Abstract
The epithelium of the mammalian intestinal mucosa is a rapidly self-renewing tissue in the body, and its homeostasis is preserved through well-controlled mechanisms. Long noncoding RNAs (lncRNAs) regulate a variety of biological functions and are intimately involved in the pathogenesis of diverse human diseases. Here we highlight the roles of several lncRNAs expressed in the intestinal epithelium, including uc.173, SPRY4-IT1, H19, and Gata6, in maintaining the integrity of the intestinal epithelium, focusing on the emerging evidence of lncRNAs in the regulation of intestinal mucosal regeneration and epithelial barrier function. We also discuss recent results that the interactions between lncRNAs with microRNAs and the RNA-binding protein HuR influence epithelial homeostasis. With rapidly advancing knowledge of lncRNAs, there is also growing recognition that lncRNAs in the intestinal epithelium might be promising therapeutic targets in our efforts to protect the integrity of the intestinal epithelium in response to stressful environments.
Keywords: gut permeability, intestinal mucosal renewal, microRNA, posttranscriptional regulation, RNA-binding proteins
INTRODUCTION
Many regions of the mammalian genome are actively transcribed into noncoding RNAs (ncRNAs). Long ncRNAs (lncRNAs) are defined as transcripts spanning >200 nucleotides in length, capable of regulating a variety of cellular processes (27). LncRNAs are dynamically expressed in tissue-, differentiation stage-, and cell type-specific manners, and they control virtually every level of gene regulation, including chromatin remodeling, transcriptional and posttranscriptional processes, and protein metabolism (6, 29). LncRNAs are distinct from other well-characterized structural and regulatory RNAs such as ribosomal RNAs, transfer RNAs, small nucleolar RNAs (snoRNAs), small interfering RNAs (siRNAs), microRNAs (miRNAs), and Piwi interacting RNAs (piRNAs), but they share structural features with mRNAs (46). Although only a small number of functional lncRNAs are well characterized in human tissues to date, dysregulation and mutations in lncRNAs have been implicated in disease processes (6, 37).
The intestinal epithelium, a rapidly self-renewing tissue in the body, acts as a physical barrier that separates mucosal tissues from luminal noxious substances and microbiota (33, 38). Intestinal stem cells (ISCs) and amplified progenitor cells replicate continuously and drive epithelial renewal, while the newly divided cells differentiate into various mature cell types as they migrate up along the crypt-villus axis (4, 36). There are a number of differentiated intestinal epithelial cells (IECs) in the epithelium, including absorptive enterocytes, mucus-secreting goblet cells, hormone-secreting enteroendocrine cells, tuft cells, and Paneth cells. These IECs perform distinct biological functions such as digestion, absorption, defense, and mucosal immunity, but they actively communicate with each other and function cooperatively (3). Homeostasis of the intestinal epithelium is essential for mucosal function and depends on a dynamic balance among cell proliferation, differentiation, migration, apoptosis, and cell-to-cell interaction (22, 33, 41). Disruptions in the integrity of the epithelium occur commonly in critical pathologies, leading to the translocation of luminal toxic substances and bacteria to the bloodstream.
Recently, evidence has emerged that lncRNAs are a novel class of master regulators of intestinal epithelium homeostasis and participate in gut mucosal growth, adaptation, and permeability (40, 46, 49). In this review, we highlight the importance of several intestinal epithelial tissue-specific lncRNAs, including uc.173, SPRY4-IT1, H19, and Gata6, in the regulation of intestinal mucosal renewal and epithelial barrier function and further discuss the molecular mechanisms by which lncRNAs regulate gene expression through interactions with miRNAs and RNA-binding proteins (RBPs).
BIOLOGICAL FUNCTIONS OF LncRNAs
High-throughput studies have revealed the complexity of the mammalian transcriptome and the fact that most (>95%) of the mammalian genome is transcribed into vast amounts of ncRNAs (7). Conversely, protein-coding transcripts only account for a minority (<4%) of the transcriptional output. NcRNAs are categorized into different classes based on their length and biological functions. LncRNAs arise from intergenic, antisense, or promoter-proximal regions and are independently transcribed with little potential to encode functional proteins. Unlike miRNAs, which are highly conserved and generally reduce mRNA stability and/or translation through specific base-pairing with target mRNAs (5), most lncRNAs are poorly conserved and regulate gene expression through a broad range of transcriptional and posttranscriptional mechanisms (42). Many lncRNAs have been identified in animal and human tissues through different methodologies; they are typically transcribed from multiexonic genes by RNA polymerase II (Pol II) and possess a 5′-methyl-guanosine cap and a 3′-poly(A) tail. On the other hand, lncRNAs lack robust open reading frames and other features (39).
LncRNAs can act as molecular scaffolds, decoys, or signals and also function through genomic targeting, cis- and trans-regulatory factors, and antisense molecules. The subcellular distribution of a lncRNA is tightly associated with its biological functions. Generally, lncRNAs localized in the nucleus are involved in gene transcription and chromatin modification, whereas lncRNAs in the cytoplasm modulate posttranscriptional processes through direct interaction with mRNAs, miRNAs, or RBPs (21). Recently, lncRNAs have been found to be important regulatory factors in many cellular processes in normal physiology, as they modulate genomic imprinting, chromosome shape, and allosterically modulate enzymatic activity (32, 39). Detailed information about lncRNA evolution, expression, and biological function has been nicely summarized in previous publications (21, 32).
LncRNAs INVOLVED IN THE REGENERATION OF THE INTESTINAL EPITHELIUM
The intestinal epithelium renews itself every 4–5 days in mice and 5–7 days in humans. The rapid turnover rate of the intestinal epithelium is tightly controlled at different levels by numerous intracellular and extracellular factors (33). Consistent with most other tissues in the body, the renewal of the intestinal mucosa is uniquely modulated by factors such as insulin, growth hormones, thyroxin, and cortisol that alter metabolism in all tissues. However, the intestinal mucosa also responds to a host of events triggered by the ingestion and presence of food within the digestive tract. Food in the intestinal lumen is one of the strongest stimulants of mucosal growth and functions by inducing the release of several gut hormones that target only mucosal tissues of the gastrointestinal tract (33, 34). In contrast, fasting in mice and the absence of food in patients with total parenteral nutrition inhibit the renewal of the intestinal epithelium and cause mucosal atrophy. Mucosal growth and wound healing in the intestine also require the supply of polyamines (spermidine, spermine, and their precursor putrescine) to the dividing cells in the crypts, whereas polyamine depletion represses epithelial regeneration and delays the repair of damaged mucosa (33).
