Abstract
MicroRNAs (miRNAs) are a class of highly conserved non-coding RNAs with 21–25 nucleotides in length and play an important role in regulating gene expression at the posttranscriptional level via base-paring with complementary sequences of the 3′-untranslated region of the target gene mRNA, leading to either transcript degradation or translation inhibition. Brain-enriched miRNAs act as versatile regulators of brain development and function, including neural lineage and subtype determination, neurogenesis, synapse formation and plasticity, neural stem cell proliferation and differentiation, and responses to insults. Herein, we summarize the current knowledge regarding the role of miRNAs in brain development and cerebrovascular pathophysiology. We review recent progress of the miRNA-based mechanisms in neuronal and cerebrovascular development as well as their role in hypoxic-ischemic brain injury. These findings hold great promise, not just for deeper understanding of basic brain biology but also for building new therapeutic strategies for prevention and treatment of pathologies such as cerebral ischemia.
Keywords: cerebrovascular development, hypoxic-ischemic brain injury, microRNAs, mitomirs, neuronal development
INTRODUCTION
MicroRNAs (miRNAs) are a class of highly conserved non-coding RNAs (ncRNAs) that are 21–25 nucleotides in length. They negatively regulate gene expression at the posttranscriptional level via base-pairing with complementary sequences of the 3′-untranslated region (3′-UTR) of the target gene mRNA, leading to either transcript degradation or translation inhibition. The regulatory function of miRNAs is determined by their seed sequences or seed regions, which consist of 6–8 nucleotides at the 5′-end of miRNAs that are essential for the binding of the miRNA to the 3′-UTR of its target mRNAs (114, 115).
The fast speed of miRNA discovery leads to a need for the management of identified miRNAs. The name miRNA is given based on the similarity of the sequence to miRNAs previously identified. The miRNA Registry acts as a guideline for the name of a newly discovered miRNA (60, 96), which is agreed on by a number of prominent miRNA scientists (2). Generally, the same name will be assigned to identical mature miRNAs (60, 61). The sequences of mature miRNAs with one or two different bases are assigned suffixes of the form -a and -b (125). Some miRNAs that come from a given species but locate at separate genomic loci are given numerical suffixes to their name, such as miR-6-1 and miR-6-2 from Drosophila melanogaster (104). Some miRNAs excised from the 5′ and 3′ arms of the hairpin precursors are assigned with -5p and -3p, respectively (60), such as miR-210-3p and miR-210-5p.
miRNAs are expressed in numerous species, including worms, flies, plants, and vertebrates. Several thousand miRNAs have been defined in humans, and these participate in the widespread control of fundamental processes by targeting thousands of genes (84). In mammals, miRNAs exert diverse functions including control and maintenance of pluripotent cell state during early embryogenesis (76), tissue-specific or organ-specific development (105), and many aspects of physiological regulation (3, 10, 72, 163, 167). Due to the diversity of cell types and functional specialization in the central nervous system, miRNAs expressed in the brain are more unique than those in other organs. Brain-enriched miRNAs act as versatile regulators of brain development and function, including neural lineage and subtype determination, neurogenesis, synapse formation and plasticity, neural stem cell (NSC) proliferation and differentiation, and responses to insults (84, 99, 102, 151, 183).
Numerous environmental, physiological and pathological stresses, such as hypoxia, hormone, infection, etc., can trigger the changes of miRNA expression profiles. The expression of miRNAs is regulated at both transcription and posttranscription levels. Changes in intragenic miRNA expression can occur due to changes in the expression of host genes (27, 202) or the methylation levels of the promoter of host or miRNA genes (89, 218). Defects in the miRNA biogenesis or processing regulate the expression of miRNAs at the posttranscription level (12, 164, 190).
BIOGENESIS OF miRNA
The biogenesis of miRNA begins with transcription from either intergenic or intragenic regions of both protein coding and non-coding genes (59, 144). Some miRNAs are transcribed as a cluster, which is a group of miRNAs with similar seed sequences (200). First, RNA polymerase II produces primary miRNAs (pri-miRNAs) in the nucleus from genomic regions, which are long segments and typically contain one to six precursors of mature miRNAs. Next, pri-miRNAs are trimmed into hairpin-shaped precursors in the length of ~70–100 nucleotides named pre-miRNAs. This processing event is conducted by a complex containing Drosha, a nuclear member of RNase type III, and DGCR8 (DiGeorge syndrome critical region gene 8), a double-stranded RNA (dsRNA)-binding protein domain (67, 111). Chaperoned by the nuclear transport receptor complex exportin-5/RanGTP, pre-miRNAs are exported to the cytoplasm, in which pre-miRNAs are further processed to form mature miRNAs (137). In mammalian cytoplasm, the terminal loop of pre-miRNAs is removed by Dicer, a double-stranded ribonuclease, to form a double-stranded miRNA duplex with ~20–22 base pairs in length, together with its dsRNA-binding partner TRBP (transactivation-response RNA-binding protein) (82, 92). The miRNA duplex is then bound to the Argonaute protein (AGO) as a dsRNA and incorporated into a RNA-induced silencing complex (RISC)-loading complex (RLC) in an ATP-dependent manner (236). One strand of the duplex is degraded by AGO, which is an endonuclease, and the remaining strand (mature miRNA) serves as a template for capturing target mRNA and silencing gene expression through a complete or partial base-paring to the 3′-UTR of target mRNAs (63, 81, 90). For each specific miRNA, the RISC-loading selection of 5p or 3p strand is a tightly controlled process that is influenced by cell types, cellular environment, and developmental stages (145). Additionally, the thermodynamic stability at the 5′-end of miRNA duplex or a 5′-UTR at nucleotide position 1 also partially influences the selection of the 5p or 3p strand (94). The extent of complementarity between miRNAs and target mRNAs determines the actions of RISC in the regulation of gene expression (12). Generally, high-affinity matching between miRNAs and mRNAs results in degradation of the target transcript, while partial complementarity may decap and deadenylate the target mRNA and thereby lead to reduced stability in inhibition of translation (162, 206, 220).
miRNAS IN NEURONAL DEVELOPMENT
MiRNAs have gained growing recognition for their significant involvement in multiple aspects of both brain development and neurodevelopmental disorders. Drosha/DGCR8 is the key element for processing pri-miRNAs, the gene of which is located within the 22q11.2 locus. Using a mouse model of schizophrenia (SCZ) with chromosome 22q11.2 deletion, Fénelon et al. (51) found that reduction of Dgcr8 levels altered short-term synaptic plasticity in prefrontal cortex and also working memory, suggesting that Dgcr8 is a potential primary candidate gene for the development of SCZ. This finding emphasizes that disturbances of miRNA biogenesis can result in abnormal brain development, and reveals an essential link between miRNAs and central nervous system (CNS) development and homeostasis. Bioinformatic evidence further demonstrates that miRNAs are abundantly expressed in the CNS and that ~70% of experimentally detectable miRNAs are enriched in the brain (23). Indeed, miRNAs in the brain are expressed in a broad diversity of expression profiles, particularly during neural differentiation and/or proliferation (101, 147, 192), and are linked to specific neurodevelopmental stages, including cell fate determination and progenitor cell migration and differentiation into neurons and glia (91, 193). To date, numerous studies have identified specialized neurodevelopmental functions of specific miRNAs in the vertebrate brain (31, 57, 126, 141, 187). Based on these findings, the critical importance of miRNA in neurogenesis, neuronal maturation, neural differentiation and maintenance, and neuroplasticity in normal and adverse conditions has been predicted.
MiR-9 is one of the most widely studied cerebral miRNAs due to its high abundance in both immature and mature vertebrate brains. Mounting evidence has revealed that miR-9 plays a prominent role in regulating neural progenitor cell proliferation and differentiation during embryonic development (34, 75, 98, 189), and is thus regarded as a core member of the gene network controlling the neural fate (237). In zebrafish, the expression of miR-9 is characteristic during late stages of embryonic CNS development, where it fine-tunes the pool of neural progenitors in the midbrain-hindbrain boundary (MHB). Paired in vivo loss- and gain-of-function experiments demonstrate that miR-9 promotes neurogenesis progression and restricts progenitor pool growth by negatively regulating the fibroblast growth factor (FGF) signaling pathway and genes regulated by antineurogenic basic helix-loop-helix (bHLH) transcription factor encoding, such as her5 and her9 (112). In addition, miR-9 interacts with the Notch signaling pathway, which is well documented for its function in stem cell maintenance. In this context, miR-9 participates in neural development in both nonvertebrate and vertebrate models, including Drosophila, zebrafish, frog, and mouse (16, 17, 35, 124). Reciprocally, the Notch signaling pathway contributes to the expression of miR-9 and its sister strand miR-9∗, and mature miR-9/miR-9∗ negatively regulate the Notch pathway by targeting the transcripts of Notch2 and HES1 to calibrate neural stem cell (NSC) proliferation and differentiation (177). In mice, miR-9 suppresses NSC proliferation and stimulates neural differentiation in the brain by inhibiting the expression of orphan nuclear receptor TLX, which is a master regulator for the self-renewal of NSCs. More interestingly, TLX also regulates miR-9 levels by suppressing the primary transcript of miR-9. This finding implies that a regulatory feedback loop involving miR-9 and TLX balances neural proliferation and differentiation (245). In addition to TLX, extensive studies confirmed that miR-9 exerts the anti-progenitor proliferative effects by negatively regulating various other transcript factors, such as forkhead transcription factor FoxG1, homeobox factor Gsx2, repressor element 1-silencing transcription factor (REST), and zinc finger transcription factor Zic5. Accordingly, these transcription factors also suppress miR-9 expression, and overexpression of them results in a miR-9-deficient-like phenotype during brain development (16, 107, 187). The significance of miR-9 in brain development has been confirmed in mutant mice lacking miR-9-2 and miR-9-3. Shibata et al. (187) found that miR-9 deficiency upregulated multiple transcription factors described above, and exhibited dysregulation of pallial and subpallial progenitor cell proliferation/differentiation in telencephalic development as well as multiple defects in the telencephalic structures. The expression of miR-9 is also regulated by other miRNAs. For example, miR-107 downregulates Dicer expression levels during zebrafish hindbrain development, which leads to a global suppression of the biogenesis of miRNAs. Among them, miR-9 exhibits the strongest response when modulating miR-107 activity during neurogenesis. Moreover, functional studies show that a gain of miR-9 function in the differentiating neuronal cells recapitulates the neurogenic defect observed in the loss of miR-107 (176).
Aside from miR-9, miR-124 is another neuron-specific miRNA extensively studied in the context of neural differentiation during brain development, which is exclusively expressed at presynaptic terminals of Aplysia (173). MiR-124 regulates alternative pre-mRNA splicing in CNS development by downregulating the expression of polypyrimidine tract-binding protein-1 (PTBP1; neural variant, nPTB). As a repressor of transcript splicing in neural cells, PTBP1 is highly expressed in nonneuronal cells but has a low expression in neurons. During neuronal differentiation, miR-124 suppresses PTBP1 and triggers a downstream switch from general to neuronal specific alternative splicing, leading to the upregulation of its neuron-enriched homolog PTBP2 (141). Overall, PTB is a versatile regulator in neuron development. In addition to regulating splicing, PTB also regulates the function of miRNAs in the process of cellular reprogramming to neuronal lineages. Diminished PTB expression boosts miR-124 effects and induces multiple neuron-specific transcription factors in nonneuronal cells, thereby promoting the conversion of fibroblasts to neurons (227). MiR-124 also regulates temporal progression of stem cell lineage in the subventricular zone (SVZ) to neurons in adult brain by suppression of the SRY-box transcription factor Sox9. Knockdown or blocking of miR-124 maintains SVZ stem cells and results in a delayed burst of neuronal formation by neurogenesis (31). During neuronal differentiation, the expression of neuron-related genes is upregulated, whereas the expression of unwanted nonneuronal genes is blocked. Repressor element 1 silencing transcription factor (REST) plays an inhibitory role in the expression of neuronal genes in nonneuronal and neural progenitor cells (NPCs). During the conversion from NPCs to mature neurons, the levels of REST are reduced, which promotes the expression of neuron-related genes (6). The opposite functions of REST and miR-124 in neurogenesis suggest that these two pathways are intricately orchestrated to regulate neuron development. Indeed, in nonneuronal cells, REST inhibits miR-124 expression, allowing transcription of nonneuronal genes, whereas in postmitotic neurons, REST dissociates from miR-124 gene loci, leading to selective degradation of nonneuronal transcripts (33). In contrast, during embryonic CNS development, miR-124 antagonizes REST functions through direct targeting of the 3′-UTR of SCP1 transcripts, which is a corepressor of REST in nonneuronal tissues that undergoing anti-neuronal differentiation (205).
Other miRNAs also play important roles in neuron development. MiR-184 has been reported to fine-tune the balance of NSC proliferation and differentiation by directly binding to the 3′-UTR of transcripts of the Numblike (Numbl) gene, which is a repressor of NSC proliferation but an enhancer of differentiation. Moreover, the miR-184/Numbl axis is epigenetically regulated by methyl-CpG-binding domain protein-1 (MBD1), which represses miR-184 expression and promotes NSC differentiation (126). In addition, miR-let-7b controls the switch of NSC proliferation and differentiation by targeting TLX signaling and the cell cycle regulator cyclin D1 (244). Other miRNAs, such as miR-20a/20b and miR-23, also inhibit cyclin D1 levels through directly binding to the 3′-UTR of cyclin D1 mRNA. Inhibition of each of these miRNAs increases cyclin D1 protein in progenitor cells in a manner specific for developmental-stage and reduces neuronal differentiation (58). Together, this evidence suggests that multiple miRNAs form a complex regulatory network to regulate progenitor fate decisions and control neuronal differentiation by modulating multiple target genes. Understanding the mechanisms regulating these key developmental events offers great potential for new insights into brain developmental disorders.
