Abstract
Adverse drug reaction is defined by the World Health Organization as any response to a drug that is noxious and unintended and occurs at a dose normally used in man. Older people are at elevated risk of adverse drug reactions—because of changes in pharmacodynamics, concurrent use of multiple medications and the related drug interactions. However, adverse drug reactions are significantly underestimated in the elderly population that is also exposed to inappropriate drugs. Amiodarone is an antiarrhythmic drug used commonly for the treatment of atrial fibrillation and is increasingly prescribed in older people. While amiodarone is an efficient drug for rhythm control, it's a carrier of different adverse reactions, and pro and cons must be carefully evaluated before its use especially in older people.
Keywords: Amiodarone, Atrial fibrillation, Drug-adverse reaction, Drug toxicity
1. Introduction
Amiodarone, a versatile class III antiarrhythmic drug, is considered one of the most effective agents in counteracting supraventricular and ventricular tachyarrhythmia. The drug, however, has a high toxicity profile involving diverse organs. The World Health Organization (WHO) defines an ADR as “any response to a drug which is noxious and unintended, and occurring at a dose normally used in man”.[1],[2]
ADRs, which are associated with the substantial expansion in the use of medications,[3] increase the risk of death, the length of hospitalization, and the cost of care.[4]
In the UK, ADRs are responsible for up to 6% of hospital admissions,[5] carry a mortality of 2%, and are associated with high costs. Similar ADRs hospital admission prevalence has been reported in studies conducted in Germany (6.5%)[6] and in Italy (6.1%).[7]
Amiodarone is widely used in patients with atrial fibrillation (AF),[8] and it's increasingly prescribed in elderly patients because of the increasing incidence of AF with age.
Older people are at elevated risk of ADRs for changes in pharmacodynamics, concurrent use of multiple medications and related interactions.
In this review, we focus on the pharmacology and adverse reactions of Amiodarone.
2. Amiodarone
2.1. General characteristics
Amiodarone (Figure 1) is an iodinated benzofuran derivative, structurally similar to thyroid hormones, containing about 37% of organic iodine that is in part (> 10%) released as free iodide in vivo. The daily maintenance dose between 100 and 600 mg, corresponding to 3.5–21 mg of iodine into the systemic circulation, equivalent to 35–140 fold excess of the iodine reference daily intake of 100–150 µg.[9]
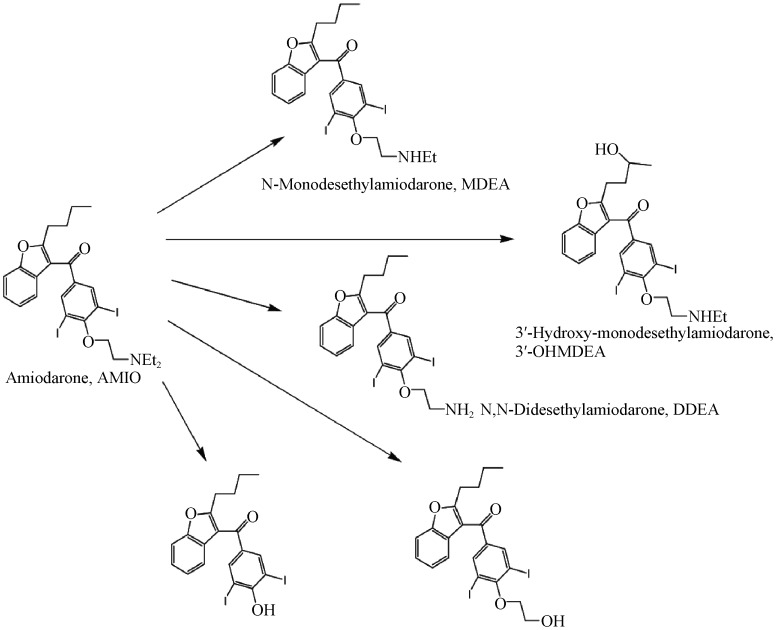
Figure 1. Amiodarone and its metabolites in human plasma.
Amiodarone shows electrophysiological characteristics of all four Vaughan Williams classes.[10] The most important effect belongs to class III antiarrhythmic, i.e., prolongation of cardiac action potential time. Class I activity on fast sodium (Na+) channels is more pronounced at rapid heart rates. In addition, Amiodarone has antisympathetic properties (class II activity), because noncompetitively inhibits α- and ß-adrenergic receptors. It possesses negative chronotropic effects in nodal tissues (class IV activity) by L-type calcium (Ca2+) channel blockade. Amiodarone also inhibits myocardial potassium (K+) channels. The anti-sympathetic activity and the blockade of Ca2+ and K+ channels slow the conduction and refractoriness in both the sinus and atrioventricular nodes.
2.2. Pharmacokinetics and metabolism
Oral Amiodarone has a slow and variable absorption rate, with a bioavailability of about 40%. After the first dose, the peak plasma levels are achieved in three to seven hours. The onset of action may take several days to a few weeks, thus loading doses help to achieve therapeutic levels more quickly. Plasma levels with repeated dosing at 100–600 mg/day are approximately dose proportional, with a mean 0.5 mg/L increase for each 100 mg/day. The steady-state plasma levels range 1.07–1.19 µg/mL but are up to 20 times more concentrated in the thyroid.[11] The bioavailability may be influenced by age, liver disease, and interactions with other drugs or substances that inhibit or induce cytochrome (CYP) 450, thus affecting efficacy. Amiodarone is a lipophilic drug, which absorption is enhanced if taken with foods high in fat content,[12] and has a large but variable volume distribution (66 L/kg of body weight). It accumulates mainly in adipose tissue (from which it's released very slowly) and in highly perfused organs such as liver, lung, and skin. Because of its long biological half-life of up to 100 days, the activity may persist months after the interruption. About 10%–30% of Amiodarone crosses the placenta and a varying amount is excreted in breast milk.[10],[13] Long-term oral treatment in a dose between 200 and 600 mg/day has minimal effects on hemodynamics. It causes a dose-related negative inotropic effect through a reduction in systemic vascular resistance. Generally, it doesn't have an effect on left ventricular ejection fraction and can be used safely even in patients with significant left ventricular dysfunction. Hypotension is rare during oral therapy (< 1%) but may occur in 16% of patients who receive intravenous treatment for refractory ventricular fibrillation or hemodynamically unstable ventricular tachycardia.[10],[13]
Amiodarone is mostly eliminated through biliary excretion in the gastrointestinal tract and minimally in urine. Hepatic metabolism proceeds mainly via N-dealkylation catalyzed by CYPP450, specifically CYP3A4 and CYP2C8. Desethyl-amiodarone (Figure 1) shows a similar structure and electrophysiological activity, although less effective, of the parent drug.[14] Amiodarone inhibits p-glycoprotein and CYP450 enzymes that alter serum levels of other drugs or substances metabolized by these routes.[15]
Because of its water insolubility, Amiodarone is dissolved in polysorbate-80, a substance that may contribute to side effects of the commercially available compound, i.e., reduction in blood pressure, heart rate, depression of atrioventricular node conduction, and increase in atrial and ventricular refractory periods.[16]
Upon infusion, a large percentage of Amiodarone binds to proteins, mainly to albumin and in a smaller percentage to beta-lipoprotein and alpha-1 acid glycoprotein.[17] Amiodarone is a strong hepatic and renal metabolism inhibitor. It inhibits different cytochrome P450 pathways, including CYP2C9, which metabolizes warfarin, CYP2D6, which metabolizes several beta-blockers and narcotics, and CYP3A4, which metabolizes cyclosporine and calcium channel blockers.[18] The most clinically relevant interaction occurs with digoxin and warfarin. Concerning warfarin, the reduced clearance leads to an increase in prothrombin time and international normalized ratio.[19] Digoxin levels double after Amiodarone co-administration, the result of P-glycoprotein transport system inhibition and consequent reduction in digoxin secretion from the renal tubules.[20] Therefore, it is recommended to halve digoxin dosage after the introduction of Amiodarone and to closely monitor plasma digoxin levels. Patients treated with Amiodarone should avoid grapefruit or grapefruit juice because it could inhibit Amiodarone conversion to the active metabolite.[21]
2.3. Indications
2.3.1. Oral administration
Oral Amiodarone was approved by the Food and Drug Administration (FDA) for the treatment of life-threatening and recurrent ventricular arrhythmias, such as ventricular fibrillation or ventricular tachycardia.[22] Prospective trials, in patients treated with Amiodarone after myocardial infarction with left ventricular systolic dysfunction, have shown improved survivability.[23],[24] Despite these results, there is some evidence that Amiodarone could adversely affect survival in patients with New York Heart Association (NYHA) functional class III heart failure.[25]
Implantable cardioverter-defibrillators are superior to Amiodarone in improving survival in patients with sustained ventricular tachycardia or ventricular fibrillation due to a non-reversible cause, and in patients at elevated risk for sudden cardiac death, i.e., selected patients after myocardial infarction or with cardiomyopathy. The molecule is the first-line antiarrhythmic drug for patients with ventricular tachycardia or ventricular fibrillation who are not candidates for an implantable cardioverter-defibrillator (ICD).[26] Amiodarone has been used up to 70% in patients with an ICD to reduce frequent arrhythmias related shocks. Moreover, it can be used also to suppress symptomatic, un-sustained ventricular tachycardia.