A novel class of lncRNAs transcribed from genomic ultraconserved regions (T-UCRs) was recently shown to play an important role in the regulation of intestinal mucosal growth and adaptation (46). UCRs are absolutely conserved between orthologous regions of human, rat, and mouse genomes, and given that they do not encode proteins, they have been named “dark matter” of the genome. A total of 481 UCRs were identified and found to be widely distributed among mammalian genomes (8). As reported (44), fasting for 48 h inhibits growth of the small intestinal mucosa in mice, as indicated by decreases in both the proliferating crypt cell population (marked by BrdU) and the lengths of villi and crypts. Genome-wide T-UCR expression profile analysis revealed 21 T-UCRs, including uc.173, differentially expressed in the small intestinal mucosa of fasted mice compared with those observed in nonfasted control animals. The expression levels of 18 T-UCRs, including uc.173, uc.481, uc.356, uc.138A, uc.46A/45A, uc.141, uc.455 (8), uc.475, uc.455 (4), and uc.144, decreased in the intestinal mucosa after a 48-h period of food starvation, while the intestinal mucosal levels of 3 T-UCRs, including uc.457(E), uc.477, and uc.457(u), increased in fasted mice. Although there are no comprehensive studies to date on the exact roles of all these T-UCRs in the regulation of intestinal mucosal growth, these findings suggest that changes in the levels of mucosal T-UCRs may participate in the maintenance of epithelial homeostasis.
uc.173
Highly expressed in the intestinal epithelium of mouse, rat, and human, uc.173 expression levels correlate closely with the proliferative status of IECs. Results obtained from studies conducted in cultured IECs, in ex vivo-prepared organoids, and in animals further revealed that uc.173 is essential for normal epithelial renewal in the small intestine (46). Ectopic uc.173 overexpression increased IEC proliferation and stimulated growth of intestinal organoids. Although systemic administration of locked nucleic acid (LNA) to antagonize uc.173 (anti-uc.173) had no acute or subchronic toxicities in mice, as reported previously (40, 44), decreasing the levels of tissue uc.173 by anti-uc.173 inhibited the growth of small intestinal mucosa in vivo. Interestingly, small intestinal mucosal tissues from patients with Crohn’s disease also exhibited a significant decrease in the levels of uc.173 compared with the levels observed in control patients (without mucosal growth inhibition, injury, or inflammation) (46). The decrease in mucosal uc.173 levels in patients was accompanied by a decrease in the proliferation and disrupted expression of tight junctions (TJs). In support of these findings, ectopic uc.173 overexpression also repressed lead-induced neuronal apoptosis and affected neurite outgrowth and axonal regeneration in the brain (30).
A reduction of uc.173 levels by anti-uc.173-mediated silencing did not alter the rates of colonic mucosal growth, although uc.173 levels are also decreased in the colonic epithelium of anti-uc.173-treated mice. The exact reasons for which uc.173 silencing failed to alter the colonic mucosal growth remain unknown, but basal mucosal turnover rate in the colon was lower than that observed in the small intestine (33). In a separate study, targeted deletion of the RBP HuR in the intestinal epithelium only inhibited mucosal growth of the small intestine without affecting regeneration of the colonic mucosa (24). These observations strongly support the notion that T-UCR uc.173 is a critical regulator of gut epithelium homeostasis, and its induction enhances renewal of the small intestinal mucosa.
uc.173 stimulated growth of the small intestinal mucosa at least partially by downregulating miR-195, a repressor of epithelial homeostasis (9, 47). uc.173 silencing specifically enhanced miR-195 expression in the intestinal epithelium in vitro as well as in vivo, but it did not alter the levels of miR-29b and miR-222 (46). Increasing the levels of endogenous miR-195 by uc.173 silencing inhibited IEC proliferation, and this effect was almost completely rescued by ectopic transfection of a miR-195 antagomir. On the other hand, the increased cell proliferation by overexpressing uc.173 was suppressed by increasing the levels of cellular miR-195 through transfection of a miR-195 precursor. These findings were partly expressed by the fact that miR-195 lowers the expression levels of several growth- and migration-promoting proteins, including cyclin-dependent kinase 4 (CDK4), CDK6, cyclin D1, and Stim1 (9, 47, 55). Along these lines, we recently reported that in cultured IECs, miR-195 represses the translation of another growth-promoting factor, the insulin-like growth factor type 2 receptor, by cooperating with CUG-binding protein 1 (CUGBP1) (53).
Gata6
LncRNA Gata6 was also found to regulate intestinal epithelial renewal by altering the function of ISCs (54). In contrast to the ubiquitous expression of uc.173 in all IECs, lncRNA Gata6 was specifically expressed in ISCs, which are positioned at the bottom of the proliferating crypts. Through transcriptome analysis conducted in sorted Lgr5+ and Lgr5- crypt cells isolated from the intestinal mucosa, Gata6 was expressed with much greater abundance in adult ISCs than in any other cell types. Gata6 knockout inhibited growth of the intestinal mucosa as indicated by shorter crypts and villus shrinkage in mice. The numbers of ISCs and crypts in the intestinal mucosa decreased in Gata6 knockout mice, and organoid formation was reduced in the crypts isolated from Gata6 knockout mice. The Gata6-deficient epithelium also exhibited delayed repair after mucosal injury caused by radiation. Mechanistically, Gata6 interacts directly with two subunits of the NURF remodeling complex and recruits the NURF complex onto the Ehf promoter, thus enhancing in ISCs the production of EHF, a protein needed for expression of LGR4/5 in ISCs, which in turn activate WNT signals (6, 54). In addition, Gata6 is also involved in tumorigenesis in the intestinal mucosa, and its expression levels are closely related to clinical severity and prognosis in patients.
