Abstract
Background
Averrhoa carambola L. is a traditional medicinal herb that has long been used to treat diabetes. Our previous studies found that 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD) isolated from A. carambola L. roots could ameliorate diabetic nephropathy (DN), but its exact mechanism remains unclear.
Methods
A DN model was established by streptozotocin (STZ, 100 mg/kg body weight) in TLR4 knockout (TLR4-/-, KO) mice and wild-type (WT) mice. Body weight and blood glucose were evaluated after oral administration of DMDD (12.5, 25, 50 mg/kg body weight/d) in diabetic mice. The levels of serum lipids, including TC, TG, HDL, and LDL and kidney function indexes Scr and BUN, were detected by biochemical equipment. The levels of inflammatory cytokines including IL-6 and TNF-α, were determined by ELISA kits. Furthermore, changes in renal ultrastructure were observed by electron microscopy. Western blot analysis and RT-PCR were used to assess the protein expression and mRNA levels of TLR4, MyD88 and NF-κB.
Results
DMDD treatment attenuated diabetic nephropathy, as a result of a decline in blood glucose, serum creatinine, and blood urine nitrogen levels and an increase in the quantity and density of podocytes, combined with improved dyslipidaemia. DMDD treatment inhibited the inflammatory response and downregulated the expression of the TLR4/MyD88/NF-κB pathway in diabetic mice, and these changes were significantly different in TLR4-/- mice.
Conclusion
DMDD alleviates diabetic nephropathy by mitigating kidney damage and inflammation via the inhibition of the TLR4/MyD88/NF-κB signalling pathway.
Keywords: 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; diabetic nephropathy; TLR4/MyD88/NF-κB signalling pathway
Introduction
Diabetes mellitus (DM), is a widespread chronic metabolic disease that encompasses a series of syndromes such as diabetic nephropathy (DN), retinopathy, and gangrene. DN is one of the risk factors leading to end-stage renal failure that increases the rate of mortality in DM patient.1
Toll-like receptors (TLRs) play important roles in the innate immune system by mediating immune and inflammatory reactions. TLR4 is activated by lipopolysaccharide (LPS) and recruits myeloid differentiation factor 88 (Myd88), subsequently increasing the expression of nuclear factor-κB (NF-κB). NF-κB is a crucial in inflammatory response that stimulates the production of TNF-α and IL-6.2,3 Researchers have demonstrated that the TLR4 signalling pathway is associated with the development of diabetic nephropathy.4
Averrhoa carambola L. is an ancient medicinal plant that is used to treat coughs, diabetes and promote digestion. In a previous study, we found that 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD) extracted from the dry roots of Averrhoa carambola L. significantly reduced the fasting blood sugar level in diabetic mice and effectively ameliorated diabetic nephropathy.5 However, the exact mechanism by which DMDD alleviates diabetic nephropathy remains unknown. In this study, we used WT and TLR4 knockout mice to investigate the mechanism of DMDD protection against diabetic nephropathy in vivo.
Materials and methods
Plant material and production of DMDD
A. carambola L. was received from Lingshan County, Guangxi Autonomous Region, China and the DMDD was isolated from 60% aq. EtOH extraction of A. carambola L. root as previously described.6 DMDD was dissolved in distilled water (2.5 mg/L) before being administered to the mice.
Animals and experimental design
TLR4 knockout mice (KO, male, 6–8 weeks old) and wild-type mice (WT, male, 6–8 weeks old) were obtained from the Model Animal Research Centre of Nanjing University (Nanjing, China). Mice were maintained in an SPF-level animal centre with a controlled temperature of 20 °C- 25 °C, 48% −52% humidity and a 12 h light-dark cycle at Guangxi Medical University. All experiments were performed with approval from the Institutional Animal Care and Use Committee of Guangxi Medical University. Animal ethics review follows the Guiding Opinions on the Treatment of Laboratory Animals issued by the Ministry of Science and Technology of the People’s Republic of China and the Laboratory Animal-Guideline for Ethical Review of Animal Welfare issued by the National Standard GB/T35892-2018 of the People’s Republic of China. After 7 days of adaptive feeding, 6 WT mice and 6 KO mice were randomly selected for WT and KO normal control groups (WT NC group and KO NC group) fed with a standard chow diet. The other mice were fed a high-fat diet. The NC groups were administered an intraperitoneal injection of physiological saline, and the other mice were injected with 100 mg/kg streptozotocin (STZ) in the abdominal cavity after 8 h of fasting. After 3 days, mice with a fasting blood glucose (FBG) level≥11.1 mmol/L, were selected as diabetic mice. Then, the WT mice and the KO mice were distributed into the following 6 groups (n=6/group) in the same form: normal control group (NC), diabetic nephropathy group (DN), gliquidone group (10 mg.kg−1.d−1, G), high dosage DMDD group (50 mg.kg−1.d−1, H), medium dosage DMDD group (25 mg.kg−1.d−1, M), and low dosage DMDD group (12.5 mg.kg−1.d−1, L). The body weight and FBG were measured every week, and the mice were sacrificed after treatment with DMDD for 4 weeks. The blood samples were collected and centrifuged separate the serum, and the kidney samples were rapidly obtained and weighed. All tissues and serum samples were cryopreserved at −80 °C until analysis.
