Abstract
Intrinsically labeled dietary proteins have been used to trace various aspects of digestion and absorption, including quantifying the contribution of dietary protein to observed postprandial amino acid and protein kinetics in human subjects. Quantification of the rate of appearance in peripheral blood of an unlabeled (tracee) amino acid originating from an intrinsically labeled protein (exogenous Ra) requires the assumption that there is no dilution of the isotope enrichment of the protein-bound amino acid in the gastrointestinal tract or across the splanchnic bed. It must also be assumed that the effective volume of distribution into which the tracer and tracee appear can be reasonably estimated by a single value and that any recycling of the tracer is minimal and thus does not affect calculated rates. We have assessed these assumptions quantitatively using values from published studies. We conclude that the use of intrinsically labeled proteins as currently described to quantify exogenous Ra systematically underestimates the true value. When used with the tracer-determined rates of amino acid kinetics, underestimation of exogenous Ra from the intrinsically labeled protein method likely translates to incorrect conclusions regarding protein breakdown, including the effect of a protein meal and the anabolic impact of the speed of digestion and absorption of amino acids. Estimation of exogenous Ra from the bioavailability of ingested protein has some advantages as compared with the intrinsically labeled protein method. We therefore conclude that the bioavailability method for estimating exogenous Ra is preferable to the intrinsically labeled protein method.
Keywords: bioavailability, gastrointestinal tract, splanchnic bed, Steele equation, tracer methodology, true ileal digestibility
INTRODUCTION
The study of the postprandial response of whole body protein synthesis and breakdown in human subjects has long been of interest but plagued by the difficulty of quantifying the contribution of absorbed amino acids from dietary protein to the observed protein kinetics. Models that involve the infusion of stable isotope tracer(s) and quantification of the rate of appearance (Ra) of an essential amino acid and its rate of oxidation have been used for decades (e.g., Ref. 34) to quantify the response of endogenous protein kinetics to meal consumption. These models have provided reliable and consistent results in the postabsorptive state in which the Ra of an essential amino acid is a direct reflection of whole body protein breakdown. The rate of protein synthesis is calculated by subtracting the rate of oxidation of the essential amino acid from its Ra, since the only two quantitatively significant fates of an essential amino acid are oxidation and protein synthesis (47). Net protein balance, which is the reflection of the extent of gain or loss of body protein, is calculated as the difference between the calculated rates of protein synthesis and breakdown (47). In theory, any essential amino acid can be used as tracer. Leucine (Leu) has been a popular choice of tracer because it is often the most abundant essential amino acid in a protein. Phenylalanine (Phe) is also a popular choice, as it is an essential amino acid whose rate of oxidation can be measured by determining the rate of hydroxylation of Phe to tyrosine, without the need to collect CO2. In the postprandial state, the total rate of appearance (total Ra) of the amino acid being traced (tracee) is the sum of the endogenous Ra and the exogenous Ra from the digested dietary protein. The challenge in the postprandial state is the quantification of the contribution of the exogenous Ra to the total Ra.
Determining the extent of digestion and absorption of amino acids in a dietary protein in human subjects is difficult. Access to the gastrointestinal tract (GIT) is limited, and once a protein is digested and the constituent amino acids enter the circulation, they are indistinguishable from their endogenous counterparts. One solution has been to use a dietary protein that contains an isotopically labeled amino acid, also called an intrinsically labeled dietary protein. Intrinsically labeled proteins have been used for 50 yr in various applications of tracer methodology (e.g., Refs. 5, 10, 32). Intrinsic labeling of a dietary protein can be accomplished in a variety of ways, including feeding chickens with a labeled amino acid and using the labeled protein in the eggs (43) or feeding a labeled amino acid to a cow and using the labeled milk (4, 42). Plant proteins can also be labeled by growing them on deuterated water (2H2O) (13). Regardless of the means of labeling or the specific protein to be labeled, the process of producing intrinsically labeled proteins in sufficient quantities to be used in human experiments is difficult and expensive. As a result, the application of this technique has been limited. Nonetheless, because of the apparent elegance of the approach, the conclusions drawn from experiments using intrinsically labeled proteins have formed a basis for our current understanding of the responses of endogenous protein metabolism to dietary protein consumption.
The intrinsically labeled protein method is predicated on the assumption that the appearance of the ingested tracer amino acid in the peripheral blood quantitatively reflects the appearance of its unlabeled counterpart. For this to be the case, enrichment of the labeled amino acid in the intrinsically labeled protein must not be diluted from the point of ingestion to the point of sampling in the peripheral blood. This means that there is no dilution of the labeled amino acid in the GIT, as well as in the transit of the absorbed amino acid across the splanchnic bed. It is also necessary to make assumptions when calculating the Ra in peripheral blood of exogenous amino acids from the intrinsically labeled protein, particularly after bolus consumption of a protein meal. Although the use of intrinsically labeled proteins to measure exogenous Ra is well established, there has not previously been an in-depth analysis of the validity of underlying assumptions. In this paper we will focus on the method described by Boirie et al. (3, 5). This approach is currently used (e.g., Ref. 16), and the method is increasingly interpreted as a standard in the determination of exogenous amino acid kinetics. Data are presented to enable the quantitative evaluation of the potential impact of errors when using this method. Similar issues arise in a variety of other applications of the intrinsically labeled protein technique.
CALCULATION OF Ra IN PERIPHERAL BLOOD OF AN AMINO ACID FROM INTRINSICALLY LABELED PROTEIN
One of the principal advantages of the intrinsically labeled protein approach described by Boirie et al. (3, 5) and used by others (e.g., 16, 30) is that it enables quantification of protein synthesis and breakdown in the nonsteady state following ingestion of a meal or a bolus ingestion of protein. This is important because it describes normal food intake. We will first describe the methodology to quantify the Ra of an intrinsically labeled protein in the blood (i.e., exogenous Ra) in the nonsteady state and later discuss the assumptions underlying the calculations and the implications of potential errors.
The conventional calculation of exogenous Ra using the intrinsically labeled protein method includes determination of total Ra by traditional tracer methodology using a primed-constant infusion of the tracer amino acid labeled differently from the protein-bound tracer. Total Ra is the sum of endogenous Ra (from protein breakdown) and exogenous Ra (from the intrinsically labeled protein). Total Ra is calculated from the tracer infusion rate divided by the resulting plasma enrichment at isotopic plateau. For example, if [1-13C]Leu is used as tracer, a reasonable tracer infusion rate would be 0.19 umol/kg/min, with a priming dose of 11.8 μmol Leu/kg. If the resulting enrichment at isotopic plateau is a tracer-to-tracee ratio (TTR; corrected for background enrichment of carbon) of 0.055, then the Leu Ra would be 3.45 μmol·kg−1·min−1 (0.19 μmol Leu·kg−1·min−1 ÷ 0.055 = 3.45 μmol Leu·kg−1·min−1) or 0.452 mg Leu·kg−1·min−1 (3.45 μmol·kg−1·min−1 × 131 μg/μmol = 0.452 mg Leu·kg−1·min−1). In the postabsorptive state, Leu Ra is a direct reflection of protein breakdown, since there is no endogenous synthesis of Leu.
A bolus ingestion of dietary protein will induce a nonsteady state in both tracer and tracee in the blood. In this case, the Steele equation (39) is used to calculate the total Ra.
| (1) |
where total Ra is the total rate of appearance; FIV is infusion rate of tracer (μmol·kg−1·min−1); pV is the effective volume of distribution of the tracer and tracee (ml/kg), assumed by Boirie et al. (5) to be P = 0.25 and V = 0.5 l/kg, or pV = 125 ml/kg; C(t) is the mean plasma concentration (μmol/l) of the tracee (Leu in Ref. 5) between two time points (μmol/l); dEiIV/dt is the change in enrichment from the intravenous (IV) infusion as a function of time; and EiIV(t) is the mean plasma tracer (i.e., Leu) enrichment of the intravenous tracer for two time points. Note that enrichment may be expressed either as TTR or mole percent excess (MPE), where MPE = TTR/(1+TTR) × 100. The use of TTR traces only the unlabeled tracee, while the use of MPE includes both the tracee and tracer. The expression of enrichment does not affect any conclusions made from our examples.
A rearrangement of the Steele equation is used to calculate exogenous Ra (5) when an intrinsically labeled protein is given (Eq. 2). The rearranged equation accounts for the fact that the tracer appearance rate is changing and unknown, whereas in Eq. 1 the tracer infusion rate FIV is constant and known. A differently labeled tracer of the same amino acid that is infused intravenously is used for the intrinsically labeled protein method.
| (2) |
where total Ra is the total rate of appearance as described above; Eipo(t) is the mean plasma enrichment for the oral tracer; dEipo/dt is the change in plasma enrichment for the oral tracer as a function of time; and Epro is the enrichment of the intrinsically labeled protein.
