Abstract
Using CRISPR-Cas9 technology, we created a 5-HT7 receptor global knockout (KO) rat, on a Sprague-Dawley background, for use in cardiovascular physiology studies focused on blood pressure regulation. A stable line carrying indels in exons 1 and 2 of the rat Htr7 locus was established and validated. Surprisingly, 5-HT7 receptor mRNA was still present in the KO rat. However, extensive cDNA and genomic sequencing of KO tissues confirmed an 11 bp deletion in exon 1 and 4 bp deletion in exon 2. The exon 1 deletion resulted in a frameshifted mRNA sequence coding for a nonfunctional protein. While the Htr1B locus was a potential off-target for the guide RNAs designed for exon 2 of Htr7, there were no off-target sequence changes at this locus in the originating founder. When the F2 generation of KO was compared with wild-type (WT) counterparts, neither the male nor female KO rats were different in body size, fat weights, or mass of organs (kidney, heart, and brain) important to blood pressure. Females were smaller in mass than their counterpart males. Clinical measures of plasma from nonfasted rats revealed largely similar values, comparing WT and KO, of glucose, blood urea nitrogen, creatinine, phosphate, calcium, and albumin to name a few. Loss of a functional 5-HT7 receptor was validated by the complete loss of relaxation to the 5-HT1/7 receptor agonist 5-carboxamidotryptamine in the isolated abdominal vena cava. This newly created 5-HT7 receptor KO rat will be of use to investigate the importance of the 5-HT7 receptor in blood pressure regulation.
Keywords: cardiovascular, 5-HT7 receptor, rat knockout
INTRODUCTION
One of the earliest observations made after identification of 5-hydroxytryptamine (5-HT) as serotonin was an acute reduction in blood pressure when 5-HT was infused in dog and human (14). Investigators later described a long-lasting hypotension upon acute (minutes) 5-HT infusion in the anesthetized cat (28), pig (22), and rat (1, 4, 21). In 1997, Terron (27) suggested that the 5-HT7 receptor mediates this acute hypotension in anesthetized rodents.
The 5-HT7 receptor is the most recently identified 5-HT receptor, cloned in 1993 (12, 18, 19). This heptahelical Gs-coupled receptor has splice variants in human, rat, and mouse. Receptors translated from all these variants are well established to activate adenylate cyclase, a stimulus resulting in smooth muscle relaxation. 5-HT7 receptors are best known for their role in central regulation of memory, sleep, and wakefulness, as well as in the gastrointestinal system (5, 6, 9, 12, 24). It was just in the last 20 yr that this receptor was discovered in the peripheral circulation. SB269970 is an established 5-HT7 receptor antagonist with a sub-nM affinity for the receptor and 1,000× greater affinity than for any other 5-HT receptor (8); SB269970 is brain penetrant. The agonists 5-carboxamidotryptamine (5-CT) and 5-HT have nM affinity for the 5-HT7 receptor (http://www.guidetopharmacology.org), with 5-CT having a 10× higher affinity for the rat 5-HT7 receptor vs. 5-HT.
Our interest in the 5-HT7 receptor lies in the role it plays in long-term blood pressure regulation. In previous studies, we extended the acute 5-HT infusion, as reported above, to a minipump-based infusion that lasted over 7 days. 5-HT, given in this manner, caused a time-dependent fall in blood pressure in conscious rats (2). The 5-HT7 receptor mediated at least the initial fall (<24 h) to 5-HT-infusion because the 5-HT7 receptor antagonist SB269970 reduced the 5-HT-induced reduction in blood pressure (23). However, we do not know the dependence of the later days (>24 h) of 5-HT infusion on 5-HT7 receptor activation. Though markedly useful, SB269970 has a short (30–45 min) half-life, making long-term administration of the drug challenging. We also desired a confirmation of the importance of this receptor in blood pressure regulation through means of a different approach. This necessitated the creation of a new tool that we report here.
We set out to create a constitutive functional knockout (KO) model of the 5-HT7 receptor. In addition to being substantially useful in testing whether the chronic drop in blood pressure during 5-HT infusion was solely dependent on 5-HT7 receptors, such a model will complement pharmacological experiments, unmask any redundancies or developmental compensation provided by other members of the 5-HT receptor family in the absence of 5-HT7 receptor, and allow for better chronic physiological and behavioral experiments. Lastly, a genetic model of 5-HT7 ablation can be a useful tool for the biodiscovery of new therapeutic compounds acting to regulate blood pressure.
The rat was our model of choice given its substantial use in the field of hypertension, other cardiovascular diseases, and our published work. The 5-HT7 receptor KO mouse has been created (9), but it is not amenable to many of the measures we ultimately hope to make, such as measuring splanchnic venous diameter in response to 5-HT (23, 29). We report here the successful creation of a 5-HT7 receptor KO rat in which the 5-HT7 receptor is nonfunctional. We follow this article with a companion paper (22a) that uses this newly created tool to test the role of the 5-HT7 receptor in long-term 5-HT-induced hypotension and normal blood pressure regulation.
METHODS
Animals.
The Michigan State University (MSU) Institutional Animal Care and Use Committee approved all protocols used in this study. MSU is an AAALAC-accredited institution (A3955-01). ARRIVE guidelines were also followed. Rats were bred in house or Sprague Dawley rats were purchased (200–350 g; Charles River Laboratories, Portage, MI). All rats were housed in a temperature-controlled room (22°C) with 12 h light-dark cycles. Animals were given standard chow and distilled water ad libitum and housed in standard cages with stainless steel wire lids and containing environmental enrichment (e.g., Nylabones, nesting materials when breeding). Each n value represents data that came from one animal. Group sizes were based on power analyses that accounted for the variability in each type of assay performed.
Creation of 5-HT7 receptor KO rat.
Htr7 KO rats were generated by CRISPR-Cas9 genome editing, as described previously (3). Briefly, ribonucleoprotein (RNP) complexes were assembled in vitro with wild-type S.p. Cas9 Nuclease 3NLS protein, synthetic tracrRNA, and crRNA (Integrated DNA Technologies, Coralville, IA). RNPs targeting both exon 1 and exon 2 of Htr7 (ENSRNOG00000018827) were electroporated into Sprague Dawley zygotes. Protospacer and PAM sequences corresponding to crRNAs were 5′ GATCGCAGGCAACTGCCTGG - TGG 3′ for exon 1, and 5′ CTACACGATCTACTCCACTG - CGG 3′ for exon 2. Fourteen founder rats were born from implanted zygotes; none died. Tail snips of founder offspring were lysed for PCR analysis with the following primers: exon 1 forward (O271) - 5′ CTG AGT GGC TTC CTA GAG GTG A 3′ and reverse (O272) - 5′ TTC CTT ACC TGT CGA TGC TGA T 3′; and exon 2 forward (O273) - 5′ TCT GCT AGG TAC CTT GGG ATC A 3′; and reverse (O274) - 5′ CAA GGT AGT GGC TGC TTT CTG T 3′. T7 Endonuclease I assay (New England BioLabs, Ipswich, MA) was used to detect indels, and then Sanger sequencing was employed to resolve exact sequence changes in exon 1 and exon 2. CRISPR guide (g)RNA selection and locus analysis were performed with the Benchling platform (Benchling, San Francisco, CA), and sequence alignments were performed with MacVector software (MacVector, Cary, NC). Off-target analysis was performed for a two-mismatch hit for the exon 2 crRNA (5′ CTACACGGTCTACTCCACGG - TGG 3′) within the Htr1b (ENSRNOG00000013042) coding region for the 5-HT1B receptor locus.
Breeding of 5-HT7 receptor KO and WT rats.
A male founder (229) chimeric for frameshift indels in both exon 1 and exon 2 on the same allele was used to establish a stable Htr7 KO (hereon termed 5-HT7 KO) line by breeding with purchased WT female rats (Sprague-Dawley, Charles River). Rats heterozygous for 5-HT7 KO were born, and male and female heterozygous rats mated to achieve the first F2 generation of wild-type (WT), Het, and KO male and female rats. Offspring were genotyped for the presence of indels at the site of double-strand DNA breaks (DSBs) in exon 1 and exon 2 and for large deletions between the two gRNA cut sites. PCR products flanking the DSB were tested for presence of indels by T7Endonuclease I and validated by Sanger sequencing. A comprehensive listing of primers is in Table 1.