Amiodarone is commonly used to treat AF although is not approved by the FDA for this purpose. The drug should be considered only after the failure of, or contraindications to, other medications because its potential organ toxicity. According to the American College of Cardiology (ACC)/American Heart Association (AHA)/Heart Rhythm Society guidelines,[27] it can be used to maintain sinus rhythm in coronaropathy patients presenting heart failure, left ventricular systolic dysfunction, left ventricular hypertrophy and/or drug-refractory symptomatic AF. Finally, some studies suggest that, Amiodarone may prevent AF related to cardiac surgery, but preoperative prophylaxis use is not universally accepted.[28]
2.3.2. Intravenous administration
Even though tissue levels rise rapidly, effective arrhythmia suppression and prevention can take days or longer. Sinus bradycardia, atrioventricular blocks and torsade de pointes can occur after intravenous loading.[29]
Intravenous administration is approved by FDA for the treatment and prophylaxis of recurrent ventricular fibrillation, hemodynamically unstable ventricular tachycardia, and to prevent ventricular fibrillation or tachycardia in patients candidate to oral Amiodarone but unable to take the oral preparation.[30]
Based on the 2010 American Heart Advanced Cardiac Life Support guidelines, intravenous administration is the first choice for persistent ventricular fibrillation or pulseless ventricular tachycardia resistant to other resuscitative measures including epinephrine.[28] Amiodarone can be used to treat lidocaine-refractory ventricular tachycardia or ventricular fibrillation after acute myocardial infarction, “electrical storm” (multiple episodes of recurrent rapid ventricular tachycardia or ventricular fibrillation requiring multiple shocks over a brief time period), and non-sustained and recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators who experience frequent device shocks.[31]
The drug administered intravenously can treat also supraventricular, most commonly acute-onset AF, including perioperative cardiovascular surgery, in intensive care units, and in emergency departments. Although it can slow the ventricular response during acute-onset AF, it is not approved by FDA for this purpose.[32]
In placebo-controlled trials, intravenous Amiodarone has not been shown to convert AF acutely.[32] Although, it can reduce the occurrence of AF after cardiac surgery, a reduction in the length of hospital stay and adverse outcomes has not been demonstrated yet.[33] Intravenous Amiodarone is reported to induce heart block or bradycardia in 4.9% of patients, and hypotension in 16%.
2.4. Contraindications
Amiodarone is contraindicated in patients with cardiogenic shock, severe sinus-node dysfunction, marked sinus bradycardia, second- or third-degree atrioventricular block, and syncope related to bradycardia. It is contraindicated in patients with a known hypersensitivity to the drug or to any of its components, including iodine.[34]
3. Side effects and adverse reactions
3.1. Cardiac adverse reactions
Amiodarone could impair sinus beat formation and conduction and may induce significant bradycardia, especially in patients with pre-existing conduction disorders, and marked prolongation of the QT interval. These events are due to its effect in blocking the potassium channels. This mechanism, moreover, can lead to dangerous polymorphic ventricular arrhythmia or torsade de pointes.[35] Physicians should be aware that QT elongation can be favoured temporary in patients on Amiodarone therapy, like severe lung disease, hypertension, concomitant antiarrhythmic drugs, antibiotics, antipsychotics, and anti-depressive drugs (Table 1).
Stramba-Badiale, et al.[36] reported that female gender may play a role in the occurrence of QT interval prolongation because sex steroid hormones may influence ventricular repolarization. In accord to this hypothesis, prolongation of QT interval during Amiodarone treatment in the female patients is found only after puberty. Notwithstanding, Amiodarone appears to have the lowest pro-arrhythmic action compared to other antiarrhythmic agents. The reason for the low incidence of pro-arrhythmia remains unclear but it's probably multifactorial.[37] Moreover, drug-induced QT prolongation most commonly occurs in patients with subclinical mutations in one of the genes causing the congenital long QT syndrome. In fact, the allelic variants of the coding region of congenital long QT syndrome, can be identified in 10 to 15% of patients with Amiodarone induced QT prolongation.[38]
Antonelli, et al.[39] showed that these patients are at higher risk of torsade de point after potassium channels blocking drugs administration, such as Amiodarone. Early episodes of torsade de point in patients on oral low dose (200 mg/day), in the absence of other predisposing factors, emphasize the importance of a careful patient monitoring.
Isoproterenol infusion is the drug of choice to increase the heart rate and to shorten the QT interval in patients with Amiodarone-associated symptomatic bradycardia and/or prolonged QT interval.
Amiodarone acutely infused intravenously can provoke hypotension by inducing vasodilation and depression of myocardial contractility. Curiously, this mechanism has been proposed to be more related to the eccipient polysorbate 80 or benzyl alcohol than Amiodarone itself.[40] In accord, experimental studies have shown that hypotension is not observed when is Amiodarone is dissolved in cyclodextrin.[40] Amiodarone may also cause signs and symptoms of allergic reactions, including angioedema, urticarial rash or even anaphylactic reactions that rapidly improve after drug discontinuation.[34]
3.2. Ocular adverse reactions
Ocular alterations represent the most frequent side effect of Amiodarone. Among this group can be found a wide spectrum of abnormalities. Typically, Amiodarone, can led to corneal microdeposits (up to 98%), corneal opacities, lenticular changes, loss of eyelashes or eyebrows, papilledema, photosensitivity, scotoma, macular degeneration and optic neuropathy or optic neuritis.[41]
Symmetrical microdeposits in the granular corneal epithelium, anterior to Bowman's membrane, occurs early and are found in nearly 100% after 1 to 2 months of treatment.[42] Asymptomatic corneal opacities are present in 50%–60% of patients. However, these changes do not affect eyesight and drug discontinuation is not warranted.[43] Retinopathy has rarely been reported.[44] Clinical findings of optic neuropathy share features with non-arteritic anterior ischemic optic neuropathy (NAION), the most common cause of visual loss from optic nerve disease among individuals over the age of 50.[45]
Mindel, et al.[46] presented the largest study with the aim to associate Amidoarone and vision loss. In their study, 1600 patients for up to 45.5 months, failed to find an association between Amiodarone and bilateral toxic vision loss. However, it must be considered that in this study, patients did not go through an ophthalmologic evaluations, but just through a nurse's query on “optic neuritis”, making unilateral or mild bilateral visual loss go undetected.
Recently, Passman, et al.[47] retrieved data from the FDA Adverse Event Reporting Systems and found 296 cases of optic neuropathy associated with drug therapy. Mansour, et al.[48] identified multiple lamellate inclusion bodies in large axons of the optic nerve, while demyelination or loss of large axons was not seen. Meanwhile, demyelination, loss of large axon and lamellate bodies are seen in the neurons and the Schwann cells of peripheral nerves in Amiodarone-associated peripheral neuropathy.[49],[50] Lamellate bodies deposits have been attributed to inhibition of lysosomal sphingomyelinases, but it's not clear whether histopathological differences are due to the type of cell or differences in the time course of ocular versus peripheral neuropathy findings before clinical symptoms develop.[44]
Amiodarone induced optic neuropathy and NAION differ with respect to optic disk appearance on funduscopic examination, sex distribution, laterality, and duration of disk oedema. Amiodarone-induced optic neuropathy occurs more often in men, tends to be insidious in onset, and two third of cases show bilateral involvement.[47] On the other hand, NAION tends to be equally sex distributed it appears often on acute onset, unilaterally, and resolves within weeks.
Moreover, whereas NAION occurs more often in patients with a small optic nerve cup-disk ratio, there is no particular cup-disk ratio associated with Amiodarone associated optic neuropathy.[51],[52]
Doubts can be related to the fact that many patients have concurrent therapy, more often with digoxin that, enhanced by Amiodarone, can decrease vision and alter colour perception, presumably as a result of optic nerve damage.[53] Ocular toxicity generally occurs within one year after starting the drug, with a median time to onset of visual symptoms within six months.
The National Institute for Health and Clinical Excellence guidelines for AF recommended Amiodarone use but are silent on recommendation relative to monitoring side effects. The Heart Rhythm Society[54] recommends an ophthalmologic evaluation for patients with pre-existing visual impairment or during follow-up if visual symptoms develop. Johnson, et al.[52] suggest equally spaced interval evaluations within the first year, followed by annual examinations to evaluate Amiodarone-induced optic neuropathy. When ocular toxicity is suspected it should be confirmed by ophthalmologic findings and reported to the appropriate surveillance system.
3.3. Pulmonary adverse reactions
3.3.1. Epidemiology and Incidence
Until 1980, there were no reports in the literature of pulmonary toxicity due to Amiodarone despite its widespread use since its introduction in the market in 1967. Rotmensch, et al.[55] hypothesized that the drug could have been responsible for pulmonary infiltrates in one of their patients. Afterward, Sobol, et al.[56] reported six cases of potential Amiodarone pulmonary toxicity (APT). All the reported patients showed interstitial fibrosis and pneumonia, and none had clear predisposing factors or pathologic findings indicative of an identifiable etiology. The risk of toxicity with Amiodarone increases with the total cumulative dose rather than with the daily dose or plasma concentration.[57] Although toxicity can occur at any time after treatment is initiated, patients considered at greatest risk are whose have received a daily dose of 400 mg or more for more than two months or a lower dose, commonly 200 mg daily, for more than two years.[58]
In the earliest descriptions of APT, when patients were usually taking 400 mg or more per day, the reported incidence of APT was between 5% and 15%.[59],[60] A lower rate of APT (1.6%) resulted in patients receiving daily Amiodarone doses of 400 mg or less.[61] Consistently, Goldschlager, et al.[62] reported an estimated rate of 2%. Polkey, et al.[63] emphasized hat there is probably no ‘safe’ dose of this medication, and this complication continues to be reported. Thus, Ott, et al.[64] noted eight cases of APT in patients receiving 200 mg/day, the dose most commonly used in clinical practice. These authors underlined the need for vigilance even with ‘low-dose’ Amiodarone therapy. Other conditions that increase the risk of pulmonary toxicity include age, pre-existing lung disease and/or an abnormal chest X-ray before starting treatment.[58] Furthermore, patients undergoing surgery and/or pulmonary angiography while on Amiodarone therapy have an increased risk for developing pulmonary adverse effects.[65] The latency time for the appearance of pulmonary toxicity is variable, usually from a few months to a year.[66] In rare cases, it can also develop from a few weeks to months after discontinuation of treatment. Acute respiratory distress syndrome (ARDS) attributable to treatment with Amiodarone has been described after general surgery, cardiac surgery, and thoracic surgery.[65] Jackevicius, et al.[67] presented a retrospective study, enrolling 57,393 patients with AF older than > 65 years, in order to investigate the risk of developing Amiodarone induced pulmonary toxicity (APT). The study showed that APT occurred in 250 of 6460 Amiodarone users (3.87%) and 676 of 50,993 nonusers (1.33%). From their results, it's found a nearly threefold higher crude incidence of APT in “users” compared to “nonusers”. The incidence of about 4% APT was in agreement with the rate of 10% reported by previous studies.[67] The risk was greater in men (nearly up to 40% increased risk of APT in men versus women), older patients, and those with chronic obstructive pulmonary and kidney disease.
