Abstract
A reduction in intestinal barrier function is currently believed to play an important role in pathogenesis of many diseases, as it facilitates passage of injurious factors such as lipopolysaccharide, peptidoglycan, whole bacteria, and other toxins to traverse the barrier to damage the intestine or enter the portal circulation. Currently available evidence in animal models and in vitro systems has shown that certain dietary interventions can be used to reinforce the intestinal barrier to prevent the development of disease. The relevance of these studies to human health is unknown. Herein, we define the components of the intestinal barrier, review available modalities to assess its structure and function in humans, and review the available evidence in model systems or perturbations in humans that diet can be used to fortify intestinal barrier function. Acknowledging the technical challenges and the present gaps in knowledge, we provide a conceptual framework by which evidence could be developed to support the notion that diet can reinforce human intestinal barrier function to restore normal function and potentially reduce the risk for disease. Such evidence would provide information on the development of healthier diets and serve to provide a framework by which federal agencies such as the US Food and Drug Administration can evaluate evidence linking diet with normal human structure/function claims focused on reducing risk of disease in the general public.
Keywords: diet, function, gut barrier structure, permeability
INTRODUCTION: OBJECTIVES
This perspective article summarizes the present scientific evidence focused on 1) the gut barrier as an important component of normal gastrointestinal (GI) structure and function in human health as well as identification of specific physiologic benefits causally associated with normal structure and function, 2) currently available modalities to describe the intestinal barrier and quantify its function in humans, and 3) providing possible associations between diet and normal gut barrier function among healthy or at-risk people. It is intended to inform translational research into dietary guidance (such as food-labeling claims) as well as address human nutrition research design decisions so that evidence is useful for making dietary guidance decisions. Despite a tremendous amount of interest in the notion that the modalities to reduce the “leaky gut” may reduce the risk for disease, there are significant limitations in our present understanding of human intestinal barrier function, as well as technical challenges that must be addressed to advance the field forward in a rigorous and reproducible scientifically based manner. Gaps and means to address them are featured throughout, with the intention to inform future translational research to serve as the basis of dietary guidance broadly.
Advances in this field of research will be fundamentally important in many practical issues that will be very impactful to human health. For example, they will have an impact on the manner in which we apply the framework established by the US Food and Drug Administration for evaluating evidence linking diet with normal human structure/function and with reducing risk of disease or medical outcomes as they are related to the general public (208, 209). This is particularly relevant, given the new definition of dietary fiber for food labeling in the United States that requires a demonstrated physiological effect that is beneficial to human health [either structure/function (e.g., laxation) or specific health-related biomarkers (e.g., inflammation)]. Table 1 describes how nutrition research evidence applies to maintaining normal gut barrier structure and function, as well as reducing the risk of gut barrier-related health conditions such as bowel diseases. Such structure/function claims are regulated by the US Food and Drug Administration: claims for conventional foods focus on effects derived from nutritive value, whereas claims for dietary supplements may focus on nonnutritive as well as nutritive effects. This review is necessitated by the recent interest in intestinal barrier function and leaky gut in diverse GI and non-GI diseases and anticipated claims of the structure/function effects of different nutrients or other remedies. This article is a distillation of presentations at a meeting organized by International Life Sciences Institute North America in December 2018.
Table 1.
Framework to evaluate scientific evidence linking diet with gut barrier structure, function, and risk reduction for adverse bowel conditions
| Evidence That Would Apply If Available | Structure and Function Claims | Health Claims |
|---|---|---|
| Examples of messages associated with structure/function claims | Dietary component X helps maintain normal gut barrier structure; dietary component X helps maintain a normally functioning gut barrier; limiting dietary component X helps maintain a normally functioning gut barrier; dietary component X helps maintain normal nutrient absorption while protecting against harmful exposures in the gut | Dietary component X helps reduce risk of [insert disease or health-related condition Y*]; for example, reduced risk of IBD among persons with a family history or [insert other conditional factors defining the relevant population] |
| Primary evidence | Human studies demonstrating that dietary component X is causally associated with maintaining or restoring normal gut barrier structure (e.g., mucus layer thickness) or function of human intestinal barrier (e.g., “normal” permeability or epithelial cell immune function); human studies demonstrating a physiological benefit to normal gut barrier permeability and gut immunological function (e.g., reduced susceptibility to food-borne/intestinal pathogens or preventing elevated endotoxins or systemic inflammation) | Strength of evidence from human studies demonstrating a clinically and statistically significant relationship between the dietary component and accepted indicators of risk for or progression to [insert specific intestinal or extraintestinal health conditions such as IBD or metabolic syndrome] |
| Background information | Animal studies that link 1) structure with normal permeability or other to-be-determined indicators of normal function (e.g., preventing chronic systemic inflammation and maintaining immune components in gut membranes) or 2) normal structure or function with a specific health benefit (such as protection from food-borne/intestinal pathogen exposure); human mechanism-of-action studies supporting a causal link between dietary component and structure/function of gut barrier (e.g., increased fermentable fiber, which protects mucin layer thickness) | Animal studies showing that dietary component X reduces risk or surrogate markers of disease or health-related condition Y; human mechanism-of-action studies associating dietary component with disease risk or surrogate marker of disease risk; human mechanism-of-action studies to support a causal relationship |
| Surrogate measure in human studies | Identify clinically accepted indicators of risk by specific condition identified above (e.g., include a gut example…similar to how elevated LDL cholesterol is recognized by the FDA as a risk factor for cardiovascular disease) |
Examples listed here require US Food and Drug Administration (FDA) review to evaluate whether they meet regulatory requirements of a disease or health-related condition: helps reduce risk of inflammatory bowel disease (IBD), helps reduce risk of celiac disease, helps reduce susceptibility to or development of food allergies, helps reduce risk of metabolic dysfunction, helps prevent low weight for age among children at risk for gastrointestinal disease-induced malnourishment, and helps reduce risk of environmental enteric dysfunction among young children exposed to environmental risks.
STRUCTURAL AND FUNCTIONAL COMPONENTS OF THE INTESTINAL BARRIER
Barrier function at a mucosal interface is an essential property of host biology that provides protection from the environment. The intestinal epithelium forms a dynamic and semipermeable barrier that allows the absorption of nutrients, electrolytes, and water, as well as antigens that play a role in immune regulation, but it also protects the host from the microbiota, as well as potentially toxic molecules in the gut lumen. Since intestinal mucosal barrier dysfunction is associated with numerous diseases, it is reasonable to postulate that its reinforcement in health may reduce the risk of disease. Disease entities where this might be the case include those that are specific to the intestinal tract such as ulceration, enteric infections, functional bowel disorders, cancer, and intestinal immune-mediated disorders such as inflammatory bowel or celiac disease. Barrier function also appears to differ with age (discussed below in potential of dietary and nondietary factors to impact components of the intestinal mucosal barrier, Diet-Independent Factors Affecting Intestinal Permeability, Age).
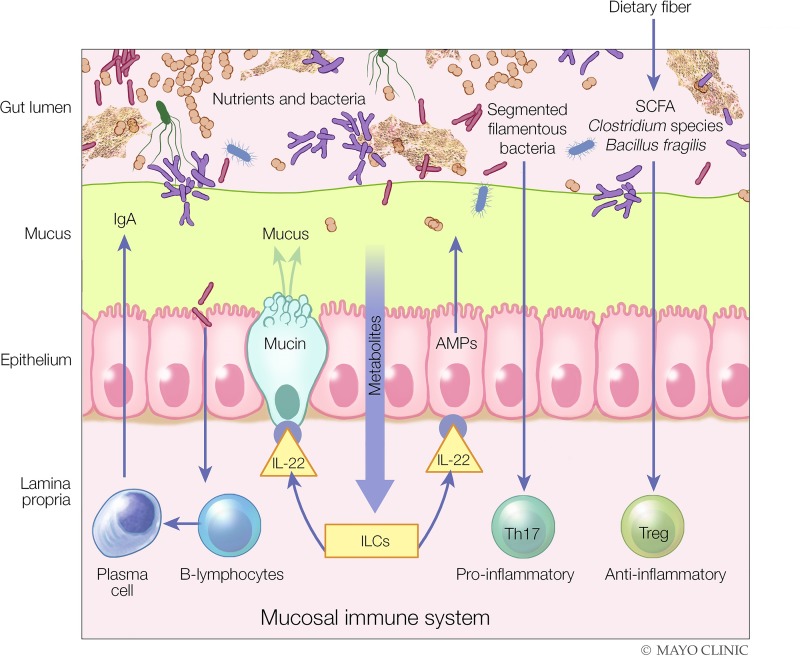
The mucosal barrier can be divided roughly into three interacting components: mucus, the intestinal epithelium, and the mucosal immune system [Fig. 1 (108)]. Intestinal mucus is a gel layer composed of complex glycoproteins important as the first-line barrier of the mucosal surface but also serving as a lubricant and aiding as a transport vehicle between luminal contents and the intestinal epithelium. In the colon, there are two mucus layers, a loose outer layer that is inhabited by the gut microbiota where it is utilized as a source of bacterial nutrition and a dense inner layer that is largely devoid of bacteria [reviewed by Johansson et al. (101)].
Fig. 1.
The three components of the intestinal mucosal barrier and the impact of diet and specific immune mechanisms involved in maintaining the integrity of the barrier. Diet can reinforce both the structure and function of the intestinal barrier, for example, through the production of short-chain fatty acids (SCFAs) by the gut microbiota, which are used by the colonic epithelium as a source of energy and can, independently, induce immune tolerance via T regulatory (Treg) cells. As another example, metabolites in diet can activate innate lymphoid cells (ILCs) to produce IL-22, which, in turn, can enhance the production of mucin and antimicrobial peptides (AMPs) by the intestinal epithelium to fortify gut barrier function. Plasma cells, a component of the mucosal immune system, can also produce IgA, which is secreted into the intestinal mucus layer. In this manner, the intestinal epithelium, the most important component of the intestinal barrier, has both structural and functional components to protect the host from the luminal contents of the intestinal tract. Th17, T helper type 17. [Modified from Kamada and Núñez (108) with permission.]
Mucus has potent antimicrobial activity because it contains antimicrobial peptides (AMPs) such as α- and β-defensins that are secreted by intestinal epithelial cell lineages such as Paneth cells (153) and secretory IgA produced by plasma cells located in the lamina propria. Secretory IgA is the most abundant immunoglobulin at mucosal surfaces (155), where it plays a significant role in maintaining barrier function within the intestinal mucus by binding to bacteria in the gut lumen with some level of specificity and preventing microbial invasion by coating bacteria, inhibiting adherence to epithelial cells, and neutralizing bacterial toxins.
The most important physical barrier at the intestinal mucosal surface lies underneath the mucus layer, the intestinal epithelium. These cells form a continuous and polarized epithelial barrier with several cellular lineages: absorptive cells, goblet cells, enteroendocrine cells, tuft cells, and Paneth cells. Each cell type has specific functions. For example, both goblet cells and Paneth cells help to fortify the mucosal barrier through the production of mucus and AMPs, respectively. Substances can pass through this barrier via two general pathways, transcellular or paracellular. Transcellular permeability is associated with solute transport predominantly regulated by selective transporters, such as the sodium-coupled transporters for glucose and fructose (85, 140), as well as neutral, dibasic, and dicarboxylic amino acids (1), whereas paracellular permeability is associated with transport in the space between epithelial cells and is regulated by intercellular complexes localized toward the apical surface of the intestinal epithelium (5, 17). These intercellular complexes are also under neurohumoral control, such as from vasoactive intestinal peptide and cholinergic neurons from the submucosal plexus (79, 82, 166). However, it is unclear whether specific transporters exist for some of the disaccharide molecules used in the measurement of intestinal permeability, such as lactulose and sucralose.
Located primarily below the intestinal epithelium, with some components situated in between, is the immunological barrier of the intestinal mucosa, known as the mucosal immune system, the largest immune organ in the human body. A complex and interconnected array of various immune cell populations maintains intestinal mucosal immune homeostasis by balancing immune activation to protect against microbial invasion while simultaneously demonstrating restraint via immune tolerance to prevent unrestrained inflammation, the hallmark of immune-mediated intestinal diseases such as inflammatory bowel diseases (147). General populations of the intestinal immune effector system include T effector and regulatory cells, B cells, dendritic cells, and components of the innate immune system such as macrophages, mast cells, and neutrophils [reviewed by Veldhoen and Brucklacher-Waldert (215)]. An important and more recently described cell population is the innate lymphoid cells (ILCs), which belong to the lymphoid lineage but lack antigen-specific T and B cell receptors (147, 215).
Interaction between all three components of the intestinal mucosal barrier is complex but is well orchestrated to maintain a homeostatic state between the host and its environment at the mucosal interface. For example, ILC3s are particularly important in the maintenance of the intestinal mucosal barrier through the secretion of IL-22 cytokine, which promotes both enhanced mucus production via goblet cells as well as AMPs. Both of these are important components of the intestinal mucus barrier. Through this multicomponent intestinal barrier system, a normally functioning gut barrier selectively enables absorption of water and essential nutrients, while protecting against adverse health effects from ingested or endogenous toxins.
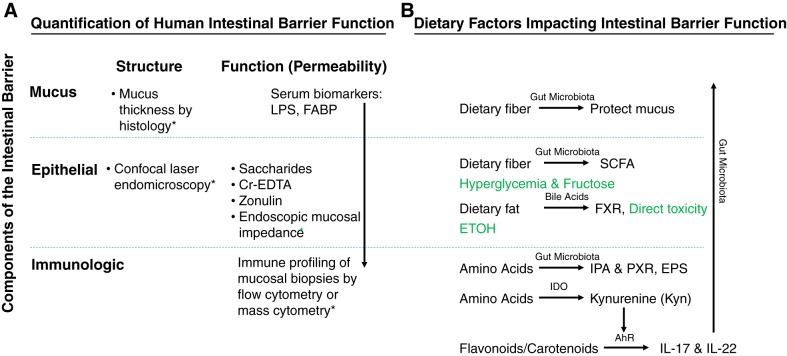
MEASUREMENTS TO CHARACTERIZE NORMAL STRUCTURE AND FUNCTION OF THE INTESTINAL BARRIER IN HUMAN NUTRITION RESEARCH
Gut permeability is a useful measure of how well the integrated system of the GI barrier (summarized as surface mucus, epithelial barrier, and immune mechanisms) is functioning. The goal in measuring intestinal permeability is to document dysfunction or leakiness of the barrier, with published research focusing on its association with risk of intestinal diseases such as celiac disease and inflammatory bowel disease, which are associated with clear morphological changes in the three components of the intestinal barrier. Increased permeability alone may not be the causal or strong contributing factor in the disease (150). It is presently unclear whether normal permeability can become “tighter” and whether doing so would enhance a healthy state.
The focus of nutrition should be on factors that maintain or restore normal gut barrier structure and function. Thus, this section addresses the optimal methods of measurement (focused on humans) and barrier function in the absence of overt mucosal inflammation or ulceration. Therefore, it is relevant to examine barrier function in health but even more in stressed conditions such as food allergy or intolerance, or irritable bowel syndrome (IBS), in which there is evidence of immune activation without significant inflammation or ulceration. This would be analogous to studying diet effects in overweight people [body mass index (BMI) 25–30 kg/m2] rather than the clinically diagnosed disease, obesity (BMI >30 kg/m2).
Intestinal Permeability Measurement
Table 2 compares methods for measuring GI structure and function in the assessment of barrier function (23). In vitro methods, such as the Ussing chamber technique, quantify mucosal to serosal fluxes of probe molecules across mucosal biopsies obtained from different regions of the gut but require multiple mucosal biopsies at different levels of the gut and are, therefore, too invasive for most human research (83). Thus, human intestinal permeability is studied predominantly by measuring the urine excretion of orally consumed probe molecules (17) and by direct measurements during endoscopic procedures.
Table 2.
Techniques for measurement of human intestinal permeability
| Barrier Function |
|||||
|---|---|---|---|---|---|
| Method | Tests Which Layer? | In vivo | In vitro | Neuroimmune Function | TJ Morphology |
| Cell monolayers (e.g., Caco-2/HT29) | Epithelium | − | + | − | − |
| Primary cell monolayers derived from organoids (human and animal model) | Epithelium | − | + | − | − |
| Ussing chambers + human mucosa | Epithelium | − | + | − | − |
| Human fecal or biopsy supernatant applied to animal mucosa | Epithelium | + | − | +/− | +/− |
| Zonula occludens-1 IHC | Epithelium | − | − | − | + |
| mRNA expression of TJ proteins | Epithelium | − | − | − | − |
| Urine excretion of oral probes | All barrier | + | − | − | − |
| Serum bacterial lipopolysaccharide and other biomarkers | All barrier | + | − | − | − |
| Duodenal mucosal impedance | Epithelium | + | − | − | − |
| Confocal endomicroscopy | Epithelium | + | − | + | − |
IHC, immunohistochemistry; TJ, tight junction; +, the method demonstrates the function; −, the method is unable to demonstrate the function; +/−, borderline ability. [Adapted from Camilleri et al. (23).]
