Abstract
Leigh syndrome French Canadian type (LSFC) is a mitochondrial disease caused by mutations in the leucine-rich pentatricopeptide repeat-containing (LRPPRC) gene leading to a reduction of cytochrome-c oxidase (COX) expression reaching 50% in skin fibroblasts. We have shown that under basal conditions, LSFC and control cells display similar ATP levels. We hypothesized that this occurs through upregulation of mechanistic target of rapamycin (mTOR)-mediated metabolic reprogramming. Our results showed that compared with controls, LSFC cells exhibited an upregulation of the mTOR complex 1 (mTORC1)/p70 ribosomal S6 kinase pathway and higher levels of hypoxia-inducible factor 1α (HIF-1α) and its downstream target pyruvate dehydrogenase kinase 1 (PDHK1), a regulator of mitochondrial pyruvate dehydrogenase 1 (PDH1). Consistent with these signaling alterations, LSFC cells displayed a 40–61% increase in [U-13C6]glucose contribution to pyruvate, lactate, and alanine formation, as well as higher levels of the phosphorylated and inactive form of PDH1-α. Interestingly, inhibition of mTOR with rapamycin did not alter HIF-1α or PDHK1 protein levels in LSFC fibroblasts. However, this treatment increased PDH1-α phosphorylation in control and LSFC cells and reduced ATP levels in control cells. Rapamycin also decreased LRPPRC expression by 41 and 11% in LSFC and control cells, respectively, and selectively reduced COX subunit IV expression in LSFC fibroblasts. Taken together, our data demonstrate the importance of mTORC1, independent of the HIF-1α/PDHK1 axis, in maintaining LRPPRC and COX expression in LSFC cells.
Keywords: COX, LRPPRC, LSFC, mTORC1, mitochondrial diseases, rapamycin
INTRODUCTION
Mitochondrial diseases, also known as oxidative phosphorylation (OXPHOS) disorders, are the most frequent inherited metabolic diseases globally with a prevalence rate of 1:5,000 (15, 25, 27). This includes Leigh syndrome French Canadian type (LSFC), a recessive neurodegenerative infantile mitochondrial disease with an incidence of 1:2,000 in the Saguenay-Lac-Saint-Jean region of Quebec, Canada (22). LSFC is caused by mutations in the nuclear gene leucine-rich pentatricopeptide repeat-containing (LRPPRC; 21). Most Quebec LSFC patients are homozygous for an A354V substitution that causes a decrease in the expression of the LRPPRC protein (21, 41). Recently, novel disease-causing mutations in this gene have also been reported outside the French Canadian population (26).
LRPPRC is a multifunctional protein that plays an important role in mRNA metabolism including posttranscriptional processes and translation (30, 32). Its deficiency produces a tissue-specific mitochondrial defect that, in fibroblasts, is characterized by an isolated respiratory chain complex IV or cytochrome-c oxidase (COX) defect (32, 33, 41). LSFC fibroblasts present several mitochondrial functional abnormalities including reduced mitochondrial membrane potential, fragmentation of the mitochondrial network, and impaired OXPHOS capacity (2). However, despite these abnormalities, LSFC fibroblasts have preserved ATP levels in basal conditions, suggesting the activation of a compensatory mechanism (2). For instance, upregulation of glycolysis and the concomitant production of lactate could potentially overcome the decrease in mitochondrial respiration.
Among the numerous signaling pathways that regulate glycolysis, mechanistic target of rapamycin (mTOR) is a key regulator of energy metabolism. mTOR exists as two functional complexes distinguished by their composition: mTOR complexes 1 (mTORC1) and 2 (mTORC2; 19). The best characterized complex, mTORC1, is sensitive to rapamycin and is regulated positively by Akt/protein kinase B (PKB) and negatively by AMP-activated protein kinase (AMPK) in response to multiple signals as well as the cellular AMP-to-ATP ratio (17, 37). In immune and cancer cells, mTORC1 regulates aerobic glycolysis through increased expression of several proteins including the transcription factor hypoxia-inducible factor 1α (HIF-1α) and its target pyruvate dehydrogenase kinase 1 (PDHK1; 4, 11, 16, 34). PDHK1 phosphorylates and inhibits pyruvate dehydrogenase 1 (PDH1), the gatekeeper of glucose oxidative metabolism, leading to a metabolic switch away from mitochondria and toward glycolysis (reviewed in Refs. 8, 34, 39).
On the basis of these findings, we hypothesize that activation of the mTORC1 pathway and its downstream targets HIF-1α/PDHK1 could be a compensatory mechanism in LSFC fibroblasts, enabling them to maintain their ATP levels. Our data demonstrate that contrary to our hypothesis, inhibition of mTORC1 does not alter the HIF-1α/PDHK1 signaling pathway or ATP levels in LSFC fibroblasts. However, our study has uncovered a potential role for mTORC1 in LRPPRC and COX expression in LSFC fibroblasts.
METHODS
Cell culture.
Skin fibroblasts from one LSFC female patient (8 yr old) homozygous for the common A354V mutation and one paired female control (5 yr old) were provided by the LSFC Consortium Biobank (Université du Québec à Chicoutimi, Chicoutimi, QC, Canada). The protocol was approved by the Research Ethics Committee of the Centre Intégré Universitaire de Santé et de Services Sociaux du Saguenay-Lac-Saint-Jean and the Montreal Heart Institute. Unless stated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). Primary fibroblasts were grown (up to passage 18) at 37°C and 5% CO2 in high-glucose (4.5 g/l) Dulbecco’s modified Eagle’s medium (DMEM; Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 10 mg/ml streptomycin (Invitrogen, Carlsbad, CA), and 1% MEM vitamin and nonessential amino acid solutions from Mediatech. For the labeling experiments, which require a larger number of cells, we used fibroblasts that were immortalized by transduction with a retroviral vector expressing the HPV-16 E7 gene and the catalytic component of human telomerase as described (42).
Cell treatment.
To examine mTORC1 function, cells were treated for 16 h with 100 nM rapamycin or vehicle (DMSO) in supplemented DMEM. On the day of the study, cells were cultured in a serum-free nonsupplemented DMEM with inhibitor or vehicle, as specified, for an additional 4 h.
Gel electrophoresis and immunoblotting.