Neuron maturation is the last step of neurogenesis, which allows new neurons to integrate into neural networks and communicate with other neurons. Numerous miRNAs are abundantly expressed in the neuronal dendritic spines and participate in neuronal maturation, including dendritic and axonal growth, dendritic spine development, and synaptogenesis (136, 191). In addition to its role in neurogenesis, miR-9 is also involved in axon development and is a functional target for brain-derived neurotrophic factor (BDNF)-dependent control of axon extension and branching by inhibiting microtubule-associated protein-1b (Map1b) translation (38). A recent study in mature mouse neurons from Sim et al. (189) found that miR-9-3p, but not miR-9-5p, regulated synaptic plasticity and memory by downregulating the long-term potentiation (LTP)-related genes Dmd and SAP97, the expression levels of which were negatively correlated with LTP. MiR-137 also modulates neuronal maturation, and overexpression of miR-137 inhibits phenotypic maturation and dendritic morphogenesis during neurodevelopment. These phenotypic deficits can be partially rescued by expression of mouse homolog of Drosophila mind bomb 1 (Mib1), a ubiquitin ligase involved in neurogenesis and neurodevelopment (194). MiR-134 levels gradually increase in rat hippocampal neurons with developmental age and reach a peak at postnatal day 13 (P13), at which time synaptic maturation occurs. Overexpression of miR-134 negatively regulates the size of dendritic spines by inhibiting the transcripts of the protein kinase Limk1 (184), whereas silencing of miR-134 diminishes hippocampal CA3 pyramidal neuron dendrite spine density (88). MiR-134 also specifically regulates dendritogenesis by downregulating the RNA-binding protein and translational repressor Pumilio2 (Pum2) (53). Similarly to miR-134, a functional screen study revealed that another brain-enriched miRNA, miR-138, also negatively regulates the size of dendritic spines in rat hippocampal neurons. MiR-138 downregulates the expression of acyl protein thioesterase-1 (APT1), an enzyme that regulates the palmitoylation status of proteins known to function at the synapse (188). MiR-132 is another brain-enriched miRNA that can be activated by neuron stimulation both in vivo and in vitro (152, 217). Using serial analysis of chromatin occupancy (SACO) technology for the genome-wide identification of target regions of cAMP response element-binding protein (CREB), Wayman et al. (217) identified miR-132 as an activity-regulated rapid response gene in hippocampal neurons regulated by the transcription factor CREB, which is a critical regulator of neuronal plasticity. Thus, as a target of CREB, miR-132 is engaged in development of activity-dependent dendritic structural and functional plasticity. Moreover, miR-132 negatively regulates the Rho family GTPase-activating protein p250GAP, because knockdown of p250GAP induces dendrite growth as does increased miR-132 expression. A subsequent study further demonstrated that the miR-132/p250GAP axis regulates synaptogenesis by modulating synapse-specific kalirin7-Rac1 signaling (83). In addition to its negative effects on dendritic spine morphology, miR-132 also regulates synaptic excitability by increasing currents mediated by the a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor- and the N-methyl-d-aspartate (NMDA) receptor- in postsynaptic cells (43, 106). In another recent study, miR-483-5p, an intragenic miRNA of the insulin-like growth factor 2 gene (Igf2), exerted an important influence on dendritic spine phenotype and neuronal maturation by negatively regulating methyl CpG-binding protein-2 (MeCP2) in the fetal brain (68).
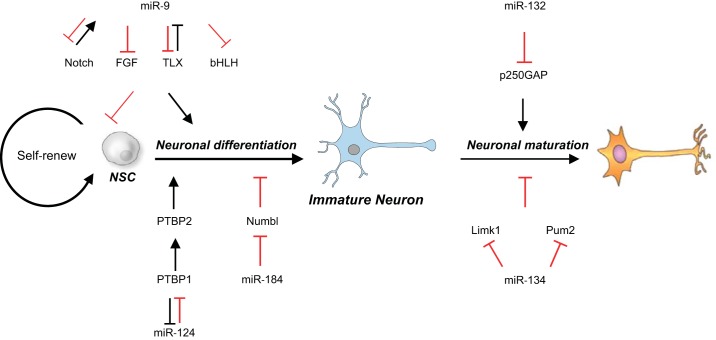
Taken together, a broad variety of studies attest to the importance of miRNA-dependent mechanisms in neuronal development and demonstrate a previously unappreciated complexity of miRNA networks in the control of neuronal differentiation, neuron maturation, dendritic spine morphogenesis, and synaptic plasticity (Fig. 1). Correspondingly, abnormal expression of miRNAs in response to adverse environmental conditions or insults have the potential to disturb neurodevelopment and contribute to long-term neurological disorders.
Fig. 1.
Regulatory effects of micro-RNAs (miR) on specific stages of neuronal development. MiR-9 inhibits neural stem cell (NSC) proliferation and promotes neuronal differentiation by negatively regulating Notch pathway, fibroblast growth factor (FGF) pathway, and transcription factors TLX and basic helix-loop-helix (bHLH). Moreover, Notch signaling pathway contributes to the expression of miR-9, whereas TLX suppresses miR-9 transcription. MiR-124 promotes neuronal differentiation by downregulating polypyrimidine tract-binding protein-1 (PTBP1). On the other hand, PTBP1 also negatively regulates miR-124 levels. MiR-184 suppresses neuronal maturation by downregulation of Numb-like (Numbl). MiR-132 promotes neuronal maturation by inhibition of p250 GTPase-activating protein (p250GAP), whereas miR-134 shows reversed effect in this event by negatively regulating LIM domain kinase-1 (Limk1) or Pumilio2 (Pum2).
miRNAS IN CEREBROVASCULAR DEVELOPMENT
In parallel with their effects on neuronal development and differentiation, miRs also influence many aspects of the development and function of the cerebral vasculature. Overall, many of the effects of miRs on cerebral vessels are similar to their effects on the extracranial vasculature, which have been extensively studied. Only recently have the effects of miRs in cerebrovascular development begun to be explored, which makes it useful to first briefly review key findings of the roles of miRs in the systemic vasculature.
miRNAs in Development of the Systemic Vasculature
Among the first of the miRs to be studied in the context of vascular biology was miR-210, which is a well-documented mediator of cellular responses to hypoxia (25). In turn, miR-210 upregulation is essential for endothelial responses to hypoxia and plays important roles in endothelial cell survival and differentiation (49). Another miR important for endothelial development and angiogenesis is miR-126, which helps mediate the response to VEGF in part by repressing negative regulators of VEGF, particularly during embryonic development (54, 213). In adults, miR-126 may also play a role in the development of atherosclerosis, perhaps by virtue of its endothelial effects (207). Within smooth muscle cells, among the first miRs recognized to play a critical role in differentiation were miR-143 and -145. Most importantly, miR-143 and miR-145 are direct transcriptional targets of both serum response factor and myocardin, which are coactivators essential for contractile differentiation of smooth muscle (160, 161). The reprogramming of fibroblasts into smooth muscle requires miR-145, which, together with miR-143, interacts with a small family of transcription factors that determine smooth muscle cell fate, including Kruppel-like factor 4 (KLF4), myocardin, and ETS-like-1 protein (ELK1) (36). Both miR-143 and miR-145 are important components of the pathways through which serum response factor regulates the expression of genes coding for cytoskeletal proteins, including during the progression of vascular disease (225). Recent studies suggest that miR-145 also can target CD40, a protein expressed by antigen-presenting cells, which is required for their activation (7). Through its inhibition of CD40 expression, miR-145 also can retard or block smooth muscle differentiation (64). Smooth muscle proliferation, however, requires miR-221 and -222 (129). Smooth muscle proliferation and growth are also regulated by miR-29b, which achieves these effects by targeting myeloid cell leukemia protein-1 (Mcl-1) and matrix metalloproteinase-2 (MMP2) (110, 207). In addition, the effects of PDGF on smooth muscle proliferation and differentiation are supported by miR-4632 (172), but are antagonized by the miR let-7g (214). Single nucleotide polymorphisms in some miRs (miR-196 and -296) may also influence the susceptibility to vascular disease (216).
miRNAs in Development of the Cerebral Vasculature
Within the cerebral circulation, a primary focus on the role of miRs has been in the context of angiogenesis. To this end, recent studies have established that miR-126, -221, and -222 can promote angiogenesis and that miR-21 can promote smooth muscle proliferation (229). Other miRs found to support cerebral angiogenesis include miRs let-7, -21, -27, -31, -130a, -296, and -378 (234). In contrast, numerous miRs appear to be antiangiogenic in the brain, including miR-15/16, -34, -92a, -137, -195*, -200b, -214, -217, -221/222, -320, -424, and -503 (78, 234). Because the effects of all the miRs studied are not the same in cerebral and systemic vessels, great care must be exercised when selecting miRs with therapeutic potential to treat ischemic vascular disease. To this end, several recent reviews have carefully compiled and detailed the effects of dozens of miRs with effects on the cerebral circulation (109, 117). An interesting result of such studies has been identification of multiple miRs that are differentially expressed in cerebral aneurysm tissues and arteriovenous malformations (109) (78), which raises the interesting possibility that intervention with antagomirs against these miRs may some day offer a therapeutic approach for the treatment of these disorders.
Another major focus on the role of miRs in the cerebral circulation has been on their involvement in endothelial biology and formation of the blood-brain barrier. Initial studies confirmed that miR-210 upregulated Notch1 signaling and stimulated endothelial migration and the early stages of capillary budding necessary for angiogenesis (134). Other studies soon established that miR-15a could promote endothelial apoptosis and that KLF11 could stimulate peroxisome proliferator-activated receptor-γ (PPARγ)-mediated suppression of miR-15a expression and thereby promote endothelial survival (233). Parallel studies further revealed that miR-101, -125, and -155 all influence endothelial biology and formation of the blood-brain barrier (235), whereas expression of miR-144 was found to accumulate with advancing age (37). Consistent with its ability to promote angiogenesis, miR-126 was also found to stimulate expression of zona occludens protein-1 (ZO-1) and other tight-junction proteins, suggesting a key role in the formation and possible maintenance of the blood-brain barrier (251). Other miRs, including miR-107 and -21 also help upregulate tight-junction proteins, but these effects can be opposed by miR-98, -150, and -181a, indicating that expression of key tight-junction proteins reflects a complex balance between multiple miRs with opposing actions (251). Ischemic conditions can depress levels of miR-126, suggesting that this effect may help explain how ischemia increases blood-brain barrier permeability (28). Cerebral ischemia can also increase endothelial expression of miR-130a, which also can increase blood-brain barrier permeability (185). Together, these results demonstrate that formation and maintenance of cerebrovascular endothelium and the blood-brain barrier depend on the integrated influence of numerous miRs. Future studies seem likely to identify even more miR molecules that govern blood-brain barrier formation and function, which again raises the possibility that some of these miRs may have therapeutic potential for treatment of an injured or dysfunctional blood-brain barrier.
miRNAS IN HYPOXIC-ISCHEMIC BRAIN INJURY
An early priority among studies of miRs in the cerebral circulation was to learn whether these small molecules could be of therapeutic value for the treatment of cerebral ischemia and other cerebrovascular injuries. Such studies vastly outnumber studies of the healthy cerebral circulation and have employed many different strategies. In addition to the roles of miRs in cell type-specific effects of cerebral ischemia, contemporary studies have expanded to examine possible involvement of miRs in cerebral ischemic depression of mitochondrial function, neural inflammation, and neural stem cells. In recognition of this diversity of studies, the following section offers a survey of main findings in each of these categories.
General Aspects of miRNAs in Cerebral Ischemia
Biomarkers.
A popular idea in early studies of miRs in cerebral ischemia was that these small regulatory molecules could serve as biomarkers for tissue injury. Initial studies quickly established miR-21 as a candidate biomarker, detectable in patient blood following a cerebral ischemic event (13, 87, 199, 203). Another possible biomarker, miR-145, was also soon identified (55, 74, 122), although the reliability of miR-145 as a marker for ischemic cerebral injury was questioned in other studies (121, 203). In turn, many other miRs have been advocated as candidate circulating biomarkers for ischemic cerebral injury including miR-17 family members (204) and miR-20a, -23a, -29b, -34a, -122, -124, -144, -146a, -155, -185, -210, -221, -223, -298, -320a, -362-3p, -376b-5p, -483, and -486 (13, 45, 87, 119, 121, 122, 174, 199, 203). A novel study by Jeon et al. (85) further suggested that polymorphisms in miR-146a were associated with elevated risk for stroke (85). Clearly, this diversity of possible biomarkers for ischemic cerebral injury suggests that this field is still evolving, due perhaps to the highly heterogeneous nature of responses to cerebral ischemia in general. Even so, this remains an active area of intense investigation and it is still hoped to develop a quick and reliable method to determine the severity of a recent cerebral ischemia insult, in much the same way that ischemic heart damage is diagnosed (140).
Microglia.
Owing to the importance of inflammation in responses to cerebral ischemia, the involvement of miRs in microglial responses to cerebral ischemic insults has also been investigated in some studies. MiR-124 promotes microglial quiescence and can attenuate neuroinflammation (116, 168), which raises the possibility that it may have therapeutic potential for attenuating postischemic neuroinflammation. Other miRs, including miR-146a and miR-424, can also suppress microglial activation and similarly have been suggested to have possible therapeutic potential for cerebral ischemia (116, 247). In contrast, miR-181c can promote microglial and neuronal apoptosis; thus, an antagomir of miR-181c may improve stroke outcomes (139). Again, this line of investigation is in its infancy but appears to offer great therapeutic potential.
Blood-brain barrier and endothelium.