Intraperitoneal glucose tolerance test (IPGTT)
After the mice were fasted overnight (with free access to drinking water) for 16 h, 25% glucose solution was administered to the mice by intraperitoneal injection, the dose was calculated according to 2 g/kg of glucose, and then blood glucose was taken for determination at 0, 15, 30, 60 and 120 min.
Insulin tolerance test (ITT)
After the mice were fasted for 4 h, 0.75 U/kg of insulin was administered by injection intraperitoneally, and blood glucose was quickly measured at 0, 30, 60, and 90 min.
Measurement of TC, TG, HDL, LDL,Scr, and BUN in the serum
The levels of TC, TG, HDL, LDL, Scr, and BUN in the serum were measured by an automatic biochemical analyser.
Biochemical index assays
TNF-α (Elabscience Biotechnology Co., Ltd, China) and IL-6 (Elabscience Biotechnology Co., Ltd, China) levels in the kidney tissue were measured according to the instructions of the ELISA kit.
Observation of renal ultrastructure
The renal tissues were immediately sheared into small pieces after removal from the mice, and then the pieces were placed in 2.5% glutaraldehyde at 4 °C for 2 h. Next, tissues were fixed in 1% osmium tetroxide for 2 h, embedded after ethanol and acetone dehydration, cut into slices, dyed, and observed by an electron microscope(HITACHI H-7650).
Real-time polymerase chain reaction (RT-PCR)
Total RNA was extracted from renal tissue by using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and the RNA was reverse transcribed to complementary DNA (cDNA) using the PrimeScript RT reagent kit and gDNA Eraser. The sequences of the primers are shown in Table 1. RT-PCR was processed by a 7300 real-time PCR detection system (Applied Biosystems, Foster City, CA, USA). β-actin was used as a reference gene. The results of the real-time PCR were calculated by the 2-△△Ct method.
Table 1.
Primer sequences used in this study
| Target gene | Forward primer | Reverse primer |
|---|---|---|
| TLR4 | TCCTGTGGACAAGGTCAGCAAC | TTACACTCAGACTCGGCACTTAGCA |
| MyD88 | TACAGGTGGCCAGAGTGGAA | GCAGTAGCGATAAAGGCATCGAA |
| NF-кB | GAACGATAACCTTTGCAGGC | TTTCGATTCCGCTATGTGTG |
| β-actin | CATCCGTAAAGACCTCTATGCCAAC | ATGGAGCCACCGATCCACA |
Western blotting analysis
Protein was extracted according to the RIPA buffer manufacturer’s instructions (Solarbio, China); the BCA method was used to calculate the concentration. Equal amounts of protein were subjected to 10% SDS-PAGE and then transferred to PVDF membranes which were incubated with antibodies including anti-TLR4 (1:1000), anti-MyD88 (1:1000), anti-NF-κBp65 (1:1000), and anti-β-actin (1:10,000) at 4 °C overnight. Next, the membranes were incubated in secondary antibody (goat anti-rabbit IgG1:10,000) for 1 h. Proteins were revealed using the eikonogen ECL method. The grey values of the protein bands were analysed by ImageJ software.
Statistical analysis
The data analysis was executed by SPSS16.0 and GraphPad Prism6.0.Statistical significance was evaluated by one-way analysis of variance (ANOVA), while multiple comparisons between the groups were performed using the S-N-K method. A value of P<0.05 indicated statistical significance.