Autoradiography studies conducted in rat jejunal tissue using radiolabeled probes of different sizes showed that the upper portion of the villus allows flux of solutes with radius up to ∼6 Å, whereas the lower villus and crypt are permeable to solutes with radii of ∼10 Å and up to 60 Å, respectively (62), being >20 Å in the crypt in some reports (5). On the basis of in vitro studies conducted in cell lines, investigators have identified a pore pathway that is permeable to molecules with radii of ∼4 Å or less [typically ions such as Na+, K+, Cl−, and and possibly urea (NH2-CO-NH2)] and the leak pathway for flux of larger noncharged solutes (210) such as the probe molecules typically used in tests of intestinal permeability. Table 3 summarizes molecular sizes of probe molecules. From a biological perspective, the pore pathway is unlikely to accommodate passage of complex molecules such as bacterial toxins that may set up immune responses.
Table 3.
Summary of molecular mass and diameter of probe molecules either published or estimated
| Molecular Diameter, Å |
|||
|---|---|---|---|
| Probe Molecule | Molecular Mass, Da | Reported | Estimated* |
| Urea | 56 | 2.3 | 4.2 |
| Erythritol | 122 | 3.2 | 6.0 |
| Rhamnose | 164 | 8.2 | 6.9 |
| Mannitol | 182 | 6.7 | 7.2 |
| Lactulose | 342 | 9.5 | 9.7 |
| Cellobiose | 342 | 10.5 | 9.7 |
| Sucralose | 398 | NA | 10.4 |
| PEG 400 | 194–634 | NA | 7.4–12.8 |
| PEG 1,000 | 634–1,338 | NA | 12.8–18.1 |
| Cr-EDTA | 340 | 10.5 | 9.6 |
| Dextran 4 kDa (e.g., FITC) and PEG 4,000 | 4,000 | NA | 30.0 |
| PEG 10,000 | 10,000 | NA | 45.7 |
| Bacterial endotoxins | 10,000–20,000 | NA | 45.7–62.8 |
| Lipopolysaccharides | 50,000–100,000 | NA | 95.7–131.7 |
| Dextran 40 kDa | 40,000 | NA | 86.4 |
| Dextran 70 kDa (e.g., rhodamine) | 70,000 | NA | 111.8 |
Flux of molecules depends on the type of molecules and the type of defects in the intestinal barrier: Ions and water pass through tight junctions, antigens pass through apoptotic leaks, and macromolecules and bacteria pass through erosions, ulcers, or transcytosis (17). 1 Å = 0.1 nm. Cr-EDTA, chromium-labeled EDTA; NA, not available; PEG, polyethylene glycol.
Calculated on the basis of the following formula: radius = 0.33 × (MM0.46), where MM is molecular mass.
Although it is claimed that monosaccharides such as mannitol or rhamnose traverse the epithelium via a paracellular “pore” pathway, and disaccharides such as lactulose via a “leak” pathway (150), examination of the molecular sizes (weight and diameter) suggests that the minor difference in molecular diameter may be associated with both monosaccharides and disaccharides traversing the epithelium via the same paracellular leak pathway. Transcellular passage occurs when there is a specialized, active transport mechanism, as occurs with glucose and fructose among saccharide molecules, but not with the probe saccharides used in testing intestinal permeability. Mucosal impedance and confocal endomicroscopic studies in humans demonstrate focal epithelial cell loss that may permit passage of larger molecules. Thus, Bischoff et al. proposed that flux of molecules depends on the type of molecules and the type of defects: Ions and water pass through tight junctions, antigens through apoptotic leaks, and macromolecules and bacteria through erosions, ulcers, or transcytosis (17).
There are major differences in in vivo measurements compared with in vitro measurements of barrier functions, as shown by the molecular size of probe molecules that can cross the epithelial barrier in humans in vivo, which is at least 10-fold smaller than in vitro. Thus, in healthy adults, ~0.1% of the mass of lactulose (molecular mass <500 Da) was recovered in urine after oral administration and passage through the entire small bowel and colon (172). Similarly, in childhood environmental enteropathy in Peru and Zambia, <1% of administered lactulose was recovered in urine (60). In contrast, molecules with molecular mass of ~4,000 Da [e.g., fluorescein isothiocyanate (FITC)-labeled dextran] easily traversed the 0.16–0.3 cm2 of human small intestinal mucosa in Ussing chamber in vitro (73).
Differences between in vivo and in vitro measurements may reflect additional functional barriers including the lamina propria, innervation (79, 82, 166) by submucosal neurons, and permeability of end-capillaries impeding passage of the probe molecules into the circulation in vivo. In addition, removing tissue from the body may reduce exposure to some factor that is involved in tissue integrity; indeed, most cell junctions in culture do not accurately reproduce the in vivo characteristics.
Methods to Assess Intestinal Permeability
Recommended probe molecules to test intestinal permeability.
In vivo permeability measurements, derived from the analysis of urinary excretion of a single or two probe molecules (typically <400-Da molecular mass) after oral ingestion, are useful for within-subject comparison, as well as for comparisons between different groups. Urea and polyethylene glycol (PEG) are not recommended because of potential false positives as a result of low molecular mass (urea), potential bacterial metabolism (urea), or insufficient sensitivity (PEG; 17). Bacteria-related assays are also used to assess intestinal permeability. These include endotoxin [lipopolysaccharide (LPS)] assay; anti-LPS antibodies or d-lactate (bacterial lactate) in plasma or serum, respectively; butyrate or hemolysin in feces; quantification of bacteria in the inner layer of mucus in the colon on biopsies; and fat content of the liver by MRI or ultrasound. In general, these methods have limited standardization or lack of specificity or are invasive or expensive (17).
saccharides.
A combination of a monosaccharide, such as mannitol or rhamnose, and a disaccharide, such as lactulose or sucralose, has been reported to distinguish mucosal barrier function in inflammatory bowel disease from IBS (172). Nevertheless, the sensitivity is limited by the low levels of disaccharide excretion in urine within 2 h (~0.1%) and within 24 h (~0.5%) of ingestion (172). Lactulose (a disaccharide) and mannitol (a monosaccharide) are easily obtainable, are nontoxic, and are accurately measured using liquid chromatography‐tandem mass spectrometry (24) or electrochemical detection (58), thus allowing low doses to be used (e.g., 0.1 g mannitol and 1 g lactulose) and thereby avoiding a laxative effect or acceleration of transit, which could potentially confound the measurement. Inadvertent dietary consumption of [12C]mannitol in food, which would interfere with the test’s interpretation, has been resolved by either using rhamnose (not generally a food contaminant; 60) or replacing [12C]mannitol with [13C]mannitol (78). Measurements of each saccharide alone or the ratio of two saccharides such as lactulose and mannitol [lactulose-to-mannitol ratio (LMR)] or lactulose and rhamnose [lactulose-to-rhamnose ratio (LRR)] are commonly used. Nevertheless, further validation, including thorough characterization of test performance and reproducibility, is required. The ratio has the potential advantage of correcting for distribution or transit between individuals.
chromium-labeled ethylenediaminetetraacetic acid.
Chromium-labeled ethylenediaminetetraacetic acid (51Cr-EDTA) is a gamma-emitting isotope with a molecular mass of 339 Da and a 27-day half-life (17). Radiation from one dose of 0.12 mSv is at the level of background radiation. 51Cr-EDTA is administered orally in 10 ml of water; timed urine samples are gamma counted and expressed as percentage excreted. Unlike the saccharides, 51Cr-EDTA is not degraded by bacteria and, therefore, can measure both colonic and small intestinal permeability, based on the timing of the urine collection (17). In view of the gamma radiation, it is generally restricted to research studies; the sensitivity of this probe for noninflammatory diseases or effects of diet is not established.
polyethylene glycols.
PEG 400 is a mixture of 11 PEGs with molecular masses ranging from 194 to 634 Da; it has been used to assess altered intestinal permeability in small intestinal diseases such as celiac disease (28), pediatric and adult allergy, acute parasitic intestinal infections, and effects of NSAIDs (57, 95, 179, 199). However, an in-depth analysis of the diverse molecular entities showed that PEG molecules were excluded in both the high- and low-molecular weight range, possibly by a combined effect of the intestinal permeability barrier and an escape to compartments other than the urine (196). Moreover, recovery of PEG 400 in urine in 24 h varied with the relative molecular mass (Mr) of each polymer from 25.9 to 68.5% (139). Larger PEG molecules with molecular weight 4,000 and 10,000 are typically used as nonabsorbable markers in intestinal perfusion studies; however, they have also been used in conditions such as alcoholic liver disease, which is associated with markedly increased permeability (158).
In summary, such probe molecule measurements are useful when timed collections correspond to the predominant location of the probe molecule within the bowel: predominantly the small intestine during the first 2 h, and almost exclusively the colon at 8–24 h, after ingestion (24, 172). These times of exposure apply almost equally in health and in stressed and disease states. However, PEG 400 has been used less frequently in recent years because of the variance in absorption and urinary excretion of the different-molecular weight entities requiring complex mathematical analysis (196).
examples of urine measurements of probe molecules to assess intestinal permeability.
Examples of urine measurements of probe molecules to assess intestinal permeability are discussed below under measurements to characterize normal structure and function of the intestinal barrier in human nutrition research, Application of Intestinal Permeability Measurements in Humans.
Serum biomarkers.
lipopolysaccharide.
Subclinical levels of serum LPS or endotoxin (the immunoadjuvant fraction of the outer cell membrane of gram-negative bacteria that is released on their lysis in the intestinal lumen) provide a marker of intestinal permeability. Impaired fasting blood glucose is associated with increased serum LPS and zonulin levels, which are independent and unrelated markers of increased intestinal permeability; the increased LPS may be biologically relevant as a trigger of in vivo platelet activation (27).
zonulin.
Zonulin, an ~47-kDa protein, regulates intestinal permeability reversibly by modulating intercellular tight junctions (59, 221); serum zonulin has been proposed as a marker of intestinal permeability (24, 182) and is strongly correlated with LMR in humans (122). Higher zonulin levels are also associated with higher waist circumference, diastolic blood pressure, and fasting glucose and increased risk of metabolic diseases (122, 151, 176), suggesting that bacterial endotoxins may play an important role in the development of the metabolic and vascular abnormalities commonly seen in obesity and diabetes-related diseases. In addition, a high-fat diet was associated with increased serum LPS (162).
intestinal fatty acid-binding proteins.
Intestinal fatty acid-binding proteins (I-FABPs) are small, unbound cytosolic proteins found mainly in enterocytes in the upper parts of small intestinal villi (161). They are sensitive markers for intestinal mucosal damage such as celiac disease (45). Serum FABP level has also been reported to increase during mild exertional heat stress or intestinal ischemia and reperfusion, though this was not always accompanied by a significant change in permeability as measured by LRR in urine or plasma, respectively (178, 184). Serum I-FABP was elevated in 5 of 20 patients with type 1 diabetes, though the mechanism and implications of this finding are unclear (84). Dietary factors such as gluten and casein supplementation in children with autism spectrum disorder did not alter urinary I-FABP (170).
examples of diet and stress effects on serum markers of intestinal permeability.
Measurements of serum markers of intestinal permeability appear promising in investigation of effects of diet on barrier function, as serum LPS levels increase after a high-fat meal in both healthy humans and humans with morbid obesity (33, 162). However, the sensitivity of serum LPS to detect effects of aspirin (a GI-damaging NSAID) is lower than 3-h urinary lactulose and LMR (77). On the other hand, there is correlation between serum levels of LPS and FABP and urine saccharide permeability measurement.
Endoscopic mucosal measurements.
These are invasive measurements that can be applied in research, typically in limited regions of the gut. Their application is relevant to small-scale clinical studies assessing stressful conditions or dietary components. Further studies and validation are required for both endoscopic-assisted methods.
mucosal impedance measurement.
Mucosal impedance measurement (112) involves passing a 2-mm-diameter catheter through the endoscope for placement on the mucosa under direct visualization with decompressed lumen and all fluid aspirated. Initial studies were performed in the esophagus and have now been extended to intestine (53, 165). Through the catheter, with two 360° circumferential sensors, 2 mm apart, placed on the mucosa, a voltage transducer produces a 10-mA current at a frequency of 2 kHz. Impedance measurements are acquired in all quadrants of the duodenum. In the one study conducted to date, measurements of duodenal impedance in patients with IBS-constipation were not different compared with healthy controls, and these results confirmed the absence of effects on the basis of tissue studies in vitro (165); in addition, measurements of small intestinal mucosal impedance were not different in patients with eosinophilic esophagitis compared with healthy controls (225). Further studies on the effect of stress or diet are awaited.
confocal laser endomicroscopy.
Confocal laser endomicroscopy with 1-µm resolution enables visualization of cellular and subcellular structures in vivo in real time. After intravenous injection of 5 ml 10% fluorescein (which is actively transported into the lumen over ~30 min) and 40 mg methylscopolamine (anticholinergic to impede contractions), the laser is activated, and measurements are acquired before and after exposure to food. In patients with IBS with suspected food intolerance, exposure to candidate food antigens caused immediate breaks, increased intervillous spaces, and increased intraepithelial lymphocytes in the intestinal mucosa (65). These changes responded to exclusion diets and correlated with clinical improvement. In the terminal ileal epithelium, median epithelial gap densities for control patients and patients with IBS were 6 and 32 gaps per 1,000 cells, respectively (P < 0.001), with numerically higher gap density in female and younger patients (207).
General pitfall with all methods of measurement of intestinal permeability.
A general pitfall applicable to all these methods is a lack of standardization of the method (including probe molecules or serum biomarkers, urine collection, and assay methods), a lack of robust normal data (including consideration of age, sex, BMI, circadian rhythm, and standardization of diet during at least the 24 h of collection of biological samples), and performance characteristics of the test including validity based on responsiveness to perturbations or treatments. In summary, at present, it is unclear what constitutes “normal” values for the diverse measurements, and each article in the literature has to assess the “altered” state (e.g., disease, treatment, or nutrient) with a healthy or placebo control.
Application of Intestinal Permeability Measurements in Humans
Illustrations of functioning mucosal barrier in noninflammatory gut conditions.
Table 4 (8, 15, 37, 41, 51, 53, 65, 74, 113, 128, 130, 137, 145, 157, 165, 172, 180, 190, 194, 205, 207, 214, 219, 237, 238) and Table 5 (16, 73, 123, 165, 167, 217, 218, 232) summarize the application of in vivo and in vitro measurements of intestinal permeability in IBS, which was selected because it is not associated with overt mucosal defects or inflammation and is more likely to reflect the magnitude of changes in permeability that might result from ingested foods or other substances. These data are, therefore, the most representative of what might occur in the general population or under conditions of stress, to provide a basis for proposing diet studies.
Table 4.
