Abstract
Purpose
We performed a retrospective, comparative study to determine if patients with aniridia and glaucoma had open angles on high-resolution anterior segment optical coherence tomography (OCT) and clinical gonioscopy.
Patients and methods
Forty-three patients (86 eyes) with aniridia had recorded anterior segment OCTs, gonioscopy, or both. Of these patients, 27 (54 eyes) were diagnosed with glaucoma and 16 (32 eyes) had no evidence of glaucoma. All patients had either anterior segment OCT, gonioscopy, or both.
Results
The 43 patients with aniridia had average age of 32±17 years, and 27 (62%) were female. Anterior segment OCT and gonioscopy were recorded in 25 (58%) of the patients and 18 (42%) of the patients had gonioscopy alone. Of the 54 eyes with aniridia and glaucoma, 4 (7%) eyes in 3 patients (11%) had partial or completely closed angles. Of the 32 eyes without glaucoma, all (100%) had open angles. The proportion of open angles in the aniridia with glaucoma eyes was not significantly different compared with the aniridia without glaucoma eyes (P=0.32). Of the 4 eyes with closed angles, all had a history of prior surgery for cataract, glaucoma, and/or keratopathy. The proportion of eyes with prior surgery was significantly higher in eyes with open-angle glaucoma and angle-closure glaucoma compared with eyes without glaucoma (P<0.001 and P=0.002, respectively).
Conclusion
The majority of eyes with aniridia and glaucoma have open anterior chamber angles, similar to patients with aniridia without glaucoma. All eyes with aniridia and glaucoma that had closed angles had a prior history of ocular surgery.
Keywords: aniridia, glaucoma, anterior segment optical coherence tomography (OCT), gonioscopy
Introduction
Aniridia is a rare congenital disorder of ocular development due to mutation of the PAX6 gene, which is inherited most commonly in an autosomal dominant fashion with variable expressivity. The incidence of aniridia is between 1:64,000 and 1:100,000.1 Patients with aniridia have a high risk for childhood glaucoma, with a median age of onset of glaucoma of 8 years with approximately half of the patients developing glaucoma.2 A proposed mechanism for aniridic glaucoma is anterior rotation of the rudimentary iris, peripheral anterior synechiae formation, and progressive angle-closure.3 We performed a retrospective, comparative study using clinical gonioscopy and high-resolution anterior segment optical coherence tomography (OCT) to assess the anterior chamber angle in aniridia patients with and without glaucoma.
Patients and methods
Patients with aniridia who had been examined by gonioscopy and/or anterior segment OCT were identified by search of electronic medical records and included in the study. The medical records from a 5-year period (May 2012 to May 2017) were assessed. Patients with congenital aniridia were included in the study (traumatic causes of aniridia were excluded). All patients had been examined and gonioscopy was performed by an experienced clinician observer (PAN). In the majority of patients, glaucoma fellows and residents had also examined the patients (AB, EB, and others). Cooperative patients were examined by gonioscopy. Patients who were unable to tolerate gonioscopy or those with a poor view of the anterior chamber angle were examined by anterior segment OCT. Patients with significant aniridic keratopathy and a poor view of the anterior chamber were imaged using anterior segment OCT.
Anterior segment OCT (Visante, Carl Zeiss Meditec, Jena, Germany) images were assessed for angle-closure using previously described methods to identify scleral spur and apposition between the iris and inner corneoscleral wall.4 Patients with open angles had no evidence of angle closure, those with partial angle closure had 1–3 quadrants of angle closure, and those with complete angle closure had completely (4 quadrants) closed anterior chamber angle. Patients with closed angle in any quadrant were classified as having partial or complete angle closure and included in the closed angle group. Patients were diagnosed with aniridic glaucoma with increased intraocular pressure, optic nerve, and/or visual field changes. All patients with diagnosis of glaucoma had been found to have increased intraocular pressure (by Goldmann or Perkins applanation tonometry, and/or rebound tonometry) and had glaucomatous optic nerve findings and/or glaucomatous visual field changes.
