Abstract
Exposure to dust in agricultural and animal environments, known as organic dust, is associated with the development of respiratory symptoms and respiratory diseases. Inflammation is a key feature of lung pathologies associated with organic dust exposure, and exposure to organic dust induces the expression of several immune and inflammatory mediators. However, information on transcription factors and cellular and molecular mechanisms controlling the production of immune and inflammatory mediators induced by organic dust is limited. In this study, we have identified STAT-3 as an important transcription factor controlling the induction of expression of immune and inflammatory mediators by poultry dust extracts in airway epithelial cells and in mouse lungs and delineated the cellular pathway for STAT-3 activation. Poultry dust extract activated STAT-3 phosphorylation in Beas2B and normal human bronchial epithelial cells and in mouse lungs. Chemical inhibition and siRNA knockdown of STAT-3 suppressed induction of immune and inflammatory mediator expression. Antioxidants suppressed the increase of STAT-3 phosphorylation induced by poultry dust extract indicating that oxidative stress [elevated reactive oxygen species (ROS) levels] is important for the activation. Chemical inhibition and siRNA knockdown experiments demonstrated that STAT-3 activation is dependent on the activation of nonreceptor tyrosine-protein kinase 2 (TYK2) and epidermal growth factor receptor (EGFR) tyrosine kinases. Our studies show that poultry dust extract controls the induction of immune and inflammatory mediator expression via a cellular pathway involving oxidative stress-mediated STAT-3 activation by TYK2 and EGFR tyrosine kinases.
Keywords: cytokines, gene regulation, inflammation, occupational lung diseases, oxidative stress
INTRODUCTION
Occupational exposure to agricultural dust is associated with increased prevalence of acute and chronic respiratory diseases (1, 60). Asthma, hypersensitivity pneumonitis, bronchitis, chronic obstructive pulmonary disease, and organic dust toxic syndrome are some of the respiratory diseases afflicting animal and agricultural workers. The practice of concentrated animal feeding operations (CAFOs; 22) and 8 h-or-longer work shifts exposes workers to significant amounts of airborne dust. The poultry production industry, a significant component of the agricultural economy, is rapidly growing worldwide and employs several hundreds of thousands of workers in the United States alone (38a, 58a). Studies have shown that poultry farmworkers are exposed to higher levels of airborne dusts compared with other animal farmworkers and experience higher prevalence and greater severity of respiratory diseases (44, 54). Poultry CAFO dust is a complex mixture of organic and inorganic materials derived from microbes, mites, bedding and feed materials, soil, and other materials found in the immediate environment (23). Poultry dust has been reported to contain endotoxin, peptidoglycan, and gases such as ammonia, methane, and hydrogen sulfide adsorbed on the particles (23).
Workers engaged in agricultural and animal farming operations experience adverse respiratory health effects due to exposure to dust, microbes, and gases in their work environment (1). Respiratory symptoms and associated lung inflammatory responses have been more extensively investigated in human subjects exposed to swine CAFO dust compared with other animal CAFO dusts. Acute exposure of naïve human volunteers to swine CAFO dust induces airway hyperresponsiveness, fever, chills, and malaise (27, 30, 62). Exposure was found to increase nasal and bronchoalveolar lavage (BAL) fluid levels of IL-6, IL-8, TNF-α, IL-1α, and IL-1β (28, 62). Increases in IL-8 levels were associated with elevated neutrophil counts in nasal and BAL fluids; however, a significant correlation between IL-8 levels and neutrophil numbers was found for nasal lavage but not for BAL (28). Markers of inflammation were more pronounced in naïve subjects compared with animal farmworkers, suggesting adaptive responses to agents present in the dust (58, 61).
Despite the high prevalence of respiratory symptoms and respiratory diseases, molecular mechanisms underlying the pathogenesis of respiratory diseases in agricultural workers are not well understood. Because inflammation is a key player in the development of lung diseases (48), we have been interested in understanding cellular and molecular mechanisms mediating production of immune and inflammatory mediators induced by organic dust. The airway epithelium acts as a frontline barrier against microbial pathogens and particulates and functions to regulate immune and inflammatory responses (36). In addition to serving as a physical barrier, airway epithelial cells produce proinflammatory cytokines and chemokines and other bioactive molecules to modulate host immune and inflammatory responses (36). Published studies have shown that organic dusts increase lung levels of inflammatory cytokines in human subjects (28, 62), experimental animals (13, 37, 43), and lung cells in vitro (20, 39, 45, 64). Pattern recognition receptors, which recognize a wide variety of microbial components, such as Toll-like receptor 2 (TLR2; 42), TLR4 (12, 57), nucleotide-binding oligomerization domain-containing protein-2 (NOD2; 41), and scavenger receptor A (SRA)/cluster of differentiation 204 (CD204; 40), have been demonstrated to be involved in the control of production of chemokines-cytokines and lung inflammatory responses in mice exposed to swine CAFO dust. Our gene expression-profiling studies showed that poultry dust extract induces the expression of chemokines, cytokines, and other inflammatory proteins in lung epithelial and THP-1 (a human monocytic leukemia cell line) monocytic cells (5). IL-8 induction by poultry dust extract is controlled primarily at the transcriptional level mediated via NF-κB and activator protein-1 (AP-1) DNA binding (20). Protease activities found in organic dust extracts (38, 46) and cellular oxidant stress via activation of protein kinase C and NF-κB (38) were found to control induction of inflammatory gene expression in airway epithelial cells.
Molecular mechanisms controlling the induction of immune and inflammatory mediators by organic dust are not fully understood. The involvement of transcription factors other than NF-κB and AP-1 in the induction of inflammatory mediators is not known. Because poultry dust extract induces several immune and inflammatory genes, it is likely that transcription factors other than NF-κB and AP-1 might also be involved in the modulation of immune and inflammatory mediator expression. We found in preliminary studies that poultry dust extracts increased signal transducer and activator of transcription 3 (STAT-3) phosphorylation indicating its activation in Beas2B cells (a human bronchial epithelial cell line). On the basis of this information, we hypothesized that STAT-3 controls the production of inflammatory mediators induced by poultry dust extracts in lung epithelial cells. In this study, we report on the identification of STAT-3 as an important transcription factor controlling the induction of inflammatory mediators in airway epithelial cells by poultry dust extracts. Furthermore, oxidant stress and activation of nonreceptor tyrosine-protein kinase 2 (TYK2) and epidermal growth factor receptor (EGFR) tyrosine kinases were found to control STAT-3 activation to modulate inflammatory mediator levels.
MATERIALS AND METHODS
Chemicals.
Stattic (STAT-3 inhibitory compound; Cayman Chemical) and AG490 (Cayman Chemical) were dissolved in dimethyl sulfoxide (DMSO). N-acetylcysteine (NAC; Sigma-Aldrich) was dissolved in serum-free cell culture medium and adjusted to pH 7.4 with sodium hydroxide. PD 153035 hydrochloride (Apex Biotechnology) and 1-(2-cyano-3, 12, 28-trioxooleana-1, 9(11)-dien-28yl)-1H-imidazole (CDDOIm) obtained from the National Cancer Institute were dissolved in DMSO. Dimethylthiourea (DMTU; Acros Organics) was prepared in cell culture medium without serum. Cremophor (Kolliphor EL) was from Sigma-Aldrich. siRNAs for human STAT-3 (sc-29493), EGFR (sc-29301), TYK2 (sc-36764), protease-activated receptor 1 (PAR-1; sc-36663), and PAR-2 (sc-36188) were obtained from Santa Cruz Biotechnology and dissolved in autoclaved water.
Dust extract preparation.
Settled broiler poultry dust collected from a commercial poultry facility located in Texas in April 2010 had been stored at −80°C. The poultry houses are constructed of solid-side walls and equipped with tunnel ventilation. Aqueous poultry dust extract was prepared by mixing dust with Kaighn’s modification of Ham’s F-12 medium (F-12K medium) containing penicillin (100 U/ml), streptomycin (100 µg/ml), and amphotericin B (0.25 µg/ml) or endotoxin-free Dulbecco’s phosphate-buffered saline (Dulbecco’s PBS) at a ratio of 1:10 (wt/vol) as described previously (20), and the concentration of the extract thus obtained was arbitrarily considered as 100%.
Cell culture.
Beas2B bronchial epithelial cells (American Type Culture Collection CRL-9609) were grown on cell culture dishes coated with bovine serum albumin, fibronectin, and bovine type I collagen in LHC-9 medium (Invitrogen). Normal human bronchial epithelial (NHBE) cells (Lonza or Lifeline Cell Technology) were maintained in bronchial epithelial growth medium (BEGM; Lonza) or BronchiaLife Complete Medium (Lifeline Cell Technology). Cells were grown at 37°C in a humidified atmosphere of 5% CO2 and 95% room air with media replaced every 2–3 days. They were grown to ~80% confluence and maintained overnight in serum-free RPMI 1640 medium containing penicillin (100 U/ml), streptomycin (100 µg/ml), and amphotericin B (0.25 µg/ml) before treatment. After treatment, cells were washed once with cold PBS and lysed in buffer containing 50 mM Tris·HCl pH 7.4, 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 15% glycerol, and 1X protease and phosphate inhibitor cocktail, and supernatants were obtained by centrifugation at 13,000 rpm at 4°C for 10 min and stored at −80°C until use.
MTS assay.
Cell viability was determined using MTS assay (Cell Titer 96 Aqueous Non-Radioactive Cell Proliferation Assay; Promega).
Animal experiments.
Female C57BL/6 mice (6–8 wk; The Jackson Laboratory) were maintained at 24 ± 1°C (SE) under a 12-h light-dark cycle and fed a standard diet and water. Animals were acclimated for 1 wk before the start of the experiment. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at Tyler. Mice were intraperitoneally injected with CDDOIm (5 mg/kg, 100 μl) dissolved in vehicle containing 10% DMSO, 10% Cremophor, and 80% PBS or vehicle alone once daily for 2 days. Mice were then anesthetized with ketamine-xylazine and intranasally administered 50 μl of PBS or dust extract (20% in PBS). After 2 h, mice were euthanized, and their lungs were homogenized in buffer containing 10 mM Tris·Cl pH 7.5, 4 M NaCl, 0.5 M EDTA, 1% Triton X-100, and 1X protease and phosphatase inhibitor cocktail. Homogenates were cleared by centrifugation at 13,000 rpm at 4°C for 10 min, and supernatants were stored at −80°C until use.
