Abstract
Background
Postoperative neurocognitive disorder (PND) is a severe postoperative complication with no effective therapy that affects up to 19–52% of senior patients. Age and surgery type have been identified as risk factors. However, what caused the increased risk in the elderly is poorly understood.
Methods
We utilized a PND model in aged mice undergoing experimental laparotomy with general anesthesia to evaluate the causal relationship between hyperhomocysteinemia and increased PND susceptibility. PND was assessed by Novel Object Tasks, Fear Conditioning Tests, and Barnes Maze Tests. Serum homocysteine (Hcy) as well as vitamin B12 and folate acid levels were tested before, immediately after surgery and from day 1 to day 29 after surgery by ELISA. The effectiveness of preventative strategy including diet supplementation of vitamin B12 + folic acid (Vit B12 + FA) and S‐adenosylmethionine (SAM) injection targeting hyperhomocysteinemia were also tested.
Results
PND in aged mice lasted for at least 2 weeks after experimental laparotomy, which was not observed in young adult mice. Serum Hcy results indicated a significant correlation between postoperative cognitive performance and perioperative Hcy level. Preoperative supplementation with VB12 and folic acid (FA) in the diet or S‐adenosylmethionine (SAM) injection reduced perioperative serum Hcy level and inhibited the development of PND in aged mice.
Conclusions
Serum homocysteine accumulation is a fundamental cause for increased susceptibility of PND in aged mice. Preoperative diet supplementation of VitB12 + FA can effectively reduce PND in aged mice, which may be a promising prophylaxis treatment in clinical settings.
Introduction
Postoperative neurocognitive disorder (PND) is a severe complication that affects the working ability including compromised attention, memory, orientation, executive function, or language fluency after surgery in patients, and is associated with increased morbidity and mortality.1 The incidence of PND in middle‐aged patients is 19.2%2 and up to 52% in senior patients.3, 4 Increased susceptibility to PND has also been found in aged animals.5, 6, 7, 8 However, the mechanisms underlying the increased susceptibility to PND in elderly individuals remained largely unknown.
Accumulative evidences have shown that serum homocysteine (Hcy) level is increased in aged population9, 10 and Hcy is associated with neurological disorders such as dementia.11, 12 Although preoperative hyperhomocysteinemia was suggested as a risk factor for developing PND in elderly patients undergoing oncological surgery,13 inconsistent conclusions was drawn on patients undergoing CABG surgery.14 No effort has been made to explore the relationship between Hcy and PND in animal researches. Thus, we hypothesized that hyperhomocysteinemia is a crucial risk for increased susceptibility of PND in aged individuals. More importantly, targeting hyperhomocysteinemia, a clinically feasible and economic preemptive approach, diet supplement with vitamin B12 (VB12) and folic acid (FA), were evaluated for its effectiveness in PND prevention in current study.
Methods
Research animals and ethical approval of study protocol
All procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The current protocols were approved by the Ethics Committee for Animal Experimentation of the Fourth Military Medical University. Two hundred and fifty male C57BL/6J mice aged 20–22 months (aged mice) and 130 aged 8–10 weeks (young adult) male mice were purchased from Chang Zhou Cavens Laboratory Animal Co.,Ltd. All mice were settled for 1 week before any intervention with access to food and water ad libitum and kept on a 12 h light/12 h dark cycle.
PND model construction
Mice were subjected to experimental laparotomy under isoflurane anesthesia to construct PND model as previously reported with slight modification.15 Anesthesia was delivered through a face mask (1.5 to 2.0% isoflurane, O2 0.5 L/min). Mice were placed on a heat pad to maintain body temperature between 36.5–37.0°C. The abdominal hair was removed and followed by skin sterilization with iodophor and 65% alcohol. Mice in surgical group had a 1‐cm abdominal midline incision. Approximately 5‐cm small intestine was exteriorized from the peritoneal cavity, covered with moist gauze, and gently rubbed for a total of 10 min. Abdominal muscle was closed with 5‐0 Vicryl sutures (PolysorbTM, USA), followed by skin closure with 4‐0 silk suture. Wound was infiltrated with 0.2% ropivacaine (300 μL) for postoperative analgesia in order to avoid the interruption of pain to cognitive assessment.16, 17 The surgery duration was fixed at 30 min. Control mice merely had their hair removed without anesthesia nor surgical intervention. Mice were allowed to recover in an incubator at 35°C for 30 min before returning to their home cages. Neurocognitive functions were assessed as below.
Neurocognitive function assessment for researched animals
Novel object recognition test
Novel object recognition test (NOR) was used to assess short‐term working memory according to previous protocol with adjustments.18. Mice were habituated on day 13 and tested on day 14 after surgery. A video tracking system (ANY‐maze, Stoelting Co., Ltd) was used to analyze exploration time ratio of the novel object as compared to an original object. For habituation, remove the mouse from its home cage and place it in the middle of the open arena. Allow the mouse to freely explore for 5 min. We conducted 2 sessions on the training day. On Training Session, place 2 same objects in opposite quadrants, then place the mouse in the center of the arena and allow the mouse to explore for 10 min. Sixty min after Training Session, we placed one of the object used during training and replaced one novel object in opposite quadrants. Finally, placed the mouse in the center of the arena and allow free exploration for 10 min. Discrimination ratio = [(Time for exploring novel object in test phase/time for exploring both objects in test phase) – (Time for exploring original object at the same location in training phase/Time for exploring both objects in training phase)] × 100%].
