Abstract
Renal denervation lowers arterial blood pressure (ABP) in multiple clinical trials and some experimental models of hypertension. These antihypertensive effects have been attributed to the removal of renal afferent nerves. The purpose of the present study was to define the function, anatomy, and contribution of mouse renal sensory neurons to a renal nerve-dependent model of hypertension. First, electrical stimulation of mouse renal afferent nerves produced frequency-dependent increases in ABP that were eliminated by ganglionic blockade. Stimulus-triggered averaging revealed renal afferent stimulation significantly increased splanchnic, renal, and lumbar sympathetic nerve activity (SNA). Second, kidney injection of wheat germ agglutinin into male C57Bl6 mice (12–14 wk; Jackson Laboratories) produced ipsilateral labeling in the T11–L2 dorsal root ganglia. Next, 2-kidney 1-clip (2K1C) hypertension was produced in male C57Bl6 mice (12–14 wk; Jackson Laboratories) by placement of a 0.5-mm length of polytetrafluoroethylene tubing around the left renal artery. 2K1C mice displayed an elevated ABP measured via telemetry and a greater fall in mean ABP to ganglionic blockade at day 14 or 21 vs. day 0. Renal afferent discharge was significantly higher in 2K1C-clipped vs. 2K1C-unclipped or sham kidneys. In addition, 2K1C-clipped vs. 2K1C-unclipped or sham kidneys had lower renal mass and higher mRNA levels of several proinflammatory cytokines. Finally, both ipsilateral renal denervation (10% phenol) or selective denervation of renal afferent nerves (periaxonal application of 33 mM capsaicin) at time of clipping resulted in lower ABP of 2K1C mice at day 14 or 21. These findings suggest mouse renal sensory neurons are activated to increase SNA and ABP in 2K1C hypertension.
NEW & NOTEWORTHY This study documents the function, anatomy, and contribution of mouse renal sensory nerves to neurogenic hypertension produced by renal stenosis. Activation of renal afferents increased sympathetic nerve activity and blood pressure. Renal afferent activity was elevated in hypertensive mice, and renal afferent denervation lowered blood pressure. Clinically, patients with renal stenosis have been excluded from clinical trials for renal denervation, but this study highlights the potential therapeutic efficacy to target renal nerves in these patients.
Keywords: autonomic, blood pressure, hypertension, sensory, sympathetic
INTRODUCTION
Renal denervation lowers arterial blood pressure (ABP) in several experimental models of hypertension, including the spontaneously hypertensive rat, deoxycorticosterone-salt model, and 2-kidney-1-clip (2K1C) model (DiBona and Kopp 1997; Osborn and Foss 2017). In addition, the majority of clinical trials report renal denervation lowered ABP in humans (Symplicity HTN-1, Symplicity HTN-2, DENERHTN, and SPYRAL) (Azizi et al. 2015; Krum et al. 2009, 2014; Townsend et al. 2017). The therapeutic effects of renal denervation have been attributed to the removal of sympathetic efferent and/or afferent fibers (Osborn and Banek 2018). First, stimulation of renal efferent nerves increases renin secretion and promotes tubular sodium reabsorption (DiBona and Kopp 1997). Second, renal denervation in humans lowered total peripheral resistance without changes in cardiac output (Ewen et al. 2015). Third, renal denervation lowered the renal resistivity index without changes in glomerular filtration rate or urinary albumin excretion (Mahfoud et al. 2012). On the other hand, stimulation of renal afferent fibers in rats and cats increased ABP (Caverson and Ciriello 1987; Simon et al. 1989; Stella et al. 1984, 1987). Dorsal rhizotomy to surgically remove all types of sensory fibers across multiple levels of the spinal cord and end-organs lowered ABP in some experimental models of hypertension (Campese and Kogosov 1995; Wyss et al. 1986). In addition, renal denervation in humans decreased muscle sympathetic nerve activity (SNA) (Grassi et al. 2015; Hering et al. 2013, 2014), reduced resting plasma glucose (Witkowski et al. 2011), and reduced cardiac arrhythmias (Linz et al. 2018). Recently, periaxonal application of capsaicin to selectively ablate renal sensory fibers reduced ABP in deoxycorticosterone-salt rat (Banek et al. 2016), but not in the Dahl salt-sensitive rat (Foss et al. 2016), angiotensin II-salt rat (Foss et al. 2018), and angiotensin II-infused mouse (Xiao et al. 2015) models of hypertension. Altogether, these observations suggest that the antihypertensive effects of renal denervation may be partly attributed to removal of renal afferent nerves in certain models of hypertension.