Mice were intraperitoneally injected with Stattic (10 mg/kg, 200 μl) dissolved in vehicle containing 10% DMSO, 10% Cremophor, and 80% PBS or vehicle alone (16 h) before administration of dust extract. On the day of the experiment, Stattic or vehicle was injected again, and after 1 h, mice were anesthetized and intranasally administered 50 μl PBS or 20% dust extract. After 3 h, mice were euthanized, lungs were perfused with PBS, and homogenates were prepared.
Protein determination.
Protein concentrations of cell lysates and mouse lung homogenates were determined by Bradford assay using bovine serum albumin as the protein standard.
Enzyme-linked immunosorbent assay.
IL-6, IL-8, keratinocyte chemoattractant (KC), and TNF-α levels in cell medium or mouse lung homogenates were determined according to the ELISA kit instructions (R&D Systems).
Western blot analysis.
Equal amounts (15–30 µg) of protein were separated by SDS-PAGE in 10% Bis-Tris gels (Thermo Fisher Scientific) and transferred to PVDF membranes (GE Healthcare Amersham) by electroblotting. Membranes were blocked for 1 h at room temperature in 5% nonfat dry milk (Fisher Scientific) and then incubated with primary antibodies against pSTAT-3 (Tyr705) (1:1,000 dilution, cat. no. 9145; Cell Signaling Technology), STAT-3 (1:1,000 dilution, cat. no. 4904; Cell Signaling Technology), ICAM-1 (1:1,000 dilution, cat. no. sc-8439, Santa Cruz Biotechnology), cyclooxygenase-2 [COX-2, also known as prostaglandin G/H synthase 2 (PTGS2), 1:1,000 dilution, cat. no. A303-600A; Bethyl Laboratories], pro-IL-1β (1:1,000 dilution, cat. no. 12703; Cell Signaling Technology), pTYK2 (1:500 dilution, cat. no. sc-11763; Santa Cruz Biotechnology), TYK2 (1:1,000 dilution, cat. no. 14193; Cell Signaling Technology), pEGFR (Tyr845) (1:1,000, cat. no. 2231; Cell Signaling Technology), EGFR (1:1,000, cat. no. 2256; Cell Signaling Technology), actin (1:1,000 dilution, cat. no. sc-47778; Santa Cruz Biotechnology), or tubulin (1:1,000 dilution, cat. no. MS-581-P0; Thermo Fisher Scientific) overnight at 4°C and then with alkaline phosphatase-conjugated secondary antibody for 1 h at room temperature. Protein bands were detected by the enhanced chemifluorescence detection method (GE HealthCare Life Sciences) after scanning with Pharos FX Plus Molecular Imager (Bio-Rad) and quantified using Quantity One software (Bio-Rad).
RNA isolation and real-time quantitative RT-PCR.
Total RNA was isolated using Direct-zol RNA MiniPrep Plus kit (Zymo Research) or TRI Reagent (Molecular Research Center), and cDNA was synthesized using an iScript Reverse Transcription kit (Bio-Rad). Levels of mRNAs and 18S rRNA were determined by TaqMan probe-based assay (Bio-Rad; Table 1) using CFX96 Real-Time PCR Detection System (Bio-Rad), and mRNA levels were normalized to 18S rRNA levels.
Table 1.
TaqMan gene expression IDs used in quantitative RT-PCR
| Gene Symbol | Gene Name | Human Assay ID |
|---|---|---|
| IL-8 | Interleukin-8 | Hs00174103_m1 |
| ICAM-1 | Intercellular adhesion molecule-1 | Hs00164932_m1 |
| IL-1β | Interleukin-1β | Hs01555410_m1 |
| IL-6 | Interleukin-6 | Hs00985639_m1 |
| CCL2 | Chemokine (C-C motif) ligand 2 | Hs00234140_m1 |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase 2/cyclooxygenase-2) | Hs00153133_m1 |
| TLR4 | Toll-like receptor 4 | Hs00152939_m1 |
| 18S | 18S ribosomal RNA | Hs99999901_s1 |
| EGFR | Epidermal growth factor receptor | Hs05062827_ft |
siRNA transfection.
siRNAs were transfected into Beas2B and NHBE cells by conventional or reverse transfection method using Lipofectamine 2000/3000 (Invitrogen) or RNAiMax (Invitrogen) according to the manufacturer’s instructions. Cells were grown for 48–72 h, at which time they were washed twice with RPMI medium without serum and subjected to treatments. The levels of mRNAs and proteins were determined by quantitative RT-PCR and Western blot analysis or ELISA, respectively.
Immunoprecipitation.
Cells were lysed in buffer containing 20 mM Tris·HCl pH 8, 137 mM NaCl, 1% Nonidet P-40, 10% glycerol, 2 mM EDTA, and protease and phosphatase inhibitor cocktail, and supernatants were obtained by centrifugation at 13,000 rpm at 4°C for 10 min. Cell lysates (500 µg) were precleared by incubating with 20 μl of protein A/G agarose beads (Pierce) at 4°C for 60 min and centrifuged at 12,000 rpm for 10 min. EGFR antibody (1:100 dilution) was added to the supernatant and incubated overnight on a rotator mixer at 4°C. Protein A/G agarose beads were added to the mixture and incubated for 4 h. Samples were centrifuged for 60 s, and the beads were washed five times with cell lysis buffer. Bound proteins were released by heating the beads in 3X loading buffer at 95–100°C for 5 min and separated by SDS-PAGE on 10% Bis-Tris gels. Phosphorylated and total EGFR levels were determined by Western blot analysis.
Immunohistochemical staining.
Lung sections were immunostained using an UltraVision Detection System kit (Thermo Scientific) according to the manufacturer’s instructions. Monoclonal antibody against STAT-3 phosphorylated at Tyr705 (cat. no. 9145; Cell Signaling Technology) at 1:200 dilution was used for immunostaining.
Luciferase reporter assay.
Beas2B cells were transiently transfected with pGL3luc vector containing human IL-8 promoter (−133/+44 bp) linked to luciferase reporter gene using Lipofectamine 2000 as described previously (20). Luciferase activities in cell lysates were measured by chemiluminescence assays (Promega) and normalized to protein content of cell lysate.
Statistical analyses.
Data shown are means ± SE. The statistical significance between two groups was analyzed by two-tailed paired t-test, and that between multiple groups was analyzed by one-way analysis of variance using Tukey’s multiple-comparison test.
RESULTS
Effect of dust extract on cell viability.
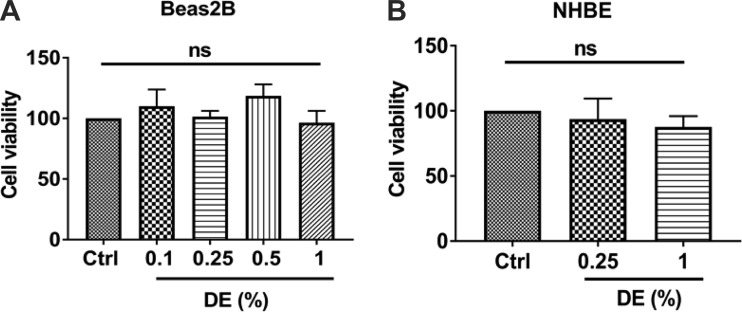
Adverse effects of treatments on cell viability are a concern that could confound interpretation of the results obtained. Therefore, we assessed the effects of treatment with dust extract on Beas2B and NHBE cell viabilities by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. We found that treatment of cells with 0.1–1% dust extract for up to 3 h had no effect on cell viability (Fig. 1) demonstrating that the concentrations and incubation times used in this study do not cause toxicity.
Fig. 1.
Effects of dust extract (DE) on the viability of Beas2B and normal human bronchial epithelial (NHBE) cells. Beas2B (A) and NHBE (B) cells were treated with medium [control (Ctrl)] or the indicated concentrations of DE for 3 h. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Absorbance in control cells was arbitrarily considered as 100, and relative levels in treated cells are shown. Data shown are means ± SE (Beas2B, n = 3–5, except n = 2 for 0.5% DE; NHBE, n = 4); ns, not significant by one-way analysis of variance using Tukey’s multiple-comparison test.
Dust extract induces STAT-3 activation.
