Abstract
The fall in mean arterial pressure (MAP) after 24 h of 5-HT infusion is associated with a dilation of the portal vein (PV) and abdominal inferior vena cava (Ab IVC); all events were blocked by the selective 5-HT7 receptor antagonist SB269970. Few studies have investigated the contribution of the 5-HT7 receptor in long-term cardiovascular control, and this requires an understanding of the chronic activation of the receptor. Using the newly created 5-HT7 receptor knockout (KO) rat, we presently test the hypothesis that continuous activation of the 5-HT7 receptor by 5-HT is necessary for the chronic (1 wk) depressor response and splanchnic venodilation. We also address if the 5-HT7 receptor contributes to endogenous cardiovascular regulation. Conscious MAP (radiotelemeter), splanchnic vessel diameter (ultrasound), and cardiac function (echocardiogram) were measured in ambulatory rats during multiday 5-HT infusion (25 μg·kg−1·min−1 via minipump) and after pump removal. 5-HT infusion reduced MAP and caused splanchnic venodilation of wild-type (WT) but not KO rats at any time point. The efficacy of 5-HT-induced contraction was elevated in the isolated abdominal inferior vena cava from the KO compared with WT rats, supporting loss of a relaxant receptor. Similarly, the efficacy of 5-HT causing an acute pressor response to higher doses of 5-HT in vivo was also increased in the KO vs. WT rat. Our work supports a novel mechanism for the cardiovascular effects of 5-HT, activation of 5-HT7 receptors mediating venodilation in the splanchnic circulation, which could prove useful in the treatment of cardiovascular disease.
Keywords: blood pressure, 5-HT7 receptor, venous circulation
INTRODUCTION
Administration of 5-hydroxytryptamine (5-HT, serotonin) is known to cause dose-dependent, depressor and pressor responses within the cardiovascular system. These outcomes depend on the level of free circulating 5-HT in the plasma as well as the location, affinity, and signaling mechanisms of the numerous 5-HT receptors found in the heart, blood vessels, brain, and autonomic nervous system (2, 11, 16, 18). Early in its discovery, 5-HT was demonstrated to cause an acute reduction in blood pressure when infused in dog and human (17). In support of these earlier findings, our laboratory has shown that chronic, low dose 5-HT (25 μg·kg−1·min−1) infused over 1 wk leads to a fall in mean arterial pressure (MAP) not only in normotensive rats, but also in mineralocorticoid-dependent hypertensive rats (>50 mmHg fall from baseline; 7) and in spontaneously hypertensive rats (fall in MAP ~30 mmHg; 27). This fall in MAP was dose dependent (23). Using a selective 5-HT7 receptor antagonist SB269970, we determined that the fall in MAP observed during the first 24 h of 5-HT infusion is dependent on 5-HT7 receptor activation (21). Our findings confirmed an earlier experiment where the acute (minutes) fall in MAP to low-dose 5-HT in rats was also mediated by 5-HT7 receptor (5, 8, 19, 24).
Moreover, we discovered a mechanism by which 5-HT may lower MAP but have only investigated this at a 24 h time point. Specifically, the fall in MAP to infused 5-HT was paralleled by a significant dilation of the portal vein (PV) and abdominal inferior vena cava (Ab IVC), but not the abdominal aorta (Ab A). Both 5-HT-stimulated venodilation and fall in MAP were blocked by the 5-HT7 selective receptor antagonist SB269970 (14, 21). This in vivo finding was consistent with in vitro findings published prior, namely that isolated rat splanchnic veins but not arteries relaxed to serotonergic agonists in a 5-HT7 receptor-dependent matter (4, 26). This was exemplified by the ability of the 5-HT1A/7 receptor agonist 5-carboxamidotryptamine (5-CT) to cause concentration-dependent venorelaxation that was antagonized by SB269970 (26).
A caveat, however, to this work is that the mechanisms of 5-HT-induced hypotension and venodilation were defined in only the relatively acute timeframe (minutes to 24 h). Linking the 5-HT7 receptor to long-term cardiovascular control and perhaps pathophysiological states (e.g., hypertension or heart failure) requires an understanding of the hormone’s more chronic effects. We do not know if and cannot assume that the mechanism(s) by which chronic (days to weeks) infusion of 5-HT causes a hypotension are the same as those responsible for acute responses. Acute hormone actions are likely to be modified under chronic conditions by well-known phenomena such as receptor downregulation, receptor desensitization, physiological compensation (e.g., baroreflex), and changes in serotonin plasma concentrations or tissue distribution. Chronic hormone actions can be challenging to explore using pharmacological tools because of concerns about the efficacy, receptor selectivity, duration of action, and off-target effects of pharmacological antagonists to the hormone. Therefore, we created a 5-HT7 receptor knockout (KO) rat to provide a different approach to understanding the acute and chronic cardiovascular actions of 5-HT mediated through the 5-HT7 receptor. Creation of this rat was reported in another paper (6). Validation that the 5-HT7 receptor was functionally ablated in the KO rat was observed by a total loss of 5-CT-induced venodilation and loss of [3H]SB269970 specific binding.
The newly created 5-HT7 KO rat allowed us to test the hypothesis, by comparison with WT littermates, that continuous activation of the 5-HT7 receptor is both necessary and sufficient for chronic 5-HT-induced depressor responses and splanchnic venodilation. We extend this hypothesis to test whether endogenous activation of the 5-HT7 receptor plays a role in basal cardiovascular regulation. Finally, we use vascular tissues from the KO rat to explore in vitro the role of the 5-HT7 receptor in control of splanchnic venous function.
METHODS
Animals
Michigan State University (MSU) Institutional Animal Care and Use Committee approved all protocols used in this study. MSU is an AAALAC-accredited institution (A3955-01). Rats were bred in house, or Sprague Dawley rats were purchased (200–350 g; Charles River Laboratories Portage, MI). All rats were housed in a temperature-controlled room (22°C) with 12 h light-dark cycles. Animals were given standard chow and distilled water ad libitum. The rats used were randomized to in vitro or in vivo studies. Each n value represents data that came from one animal.
Creation, validation, breeding, biological description, and genotyping of the 5-HT7 receptor KO rat are described in Demireva et al. (6). WT and KO littermates from several heterozygous breeding pairs are represented in this paper. Rats were randomized to the experiments performed.
In Vivo Protocols
Telemetry and pump implantation.