Table 1.
Primers used for genotyping, mutation validation, sequencing, and off-target analyses
| Primer Name | Primer Sequence |
|---|---|
| O271_rHtr7_ex1_geno_F | CTGAGTGGCTTCCTAGAGGTGA |
| O272_rHtr7_ex1_geno_R | TTCCTTACCTGTCGATGCTGAT |
| O273_rHtr7_ex2_geno_F | TCTGCTAGGTACCTTGGGATCA |
| O274_rHtr7_ex2_geno_R | CAAGGTAGTGGCTGCTTTCTGT |
| O407_r5HT7_ex1WT_R | TCACCACCAGGCAGTTGCC |
| O428_r5HT7_ex1indel_R | TCACCACCTGCGATCGT |
| O409_rHtr1B_offtarget_F | ATGCGGTGGACTATTCTGCT |
| O410_rHtr1B_offtarget_R | AGAGCGGGCTTCCACATAGAT |
| O552_r5HT7-ex1_seq_F2 | GAGCGGGCAAGGTGAATCCA |
| O553_r5HT7-ex1_seq_R2 | GCTTGGTTGGTGTAGGGGTG |
| O554_r5HT7-ex2_seq_F1 | CTGGCTGGTAGCACTCCGT |
| O555_r5HT7-ex2_seq_R1 | TCTAGCCACTGATTCACGGGG |
| O566_r5HT7_cds-seq_F1 | AGCGGGCAAGGTGAATCC |
| O567_r5HT7_cds-seq_R1 | CCAGGGTTCCACTCTGGATCAT |
| O598_5-HT7_cDNA 3′ out seq F | CTGAGGACCACCTATCGTAGC |
Referred to by name (e.g., O409) in the text and figure legends.
Standard genotyping.
At weaning, ear and tail tips were taken for independent genotyping by two different laboratories at MSU. All genotyping was done in a blinded fashion and using a WT and KO parallel control in all tests (established through the original CRISPR/Cas animals). Standard PCR conditions were used: 95°C 2 min (1 cycle); 95°C 15 s, 61°C 15 s, 72°C 30 s; repeated for 35 cycles; 72°C 4 min (1 cycle); 4°C hold. Primers were used in two separate PCR experiments to investigate 1) WT gene; and 2) KO gene. The following were used in these two separate PCR reactions with different allele-specific reverse primers: 5-HT7 Exon 1 forward (O271): 5′ CTG AGT GGC TTC CTA GAG GTG A 3′, 5-HT7 Exon 1 WT reverse (O407): 5′ TCA CCA CCA GGC AGT TGC C 3′; 5-HT7 Exon 1 KO Rev (O428): 5′ TCA CCA CCT GCG ATC GT 3′. All products were run on a 1.5% agarose gel, and WT and KO runs were compared on the same gel, in parallel. The expected product was observed in the WT gel and not KO gel only if the sample was from a WT rat, in both gels if the animal was a Het, and only in the KO gel if the sample was from a KO rat. The two independent laboratories, blinded to sample genotype, that conducted initial genotyping agreed 100% on outcomes for over 20 sampled rats.
Quantitative PCR for 5-HT7 receptor mRNA.
Cerebellar samples from anesthetized (Fatal Plus, 60–80 mg/kg of pentobarbital ip) 5-HT7 WT and KO rats were isolated and homogenized with the Omni Bead Ruptor (Omni, Kennesaw, GA) in 2.0 ml tubes (Omni) with 1.4 mm ceramic bead media (Cat. #19-645, Omni). RNA was isolated using the Zymo Quick-RNA Mini Prep Kit (Cat. #R1054, Zymo Research, Irvine, CA) and quantified on a NanoDrop 2000c (Thermo Scientific). mRNA was reverse-transcribed into cDNA using the High Capacity cDNA kit (Cat. #4368814, Thermo Fisher Scientific). SYBR Green RT-PCR was performed on an ABI 7500 FAST Real Time PCR system (Life Technologies, Carlsbad, CA) using Fast SYBR Green Master Mix (Cat. #4385612, Thermo Fisher Scientific) with the following parameters: 95°C for 10 min; 95°C for 15 s, and 60°C for 1 min for 40 cycles. 5-HT7 receptor primers used were from SABiosciences [600–800 region (RT2 qPCR primer assay Cat. #PPR06826A)] and RealTimePrimers.com [1,200–1,400 region (Cat. VRPS-2846, set #1, Elkins Park, PA; forward primer = 5′-TGGGCTATGCAAACTCTCTC-3′, reverse primer = 5′-CAGCACAAACTCGGATCTCT-3′)]. Values were normalized to beta-2-microglobulin (RT2 qPCR primer assay Cat. #PPR42607A, SABiosciences). TaqMan RT-PCR was performed on a QuantStudio 7 Flex Real-Time PCR system using PerfeCTa FastMix II ROX (Cat. #95119; Quanta Bio, Beverly, MA) with the following parameters: 95°C for 20 s; 95°C for 1 s, and 60°C for 20 s for 40 cycles. Thermo Fisher Scientific 5HT7 primers [exons 1–2, assay location 655 (Cat. #4331182, assay ID #Rn00576048_m1)] were used and normalized with beta actin (Cat. #4331182, assay ID #Rn00667869_m1). A melt curve for SYBR Green-based experiments was performed following each RT-PCR to determine the presence of a single PCR product. Data were analyzed with the 2-ΔCT method in which CT is the threshold cycle.
Histochemistry.
Tissues were removed from anesthetized animals. Tissues were formaldehyde-fixed. Paraffin-embedded sections (8 micron) were cut, dewaxed, and taken through a standard protocol using the appropriate species-based Vector kit (Vector Laboratories, Burlingame, CA). Vessel sections were incubated 24 h with 5-HT7 receptor antibody as designated in Table 2 or no primary antibody. Sections were developed according to manufacturer’s instructions using a DAB (3, 3-diaminobenzidine) developing solution (Vector Laboratories). Binding was observed as a dark brown/black precipitate and specific binding of the primary antibody determined as that which was absent in parallel sections incubated without primary antibody. All slides were counterstained with Vector hematoxylin (30–45 s), with nuclei stained blue. Sections were dried, coverslipped, and photographed on a Nikon TE2000 inverted microscope using MMI Cellcut software.
Table 2.
Listing of commercially available antibodies used in either Western or immunohistochemical analysis on samples (specified in column) from WT and KO 5-HT7 receptor rats
| Antibody, RRID Code; Epitope (AA) of 5-HT7 protein | Application | WT, KO Samples Tested |
|---|---|---|
| Abcam Cat. #ab128892, RRID:AB_11139629; AA 1–30, monoclonal | Western | multiple vessels |
| Thermo Fisher Scientific Cat. #PA1-41122, RRID:AB_2122687; AA 13–28, polyclonal | Western | brain |
| ImmunoStar Cat. #24430, RRID:AB_572214; AA 8–23, polyclonal | IHC | brain, multiple vessels |
| LifeSpan Cat. #LS-A7991-50, RRID:AB_902115; AA 301–350, polyclonal | Western | multiple vessels |
| LifeSpan Cat. #LS-A7991-50, RRID:AB_902115; AA 301–350 polyclonal | IHC | multiple vessels, liver |
| Novus Cat. #NBP1-46598, RRID:AB_10009834; AA 300–350, polyclonal | Western | multiple vessels |
| Novus Cat. #NBP1-46598, RRID:AB_10009834; AA 300–350, polyclonal | IHC | multiple vessels, liver |
| Novus Cat. #WB, RRID:AB_2122703; COOH terminus, polyclonal | Western | multiple vessels |
| Abcam Cat. #ab129559, RRID:AB_11154850; AA 404–433, polyclonal | Western | brain |
| Acris Antibodies Cat. #AP07036PU-N, RRID:AB_1611252 COOH terminus, polyclonal | IHC | liver, femoral vessels, portal vein |
WT, wild type; KO, knockout; AA, amino acids; IHC, immunohistochemistry.
Western blot analysis.