When total and exogenous Ra are known, then:
| (3) |
Total Ra, exogenous Ra, and endogenous Ra values are expressed as rates, conventionally as micromoles per kilograms per minutes.
EVALUATION OF ASSUMPTIONS IMPLICIT IN THE USE OF AN INTRINSICALLY LABELED PROTEIN
There are three potential problems with the intrinsically labeled protein method 1) dilution of tracer in the GIT as a result of the process of digestion; 2) dilution of tracer across the splanchnic bed; and 3) calculation of the appearance of exogenous amino acid in the peripheral circulation. In discussing each issue, we will use hypothetical numerical examples.
Dilution of Tracer in the Process of Digestion.
Studies to date have assumed that the enrichment of the tracer amino acid in an intrinsically labeled protein (Epro in Eq. 2) is equal to the enrichment of the absorbed tracer amino acid. However, any tracee amino acid arising from the digestion of endogenous protein secreted into the GIT will dilute the enrichment of tracer derived from the digestion of the intrinsically labeled protein. Previous studies of the rate of endogenous protein secreted into the GIT allow for an approximation of the extent of the resultant dilution of tracer (28, 29, 36).
The amount of endogenous protein secreted into the GIT lumen of the adult human has been estimated using a factorial approach (37). In this model, the absolute protein synthesis rate (in g/day) in the upper GIT was estimated using organ weights, percent protein content, and protein fractional synthetic rates (FSRs) for both the growing pig and adult human. Since direct measures of the rate of endogenous protein secretion into the human GIT do not exist, data quantifying the secretion rate of endogenous protein in the upper GIT of the growing pig were used. The rate of endogenous protein secreted in the upper GIT was expressed as a fraction of total upper GIT protein synthesized. This relationship was applied to the absolute rate of protein synthesis in the adult human upper GIT to estimate endogenous protein secretion into the lumen of the upper GIT, with the assumption that there is no net change in tissue mass, i.e., the rates of protein synthesis and breakdown are equal. In addition, the model made use of direct estimates of the endogenous amino acid losses from the human upper digestive tract (i.e., amino acids entering the colon) (8, 15, 28). With the use of this model, it was determined that ~33 g/day of endogenous protein is secreted into the upper GIT (for the 70-kg adult human) (37). By way of comparison, the recommended dietary allowance for protein intake for a 70-kg adult is 56 g protein/day (19). Thus, in a general sense, it is evident that at least some dilution of the enrichment of intrinsically labeled protein occurs in the process of digestion.
It is possible to make a quantitative estimate of the extent of dilution of the enrichment of an intrinsically labeled protein. About 85% of amino acids (i.e., 28.05 g) resulting from the digestion of endogenous protein are reabsorbed (22, 38). Multiplying this amount by the contribution of the tracee amino acid (e.g., Leu) to the composition of endogenous protein in the ileal digesta (9% in the case of Leu) gives a mean flow of Leu across the upper GIT followed by reabsorption of 2.52 g/day, or 0.105 g/h if considered over 24 h. However, since much of the Leu flow across the upper GIT is in the postprandial state, it is more reasonable to divide the total value (i.e., 2.52 g) by 16 h rather than 24 h. This would mean endogenous Leu is taken up from the GIT at a rate of ~0.158 g/h in the postprandial state.
Labeled amino acid from the digestion of intrinsically labeled protein will be diluted by the amount of unlabeled amino acid coming from the digestion of the endogenous protein secreted into the GIT. For the purpose of illustrating the effect of secretion of endogenous protein on the enrichment of labeled Leu in an intrinsically labeled protein, we will assume a constant value of 0.158 g/h of endogenously derived Leu in the GIT. While the value for endogenous Leu appearance in the GIT may be greater in the initial phase of absorption due to a greater rate of enzyme secretion, it would be balanced by a slowing of enzyme secretion as absorption of the intrinsically labeled protein slows. Therefore, if we calculate the total response over 4 h after ingestion of the protein, then we should roughly account for any high and low points of endogenous Leu flow into the GIT.
For this example, we will assume that 20 g of protein intrinsically labeled with [5,5,5-2H3]Leu are ingested. Multilabeling with deuterium has been selected for this example so that the naturally occurring isotope with the same mass as the tracer can be ignored since an insignificant amount of naturally occurring Leu is 3 mass units greater than the lowest molecular weight of Leu. We will assume that Leu comprises ~9% of the intrinsically labeled dietary protein. In this case, 1.8 g of Leu are ingested. Since the true ileal digestibility of Leu in whey protein is ~90% (36), ~1.62 g of Leu will be absorbed when 1.8 g of protein-bound Leu are ingested. We will assume the TTR of the Leu in the intrinsically labeled protein to be 0.05, meaning that ~1.543 g of unlabeled Leu and 0.077 g of labeled Leu are absorbed when 20 g of the protein are consumed. Furthermore, we will assume that Leu from the exogenous protein is absorbed over a 4-h period. If we use the estimated value of 0.158 g/h as the rate of endogenous Leu flow across the upper GIT, then over 4 h a total of 0.632 g of endogenous Leu are reabsorbed, thereby diluting the isotopic enrichment of the Leu from the intrinsically labeled protein. In this example, 1.543 g of unlabeled Leu from the intrinsically labeled protein are absorbed over 4 h, and 0.632 g of Leu from endogenous protein are absorbed. Thus a total of 2.175 g of unlabeled Leu is absorbed from combined exogenous and endogenous sources. The amount of labeled Leu that is absorbed (0.077 g) is not affected by the endogenous Leu entering the GIT. In this example, although the TTR of the Leu in the intrinsically labeled protein is 0.05, the TTR at the point of absorption into the body would be 0.077/2.175 = 0.0354, which represents a 29% dilution of the TTR.
This example makes the simplifying assumption that tracer from the intrinsically labeled protein does not recycle into the GIT in the time frame of the study. If this type of recycling occurs, which appears to be the case (23), then the recycled tracer amino acid would be reabsorbed, but it would not be from the dietary source but from the digestion of the endogenous protein secreted into the GIT. This type of recycling would further complicate the interpretation of the calculation of exogenous Ra, as not all of the tracer appearing in the circulation would be from the dietary source. In our example we have made the simplifying assumption that the flow of Leu from the digestion of endogenous protein into the GIT is constant, in which case the extent of the dilution of the tracer derived from the intrinsically labeled protein depends on the amount of protein ingested. Alternatively, the flow of endogenous Leu into the GIT may be a constant function of the amount of protein eaten, as much of the flow of endogenous Leu into the GIT is in the form of digestive enzymes. If endogenous flow of Leu into the GIT is a direct function of the amount of protein ingested, then the extent of dilution of the Leu tracer from the intrinsically labeled protein would be relatively constant, regardless of the amount of protein ingested. A number of studies (11, 18, 28, 33) have demonstrated an increase in ileal endogenous amino acid loss with increases in the amount of dietary protein. Finally, we have assumed that the intrinsically labeled dietary protein is entirely digested to free amino acids. If some part of the dietary protein is absorbed as peptides, the enrichment of the peptide-bound tracer that originated from partial digestion of an intrinsically labeled protein will be diluted only to the extent that endogenously secreted proteins are also absorbed as peptides.
From the above example, it is likely that the enrichment of an intrinsically labeled protein is diluted 20–30% in the GIT. This estimation is consistent with the results of previous studies demonstrating the rapid turnover of gut proteins. When the extent of recycling of a labeled dietary amino acid into gut endogenous protein was determined in frequently fed growing rats, more than half of the endogenous nitrogen (N) was found to be recycled dietary N (8). In the meal-fed growing pig, ~25% of the endogenous N is recycled dietary N (8, 28), and for the meal-fed adult human, 11% of ileal endogenous N is from recycled dietary N (8). The dilution of tracer in the GIT means that the true value for the enrichment of the absorbed tracer amino acid is lower than the value (Epro) in the intrinsically labeled protein. Since the term Epro is in the denominator of Eq. 2, an overestimation of the value for Epro resulting from failure to account for dilution in the GIT will result in an underestimation of the correct value for exogenous Ra.
Dilution of Tracer Across the Splanchnic Bed
The calculated rate of exogenous Ra using an intrinsically labeled protein should account for any protein-bound tracer that is not absorbed as well as any splanchnic uptake that occurs before the amino acid appears in the peripheral circulation. Each of these processes should not distinguish between tracer and tracee, meaning that there should be no dilution of the isotopic enrichment of amino acid tracer in the intrinsically labeled protein (Epro) from the mouth to appearance in peripheral blood. As discussed above, the process of digestion dilutes the tracer to some extent. In addition, tracer enrichment may also be diluted across the splanchnic bed.