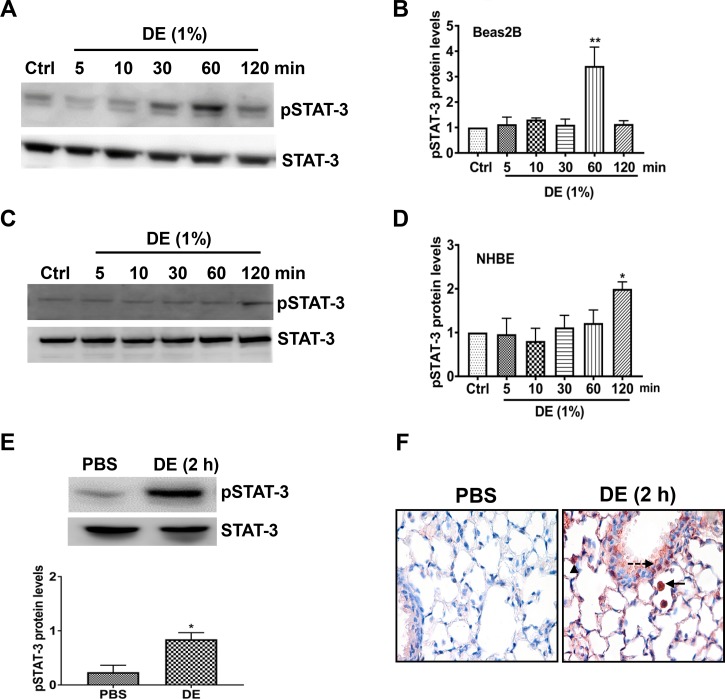
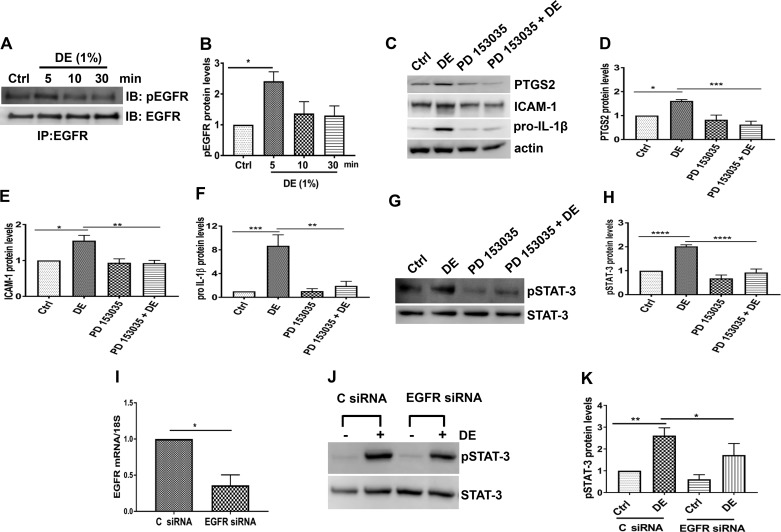
Cytokines and growth factors activate receptor and nonreceptor kinases to phosphorylate a specific tyrosine residue within STAT proteins leading to their dimerization and translocation to the nucleus, where they bind to their cognate DNA elements to modulate gene transcription. Activation of STAT proteins plays critical roles in the control of innate immune and inflammatory responses (24). Among the various STAT proteins, STAT-3 activation has been implicated in the development of acute and chronic lung injury (18, 52). To determine whether poultry CAFO dust extract (hereinafter termed “dust extract”) activates STAT-3, we examined the most commonly studied STAT-3 tyrosine phosphorylation site at Tyr705 at various time points of treatment in Beas2B (Fig. 2, A and B) and NHBE (Fig. 2, C and D) cells by Western blot analysis. Treatment with 1% dust extract increased STAT-3 tyrosine phosphorylation in Beas2B (Fig. 2, A and B) and NHBE (Fig. 2, C and D) cells in a time-dependent manner. In Beas2B cells, STAT-3 phosphorylation peaked at 1 h and subsided thereafter, whereas in NHBE cells increase in STAT-3 phosphorylation was not evident until 2 h. Treatment of Beas2B cells with 0.25% dust extract increased STAT-3 phosphorylation to a lesser degree (~50%) compared with 1% dust extract (data not shown). Intranasal administration of 50 μl of 20% dust extract increased phosphorylated STAT-3 protein level and immunostaining in mouse lung (Fig. 2, E and F). Increased phosphorylated STAT-3 staining was found in bronchial and alveolar epithelial and inflammatory cells (Fig. 2F).
Fig. 2.
Dust extract (DE) induces STAT-3 activation in human bronchial epithelial cells and in mice. Beas2B (A and B) and normal human bronchial epithelial (NHBE, C and D) cells were treated with medium [control (Ctrl)] or 1% DE for the indicated times, and levels of pSTAT-3 and total STAT-3 were determined by Western blot analysis. Levels of pSTAT-3 were normalized to total STAT-3 levels. Normalized pSTAT-3 in control cells was arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4 for Beas2B, except n = 3 for 120-min treatment; n = 5 for NHBE). *P < 0.05, **P < 0.01 compared with cells treated with medium alone, according to one-way analysis of variance using Tukey’s multiple-comparison test. E: mice were administered 50 μl PBS or 50 μl 20% DE via intranasal administration, and 2 h later, pSTAT-3 and STAT-3 levels in lung homogenates were determined by Western blot analysis. Levels of pSTAT-3 were normalized to total STAT-3 levels, and relative levels in DE-treated mice are shown. Data shown are means ± SE (n = 4). *P < 0.05 compared with mice treated with PBS according to paired t-test. F: immunohistochemical detection of pSTAT-3 levels in lung sections of mice treated with PBS or 20% DE is shown. Arrow, inflammatory cell; dashed arrow, bronchiolar epithelial cell; arrowhead, alveolar type II cell. Magnification, ×400.
Inhibition of STAT-3 suppresses dust extract induction of expression of inflammatory mediators in Beas2B and NHBE cells.
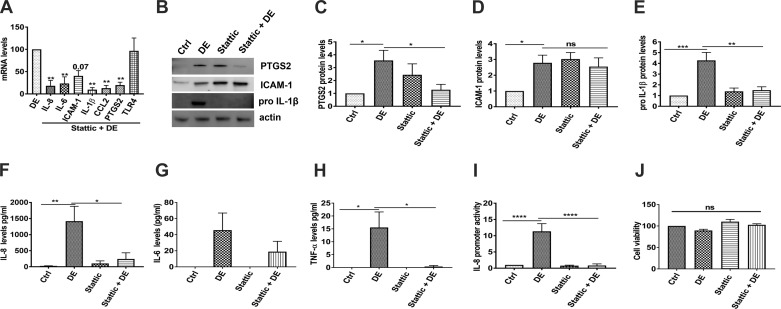
Our studies have shown that poultry dust extracts induce various immune and inflammatory mediators in lung epithelial cells in vitro (20) and in mouse lungs in vivo (5, 37). Our fractionation studies using centrifugal filters with cutoffs of 3,000, 10,000, and 30,000 molecular weight (Amicon) showed that the inducing activity of dust extracts is confined to the retentate fraction indicating the macromolecular nature of the inducing activity (unpublished observations). To determine the involvement of STAT-3 activation, we first investigated the effects of Stattic (49), a nonpeptidic inhibitor that inhibits dimerization and DNA binding of STAT-3, on dust extract induction of inflammatory mediators in Beas2B cells. We found that Stattic significantly suppressed the induction of IL-8, IL-6, ICAM-1, IL-1β, chemokine (C-C motif) ligand 2 (CCL2), and PTGS2 mRNAs but had no effect on TLR4 mRNA levels (Fig. 3A). Inhibition of IL-8, IL-6, IL-1β, and PTGS2 mRNA expression was associated with inhibition of expression of the corresponding proteins (Fig. 3, B, C, and E–G). Induction of ICAM-1 protein expression was not inhibited by Stattic, as Stattic by itself induced ICAM-1 expression (Fig. 3D). We also found that Stattic suppressed induction of TNF-α protein levels by dust extract (Fig. 3H). We could not detect mature IL-1β in control or treated Beas2B and NHBE cells under these conditions. Stattic did not affect the viability of Beas2B cells by itself or in combination with dust extract (Fig. 3J). To further understand mechanisms mediating STAT-3 induction of IL-8 gene expression, we studied the effects of Stattic on IL-8 promoter activity in Beas2B cells. Our results showed that dust extract induction of IL-8 promoter activity was inhibited by Stattic, indicating that STAT-3 activation controls increase of IL-8 gene transcription (Fig. 3I).
Fig. 3.
Effects of Stattic, a STAT-3 inhibitor, on the induction of inflammatory mediators in Beas2B cells. Beas2B cells were incubated with medium [control (Ctrl)] or medium containing 5 µM Stattic for 1 h and then treated with or without dust extract (DE, 0.25%) for 3 h. A: levels of mRNAs were determined by real-time quantitative RT-PCR and normalized to 18S rRNA levels. Levels of mRNAs in DE-treated cells were arbitrarily considered as 100, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4). **P < 0.01 compared with cells treated with DE alone according to one-way analysis of variance using Tukey’s multiple-comparison test. CCL2, chemokine (C-C motif) ligand 2; TLR4, Toll-like receptor 4. B–E: effects of Stattic on the protein levels of prostaglandin G/H synthase 2 (PTGS2), ICAM-1, and pro-IL-1β were determined by Western blot analysis, and protein levels were normalized to actin. Protein levels in control cells were arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001, ns, not significant, according to one-way analysis of variance using Tukey’s multiple-comparison test. F–H: IL-8, IL-6, and TNF-α protein levels in cell medium were determined by ELISA. Data shown are means ± SE (n = 5 for IL-8 and TNF-α; n = 4 for IL-6). *P < 0.05 and **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test. I: Beas2B cells were transfected with IL-8 promoter plasmid containing −133/+44 bp of human IL-8 promoter sequence linked to luciferase reporter gene. Transfected cells were incubated first with medium (Ctrl) or Stattic (5 µM) for 1 h and then treated with or without DE (0.25%) for 6 h. Luciferase activities in cell extracts were normalized to total cell protein. Luciferase activity in control cells was arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 3). ****P < 0.0001 according to one-way analysis of variance using Tukey’s multiple-comparison test. J: effects of Stattic on cell viability of DE-treated Beas2B cells. Beas2B cells were incubated with medium or medium containing 5 µM Stattic for 1 h and then treated with 0.25% DE for 3 h. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Absorbance in control cells was arbitrarily considered as 100, and relative levels in treated cells are shown. Data shown are means ± SE (n = 5); ns, not significant according to one-way analysis of variance using Tukey’s multiple-comparison test.
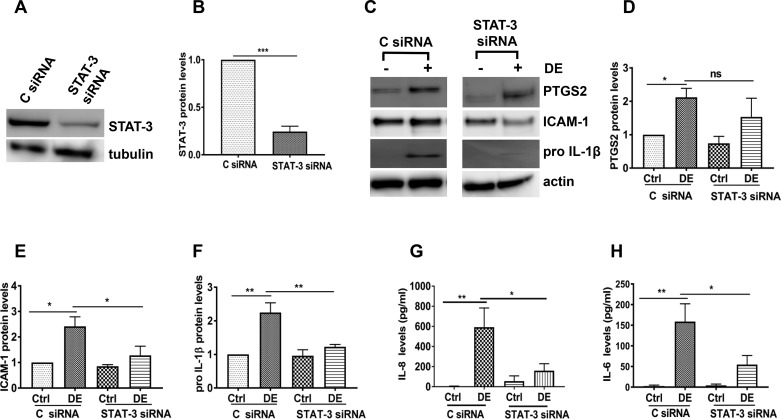
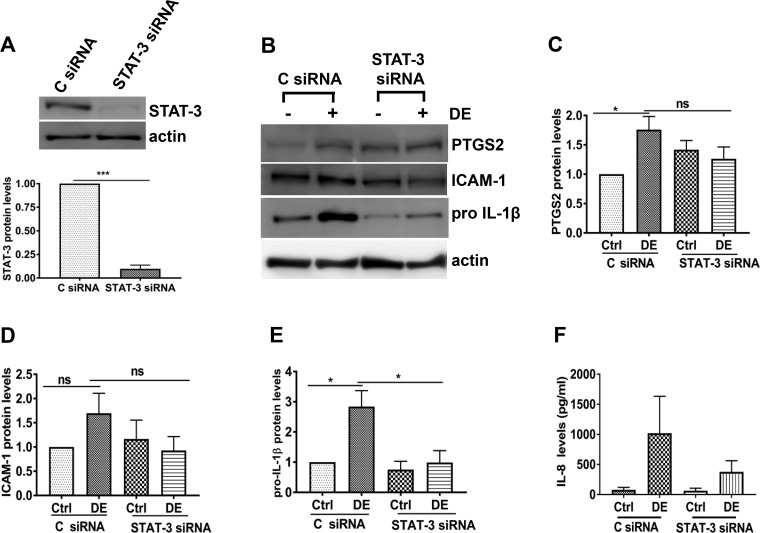
To further confirm the involvement of STAT-3 activation, we determined the effects of siRNA-mediated knockdown of STAT-3 on dust extract induction of inflammatory mediators in Beas2B cells. In agreement with the effects of Stattic, knockdown of STAT-3 (Fig. 4, A and B) caused a significant decrease in the expression of ICAM-1, pro-IL-1β, IL-6, and IL-8 proteins induced by dust extract (Fig. 4, C and E–H); however, effects on PTGS2 levels were not significant (Fig. 4D). In contrast to the lack of effects of Stattic on ICAM-1 protein levels, knockdown of STAT-3 suppressed dust extract induction of ICAM-1 protein expression (Fig. 4, C and E). Knockdown of STAT-3 in NHBE cells (Fig. 5A) produced effects similar to those in Beas2B cells (Fig. 4, A and B). STAT-3 knockdown suppressed the inductive effects of dust extract on pro-IL-1β and PTGS2 (Fig. 5, B, C, and E) and IL-8 protein levels (Fig. 5F). Although dust extract did not significantly induce ICAM-1 protein in NHBE cells, STAT-3 knockdown suppressed ICAM-1 induction (Fig. 5, B and D). IL-6 and TNF-α levels in control or treated NHBE cell medium were below the detection limit.