Following elucidation of the ability of miR-210 to induce endothelial migration and angiogenic activity (134), it was quickly appreciated that miR-210 was a key component of endothelial responses to hypoxia and ischemia. Similarly, the ability of PPARγ to suppress expression of miR-15a, and thereby attenuate endothelial apoptosis, was recognized as a possible strategy for blood brain barrier protection following ischemia (233). Other endothelial miRs were also associated with cerebral ischemia, including miR-15a, -139, -199, and -320, but the therapeutic potential of these molecules has been less obvious (235). Efforts to understand which miRs may be beneficial for the endothelium after stroke suggest that administration of miR-146a/b (196), or inhibition of miR-155 (178), can improve stroke outcomes. Another study by Matsuoka, et al. (143) demonstrated with both in vitro and in vivo approaches that miR-124 antagonizes translation and expression of the tight-junction complex protein claudin domain containing 1 (CLDND1) in stroke-prone spontaneously hypertensive rats and thus might potentially contribute to the elevated incidence of stroke in this rat strain. A primary limitation of these approaches, however, is that most miRs affect multiple gene transcripts; thus, off-target effects are likely. For example, miR-155 affects many different cell types other than endothelium, including vascular smooth muscle cells and neurons, means that cell type-specific targeting will be essential for success of this and many other miR-based therapeutic approaches to ameliorate the effects of cerebral ischemia.
Angiogenesis.
Studies of post-stroke angiogenesis have offered many potential miR candidates for intervention in ischemic injury, including the miR-17–92 cluster and miR-21, -27b, -92a, -107, -126, -150 -221, -222, -493, and many others (73, 120, 123, 229, 234, 238). Relatively few studies, however, have performed interventional experiments that manipulated miR levels and measured the corresponding effects on angiogenesis. In one of these, a traditional Chinese medicine (Buyang Huanwu decoction) was found to simultaneously increase miR-126 and decrease miR-221 and -222, resulting in increased angiogenesis in postischemic rat brain (230). In another study, overexpression of miR-296 improved angiogenesis following ischemia (52). Administration of an antagomir of miR-493 has also been shown to improve capillary density following middle cerebral artery occlusion in rats through effects related to macrophage migration inhibitory factor (120). Not unexpectedly, overexpression of miR-210 via lentiviral transfection also enhanced microvessel density and postischemic outcomes in mouse brain (240). Using an in vitro approach, Zhao et al. (248) demonstrated that miR-195 overexpression attenuated angiogenic activity, whereas downregulation improved such activity. More recently, knockdown of miR-377 was shown to promote angiogenesis following middle cerebral artery occlusion in rats (47). In another recent study, transfection with miR-27b increased angiogenesis in the penumbra after middle cerebral artery occlusion, which promoted postischemic recovery and improved neurobehavioral performance (238). Together, these studies demonstrate that manipulation of angiogenic miRs is feasible and produces beneficial results on post-ischemic angiogenesis.
Cerebral vasculature.
Whereas the influence of miRs in the systemic circulation has been explored extensively (56), particularly in relation to endothelial and smooth muscle involvement in the etiology of atherosclerosis and restenosis (181), relatively little effort has been invested thus far in the roles of miRs in cerebrovascular pathophysiology. Owing to the intense interest in mechanisms mediating brain injury secondary to cerebral ischemia, most studies of miRs in the cerebral circulation have identified numerous miRs that change post-insult, including miR-30a, miR-143, and many others (149, 207). However, in one recent study, inhibition of miR-155 expression helped maintain vascular function after ischemia and improved recovery (21). More studies that focus on changes in miR expression within cerebrovascular smooth muscle are needed to better understand how miR responses to ischemia vary among the different cell types within the CNS. Specifically, an important goal of future studies should be to identify which cerebrovascular cell types and locations actively produce miRs in response to cerebral ischemia and whether or not these cells release miRs to act on neighboring cells in a paracrine fashion (204, 224).
Neurons and glia.
A major fraction of studies of the effects of cerebral ischemia on miRs in the CNS have focused on the ability of miRs to influence post-ischemia neuronal apoptosis. Abundant evidence demonstrates that miR-210 can target Bcl-2 and thereby induce neuronal apoptosis (32). Multiple studies also document the neuroprotective effects of the miR-29 family (71), which has been attributed to inhibition of apoptotic neuronal death (93), and astrocytic death as well (159), by targeting the proapoptotic Bcl2. Hypoxic-ischemic stress can increase miR-1, which in turn can interact with heat shock protein-70 (HSP70) to activate the intrinsic Bax-caspase pathway; knockdown of miR-1 correspondingly attenuates neuronal cell death (26). The neuroprotective profile of miR-21 has been attributed to its antiapoptotic characteristics (150). Overexpression of miR-99a has been shown to attenuate postischemic neuronal apoptosis through regulation of cell cycle progression (201). Injection of bone mesenchymal stem cells transfected with miR-705 also can attenuate postischemic neuronal apoptosis through enhancement of BDNF and VEGF expression (86). Other studies have detailed how cerebral ischemia affects miR expression and have identified numerous miRs, such as miR-347, that regulate postischemic neuronal death (62). Interestingly, the neuroprotective and antiapoptotic effects of isosteviol sodium also have been attributed recently to downregulation of miR-181b (241), whereas administration of miR-181c via transfection can exert neuroprotective effects (48). Given that miR-181c has been shown to promote postischemic neuronal apoptosis (139), it is clear that the cellular environment in which change in miR levels occur greatly influence outcomes.
Upstream of apoptosis, many miRs exert indirect effects on cell death via molecules such as p53 (130), HSP70 (155), Hhypoxia-inducible factor-α (HIF1α) (127), Toll-like receptor 4/myeloid differentiation primary response protein-88 (TLR4)/MyD88) (219), and enzymes involved in oxidative stress (128, 246). This diverse array of effects, in concert with the many miRs involved in direct regulation of apoptosis, clearly demonstrates both the complexity and regulatory importance of miRs in neuronal and glial apoptotic responses to ischemic injury.
miRNAs in Cerebral Mitochondria
General aspects of mitochondrial miRNA.
Mitochondria mediate the internal caspase-dependent pathway of apoptosis in all cell types during times of stress, but more importantly, mitochondria synthesize ATP and regulate cell metabolism under nominal conditions. Because mitochondria possess their own unique genome, considerable interest has been focused on how mitochondrial activity is coordinated with the overall activities of the host cell. Not long after the discovery of miRNA, these small molecules were considered as possible mediators of cellular and nuclear influences in mitochondrial activity and patterns of gene expression. Early studies demonstrated that miR-338 is brain specific and regulates cytochrome oxidase IV in the axons of sympathetic nerves (4). Purified live mitochondria were found to contain 15 miRs of nuclear origin, some of which targeted proteins involved in apoptosis and cell proliferation and differentiation, leading to the creative speculation that mitochondria may serve as reservoirs for key regulatory miRs (100). In mitochondria from a cancer cell line, miR-210 was found to depress overall mitochondrial function and upregulate glycolysis, thereby producing the unique metabolic characteristics of cancer cells (30). In HeLa cells, dozens of miRs were differentially expressed in the mitochondrial and cytosolic compartments, based in part on structural characteristics (8). Perhaps more importantly, Argonaute-2 was also found in the mitochondria, suggesting cross-talk between the nucleus and mitochondria and the possibility that nuclear signals can regulate mitochondrial biogenesis. In human myoblasts, mitochondria were found to contain pre-miRs, suggesting that miR-processing components must be synthesized in the parent cell cytoplasm and translocated into the mitochondria through unknown mechanisms (9). In cardiac myocytes, miR-181c was found within mitochondria, where it bound the 3′-UTR of mitochondrial (mt-) cyclooxygenase-1 (COX1), indicating regulation of the mitochondrial genome (39). Recent studies have further revealed that mitochondrial miRs (“mitomirs”) can be identified in numerous vertebrates, particularly those that are anoxia tolerant (175).
Cerebral mitochondrial miRNA.
Much of the initial work on miRs in cerebral mitochondria has focused on neuroblastoma, glioma, and glioblastoma cells (221, 222, 226, 228). This work has largely shown that miR-125b, -135a, 302b, and -497 all target and attenuate mitochondrial pathways of apoptosis and that other miRs target mitochondrial proteins involved in ATP synthesis. In dopaminergic cell models of Parkinson’s disease, tumor necrosis factor-α (TNFα) altered the levels of multiple miRs, including miR-5701, that in turn altered mitochondrial function and cell vulnerability (169, 170). In mouse models of Alzheimer’s disease, miR-195 has been shown to bind to mitofusin 2 and decrease mitochondrial membrane potential, and miR-98 was able to bind HEY2, activate Notch signaling, reduce oxidative stress, and improve mitochondrial function (29, 243). In more normal cells, miR-743a has been shown in hippocampal neurons to mediate the effects of oxidative stress by targeting and downregulating the mitochondrial TCA cycle gene Mdh2 (186). In cerebrovascular endothelial cells, miR-34a targets several mitochondrial genes and targets cytochrome c, leading to decreases in ATP production and increases in blood-brain barrier permeability (18). Whereas the ability to study the independent and direct effects of miRs on mitochondrial function is experimentally limited by the inability to transfect or transplant individual mitochondria, the findings obtained thus far emphasize the value of this line of investigation and encourage innovation to improve elucidation of mechanisms and causality in the interactions of miRs with cerebral mitochondria.
Effects of ischemia on cerebral mitochondrial miRNA.
Despite the technical limitations inherent in studies of mitochondrial miRs, several studies have thus far provided convincing involvement for miRs in cerebral responses to ischemia. Specifically, miR-181a targets multiple members of the Bcl-2 family but also induces mitochondrial dysfunction and loss of mitochondrial membrane potential in astrocytes after cerebral ischemia (118, 156, 158). In tandem with miR-181 family members, molecules in the miR-29 family also modulate astrocytic responses to ischemia (118, 158). MiR-29a targets the mitochondrial voltage-dependent cation channel 1 transcript in astrocytes and thereby can improve mitochondrial membrane potential and function following cerebral ischemia (118, 195). Other studies in neurons have shown that miR-338 regulates mitochondrial mRNA transcripts that code for components of the oxidative phosphorylation cascade (157). In a traumatic brain injury model, both miR-155 and miR-223 can be found in hippocampal mitochondria, where they have the potential to modulate levels of mitochondrial transcripts and mediate the effects of ischemic injury (215). In a rat model of focal ischemia, administration of miR-7a-5p reduced postischemic levels of α-synuclein, a protein that controls mitochondrial fragmentation, and correspondingly reduced lesion volume and behavioral outcomes (95). As a whole, the results to date constitute only a first step toward understanding how miRs contribute to the effects of ischemia on mitochondrial function. Even so, they strongly support the idea that further studies of miRs that influence and regulate mitochondrial function and abundance have great potential to identify new therapeutic approaches for the treatment of cerebral ischemia (77).
miRNAs in Neuroinflammation
Neonatal hypoxic-ischemia (HI) induces a robust inflammatory response that is a major contributor to subsequent secondary brain injury. The inflammatory response begins within minutes after an HI insult and includes activation of brain resident cells and infiltration of peripheral leukocytes into the brain parenchyma across the blood-brain barrier (14, 44, 65, 66, 116). The activated microglia and astrocytes, together with infiltrated peripheral innate immune cells, release proinflammatory cytokines, reactive oxygen species (ROS) and other neurotoxic agents, leading to neuronal death. In addition, growing evidence suggests that the adaptive immune system also contributes to brain injury after HI. It has been reported that regulatory T cells promote ischemic brain injury in the acute phase (97), but invading T cells are involved in neural repair processes during the chronic phase after stroke (223). Relevant to these processes, miRs play important roles in modulating proliferation, early differentiation, maturation, and maintenance of immune cells, particularly during brain development. In turn, deregulation of miRNAs contributes to neuroinflammation under various pathological conditions, such as infection, neurodegeneration, autoimmunity diseases, ischemic stroke, etc. (13, 79, 153, 182). A microarray assay using umbilical cord blood samples from infants with hypoxic-ischemic encephalopathy (HIE) indicated that neonatal HIE alters the expression patterns of numerous miRNAs (133). However, few studies address the potential influences of miRNAs in regulating inflammatory responses in neonatal HIE. Further studies are needed to better understand the roles of specific miRNAs in HI-induced neuroinflammation so that promising therapeutic strategies can be identified.
MiR-210 is regarded as the master hypoxamir among a specific group of miRs regulated by hypoxia. MiR-210 regulates multiple diverse cellular events, including angiogenesis, mitochondrial metabolism, and apoptotic cell death (24, 25, 32, 138). Increasing evidence shows that miR-210 also acts as a key regulator during inflammatory responses under high-stress conditions. A recent study revealed the role of miR-210 in the regulation of the activation and differentiation of T cells under low oxygen conditions. Wang et al. (212) found that the levels of miR-210 were significantly upregulated in stimulated T cells, especially the TH17 lineage of helper T cells during hypoxia. Further experiment demonstrated that this upregulation of miR-210 was controlled by the T cell antigen receptor (TCR) and its coreceptor CD28. Moreover, miR-210 deficiency promoted TH17 differentiation during hypoxia, and this event was mediated by a negative feedback loop in which miR-210 inhibited HIF-1α expression. In addition to hypoxia, lipopolysaccharide (LPS) also promotes miR-210 upregulation in murine macrophages. Qi et al. (171) reported that miR-210 regulated LPS-induced inflammatory responses by targeting NF-κB, thereby reducing the expression of proinflammatory cytokines. We have investigated the role of miR-210 in HI brain injury in neonatal and adult rodents and found that brain miR-210 levels were significantly upregulated after insult (79, 138). In an ischemic stroke model in adult mice, miR-210 inhibition significantly reduced the expression of pro-inflammatory cytokines (TNFα, IL-1β, and IL-6) and chemokines C-C motif ligand 2 (CCL2) and CCL3 (79). We also found that miR-210 suppressed glucocorticoid receptor (GR) levels in HI brain injury in rat pups (138) and in cardiomyocytes isolated from fetal rat heart (142) by directly binding to the 3′-UTR of the GR transcript. GR is well known for its function in upregulating the expression of anti-inflammatory proteins in the nucleus or repressing proinflammatory proteins in the cytosol (11, 41). Thus, abundant evidence suggests that miR-210 potently regulates inflammatory responses in the neonatal brain after HI insults.