Contextual fear conditioning test
Mice were submitted to a contextual fear conditioning paradigm in a certain context (Xeye Instruments, Beijing MacroAmbition S&T Development Co., Ltd) with slight modification.19 Contextual fear conditioning test contained three sessions: habituation phase (day 13), training phase (day 14), and test phase (day 15). On the habituation day, mice were placed in the training context for 10 min free moving. On training day, a total of five foot‐shocks were delivered (current: 0.7 mA, 2 sec; inter foot‐shocks interval: 35–60 sec). Twenty‐four hours later, mice were tested for contextual memory retrieval in the same context without aversive stimuli for 5 min. The animals were considered freezing if no movement was detected for 2 sec (Freezing time ratio = Freezing time/300 s × 100%).
Barnes maze test
Barnes maze test was carried out as described before with modifications.20 Barnes maze (Global Biotech Co.,Ltd, Shanghai) test was conducted in a dim room and included 5 consecutive days with training phase (days 12–15 after surgery) and test phase (day 16 after surgery). A video camera was set above and connected to the Any‐Maze animal tracking system (Stoelting Co., Ltd, USA). The escape latency and time spent in the target quadrant were analyzed. On day 11, after surgery, mice were firstly habituated in the testing room for 1 hour, then placed on the platform for 2 min and guided toward the escape box for another 3 min. During the training period, bright light (200 W) and white noise (85 dB) were given as aversive stimulation. Once reaching the escape box, the buzzer was turned off. Mice were allowed to remain in the escape box for 1 min before returned back to their home cages. The training phase consisted of 15 trials: three trials on the first day and four trials per day on the next 3 days (3 min each trial and 15 min intervals). Odor cues were removed through ethanol wipes between sessions. During the testing period, the escape box was removed, and then mice were allowed to move freely for 2 min with same aversive stimuli. The increases in escape latency, and decrease in time in target quadrant in the Barnes maze suggests cognitive impairment of the mice. (Time ratio in the target quadrant = time spent in the target quadrant in the test session/120s × 100%).
Vitamin supplementation and drug delivery
Diet supplementation of Vitamin B12 and/or folic acid
The control diet was AIN93G (contained Vitamin Mix V10037, folic acid 2 mg/kg, Vitamin B12 25 μg/kg, Open SourceTM, China). For vitamin‐rich diet, diet containing five times normal dose of vitamin B12 (125 μg/kg) and folic acid (10 mg/kg) were given to aged mice for 2 weeks before surgery till the end of study.
Homocysteine and SAM administration
S‐adenosylmethionine (SAM, Shanghai Macklin Biochemical Co., Ltd) was used as an allosteric activator for cystathionine beta synthase (CBS)21 to enhance sulfur transfer pathway metabolism of homocysteine. Aged mice were injected with different doses of SAM (0.4, 0.8, 4, 8, or 16 mg/kg) to determine the appropriate dosage for serum homocysteine reduction. Follow‐up study adopted 4 mg/kg SAM i.p. injection (or equal volume of saline) per day for 1 week before surgery.
To reverse the homocysteine‐lowering effect of VB12 and FA, homocysteine was given intraperitoneally once per day for 7 days before surgery in aged mice already fed on VB12 + FA rich diet 1 week prior to Hcy injection. The VB12 + FA diet was maintained throughout the perioperative period. Different doses of homocysteine (0.5, 5, 10, or 20 mg/kg, Sigma‐Aldrich Trading Co., Ltd, Shanghai) were used. Thus, the follow‐up study used 10 mg/kg homocysteine i.p. injection (or equal volume of saline) for 1 week as protocol for hyperhomocysteinemia.
In order to induce hyperhomocysteinemia in young adult mice, 10 mg/kg extragenic homocysteine was administered once per day for 1 week, which mimicked the change of serum homocysteine in aged mice. Serum homocysteine were detected at different times pre‐ and post‐surgery.
Blood tests for levels of homocysteine, vitamin B12, and folic acid
Before and after a 30‐min surgery, animals were maintained on anesthesia and blood samples were collected directly from the left ventricle before sacrifice. The time point is around 35 min since the onset of anesthesia.
Commercially available ELISA kits for measuring serum homocysteine, vitamin B12, and folic acid of mice (WESTANG BIO‐TECH Co., Ltd, Shanghai, China) were used according to manufacturers’ instructions. Triple tests for each sample were performed. Absorbances (450 nm) were read using an ELISA microplate reader (DENLEY DRAGON Wellscan MK 3, Thermo, Finland).
Statistic analysis
Statistical analysis was performed using the SPSS version 20.0 program (SPSS Inc., Chicago, IL) or GraphPad Prism 7.0 software. For comparison of serological tests (serum Hcy, VB12 and folic acid) and neurocognitive tests between two groups, student's t‐test was used. And a one‐way ANOVA analysis with post hoc Tukey's test was used when more than two groups were compared at different time points. Besides, freezing time ratio was analyzed with Mann–Whitney U test when comparing two groups, while Kruskal–Wallis test with Dunn’s post hoc test was used when comparing four groups. For spatial learning (escape latency), a two‐way ANOVA analysis was used to determine statistical significance among groups at different time points with Sidak’s multiple comparison. P < 0.05 was considered significance.