Isolated vessels were cleaned, frozen, and ground into a powder with a mortar and a pestle. Homogenization buffer [125 mM Tris (pH 6.8), 4% SDS, 20% glycerol, 0.5 mM phenylmethylsulfonyl fluoride, 1 mM orthovanadate, 10 μg/ml aprotinin, 10 μg/ml leupeptin] was added, and the homogenates were vortexed and sonicated for 5 cycles and centrifuged. Supernatants were collected and protein concentration was determined with the BCA protein kit (catalog #BCA1; Sigma Chemical, St. Louis, MO). SDS-PAGE separation of proteins in tissue homogenates (50 μg) was performed, and proteins transferred to PVDF. Controls [rat brain for 5-HT7 receptor (50 μg)] were run in parallel lanes along with molecular weight markers. Blots were stained with Total Protein Stain (#926-11021; Li-Cor, Lincoln, NE) and imaged on the 700 channel; stain was then removed with REVERT (#926-11013, Li-Cor). Blots were blocked for 3 h with Li-Cor Blocker (#927-50100) before being incubated overnight at 4°C with 5-HT7 primary antibody (as listed in Table 2) after which they were washed three times with Tris-buffered solution (TBS) and incubated with the appropriate 800 CW secondary antibody (goat anti-mouse #926-32210, goat anti-rabbit #926-32211; Li-Cor) for 1 h. Secondary antibody was then washed off with three 10 min TBS washes, and blots were imaged with Li-Cor Odyssey CLx Infrared Imaging and quantified with Image Studio (5.2.5, Li-Cor).
Autoradiography.
Eight brains (4 WT and 4 KO, both male and female) were shipped frozen to Gifford Bioscience (Birmingham, UK) for measurement of 5-HT7 receptor binding using [3H]SB269970. Experiments were done blinded as to the animal genotype, and key-broken after final autoradiograms were produced. Brains were stored at −80°C until sectioning. Brain tissue was trimmed, orientated, and mounted on a cryostat chuck in a cryostat (Bright OTF 5000). Tissue sections were cut at a thickness of 20 µm in a coronal orientation. Sections were then thaw-mounted onto Superfrost slides. Three sections were placed on each slide, with a total of two slides from each brain (16 slides total) and three slides for defining nonspecific binding. Following sectioning, slides were stored at 4°C together with silica desiccant.
The slide box was removed from the refrigerator and allowed to warm fully to room temperature before being opened to avoid formation of condensation. Slides were incubated in wash buffer [50 mM Tris (pH 7.4), 4 mM CaCl2, and 1 mM ascorbic acid] for 15 min. Slides were then placed horizontally in a humidified box, and 0.7 ml of [3H]SB269970 (5 nM; PerkinElmer NET1198U250UC SBF3) in incubation assay buffer [50 mM Tris (pH 7.4), 4 mM CaCl2, and 1 mM ascorbic acid] was layered over each slide. Slides for nonspecific binding additionally contained unlabeled SB269970 (10 µM) in the incubation buffer. Sections were incubated in the radioligand solution for 90 min at room temperature. The radioligand solution was then rapidly aspirated off, and the slides briefly placed in ice-cold wash buffer, followed by three washes (10 min each) in ice-cold wash buffer. After the final wash, the slides were dipped briefly in distilled water and dried under a stream of air. After drying, sections were placed over a tritium-sensitive phosphor screen (GE Healthcare) together with autoradiographic standards. The screen was exposed for 7 days and then scanned on a PerkinElmer Cyclone scanner.
Clinical measures and weights of WT and 5-HT7 KO rats.
At 14–16 wk of age, one pair of 5-HT7 WT and KO male or WT and KO female rats was anesthetized with isoflurane (4% in oxygen) and euthanized by a bilateral pneumothorax. Animals were always paired in this way, and one pair was experimented on each day. The final weights of rats were taken. Weights are reported in absolute magnitude and normalized to body weight given that males and females differed substantially in their body weight. Blood was collected from the aorta above the kidneys into a lithium heparin blood collection tube and centrifuged at 4°C for 20 min at 2,000 g, and plasma was collected for measurement on an IDEXX clinical analyzer (IDEXX Catalyst One, Westbrook, ME) using Chem 15 Clip plus Lyte 4 Clip. Clip cassettes were used to measure, at one time, the following different parameters in every animal: glucose, blood urea nitrogen, creatinine, phosphate, calcium, albumin, globulin, alanine aminotransferase, alkaline phosphatase, gamma glutamyltransferase, total bilirubin, cholesterol, triglycerides, sodium, potassium, chloride, and osmolality. Tissues (retroperitoneal fat, perigonadal fat, kidney, liver, spleen, heart, and brain) were also dissected and weighed with weights reported as absolute magnitude or a percentage of body weight.
Isometric contractility for validation of loss of functional 5-HT7 receptor.
From the same animals described above, the abdominal vena cava and paired abdominal aorta were dissected from just above the iliac bifurcation up to the kidneys. Tissue dissection took place under a stereomicroscope and in a silastic-coated dish filled with physiological salt solution (PSS) containing (in mM): NaCl 130; KCl 4.7; KH2PO4 1.18; MgSO4 · 7H2O 1.17; NaHCO3 14.8; dextrose 5.5; CaNa2EDTA 0.03, CaCl2 1.6 (pH 7.2). The abdominal aorta was stabilized on a guiding wire in a silastic-impregnated dish, and the abdominal inferior vena cava (Ab IVC) was dissected away from the abdominal aorta. Once isolated, the Ab IVC was guided individually onto the stabilizing wire and cleaned of fat. Cleaned veins were cut into rings (~3 mm wide) for measurement of isometric contractile force. Rings were mounted in warmed (37°C) and aerated (95% O2, 5% CO2) tissue baths (30 ml PSS) on Grass isometric transducers (FT03; Grass instruments, Quincy, MA) connected to a four-channel PowerLab (ADInstruments, Colorado Springs, CO). All tissues were randomized in their placement in one of four tissue baths in each experiment (WT vena cava, KO vena cava). Tissues were placed under optimal resting tension (1,000 mg) and allowed to equilibrate for 1 h before an initial challenge with a maximal concentration of norepinephrine (NE; 10−5 M).
All tissues underwent the following challenge. A submaximal concentration of endothelin-1 (ET-1; 10−9) was used to contract tissues. The magnitudes of contraction to ET-1 are reported in the figure legends. Once ET-1-induced contraction was stable (~12–15 min), the serotonergic agonist 5-CT (10−6 M) was added to test agonist-induced relaxation. When a maximum response (contraction, relaxation) was achieved, the 5-HT7 receptor antagonist SB269970 (1 μM) was added. When this response stabilized, tissues were incubated with the adenylate cyclase stimulator forskolin (10−6 M).
Materials (general).
Acetylcholine chloride, forskolin, 5-HT creatinine sulfate, and NE hydrochloride were obtained from Sigma Chemical Company (St. Louis, MO). SB269970 and 5-CT maleate were purchased from Tocris (R & D, Minneapolis, MN) or Abcam (Cambridge, MA). ET-1 (1–21) was purchased from Bachem (Torrance, CA).
Data and statistical analyses.
Quantitative data are reported as means ± SE for number of animals in parentheses. WT and KO were littermates. No outliers were removed from any of the data presented. The whole gels from experiments are shown; no manipulation to the images were made. Weights (whole body, tissue) were taken on a standard 4 decimal top loader scale and are presented as either raw measures or as a percentage of whole-body weight. For autoradiography, image files (16-bit tiff files) from the phosphorimager were analyzed using ImageJ (NIH). For each section, regions-of-interest (ROIs) were drawn over the thalamus. Radiotracer binding in the ROIs was quantified as digital light units (DLU) per pixel and averaged over the area of the ROI. Nonspecific binding was defined using a receptor-saturating (10 µM) concentration of “cold” SB269970. The ROI values for the three nonspecific slides were averaged, and this mean value (which also includes the phosphor-imager screen background) was subtracted from total binding ([3H]SB269970 only) to give the specifically bound radioligand (in DLU/pixel). Clinical measures are reported in their standard SI units and as means ± SE. For in vitro measures, t-tests to compare agonist-induced maximal responses were used. In in vitro experiments where three or more groups were used, an ANOVA with post hoc (Tukey’s test) was used. In all cases, a P value of <0.05 was considered significant. If Bartlett’s test revealed significant variance, then a Kruskal-Wallis test was used for multiple group comparisons.
RESULTS
Generation of 5-HT7 receptor KO rat.