3.3.2. Pathogenesis
The side effects of Amiodarone, including its main derivate desethyl-amiodarone seems related the highly lipophilic characteristics and the concurrent long half-life, which is wide variable (35–100 days), and accumulation in the different tissues of the body, including the lung.[68] Although still uncertain, the pathogenesis of lung injury seems to be a direct toxic effect on the turnover of intracellular phospholipids.[60] In patients with APT, tissue sections show infiltration of polymorphonuclear cells and lymphocytes, as a proof of inflammation and a possible immune reaction to the drug.[60] Jessurun, moreover, showed type II Pneumocytes hyperplasia and widening of alveolar septa with varying degrees of interstitial fibrosis.[59] A typical finding is the accumulation of lipid-laden macrophages in the alveolar space. Ultrastructural examination of these cells shows membrane-bound lamellar bodies similar to surfactant.[69] It should be noted, however, that foamy macrophages, sometimes with lamellate inclusion bodies, have been reported in patients receiving chronic Amiodarone therapy in the absence of APT.[70] Bronchoalveolar lavage fluid is reportedly containing cytotoxic T cells supporting a role of an immune-mediated reaction playing a role in APT.
In some cases, there is little evidence of an inflammatory process and the predominant finding is nonspecific pulmonary fibrosis. This may be found after an acute episode of typical APT or diagnosed lately in latent forms.[71] On light microscopy, vacuolization of the cytoplasm is seen in alveolar Pneumocytes, bronchial epithelial cells, and endothelial cells.[69],[72] Less frequent pathological manifestations of APT include patchy bronchiolitis, obliterans organizing pneumonia or a severe diffuse alveolar damage with hyaline membrane formation.[73]
3.3.3. Clinical and lab findings
The patterns of lung involvement in patients with APT are variable and include chronic interstitial pneumonia with or without fibrosis, bronchiolitis with or without organizing pneumonia, and ARDS. Individuals usually present with progressive shortness of breath, non-productive cough, malaise, fever, and occasionally pleuritic chest pain.[59],[64],[73] Physical examination may be unremarkable in milder cases, but in more severely affected individuals, diffuse rales, hypoxemia, and respiratory distress may be noted. Having no clinical and histological features, the diagnosis of APT is of exclusion. Laboratory data may reveal a leucocytosis that is rarely due to eosinophilia. A nonspecific elevation in lactic dehydrogenase or serum KL-6, a mucin-like glycoprotein, is often present but does not distinguish APT from other interstitial lung diseases.[73],[74]
Pulmonary function tests usually reveals low lung volumes and a restrictive pattern in these patients. The diffusion lung capacity for carbon monoxide (DLCO) should always be performed before starting therapy, particularly in patients with previous lung disease. In fact, an isolated, small reduction in DLCO is present in most patients in chronic therapy, and it should not be considered a sign of pulmonary toxicity but of drug exposure.[75],[76] This is attributed to an increase in phospholipids and pulmonary surfactant. The Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy study,[75] reported a reduction in DLCO by 6% after 1 year of treatment with Amiodarone, compared to patients treated with placebo (P = 0.02). In the subgroup of patients with COPD, the DLCO was reduced of 11.4% versus no variations in patients treated with placebo. This finding highlights the toxicity of the drug that increases in the presence of lung injury.
Moreover, these patients can develop different degrees of hypoxemia related their chronic lung disease worsened by the Amiodarone pulmonary toxicity.
3.3.4. Radiographic features
High-resolution computed tomography (HRCT) scanning has greater sensibility than chest X-ray to highlight the pulmonary injury. The HRTC images usually reveal bilateral interstitial, alveolar, or mixed interstitial and alveolar infiltrates.[77] Pleural thickening is commonly seen, especially in areas where the infiltrates are densest, while pleural effusion is a less common finding. High attenuation may be noted incidentally during HRCT on views of the liver and spleen, related to the accumulation of Amiodarone and its metabolites in tissue macrophages. This latter finding is not necessarily associated with APT.
Gallium scanning might demonstrate increased, non-specific, parenchymal activity and may help to differentiate infiltrates from oedema due to congestive heart failure, a condition frequently present in patients taking Amiodarone.[78] A more unusual radiographic finding in patients with APT is the presence of single or multiple pulmonary nodules, or mass-like opacities.[27] They are most often seen in the upper lobes, frequently peripherally adjacent to the pleura. These nodules are due to localized drug deposits in areas of previous inflammation and usually have high attenuation for the presence of iodine-rich Amiodarone in type II pneumocytes within the lesions.[79],[80] It is important to know that these nodules/masses have increased uptake on fluorodeoxyglucose positron emission tomography scanning, falsely suggesting a lung neoplasm.[81] The most dramatic manifestation of APT is a rapidly progressing diffuse pneumonitis with acute respiratory failure and features typical of ARDS. This condition has been reported after contrast infusion for pulmonary angiography, and particularly in patients undergoing cardiac or pulmonary surgery, especially pneumonectomy.[73],[79],[82] Van Meighen, et al.[30] reported an incidence of ARDS of 11% after pulmonary surgery in patients treated with Amiodarone, compared with 1.8% in those without. Susceptibility to Amiodarone-induced lung damage may be particularly relevant in patients undergoing major cardiothoracic surgery, in relation to the presence of chronic lung disease and the concomitant use of mechanical ventilation in the perioperative period. Moreover, some authors hypothesized that Amiodarone is a potential, unrecognized cause of acute respiratory failure in the intensive care units.[82] These data suggest that physicians should careful doublethink of starting Amiodarone in such a high risk population as chronic lung disease patients.
3.3.5. Diagnosis
The Amiodarone pulmonary toxicity must be considered for all patients treated with Amiodarone presenting new or worsening pulmonary symptoms such as a non-productive cough, shortness of breath, malaise, fever, pleuritic chest pain, and new infiltrates on chest X-ray.[73] The physical examination often reveals bilateral inspiratory crackles.[83] In most cases, the diagnosis is made by exclusion because these patients often have associated morbidity that exposes them to the risk of pulmonary oedema, pneumonia, pulmonary embolism or other acute events, which complicate the diagnosis. Isolated, small reduction in DLCO without clinical evidence of disease is not specific and not diagnostic for APT.[84] Fibre optic bronchoscopy with bronchoalveolar lavage (BAL) and transbronchial biopsy can be extremely useful in ruling out other causes of diffuse lung disease. BAL cells may reveal evidence of an inflammatory or immune response,[59],[52] but is not diagnostic for Amiodarone toxicity. Thus, total or differential BAL cell count, while they may suggest the presence of pneumonitis, do not allow Amiodarone to be directly implicated. Likewise, lung biopsy, though it may be helpful and may show a characteristic lipoid pneumonitis, is more often non-diagnostic and may reveal just aspecific inflammatory changes.[85] Plasma levels of Amiodarone are non-diagnostic, but elevated levels of its metabolite, desethyl-amiodarone, have been found to be more frequent in patients with a diagnosed pulmonary toxicity.[86]
3.3.6. Treatment
Once the diagnosis of APT is considered plausible, the drug should be discontinued. After that, clinical and radiological improvement can be slow in relation to the long half-life of the accumulated drug in the lung. It has been suggested that the more insidious the onset of the disease, the slower the resolution.[59] The general consensus is that corticosteroid should be given to patients showing extensive involvement on imaging or hypoxemia in the attempt to accelerate the recovery process and possibly to minimize the likelihood of lung fibrosis.[83] Prednisone is started at doses of 40 mg/day to 60 mg/day orally and tapered slowly. Again, the pharmacodynamics of Amiodarone dictates treatment for 4 to 12 months.[58] Cases of relapse on early steroid withdrawal have been reported. Okayasu, et al.[87] suggested that patients with excess adipose tissue, as measured by a high body mass index, are more susceptible to recurrences with steroid tapering because of the high concentration of lipophilic Amiodarone within their adipocytes.
Things get complicated when patients develop APT and are unable to take off Amiodarone due to the complexity of the arrhythmias. Zaher, et al.[88] proposed that an APT evaluation should take place in order to understand its severity in these patients. No life-threatening, and Amiodarone alternative is to reduce the dosage to the minimum effective dose in association with low-dose of steroids.
3.3.7. Screening and follow-up
Recommendations to reduce the risk of Amiodarone toxicity suggest chest X-ray and pulmonary function tests, including DLCO, obtained before therapy and a yearly chest X-ray as long as patients remain on Amiodarone treatment.[61] An immediate chest X-ray and pulmonary function testing should be performed if there is clinical suspicion of pulmonary toxicity.