Renal afferent nerves densely innervate the pelvic wall and, to a lesser extent, the renal vasculature (DiBona and Kopp 1997; Kopp 2015). Direct neurophysiological recordings have demonstrated renal afferent fibers are classified into mechano-, chemo-, or nociceptive units (Recordati et al. 1978, 1980, 1981) and are responsive to changes in renal perfusion pressure, hypoxia, and renal pelvic pressure as well as a number of local factors (Kopp 2015; Recordati et al. 1978, 1980, 1981). In turn, studies in rats and cats suggest renal afferent fibers project centrally to cell bodies located in the lower thoracic and upper lumbar dorsal root ganglia (DRG) to impact SNA and ABP (Ciriello and Calaresu 1983; Donovan et al. 1983; Kuo et al. 1983).
The majority of data regarding renal sensory nerves originate from studies performed in rats or larger animal models. Although mice currently represent the most common biomedical research model and transgenic lines provide a platform to address unique research questions, no information is currently available of mouse renal sensory nerves regarding the function, anatomical location of cell bodies, and contribution to neurogenic hypertension. Therefore, the purpose of the present study was to lay a foundational framework of mouse renal sensory nerves and then test the contribution of these nerves in an established renal nerve-dependent model of hypertension. The current study utilized an array of approaches including renal afferent nerve stimulation and simultaneous sympathetic nerve recordings, neuronal anatomical tracing, direct renal afferent nerve recording, and total vs. selective afferent renal nerve denervation in a 2K1C model of hypertension. This model has important clinical implications because renal stenosis has been an exclusion criterion for every clinical trial to assess the therapeutic efficacy of renal nerve denervation. Thus there are limited data to directly assess the antihypertensive effect of renal denervation (whether efferent or afferent) in this cohort (Azizi et al. 2015; Krum et al. 2009, 2014; Townsend et al. 2017).
METHODS
All of the experimental procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh School of Medicine. Male C57Bl6J mice (8–12 wk of age, strain 00664; Jackson Laboratories) were housed in a temperature-controlled room (22 ± 1°C), fed 0.1% NaCl chow (D17020; Research Diets), and given access to deionized water.
Experiment 1 investigated whether activation of mouse renal sensory nerves increases SNA and ABP. Mice (n = 6) were anesthetized with isoflurane and instrumented with femoral arterial and venous catheters. Body temperature was maintained at 37.0 ± 0.2°C through a servo-controlled temperature controller and rectal probe (CWE). Through a retroperitoneal incision, the right renal nerve was isolated, placed on bipolar stainless steel electrodes, cut distally, and insulated with KWIK-SIL. After the mouse stabilized for >10 min at 1.8% isoflurane, the renal afferent nerves were stimulated electrically (1-ms pulse, 10-s train, 200 μA) at various frequencies (2, 5, 10, 20, 30, 50 Hz) in a random order separated by 5 min. To test the contribution of the sympathetic nervous system, the stimulation was repeated after administration of the ganglionic blocker chlorisondamine (5 mg/kg iv). The contribution of vasopressin was tested in half of the animals by administration of [β-mercapto-β,β-cycloptenamethyleneproionyl1, O-me-Tyr2,Arg8]-vasopressin (10 μg/kg iv) before chlorisondamine. In a second set of mice anesthetized with isoflurane (n = 4), the left splanchnic, renal, and lumbar sympathetic nerves were isolated, placed on bipolar stainless steel electrodes, and insulated with KWIK-SIL. Signals were filtered (100–1,000 Hz), digitized (2 kHz), rectified, and integrated (10-ms time constant) using a Micro1401 data acquisition unit and Spike2 software (Cambridge Electronic Design). Because of electrical artifacts produced by electrical stimulation of the renal afferent nerves, stimulus-triggered averaging was performed before and after a single electrical pulse was applied to the renal afferent nerve (1-ms duration, 30 sweeps, 100 μA). To evaluate whether the type of anesthesia impacted these cardiovascular responses, renal afferent stimulation was repeated in mice (n = 3) anesthetized with urethane (1.2 mg/kg body wt iv) after induction with isoflurane.