Fig. 4.
Effects of STAT-3 knockdown on the induction of inflammatory mediators in Beas2B cells. Control siRNA (C siRNA) and STAT-3 siRNA were transfected into cells, and 72 h later, cells were treated with medium [control (Ctrl)] or 0.25% dust extract (DE) for 3 h. A and B: STAT-3 levels were determined by Western blot analysis and normalized to tubulin or actin levels. STAT-3 levels in C siRNA-transfected control cells were arbitrarily considered as 1, and relative levels in STAT-3 siRNA-transfected cells are shown. Data shown are means ± SE (n = 4). ***P < 0.001 according to two-tailed paired t-test. C–F: prostaglandin G/H synthase 2 (PTGS2), ICAM-1, and pro-IL-1β levels were determined by Western blot analysis and normalized to actin. Levels in control siRNA-transfected control cells were arbitrarily considered as 1, and relative levels in other treatments are shown. Data shown are means ± SE (n = 4); ns, not significant, *P < 0.05, **P < 0.01 according to one-way analysis of variance with Tukey’s multiple-comparison test. G and H: IL-8 and IL-6 protein levels in cell medium were determined by ELISA. Data shown are means ± SE (n = 4 for IL-8 and n = 5 for IL-6). *P < 0.05, **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test.
Fig. 5.
Effects of STAT-3 knockdown on the induction of inflammatory mediators in normal human bronchial epithelial cells. Control siRNA (C siRNA) and STAT-3 siRNA were transfected into cells, and 72 h later, cells were treated with medium [control (Ctrl)] or 0.25% dust extract (DE) for 3 h. A: STAT-3 protein levels were determined by Western blot analysis and normalized to actin levels. STAT-3 protein levels in C siRNA-transfected control cells were arbitrarily considered as 1, and data shown are means ± SE (n = 4). ***P < 0.001 according to two-tailed paired t-test. B–E: protein levels of prostaglandin G/H synthase 2 (PTGS2), ICAM-1, and pro-IL-1β were determined by Western blot analysis and normalized to actin levels. Protein levels in C siRNA-transfected control cells were arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4). *P < 0.05; ns, not significant according to one-way analysis of variance with Tukey’s multiple-comparison test. F: IL-8 protein levels in cell medium were determined by ELISA. Data shown are means ± SE (n = 4).
Effects of Stattic on dust extract induction of inflammatory mediator expression in mice.
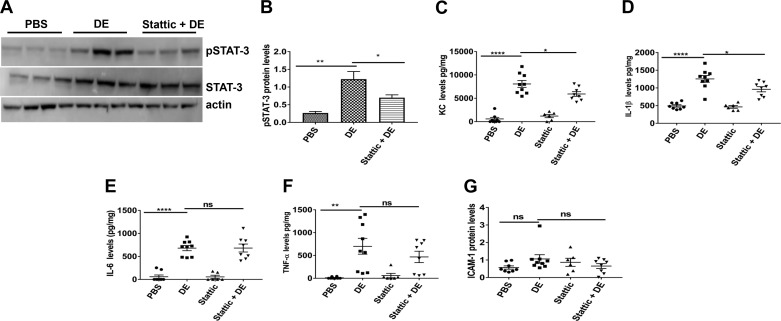
We found that the STAT-3 inhibitor Stattic and/or the silencing of STAT-3 in Beas2B and NHBE cells suppressed induction of inflammatory mediators by dust extract. We also found that dust extract activated STAT-3 by increasing Tyr705 phosphorylation both in vitro and in vivo. To determine whether STAT-3 activation is involved in the induction of inflammatory mediators in vivo, we determined the effects of Stattic on dust extract induction of inflammatory mediators in mouse lungs in vivo. We previously found that intranasal administration of 50 μl of 20% dust extract reproducibly induced lung expression of KC, TNF-α, and IL-6 after 2 h in mice (37). We therefore used this dose to determine the effects of Stattic on the induction of inflammatory mediators in mice. Assessment of toxicity by lactate dehydrogenase assay of BAL samples from control mice and mice treated with 20% dust extract did not show any toxicity (absorbance at 490-nm wavelength: control, 0.075 ± 0.032; dust extract, 0.062 ± 0.017, 2-tailed P = 0.72, n = 6–7). We found that Stattic reduced dust extract-induced phosphorylation of STAT-3 by ~50% (Fig. 6, A and B) and reduced dust extract induction of KC (Fig. 6C) and IL-1β (Fig. 6D) protein expression; however, IL-6 levels were not affected (Fig. 6E). Stattic treatment appeared to reduce induction of TNF-α (Fig. 6F) and ICAM-1 expression (Fig. 6G); however, the decreases were not statistically significant.
Fig. 6.
Effects of Stattic, a STAT-3 inhibitor, on STAT-3 activation and induction of inflammatory mediators in mouse lungs. Female C57BL/6 mice received vehicle or Stattic via intraperitoneal injections 16 and 1 h before intranasal administration of 50 μl of PBS or 20% dust extract (DE). After 3 h, mice were euthanized, and lungs were cleared of blood by perfusing with PBS and homogenized. A and B: levels of pSTAT-3, STAT-3, and actin were determined by Western blot analysis, and pSTAT-3 level was normalized to STAT-3, which had been normalized to actin. Data shown are means ± SE (n = 5 for PBS, n = 6 for DE and Stattic + DE). *P < 0.05, **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test. C–F: levels of keratinocyte chemoattractant (KC), IL-1β, IL-6, and TNF-α were determined by ELISA and normalized to total protein levels. G: ICAM-1 levels were determined by Western blot analysis and normalized to actin levels. Data shown are means ± SE (n = 6–9). *P < 0.05, **P < 0.01, ****P < 0.0001; ns, not significant according to one-way analysis of variance using Tukey’s multiple-comparison test.
Antioxidants suppress STAT-3 activation.
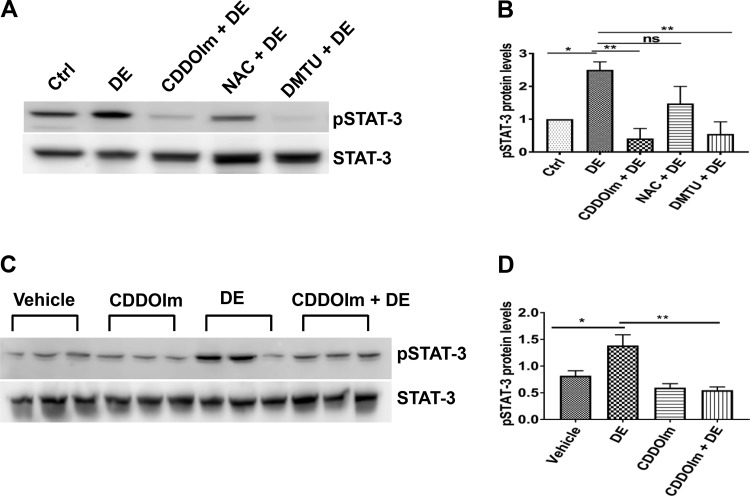
Inhibition of STAT-3 activation using Stattic inhibitor or siRNA-mediated knockdown reduced dust extract induction of inflammatory mediators indicating that it serves as an important transcription factor controlling the induction of expression of inflammatory genes. Previously, our (20) and other studies (34, 59) showed that NF-κB and AP-1 are important for the induction of IL-6 and IL-8 expression by different organic dusts. Our studies also showed that poultry dust extract induction of inflammatory mediators in lung epithelial cells in vitro is associated with oxidative stress, and antioxidants suppressed induction of inflammatory mediators underscoring the importance of oxidative stress in the induction (38). To determine whether dust extract-induced oxidative stress is a mediator of STAT-3 activation, we investigated the effects of the antioxidants CDDOIm, NAC, and DMTU on STAT-3 activation in Beas2B cells. We found that CDDOIm, NAC, and DMTU reduced STAT-3 activation (Fig. 7, A and B) indicating that oxidative stress is an important regulator of STAT-3 activation by dust extract. Administration of CDDOIm to mice reduced STAT-3 phosphorylation induced by dust extract (Fig. 7, C and D) further supporting a role for oxidative stress for STAT-3 activation.
Fig. 7.