Groups were compared using the comparison of proportions test and the unpaired t-test. P-values less than 0.05 were considered statistically significant. The study was approved by the University of Virginia Institutional Review Board (IRB) and conformed to the requirements of the Declaration of Helsinki and the United States Health Insurance Portability and Privacy Act (HIPPA).
Results
Eighty-six eyes (N=43) patients with aniridia were included in the study (Table 1). In the 43 included patients with aniridia, anterior segment OCT was recorded in 23 patients (53%), both gonioscopy and anterior segment OCT were recorded in 2 patients (5%), and gonioscopy alone was performed in 18 patients (42%). Of these patients, 27 (54 eyes) were diagnosed with glaucoma and 16 (32 eyes) had no evidence of glaucoma. Twenty-five patients were female. The average age was 30.8±17.9 years. The average age of aniridics with glaucoma (30±17 years) was not significantly different compared with aniridics without glaucoma (36±17 years, P=0.27). Nearly half of the patients (47%) had familial aniridia, and approximately half (53%) had sporadic aniridia.
Table 1.
Characteristics of aniridia patients (N=43)
| Number of patients | 43 |
| Number of eyes | 86 |
| Gender, male/female, N (%) | 18/25 (42/58%) |
| Mean age±SD (median age), years | 30.8±17.9 |
| Race, Caucasian/Asian, N (%) | 42/1 (98/2%) |
| Glaucoma, yes/no, N (%) | 27/16 (63/37%) |
| Aniridia | |
| Familial, N (%) | 20 (47%) |
| Sporadic, N (%) | 23 (53%) |
| WAGR, N (%) | 0 (0%) |
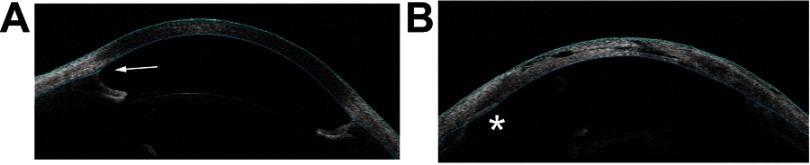
The anterior chamber angle was assessed for angle closure with OCT and gonioscopy, with representative OCT images shown in Figure 1. Of the 54 eyes with aniridia and glaucoma, 4 (7%) eyes in 3 of 27 patients with glaucoma (11%) had partial or completely closed angles (Table 2). Of the 32 eyes without glaucoma, all (100%) had open angles. The proportion of open angles in the aniridia with glaucoma eyes was not significantly different compared with the aniridia without glaucoma eyes (P=0.32). Of the 4 eyes in 3 patients with closed angles, all had a history of prior surgery for cataract, glaucoma, and/or keratopathy. A significantly higher proportion of eyes with open-angle glaucoma and angle-closure glaucoma had prior surgery compared with eyes without glaucoma (P<0.001 and P=0.002, respectively).
Figure 1.
Anterior segment OCT images showing open- and closed-angle findings in aniridia patients. (A) A 12-year-old aniridia patient with glaucoma and open-angle (arrow). (B) A 21-year-old aniridia patient with glaucoma, aphakia, and closed-angle. The asterisk shows an area of angle closure, with iris tissue appositional to the cornea. This patient was treated with lensectomy and goniotomy as an infant, and subsequently with glaucoma drainage implant.
Table 2.