Cells were washed twice with ice-cold PBS. They were then lysed in buffer containing 25 mM Tris·HCl pH 7.4, 150 mM NaCl, 1 mM sodium orthovanadate, 20 mM sodium fluoride, 10 mM sodium pyrophosphate, 2 mM EGTA, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 0.1% sodium dodecyl sulfate (SDS), 1.5% dodecyl maltoside, and protease inhibitors. Lysates were kept on ice for 30 min and then centrifuged at 20,000 g for 30 min at 4°C to remove insoluble material. An equal amount of protein was resolved on 8 or 12% SDS-PAGE gels, as appropriate, and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) for 1 h at 4°C. Membranes were blocked for 1 h with 5% (wt/vol) nonfat dry milk in TBST (25 mM Tris·HCl pH 7.6, 150 mM NaCl, and 0.05% Tween 20) and then incubated overnight at 4°C with the following primary antibodies: AMPK, phospho-AMPK (Thr172), acetyl-CoA carboxylase (ACC), phospho-ACC (Ser79), mTOR, phospho-mTOR (Ser2448), Akt, phospho-Akt (Ser473), p70 ribosomal S6 kinase (p70S6K), phospho-p70S6K (Thr389), eukaryotic translation initiation factor 4E-binding protein-1 (4E-BP1), phospho-4E-BP1 (Thr37/46), HIF-1α, PDHK1, COX subunit IV (COX IV), and NADH dehydrogenase (ubiquinone) iron-sulfur protein-2, mitochondrial (NDUFS2), from Cell Signaling Technology (Danvers, MA); β-actin from Santa Cruz Biotechnology (Dallas, TX); PDH1-α, phospho-PDH1-α (Ser293), and an OXPHOS cocktail from Abcam (Toronto, ON, Canada); and a polyclonal rabbit anti-LRPPRC antibody raised against a 22-amino acid peptide having the sequence CEPPESFEFYAQQLRKLRENSS (antibody 295-313; Zymed Laboratories, San Francisco, CA). Membranes were then incubated at room temperature for 1.5 h with the appropriate horseradish peroxidase-conjugated secondary antibody from Cell Signaling Technology. Immunoreactive bands were then visualized using chemiluminescence and quantified using ImageJ software (http://imagej.net/Downloads). All protein levels were expressed in arbitrary units and normalized to β-actin, which was used as loading control. For treatment conditions, results were presented as fold change over basal conditions.
ATP levels.
ATP levels were measured with the ATPlite luminescence ATP detection assay system from PerkinElmer (Waltham, MA), according to the manufacturer’s instructions. Fibroblasts were seeded in opaque 96-well plates at a density of 10,000 cells per well. Determinations were performed in triplicate. Luminescence was quantified using a Synergy 2 Alpha Microplate Reader (BioTek Instruments, Winooski, VT).
Cell treatments and [U-13C6]glucose labeling.
The relative contribution of exogenous glucose to the formation of intracellular pyruvate, lactate, and alanine was assessed using modifications of previously published methods (18, 28). For these studies, confluent immortalized fibroblasts were cultured in supplemented DMEM with the following modifications: 5 mM glucose, 0.2 mM pyruvate, and 0.5 mM glutamine. On the day of the study, cells were washed with PBS and then incubated in a serum-free nonsupplemented DMEM containing 5 mM [U-13C6]glucose, 0.2 mM pyruvate, and 0.5 mM glutamine for 4 h. Cells were washed again with PBS and recovered in a 70% methanol buffer. Hydroxylamine was added to the supernatants to a final concentration of 25 mM. Labeled metabolites (pyruvate, lactate, and alanine) were extracted with 100% methanol, sonicated for 1 min, incubated at 70°C for 15 min, and then centrifuged at 14,000 rpm at 4°C for 15 min. The resulting supernatants were dried, taken up in 25 μl pyridine, incubated at 45°C for 90 min, derivatized in 100 μl N-methyl-N-tert-butyldimethylsilyltrifluoroacetamide (MtBSTFA), and then incubated at 90°C for 4 h. Metabolites were then measured by gas chromatography-mass spectrometry (GC-MS). Details for the measurement of 13C enrichment of lactate, pyruvate, and alanine by GC-MS, as well as the equations used to calculate the relative contribution of exogenous glucose to the formation of these metabolites, have been previously described (28, 29).
Statistical analysis.
Statistical analysis was performed using GraphPad Prism 5 software. Significant differences were determined using a paired two-tailed Student’s t-test. Data were log transformed (base 10) for analysis when necessary. The results are expressed as means ± SE, and significance was accepted at P ≤ 0.05.
RESULTS
Increased contribution of exogenous glucose to the formation of pyruvate, lactate, and alanine in LSFC fibroblasts.
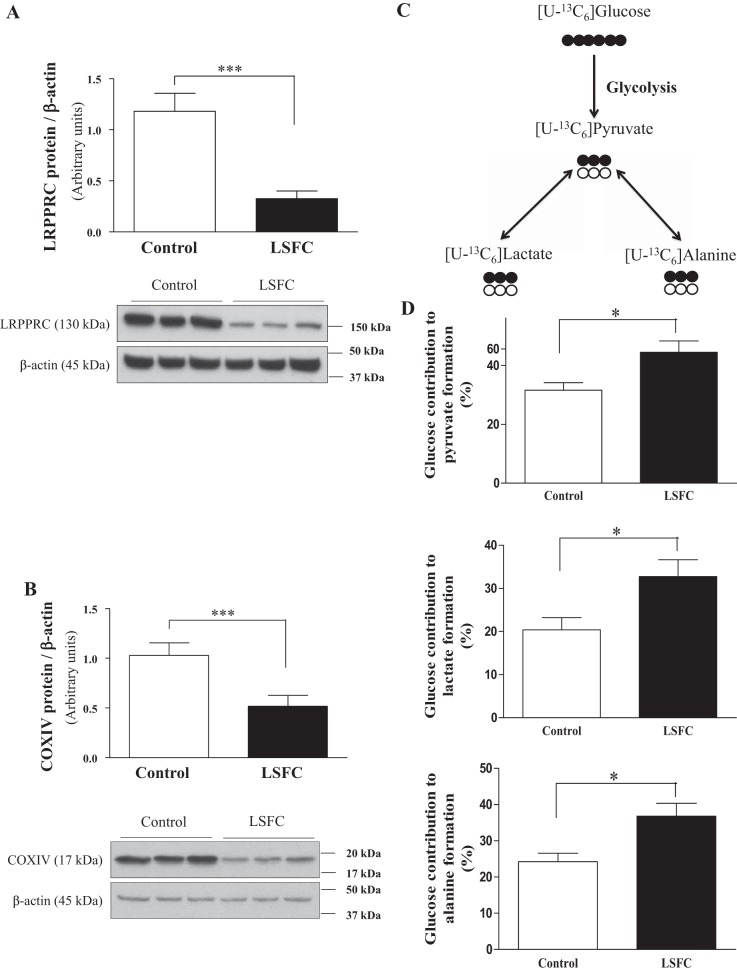
As previously reported (32), compared with controls, LSFC fibroblasts displayed a reduction of LRPPRC and COX IV expression by 72 and 50%, respectively (Fig. 1, A and B). Despite mitochondrial dysfunction and COX deficiency, control and LSFC fibroblasts had similar ATP levels under basal conditions (2). We hypothesized that this was the result of increased glycolytic flux. As a first step to address this question, we evaluated the relative contribution of exogenous glucose to pyruvate, lactate, and alanine using [U-13C6]glucose (Fig. 1C) as described in the methods section. In control fibroblasts, 32% of labeled pyruvate derives from [U-13C6]glucose, while the remainder comes from unlabeled substrates, most likely extracellular pyruvate (Fig. 1D). Furthermore, the formation of 13C-labeled pyruvate, lactate, and alanine from [U-13C6]glucose was increased by 41%, 61%, and 52%, respectively, in LSFC cells compared with control cells (P ≤ 0.05).