Results
We used both aged and young adult mice to investigate the effect of serum Hcy level on postoperative cognitive function. Serum Hcy levels were higher (Fig. 1A, P < 0.0001), and VB12 levels were lower (Fig. 1B, P = 0.0005) in aged mice than in younger controls, while FA levels remained similar throughout the perioperative period (Fig. 1C). Serum Hcy level decreased after surgery but rose back to the preoperative level at 1 day after surgery in both groups. On postoperative day 4, the serum Hcy levels of aged mice were significantly higher than those of young adults (Fig. 1A, P = 0.018). VB12 levels were significantly reduced in both young adult and aged animals after surgery (Fig. 1B). This reduction lasted for at least 4 days in aged mice and 8 days in young ones.
Figure 1.
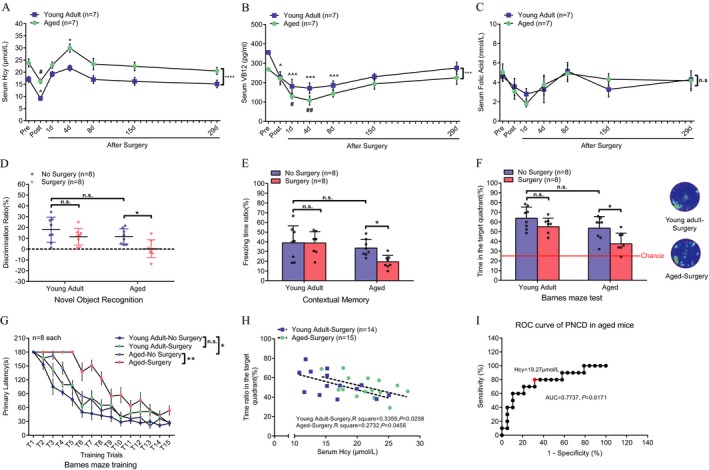
Serum homocysteine, vitamin B12, and folic acid levels and neurocognitive performance in aged and young adult mice before and after surgery. (A) Time course of serum Hcy levels in adult and aged mice throughout the perioperative period until 29 days after surgery. ****P < 0.0001; *P = 0.018 versus young adult mice at the same time point. # P = 0.0498, post‐Hcy versus pre‐Hcy in aged mice, and ^ P = 0.0324, post‐Hcy versus pre‐Hcy in young adult mice. (B) Perioperative vitamin B12 level. ***P = 0.0005; # P = 0.0147, ## P = 0.0016 versus pre‐Aged mice; ^ P = 0.0394, ^^^ P = 0.0003, P = 0.0001 and P = 0.0005 at 1 day, 4 days, and 8 days versus preoperatively in young adult mice. (C) Perioperative folic acid level. P = 0.9763. For a, b, and c, Two‐way ANOVA between aged and young adult mice and Sidak's multiple comparison between groups at each time point and among time points within the same group. N = 7 at each time point for each group, mean ± SEM. (D–G) Differences in cognitive function between aged and young adult mice revealed by the novel object recognition test (D), unpaired t‐test, *P = 0.0346, n = 8 each, mean ± SD), contextual fear conditioning test (E), Mann–Whitney U test, *P = 0.0458, n = 8 each, mean ± SD), Barnes maze test (F), unpaired t test, *P = 0.0114, n = 8 each, mean ± SD) and Barnes maze training (G), two‐way ANOVA, n = 8 each, mean ± SEM) in mice without surgery or mice at 14 days after laparotomy. *P < 0.05, **P < 0.01 compared to aged mice without surgery. (H) The correlation between serum Hcy levels and spatial memory performance at 14 days after surgery (linear regression, n = 14 for young adult mice: R 2 = 0.3359, P = 0.0298 and n = 15 for aged mice: R 2 = 0.2732, P = 0.0456). (I) ROC curve of post‐Hcy levels for PND in aged mice (spatial memory reduction of at least 1.5 SD of sham‐aged mice were considered a cognitive decline). AUC = 0.7737, P = 0.0171
Compared with nonsurgical controls, aged animals showed significant deterioration at 14 days after surgery in working memory tested with novel object recognition (Fig. 1D, P = 0.0346), contextual fear memory (Fig. 1E, P = 0.0458), and spatial memory tested with a Barnes maze (Figs. 1F, P = 0.0114 and 1G, P < 0.0001 between no‐surgery and surgery groups of age mice). However, no significant cognitive decline was observed after surgery in young animals. Strong negative correlations between postoperative serum Hcy levels and spatial memory were observed at 14 days after surgery in both aged and young adult mice (Fig. 1H, for aged mice: R 2 = 0.2732, P = 0.0456; for young adult mice: R 2 = 0.3359, P = 0.0298).
We further generated a receiver operating characteristic (ROC) curve of post‐Hcy for PND in aged mice by dividing the mice into impaired cognition and normal cognition groups, using the mean‐1.5 × standard deviation (SD) of time spent in the target quadrant of sham‐controlled aged mice as the standard. A post‐Hcy level of 19.27 μmol/L had an 80% sensitivity and a 68.42% specificity for diagnosing postoperative spatial memory decline in aged mice (Fig. 1I, AUC = 0.7737, P = 0.0171).