Experiment 2 identified the location of renal sensory neurons. Mice (n = 4) were anesthetized with isoflurane. After a midline laparotomy to visualize the kidneys, a glass micropipette (outer diameter 20–40 µm) containing wheat germ agglutinin (WGA tagged with Alexa Fluor 488 or 594, 10%) was lowered into the kidneys using coordinates relative to the renal hillus: 1.5–2.0 mm lateral and 1.5–1.7 mm ventral to the surface of the kidney. The injection volume was 500 nl per site. Two additional injections per kidney were performed in which the pipette was moved 1.5–2.0 mm toward each pole, using the same depth. These sites were targeted because prior studies in rats indicated the sensory nerves largely innervate the renal pelvis (Marfurt and Echtenkamp 1991). Each kidney within the same mouse received a different WGA-Alexa Fluor tracer. At 5–7 days later, animals were anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde (in 10 mM PBS, pH 7.3). The DRG at T5–L3 and nodose ganglia were harvested, postfixed in 4% paraformaldehyde for 1 h at 4°C, immersed in 30% sucrose overnight, and sectioned at 20 µm using a cryostat. Labeled cells were visualized and quantified using a Leica DM6000b epifluorescence microscope at every other section by two independent researchers. To ensure the labeling was dependent on renal nerve transport vs. leakage into the bloodstream, the renal tracer injections were performed in an additional set of mice (n = 3) after total renal nerve denervation by surgical removal of the renal nerve and 10% phenol application to the renal vessels (see denervation protocol described below).
Experiment 3 examined the time course and contribution of the sympathetic nervous system to 2K1C hypertension. Mice were anesthetized with isoflurane (2–3% in 100% oxygen) and instrumented with PA-C10 telemetry units (Data Sciences) advanced 1.5 cm into the femoral artery with the transmitter body secured subcutaneously in the flank. Mice were treated with buprenorphine (Buprenex; 0.03 mg/kg sc, twice per day for 48 h) and enrofloxacin (2 mg/kg sc), returned to home cages, and given 1 wk to recover before experiments began. Telemetry data were analyzed beat to beat for systolic and diastolic ABP. Mean ABP was calculated by diastolic plus one-third of the pulse pressure. Data were analyzed between 10:00 AM and 4:00 PM and between 10:00 PM and 4:00 AM (lights on 7:00 AM–7:00 PM). After a 7-day recovery and 3-day baseline recording, the 2K1C model of hypertension was produced using methods described previously (Kashyap et al. 2016; Warner et al. 2012). Mice were anesthetized with isoflurane (2–3% in 100% oxygen). After a retroperitoneal incision, the left kidney was gently retracted. A small section at the midpoint of the renal artery was isolated free from the renal vein and/or renal nerves. A 0.5-mm length polytetrafluoroethylene (PTFE) tubing (inner diameter 0.008 in. × outer diameter 0.014 in., item no. SUBL 140; Braintree Scientific) was cut longitudinally, placed around the renal artery, and secured by two 10–0 circumferential sutures. In control mice, the left kidney and renal artery were exposed, but no clip was implanted. The incisions were closed with suture and the animals treated with Buprenex and enrofloxacin. Ganglionic blockade with hexamethonium (30 mg/kg ip) was performed on day 0 (before the renal clip surgery), 14, and 21 relative to 2K1C surgery. On day 22–23, mice were anesthetized with isoflurane (3% in 100% oxygen) and perfused transcardially with saline and 4% paraformaldehyde (in 10 mM PBS, pH 7.3). The kidneys were harvested and weighed. A subset of kidneys was embedded in paraffin, sectioned at 3 µm, and counterstained with hematoxylin-eosin. The remaining kidneys were processed for immunofluorescence visualization of tyrosine hydroxylase and transient receptor potential vanilloid type 1 (TRPV1) as described below (experiment 5).