lncRNA IMPLICATED IN INTESTINAL EPITHELIAL BARRIER FUNCTION
The intestinal epithelium establishes a highly selective permeable barrier that supports nutrient absorption and protects the epithelium from intrusion by luminal noxious substances, allergens, and microbial pathogens (3). The effectiveness and integrity of the barrier function depend on specialized structures that consist of TJs and adherens junctions (AJs). The TJ is the most apical of these junctional complexes, and it fences the paracellular space in the intestinal epithelium and prevents even small molecules from leaking between cells (40, 51). The assembly and stability of the TJ complex are essential for barrier function and are involved in strong apical cell-to-cell adhesion. Immediately below the TJs are the cadherin-rich AJs that provide strong cell-to-cell adhesion and play functional roles in forming and regulating the barrier function (31). The AJ E-cadherin integrates cellular signals and is crucial for the control of gut permeability. TJs and AJs are highly dynamic and undergo continuous remodeling, and their constituent proteins turn over at a rapid pace. The disrupted expression of TJs and AJs and subsequent barrier dysfunction occur commonly in various pathologies.
SPRY4-IT1
SPRY4-IT1 was the first lncRNA found to regulate gut permeability by altering TJ expression at the posttranscription level (43). SPRY4-IT1 is a 706-bp lncRNA transcribed from the intronic region of the SPRY4 gene, but SPRY4 mRNA and SPRY4-IT1 are two totally separate transcripts (19). SPRY4-IT1 is highly expressed in the intestinal epithelium and distributed in both the cytoplasm and nucleus in IECs. Expression levels of SPRY4-IT1 were found to decrease in the colonic mucosa obtained from patients diagnosed with leaky gut (43). Decreased SPRY4-IT1 was associated with an inhibition of TJ expression, as shown by a decrease in immunostaining signals for claudin-1, claudin-3, occludin, and JAM-1. In cultured IECs, decreasing the levels of cellular SPRY4-IT1 inhibited expression of these TJ proteins, although it did not alter the levels of TJ zonula occludens-1 (ZO-1) and AJ proteins E-cadherin, α-catenin, and β-catenin. SPRY4-IT1 silencing also caused epithelial barrier dysfunction, as indicated by increased paracellular flux of fluorescein isothiocyanate (FITC)-dextran in an in vitro model. In vivo studies showed that increasing the levels of SPRY4-IT1 in the intestinal mucosa by the recombinant lentiviral SPRY4-IT1 expression vector protected the gut barrier function in mice exposed to septic stress induced by cecal ligation and puncture (CLP). The levels of claudin-1, claudin-3, occludin, and JAM-1 proteins decreased in the small intestinal mucosa after CLP, but this inhibition was either abolished or decreased by elevating mucosal SPRY4-IT1 levels. Further studies showed that SPRY4-IT1 regulated the expression levels of TJ proteins by both stabilizing and promoting the translation of mRNAs encoding TJ factors. Since SPRY4-IT1 is associated with polyribosomes (6), it is likely that SPRY4-IT1 enhanced TJ protein levels posttranscriptionally by increasing the recruitment of the corresponding mRNAs to polyribosomes. In fact, SPRY4-IT1 was enriched in actively translating fractions prepared from polysomal gradients, whereas SPRY4-IT1 silencing resulted in a shift of the TJ mRNAs from actively translating fractions to low-translating fractions of polyribosomes (43).
H19
The lncRNA H19 is a 2.3-kb transcript derived from the evolutionarily conserved imprinted H19/igf2 gene cluster and is also involved in regulating the epithelial barrier function (17, 23). The levels of H19 increase dramatically in extraembryonic tissues, the embryo itself, and most fetal tissues during embryogenesis but decrease remarkably after birth (12). Elevation of H19 abundance inhibits embryonic placental growth and also modulates a network of imprinted genes during fetal development. Targeted deletion of global H19 induces an overgrowth phenotype and increases body weight, whereas transgenic reexpression of the H19 gene prevents the increased growth in mice with ablated H19 (12, 13). H19 levels are commonly elevated in a broad spectrum of conditions, including malignancies and following exposure to hypoxia or estrogens (26, 48). It was recently reported that there are significant increases in the levels of H19 in the inflamed human and murine intestinal mucosa, which results predominantly from an increase in the inflammatory cytokine IL-22 (14). Our study revealed that increasing the levels of H19 inhibited expression of ZO-1 and E-cadherin, as well as disrupted epithelial barrier function in vitro (56). H19 reduced production of ZO-1 and E-cadherin by decreasing stability and translation of the ZO-1 and E-cadherin mRNAs. Since two highly conserved miRNAs, miR-675–5p and miR-675–3p, are embedded in H19 exon 1 (11), we investigated the possibility that the function of H19 might be mediated by miR-675. Interestingly, ectopic overexpression of H19 induced the levels of both miR-675-5p and miR-675-3p, while increasing the levels of either miR-675-3p or miR-675-5p alone lowered production of ZO-1 and E-cadherin and compromised the epithelial barrier. H19 also functions as a molecular sponge to decrease the bioavailability of let-7 (48), but decreasing the levels of cellular let-7 failed to inhibit expression of ZO-1 and E-cadherin in IECs (56), suggesting that the interaction of H19 with let-7 did not influence H19-induced inhibition of ZO-1 and E-cadherin expression or barrier dysfunction.