To rapidly generate a 5-HT7 receptor KO, we targeted the rat Htr7 locus by using CRISPR-Cas9 genome editing. We selected two gRNA, targeting exon 1 and exon 2 of the locus (Fig. 1B), and electroporated them simultaneously in Sprague Dawley rat embryos together with Cas9 protein. A total of 40 electroporated embryos were implanted and resulted in the birth of 14 founder offspring, which were analyzed for edits at both exons. Eleven (11) out of 14 pups were edited on at least one allele (Supplemental Fig. S1 and Supplemental Table S1; supplemental material is available at: https://figshare.com/articles/Demireva_et_al_5-HT7_KO_Supplementary_Material/7938404).
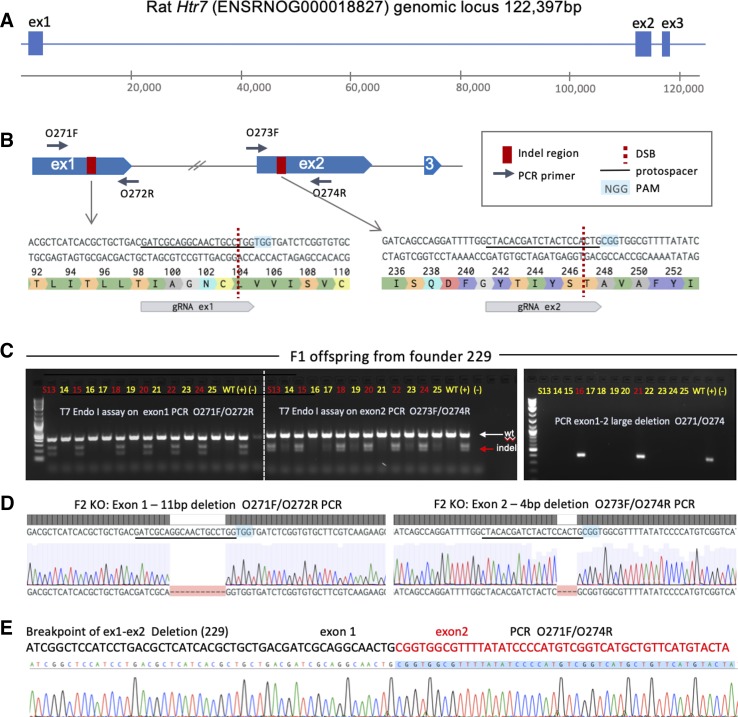
Fig. 1.
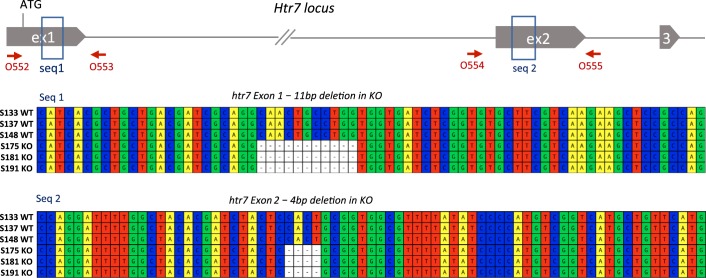
Targeting of the Htr7 locus. A: the rat Htr7 genomic locus (exon size not to scale), exon 1 and exon 2 were targeted by CRISPR-Cas9. B: location and exact sequence of gRNA targets within exon 1 and exon 2, primer design to detect indels, as well as large deletion are indicated. Location of DSBs and PAMs are indicated. C: raw gel electrophoresis images of T7 Endonuclease I (T7 Endo I) digestion analysis of F1 offspring from founder 229 carrying ex1 and ex2 indels on one allele, and a large deletion between ex1 and ex2 DSB sites on the second allele. D: individual gene edits from founder 229 and its offspring were characterized by Sanger sequencing of indicated PCR products: an 11 bp deletion in exon 1 and a 4 bp deletion in exon 2 were identified in F2 homozygous knockout (KO) animals. Location of deletions with respect to gRNA protospacer and PAM are indicated. E: exact sequence and breakpoint of a large deletion between exon 1 and exon 2 DSB sites are indicated. DSB, double-strand DNA break; PAM, protospacer adjacent motif.
The overall editing efficiencies for exon 1 and exon 2 were 36% (5/14) and 79% (11/14), respectively, indicating that the gRNA for exon 2 had higher editing efficiency. One founder had a homozygous deletion of 43 bp in exon 2, while the remaining founders were heterozygous for one or more edits. Three founders exhibited a large deletion between exon 1 and exon 2 target sites, removing intron 1 (~112 kb) entirely.
A male founder (229), chimeric for a large deletion and a double indel, was bred with a WT female to segregate the alleles in the next generation (Fig. 1C). Heterozygous animals from the resultant F1 offspring were genotyped and confirmed to have indels in exon 1 and exon 2 on the same allele and were used for subsequent breeding. A large deletion was detected on the opposite allele (Fig. 1E). HET × HET breeding generated experimental and control cohorts for testing. The two indels were further characterized in KO animals to be an 11 bp deletion in exon 1 and a 4 bp deletion in exon 2 (Fig. 1D). 5-HT7 KO rats from founder 229 were established as a stable line and reproducibly genotyped (Table 1, primers).
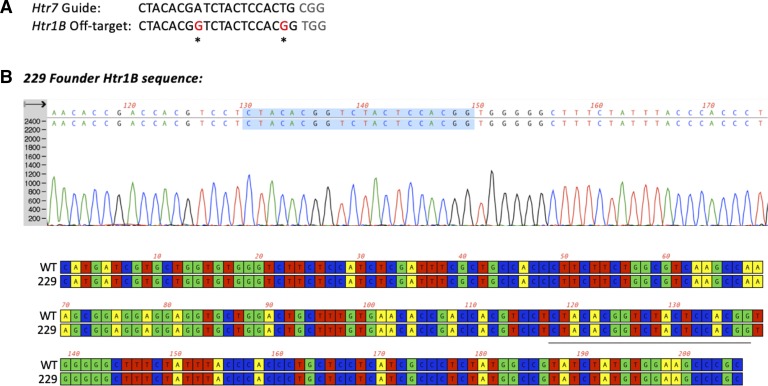
Off-target analysis for the gRNAs used revealed one potential hit of the exon 2 gRNA within the coding sequence of the 5-HT1B receptor gene. Sequencing of the off-target amplicon of Htr1B, revealed that it was intact in the originating founder (229) for the 5-HT7 receptor KO rat (Fig. 2).
Fig. 2.
Off-target analysis. A: gRNA targeting exon 2 of the Htr7 locus also recognizes a target in the Htr1B locus with two mismatches (CFD off-target score: 0.183, MIT off-target score: 2.93, position: chr9:81631922–81631944). To rule out that an off-target hit occurred in the Htr1B locus during Htr7 editing, we performed PCR and Sanger sequencing of the amplicon surrounding the potential off-target site, with primers O409 and O410 (Table 1). Genomic DNA from founder 229 was used for PCR amplification. B: Sanger sequencing and alignment between wild-type (WT) and 5-HT7 KO 229 founder revealed no changes at the predicted cut site or nearby bases, indicating that no off-target changes at the Htr1B locus have occurred. Protospacer of off-target gRNA is underlined or highlighted in sequence and alignment.
Validation of 5-HT7 receptor KO.
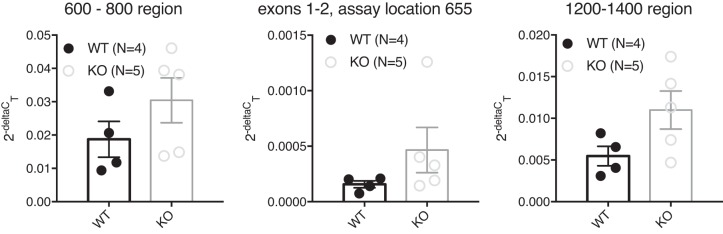
Next, we performed validation of the 5-HT7 KO animals at a genomic, mRNA, protein, and functional level. First mRNA was isolated from cerebellum, reverse-transcribed into cDNA, and quantified by quantitative PCR. Quantitative PCR analysis using primers that covered appropriate regions of Htr7 revealed that mRNA for 5-HT7 was still produced in the mutant rats, as we did not observe a significant decrease of mRNA levels in KO animals compared with WT controls (Fig. 3). Based on these data, mRNA is still produced in the 5-HT7 KO animals.