A second set of 2K1C and sham mice (n = 4 per group) without telemetry units were produced to assess renal cytokine expression using quantitative PCR (qPCR). At 3 wk after surgery, mice were anesthetized with isoflurane (2–3% in 100% oxygen). The kidneys were harvested, the poles were excised, and the middle portion containing the pelvis and cortex was homogenized in TRIzol. RNA was converted to cDNA using a reverse transcription kit (catalog no. K1691; ThermoFisher). Primers were designed using Primar-BLAST (NIH) and purchased from ThermoFisher (see Table 1 for sequences). cDNA, diluted primers, and SYBR Green master mix (catalog no. 1725121; Bio-Rad) were combined in a 96-well plate and analyzed in a CFX Connect real-time PCR detection system. Annealing temperature was set to 60°C for all primers, and melt curves were generated to assess primer specificity. Gene expression was normalized to β-actin and then quantified using the comparative CT method (ΔΔCT).
Table 1.
Primers for quantitative PCR of kidney samples
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| IL-1β | TGCCACCTTTTGACAGTGATG | ATGTGCTGCTGCGAGATTTG |
| IL-2 | CAAGCAGGCCACAGAATTGAAA | GGCACTCAAATGTGTTGTCAGA |
| IL-4 | ACTTGAGAGAGATCATCGGCA | AGCTCCATGAGAACACTAGAGTT |
| IL-5 | AGGCTTCCTGTCCCTACTCAT | CGCCACACTTCTCTTTTTGGC |
| IL-6 | CTTGGGACTGATGCTGGTG | TCCACGATTTCCCAGAGAAC |
| IL-10 | ACTTTAAGGGTTACTTGGGTTGC | TTCAGCTTCTCACCCAGGGA |
| IL-17F | CGTGAAACAGCCATGGTCAAG | GGGACAGAAATGCCCTGGTT |
| IL-23 | ACCAGCGGGACATATGAATCT | AGACCTTGGCGGATCCTTTG |
| TNF-α | TCGTAGCAAACCACCAAGTG | CCTTGAAGAGAACCTGGGAG |
| MCP1 | CCCACTCACCTGCTGCTAC | TTCTTGGGGTCAGCACAGA |
| Collagen 1 | ATCTCCTGGTGCTGATGGAC | ACCTTGTTTGCCAGGTTCAC |
| Fibronectin | CGAGGTGACAGAGACCACAA | CTGGAGTCAAGCCAGACACA |
| TGF-β | GTGGAAATCAACGGGATCAG | GTTGGTATCCAGGGCTCTCC |
| IFN-γ | AGACAATCAGGCCATCAGCAA | TGTGGGTTGTTGACCTCAAACT |
| β-Actin | CAGCTGAGAGGGAAATCGTG | CGTTGCCAATAGTGATGACC |
MCP1, Monocyte chemoattractant protein 1; TGF-β, transforming growth factor.
Experiment 4 determined whether renal afferent nerve activity differed between kidneys of 2K1C or sham mice. 2K1C and sham mice without telemetry units were produced as described above. At 3 wk later, mice were anesthetized with isoflurane and instrumented with femoral catheters as described above. Through a retroperitoneal incision, the left and right renal nerves were isolated, placed on a bipolar stainless steel electrodes, cut proximally, and insulated with KWIK-SIL. Signals were filtered (300–1,000 Hz), digitized (2 kHz), full-wave rectified, and integrated (1-s time constant) using a Micro1401 data acquisition unit and Spike2 software (Cambridge Electronic Design). The raw signal was also analyzed by a window discriminator (FHC) to quantify discharge rate. The window was set immediately above the estimated noise level and confirmed to produce no events postmortem. After mice stabilized at 1.8% isoflurane for 20 min, variables were recorded for 20 min. Animals were then euthanized, noise was confirmed for both rectified/integrated signals and the window discriminator using a 5-min average, and the kidneys were harvested and weighed.