Anterior chamber angle and prior surgery in eyes with aniridia (N=86)
| Open angle with glaucoma, N (%) | 50/54 (93%) | P=0.32 |
| Open angle without glaucoma, N (%) | 32/32 (100%) | P<0.001 |
| Angle closure with glaucoma, N (%) | 4/54 (7%) | |
| Prior surgery with open-angle glaucoma, N (%) | 35/50 (70%) | P<0.001 |
| Prior surgery without glaucoma, N (%) | 5/32 (16%) | P=0.002 |
| Prior surgery with angle-closure glaucoma, N (%) | 4/4 (100%) |
One patient with both angles classified as closed had trabeculotomy in both eyes shortly after birth, glaucoma drainage implant surgery in both eyes at age 1–2 years, penetrating keratoplasty and keratolimbal allograft in the left eye at age 5 years, cyclophotocoagulation in the left eye at age 16–17 years, cataract surgery without intraocular lens in the right eye at age 18 years, and cyclophotocoagulation in the right eye at age 19 years. One patient with one angle classified as closed had cataract surgery with posterior chamber intraocular lens implantation at age 43 years, glaucoma drainage implant at age 44 years, Descemet stripping automated endothelial keratoplasty with keratolimbal allograft at age 54 years, and keratoprosthesis at age 56 years. One patient with one angle classified as closed had cataract surgery with posterior chamber intraocular lens and iris prosthesis implantation at age 5 years, iris prosthesis revision at age 7 years with hyphema and aqueous misdirection, medical therapy for glaucoma, and aniridia fibrosis syndrome treated with vitrectomy and membrane removal at age 9 years.
Of the 4 eyes with closed angles, 3 eyes had partial angle closure (1 eye with approximately 1 quadrant closure, 2 eyes with 2–3 quadrants closure) and 1 eye had complete angle closure. All eyes with open angles had subjective estimation of Shaffer grade 3–4 angular width. Eyes with closed angles (partial and complete) had subjective description of angle closure due to peripheral anterior synechiae.
Discussion
Congenital aniridia is a panocular malformation of the eye, including findings in the anterior and posterior segment of the eye. Glaucoma is a common problem in aniridia, which may cause progressive vision loss.1 A proposed mechanism for aniridic glaucoma is peripheral anterior synechiae formation and progressive angle closure.3 In this study, we found that the majority of eyes with aniridia and glaucoma have open anterior chamber angles, similar to patients with aniridia without glaucoma. In our patients, the small proportion (7%) of eyes with aniridia and glaucoma that had closed angles had a prior history of ocular surgery.
Sakata et al reported that angle closure in non-aniridic patients was detected more often with anterior segment OCT compared with gonioscopy (59% and 33% of the eyes had angle closure observed in at least 1 quadrant by OCT and gonioscopy, respectively).5 OCT may not substitute for gonioscopy because gonioscopy remains useful for evaluation of pigment and neovascularization of the angle, as well as assessment of peripheral anterior synechiae (PAS) with indentation gonioscopy. In aniridia, the visibility of the anterior chamber angle can be compromised because of aniridic keratopathy and nystagmus. In our study, anterior segment OCT was performed in the majority of patients (58%).
A report of treatment of one aniridia patient with goniotomy by Barkan in 1953 described control of intraocular pressure during 9 months follow-up.6 Progressive angle closure has been proposed as a mechanism for aniridic glaucoma, based on gonioscopic evaluation of a group of patients.3 A possible degeneration of the corneoscleral angle was proposed, with the development of a contractile membrane between the surface of the iris and the angle wall playing a role in the gradual obstruction or closure of the angle.3 The findings in our study do not exclude this possible mechanism but suggest that it is not common in previously untreated eyes. Our findings are consistent with Nelson and colleagues7 and Margo8 who did not find progressive angle closure in aniridia patients clinically or histologically.
In our study, 4 eyes with glaucoma (7%) in 3 patients with glaucoma (11%) had partial or completely closed angles, all of whom had a history of prior surgery for cataract, glaucoma, and/or keratopathy. We found a significantly higher proportion of prior ocular surgery in eyes with angle closure compared with eyes without glaucoma (P=0.002). Aniridia is a profibrotic syndrome,9 which may account for this observation. One patient with closed angle in our study was diagnosed with aniridia fibrosis syndrome after several intraocular procedures. The only known risk factor for aniridia fibrosis syndrome is prior ocular surgery,9 although it is possible there is an unrecognized relationship with the specific type of PAX6 mutation. Possible risk of fibrosis and glaucoma may be discussed with the decision for any type of primary ocular surgery in aniridia patients.