The assumption that a tracer accurately traces the tracee is fundamental to all tracer methods. However, that assumption is not necessarily upheld when a tracer is used to quantify splanchnic amino acid uptake. For the purpose of illustrating this point, we will focus on the effect of protein turnover in the liver on tracer enrichment during transit from the portal vein to the hepatic vein, with the understanding that the liver is only one potential site of tracer dilution in the splanchnic bed. Other potential sites of dilution as a result of protein turnover include enterocytes, the pancreas, and goblet cells. For simplification, we will ignore the role of these other tissues and organs on tracer enrichment. We will also ignore hepatic arterial flow, as the hepatic artery will contain tracer in amounts that vary over time, thereby resulting in a complex time dependency of the amount of tracer and tracee perfusing the liver. The general point of our example applies if account was to be taken of all the other complicating factors in the transit across the splanchnic bed, although the quantitative aspects would likely differ.
Liver protein is in a constant state of turnover, meaning that protein synthesis and breakdown are occurring simultaneously. Amino acids are taken up from blood as precursors for protein synthesis, while amino acids are released into the blood as a consequence of protein breakdown. Over a 24-h period, these processes balance each other to maintain a constant organ protein mass. In the case of our example of consumption of a dietary protein intrinsically labeled with Leu tracer, the uptake of Leu from the blood by the liver does not distinguish between labeled and unlabeled Leu. Because of the relatively large protein pool of the liver compared with its turnover rate (Refs. 14 and 45), we will assume that there is no enrichment of the liver protein from the intrinsically labeled protein in the time frame of an acute study of 4–5 h. Since there is virtually no label in the liver protein, the protein can only be taken up at a rate approximately equal to the rate at which both labeled and unlabeled amino acids are taken up from the blood and incorporated into protein. Since both labeled and unlabeled Leu are taken up by the liver from the portal vein while only unlabeled is released from the liver into the hepatic vein, this represents a dilution of Leu tracer in the plasma that is not representative of net Leu uptake.
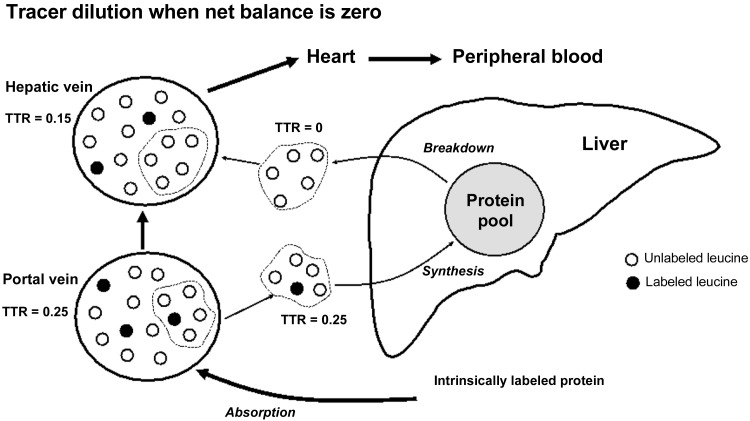
A simple schematic representation can illustrate the dissociation between the net uptake of tracee and tracer. In Fig. 1, we consider the situation of a labeled amino acid absorbed from the digestion of an intrinsically labeled protein as it passes from the portal vein to the hepatic vein. In Fig. 1, it is assumed that the rate of liver protein synthesis equals the rate of liver protein breakdown, meaning that there is neither uptake nor release of the amino acid across the liver on the net basis (i.e., liver net Leu balance = 0). We assume a blood flow rate of 1 ml/min and a TTR of 0.25 in the portal vein blood. Again, for the purpose of a convenient example, we assume that 33% of the amino acid in the portal vein is taken up by the liver and incorporated into liver protein. In Fig. 1, this is represented by the uptake of 5 units, 1 unit of which is labeled. The other amino acids in the portal vein (8 unlabeled and 2 labeled) pass through directly to the hepatic vein. Since the rate of protein synthesis is assumed to be equal to the rate of breakdown, 5 units arising from protein breakdown will be released into the blood to match the 5 units that are taken up by the liver. All of the units released will be unlabeled because there is essentially no label in liver protein. Therefore, the hepatic vein will contain the 8 unlabeled plus 2 labeled units that passed directly from the portal vein to the hepatic vein, plus the 5 unlabeled units released by protein breakdown. The resulting enrichment in the hepatic vein is a TTR of 0.15. The enrichment of the labeled amino acid from the intrinsically labeled protein is reduced by 40% in the circumstance in which there is no net uptake of the amino acid. Furthermore, the amount of the tracer released into the peripheral blood is decreased so that the measured value of Eipo(t) will be less than the correct value. Since the calculation of exogenous Ra (Eq. 2) includes the value Eipo(t) in the numerator, dilution of the amount of tracer in the blood from the hepatic vein entering the peripheral blood will be reflected in an underestimation of the true value for exogenous Ra, as account is not taken of dilution of Epro in the GIT or splanchnic bed.
Fig. 1.
Schematic representation of tracer (solid circles) and tracee (empty circles) uptake by the liver and release into blood as a result of liver protein synthesis and protein breakdown. In this example it is assumed that protein synthesis = protein breakdown, i.e., there is no net uptake or release of tracee (i.e., net balance = 0). Nonetheless, uptake from the portal vein includes both tracer and tracee, while only tracee is released by protein breakdown. This is because uptake does not distinguish between the tracer and tracee, while there is no tracer in the liver protein at the outset of the experiment. The result of both tracer and tracee uptake and only tracee release is a reduction in enrichment from tracer-to-tracee ratio (TTR) from 0.25 in the portal vein to 0.15 in the hepatic vein in the absence of any change in actual net uptake of tracee and tracer.
In our simple example, we have assumed that the rate of protein breakdown is equal to the rate of protein synthesis. Alternatively, if there is a complete suppression of protein breakdown then the flow from the hepatic vein to the peripheral circulation will accurately reflect the flow of both tracee and tracer. Also, if the intake of intrinsically labeled protein is maintained for a sufficient time, the hepatic proteins will eventually become labeled at the same enrichment as the blood entering the liver, in which case protein turnover will not dilute the enrichment of tracer in the blood between the portal and hepatic veins. In the case of a suppression of protein breakdown, the net uptake of tracer would be reflected by the reduction in the amount of tracer in the hepatic vein, while the enrichment would not be reduced, as there would be no release of unlabeled tracee from the liver. This is an extremely unlikely scenario under normal physiological circumstances. However, in a general sense, a large positive Leu balance after protein ingestion is possible. The greater the positive net balance, the closer the calculated rate of outflow of tracer from the portal vein will reflect net uptake. If a steady-state condition is created by sip feeding, then the Leu enrichment of the liver protein will eventually equal the enrichment of the Leu entering the liver (i.e., the enrichment of the liver protein would plateau at the enrichment of the precursor). In this case, the release of amino acids from protein breakdown would equal the precursor enrichment entering the liver and therefore would not dilute the enrichment. Despite these possibilities, when the intrinsically labeled protein method is used conventionally, a certain amount of dilution of Leu across the splanchnic bed is inevitable since our example has considered only protein turnover in the liver, and the same mechanisms of tracer dilution will also occur in the other tissues and organs of the splanchnic bed.
The schematic example shown in Fig. 1 demonstrates the principle that changes in tracer enrichment across an organ or tissue do not necessarily correspond to changes in the net uptake of tracee. A more realistic numerical example is useful to assess the potential magnitude of errors encountered in a more physiological setting as a result of isotope exchange. The human liver is ~1.4 kg and ~24% of that weight is protein (27, 45). Therefore, we can assume that there is ~336 g of protein (i.e., 24% of 1.4 kg) in the liver. The FSR of human liver protein is 1%/h (2), meaning that 3.36 g of liver protein (1% of 336 g) is produced per hour. For the purpose of consistency with the schematic example in Fig. 1, we will assume a circumstance in which the rate of liver protein breakdown equals the rate of liver protein synthesis and the precursor for protein synthesis is derived from the blood. If we assume that 7.0% of liver protein is Leu (48), then the turnover rate (i.e., incorporation into protein and release from breakdown) of Leu is 0.235 g Leu (7% of 3.36 g)/h, or 0.941 g/4 h. We will assume that 20 g of intrinsically labeled protein containing Leu with a TTR of 0.05 (Epro) is consumed. If the original Epro was 0.05, dilution in the GIT would reduce the TTR to 0.0354 in the hepatic vein using the example above. If we assume complete absorption of the intrinsically labeled protein and that 9% of the intrinsically labeled protein is Leu (5), then a total of 1.8 g of Leu from the intrinsically labeled protein would be absorbed. If the TTR of the absorbed Leu is 0.0354 and then ~0.0615 g of labeled Leu and 1.739 g of unlabeled Leu would be absorbed over 4 h and delivered to the liver via the portal vein, ignoring any dilution that may occur from the point of absorption to the portal vein. Since the assumed amount of Leu incorporated into liver protein (0.235 g Leu/h × 4 h) over 4 h is 0.941 g, then 52% of absorbed Leu would be incorporated into liver protein [(0.0941 g/1.8 g) × 100]. This would mean that 0.941 g of Leu (0.032 g of labeled and 0.908 g unlabeled Leu) would be cleared by the liver, and the same amount (i.e., 0.941 g) unlabeled would be released by the liver into the hepatic vein. As a result, the hepatic vein would contain the labeled and unlabeled Leu that was not cleared by the liver (0.859 g Leu = 0.029 g labeled Leu and 0.830 g unlabeled) over 4 h, plus the amount released by the liver (0.941 g of unlabeled Leu). The result would be 0.029 g of labeled Leu and 1.771 g of unlabeled (0.941 g released + 0.830 g not cleared) Leu. In this example, TTR in the hepatic vein would therefore be reduced to 0.0164 as compared with the corresponding value of 0.0354 in the portal vein. This represents a 54% dilution of the enrichment in the absence of any net uptake of Leu. Although this example considers the extreme case in which there is no labeling of liver protein, the general principle of tracer dilution as a result of protein turnover applies at any time point until an isotopic equilibrium is reached in the protein pool. Again, dilution of tracer translates directly to an underestimation of exogenous Ra.