Experiment 5 determined whether total renal nerve denervation or selective afferent renal denervation lowered ABP in 2K1C mice. Mice were implanted with telemetry units as described above. After a 2-wk recovery, the left renal artery was isolated and clipped as described above. Immediately before clipping, total renal nerve denervation was performed unilaterally to the same kidney by stripping the artery and vein of nerves and connective tissue and painting the vessels with 10% phenol (in 90% ethanol). Selective afferent nerve denervation was performed unilaterally to the same kidney by carefully removing connective tissue overlying the renal artery and vein and painting the vessels with capsaicin (33 mM in 5% ethanol-95% saline) for 10 min using a small piece of cotton swab soaked in the capsaicin solution. ABP and heart rate were recorded 3 days before and 21 days after 2K1C surgery. Ganglionic blockade with hexamethonium (30 mg/kg ip) was performed on days 0, 14, and 21 relative to 2K1C surgery. On day 22–23, mice were anesthetized with isoflurane and perfused transcardially with 4% paraformaldehyde. Kidneys were harvested, weighed, and sectioned at 10 µm using a cryostat. Alternate sections were incubated with polyclonal antibodies for tyrosine hydroxylase (1:2,000, catalog no, AB152; EMD Millipore) or TRPV1 (1:500, catalog no. ACC-030; Alomone Laboratories) overnight at 4°C with 1% donkey serum (ThermoScientific) and visualized after with donkey anti-rabbit Alexa Fluor 647 (1:250; Invitrogen; 4°C overnight incubation). Sections were qualitatively assessed for the presence or absence of immunofluorescent signals by an individual blind to the experimental group. A subset of kidneys from experiment 1 were included as a positive control.
All data are presented as means ± SE, plus individual data points when possible. Telemetry, ganglionic blockade, and renal mass were analyzed using two-way ANOVA (group and kidney). When significant F values were obtained, independent or paired t-tests with a layered Bonferroni correction were performed to assess group or time differences. The depressor response to ganglionic blockade was calculated by the peak drop (1 s) vs. baseline (300 s) value of mean ABP. qPCR analysis of renal cytokine expression was compared using two-way ANOVA (group and kidney) with post hoc testing described above. Responses to stimulation of renal afferents were analyzed by comparing baseline (30 s) vs. peak (1 s) segments. SNA responses during spike-triggered average were compared using a baseline (100 ms) vs. peak (10 ms) segment. Data were analyzed by two-way ANOVA (frequency × drug-chlorisondamine-Manning compound). Renal afferent discharge, rectified and integrated (1-s time constant) voltage (signal − noise), and ABP were averaged over a 20-min period and analyzed using a two-way ANOVA (group and kidney).
RESULTS
Experiment 1: Renal afferent stimulation in mice produces a sympathetically mediated increase in ABP.
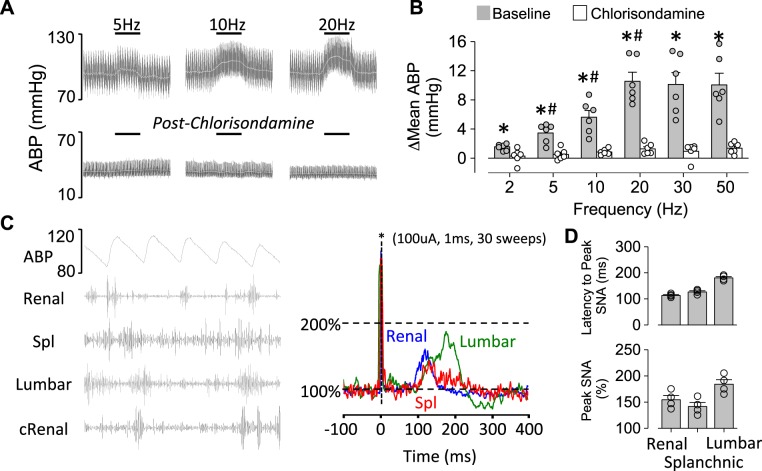
Baseline mean ABP and heart rate of mice anesthetized with isoflurane and prepared for recordings were 88 ± 4 mmHg and 603 ± 20 beats/min, respectively. Electrical stimulation of the right renal afferent nerve produced frequency-dependent increases in ABP (Fig. 1, A and B). Heart rate decreased (2 Hz: −1 ± 1, 5 Hz: −3 ± 1, 10 Hz: −18 ± 4, 20 Hz: −19 ± 5, 30 Hz: −17 ± 4, and 50 Hz: −24 ± 5 beats/min; n = 6; P < 0.05 baseline vs. 10, 20, 30, and 50 Hz). Administration of chlorisondamine decreased baseline ABP from 86 ± 3 to 41 ± 4 mmHg (P < 0.01) and largely eliminated these responses (Fig. 1, A and B). On the other hand, blockade of vasopressin receptors did not significantly alter the pressor response (2 Hz: 1 ± 1, 5 Hz: 3 ± 1, 10 Hz: 5 ± 1, 20 Hz: 9 ± 1, 30 Hz: 10 ± 2, and 50 Hz: 10 ± 2 mmHg; n = 3; P > 0.7 from overall ANOVA).