Under isoflurane anesthesia (2% in oxygen), radiotelemeter transmitters (HD-S10; Data Sciences International) were implanted subcutaneously through a 1–1.5 cm incision in the left inguinal area. The radiotelemeter catheters were introduced into the left femoral artery and advanced into the abdominal aorta below the renal artery. Animals received a dose of enrofloxacin (Baytril, 2.5 mg/kg im) and carprofen (Rimadyl, 5 mg/kg sc). After 5 days of postoperative recovery, baseline cardiovascular measurements were recorded for 5 days. MAP and heart rate (HR) were measured for 10 s every 10 min throughout the duration of the study. Under isoflurane anesthesia, osmotic pumps (model 2ML1, Alzet osmotic pumps) containing 5-HT (25 μg·kg−1·min−1 in 1% ascorbate in sterile saline, pH balanced to pH 6–7) were implanted subcutaneously between the scapulae. On the completion of the final day of infusion, pumps were removed from rats under isoflurane anesthesia and cardiovascular parameters were monitored for 6 additional days (termed recovery). The weight of the 5-HT minipumps was recorded before implantation and after removal to confirm drug delivery.
Ultrasound imaging of vessel diameter and echocardiograms.
This technique was originally reported in (20). Both WT and KO, male and female, rats (isoflurane-anesthetized, 1–2%) were positioned supine on a warmed platform (Vevo 2100 Imaging System; Visualsonics, Toronto, Canada). Ultrasound gel was applied to the prepared abdominal skin, just below the xiphoid process to couple the transducer (21 MHz probe; MS250) before imaging in B-mode. Real-time images were scanned at 25 frames per second. Images were taken at the location of the PV exiting the liver, which provides images of the Ab A, Ab IVC, and PV. In addition, an echocardiogram was performed to assess cardiac structure and function. A Doppler probe in the parasternal long axis view was used to obtain cardiovascular systemic measurements of stroke volume (SV), ejection fraction (EF), and cardiac output (CO). The Vevo 2100 imaging system software derived each of the above cardiovascular parameters. Each session took ~10 min per rat, minimizing the amount of time each animal was under anesthesia. Images for both vessel diameter and echocardiogram were taken at baseline for both sexes in KO and WT animals. Male and female rats showed similar 5-HT-induced depressor responses and analogous outcomes to 5-HT during in vitro contractility. Due to the similarities of 5-HT responses between male and females, the vessel diameter image measurements and echocardiogram response to 24 h of 5-HT infusion, day 4 of 5-HT infusion and recovery (pump removed) data were obtained in only female KO and female WT rats.
Acute 5-HT administration and blood pressure measurement.
Paired male WT and KO littermates were instrumented with radiotelemetry and intravenous femoral catheters for administration of 5-HT in the conscious state. Cumulative doses of 5-HT (12.5–100 μg/kg) were given with each dose administered over 20 min during which time MAP reached a stable plateau. After several days of washout, these same animals were given a constant infusion of 5-CT (1 μg·kg−1·min−1).
In Vitro Protocol
Isolated tissue bath measurement of isometric contraction.
Before terminal tissue removal, rats (both male and female) were given pentobarbital as a deep anesthetic (80 mg/kg ip). A bilateral pneumothorax was created before vessel dissection. The Ab IVC and paired Ab A were dissected from just above the iliac bifurcation up to the kidneys. Tissue dissection took place under a stereomicroscope and in a Silastic-coated dish filled with physiological salt solution (PSS) containing [mM: NaCl 130; KCl 4.7; KH2PO4 1.18; MgSO4·7H2O 1.17; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03, CaCl2 1.6 (pH 7.2)]. The Ab A and the Ab IVC were separated and guided individually onto stabilizing wire, cleaned of fat, and used in one of the protocols described below. The endothelium was left intact. Cleaned vessels were cut into rings (~3 mm wide) for measurement of isometric contractile force. Rings were mounted in warmed (37°C) and aerated (95% O2, 5% CO2) tissue baths (30 ml PSS) on Grass isometric transducers (FT03; Grass Instruments, Quincy, MA) connected to a four-channel PowerLab (ADInstruments, Colorado Springs, CO). All tissues were randomized in their placement in one of four tissue baths in each experiment (WT Ab IVC, KO Ab IVC, WT Ab A, and KO Ab A). Tissues were placed under optimal resting tension (Ab IVC = 1,000 mg; Ab A = 4,000 mg) and allowed to equilibrate for 1 h before an initial challenge with a maximal concentration of norepinephrine (NE; 10−5 M). The magnitude of contractions to NE are reported in the figure legends.
After this challenge, tissues were washed until tone returned to baseline. Cumulative concentration response curves were generated to NE and 5-HT (1×10−9 to 3×10−5 M) in all tissues. Tissues were washed out for at least 90 min between curves with buffer exchanges occurring every 5 min. In a separate set of experiments, Ab IVC from female KO rats were studied. We incubated tissues with either vehicle or the 5-HT2A/2C receptor antagonist ketanserin (100 nM) for 1 h before constructing a cumulative concentration response curve to 5-HT.
Materials
Acetylcholine chloride, forskolin, 5-HT creatinine sulfate, and norepinephrine hydrochloride were obtained from Sigma Chemical Company (St. Louis, MO). 5-CT maleate was purchased from Tocris (R & D, Minneapolis, MN) or Abcam (Cambridge, MA).
Data and Statistical Analyses
Quantitative data are reported as means ± SE for number of animals in parentheses. WT and KO were littermates. No outliers were removed from any of the data presented. For both ultrasound vessel diameter images and echocardiogram measurements, a trained user/reader of the Vevo Imaging system, blinded to the nature of the rat (KO or WT) or type of measure (baseline, 24 h, day 4, recovery), analyzed each vessel diameter and heart parameter. All vessel diameter measurements were controlled for respiration and cardiac cycles and are reported in millimeters (mm). The echocardiogram generated the following cardiovascular measurements: CO (ml/min), EF (%), and SV (μl). For isometric contractile measures, contraction is reported in milligrams or as a percentage initial NE-induced contraction. Magnitudes of contraction to these agonists for each experimental group are reported in the appropriate figure legend. Agonist potencies were calculated using a nonlinear regression (curve fit) within GraphPad Prism 7.0 (La Jolla, CA) and are reported as –log EC50 values [M]. Maximums are reported as the maximal effect achieved. For in vitro measures, t-tests to compare agonist-induced maximal responses agonist or potencies were used. For in vivo measures, we used a repeated-measures ANOVA when comparing values from baseline or within a group (GraphPad Prism 7) when the F value achieved statistical significance and there was no significant variance in homogeneity as tested by Bartlett’s. When comparing between groups, we used a t-test. In all cases, a P value of <0.05 was considered significant.