Results
Effects of DMDD on the body weight of diabetic mice
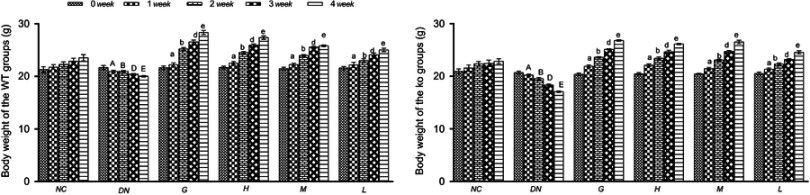
Body weight is an important index of diabetes. The body weight of normal mice gradually increased compared with that of the DN group mice, and the body weight of the diabetic nephropathy mice significantly decreased from 1 week. Compared with the untreated model mice, the mice administered gliquidone and DMDD displayed increased body weight (Figure 1).
Figure 1.
Effect of DMDD on the body weight of the WT and KO groups (n=6). NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). The data are presented as the mean ± SEM. A, B, D, and E: compared with those of the same period of the normal control group (P<0.05); a, b, d, and e: compared with those of the same period of the diabetic nephropathy group (P<0.05).
Abbreviations: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; WT, wild type; KO, knockout.
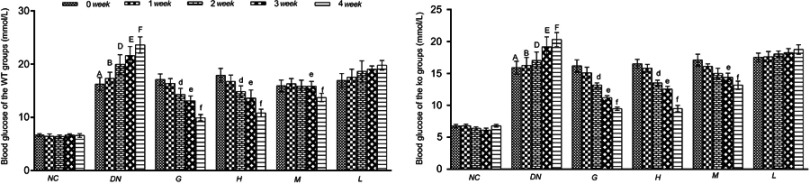
Effects of DMDD on the blood glucose levels of diabetic mice
As shown in the Figure 2, the blood glucose levels of diabetic mice were increased compared with those of normal mice, while the mice administered DMDD and gliquidone had significantly reduced blood glucose levels compared to those of diabetic mice.
Figure 2.
Effect of DMDD on the blood glucose level of the WT and KO groups (n=6). NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). The data are presented as the mean ± SEM. A, B, D, E, and F: compared with those of the same period of the normal control group (P<0.05). d, e, and f: compared with those of the same period of the diabetic nephropathy group (P<0.05).
Abbreviations: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; WT, wild type; KO, knockout.
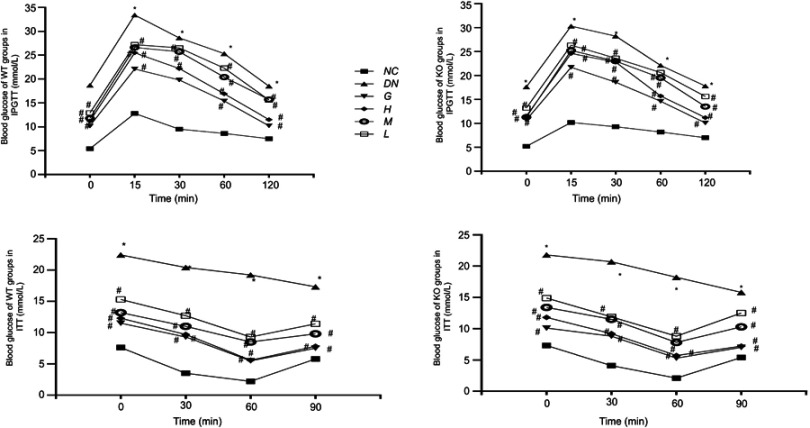
Effect of DMDD on IPGTT and ITT
After injection of 25% glucose solution, the blood glucose levels of the DN groups were higher than those of the NC groups. The blood glucose levels of the diabetic mice treated with DMDD were significantly reduced compared with those of the model group.
The insulin tolerance results are shown in Figure 3. After the intraperitoneal injection of insulin, the blood glucose level of the normal mice began to rise after 60 min. The model group maintained a downward trend. Compared with that of the DN group mice, the blood glucose level of the mice treated with DMDD increased from 60 min.
Figure 3.
Effect of DMDD on IPGTT and ITT (n=6). NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). *P<0.05: compared with the normal control groups. #P<0.05: compared with the diabetic nephropathythy groups.