This numerical example is only for the point of illustrating the potential problem of dilution of tracer enrichment across the splanchnic bed caused by protein turnover. We made the simplifying assumption that recirculating blood would contain only unlabeled Leu and that is not the case. In reality, recirculating blood will contain both labeled and unlabeled Leu and the amount of recirculating labeled Leu will be time dependent. Our assumption that all liver FSR is derived from blood-borne precursors overestimates true hepatic uptake of GIT-absorbed amino acid. We also did not consider the contribution of the hepatic artery to liver amino acid balance. In addition, we did not consider the impact of protein turnover in tissues and organs drained by the portal vein. There is evidence that the type of tracer dilution described above for the liver occurs to a similar extent across the enterocytes (37). All of these factors further complicate the quantification of the extent of label dilution as the tracer passes through the splanchnic bed. Most importantly, we assumed a net zero uptake of Leu by the liver, which is not likely the case following ingestion of a protein containing Leu. If there is a net uptake of Leu, then the dilution of tracer will be less because not as much unlabeled Leu would be released back into the hepatic vein. When considering all of these factors that may affect the extent of tracer dilution, it is clear that some dilution of the tracer from an intrinsically labeled protein will occur across the splanchnic bed, but the exact extent of dilution is uncertain. The uncertainty of the quantitative impact of our assumptions notwithstanding, the salient point of our example relative to the use of intrinsically labeled protein is that the change in tracer enrichment across a tissue or organ does not necessarily reflect the net uptake of the corresponding tracee and that the error involved in assuming that there is no dilution of tracer may be significant. Furthermore, the magnitude of dilution of tracer cannot be estimated with any certainty given the many dynamic factors that may affect the measured enrichment. As in the case of dilution of tracer enrichment in the GIT, the dilution of tracer by protein turnover in the splanchnic bed causes a systematic underestimation of the true value of exogenous Ra.
Assessment of the Steele Equation to Calculate Total Ra and Exogenous and Endogenous Ra with Intrinsically Labeled Dietary Proteins
The central challenge of using an isotopic tracer of an amino acid in a nonsteady state, such as occurs after ingestion of a protein meal, is that samples are drawn from blood, but the tracee may be distributed more extensively throughout the body. The consequences of the distribution of the tracee throughout the body have been most closely evaluated in the case of glucose (1), which is distributed throughout blood and interstitial fluid. Therefore, values obtained from the blood or plasma in the nonsteady state may not reflect the values throughout the entire volume of distribution because complete mixing is not instantaneous. Steele (39) described an equation to address this problem with regard to the calculation of nonsteady-state glucose kinetics by tracer dilution. His innovation was the simplifying assumption that in a nonsteady state the tracee and tracer are distributed in some constant fraction (p) of their total volume of distribution (V). Subsequent research has shown that there is no unique assumed value of volume of distribution (pV) for glucose that yields the correct answer in all nonsteady-state circumstances. Rather, the appropriate volume of distribution is time dependent (1, 47). For example, in the case of a step increase in the Ra of glucose, a blood sample early after the change in Ra reflects a very small fraction of the total V. In this phase of the response, a value of pV of 40 ml/kg, which roughly corresponds to the plasma volume, will provide the most accurate value for Ra (1, 47). The fact that initial changes in enrichment and concentration following a step increase in glucose Ra would be distributed primarily in plasma is physiologically reasonable, since the plasma is rapidly mixed. In contrast, distribution throughout the interstitial fluid occurs by diffusion and much more time is required before a blood sample would reflect the concentrations and enrichments of glucose in the interstitial fluid. As time proceeds after a step increase in Ra, a new steady state (i.e., dEiIV/dt = 0) will be approached. This will involve a more uniform distribution of tracer and tracee throughout its entire volume of distribution, and a value of pV reflecting a large percentage of the entire volume of distribution results in a more accurate calculation of glucose Ra (1, 47). When a new steady state is established, the term [pV·C(t)·dEiIV/dt] in Eq. 1 becomes 0.
There is significant literature addressing the most appropriate value of pV for glucose (6); however, choice of the most appropriate value of pV for amino acids is more complicated. Free amino acids are distributed throughout the plasma, interstitial fluid, and also in the intracellular space. To further complicate the issue, exchange between the interstitial fluid and the intracellular space is governed by active transport, with the result that intracellular concentrations differ from the corresponding extracellular fluid concentrations. When the Steele equation is used to calculate the Ra of an amino acid tracee in the nonsteady state by tracer dilution, the uncertainty about the most appropriate value for the volume of distribution (pV in Eq. 1) becomes more problematic. The more rapid the increase in Ra of an amino acid, the greater the overestimation of the true value of Ra by the Steele equation (1, 47). This error results from the use of a constant value for pV, while the correct value for Ra could only be calculated with a time-dependent changing value of pV. The initial response to an increase in Ra reflects a change in enrichment of only a very small portion of the total pool size of the tracee, with the result that the effective value for pV is much smaller than the value of 125 ml/kg assumed in Ref. 5 (1, 47). An overestimation of the correct value of pV will result in a corresponding overestimation of the true Ra (Eq. 1). The problems of calculating the total Ra using the Steele equation in the physiological nonsteady state can be mitigated by adding to the exogenous source of Ra as is being infused intravenously (20, 47). By doing so, the changes in enrichment from the basal equilibrium value are minimized, and the expression [pV·C(t)·dEipo/dt] in Eq. 1 approaches zero, thereby eliminating the need for an assumed value for pV.
The rearrangement of the Steele equation to calculate exogenous Ra from an intrinsically labeled protein (Eq. 2) introduces additional problems beyond what are encountered with using the Steele equation as originally derived and commonly applied. In contrast to the conventional application of the Steele equation, which is used to measure total Ra, the tracer in the intrinsically labeled protein (which is the same amino acid that is infused but labeled differently) is not infused but appears in the blood at a rate that is neither constant nor known. Rather, the appearance of tracer from the intrinsically labeled protein [total Ra·Eipo(t) in Eq. 2] is a measured variable. Furthermore, in contrast to the traditional use of the Steele equation whereby baseline steady-state enrichment is established before any perturbation occurs, there is no tracer in the blood before ingestion of the intrinsically labeled protein. The absence of any tracer in the blood before ingestion of the intrinsically labeled protein means that a nonsteady state of enrichment resulting from the absorbed tracer is inevitable. Since the starting point of the change in enrichment following ingestion of an intrinsically labeled protein is not a preexistent isotopic plateau, the requirement to assume a constant value for pV cannot be avoided when calculating exogenous Ra by Eq. 2.
The problems stemming from the inevitable isotopic nonsteady state following the bolus ingestion of an intrinsically labeled protein can be circumvented by providing an intrinsically labeled protein in small doses over many hours (sip feeding). With sip feeding, the intrinsically labeled protein establishes a steady state in tracer enrichment and Eq. 2 reduces to exogenous Ra = total Ra·Eipo(t)/Epro. This is a valid application of the equation because there is a steady inflow of tracer. However, since food is conventionally eaten as meals rather than ingested continuously over many hours, sip feeding has limited practical relevance.