RESULTS
Lack of Functional 5-HT7 Receptor Does Not Modify Basal MAP
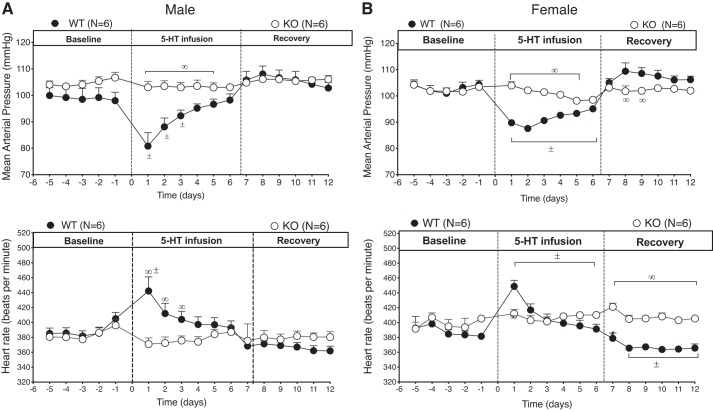
All baseline cardiovascular measurements are reported in Table 1. 5-HT7 receptor KO and WT rats were implanted with telemetry for measurement of cardiovascular parameters. Figure 1 shows 24 h average MAP and HR for the males (Fig. 1A) and the females (Fig. 1B). At baseline (before first dashed vertical line), MAP was not significantly different between KO and WT controls, nor was there a difference in HR. This was true in both sexes.
Table 1.
Baseline cardiovascular measurements for KO and WT rats, both male and female
| Female (n = 6) |
Male (n = 6) |
|||
|---|---|---|---|---|
| WT | KO 5-HT7 receptor | WT | KO 5-HT7 receptor | |
| Body weight, g | 328 ± 14 | 285 ± 14* | 480 ± 9.2 | 469 ± 9.2 |
| MAP, mmHg | 102.8 ± 1 | 103 ± 1 | 99.2 ± 3 | 104.2 ± 1 |
| HR, beats/min | 390.3 ± 6 | 400.6 ± 6 | 384.6 ± 7 | 381 ± 6 |
| DIA, mmHg | 86.3 ± 0.2 | 88.7 ± 1 | 83.4 ± 2 | 86.8 ± 1 |
| SYS, mmHg | 124.2 ± 0.3 | 129.9 ± 1 | 120.6 ± 5 | 127.8 ± 2 |
| AbIVC, mm | 2.96 ± 0.3 (0.009) | 2.92 ± 0.1 (0.01) | 6.3 ± 0.6 (0.013) | 6.5 ± 0.5 (0.013) |
| PV, mm | 1.7 ± 0.09 (0.005) | 1.8 ± 0.04 (0.006) | 2.9 ± 0.1 (0.006) | 2.5 ± 0.1 (0.005) |
| AbA, mm | 2.1 ± 0.04 (0.006) | 1.9 ± 0.07 (0.006) | 2.2 ± 0.05 (0.004) | 2.1 ± 0.03* (0.004) |
| SV, μl | 222 ± 7 (0.68) | 195 ± 15 (0.68) | 295 ± 12 (0.61) | 277 ± 9 (0.59) |
| CO, ml/min | 80 ± 4 (0.24) | 67 ± 6 (0.23) | 106 ± 7 (0.22) | 92 ± 2 (0.20) |
| EF, % | 64.3 ± 1 (0.20) | 63.6 ± 3 (0.22) | 67.4 ± 2 (0.14) | 65.8 ± 3 (0.14) |
Values represent means ± SE for 6 rats per group. Values in parentheses represent individual cardiovascular values indexed to body weight.
P < 0.05 between same sex vs. WT use an unpaired t-test. KO, knockout; WT, wild type; MAP, mean arterial pressure; HR, heart rate; DIA, diastolic pressure; SYS, systolic pressure; Ab IVC, abdominal inferior vena cava; PV, portal vein; Ab A, abdominal aorta; SV, stroke volume; CO, cardiac output; EF, ejection fraction.
Fig. 1.
Time course of 5-hydroxytryptamine (5-HT)-induced changes in mean arterial blood pressure (top) and heart rate (bottom) in male (A; n = 6) and female (B; n = 6) wild-type (WT) and knockout (KO) rats during baseline, 5-HT-infusion, and recovery postpump removal. Points represent means ± SE. ±, Significant differences in WT values compared with own baseline (repeated-measures ANOVA); ∞, significant differences between WT vs. KO rats (ANOVA). P < 0.05. First hatched vertical line = pump implantation, second line = pump removal (recovery).
One-week Infusion of 5-HT Did Not Reduce MAP in the Conscious 5-HT7 Receptor KO Rat
After 5 days of baseline cardiovascular data collection, a minipump containing 5-HT (25 μg kg·min−1) was implanted into all rats. In both WT males and females, infused 5-HT caused a fall in MAP that reached its nadir within the first day (male WT baseline 99.2 ± 3 mmHg vs with 5-HT 80.8 ± 5.1 mmHg; female WT baseline 102.8 ± 1 mmHg vs with 5-HT 89.7 ± 0.7 mmHg) (Fig. 1, top, A and B). The WT male rats maintained significantly reduced MAP during the first 3 days of 5-HT infusion compared with their baseline values. Female WT rats maintained significantly reduced MAP for the entire infusion period, then MAP returned to baseline value once the 5-HT pump was removed. By contrast, neither male nor female KO rats showed a change in MAP during the duration of 5-HT administration. This lack of MAP response to 5-HT infusion was paralleled by no change in HR (Fig. 1, bottom, A and B). The WT rats, both male and female, had an increase in HR concurrent with the corresponding fall in MAP, likely a baroreflex response. After removal of the 5-HT pumps, only the WT female rats had a significant overshoot of MAP, which corresponded with a reduction in HR. The weight of the 5-HT pumps upon removal were all similar among groups indicating comparable drug delivery in all groups.