Fig. 1.
Higher contribution of exogenous glucose to pyruvate formation in Leigh syndrome French Canadian type (LSFC) fibroblasts. A and B: immunoblot and densitometric analysis of leucine-rich pentatricopeptide repeat-containing (LRPPRC, n = 12; A) and cytochrome-c oxidase subunit IV (COX IV, n = 9; B) protein levels in primary fibroblasts from LSFC and control donors. C and D: immortalized control and LSFC fibroblasts were incubated with 5 mM [U-13C6]glucose, 0.2 mM pyruvate, and 0.5 mM glutamine for 4 h in serum-free DMEM, and the relative contribution of glucose to the formation of pyruvate, lactate, and alanine was analyzed by GC-MS (n = 7). Results represent means ± SE. Difference between control and LSFC cells was assessed with a paired Student’s t-test. *P ≤ 0.05; ***P ≤ 0.001.
Upregulation of the Akt/mTORC1 signaling pathway in LSFC fibroblasts.
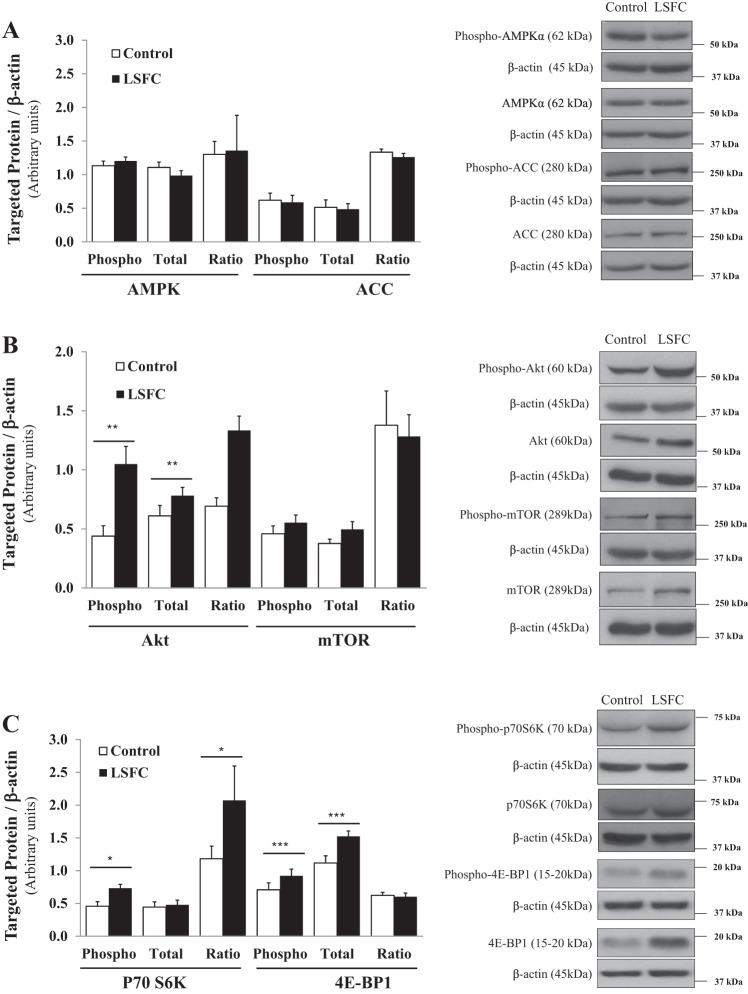
We next examined the AMPK and Akt/mTORC1 signaling pathways, two master regulators of cellular energy homeostasis (4, 7, 38). As shown in Fig. 2A, we did not detect any changes in the phosphorylation levels of AMPKα or its downstream target ACC in LSFC fibroblasts, which is consistent with their unchanged ATP levels under basal conditions. Next, we determined the activity of the Akt/mTOR signaling pathway and found an increase of total (28%; P ≤ 0.01) and phospho-Akt (138%; P ≤ 0.01) resulting in an unchanged ratio. We did not detect any changes in total or phospho-mTOR (Fig. 2B). However, mTOR regulation is complex, and it has been suggested that activation of downstream targets should be used as proxy for its activation. As shown in Fig. 2C, we observed enhanced phosphorylation of p70S6K and 4E-BP1 by 59% (P ≤ 0.05) and 29% (P ≤ 0.001), respectively (Fig. 2C), as well as a 36% increase in total 4E-BP1 (P ≤ 0.001). This resulted in a 75% increase in the ratio of phosphorylated to total protein for p70S6K (P ≤ 0.05) but an unchanged ratio for 4E-BP1 in LFSC fibroblasts compared with controls.
Fig. 2.
Upregulation of the Akt/mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) signaling pathway in Leigh syndrome French Canadian type (LSFC) fibroblasts. A: AMPK activity of primary fibroblasts from control and LSFC donors was assessed by immunoblot and densitometric analysis of total and phosphorylated levels of AMPK (n = 7) and its downstream target acetyl-CoA carboxylase (ACC; n = 6). B and C: activity of the mTORC1 signaling pathway in control and LSFC primary fibroblasts was assessed by immunoblot and densitometric analysis of total and phosphorylated levels of Akt (n = 10), mTOR (n = 13), and their downstream targets p70 ribosomal S6 kinase (p70S6K, n = 9) and eukaryotic translation initiation factor 4E-binding protein-1 (4E-BP1, n = 12). Results represent means ± SE. Difference between control and LSFC cells was assessed with a paired Student’s t-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
PDH1 is inhibited in LSFC fibroblasts.