Summary of in vivo measurements of intestinal permeability in humans, focusing on studies that include noninflammatory disease
| Reference | Year | Method | Patients with IBS and Controls, n | IP of Patients with IBS, % above normal or LMR | Comments |
|---|---|---|---|---|---|
| Strobel et al. (194) | 1984 | C/M | 15 IBS and 10 controls | Mean ratio: 0.024 (normal 0.037) | Nonbiopsied volunteers as controls |
| Lobley et al. (130) | 1990 | Raffinose/l-arabinose | 62 IBS and 40 controls | Mean ratio: 0.016 (normal 0.015) | No significant difference in IP between IBS and controls |
| Barau and Dupont (8) | 1990 | L/M | 17 IBS and 39 controls (children) | 47 vs. 0% above normal for IBS vs. controls, respectively (normal <0.0245) | Threshold of normal defined by a control group of children without IBS |
| Vogelsang et al. (219) | 1995 | L/M | 40 symptomatic and 30 controls | 30% of symptomatic patients above normal (>0.030) | Patients with “nonspecific” GI symptoms |
| Dainese et al. (37) | 1999 | L/M | 33 IBS and 0 controls | 12% IBS above normal (>0.025) | IP normal in 88% of subjects |
| Berstad et al. (15) | 2000 | 51Cr-EDTA | 18 IBS and 0 controls | Excretion: 0.07% in IBS | Patients with IBS (abdominal pain and/or diarrhea) used as controls in IBD study |
| Spiller et al. (190) | 2000 | L/M | 10 PI-IBS, 21 acute Campylobacter enteritis, and 12 controls | 50% IBS vs. 12 controls; mean LMR: 0.060; range: 0.008–0.22 (normal <0.03) | Increased IP in subset of patients with PI-IBS compared with asymptomatic controls |
| Tibble et al. (205) | 2002 | L/R | 339 IBS and 263 organic disease | Mean ratio: 0.028; range: 0.005–0.216 (normal <0.05) | Permeability of small intestine close to normal in IBS |
| Marshall et al. (137) | 2004 | L/M | 132 IBS and 86 controls | 35.6 vs. 18.6% above normal for IBS vs. controls, respectively (>0.020 LMR) | After outbreak of acute gastroenteritis, SB IP was slightly elevated in IBS (no difference between PI-IBS and non-PI-IBS) |
| Dunlop et al. (51) | 2006 | 51Cr-EDTA | 15 IBS-D + 15 IBS-C with 15 controls and 15 PI-IBS + 15 non-PI-IBS with 12 controls | Excretion: in proximal SB: 0.19% IBS-D, 0.085% IBS-C, 0.07% controls; in SB: 0.43% PI-IBS, 0.84% non-PI-IBS, 0.27% controls | There were 2 studies: 1 comparing IBS-D and IBS-C vs. controls and 1 comparing PI-IBS and non-PI-IBS with IBS-D vs. controls; there may be subtle differences in IP between IBS subgroups |
| Shulman et al. (180) | 2008 | L/M and S/L | 109 Children with IBS or functional abdominal pain and 66 controls | Increased SB and colonic permeability | No correlation between GI permeability and pain-related symptom or stool form |
| Park et al. (157) | 2009 | PEG 3,350-to-PEG 400 ratio by HPLC | 38 IBS (all subtypes) and 12 healthy controls | Increased in whole IBS group | No relationship of increased permeability and positive L breath test |
| Zhou et al. (238) | 2009 | L/M | 54 IBS-D and 22 controls | Increased LMR in 39% of patients | Relationship to increased abdominal pain and visceral and thermal sensitivity |
| Kerckhoffs et al. (113) | 2010 | PEG | 14 IBS (all subtypes) and 15 healthy controls | No difference between IBS and healthy controls | NSAIDs increase permeability more in IBS than in healthy controls |
| Zhou et al. (237) | 2010 | L/M | 19 IBS-D and 10 controls | Increased in 42% of patients | |
| Rao et al. (172) | 2011 | L/M | 12 IBS-D, 12 healthy, and 10 inactive or treated UC or microscopic colitis | Increased urine M excretion at 0–2 and 2–8 h and L excretion at 8–24 h in IBS-D | Demonstrated validity of individual sugar excretion as well as LMR |
| Gecse et al. (74) | 2012 | 51Cr-EDTA | 18 IBS-D, 12 IBS-C, 13 inactive UC, and 10 healthy | Decreased in proximal small intestine of IBS-C; increased in colon of IBS-D | Elevated gut permeability is localized to the colon both in IBS-D and in inactive UC |
| Vazquez-Roque et al. (214) | 2013 | L/M | 45 IBS-D: trial of ±gluten diets | GCD increased SB permeability (based on M and LMR); no increase in colon permeability | GCD significantly decreased expression of ZO-1, claudin-1, and occludin in rectosigmoid mucosa; all effects of gluten were greater in patients positive for HLA DQ2/8 |
| Del Valle-Pinero et al. (41) | 2013 | 4 probes: S, sucrose, M, and L | 20 IBS and 39 matched healthy controls | Colonic permeability significantly lower in IBS compared with healthy controls, shown by lower S excretion in IBS compared with controls | IBS subgroups not specified |
| Turcotte et al. (207) | 2013 | Confocal laser endomicroscopy | 16 IBS and 18 healthy controls | Median epithelial gap densities for controls and IBS were 6 and 32 gaps per 1,000 epithelial cells, respectively | Median difference in gap density between IBS and controls was 26 (95% CI: 12–39) gaps per 1,000 cells; small effects of age and sex |
| Fritscher-Ravens et al. (65) | 2014 | Confocal laser endomicroscopy | 36 IBS with suspected food intolerance | No overall differences, but positive results in 22 of 36 patients: increased number of IELs, formation of epithelial leaks/gaps, and intervillous spaces widened | Diluted food antigens administered directly to the duodenal mucosa; however, no correlation with conventional histology |
| Mujagic et al. (145) | 2014 | Sucrose excretion and LRR in 0–5-h urine; 0–24- and 5–24-h S-to-erythritol ratio | 34 IBS-D, 21 IBS-C, 30 IBS-M, 6 IBS-U, and 94 healthy controls | The 0–5-h LRR only different in IBS-D vs. healthy controls; no other differences in gastroduodenal or colonic permeability | Analysis adjusted for age, sex, BMI, anxiety or depression, smoking, alcohol intake, and use of medication |
| Peters et al. (165) | 2017 | L/13C-M, mucosal impedance, and serum LPS | 19 IBS-C and 18 healthy volunteers | Normal SB and colonic permeability in IBS-C | Concordant results (normal) using duodenal mucosal impedance, ex vivo barrier measurements, and colonic mucosal expression of occludin, ZO-1, 2, and 3, and claudin genes |
| Edogawa et al. (53) | 2018 | L/13C-M | 9 healthy volunteers | Increased L SB permeability by indomethacin, recovered to baseline 4–6 wk later | Only women demonstrated decreased fecal microbial diversity, including an increase in Prevotella abundance, after indomethacin |
| Linsalata et al. (128) | 2018 | urinary sucrose, L, and M over 5 h and circulating biomarkers | 39 IBS-D and 20 healthy volunteers | There were 2 distinct IBS-D subtypes identified, 1 with increased L, sucrose excretion, and I-FABP and DAO levels, suggesting increased permeability of small intestine | Inflammatory parameters and markers of bacterial translocation (IL-6 and LPS) were significantly higher in IBS-D with increased permeability of small intestine |
Here, n = no. of subjects. BMI, body mass index; C, cellobiose; CI, confidence interval; 13C-M, [13C]mannitol; 51Cr-EDTA, chromium-labeled EDTA; DAO, diamine oxidase; GCD, gluten-containing diet; GI, gastrointestinal; HLA DQ2/8, human leukocyte antigen DQ2 or DQ8; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IBS-C, IBS with constipation; IBS-D, IBS with diarrhea; IBS-M, IBS with mixed bowel habits; IBS-U, unsubtyped IBS; IELs, intraepithelial lymphocytes; I-FABP, intestinal fatty acid-binding protein; IP, intestinal permeability; L, lactulose; LMR, lactulose-to-mannitol ratio; LRR, lactulose-to-rhamnose ratio; M, mannitol; PEG, polyethylene glycol; PI-IBS, postinfectious IBS; R, rhamnose; S, sucralose; SB, small bowel; UC, ulcerative colitis; ZO-1, zonula occludens-1.
Table 5.
In vitro effects of soluble factors on barrier function and tissue expression in studies including noninflammatory disease
| Reference | Year | Method | IBS Group, n | Permeability | Comments |
|---|---|---|---|---|---|
| Gecse et al. (73) | 2008 | FSN applied to murine colonic strips mounted in Ussing chambers; FITC-dextran transfer | 52 All IBS subtypes and 25 controls | Increased with IBS-D supernatants, no difference with IBS-C | FSN also rapidly increased phosphorylation of myosin light chain and delayed redistribution of ZO-1 in colonocytes |
| Piche et al. (167) | 2009 | Colonic biopsies mounted in Ussing chambers; fluorescein-5-(and-6)-sulfonic acid as probe and ZO-1 and occludin expression | 51 IBS, all subtypes, and 14 controls | Increased FITC paracellular permeability in all IBS subtypes; reduced ZO-1 expression | No difference in occludin expression; increase in FITC-dextran in Caco-2 cell monolayer, which correlated with abdominal pain score |
| Lee et al. (123) | 2010 | Colonic biopsies in Ussing chambers; horseradish peroxidase as probe | 20 IBS-D and 30 controls | Increased in IBS-D compared with controls | Increased permeability decreased with the mast cell tryptase inhibitor nafamostat |
| Bertiaux-Vandaële et al. (16) | 2011 | Colonic mucosal biopsies and ZO-1, occludin, and claudin-1 expression | 50 IBS (-C, -D, -A, or -U) and 31 controls | Occludin and claudin-1 expression decreased in IBS-D but not in IBS-C/A | Occludin (r = 0.40) and claudin-1 (r = 0.46) expression significantly correlated with duration of symptoms |
| Vivinus-Nébot et al. (217) | 2012 | Colonic biopsies mounted in Ussing chambers; fluorescein-5-(and-6)-sulfonic acid | 34 IBS, all subtypes, and 15 controls | Increased in all IBS subtypes | Also higher number of mast cells, and spontaneous release of tryptase; worse in IBS with allergic factors |
| Vivinus-Nébot et al. (218) | 2014 | Cecal biopsies: Ussing chambers, FITC-sulfonic acid as probe, and mRNA expression of TJ proteins (ZO-1, α-catenin, and occludin) | 49 inactive IBD (IBS), 51 IBS, and 27 controls | Increased permeability and lower expression of ZO-1 and α-catenin in both inactive IBD and IBS | Persistent increase in TNF-α in colonic mucosa may contribute to the epithelial barrier defects in quiescent (inactive) IBD but not in IBS |
| Peters et al. (165) | 2017 | TMR and FITC-dextran flux (4 kDa) | 19 IBS-C and 18 controls | No differences | Results consistent with in vivo permeability measurements |
| Wu et al. (232) | 2017 | H&E and semiquantitative immunohistochemistry for phosphorylated MLC, MLC kinase, and claudins-2, -8, and -15 | 27 IBS-D ±gluten diet | Increased MLC phosphorylation and colonocyte expression of the paracellular Na+ channel claudin-15 by GCD | Small intestine MLC phosphorylation increased by GCD correlated with increased intestinal permeability |
Here, n = no. of subjects. FITC, fluorescein isothiocyanate; FSN, fecal supernatant; GCD, gluten-containing diet; H&E, hematoxylin-eosin; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IBS-A, IBS with alternating constipation and diarrhea; IBS-C, IBS with constipation; IBS-D, IBS with diarrhea; IBS-U, unsubtyped IBS; MLC, myosin II regulatory light chain; TJ, tight junction; TMR, transmucosal resistance; ZO-1, zonula occludens-1.
Although there is mention of leaky gut in various conditions, from allergies to chronic fatigue syndromes, it is important to note that the evidence is often based on serum IgA and IgM responses directed against commensal bacteria, suggesting bacterial translocation across the intestinal barrier. On the other hand, permeability probes of lower molecular weight than bacteria show perturbations in barrier function such as those due to NSAIDs (20), and their effect is reversed with lubiprostone (36, 111). These intriguing results require replication. Thus, it is conceivable that under stressful or dietary stimuli, there may be alterations in mucosal permeability in the healthy state in addition to the presence of circulating immunoglobulins against commensal bacteria that might reflect the effects of a pathological state.
Application of oral probes to measure barrier changes among healthy subjects exposed to different diets.
In healthy subjects, there are two relevant scenarios. First, normal gut barrier function can be exposed to diets or dietary components to determine whether permeability is increased. For example, when fasting, normal subjects were given 300-cal drinks of either glucose, saturated fat such as cream, orange juice, or only water; only cream caused an increase in LPS concentration (suggesting increased permeability) and Toll-like receptor 4 expression indicative of cellular inflammation (44). Coadministration of fiber reversed the effects of the high-fat, high-calorie meal (76).
Dysfunction of gut mucosal barrier due to stressors and effects of nondrug interventions.
The normal gut barrier function can be stressed so that diets or dietary components can be assessed for their role in restoring permeability to baseline normal for that individual. Examples of such stressors resulting in mucosal perturbation are exposure to NSAIDs (18, 144, 181) or endurance exercise with ischemia-induced changes in permeability. Although the effects of NSAIDs on permeability are reversible pharmacologically with lubiprostone (36, 111) and demonstrate that prostaglandins may restore normal barrier function, the examples in Table 6 (18, 103, 109, 119, 131, 144, 168, 181, 213) illustrate the efficacy of substances in the diet to reverse these effects.
Table 6.
Examples of stressors altering permeability in health and reversal by dietary intervention
| Effects on Barrier Function |
|||||||
|---|---|---|---|---|---|---|---|
| Barrier Stressor and Clinical Scenario | Specific Study | Intestinal permeability | TJ | Mucosal damage | Other effects | Dietary Intervention | Reference(s) |
| Endurance exercise; marathon runners with fecal occult blood or bloody diarrhea | Biking challenge | Urine iohexol (MM 821 Da) ↑ | ND | Serum I-FABP ↑, zonulin ↓ | ND | Noninterventional study | (109) |
| Running challenge | LRR ↑ and correlated with core temperature (e.g., >39°C) | ND | ND | ND | ND | (168) | |
| Biking challenge | LRR not different with citrulline Rx | ND | Serum I-FABP ↑ reversed with citrulline | Gastric hypoperfusion reversed with citrulline | Citrulline vs. alanine | (213) | |
| Biking challenge | ND | ND | Serum I-FABP ↑ reversed with sucrose | Gastric hypoperfusion not different with sucrose | Sucrose vs. nitrate | (103) | |
| NSAID enteropathy; NSAIDs cause small bowel ulcers and inflammation | Diverse NSAIDs including indomethacin | 51Cr-EDTA, saccharides | ND | ND | ND | Noninterventional studies | (18, 144, 181) |
| Indomethacin | LRR reduced with zinc carnosine | ND | ND | HT29 cell proliferation ↑ with zinc carnosine vs. ZnSO4 | Zinc carnosine vs. placebo | (131) | |
| Aspirin | Bifidobacterium lactis BB-12 and GOS reduce colonic permeability: urine SLR and sucralose | ND | ND | Bifidobacterium strains (B. lactis BB-12 and B. adolescentis IVS-1) and GOS prebiotic | (119) | ||
Cr-EDTA, chromium-labeled EDTA; GOS, galactooligosaccharide; I-FABP, intestinal fatty acid-binding protein; LRR, lactulose-to-rhamnose ratio; MM, molecular mass; ND, not determined; Rx, prescription; SLR, sucralose-to-lactulose ratio; TJ, tight junction expression; ↑, increase; ↓, decrease.
Illustration of dietary activation of local protective immune or barrier mechanisms in disease states.
Although not the main focus of this article, it is intriguing that indigo naturalis, through the aryl hydrocarbon receptor (AhR), was able to stimulate the protective IL-22 and resulted in mucosal healing in a randomized, controlled trial in patients with ulcerative colitis (146). Other examples pertaining to dietary interventions in severe burns, nutritional depletion, and Crohn’s disease are shown in Table 7 (11, 90, 142, 163, 239).
Table 7.
Examples from the literature of in vivo human studies showing alterations in intestinal permeability as a result of gut-directed therapy
| Reference | Year | Therapy Studied | Comments |
|---|---|---|---|
| Hulsewé et al. (90) | 2004 | Glutamine intravenous | Patients with nutritional depletion and increased IP did not improve after glutamine-enriched parenteral nutrition |
| Zhou et al. (239) | 2003 | Glutamine enteral | Patients with 50–80% burns: urinary LMR in enteral glutamine group was lower than standard enteral formula |
| Peng et al. (163) | 2004 | Glutamine enteral | Patients with 30–75% burns: plasma DAO activity and urinary LMR in enteral glutamine group were lower than in untreated burn group |
| Benjamin et al. (11) | 2012 | Glutamine | In patients with Crohn’s disease, glutamine and active control (whey) both reduced LMR |
| Meng et al. (142) | 2011 | Rhubarb | Patients day 3 postburns: plasma DAO activity in rhubarb-treated group was lower than in controls |
DAO, diamine oxidase; IP, intestinal permeability; LMR, lactulose-to-mannitol ratio.
Conclusions on Testing of Intestinal Permeability
Normal values vary for each probe molecule, and further validations of normal values are required. There is lower intraindividual coefficient of variation compared with interindividual variation. Hence, intraindividual comparisons of diets or other perturbations appear to be preferable in humans when using urinary recoveries after oral administration. However, with careful test performance, individual saccharide excretion and ratio of disaccharide to monosaccharide excretion provide valid information on intestinal permeability in noninflammatory conditions and, therefore, could potentially serve as useful probes to study effects of diet on either increasing permeability or restoring to normal after a stressor.
POTENTIAL OF DIETARY AND NONDIETARY FACTORS TO IMPACT COMPONENTS OF THE INTESTINAL MUCOSAL BARRIER
Maintenance of a healthy gut barrier requires the functional integration and homeostasis of the gut microbiota, mucus production, intestinal epithelial cells, and the mucosal immune system. Each of these components is continually responding to cues from the other components, as well as to signals derived from the intestinal lumen. Food-derived compounds and their metabolites have broad immunomodulatory and physiological effects and can act to modulate cellular and barrier function by direct interactions with immune and epithelial cells and indirectly by altering gut microbial composition and/or function (39, 231). The gut microbiota, including archaea, eukaryotes, bacteria, and viruses, can reach densities exceeding 1011 cells per gram in the colon (118). An altered composition of the gut microbiota has been documented in diverse human diseases, and a causal role of the gut microbiota in metabolic and inflammatory diseases has been demonstrated repeatedly in animal models (186, 188). Many dietary compounds serve as chemical precursors that feed into a complex network of still incompletely understood microbial and host metabolic pathways (50). The small-molecule metabolites that result from these pathways can be absorbed into the bloodstream and circulate throughout the body and may interact with tissues and be cometabolized into additional chemical forms or eventually excreted (148).