In contrast to miR-210, accumulating evidence suggests that miR-126 provides neuroprotection after ischemic stroke through inhibition of inflammatory responses. Harris et al. (69) found that miR-126 downregulated vascular cell adhesion molecule 1 (VCAM-1) expression by targeting a miR-126-binding site in the VCAM-1 3′-UTR, which mediates endothelial cell activation and leukocyte infiltration under high-stress conditions. Further experiments confirmed that downregulation of miR-126 leads to increased leukocyte adherence to the vasculature. In addition, in a mouse xenograft model of breast cancer, miR-126 inhibited the transmigration of monocytes by direct inhibition of stromal cell-derived factor-1α (SDF-1α) expression, thereby indirectly repressing the expression of chemokine CCL2 (233). In addition to miR-126, functional participation of miR-146a has also been identified in inflammatory responses. Taganov et al. (198) found that miR-146a was a NF-κB-dependent gene and regulated the onset and termination of innate immune response by directly downregulating protein levels of IL-1 receptor-associated kinase-1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6). In addition, a study from Zhang et al. (242) investigated the role of the miR-146a/IRAK1 axis in the regulation of inflammatory responses in a focal ischemic stroke model. They found that combined treatment of focal ischemic stroke with Velcade and tissue plasminogen activator (tPA) increased endothelial miR-146a levels but downregulated IRAK1. Moreover, this boost in endothelial miR-146a levels provided neuroprotection by repressing the TLR/NF-κB pathway, ultimately leading to a reduction of infarct size and neurological deficits.
MiR-124 is another miRNA that potentially regulates neuroinflammatory responses after neonatal HIE. In general, miR-124 is highly expressed in microglia, where it helps maintain a resting state, but is downregulated in activated microglia. MiR-124 also can regulate microglial quiescence by downregulating the transcription factors CCAAT enhancer-binding protein-α (C/EBPα) and PU.1 (168). Moreover, overexpression of miR-124 in macrophages promotes the phenotype switch from M1 to M2 by downregulation of the M1 markers TNFα and CD86, and upregulation of the M2 markers transforming growth factor- β1 (TGFβ1) and arginase-I (168). This latter finding suggests that miR-124 may reduce neuroinflammation by controlling the balance of M1/M2 phenotypes after neonatal HI brain injury. MiR-155 also has been reported to regulate post-stroke inflammatory responses in ischemic stroke. Inhibition of miR-155 can significantly reduce the expression of CCL12 and C-X-C motif ligand 3 (CXCL3) cytokines in the brain (165) and may contribute to brain tissue repair after ischemic stroke (21). Moreover, miR-155 acts as an inhibitor of the alternative M2 phenotype by downregulating the TGFβ signaling pathway and transcription factor C/EBPβ mediated anti-inflammatory gene expression (135, 154). These findings suggest that miR-155 plays a potentially detrimental role in ischemic stroke. Considerable evidence highlights the regulatory roles of miRNAs in inflammatory responses of the ischemic adult brain, which are also commonly induced in neonatal brain by HI insults, and suggest a potential role of miRNAs in neonatal HI-induced neuroinflammation.
miRNAs in Neural Stem Cells
Neural stem cells (NSCs) and oligodendrocyte precursor cells (OPCs) are particularly vulnerable to HI insults in the immature brain. Neonatal HI brain injury causes loss of ~20% of the total cell population in the SVZ of the rat brain due to early necrotic and late apoptotic cell death, which three weeks later leads to a smaller SVZ with reduced stem/progenitor cell numbers. Immunostaining results indicate that many of the apoptotic cells are positive for a marker of immature oligodendrocytes (OL) (113). Back et al. (5) further investigated the vulnerability of OL lineages at different maturation stages to HI insults. They found that late OL progenitors were more vulnerable to HI-induced apoptotic cell death, whereas early OL progenitors and more mature OLs exhibited high resistance to HI insults. In another study investigating cell death pathways in progenitor cells during neonatal HI, activated calpains and caspase-3 accumulated mainly in the cells of the lateral SVZ, which coincided with progenitor cell death, but not in the medial SVZ (179). One explanation is that NSCs in the medial SVZ reside in an area of physiological hypoxia and thus adapt to hypoxic conditions and exhibit resistance when exposed to HI insults. In addition to caspase-dependent pathways, an in vitro study found that hypoxia-induced death of NPCs was also mediated by Bcl-2/adenovirus E1B 19-kDa interacting protein-3 (BNIP3) (209). However, other studies reported opposite results; HI insults expanded the ipsilateral SVZ and increased cell proliferation of both doublecortin (DCX)-positive neuroblasts and glial fibrillary acidic protein (GFAP)/neural/glial antigen 2 (NG2)-positive glial cells (70, 166). Using multimarker flow cytometry to sort progenitor cells from the SVZ, Buono et al. (20) demonstrated that HI insults diminished NSCs but remarkably amplified two types of multipotential progenitors and three types of unique glial-restricted precursors. Similar to NSCs in SVZ, in the dentate gyrus subgranular zone (SGZ), HI insults also induced apoptotic cell death in doublecortin-positive NPCs (103, 146), but activated cell proliferation in nestin-positive NSCs/NPCs (146). These results have helped give rise to controversy regarding the survival of newly generated neurons in HI-injured brain. Some studies have demonstrated that young neurons in the striatum survived less than two weeks (166), whereas others found that new neurons lived up to five weeks and died before maturation (50, 232). These inconsistent findings may reflect different durations of hypoxia treatment and varying extents of damage to the immature brain.
miRNAs are heavily engaged in the regulation of cellular behavior in neonatal brain responses to HI insults. Delaloy et al. (40) investigated the actions of miR-9 on the proliferation and migration of human NPCs. They found that the expression of miR-9 was upregulated in human NPCs, which promoted NPC proliferation but delayed the migration of early human NPCs in vitro. This finding was further confirmed in a mouse model of stroke, in which miR-9-deficient NPCs transplanted into the ipsilateral striatum of the brain exhibited enhanced migration to the neocortex. Inhibition of stathmin, a potential target of miR-9, blocked the effect of miR-9 depletion on NPC proliferation and migration (40). In addition to its involvement in early-life neurodevelopment events, miR-124 also can regulate neuronal differentiation of SVZ cells in the adult brain (31), suggesting a potential role in neuromodeling processes after HI brain injury. Indeed, ischemic stroke in adult rats leads to substantial reduction of brain miR-124 levels in the NPCs of SVZ, whereas overexpression of miR-124 in NPCs promotes neurogenesis but inhibits proliferation. Regarding mechanism, further study indicated that the function of miR-124 was inversely associated with Notch signaling and directly suppressed Jagged-1 (JAG1), a Notch ligand that signals activation (132). Yang et al. (231) used exosomes (RVG-exosomes) modified with rabies virus glycoprotein (RVG) to deliver miR-124 into the infarct site of the adult brain after ischemic stroke. They found that miR-124 promoted cortical neurogenesis and protected the brain against ischemic insults.
MiR-210 is a well-known miRNA induced by HI insults. However, few studies elucidate its role in neurogenesis after brain injury. Zeng et al. (239) transfected adult ischemic brain with lentiviral vector carrying miR-210 (LV-miR-210) and found increased NPCs in the SVZ after four weeks. Another recent study also identified a potential role for miR-210 in neurogenesis, in which inhibition of miR-210 protects young neurons from injury secondarily to inflammation but attenuates NSC proliferation (208). These findings suggest that miR-210 may participate in postischemic brain repair.
The miR cluster miR-17–92 mediates neural progenitor cell survival in ischemic brain. Upregulation of the miR-17–92 cluster enhances SVZ cell proliferation after stroke by downregulating phosphatase and tensin homolog (PTEN) (131). Moreover, administration of miR-17–92 cluster-enriched exosomes enhances neurogenesis and oligodendrogenesis after stroke, suggesting that miR-17–92 cluster is involved in the neuroplasticity and brain recovery after stroke (224).
In the brain of premature infants, immature OPCs localize predominantly to the periventricular white matter, and the loss of oligodendrocytes is a hallmark of periventricular leukomalacia (PVL), a common occurrence in preterm infants with HIE. Several miRNAs have been reported to specifically regulate OL differentiation and myelination. Dugas et al. (42) reported that deleting Dicer 1 from OPCs in mice disrupted CNS myelination, and blocked the normal differentiation of OPCs to OLs, in vitro. Moreover, miR-219, miR-138, and miR-338 were induced during OPC differentiation. Among these miRs, miR-219 was the most potent regulator of OPC differentiation and directly downregulated OPC proliferation-related proteins, including PDGF receptor-α (PDGFRα), SRY-Box 6 (Sox6), forkhead box J3 (FoxJ3), and zinc finger protein 238 (ZFP238). Furthermore, transplantation of miR-219-overexpressing OPCs promoted remyelination and improved cognitive functions, suggesting therapeutic potential for the treatment of demyelinating diseases (46). In addition, miR-219 and miR-338 promote OL differentiation in spinal cord partially by suppressing expression of the transcription factors Sox6 and Hes5, which are repressors of OL differentiation (249).
To investigate the role of miRNA biogenesis in neonatal HI-induced white matter injury, Birch and colleagues used conditional knockout mice with a Dicer deletion from NG2-positive progenitor cells. They found that, relative to wild-type mice, Dicer knockout after HI increased the expression of myelin basic protein (MBP) and increased numbers of mature oligodendrocytes within the corpus callosum. In wild-type mice, miR-138 and miR-338 were significantly increased following neonatal HI brain injury (15), suggesting that miRNAs may mediate the cellular responses to HI insults in OL lineage cells. However, NG2-positive progenitor cells are not restricted to OL but also exhibit an astrocyte fate (80, 250), Thus, this study cannot rule out the effect of astrocyte-derived miRNAs on OL differentiation and white matter injury after HI.
MiR-9 is a versatile regulator of NSC proliferation and differentiation, but there is no direct evidence supporting a role for miR-9 in oligodendrocyte development in neonatal brain. In contrast, following ischemic stroke in adults, miR-9 can regulate OPC differentiation in the corpus callosum in cooperation with miR-200. Correspondingly, overexpression of miR-9 and miR-200 inhibits OPC differentiation by downregulating serum response factor, which promotes OPC differentiation into oligodendrocytes (19). These studies provide fundamental knowledge about the involvement of miRNAs in neural stem cell development during pathological states. Hopefully, future studies will focus on the specific effects of additional miRNAs in the regulation of stem/progenitor cells, within the context of neonatal HI-induced brain injury.
CONCLUDING REMARKS
A key early experiment in efforts to understand the physiological importance of miRs involved selective deletion of Dicer, the rate-limiting enzyme in miRNA synthesis (1). This deletion was embryonically lethal due to blood vessel rupture and extensive internal hemorrhage. Similar studies with an endothelium-specific deletion of Dicer survived but exhibited defective angiogenesis, leading to the term “angiomirs” to denote miRs essential for vessel development and function (213). Work completed since publication of those early findings has served to reinforce the idea that miRs are deeply involved in virtually all cellular processes. The literature focusing on miR biology is growing rapidly and currently totals more than 80,000 manuscripts, with more than 10,000 studies published in 2018 alone. The intensity of interest in miRs has also spawned interest in cognate non-coding RNA molecules, such as Piwi-interacting RNAs (piRNA) (108), circular RNA (180), and long non-coding RNA (lncRNA) (210). Clearly, we are in the early days of exploration of these new mechanisms of regulation of genomic expression. From an even broader perspective, miRs can interact with other epigenetic mechanisms, including DNA methylation (22) and histone modification (197), giving the cell exquisite control of how and when each gene product is expressed, how metabolic activity is coordinated both within and between cells, and how cells respond to stress and injury. At the center of this regulatory complexity, however, is the simple concept that non-coding RNAs make up a very large proportion of the transcriptome. As discussed in this review, miRs are a critically important component of the non-coding transcriptome, whose functions are just beginning to be understood. Although a broad variety of studies have convincingly shown that circulating miRs can serve as important biomarkers for certain human pathologies (148, 211), the therapeutic administration of miRs has yet to be attempted. Even so, it is clear that future work in this growing field holds great promise, not just for deeper understanding of basic biology but also for building new therapeutic strategies for prevention and treatment of pathologies such as cerebral ischemia. Much exciting work lies ahead.
GRANTS
This work was supported in part by National Institutes of Health Grants HD-083132 and NS-103017 and by American Heart Association Beginning Grant-in-Aid 15BGIA25750063.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.M. prepared figures; L.Z., Q.M., and W.J.P. drafted manuscript; L.Z., Q.M., and W.J.P. edited and revised manuscript; L.Z., Q.M., and W.J.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We apologize to the authors whose excellent studies covered by the scope of this review were unable to be cited due to space restrictions.