Fig. 1.
A: raw traces of arterial blood pressure (ABP) and mean ABP (white) during electrical stimulation of the right renal sensory nerve (1-ms pulse, 200 μA) at 5, 10, and 20 Hz before and after the ganglionic blocker chlorisondamine. B: means ± SE and individual data points of the peak change (Δ) in mean ABP. *P < 0.01 vs. chlorisondamine. #P < 0.05 vs. smaller frequency. C, left: simultaneous recording of ABP and renal, splanchnic (Spl), lumbar, and contralateral renal (cRenal) sympathetic nerve activity (SNA) in a control mouse. Note pulse-synchronous activity in all 4 sympathetic nerves. Right: example of stimulus-triggered averaging during electrical stimulation of the contralateral renal afferent nerve (1 ms, 1 Hz, 100 μA, 30 sweeps) revealed an increase in renal, Spl, and lumbar SNA between 100 and 200 ms. D: means ± SE and individual data points of latency to peak (ms) and peak SNA (%) during stimulus-triggered averaging. Baseline nerve activity was normalized to 100%.
In a subset of mice (n = 4), we performed simultaneous recordings of splanchnic, renal, and lumbar SNA and applied single pulses to the contralateral renal nerve to determine whether activation of renal afferent nerves increases SNA. Figure 1C illustrates the simultaneous SNA recordings and a representative example of the stimulus-triggered averaging of SNA. Stimulation of renal afferents significantly increased contralateral renal, splanchnic, and lumbar SNA with a latency to peak of ~100–200 ms (Fig. 1D).
A final set of experiments repeated these stimulations in mice anesthetized with urethane. Renal afferent stimulation produced frequency-dependent pressor responses (2 Hz: −1 ± 1, 5 Hz: 3 ± 1, 10 Hz: 7 ± 1, 20 Hz: 11 ± 2, 30 Hz: 12 ± 3, and 50 Hz: 12 ± 3 mmHg; n = 3), which were also blocked by chlorisondamine (2 Hz: 0 ± 0, 5 Hz: 0 ± 0, 10 Hz: 1 ± 1, 20 Hz: 0 ± 1, 30 Hz: 1 ± 1, and 50 Hz: 2 ± 1 mmHg; n = 3). Baseline mean ABP and heart rate in urethane-anesthetized mice were 79 ± 4 mmHg and 658 ± 16 beats/min, respectively.
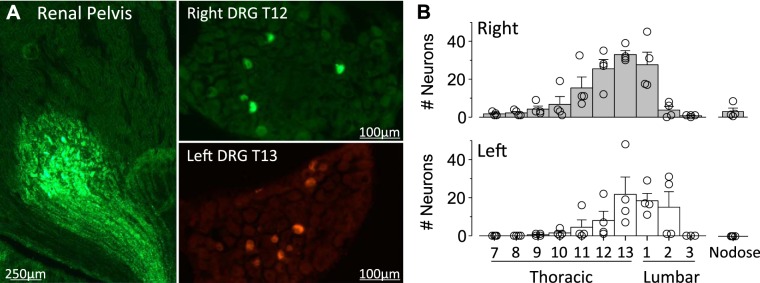
Experiment 2: Location of renal sensory neurons in the dorsal root ganglia.