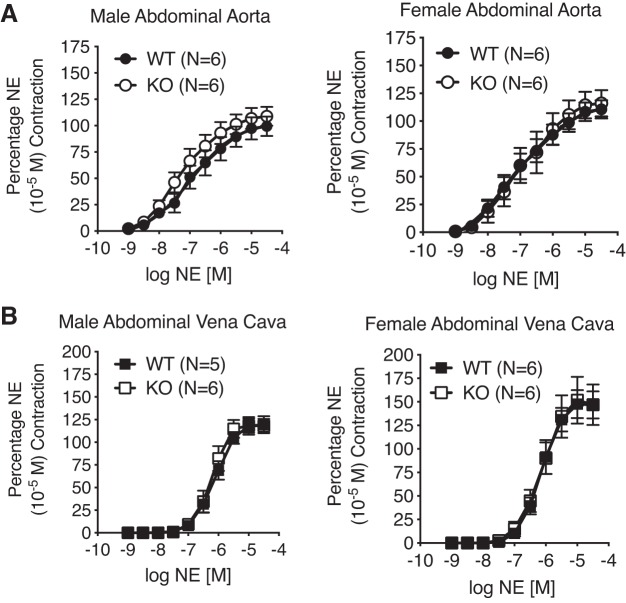
Vessel Contractility to NE Was Not Modified in the 5-HT7 Receptor KO Rat
Paired arteries and veins from WT and KO, male and female, rats were used to investigate contraction to both nonserotonergic and serotonergic stimuli. Figure 2 demonstrates that in both the isolated Ab A (Fig. 2A) and Ab IVC (Fig. 2B), contraction to the adrenergic agonist NE was not modified in potency or in efficacy (pharmacological parameters in Table 2). This validates that lack of a 5-HT7 receptor did not change arterial reactivity to NE.
Fig. 2.
Concentration-dependent contraction to norepinephrine (NE) of isolated abdominal aorta (A) and vena cava (B) from the male (left; n = 5–6) and female (right; n = 6) WT and KO rats. One male WT vena cava ring did not wake up to NE and was not included in analysis. Points represent means ± SE. NE maximum contraction (used for normalization): male abdominal aorta: WT = 1504 ± 193 mg, KO = 1648 ± 153 mg; female abdominal aorta: WT = 1936 ± 129 mg, KO = 1323 ± 100 mg; male abdominal vena cava: WT = 655 ± 189 mg, KO = 608 ± 81 mg; female abdominal vena cava: WT = 381 ± 105 mg, KO = 381 ± 105 mg.
Table 2.
Potency and efficacy of NE and 5-HT in abdominal aorta and vena cava male and female WT and KO rats
| NE | −Log EC50 [M] | Efficacy (% NE contraction) |
|---|---|---|
| Abdominal Aorta | ||
| Male WT | 6.95 ± 0.26 | 99.4 ± 9.2 |
| Male KO | 7.38 ± 0.28 | 108.9 ± 9.00 |
| Female WT | 7.20 ± 0.33 | 110.6 ± 8.3 |
| Female KO | 7.01 ± 0.35 | 116.1 ± 11.9 |
| Abdominal Vena Cava | ||
| Male WT | 6.11 ± 0.07 | 119.5 ± 9.4 |
| Male KO | 6.24 ± 0.07 | 118.1 ± 7.7 |
| Female WT | 6.16 ± 0.08 | 146.7 ± 14.3 |
| Female KO | 6.16 ± 0.13 | 146.9 ± 21.5 |
| 5-HT | −log EC50 [M] | Efficacy (% NE contraction) |
| Abdominal Aorta | ||
| Male WT | 5.71 ± 0.19 | 105.8 ± 11.6 |
| Male KO | 5.67 ± 0.12 | 116.3 ± 9.0 |
| Female WT | 5.95 ± 0.15 | 100.6 ± 13.2 |
| Female KO | 5.84 ± 0.09 | 117.4 ± 9.0 |
| Abdominal Vena Cava | ||
| Male WT | 6.01 ± 0.14 | 19.4 ± 5.4 |
| Male KO | 5.83 ± 0.14 | 80.0 ± 17.2* |
| Female WT | 6.25 ± 0.29 | 7.9 ± 3.7 |
| Female KO | 5.80 ± 0.26 | 68.6 ± 13.9* |
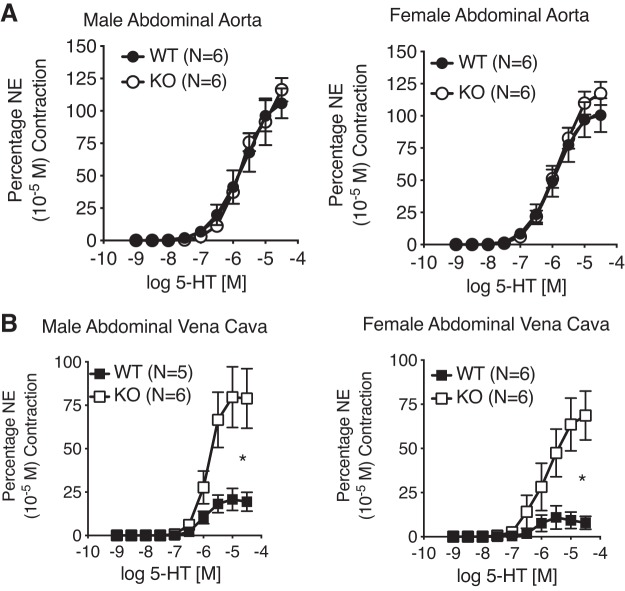
Venous but Not Arterial Contraction to 5-HT Was Upregulated in the 5-HT7 Receptor KO Rat
By contrast to the normal contraction to NE observed in all vessels, 5-HT-induced efficacy of contraction of the Ab IVC from the KO rat (male and female) was markedly increased vs. the WT male and female (Fig. 3B). This upregulated contraction was mediated by the 5-HT2A receptor given the ability of the 5-HT2A/2C receptor antagonist ketanserin to cause a rightward shift to the 5-HT-induced contraction in Ab IVC from KO rats (female; Fig. 4). Contraction to 5-HT was not modified in the Ab A of either the WT or KO, reaffirming the lack of a functional 5-HT7 receptor in this tissue (Fig. 3A).
Fig. 3.