Dietary Compounds and Metabolites
Dietary metabolites can interact directly with host cells via surface transmembrane G protein-coupled receptors (GPCRs) or through transcription factors such as the AhR. Metabolites can also have transcriptional and epigenetic effects via acetylation and deacetylation of histone proteins. Dietary metabolites can thus elicit immediate responses through altering cellular signaling and also have long-term effects through epigenetic modifications (200). GPCRs interact with food-derived metabolites such as short-, medium-, and long-chain fatty acids, as well as products of other metabolic pathways such as those involved in tryptophan (Trp) metabolism. GPCRs have a major role in promoting anti-inflammatory responses, inducing immune tolerance, and controlling metabolic function. The AhR is a cytosolic ligand-activated transcription factor expressed by intestinal epithelial cells and most immune cells (193). AhR can be activated and/or inhibited by numerous dietary molecules including flavonoids and carotenoids. AhR activity is critical for T helper type 22 (Th22) and Th17 cell activation and IL-22 production (234). IL-22 has a critical role in barrier defense through enhancing epithelial regeneration, increasing mucus production, stimulating wound healing, reinforcing epithelial tight junctions, and enhancing production of AMPs (56). A lack of IL-22 has been linked to host pathologies including infections and metabolic disorders (222, 227). Some evidence from randomized, controlled trials and epidemiological studies suggests that consumption of flavonoid- and carotenoid-rich foods may be beneficial for maintaining metabolic and cardiovascular health (223), but whether this is due to enhanced signaling through AhR or through effects on gut barrier function is not known (72).
Dietary fiber and barrier function.
It is notable that the only present evidence for a dietary intervention that may help to fortify intestinal barrier function in healthy humans, from a conceptual standpoint, is the consumption of dietary fiber. Microbiota-accessible carbohydrates (MACs) found within dietary fiber (187) are complex carbohydrates that are indigestible by host enzymes but can be fermented by gut microbiota primarily in the colon. The association between consumption of dietary MACs and maintenance of a functional intestinal barrier (132) can be, at least partly, attributed to the production of short-chain fatty acids (SCFAs) in the gut lumen. SCFAs support intestinal epithelial cell proliferation (93, 115, 175, 197) and protect intestinal barrier integrity (31, 67, 191) via a combination of mechanisms. They constitute the primary energy source for the colonocytes, induce mucus secretion, alter epigenetic processes by inhibiting histone deacetylase, and activate free fatty acid receptors 2 and 3 and GPCRs on epithelial cells (25, 42, 116).
The importance of mucus integrity to host health is evidenced by spontaneous colitis that develops in mice when components of the intestinal mucus have been genetically compromised (13, 66, 228). Many bacterial members of the gut microbiota have the capacity to degrade and consume portions of the mucus layer, but in a healthy gut, microbes are excluded from the tight, inner layer of mucus closest to the epithelium (101). Highly controlled experiments in animal models have revealed that mucus consumption by gut microbes increases when dietary fiber becomes scarce in the diet, and mucus glycans appear to serve an important role as a backup food source for many fiber-degrading microbes (138, 189, 198). As microbial consumption of host-secreted mucus glycans increases in fiber-scarce dietary conditions, the mucus layers become thinner, as determined through quantitative measurements of the mucus layer in mouse models (46, 52). Furthermore, markers of mucosal and systemic inflammation increase concomitantly with the low fiber-induced mucosal thinning (46, 52). These diet-driven modifications of the mucus layer and induction of inflammation are consistent with findings in mouse colitis models and in humans with inflammatory bowel diseases; microbial encroachment of the inner mucus layer is linked to intestinal inflammatory states (100). Similarly, a high-fat diet (60% of energy) induced microbiota encroachment and low-grade inflammation in mice that was ameliorated with the supplementation of the dietary MAC inulin (20% wt/wt; 241). Together, these findings reveal that aspects of the Western diet and, specifically, low dietary fiber content, alter the relationship between the human gut microbiota and the gut mucus layer and may predispose those ingesting a fiber-poor diet to increased intestinal inflammation.
Dietary fiber leading to the production of SCFAs can also help regulate the intestinal mucosal immune barrier. SCFAs modulate the size and function of the T cell network in the gut through G protein-coupled receptor 43 (GPR43)-dependent mechanisms and through histone deacetylase inhibition (4, 68, 104, 105, 183). Disruption of the homeostasis of regulatory T cells (which regulate Th cells) results in the loss of immune tolerance and development of aberrant effector responses, in particular Th17 cells (10). Th17 cells have been implicated in the development of inflammatory bowel disease (71).
Administration of MAC to healthy animals (143) or animals with a compromised gut barrier (91, 92) consistently showed improved colonic barrier function as evidenced by increased expression of colonic tight junction proteins. Other interventions with SCFAs have also yielded positive results on intestinal permeability (61, 89). However, in healthy subjects, only one study reported improved small intestinal permeability after consumption of inulin-enriched pasta (11.0 g/day of fructans) for 8 wk compared with control pasta (1.4 g/day of fructans; 174). In contrast, intestinal permeability was not affected by synbiotic or prebiotic supplementation in healthy men (202, 229, 230) or men with well-controlled type 2 diabetes (160). The dose of oligosaccharide (fructooligosaccharide or galactooligosaccharide) in these studies varied between 100 mg/day and 20 g/day.
Amino acids, proteins, and barrier function.
One paradigm by which a dietary nutrient plays a role in the regulation of intestinal barrier immune function is through Trp, an essential amino acid that must be supplied exogenously in the diet. Trp can be directly transformed into indoles and derivatives or enter the kynurenine (Kyn) pathway in immune and epithelial cells via indoleamine 2,3-dioxygenase 1 (IDO1). Trp can also pass through the serotonin (5-hydroxytryptamine pathway) via Trp hydroxylase (49, 70, 96, 121). The integrity of the barrier in a mouse model can be directly affected by metabolites produced by gut microbes such as indole-3-propionic acid (IPA), a microbial metabolite of the amino acid Trp that binds to the pregnane X receptor and influences immunity and barrier integrity (49, 216). Alterations in Trp metabolism have been linked with several human diseases (2, 72). Genetic susceptibility to inflammatory bowel disease due to deletion of the caspase recruitment domain family member 9 (Card9) gene is associated with an altered microbiota that has an impaired capacity to metabolize Trp to AhR ligands (121).
Kyn is an AhR agonist generated from Trp by IDO1 and tryptophan 2,3-dioxygenase (TDO). An increased activity of IDO1 and increased serum Kyn levels are seen in individuals with metabolic syndrome, and correlations between the Kyn-to-Trp ratio and obesity, metabolic syndrome, BMI, and blood triglycerides have been described (133). Patients with type 2 diabetes have been shown to have increased gut permeability associated with metabolic endotoxemia (86). Bariatric surgery improves metabolic disease and also reduces gut permeability in conjunction with altering gut microbiota and increasing levels of IPA (96, 206). Experimental studies have shown that bacterial-derived IPA reduces intestinal permeability, suggesting a role for Trp metabolites in the control of metabolism and gut permeability (96).
Circulating concentrations of 4-ethylphenylsulfate (EPS), a microbial-host cometabolite of dietary tyrosine, were 46-fold elevated in a mouse model of autism, where the animals had reduced gut barrier function. Oral treatment with Bacteroides fragilis (1010 colony-forming units/48 h) to improve barrier function completely normalized EPS concentrations and improved autism-like behavior (88).
There is some epidemiological evidence showing an increased risk of developing inflammatory bowel disease in subjects with a high protein intake, specifically with meat protein [reviewed by Kakodkar and Mutlu (107)]. A mechanism that may help explain this association lies in the interactions between high protein levels reaching the colonic microbiota and the fermentation by these microbes, which releases toxic compounds such as ammonia, phenols, branched-chain amino acids, and hydrogen sulfide (127, 233). An elegant study examining effects of altering diet composition on intestinal permeability and colitis development in mice clearly demonstrated that increasing protein in the diet increased gut permeability and also the severity of colitis in a microbiota-dependent and microbiota-independent manner (129).
Sugar consumption and barrier function.
Western diets high in fat and sugars have been associated with impaired intestinal barrier function in animal models (81, 220). Intestinal glucose absorption occurs primarily through sodium/glucose cotransporter 1 (SGLT1) but can also occur through tight junctions at very high intraluminal glucose concentrations and a sufficient osmotic gradient to promote volume flow (7). However, given the very rapid absorption of monosaccharides in the proximal 70 cm of the small intestine (21, 99), it is unclear whether intraluminal concentrations of glucose are sufficient to alter intestinal permeability. However, Thaiss et al. recently demonstrated in an elegant set of in vitro and mouse experiments that hyperglycemia, even in the absence of obesity, could increase gut permeability via alterations in tight junction and adherens junction integrity (203). Another study in middle-aged human subjects showed that those individuals with dysglycemia had encroachment of bacteria into the normally sterile inner colonic mucus layer, representing a loss of barrier function (30).
In a mouse model, high levels of dietary fructose in excess of glucose have also been shown to increase gut permeability and allow for enhanced translocation of LPS due to effects on tight junctions, reduced mucus thickness, and a reduced expression of antimicrobial proteins (220). A link between fructose consumption and the development of metabolic syndrome and/or nonalcoholic fatty liver disease in humans has been demonstrated in several human studies [154, 164, 191; reviewed by Jensen et al. (97)]. It has been suggested that consumption of high levels of dietary fructose increases systemic endotoxin levels by increasing intestinal permeability through both direct and indirect methods (98, 204). These include the effects of fructose metabolism within gut enterocytes and hepatocytes. In hepatocytes, phosphorylation of fructose can result in transient intracellular phosphate and ATP depletion (9, 35). This, in turn, causes a transient reduction in protein synthesis, mitochondrial dysfunction, an increase in uric acid production, and resultant enhanced systemic oxidative stress that can then increase gut permeability [reviewed by Zhang et al. (236)]. A similar mechanism has been proposed to directly occur in gut epithelial cells. Fructose is absorbed through glucose transporter 5 (GLUT5) and GLUT2-mediated facilitative diffusion. Given that dietary fructose is often associated with glucose in humans, the active fructose uptake through GLUT2 reduces the luminal concentration of fructose; therefore, the potential effects of dietary fructose may be different from those observed in experimental animals fed fructose alone. Therefore, the potential effects of dietary fructose may be different from those observed in animal and human experimental models fed fructose relative to glucose at levels not typically consumed in the human diet. Studies in mice have demonstrated that increased dietary fructose results in a decrease in occludin and zonula occludens-1 (ZO-1) gene expression in small intestinal epithelium, suggesting increased permeability (102). Indeed, these changes were not seen in fructokinase A/C knockout mice, indicating that the changes in epithelial tight junctions were dependent on fructose metabolism through fructokinase.
High-fat diets and bile acids.
The fat content of the diet has a significant influence on bile acid secretion. In addition to their role in dietary lipid absorption and cholesterol homeostasis, bile acids act as signaling molecules via two major signaling pathways: G protein-coupled bile acid receptor (GPBAR1, or TGR5) and members of the nuclear hormone receptor superfamily including the farnesoid X receptor (FXR; 63). Animal studies indicate that both TGR5 and FXR contribute to the integrity of the intestinal barrier (32, 48, 69, 94). Alternatively, some bile acids exert direct, receptor-independent toxicity toward intestinal epithelial cells (34), based on the detergent properties of bile acids (149), induction of apoptosis (3), and induction of changes in tight junction proteins (171). Finally, some unconjugated bile acids may provoke colonic mucus secretion, probably through a direct action on mucus-secreting cells.
High-fat feeding and bile acids also induce intestinal inflammation and alter the gut microbiota composition (40, 159). Levels of Akkermansia muciniphila were 100-fold lower in mice fed a high-fat diet compared with mice on a control diet (55), and gut barrier dysfunction in these animals was reduced by administration of A. muciniphila for 4 wk, probably by stimulating the growth of the intestinal mucus layer. Other studies have linked high-fat diets to increases in sulfate-reducing bacteria and barrier dysfunction through the inhibition of butyrate oxidation in colonocytes (6, 26, 43, 173, 235). In animal models, it has been shown that fat from milk increases the secretion of taurocholic acid in bile, which serves as a substrate for Bilophila wadsworthia, another hydrogen sulfide-producing species disrupting barrier function (47).
Emulsifiers and surfactants.
Emulsifiers are detergent-like molecules that are used extensively in processed foods. Studies in mice have shown that two commonly used emulsifiers, carboxymethylcellulose and polysorbate-80, induced metabolic syndrome and low-grade inflammation in conjunction with gut barrier dysfunction (29). Other surfactants that are used in the food industry such as monoglycerides, lecithins, glycolipids, fatty alcohols, fatty acids, and organic solvents used in food and beverage preparation (117) disrupt the intestinal barrier in animal models (80), as well as opening tight junctions in cell culture (124).
Although these observations suggest putative roles for these agents, it is unclear whether the mass ingested by humans is sufficient to be biologically or clinically relevant, particularly as they are diluted by the ~2 liters of aqueous solutions entering the GI tract with every meal and because of sequestration of fats in micelles and their ultimate absorption, preventing direct contact with the epithelium to exert their surface-active properties.
Alcohol.
Both acute and long-term intake of alcohol consistently increased small intestinal epithelial barrier permeability (19, 192, 224), but no data are available on the effects of ethanol on the human colonic barrier. Although the exact mechanism of increased permeability remains largely unknown, direct damage to epithelial cells, changes in the expression of the tight junction-associated and adherens junction proteins, and changes in the intestinal microbiota have been shown to be involved (54). Gut permeability to PEG 1,500, 4,000, and 10,000 is increased in people with alcohol use disorder, allowing large macromolecules through the intestinal barrier, and plasma LPS (endotoxin) levels increased in parallel with increases in gut permeability (158). There is also evidence of a marked increase in lactulose absorption as well as in urinary LMR in people with alcoholism and chronic liver disease compared with people with alcoholism and no liver disease and nonalcoholics with liver disease (114).
Nutrients in combination.
One example of effects of nutrients in combination is illustrated by a randomized, double-blind, placebo-controlled trial in 120 children, aged 2 mo to 9 yr, from an urban shanty community, that showed that glutamine alone or glutamine plus vitamin A and zinc (both gut-trophic nutrients) reduced intestinal permeability (%lactulose excretion and LMR) compared with placebo (zinc plus vitamin A vehicle; 126). Beneficial effects of other gut-trophic nutrients (such as arginine, dietary fiber, glutamine, glutathione, SCFAs, vitamin A, and zinc) on intestinal growth, adaptation, and barrier function have also been described. These are reviewed elsewhere (240).
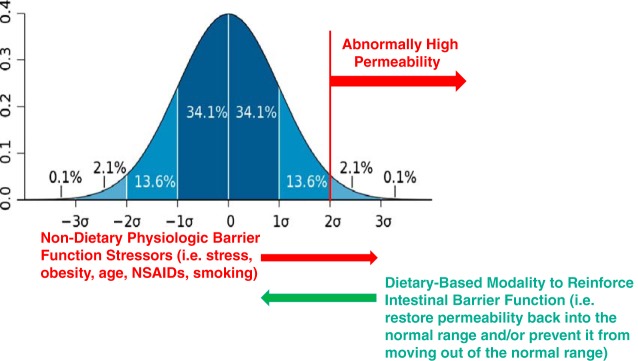
Diet-Independent Factors Affecting Intestinal Permeability
Diet is an important, but not the only, factor that affects the intestinal barrier. Other lifestyle-associated factors, including stress, obesity (BMI), aging, smoking habits, and use of medication, should at least be reported in human diet-gut barrier studies and eventually be considered as confounding factors in statistical analysis. Unfortunately, most studies generally do not control for dietary intake. Therefore, it cannot be excluded that differences in barrier function between target groups and controls are due to differences in dietary intake rather than the lifestyle factors under consideration. Additionally, it is conceivable that studies focused on healthy humans, where diet-independent factors that have an influence on the intestinal epithelial barrier (described below), could be used to test dietary-based strategies. Such studies could fortify intestinal barrier function as a method to maintain or restore health and reduce risk of disease.
Stress.
Limited data are available on the impact of psychological stress on barrier function in humans. Acute stress induced by a public speech test in healthy subjects increased intestinal permeability versus control conditions in an LMR test (0.059 ± 0.040 vs. 0.030 ± 0.022, mean ± SE; P < 0.01) in a subgroup of subjects that exhibited increased salivary cortisol. Exogenous corticotropin-releasing hormone mimicked these effects, whereas prior administration of a mast cell stabilizer abolished them (211). Strenuous exercise is believed to induce a combination of physical and psychological stress. In addition to the effect mentioned above, exercise leads to redistribution of blood away from the splanchnic area, resulting in intestinal hypoperfusion and rapid reperfusion leading to epithelial cell damage and loss of epithelial integrity (212). Increased intestinal permeability has been confirmed in athletes (136, 152, 156) and in combat soldiers (110, 125). Whether stress-induced permeability can be prevented or abolished by dietary strategies and whether such intervention would result in relevant end points such as a reduction in stress-associated symptoms remain to be tested.
Obesity.