3.3.8. Prognosis
When diagnosed early, the prognosis of Amiodarone-related lung disease is usually favourable. However, the more advanced disease may be fatal or result in pulmonary fibrosis.[58] Clinical improvement and clearing of pulmonary opacities typically require 1–3 months.[83] Radiological follow-up shows complete clearing in about 85% of patients while residual opacities persist in the remaining patients.[89] A mortality rate of 5%–10% due to pulmonary has been attributed to Amiodarone doses hiogher than 400 mg/day.[59] Yet in patients who develop acute respiratory distress syndrome and require mechanical ventilation, mortality is as high as 50% to 100%.[90]
3.4. Hepatotoxicity due to intravenous Amiodarone Infusion
Approximately 25% of patients taking Amiodarone develop a transient asymptomatic rise in serum aminotransferase levels. Symptomatic hepatitis, cirrhosis, and hepatic failure are less common complications that involve less than 3% of patients under Amiodarone chronic, oral treatment.[91] Amiodarone-associated hepatitis shares histological features with alcoholic hepatitis and includes steatosis, fibrosis, and phospholipid laden lysosomal lamellar bodies.[92] Hepatitis due to intravenous Amiodarone infusion (HIVAD) is extremely rare and only 34 cases, of which six, have been reported in the literature.[93] The pathophysiological mechanism is still unknown. HIVAD shares many characteristics that are seen in ischemic hepatitis. Gluck, et al.[94] proposed that acute liver injury is related to liver ischemia, rather than direct drug toxicity. The majority of cases reported in the literature show evidence of poor forward output, hepatic venous congestion, impaired circulation, and acute kidney injury that in turn facilitate ischemic hepatitis. Drug infusion is associated with elevation of transaminases within 24 h. The role of polysorbate 80, which is used to solubilize Amiodarone for i.v. use, cannot be excluded.[95] Polysorbate 80 has been implicated in the E-ferol syndrome, described in infants, characterized by hepatomegaly, splenomegaly, cholestatic jaundice, renal failure, and thrombocytopenia. Liver histology of this syndrome shows Kupffer cell exfoliation, centrilobular accumulation of cellular debris, and panlobular congestion.[96] Lahbabi, et al.[95] published a case report of acute hepatitis during Amiodarone infusion but showing normalization of liver function tests thereafter during oral Amiodarone treatment. This observation supports the concept that HIVAD could be related to the diluent rather than the drug.
Whatever the cause of HIVAD, physicians should be aware of this possible mechanism and regularly obtain a liver function panel subsequent to parenteral drug infusion, and proceed with caution in the setting of heart failure and hepatic congestion. The role of polysorbate in inducing the acute liver effects could not be excluded.[97]
3.5. Amiodarone-induced dermatologic side effects
Dermatologic side effects include photosensitivity (24%–57%) and blue-gray skin discoloration (1%–7%).[98] The mechanism of the reaction seems to be related to the active metabolites generated by UV radiation and oxygen free radicals. Phototoxic effects of Amiodarone and desethyl-amiodarone were shown in vitro tests related to the use of photohemolysis, DNA synthesis inhibition assay in phytohaemoagglutinin-stimulated lymphocytes and finally the killing of macrophages obtained from mouse peritoneal fluid.[99] Skin lesions, which usually occur after at least four months of therapy and with the minimal cumulative dose of 40 g,[100] are typically erythematous or eczematous in appearance, iching and sun exposed areas, i.e., hands, face, and neck. Rare cases of toxic epidermal necrolysis, exfoliative dermatitis, vasculitis, polyserositis, bullous dermatosis, and pustular psoriasis have also been described.[101]
Few cases of Amiodarone-induced air-loss and alopecia have been reported in the literature.[102] Samanta, et al.[103] described a case of complete alopecia resolving eight months after Amiodarone discontinuation. Despite this side effect is considered to be very rare, it has been supported by two studies where drug re-challenge led to the recurrence of alopecia.[104] Although alopecia doesn't seems to be the most dangerous side effects of Amiodarone, it can be related to a reduction in self-confident through psychological issue that can be even be worse in female patients.
3.6. Amiodarone induced epididymitis
About 20 cases of Amiodarone-induced epididymitis have been reported.[105],[106] Amiodarone-induced epididymitis is a chronic condition that usually appears bilaterally without fever or leucocytosis. In these patients, discrete amount of Amiodarone metabolites have been found toaccumulate locally. Histological examination reveals focal epididymal fibrosis and lymphocyte infiltration.[105] The time course of drug therapy and the reported urological symptoms varies from 4 to 71 months.[107] These symptoms can resolve upon Amiodarone discontinuation within 10 days to 3 months. Finally, in patients unresponsive to painkiller and unable to discontinue medication epididymectomy could be considered.[108]
3.7. Hematological side effects
Pancytopenia, hemolytic anaemia, and aplastic anaemia are rare hematologic side effect reported in patients on Amiodarone. Ten cases of bone marrow granulomas have been reported in the literature.[109],[110] These patients–presenting with severe thrombocythemia, paraproteinemia, and anemia or pancytopenia, underwent bone marrow biopsy that revealed normocellular marrow with multiple non-caseating granulomas negative for acid-fast bacteria and fungi.[111] No data were presented about discontinuation and rechallenge, but the time on medication and the absence of other possible ematological causes, led to be amidoarone the first suspect. Physicians should consider Amiodarone as a cause of bone marrow granuloma in patients taking this antiarrhythmic drug and presenting with cytopenia.[110]
3.8. Psychiatric side effects
Only few cases of altered mental status[112] related to Amiodarone therapy have been reported. These cases included catatonic depression,[113] hallucinations and delirium.[114] Although the prevalence of this disorder is extremely low, it is worthy of note the time-dependent relation between the onset of therapy and the beginning of psychiatric symptoms. Barry, et al.[115] reported a case in which symptoms appeared after four days of Amiodarone therapy, decreased after discontinuation, and appear again after four days re-challenge. Despite the casuistry is very small, discontinuation and re-challenge are the strongest factors to ADR diagnosis.
Moreover, it has been reported that blood drug concentration is associated to neuropsychiatric disorders like, tremor, asthenia, hallucinations, and agitation.[112] The effects of Amiodarone on thyroid functionality could explain the humour variability seen among different patients. However, the few cases of Amiodarone-induced catatonic depression presented with poorly increased TSH levels are not enough to justify this theory.[113] Delirium and altered mental status are frequent features of in-hospital, chronic heart disease old people. These patients are the best candidates to start oral Amiodarone therapy.
It is plausible to think, that new Amiodarone medication could have led to plenty of undiagnosed in-hospital altered mental status.
3.9. Amiodarone induced neuromuscular disorders
Amiodarone-induced neurologic side effects have a prevalence of 3%–30%[58] include tremor, ataxia, peripheral neuropathies, gait disturbances, myopathies, paraesthesia, vestibulopathies and insomnia.[44],[48],[116] Flanagan, et al.[117] reported that patients with neuro-myopathy showed clinical features of proximal and distal muscle weakness, dysphagia, ataxia, and poly-myoclonus. Electromyography revealed short duration, low amplitude, rapidly recruited motor unit potentials in the proximal muscles. Muscle biopsies revealed features of necrotizing myopathy and vacuolar myopathy. After Amiodarone discontinuation, patients improved in 2–6 months, with a decrease in creatine kinase blood levels. Amiodarone is an amphiphilic cation and could interact with anionic phospholipids of organelles, which may predispose to the formation of autophagic vacuoles seen in muscle biopsies; meanwhile, its metabolite desethyl-amiodarone may accumulate in muscle tissue.[118] In the study of Abarbanel, et al.,[49] Amiodarone therapy was associated to a peripheral neuropathy characterized by demyelination, loss of large axons and deposits of lamellated bodies, attributed to inhibition of lysosomal sphingomyelinases.
3.10. Thyroid adverse effects
Thyroid adverse effects related to chronic Amiodarone therapy are frequent. Functional thyroid disorders are caused by its high iodine contents, that is 37 mg/100 mg, whose 10% is available for metabolic processes of deiodination.[119] Thyroid abnormalities have been reported in up to 14%–18% of patients receiving long-term Amiodarone therapy.[120] Based on a meta-analysis, Amiodarone lower oral dose (150–330 mg) is associated to thyroid dysfunction in 3.7% of patients.[13] The clinical presentation may reveal hyperthyroidism, which is more frequent in geographical areas with low iodine intake, such as Europe - or hypothyroidism, which is more frequent in iodine-replete areas, such as the United States.[13],[120],[121]
Amiodarone is recognized to act at different enzymatic and non-enzymatic levels in the thyroid:[122]
It inhibits type 1 5′-deiodinase enzyme activity thereby it decreases the peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and reduces the clearance of both T4 and reverse-T3. Consequently, the serum levels of T4 and rT3 increase and the serum levels of T3 decrease by 20%–25%.
Amidoarone, inhibits type 2 5′-deiodinase enzyme activity in the pituitary gland due to feedback regulation. It occurs in the first 1-3 months of therapy and leads to an increase in thyroid-stimulating hormone (TSH) levels. This is not an indication for T4 replacement in these patients. Serum TSH levels are normalized in 2–3 months when T4 concentration rises sufficiently to overcome the partial block in T3 production.
It inhibits entry of T4 and T3 into the peripheral tissue. Serum T4 levels increase by an average of 40% above pre-treatment levels after 1–4 months of treatment with Amiodarone, but it does not constitute evidence of hyperthyroidism. T4 levels can be clearly elevated or at the upper limit of normal range.
Amiodarone and its metabolites show a direct, dose-dependent cytotoxic effect on the thyroid follicular cells, by an iodine-independent mechanism, causing destructive thyroiditis consisting in areas of necrosis, macrophage infiltrations, and inclusion bodies. These effects are present also in other organs, such as liver, skin, lung, heart, cornea and peripheral nerves. Amiodarone and its mean metabolically metabolite desethyl-amiodarone can act as a competitive antagonist of T3 on the cardiac cells.
The excess of iodine caused by Amiodarone inhibits thyroid hormone synthesis, leading to hypothyroidism in through the Wolff-Chaikoff effect.
Amiodarone and desethyl-amiodarone block T3 binding to nuclear thyroid receptors and decrease expression of some thyroid hormone-related genes such as α-myosin heavy chain and the low-density lipoprotein receptor. In summary, serum T4 levels rise by 20%–40% during the first month of therapy and then gradually fall toward high normal. Serum T3 levels decrease by up to 30% within the first few weeks of therapy and remain slightly decreased or low normal. Serum rT3 levels increase by 20% soon afterward and remain increased. Serum thyrotropin (TSH) levels usually rise after the start of therapy but return to normal in 2–3 months.
The most likely mechanism of Amiodarone-induced hypothyroidism (AIH) is an increased susceptibility to the inhibitory effect of iodine and the inability of the thyroid gland to escape from the Wolff-Chaikoff effect after an iodine load in patients with pre-existing Hashimoto thyroiditis. In addition, iodine-induced damage to the thyroid follicles may accelerate the natural trend of Hashimoto thyroiditis toward hypothyroidism. It is likely that patients without underlying thyroid abnormalities have subtle defects in iodine organification that lead to decreased thyroid hormone synthesis, peripheral down-regulation of thyroid hormone receptors, and subsequent hypothyroidism.