REFERENCES
- 1.Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol 30: 1118–1126, 2010. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. RNA 9: 277–279, 2003. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambros V, Lee RC. Identification of microRNAs and other tiny noncoding RNAs by cDNA cloning. Methods Mol Biol 265: 131–158, 2004. doi: 10.1385/1-59259-775-0:131. [DOI] [PubMed] [Google Scholar]
- 4.Aschrafi A, Schwechter AD, Mameza MG, Natera-Naranjo O, Gioio AE, Kaplan BB. MicroRNA-338 regulates local cytochrome c oxidase IV mRNA levels and oxidative phosphorylation in the axons of sympathetic neurons. J Neurosci 28: 12581–12590, 2008. doi: 10.1523/JNEUROSCI.3338-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Back SA, Han BH, Luo NL, Chricton CA, Xanthoudakis S, Tam J, Arvin KL, Holtzman DM. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci 22: 455–463, 2002. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell 121: 645–657, 2005. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Bazan F, Blanchard D, Brière F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol 12: 881–922, 1994. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- 8.Bandiera S, Rüberg S, Girard M, Cagnard N, Hanein S, Chrétien D, Munnich A, Lyonnet S, Henrion-Caude A. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One 6: e20746, 2011. doi: 10.1371/journal.pone.0020746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrey E, Saint-Auret G, Bonnamy B, Damas D, Boyer O, Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS One 6: e20220, 2011. doi: 10.1371/journal.pone.0020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel F, Schulz J, Blümke K, Kappler M, Bache M, Schmidt H, Taubert H. [HDMX amplification and high levels of HDMX-S splice variant are correlated with a poor prognosis in soft tissue sarcomas] [in German]. Verh Dtsch Ges Pathol 88: 199–206, 2004. doi: 10.1016/S0344-0338(04)80554-5. [DOI] [PubMed] [Google Scholar]
- 11.Beck IM, Vanden Berghe W, Vermeulen L, Yamamoto KR, Haegeman G, De Bosscher K. Crosstalk in inflammation: the interplay of glucocorticoid receptor-based mechanisms and kinases and phosphatases. Endocr Rev 30: 830–882, 2009. doi: 10.1210/er.2009-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behm-Ansmant I, Rehwinkel J, Izaurralde E. MicroRNAs silence gene expression by repressing protein expression and/or by promoting mRNA decay. Cold Spring Harb Symp Quant Biol 71: 523–530, 2006. doi: 10.1101/sqb.2006.71.013. [DOI] [PubMed] [Google Scholar]
- 13.Bhalala OG, Srikanth M, Kessler JA. The emerging roles of microRNAs in CNS injuries. Nat Rev Neurol 9: 328–339, 2013. doi: 10.1038/nrneurol.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhalala US, Koehler RC, Kannan S. Neuroinflammation and neuroimmune dysregulation after acute hypoxic-ischemic injury of developing brain. Front Pediatr 2: 144, 2015. doi: 10.3389/fped.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birch D, Britt BC, Dukes SC, Kessler JA, Dizon MLV. MicroRNAs participate in the murine oligodendroglial response to perinatal hypoxia-ischemia. Pediatr Res 76: 334–340, 2014. doi: 10.1038/pr.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonev B, Pisco A, Papalopulu N. MicroRNA-9 reveals regional diversity of neural progenitors along the anterior-posterior axis. Dev Cell 20: 19–32, 2011. doi: 10.1016/j.devcel.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonev B, Stanley P, Papalopulu N. MicroRNA-9 modulates Hes1 ultradian oscillations by forming a double-negative feedback loop. Cell Reports 2: 10–18, 2012. doi: 10.1016/j.celrep.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bukeirat M, Sarkar SN, Hu H, Quintana DD, Simpkins JW, Ren X. MiR-34a regulates blood-brain barrier permeability and mitochondrial function by targeting cytochrome c. J Cereb Blood Flow Metab 36: 387–392, 2016. doi: 10.1177/0271678X15606147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buller B, Chopp M, Ueno Y, Zhang L, Zhang RL, Morris D, Zhang Y, Zhang ZG. Regulation of serum response factor by miRNA-200 and miRNA-9 modulates oligodendrocyte progenitor cell differentiation. Glia 60: 1906–1914, 2012. doi: 10.1002/glia.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buono KD, Goodus MT, Guardia Clausi M, Jiang Y, Loporchio D, Levison SW. Mechanisms of mouse neural precursor expansion after neonatal hypoxia-ischemia. J Neurosci 35: 8855–8865, 2015. doi: 10.1523/JNEUROSCI.2868-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, Bragin D, Yang Y, Erhardt EB, Roitbak T. In vivo inhibition of miR-155 promotes recovery after experimental mouse stroke. J Neurosci 35: 12446–12464, 2015. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao C, Zhang H, Zhao L, Zhou L, Zhang M, Xu H, Han X, Li G, Yang X, Jiang Y. miR-125b targets DNMT3b and mediates p53 DNA methylation involving in the vascular smooth muscle cells proliferation induced by homocysteine. Exp Cell Res 347: 95–104, 2016. doi: 10.1016/j.yexcr.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 23.Cao X, Yeo G, Muotri AR, Kuwabara T, Gage FH. Noncoding RNAs in the mammalian central nervous system. Annu Rev Neurosci 29: 77–103, 2006. doi: 10.1146/annurev.neuro.29.051605.112839. [DOI] [PubMed] [Google Scholar]
- 24.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation 19: 215–223, 2012. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang CY, Lui TN, Lin JW, Lin YL, Hsing CH, Wang JJ, Chen RM. Roles of microRNA-1 in hypoxia-induced apoptotic insults to neuronal cells. Arch Toxicol 90: 191–202, 2016. doi: 10.1007/s00204-014-1364-x. [DOI] [PubMed] [Google Scholar]
- 27.Chaulk SG, Ebhardt HA, Fahlman RP. Correlations of microRNA:microRNA expression patterns reveal insights into microRNA clusters and global microRNA expression patterns. Mol Biosyst 12: 110–119, 2016. doi: 10.1039/C5MB00415B. [DOI] [PubMed] [Google Scholar]
- 28.Chen F, Du Y, Esposito E, Liu Y, Guo S, Wang X, Lo EH, Xing C, Ji X. Effects of focal cerebral ischemia on exosomal versus serum miR126. Transl Stroke Res 6: 478–484, 2015. doi: 10.1007/s12975-015-0429-3. [DOI] [PubMed] [Google Scholar]
- 29.Chen FZ, Zhao Y, Chen HZ. MicroRNA-98 reduces amyloid β-protein production and improves oxidative stress and mitochondrial dysfunction through the Notch signaling pathway via HEY2 in Alzheimer’s disease mice. Int J Mol Med 43: 91–102, 2019. doi: 10.3892/ijmm.2018.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29: 4362–4368, 2010. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 31.Cheng LC, Pastrana E, Tavazoie M, Doetsch F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nat Neurosci 12: 399–408, 2009. doi: 10.1038/nn.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chio CC, Lin JW, Cheng HA, Chiu WT, Wang YH, Wang JJ, Hsing CH, Chen RM. MicroRNA-210 targets antiapoptotic Bcl-2 expression and mediates hypoxia-induced apoptosis of neuroblastoma cells. Arch Toxicol 87: 459–468, 2013. doi: 10.1007/s00204-012-0965-5. [DOI] [PubMed] [Google Scholar]
- 33.Conaco C, Otto S, Han JJ, Mandel G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc Natl Acad Sci USA 103: 2422–2427, 2006. doi: 10.1073/pnas.0511041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coolen M, Katz S, Bally-Cuif L. miR-9: a versatile regulator of neurogenesis. Front Cell Neurosci 7: 220, 2013. doi: 10.3389/fncel.2013.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coolen M, Thieffry D, Drivenes Ø, Becker TS, Bally-Cuif L. miR-9 controls the timing of neurogenesis through the direct inhibition of antagonistic factors. Dev Cell 22: 1052–1064, 2012. doi: 10.1016/j.devcel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460: 705–710, 2009. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro-angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol 307: H292–H306, 2014. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dajas-Bailador F, Bonev B, Garcez P, Stanley P, Guillemot F, Papalopulu N. microRNA-9 regulates axon extension and branching by targeting Map1b in mouse cortical neurons. Nat Neurosci 15: 697–699, 2012. doi: 10.1038/nn.3082. [DOI] [PubMed] [Google Scholar]
- 39.Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, Steenbergen C. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res 110: 1596–1603, 2012. doi: 10.1161/CIRCRESAHA.112.267732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaloy C, Liu L, Lee JA, Su H, Shen F, Yang GY, Young WL, Ivey KN, Gao FB. MicroRNA-9 coordinates proliferation and migration of human embryonic stem cell-derived neural progenitors. Cell Stem Cell 6: 323–335, 2010. doi: 10.1016/j.stem.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desmet SJ, De Bosscher K. Glucocorticoid receptors: finding the middle ground. J Clin Invest 127: 1136–1145, 2017. doi: 10.1172/JCI88886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dugas JC, Cuellar TL, Scholze A, Ason B, Ibrahim A, Emery B, Zamanian JL, Foo LC, McManus MT, Barres BA. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 65: 597–611, 2010. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65: 373–384, 2010. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ek CJ, D’Angelo B, Baburamani AA, Lehner C, Leverin A-L, Smith PLP, Nilsson H, Svedin P, Hagberg H, Mallard C. Brain barrier properties and cerebral blood flow in neonatal mice exposed to cerebral hypoxia-ischemia. J Cereb Blood Flow Metab 35: 818–827, 2015. doi: 10.1038/jcbfm.2014.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eyileten C, Wicik Z, De Rosa S, Mirowska-Guzel D, Soplinska A, Indolfi C, Jastrzebska-Kurkowska I, Czlonkowska A, Postula M. MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke-a comprehensive review and bioinformatic analysis. Cells 7: 249, 2018. doi: 10.3390/cells7120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan HB, Chen LX, Qu XB, Ren CL, Wu XX, Dong FX, Zhang BL, Gao DS, Yao RQ. Transplanted miR-219-overexpressing oligodendrocyte precursor cells promoted remyelination and improved functional recovery in a chronic demyelinated model. Sci Rep 7: 41407, 2017. doi: 10.1038/srep41407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Y, Ding S, Sun Y, Zhao B, Pan Y, Wan J. MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem 119: 327–337, 2018. doi: 10.1002/jcb.26181. [DOI] [PubMed] [Google Scholar]
- 48.Fang C, Li Q, Min G, Liu M, Cui J, Sun J, Li L. MicroRNA-181c ameliorates cognitive impairment induced by chronic cerebral hypoperfusion in rats. Mol Neurobiol 54: 8370–8385, 2017. doi: 10.1007/s12035-016-0268-6. [DOI] [PubMed] [Google Scholar]
- 49.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Felling RJ, Snyder MJ, Romanko MJ, Rothstein RP, Ziegler AN, Yang Z, Givogri MI, Bongarzone ER, Levison SW. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci 26: 4359–4369, 2006. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fénelon K, Xu B, Lai CS, Mukai J, Markx S, Stark KL, Hsu PK, Gan WB, Fischbach GD, MacDermott AB, Karayiorgou M, Gogos JA. The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion. J Neurosci 33: 14825–14839, 2013. doi: 10.1523/JNEUROSCI.1611-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng J, Huang T, Huang Q, Chen H, Li Y, He W, Wang GB, Zhang L, Xia J, Zhang N, Liu Y. Pro-angiogenic microRNA-296 upregulates vascular endothelial growth factor and downregulates Notch1 following cerebral ischemic injury. Mol Med Rep 12: 8141–8147, 2015. doi: 10.3892/mmr.2015.4436. [DOI] [PubMed] [Google Scholar]
- 53.Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, Greenberg ME, Schratt G. Mef2-mediated transcription of the miR379-410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J 28: 697–710, 2009. doi: 10.1038/emboj.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell 15: 272–284, 2008. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gan CS, Wang CW, Tan KS. Circulatory microRNA-145 expression is increased in cerebral ischemia. Genet Mol Res 11: 147–152, 2012. doi: 10.4238/2012.January.27.1. [DOI] [PubMed] [Google Scholar]
- 56.Gandhi S, Ruehle F, Stoll M. Evolutionary patterns of non-coding rna in cardiovascular biology. Noncoding RNA 5: 15, 2019. doi: 10.3390/ncrna5010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 466: 1105–1109, 2010. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghosh T, Aprea J, Nardelli J, Engel H, Selinger C, Mombereau C, Lemonnier T, Moutkine I, Schwendimann L, Dori M, Irinopoulou T, Henrion-Caude A, Benecke AG, Arnold SJ, Gressens P, Calegari F, Groszer M. MicroRNAs establish robustness and adaptability of a critical gene network to regulate progenitor fate decisions during cortical neurogenesis. Cell Reports 7: 1779–1788, 2014. doi: 10.1016/j.celrep.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 59.Griffiths-Jones S. Annotating noncoding RNA genes. Annu Rev Genomics Hum Genet 8: 279–298, 2007. doi: 10.1146/annurev.genom.8.080706.092419. [DOI] [PubMed] [Google Scholar]
- 60.Griffiths-Jones S. The microRNA registry. Nucleic Acids Res 32: D109–D111, 2004. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res 34: D140–D144, 2006. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gubern C, Camós S, Ballesteros I, Rodríguez R, Romera VG, Cañadas R, Lizasoain I, Moro MA, Serena J, Mallolas J, Castellanos M. miRNA expression is modulated over time after focal ischaemia: up-regulation of miR-347 promotes neuronal apoptosis. FEBS J 280: 6233–6246, 2013. doi: 10.1111/febs.12546. [DOI] [PubMed] [Google Scholar]
- 63.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840, 2010. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo X, Li D, Chen M, Chen L, Zhang B, Wu T, Guo R. miRNA-145 inhibits VSMC proliferation by targeting CD40. Sci Rep 6: 35302, 2016. doi: 10.1038/srep35302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagberg H, Gressens P, Mallard C. Inflammation during fetal and neonatal life: implications for neurologic and neuropsychiatric disease in children and adults. Ann Neurol 71: 444–457, 2012. doi: 10.1002/ana.22620. [DOI] [PubMed] [Google Scholar]