The association between obesity and compromised gut barrier in humans remains an open question. Teixeira et al. observed a slightly, nonsignificantly higher permeability in subjects with obesity (BMI: 35.04 ± 3.98 kg/m2, mean ± SE; 201), whereas others found no difference between subjects with overweight/obesity and lean controls (22, 120). Perhaps, intestinal permeability is disturbed only in subjects with morbid obesity. Indeed, subjects with obesity and a BMI ≥40 kg/m2 had an elevated intestinal permeability in an LMR test (0.080; 95% confidence interval: 0.073, 0.093) that returned to normal ranges (0.027; 95% confidence interval: 0.024, 0.034) after weight loss despite the subjects still being obese (BMI: 35 kg/m2 on average; 38). An alternative explanation for the discrepant results is a lack of sensitivity of the currently used permeability tests. In a recent study, LMRs were not different in a large cohort (n = 122) of subjects with severe obesity and nonobese subjects, although ex vivo investigations pointed to subtly compromised barrier function in the subjects with obesity (75).
Age.
Impaired barrier function is believed to be a feature of normal aging and to contribute to the increased systemic inflammation observed in elderly people (64). Ex vivo analyses in terminal ileal biopsy tissues from healthy aged (66–77 yr), young (7–12 yr), and adult (20–40 yr) subjects suggested increased small intestinal permeability to solutes in the aged subjects (based on a 2-fold reduction of the transepithelial electric resistance across ileal biopsies in Ussing chambers) with unaffected permeability to macromolecules (134). Nevertheless, urinary excretion of lactulose and mannitol was only slightly lower with increasing age, and the LMR was not different between old and young subjects (141).
Smoking.
The effect of cigarette smoking on intestinal barrier function has mainly been investigated in the field of inflammatory bowel diseases (14). Although an initial study suggested that smoking tightens the gut (169), these results were not confirmed in later studies using 51Cr-EDTA (12, 169, 195) or PEG (169, 185) as probes.
Drugs: NSAIDs and proton pump inhibitors.
NSAIDs are well known to increase intestinal permeability (20). Proton pump inhibitors (PPIs) often are prescribed as a prophylactic treatment against NSAID-induced GI toxicities (177). However, capsule endoscopy revealed exacerbation of NSAID-induced small bowel injury by a PPI (rabeprazole) in 57 healthy subjects (226). In contrast, use of a PPI was not associated with incidence of bloodstream infections in the intensive care unit (data from 24,774 patients), suggesting that PPIs do not meaningfully alter permeability (34).
HOW CAN WE STRENGTHEN THE PRESENT EVIDENCE THAT DIET IS AN INFLUENCING FACTOR IN THE FUNCTION OF THE INTESTINAL BARRIER IN HUMANS?
The integrated intestinal gut barrier system modulates intestinal exposures so that nutrients, electrolytes, and water are absorbed while simultaneously serving as a first line of defense to protect the host from components found in the environment of the intestinal lumen including both secreted and ingested molecules, as well as gut microbiota and their metabolites. A normal-functioning gut barrier is characterized by a robust physical mucus barrier, intestinal epithelium supporting transcellular and paracellular nutrient transport, and multiple immune factors active in the mucus, in the epithelium, and below the epithelium. Normal function results in nutrient absorption within normal ranges of bioavailability with a balanced immune response such that endogenous and external toxins do not cross the gut barrier, while modulating the immune response to prevent unrestrained inflammation, which results in systemic and bowel inflammatory conditions. Asthma, food allergies, and inflammatory bowel diseases in humans have been associated with the consumption of a Western-style diet high in processed foods and low in fiber (87, 106, 135), suggesting that diet-induced alterations in intestinal barrier function may be playing a role. It remains unknown how much of this dysregulation is due to a definable mechanism that can be linked to barrier function and maintenance of health or subsequent autoimmune and inflammatory chronic diseases in humans.
To identify a pathway forward to strengthen the evidence that diet can be used as a modality to strengthen human intestinal barrier function to reduce the risk of disease, it is fundamentally important to consider the numerous gaps in knowledge that represent hurdles and challenges to this paradigm. On the basis of the evidence described in the previous sections of this article, we provide a summary based on three categories in Table 8.
Table 8.
Overall concepts and challenges to the paradigm of gut barrier dysfunction
| Concept | Challenges to Establishing Role of Gut Barrier Dysfunction |
|---|---|
| Establishing gut barrier as essential to normal gastrointestinal structure and function in human health |
|
| Recommending feasible methods to measure normal barrier in human research |
|
| Linking diet to normal gut barrier function among healthy or at-risk people |
|
IBD, inflammatory bowel disease.
Since there are technological limitations in quantifying intestinal barrier function in humans, there is a lack of evidence that reduction of human intestinal barrier function contributes to disease pathogenesis, and there is a lack of evidence that the fortification of barrier function can be used to treat human disease, it is currently tenuous to suggest that diet can be used to fortify human intestinal barrier function with the intent of reducing disease risk.
Given these challenges and gaps in knowledge, what is the approach by which scientific investigation can provide evidence that present notions of how animal model data showing how intestinal barrier function can be reinforced by diet (described in potential of dietary and nondietary factors to impact components of the intestinal mucosal barrier and summarized in Fig. 2) may be relevant to human biology using currently available technologies to quantify human intestinal barrier function (described in measurements to characterize normal structure and function of the intestinal barrier in human nutrition research and summarized in Fig. 2)?
Fig. 2.
Components of the intestinal barrier. A: currently available methods to quantify human intestinal barrier function. *Invasive testing. B: dietary factors impacting intestinal barrier function. Green text indicates reduced barrier function with demonstration of relevance in humans. AhR, aryl hydrocarbon receptor; Cr-EDTA, chromium-labeled EDTA; EPS, 4-ethylphenylsulfate; ETOH, ethanol; FABP, fatty acid-binding protein; FXR, farnesoid X receptor; IDO, indoleamine 2,3-dioxygenase 1; IPA, indole-3-propionic acid; PXR, pregnane X receptor; SCFA, short-chain fatty acid.
We suggest that the first step is to perform studies to define the boundaries of normal human intestinal barrier function. A reference range defining normal as 95% of values for a test of intestinal permeability using saccharide-based methods (described in measurements to characterize normal structure and function of the intestinal barrier in human nutrition research) in a defined population free from disease would be one approach, if the values fall along a Gaussian distribution. Individuals whose values lay either above or below 2 standard deviations from the mean would be defined as having “abnormal” values (Fig. 3).
Fig. 3.
Defining normal boundaries of intestinal permeability as a functional biomarker of barrier function in humans. Studies could be performed in healthy individuals to determine whether the intestinal barrier can be reinforced by diet.
However, whether or not this is indicative of a physiologically relevant process (i.e., disease) would need to be established by additional studies designed to determine the sensitivity, specificity, and precision of the test in humans, both with and without disease, known to have an effect on intestinal barrier function as defined by permeability testing. Although individuals with intestinal permeability values that are abnormally high might have an increased risk for the presence or risk of a disease state, the physiological interpretation of an abnormally low permeability measure would be unknown. Assuming that the test was properly performed, perhaps these individuals would have a lower risk for certain disease states, or maybe these individuals should be considered to be normal.
Once the reference range for intestinal permeability has been determined, studies could be designed to assess whether or not specific dietary interventions could prevent, reverse, or improve abnormally high intestinal permeability associated with conditions such as psychological or physical stress, exposure to NSAIDs, age, obesity, and smoking (see potential of dietary and nondietary factors to impact components of the intestinal mucosal barrier and Fig. 3). A positive outcome in such a study might provide evidence that diet can be used to reinforce human intestinal barrier function with the notion that it might reduce the risk for certain diseases. In this fashion, a specific intestinal permeability test could be considered to be a surrogate biomarker of health or disease that is sensitive to diet. Of course, much larger longitudinal prospective cohort studies would need to be performed to validate this notion.
There are many analogies to the approach described above and standard surrogate biomarkers for disease states such as hypertension and blood lipid profiles for the risk of cardiovascular disease and fasting blood glucose and HbA1c levels for type 2 diabetes mellitus. In the latter scenario, abnormally elevated blood glucose and HbA1c levels are associated with either the risk or diagnosis of type 2 diabetes mellitus. Treatment of the prediabetic state or frank diabetes reduces the levels of these two surrogate biomarkers, which is indicative of successful treatment, thereby reducing the risk for morbidity and mortality.
Future research should consider approaches that determine whether dietary interventions help to maintain or restore normal function in healthy populations when exposed to a challenge that affects gut barrier permeability.
GRANTS
This work was supported by National Institutes of Health Grant R01-DK-115950 to M. Camilleri and by the North American branch of the International Life Sciences Institute (ILSI North America). ILSI North America is a public, nonprofit foundation that provides a forum to advance understanding of scientific issues related to the nutritional quality and safety of the food supply by sponsoring research programs, educational seminars and workshops, and publications. It receives support primarily from its industry membership.
DISCLAIMERS
The opinions expressed herein are those of the authors and do not necessarily represent the views of the funding organization and the authors’ employers.
DISCLOSURES
B. J. Lyle provides technical advice and scientific writing consultancy to a range of clients in the private for profit as well as non-profit food and research sector. She is a paid nutrition advisor to the ILSI NA committee that sponsored this project. M. Camilleri: relevant disclosures related to IBS-diarrhea: NIH funding R01-DK115950 and research grants from Novartis (research studies on CLN452) and Allergan (research studies on elobixibat). G. D. Wu: Relevant disclosures related to the gut microbiome and diet include research funding from Seres Therapeutics, Intercept Pharmaceuticals, and Takeda; scientific advisory boards include Danone and Biocodex; consultant agreement with Hitachi. J. Sonnenburg: Cofounder of Novome Biotechnologies, Inc., January, Inc.; scientific advisory board of Clorox/Renew Life, Kaleido Biotechnologies, Second Genome, Gnubiotics Sciences; research funding from Second Genome, Clorox/Renew Life, Abbott Laboratories. Neither of the other authors has any conflicts of interest, financial or otherwise, to report.
AUTHOR CONTRIBUTIONS
M.C., B.J.L., K.L.M., J.S., K.V., and G.D.W. conceived and designed research; K.L.M. interpreted results of experiments; M.C., B.J.L., K.L.M., J.S., K.V., and G.D.W. prepared figures; M.C., B.J.L., K.L.M., J.S., K.V., and G.D.W. drafted manuscript; M.C., B.J.L., K.L.M., J.S., K.V., and G.D.W. edited and revised manuscript; M.C., B.J.L., K.L.M., J.S., K.V., and G.D.W. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cindy Stanislav for excellent secretarial assistance.
REFERENCES
- 1.Addison JM, Burston D, Matthews DM. Evidence for active transport of the dipeptide glycylsarcosine by hamster jejunum in vitro. Clin Sci 43: 907–911, 1972. doi: 10.1042/cs0430907. [DOI] [PubMed] [Google Scholar]
- 2.Agus A, Planchais J, Sokol H. Gut microbiota regulation of trytptophan metabolism in health and disease. Cell Host Microbe 23: 716–724, 2018. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Araki Y, Fujiyama Y, Andoh A, Nakamura F, Shimada M, Takaya H, Bamba T. Hydrophilic and hydrophobic bile acids exhibit different cytotoxicities through cytolysis, interleukin-8 synthesis and apoptosis in the intestinal epithelial cell lines. IEC-6 and Caco-2 cells. Scand J Gastroenterol 36: 533–539, 2001. doi: 10.1080/003655201750153430. [DOI] [PubMed] [Google Scholar]
- 4.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 55: 1512–1520, 2006. doi: 10.1136/gut.2005.085373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babidge W, Millard S, Roediger W. Sulfides impair short chain fatty acid beta-oxidation at acyl-CoA dehydrogenase level in colonocytes: implications for ulcerative colitis. Mol Cell Biochem 181: 117–124, 1998. doi: 10.1023/A:1006838231432. [DOI] [PubMed] [Google Scholar]
- 7.Ballard ST, Hunter JH, Taylor AE. Regulation of tight-junction permeability during nutrient absorption across the intestinal epithelium. Annu Rev Nutr 15: 35–55, 1995. doi: 10.1146/annurev.nu.15.070195.000343. [DOI] [PubMed] [Google Scholar]
- 8.Barau E, Dupont C. Modifications of intestinal permeability during food provocation procedures in pediatric irritable bowel syndrome. J Pediatr Gastroenterol Nutr 11: 72–77, 1990. doi: 10.1097/00005176-199007000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Bawden SJ, Stephenson MC, Ciampi E, Hunter K, Marciani L, Macdonald IA, Aithal GP, Morris PG, Gowland PA. Investigating the effects of an oral fructose challenge on hepatic ATP reserves in healthy volunteers: a 31P MRS study. Clin Nutr 35: 645–649, 2016. doi: 10.1016/j.clnu.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Belkaid Y, Harrison OJ. Homeostatic immunity and the microbiota. Immunity 46: 562–576, 2017. doi: 10.1016/j.immuni.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benjamin J, Makharia G, Ahuja V, Anand Rajan KD, Kalaivani M, Gupta SD, Joshi YK. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig Dis Sci 57: 1000–1012, 2012. doi: 10.1007/s10620-011-1947-9. [DOI] [PubMed] [Google Scholar]
- 12.Benoni C, Prytz H. Effects of smoking on the urine excretion of oral 51Cr EDTA in ulcerative colitis. Gut 42: 656–658, 1998. doi: 10.1136/gut.42.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergstrom K, Fu J, Johansson ME, Liu X, Gao N, Wu Q, Song J, McDaniel JM, McGee S, Chen W, Braun J, Hansson GC, Xia L. Core 1- and 3-derived O-glycans collectively maintain the colonic mucus barrier and protect against spontaneous colitis in mice. Mucosal Immunol 10: 91–103, 2017. doi: 10.1038/mi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz L, Schultz BM, Salazar GA, Pardo-Roa C, Sebastián VP, Álvarez-Lobos MM, Bueno SM. Impact of cigarette smoking on the gastrointestinal tract inflammation: opposing effects in Crohn’s disease and ulcerative colitis. Front Immunol 9: 74, 2018. doi: 10.3389/fimmu.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berstad A, Arslan G, Folvik G. Relationship between intestinal permeability and calprotectin concentration in gut lavage fluid. Scand J Gastroenterol 35: 64–69, 2000. doi: 10.1080/003655200750024551. [DOI] [PubMed] [Google Scholar]
- 16.Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, Leroi AM, Déchelotte P, Ménard JF, Ducrotté P, Coëffier M. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol 106: 2165–2173, 2011. doi: 10.1038/ajg.2011.257. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff SC, Barbara G, Buurman W, Ockhuizen T, Schulzke JD, Serino M, Tilg H, Watson A, Wells JM. Intestinal permeability: a new target for disease prevention and therapy. BMC Gastroenterol 14: 189, 2014. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of damage to the gastrointestinal tract from nonsteroidal anti-inflammatory drugs. Gastroenterology 154: 500–514, 2018. doi: 10.1053/j.gastro.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Bjarnason I, Ward K, Peters TJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 323: 179–182, 1984. doi: 10.1016/S0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 20.Bjarnason I, Williams P, Smethurst P, Peters TJ, Levi AJ. Effect of non-steroidal anti-inflammatory drugs and prostaglandins on the permeability of the human small intestine. Gut 27: 1292–1297, 1986. doi: 10.1136/gut.27.11.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgström B, Dahlqvist A, Lundh G, Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest 36: 1521–1536, 1957. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brignardello J, Morales P, Diaz E, Romero J, Brunser O, Gotteland M. Pilot study: alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment Pharmacol Ther 32: 1307–1314, 2010. doi: 10.1111/j.1365-2036.2010.04475.x. [DOI] [PubMed] [Google Scholar]
