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan SK, & Strauss GP (2017). Electrophysiological evidence for detrimental impact of a reappraisal emotion regulation strategy on subsequent cognitive control in schizophrenia. Journal of abnormal psychology, 126(5), 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurel A, & Gentaz E (2018). The regulation of emotions in adolescents: Age differences and emotion-specific patterns. PloS One, 13(6), e0195501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Swart M, van der Velde J, Pijnenborg G, Wiersma D, Bruggeman R, & Aleman A (2014). Neural correlates of emotion regulation in patients with schizophrenia and non-affected siblings. PLoS One, 9(6), e99667. doi: 10.1371/journal.pone.0099667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, van’t Wout M, & Aleman A (2009). Emotion regulation strategies in patients with schizophrenia. Psychiatry Research, 170(2–3), 108–113. doi: 10.1016/j.psychres.2009.07.010 [DOI] [PubMed] [Google Scholar]
- Walker E, Mittal V, & Tessner K (2008). Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annual Review of Clinical Psychology, 4, 189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248 [DOI] [PubMed] [Google Scholar]
- Walker EF, Trotman HD, Pearce BD, Addington J, Cadenhead KS, Cornblatt BA,… & Tsuang MT. (2013). Cortisol levels and risk for psychosis: Initial findings from the North American Prodrome Longitudinal Study. Biological psychiatry, 74(6), 410–417. doi: 10.1016/j.biopsych.2013.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, & McGorry PD (1996). The initial prodrome in psychosis: Descriptive and qualitative aspects. Australian and New Zealand Journal of Psychiatry, 30(5), 587–599. doi: 10.3109/00048679609062654 [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, & McGorry PD (2004). Risk factors for psychosis in an ultra high-risk group: Psychopathology and clinical features. Schizophrenia Research, 67(2–3), 131–142. doi: 10.1016/s0920-9964(03)00192-0 [DOI] [PubMed] [Google Scholar]