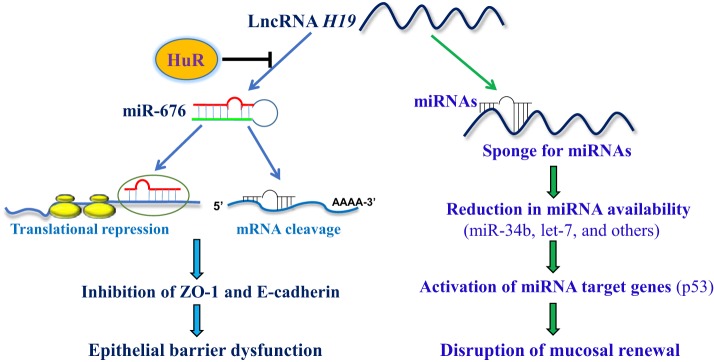
Interestingly, the RBP HuR directly interacts with H19 (12, 17). Ectopically expressed HuR decreased miR-675 levels by inhibiting its processing, rescued the expression of ZO-1 and E-cadherin, and prevented the barrier dysfunction in the epithelium overexpressing H19 (56). In contrast, intestinal epithelial tissue-specific deletion of HuR in mice induced miR-675 production and delayed the recovery of the epithelial barrier function after exposure to mesenteric ischemia-reperfusion. Taken together, the findings from our group and others suggest a model by which H19 associates with HuR to control epithelial barrier function and defense under pathophysiological conditions (Fig. 1). According to this model, elevating H19 levels represses ZO-1 and E-cadherin expression by decreasing the stability and translation of ZO-1 and E-cadherin mRNAs, and thus causes barrier dysfunction through the release of miR-675. On the other hand, HuR suppresses the processing of miR-675 from H19 and enhances the barrier function by increasing the expression of ZO-1 and E-cadherin. Given that H19 also functions as an RNA decoy for miR-34b and let-7, two microRNAs involved in controlling the expression of p53 and other apoptosis-associated proteins (10, 27, 28, 48), it is likely that H19 can also affect epithelium defense by altering the availability of miR-34b and let-7. These exciting findings and proposed model represent a conceptual advance by linking lncRNA H19 and miR-675 with the control of epithelial barrier function, and highlight the importance of the H19/miR-675 axis by HuR for maintaining homeostasis of the intestinal epithelium.
Fig. 1.
Model proposed to explain the role of H19 in intestinal epithelial homeostasis by interaction with HuR and microRNAs (miRNAs). Increased H19 decreases the stability and translation of mRNAs encoding zonula occludens-1 (ZO-1) and E-cadherin via release of miR-675, thus disrupting epithelial barrier function. HuR prevents miR-675 processing from H19, rescues expression of ZO-1 and E-cadherin, and enhances barrier function. H19 also functions as an RNA decoy for miRNAs such as miR-34b and let-7 and regulates mucosal renewal by altering the expression levels of miRNA targets such as the p53 protein.
uc.173
Besides its role in intestinal mucosal renewal (46), uc.173 also regulates intestinal epithelial barrier function by enhancing the production of the TJ protein claudin-1. Decreasing the levels of uc.173 specifically inhibited expression of claudin-1 and weakened epithelial barrier function in vitro (40). Reducing uc.173 abundance in mice by administering systemically the LNA-modified anti-uc.173 increased the vulnerability of the gut barrier to septic stress induced by CLP. Although CLP led to barrier dysfunction in both anti-uc.173-treated mice and controls, gut permeability following CLP in mice treated with anti-uc.173 was much higher than that observed in control mice. CLP stress also decreased the levels of claudin-1 and claudin-3 in the intestinal mucosa, but inhibition of claudin-1 was amplified by decreasing the levels of uc.173 in anti-uc.173-treated mice. Mechanistically, uc.173 enhances translation of claudin-1 by associating with miR-29b rather than directly binding to claudin-1 mRNA. Subsequent studies showed that uc.173 functioned as a decoy RNA for miR-29b and that miR-29b directly bound to claudin-1 mRNA via its 3′-untranslated region (UTR) and repressed claudin-1 translation. Increasing the levels of cellular uc.173 abolished the association of miR-29b with claudin-1 mRNA and rescued claudin-1 expression in cells overexpressing miR-29b, thus restoring epithelial barrier function. Taken together these results support the view that uc.173 promotes epithelial barrier function by antagonizing miR-29b.
MECHANISMS OF lncRNAs IN INTESTINAL EPITHELIUM HOMEOSTASIS
Given that lncRNAs modulate a variety of biological functions, the mechanisms whereby lncRNAs control gene expression programs have been investigated extensively. The myriad functions of lncRNAs have been grouped into four archetypes of molecular mechanisms: signals, decoys, guides, and scaffolds (21, 42), providing a useful framework for predictions and explanations of the physiological outcomes of various lncRNAs. An increasing body of evidence also indicates that interactions of lncRNAs with miRNAs and/or RBPs play an important role in intestinal epithelial homeostasis (41, 43, 45). Through these complex molecular interactions with miRNAs and/or RBPs, lncRNAs enable IECs in the intestinal epithelium to adapt and respond to different stressful environments. Through cooperative and competitive interaction paradigms, lncRNAs and miRNAs/RBPs implement gene-regulatory programs to maintain tissue homeostasis in different conditions.
LncRNA Interactions with miRNAs
Similar to lncRNAs, the expression patterns of many miRNAs are also tissue and cell lineage specific (10). MiRNAs typically function by base pairing with the 3′-UTRs of target mRNAs and inhibiting mRNA stability and/or translation. Several studies conducted in mice and human tissue samples have shown the importance of miRNAs in gut permeability, and detailed information regarding these results has been discussed in depth in previous publications (41, 49). It has been widely recognized that lncRNAs can execute many cellular functions by interacting with miRNAs as inhibitors or RNA decoys to reduce miRNA production and availability.
uc.173 was found to interact with and destabilize primary (pri-)miR-195 without affecting its transcription in the intestinal epithelium (46). Pri-miR-195 shows perfect sequence complementarity with uc.173 at seven nucleotides situated in the central stem region of pri-miR-195. RNA pull-down assays revealed a direct association of uc.173 with pri-miR-195, whereas mature miR-195 was not complementary to uc.173 nor did it pull down uc.173. Ectopically expressed uc.173 enhanced the degradation of pri-miR-195, since the half-life of pri-miR-195 declined in cells overexpressing uc.173. On the other hand, uc.173 overexpression in IECs did not affect the stability of pri-miR-29b or pri-miR-222. It was also reported that downregulation of pri-miRNA processing was dependent on direct RNA-RNA interaction between the lower stem region of the pri-miR-195 and specific sequences located in the T-UCR uc.283+A (25). This interaction of uc.283+A with pri-miR-195 blocked optimal recognition of the region by the RBP DGCR8 and consequently repressed Drosha-mediated pri-miRNA processing to precursor miRNA. The complementary sequence that binds to the uc.173 is present in the central stem region (upstream of the 3′ terminus) of the pri-miR-195, suggesting an essential role for this region in the regulation of pri-miRNA stability.