Concentration-dependent contraction to 5-HT of isolated abdominal aorta (A) and vena cava (B) from the male (left; n = 5–6) and female (right; n = 6) WT and KO rats. One male WT vena cava ring did not wake up to NE and was not included in analysis. Points represent means ± SE *Significant differences (P < 0.05) in the maximums between WT and KO as determined through an unpaired t-test. Absolute magnitudes of NE maximums as reported in legend for Fig. 2.
Fig. 4.
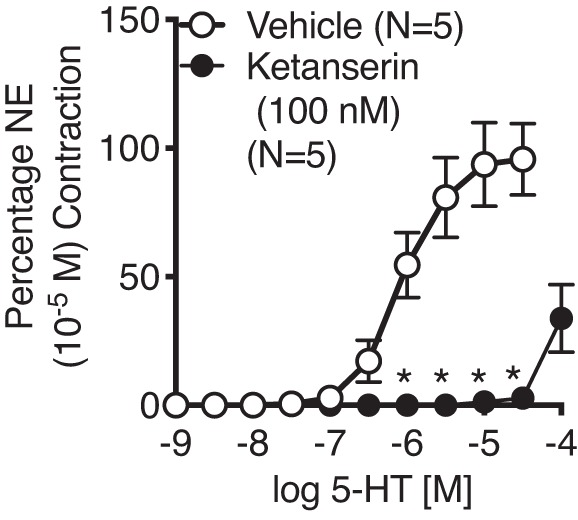
Ability of the 5-HT2A/2C receptor antagonist ketanserin (100 nM) to antagonize the 5-HT-induced contraction of the vena cava isolated from the KO female rat (n = 5). Points represent means ± SE for the number of animals indicated in parentheses. *Significant differences between vehicle- and ketanserin-incubated values as determined through unpaired t-tests with Bonferroni corrections. NE maximums: vehicle = 394 ± 79 mg; ketanserin = 508 ± 81 mg.
Lack of Splanchnic Venodilation or Changes in Cardiac Hemodynamics in Female 5-HT7 Receptor KO Rats after 1 wk Infusion of 5-HT
Thus far, data generated in vivo and in vitro have shown no difference in response to 5-HT between the male and female rats. As such, only female KO and WT rats were used during this next portion of the study. Baseline diameters of the observed vessels and cardiovascular parameters were similar between groups in the female rats as reported in Table 1. Splanchnic vessel diameters, as well as the cardiac parameters of SV, CO, and EF, were measured in WT and 5-HT7 receptor KO rats at baseline and then again, in the same animals, 24 h and 4 days after beginning 5-HT-infusion through minipump implantation. Recovery measures with pump removal were also made in the same rats.
In WT female rats, both the PV and Ab IVC but not the Ab A dilated in the presence of 5-HT, which was significant during first 24 h of 5-HT infusion (Fig. 5, A–C). Once the 5-HT stimulus was removed, diameters of the WT PV and Ab IVC were restored to near baseline levels. In contrast, the diameter of neither the veins (PV or Ab IVC) nor Ab A of the female KO rat was changed by 5-HT infusion. In this same set of rats, SV was elevated 24 h post-5-HT infusion in the WT rats only compared with its own baseline values and the value of 24 h post-5-HT KO rat (Fig. 6A). Surprisingly, CO remained increased in the WT rats during the duration of 5-HT infusion and after pump removal (Fig. 6B). EF was similar between KO and WT throughout the duration of the study (Fig. 6C). None of the cardiac measures changed in the KO rat when 5-HT was infused.
Fig. 5.
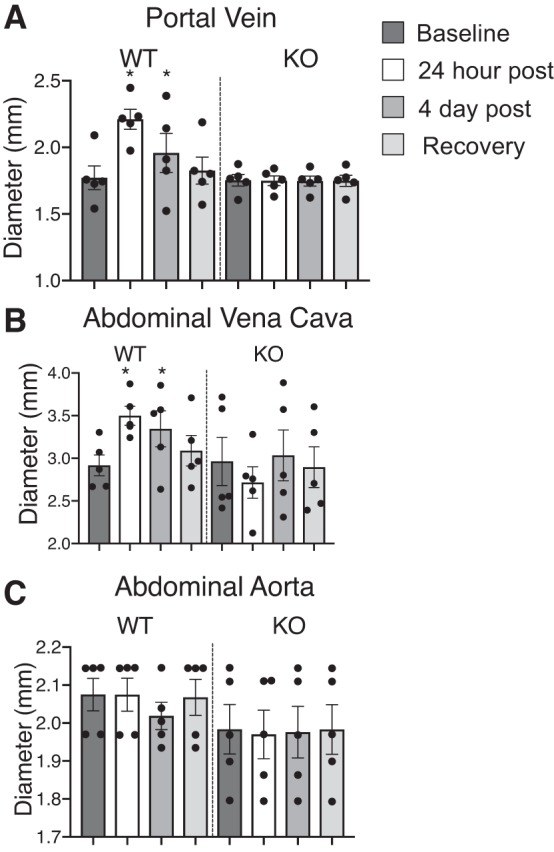
Dilation of portal vein (A) and abdominal vena cava (B) but not abdominal aorta (C) in the WT but not the KO female rats (n = 5) with 5-HT infusion over 1 wk; measures were made at baseline, 24 postinfusion, 4 days postinfusion. and 5 days after pump removal. Bars represent means ± SE around which points are scattered. *Significant differences (P < 0.05) in WT values compared with baseline using ANOVA.
Fig. 6.