We then investigated whether lowering serum Hcy levels by diet supplementation with VB12 and FA before surgery could inhibit the development of PND. The results showed that preoperative supplementation with VB12 and FA in the diet for 2 weeks significantly reduced serum Hcy levels throughout the perioperative period (Fig. 2A, P < 0.0001) and could prevent postoperative neurocognitive dysfunction in aged mice (Fig. 2D–G). Hcy could be eliminated through trans‐sulfur pathway by supplementing SAM, an allosteric activator of cystathionine beta synthase (CβS). Thus, we assessed the effects of SAM intraperitoneal injection on serum Hcy level and found that 4.0–16.0 mg/kg per day SAM injection for 7 days significantly reduced serum Hcy levels in aged mice (Fig. 2H, P < 0.01). Preoperative administration of SAM (4 mg/kg) to aged mice significantly blocked the spatial memory, contextual memory, and working memory decline at 14 days after surgery (Fig. 2I, J and K, P = 0.0136, P = 0.0204, and P = 0.0385, respectively). We also analyzed the correlation between serum Hcy, VB12, or FA with postoperative spatial memory performance. The results again showed that in aged animals with or without VB12 + FA supplementation, the spatial memory was negatively correlated to Hcy level (R 2 = 0.3472, P = 0.0438 for control group; R 2 = 0.3907, P = 0.0298 for VB12 + FA group) and positively correlated to VB12 level (R 2 = 0.3450, P = 0.045 for control group; R 2 = 0.3659, P = 0.037 for VB12 + FA group) but not serum FA level.
Figure 2.
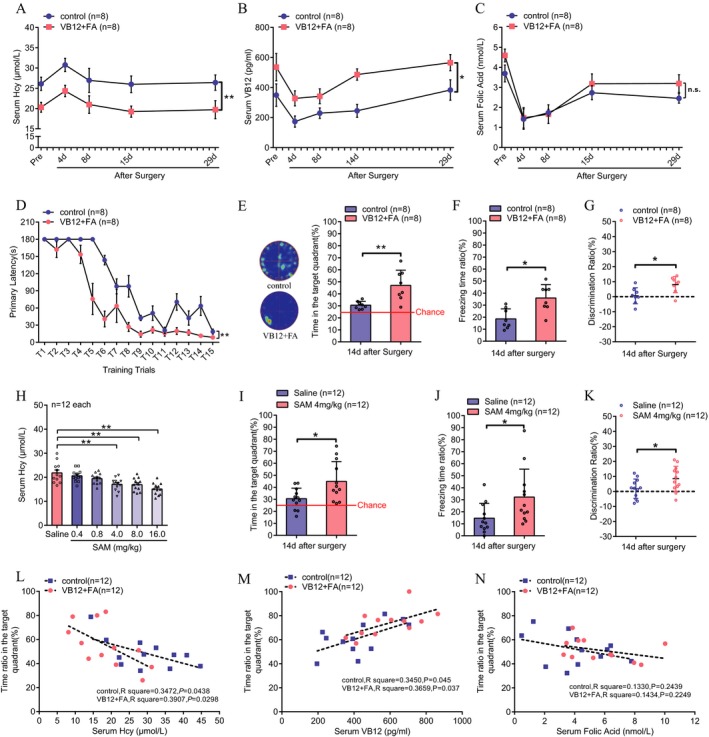
Both preoperative supplementation with dietary vitamin B12 plus folic acid and preoperative Sadenosylmethionine injection reduced serum homocysteine levels and prevented the development of postoperative neurocognitive deficits in aged mice. (A) Serum Hcy levels, (B) vitamin B12 levels, and (C) folic acid levels (P = 0.207) at different time points throughout the perioperative period in aged mice fed a control diet or supplemented with a vitamin B12 and folic acid‐rich diet for 2 weeks before surgery (two‐way ANOVA, n = 8 each, mean ± SEM, P < 0.0001, P = 0.01, P = 0.207 respectively). Postoperative cognitive function in these two groups was assessed at 14 days after surgery. (D) and (E) show the statistical results for spatial learning (*P = 0.0027, two‐way ANOVA, n = 8 each, mean ± SEM) and spatial memory (*P = 0.0032, unpaired t‐test, n = 8 each, mean ± SD) in the Barnes maze test. (F) Contextual memory (*P = 0.0047, Mann–Whitney U test, n = 8 each, mean ± SD) and (G) working memory (*P = 0.0148, unpaired t test, n = 8 each, mean ± SD) were also evaluated. (H) The dose‐effect of intraperitoneal injection of SAM induced a reduction in Hcy levels (one‐way ANOVA, n = 12 each, mean ± SEM, **P < 0.01). (I–K) Postoperative cognitive function in the SAM or saline group was assessed 14 days after surgery. (I) Spatial memory (*P = 0.0136, unpaired t‐test, n = 12 each, mean ± SD). (J) Contextual memory (*P = 0.0204, Mann–Whitney U test, n = 12 each, mean ± SD). (K) Working memory (*P = 0.0385, unpaired t‐test, n = 12 each, mean ± SD). (L–N) Correlation analysis between postoperative spatial memory performance at 14 days after surgery and postoperative serum Hcy levels (L), vitamin B12 levels (M) or folic acid levels (N) and (linear regression, n = 12 each) in mice from both the control diet group and the VB12 + FA supplementation diet group
To identify the causal effect of hyperhomocysteinemia on PND susceptibility, we recreated hyperhomocysteinemia by intraperitoneal injection of Hcy (0.5 to 20 mg/kg for 7 days) in aged mice that were supplemented with B12 + FA in the diet (Fig. 3A, P < 0.01). Perioperative Hcy and VB12 levels were also evaluated to exclude the influence of Hcy injection on serum VB12. Results showed that Hcy injection partly reversed the Hcy lowering effect of VB12 + FA during the perioperative period (Fig. 3B, P = 0.0035, VB12 + FA + Hcy vs. VB12 + FA + saline group), while their serum VB12 level maintained higher than aged control mice and as high as mice in VB12 + FA supplementation only group (Fig. 3C, P = 0.004, VB12 + FA + Hcy vs. Control group. P = 0.1094, VB12 + FA + Hcy vs. B12 + FA + saline group). These results indicated that preoperative Hcy injection for a week did not affect serum VB12 elevation induced by diet supplementation.