Fig. 3.
Quantitative PCR analysis of 5-HT7 mRNA levels. 5-HT7 mRNA measures using three different gene regions (600–800, 655, and 1200–1400 in the COOH terminus) in cerebellar samples from 5-HT7 WT and KO male rats. One WT sample was lost in processing, hence n = 4 for WT and n = 5 for KO. PCR primers are listed in methods. Bars are means ± SE with values scattered around the mean. An unpaired t-test was used to compare values of WT and KO. P > 0.05 in all comparisons using an unpaired t-test.
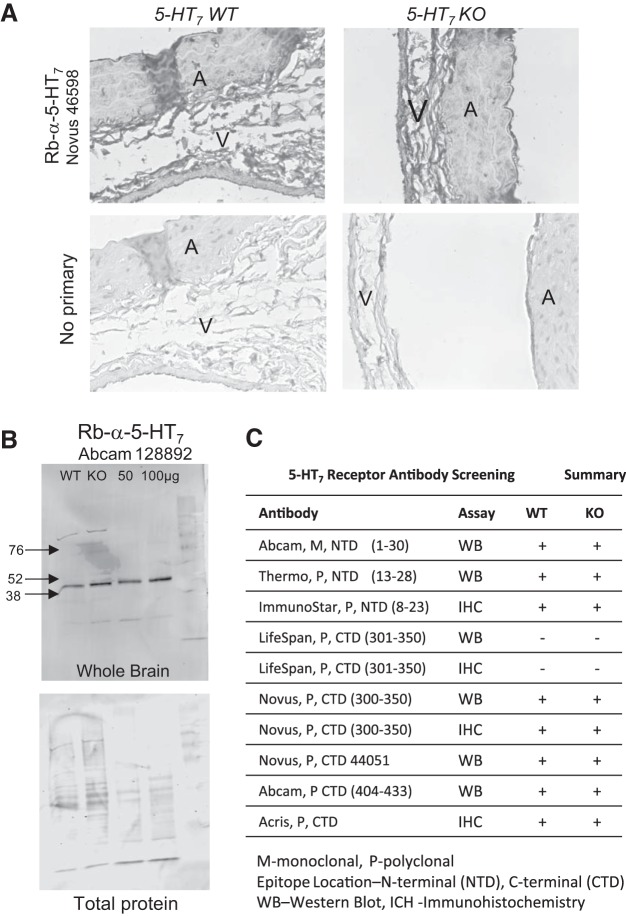
We attempted to reveal loss of 5-HT7 receptor protein by immunohistochemistry and Western blot with antibodies from several vendors but were unable to show differences in signal between KO and WT in either analysis. Figure 4 shows one example of an antibody used for immunohistochemistry (Fig. 4A) and Western analyses (Fig. 4B). Table 2 lists 10 commercially available antibodies, stated in their product sheets to cover antigenic sequences from the NH2 (top of table) to the COOH (bottom) terminus of the 5-HT7 receptor protein; these are summarized in Fig. 4C. None of the antibodies reported in this table were able to distinguish a signal loss in the KO tissues.
Fig. 4.
5-HT7 antibodies do not discriminate between 5-HT7 WT and KO tissue. One example of the failure of available 5-HT7 receptor antibodies to detect differences in 5-HT7 receptor expression between tissues from male WT and KO rats by immunohistochemistry in the isolated abdominal aorta (A) and vena cava (V) (A; Novus 46598) and Western analyses of cerebellar homogenates (B; Abcam 128892). Representative of at least three tissues (male and female) for each procedure. C: summary of all anti-5-HT7 antibodies tested in WT and KO; location of epitope and assay used are indicated.
The lack of mRNA loss and inability to show loss of protein prompted us to completely sequence exon 1 and exon 2 at the genomic level and the entire coding region at the cDNA level. Sequencing analysis confirmed the frameshift-causing deletion in exon 1 was followed by three premature termination codons (PTCs) at both the genomic and cDNA level (Figs. 5 and 6). Sequences for all three exons were identified as part of the mutant cDNA, and no secondary mutations, splicing changes, or exon skipping were detected (11). Based on this analysis, the mutant mRNA that is produced is frameshifted starting at N102, and the first PTC occurs at amino acid position 106, followed by two more PTC at positions 138 and 227. Therefore, if a protein fragment from the mutant mRNA was translated, only the first 101 amino acids would be of the correct protein sequence. The first 101 amino acids of the protein correspond to the extracellular NH2-terminal domain [1–84 amino acids (aa)], and a portion of the first transmembrane helix (85–101 aa), which is at the start of the 7-transmembrane GPCR, rhodopsin-like domain (85–390 aa). Based on these sequencing results, only a nonfunctional protein can be produced from the mutant mRNA.
Fig. 5.
Validation of editing of the Htr7 genomic locus. Genomic DNA from coding region of exon 1 and exon 2 in WT and 5-HT7 KO [n = 3 per group for S### as indicated at left; 2 male (M) and 1 female (F) in WT; 1 M and 2 F in KO] animals were sequenced completely to confirm that no other mutations near the edit sites were present. Genomic DNA was amplified with primers O552 and O553 for exon1 and O554 and O555 for exon 2 (Table 1), and sequenced. A 11 bp deletion in exon 1, and a 4 bp deletion in exon 2 were confirmed. Alignment of sequences from WT and KO animals (n = 3 each) are shown for edited regions.
Fig. 6.
Mutations within Htr7 locus result in a frameshifted mRNA sequence coding for a nonfunctional protein. A: cDNA from WT (n = 5) and homozygous 5-HT7 KO (n = 5) animals was reverse-transcribed from cerebellar mRNA and amplified with primers O566 and O567 (Table 1). The entire coding region of the cDNA was sequenced with primers O566, O567, and O598; 11 bp and 4 bp deletions were confirmed corresponding to those in the genomic DNA (red boxes). Sequencing confirmed that a frameshift begins at amino acid 102 and is followed by 3 premature STOP codons. No other mutations or unexpected splicing changes in the cDNA were detected. B: based on the coding region of the cDNA, protein sequences and alignments were examined. Start and end of frameshift caused by the deletions in exon 1 and exon 2 are denoted by red arrows. STOP codons are denoted by asterisks (*). Three premature STOP codons were identified after the frameshift in exon1 (red rectangles).
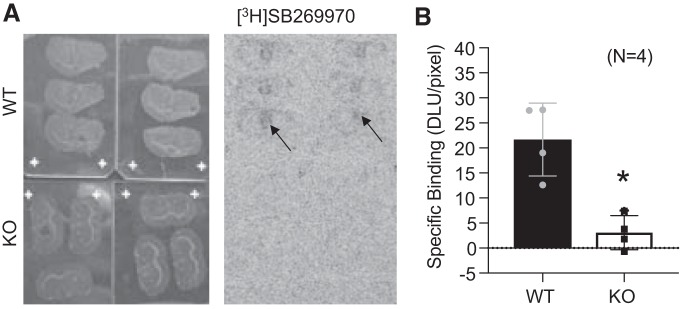
Next, we performed an autoradiographic analysis of the binding site of SB269970, hypothesizing that specific radioligand binding should be absent in the KO compared with the WT. Figure 7 depicts representative coronal slices (Fig. 7A) and their corresponding autoradiograph) when incubated with [3H]SB269970 as a 5-HT7 receptor ligand. Thalamic binding of [3H]SB269970 was present in all sections from the WT animals, but no binding was observed in the KO. We determined nonspecific binding by incubating slides from WT with [3H]SB269970 + unlabeled SB269970 to displace the radioligand (not shown). Specific binding in the thalamus was quantified in both the WT and the KO sections and is shown in Fig. 7B. There was virtually no detectable specific binding of [3H]SB269970 in the KO brain, indicating that the genetic disruption of Htr7 resulted in loss of the 5-HT7 receptor protein to which SB269970 binds. This experiment provided the strongest evidence that we have a 5-HT7 receptor KO rat.
Fig. 7.