Regardless of the mechanism of glaucoma, patients with aniridia are at risk for childhood glaucoma, with approximately half of the patients developing glaucoma by age 8 years old.2 Prophylactic goniotomy has been proposed as a possible treatment for eyes with aniridia that are developing progressive closure of the angle,10 while our results suggest a limited role for this approach. Therapeutic goniotomy is usually not helpful for aniridia patients, although the procedure may be considered in the uncommon situation of an infant with aniridia and glaucoma.1 Surgical approaches that remove tissue from the angle such as Trabectome (Neomedix, Tustin, CA) or Kahook Dual Blade (New World Medical, Rancho Cucamonga, CA) are under evaluation. Often clinicians will treat with glaucoma drainage implant in aniridia patients who develop intractable elevation of intraocular pressure despite medical and surgical therapy.
In our aniridia patients, all (100%) eyes without glaucoma had open anterior chamber angles. The proportion of open angles in aniridia with glaucoma eyes was not significantly different compared with the aniridia without glaucoma eyes (P=0.32). Viestenz and coworkers reported that the mean distance between anterior chamber angle and Schlemms canal was 1.3±0.4 mm (range: 0.5–2.1 mm) in 23 eyes (17 patients) with aniridia.11 Ultrasound biomicroscopy images of two aniridia patients show open anterior chamber angles, while summary data regarding the proportion with open angles and previous surgery are not shown.11 In another ultrasound biomicroscopy study, the trabecular-iris angle of aniridics with glaucoma was not significantly different from that of eyes without glaucoma, suggesting that open-angle configuration is more common than closed-angle in aniridic glaucoma.12
Animal models of aniridia have shown some variability in anterior segment findings. In the mouse model for aniridia, the anterior chamber angle may be closed, but these mutant mice have a smaller eye than normal, a more crowded anterior chamber angle, and a more severe narrow-angle phenotype in the anterior chamber than in humans.13–15 In an aniridia model in the amphibian Xenopus tropicalis,16 mutant frogs have no change in eye size compared with normal, and an open anterior chamber angle (Nakayama, Netland and Grainger, unpublished). In general, the frog eye phenotype is more similar than mouse to human;16 specifically, eye size is unchanged in the pax6 heterozygous frog and human eyes.
Limitations of this study include potential for investigator bias in the selection and review of cases, which is a general problem in retrospective studies. The search of electronic records may not have identified all patients with aniridia who had an assessment of the anterior chamber angle. The number of patients may not have been sufficiently large to detect differences between groups. Our criteria for angle closure were based on findings of apposition of the iris remnant and the inner corneoscleral wall, which could have strengthened by quantitative measurement of the depth of the iridocorneal angle. The small number of patients with angle closure and prior ocular surgery did not have documentation of the pre-operative status of their anterior chamber angles, and it is not known whether surgery contributed to their angle closure or if angle closure caused glaucoma for which surgery was performed.
Conclusion
The majority of our patients with aniridia and glaucoma have open anterior chamber angles, similar to patients with aniridia without glaucoma. We also found that eyes with aniridic glaucoma and closed angles had a prior history of ocular surgery, which may have contributed to the development of angle closure.