Figure 3.
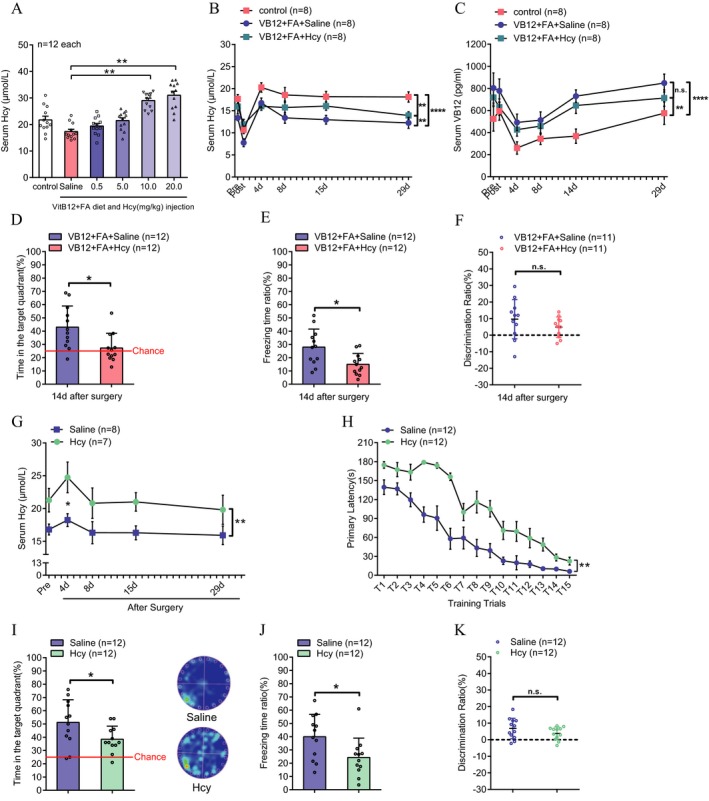
Hyperhomocysteinemia is responsible for increased susceptibility of PND in both aged and young adult mice. (A) Effect of different doses of intraperitoneal injection of Hcy on serum Hcy levels in aged mice fed a high vitamin B12 and folic acid diet. **P = 0.007, 10 mg/kg Hcy versus saline group; **P = 0.0012, 20 mg/kg Hcy versus saline group (one‐way ANOVA, n = 12 each, mean ± SEM). (B) Perioperative serum Hcy level of animals in three groups. **P = 0.0017 and ****P < 0.0001 versus control group; **P = 0.0035, VB12 + FA + Hcy versus VB12 + FA + saline group. (C) Perioperative serum VB12 level of animals in three groups. **P = 0.004 and ****P < 0.0001 versus control group. P = 0.1094, VB12 + FA + Hcy versus VB12 + FA + saline group. (D) Postoperative spatial memory (*P = 0.0105, unpaired t‐test, n = 12 each, mean ± SD). (E) contextual memory (*P = 0.0145, Mann–Whitney U test, n = 12 each, mean ± SD) and (F) working memory (P = 0.2424, unpaired t‐test, n = 12 each, mean ± SD) were compared between mice in the VB12 + FA group and the VB12 + FA + Hcy group at 14 days after surgery. (G) Serum Hcy levels at different time points in young adult mice treated with saline or 10 mg/kg·day Hcy administration for 7 days before surgery (two‐way ANOVA, *P < 0.0434 versus serum Hcy level before surgery in saline group. **P < 0.0001 between Hcy and saline groups. n = 12 each, mean ± SEM). Postoperative cognitive function in these two groups were assessed 14 days after surgery. (H) The ability of spatial learning (**P = 0.0056, two‐way ANOVA, n = 12 each, mean ± SEM) and (I) spatial memory (*P = 0.037, unpaired t test, n = 12 each, mean ± SD) were assessed by the Barnes maze test. (J) Contextual memory (*P = 0.0346, Mann–Whitney U test, n = 12 each, mean ± SD) was assessed by the fear conditioning test. (K) Working memory was assessed by a novel object recognition test (P = 0.1733, unpaired t‐test, n = 12 each, mean ± SD)
Preoperative Hcy injection (10 mg/kg) increased serum Hcy levels and significantly abolished the beneficial effect of B12 + FA supplementation against PND in spatial and contextual memory (Fig. 3D, P = 0.0105 and Fig. 3E, P = 0.0145) but did not result in the reversal of working memory preservation (Fig. 3F, P = 0.2424). We then administered Hcy to young adult mice (10 mg/kg once per day for 7 days) and found that Hcy administration increased serum Hcy levels throughout the perioperative period (Fig. 3G, P < 0.0001), which led to deterioration in postoperative spatial learning, spatial memory, and contextual fear memory (Fig. 3H–J, P = 0.0056, P = 0.037, and P = 0.0346, respectively). However, the difference in working memory between the two groups was not significant (Fig. 3K, P = 0.1733).
Discussion
In the current study, we firstly provided evidences to suggest that elevated serum Hcy was a key mediator for increased susceptibility of PND in aged animals. Targeting hyperhomocysteinemia by activating Hcy trans‐methylation metabolism with preoperative vitamin B12 + folic acid supplementation or activating trans‐sulfuration metabolism with preoperative SAM injection both produced an effective prevention for PND in aged mice.