Several lncRNAs have been also defined as miRNA sponges that reduce the activity of the target miRNAs without affecting their biogenesis (10, 16). In addition, lncRNA-miRNA associations can negatively regulate target lncRNAs, as demonstrated in a zebrafish model (20). uc.173 failed to interact with the mature miR-195, and therefore it did not compete with miR-195 to affect the binding of miR-195 to target mRNAs. These findings support the view that uc.173 inhibits miR-195 expression predominantly by destabilizing the pri-miR-195 transcript.
uc.173 also functioned as a molecular sponge for miR-29b and reduced the availability of miR-29b to targets such as claudin-1 mRNA (40). uc.173 directly bound to miR-29b as measured by using biotinylated miR-29b. Elevation of cellular uc.173 levels prevented the association of miR-29b with claudin-1 mRNA and restored claudin-1 expression in cells overexpressing miR-29b. These findings indicated that uc.173 stimulates claudin-1 expression and enhanced epithelial barrier function by decreasing the levels of the miR-29b/claudin-1 mRNA complex. We also reported that miR-29b potently repressed intestinal mucosal growth and that elevating miR-29b abundance inhibited mucosal renewal and disrupted the integrity of the intestinal epithelium (44). These results strongly suggest that uc.173-mediated inhibition of miR-29b also contributes to the stimulation of intestinal mucosal growth by uc.173. On the other hand, miR-195 inhibited IEC migration over the wounded area by destabilizing Stim1 mRNA, leading to an inhibition of intestinal epithelial repair after acute injury (55). Decreased levels of miR-195 by uc.173 also participated in the uc.173-mediated protection of epithelial barrier function. Therefore, the enhancement in mucosal growth and epithelial barrier function by uc.173 was mediated by antagonizing both miR-29b and miR-195 via distinct mechanisms (Fig. 2). Because the basal levels of uc.173 in the intestinal mucosa are high and its levels change dramatically in response to stress, the interactions between uc.173 and miR-195/29b are crucial for maintaining intestinal epithelium homeostasis.
Fig. 2.
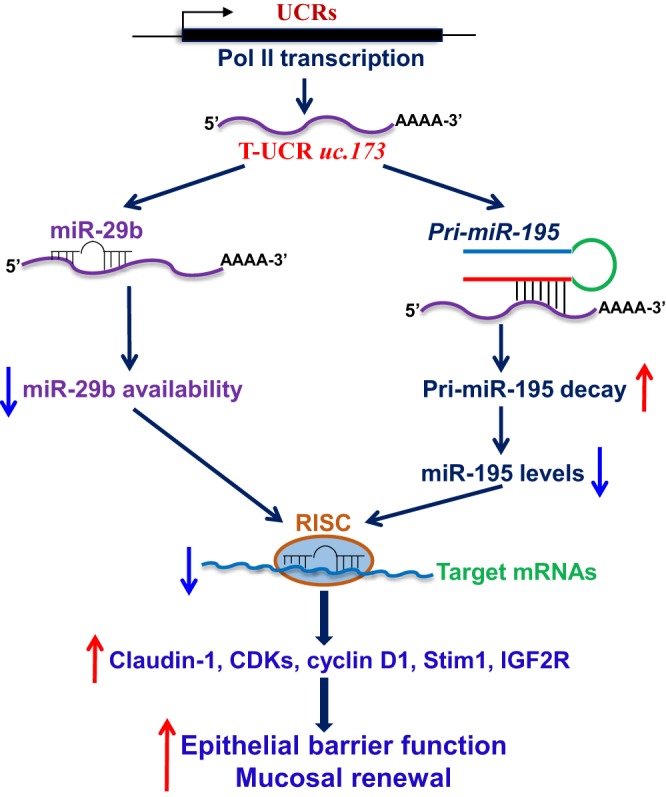
Role of uc.173 in homeostasis of the intestinal epithelium. uc.173 functions as a specific repressor of miR-195 and also acts as an RNA decoy for miR-29b in the intestinal epithelium. Increased levels of uc.173 enhance the epithelial barrier function by reducing the availability of miR-29b, but it promotes renewal of the intestinal mucosa by enhancing the degradation of primary miR-195. See the text for additional definitions.
H19 plays multiple cellular functions by acting on specific genes through different molecular mechanisms, depending on the biological and spatiotemporal context during development and disease states. As pointed out, H19 can act through the processing of miR-675 to inhibit placental growth (18), promote skeletal muscle differentiation and regeneration (11), and downregulate the intestinal epithelial barrier (56). H19 also regulates muscle glucose metabolism by acting as a molecular sponge of the miRNA let-7, thereby inhibiting expression of key metabolic genes such as Insr and Lpl (48). H19 associates with EZH2 (a key component of polycomb repressive complex 2) and represses transcription of select genes, thus increasing bladder cancer metastasis (26) and glioma cell invasion (35). Moreover, expression of the Igf2 gene was affected by targeted deletion of the H19 gene; eight other genes belonging to an Imprinted Gene Network also showed higher expression levels in the absence of H19 (28). In the intestinal epithelium, H19 inhibited expression of ZO-1 and E-cadherin by acting as a regulatable reservoir of miR-675 (56).
LncRNA Interactions with RBPs
RBPs contain structural motifs such as an RNA recognition motif (RRM) and dsRNA binding domain and can bind to target mRNAs via AU-rich or GU-rich elements located in the 3′-UTRs of labile transcripts (1, 45). RBPs regulate gene expression by controlling mRNA splicing, polyadenylation, localization, stabilization, and translation (15). Some RBPs such as AUF1, CUGBP1, and TIAR induce mRNA decay and translational repression through the recruitment of given mRNAs to sites of mRNA degradation such as the exosome, proteasome, or processing bodies, while other RBPs such as HuR stabilize target mRNAs and increase translation (49). HuR is one of the best studied RBPs and contains three RRMs through which it binds to target mRNAs with high affinity and specificity. HuR can function together with ncRNAs, including lncRNAs and miRNAs, and jointly regulates expression of shared target mRNAs (43, 56). For example, HuR binds and recruits lncRNA-p21 to let-7/Ago, leading to the destabilization of lncRNA-p21 (50), and it also competes with ncRNA 7SL to regulate translation of p53 (2). Our study shows that increased HuR blocks H19-induced inhibition of ZO-1 and E-cadherin and enhances epithelial barrier function by preventing miR-675 processing via direct interaction with H19 (56).