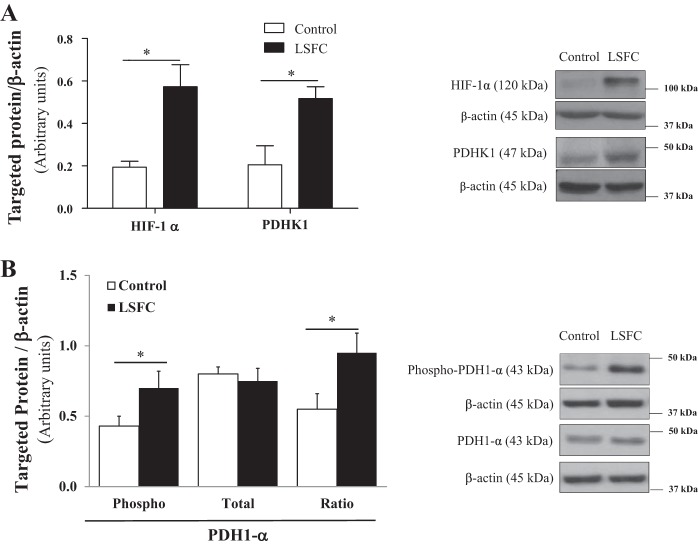
In cancer cells, mTORC1-mediated metabolic remodeling has been shown to occur, in part, through activation of the HIF-1α/PDHK1 axis (8, 11). Thus, we examined whether this pathway was upregulated in LSFC cells. As illustrated in Fig. 3A, the expression of HIF-1α and PDHK1 was increased by 200% (P ≤ 0.05) and 155% (P ≤ 0.05), respectively, in LSFC fibroblasts. Consistent with this result, the phosphorylated and inhibited form of PDH1-α (phospho-PDH1-α) was increased by 63% (P ≤ 0.05) in LSFC fibroblasts compared with controls, whereas total PDH1-α protein levels remained unchanged (Fig. 3B). This leads to a 72% increase in the ratio of phosphorylated to total PDH1-α (P ≤ 0.05) in LFSC fibroblasts compared with controls.
Fig. 3.
Pyruvate dehydrogenase 1 (PDH1) is inhibited in Leigh syndrome French Canadian type (LSFC) fibroblasts. Immunoblot and densitometric analysis of total and/or phosphorylated levels of hypoxia-inducible factor 1α (HIF-1α, n = 5) and pyruvate dehydrogenase kinase 1 (PDHK1, n = 5; A) and PDH1-α (n = 8; B) in primary fibroblasts from control and LSFC donors. Results represent means ± SE. Difference between control and LSFC cells was assessed with a paired Student’s t-test. *P ≤ 0.05.
Suppression of mTORC1 activity does not alter HIF-1α and PDHK1 levels.
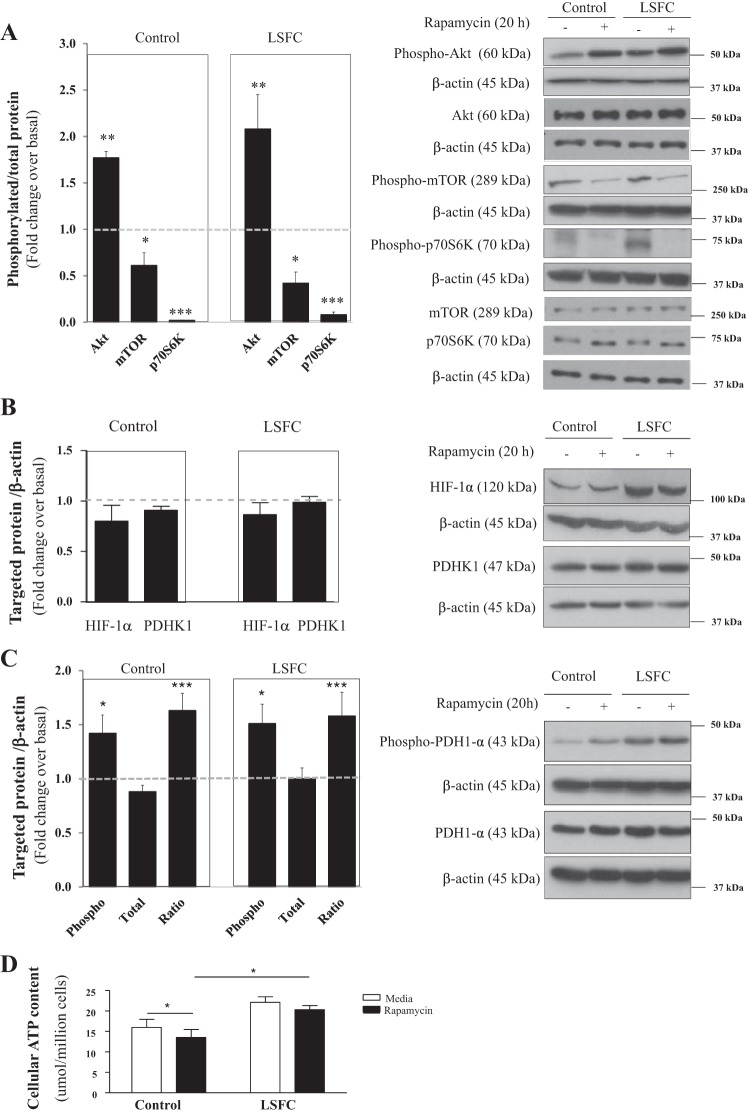
To further our understanding of mTORC1’s role, cells were treated with 100 nM rapamycin for 20 h. As anticipated, rapamycin reduced the ratio of phosphorylated to total mTOR by 58 and 49% (P ≤ 0.05) in LSFC and control fibroblasts, respectively (Fig. 4A). This resulted in a 98% and 92% reduction (P ≤ 0.001) in the ratio of phosphorylated to total p70S6K in control and LSFC fibroblasts (Fig. 4A), respectively. It has been shown that inhibition of p70S6K prevents its negative feedback on phosphatidylinositol 3-kinase leading to mTORC2 activation and phosphorylation of its downstream target Akt (31, 47). Consistent with this, rapamycin treatment was associated with a 1.8- and 2.1-fold increase in the ratio of phosphorylated to total Akt (P ≤ 0.01) in control and LSFC fibroblasts (Fig. 4A), respectively. However, contrary to our hypothesis, rapamycin did not affect HIF-1α and PDHK1 levels either in LSFC or in control fibroblasts (Fig. 4B). On the other hand, PDH1-α phosphorylation increased by 1.42- (P ≤ 0.05) and 1.51-fold (P ≤ 0.01) in control and LSFC fibroblasts, respectively, in response to rapamycin (Fig. 4C). This occurs in the absence of any change in total PDH1-α protein levels leading to a 1.6-fold increase in the ratio of phosphorylated to total PDH1-α in control and LSFC fibroblasts (Fig. 4C). In light of these results, we determined whether mTOR inhibition alters ATP levels. As illustrated in Fig. 4D, rapamycin reduced ATP levels by 15% (P ≤ 0.05) in control cells but did not provoke any significant changes in LSFC cells. Thus, in rapamycin-treated cells, ATP levels were higher in LSFC fibroblasts compared with controls (P ≤ 0.05).