Antioxidants suppress STAT-3 activation. A and B: Beas2B cells were first incubated with medium [control (Ctrl)] or medium containing 0.5 µM 1-(2-cyano-3, 12, 28-trioxooleana-1, 9(11)-dien-28yl)-1H-imidazole (CDDOIm) or 15 mM N-acetylcysteine (NAC) for 3 h or 30 mM dimethylthiourea (DMTU) for 1 h and then treated with or without 1% dust extract (DE) for 1 h. Levels of pSTAT-3 and STAT-3 were determined by Western blot analysis, and pSTAT-3 level was normalized to STAT-3. Levels of pSTAT-3 in control cells were arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4). *P < 0.05, **P < 0.01; ns, not significant, according to one-way analysis of variance with Tukey’s multiple-comparison test. C and D: female C57BL/6 mice (n = 3) received vehicle or CDDOIm (5 mg/kg) once daily for 2 days via intraperitoneal injections before intranasal administration of 50 μl of PBS or 20% DE. After 2 h, mice were euthanized, and lung homogenates were prepared. Levels of pSTAT-3 and STAT-3 were determined by Western blot analysis, and pSTAT-3 level was normalized to STAT-3. Data shown are means ± SE. *P < 0.05, **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test.
Dust extract-induced STAT-3 activation is linked to TYK2.
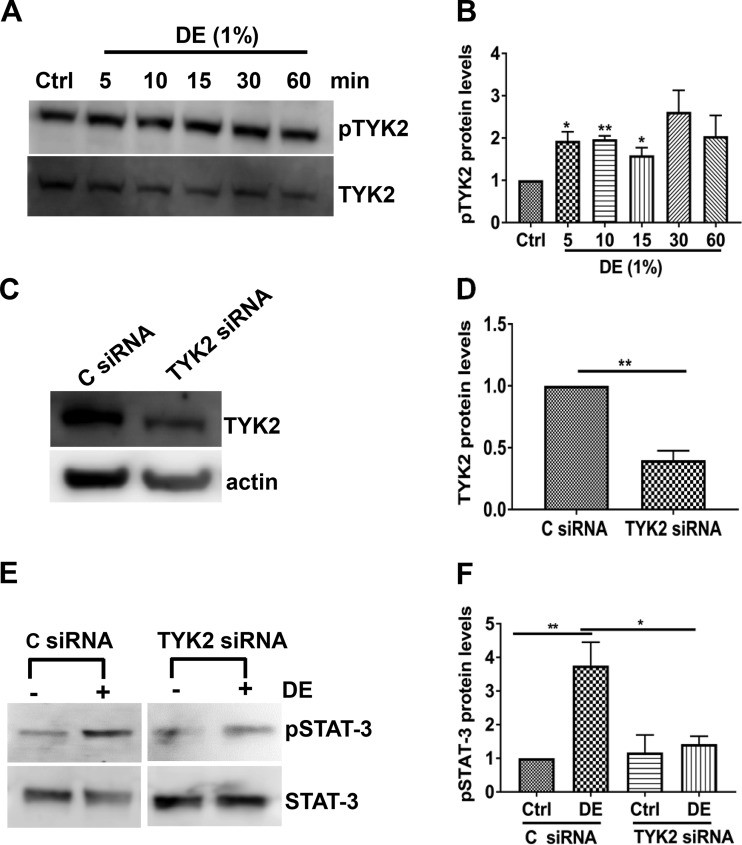
Receptor and nonreceptor tyrosine kinases are known to activate STAT-3 via phosphorylation (56, 65). Among the nonreceptor tyrosine kinases, the Janus kinase family of tyrosine kinases, JAK1, JAK2, and JAK3, TYK2, and Src kinases phosphorylate the STAT family of proteins (65). Because JAK2 and TYK2 are activated in lung injury (4, 50), we examined their role in the control of STAT-3 phosphorylation induced by poultry dust extract. Western blot analysis of Beas2B and NHBE cells treated with dust extract for different lengths of time did not detect phosphorylated JAK2 (data not shown); however, phosphorylated TYK2 could be readily detected. Treatment of Beas2B cells increased TYK2 phosphorylation after 5 min, and elevated TYK2 phosphorylation was evident until 60 min (Fig. 8, A and B). To understand the role of TYK2 in STAT-3 activation, we determined the effects of siRNA knockdown of TYK2 on STAT-3 phosphorylation. Transfection with siRNA significantly reduced TYK2 levels (Fig. 8, C and D), and dust extract failed to increase STAT-3 phosphorylation (Fig. 8, E and F) indicating that TYK2 mediates STAT-3 phosphorylation. The Src kinase inhibitor 4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine (PP2) did not suppress dust extract increase of STAT-3 phosphorylation indicating that STAT-3 phosphorylation occurs independently of Src kinases (data not shown).
Fig. 8.
Dust extract (DE) activates STAT-3 via nonreceptor tyrosine-protein kinase 2 (TYK2). A and B: Beas2B cells were treated with medium [control (Ctrl)] or 1% DE for the indicated times. Levels of phospho-TYK2 (pTYK2) and total TYK2 were determined by Western blot analysis, and pTYK2 levels were normalized to total TYK2 levels. Levels of pTYK2 in control cells were arbitrarily considered as 1, and relative levels in treated cells are shown. Data are means ± SE (n = 4 for all treatments, except n = 3 for 10 min). *P < 0.05, **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test. C–F: control siRNA (C siRNA) or TYK2 siRNA was transfected into Beas2B cells, and after 72 h, cells were treated with medium (Ctrl) or 1% DE for 1 h. Levels of TYK2, pSTAT-3, total STAT-3, and actin were determined by Western blot analysis. Levels of TYK2 and pSTAT-3 were normalized to actin and total STAT-3, respectively. TYK2 and pSTAT-3 levels in C siRNA-transfected control cells were arbitrarily considered as 1, and relative levels in other treatments are shown. Data shown are means ± SE (n = 5). *P < 0.05, **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test.
EGFR activates STAT-3.
Recent studies have shown that activation of EGFR controls induction of IL-6 and IL-8 by swine CAFO dust extract in airway epithelial cells (16). Because EGFR is known to activate STAT-3 and oxidative stress is a mediator of EGFR activation (3, 17), we investigated the involvement of EGFR in STAT-3-mediated induction of immune and inflammatory mediators by poultry dust extract. We found that treatment with dust extract increased EGFR phosphorylation rapidly in Beas2B cells (Fig. 9, A and B) and PD 153035, a highly selective EGFR inhibitor, suppressed dust extract induction of STAT-3 phosphorylation (Fig. 9, G and H) and induction of PTGS2, ICAM-1, and pro-IL-1β (Fig. 9, C–F). Knockdown of EGFR (Fig. 9I) by siRNA transfection reduced dust extract-induced increase of STAT-3 phosphorylation (Fig. 9, J and K) consistent with data obtained with PD 153035.
Fig. 9.
Dust extract (DE) activates STAT-3 via epidermal growth factor receptor (EGFR), and chemical inhibition or siRNA knockdown of EGFR suppresses inflammatory mediators and STAT-3 phosphorylation. A and B: Beas2B cells were treated with medium [control (Ctrl)] or 1% DE for the indicated times, and the levels of phospho-EGFR (pEGFR) and total EGFR were determined by immunoprecipitation (IP) followed by Western blot analysis. Data shown are means ± SE (n = 4); *P < 0.05 according to one-way analysis of variance using Tukey’s multiple-comparison test. IB, immunoblot. C–F: Beas2B cells were treated with medium (Ctrl) or PD 153035 (10 µM) for 1 h and then treated with or without 0.25% DE for 3 h. Levels of prostaglandin G/H synthase 2 (PTGS2), ICAM-1, and pro-IL-1β were determined by Western blot analysis and normalized to actin. Their levels in control cells were arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 according to one-way analysis of variance using Tukey’s multiple-comparison test. G and H: Beas2B cells were treated with medium (Ctrl) or PD 153035 (10 µM) for 1 h and then treated with or without 1% DE for 1 h. Levels of pSTAT-3 and total STAT-3 were determined by Western blot analysis, and pSTAT-3 level was normalized to total STAT-3. Levels of pSTAT-3 in control cells were arbitrarily considered as 1, and relative levels in treated cells are shown. Data shown are means ± SE (n = 4). ****P < 0.0001 according to one-way analysis of variance with Tukey’s multiple-comparison test. I–K: control siRNA (C siRNA) and EGFR siRNA were transfected into Beas2B cells, and after 72 h, cells were treated with medium (Ctrl) or 1% DE for 1 h. I: EGFR mRNA levels were determined by real-time quantitative RT-PCR and normalized to 18S rRNA levels. EGFR mRNA levels in C siRNA-transfected control cells were arbitrarily considered as 1, and relative level in EGFR siRNA-transfected cells is shown. Data shown are means ± SE (n = 4). *P < 0.05 according to paired t-test. J and K: levels of pSTAT-3 and total STAT-3 were determined by Western blot analysis, and pSTAT-3 levels were normalized to STAT-3. pSTAT-3 level in C siRNA-transfected control cells was arbitrarily considered as 1, and relative levels in other samples are shown. Data shown are means ± SE (n = 5). *P < 0.05, **P < 0.01 according to one-way analysis of variance using Tukey’s multiple-comparison test.
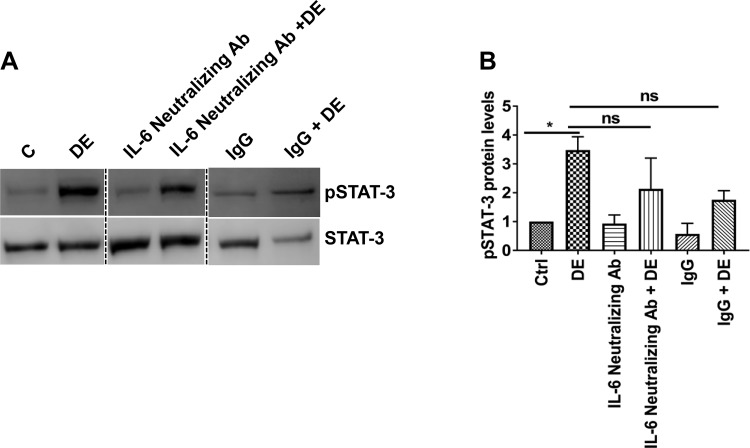
Because IL-6 is a major activator of STAT-3 (67), we examined whether dust extract activation of STAT-3 is linked to IL-6 production by blocking IL-6 activity with anti-IL-6 neutralizing antibodies. We found that anti-IL-6 neutralizing antibody did not completely suppress STAT-3 phosphorylation (Fig. 10) indicating that in addition to IL-6, other mechanisms are involved in dust extract activation of STAT-3.
Fig. 10.