Abbreviations: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; IPGTT, intraperitoneal glucose tolerance test; ITT, insulin tolerance test.
From the results of IPGTT and ITT analysis, it was found that DMDD is a powerful regulator of glucose and insulin intolerance.
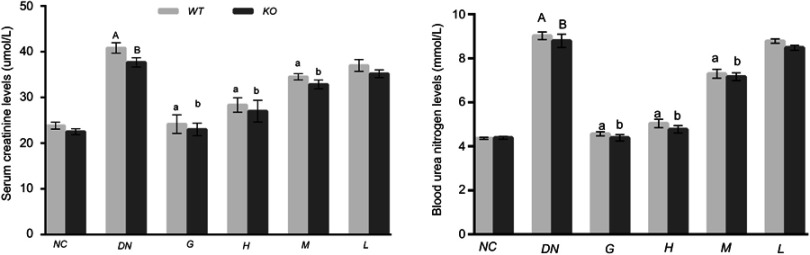
Effect of DMDD on the renal function of diabetic mice
The renal function parameters, including serum creatine (Scr) and blood urea nitrogen (BUN) levels, were measured by an automatic biochemical analyser. The levels of Scr and BUN were higher in diabetic nephropathy mice than in the normal control mice, and these levels were significantly abated in the mice treated with DMDD. Compared with that in the high-dosage DMDD group of WT mice, the level of Scr and BUN declined in KO mice without significant differences (Figure 4). These results indicate that DMDD treatment efficaciously improves diabetic nephropathy.
Figure 4.
Effect of DMDD on the renal function of diabetic mice (n=6). NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). A and B compared with the normal control groups (P<0.05); a, b: compared with the diabetic nephropathy groups (P<0.05).
Abbreviation: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione.
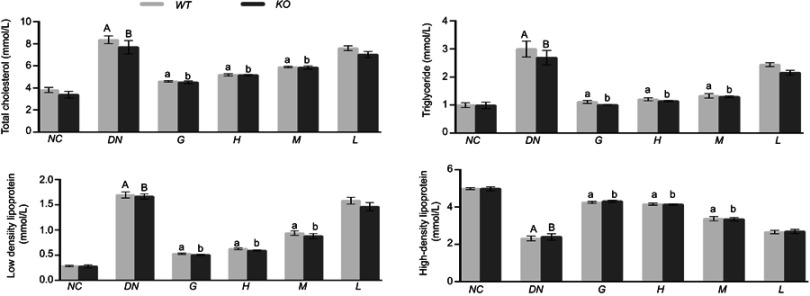
Effect of DMDD on the levels of TC, TG, HDL, and LDL in the serum
The levels of TC, TG and LDL were higher in diabetic mice than in normal mice, while the HDL was lower in diabetic mice. After treatment with DMDD, the levels of TC, TG and LDL were decreased dramatically accompanied by an increase in HDL in the diabetic mice (Figure 5). Therefore, DMDD treatment effectively improved dyslipidaemia.
Figure 5.
Effect of DMDD on the levels of TC, TG, HDL, and LDL in the serum (n=6). NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). A and B: compared with the normal control groups (P<0.05); a, b: compared with the diabetic nephropathy groups (P<0.05).
Abbreviation: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione.
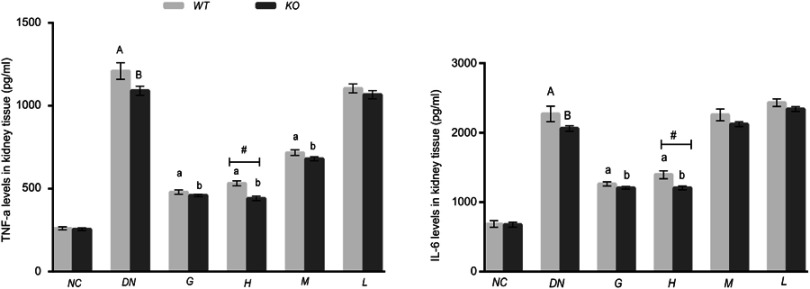
Effect of DMDD on the levels of TNF-α and IL-6 in kidney tissue
It has been demonstrated that diabetic nephropathy is related to the inflammatory reaction. In this study, we assessed the levels of the inflammatory factors TNF-α and IL-6 in kidney tissue by ELISA kits. The results revealed that the levels of TNF-α and IL-6 were elevated in the mice injected with STZ compared with those in normal mice. However, the levels of IL-6 and TNF-α were distinctly inhibited after treatment with DMDD (50 or 25 mg/kg). In addition, the production of IL-6 and TNF-α was evidently higher in the WT high-dosage DMDD group than in the equivalent KO group (Figure 6). Therefore, these results demonstrate that DMDD attenuated inflammation in diabetic mice and that TLR4 deficiency could alleviate the inflammatory response.