Fig. 4.
Suppression of mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) activity does not alter hypoxia-inducible factor 1α (HIF-1α) and pyruvate dehydrogenase kinase 1 (PDHK1) levels. Primary fibroblasts from control and Leigh syndrome French Canadian type (LSFC) donors were treated for 16 h with 100 nM rapamycin or vehicle (DMSO) in supplemented DMEM. On the day of the study, cells were cultured in a serum-free nonsupplemented DMEM, with inhibitor or vehicle as indicated, for an additional 4 h. A: suppression of mTORC1 activity was confirmed by immunoblot and densitometric analysis of phosphorylated levels of mTOR (n = 3), p70 ribosomal S6 kinase (p70S6K, n = 3), and Akt (n = 3). B and C: immunoblot and densitometric analysis of total levels of HIF-1α (n = 7) and PDHK1 (n = 8; B) and phosphorylated pyruvate dehydrogenase 1α (PDH1-α, n = 7; C) in response to rapamycin treatment. D: primary fibroblasts were cultured with inhibitor or vehicle, as indicated, in opaque 96-well plates at a density of 10,000 cells per well in triplicate. Cellular ATP levels were measured with the ATPlite luminescence ATP detection assay system (n = 4). Results represent means ± SE. Difference between baseline and rapamycin treatment was assessed with a paired Student’s t-test. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
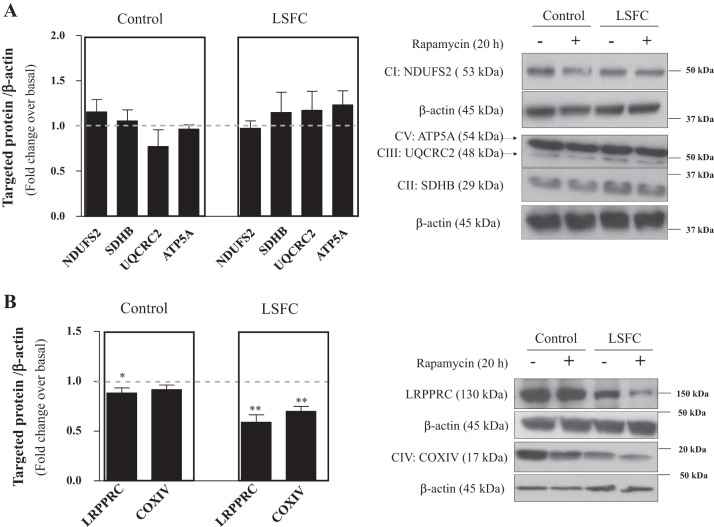
Suppression of mTORC1 activity decreases LRPPRC and COX IV expression in LSFC fibroblasts.
Since mTORC1 also regulates mitochondrial protein expression (23, 24), we investigated the effect of rapamycin on the expression of OXPHOS complexes. Whereas no effect on OXPHOS complexes I, II, III, and V was observed in control or LSFC fibroblasts (Fig. 5A), there was a selective reduction of COX IV expression by 32% (P ≤ 0.01) in LSFC fibroblasts (Fig. 5B). Given the link between COX and LRPPRC, we examined the effect of mTORC1 inhibition on the latter. Rapamycin decreased LRPPRC expression by 11% (P ≤ 0.05) in control fibroblasts but by 41% (P ≤ 0.01) in LSFC fibroblasts (Fig. 5B). This supports a potential role for mTORC1 in the regulation of LRPPRC and COX expression.
Fig. 5.
Suppression of mechanistic target of rapamycin complex 1 (mTORC1) activity decreases leucine-rich pentatricopeptide repeat-containing (LRPPRC) and cytochrome-c oxidase (COX) expression in Leigh syndrome French Canadian type (LSFC) fibroblasts. Primary fibroblasts from control and LSFC donors were treated for 16 h with 100 nM rapamycin or vehicle (DMSO) in supplemented DMEM. On the day of the study, cells were cultured in serum-free nonsupplemented DMEM, with inhibitor or vehicle as indicated for an additional 4 h. A: immunoblot and densitometric analysis of mitochondrial complex levels [NADH dehydrogenase (ubiquinone) iron-sulfur protein-2, mitochondrial (NDUFS2, n = 5), succinate dehydrogenase (ubiquinone) iron-sulfur subunit, mitochondrial (SDHB, n = 3), cytochrome–b-c1 complex subunit 2, mitochondrial (UQCRC2, n = 4), and ATP synthase subunit-α, mitochondrial (ATP5A, n = 5)] in response to rapamycin treatment. B: immunoblot and densitometric analysis of LRPPRC (n = 8) and COX subunit IV (COX IV, n = 11) content in response to rapamycin treatment. Results represent means ± SE. CI–CV, oxidative phosphorylation complexes I–V. Difference between baseline and rapamycin treatment was assessed with a paired Student’s t-test. *P ≤ 0.05; **P ≤ 0.01.
DISCUSSION
LSFC is a metabolic disease characterized by decreased COX activity and mitochondrial dysfunction, in the absence of any changes in ATP levels under basal conditions (2). This suggests that activation of compensatory pathways can maintain ATP production. Our aim was to examine the remodeling of signaling pathways in LSFC fibroblasts. Our data demonstrate that LSFC fibroblasts present a series of adaptations including increased contribution of glucose to lactate formation, upregulation of the mTORC1/p70S6K/HIF-1α/PDHK1 signaling pathway, and increased PDH1-α phosphorylation. However, contrary to expectation, inhibition of mTORC1 with rapamycin was associated with increased phospho-PDH1-α in the absence of any changes in HIF-1α/PDHK1 levels. Importantly, rapamycin treatment further decreased LRPPRC and COX IV expression in LSFC fibroblasts. To our knowledge, this is the first time that LSFC metabolic signaling alterations have been reported and that mTORC1 regulation of LRPPRC levels has been revealed.
Our results demonstrate that the relative contribution of exogenous glucose to intracellular pyruvate, lactate, and alanine formation is enhanced in LSFC fibroblasts. This is consistent with the clinical phenotype of LSFC patients, which shows elevated plasma levels of these metabolites (40). A redirection of intracellular pyruvate toward the formation of cytosolic lactate and alanine, to the detriment of its mitochondrial metabolism, could be due to reduced pyruvate entry into the mitochondria due to PDH1 inactivation as well as reduced COX IV levels. Another possibility is enhanced glycolysis. Interestingly, an increase in basal extracellular acidification rate has been reported in fibroblasts of patients with novel LRPPRC mutations (not the French Canadian mutations; 26). Increased glycolysis in other models of mitochondrial diseases is also supported by indirect measurements such as higher levels of tissue glycolytic intermediates or use of inhibitors (6, 14, 26). In cancer cells, glycolysis can be upregulated as much as 200-fold allowing for rapid cell proliferation (reviewed in Ref. 7). A metabolic shift including enhanced glycolysis would enable greater cytosolic ATP production and could possibly explain the maintenance of ATP levels in LSFC fibroblasts.