Methionine and folic acid metabolism interact to promote methylation–demethylation cycle that affects DNA, protein, phospholipid, and neurotransmitter synthesis, which are key components for maintaining normal neurocognitive function. Hcy, an intermediate product of the methylation cycle, has been reported to be correlated with neurocognitive decline in the elderly population.12 Moderately elevated plasma total homocysteine is a risk factor for vascular dementia, Alzheimer's disease, white matter damage, brain atrophy, neurofibrillary tangles, etc.22 However, few studies illustrated the relationship between Hcy and PND, and no attempt has been made using vitamin B12 and FA as Hcy pharmacological clearance in the perioperative period to prevent PND. In the current study, aged but not young adult mice exhibited postoperative neurocognitive decline at 14 days after surgery. In the meantime, serum Hcy level was higher in aged mice as compared to young adult mice during the whole perioperative period, suggesting that aged animals with higher serum Hcy levels have greater susceptibility to PND. We further analyzed the correlation of post‐Hcy to postoperative spatial memory at 14 days after surgery, significant negative correlation was found in both adult and aged animals (Fig. 1H). And a post‐Hcy level over 19.27 μmol/L is able to sensitively diagnose postoperative spatial memory decline in aged mice. Since neuropsychological tests for PND diagnosis were very complicated and time consuming, our results suggested that postoperative serum Hcy level may be a valuable biomarker for postoperative cognitive performances.
The present study revealed a sharp reduction of serum Hcy level immediately after surgery in both young adult and aged mice. To our knowledge, no literature has observed an immediate reduction of serum Hcy level after surgery and anesthesia exposure. We also observed a significant increase of Hcy in aged mice at 4 days after surgery as compared to their preoperative level, but this change was not significant in young adult mice. On the other hand, serum vitamin B12 level dropped in both aged and young animals, the reduction remained even longer (until day 8 after surgery) in young adult mice. But serum folic acid level change was not obvious throughout perioperative period. These results together with the phenotype of cognitive deficiency in aged but not young adult mice put high level of homocysteine in a more pivotal stand then vitamin B12 deficiency regarding PND. The anesthetic used in current study is isoflurane. Different from our observation, another general anesthetic, nitrous oxide, was reported to induce Hcy elevation in patients both immediately (upon arrival to the postanesthesia unit),23 and at 24 h–48 h after surgery.23, 24 The possible mechanisms for this immediate reduction we observed include protein expression and activity changes in enzymes that are involved in the metabolism of Hcy such as cystathionine beta synthase (CBS), methylene tetrahydrofolate reductase (MTHFR), methionine synthase (MS), betaine homocysteine methyltransferase (BHMT), and S‐adenosyl‐L‐homocysteine hudrolase (SAHH). Another possibility lies in the alteration of Hcy distribution, reduced extracellular secretion, or increased accumulation in certain organs. However, the exact mechanism for this sudden and profound reduction of serum Hcy level after surgery and anesthesia merits further exploration.
There is no effective prevention or treatment for PND yet. Several drugs/agents were suggested as candidates that may reduce or ameliorate PND. These candidates include anti‐inflammatory agents such as RAGE antagonist FPSZM1,25 berberine,26 microglia activation inhibitor minocyclin,27 anti‐inflammatory cytokine IL‐4,8 and glycyrrhizin, a natural high‐mobility group box 1 (HMGB1) inhibitor.28 Another type of agents are oxidative stress inhibitors such as curcumin,29 edaravone,30 and apocynin.31 In clinical study, intraoperative dexmedetomidine was reported to reduce the incidence of PND along with decreased levels of β‐amyloid and Tau protein accumulation.32 The present study showed that both preoperative VB12 + FA or SAM supplementation can reduce serum Hcy while ameliorating the neurocognitive decline in aged mice after laparotomy. Since VB12 and FA are widely used, both safe and economic, our discovery advocates further clinical trials to confirm their efficacy.
Vitamin B supplementation has been widely used for treating neurologic diseases in clinical settings. However, the reported efficacies were controversial. Regular vitamin intake in middle‐aged asymptomatic individuals were associated with improved neurocognitive performance for early dementia‐sensitive neurocognitive changes.33 In an Alzheimer's disease model induced by hypoxia exposure, multiple vitamin supplementation (vitamin B6, B12, folate, and choline) in young adult mice ameliorated the spatial memory deterioration.34 However, in older patients with mild to moderate dementia, 10‐months of vitamin B12 supplementation did not improve neurocognitive function.35 These results indicated that the effectiveness of vitamin B group on cognitive performance relies on the timing of supplementation, more prophylaxis than treatment. When cognition decline has already taken place, supplementation of VB12 could not reverse this course, which also support our results that preoperative VB12 + FA supplementation reduces PND susceptibility. These results also indicated that VB12 is not a rescue plan, it has to work through risk reduction, which led us to believe that accumulation of Hcy is the fundamental cause of increased PND susceptibility.
The current study showed linear correlation between spatial memory performance and serum Hcy level in young or aged mice with and without VB12 + FA supplementation after surgery (Figs. 2H and 3L). Spatial memory in aged mice after surgery also correlated to postoperative serum vitamin B12 level (Fig. 3M), but not folic acid level (Fig. 3N). Vitamin B12 has a variety of physiological effects other than promoting homocysteine metabolism. In order to verify whether the fundamental cause of increased PND susceptibility in aged mice was either VB12 deficiency or Hcy accumulation, we then subjected additional L‐Hcy injection in aged mice during VB12 + FA supplementation. The results revealed that homocysteine level elevation significantly reversed the prophylaxis effect of vitamin B12 supplementation against PND (Fig. 3B–D), which indicated that homocysteine reduction is the key factor in vitamin B12 induced PND prevention. More importantly, the Hcy injection did not affect serum VB12 level increase induced by diet supplementation in aged mice, which further confirmed that cognitive function was more likely to be affected by hyperhomocysteinemia but not VB12 deficiency. We also generated hyperhomocysteinemia in young adult animals by i.p. injection of L‐Hcy for a week prior to surgery. Elevated homocysteine impaired postoperative spatial learning, spatial memory, and contextual fear memory in young adult mice (Fig. 3E–I). These results suggested that higher homocysteine alone is able to induce increased PND susceptibility.