Figure 6.
Effect of DMDD on the levels of IL-6 and TNF-α in kidney tissue (n=6). NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). A and B: compared with the normal control groups (P<0.05); a and b :compared with the diabetic nephropathy groups (P<0.05). #: compared with WT mice( P<0.05).
Abbreviation: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione.
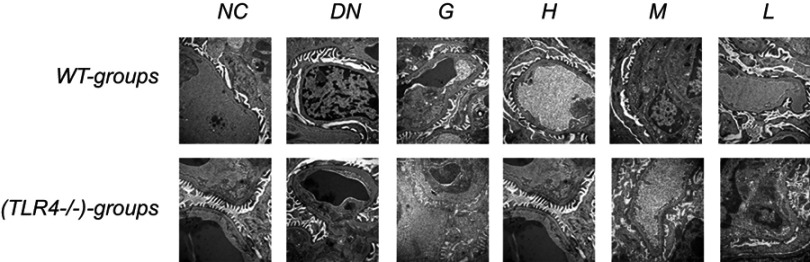
Observation of renal ultrastructure
The ultrastructure of the renal tissue was observed by electron microscopy. Compared with normal mouse renal tissue, DN tissue displayed increase incrassation of the glomerular basement membrane with podocytes exhibiting significant fusion significantly . The thickened glomerular basement membrane and the cytomixis of podocytes was attenuated by treatment with DMDD especially in a high dosage (Figure 7).
Figure 7.
Effect of DMDD on the ultrastructural changes in the renal tissue of WT and KO mice. NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1).
Abbreviations: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; WT, wild type; KO, knockout.
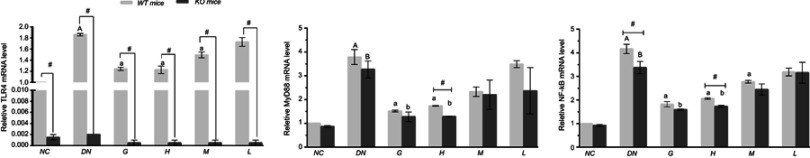
Effects of DMDD on the level of TLR4, MyD88 and NF-кb mRNA
The mRNA levels of TLR4, Myd88 and NF-кB in diabetic mice were significantly increased compared with those in the normal control mice. However, the TLR4, Myd88 and NF-кB mRNA levels were notably decreased by DMDD treatment. The TLR4 knockout mice displayed lower TLR4 mRNA levels than the wild-type diabetic mice. Interestingly, the mRNA level of NF-кB was markedly decreased in the TLR4 knockout diabetic mice compared with those in the wild-type mice after administration of DMDD (Figure 8).
Figure 8.
Effect of DMDD on the mRNA levels of TLR4, MyD88, and NF-κB. NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). A and B: compared with the WT and KO normal controls (P<0.05); a and b: compared with the WT and KO diabetic nephropathy groups (P<0.05); and #: compared with WT mice (P<0.05).
Abbreviations: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; WT, wild type; KO, knockout.