IL-6 is not solely responsible for STAT-3 activation. A and B: Beas2B cells were treated with 1 µg/ml of anti-IL-6 neutralizing antibody or matching control antibody for 1 h and then treated with 1% dust extract (DE) for 1 h. Levels of pSTAT-3 and total STAT-3 were determined by Western blot analysis, and pSTAT-3 level was normalized to total STAT-3. Dashed lines show reassembly of noncontiguous lanes. pSTAT-3 level in control (C/Ctrl) cells was arbitrarily considered as 1, and relative levels in other samples are shown. Data shown are means ± SE (n = 5); ns, not significant, *P < 0.05 according to one-way analysis of variance using Tukey’s multiple-comparison test.
DISCUSSION
Exposure to organic dust is strongly linked to the development of lung diseases such as asthma, bronchitis, organic dust toxic syndrome, and chronic obstructive pulmonary disease (1, 60). These diseases are characterized by chronic inflammation and increased production of immune and inflammatory mediators. Although elevated production of immune and inflammatory mediators has been demonstrated in human subjects (28, 62), experimental animals (13, 37, 43), and lung cells in vitro (20, 39, 45, 64), molecular mechanisms controlling their production are not well understood. A limited number of studies have shown that NF-κB and AP-1 are necessary for organic dust induction of IL-6 and IL-8 promoter activities with NF-κB playing a dominant role (20, 34). However, very little is known about the involvement of other transcription factors controlling the production of inflammatory mediators induced by organic dust. Because exposure to organic dust induces the expression of several immune and inflammatory genes (5), it is likely that transcription factors other than NF-κB and AP-1 are also involved in achieving regulated expression.
STATs are a family of transcription factors that mediate cellular signaling by cytokines and growth factors (14). In their latent state, STATs are found in the cytosol, and upon activation by phosphorylation of conserved tyrosine residues they form homodimers and heterodimers that translocate to the nucleus, where they bind to their cognate DNA elements to modulate gene transcription (14). In the present study, we found that dust extracts increased STAT-3 phosphorylation in lung epithelial cells and in mouse lungs. In Beas2B cells, STAT-3 phosphorylation peaked at 60 min after treatment and dropped to control levels at 120 min, whereas in NHBE cells, increase of STAT-3 phosphorylation was not evident until 120 min (Fig. 2, A–D). Delayed and prolonged STAT-3 activation has been previously observed in airway epithelial cells. In NHBE cells treated with diesel exhaust particles, increased STAT-3 phosphorylation was detectable at 1 h after treatment and continued to increase up to 4 h (9). In Beas2B cells treated with urokinase, increased STAT-3 phosphorylation was not evident until 12 h after treatment and was sustained up to 24 h (51). Activation of STATs is controlled by protein tyrosine phosphatases, which are subject to redox regulation (8). The differences in STAT-3 activation by dust extract between Beas2B and NHBE cells could be due to differential control of protein tyrosine phosphatases responsible for STAT-3 dephosphorylation.
Chemical or genetic inhibition of STAT-3 in Beas2B cells significantly suppressed dust extract induction of pro-IL-1β, PTGS2, IL-8, IL-6, and TNF-α expression (Fig. 3, B, C, and E–H) indicating the importance of STAT-3 activation in the induction of these genes. In Beas2B cells, Stattic suppression of ICAM-1 mRNA was not associated with inhibition of ICAM-1 protein expression as Stattic by itself increased ICAM-1 protein levels (Fig. 3, B and D). At variance with these results, siRNA knockdown of STAT-3 suppressed induction of ICAM-1 protein expression (Fig. 4, C and E), suggesting that the lack of effects of Stattic could be due to nontarget actions. As in the case of Beas2B cells, knockdown of STAT-3 in NHBE cells (Fig. 5) also resulted in the suppression of pro-IL-1β, IL-8, and ICAM-1 protein levels. Stattic suppressed STAT-3 activation and induction of KC and IL-1β expression (Fig. 6, C and D) by dust extract in mice but had no effect on IL-6 levels (Fig. 6E). Stattic appeared to decrease induction of TNF-α and ICAM-1 expression (Fig. 6, F and G), but the changes were not statistically significant. Although dust extracts significantly induced KC, IL-1β, IL-6, and TNF-α expression at 3 h after dust extract administration in mice, the inductive effect on ICAM-1 levels was weak. The lack of effect on ICAM-1 levels could be due to the relatively short time period of exposure to dust extract, and longer exposure times may be required to achieve higher ICAM-1 levels. The reasons for the differential inhibitory effects of Stattic are not clear, but they could be due to species-specific differences between humans and mice in regard to STAT-3-dependent regulation of IL-6 and IL-1β gene expression. Although humans and mice share many similarities with regard to immune and inflammatory responses to pathogenic agents, significant differences between the species exist (68).
STAT-3 target genes include transcription factors, metalloproteinases, cytokines, and apoptotic proteins (11). STAT-3 directly binds to the promoters of COX-2, ICAM-1, IL-6, IL-8, TNF-α, transforming growth factor-β, and regulated on activation, normal T cell expressed, and secreted (RANTES) to increase gene transcription (11). Our and other studies have shown that NF-κB and AP-1 control organic dust induction of IL-8 and IL-6 promoter activities (20, 34). TLR2 and TLR4 signaling pathways mediate airway inflammatory mediator production and airway inflammatory responses in mice exposed to swine CAFO dust (12, 42, 57). The activation of the TLR signaling pathway results in the phosphorylation and subsequent degradation of inhibitory κB proteins culminating in the activation of NF-κB (26). In our study, identification of STAT-3 as playing a positive role suggests potential interactions between STAT-3 and NF-κB in the modulation of expression of immune and inflammatory mediator genes by organic dust. Indeed, cooperation between STAT-3 and NF-κB has been reported to be responsible for the induction of a subset of genes by TNF-α (21). Genes encoding chemokines, antiapoptotic proteins, and cell cycle control proteins are modulated by the collaborative actions of NF-κB and STAT-3. Apart from interactions between STAT-3 and NF-κB on the promoter, direct interactions between them in the modulation of gene expression have also been reported. For example, activation of NF-κB by displacement of IκB from the NF-κB complex after binding of STAT-3 (31) and IκB binding to STAT-3 to prevent STAT-3 binding to its DNA element have been reported (63). Although STATs can be phosphorylated by nonreceptor kinases such as JAK or Src family kinases or receptor kinases such as EGFR or platelet-derived growth factor receptor, cytokine receptors preferentially use JAKs as signaling effectors (2). Among STAT family members, STAT-3 has been implicated in the development of acute and chronic lung inflammation. Administration of LPS rapidly activated lung STAT-1 and STAT-3 in mice (50). Airway epithelial STAT-3 was found to mediate house dust mite extract-induced lung inflammation in mice and regulate the levels of IL-4, IL-5, IL-13, TNF-α, and KC, but not IFN-γ or eotaxin levels (52). Diesel exhaust particle activation of STAT-3 in airway epithelial cells is dependent on EGFR and Src kinase suggesting that STAT-3 might be involved in the control of lung inflammation (9). STAT-3 activation was demonstrated to mediate streptococcal M1 protein-induced lung neutrophilia via increase in C-X-C motif chemokine levels (66).
We previously reported that oxidative stress and protein kinase and NF-κB signaling control induction of inflammatory mediator expression by poultry dust extracts (38). In the present study, antioxidants such as CDDOIm, DMTU, and NAC suppressed dust extract-induced phosphorylation of STAT-3 (Fig. 7) indicating that oxidative stress is involved in STAT-3 activation. Changes in the cellular redox state are known to control STAT-3 activation. Cellular oxidative stress caused by hydrogen peroxide (10, 53), respiratory syncytial virus infection (35), diesel exhaust particles (9), and oxidized phospholipids (19) has been demonstrated to activate STAT-3.
In our study, STAT-3 phosphorylation induced by poultry dust extract was found to be dependent on the activation of TYK2 and EGFR (Figs. 8 and 9), but not JAK2. It is not yet known whether dust extract-induced oxidative stress controls activation of TYK2 and EGFR to increase STAT-3 phosphorylation. Oxidative stress is known to differentially control the activation of tyrosine kinases responsible for STAT-3 phosphorylation. In the case of respiratory syncytial virus infection, reactive oxygen species (ROS)-dependent STAT-3 activation was not ascribed to JAK or Src kinases, whereas EGFR activation of STAT-3 was found to be dependent on ROS inhibition of tyrosine phosphatase activity (35). Induction of IL-8 transcription by oxidized phospholipids was demonstrated to be due to JAK2-mediated activation of STAT-3 (19). Tyrosine phosphatases have been reported to be highly sensitive to inactivation by oxidation because of the presence of a critical thiol group in the active site of the enzyme (47). Although oxidative stress has been found to activate the JAK-STAT pathway, it is also known to inhibit the cytokine-induced JAK-STAT pathway (15, 25). The mechanisms underlying the inhibition of activation of the JAK-STAT pathway by oxidative stress are not known; however, direct oxidation of JAK and STAT proteins leading to their inactivation is a possibility. The kinase activity of JAK2 has been reported to be sensitive to oxidation of cysteine residues in the catalytic domain (55), and STAT-3 DNA binding and transcriptional activity can be inhibited via oxidation of conserved cysteine residues directly inactivated by oxidants (32, 33). IL-6 is a well-known activator of STAT-3 phosphorylation (67). We found that neutralization of IL-6 activity with anti-IL-6 neutralizing antibody did not completely abolish STAT-3 phosphorylation induced by dust extract indicating that mechanisms independent of IL-6 are also involved.
Poultry dust extracts prepared in our laboratory contain endotoxin and low amounts of muramic acid (20), a marker of peptidoglycan and serine protease-like activity (38). Endotoxin in poultry dust extracts does not appear to be a primary driver of induction of IL-8 as polymyxin B failed to suppress induction of IL-8 mRNA in lung epithelial and THP-1 cells in vitro (20). Similar results showing a lack of inductive effects of endotoxin on the release of IL-6 and IL-8 were reported for swine CAFO dust extracts cleared of endotoxin using a polymyxin B column (45). These data, combined with data of the weaker stimulatory effects of LPS (20, 45) and peptidoglycan (45) on IL-6 and IL-8 levels, indicate that endotoxin and peptidoglycan are not primarily responsible for the induction of IL-6 and IL-8 expression. However, it remains to be determined whether endotoxin in poultry dust extracts has any role in the induction of IL-1β, PTGS2, ICAM-1, and TNF-α expression. We (38) and others (46) recently found that protease activity in organic dust extracts is involved in the induction of inflammatory mediators. We previously found that poultry dust extracts increase oxidant stress to induce inflammatory mediator expression in lung epithelial cells (38). An agent(s) in poultry dust extracts that increases oxidant stress and the underlying mechanisms are not known. Considering that endotoxin and peptidoglycan in organic dust extracts are weak stimulators of IL-6 and IL-8 levels and that protease activity is a major inducer of inflammatory mediator expression, the link between protease activity and oxidant stress would be interesting to study in the future.