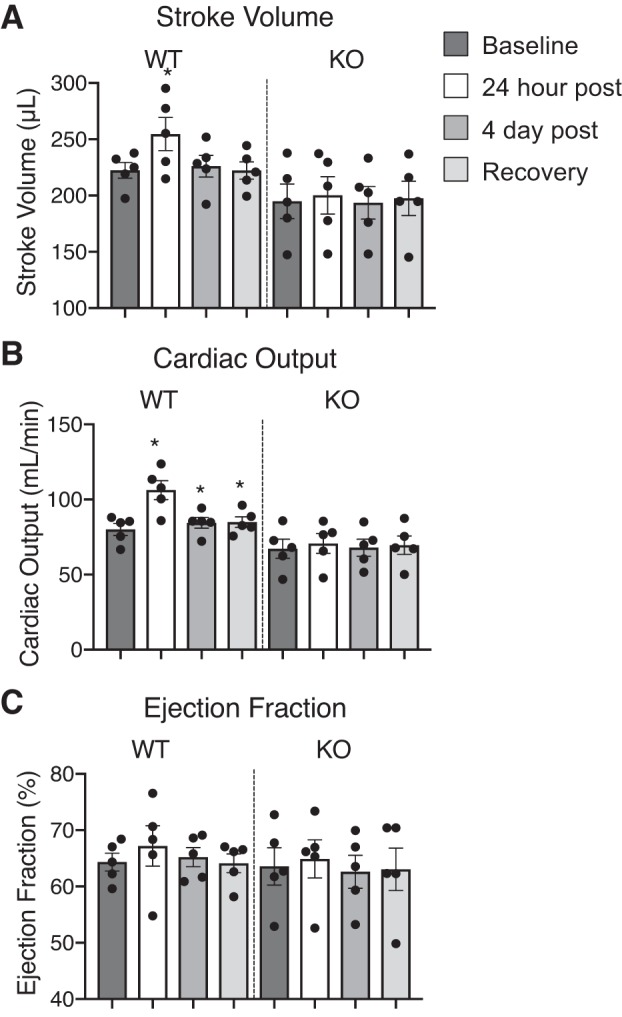
Echocardiogram measurements of stroke volume (A), cardiac output (B), and ejection fraction (C) in the KO and WT female rats (n = 5) with 1 wk infusion of 5-HT; measures were made at baseline, 24 postinfusion, 4 days postinfusion, and 5 days after pump removal. Bars represent means ± SE for number of animals in parentheses and each dot represents an individual rat. *Significant differences (P < 0.05) in WT values compared with own baseline using ANOVA.
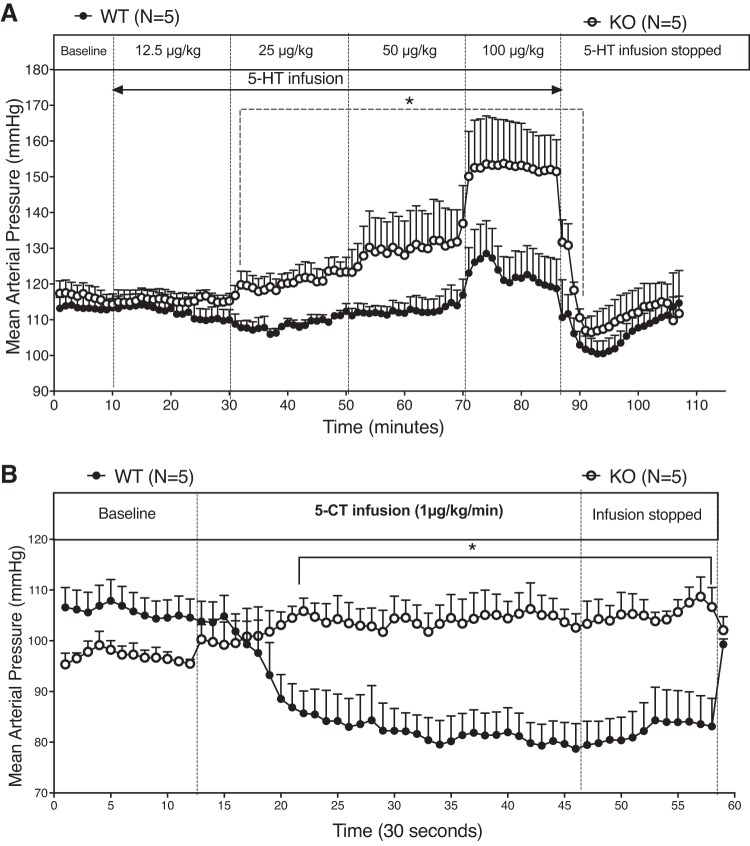
Potency and Efficacy of 5-HT as a Vasopressor Is Increased in the KO; KO Shows Complete Loss of Response to 5-CT In Vivo
We tested whether loss of a functional 5-HT7 receptor modified the acute pressor response to 5-HT by infusing 5-HT in a dose response manner (0–100 μg/kg) in conscious male rats. This is an experiment similar in intent to that of the isolated Ab IVC (Fig. 3) in which the efficacy of 5-HT was increased in the KO vs. the WT. Consistent with findings from this in vitro experiment, 5-HT was more efficacious in raising MAP in the KO vs. WT rat (Fig. 7A). Finally, the KO rat had no vasodepressor response to acute administration of 5-CT, while a reduction in blood pressure was observed in the WT rat (Fig. 7B).
Fig. 7.
Response of conscious male WT and KO rats (n = 5) to increasing doses of 5-HT (A) and individual dose of 5-CT (B). Points represent means ± SE for the number of animals indicated in parentheses. *Significant differences (P < 0.05) between WT and KO response using ANOVA.
DISCUSSION
The use of the newly created 5-HT7 receptor KO rat allowed us new insights into the contributions made by the 5-HT7 receptor in chronic regulation of blood pressure. Although our previous use of SB269970 provides one approach to demonstrating the importance of the 5-HT7 receptor in 5-HT-induced hypotension, it is challenging to use for long-term studies due to its short half-life and poor parenteral bioavailability. This led us to develop a 5-HT7 receptor KO rat model with CRISPR-Cas 9 technology (6).
Concentration/Dose Matters for Experimental Outcome
It is noteworthy that 5-HT can cause both vasoconstriction and vasodilation, as well as elevation or depression of blood pressure, depending on the concentration achieved in the circulating plasma, and the specific 5-HT receptor(s) activated. The infusion rate of 5-HT used in vivo (25 μg·kg−1·min−1) achieves an elevation of free circulating 5-HT to concentrations that are in the midnanomolar range (7). This concentration of free 5-HT in the plasma is insufficient to fully activate the vasoconstrictor 5-HT2A receptors, for which 5-HT possesses affinity (Ki) that is ~300–1,000 nM but is sufficient to nearly maximally activate 5-HT7 receptors (Ki ~1–10 nM) (https://pdsp.unc.edu/databases).