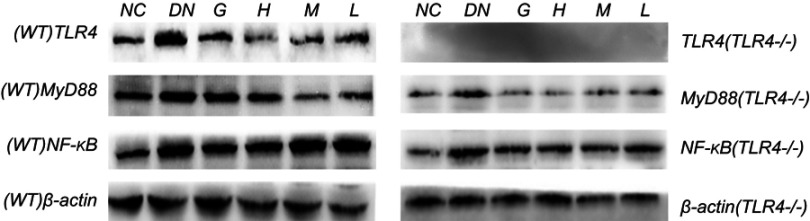
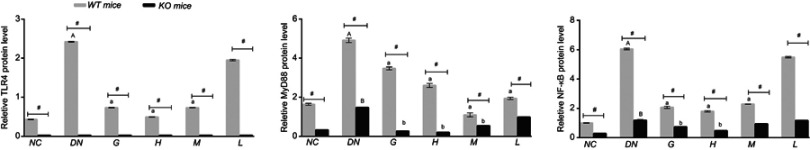
Effects of DMDD on the protein expression level of TLR4, MyD88, and NF-κb
The protein of TLR4/MyD88/NF-κB pathway was significantly elevated in the diabetic mice compared with normal mice and the levels of TLR4, MyD88, and NF-κB were decreased in diabetic mice after supplementation with DMDD in WT mice. On the other hand, the levels of MyD88 and NF-κB were declined by treatment with DMDD in diabetic mice in KO mice. Simultaneously, the expression levels of TLR4, MyD88 and NF-κB were higher in WT mice than in KO mice (Figures 9 and 10).
Figure 9.
Effect of DMDD on the expression of proteins in the TLR4/MyD88/NF-κB pathway. NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1).
Abbreviation: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione.
Figure 10.
Effect of DMDD on the expression of proteins in the TLR4/MyD88/NF-κB pathway. NC: normal control, DN: diabetic nephropathy group, G: gliquidone group (10 mg.kg−1.d−1), H: high dosage of DMDD group (50 mg.kg−1.d−1), M: medium dosage of DMDD group (25 mg.kg−1.d−1), L: low dosage of DMDD group (12.5 mg.kg−1.d−1). A and B: compared with the WT and KO normal controls (P<0.05); a and b: compared with the WT and KO diabetic nephropathy groups (P<0.05); and #: compared with WT mice (P<0.05).
Abbreviations: DMDD, 2- dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione; WT, wild type; KO, knockout.
Discussion
Diabetes mellitus (DM), a chronic metabolic disease, has become a global health issue with more than 80% of patients with diabetes living in developing countries; the number of people with diabetes may reach 592 million in the next 20 years.7,8 Diabetic nephropathy that develops into end-stage renal disease is one of the severe complications of diabetes. Diabetic nephropathy is prone to produce kidney damage with albuminuria in the USA and China.9 Hyperglycaemia contributes to the progression of DN; it generates glomerular hypertrophy leading to inflammation of glomeruli and also reduces cell numbers and the accumulation of extracellular matrix.10 Accumulating studies have shown that inflammation and innate immunity are essential processes in the development of renal damage from diabetes.11,12 The inflammatory response affects kidney cells through Toll-like receptors (TLRs), especially TLR4, and nuclear factor-κB(NF-κB),proinflammatory cytokines, and the induction of kidney that accelerates DN exacerbation.10,13 Recent studies utilizing TLR4 ablation have demonstrated that TLR4 is involved in renal injuries and alleviates the inflammatory response in diabetes.14–18 TLR4 signalling pathways are divided into classical myeloid differentiation factor 88 (MyD88)-dependent and MyD88-independent pathways. TLR4 binds to its major adaptor protein MyD88, in turn leading to the phosphorylation of the NF-κB inhibitory protein inhibitor of NF-κB (IκB). Phosphorylated IκB separates and transfers NF-κB in the cytoplasmic resting state into the nucleus, which activates the release of various inflammatory factors.19,20 Studies have shown that TLR4, MyD88 and NF-κB are highly expressed in the renal tissues of DN mice, and their expression levels are positively correlated with renal tissue inflammation and renal tubular epithelial-mesenchymal transdifferentiation; therefore, the TLR4/MyD88/NF-κB pathway is considered to be a major factor in promoting micro-inflammation and interstitial fibrosis in DN kidneys.16,21–24 Although, we understand more about DN, the treatment of DN remains an unresolved matter.25
Chinese herbal medicine has existed for thousands of years and plays important roles in the treatment of diseases in China. A. carambola L., which is mainly distributed in the tropics, is a sorrel plant and provides a wide range of pharmacological effects, such as improving digestion, treating coughs and diabetes.26,27 In the past 10 years, we have investigated the effects of A. carambola L. in the treatment of diabetes and its complications. The compounds of an ethanol extract and polysaccharide isolated from roots of A. carambola L. reduced blood glucose and resisted oxidative stress, and we successfully extracted 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (DMDD) from the ethanol-extracted compounds. Recently, we used a high-sugar and high-fat diet integrated with low-dosage STZ to generate a diabetic nephropathy mouse model and found that DMDD significantly relieved renal pathological damage, blood urea nitrogen, serum creatinine, blood urea and urine protein levels. Experiments showed that DMDD significantly attenuated the apoptosis of MIN6 cells which induced by Palmitic Acid through inhibiting the TLR4/MyD88/NF-κB signalling pathway.5,28,29 Now, we intend to investigate TLR4 as a target to explore the mechanism of DMDD-mediated protection in diabetic nephropathy, providing experimental basis and theoretical support for DMDD treatment of DN.