- 23.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 303: G775–G785, 2012. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri M, Nadeau A, Lamsam J, Nord SL, Ryks M, Burton D, Sweetser S, Zinsmeister AR, Singh R. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 22: e15–e26, 2010. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11: 577–591, 2015. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 26.Carbonero F, Benefiel AC, Alizadeh-Ghamsari AH, Gaskins HR. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front Physiol 3: 448, 2012. doi: 10.3389/fphys.2012.00448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carnevale R, Pastori D, Nocella C, Cammisotto V, Baratta F, Del Ben M, Angelico F, Sciarretta S, Bartimoccia S, Novo M, Targher G, Violi F. Low-grade endotoxemia, gut permeability and platelet activation in patients with impaired fasting glucose. Nutr Metab Cardiovasc Dis 27: 890–895, 2017. doi: 10.1016/j.numecd.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Chadwick VS, Phillips SF, Hofmann AF. Measurements of intestinal permeability using low molecular weight polyethylene glycols (PEG 400). II. Application to normal and abnormal permeability states in man and animals. Gastroenterology 73: 247–251, 1977. doi: 10.1016/S0016-5085(19)32197-3. [DOI] [PubMed] [Google Scholar]
- 29.Chassaing B, Koren O, Goodrich JK, Poole AC, Srinivasan S, Ley RE, Gewirtz AT. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519: 92–96, 2015. [Erratum in Nature 536: 238, 2016.] doi: 10.1038/nature14232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chassaing B, Raja SM, Lewis JD, Srinivasan S, Gewirtz AT. Colonic microbiota encroachment correlates with dysglycemia in humans. Cell Mol Gastroenterol Hepatol 4: 205–221, 2017. doi: 10.1016/j.jcmgh.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T, Kim CY, Kaur A, Lamothe L, Shaikh M, Keshavarzian A, Hamaker BR. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct 8: 1166–1173, 2017. doi: 10.1039/C6FO01532H. [DOI] [PubMed] [Google Scholar]
- 32.Cipriani S, Mencarelli A, Chini MG, Distrutti E, Renga B, Bifulco G, Baldelli F, Donini A, Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One 6: e25637, 2011. [Erratum in PLoS One 8: 10.1371/annotation/55febddb-0209-4a48-9c14-23df882126a2, 2013.] doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clemente-Postigo M, Queipo-Ortuño MI, Murri M, Boto-Ordoñez M, Perez-Martinez P, Andres-Lacueva C, Cardona F, Tinahones FJ. Endotoxin increase after fat overload is related to postprandial hypertriglyceridemia in morbidly obese patients. J Lipid Res 53: 973–978, 2012. doi: 10.1194/jlr.P020909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen ME, Hathway JM, Salmasian H, Liu J, Terry M, Abrams JA, Freedberg DE. Prophylaxis for stress ulcers with proton pump inhibitors is not associated with increased risk of bloodstream infections in the intensive care unit. Clin Gastroenterol Hepatol 15: 1030–1036.e1, 2017. doi: 10.1016/j.cgh.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. JAMA 282: 1659–1664, 1999. doi: 10.1001/jama.282.17.1659. [DOI] [PubMed] [Google Scholar]
- 36.Cuppoletti J, Blikslager AT, Chakrabarti J, Nighot PK, Malinowska DH. Contrasting effects of linaclotide and lubiprostone on restitution of epithelial cell barrier properties and cellular homeostasis after exposure to cell stressors. BMC Pharmacol 12: 3, 2012. doi: 10.1186/1471-2210-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dainese R, Galliani EA, De Lazzari F, Di Leo V, Naccarato R. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol 94: 1892–1897, 1999. doi: 10.1111/j.1572-0241.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 38.Damms-Machado A, Louis S, Schnitzer A, Volynets V, Rings A, Basrai M, Bischoff SC. Gut permeability is related to body weight, fatty liver disease, and insulin resistance in obese individuals undergoing weight reduction. Am J Clin Nutr 105: 127–135, 2017. doi: 10.3945/ajcn.116.131110. [DOI] [PubMed] [Google Scholar]
- 39.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010. doi: 10.1152/ajpgi.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Valle-Pinero AY, Van Deventer HE, Fourie NH, Martino AC, Patel NS, Remaley AT, Henderson WA. Gastrointestinal permeability in patients with irritable bowel syndrome assessed using a four probe permeability solution. Clin Chim Acta 418: 97–101, 2013. doi: 10.1016/j.cca.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340, 2013. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Den Hond E, Hiele M, Evenepoel P, Peeters M, Ghoos Y, Rutgeerts P. In vivo butyrate metabolism and colonic permeability in extensive ulcerative colitis. Gastroenterology 115: 584–590, 1998. doi: 10.1016/S0016-5085(98)70137-4. [DOI] [PubMed] [Google Scholar]
- 44.Deopurkar R, Ghanim H, Friedman J, Abuaysheh S, Sia CL, Mohanty P, Viswanathan P, Chaudhuri A, Dandona P. Differential effects of cream, glucose, and orange juice on inflammation, endotoxin, and the expression of Toll-like receptor-4 and suppressor of cytokine signaling-3. Diabetes Care 33: 991–997, 2010. doi: 10.2337/dc09-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derikx JP, Vreugdenhil AC, Van den Neucker AM, Grootjans J, van Bijnen AA, Damoiseaux JG, van Heurn LW, Heineman E, Buurman WA. A pilot study on the noninvasive evaluation of intestinal damage in celiac disease using I-FABP and L-FABP. J Clin Gastroenterol 43: 727–733, 2009. doi: 10.1097/MCG.0b013e31819194b0. [DOI] [PubMed] [Google Scholar]
- 46.Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter M, Pudlo NA, Kitamoto S, Terrapon N, Muller A, Young VB, Henrissat B, Wilmes P, Stappenbeck TS, Núñez G, Martens EC. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 167: 1339–1353.e21, 2016. doi: 10.1016/j.cell.2016.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487: 104–108, 2012. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding L, Yang L, Wang Z, Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B 5: 135–144, 2015. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dodd D, Spitzer MH, Van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, Sonnenburg JL. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature 551: 648–652, 2017. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donia MS, Fischbach MA. Small molecules from the human microbiota. Science 349: 1254766, 2015. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunlop SP, Hebden J, Campbell E, Naesdal J, Olbe L, Perkins AC, Spiller RC. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 101: 1288–1294, 2006. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 52.Earle KA, Billings G, Sigal M, Lichtman JS, Hansson GC, Elias JE, Amieva MR, Huang KC, Sonnenburg JL. Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18: 478–488, 2015. doi: 10.1016/j.chom.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edogawa S, Peters SA, Jenkins GD, Gurunathan SV, Sundt WJ, Johnson S, Lennon RJ, Dyer RB, Camilleri M, Kashyap PC, Farrugia G, Chen J, Singh RJ, Grover M. Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. FASEB J 32: 6615–6625, 2018. doi: 10.1096/fj.201800560R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elamin EE, Masclee AA, Dekker J, Jonkers DM. Ethanol metabolism and its effects on the intestinal epithelial barrier. Nutr Rev 71: 483–499, 2013. doi: 10.1111/nure.12027. [DOI] [PubMed] [Google Scholar]
- 55.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol 47: 607–614, 2017. doi: 10.1002/eji.201646723. [DOI] [PubMed] [Google Scholar]
- 57.Fälth-Magnusson K, Kjellman NI, Magnusson KE, Sundqvist T. Intestinal permeability in healthy and allergic children before and after sodium-cromoglycate treatment assessed with different-sized polyethyleneglycols (PEG 400 and PEG 1000). Clin Allergy 14: 277–286, 1984. doi: 10.1111/j.1365-2222.1984.tb02207.x. [DOI] [PubMed] [Google Scholar]
- 58.Farhadi A, Keshavarzian A, Fields JZ, Sheikh M, Banan A. Resolution of common dietary sugars from probe sugars for test of intestinal permeability using capillary column gas chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 836: 63–68, 2006. doi: 10.1016/j.jchromb.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 59.Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci 915: 214–222, 2000. doi: 10.1111/j.1749-6632.2000.tb05244.x. [DOI] [PubMed] [Google Scholar]
- 60.Faubion WA, Camilleri M, Murray JA, Kelly P, Amadi B, Kosek MN, Enders F, Larson J, Grover M, Boe G, Dyer R, Singh R. Improving the detection of environmental enteric dysfunction: a lactulose, rhamnose assay of intestinal permeability in children aged under 5 years exposed to poor sanitation and hygiene. BMJ Glob Health 1: e000066, 2016. [Erratum in BMJ Global Health 2: e000066corr1, 2017.] doi: 10.1136/bmjgh-2016-000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferreira TM, Leonel AJ, Melo MA, Santos RR, Cara DC, Cardoso VN, Correia MI, Alvarez-Leite JI. Oral supplementation of butyrate reduces mucositis and intestinal permeability associated with 5-Fluorouracil administration. Lipids 47: 669–678, 2012. doi: 10.1007/s11745-012-3680-3. [DOI] [PubMed] [Google Scholar]
- 62.Fihn BM, Sjöqvist A, Jodal M. Permeability of the rat small intestinal epithelium along the villus-crypt axis: effects of glucose transport. Gastroenterology 119: 1029–1036, 2000. doi: 10.1053/gast.2000.18148. [DOI] [PubMed] [Google Scholar]
- 63.Fiorucci S, Mencarelli A, Palladino G, Cipriani S. Bile-acid-activated receptors: targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends Pharmacol Sci 30: 570–580, 2009. doi: 10.1016/j.tips.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69, Suppl 1: S4–S9, 2014. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- 65.Fritscher-Ravens A, Schuppan D, Ellrichmann M, Schoch S, Röcken C, Brasch J, Bethge J, Böttner M, Klose J, Milla PJ. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology 147: 1012–20.e4, 2014. doi: 10.1053/j.gastro.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 66.Fu J, Wei B, Wen T, Johansson ME, Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, McDaniel JM, Sferra TJ, Turner JR, Chen H, Hansson GC, Braun J, Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J Clin Invest 121: 1657–1666, 2011. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469: 543–547, 2011. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 68.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013. [Erratum in Nature 506: 254, 2014.] doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 69.Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta 1801: 683–692, 2010. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Galligan JJ. Beneficial actions of microbiota-derived tryptophan metabolites. Neurogastroenterol Motil 30: e13283, 2018. doi: 10.1111/nmo.13283. [DOI] [PubMed] [Google Scholar]
- 71.Gálvez J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm 2014: 928461, 2014. doi: 10.1155/2014/928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by tryptophan metabolism. Front Cell Infect Microbiol 8: 13, 2018. doi: 10.3389/fcimb.2018.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gecse K, Róka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztóczy A, Izbéki F, Fioramonti J, Wittmann T, Bueno L. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut 57: 591–599, 2008. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- 74.Gecse K, Róka R, Séra T, Rosztóczy A, Annaházi A, Izbéki F, Nagy F, Molnár T, Szepes Z, Pávics L, Bueno L, Wittmann T. Leaky gut in patients with diarrhea-predominant irritable bowel syndrome and inactive ulcerative colitis. Digestion 85: 40–46, 2012. doi: 10.1159/000333083. [DOI] [PubMed] [Google Scholar]
- 75.Genser L, Aguanno D, Soula HA, Dong L, Trystram L, Assmann K, Salem JE, Vaillant JC, Oppert JM, Laugerette F, Michalski MC, Wind P, Rousset M, Brot-Laroche E, Leturque A, Clément K, Thenet S, Poitou C. Increased jejunal permeability in human obesity is revealed by a lipid challenge and is linked to inflammation and type 2 diabetes. J Pathol 246: 217–230, 2018. doi: 10.1002/path.5134. [DOI] [PubMed] [Google Scholar]
- 76.Ghanim H, Batra M, Abuaysheh S, Green K, Makdissi A, Kuhadiya ND, Chaudhuri A, Dandona P. Antiinflammatory and ROS suppressive effects of the addition of fiber to a high-fat high-calorie meal. J Clin Endocrinol Metab 102: 858–869, 2017. doi: 10.1210/jc.2016-2669. [DOI] [PubMed] [Google Scholar]
- 77.Gnauck A, Lentle RG, Kruger MC. Aspirin-induced increase in intestinal paracellular permeability does not affect the levels of LPS in venous blood of healthy women. Innate Immun 21: 537–545, 2015. doi: 10.1177/1753425914557101. [DOI] [PubMed] [Google Scholar]
- 78.Grover M, Camilleri M, Hines J, Burton D, Ryks M, Wadhwa A, Sundt W, Dyer R, Singh RJ. 13C mannitol as a novel biomarker for measurement of intestinal permeability. Neurogastroenterol Motil 28: 1114–1119, 2016. doi: 10.1111/nmo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hällgren A, Flemström G, Nylander O. Interaction between neurokinin A, VIP, prostanoids, and enteric nerves in regulation of duodenal function. Am J Physiol Gastrointest Liver Physiol 275: G95–G103, 1998. doi: 10.1152/ajpgi.1998.275.1.G95. [DOI] [PubMed] [Google Scholar]
- 80.Hamid KA, Katsumi H, Sakane T, Yamamoto A. The effects of common solubilizing agents on the intestinal membrane barrier functions and membrane toxicity in rats. Int J Pharm 379: 100–108, 2009. doi: 10.1016/j.ijpharm.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 81.Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet-fed rats are dynamic and region dependent. Am J Physiol Gastrointest Liver Physiol 308: G840–G851, 2015. doi: 10.1152/ajpgi.00029.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayden UL, Carey HV. Neural control of intestinal ion transport and paracellular permeability is altered by nutritional status. Am J Physiol Regul Integr Comp Physiol 278: R1589–R1594, 2000. doi: 10.1152/ajpregu.2000.278.6.R1589. [DOI] [PubMed] [Google Scholar]
- 83.Herrmann JR, Turner JR. Beyond Ussing’s chambers: contemporary thoughts on integration of transepithelial transport. Am J Physiol Cell Physiol 310: C423–C431, 2016. doi: 10.1152/ajpcell.00348.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoffmanová I, Sánchez D, Hábová V, Anděl M, Tučková L, Tlaskalová-Hogenová H. Serological markers of enterocyte damage and apoptosis in patients with celiac disease, autoimmune diabetes mellitus and diabetes mellitus type 2. Physiol Res 64: 537–546, 2015. [DOI] [PubMed] [Google Scholar]
- 85.Holdsworth CD, Dawson AM. The absorption of monosaccharides in man. Clin Sci 27: 371–379, 1964. [PubMed] [Google Scholar]
- 86.Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51Cr)-EDTA in human type 2 diabetes. Diabet Med 31: 559–563, 2014. doi: 10.1111/dme.12360. [DOI] [PubMed] [Google Scholar]
- 87.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 106: 563–573, 2011. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 88.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155: 1451–1463, 2013. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, Song P, Fan P, Hou C, Thacker P, Ma X. Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr 145: 2774–2780, 2015. doi: 10.3945/jn.115.217406. [DOI] [PubMed] [Google Scholar]
- 90.Hulsewé KW, van Acker BA, Hameeteman W, van der Hulst RR, Vainas T, Arends JW, van Kreel BK, von Meyenfeldt MF, Soeters PB. Does glutamine-enriched parenteral nutrition really affect intestinal morphology and gut permeability? Clin Nutr 23: 1217–1225, 2004. doi: 10.1016/j.clnu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 91.Hung TV, Suzuki T. Dietary fermentable fiber reduces intestinal barrier defects and inflammation in colitic mice. J Nutr 146: 1970–1979, 2016. doi: 10.3945/jn.116.232538. [DOI] [PubMed] [Google Scholar]
- 92.Hung TV, Suzuki T. Dietary fermentable fibers attenuate chronic kidney disease in mice by protecting the intestinal barrier. J Nutr 148: 552–561, 2018. doi: 10.1093/jn/nxy008. [DOI] [PubMed] [Google Scholar]
- 93.Ichikawa H, Sakata T. Stimulation of epithelial cell proliferation of isolated distal colon of rats by continuous colonic infusion of ammonia or short-chain fatty acids is nonadditive. J Nutr 128: 843–847, 1998. doi: 10.1093/jn/128.5.843. [DOI] [PubMed] [Google Scholar]
- 94.Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, Mangelsdorf DJ, Kliewer SA. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci USA 103: 3920–3925, 2006. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jackson PG, Baker RW, Lessof MH, Ferrett J, MacDonald DM. Intestinal permeability in patients with eczema and food allergy. Lancet 317: 1285–1286, 1981. doi: 10.1016/S0140-6736(81)92459-4. [DOI] [PubMed] [Google Scholar]
- 96.Jennis M, Cavanaugh CR, Leo GC, Mabus JR, Lenhard J, Hornby PJ. Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol Motil 30: e13178, 2018. doi: 10.1111/nmo.13178. [DOI] [PubMed] [Google Scholar]