Equation 2 also suffers from potential issues stemming from recirculation of tracer. The Steele equation is based on the determination of Ra by dilution of a constant infusion of tracer by the appearance of unlabeled tracee. It is a fundamental premise of tracer dilution that at physiological and isotopic steady-state rates of appearance and disappearance of tracer and tracee are equal. In contrast to the conventional use of the Steele equation, Eq. 2 is based on changes in the Ra of tracer. The fact that the tracer Ra is a measured variable rather than a known constant value is not only complicated in the nonsteady state by the issues discussed above but also by the fact that the tracer enrichment in the plasma at any time reflects a combination of a changing Ra of the tracer from absorption and recirculation of previously absorbed tracer. The recirculation of tracer is evident in the decay curve following injection of a mixture of labeled plus unlabeled Leu (14). Two hours after the intravenous bolus injection of labeled Leu, the enrichment in plasma is 50% of the enrichment at t = 0 (14), even though no new tracer is entering the circulation. The problem potentially created in the nonsteady state after ingestion of intrinsically labeled protein can be conceptualized by considering the circumstance after all oral tracer has been absorbed. Tracer will continue to circulate despite the absence of newly absorbed tracer because of the time needed to fully clear the tracer from the blood. As a result, values for exogenous Ra can be overestimated as calculated by Eq. 2. The problem caused by recycling of tracer cannot be overcome by calculating the area under the enrichment versus time curve because the tracer does not enter the circulation as a bolus, but rather the tracer appears over time at a nonsteady rate. Thus taking the area under the curve for exogenous Ra for 4 h will yield unreasonably large values relative to the amount of protein ingested.
The errors resulting from calculating exogenous Ra in the nonsteady state following a bolus ingestion of an intrinsically labeled protein may be sufficient to have physiological implications. The example we will consider is the comparison of the effect of speed of digestion and absorption of a “fast” digested protein (whey protein) and a “slowly” digested protein (casein) on the anabolic impact of a dietary protein (3). Consumption of whey protein causes a more rapid and higher peak increase in essential amino acid concentrations in plasma than consumption of casein (3, 31), and the faster digestion of whey protein is associated with a greater whole body anabolic response according to the intrinsically labeled protein approach (3). Since the amino acid contents of whey and casein are similar, it was concluded that the faster digestion of whey was responsible for its greater anabolic effect (3). While the conclusion that speed of digestion and absorption affect the anabolic response may be valid, there is a reason to question the results from the intrinsically labeled protein study. The predicted errors in calculation of exogenous Ra would favor an overestimation of the anabolic effect of whey as compared with casein. It would be expected that when a constant value for pV is used to calculate the exogenous Ra, a more rapid increase in Leu enrichment and concentration following whey protein ingestion would result in a greater overestimation of exogenous Ra as compared with casein consumption, which results in a smaller disruption of the steady state. An overestimation of exogenous Ra of Leu from whey, relative to casein, would translate to the calculation of a greater anabolic response because endogenous Ra (i.e., protein breakdown) would be underestimated, even if the anabolic responses to whey and casein were actually similar. Whether or not whey protein is more anabolic than casein is uncertain, but results comparing the anabolic responses to whey and casein using other approaches suggest that the conclusion regarding the greater anabolic response to whey protein found with the intrinsically labeled protein may be in error. For example, when the anabolic responses to whey and casein were evaluated during sip feeding of the two proteins to avoid the complications of the nonsteady state, casein, rather than whey, was found to have the greater anabolic effect (9). It could be argued that sip feeding does not address the issue of the effect of speed of absorption, since the experimental protocol eliminates speed of digestion as a factor. However, consistent with the sip-feeding results, in an amino acid balance study across the leg the anabolic response to the bolus ingestion of casein was numerically (but not statistically) greater than the response to whey protein (41). Furthermore, the anabolic effects of whey and casein protein supplementation were indistinguishable in an 8-wk trial involving resistance training (46). Thus, while the importance of the speed of absorption of dietary protein on whole body protein kinetics remains unresolved, the available data are consistent with the possibility that the calculation of exogenous Ra in a rapidly changing nonsteady state (as exists after whey protein ingestion) systematically overestimates the anabolic response to a rapidly digested protein such as whey protein.
Quantitative Estimation of the Errors in the Calculation of Exogenous Ra Using an Intrinsically Labeled Protein
We have discussed how dilution of tracer in the GIT and across the splanchnic bed causes an underestimation of exogenous Ra. Countering this to an unknown extent, the application of the rearrangement of the Steele equation may result in the overestimation of exogenous Ra following a bolus ingestion of protein due to the use of a constant value for pV in the nonsteady state and to the recycling of tracer. It is difficult to estimate the net effect of all of these potential sources of errors. For this reason, the calculated value of exogenous Ra is almost certainly inaccurate.
It is likely that errors in the calculation of exogenous Ra will have a significant consequence in terms of assessing the physiological response to dietary protein. Total Ra is determined by the sum of the exogenous and endogenous Ra (Eq. 3). Endogenous Ra represents the rate of whole body protein breakdown. We will illustrate how underestimating exogenous Ra due to a failure to account for the dilution of tracer in the GIT and across the splanchnic bed will lead to an underestimation of the suppressive effect of dietary protein intake on protein breakdown.
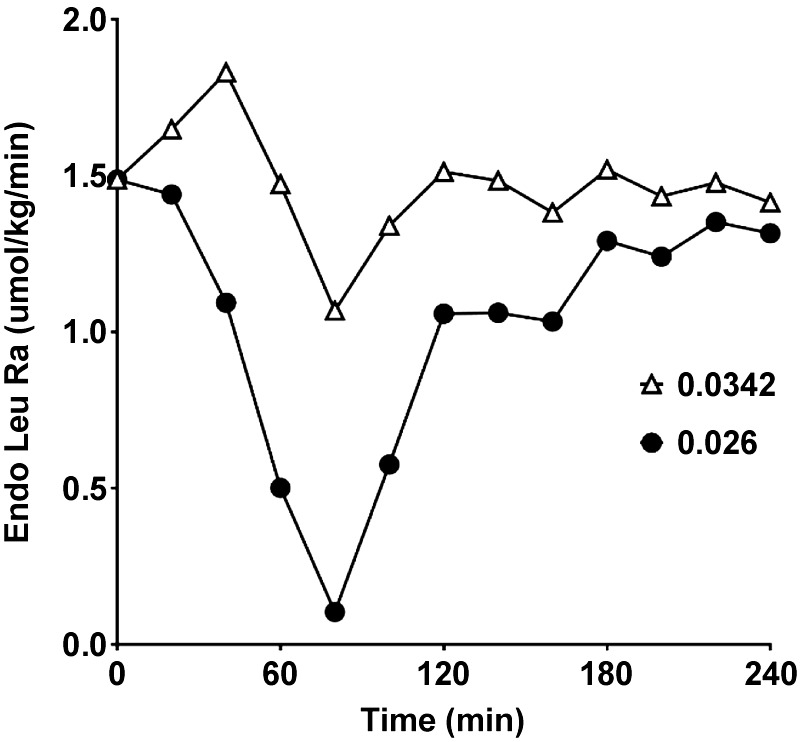
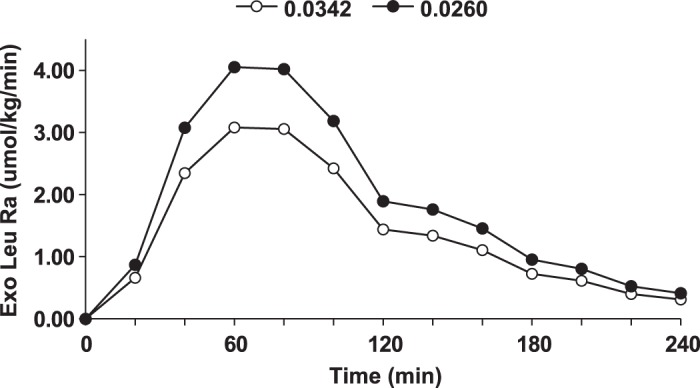
For the purpose of example, we have used published data resulting from the ingestion of an intrinsically labeled whey protein (3). In that study, the enrichment (expressed as MPE) of the intrinsically labeled protein was 0.0342. Our recalculation of exogenous Ra and endogenous Ra using the value of 0.0342 and the data of Leu concentrations and enrichments presented in their paper confirms their calculated values. However, if we make a conservative estimate of the reduction in enrichment resulting from tracer dilution in the GIT and across the splanchnic bed, then the calculated exogenous Ra is underestimated. The dilution of the tracer means that Eipo in Eq. 2 is overestimated. The overestimation of Eipo is reflected in an underestimation of exogenous Ra. In Figs. 2 and 3, we present the results for exogenous Ra and endogenous Ra respectively, calculated from the values for Eipro in Ref. 3 and also using values for Eipro that reflect a 24% overestimation of the correct value for Eipro that accounts for tracer dilution. The assumed dilution of tracer of 24% is conservative as compared with the sum of the values estimated above for dilution of tracer in the GIT plus dilution across the splanchnic bed. We have used this conservative estimate because of the likely overestimation of exogenous Ra resulting from the nonsteady-state kinetics. As explained conceptually above, if tracer dilution is ignored (Fig. 2), the true value for exogenous Ra is underestimated as compared with the exogenous Ra when account is taken of tracer dilution (Fig. 2). Since endogenous Ra is a direct reflection of protein breakdown, the conclusion from the data derived, without taking account of dilution of the tracer in the intrinsically labeled protein, is that dietary protein has little effect on protein breakdown (Fig. 3). On the other hand, when account is taken of tracer dilution, it is concluded that the major site of action of ingested protein on endogenous protein metabolism is a suppression of protein breakdown (Fig. 3).