The lncRNA SPRY4-IT1 also associated with HuR, and this interaction enhanced the binding of SPRY4-IT1 to the mRNAs encoding TJs (43). HuR was found to interact with the 3′-UTRs of claudin-1, claudin-3, occludin, and JAM-1 mRNAs and enhanced their translation and stability (52). Since both SPRY4-IT1 and HuR regulate TJ expression, we studied whether SPRY4-IT1 affected the interaction of HuR with mRNAs encoding TJ proteins in the intestinal epithelium. Biotinylated SPRY4-IT1 specifically interacted with HuR but not with other RBPs such as CUGBP1, TIAR, or AUF1. SPRY4-IT1 silencing reduced the association of HuR with the TJ mRNAs encoding claudin-1, claudin-3, occludin, and JAM-1 but did not alter whole-cell HuR levels or subcellular distribution. On the other hand, SPRY4-IT1 also directly associated with these TJ mRNAs, since there are multiple predicted SPRY4-IT1 binding sites in the 3′-UTRs of these TJ transcripts. Lowering the levels of cellular HuR only decreased, but did not totally block, the association of SPRY4-IT1 to TJ mRNAs. In sum, SPRY4-IT1 and HuR stimulated TJ expression and promoted epithelial barrier function synergistically.
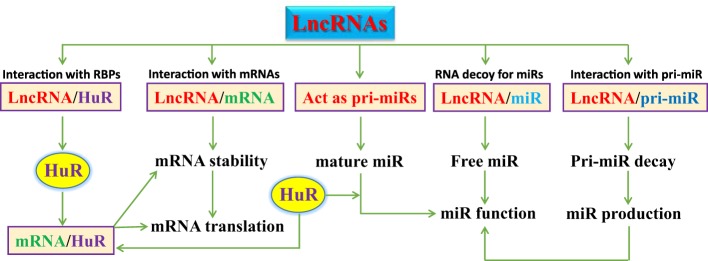
Taken together, lncRNAs can directly interact with RBPs and mRNAs, act as pri-miRNAs, function as natural decoys for miRNAs, and enhance pri-miRNA degradation in the intestinal epithelium (Fig. 3), thus altering expression levels of various genes. These examples illustrate the rich interplay among lncRNAs and miRNAs whereby HuR helps to maintain the homeostasis of the intestinal epithelium.
Fig. 3.
Schematic diagram depicting interactions of long noncoding RNAs (lncRNAs) with RNA-binding proteins (RBPs), mRNAs, and miRNAs. LncRNAs modulate the expression levels of target genes by directly interacting with the RBP HuR, mRNAs, and miRNAs, thereby influencing the protein expression programs that govern intestinal epithelium homeostasis.
CONCLUSIONS AND FUTURE PERSPECTIVES
The identification of lncRNAs in the intestinal mucosa represents a new layer of complexity driving epithelial homeostasis and disease. LncRNAs and their interactions with miRNAs and/or RBPs have a vast spectrum of functions in the physiology of the gut mucosa, although most lncRNAs in the intestinal epithelium have not been verified or investigated fully yet. The results summarized here provide evidence that several lncRNAs expressed in the intestinal epithelium participate in a wide repertoire of biological processes and play an important role in the regulation of mucosal renewal and epithelial barrier function under pathophysiological conditions. Intestinal epithelial homeostasis and integrity are tightly controlled by lncRNAs through posttranscriptional regulation of expression of various genes involved in proliferation, migration, and cell-to-cell interactions. Some lncRNAs, including uc.173, SPRY4-IT1, and Gata6, increase growth of the intestinal mucosa and enhance barrier function, thus promoting epithelium homeostasis. In contrast, lncRNAs such as H19 destabilize and/or inhibit translation of target mRNAs, leading to disruption of epithelial integrity. The RBP HuR interacts with SPRY4-IT1 to enhance barrier function synergistically and inhibits the processing of miR-675 from H19, in turn preventing barrier dysfunction induced by H19 overexpression. Thus homeostasis of the intestinal epithelium is dependent on a dynamic balance between the actions of diverse lncRNAs and their interactions with miRNAs and/or RBPs.
However, there are still many gaps in our understanding of lncRNA functions in the intestinal epithelium. The molecular processes controlling the expression levels of these intestinal epithelial tissue-specific lncRNAs in response to stressful environments remain largely unknown. Current efforts employ state-of-the-art techniques to image lncRNAs at high resolution in vivo and to identify lncRNAs using high-throughput technologies. The development of tissue-specific genetic mouse models will also provide critical information on the functions of specific lncRNAs in the intestinal epithelium in vivo. Future experiments must also define the mechanism by which mutations in lncRNA functional motifs affect their regulatory domains and their ability to interact with miRNAs and/or RBPs and influence intestinal epithelium homeostasis. Finally, studies using human mucosal samples from patients with disrupted epithelial renewal/adaptation and gut barrier dysfunction will be essential to establish the impact of lncRNAs on disease pathogenesis and devise therapeutic venues.
GRANTS
This work was supported by a Merit Review Award from US Department of Veterans Affairs (to J.-Y. Wang) and by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-57819, DK-61972, and DK-68491 (to J.-Y. Wang). J.-Y. Wang is a Senior Research Career Scientist, Biomedical Laboratory Research & Development Service, US Department of Veterans Affairs. M. Gorospe was supported by the National Institute on Aging Intramural Research Program, NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.X. prepared figures; L.X. and J.-Y.W. drafted manuscript; M.G. and J.-Y.W. edited and revised manuscript; M.G. and J.-Y.W. approved final version of manuscript.