In summary, homocysteine is a key mediator for the increased susceptibility of postoperative neurocognitive impairment in the aged mice. Preoperative supplementation of vitamin B12 and folic acid, or SAM, can prevent PND by reducing serum Hcy. Further clinical trials and neural mechanism studies are needed to explore whether this simple method could help reducing PND in elderly surgical patients.
Author Contributions
G.C.Z., J.D., and Y.S. contributed to acquisition and analysis of data and drafting of the manuscript. P.Z. contributed to the animal experiments. H.L.D. contributed to the experimental design and drafting of the manuscript. Z.X. contributed to conception and design of the clinical study, and revision of the manuscript. L.X. contributed to conception and design of the study, and manuscript revision.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by a key project of the National Natural Science Foundation of China (No.81730032) to Dr. Lize Xiong, an Elite Team Grant of the Fourth Military Medical University to Dr. Lize Xiong, a key project of International Cooperation and Exchanges of the National Natural Science Foundation of China (No.8172018012) to Dr. Yuan Shen, an International Cooperation Grant from Shanghai Natural Science Foundation (No. 16410724500) to Dr. Yuan Shen from the National Institutes of Health to Zhongcong Xie, and Henry K. Beech Professorship from Harvard University to Zhongcong Xie. And a National Natural Science Foundation of China (No.81771421) to Dr. Jiao Deng.
Guangchao Zhao, Jiao Deng and Yuan Shen are contributed equally to this research.
Funding Information
This work was supported by a key project of the National Natural Science Foundation of China (No.81730032) to Dr. Lize Xiong, an Elite Team Grant of the Fourth Military Medical University to Dr. Lize Xiong, a key project of International Cooperation and Exchanges of the National Natural Science Foundation of China (No.8172018012) to Dr. Yuan Shen, an International Cooperation Grant from Shanghai Natural Science Foundation (No. 16410724500) to Dr. Yuan Shen. Dr. Zhongcong Xie was partially supported by the Henry K. Beecher Professorship from Harvard University, Cambridge, MA. A National Natural Science Foundation of China (No.81771421) to Dr. Jiao Deng
Funding Statement
This work was funded by National Institutes of Health grant ; Elite Team Grant of the Fourth Military Medical University grant ; National Natural Science Foundation of China grants 8172018012, 81730032, and 81771421; Henry K. Beecher Professorship from Harvard University grant ; Shanghai Natural Science Foundation grant 16410724500.
Contributor Information
Zhongcong Xie, Email: zxie@mgh.harvard.edu.
Lize Xiong, Email: mzkxlz@126.com.
References
- 1. Vutskits L, Xie Z. Lasting impact of general anaesthesia on the brain: mechanisms and relevance. Nat Rev Neurosci 2016;17:705–717. [DOI] [PubMed] [Google Scholar]
- 2. Johnson T, Monk T, Rasmussen LS, et al. Postoperative cognitive dysfunction in middle‐aged patients. Anesthesiology 2002;96:1351–1357. [DOI] [PubMed] [Google Scholar]
- 3. Meybohm P, Renner J, Broch O, et al. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double‐blind randomized controlled pilot study. PLoS ONE 2013;8:e64743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008;108:18–30. [DOI] [PubMed] [Google Scholar]
- 5. Le Y, Liu S, Peng M, et al. Aging differentially affects the loss of neuronal dendritic spine, neuroinflammation and memory impairment at rats after surgery. PLoS ONE 2014;9:e106837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hovens IB, Schoemaker RG, van der Zee EA, et al. Surgery‐induced behavioral changes in aged rats. Exp Gerontol 2013;48:1204–1211. [DOI] [PubMed] [Google Scholar]
- 7. Wang Z, Meng S, Cao L, et al. Critical role of NLRP3‐caspase‐1 pathway in age‐dependent isoflurane‐induced microglial inflammatory response and cognitive impairment. J Neuroinflam 2018;15:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Liu F, Ma H, et al. Age exacerbates surgery‐induced cognitive impairment and neuroinflammation in Sprague‐Dawley rats: the role of IL‐4. Brain Res 2017;1665:65–73. [DOI] [PubMed] [Google Scholar]
- 9. Ardawi MS, Rouzi AA, Qari MH, et al. Influence of age, sex, folate and vitamin B12 status on plasma homocysteine in Saudis. Saudi Med J 2002;23:959–968. [PubMed] [Google Scholar]
- 10. Strassburg A, Krems C, Luhrmann PM, et al. Effect of age on plasma homocysteine concentrations in young and elderly subjects considering serum vitamin concentrations and different lifestyle factors. Int J Vitam Nutr Res 2004;74:129–136. [DOI] [PubMed] [Google Scholar]
- 11. Song JH, Park MH, Han C, et al. Serum homocysteine and folate levels are associated with late‐life dementia in a Korean population. Osong Public Health Res Perspect 2010;1:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ravaglia G, Forti P, Maioli F, et al. Homocysteine and cognitive performance in healthy elderly subjects. Arch Gerontol Geriatr Suppl 2004; 38:349–357. [DOI] [PubMed] [Google Scholar]