3.10.1. Amiodarone induced thyrotoxicosis
Amiodarone-induced thyrotoxicosis (AIT), contrarily to spontaneous hyperthyroidism, is more common in men than in women,[10] with a male-to-female ratio of 3: 1. Although Amiodarone-induced hypothyroidism poses no particular problem, Amiodarone-induced thyrotoxicosis is a diagnostic and therapeutic challenge. There are two main forms of AIT: (1) type 1, an iodine-induced hyperthyroidism, that usually occurs in abnormal thyroid glands; (2) and type 2, a drug-induced destructive thyroiditis, that develops in apparently normal thyroid glands (or small goiters) and that leads to the release of preformed thyroid hormones from the damaged thyroid follicular cells.[10] AIT can develop early or late during Amiodarone treatment, or even several months after drug withdrawal.[123] Total body iodine stores do not decrease up to 9 months after drug discontinuation. Type 2 AIT is associated with increased mortality risk, especially in elderly patients with impaired left ventricular function.[124] The onset of AIT is often sudden and explosive and may occur early after starting Amiodarone. AIT might present heavily symptomatic, especially in younger patients, whose clinical manifestations are indistinguishable from those of spontaneous hyperthyroidism. It's crucial to promptly identify the two subtypes of AIT and start appropriate therapeutic approach.[125] Classical symptoms may be absent for the anti-adrenergic action of drug and the impairment of T4 to T3 conversion, and the syndrome can be afforded after worsening of the underlying cardiac disorder, with occurrence of heart failure, tachyarrhythmias, angina, and weight loss.[10],[126]
Thyroid ultrasonography shows the presence or absence of a diffuse or a nodular goiter. Color flow doppler sonography (CFDS) of the thyroid is an important diagnostic tool to differentiate the two subtypes because type 2 AIT is characterized by absent hypervascularity (pattern 0), instead in type 1 usually there is an increased vascularity (patterns 1–3) and blood flow velocity.[127]
Search for thyroid-directed auto-antibodies, particularly TSH receptor autoantibody, is relevant only in AIT patients with an underlying and pre-existing Graves' disease.
3.10.2. Amiodarone induced hypothyroidism
Amiodarone-induced hypothyroidism (AIH) occurs more frequently than AIT in iodine-sufficient areas. In contrast to AIT, AIH is slightly more frequent in female patients, female to male ratio of 1.5: 1, who are older than AIT patients. AIH usually develops earlier than AIT, both in patients with apparently normal thyroid glands and in patients with pre-existing thyroid abnormalities.[10] The most frequent pre-existing abnormality is the presence of circulating thyroid peroxidase antibodies. It has been reported that the combination of female sex and antithyroid antibodies increase 13.5 times the risk of AIH.[128] Thus, pre-existing Hashimoto's thyroiditis is an established risk factor for the occurrence of hypothyroidism in Amiodarone-treated patients that have an enhanced susceptibility to develop myxedema. It is also possible that the cytotoxicity induced by Amiodarone would decrease the possibility of a developing goiter while receiving Amiodarone.[129]
The most likely pathogenic mechanism is that the thyroid gland of these patients, damaged by pre-existing Hashimoto's thyroiditis, is unable to avoid the acute Wolff-Chaikoff effect after iodine load and to resume normal thyroid hormone synthesis for a subtle defect in thyroid hormonogenesis.[130] This is supported by a positive perchlorate discharge test, indicating a defect in iodine organification. In addition, AIH accelerates the natural trend of the pre-existing Hashimoto's thyroiditis toward hypothyroidism.[130] It has been reported that AIH may spontaneously remit. Clinically, AIH is very similar to spontaneous hypothyroidism and arises with vague symptoms and signs, such as fatigue, cold intolerance, mental sluggishness, and dry skin.[122] A case of myxoedema coma occurring during long-term Amiodarone therapy has been reported.[131] In patients already on T4 replacement therapy, the dose of T4 may need to be increased to slow down the generation of T3 from T4 induced by Amiodarone.
Laboratory findings are similar to those in spontaneous hypothyroidism, such a decreased serum free T4 and increased serum TSH concentrations. However, high TSH levels are not useful in the first three months of Amiodarone therapy because are common features related to the medication. Serum TSH values > 20 mIU/L are commonly seen in patients with AIH but rarely in euthyroid patients early in the course of treatment.[13] Serum thyroglobulin is often increased, probably related to the increased thyroid stimulation by TSH. The presence of circulating thyroid peroxidase antibodies increased the risk of permanent hypothyroidism.[122]
3.10.3. Treatment
If it is necessary to continue Amiodarone for the underlying cardiac disorder, it can be continued in association with L-T4 replacement, given only once-daily administration. T4 replacement is almost always considered for patients with TSH > 20 mU/L.[128] If discontinuation of Amiodarone therapy is feasible, spontaneous remission of hypothyroidism often occurs, particularly in patients without underlying thyroid abnormalities, while this outcome is less likely to occur in patients with Hashimoto's thyroiditis. The dose of T4 needed to normalize serum TSH may be higher in Amiodarone-treated patients, compared with conventional hypothyroid patients, an event that can be explained by Amiodarone-dependent inhibition of pituitary type 2 5-deiodinase intra-pituitary with a consequent decrease in T3 production.[13]
3.11. Amiodarone and the Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH)
SIADH is a rare but serious side effect of Amiodarone treatment, which physicians should be aware of particularly after the loading dose.[132] Amiodarone-induced SIADH may occur during the initial loading period and may be counteracted by reducing the dose without discontinuing the drug.
The mechanism of this adverse drug reaction is not known and could involve the channel modulating properties of Amiodarone at renal or brain level.[133] There is a clear temporal and dose-related association between hyponatremia and the loading phase, therefore serum electrolyte monitoring is mandatory during Amiodarone loading, and with some regularity thereafter. When SIADH occurs, Amiodarone dose must be reduced at first glance or otherwise discontinued to bring sodium level into normal range.[134],[135]
Identifying the exact cause of hyponatremia often is difficult. Most drugs that cause SIADH act by sensitizing the kidney and/or stimulating the release of antidiuretic hormone.[136] Whether Amiodarone acts by one of these mechanisms is not known. Hyponatremia resolves rapidly within 3–5 days after Amiodarone discontinuation, which is surprising because of the long half-life of Amiodarone.
4. Conclusions
Amiodarone is one of the most effective antiarrhythmic drugs but unfortunately carries a high toxicity profile. Adverse Amiodarone reactions—which involve different organs, including lung, thyroid, eye, and liver, can be life-threatening and pose challenges when Amiodarone is used to prevent ventricular arrhythmias and there are no alternative options. On the other hand, Amiodarone is widely prescribed in the elderly for the high incidence of AF in this population, notwithstanding there is no support from the current guidelines.
Being the elderly population at risk for adverse drug reactions, the choice of introducing Amiodarone in the geriatric population has, therefore, to be cautiously evaluated by internal medicine and geriatric specialist doctors, considering long and short-term side effects, interaction with other drugs and patient compliance.
It's advisable that patients under Amiodarone treatment are strictly monitored to intercept any potential insurgent side effects.
Additional data are needed to evaluate the impact of the rarest side effects reported in the literature to fully understand its benefits and disadvantages.
Acknowledgments
This work was supported in part by Sapienza University grant # C26A15M78W (to IL).
References
- 1.Raviña T, Gutierrez J. Amiodarone-induced AV block and ventricular standstill. A forme fruste of an idiopathic long QT syndrome. Int J Cardiol. 2000;75:105–108. doi: 10.1016/s0167-5273(00)00295-3. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitz H, Berkowitch M, Golik A, Shani S. [Adverse drug reactions definitions and terminology] Harefuah. 2001;140:1181–1186. 1228. [Article in Hebrew] [PubMed] [Google Scholar]
- 3.Moore TJ, Cohen MR, Furberg CD. Serious adverse drug events reported to the Food and Drug Administration, 1998–2005. Arch Intern Med. 2007;167:1752–1759. doi: 10.1001/archinte.167.16.1752. [DOI] [PubMed] [Google Scholar]
- 4.Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277:301–306. [PubMed] [Google Scholar]
- 5.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18820 patients. BMJ. 2004;329:15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schurig AM, Böhme M, Just KS, et al. Adverse Drug Reactions (ADR) and emergencies. Dtsch Arztebl Int. 2018;115:251–258. doi: 10.3238/arztebl.2018.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ognibene S, Vazzana N, Giumelli C, et al. Hospitalisation and morbidity due to adverse drug reactions in elderly patients: a single-centre study. Intern Med J. 2018;48:1192–1197. doi: 10.1111/imj.13961. [DOI] [PubMed] [Google Scholar]
- 8.Doyle JF, Ho KM. Benefits and risks of long-term amiodarone therapy for persistent atrial fibrillation: a meta-analysis. Mayo Clin Proc. 2009;84:234–242. doi: 10.4065/84.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Trace elements in human nutrition and health. Geneva: Macmillian Ceuterick; 1996. p. p62. [Google Scholar]
- 10.Martino E, Bartalena L, Bogazzi F, Braverman LE. The effects of amiodarone on the thyroid. Endocr Rev. 2001;22:240–254. doi: 10.1210/edrv.22.2.0427. [DOI] [PubMed] [Google Scholar]
- 11.Hrudikova Vyskocilova E, Grundmann M, et al. Therapeutic monitoring of amiodarone: pharmacokinetics and evaluation of the relationship between effect and dose/concentration. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:134–143. doi: 10.5507/bp.2017.016. [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Mojaverian P, Doedee M, et al. Bioavailability of amiodarone tablets administered with and without food in healthy subjects. Am J Cardiol. 2001;87:432–435. doi: 10.1016/s0002-9149(00)01396-5. [DOI] [PubMed] [Google Scholar]
- 13.Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med. 2005;118:706–714. doi: 10.1016/j.amjmed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Aronow WS. Management of the older person with atrial fibrillation. J Gerontol A Biol Sci Med Sci. 2002;57:M352–M363. doi: 10.1093/gerona/57.6.m352. [DOI] [PubMed] [Google Scholar]
- 15.Soyama A, Hanioka N, Saito Y, et al. Amiodarone N-deethylation by CYP2C8 and its variants, CYP2C8*3 and CYP2C8 P404A. Pharmacol Toxicol. 2002;91:174–178. doi: 10.1034/j.1600-0773.2002.910404.x. [DOI] [PubMed] [Google Scholar]
- 16.Torres-Arraut E, Singh S, Pickoff AS. Electrophysiologic effects of tween 80 in the myocardium and specialized conduction system of the canine heart. J Electrocardiol. 1984;17:145–151. doi: 10.1016/s0022-0736(84)81088-2. [DOI] [PubMed] [Google Scholar]
- 17.Veronese ME, McLean S, Hendriks R. Plasma protein binding of amiodarone in a patient population: measurement by erythrocyte partitioning and a novel glass-binding method. Br J Clin Pharmacol. 1988;26:721–731. doi: 10.1111/j.1365-2125.1988.tb05311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bohets H, Lavrijsen K, Hendrickx J, et al. Identification of the cytochrome P450 enzymes involved in the metabolism of cisapride: in vitro studies of potential co-medication interactions. Br J Pharmacol. 2000;129:1655–1667. doi: 10.1038/sj.bjp.0703246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanoski CA, Bauman JL. Clinical observations with the amiodarone/warfarin interaction: dosing relationships with long-term therapy. Chest. 2002;121:19–23. doi: 10.1378/chest.121.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Hori R, Okamura N, Aiba T, Tanigawara Y. Role of P-glycoprotein in renal tubular secretion of digoxin in the isolated perfused rat kidney. J Pharmacol Exp Ther. 1993;266:1620–1625. [PubMed] [Google Scholar]
- 21.Amiodarone—FDA prescribing information, side effects and uses'. [(Accessed August 9, 2018)]. https://www.drugs.com/pro/amiodarone.html.