In this study, we demonstrated the protective effect of DMDD against diabetic nephropathy by increasing the body weight of diabetic mice, decreasing fasting blood glucose levels and improving kidney functions as well as damage to podocytes. Next, we detected the levels of inflammatory cytokines, observing that the levels of IL-6 and TNF-α were markedly increased in diabetic nephropathy mice but markedly decreased after administration of DMDD, especially at high doses. Finally, we analysed the protein expression levels of TLR4, MyD88, and NF-кB to verify whether TLR4 signalling is the mechanism underlying DMDD-mediated protection against DN. From the PCR and Western blot results, we found that TLR4, MyD88, and NF-кB levels were markedly increased in diabetic mice and decreased in mice treated with DMDD. Interestingly, not only the inflammation reaction but also the proteins of the TLR4/MyD88/NF-кB signalling pathway were lower in KO diabetic mice than in WT diabetic mice.
In summary, we propose that DMDD protects mice against diabetic nephropathy by inhibiting the TLR4/MyD88/NF-κB signalling pathway.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81760665, 81460205, 81360129), Innovation Project of Guangxi Graduate Education (YCBZ2018043, YCBZ2017043), and the Postdoctoral Science Foundation of China (No. 2017M613271XB).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Allison SJ. Acute kidney injury:Mechanism of AKI sensitivity in diabetic nephropathy. Nat Rev Nephrol. 2014;10(9):484. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13(11):460–469. doi: 10.1016/j.molmed.2007.09.002 [DOI] [PubMed] [Google Scholar]
- 4.Kaur H, Chien A, Jialal I. Hyperglycemia induces Toll like receptor 4 expression and activity in mouse mesangial cells: relevance to diabetic nephropathy. Am J Physiol Renal Physiol. 2012;303(8):F1145–F1150. doi: 10.1152/ajprenal.00319.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng N, Lin X, Wen Q, et al. Effect of 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione, isolated from Averrhoa carambola L. (Oxalidaceae) roots, on advanced glycation end-product-mediated renal injury in type 2 diabetic KKAy mice. Toxicol Lett. 2013;219(1):77–84. doi: 10.1016/j.toxlet.2013.03.001 [DOI] [PubMed] [Google Scholar]
- 6.Wen Q, Lin X, Liu Y, et al. Phenolic and lignan glycosides from the butanol extract of Averrhoa carambola L. root. Molecules. 2012;17(10):12330–12340. doi: 10.3390/molecules171012330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8 [DOI] [PubMed] [Google Scholar]
- 8.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Je S. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi: 10.1016/j.diabres.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 9.Bhalla V, Zhao B, Azar KM, et al. Racial/ethnic differences in the prevalence of proteinuric and nonproteinuric diabetic kidney disease. Diabetes Care. 2013;36(5):1215–1221. doi: 10.2337/dc12-0951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivero A, Mora C, Muros M, Garcia J, Herrera H, Navarro-Gonzalez JF. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci. 2009;116(6):479–492. doi: 10.1042/CS20080394 [DOI] [PubMed] [Google Scholar]
- 11.Mora C, Navarro JF. Inflammation and diabetic nephropathy. Curr Diab Rep. 2006;6(6):463–468. [DOI] [PubMed] [Google Scholar]
- 12.Tuttle KR. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Society Nephrol. 2005;16(6):1537–1538. doi: 10.1681/ASN.2005040393 [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Gonzalez JF, Mora-Fernandez C, Muros de Fuentes M, Garcia-Perez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nature Rev Endocrinol. 2011;7(6):327–340. doi: 10.1038/nrneph.2011.51 [DOI] [PubMed] [Google Scholar]