Two major signaling pathways regulate glucose homeostasis, namely, AMPK and mTORC1. Whereas we did not observe any changes in AMPK activity, we found that LSFC fibroblasts display an upregulation of the mTORC1/p70S6K signaling cascade. mTORC1 activation has also been reported in Ndufs4 knockout mice, a model of Leigh syndrome (14), as well as in cell lines from patients with primary respiratory chain diseases such as mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS), Leigh syndrome, and mitochondrial myopathy (13, 46). Thus, mTORC1 activation may represent an adaptive response to electron transport chain defects.
Our data show that LSFC cells overexpressed HIF-1α, an important regulator of both glycolysis and glucose oxidation. Consistent with results obtained in other cells, upregulation of HIF-1α was found to be associated with increased PDHK1 expression and phosphorylation (i.e., inactivation) of PDH1 (16, 34). Increased HIF-1α expression may be a compensatory mechanism to palliate the mitochondrial deficit of LSFC cells. Indeed, HIF-1α stabilization improved survival of zebrafish embryos exposed to the complex III inhibitor antimycin (12). This study also showed that Ndufs4 knockout mice appeared healthier after chronic hypoxia (12). In cancer cells, HIF-1α impairs fatty acid oxidation through decreased medium- and long-chain acyl-CoA dehydrogenase levels (10). Interestingly, LSFC patients display elevated plasma levels of medium- and long-chain acyl-carnitines. The latter may be due to an electron chain defect, which reduces the mitochondrial capacity to metabolize acetyl-CoA produced by β-oxidation leading to increased acyl-carnitine levels (40). A reduction of both glucose and fatty acid oxidation (3, 16, 36) in LSFC cells may be protective in view of the reduced mitochondrial oxidative capacity (2, 26). However, although these mechanisms may compensate for mitochondrial dysfunction under basal conditions, we have previously shown that LSFC fibroblasts are more susceptible to cell death when exposed to high levels of palmitate (2) suggesting that these adaptive mechanisms may not be sufficient in cells exposed to nutrient overload.
Although the mechanism underlying HIF-1α expression in LSFC cells is unknown at present, numerous mechanisms have been shown to contribute to increased HIF-1α levels including lactate production, activation of the mTORC1 pathway, or reduction of succinate dehydrogenase activity (16a, 35). Furthermore, HIF-1α expression can be regulated at multiple levels including transcription, translation, or degradation (reviewed in Ref 16a). It is unlikely that the increase in HIF-1α levels is due to increased transcription since we did not observe any change in HIF-1α mRNA levels in LSFC fibroblasts compared with controls (data not shown). Numerous studies have highlighted the role of mTORC1 in HIF-1α activation (9, 11, 43). Surprisingly, inhibition of mTORC1 did not affect the expression of HIF-1α or its downstream target PDHK1 in LSFC cells, suggesting that upregulation of HIF-1α is independent of this pathway. Potentially, HIF-1α upregulation may result from inhibition of its degradation or increased translation. It should be noted that HIF-1α protein can be stabilized by end products of glycolysis, such as lactate and pyruvate, both of which are increased in LSFC fibroblasts (20). Further studies should explore the mechanisms underlying HIF-1α expression in LSFC cells.
Our results demonstrated that rapamycin increased PDH1 phosphorylation in both LSFC and control cells. A similar response to mTOR inhibition has also been observed in head and neck squamous cell carcinoma cells (3); however, this effect was attributed to Akt inhibition. In contrast, we observed an increase in phospho-Akt in rapamycin-treated cells, which probably occurs through activation of its upstream kinase, mTORC2 (31, 47). Since we did not observe any change in PDHK1 expression, rapamycin treatment may lead to overexpression of another PDHK isoenzyme (45) or, alternatively, modulate the expression or activity of phosphatases (reviewed in Ref. 1).
An important finding of our study is that mTORC1 inhibition resulted in a reduction of COX IV expression in LSFC cells. Morita et al. demonstrated that mTORC1 regulates the translation of a subset of mitochondrial proteins including complexes I and V (23). Since COX IV expression is dependent on LRPPRC, we hypothesized that mTORC1 may modulate COX IV expression through changed LRPPRC levels. Consistent with our hypothesis, rapamycin reduced LRPPRC levels in both control and LSFC cells. To our knowledge, this is the first report of LRPPRC regulation by mTORC1. Data on the regulation of LRPPRC expression are scarce. Cooper et al. have shown reduced LRPPRC levels in PGC-1α and PGC-1β double-deficient cells, suggesting that LRPPRC is downstream of these proteins (5). Future studies should investigate the underlying mechanism leading to the reduction of LRPPRC and COX IV in response to rapamycin. Interestingly, rapamycin treatment also resulted in reduction of ATP levels in control but not in LSFC cells. Furthermore, despite reduced COX IV expression, ATP levels of LSFC fibroblasts remained elevated compared with controls in rapamycin-treated cells. This strengthens the hypothesis that LSFC fibroblasts depend on extramitochondrial metabolism to maintain ATP levels.
Although patient fibroblasts are commonly used as a research model when studying mitochondrial diseases, the consequences of reduced LRPPRC expression are tissue specific (33). Thus, although our data cannot be extrapolated to tissues such as brain and liver, which have different energetic metabolism, they provide a better understanding of the adaptive molecular mechanisms for energy homeostasis that should be considered in future studies of mitochondrial diseases as well as important new information related to the potential role of mTORC1 in regulating LRPPRC expression.
In conclusion, we demonstrated that LSFC fibroblasts display molecular adaptation, including an mTORC1-independent upregulation of the HIF-1α/PDHK1 axis. However, mTORC1 upregulation appears to be required for LRPPRC and COX IV expression in LSFC fibroblasts. Further studies should examine in detail the effect of mTOR inhibition on LRPPRC and mitochondrial function in brain and liver, the two primary tissues affected by the disease.
GRANTS
This work was supported by a Canadian Institutes of Health Research Emerging Team Grant on LSFC (CPG 102168) and by the Fondation du Grand défi Pierre Lavoie. Y. Mukaneza was supported by a studentship from the Université de Montréal and Corporation de Recherche et d’Action sur les Maladies Héréditaires.