If the dose of 5-HT infused is elevated above 25 μg·kg−1·min−1, then a pressor response, likely mediated by 5-HT2A receptors, is stimulated; data in Fig. 7 of the present paper illustrate this point. Thus, the dose of 5-HT matters significantly when interpreting experimental outcomes. 5-HT7 receptor KO rats, both male and female, showed no hypotensive response to our standard rate of 5-HT (25 μg·kg−1·min−1) throughout a week-long infusion, whereas a typical hypotensive response was seen in WT rats. There was a small but insignificant fall in blood pressure of the female KO when given 5-HT. We must pay attention to this in future cohorts to determine whether there is a potential for differences in blood pressure response by the male and female. A pressor response was not observed in the KO rats, confirming that our typical infusion rate produces plasma concentrations of 5-HT that activate 5-HT7 but likely not other subtypes of 5-HT receptors. Another important conclusion is that 5-HT7 receptor activation up to a week can maintain a reduced blood pressure to some degree. The hypotension response wanes in magnitude, likely due to both pharmacological and physiological compensatory responses. This recovery of blood pressure is not because of degradation of 5-HT within the pump (7), and veins from animals infused with 5-HT continue to venodilate (not shown). Males appeared to recover more rapidly than females relative to their own baseline blood pressure; we have no good explanation for this difference. Similarly, the rebound in blood pressure in the females after 5-HT removal, which was accompanied by a sustained fall in HR, is an observation for which we have no mechanistic explanation. Altogether, the 5-HT7 receptor could function as a long-term regulator of cardiovascular function that could be taken advantage of therapeutically.
Basal Function of the 5-HT7 Receptor in Blood Pressure Regulation?
Another question we hoped to answer is whether the 5-HT7 receptor was important to basal blood pressure regulation. If the 5-HT7 receptor is activated constitutively by endogenous 5-HT, this would lower blood pressure. Thus, removal of the 5-HT7 receptor would likely be accompanied by an elevated blood pressure. We did not, however, observe an elevated MAP in the KO vs. WT rat in either males or females. Interestingly, CO and SV were globally lower in the KO rats in both sexes, but these measures were not significantly different from WT. Even in isolated blood vessels with functional 5-HT7 receptors (splanchnic veins), the loss of the 5-HT7 receptor did not affect the contractile response to NE. These collective data suggest that 1) it is unlikely endogenous 5-HT is high enough in normal physiology to activate a sufficient cadre of 5-HT7 receptors peripherally to cause a biological effect; and 2) the 5-HT7 receptor does not functionally interact with the α-adrenergic receptor. This is an important point to make as it divorces the hypotension caused by activation of the 5-HT7 receptor from the functional control of the α-adrenergic receptors, which are undeniably important for elevation of total peripheral resistance and blood pressure.
Impact of 5-HT7 Receptor on Venous Function
Infusion of 5-HT caused both the PV and Ab IVC to dilate in WT rats but not KO rats. There were no changes in the Ab A diameter during the infusion in either the WT or KO rats. These results confirm earlier pharmacological studies that activation of the 5-HT7 receptor is more important to venous vs. arterial function, at least in vessels from the splanchnic circulation. The veins within the splanchnic region have large capacitance capability. They store ~70% of the total blood volume. Constriction of small veins in the splanchnic region causes an increase in mean circulatory filling pressure (MCFP) moving unstressed volume into the circulation. Constriction of larger splanchnic veins cause an increase in the resistance to venous return (RVR). Both variables are critical regulators of CO (13). Therefore, changes in the diameter of these splanchnic veins are influential in blood pressure regulation mainly due to changes in SV and CO. In the present and in a previously published work (4), CO and SV were increased with a dilation of the Ab IVC during chronic 5-HT infusion. These results suggest that the main function of splanchnic venous 5-HT7 receptors is to control RVR rather than MCFP. The lack of venodilation and depressor response observed in the KO rats during 5-HT infusion suggests that venodilation contributes to the chronic depressor response to 5-HT. It is notable that the hypotension stimulated by 5-HT occurred in the absence of any receptor antagonism, and this fact contributes to the importance of the finding. Published studies have used receptor antagonists to uncover a vasodepressor response to 5-HT (24, 25), but this was not necessary with our intervention.
Interestingly, there was a markedly increased efficacy of 5-HT in the Ab IVC KO compared with WT. The arterial pair to this vessel, the Ab A, showed no change in 5-HT-induced contraction in KO vs WT. The enhanced 5-HT-induced contraction in the KO Ab IVC was likely mediated by the 5-HT2A receptor because the 5-HT2A receptor antagonist ketanserin antagonized this contraction. This finding suggests an antagonism, physiological or pharmacological, between the 5-HT2A (pressor) and 5-HT7 (depressor) receptor in the AB IVC. Loss of the 5-HT7 receptor allowed 5-HT to be a more efficacious venoconstrictor. It has not yet been possible to measure receptor number (e.g., does loss of 5-HT7 increase 5-HT2A?) because of poor antibody selectivity. By contrast, in the large artery, the 5-HT7 receptor is not normally functional in modifying smooth muscle tone such that the removal of the receptor does not affect normal contractility. Ultrasound imaging of these same vessels in vivo supports these in vitro findings.
Conclusion and Clinical Relevance
This study provides insight into the unique cardiovascular pharmacology of the 5-HT7 receptor, a member of the 5-HT receptor family whose cardiovascular actions have been little studied (12). Our work here confirms that the 5-HT7 receptor mediates the hypotension to a low dose of 5-HT infused over days. In addition, this work provides novel insight to the significance of this receptor to controlling the splanchnic veins. The relevance of this work is grounded in intriguing studies in which 5-HT or 5-hydroxytryptophan (precursor to 5-HT) infusion reversed and/or delayed development of multiple forms of hypertension in the experimental animal models (1, 3, 9, 10, 15). Collectively our findings suggest that the 5-HT7 receptor may be a novel and useful target for creating therapies to manage cardiovascular diseases (22).
GRANTS
Support from National Heart, Lung, and Blood Institute Grant HL-107495.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
B.M.S., E.Y.D., H.X., G.D.F., and S.W.W. conceived and designed research; B.M.S., T.K.-B., W.M.B., and S.W.W. performed experiments; B.M.S., T.K.-B., W.M.B., and S.W.W. analyzed data; B.M.S., G.D.F., T.K.-B., W.M.B., and S.W.W. interpreted results of experiments; B.M.S. and S.W.W. prepared figures; B.M.S. and S.W.W. drafted manuscript; B.M.S., E.Y.D., H.X., G.D.F., T.K.-B., W.M.B., and S.W.W. edited and revised manuscript; B.M.S., E.Y.D., H.X., G.D.F., T.K.-B., W.M.B., and S.W.W. approved final version of manuscript.