[3H]SB269970 radioligand binding site is lost in the 5-HT7 KO rat. Representative brain slides and correlated autoradiogram (A) and quantitation of the thalamus as a region of interest (arrows) for [3H]SB269970-specific binding in brain sections of the WT and KO rat (B). Bars represent means ± SE for number of points scattered around the mean. Four different rats, including male and female, are represented in this measure. An unpaired t-test was used to compare values of WT and KO. *P > 0.05 in all comparisons between WT and 5-HT7 KO tissues. DLU, digital light units.
Characterization of 5-HT7 receptor KO rat.
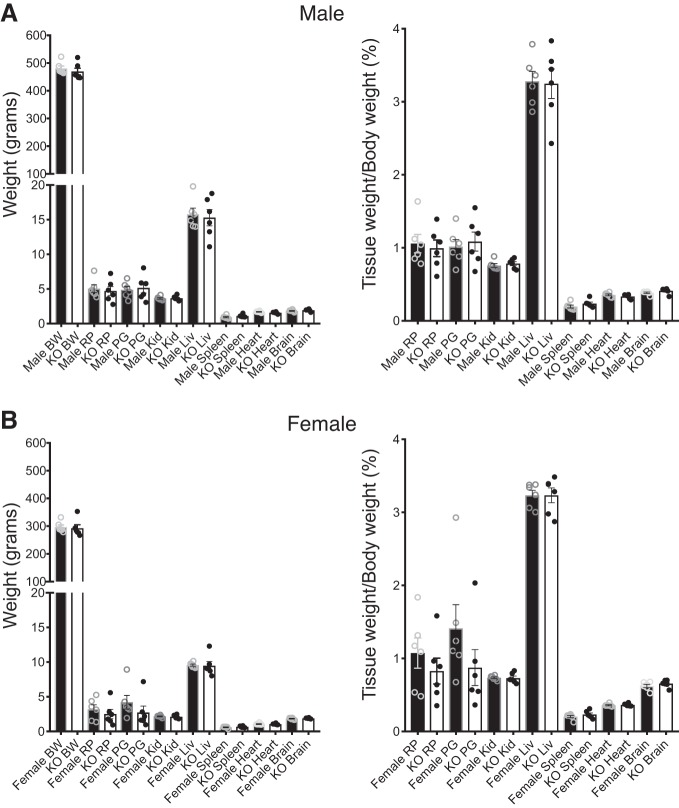
WT, Het, and KO animals for the 5-HT7 receptor were generated at close to Mendelian ratios, and homozygous KO animals were viable and reached adulthood without any overt phenotypes [out of 300 rats: male (M) WT (36/300); M Het (84/300); M KO (35/300); female (F) WT (40/300); F Het (68/300); F KO (37/300)]. Figure 8 depicts weights of animals and organs at ~16 wk of age. While there were no differences between the WT and KO body weights within a sex, the male rats were significantly larger than their female littermates. Similarly, there were no differences in the tissue weights between WT and KO in either sex when considered as a raw measure (Fig. 8, left) or as a percentage of body weight (Fig. 8, right). Table 3 lists values for basic clinical parameters of nonfasted male and female WT and KO rats as compared with reference values for similarly aged Sprague Dawley rats (13–22 wk). The male KO had lower cholesterol, triglycerides, and alkaline phosphatase than WT. The female KO showed an elevated gamma-glutamyltransferase vs. WT. These were the only statistical differences observed when comparing genotypes within a sex. Where the values fall out of the range indicated by Charles River, they are marked as high or low for each sex in the column immediately to the right (Table 3). Creatinine, phosphate, albumin, and potassium in all the WTs and KO rats were lower than that reported for Sprague-Dawley rats, while alanine aminotransferase was higher.
Fig. 8.
Body and tissue weights of 5-HT7 WT and KO male and female rats. Body and tissue weights of male (A) and female (B) WT and KO rats ~16 wk of age. Bars represent means ± SE for n = 6 rats. BW, body weight; RP, retroperitoneal fat; PG, perigonadal fat; Kid, kidney; Liv, liver. An unpaired t-test was used to compare values of WT and KO. P > 0.05 in all comparisons between WT and 5-HT7 KO tissues.
Table 3.
Clinical values derived from plasma from the WT and KO male and female rats
| Male |
Female |
|||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Glucose, mmol/l | 10.00 ± 0.42 | 9.64 ± 0.63 | 8.89 ± 0.24* | 9.45 ± 0.19 |
| BUN, mmol/l | 6.01 ± 0.56 | 5.47 ± 0.41 | 5.21 ± 0.43 | 6.19 ± 0.24 |
| Creatinine, µmol/l | 14.73 ± 1.86 | 17.68 ± 0.0L | 17.68 ± 0.0 | 17.68 ± 0.0L |
| Phosphate, mmol/l | 1.83 ± 0.07 | 1.90 ± 0.07L | 1.54 ± 0.08‡ | 1.55 ± 0.08†L |
| Calcium, mmol /l | 2.38 ± 0.02 | 2.38 ± 0.01 | 2.38 ± 0.04 | 2.38 ± 0.03 |
| Albumin, g/l | 25.80 ± 0.40 | 25.60 ± 0.50L | 28.20 ± 1.3 | 28.10 ± 1.4L |
| Globulin, g/l | 31.16 ± 0.54 | 32.66 ± 0.50 | 31.80 ± 0.40 | 32.30 ± 0.40 |
| ALT, U/l | 51.16 ± 5.41 | 50.83 ± 3.70H | 41.0 ± 4.32 | 84.33 ± 26.68H |
| ALKP, U/l | 279.8 ± 45.35 | 201.3 ± 42.92* | 160.4 ± 30.09 | 155.16 ± 25.96 |
| GGT, U/l | 0.60 ± 0.33 | 0.17 ± 0.166 | 0.0 ± 0.0 | 1.33 ± 0.33*† |
| T. Bilirubin, µmol/l | 2.28 ± 0.57 | 1.99+00.28 | 2.39 ± 0.42 | 3.13 ± 0.68 |
| Cholesterol, mmol/l | 1.73 ± 0.22 | 1.48 ± 0.18* | 1.84 ± 0.16 | 1.63 ± 0.19 |
| Triglycerides, mmol/l | 0.83 ± 0.22 | 0.48 ± 0.08*L | 0.77 ± 0.09 | 0.64 ± 0.12 |
| Sodium, mmol/l | 139.6 ± 0.42 | 142.5 ± 1.17 | 141.8 ± 0.58 | 140.6 ± 0.91 |
| Potassium, mmol/l | 4.33 ± 0.15 | 4.00 ± 0.11L | 3.74 ± 0.08† | 3.78 ± 0.10‡L |
| Chloride, mmol/l | 101.83 ± 0.31 | 103.0 ± 0.97 | 101.6 ± 0.67 | 102.16 ± 0.75 |
| Osm Calc, mmol/kg | 284.6 ± 0.33 | 287.3 ± 2.75 | 284.8 ± 1.02 | 284.0 ± 1.98 |
Values are averages ± SE for n = 5–6. BUN, blood urea nitrogen; ALT, alanine aminotransferase; ALKP, alkaline phosphatase; GGT = gamma glutamyl transferase; H or L, outside the range reported for 13–22 wk old Charles River rats (https://www.criver.com/files/pdfs/rms/cd/rm_rm_r_clinical_parameters_cd_rat_06.aspx).
Significant differences between genotype but same sex;
significant differences between different sex of same genotype;
significant difference between different sex and different genotype. Determined by ANOVA with Tukey’s post hoc.
Loss of 5-HT7 receptor mediated venous relaxation in the KO vs. WT rat.