- 66.Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P. The role of inflammation in perinatal brain injury. Nat Rev Neurol 11: 192–208, 2015. doi: 10.1038/nrneurol.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev 18: 3016–3027, 2004. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, Silva AJ, Takano T, Goldman SA, Nedergaard M. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell 12: 342–353, 2013. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 105: 1516–1521, 2008. doi: 10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hayashi T, Iwai M, Ikeda T, Jin G, Deguchi K, Nagotani S, Zhang H, Sehara Y, Nagano I, Shoji M, Ikenoue T, Abe K. Neural precursor cells division and migration in neonatal rat brain after ischemic/hypoxic injury. Brain Res 1038: 41–49, 2005. doi: 10.1016/j.brainres.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 71.He J, Gao Y, Wu G, Lei X, Zhang Y, Pan W, Yu H. Bioinformatics analysis of microarray data to reveal the pathogenesis of brain ischemia. Mol Med Rep 18: 333–341, 2018. doi: 10.3892/mmr.2018.9000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet 5: 522–531, 2004. [Erratum in Nat Rev Genet 5: 631, 2004.] doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 73.He QW, Li Q, Jin HJ, Zhi F, Suraj B, Zhu YY, Xia YP, Mao L, Chen XL, Hu B. MiR-150 regulates poststroke cerebral angiogenesis via vascular endothelial growth factor in rats. CNS Neurosci Ther 22: 507–517, 2016. doi: 10.1111/cns.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He W, Chen S, Chen X, Li S, Chen W. Bioinformatic analysis of potential microRNAs in ischemic stroke. J Stroke Cerebrovasc Dis 25: 1753–1759, 2016. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 75.Heimberg AM, Cowper-Sal·lari R, Sémon M, Donoghue PC, Peterson KJ. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci USA 107: 19379–19383, 2010. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Houbaviy HB, Murray MF, Sharp PA. Embryonic stem cell-specific microRNAs. Dev Cell 5: 351–358, 2003. doi: 10.1016/S1534-5807(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 77.Hu Y, Deng H, Xu S, Zhang J. MicroRNAs regulate mitochondrial function in cerebral ischemia-reperfusion injury. Int J Mol Sci 16: 24895–24917, 2015. doi: 10.3390/ijms161024895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang J, Song J, Qu M, Wang Y, An Q, Song Y, Yan W, Wang B, Wang X, Zhang S, Chen X, Zhao B, Liu P, Xu T, Zhang Z, Greenberg DA, Wang Y, Gao P, Zhu W, Yang GY. MicroRNA-137 and microRNA-195* inhibit vasculogenesis in brain arteriovenous malformations. Ann Neurol 82: 371–384, 2017. doi: 10.1002/ana.25015. [DOI] [PubMed] [Google Scholar]
- 79.Huang L, Ma Q, Li Y, Li B, Zhang L. Inhibition of microRNA-210 suppresses pro-inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp Neurol 300: 41–50, 2018. doi: 10.1016/j.expneurol.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia 62: 896–913, 2014. doi: 10.1002/glia.22648. [DOI] [PubMed] [Google Scholar]
- 81.Huntzinger E, Izaurralde E. Gene silencing by microRNAs: contributions of translational repression and mRNA decay. Nat Rev Genet 12: 99–110, 2011. doi: 10.1038/nrg2936. [DOI] [PubMed] [Google Scholar]
- 82.Hutvágner G, McLachlan J, Pasquinelli AE, Bálint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 293: 834–838, 2001. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 83.Impey S, Davare M, Lesiak A, Fortin D, Ando H, Varlamova O, Obrietan K, Soderling TR, Goodman RH, Wayman GA. An activity-induced microRNA controls dendritic spine formation by regulating Rac1-PAK signaling. Mol Cell Neurosci 43: 146–156, 2010. doi: 10.1016/j.mcn.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson RJ, Standart N. How do microRNAs regulate gene expression? Sci STKE 2007: re1, 2007. doi: 10.1126/stke.3672007re1. [DOI] [PubMed] [Google Scholar]
- 85.Jeon YJ, Kim OJ, Kim SY, Oh SH, Oh D, Kim OJ, Shin BS, Kim NK. Association of the miR-146a, miR-149, miR-196a2, and miR-499 polymorphisms with ischemic stroke and silent brain infarction risk. Arterioscler Thromb Vasc Biol 33: 420–430, 2013. doi: 10.1161/ATVBAHA.112.300251. [DOI] [PubMed] [Google Scholar]
- 86.Ji M, Wang W, Li S, Hu W. Implantation of bone mesenchymal stem cells overexpressing miRNA-705 mitigated ischemic brain injury. Mol Med Rep 16: 8323–8328, 2017. doi: 10.3892/mmr.2017.7626. [DOI] [PubMed] [Google Scholar]
- 87.Jia L, Hao F, Wang W, Qu Y. Circulating miR-145 is associated with plasma high-sensitivity C-reactive protein in acute ischemic stroke patients. Cell Biochem Funct 33: 314–319, 2015. doi: 10.1002/cbf.3116. [DOI] [PubMed] [Google Scholar]
- 88.Jimenez-Mateos EM, Engel T, Merino-Serrais P, McKiernan RC, Tanaka K, Mouri G, Sano T, O’Tuathaigh C, Waddington JL, Prenter S, Delanty N, Farrell MA, O’Brien DF, Conroy RM, Stallings RL, DeFelipe J, Henshall DC. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med 18: 1087–1094, 2012. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiménez-Wences H, Martínez-Carrillo DN, Peralta-Zaragoza O, Campos-Viguri GE, Hernández-Sotelo D, Jiménez-López MA, Muñoz-Camacho JG, Garzón-Barrientos VH, Illades-Aguiar B, Fernández-Tilapa G. Methylation and expression of miRNAs in precancerous lesions and cervical cancer with HPV16 infection. Oncol Rep 35: 2297–2305, 2016. doi: 10.3892/or.2016.4583. [DOI] [PubMed] [Google Scholar]
- 90.Kane NM, Howard L, Descamps B, Meloni M, McClure J, Lu R, McCahill A, Breen C, Mackenzie RM, Delles C, Mountford JC, Milligan G, Emanueli C, Baker AH. Role of microRNAs 99b, 181a, and 181b in the differentiation of human embryonic stem cells to vascular endothelial cells. Stem Cells 30: 643–654, 2012. doi: 10.1002/stem.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kapsimali M, Kloosterman WP, de Bruijn E, Rosa F, Plasterk RH, Wilson SW. MicroRNAs show a wide diversity of expression profiles in the developing and mature central nervous system. Genome Biol 8: R173, 2007. doi: 10.1186/gb-2007-8-8-r173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev 15: 2654–2659, 2001. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khanna S, Rink C, Ghoorkhanian R, Gnyawali S, Heigel M, Wijesinghe DS, Chalfant CE, Chan YC, Banerjee J, Huang Y, Roy S, Sen CK. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab 33: 1197–1206, 2013. doi: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khvorova A, Reynolds A, Jayasena SD. Functional siRNAs and miRNAs exhibit strand bias. Cell 115: 209–216, 2003. doi: 10.1016/S0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 95.Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, Sullivan R, Kim HT, Cook TD, Kim JY, Kim H, Kim C, Vemuganti R. The microRNA miR-7a-5p ameliorates ischemic brain damage by repressing α-synuclein. Sci Signal 11: eaat4285, 2018. doi: 10.1126/scisignal.aat4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 6: 376–385, 2005. doi: 10.1038/nrm1644. [DOI] [PubMed] [Google Scholar]
- 97.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Göbel K, Schuhmann MK, Langhauser F, Helluy X, Schwarz T, Bittner S, Mayer CT, Brede M, Varallyay C, Pham M, Bendszus M, Jakob P, Magnus T, Meuth SG, Iwakura Y, Zernecke A, Sparwasser T, Nieswandt B, Stoll G, Wiendl H. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 121: 679–691, 2013. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 11: 441–450, 2006. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 99.Kosik KS. The neuronal microRNA system. Nat Rev Neurosci 7: 911–920, 2006. doi: 10.1038/nrn2037. [DOI] [PubMed] [Google Scholar]
- 100.Kren BT, Wong PY, Sarver A, Zhang X, Zeng Y, Steer CJ. MicroRNAs identified in highly purified liver-derived mitochondria may play a role in apoptosis. RNA Biol 6: 65–72, 2009. doi: 10.4161/rna.6.1.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS. A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9: 1274–1281, 2003. doi: 10.1261/rna.5980303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krol J, Busskamp V, Markiewicz I, Stadler MB, Ribi S, Richter J, Duebel J, Bicker S, Fehling HJ, Schübeler D, Oertner TG, Schratt G, Bibel M, Roska B, Filipowicz W. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell 141: 618–631, 2010. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 103.Kwak M, Lim S, Kang E, Furmanski O, Song H, Ryu YK, Mintz CD. Effects of neonatal hypoxic-ischemic injury and hypothermic neuroprotection on neural progenitor cells in the mouse hippocampus. Dev Neurosci 37: 428–439, 2015. doi: 10.1159/000430862. [DOI] [PubMed] [Google Scholar]
- 104.Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science 294: 853–858, 2001. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 105.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Curr Biol 12: 735–739, 2002. doi: 10.1016/S0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 106.Lambert TJ, Storm DR, Sullivan JM. MicroRNA132 modulates short-term synaptic plasticity but not basal release probability in hippocampal neurons. PLoS One 5: e15182, 2010. doi: 10.1371/journal.pone.0015182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laneve P, Gioia U, Andriotto A, Moretti F, Bozzoni I, Caffarelli E. A minicircuitry involving REST and CREB controls miR-9-2 expression during human neuronal differentiation. Nucleic Acids Res 38: 6895–6905, 2010. doi: 10.1093/nar/gkq604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le Thomas A, Tóth KF, Aravin AA. To be or not to be a piRNA: genomic origin and processing of piRNAs. Genome Biol 15: 204, 2014. doi: 10.1186/gb4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee HJ, Yi JS, Lee HJ, Lee IW, Park KC, Yang JH. Dysregulated expression profiles of MicroRNAs of experimentally induced cerebral aneurysms in rats. J Korean Neurosurg Soc 53: 72–76, 2013. doi: 10.3340/jkns.2013.53.2.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee J, Lim S, Song BW, Cha MJ, Ham O, Lee SY, Lee C, Park JH, Bae Y, Seo HH, Seung M, Choi E, Hwang KC. MicroRNA-29b inhibits migration and proliferation of vascular smooth muscle cells in neointimal formation. J Cell Biochem 116: 598–608, 2015. doi: 10.1002/jcb.25011. [DOI] [PubMed] [Google Scholar]
- 111.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419, 2003. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 112.Leucht C, Stigloher C, Wizenmann A, Klafke R, Folchert A, Bally-Cuif L. MicroRNA-9 directs late organizer activity of the midbrain-hindbrain boundary. Nat Neurosci 11: 641–648, 2008. doi: 10.1038/nn.2115. [DOI] [PubMed] [Google Scholar]
- 113.Levison SW, Rothstein RP, Romanko MJ, Snyder MJ, Meyers RL, Vannucci SJ. Hypoxia/ischemia depletes the rat perinatal subventricular zone of oligodendrocyte progenitors and neural stem cells. Dev Neurosci 23: 234–247, 2001. doi: 10.1159/000046149. [DOI] [PubMed] [Google Scholar]
- 114.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20, 2005. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 115.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell 115: 787–798, 2003. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 116.Li B, Concepcion K, Meng X, Zhang L. Brain-immune interactions in perinatal hypoxic-ischemic brain injury. Prog Neurobiol 159: 50–68, 2017. doi: 10.1016/j.pneurobio.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li G, Morris-Blanco KC, Lopez MS, Yang T, Zhao H, Vemuganti R, Luo Y. Impact of microRNAs on ischemic stroke: from pre- to post-disease. Prog Neurobiol 163-164: 59–78, 2018. doi: 10.1016/j.pneurobio.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li L, Stary CM. Targeting glial mitochondrial function for protection from cerebral ischemia: relevance, mechanisms, and the role of microRNAs. Oxid Med Cell Longev 2016: 6032306, 2016. doi: 10.1155/2016/6032306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li LJ, Huang Q, Zhang N, Wang GB, Liu YH. miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol Med Rep 10: 527–535, 2014. doi: 10.3892/mmr.2014.2172. [DOI] [PubMed] [Google Scholar]
- 120.Li Q, He Q, Baral S, Mao L, Li Y, Jin H, Chen S, An T, Xia Y, Hu B. MicroRNA-493 regulates angiogenesis in a rat model of ischemic stroke by targeting MIF. FEBS J 283: 1720–1733, 2016. doi: 10.1111/febs.13697. [DOI] [PubMed] [Google Scholar]
- 121.Li SH, Su SY, Liu JL. Differential regulation of microRNAs in patients with ischemic stroke. Curr Neurovasc Res 12: 214–221, 2015. doi: 10.2174/1567202612666150605121709. [DOI] [PubMed] [Google Scholar]
- 122.Li WY, Jin J, Chen J, Guo Y, Tang J, Tan S. Circulating microRNAs as potential non-invasive biomarkers for the early detection of hypertension-related stroke. J Hum Hypertens 28: 288–291, 2014. doi: 10.1038/jhh.2013.94. [DOI] [PubMed] [Google Scholar]
- 123.Li Y, Mao L, Gao Y, Baral S, Zhou Y, Hu B. MicroRNA-107 contributes to post-stroke angiogenesis by targeting Dicer-1. Sci Rep 5: 13316, 2015. doi: 10.1038/srep13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y, Wang F, Lee JA, Gao FB. MicroRNA-9a ensures the precise specification of sensory organ precursors in Drosophila. Genes Dev 20: 2793–2805, 2006. doi: 10.1101/gad.1466306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science 299: 1540, 2003. doi: 10.1126/science.1080372. [DOI] [PubMed] [Google Scholar]
- 126.Liu C, Teng ZQ, Santistevan NJ, Szulwach KE, Guo W, Jin P, Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell 6: 433–444, 2010. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu FJ, Kaur P, Karolina DS, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K. MiR-335 regulates Hif-1α to reduce cell death in both mouse cell line and rat ischemic models. PLoS One 10: e0128432, 2015. doi: 10.1371/journal.pone.0128432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao L, Yan F, Liu X, Yu S, Ji X, Luo Y. MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 46: 513–519, 2015. doi: 10.1161/STROKEAHA.114.007482. [DOI] [PubMed] [Google Scholar]
- 129.Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 104: 476–487, 2009. doi: 10.1161/CIRCRESAHA.108.185363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu X, Li F, Zhao S, Luo Y, Kang J, Zhao H, Yan F, Li S, Ji X. MicroRNA-124-mediated regulation of inhibitory member of apoptosis-stimulating protein of p53 family in experimental stroke. Stroke 44: 1973–1980, 2013. doi: 10.1161/STROKEAHA.111.000613. [DOI] [PubMed] [Google Scholar]