DISCLAIMERS
The funders had no role in study design, data collection and analysis, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.M., J.T., C.L., C.D.R., and L. Coderre conceived and designed research; Y.M., A.C., M.-E.R., and S.D. performed experiments; Y.M., A.C., M.-E.R., S.D., and L. Coderre analyzed data; Y.M., C.D.R., and L. Coderre interpreted results of experiments; Y.M. prepared figures; Y.M. drafted manuscript; Y.M., M.R., C.L., C.D.R., and L. Coderre edited and revised manuscript; Y.M., A.C., M.-E.R., J.T., S.D., M.R., LSFC Consortium, C.L., C.D.R., and L. Coderre approved final version of manuscript.
ACKNOWLEDGMENTS
We thank N. El Zir for the labeling studies. The authors are grateful to the Association de l’Acidose Lactique, the patients, and their families for support and tissue samples.
The LSFC Consortium is a group of basic and clinical researchers dedicated to investigating the causes and developing treatments for LSFC. At the time of this study, the members were, in alphabetical order: Bruce G. Allen, Claudine Beauchamp, Chantal Bemeur, Yan Burelle, Guy Charron, Lise Coderre, Christine Des Rosiers, Sonia Deschênes, François Labarthe, Jeannine Landry, Catherine Laprise, Geneviève Lavallée, Pierre Lavoie, Sylvie Lesage, Bruno Maranda, Charles Morin, Yvette Mukaneza, John D. Rioux, Marie-Ève Rivard, Matthieu Ruiz, Eric A. Shoubridge, Jessica Tardif, Julie Thompson Legault, Nancy Tremblay, Vanessa Tremblay-Vaillancourt, Luc Vachon, and Josée Villeneuve.
Present address of A. Cohen: The Alexander Grass Center for Bioengineering, The Hebrew University of Jerusalem, Silberman 3-512, Jerusalem, 91904, Israel.
REFERENCES
- 1.Alessi DR, Pearce LR, García-Martínez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal 2: pe27, 2009. doi: 10.1126/scisignal.267pe27. [DOI] [PubMed] [Google Scholar]
- 2.Burelle Y, Bemeur C, Rivard ME, Thompson Legault J, Boucher G, Morin C, Coderre L, Des Rosiers C; LSFC Consortium . Mitochondrial vulnerability and increased susceptibility to nutrient-induced cytotoxicity in fibroblasts from Leigh syndrome French Canadian patients. PLoS One 10: e0120767, 2015. doi: 10.1371/journal.pone.0120767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cerniglia GJ, Dey S, Gallagher-Colombo SM, Daurio NA, Tuttle S, Busch TM, Lin A, Sun R, Esipova TV, Vinogradov SA, Denko N, Koumenis C, Maity A. The PI3K/Akt pathway regulates oxygen metabolism via pyruvate dehydrogenase (PDH)-E1α phosphorylation. Mol Cancer Ther 14: 1928–1938, 2015. doi: 10.1158/1535-7163.MCT-14-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, Manjeri GR, Li Y, Ifrim DC, Arts RJ, van der Veer BM, Deen PM, Logie C, O’Neill LA, Willems P, van de Veerdonk FL, van der Meer JW, Ng A, Joosten LA, Wijmenga C, Stunnenberg HG, Xavier RJ, Netea MG. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 345: 1250684, 2014. [Erratum in Science 346: aaa1503, 2014.] doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper MP, Uldry M, Kajimura S, Arany Z, Spiegelman BM. Modulation of PGC-1 coactivator pathways in brown fat differentiation through LRP130. J Biol Chem 283: 31960–31967, 2008. doi: 10.1074/jbc.M805431200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Andrade PB, Rubi B, Frigerio F, van den Ouweland JM, Maassen JA, Maechler P. Diabetes-associated mitochondrial DNA mutation A3243G impairs cellular metabolic pathways necessary for beta cell function. Diabetologia 49: 1816–1826, 2006. doi: 10.1007/s00125-006-0301-9. [DOI] [PubMed] [Google Scholar]
- 7.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7: 11–20, 2008. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Dupuy F, Tabariès S, Andrzejewski S, Dong Z, Blagih J, Annis MG, Omeroglu A, Gao D, Leung S, Amir E, Clemons M, Aguilar-Mahecha A, Basik M, Vincent EE, St-Pierre J, Jones RG, Siegel PM. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab 22: 577–589, 2015. doi: 10.1016/j.cmet.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Harada H, Itasaka S, Kizaka-Kondoh S, Shibuya K, Morinibu A, Shinomiya K, Hiraoka M. The Akt/mTOR pathway assures the synthesis of HIF-1α protein in a glucose- and reoxygenation-dependent manner in irradiated tumors. J Biol Chem 284: 5332–5342, 2009. doi: 10.1074/jbc.M806653200. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Li T, Li X, Zhang L, Sun L, He X, Zhong X, Jia D, Song L, Semenza GL, Gao P, Zhang H. HIF-1-mediated suppression of acyl-CoA dehydrogenases and fatty acid oxidation is critical for cancer progression. Cell Reports 8: 1930–1942, 2014. doi: 10.1016/j.celrep.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol 22: 7004–7014, 2002. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, Goldberger O, Peng J, Shalem O, Sanjana NE, Zhang F, Goessling W, Zapol WM, Mootha VK. Hypoxia as a therapy for mitochondrial disease. Science 352: 54–61, 2016. doi: 10.1126/science.aad9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji K, Zheng J, Lv J, Xu J, Ji X, Luo YB, Li W, Zhao Y, Yan C. Skeletal muscle increases FGF21 expression in mitochondrial disorders to compensate for energy metabolic insufficiency by activating the mTOR-YY1-PGC1α pathway. Free Radic Biol Med 84: 161–170, 2015. doi: 10.1016/j.freeradbiomed.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SC, Yanos ME, Kayser EB, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342: 1524–1528, 2013. doi: 10.1126/science.1244360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan NA, Govindaraj P, Meena AK, Thangaraj K. Mitochondrial disorders: challenges in diagnosis & treatment. Indian J Med Res 141: 13–26, 2015. doi: 10.4103/0971-5916.154489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 16a.Koh MY, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci 33: 526–534, 2008. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 149: 274–293, 2012. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauzier B, Vaillant F, Merlen C, Gélinas R, Bouchard B, Rivard ME, Labarthe F, Dolinsky VW, Dyck JR, Allen BG, Chatham JC, Des Rosiers C. Metabolic effects of glutamine on the heart: anaplerosis versus the hexosamine biosynthetic pathway. J Mol Cell Cardiol 55: 92–100, 2013. doi: 10.1016/j.yjmcc.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liko D, Hall MN. mTOR in health and in sickness. J Mol Med (Berl) 93: 1061–1073, 2015. doi: 10.1007/s00109-015-1326-7. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem 277: 23111–23115, 2002. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 21.Mootha VK, Lepage P, Miller K, Bunkenborg J, Reich M, Hjerrild M, Delmonte T, Villeneuve A, Sladek R, Xu F, Mitchell GA, Morin C, Mann M, Hudson TJ, Robinson B, Rioux JD, Lander ES. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc Natl Acad Sci USA 100: 605–610, 2003. doi: 10.1073/pnas.242716699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morin C, Mitchell G, Larochelle J, Lambert M, Ogier H, Robinson BH, De Braekeleer M. Clinical, metabolic, and genetic aspects of cytochrome C oxidase deficiency in Saguenay-Lac-Saint-Jean. Am J Hum Genet 53: 488–496, 1993. [PMC free article] [PubMed] [Google Scholar]