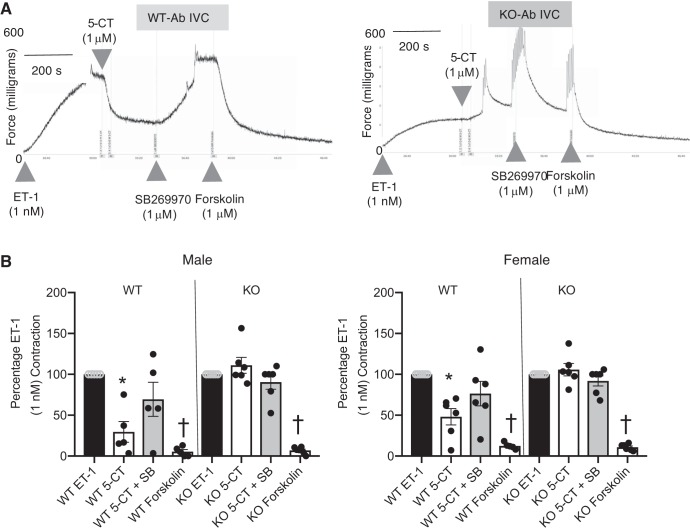
The lack of a functional 5-HT7 receptor in the KO was validated by in vitro experiments using the isolated Ab IVC, validated to relax to 5-HT in a 5-HT7 receptor-dependent manner (29). Tissues were stably contracted with ET-1, and the 5-HT1/7 receptor agonist 5-carboxamidotryptamine (5-CT; 1 μM) was added. In the vena cava from a WT rat, 5-CT caused a significant relaxation (~70% in WT) that was reversed, in part, by the 5-HT7 receptor antagonist SB269970 (Fig. 9A). By contrast, 5-CT caused no relaxation in the vena cava of the KO; in some, a constriction was observed [KO male 5-CT = 116 ± 3.4%; (Fig. 9A, right)]. SB269970 did not modify venous tone in the presence of 5-CT in the vena cava from the KO rat. These data confirm the lack of a functional receptor in 5-HT7 KO animals. All tissues relaxed to the adenylate cyclase stimulant forskolin, indicating that a lack of relaxation to 5-CT was not because vessels cannot relax to any stimulus, specifically one predicted to activate adenylate cyclase, a recognized 5-HT7 receptor signal transducer. These data are quantified in Fig. 9B.
Fig. 9.
Lack of a functional 5-HT7 receptor leads to complete loss of venous relaxation to 5-CT. Representative tracings of abdominal inferior vena cava (Ab IVC) from the WT and 5-HT7 receptor KO rat (A) in response to 5-CT (1 μM), followed by the 5-HT7 receptor antagonist SB269970 (1 μM), and adenylate cyclase activator forskolin (1 μM) after an established ET-1 contraction. B: quantitation of 5-CT-induced changes in isometric tone in the abdominal vena cava and aorta from male (left) and female (right) WT and KO rats, followed by acute antagonism with SB269970 and forskolin addition. Bars represent means ± SE for n = 5–6 around which individual values are scattered. *Significant differences between ET-1-induced contraction; †from SB269970-incubated responses as determined through ANOVA with Tukey’s post hoc test. ET-1 maximums: male abdominal vena cava: WT = 261 ± 72 mg, KO = 272 ± 75 mg; female abdominal vena cava: WT = 302 ± 109 mg, KO = 272 ± 75 mg.
DISCUSSION
The 5-HT7 receptor is among the most recent 5-HT receptors to be cloned, an event that placed this molecule in a new 5-HT receptor class (19). Only 4 yr after this discovery, Terron (27) suggested the 5-HT7 receptor was responsible for mediating the observed hypotension upon acute (minutes) infusion of 5-HT to the anesthetized, pithed, and ketanserin-treated rat. This important paper and others that supported acute 5-HT-induced hypotension in the rat and other species (1, 4, 21, 22, 28) opened the doors for considering the 5-HT7 receptor as relevant to blood pressure regulation, in addition to being involved in the modulation of circadian rhythm and mood (6, 7). The creation of a rat strain with genetic ablation of the 5-HT7 receptor was important as a tool to examine whether activation of the 5-HT7 receptor was essential to not only the initial 5-HT-induced hypotension (23), but through the whole week of infusion. Because the creation of this animal led to unexpected findings and experimental obstacles, we report this work in this paper, separate from the study dedicated to cardiovascular experiments.
Creation of the 5-HT7 receptor KO rat.
The KO was successful in that the 5-HT7 receptor function was abolished, but the KO rat and its creation was unusual in at least two ways. Although CRISPR technology is now widely used to inactivate genes, the effects of resultant indel mutations on mRNA processing and degradation have been examined in detail by only a few studies. Under normal circumstances, an indel that is not a multiple of three results in a frameshifted mRNA that will contain multiple PTCs and such mRNAs molecules will be detected by the nonsense-mediated decay (NMD) surveillance machinery and rapidly degraded (15). NMD prevents the translation of truncated proteins from mutant mRNAs that may have deleterious effects such as gain of function or aberrant aggregation properties. Furthermore, indels resulting in PTCs can also be subject to nonsense-associated alternative splicing, which results in exon skipping. If the skipped exon is in-frame, it can still produce full length transcripts that evade the NMD and are translated into proteins. To date, a number of studies have reported a high frequency of exon skipping following genome editing using CRISPR/Cas in several mammalian and nonmammalian systems (10, 13, 16, 25).
The mRNA for the Htr7 gene was present in the KO and the WT, despite the fact that CRISPR editing resulted in an indel in exon 1, which created a frameshift starting at amino acid 102 and was followed by three PTCs. This led us to conclude that the mutant 5-HT7 mRNA is not sensitive to NMD degradation. We next tested whether exon skipping could have played a role in the evasion of NMD by the mutant transcript. We fully sequenced the coding region of the cDNA and found that all exons (1–3) were part of the transcript and the indels were at the expected locations. Based on this information, the mutant transcript is not subject to either NMD or exon skipping.
Our analysis of protein levels of 5-HT7 did not verify loss of protein because of significant limitations in 5-HT7 receptor antibody specificity. Antibodies against both NH2-terminal and COOH-terminal epitopes were tested, and all failed to detect a difference between WT and KO. While the lack of success in antibody-based experiments could indicate that the 5-HT7 protein may still be immunologically detectable, this is unlikely given that three stop codons were introduced into the gene such that the transmembrane domain and the COOH terminus should be missing. If a truncated protein is produced from the mutant messages, we predict that it would be a 137-amino acid protein fragment with only the first 101 amino acids of the sequence being correct. Such a protein fragment cannot be localized to the membrane because it lacks a TM domain. Furthermore, Western blot analysis using NH2 terminus-specific antibodies did not detect a specific band in the KO with a molecular weight (~15 kDa) corresponding to such a fragment. If such a protein product is produced, it may be unstable or degraded; alternatively, the antibodies used may not be sensitive enough to detect such a protein fragment. It is most likely these antibodies, some of which are directed to the COOH terminus, which should be absent, are not specific in their detection of the 5-HT7 receptor. Lack of specificity is a common observation for many antibodies generated against heptahelical receptors. However, the loss of [3H]SB269970 binding in the KO vs. WT strongly supports the lack of expression of the normal 5-HT7 receptor protein.
We reviewed several previously reported KO mice of different 5-HT receptors and found that for two (the 5-HT3A and 5-HT2A) loss or reduction for mRNA was reported (30, 31), but for four others (5-HT1A, 5-HT1B, 5-HT2C, and 5-HT7) it was either not reported or was not observed (9, 17, 20, 26). Specifically, disruption of the mouse Htr7 locus by insertion of a KO cassette within exon 2 produced a mutant mRNA but not at reduced levels compared with WT (9). It is important therefore to examine the presence of mutant mRNA abundance in KOs, especially considering that CRISPR-induced mutations are subtle and may leave frameshifted mRNAs intact. What is quite clear is that the function of the 5-HT7 receptor has been lost in the KO vs. the WT, evidenced by a total loss of 5-CT-induced venous relaxation.
Broader and Clinical Implications
This new rat model adds to the arsenal of genetic tools to study 5-HT receptor biology more broadly, especially since significantly fewer rat KOs are available compared with mice. This rat will be useful in addressing the role of the 5-HT7 receptor in central nervous system-mediated functions such as circadian rhythm regulation and neuropsychiatric disorders related behaviors. These include sleep and behavior, two phenotypes that we have not investigated formally. With respect to smooth muscle relaxation and effects on blood pressure, this model is of clinical relevance specifically because it could be used to test compounds with potential therapeutic applications. The article accompanying this one shares the studies performed that were the drive for the creation of this new KO rat (22a). Use of this model would be amenable for drug discovery.
GRANTS
This work was funded by National Heart, Lung, and Blood Institute Grant HL-107495 (S. W. Watts).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
E.Y.D. and S.W.W. conceived and designed research; E.Y.D., H.X., E.D.F., J.M.T., B.M.S., and S.W.W. performed experiments; E.Y.D., H.X., E.D.F., J.M.T., B.M.S., and S.W.W. analyzed data; E.Y.D., H.X., E.D.F., J.M.T., and S.W.W. interpreted results of experiments; E.Y.D., E.D.F., J.M.T., and S.W.W. prepared figures; E.Y.D. and S.W.W. drafted manuscript; E.Y.D., H.X., E.D.F., J.M.T., B.M.S., and S.W.W. edited and revised manuscript; E.Y.D., H.X., E.D.F., J.M.T., B.M.S., and S.W.W. approved final version of manuscript.