Fig. 2.
Exogenous (Exo) rate of appearance (Ra) of Leu calculated using data from Ref. 3 in which an intrinsically labeled protein was used. In one case the uncorrected value for enrichment of the intrinsically labeled protein (Eipro) in Ref. 3 was used (open circles) (0.0342) and in the other case the value for Eipro was reduced 24% (0.026) to account for an assumed amount of tracer dilution (closed circles). Mole percent excess was used for the calculations.
Fig. 3.
Calculated values for endogenous (Endo) rate of appearance (Ra) of Leu using data from Ref. 3 in which an intrinsically labeled protein was used. In one case the uncorrected value for enrichment of the intrinsically labeled protein (Eipro) in Ref. 3 was used (open triangles) (0.0342), and in the other case the value for Eipro was reduced 24% (0.026) to account for an assumed amount of tracer dilution (closed circles). As shown, endogenous Ra is a direct reflection of whole body protein breakdown. Mole percent excess (MPE) was used for the calculations.
We have also discussed errors in using the rearranged Steele equation. The errors are more unpredictable than in the case of tracer dilution but may mitigate to some extent the errors caused by not taking account of tracer dilution. Regardless, it is reasonable to presume that errors stemming from use of this equation raise further concern regarding the validity of the intrinsically labeled protein method.
IS THERE AN ALTERNATIVE TO INTRINSICALLY LABELED PROTEINS?
Endogenous Ra is determined by subtracting exogenous Ra from the measured total Ra. In the absence of an intrinsically labeled protein, exogenous Ra is calculated from the total amount of protein ingested, the content of the tracee (e.g., Leu or Phe) in that protein, and the bioavailability of the tracee in the digested protein. The bioavailability approach has been used by many investigators for decades (e.g., Ref. 34).
There are limitations to the bioavailability approach. The nature of the data obtained is limited to the total exogenous appearance, so that a minute-by-minute tracking of the response of endogenous Ra is not possible. In addition, the bioavailability method requires assumptions. First, the true ileal digestibility of the dietary protein must be assumed. True ileal digestibility has been experimentally determined in humans for some proteins, but more commonly digestibility is determined by the difference between the amount of N ingested and the amount of fecal N loss (fecal digestibility). Fecal digestibility values are significantly higher than ileal digestibility for a number (but not all) of amino acids, as well as proteins, because N is absorbed from the colon in forms other than the ingested amino acids (35). Fecal digestibility overestimates exogenous Ra by the difference between fecal digestibility and true ileal digestibility. Actual measurements of true ileal digestibility can be obtained from the literature (e.g., Refs. 29, 36) or can be determined experimentally. Although limited ileal digestibility data are available from human subjects, determination of ileal digestibility in pigs or rats yields values similar to humans (13).
The bioavailability method should provide an accurate estimate of total absorption of unlabeled tracee from dietary protein. However, that value may overestimate the amount of absorbed tracee that appears in the peripheral circulation, because account is not taken of any first-pass net uptake of absorbed tracee in the splanchnic bed. Splanchnic uptake can occur either as a result of oxidation or a positive net balance between protein synthesis and protein breakdown. The extent to which absorbed tracee is cleared on the first pass through the splanchnic bed will be reflected in the calculation of total Ra because the tracer enrichment in the peripheral circulation (where sampling occurs) will not be diluted by exogenous appearance of unlabeled tracee to the same extent as if there was no first-pass splanchnic clearance. The calculated value of total Ra is inversely related to the isotopic enrichment in plasma (e.g., Ref. 1). Thus total Ra calculated from the tracee enrichment in the peripheral circulation will not include any absorbed tracee cleared by the splanchnic bed. Since protein breakdown is calculated as the difference between total and exogenous Ra, the value for protein breakdown will be underestimated by the extent of first-pass splanchnic uptake unless there is appropriate correction of exogenous Ra. Correction of exogenous Ra for first-pass irreversible loss in the splanchnic bed can be accomplished when using Phe/Tyr tracers, since Phe hydroxylation to Tyr occurs in the liver. Thus the difference between the total and corrected exogenous Ra will be a good reflection of protein breakdown, with the assumption that there is a balance between protein synthesis and breakdown in the splanchnic bed.
Protein synthesis is calculated as the difference between total Ra and tracee oxidation/hydroxylation. Since both measures are based on the enrichment of tracer in the peripheral circulation, first-pass splanchnic uptake should not affect the difference between total Ra and tracee oxidation. Consequently, the calculation of protein synthesis (and therefore net protein balance) should be accurate.
Although assumptions of uncertain validity are required to estimate bioavailability, these assumptions do not result in systematic over- or under-estimations of exogenous Ra. The bioavailability approach has the further advantage of being applicable to a mixed meal comprised of different protein food sources. For these reasons, the bioavailability method has distinct advantages over the intrinsically labeled protein approach.
PHYSIOLOGICAL IMPLICATIONS OF UNDERESTIMATION OF EXOGENOUS Ra BY THE INTRINSICALLY LABELED PROTEIN METHOD
The conclusion from our preceding discussion is that the intrinsically labeled protein method underestimates exogenous Ra. This perspective is supported by the results of a recent paper in which the intrinsically labeled protein method was used in conjunction with the measurement of arterial-venous balance across the leg and the measurement of muscle protein synthesis using the direct incorporation technique to determine the FSR of muscle protein (16). The intrinsically labeled protein method determined that 55.3% of ingested protein (20 g) appeared in the peripheral circulation and that 20% of that amount was incorporated into muscle protein on the basis of the arterial-venous balance across the muscle (16). This means that ~2.2 g of ingested protein was incorporated into muscle protein or ~11% of the total ingested protein. However, that result is at odds with the simultaneously determined muscle FSR, which increased from 0.029 to 0.044%/h or 0.075% over the 5-h measurement period. For comparative purposes, it is necessary to extrapolate the FSR data to the total amount of muscle protein produced. Skeletal muscle mass is ~40–45% of body weight, depending on gender and body type (7, 17), and ~20% of that weight is muscle protein (7). If we assume that skeletal muscle mass constituted 40% of body weight, then the average skeletal muscle protein of the subjects (average body weight = 72.5 kg) was ~5.8 kg (72.5 kg × 40% skeletal muscle × 20% protein). Over the 5 h of the study after ingestion of protein, muscle protein synthesis thus increased 4.35 g (0.00075 × 5.8 kg). This value is 22% of the ingested 20 g casein protein, accounting for the bioavailability of Leu. The estimation of 22% of the absorbed Leu is approximately double the value of 11% based on the estimation of exogenous Ra using the intrinsically labeled protein approach. The higher value determined by the FSR method is consistent with the notion that the intrinsically labeled protein method underestimates exogenous Ra.
The underestimation of exogenous Ra by the intrinsically labeled protein method has important implications with regard to assessing the metabolic response to dietary protein intake at the whole body level. An underestimation of exogenous Ra will result in an overestimation of endogenous Ra. Since endogenous Ra of an essential amino acid tracee is a direct reflection of protein breakdown, use of the intrinsically labeled protein method will potentially obscure a suppressive effect of dietary protein intake on protein breakdown. We have recently discussed evidence that the suppression of protein breakdown is an important aspect of the anabolic response to dietary protein intake (21). The direct suppressive effect on muscle protein breakdown of a large increase in the blood concentrations of amino acids has been well established for almost 30 yr (12, 24, 25,). In contrast, studies utilizing the intrinsically labeled protein method to quantify exogenous Ra have concluded that dietary protein intake does not suppress protein breakdown (e.g., 5).
In contrast to the potential misinterpretation of the physiological response of whole body protein breakdown to dietary protein intake resulting from the use of the intrinsically labeled protein method, the calculation of whole body protein synthesis is not affected by the estimation of exogenous Ra. Whole body protein synthesis is calculated from the difference between total Ra of an essential amino acid and its rate of oxidation, with the assumption that what is not oxidized is incorporated into protein.