- 22.Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468–475. doi: 10.1016/j.amjmed.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 23.Doval HC, Nul DR, Grancelli HO, et al. Randomised trial of low-dose amiodarone in severe congestive heart failure. Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) Lancet. 1994;344:493–498. doi: 10.1016/s0140-6736(94)91895-3. [DOI] [PubMed] [Google Scholar]
- 24.Garguichevich JJ, Ramos J L, Gambarte A, et al. Effect of amiodarone therapy on mortality in patients with left ventricular dysfunction and asymptomatic complex ventricular arrhythmias: Argentine Pilot Study of Sudden Death and Amiodarone (EPAMSA) Am Heart J. 1995;130:494–500. doi: 10.1016/0002-8703(95)90357-7. [DOI] [PubMed] [Google Scholar]
- 25.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 26.Zipes DP, Prystowsky EN, Heger JJ. Amiodarone: electrophysiologic actions, pharmacokinetics and clinical effects. J Am Coll Cardiol. 1984;3:1059–1071. doi: 10.1016/s0735-1097(84)80367-8. [DOI] [PubMed] [Google Scholar]
- 27.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 28.Chatterjee S, Sardar P, Mukherjee D, et al. Timing and route of amiodarone for prevention of postoperative atrial fibrillation after cardiac surgery: a network regression meta-analysis. Pacing Clin Electrophysiol. 2013;36:1017–1023. doi: 10.1111/pace.12140. [DOI] [PubMed] [Google Scholar]
- 29.Dohadwala M, Kamili F, Estes NM, Homoud M. Atrioventricular block and pause-dependent torsade de pointes. HeartRhythm Case Rep. 2017;3:115–119. doi: 10.1016/j.hrcr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Mieghem W, Coolen L, Malysse I, et al. Amiodarone and the development of ARDS after lung surgery. Chest. 1994;105:1642–1645. doi: 10.1378/chest.105.6.1642. [DOI] [PubMed] [Google Scholar]
- 31.Anastasiou-Nana MI, Nanas JN, Nanas SN, et al. Effects of amiodarone on refractory ventricular fibrillation in acute myocardial infarction: experimental study. J Am Coll Cardiol. 1994;23:253–258. doi: 10.1016/0735-1097(94)90528-2. [DOI] [PubMed] [Google Scholar]
- 32.Galve E, Rius T, Ballester R, et al. Intravenous amiodarone in treatment of recent-onset atrial fibrillation: results of a randomized, controlled study. J Am Coll Cardiol. 1996;27:1079–1082. doi: 10.1016/0735-1097(95)00595-1. [DOI] [PubMed] [Google Scholar]
- 33.Alawami M, Chatfield A, Ghashi R, Walker L. Atrial fibrillation after cardiac surgery: prevention and management: the Australasian experience. J Saudi Heart Assoc. 2018;30:40–46. doi: 10.1016/j.jsha.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cordarone (Amiodarone Hydrochloride)—Drug Interactions, Contraindications, Other Rx Info. [(Accessed August 9, 2018)]. http://www.druglib.com/druginfo/cordarone/interactions_overdosage_contraindications/
- 35.Nafrialdi N, Kurniawan TG, Setiawati A, Makmun LH. QT interval prolongation associated with amiodarone use in Cipto Mangunkusumo Hospital, Jakarta. Acta Med Indones. 2014;46:292–297. [PubMed] [Google Scholar]
- 36.Stramba-Badiale M, Locati EH, Martinelli A, et al. Gender and the relationship between ventricular repolarization and cardiac cycle length during 24-h Holter recordings. Eur Heart J. 1997;18:1000–1006. doi: 10.1093/oxfordjournals.eurheartj.a015357. [DOI] [PubMed] [Google Scholar]
- 37.Lim HE, Pak HN, Ahn JC, et al. Torsade de pointes induced by short-term oral amiodarone therapy. Europace. 2006;8:1051–1053. doi: 10.1093/europace/eul118. [DOI] [PubMed] [Google Scholar]
- 38.Hoffmann C, Falzone E, Augé M, et al. Long QT syndrome, amiodarone use, and the mechanism underlying lidocaine toxicity. Anesth Analg. 2012;115:1253–1254. doi: 10.1213/ANE.0b013e31826b4789. [DOI] [PubMed] [Google Scholar]
- 39.Antonelli D, Atar S, Freedberg NA, Rosenfeld T. Torsade de pointes in patients on chronic amiodarone treatment: contributing factors and drug interactions. Isr Med Assoc J. 2005;7:163–165. [PubMed] [Google Scholar]
- 40.Doshi D, Jayawardana R. Amiodarone-induced life-threatening refractory hypotension. Am J Case Rep. 2015;16:617–620. doi: 10.12659/AJCR.893920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ingram DV, Jaggarao NS, Chamberlain DA. Ocular changes resulting from therapy with amiodarone. Br J Ophthalmol. 1982;66:676–679. doi: 10.1136/bjo.66.10.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shukla R, Jowett NI, Thompson DR, Pohl JE. Side effects with amiodarone therapy. Postgrad Med J. 1994;70:492–498. doi: 10.1136/pgmj.70.825.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park HS, Kim YN. Adverse effects of long-term amiodarone therapy. Korean J Intern Med. 2014;29:571–573. doi: 10.3904/kjim.2014.29.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mäntyjärvi M, Tuppurainen K, Ikäheimo K. Ocular side effects of amiodarone. Surv Ophthalmol. 1998;42:360–366. doi: 10.1016/s0039-6257(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 45.Rucker JC, Biousse V, Newman NJ. Ischemic optic neuropathies. Curr Opin Neurol. 2004;17:27–35. doi: 10.1097/00019052-200402000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Mindel JS, Anderson J, Johnson G, et al. Absence of bilateral vision loss from amiodarone: a randomized trial. Am Heart J. 2007;153:837–842. doi: 10.1016/j.ahj.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Passman RS, Bennett CL, Purpura JM, et al. Amiodarone-associated optic neuropathy: a critical review. Am J Med. 2012;125:447–453. doi: 10.1016/j.amjmed.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansour AM, Puklin JE, O'Grady R. Optic nerve ultrastructure following amiodarone therapy. J Clin Neuroophthalmol. 1988;8:231–237. [PubMed] [Google Scholar]
- 49.Abarbanel JM, Osiman A, Frisher S, Herishanu Y. Peripheral neuropathy and cerebellar syndrome associated with amiodarone therapy. Isr J Med Sci. 1987;23:893–895. [PubMed] [Google Scholar]
- 50.Costa-Jussà FR, Jacobs JM. The pathology of amiodarone neurotoxicity. II. Peripheral neuropathy in man. Brain. 1985;108:753–769. doi: 10.1093/brain/108.3.753. [DOI] [PubMed] [Google Scholar]
- 51.Beck RW, Servais GE, Hayreh SS. Anterior ischemic optic neuropathy. IX. Cup-to-disc ratio and its role in pathogenesis. Ophthalmology. 1987;94:1503–1508. [PubMed] [Google Scholar]
- 52.Johnson LN, Krohel GB, Thomas ER. The clinical spectrum of amiodarone-associated optic neuropathy. J Natl Med Assoc. 2004;96:1477–1491. [PMC free article] [PubMed] [Google Scholar]
- 53.Venkatesh N, Singh BN, Al-Sarraf L, Kannan R. Digoxin-desethylamiodarone interaction in the rat: comparison with the effects of amiodarone. J Cardiovasc Pharmacol. 1986;8:309–313. doi: 10.1097/00005344-198603000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e190–e252. doi: 10.1016/j.hrthm.2017.10.035. [DOI] [PubMed] [Google Scholar]
- 55.Rotmensch HH, Liron M, Tupilski M, Laniado S. Possible association of pneumonitis with amiodarone therapy. Am Heart J. 1980;100:412–413. doi: 10.1016/0002-8703(80)90165-9. [DOI] [PubMed] [Google Scholar]
- 56.Sobol SM, Rakita L. Pneumonitis and pulmonary fibrosis associated with amiodarone treatment: a possible complication of a new antiarrhythmic drug. Circulation. 1982;65:819–824. doi: 10.1161/01.cir.65.4.819. [DOI] [PubMed] [Google Scholar]
- 57.Rotmensch HH, Belhassen B, Swanson BN, et al. Steady-state serum amiodarone concentrations: relationships with antiarrhythmic efficacy and toxicity. Ann Intern Med. 1984;101:462–469. doi: 10.7326/0003-4819-101-4-462. [DOI] [PubMed] [Google Scholar]
- 58.Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16:43–48. doi: 10.1155/2009/282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martin WJ, Rosenow EC. Amiodarone pulmonary toxicity. Recognition and pathogenesis (Part 2) Chest. 1988;93:1242–1248. doi: 10.1378/chest.93.6.1242. [DOI] [PubMed] [Google Scholar]