- 13. Weerink LB, van Leeuwen BL, Gernaat SA, et al. Vitamin status and the development of postoperative cognitive decline in elderly surgical oncologic patients. Ann Surg Oncol 2018;25:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Silbert B, Evered L, Scott DA, et al. Homocysteine and C‐reactive protein are not markers of cognitive impairment in patients with major cardiovascular disease. Dement Geriatr Cogn Disord 2008;25:309–316. [DOI] [PubMed] [Google Scholar]
- 15. Kawano T, Eguchi S, Iwata H, et al. Impact of preoperative environmental enrichment on prevention of development of cognitive impairment following abdominal surgery in a rat model. Anesthesiology 2015;123:160–170. [DOI] [PubMed] [Google Scholar]
- 16. Gong GL, Liu B, Wu JX, et al. Postoperative cognitive dysfunction induced by different surgical methods and its risk factors. Am Surg 2018;84:1531–1537. [PubMed] [Google Scholar]
- 17. Gu H, Deng X, Lv Y, et al. Preoperational chronic pain impairs the attention ability before surgery and recovery of attention and memory abilities after surgery in non‐elderly patients. J Pain Res 2019;12:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 2017;30:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu C, Krabbe S, Gründemann J, et al. Distinct hippocampal pathways mediate dissociable roles of context in memory retrieval. Cell 2016;167:961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qiu LL, Gu C, Mandeville ET, et al. Anesthesia and surgery impair blood–brain barrier and cognitive function in mice. Front Immunol 2017;8:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pey AL, Majtan T, Sanchez‐Ruiz JM, Kraus JP. Human cystathionine beta‐synthase (CBS) contains two classes of binding sites for S‐adenosylmethionine (SAM): complex regulation of CBS activity and stability by SAM. Biochem J 2013;449:109–121. [DOI] [PubMed] [Google Scholar]
- 22. Smith AD, Refsum H. Homocysteine, B vitamins, and cognitive impairment. Annu Rev Nutr 2016;36:211–239. [DOI] [PubMed] [Google Scholar]
- 23. Badner NH, Beattie WS, Freeman D, Spence JD. Nitrous oxide‐induced increased homocysteine concentrations are associated with increased postoperative myocardial ischemia in patients undergoing carotid endarterectomy. Anesth Analg 2000;91:1073–1079. [DOI] [PubMed] [Google Scholar]
- 24. Myles PS, Chan MT, Kaye DM, et al. Effect of nitrous oxide anesthesia on plasma homocysteine and endothelial function. Anesthesiology 2008;109:657–663. [DOI] [PubMed] [Google Scholar]
- 25. Zhou H, Luo T, Wei C, et al. RAGE antagonism by FPSZM1 attenuates postoperative cognitive dysfunction through inhibition of neuroinflammation in mice. Mol Med Rep 2017;16:4187–4194. [DOI] [PubMed] [Google Scholar]
- 26. Zhang Z, Li X, Li F, An L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int Immunopharmacol 2016;38:426–433. [DOI] [PubMed] [Google Scholar]
- 27. Wang HL, Liu H, Xue ZG, et al. Minocycline attenuates post‐operative cognitive impairment in aged mice by inhibiting microglia activation. J Cell Mol Med 2016;20:1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kong ZH, Chen X, Hua HP, et al. The oral pretreatment of glycyrrhizin prevents surgery‐induced cognitive impairment in aged mice by reducing neuroinflammation and Alzheimer's‐related pathology via HMGB1 inhibition. J Mol Neurosci 2017;63:385–395. [DOI] [PubMed] [Google Scholar]
- 29. Wu X, Chen H, Huang C, et al. Curcumin attenuates surgery‐induced cognitive dysfunction in aged mice. Metab Brain Dis 2017;32:789–798. [DOI] [PubMed] [Google Scholar]
- 30. Tian A, Ma H, Zhang R, et al. Edaravone improves spatial memory and modulates endoplasmic reticulum stress‐mediated apoptosis after abdominal surgery in mice. Exp Ther Med 2017;14:355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang J. Nox‐2‐mediated phenotype loss of hippocampal parvalbumin interneurons might contribute to postoperative cognitive decline in aging mice. Front Aging Neurosci 2016;8:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xu G, Li LL, Sun ZT, et al. Effects of dexmedetomidine on postoperative cognitive dysfunction and serum levels of b‐amyloid and neuronal microtubule‐associated protein in orthotopic liver transplantation patients. Ann Transplant 2016;21:508–515. [DOI] [PubMed] [Google Scholar]
- 33. Flitton M, Macdonald IA, Knight HM. Vitamin intake is associated with improved visuospatial and verbal semantic memory in middle‐aged individuals. Nutr Neurosci 2017;22:401–8. [DOI] [PubMed] [Google Scholar]
- 34. Yu L, Chen Y, Wang W, et al. Multi‐vitamin B supplementation reverses hypoxia‐induced tau hyperphosphorylation and improves memory function in adult mice. J Alzheimers Dis 2016;54:297–306. [DOI] [PubMed] [Google Scholar]
- 35. Kwok T, Lee J, Lam L, Woo J. Vitamin B(12) supplementation did not improve cognition but reduced delirium in demented patients with vitamin B(12) deficiency. Arch Gerontol Geriat 2008;46:273–282. [DOI] [PubMed] [Google Scholar]