- 131.Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, Zhang ZG. MicroRNA-17-92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. J Biol Chem 288: 12478–12488, 2013. doi: 10.1074/jbc.M112.449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu XS, Chopp M, Zhang RL, Tao T, Wang XL, Kassis H, Hozeska-Solgot A, Zhang L, Chen C, Zhang ZG. MicroRNA profiling in subventricular zone after stroke: MiR-124a regulates proliferation of neural progenitor cells through Notch signaling pathway. PLoS One 6: e23461, 2011. doi: 10.1371/journal.pone.0023461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Looney AM, Walsh BH, Moloney G, Grenham S, Fagan A, O’Keeffe GW, Clarke G, Cryan JF, Dinan TG, Boylan GB, Murray DM. Downregulation of umbilical cord blood levels of miR-374a in neonatal hypoxic ischemic encephalopathy. J Pediatr 167: 269–73.e2, 2015. doi: 10.1016/j.jpeds.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 134.Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF. miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Mol Cell Biochem 370: 45–51, 2012. doi: 10.1007/s11010-012-1396-6. [DOI] [PubMed] [Google Scholar]
- 135.Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-beta. J Biol Chem 285: 41328–41336, 2010. doi: 10.1074/jbc.M110.146852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lugli G, Torvik VI, Larson J, Smalheiser NR. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J Neurochem 106: 650–661, 2008. doi: 10.1111/j.1471-4159.2008.05413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science 303: 95–98, 2004. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 138.Ma Q, Dasgupta C, Li Y, Bajwa NM, Xiong F, Harding B, Hartman R, Zhang L. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis 89: 202–212, 2016. doi: 10.1016/j.nbd.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma Q, Zhao H, Tao Z, Wang R, Liu P, Han Z, Ma S, Luo Y, Jia J. MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging Dis 7: 705–714, 2016. doi: 10.14336/AD.2016.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mair J, Apple F. Progress in myocardial damage detection: new biochemical markers for clinicians. Crit Rev Clin Lab Sci 34: 1–66, 1997. doi: 10.3109/10408369709038215. [DOI] [PubMed] [Google Scholar]
- 141.Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The MicroRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell 27: 435–448, 2007. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martinez SR, Ma Q, Dasgupta C, Meng X, Zhang L. MicroRNA-210 suppresses glucocorticoid receptor expression in response to hypoxia in fetal rat cardiomyocytes. Oncotarget 8: 80249–80264, 2017. doi: 10.18632/oncotarget.17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matsuoka H, Tamura A, Kinehara M, Shima A, Uda A, Tahara H, Michihara A. Levels of tight junction protein CLDND1 are regulated by microRNA-124 in the cerebellum of stroke-prone spontaneously hypertensive rats. Biochem Biophys Res Commun 498: 817–823, 2018. doi: 10.1016/j.bbrc.2018.03.063. [DOI] [PubMed] [Google Scholar]
- 144.Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet 14: R121–R132, 2005. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- 145.Meijer HA, Smith EM, Bushell M. Regulation of miRNA strand selection: follow the leader? Biochem Soc Trans 42: 1135–1140, 2014. doi: 10.1042/BST20140142. [DOI] [PubMed] [Google Scholar]
- 146.Miles DK, Kernie SG. Hypoxic-ischemic brain injury activates early hippocampal stem/progenitor cells to replace vulnerable neuroblasts. Hippocampus 18: 793–806, 2008. doi: 10.1002/hipo.20439. [DOI] [PubMed] [Google Scholar]
- 147.Miska EA, Alvarez-Saavedra E, Townsend M, Yoshii A, Sestan N, Rakic P, Constantine-Paton M, Horvitz HR. Microarray analysis of microRNA expression in the developing mammalian brain. Genome Biol 5: R68, 2004. doi: 10.1186/gb-2004-5-9-r68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105: 10513–10518, 2008. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Müller AH, Povlsen GK, Bang-Berthelsen CH, Kruse LS, Nielsen J, Warfvinge K, Edvinsson L. Regulation of microRNAs miR-30a and miR-143 in cerebral vasculature after experimental subarachnoid hemorrhage in rats. BMC Genomics 16: 119, 2015. doi: 10.1186/s12864-015-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nampoothiri SS, Menon HV, Das D, Krishnamurthy RG. ISCHEMIRs: finding a way through the obstructed cerebral arteries. Curr Drug Targets 17: 800–810, 2016. doi: 10.2174/1389450116666150518102404. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen LS, Fregeac J, Bole-Feysot C, Cagnard N, Iyer A, Anink J, Aronica E, Alibeu O, Nitschke P, Colleaux L. Role of miR-146a in neural stem cell differentiation and neural lineage determination: relevance for neurodevelopmental disorders. Mol Autism 9: 38, 2018. doi: 10.1186/s13229-018-0219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus 20: 492–498, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O’Connell RM, Kahn D, Gibson WS, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D. MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33: 607–619, 2010. doi: 10.1016/j.immuni.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.O’Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med 205: 585–594, 2008. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ouyang YB, Giffard RG. MicroRNAs regulate the chaperone network in cerebral ischemia. Transl Stroke Res 4: 693–703, 2013. doi: 10.1007/s12975-013-0280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ouyang YB, Lu Y, Yue S, Giffard RG. miR-181 targets multiple Bcl-2 family members and influences apoptosis and mitochondrial function in astrocytes. Mitochondrion 12: 213–219, 2012. doi: 10.1016/j.mito.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ouyang YB, Stary CM, White RE, Giffard RG. The use of microRNAs to modulate redox and immune response to stroke. Antioxid Redox Signal 22: 187–202, 2015. doi: 10.1089/ars.2013.5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ouyang YB, Xu L, Liu S, Giffard RG. Role of astrocytes in delayed neuronal death: GLT-1 and its novel regulation by microRNAs. Adv Neurobiol 11: 171–188, 2014. doi: 10.1007/978-3-319-08894-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ouyang YB, Xu L, Lu Y, Sun X, Yue S, Xiong XX, Giffard RG. Astrocyte-enriched miR-29a targets PUMA and reduces neuronal vulnerability to forebrain ischemia. Glia 61: 1784–1794, 2013. doi: 10.1002/glia.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801, 2004. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 161.Parmacek MS. Myocardin-related transcription factors: critical coactivators regulating cardiovascular development and adaptation. Circ Res 100: 633–644, 2007. doi: 10.1161/01.RES.0000259563.61091.e8. [DOI] [PubMed] [Google Scholar]
- 162.Pasquinelli AE. Molecular biology. Paring miRNAs through pairing. Science 328: 1494–1495, 2010. doi: 10.1126/science.1191531. [DOI] [PubMed] [Google Scholar]
- 163.Pasquinelli AE, Hunter S, Bracht J. MicroRNAs: a developing story. Curr Opin Genet Dev 15: 200–205, 2005. doi: 10.1016/j.gde.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 164.Pawlicki JM, Steitz JA. Primary microRNA transcript retention at sites of transcription leads to enhanced microRNA production. J Cell Biol 182: 61–76, 2008. doi: 10.1083/jcb.200803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T, Roitbak T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation 13: 287, 2016. doi: 10.1186/s12974-016-0753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis 16: 585–595, 2004. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 167.Plasterk RH. Micro RNAs in animal development. Cell 124: 877–881, 2006. doi: 10.1016/j.cell.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 168.Ponomarev ED, Veremeyko T, Barteneva N, Krichevsky AM, Weiner HL. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat Med 17: 64–70, 2011. doi: 10.1038/nm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Prajapati P, Sripada L, Singh K, Bhatelia K, Singh R, Singh R. TNF-α regulates miRNA targeting mitochondrial complex-I and induces cell death in dopaminergic cells. Biochim Biophys Acta 1852: 451–461, 2015. doi: 10.1016/j.bbadis.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 170.Prajapati P, Sripada L, Singh K, Roy M, Bhatelia K, Dalwadi P, Singh R. Systemic analysis of miRNAs in PD stress condition: miR-5701 modulates mitochondrial-lysosomal cross talk to regulate neuronal death. Mol Neurobiol 55: 4689–4701, 2018. doi: 10.1007/s12035-017-0664-6. [DOI] [PubMed] [Google Scholar]
- 171.Qi J, Qiao Y, Wang P, Li S, Zhao W, Gao C. microRNA-210 negatively regulates LPS-induced production of proinflammatory cytokines by targeting NF-κB1 in murine macrophages. FEBS Lett 586: 1201–1207, 2012. doi: 10.1016/j.febslet.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 172.Qian Z, Li Y, Chen J, Li X, Gou D. miR-4632 mediates PDGF-BB-induced proliferation and antiapoptosis of human pulmonary artery smooth muscle cells via targeting cJUN. Am J Physiol Cell Physiol 313: C380–C391, 2017. doi: 10.1152/ajpcell.00061.2017. [DOI] [PubMed] [Google Scholar]
- 173.Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T, Kandel E. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63: 803–817, 2009. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ren X, Engler-Chiurazzi EB, Russell AE, Sarkar SN, Rellick SL, Lewis S, Corbin D, Clapper J, Simpkins JW. MiR-34a and stroke: assessment of non-modifiable biological risk factors in cerebral ischemia. Neurochem Int. In press. doi: 10.1016/j.neuint.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Riggs CL, Summers A, Warren DE, Nilsson GE, Lefevre S, Dowd WW, Milton S, Podrabsky JE. Small non-coding RNA expression and vertebrate anoxia tolerance. Front Genet 9: 230, 2018. doi: 10.3389/fgene.2018.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ristori E, Lopez-Ramirez MA, Narayanan A, Hill-Teran G, Moro A, Calvo CF, Thomas JL, Nicoli S. A Dicer-miR-107 interaction regulates biogenesis of specific miRNAs crucial for neurogenesis. Dev Cell 32: 546–560, 2015. doi: 10.1016/j.devcel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roese-Koerner B, Stappert L, Berger T, Braun NC, Veltel M, Jungverdorben J, Evert BO, Peitz M, Borghese L, Brüstle O. Reciprocal regulation between bifunctional miR-9/9(∗) and its transcriptional modulator notch in human neural stem cell self-renewal and differentiation. Stem Cell Reports 7: 207–219, 2016. doi: 10.1016/j.stemcr.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roitbak T. Silencing a multifunctional microRNA is beneficial for stroke recovery. Front Mol Neurosci 11: 58, 2018. doi: 10.3389/fnmol.2018.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Romanko MJ, Zhu C, Bahr BA, Blomgren K, Levison SW. Death effector activation in the subventricular zone subsequent to perinatal hypoxia/ischemia. J Neurochem 103: 1121–1131, 2007. doi: 10.1111/j.1471-4159.2007.04820.x. [DOI] [PubMed] [Google Scholar]
- 180.Rong D, Sun H, Li Z, Liu S, Dong C, Fu K, Tang W, Cao H. An emerging function of circRNA-miRNAs-mRNA axis in human diseases. Oncotarget 8: 73271–73281, 2017. doi: 10.18632/oncotarget.19154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Santulli G. MicroRNAs distinctively regulate vascular smooth muscle and endothelial cells: functional implications in angiogenesis, atherosclerosis, and in-stent restenosis. Adv Exp Med Biol 887: 53–77, 2015. doi: 10.1007/978-3-319-22380-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Satoorian T, Li B, Tang X, Xiao J, Xing W, Shi W, Lau KH, Baylink DJ, Qin X. MicroRNA223 promotes pathogenic T-cell development and autoimmune inflammation in central nervous system in mice. Immunology 148: 326–338, 2016. doi: 10.1111/imm.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schratt G. microRNAs at the synapse. Nat Rev Neurosci 10: 842–849, 2009. doi: 10.1038/nrn2763. [DOI] [PubMed] [Google Scholar]
- 184.Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature 439: 283–289, 2006. [Erratum in Nature 441: 902, 2006.] doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- 185.Shekhar S, Cunningham MW, Pabbidi MR, Wang S, Booz GW, Fan F. Targeting vascular inflammation in ischemic stroke: Recent developments on novel immunomodulatory approaches. Eur J Pharmacol 833: 531–544, 2018. doi: 10.1016/j.ejphar.2018.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Shi Q, Gibson GE. Up-regulation of the mitochondrial malate dehydrogenase by oxidative stress is mediated by miR-743a. J Neurochem 118: 440–448, 2011. doi: 10.1111/j.1471-4159.2011.07333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shibata M, Nakao H, Kiyonari H, Abe T, Aizawa S. MicroRNA-9 regulates neurogenesis in mouse telencephalon by targeting multiple transcription factors. J Neurosci 31: 3407–3422, 2011. doi: 10.1523/JNEUROSCI.5085-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, Hübel K, Dekker F, Hedberg C, Rengarajan B, Drepper C, Waldmann H, Kauppinen S, Greenberg ME, Draguhn A, Rehmsmeier M, Martinez J, Schratt GM. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol 11: 705–716, 2009. doi: 10.1038/ncb1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sim SE, Lim CS, Kim JI, Seo D, Chun H, Yu NK, Lee J, Kang SJ, Ko HG, Choi JH, Kim T, Jang EH, Han J, Bak MS, Park JE, Jang DJ, Baek D, Lee YS, Kaang BK. The brain-enriched microRNA miR-9-3p regulates synaptic plasticity and memory. J Neurosci 36: 8641–8652, 2016. doi: 10.1523/JNEUROSCI.0630-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Slezak-Prochazka I, Kluiver J, de Jong D, Kortman G, Halsema N, Poppema S, Kroesen BJ, van den Berg A. Cellular localization and processing of primary transcripts of exonic microRNAs. PLoS One 8: e76647, 2013. doi: 10.1371/journal.pone.0076647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Smalheiser NR, Lugli G. microRNA regulation of synaptic plasticity. Neuromolecular Med 11: 133–140, 2009. doi: 10.1007/s12017-009-8065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Smirnova L, Gräfe A, Seiler A, Schumacher S, Nitsch R, Wulczyn FG. Regulation of miRNA expression during neural cell specification. Eur J Neurosci 21: 1469–1477, 2005. doi: 10.1111/j.1460-9568.2005.03978.x. [DOI] [PubMed] [Google Scholar]