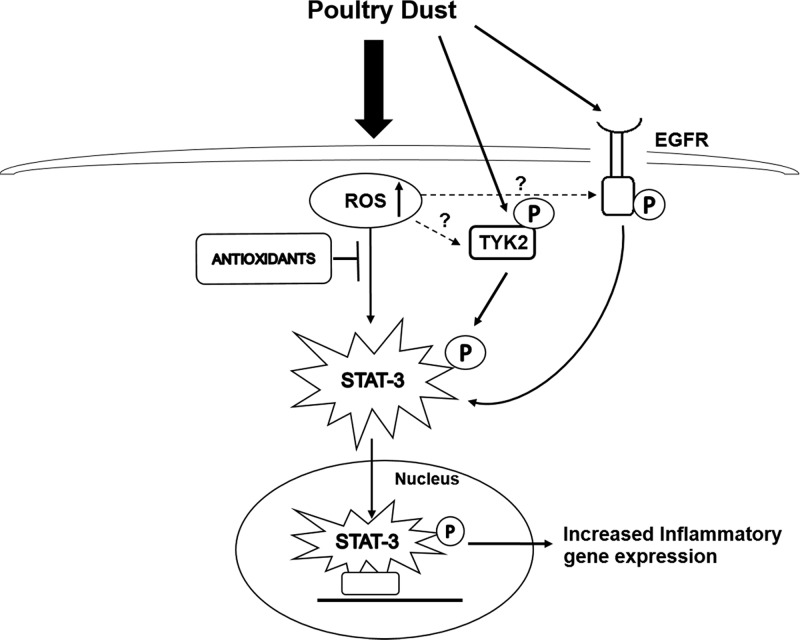
In summary, our studies have revealed that STAT-3 is an important transcription factor controlling the induction of lung immune and inflammatory genes by organic dust extract. Interactions between STAT-3, NF-κB, and AP-1 likely determine the regulated expression of various immune and inflammatory mediators. Furthermore, our studies delineated a cellular signaling pathway involving oxidative stress-dependent activation of STAT-3 mediated by TYK2 and EGFR tyrosine kinases (Fig. 11).
Fig. 11.
Mechanism for dust extract induction of inflammatory gene expression. Treatment of lung epithelial cells with dust extracts increases intracellular reactive oxygen species (ROS) levels causing oxidative stress and leading to STAT-3 activation mediated by nonreceptor tyrosine-protein kinase 2 (TYK2) and epidermal growth factor receptor (EGFR) tyrosine kinases. Activated STAT-3 is translocated to the nucleus, where it binds to cognate promoter DNA elements to increase transcription of immune and inflammatory genes. The effects of ROS induced by dust extract on activation of TYK2 and EGFR are not known. The “P” inside an oval represents phosphorylation.
GRANTS
This research was supported by the Centers for Disease Control and National Institute for Occupational Safety and Health Grant U54-OH-007541.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.B. conceived and designed research; K.N., V.M., and C.M. performed experiments; K.N., V.M., C.M., and V.B. analyzed data; K.N. and V.B. interpreted results of experiments; K.N. prepared figures; K.N. drafted manuscript; K.N. and V.B. edited and revised manuscript; K.N., V.M., C.M., and V.B. approved final version of manuscript.
REFERENCES
- 1.American Thoracic Society Respiratory health hazards in agriculture. Am J Respir Crit Care Med 158, Suppl 1: S1–S76, 1998. doi: 10.1164/ajrccm.158.supplement_1.rccm1585s1. [DOI] [PubMed] [Google Scholar]
- 2.Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J 462: 1–13, 2014. doi: 10.1042/BJ20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 272: 217–221, 1997. doi: 10.1074/jbc.272.1.217. [DOI] [PubMed] [Google Scholar]
- 4.Berg J, Zscheppang K, Fatykhova D, Tönnies M, Bauer TT, Schneider P, Neudecker J, Rückert JC, Eggeling S, Schimek M, Gruber AD, Suttorp N, Hippenstiel S, Hocke AC. Tyk2 as a target for immune regulation in human viral/bacterial pneumonia. Eur Respir J 50: 1601953, 2017. doi: 10.1183/13993003.01953-2016. [DOI] [PubMed] [Google Scholar]
- 5.Boggaram V, Loose DS, Gottipati KR, Natarajan K, Mitchell CT. Gene expression profiling of the effects of organic dust in lung epithelial and THP-1 cells reveals inductive effects on inflammatory and immune response genes. Physiol Genomics 48: 281–289, 2016. doi: 10.1152/physiolgenomics.00096.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böhmer FD, Friedrich K. Protein tyrosine phosphatases as wardens of STAT signaling. JAK-STAT 3: e28087, 2014. doi: 10.4161/jkst.28087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao D, Tal TL, Graves LM, Gilmour I, Linak W, Reed W, Bromberg PA, Samet JM. Diesel exhaust particulate-induced activation of Stat3 requires activities of EGFR and Src in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L422–L429, 2007. doi: 10.1152/ajplung.00204.2006. [DOI] [PubMed] [Google Scholar]
- 10.Carballo M, Conde M, El Bekay R, Martín-Nieto J, Camacho MJ, Monteseirín J, Conde J, Bedoya FJ, Sobrino F. Oxidative stress triggers STAT-3 tyrosine phosphorylation and nuclear translocation in human lymphocytes. J Biol Chem 274: 17580–17586, 1999. doi: 10.1074/jbc.274.25.17580. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter RL, Lo HW. STAT-3 target genes relevant to human cancers. Cancers (Basel) 6: 897–925, 2014. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charavaryamath C, Juneau V, Suri SS, Janardhan KS, Townsend H, Singh B. Role of Toll-like receptor 4 in lung inflammation following exposure to swine barn air. Exp Lung Res 34: 19–35, 2008. doi: 10.1080/01902140701807779. [DOI] [PubMed] [Google Scholar]
- 13.Cleave J, Willson PJ, Town J, Gordon JR. Fractionation of swine barn dust and assessment of its impact on the respiratory tract following repeated airway exposure. J Toxicol Environ Health A 73: 1090–1101, 2010. doi: 10.1080/15287394.2010.482916. [DOI] [PubMed] [Google Scholar]
- 14.Darnell JE., Jr STATs and gene regulation. Science 277: 1630–1635, 1997. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 15.Di Bona D, Cippitelli M, Fionda C, Cammà C, Licata A, Santoni A, Craxì A. Oxidative stress inhibits IFN-α-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol 45: 271–279, 2006. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 16.Dodmane PR, Schulte NA, Heires AJ, Band H, Romberger DJ, Toews ML. Airway epithelial epidermal growth factor receptor mediates hogbarn dust-induced cytokine release but not Ca2+ response. Am J Respir Cell Mol Biol 45: 882–888, 2011. doi: 10.1165/rcmb.2010-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gamou S, Shimizu N. Hydrogen peroxide preferentially enhances the tyrosine phosphorylation of epidermal growth factor receptor. FEBS Lett 357: 161–164, 1995. doi: 10.1016/0014-5793(94)01335-X. [DOI] [PubMed] [Google Scholar]
- 18.Gao H, Guo RF, Speyer CL, Reuben J, Neff TA, Hoesel LM, Riedemann NC, McClintock SD, Sarma JV, Van Rooijen N, Zetoune FS, Ward PA. Stat3 activation in acute lung injury. J Immunol 172: 7703–7712, 2004. doi: 10.4049/jimmunol.172.12.7703. [DOI] [PubMed] [Google Scholar]
- 19.Gharavi NM, Alva JA, Mouillesseaux KP, Lai C, Yeh M, Yeung W, Johnson J, Szeto WL, Hong L, Fishbein M, Wei L, Pfeffer LM, Berliner JA. Role of the Jak/STAT pathway in the regulation of interleukin-8 transcription by oxidized phospholipids in vitro and in atherosclerosis in vivo. J Biol Chem 282: 31460–31468, 2007. doi: 10.1074/jbc.M704267200. [DOI] [PubMed] [Google Scholar]
- 20.Gottipati KR, Bandari SK, Nonnenmann MW, Levin JL, Dooley GP, Reynolds SJ, Boggaram V. Transcriptional mechanisms and protein kinase signaling mediate organic dust induction of IL-8 expression in lung epithelial and THP-1 cells. Am J Physiol Lung Cell Mol Physiol 308: L11–L21, 2015. doi: 10.1152/ajplung.00215.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivennikov SI, Karin M. Dangerous liaisons: STAT-3 and NF-κB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev 21: 11–19, 2010. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heederik D, Sigsgaard T, Thorne PS, Kline JN, Avery R, Bønløkke JH, Chrischilles EA, Dosman JA, Duchaine C, Kirkhorn SR, Kulhankova K, Merchant JA. Health effects of airborne exposures from concentrated animal feeding operations. Environ Health Perspect 115: 298–302, 2007. doi: 10.1289/ehp.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Just N, Duchaine C, Singh B. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J Occup Med Toxicol 4: 13, 2009. doi: 10.1186/1745-6673-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan MH. STAT signaling in inflammation. JAK-STAT 2: e24198, 2013. doi: 10.4161/jkst.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaur N, Lu B, Monroe RK, Ward SM, Halvorsen SW. Inducers of oxidative stress block ciliary neurotrophic factor activation of Jak/STAT signaling in neurons. J Neurochem 92: 1521–1530, 2005. doi: 10.1111/j.1471-4159.2004.02990.x. [DOI] [PubMed] [Google Scholar]
- 26.Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors. Trends Mol Med 13: 460–469, 2007. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Larsson BM, Larsson K, Malmberg P, Mártensson L, Palmberg L. Airway responses in naive subjects to exposure in poultry houses: comparison between cage rearing system and alternative rearing system for laying hens. Am J Ind Med 35: 142–149, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 28.Larsson BM, Palmberg L, Malmberg PO, Larsson K. Effect of exposure to swine dust on levels of IL-8 in airway lavage fluid. Thorax 52: 638–642, 1997. doi: 10.1136/thx.52.7.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson K, Malmberg P, Eklund A. Acute exposure to swine dust causes airway inflammation and bronchial hyperresponsiveness. Am J Ind Med 25: 57–58, 1994. doi: 10.1002/ajim.4700250115. [DOI] [PubMed] [Google Scholar]
- 30.Larsson KA, Eklund AG, Hansson LO, Isaksson BM, Malmberg PO. Swine dust causes intense airways inflammation in healthy subjects. Am J Respir Crit Care Med 150: 973–977, 1994. doi: 10.1164/ajrccm.150.4.7921472. [DOI] [PubMed] [Google Scholar]
- 31.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, Forman S, Jove R, Pardoll DM, Yu H. Persistently activated Stat3 maintains constitutive NF-κB activity in tumors. Cancer Cell 15: 283–293, 2009. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Cheung SH, Evans EL, Shaw PE. Modulation of gene expression and tumor cell growth by redox modification of STAT-3. Cancer Res 70: 8222–8232, 2010. doi: 10.1158/0008-5472.CAN-10-0894. [DOI] [PubMed] [Google Scholar]