- 60.Jessurun GA, Crijns HJ. Amiodarone pulmonary toxicity. BMJ. 1997;314:619–620. doi: 10.1136/bmj.314.7081.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sunderji R, Kanji Z, Gin K. Pulmonary effects of low dose amiodarone: a review of the risks and recommendations for surveillance. Can J Cardiol. 2000;16:1435–1440. [PubMed] [Google Scholar]
- 62.Goldschlager N, Epstein AE, Naccarelli GV, et al. A practical guide for clinicians who treat patients with amiodarone: 2007. Heart Rhythm. 2007;4:1250–1259. doi: 10.1016/j.hrthm.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 63.Polkey MI, Wilson PO, Rees PJ. Amiodarone pneumonitis: no safe dose. Respir Med. 1995;89:233–235. doi: 10.1016/0954-6111(95)90254-6. [DOI] [PubMed] [Google Scholar]
- 64.Ott MC, Khoor A, Leventhal JP, et al. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123:646–651. doi: 10.1378/chest.123.2.646. [DOI] [PubMed] [Google Scholar]
- 65.Karnatovskaia LV, Festic E, Gajic O, et al. Prehospital amiodarone may increase the incidence of acute respiratory distress syndrome among patients at risk. J Crit Care. 2012;27:447–453. doi: 10.1016/j.jcrc.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sauler M, Gulati M. Newly recognized occupational and environmental causes of chronic terminal airways and parenchymal lung disease. Clin Chest Med. 2012;33:667–680. doi: 10.1016/j.ccm.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705–710. doi: 10.1016/j.amjcard.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 68.Holt DW, Tucker GT, Jackson PR, Storey GC. Amiodarone pharmacokinetics. Am Heart J. 1983;106:840–847. doi: 10.1016/0002-8703(83)90006-6. [DOI] [PubMed] [Google Scholar]
- 69.Kratz A, Lewandrowski KB. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Normal reference laboratory values. N Engl J Med. 1998;339:1063–1072. doi: 10.1056/NEJM199810083391508. [DOI] [PubMed] [Google Scholar]
- 70.Kennedy JI, Myers JL, Plumb VJ, Fulmer JD. Amiodarone pulmonary toxicity. Clinical, radiologic, and pathologic correlations. Arch Intern Med. 1987;147:50–55. doi: 10.1001/archinte.147.1.50. [DOI] [PubMed] [Google Scholar]
- 71.Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis. Bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102:1005–1012. doi: 10.1378/chest.102.4.1005. [DOI] [PubMed] [Google Scholar]
- 72.Marchlinski FE, Gansler TS, Waxman HL, Josephson ME. Amiodarone pulmonary toxicity. Ann Intern Med. 1982;97:839–845. doi: 10.7326/0003-4819-97-6-839. [DOI] [PubMed] [Google Scholar]
- 73.Camus P, Martin WJ, Rosenow EC. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25:65–75. doi: 10.1016/S0272-5231(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 74.Endoh Y, Hanai R, Uto K, et al. [Diagnostic usefulness of KL-6 measurements in patients with pulmonary complications after administration of amiodarone] J Cardiol. 2000;35:121–127. [PubMed] [Google Scholar]
- 75.Singh S, Fletcher RD, Fisher S, et al. Congestive heart failure: survival trial of antiarrhythmic therapy (CHF STAT). The CHF STAT Investigators. Control Clin Trials. 1992;13:339–350. doi: 10.1016/0197-2456(92)90036-y. [DOI] [PubMed] [Google Scholar]
- 76.Sweidan AJ, Singh NK, Dang N, et al. Amiodarone-induced pulmonary toxicity—a frequently missed complication. Clin Med Insights Case Rep. 2016;9:91–94. doi: 10.4137/CCRep.S39809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kuhlman JE, Teigen C, Ren H, et al. Amiodarone pulmonary toxicity: CT findings in symptomatic patients. Radiology. 1990;177:121–125. doi: 10.1148/radiology.177.1.2399310. [DOI] [PubMed] [Google Scholar]
- 78.Zhu YY, Botvinick E, Dae M, et al. Gallium lung scintigraphy in amiodarone pulmonary toxicity. Chest. 1988;93:1126–1131. doi: 10.1378/chest.93.6.1126. [DOI] [PubMed] [Google Scholar]
- 79.Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176:1411–1413. doi: 10.1503/cmaj.061102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Azzam I, Tov N, Elias N, Naschitz JE. Amiodarone toxicity presenting as pulmonary mass and peripheral neuropathy: the continuing diagnostic challenge. Postgrad Med J. 2006;82:73–75. doi: 10.1136/pgmj.2005.040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Halley A, Hugentobler A, Icard P, et al. Efficiency of 18F-FDG and 99mTc-depreotide SPECT in the diagnosis of malignancy of solitary pulmonary nodules. Eur J Nucl Med Mol Imaging. 2005;32:1026–1032. doi: 10.1007/s00259-005-1812-1. [DOI] [PubMed] [Google Scholar]
- 82.Ashrafian H, Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120:275–282. doi: 10.1378/chest.120.1.275. [DOI] [PubMed] [Google Scholar]
- 83.Schwaiblmair M, Berghaus T, Haeckel T, et al. Amiodarone-induced pulmonary toxicity: an under-recognized and severe adverse effect? Clin Res Cardiol. 2010;99:693–700. doi: 10.1007/s00392-010-0181-3. [DOI] [PubMed] [Google Scholar]
- 84.Gleadhill IC, Wise RA, Schonfeld SA, et al. Serial lung function testing in patients treated with amiodarone: a prospective study. Am J Med. 1989;86:4–10. doi: 10.1016/0002-9343(89)90221-0. [DOI] [PubMed] [Google Scholar]
- 85.Reyes CV, Thompson KS, Jensen J. Multivesiculated macrophages: their implication in fine-needle aspiration cytology of lung mass lesions. Diagn. Cytopathol. 1998;19:98–101. doi: 10.1002/(sici)1097-0339(199808)19:2<98::aid-dc5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 86.Dusman RE, Stanton MS, Miles WM, et al. Clinical features of amiodarone-induced pulmonary toxicity. Circulation. 1990;82:51–59. doi: 10.1161/01.cir.82.1.51. [DOI] [PubMed] [Google Scholar]
- 87.Okayasu K, Takeda Y, Kojima J, et al. Amiodarone pulmonary toxicity: a patient with three recurrences of pulmonary toxicity and consideration of the probable risk for relapse. Intern Med. 2006;45:1303–1307. doi: 10.2169/internalmedicine.45.1800. [DOI] [PubMed] [Google Scholar]
- 88.Zaher C, Hamer A, Peter T, Mandel W. Low-dose steroid therapy for prophylaxis of amiodarone-induced pulmonary infiltrates. N Engl J Med. 1983;308:779. doi: 10.1056/NEJM198303313081317. [DOI] [PubMed] [Google Scholar]
- 89.Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11:1697–1703. doi: 10.1007/s003300000809. [DOI] [PubMed] [Google Scholar]
- 90.Iskandar SB, Abi-Saleh B, Keith RL, et al. Amiodarone-induced alveolar hemorrhage. South Med J. 2006;99:383–387. doi: 10.1097/01.smj.0000208971.43461.bb. [DOI] [PubMed] [Google Scholar]
- 91.Lewis JH, Ranard RC, Caruso A, et al. Amiodarone hepatotoxicity: prevalence and clinicopathologic correlations among 104 patients. Hepatology. 1989;9:679–685. doi: 10.1002/hep.1840090504. [DOI] [PubMed] [Google Scholar]
- 92.Guigui B, Perrot S, Berry JP, et al. Amiodarone-induced hepatic phospholipidosis: a morphological alteration independent of pseudoalcoholic liver disease. Hepatology. 1988;8:1063–1068. doi: 10.1002/hep.1840080514. [DOI] [PubMed] [Google Scholar]
- 93.Desai AD, Chun S, Sung RJ. The role of intravenous amiodarone in the management of cardiac arrhythmias. Ann Intern Med. 1997;127:294–303. doi: 10.7326/0003-4819-127-4-199708150-00007. [DOI] [PubMed] [Google Scholar]
- 94.Gluck N, Fried M, Porat R. Acute amiodarone liver toxicity likely due to ischemic hepatitis. Isr Med Assoc J. 2011;13:748–752. [PubMed] [Google Scholar]
- 95.Lahbabi M. Acute hepatitis secondary to parenteral amiodarone does not preclude subsequent oral therapy. World J Hepatol. 2012;4:196. doi: 10.4254/wjh.v4.i6.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rätz Bravo AE, Drewe J, Schlienger RG, et al. Hepatotoxicity during rapid intravenous loading with amiodarone: Description of three cases and review of the literature. Crit Care Med. 2005;33:128–34. discussion 245. doi: 10.1097/01.ccm.0000151048.72393.44. [DOI] [PubMed] [Google Scholar]
- 97.Chen CC, Wu CC. Acute hepatotoxicity of intravenous amiodarone: case report and review of the literature. Am J Ther. 2016;23:e260–e263. doi: 10.1097/MJT.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 98.Enseleit F, Wyss CA, Duru F, et al. Images in cardiovascular medicine. The blue man: amiodarone-induced skin discoloration. Circulation. 2006;113:e63. doi: 10.1161/CIRCULATIONAHA.105.554303. [DOI] [PubMed] [Google Scholar]