- 14.Lin M, Yiu WH, Wu HJ, et al. Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Society Nephrol. 2012;23(1):86–102. doi: 10.1681/ASN.2010111210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devaraj S, Tobias P, Jialal I. Knockout of toll-like receptor-4 attenuates the pro-inflammatory state of diabetes. Cytokine. 2011;55(3):441–445. doi: 10.1016/j.cyto.2011.03.023 [DOI] [PubMed] [Google Scholar]
- 16.Lin M, Yiu WH, Li RX, et al. The TLR4 antagonist CRX-526 protects against advanced diabetic nephropathy. Kidney Int. 2013;83(5):887–900. doi: 10.1038/ki.2013.11 [DOI] [PubMed] [Google Scholar]
- 17.Kuwabara T, Mori K, Mukoyama M, et al. Exacerbation of diabetic nephropathy by hyperlipidaemia is mediated by Toll-like receptor 4 in mice. Diabetologia. 2012;55(8):2256–2266. doi: 10.1007/s00125-012-2578-1 [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Yang S, Ma Y, Bai XY, Chen X. Role of Toll-like receptors in diabetic renal lesions in a miniature pig model. Sci Adv. 2015;1(5):e1400183. doi: 10.1126/sciadv.1400183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee S, Karmakar S, Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016;20(2):193–204. doi: 10.1016/j.bjid.2015.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brancato SK, Thomay AA, Daley JM, et al. Toll-like receptor 4 signaling regulates the acute local inflammatory response to injury and the fibrosis/neovascularization of sterile wounds. Wound Repair Regener. 2013;21(4):624–633. doi: 10.1111/wrr.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng KW, Zhang T, Fu H, Liu GX, Wang XM. Schisandrin B exerts anti-neuroinflammatory activity by inhibiting the Toll-like receptor 4-dependent MyD88/IKK/NF-kappaB signaling pathway in lipopolysaccharide-induced microglia. Eur J Pharmacol. 2012;692(1–3):29–37. doi: 10.1016/j.ejphar.2012.05.030 [DOI] [PubMed] [Google Scholar]
- 22.Ma J, Chadban SJ, Zhao CY, et al. TLR4 activation promotes podocyte injury and interstitial fibrosis in diabetic nephropathy. PLoS One. 2014;9(5):e97985. doi: 10.1371/journal.pone.0097985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. doi: 10.1172/JCI28898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu P, Li F, Qiu M, He L. Expression and cellular distribution of TLR4, MyD88, and NF-kappaB in diabetic renal tubulointerstitial fibrosis, in vitro and in vivo. Diabetes Res Clin Pract. 2014;105(2):206–216. doi: 10.1016/j.diabres.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 25.Cooper ME. Diabetes: treating diabetic nephropathy-still an unresolved issue. Nature Rev Endocrinol. 2012;8(9):515–516. doi: 10.1038/nrendo.2012.125 [DOI] [PubMed] [Google Scholar]
- 26.Cazarolli LH, Kappel VD, Pereira DF, et al. Anti-hyperglycemic action of apigenin-6-C-beta-fucopyranoside from Averrhoa carambola. Fitoterapia. 2012;83(7):1176–1183. doi: 10.1016/j.fitote.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 27.Carolino RO, Beleboni RO, Pizzo AB, et al. Convulsant activity and neurochemical alterations induced by a fraction obtained from fruit Averrhoa carambola (Oxalidaceae: geraniales). Neurochem Int. 2005;46(7):523–531. doi: 10.1016/j.neuint.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 28.Xie Q, Zhang S, Chen C, et al. Protective Effect of 2-dodecyl-6-methoxycyclohexa-2, 5-diene-1, 4-dione, isolated from averrhoa carambola L., Against palmitic acid-induced inflammation and apoptosis in Min6 cells by inhibiting the TLR4-MyD88-NF-kappaB Signaling Pathway. Cell Physiol Biochem. 2016;39(5):1705–1715. doi: 10.1159/000447871 [DOI] [PubMed] [Google Scholar]
- 29.Li J, Wei X, Xie Q, et al. Protective Effects of 2-dodecyl-6-methoxycyclohexa-2,5 -diene-1,4-dione isolated from Averrhoa Carambola L. (Oxalidaceae) roots on high-fat diet-induced obesity and insulin resistance in Mice. Cell Physiol Biochem. 2016;40(5):993–1004. doi: 10.1159/000453156 [DOI] [PubMed] [Google Scholar]