REFERENCES
- 1.De Vries P, De Visser PA, Heiligers JPC, Villalón CM, Saxena PR. Changes in systemic and regional haemodynamics during 5-HT7 receptor-mediated depressor responses in rats. Naunyn Schmiedebergs Arch Pharmacol 359: 331–338, 1999. doi: 10.1007/PL00005359. [DOI] [PubMed] [Google Scholar]
- 2.Diaz J, Ni W, Thompson J, King A, Fink GD, Watts SW. 5-Hydroxytryptamine lowers blood pressure in normotensive and hypertensive rats. J Pharmacol Exp Ther 325: 1031–1038, 2008. doi: 10.1124/jpet.108.136226. [DOI] [PubMed] [Google Scholar]
- 3.Ding D, Liu J, Dong K, Midic U, Hess RA, Xie H, Demireva EY, Chen C. PNLDC1 is essential for piRNA 3′ end trimming and transposon silencing during spermatogenesis in mice. Nat Commun 8: 819, 2017. doi: 10.1038/s41467-017-00854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding XR, Stier CT Jr, Itskovitz HD. Serotonin and 5-hydroxytryptophan on blood pressure and renal blood flow in anesthetized rats. Am J Med Sci 297: 290–293, 1989. doi: 10.1097/00000441-198905000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Gardani M, Biello SM. The effects of photic and nonphotic stimuli in the 5-HT7 receptor knockout mouse. Neuroscience 152: 245–253, 2008. doi: 10.1016/j.neuroscience.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 6.Gellynck E, Heyninck K, Andressen KW, Haegeman G, Levy FO, Vanhoenacker P, Van Craenenbroeck K. The serotonin 5-HT7 receptors: two decades of research. Exp Brain Res 230: 555–568, 2013. doi: 10.1007/s00221-013-3694-y. [DOI] [PubMed] [Google Scholar]
- 7.Guseva D, Wirth A, Ponimaskin E. Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front Behav Neurosci 8: 306, 2014. doi: 10.3389/fnbeh.2014.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hagan JJ, Price GW, Jeffrey P, Deeks NJ, Stean T, Piper D, Smith MI, Upton N, Medhurst AD, Middlemiss DN, Riley GJ, Lovell PJ, Bromidge SM, Thomas DR. Characterization of SB-269970-A, a selective 5-HT(7) receptor antagonist. Br J Pharmacol 130: 539–548, 2000. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci USA 100: 1375–1380, 2003. doi: 10.1073/pnas.0337340100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kapahnke M, Banning A, Tikkanen R. Random Splicing of Several Exons Caused by a Single Base Change in the Target Exon of CRISPR/Cas9 Mediated Gene Knockout. Cells 5: E45, 2016. doi: 10.3390/cells5040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lalonde S, Stone OA, Lessard S, Lavertu A, Desjardins J, Beaudoin M, Rivas M, Stainier DYR, Lettre G. Frameshift indels introduced by genome editing can lead to in-frame exon skipping. PLoS One 12: e0178700, 2017. doi: 10.1371/journal.pone.0178700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monti JM, Jantos H. The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev Neurosci 25: 429–437, 2014. doi: 10.1515/revneuro-2014-0016. [DOI] [PubMed] [Google Scholar]
- 13.Mou H, Smith JL, Peng L, Yin H, Moore J, Zhang XO, Song CQ, Sheel A, Wu Q, Ozata DM, Li Y, Anderson DG, Emerson CP, Sontheimer EJ, Moore MJ, Weng Z, Xue W. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol 18: 108, 2017. doi: 10.1186/s13059-017-1237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page IH, McCubbin JW. The variable arterial pressure response to serotonin in laboratory animals and man. Circ Res 1: 354–362, 1953. doi: 10.1161/01.RES.1.4.354. [DOI] [PubMed] [Google Scholar]
- 15.Popp MW, Maquat LE. Leveraging Rules of Nonsense-Mediated mRNA Decay for Genome Engineering and Personalized Medicine. Cell 165: 1319–1322, 2016. doi: 10.1016/j.cell.2016.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prykhozhij SV, Steele SL, Razaghi B, Berman JN. A rapid and effective method for screening, sequencing and reporter verification of engineered frameshift mutations in zebrafish. Dis Model Mech 10: 811–822, 2017. doi: 10.1242/dmm.026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, Mann JJ, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci USA 95: 14476–14481, 1998. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts AJ, Hedlund PB. The 5-HT(7) receptor in learning and memory. Hippocampus 22: 762–771, 2012. doi: 10.1002/hipo.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA 90: 8547–8551, 1993. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saudou F, Amara DA, Dierich A, LeMeur M, Ramboz S, Segu L, Buhot MC, Hen R. Enhanced aggressive behavior in mice lacking 5-HT1B receptor. Science 265: 1875–1878, 1994. doi: 10.1126/science.8091214. [DOI] [PubMed] [Google Scholar]
- 21.Saxena PR, Lawang A. A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors. Arch Int Pharmacodyn Ther 277: 235–252, 1985. [PubMed] [Google Scholar]
- 22.Saxena PR, Villalón CM. Cardiovascular effects of serotonin agonists and antagonists. J Cardiovasc Pharmacol 15, Suppl 7: S17–S34, 1990. doi: 10.1097/00005344-199001001-00004. [DOI] [PubMed] [Google Scholar]
- 22a.Seitz BM, Demireva EY, Xie H, Fink GD, Krieger-Burke T, Burke WM, Watts SW. 5-HT does not lower blood pressure in the 5-HT7 knockout rat. Physiol Genomics, 2019. doi: 10.1152/physiolgenomics.00031.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seitz BM, Orer HS, Krieger-Burke T, Darios ES, Thompson JM, Fink GD, Watts SW. 5-HT causes splanchnic venodilation. Am J Physiol Heart Circ Physiol 313: H676–H686, 2017. doi: 10.1152/ajpheart.00165.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sprouse J, Li X, Stock J, McNeish J, Reynolds L. Circadian rhythm phenotype of 5-HT7 receptor knockout mice: 5-HT and 8-OH-DPAT-induced phase advances of SCN neuronal firing. J Biol Rhythms 20: 122–131, 2005. doi: 10.1177/0748730404273432. [DOI] [PubMed] [Google Scholar]
- 25.Sui T, Song Y, Liu Z, Chen M, Deng J, Xu Y, Lai L, Li Z. CRISPR-induced exon skipping is dependent on premature termination codon mutations. Genome Biol 19: 164, 2018. doi: 10.1186/s13059-018-1532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature 374: 542–546, 1995. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- 27.Terrón JA. Role of 5-ht7 receptors in the long-lasting hypotensive response induced by 5-hydroxytryptamine in the rat. Br J Pharmacol 121: 563–571, 1997. doi: 10.1038/sj.bjp.0701134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villalón CM, Centurión D, Bravo G, De Vries P, Saxena PR, Ortiz MI. Further pharmacological analysis of the orphan 5-HT receptors mediating feline vasodepressor responses: close resemblance to the 5-HT7, receptor. Naunyn Schmiedebergs Arch Pharmacol 361: 665–671, 2000. doi: 10.1007/s002100000256. [DOI] [PubMed] [Google Scholar]
- 29.Watts SW, Darios ES, Seitz BM, Thompson JM. 5-HT is a potent relaxant in rat superior mesenteric veins. Pharmacol Res Perspect 3: e00103, 2015. doi: 10.1002/prp2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisstaub NV, Zhou M, Lira A, Lambe E, González-Maeso J, Hornung JP, Sibille E, Underwood M, Itohara S, Dauer WT, Ansorge MS, Morelli E, Mann JJ, Toth M, Aghajanian G, Sealfon SC, Hen R, Gingrich JA. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science 313: 536–540, 2006. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- 31.Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22: 1010–1019, 2002. doi: 10.1523/JNEUROSCI.22-03-01010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]