- 97.Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, Nakagawa T, Kuwabara M, Sato Y, Kang DH, Tolan DR, Sanchez-Lozada LG, Rosen HR, Lanaspa MA, Diehl AM, Johnson RJ. Fructose and sugar: a major mediator of non-alcoholic fatty liver disease. J Hepatol 68: 1063–1075, 2018. doi: 10.1016/j.jhep.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin R, Willment A, Patel SS, Sun X, Song M, Mannery YO, Kosters A, McClain CJ, Vos MB. Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. Int J Hepatol 2014: 560620, 2014. doi: 10.1155/2014/560620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Johansson C. Studies of gastrointestinal interactions. VII. Characteristics of the absorption pattern of sugar, fat and protein from composite meals in man. A quantitative study. Scand J Gastroenterol 10: 33–42, 1975. [PubMed] [Google Scholar]
- 100.Johansson ME, Gustafsson JK, Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63: 281–291, 2014. doi: 10.1136/gutjnl-2012-303207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johansson ME, Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA 108, Suppl 1: 4659–4665, 2011. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnson RJ, Rivard C, Lanaspa MA, Otabachian-Smith S, Ishimoto T, Cicerchi C, Cheeke PR, MacIntosh B, Hess T. Fructokinase, fructans, intestinal permeability, and metabolic syndrome: an equine connection? J Equine Vet Sci 33: 120–126, 2013. doi: 10.1016/j.jevs.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jonvik KL, Lenaerts K, Smeets JS, Kolkman JJ, Van Loon LJ, Verdijk LB. Sucrose but not nitrate ingestion reduces strenuous cycling-induced intestinal injury. Med Sci Sports Exerc 51: 436–444, 2019. doi: 10.1249/MSS.0000000000001800. [DOI] [PubMed] [Google Scholar]
- 104.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 30: 531–564, 2012. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399, 2012. doi: 10.1038/nature10772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Julia V, Macia L, Dombrowicz D. The impact of diet on asthma and allergic diseases. Nat Rev Immunol 15: 308–322, 2015. doi: 10.1038/nri3830. [DOI] [PubMed] [Google Scholar]
- 107.Kakodkar S, Mutlu EA. Diet as a therapeutic option for adult inflammatory bowel disease. Gastroenterol Clin North Am 46: 745–767, 2017. doi: 10.1016/j.gtc.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology 146: 1477–1488, 2014. doi: 10.1053/j.gastro.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Karhu E, Forsgård RA, Alanko L, Alfthan H, Pussinen P, Hämäläinen E, Korpela R. Exercise and gastrointestinal symptoms: running-induced changes in intestinal permeability and markers of gastrointestinal function in asymptomatic and symptomatic runners. Eur J Appl Physiol 117: 2519–2526, 2017. doi: 10.1007/s00421-017-3739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y, Hoke AV, Levangie MW, Kumar R, Chakraborty N, Gautam A, Hammamieh R, Martini S, Montain SJ, Pasiakos SM. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol 312: G559–G571, 2017. doi: 10.1152/ajpgi.00066.2017. [DOI] [PubMed] [Google Scholar]
- 111.Kato T, Honda Y, Kurita Y, Iwasaki A, Sato T, Kessoku T, Uchiyama S, Ogawa Y, Ohkubo H, Higurashi T, Yamanaka T, Usuda H, Wada K, Nakajima A. Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: a prospective randomized pilot study in healthy volunteers. PLoS One 12: e0175626, 2017. doi: 10.1371/journal.pone.0175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Katzka DA, Ravi K, Geno DM, Smyrk TC, Iyer PG, Alexander JA, Mabary JE, Camilleri M, Vaezi MF. Endoscopic mucosal impedance measurements correlate with eosinophilia and dilation of intercellular spaces in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol 13: 1242–1248.e1, 2015. doi: 10.1016/j.cgh.2014.12.032. [DOI] [PubMed] [Google Scholar]
- 113.Kerckhoffs AP, Akkermans LM, de Smet MB, Besselink MG, Hietbrink F, Bartelink IH, Busschers WB, Samsom M, Renooij W. Intestinal permeability in irritable bowel syndrome patients: effects of NSAIDs. Dig Dis Sci 55: 716–723, 2010. doi: 10.1007/s10620-009-0765-9. [DOI] [PubMed] [Google Scholar]
- 114.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol 94: 200–207, 1999. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 115.Kim CH. Immune regulation by microbiome metabolites. Immunology 154: 220–229, 2018. doi: 10.1111/imm.12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165: 1332–1345, 2016. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 117.Kralova I, Sjöblom J. Surfactants used in food industry: a review. J Dispers Sci Technol 30: 1363–1383, 2009. doi: 10.1080/01932690902735561. [DOI] [Google Scholar]
- 118.Kriss M, Hazleton KZ, Nusbacher NM, Martin CG, Lozupone CA. Low diversity gut microbiota dysbiosis: drivers, functional implications and recovery. Curr Opin Microbiol 44: 34–40, 2018. doi: 10.1016/j.mib.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krumbeck JA, Rasmussen HE, Hutkins RW, Clarke J, Shawron K, Keshavarzian A, Walter J. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome 6: 121, 2018. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuzma JN, Cromer G, Hagman DK, Breymeyer KL, Roth CL, Foster-Schubert KE, Holte SE, Weigle DS, Kratz M. No differential effect of beverages sweetened with fructose, high-fructose corn syrup, or glucose on systemic or adipose tissue inflammation in normal-weight to obese adults: a randomized controlled trial. Am J Clin Nutr 104: 306–314, 2016. doi: 10.3945/ajcn.115.129650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, Brot L, Taleb S, Couturier-Maillard A, Nion-Larmurier I, Merabtene F, Seksik P, Bourrier A, Cosnes J, Ryffel B, Beaugerie L, Launay JM, Langella P, Xavier RJ, Sokol H. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med 22: 598–605, 2016. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lassenius MI, Pietiläinen KH, Kaartinen K, Pussinen PJ, Syrjänen J, Forsblom C, Pörsti I, Rissanen A, Kaprio J, Mustonen J, Groop PH, Lehto M; FinnDiane Study Group . Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34: 1809–1815, 2011. doi: 10.2337/dc10-2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee JW, Park JH, Park DI, Park JH, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI. Subjects with diarrhea-predominant IBS have increased rectal permeability responsive to tryptase. Dig Dis Sci 55: 2922–2928, 2010. doi: 10.1007/s10620-009-1094-8. [DOI] [PubMed] [Google Scholar]
- 124.Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev 14: 479–489, 2015. doi: 10.1016/j.autrev.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 125.Li X, Kan EM, Lu J, Cao Y, Wong RK, Keshavarzian A, Wilder-Smith CH. Combat-training increases intestinal permeability, immune activation and gastrointestinal symptoms in soldiers. Aliment Pharmacol Ther 37: 799–809, 2013. doi: 10.1111/apt.12269. [DOI] [PubMed] [Google Scholar]
- 126.Lima AA, Anstead GM, Zhang Q, Figueiredo ÍL, Soares AM, Mota RM, Lima NL, Guerrant RL, Oriá RB. Effects of glutamine alone or in combination with zinc and vitamin A on growth, intestinal barrier function, stress and satiety-related hormones in Brazilian shantytown children. Clinics (São Paulo) 69: 225–233, 2014. doi: 10.6061/clinics/2014(04)02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Linden DR. Hydrogen sulfide signaling in the gastrointestinal tract. Antioxid Redox Signal 20: 818–830, 2014. doi: 10.1089/ars.2013.5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Linsalata M, Riezzo G, D’Attoma B, Clemente C, Orlando A, Russo F. Noninvasive biomarkers of gut barrier function identify two subtypes of patients suffering from diarrhoea predominant-IBS: a case-control study. BMC Gastroenterol 18: 167, 2018. doi: 10.1186/s12876-018-0888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Llewellyn SR, Britton GJ, Contijoch EJ, Vennaro OH, Mortha A, Colombel JF, Grinspan A, Clemente JC, Merad M, Faith JJ. Interactions between diet and the intestinal microbiota alter intestinal permeability and colitis severity in mice. Gastroenterology 154: 1037–1046.e2, 2018. doi: 10.1053/j.gastro.2017.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lobley RW, Burrows PC, Warwick R, Dawson DJ, Holmes R. Simultaneous assessment of intestinal permeability and lactose tolerance with orally administered raffinose, lactose and l-arabinose. Clin Sci (Lond) 79: 175–183, 1990. doi: 10.1042/cs0790175. [DOI] [PubMed] [Google Scholar]
- 131.Mahmood A, FitzGerald AJ, Marchbank T, Ntatsaki E, Murray D, Ghosh S, Playford RJ. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut 56: 168–175, 2007. doi: 10.1136/gut.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Makki K, Deehan EC, Walter J, Bäckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23: 705–715, 2018. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 133.Mallmann NH, Lima ES, Lalwani P. Dysregulation of tryptophan catabolism in metabolic syndrome. Metab Syndr Relat Disord 16: 135–142, 2018. doi: 10.1089/met.2017.0097. [DOI] [PubMed] [Google Scholar]
- 134.Man AL, Bertelli E, Rentini S, Regoli M, Briars G, Marini M, Watson AJ, Nicoletti C. Age-associated modifications of intestinal permeability and innate immunity in human small intestine. Clin Sci (Lond) 129: 515–527, 2015. doi: 10.1042/CS20150046. [DOI] [PubMed] [Google Scholar]
- 135.Manzel A, Muller DN, Hafler DA, Erdman SE, Linker RA, Kleinewietfeld M. Role of “Western diet” in inflammatory autoimmune diseases. Curr Allergy Asthma Rep 14: 404, 2014. doi: 10.1007/s11882-013-0404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marchbank T, Davison G, Oakes JR, Ghatei MA, Patterson M, Moyer MP, Playford RJ. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol 300: G477–G484, 2011. doi: 10.1152/ajpgi.00281.2010. [DOI] [PubMed] [Google Scholar]
- 137.Marshall JK, Thabane M, Garg AX, Clark W, Meddings J, Collins SM; WEL Investigators . Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther 20: 1317–1322, 2004. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 138.Martens EC, Chiang HC, Gordon JI. Mucosal glycan foraging enhances fitness and transmission of a saccharolytic human gut bacterial symbiont. Cell Host Microbe 4: 447–457, 2008. doi: 10.1016/j.chom.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Maxton DG, Bjarnason I, Reynolds AP, Catt SD, Peters TJ, Menzies IS. Lactulose, 51Cr-labelled ethylenediaminetetra-acetate, l-rhamnose and polyethyleneglycol 400 [corrected] as probe markers for assessment in vivo of human intestinal permeability. Clin Sci (Lond) 71: 71–80, 1986. doi: 10.1042/cs0710071. [DOI] [PubMed] [Google Scholar]
- 140.McMichael HB. A second intestinal glucose carrier. Gut 14: 428–429, 1973. [PubMed] [Google Scholar]
- 141.Meier J, Sturm A. The intestinal epithelial barrier: does it become impaired with age? Dig Dis 27: 240–245, 2009. doi: 10.1159/000228556. [DOI] [PubMed] [Google Scholar]
- 142.Meng YB, Lei J, Hao ZM, Cao RL. [Influence of rhubarb on gastrointestinal motility and intestinal mucosal barrier in patients with severe burn]. Zhonghua Shao Shang Za Zhi 27: 337–340, 2011. doi: 10.3760/cma.j.issn.1009-2587.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 143.Monk JM, Lepp D, Wu W, Pauls KP, Robinson LE, Power KA. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J Nutr Biochem 49: 89–100, 2017. doi: 10.1016/j.jnutbio.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 144.Moore A, Bjarnason I, Cryer B, Garcia-Rodriguez L, Goldkind L, Lanas A, Simon L. Evidence for endoscopic ulcers as meaningful surrogate endpoint for clinically significant upper gastrointestinal harm. Clin Gastroenterol Hepatol 7: 1156–1163, 2009. doi: 10.1016/j.cgh.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 145.Mujagic Z, Ludidi S, Keszthelyi D, Hesselink MA, Kruimel JW, Lenaerts K, Hanssen NM, Conchillo JM, Jonkers DM, Masclee AA. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders. Aliment Pharmacol Ther 40: 288–297, 2014. doi: 10.1111/apt.12829. [DOI] [PubMed] [Google Scholar]
- 146.Naganuma M, Sugimoto S, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, Tanaka S, Andoh A, Ohmiya N, Saigusa K, Yamamoto T, Morohoshi Y, Ichikawa H, Matsuoka K, Hisamatsu T, Watanabe K, Mizuno S, Suda W, Hattori M, Fukuda S, Hirayama A, Abe T, Watanabe M, Hibi T, Suzuki Y, Kanai T, Naganuma M, Sugimoto S, Mizuno S, Nakazato Y, Fukuda T, Teratani T, Ogata H, Iwao Y, Kanai T, Yamasaki H, Mitsuyama K, Kobayashi T, Toyonaga T, Nakano M, Hibi T, Yoshimura N, Sameshima Y, Ohi H, Hayashi R, Ueno Y, Tanaka S, Bamba S, Andoh A, Matsuoka K, Watanabe M, Saigusa K, Nakazawa A, Morohoshi Y, Koike Y, Imai J, Ichikawa H, Shimoyama T, Yamamoto T, Takeuchi K, Suzuki Y, Nagasaka M, Ohmiya N, Kitano A, Ashizuka S, Inatsu H, Onodera K, Nakase H, Kitamura K, Ikeya K, Hanai H, Watanabe C, Hokari R, Hirai F, Naito Y, Hoshi N, Kinjo F, Ishiguro Y, Sasaki M, Matsumoto T, Watanabe K, Hisamatsu T, Sano F, Roberts R, Abe T, Suda W, Hattori M, Fukuda S, Hirayama A; INDIGO Study Group . Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology 154: 935–947, 2018. doi: 10.1053/j.gastro.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 147.Ni J, Wu GD, Albenberg L, Tomov VT. Gut microbiota and IBD: causation or correlation? Nat Rev Gastroenterol Hepatol 14: 573–584, 2017. doi: 10.1038/nrgastro.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science 336: 1262–1267, 2012. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 149.O’Connor CJ, Wallace RG, Iwamoto K, Taguchi T, Sunamoto J. Bile salt damage of egg phosphatidylcholine liposomes. Biochim Biophys Acta 817: 95–102, 1985. doi: 10.1016/0005-2736(85)90072-0. [DOI] [PubMed] [Google Scholar]
- 150.Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol 11: 1075–1083, 2013. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ohlsson B, Orho-Melander M, Nilsson PM. Higher levels of serum zonulin may rather be associated with increased risk of obesity and hyperlipidemia, than with gastrointestinal symptoms or disease manifestations. Int J Mol Sci 18: 582, 2017. doi: 10.3390/ijms18030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Øktedalen O, Lunde OC, Opstad PK, Aabakken L, Kvernebo K. Changes in the gastrointestinal mucosa after long-distance running. Scand J Gastroenterol 27: 270–274, 1992. doi: 10.3109/00365529209000073. [DOI] [PubMed] [Google Scholar]
- 153.Ouellette AJ. Paneth cells and innate mucosal immunity. Curr Opin Gastroenterol 26: 547–553, 2010. doi: 10.1097/MOG.0b013e32833dccde. [DOI] [PubMed] [Google Scholar]
- 154.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 48: 993–999, 2008. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol 12: 821–832, 2012. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 156.Pals KL, Chang RT, Ryan AJ, Gisolfi CV. Effect of running intensity on intestinal permeability. J Appl Physiol (1985) 82: 571–576, 1997. doi: 10.1152/jappl.1997.82.2.571. [DOI] [PubMed] [Google Scholar]
- 157.Park JH, Park DI, Kim HJ, Cho YK, Sohn CI, Jeon WK, Kim BI, Won KH, Park SM. The relationship between small-intestinal bacterial overgrowth and intestinal permeability in patients with irritable bowel syndrome. Gut Liver 3: 174–179, 2009. doi: 10.5009/gnl.2009.3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol 32: 742–747, 2000. doi: 10.1016/S0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- 159.Pavlidis P, Powell N, Vincent RP, Ehrlich D, Bjarnason I, Hayee B. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment Pharmacol Ther 42: 802–817, 2015. doi: 10.1111/apt.13333. [DOI] [PubMed] [Google Scholar]
- 160.Pedersen C, Gallagher E, Horton F, Ellis RJ, Ijaz UZ, Wu H, Jaiyeola E, Diribe O, Duparc T, Cani PD, Gibson GR, Hinton P, Wright J, La Ragione R, Robertson MD. Host-microbiome interactions in human type 2 diabetes following prebiotic fibre (galacto-oligosaccharide) intake. Br J Nutr 116: 1869–1877, 2016. doi: 10.1017/S0007114516004086. [DOI] [PubMed] [Google Scholar]
- 161.Pelsers MM, Namiot Z, Kisielewski W, Namiot A, Januszkiewicz M, Hermens WT, Glatz JF. Intestinal-type and liver-type fatty acid-binding protein in the intestine. Tissue distribution and clinical utility. Clin Biochem 36: 529–535, 2003. doi: 10.1016/S0009-9120(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 162.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology 142: 1100–1101.e2, 2012. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Peng X, Yan H, You Z, Wang P, Wang S. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns 30: 135–139, 2004. doi: 10.1016/j.burns.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 164.Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: role of uric acid in the hypertensive response. Int J Obes 34: 454–461, 2010. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 165.Peters SA, Edogawa S, Sundt WJ, Dyer RB, Dalenberg DA, Mazzone A, Singh RJ, Moses N, Smyrk TC, Weber C, Linden DR, MacNaughton WK, Turner JR, Camilleri M, Katzka DA, Farrugia G, Grover M. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function. Am J Gastroenterol 112: 913–923, 2017. doi: 10.1038/ajg.2017.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Phillips TE, Phillips TL, Neutra MR. Macromolecules can pass through occluding junctions of rat ileal epithelium during cholinergic stimulation. Cell Tissue Res 247: 547–554, 1987. doi: 10.1007/BF00215748. [DOI] [PubMed] [Google Scholar]
- 167.Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, Cremon C, Stanghellini V, De Giorgio R, Galmiche JP, Neunlist M. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 58: 196–201, 2009. doi: 10.1136/gut.2007.140806. [DOI] [PubMed] [Google Scholar]