- 23.Morita M, Gravel SP, Chénard V, Sikström K, Zheng L, Alain T, Gandin V, Avizonis D, Arguello M, Zakaria C, McLaughlan S, Nouet Y, Pause A, Pollak M, Gottlieb E, Larsson O, St-Pierre J, Topisirovic I, Sonenberg N. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab 18: 698–711, 2013. doi: 10.1016/j.cmet.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Morita M, Gravel SP, Hulea L, Larsson O, Pollak M, St-Pierre J, Topisirovic I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 14: 473–480, 2015. doi: 10.4161/15384101.2014.991572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell 148: 1145–1159, 2012. doi: 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oláhová M, Hardy SA, Hall J, Yarham JW, Haack TB, Wilson WC, Alston CL, He L, Aznauryan E, Brown RM, Brown GK, Morris AA, Mundy H, Broomfield A, Barbosa IA, Simpson MA, Deshpande C, Moeslinger D, Koch J, Stettner GM, Bonnen PE, Prokisch H, Lightowlers RN, McFarland R, Chrzanowska-Lightowlers ZM, Taylor RW. LRPPRC mutations cause early-onset multisystem mitochondrial disease outside of the French-Canadian population. Brain 138: 3503–3519, 2015. doi: 10.1093/brain/awv291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion 30: 105–116, 2016. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riazi R, Khairallah M, Cameron JM, Pencharz PB, Des Rosiers C, Robinson BH. Probing pyruvate metabolism in normal and mutant fibroblast cell lines using 13C-labeled mass isotopomer analysis and mass spectrometry. Mol Genet Metab 98: 349–355, 2009. doi: 10.1016/j.ymgme.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz M, Gélinas R, Vaillant F, Lauzier B, Des Rosiers C. Metabolic tracing using stable isotope-labeled substrates and mass spectrometry in the perfused mouse heart. Methods Enzymol 561: 107–147, 2015. doi: 10.1016/bs.mie.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 30.Ruzzenente B, Metodiev MD, Wredenberg A, Bratic A, Park CB, Cámara Y, Milenkovic D, Zickermann V, Wibom R, Hultenby K, Erdjument-Bromage H, Tempst P, Brandt U, Stewart JB, Gustafsson CM, Larsson NG. LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J 31: 443–456, 2012. doi: 10.1038/emboj.2011.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307: 1098–1101, 2005. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 32.Sasarman F, Brunel-Guitton C, Antonicka H, Wai T, Shoubridge EA; LSFC Consortium . LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol Biol Cell 21: 1315–1323, 2010. doi: 10.1091/mbc.e10-01-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasarman F, Nishimura T, Antonicka H, Weraarpachai W, Shoubridge EA, Allen B, Burelle Y, Charron G, Coderre L, DesRosiers C, Laprise C, Morin C, Rioux J, Shoubridge EA; LSFC Consortium . Tissue-specific responses to the LRPPRC founder mutation in French Canadian Leigh syndrome. Hum Mol Genet 24: 480–491, 2015. doi: 10.1093/hmg/ddu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: an old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer 138: 809–817, 2016. doi: 10.1002/ijc.29564. [DOI] [PubMed] [Google Scholar]
- 35.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7: 77–85, 2005. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev 20: 51–56, 2010. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40: 310–322, 2010. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev 89: 1025–1078, 2009. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 39.Tan Z, Xie N, Cui H, Moellering DR, Abraham E, Thannickal VJ, Liu G. Pyruvate dehydrogenase kinase 1 participates in macrophage polarization via regulating glucose metabolism. J Immunol 194: 6082–6089, 2015. doi: 10.4049/jimmunol.1402469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson Legault J, Strittmatter L, Tardif J, Sharma R, Tremblay-Vaillancourt V, Aubut C, Boucher G, Clish CB, Cyr D, Daneault C, Waters PJ, Vachon L, Morin C, Laprise C, Rioux JD, Mootha VK, Des Rosiers C, Aliskashani A, Allen BG, Aubut C, Beauchamp C, Bemeur C, Burelle Y, Charron G, Coderre L, Des Rosiers C, Deschênes S, Labarthe F, Landry J, Laprise C, Lavallée G, Lavoie P, Maranda B, Morin C, Mukaneza Y, Nishimura T, Rioux JD, Rivard MÈ, Sasarman F, Shoubridge EA, Tardif J, Thompson Legault J, Tremblay N, Tremblay-Vaillancourt V, Vachon L, Villeneuve J; LSFC Consortium . A metabolic signature of mitochondrial dysfunction revealed through a monogenic form of Leigh syndrome. Cell Reports 13: 981–989, 2015. doi: 10.1016/j.celrep.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu F, Morin C, Mitchell G, Ackerley C, Robinson BH. The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem J 382: 331–336, 2004. doi: 10.1042/BJ20040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao J, Shoubridge EA. Expression and functional analysis of SURF1 in Leigh syndrome patients with cytochrome c oxidase deficiency. Hum Mol Genet 8: 2541–2549, 1999. doi: 10.1093/hmg/8.13.2541. [DOI] [PubMed] [Google Scholar]
- 43.Yasinska IM, Gibbs BF, Lall GS, Sumbayev VV. The HIF-1 transcription complex is essential for translational control of myeloid hematopoietic cell function by maintaining mTOR phosphorylation. Cell Mol Life Sci 71: 699–710, 2014. doi: 10.1007/s00018-013-1421-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo H, Kang JW, Lee DW, Oh SH, Lee YS, Lee EJ, Cho J. Pyruvate metabolism: a therapeutic opportunity in radiation-induced skin injury. Biochem Biophys Res Commun 460: 504–510, 2015. doi: 10.1016/j.bbrc.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Tsukikawa M, Peng M, Polyak E, Nakamaru-Ogiso E, Ostrovsky J, McCormack S, Place E, Clarke C, Reiner G, McCormick E, Rappaport E, Haas R, Baur JA, Falk MJ. Primary respiratory chain disease causes tissue-specific dysregulation of the global transcriptome and nutrient-sensing signaling network. PLoS One 8: e69282, 2013. doi: 10.1371/journal.pone.0069282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by association with the ribosome. Cell 144: 757–768, 2011. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]