- 33.Li L, Shaw PE. A STAT-3 dimer formed by inter-chain disulphide bridging during oxidative stress. Biochem Biophys Res Commun 322: 1005–1011, 2004. doi: 10.1016/j.bbrc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 34.Lidén J, Ek A, Palmberg L, Okret S, Larsson K. Organic dust activates NF-κB in lung epithelial cells. Respir Med 97: 882–892, 2003. doi: 10.1016/S0954-6111(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 35.Liu T, Castro S, Brasier AR, Jamaluddin M, Garofalo RP, Casola A. Reactive oxygen species mediate virus-induced STAT activation: role of tyrosine phosphatases. J Biol Chem 279: 2461–2469, 2004. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 36.Martin LD, Rochelle LG, Fischer BM, Krunkosky TM, Adler KB. Airway epithelium as an effector of inflammation: molecular regulation of secondary mediators. Eur Respir J 10: 2139–2146, 1997. doi: 10.1183/09031936.97.10092139. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell CT. Organic Dust Induced Lung Inflammatory Responses in Mice (Master’s thesis). Tyler, TX: University of Texas Health Science Center at Tyler, 2015. [Google Scholar]
- 38.Natarajan K, Gottipati KR, Berhane K, Samten B, Pendurthi U, Boggaram V. Proteases and oxidant stress control organic dust induction of inflammatory gene expression in lung epithelial cells. Respir Res 17: 137, 2016. doi: 10.1186/s12931-016-0455-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38a.National Center for Farmworker Health Poultry Workers. Buda, TX: National Center for Farmworker Health, 2014. [Google Scholar]
- 39.Palmberg L, Larsson BM, Malmberg P, Larsson K. Induction of IL-8 production in human alveolar macrophages and human bronchial epithelial cells in vitro by swine dust. Thorax 53: 260–264, 1998. doi: 10.1136/thx.53.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole JA, Anderson L, Gleason AM, West WW, Romberger DJ, Wyatt TA. Pattern recognition scavenger receptor A/CD204 regulates airway inflammatory homeostasis following organic dust extract exposures. J Immunotoxicol 12: 64–73, 2015. doi: 10.3109/1547691X.2014.882449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole JA, Kielian T, Wyatt TA, Gleason AM, Stone J, Palm K, West WW, Romberger DJ. Organic dust augments nucleotide-binding oligomerization domain expression via an NF-κB pathway to negatively regulate inflammatory responses. Am J Physiol Lung Cell Mol Physiol 301: L296–L306, 2011. doi: 10.1152/ajplung.00086.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, Golden G, West WW, Sisson JH, Romberger DJ. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am J Respir Cell Mol Biol 45: 711–719, 2011. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poole JA, Wyatt TA, Oldenburg PJ, Elliott MK, West WW, Sisson JH, Von Essen SG, Romberger DJ. Intranasal organic dust exposure-induced airway adaptation response marked by persistent lung inflammation and pathology in mice. Am J Physiol Lung Cell Mol Physiol 296: L1085–L1095, 2009. doi: 10.1152/ajplung.90622.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radon K, Weber C, Iversen M, Danuser B, Pedersen S, Nowak D. Exposure assessment and lung function in pig and poultry farmers. Occup Environ Med 58: 405–410, 2001. doi: 10.1136/oem.58.6.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romberger DJ, Bodlak V, Von Essen SG, Mathisen T, Wyatt TA. Hog barn dust extract stimulates IL-8 and IL-6 release in human bronchial epithelial cells via PKC activation. J Appl Physiol (1985) 93: 289–296, 2002. doi: 10.1152/japplphysiol.00815.2001. [DOI] [PubMed] [Google Scholar]
- 46.Romberger DJ, Heires AJ, Nordgren TM, Souder CP, West W, Liu XD, Poole JA, Toews ML, Wyatt TA. Proteases in agricultural dust induce lung inflammation through PAR-1 and PAR-2 activation. Am J Physiol Lung Cell Mol Physiol 309: L388–L399, 2015. doi: 10.1152/ajplung.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salmeen A, Barford D. Functions and mechanisms of redox regulation of cysteine-based phosphatases. Antioxid Redox Signal 7: 560–577, 2005. doi: 10.1089/ars.2005.7.560. [DOI] [PubMed] [Google Scholar]
- 48.Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun 9: 3261, 2018. doi: 10.1038/s41467-018-05800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT-3 activation and dimerization. Chem Biol 13: 1235–1242, 2006. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, Cochran BH, Simon AR. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol 286: L1282–L1292, 2004. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- 51.Shetty S, Rao GN, Cines DB, Bdeir K. Urokinase induces activation of STAT-3 in lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L772–L780, 2006. doi: 10.1152/ajplung.00476.2005. [DOI] [PubMed] [Google Scholar]
- 52.Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT-3 is required for allergic inflammation in a murine model of asthma. J Immunol 178: 6191–6199, 2007. doi: 10.4049/jimmunol.178.10.6191. [DOI] [PubMed] [Google Scholar]
- 53.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol Cell Physiol 275: C1640–C1652, 1998. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 54.Simpson JC, Niven RM, Pickering CA, Fletcher AM, Oldham LA, Francis HM. Prevalence and predictors of work related respiratory symptoms in workers exposed to organic dusts. Occup Environ Med 55: 668–672, 1998. doi: 10.1136/oem.55.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JK, Patil CN, Patlolla S, Gunter BW, Booz GW, Duhé RJ. Identification of a redox-sensitive switch within the JAK2 catalytic domain. Free Radic Biol Med 52: 1101–1110, 2012. doi: 10.1016/j.freeradbiomed.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Song L, Turkson J, Karras JG, Jove R, Haura EB. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene 22: 4150–4165, 2003. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 57.Staab E, Thiele GM, Clarey D, Wyatt TA, Romberger DJ, Wells AD, Dusad A, Wang D, Klassen LW, Mikuls TR, Duryee MJ, Poole JA. Toll-like receptor 4 signaling pathway mediates inhalant organic dust-induced bone loss. PLoS One 11: e0158735, 2016. doi: 10.1371/journal.pone.0158735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sundblad BM, von Scheele I, Palmberg L, Olsson M, Larsson K. Repeated exposure to organic material alters inflammatory and physiological airway responses. Eur Respir J 34: 80–88, 2009. doi: 10.1183/09031936.00105308. [DOI] [PubMed] [Google Scholar]
- 58a.US Department of Agriculture Poultry and Eggs. Washington, DC: USDA Economic Research Service, 2018. [Google Scholar]
- 59.Vogel CF, Garcia J, Wu D, Mitchell DC, Zhang Y, Kado NY, Wong P, Trujillo DA, Lollies A, Bennet D, Schenker MB, Mitloehner FM. Activation of inflammatory responses in human U937 macrophages by particulate matter collected from dairy farms: an in vitro expression analysis of pro-inflammatory markers. Environ Health 11: 17, 2012. doi: 10.1186/1476-069X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Von Essen S, Donham K. Illness and injury in animal confinement workers. Occup Med 14: 337–350, 1999. [PubMed] [Google Scholar]
- 61.Von Essen S, Romberger D. The respiratory inflammatory response to the swine confinement building environment: the adaptation to respiratory exposures in the chronically exposed worker. J Agric Saf Health 9: 185–196, 2003. doi: 10.13031/2013.13684. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Larsson K, Palmberg L, Malmberg P, Larsson P, Larsson L. Inhalation of swine dust induces cytokine release in the upper and lower airways. Eur Respir J 10: 381–387, 1997. doi: 10.1183/09031936.97.10020381. [DOI] [PubMed] [Google Scholar]
- 63.Wu Z, Zhang X, Yang J, Wu G, Zhang Y, Yuan Y, Jin C, Chang Z, Wang J, Yang X, He F. Nuclear protein IκB-ζ inhibits the activity of STAT-3. Biochem Biophys Res Commun 387: 348–352, 2009. doi: 10.1016/j.bbrc.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 64.Wyatt TA, Slager RE, Devasure J, Auvermann BW, Mulhern ML, Von Essen S, Mathisen T, Floreani AA, Romberger DJ. Feedlot dust stimulation of interleukin-6 and -8 requires protein kinase Cε in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 293: L1163–L1170, 2007. doi: 10.1152/ajplung.00103.2007. [DOI] [PubMed] [Google Scholar]
- 65.Yamaoka K, Saharinen P, Pesu M, Holt VE III, Silvennoinen O, O’Shea JJ. The Janus kinases (Jaks). Genome Biol 5: 253, 2004. doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang S, Hwaiz R, Luo L, Herwald H, Thorlacius H. STAT-3-dependent CXC chemokine formation and neutrophil migration in streptococcal M1 protein-induced acute lung inflammation. Am J Physiol Lung Cell Mol Physiol 308: L1159–L1167, 2015. doi: 10.1152/ajplung.00324.2014. [DOI] [PubMed] [Google Scholar]
- 67.Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 264: 95–98, 1994. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 68.Zschaler J, Schlorke D, Arnhold J. Differences in innate immune response between man and mouse. Crit Rev Immunol 34: 433–454, 2014. doi: 10.1615/CritRevImmunol.2014011600. [DOI] [PubMed] [Google Scholar]