REFERENCES
- 1.Baron A, Riesselmann A, Fregly MJ. Reduction in the elevated blood pressure of Dahl salt-sensitive rats treated chronically with L-5-hydroxytryptophan. Pharmacology 42: 15–22, 1991. doi: 10.1159/000138763. [DOI] [PubMed] [Google Scholar]
- 2.Cade JR, Fregly MJ, Privette M. Effect of tryptophan and 5-hydroxytryptophan on the blood pressure of patients with mild to moderate hypertension. Amino Acids 2: 133–142, 1992. doi: 10.1007/BF00806084. [DOI] [PubMed] [Google Scholar]
- 3.Dalton DW, Feniuk W, Humphrey PP. An investigation into the mechanisms of the cardiovascular effects of 5-hydroxytryptamine in conscious normotensive and DOCA-salt hypertensive rats. J Auton Pharmacol 6: 219–228, 1986. doi: 10.1111/j.1474-8673.1986.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis RP, Pattison J, Thompson JM, Tiniakov R, Scrogin KE, Watts SW. 5-hydroxytryptamine (5-HT) reduces total peripheral resistance during chronic infusion: direct arterial mesenteric relaxation is not involved. BMC Pharmacol 12: 4, 2012. doi: 10.1186/1471-2210-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Vries P, De Visser PA, Heiligers JPC, Villalón CM, Saxena PR. Changes in systemic and regional haemodynamics during 5-HT7 receptor-mediated depressor responses in rats. Naunyn Schmiedebergs Arch Pharmacol 359: 331–338, 1999. doi: 10.1007/PL00005359. [DOI] [PubMed] [Google Scholar]
- 6.Demireva EY, Xie H, Flood ED, Thompson JM, Seitz BM, Watts SW. Creation of the 5-hydroxytryptamine receptor 7 knockout (5-HT7KO) Rat as a Tool for Cardiovascular Research. Physiol Genomics physiolgenomics.00030.2019, 2019. doi: 10.1152/physiolgenomics.00030.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz J, Ni W, Thompson J, King A, Fink GD, Watts SW. 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J Pharmacol Exp Ther 325: 1031–1038, 2008. doi: 10.1124/jpet.108.136226. [DOI] [PubMed] [Google Scholar]
- 8.Ding XR, Stier CT Jr, Itskovitz HD. Serotonin and 5-hydroxytryptophan on blood pressure and renal blood flow in anesthetized rats. Am J Med Sci 297: 290–293, 1989. doi: 10.1097/00000441-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Echizen H, Freed CR. Long-term infusion of L-5-hydroxytryptophan increases brain serotonin turnover and decreases blood pressure in normotensive rats. J Pharmacol Exp Ther 220: 579–584, 1982. [PubMed] [Google Scholar]
- 10.Fregly MJ, Lockley OE, Sumners C. Chronic treatment with L-5-hydroxytryptophan prevents the development of DOCA-salt-induced hypertension in rats. J Hypertens 5: 621–628, 1987. doi: 10.1097/00004872-198710000-00018. [DOI] [PubMed] [Google Scholar]
- 11.Gamoh S, Hisa H, Yamamoto R. 5-hydroxytryptamine receptors as targets for drug therapies of vascular-related diseases. Biol Pharm Bull 36: 1410–1415, 2013. doi: 10.1248/bpb.b13-00317. [DOI] [PubMed] [Google Scholar]
- 12.Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, Van Craenenbroeck K. The serotonin 5-HT7 receptors: two decades of research. Exp Brain Res 230: 555–568, 2013. doi: 10.1007/s00221-013-3694-y. [DOI] [PubMed] [Google Scholar]
- 13.Green JF. Circulatory mechanics. In: Fundamental Cardiovascular and Pulmonary Physiology. Philadelphia, PA: Lea & Febiger, pp. 1–110, 1982. [Google Scholar]
- 14.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 130: 539–548, 2000. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Itskovitz HD, Werber JL, Sheridan AM, Brewer TF, Stier CT Jr. 5-Hydroxytryptophan and carbidopa in spontaneously hypertensive rats. J Hypertens 7: 311–315, 1989. doi: 10.1097/00004872-198904000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Kaumann AJ, Levy FO. 5-hydroxytryptamine receptors in the human cardiovascular system. Pharmacol Ther 111: 674–706, 2006. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Page IH, McCubbin JW. The variable arterial pressure response to serotonin in laboratory animals and man. Circ Res 1: 354–362, 1953. doi: 10.1161/01.RES.1.4.354. [DOI] [PubMed] [Google Scholar]
- 18.Ramage AG, Villalon CM. 5-Hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci 29: 472–481, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Saxena PR, Lawang A. A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors. Arch Int Pharmacodyn Ther 277: 235–252, 1985. [PubMed] [Google Scholar]
- 20.Seitz BM, Krieger-Burke T, Fink GD, Watts S. Serial measurements of splanchnic vein diameters in rats using high-frequency ultrasound. Front Pharmacol 7:116, 2016. doi: 10.3389/fphar.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seitz BM, Orer HS, Krieger-Burke T, Darios ES, Thompson JM, Fink GD, Watts SW. 5-HT causes splanchnic venodilation. Am J Physiol Heart Circ Physiol 313: H676–H686, 2017. doi: 10.1152/ajpheart.00165.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selim AM, Sarswat N, Kelesidis I, Iqbal M, Chandra R, Zolty R. Plasma serotonin in heart failure: possible marker and potential treatment target. Heart Lung Circ, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Tan T, Watts SW, Davis RP. Drug delivery: enabling technology for drug discovery and development. iPRECIO micro infusion pump: programmable, refillable, and implantable. Front Pharmacol 2: 44, 2011. doi: 10.3389/fphar.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Terrón JA. Role of 5-ht7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br J Pharmacol 121: 563–571, 1997. doi: 10.1038/sj.bjp.0701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Villalón CM, Centurión D, Bravo G, De Vries P, Saxena PR, Ortiz MI. Further pharmacological analysis of the orphan 5-HT receptors mediating feline vasodepressor responses: close resemblance to the 5-HT7, receptor. Naunyn Schmiedebergs Arch Pharmacol 361: 665–671, 2000. doi: 10.1007/s002100000256. [DOI] [PubMed] [Google Scholar]
- 26.Watts SW, Darios ES, Seitz BM, Thompson JM. 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect 3: e00103, 2015. doi: 10.1002/prp2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388, 2012. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]