- 99.Collins P, Ferguson J. Narrow-band UVB (TL-01) phototherapy: an effective preventative treatment for the photodermatoses. Br J Dermatol. 1995;132:956–963. doi: 10.1111/j.1365-2133.1995.tb16955.x. [DOI] [PubMed] [Google Scholar]
- 100.Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369–2372. doi: 10.12659/MSM.890881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dootson G, Byatt C. Amiodarone-induced vasculitis and a review of the cutaneous side-effects of amiodarone. Clin Exp Dermatol. 1994;19:422–424. doi: 10.1111/j.1365-2230.1994.tb02701.x. [DOI] [PubMed] [Google Scholar]
- 102.Korantzopoulos P, Kyrlas K, Goudevenos JA. Amiodarone-induced hair loss: case report and review of the literature. QJM. 2015;108:325–327. doi: 10.1093/qjmed/hcs168. [DOI] [PubMed] [Google Scholar]
- 103.Samanta A, Jones GR, Burden AC. Adverse reactions during treatment with amiodarone hydrochloride. Br Med J (Clin Res Ed) 1983;287:503. doi: 10.1136/bmj.287.6390.503-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmad S. Amiodarone and reversible alopecia. Arch Intern Med. 1995;155:1106. [PubMed] [Google Scholar]
- 105.Gasparich JP, Mason JT, Greene HL, et al. Amiodarone-associated epididymitis: drug-related epididymitis in the absence of infection. J Urol. 1985;133:971–972. doi: 10.1016/s0022-5347(17)49338-4. [DOI] [PubMed] [Google Scholar]
- 106.Hamoud K, Kaneti J, Smailowitz Z, Lissmer L. Amiodarone-induced epididymitis. Report of 2 cases. Eur Urol. 1996;29:497–498. [PubMed] [Google Scholar]
- 107.Sadek I, Biron P, Kus T. Amiodarone-induced epididymitis: report of a new case and literature review of 12 cases. Can J Cardiol. 1993;9:833–836. [PubMed] [Google Scholar]
- 108.Kirkali Z. Recurrent bilateral amiodarone induced epididymitis. J Urol. 1999;162:808–809. doi: 10.1097/00005392-199909010-00063. [DOI] [PubMed] [Google Scholar]
- 109.Yamreudeewong W, McIntyre WW, Sun TJ, Ranelli PL. Bone marrow granulomas possibly associated with amiodarone. Pharmacotherapy. 2000;20:855–859. doi: 10.1592/phco.20.9.855.35204. [DOI] [PubMed] [Google Scholar]
- 110.Mohamed T, Sanjay R, Sycheva T, et al. Amiodarone-associated bone marrow granulomas: a report of 2 cases and review of the literature. Int J Hematol. 2007;85:101–104. doi: 10.1532/IJH97.NA0608. [DOI] [PubMed] [Google Scholar]
- 111.Mukhopadhyay S, Mukhopadhyay S, Abraham NZ, et al. Unexplained bone marrow granulomas: Is amiodarone the culprit? A report of 2 cases. World J Hepatol. 2004;75:110–112. doi: 10.1002/ajh.10465. [DOI] [PubMed] [Google Scholar]
- 112.Salouage I, Klouz A, Trabelsi S, et al. Neuro-psychiatric disorders induced by Amiodarone high levels (Cordarone) Therapie. 2007;62:357–359. doi: 10.2515/therapie:2007057. [DOI] [PubMed] [Google Scholar]
- 113.Rajagopal S. Catatonic depression precipitated by amiodarone prescribed for atrial fibrillation. Ind J Psychiatry. 2015;57:105. doi: 10.4103/0019-5545.148545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Athwal H, Murphy G, Chun S. Amiodarone-induced delirium. Am J Geriatr Psychiatry. 2003;11:696–697. doi: 10.1176/appi.ajgp.11.6.696. [DOI] [PubMed] [Google Scholar]
- 115.Barry JJ, Franklin K. Amiodarone-induced delirium. Am J Psychiatry. 1999;156:1119. doi: 10.1176/ajp.156.7.1119. [DOI] [PubMed] [Google Scholar]
- 116.Vorperian VR, Havighurst TC, Miller S, January CT. Adverse effects of low dose amiodarone: a meta-analysis. J Am Coll Cardiol. 1997;30:791–798. doi: 10.1016/s0735-1097(97)00220-9. [DOI] [PubMed] [Google Scholar]
- 117.Flanagan EP, Harper CM, St Louis EK, et al. Amiodarone-associated neuromyopathy: a report of four cases: Letter to the Editor. Eur J Neurol. 2012;19:e50–e51. doi: 10.1111/j.1468-1331.2012.03678.x. [DOI] [PubMed] [Google Scholar]
- 118.Carella F, Riva E, Morandi L, et al. Myopathy during amiodarone treatment: a case report. Ital J Neurol Sci. 1987;8:605–608. doi: 10.1007/BF02333668. [DOI] [PubMed] [Google Scholar]
- 119.Narayana SK, Woods DR, Boos CJ. Management of amiodarone-related thyroid problems. Ther Adv Endocrinol Metab. 2011;2:115–126. doi: 10.1177/2042018811398516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tsang W, Houlden RL. Amiodarone-induced thyrotoxicosis: a review. Can J Cardiol. 2009;25:421–424. doi: 10.1016/s0828-282x(09)70512-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eskes SA, Wiersinga WM. Amiodarone and thyroid. Best Pract. Res Clin Endocrinol Metab. 2009;23:735–751. doi: 10.1016/j.beem.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 122.Han TS, Williams GR, Vanderpump MPJ. Benzofuran derivatives and the thyroid. Clin Endocrinol (Oxf) 2009;70:2–13. doi: 10.1111/j.1365-2265.2008.03350.x. [DOI] [PubMed] [Google Scholar]
- 123.Kurt IH, Yigit T, Karademir BM. Atrial fibrillation due to late amiodarone-induced thyrotoxicosis. Clin Drug Investig. 2008;28:527–531. doi: 10.2165/00044011-200828080-00008. [DOI] [PubMed] [Google Scholar]
- 124.O'Sullivan AJ. Amiodarone-induced thyrotoxicosis: left ventricular dysfunction is associated with increased mortality. Eur J Endocrinol. 2006;154:533–536. doi: 10.1530/eje.1.02122. [DOI] [PubMed] [Google Scholar]
- 125.Bogazzi F, Bartalena L, Martino E. Approach to the patient with amiodarone-induced thyrotoxicosis. J Clin Endocrinol Metab. 2010;95:2529–2535. doi: 10.1210/jc.2010-0180. [DOI] [PubMed] [Google Scholar]
- 126.Piga M, Cocco MC, Serra A, et al. The usefulness of 99mTc-sestaMIBI thyroid scan in the differential diagnosis and management of amiodarone-induced thyrotoxicosis. Eur J Endocrinol. 2008;159:423–429. doi: 10.1530/EJE-08-0348. [DOI] [PubMed] [Google Scholar]
- 127.Bogazzi F, Martino E, Dell'Unto E, et al. Thyroid color flow doppler sonography and radioiodine uptake in 55 consecutive patients with amiodarone-induced thyrotoxicosis. J Endocrinol Invest. 2003;26:635–640. doi: 10.1007/BF03347021. [DOI] [PubMed] [Google Scholar]
- 128.Trip MD, Wiersinga W, Plomp TA. Incidence, predictability, and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med. 1991;91:507–511. doi: 10.1016/0002-9343(91)90187-3. [DOI] [PubMed] [Google Scholar]
- 129.Bongard V, Marc D, Philippe V, et al. Incidence rate of adverse drug reactions during long-term follow-up of patients newly treated with amiodarone. Am J Ther. 2006;13:315–319. doi: 10.1097/00045391-200607000-00007. [DOI] [PubMed] [Google Scholar]
- 130.Martino E, Aghini-Lombardi F, Bartalena L, et al. Enhanced susceptibility to amiodarone-induced hypothyroidism in patients with thyroid autoimmune disease. Arch Intern Med. 1994;154:2722–2726. doi: 10.1001/archinte.1994.00420230115013. [DOI] [PubMed] [Google Scholar]
- 131.Mazonson PD, Williams ML, Cantley LK, et al. Myxedema coma during long-term amiodarone therapy. Am J Med. 1984;77:751–754. doi: 10.1016/0002-9343(84)90379-6. [DOI] [PubMed] [Google Scholar]
- 132.Afshinnia F, Sheth N, Perlman R. Syndrome of inappropriate antidiuretic hormone in association with amiodarone therapy: a case report and review of literature. Ren Fail. 2011;33:456–458. doi: 10.3109/0886022X.2011.565138. [DOI] [PubMed] [Google Scholar]
- 133.Pham L, Shaer AJ, Marnejon T. Hyponatremia—a rare but serious complication of amiodarone: a case report and review of the literature. Case Rep Nephrol Urol. 2013;3:46–50. doi: 10.1159/000350910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Aslam MK, Gnaim C, Kutnick J, et al. Syndrome of inappropriate antidiuretic hormone secretion induced by amiodarone therapy. Pacing Clin Electrophysiol. 2004;27:831–832. doi: 10.1111/j.1540-8159.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 135.Patel GP, Kasiar JB. Syndrome of inappropriate antidiuretic hormone-induced hyponatremia associated with amiodarone. Pharmacotherapy. 2002;22:649–651. doi: 10.1592/phco.22.8.649.33206. [DOI] [PubMed] [Google Scholar]
- 136.Mulloy AL, Caruana RJ. Hyponatremic emergencies. Med Clin North Am. 1995;79:155–168. doi: 10.1016/s0025-7125(16)30089-x. [DOI] [PubMed] [Google Scholar]