- 193.Smith B, Treadwell J, Zhang D, Ly D, McKinnell I, Walker PR, Sikorska M. Large-scale expression analysis reveals distinct microRNA profiles at different stages of human neurodevelopment. PLoS One 5: e11109, 2010. doi: 10.1371/journal.pone.0011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, Jin P, Zhao X. MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells 28: 1060–1070, 2010. doi: 10.1002/stem.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Stary CM, Sun X, Ouyang Y, Li L, Giffard RG. miR-29a differentially regulates cell survival in astrocytes from cornu ammonis 1 and dentate gyrus by targeting VDAC1. Mitochondrion 30: 248–254, 2016. doi: 10.1016/j.mito.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Su ZF, Sun ZW, Zhang Y, Wang S, Yu QG, Wu ZB. Regulatory effects of miR-146a/b on the function of endothelial progenitor cells in acute ischemic stroke in mice. Kaohsiung J Med Sci 33: 369–378, 2017. doi: 10.1016/j.kjms.2017.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Swierczynski S, Klieser E, Illig R, Alinger-Scharinger B, Kiesslich T, Neureiter D. Histone deacetylation meets miRNA: epigenetics and post-transcriptional regulation in cancer and chronic diseases. Expert Opin Biol Ther 15: 651–664, 2015. doi: 10.1517/14712598.2015.1025047. [DOI] [PubMed] [Google Scholar]
- 198.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 103: 12481–12486, 2006. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PLoS One 4: e7689, 2009. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tanzer A, Stadler PF. Molecular evolution of a microRNA cluster. J Mol Biol 339: 327–335, 2004. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 201.Tao Z, Zhao H, Wang R, Liu P, Yan F, Zhang C, Ji X, Luo Y. Neuroprotective effect of microRNA-99a against focal cerebral ischemia-reperfusion injury in mice. J Neurol Sci 355: 113–119, 2015. doi: 10.1016/j.jns.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 202.Truscott M, Islam AB, Frolov MV. Novel regulation and functional interaction of polycistronic miRNAs. RNA 22: 129–138, 2016. doi: 10.1261/rna.053264.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT, Juo SH. Serum microRNA-21 and microRNA-221 as potential biomarkers for cerebrovascular disease. J Vasc Res 50: 346–354, 2013. doi: 10.1159/000351767. [DOI] [PubMed] [Google Scholar]
- 204.van Kralingen JC, McFall A, Ord ENJ, Coyle TF, Bissett M, McClure JD, McCabe C, Macrae IM, Dawson J, Work LM. Altered extracellular vesicle microrna expression in ischemic stroke and small vessel disease. Transl Stroke Res. In press. doi: 10.1007/s12975-018-0682-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Visvanathan J, Lee S, Lee B, Lee JW, Lee SK. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev 21: 744–749, 2007. doi: 10.1101/gad.1519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Vohradsky J, Panek J, Vomastek T. Numerical modelling of microRNA-mediated mRNA decay identifies novel mechanism of microRNA controlled mRNA downregulation. Nucleic Acids Res 38: 4579–4585, 2010. doi: 10.1093/nar/gkq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Volný O, Kašičková L, Coufalová D, Cimflová P, Novák J. microRNAs in cerebrovascular disease. Adv Exp Med Biol 888: 155–195, 2015. doi: 10.1007/978-3-319-22671-2_9. [DOI] [PubMed] [Google Scholar]
- 208.Voloboueva LA, Sun X, Xu L, Ouyang YB, Giffard RG. Distinct effects of miR-210 reduction on neurogenesis: increased neuronal survival of inflammation but reduced proliferation associated with mitochondrial enhancement. J Neurosci 37: 3072–3084, 2017. doi: 10.1523/JNEUROSCI.1777-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Walls KC, Ghosh AP, Ballestas ME, Klocke BJ, Roth KA. bcl-2/Adenovirus E1B 19-kd interacting protein 3 (BNIP3) regulates hypoxia-induced neural precursor cell death. J Neuropathol Exp Neurol 68: 1326–1338, 2009. doi: 10.1097/NEN.0b013e3181c3b9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wang C, Wang L, Ding Y, Lu X, Zhang G, Yang J, Zheng H, Wang H, Jiang Y, Xu L. LncRNA structural characteristics in epigenetic regulation. Int J Mol Sci 18: 2659, 2017. doi: 10.3390/ijms18122659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wang GK, Zhu JQ, Zhang JT, Li Q, Li Y, He J, Qin YW, Jing Q. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J 31: 659–666, 2010. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 212.Wang H, Flach H, Onizawa M, Wei L, McManus MT, Weiss A. Negative regulation of Hif1a expression and TH17 differentiation by the hypoxia-regulated microRNA miR-210. Nat Immunol 15: 393–401, 2014. doi: 10.1038/ni.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang S, Olson EN. AngiomiRs—key regulators of angiogenesis. Curr Opin Genet Dev 19: 205–211, 2009. doi: 10.1016/j.gde.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Wang TM, Chen KC, Hsu PY, Lin HF, Wang YS, Chen CY, Liao YC, Juo SH. microRNA let-7g suppresses PDGF-induced conversion of vascular smooth muscle cell into the synthetic phenotype. J Cell Mol Med 21: 3592–3601, 2017. doi: 10.1111/jcmm.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang WX, Visavadiya NP, Pandya JD, Nelson PT, Sullivan PG, Springer JE. Mitochondria-associated microRNAs in rat hippocampus following traumatic brain injury. Exp Neurol 265: 84–93, 2015. doi: 10.1016/j.expneurol.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang Y, Li Q, Mambiya M, Zhang K, Yang L, Zhang Q, Liu S, Liu M, Yin J, Liu W. A meta-analysis of the association between microrna-196a2 and risk of ischemic stroke and coronary artery disease in Asian population. J Stroke Cerebrovasc Dis 27: 3008–3019, 2018. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 217.Wayman GA, Davare M, Ando H, Fortin D, Varlamova O, Cheng HY, Marks D, Obrietan K, Soderling TR, Goodman RH, Impey S. An activity-regulated microRNA controls dendritic plasticity by down-regulating p250GAP. Proc Natl Acad Sci USA 105: 9093–9098, 2008. doi: 10.1073/pnas.0803072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wee EJ, Peters K, Nair SS, Hulf T, Stein S, Wagner S, Bailey P, Lee SY, Qu WJ, Brewster B, French JD, Dobrovic A, Francis GD, Clark SJ, Brown MA. Mapping the regulatory sequences controlling 93 breast cancer-associated miRNA genes leads to the identification of two functional promoters of the Hsa-mir-200b cluster, methylation of which is associated with metastasis or hormone receptor status in advanced breast cancer. Oncogene 31: 4182–4195, 2012. doi: 10.1038/onc.2011.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wen Y, Zhang X, Dong L, Zhao J, Zhang C, Zhu C. Acetylbritannilactone modulates microRNA-155-mediated inflammatory response in ischemic cerebral tissues. Mol Med 21: 197–209, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA 103: 4034–4039, 2006. doi: 10.1073/pnas.0510928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu N, Lin X, Zhao X, Zheng L, Xiao L, Liu J, Ge L, Cao S. MiR-125b acts as an oncogene in glioblastoma cells and inhibits cell apoptosis through p53 and p38MAPK-independent pathways. Br J Cancer 109: 2853–2863, 2013. doi: 10.1038/bjc.2013.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Wu S, Lin Y, Xu D, Chen J, Shu M, Zhou Y, Zhu W, Su X, Zhou Y, Qiu P, Yan G. MiR-135a functions as a selective killer of malignant glioma. Oncogene 31: 3866–3874, 2012. doi: 10.1038/onc.2011.551. [DOI] [PubMed] [Google Scholar]
- 223.Xie L, Li W, Hersh J, Liu R, Yang SH. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow Metab X18792372, 2018. doi: 10.1177/0271678X18792372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke 48: 747–753, 2017. [Erratum in Stroke 48: e137, 2017.] doi: 10.1161/STROKEAHA.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Xin M, Small EM, Sutherland LB, Qi X, McAnally J, Plato CF, Richardson JA, Bassel-Duby R, Olson EN. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev 23: 2166–2178, 2009. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xu G, Li JY. ATP5A1 and ATP5B are highly expressed in glioblastoma tumor cells and endothelial cells of microvascular proliferation. J Neurooncol 126: 405–413, 2016. doi: 10.1007/s11060-015-1984-x. [DOI] [PubMed] [Google Scholar]
- 227.Xue Y, Ouyang K, Huang J, Zhou Y, Ouyang H, Li H, Wang G, Wu Q, Wei C, Bi Y, Jiang L, Cai Z, Sun H, Zhang K, Zhang Y, Chen J, Fu XD. Direct conversion of fibroblasts to neurons by reprogramming PTB-regulated microRNA circuits. Cell 152: 82–96, 2013. doi: 10.1016/j.cell.2012.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, Parmar D. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol Chem 286: 37347–37357, 2011. doi: 10.1074/jbc.M111.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Yan H, Fang M, Liu XY. Role of microRNAs in stroke and poststroke depression. ScientificWorldJournal 2013: 459692, 2013. doi: 10.1155/2013/459692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yang J, Gao F, Zhang Y, Liu Y, Zhang D. Buyang Huanwu Decoction (BYHWD) enhances angiogenic effect of mesenchymal stem cell by upregulating VEGF expression after focal cerebral ischemia. J Mol Neurosci 56: 898–906, 2015. doi: 10.1007/s12031-015-0539-0. [DOI] [PubMed] [Google Scholar]
- 231.Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol Ther Nucleic Acids 7: 278–287, 2017. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience 139: 555–564, 2006. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 233.Yin KJ, Fan Y, Hamblin M, Zhang J, Zhu T, Li S, Hawse JR, Subramaniam M, Song CZ, Urrutia R, Lin JD, Chen YE. KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain 136: 1274–1287, 2013. doi: 10.1093/brain/awt002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yin KJ, Hamblin M, Chen YE. Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc Pharmacol 13: 352–365, 2015. doi: 10.2174/15701611113119990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yin KJ, Hamblin M, Chen YE. Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke. Neurochem Int 77: 9–16, 2014. doi: 10.1016/j.neuint.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Yoda M, Kawamata T, Paroo Z, Ye X, Iwasaki S, Liu Q, Tomari Y. ATP-dependent human RISC assembly pathways. Nat Struct Mol Biol 17: 17–23, 2010. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476: 228–231, 2011. doi: 10.1038/nature10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yuan Y, Zhang Z, Wang Z, Liu J. MiRNA-27b regulates angiogenesis by targeting AMPK in mouse ischemic stroke model. Neuroscience 398: 12–22, 2019. doi: 10.1016/j.neuroscience.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 239.Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Wang Y, Fu Y, Yang GY. MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene Ther 21: 37–43, 2014. doi: 10.1038/gt.2013.55. [DOI] [PubMed] [Google Scholar]
- 240.Zeng LL, He XS, Liu JR, Zheng CB, Wang YT, Yang GY. Lentivirus-mediated overexpression of MicroRNA-210 improves long-term outcomes after focal cerebral ischemia in mice. CNS Neurosci Ther 22: 961–969, 2016. doi: 10.1111/cns.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhang H, Zhong K, Lu M, Mei Y, Tan E, Sun X, Tan W. Neuroprotective effects of isosteviol sodium through increasing CYLD by the downregulation of miRNA-181b. Brain Res Bull 140: 392–401, 2018. doi: 10.1016/j.brainresbull.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 242.Zhang L, Chopp M, Liu X, Teng H, Tang T, Kassis H, Zhang ZG. Combination therapy with VELCADE and tissue plasminogen activator is neuroprotective in aged rats after stroke and targets microRNA-146a and the toll-like receptor signaling pathway. Arterioscler Thromb Vasc Biol 32: 1856–1864, 2012. doi: 10.1161/ATVBAHA.112.252619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhang R, Zhou H, Jiang L, Mao Y, Cui X, Xie B, Cui D, Wang H, Zhang Q, Xu S. MiR-195 dependent roles of mitofusin2 in the mitochondrial dysfunction of hippocampal neurons in SAMP8 mice. Brain Res 1652: 135–143, 2016. doi: 10.1016/j.brainres.2016.09.047. [DOI] [PubMed] [Google Scholar]
- 244.Zhao C, Sun G, Li S, Lang MF, Yang S, Li W, Shi Y. MicroRNA let-7b regulates neural stem cell proliferation and differentiation by targeting nuclear receptor TLX signaling. Proc Natl Acad Sci USA 107: 1876–1881, 2010. doi: 10.1073/pnas.0908750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhao C, Sun G, Li S, Shi Y. A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol 16: 365–371, 2009. doi: 10.1038/nsmb.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhao H, Tao Z, Wang R, Liu P, Yan F, Li J, Zhang C, Ji X, Luo Y. MicroRNA-23a-3p attenuates oxidative stress injury in a mouse model of focal cerebral ischemia-reperfusion. Brain Res 1592: 65–72, 2014. doi: 10.1016/j.brainres.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 247.Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 44: 1706–1713, 2013. doi: 10.1161/STROKEAHA.111.000504. [DOI] [PubMed] [Google Scholar]
- 248.Zhao WJ, Zhang HF, Su JY. Downregulation of microRNA-195 promotes angiogenesis induced by cerebral infarction via targeting VEGFA. Mol Med Rep 16: 5434–5440, 2017. doi: 10.3892/mmr.2017.7230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 249.Zhao X, He X, Han X, Yu Y, Ye F, Chen Y, Hoang T, Xu X, Mi Q-S, Xin M, Wang F, Appel B, Lu QR. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron 65: 612–626, 2010. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhu X, Bergles DE, Nishiyama A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135: 145–157, 2008. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- 251.Zhuang Y, Peng H, Mastej V, Chen W. MicroRNA regulation of endothelial junction proteins and clinical consequence. Mediators Inflamm 2016: 5078627, 2016. doi: 10.1155/2016/5078627. [DOI] [PMC free article] [PubMed] [Google Scholar]