- 168.Pires W, Veneroso CE, Wanner SP, Pacheco DAS, Vaz GC, Amorim FT, Tonoli C, Soares DD, Coimbra CC. Association between exercise-induced hyperthermia and intestinal permeability: a systematic review. Sports Med 47: 1389–1403, 2017. doi: 10.1007/s40279-016-0654-2. [DOI] [PubMed] [Google Scholar]
- 169.Prytz H, Benoni C, Tagesson C. Does smoking tighten the gut? Scand J Gastroenterol 24: 1084–1088, 1989. doi: 10.3109/00365528909089259. [DOI] [PubMed] [Google Scholar]
- 170.Pusponegoro HD, Ismael S, Firmansyah A, Sastroasmoro S, Vandenplas Y. Gluten and casein supplementation does not increase symptoms in children with autism spectrum disorder. Acta Paediatr 104: e500–e505, 2015. doi: 10.1111/apa.13108. [DOI] [PubMed] [Google Scholar]
- 171.Raimondi F, Santoro P, Barone MV, Pappacoda S, Barretta ML, Nanayakkara M, Apicella C, Capasso L, Paludetto R. Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am J Physiol Gastrointest Liver Physiol 294: G906–G913, 2008. doi: 10.1152/ajpgi.00043.2007. [DOI] [PubMed] [Google Scholar]
- 172.Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol 301: G919–G928, 2011. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 47: 241–259, 2006. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 174.Russo F, Linsalata M, Clemente C, Chiloiro M, Orlando A, Marconi E, Chimienti G, Riezzo G. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr Res 32: 940–946, 2012. doi: 10.1016/j.nutres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 175.Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: a possible explanation for trophic effects of fermentable fibre, gut microbes and luminal trophic factors. Br J Nutr 58: 95–103, 1987. doi: 10.1079/BJN19870073. [DOI] [PubMed] [Google Scholar]
- 176.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, Iafusco D, Prisco F, Laghi F, Riegler G, Carratu R, Counts D, Fasano A. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 55: 1443–1449, 2006. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 177.Scheiman JM. The use of proton pump inhibitors in treating and preventing NSAID-induced mucosal damage. Arthritis Res Ther 15, Suppl 3: S5, 2013. doi: 10.1186/ar4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schellekens DH, Hundscheid IH, Leenarts CA, Grootjans J, Lenaerts K, Buurman WA, Dejong CH, Derikx JP. Human small intestine is capable of restoring barrier function after short ischemic periods. World J Gastroenterol 23: 8452–8464, 2017. doi: 10.3748/wjg.v23.i48.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Serrander R, Magnusson KE, Sundqvist T. Acute infections with Giardia lamblia and rotavirus decrease intestinal permeability to low-molecular weight polyethylene glycols (PEG 400). Scand J Infect Dis 16: 339–344, 1984. doi: 10.3109/00365548409073958. [DOI] [PubMed] [Google Scholar]
- 180.Shulman RJ, Eakin MN, Czyzewski DI, Jarrett M, Ou CN. Increased gastrointestinal permeability and gut inflammation in children with functional abdominal pain and irritable bowel syndrome. J Pediatr 153: 646–650, 2008. doi: 10.1016/j.jpeds.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sigthorsson G, Tibble J, Hayllar J, Menzies I, Macpherson A, Moots R, Scott D, Gumpel MJ, Bjarnason I. Intestinal permeability and inflammation in patients on NSAIDs. Gut 43: 506–511, 1998. doi: 10.1136/gut.43.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smecuol E, Sugai E, Niveloni S, Vázquez H, Pedreira S, Mazure R, Moreno ML, Label M, Mauriño E, Fasano A, Meddings J, Bai JC. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol 3: 335–341, 2005. doi: 10.1016/S1542-3565(04)00778-5. [DOI] [PubMed] [Google Scholar]
- 183.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Snipe RM, Khoo A, Kitic CM, Gibson PR, Costa RJS. The impact of mild heat stress during prolonged running on gastrointestinal integrity, gastrointestinal symptoms, systemic endotoxin and cytokine profiles. Int J Sports Med 39: 255–263, 2018. doi: 10.1055/s-0043-122742. [DOI] [PubMed] [Google Scholar]
- 185.Söderholm J, Olaison G, Sjödahl R, Tagesson C. Smoking and intestinal absorption of oral polyethylene glycols in Crohn’s disease. Scand J Gastroenterol 28: 163–167, 1993. doi: 10.3109/00365529309096064. [DOI] [PubMed] [Google Scholar]
- 186.Sommer F, Bäckhed F. The gut microbiota: masters of host development and physiology. Nat Rev Microbiol 11: 227–238, 2013. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 187.Sonnenburg ED, Sonnenburg JL. Starving our microbial self: the deleterious consequences of a diet deficient in microbiota-accessible carbohydrates. Cell Metab 20: 779–786, 2014. doi: 10.1016/j.cmet.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 535: 56–64, 2016. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sonnenburg JL, Xu J, Leip DD, Chen CH, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307: 1955–1959, 2005. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]
- 190.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, Neal KR. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut 47: 804–811, 2000. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119: 1322–1334, 2009. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stärkel P, Leclercq S, de Timary P, Schnabl B. Intestinal dysbiosis and permeability: the yin and yang in alcohol dependence and alcoholic liver disease. Clin Sci (Lond) 132: 199–212, 2018. doi: 10.1042/CS20171055. [DOI] [PubMed] [Google Scholar]
- 193.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol 32: 403–432, 2014. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 194.Strobel S, Brydon WG, Ferguson A. Cellobiose/mannitol sugar permeability test complements biopsy histopathology in clinical investigation of the jejunum. Gut 25: 1241–1246, 1984. doi: 10.1136/gut.25.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Suenaert P, Bulteel V, Den Hond E, Hiele M, Peeters M, Monsuur F, Ghoos Y, Rutgeerts P. The effects of smoking and indomethacin on small intestinal permeability. Aliment Pharmacol Ther 14: 819–822, 2000. doi: 10.1046/j.1365-2036.2000.00754.x. [DOI] [PubMed] [Google Scholar]
- 196.Sundqvist T, Magnusson KE, Sjödahl R, Stjernström I, Tagesson C. Passage of molecules through the wall of the gastrointestinal tract. II. Application of low-molecular weight polyethyleneglycol and a deterministic mathematical model for determining intestinal permeability in man. Gut 21: 208–214, 1980. doi: 10.1136/gut.21.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 100: 297–305, 2008. doi: 10.1017/S0007114508888733. [DOI] [PubMed] [Google Scholar]
- 198.Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet 6: 81, 2015. doi: 10.3389/fgene.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Takamatsu N, Welage LS, Idkaidek NM, Liu DY, Lee PI, Hayashi Y, Rhie JK, Lennernäs H, Barnett JL, Shah VP, Lesko L, Amidon GL. Human intestinal permeability of piroxicam, propranolol, phenylalanine, and PEG 400 determined by jejunal perfusion. Pharm Res 14: 1127–1132, 1997. doi: 10.1023/A:1012134219095. [DOI] [PubMed] [Google Scholar]
- 200.Tan JK, McKenzie C, Mariño E, Macia L, Mackay CR. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol 35: 371–402, 2017. doi: 10.1146/annurev-immunol-051116-052235. [DOI] [PubMed] [Google Scholar]
- 201.Teixeira TF, Souza NC, Chiarello PG, Franceschini SC, Bressan J, Ferreira CL, Peluzio MC. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr 31: 735–740, 2012. doi: 10.1016/j.clnu.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 202.Ten Bruggencate SJ, Bovee-Oudenhoven IM, Lettink-Wissink ML, Katan MB, van der Meer R. Dietary fructooligosaccharides affect intestinal barrier function in healthy men. J Nutr 136: 70–74, 2006. doi: 10.1093/jn/136.1.70. [DOI] [PubMed] [Google Scholar]
- 203.Thaiss CA, Levy M, Grosheva I, Zheng D, Soffer E, Blacher E, Braverman S, Tengeler AC, Barak O, Elazar M, Ben-Zeev R, Lehavi-Regev D, Katz MN, Pevsner-Fischer M, Gertler A, Halpern Z, Harmelin A, Aamar S, Serradas P, Grosfeld A, Shapiro H, Geiger B, Elinav E. Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection. Science 359: 1376–1383, 2018. doi: 10.1126/science.aar3318. [DOI] [PubMed] [Google Scholar]
- 204.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Königsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 138: 1452–1455, 2008. doi: 10.1093/jn/138.8.1452. [DOI] [PubMed] [Google Scholar]
- 205.Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 123: 450–460, 2002. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 206.Tuomi K, Logomarsino JV. Bacterial lipopolysaccharide, lipopolysaccharide-binding protein, and other inflammatory markers in obesity and after bariatric surgery. Metab Syndr Relat Disord 14: 279–288, 2016. doi: 10.1089/met.2015.0170. [DOI] [PubMed] [Google Scholar]
- 207.Turcotte JF, Kao D, Mah SJ, Claggett B, Saltzman JR, Fedorak RN, Liu JJ. Breaks in the wall: increased gaps in the intestinal epithelium of irritable bowel syndrome patients identified by confocal laser endomicroscopy (with videos). Gastrointest Endosc 77: 624–630, 2013. doi: 10.1016/j.gie.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 208.US Food and Drug Administration Guidance for Industry: Evidence-Based Review System for Scientific Evaluation of Health Claims (Online). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-evidence-based-review-system-scientific-evaluation-health-claims. [9 December 2018].
- 209.US Food and Drug Administration Guidance for Industry: Substantiation for Dietary Supplement Claims Made Under Section 403(r) (6) of the Federal Food, Drug, and Cosmetic Act (Online). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-substantiation-dietary-supplement-claims-made-under-section-403r-6-federal-food. [9 December 2018].
- 210.Van Itallie CM, Holmes J, Bridges A, Gookin JL, Coccaro MR, Proctor W, Colegio OR, Anderson JM. The density of small tight junction pores varies among cell types and is increased by expression of claudin-2. J Cell Sci 121: 298–305, 2008. doi: 10.1242/jcs.021485. [DOI] [PubMed] [Google Scholar]
- 211.Vanuytsel T, van Wanrooy S, Vanheel H, Vanormelingen C, Verschueren S, Houben E, Salim Rasoel S, Tόth J, Holvoet L, Farré R, Van Oudenhove L, Boeckxstaens G, Verbeke K, Tack J. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut 63: 1293–1299, 2014. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- 212.van Wijck K, Lenaerts K, Grootjans J, Wijnands KA, Poeze M, van Loon LJ, Dejong CH, Buurman WA. Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: strategies for evaluation and prevention. Am J Physiol Gastrointest Liver Physiol 303: G155–G168, 2012. doi: 10.1152/ajpgi.00066.2012. [DOI] [PubMed] [Google Scholar]
- 213.van Wijck K, Wijnands KA, Meesters DM, Boonen B, van Loon LJ, Buurman WA, Dejong CH, Lenaerts K, Poeze M. L-citrulline improves splanchnic perfusion and reduces gut injury during exercise. Med Sci Sports Exerc 46: 2039–2046, 2014. doi: 10.1249/MSS.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 214.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D, Burton D, Zinsmeister AR. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144: 903–911.e3, 2013. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Veldhoen M, Brucklacher-Waldert V. Dietary influences on intestinal immunity. Nat Rev Immunol 12: 696–708, 2012. doi: 10.1038/nri3299. [DOI] [PubMed] [Google Scholar]
- 216.Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41: 296–310, 2014. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Vivinus-Nébot M, Dainese R, Anty R, Saint-Paul MC, Nano JL, Gonthier N, Marjoux S, Frin-Mathy G, Bernard G, Hébuterne X, Tran A, Theodorou V, Piche T. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol 107: 75–81, 2012. doi: 10.1038/ajg.2011.315. [DOI] [PubMed] [Google Scholar]
- 218.Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, Filippi J, Saint-Paul MC, Tulic MK, Verhasselt V, Hébuterne X, Piche T. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut 63: 744–752, 2014. doi: 10.1136/gutjnl-2012-304066. [DOI] [PubMed] [Google Scholar]
- 219.Vogelsang H, Wyatt J, Penner E, Lochs H. Screening for celiac disease in first-degree relatives of patients with celiac disease by lactulose/mannitol test. Am J Gastroenterol 90: 1838–1842, 1995. [PubMed] [Google Scholar]
- 220.Volynets V, Louis S, Pretz D, Lang L, Ostaff MJ, Wehkamp J, Bischoff SC. Intestinal barrier function and the gut microbiome are differentially affected in mice fed a Western-style diet or drinking water supplemented with fructose. J Nutr 147: 770–780, 2017. doi: 10.3945/jn.116.242859. [DOI] [PubMed] [Google Scholar]
- 221.Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 113: 4435–4440, 2000. [DOI] [PubMed] [Google Scholar]
- 222.Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature 514: 237–241, 2014. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 223.Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 349: g4490, 2014. [Erratum in BMJ 349: g5472, 2014.] doi: 10.1136/bmj.g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang Y, Tong J, Chang B, Wang B, Zhang D, Wang B. Effects of alcohol on intestinal epithelial barrier permeability and expression of tight junction-associated proteins. Mol Med Rep 9: 2352–2356, 2014. doi: 10.3892/mmr.2014.2126. [DOI] [PubMed] [Google Scholar]
- 225.Warners MJ, Vlieg-Boerstra BJ, Verheij J, van Hamersveld PH, van Rhijn BD, Van Ampting MT, Harthoorn LF, de Jonge WJ, Smout AJ, Bredenoord AJ. Esophageal and small intestinal mucosal integrity in eosinophilic esophagitis and response to an elemental diet. Am J Gastroenterol 112: 1061–1071, 2017. doi: 10.1038/ajg.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Washio E, Esaki M, Maehata Y, Miyazaki M, Kobayashi H, Ishikawa H, Kitazono T, Matsumoto T. Proton pump inhibitors increase incidence of nonsteroidal anti-inflammatory drug-induced small bowel injury: a randomized, placebo-controlled trial. Clin Gastroenterol Hepatol 14: 809–815.e1, 2016. doi: 10.1016/j.cgh.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 227.Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol 8: 477–512, 2013. doi: 10.1146/annurev-pathol-011110-130318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Wenzel UA, Magnusson MK, Rydström A, Jonstrand C, Hengst J, Johansson ME, Velcich A, Öhman L, Strid H, Sjövall H, Hansson GC, Wick MJ. Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis. PLoS One 9: e100217, 2014. doi: 10.1371/journal.pone.0100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.West NP, Pyne DB, Cripps AW, Christophersen CT, Conlon MA, Fricker PA. Gut Balance, a synbiotic supplement, increases fecal Lactobacillus paracasei but has little effect on immunity in healthy physically active individuals. Gut Microbes 3: 221–227, 2012. doi: 10.4161/gmic.19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wilms E, Gerritsen J, Smidt H, Besseling-van der Vaart I, Rijkers GT, Garcia Fuentes AR, Masclee AA, Troost FJ. Effects of supplementation of the Synbiotic Ecologic® 825/FOS P6 on intestinal barrier function in healthy humans: a randomized controlled trial. PLoS One 11: e0167775, 2016. doi: 10.1371/journal.pone.0167775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108, 2011. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wu RL, Vazquez-Roque MI, Carlson P, Burton D, Grover M, Camilleri M, Turner JR. Gluten-induced symptoms in diarrhea-predominant irritable bowel syndrome are associated with increased myosin light chain kinase activity and claudin-15 expression. Lab Invest 97: 14–23, 2017. doi: 10.1038/labinvest.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yao CK, Muir JG, Gibson PR. Review article: insights into colonic protein fermentation, its modulation and potential health implications. Aliment Pharmacol Ther 43: 181–196, 2016. doi: 10.1111/apt.13456. [DOI] [PubMed] [Google Scholar]
- 234.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39: 372–385, 2013. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 235.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, Mao Y, Zhang X, Pang X, Wei C, Zhao G, Chen Y, Zhao L. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME J 4: 232–241, 2010. [Erratum in ISME J 4: 312–313, 2010.] doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 236.Zhang DM, Jiao RQ, Kong LD. High dietary fructose: direct or indirect dangerous factors disturbing tissue and organ functions. Nutrients 9: 335, 2017. doi: 10.3390/nu9040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhou Q, Souba WW, Croce CM, Verne GN. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 59: 775–784, 2010. doi: 10.1136/gut.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhou Q, Zhang B, Verne GN. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome. Pain 146: 41–46, 2009. doi: 10.1016/j.pain.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhou YP, Jiang ZM, Sun YH, Wang XR, Ma EL, Wilmore D. The effect of supplemental enteral glutamine on plasma levels, gut function, and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN J Parenter Enteral Nutr 27: 241–245, 2003. doi: 10.1177/0148607103027004241. [DOI] [PubMed] [Google Scholar]
- 240.Ziegler TR, Evans ME, Fernández-Estívariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr 23: 229–261, 2003. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 241.Zou J, Chassaing B, Singh V, Pellizzon M, Ricci M, Fythe MD, Kumar MV, Gewirtz AT. Fiber-mediated nourishment of gut microbiota protects against diet-induced obesity by restoring IL-22-mediated colonic health. Cell Host Microbe 23: 41–53.e4, 2018. doi: 10.1016/j.chom.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]