REFERENCES
- 1.Abdelmohsen K, Gorospe M. RNA-binding protein nucleolin in disease. RNA Biol 9: 799–808, 2012. doi: 10.4161/rna.19718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelmohsen K, Panda AC, Kang MJ, Guo R, Kim J, Grammatikakis I, Yoon JH, Dudekula DB, Noh JH, Yang X, Martindale JL, Gorospe M. 7SL RNA represses p53 translation by competing with HuR. Nucleic Acids Res 42: 10099–10111, 2014. doi: 10.1093/nar/gku686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad R, Sorrell MF, Batra SK, Dhawan P, Singh AB. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal Immunol 10: 307–317, 2017. doi: 10.1038/mi.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233, 2009. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell 152: 1298–1307, 2013. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 96: 1297–1325, 2016. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 8.Bejerano G, Pheasant M, Makunin I, Stephen S, Kent WJ, Mattick JS, Haussler D. Ultraconserved elements in the human genome. Science 304: 1321–1325, 2004. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya A, Schmitz U, Wolkenhauer O, Schönherr M, Raatz Y, Kunz M. Regulation of cell cycle checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene 32: 3175–3183, 2013. doi: 10.1038/onc.2012.324. [DOI] [PubMed] [Google Scholar]
- 10.Bracken CP, Scott HS, Goodall GJ. A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 17: 719–732, 2016. doi: 10.1038/nrg.2016.134. [DOI] [PubMed] [Google Scholar]
- 11.Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev 28: 491–501, 2014. doi: 10.1101/gad.234419.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabory A, Jammes H, Dandolo L. The H19 locus: role of an imprinted non-coding RNA in growth and development. BioEssays 32: 473–480, 2010. doi: 10.1002/bies.200900170. [DOI] [PubMed] [Google Scholar]
- 13.Gabory A, Ripoche MA, Le Digarcher A, Watrin F, Ziyyat A, Forné T, Jammes H, Ainscough JF, Surani MA, Journot L, Dandolo L. H19 acts as a trans regulator of the imprinted gene network controlling growth in mice. Development 136: 3413–3421, 2009. doi: 10.1242/dev.036061. [DOI] [PubMed] [Google Scholar]
- 14.Geng H, Bu HF, Liu F, Wu L, Pfeifer K, Chou PM, Wang X, Sun J, Lu L, Pandey A, Bartolomei MS, De Plaen IG, Wang P, Yu J, Qian J, Tan XD. In inflamed intestinal tissues and epithelial cells, interleukin 22 signaling increases expression of H19 long noncoding RNA, which promotes mucosal regeneration. Gastroenterology 155: 144–155, 2018. doi: 10.1053/j.gastro.2018.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorospe M, Tominaga K, Wu X, Fähling M, Ivan M. Post-transcriptional control of the hypoxic response by RNA-binding proteins and microRNAs. Front Mol Neurosci 4: 7, 2011. doi: 10.3389/fnmol.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16: 421–433, 2015. doi: 10.1038/nrg3965. [DOI] [PubMed] [Google Scholar]
- 17.Juan V, Crain C, Wilson C. Evidence for evolutionarily conserved secondary structure in the H19 tumor suppressor RNA. Nucleic Acids Res 28: 1221–1227, 2000. doi: 10.1093/nar/28.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G, Reik W. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol 14: 659–665, 2012. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khaitan D, Dinger ME, Mazar J, Crawford J, Smith MA, Mattick JS, Perera RJ. The melanoma-upregulated long noncoding RNA SPRY4-IT1 modulates apoptosis and invasion. Cancer Res 71: 3852–3862, 2011. doi: 10.1158/0008-5472.CAN-10-4460. [DOI] [PubMed] [Google Scholar]
- 20.Kleaveland B, Shi CY, Stefano J, Bartel DP. A network of noncoding regulatory RNAs acts in the mammalian brain. Cell 174: 350–362.e17, 2018. doi: 10.1016/j.cell.2018.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp F, Mendell JT. Functional classification and experimental dissection of long noncoding RNAs. Cell 172: 393–407, 2018. doi: 10.1016/j.cell.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol 8: 1327–1336, 2006. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 23.Leighton PA, Ingram RS, Eggenschwiler J, Efstratiadis A, Tilghman SM. Disruption of imprinting caused by deletion of the H19 gene region in mice. Nature 375: 34–39, 1995. doi: 10.1038/375034a0. [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Christodoulou-Vafeiadou E, Rao JN, Zou T, Xiao L, Chung HK, Yang H, Gorospe M, Kontoyiannis D, Wang JY. RNA-binding protein HuR promotes growth of small intestinal mucosa by activating the Wnt signaling pathway. Mol Biol Cell 25: 3308–3318, 2014. doi: 10.1091/mbc.e14-03-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liz J, Portela A, Soler M, Gómez A, Ling H, Michlewski G, Calin GA, Guil S, Esteller M. Regulation of pri-miRNA processing by a long noncoding RNA transcribed from an ultraconserved region. Mol Cell 55: 138–147, 2014. doi: 10.1016/j.molcel.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Luo M, Li Z, Wang W, Zeng Y, Liu Z, Qiu J. Long non-coding RNA H19 increases bladder cancer metastasis by associating with EZH2 and inhibiting E-cadherin expression. Cancer Lett 333: 213–221, 2013. doi: 10.1016/j.canlet.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159, 2009. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 28.Monnier P, Martinet C, Pontis J, Stancheva I, Ait-Si-Ali S, Dandolo L. H19 lncRNA controls gene expression of the Imprinted Gene Network by recruiting MBD1. Proc Natl Acad Sci USA 110: 20693–20698, 2013. doi: 10.1073/pnas.1310201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris KV, Mattick JS. The rise of regulatory RNA. Nat Rev Genet 15: 423–437, 2014. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nan A, Zhou X, Chen L, Liu M, Zhang N, Zhang L, Luo Y, Liu Z, Dai L, Jiang Y. A transcribed ultraconserved noncoding RNA, Uc.173, is a key molecule for the inhibition of lead-induced neuronal apoptosis. Oncotarget 7: 112–124, 2016. doi: 10.18632/oncotarget.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153, 2014. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 32.Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet 17: 47–62, 2016. doi: 10.1038/nrg.2015.10. [DOI] [PubMed] [Google Scholar]