OTHER USES OF INTRINSICALLY LABELED PROTEINS
Our assessment of the validity of the intrinsically labeled protein is specific to the quantification of the rate of whole body protein synthesis and breakdown. There are alternative uses of an intrinsically labeled protein or ingestion of a labeled free amino acid. One such application is the calculation of hepatic or gut “first-pass” clearance (26, 40, 44). The principle of this method is similar to the methodology we have discussed, but the oral tracer is given as a free amino acid rather than a protein-bound amino acid. The oral tracer has the same problems of tracer dilution discussed for the intrinsically labeled protein method and will therefore overestimate first-pass clearance. Other applications of intrinsically labeled proteins may account for some of the issues we have discussed. For example, use of two different intrinsically labeled proteins, one of which is a standard, to quantify relative rates of absorption (e.g., Ref. 10) avoids some of the problems caused by tracer dilution in the GIT and splanchnic bed. Similarly, use of sip feeding to establish a steady state of dietary intake (9) can bypass the problems caused by the nonsteady state, although the physiological response to the more common meal feeding pattern may differ from the response to sip feeding. One of the earliest uses of intrinsically labeled protein methodology was to label the body N pool (32), in which case the dilution of ingested tracer is not relevant to the calculation. 15N-labeled proteins have also been used to trace the metabolism of absorbed nitrogen, and to determine the utilization of dietary proteins.
CONCLUSION
The use of intrinsically labeled proteins to quantify the contribution of amino acids absorbed from digested dietary protein to the plasma pool of amino acids (i.e., exogenous Ra) is unreliable. The methodology requires the incorrect assumptions that there is no dilution of tracer in the GIT, that all uptake of tracer in the splanchnic bed corresponds to net uptake of ingested amino acid, that there is no recycling of tracer, and (in the case of a nonsteady state following ingestion of a protein meal) that measured changes in concentration and enrichment over time in peripheral blood are distributed uniformly in a unique volume of distribution. Failure to account for the dilution of the tracer amino acid from the exogenous protein in the processes of digestion and absorption, as well as transit through the splanchnic bed, will result in an underestimation of exogenous Ra, which in turn leads to an overestimation of the true rate of protein breakdown (i.e., endogenous Ra). An overestimation of the rate of protein breakdown may lead to a mistaken conclusion that the anabolic effect of dietary protein is entirely due to a stimulation of protein synthesis rather than a combination of stimulated protein synthesis and inhibited protein breakdown. Furthermore, complications caused by nonsteady-state kinetics of a rapidly absorbed protein (e.g., whey), as opposed to slowly absorbed protein (e.g., casein), could potentially explain the apparent importance of speed of digestion on calculated differences in anabolic responses. As a result of these considerations, it is our contention that the intrinsically labeled protein approach should not be used to quantify the exogenous Ra in plasma of amino acids contained in dietary protein.
In contrast to the intrinsically labeled protein approach, the bioavailability approach involves no systematic under- or over-estimation of exogenous Ra, so reasonable values for protein synthesis and breakdown can be estimated. While the bioavailability approach does not enable a minute-by-minute tracking of the response of protein synthesis and breakdown to a protein meal, the overall response can be calculated without bias. Consequently, in our view the bioavailability method provides a better estimation of the contribution of exogenous Ra than the intrinsically labeled protein approach.
GRANTS
This work was supported by National Cattlemen’s Beef Association, National Pork Board. American Egg Board/Egg Nutrition Center, National Institute on Aging Grant 2R42-AG0-50375, and Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2018R1D1AB07051053).
DISCLOSURES
R. R. Wolfe has received honoraria and research grants from the National Cattleman’s Beef Association and is a shareholder in Essential Blends, LLC. R. R. Wolfe is a consultant for Trivita, LLC and Accella, LLC.
AUTHOR CONTRIBUTIONS
R.R.W., A.A.F., and P.J.M. conceived and designed research; R.R.W., S.P., and I.-Y.K. performed experiments; R.R.W., S.P., I.-Y.K., C.S., A.A.F., and P.J.M. analyzed data; R.R.W., S.P., I.-Y.K., C.S., A.A.F., and P.J.M. interpreted results of experiments; R.R.W., S.P., and I.-Y.K. prepared figures; R.R.W. drafted manuscript; R.R.W., S.P., I.-Y.K., C.S., B.J.M., A.A.F., and P.J.M. edited and revised manuscript; R.R.W., S.P., I.-Y.K., C.S., B.J.M., A.A.F., and P.J.M. approved final version of manuscript.
REFERENCES
- 1.Allsop JR, Wolfe RR, Burke JF. The realiability of rates of glucose appearance in vivo calculated from constant tracer infusions. Biochem J 172: 407–416, 1978. doi: 10.1042/bj1720407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barle H, Nyberg B, Essén P, Andersson K, McNurlan MA, Wernerman J, Garlick PJ. The synthesis rates of total liver protein and plasma albumin determined simultaneously in vivo in humans. Hepatology 25: 154–158, 1997. doi: 10.1053/jhep.1997.v25.pm0008985282. [DOI] [PubMed] [Google Scholar]
- 3.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci USA 94: 14930–14935, 1997. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boirie Y, Fauquant J, Rulquin H, Maubois JL, Beaufrère B. Production of large amounts of [13C]leucine-enriched milk proteins by lactating cows. J Nutr 125: 92–98, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Boirie Y, Gachon P, Corny S, Fauquant J, Maubois JL, Beaufrère B. Acute postprandial changes in leucine metabolism as assessed with an intrinsically labeled milk protein. Am J Physiol Endocrinol Metab 271: E1083–E1091, 1996. doi: 10.1152/ajpendo.1996.271.6.E1083. [DOI] [PubMed] [Google Scholar]
- 6.Butler PC, Caumo A, Zerman A, O’Brien PC, Cobelli C, Rizza RA. Methods for assessment of the rate of onset and offset of insulin action during nonsteady state in humans. Am J Physiol 264: E548–E560, 1993. doi: 10.1152/ajpendo.1993.264.4.E548. [DOI] [PubMed] [Google Scholar]
- 7.Brooks GA, Fahey TD, Baldwin KM. Exercise Physiology: Human Bioenergetics and its Applications (4th ed) New York: McGraw-Hill, 2005. p. 363. [Google Scholar]
- 8.Deglaire A. Gut Endogenous Protein Flows and Postprandial Metabolic Utilization of Dietary Amino Acids in Simple-Stomached Animals and Humans (PhD Thesis) Palmerston North, New Zealand: Massey University, 2008. [Google Scholar]
- 9.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Casein protein results in higher prandial and exercise induced whole body protein anabolism than whey protein in chronic obstructive pulmonary disease. Metabolism 61: 1289–1300, 2012. doi: 10.1016/j.metabol.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelen MP, Com G, Anderson PJ, Deutz NE. New stable isotope method to measure protein digestibility and response to pancreatic enzyme intake in cystic fibrosis. Clin Nutr 33: 1024–1032, 2014. doi: 10.1016/j.clnu.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eklund M, Mosenthin R, Piepho HP, Rademacher M. Effect of dietary crude protein level on basal ileal endogenous losses and standardized ileal digestibilities of crude protein and amino acids in newly weaned pigs. J Anim Physiol Anim Nutr (Berl) 92: 578–590, 2008. doi: 10.1111/j.1439-0396.2007.00751.x https://www.ncbi.nlm.nih.gov/pubmed/19012602. [DOI] [PubMed] [Google Scholar]
- 12.Ferrando AA, Williams BD, Stuart CA, Lane HW, Wolfe RR. Oral branched-chain amino acids decrease whole-body proteolysis. JPEN J Parenter Enteral Nutr 19: 47–54, 1995. doi: 10.1177/014860719501900147. [DOI] [PubMed] [Google Scholar]
- 13.Food and Agriculture Organization of the United Nations Research approaches and methods for evaluating the protein quality of human foods. FAO Food and Nutrition. In: Report of a FAO Expert Working Group. Bangalore, India: Food and Agriculture Organization of the United Nations, 2014. [Google Scholar]