Acknowledgments
The authors acknowledge Jill Nerby and Aniridia Foundation International (AFI) for patient support (PAN, RMG) and Vision for Tomorrow (VFT) for aniridia research support (RMG). The abstract of this paper was presented at The Association for Research in Vision and Ophthalmology (ARVO) 2017 Annual Meeting as a poster with interim findings (Baltimore, Maryland, May 8, 2017).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Netland PA. Aniridia: recent developments in scientific and clinical research In: Parekh M, Poli B, Ferrari S, Teofili C, Ponzin D, editors. Management of Glaucoma in Congenital Aniridia. London: Springer; 2015:27–37. [Google Scholar]
- 2.Netland PA, Scott ML, Boyle JW, Lauderdale JD. Ocular and systemic findings in a survey of aniridia subjects. JAAPOS. 2011;15:562–566. [DOI] [PubMed] [Google Scholar]
- 3.Grant WM, Walton DS. Progressive changes in the angle in congenital aniridia, with development of glaucoma. Am J Ophthalmol. 1974;78:842–847. doi: 10.1016/0002-9394(74)90308-0 [DOI] [PubMed] [Google Scholar]
- 4.Nolan WP, See JL, Chew PTK, et al. Detection of primary angle closure using anterior segment optical coherence tomography in Asian eyes. Ophthalmology. 2007;114:33–39. doi: 10.1016/j.ophtha.2006.05.073 [DOI] [PubMed] [Google Scholar]
- 5.Sakata LM, Lavanya R, Friedman DS, et al. Comparison of gonioscopy and anterior segment ocular coherence tomography in detecting angle closure in different quadrants of the anterior chamber angle. Ophthalmology. 2008;115:769–774. doi: 10.1016/j.ophtha.2007.06.030 [DOI] [PubMed] [Google Scholar]
- 6.Barkan O. Goniotomy for glaucoma associated with aniridia. AMA Arch Ophthalmol. 1953;49:1–5. [DOI] [PubMed] [Google Scholar]
- 7.Nelson LB, Spaeth GL, Nowinski TS, Margo CE, Jackson L. Aniridia, a review. Surv Ophthalmol. 1984;28:621–642. [DOI] [PubMed] [Google Scholar]
- 8.Margo CE. Congenital aniridia: a histopathologic study of the anterior segment in children. J Pediatr Ophthalmol Strabismus. 1983;20:192–198. [DOI] [PubMed] [Google Scholar]
- 9.Tsai JH, Freeman JM, Chan CC, et al. A progressive anterior fibrosis syndrome in patients with postsurgical congenital aniridia. Am J Ophthalmol. 2005;140:1075–1079. doi: 10.1016/j.ajo.2005.07.035 [DOI] [PubMed] [Google Scholar]
- 10.Chen TC, Walton DS. Goniosurgery for prevention of aniridic glaucoma. Arch Ophthalmol. 1999;117:1144–1148. doi: 10.1001/archopht.117.9.1144 [DOI] [PubMed] [Google Scholar]
- 11.Viestenz A, Seitz B, Deland E, et al. Clinical anatomy of the anterior chamber angle in congenital aniridia and consequences for trabeculotomy/cyclophotocoagulation. Clin Anat. 2018;31:64–67. doi: 10.1002/ca.22935 [DOI] [PubMed] [Google Scholar]
- 12.Okamoto F, Nakano S, Okamoto C, Hommura S, Oshika T. Ultrasound biomicroscopic findings in aniridia. Am J Ophthalmol. 2004;137:858–862. doi: 10.1016/j.ajo.2003.12.014 [DOI] [PubMed] [Google Scholar]
- 13.Baulmann DC, Ohlmann A, Flügel-Koch C, Gaswami S, Cvekl A, Tamm ER. Pax6 heterozygous eye show defects in chamber angle differentiation that are associated with a wide spectrum of other anterior eye segment abnormalities. Mech Dev. 2002;118:3–17. [DOI] [PubMed] [Google Scholar]
- 14.Ramaesh T, Collinson JM, Ramaesh K, Kaufman MH, West JD, Dhillon B. Corneal abnormalities in Pax6± small eye mice mimic human aniridia-related keratopathy. Invest Opthalmol Vis Sci. 2003;44:1871–1878. doi: 10.1167/iovs.02-0576 [DOI] [PubMed] [Google Scholar]
- 15.Kroeber M, Davis N, Holzmann S, et al. Reduced expression of Pax6 in lens and cornea of mutant mice leads to failure of chamber angle development and juvenile glaucoma. Hum Mol Genet. 2010;19:3332–3342. doi: 10.1093/hmg/ddq237 [DOI] [PubMed] [Google Scholar]
- 16.Nakayama T, Fisher M, Nakajima K, et al. Xenopus pax6 mutants affect eye development and other organ systems, and have phenotypic similarities to human aniridia patients. Dev Biol. 2015;408:328–344. doi: 10.1016/j.ydbio.2015.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]