- 33.Rao JN, Wang JY. Regulation of Gastrointestinal Mucosal Growth (2nd ed.), edited by Granger RN, Granger GJ. Williston, VT: Morgan & Claypool Life Sciences, 2017. [PubMed] [Google Scholar]
- 34.Ray RM, Johnson LR. Regulation of intestinal mucosal growth by amino acids. Amino Acids 46: 565–573, 2014. doi: 10.1007/s00726-013-1565-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y, Wang Y, Luan W, Wang P, Tao T, Zhang J, Qian J, Liu N, You Y. Long non-coding RNA H19 promotes glioma cell invasion by deriving miR-675. PLoS One 9: e86295, 2014. doi: 10.1371/journal.pone.0086295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons BD, Clevers H. Stem cell self-renewal in intestinal crypt. Exp Cell Res 317: 2719–2724, 2011. doi: 10.1016/j.yexcr.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Tsai MC, Spitale RC, Chang HY. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res 71: 3–7, 2011. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 39.Ulitsky I. Evolution to the rescue: using comparative genomics to understand long non-coding RNAs. Nat Rev Genet 17: 601–614, 2016. doi: 10.1038/nrg.2016.85. [DOI] [PubMed] [Google Scholar]
- 40.Wang JY, Cui YH, Xiao L, Chung HK, Zhang Y, Rao JN, Gorospe M, Wang JY. Regulation of intestinal epithelial barrier function by long noncoding RNA uc.173 through interaction with microRNA 29b. Mol Cell Biol 38: e00010–e00018, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang JY, Xiao L, Wang JY. Posttranscriptional regulation of intestinal epithelial integrity by noncoding RNAs. Wiley Interdiscip Rev RNA 8: e1399, 2017. doi: 10.1002/wrna.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell 43: 904–914, 2011. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao L, Rao JN, Cao S, Liu L, Chung HK, Zhang Y, Zhang J, Liu Y, Gorospe M, Wang JY. Long noncoding RNA SPRY4-IT1 regulates intestinal epithelial barrier function by modulating the expression levels of tight junction proteins. Mol Biol Cell 27: 617–626, 2016. doi: 10.1091/mbc.E15-10-0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao L, Rao JN, Zou T, Liu L, Cao S, Martindale JL, Su W, Chung HK, Gorospe M, Wang JY. miR-29b represses intestinal mucosal growth by inhibiting translation of cyclin-dependent kinase 2. Mol Biol Cell 24: 3038–3046, 2013. doi: 10.1091/mbc.e13-05-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao L, Wang JY. RNA-binding proteins and microRNAs in gastrointestinal epithelial homeostasis and diseases. Curr Opin Pharmacol 19: 46–53, 2014. doi: 10.1016/j.coph.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao L, Wu J, Wang JY, Chung HK, Kalakonda S, Rao JN, Gorospe M, Wang JY. Long noncoding RNA uc.173 promotes renewal of the intestinal mucosa by inducing degradation of microRNA 195. Gastroenterology 154: 599–611, 2018. doi: 10.1053/j.gastro.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP, Zhuang SM. MicroRNA-195 suppresses tumorigenicity and regulates G1/S transition of human hepatocellular carcinoma cells. Hepatology 50: 113–121, 2009. doi: 10.1002/hep.22919. [DOI] [PubMed] [Google Scholar]
- 48.Yan L, Zhou J, Gao Y, Ghazal S, Lu L, Bellone S, Yang Y, Liu N, Zhao X, Santin AD, Taylor H, Huang Y. Regulation of tumor cell migration and invasion by the H19/let-7 axis is antagonized by metformin-induced DNA methylation. Oncogene 34: 3076–3084, 2015. doi: 10.1038/onc.2014.236. [DOI] [PubMed] [Google Scholar]
- 49.Yang H, Rao JN, Wang JY. Posttranscriptional regulation of intestinal epithelial tight junction barrier by RNA-binding proteins and microRNAs. Tissue Barriers 2: e28320, 2014. doi: 10.4161/tisb.28320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoon JH, Abdelmohsen K, Srikantan S, Yang X, Martindale JL, De S, Huarte M, Zhan M, Becker KG, Gorospe M. LincRNA-p21 suppresses target mRNA translation. Mol Cell 47: 648–655, 2012. [Erratum in Mol Cell 50: 303, 2013.] doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu TX, Rao JN, Zou T, Liu L, Xiao L, Ouyang M, Cao S, Gorospe M, Wang JY. Competitive binding of CUGBP1 and HuR to occludin mRNA controls its translation and modulates epithelial barrier function. Mol Biol Cell 24: 85–99, 2013. doi: 10.1091/mbc.e12-07-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu TX, Wang PY, Rao JN, Zou T, Liu L, Xiao L, Gorospe M, Wang JY. Chk2-dependent HuR phosphorylation regulates occludin mRNA translation and epithelial barrier function. Nucleic Acids Res 39: 8472–8487, 2011. doi: 10.1093/nar/gkr567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, Zhang Y, Xiao L, Yu TX, Li JZ, Rao JN, Turner DJ, Gorospe M, Wang JY. Cooperative repression of insulin-like growth factor type 2 receptor translation by microRNA 195 and RNA-binding protein CUGBP1. Mol Cell Biol 37: e00225-17, 2017. doi: 10.1128/MCB.00225-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu P, Wu J, Wang Y, Zhu X, Lu T, Liu B, He L, Ye B, Wang S, Meng S, Fan D, Wang J, Yang L, Qin X, Du Y, Li C, He L, Ren W, Wu X, Tian Y, Fan Z. LncGata6 maintains stemness of intestinal stem cells and promotes intestinal tumorigenesis. Nat Cell Biol 20: 1134–1144, 2018. doi: 10.1038/s41556-018-0194-0. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang R, Rao JN, Zou T, Liu L, Xiao L, Cao S, Hansraj NZ, Gorospe M, Wang JY. miR-195 competes with HuR to modulate stim1 mRNA stability and regulate cell migration. Nucleic Acids Res 41: 7905–7919, 2013. doi: 10.1093/nar/gkt565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zou T, Jaladanki SK, Liu L, Xiao L, Chung HK, Wang JY, Xu Y, Gorospe M, Wang JY. H19 long noncoding RNA regulates intestinal epithelial barrier function via microRNA 675 by interacting with RNA-binding protein HuR. Mol Cell Biol 36: 1332–1341, 2016. doi: 10.1128/MCB.01030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]