- 14.Garlick PJ, Wernerman J, McNurlan MA, Essen P, Lobley GE, Milne E, Calder GA, Vinnars E. Measurement of the rate of protein synthesis in muscle of postabsorptive young men by injection of a ‘flooding dose’ of [1-13C]leucine. Clin Sci (Lond) 77: 329–336, 1989. doi: 10.1042/cs0770329. [DOI] [PubMed] [Google Scholar]
- 15.Gaudichon C, Bos C, Morens C, Petzke KJ, Mariotti F, Everwand J, Benamouzig R, Daré S, Tomé D, Metges CC. Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology 123: 50–59, 2002. doi: 10.1053/gast.2002.34233. [DOI] [PubMed] [Google Scholar]
- 16.Groen B, Hamer HM, van Kranenburg, Bierau J, Poeze M, Wodzig WK, Rasmussen BB, van Loon LJ. Post-prandial protein handling: you are what you just ate. PLoS One 10: e0141582, 2015. doi: 10.1371/journal.pone.0141582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall JE. Guyton and Hall Textbook of Medical Physiology 13E. Philadelphia, PA: Elsevier, 2016, p. 75. [Google Scholar]
- 18.Hodgkinson SM, Moughan PJ, Reynolds GW, James KA. The effect of dietary peptide concentration on endogenous ileal amino acid loss in the growing pig. Br J Nutr 83: 421–430, 2000. doi: 10.1017/S0007114500000520. [DOI] [PubMed] [Google Scholar]
- 19.Institute of Medicine of the National Academies Dietary Reference Intakes For Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids (Macronutrients). Washington, DC: National Academies Press, 2002–2005 [Google Scholar]
- 20.Jonker R, Deutz NE, Schols AM, Veley EA, Harrykissoon R,Zachria AJ, Engelen MP. Whole body protein anabolism in COPD patients and healthy older adults is not enhanced by adding eithercarbohydrates or leucine to a serving of protein. Clin Nutr. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim IY, Deutz NEP, Wolfe RR. Update on maximal anabolic response to dietary protein. Clin Nutr 37: 411–418, 2018. doi: 10.1016/j.clnu.2017.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krawielitzki K, Zebrowska T, Schadereit R, Kowalczyk J, Hennig U, Wünsche J, Herrmann U. Determining of nitrogen absorption and nitrogen secretion in different sections of the pig’s intestine by digesta exchange between 15N labelled and unlabelled animals. Arch Tierernahr 40: 25–37, 1990. doi: 10.1080/17450399009428378. [DOI] [PubMed] [Google Scholar]
- 23.Leterme P, Théwis A, François E, Van Leeuwen P, Wathelet B, Huisman J. The use of 15N-labeled dietary proteins for determining true ileal amino acid digestibilities is limited by their rapid recycling in the endogenous secretions of pigs. J Nutr 126: 2188–2198, 1996. doi: 10.1093/jn/126.9.2188. [DOI] [PubMed] [Google Scholar]
- 24.Louard RJ, Barrett EJ, Gelfand RA. Effect of infused branched-chain amino acids on muscle and whole-body amino acid metabolism in man. Clin Sci (Lond) 79: 457–466, 1990. doi: 10.1042/cs0790457. [DOI] [PubMed] [Google Scholar]
- 25.Louard RJ, Barrett EJ, Gelfand RA. Overnight branched-chain amino acid infusion causes sustained suppression of muscle proteolysis. Metabolism 44: 424–429, 1995. doi: 10.1016/0026-0495(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DE, Marano MA, Campbell RG. Splanchnic bed utilization of leucine and phenylalanine in humans. Am J Physiol Endocrinol Metab 264: 27: E109–E118, 1993. doi: 10.1152/ajpendo.1993.264.1.E109. [DOI] [PubMed] [Google Scholar]
- 27.Molina DK, Dimaio VJ. Normal organ weights in men part IIV the brain, lungs, liver, spleen, and kidneys. Am J Forensic Med Pathol 33: 368–372, 2012. doi: 10.1097/PAF.0b013e31823d29ad. [DOI] [PubMed] [Google Scholar]
- 28.Moughan PJ, Butts CA, Rowan AM, Deglaire A. Dietary peptides increase endogenous amino acid losses from the gut in adults. Am J Clin Nutr 81: 1359–1365, 2005. doi: 10.1093/ajcn/81.6.1359. [DOI] [PubMed] [Google Scholar]
- 29.Moughan P, Miner-Williams W, Has R. The digestionof protein-amino acid digstibility. In: Feed Evaluation Science. Moughan P, Hendriks W. Wageningen, The Netherlands: Wageningen Academic Publishers, 2018, p. 173–217. [Google Scholar]
- 30.Pennings B, Pellikaan WF, Senden JM, van Vuuren AM, Sikkema J, van Loon LJC. The production of intrinsically labeled milk and meat protein is feasible and provides functional tools for human nutrition research. J Dairy Sci 94: 4366–4373, 2011. doi: 10.3168/jds.2011-4451. [DOI] [PubMed] [Google Scholar]
- 31.Pennings B, Boirie Y, Senden JM, Gijsen AP, Kuipers H, van Loon LJ. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am J Clin Nutr 93: 997–1005, 2011. doi: 10.3945/ajcn.110.008102. [DOI] [PubMed] [Google Scholar]
- 32.Picou D, Taylor-Roberts T. The measurement of total protein synthesis and catabolism and nitrogen turnover in infants in different nutritional states and receiving different amounts of dietary protein. Clin Sci 36: 283–296, 1969. [PubMed] [Google Scholar]
- 33.Ravindran V, Morel PC, Rutherfurd SM, Thomas DV. Endogenous flow of amino acids in the avian ileum as influenced by increasing dietary peptide concentrations. Br J Nutr 101: 822–828, 2009. doi: 10.1017/S0007114508039974 https://www.ncbi.nlm.nih.gov/pubmed/18662428. [DOI] [PubMed] [Google Scholar]
- 34.Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci (Lond) 63: 519–523, 1982. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- 35.Rowan AM, Moughan PJ, Wilson MN, Maher K, Tasman-Jones C. Comparison of the ileal and faecal digestibility of dietary amino acids in adult humans and evaluation of the pig as a model animal for digestion studies in man. Br J Nutr 71: 29–42, 1994. doi: 10.1079/BJN19940108. [DOI] [PubMed] [Google Scholar]
- 36.Rutherfurd SM, Moughan PJ. The digestible amino acid composition of several milk proteins: application of a new bioassay. J Dairy Sci 81: 909–917, 1998. doi: 10.3168/jds.S0022-0302(98)75650-4. [DOI] [PubMed] [Google Scholar]
- 37.Starck CS, Wolfe RR, Moughan PJ. Endogenous amino acid losses from the gastrointestinal tract of the adult human — a quantitative model. J Nutr 148: 1871–1881, 2018. doi: 10.1093/jn/nxy162. [DOI] [PubMed] [Google Scholar]
- 38.Souffrant WB, Rérat A, Laplace JP, Darcy-Vrillon B, Köhler R, Corring T, Gebhardt G, Bernard F, Jähnichen ML, Schneider B, Cointepas F. Exogenous and endogenous contributions to nitrogen fluxes in the digestive tract of pigs fed a casein diet. III. Recycling of endogenous nitrogen. Reprod Nutr Dev 33: 373–382, 1993. doi: 10.1051/rnd:19930406. [DOI] [PubMed] [Google Scholar]
- 39.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci 82: 420–430, 1959. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 40.Stoll B, Burrin DG, Henry J, Jahoor F, Reeds PJ. Phenylalanine utilization by the gut and liver measured with intravenous and intragastric tracers in pigs. Am J Physiol Gastrointest Liver Physiol 273: G1208–G1217, 1997. doi: 10.1152/ajpgi.1997.273.6.G1208. [DOI] [PubMed] [Google Scholar]
- 41.Tipton KD, Elliott TA, Cree MG, Wolf SE, Sanford AP, Wolfe RR. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med Sci Sports Exerc 36: 2073–2081, 2004. doi: 10.1249/01.MSS.0000147582.99810.C5. [DOI] [PubMed] [Google Scholar]
- 42.van Loon LJ, Boirie Y, Gijsen AP, Fauquant J, de Roos AL, Kies AK, Lemosquet S, Saris WH, Koopman R. The production of intrinsically labeled milk protein provides a functional tool for human nutrition research. J Dairy Sci 92: 4812–4822, 2009. doi: 10.3168/jds.2009-2317. [DOI] [PubMed] [Google Scholar]
- 43.van Vliet S, Beals JW, Parel JT, Hanna CD, Utterback PL, Dilger AC, Ulanov AV, Li Z, Paluska SA, Moore DR, Parsons CM, Burd NA. Development of Intrinsically Labeled Eggs and Poultry Meat for Use in Human Metabolic Research. J Nutr 146: 1428–1433, 2016. doi: 10.3945/jn.115.228338. [DOI] [PubMed] [Google Scholar]
- 44.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab 277: E513–E520, 1999. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 45.Waterlow JC, Weisz T. The fat, protein and nucleic acid content of the liver in malnourished human infants. J Clin Invest 35: 346–354, 1956. doi: 10.1172/JCI103284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilborn CD, Taylor LW, Outlaw J, Williams L, Campbell B, Foster CA, Smith-Ryan A, Urbina S, Hayward S. The effects of pre- and post-exercise whey vs. casein protein consumption on body composiiton and performance measures in collegiate female athletes. J Sports Sci Med 12: 74–79, 2013. [PMC free article] [PubMed] [Google Scholar]
- 47.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. New York: Wiley-Liss, 2005. [Google Scholar]
- 48.Wolfe RR, Wolfe MH, Nadel ER, Shaw JH. Isotopic determination of amino acid-urea interactions in exercise in humans. J Appl Physiol 56: 221–229, 1984. doi: 10.1152/jappl.1984.56.1.221. [DOI] [PubMed] [Google Scholar]