To anatomically map the location of renal sensory neurons in the DRG, mice received an injection of WGA-Alexa Fluor 488 or 594 into the right and left kidney, respectively. Figure 2A illustrates a representative single injection located on the pelvic wall with no tracer deposited in the cortex. Each injection site produced WGA-positive nerve fibers projecting along the pelvic wall. Figure 2A also illustrates examples of labeled cells in the right and left DRG. Figure 2B illustrates the greatest number of labeled cells were located at T11–L1 and T12–L2 for the right and left kidney, respectively. No cells were observed at T5 or T6 levels (data not shown), and few cells were labeled in the contralateral DRG (0.7 ± 0.2 cells per DRG at T11–L2). In addition, the nodose ganglia did not contain labeled cells in three of four mice (Fig. 2B). The only mouse that produced labeling in the nodose ganglia resulted in a few cells on the right side only. Finally, total renal nerve denervation with 10% phenol eliminated the number of labeled cells in the DRG at any level (0 ± 0 cells per DRG at T11–L2, n = 3).
Fig. 2.
A: digital images of a single kidney injection site and labeled dorsal root ganglion (DRG) neurons. B: means ± SE and individual data points of the number of labeled DRG neurons per spinal level after injection of wheat germ agglutinin-Alexa Fluor 488 or 594 into the right and left kidney, respectively. Total renal nerve denervation eliminated the number of labeled cells in the DRG at any level (0 ± 0 cells per DRG at T11–L2, n = 3).
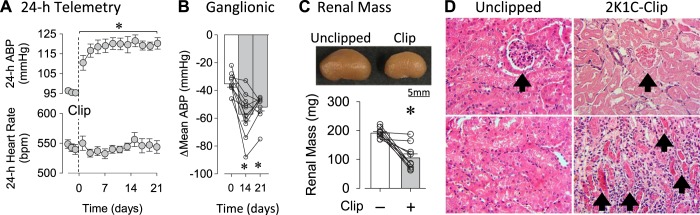
Experiment 3: 2K1C hypertension model in mice.
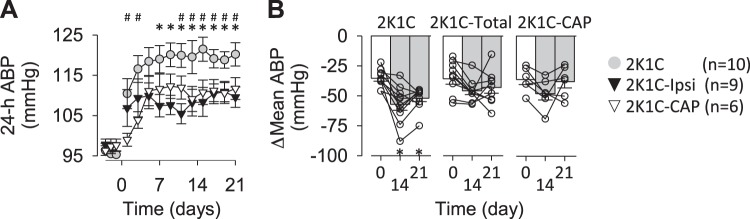
An initial set of experiments established the 2K1C mouse model of neurogenic hypertension. Unilateral placement of a PTFE clip to model renal stenosis significantly increased mean ABP without changes in heart rate (Fig. 3A). These differences were present at day and nighttime periods (data not shown). Ganglionic blockade with hexamethonium produced significantly greater falls in mean ABP at days 14 and 21 vs. day 0 (Fig. 3B). Renal mass of 2K1C mice was significantly less in clipped vs. unclipped kidneys (Fig. 3C). Qualitative analysis of renal histology indicated the presence of damaged glomeruli and renal casts in 2K1C-clipped vs. 2K1C-unclipped or sham mice. qPCR analysis of renal samples revealed significant increases in mRNA for various cytokines in 2K1C-clipped vs. 2K1C-unclipped or sham kidneys (Table 2).
Fig. 3.
A: means ± SE of 24-h mean arterial blood pressure (ABP) and heart rate before and after unilateral clipping of the left renal artery. *P < 0.05 vs. average baseline (1-way ANOVA and paired t-tests with layered Bonferroni correction). B: means ± SE and individual data points of changes (Δ) in mean ABP in response to ganglionic blocker hexamethonium (30 mg/kg ip). *P < 0.05 vs. day 0 (paired t-test). C: images of 2-kidney, 1-clip (2K1C) unclipped vs. clipped kidneys illustrate reduction in renal mass after unilateral clipping. Graph shows means ± SE and individual data points of renal mass in unclipped and clipped kidneys in 2K1C mice. *P < 0.05 vs unclipped. D: renal histology revealed the presence of damaged glomeruli (arrow, top) and renal casts (arrows, bottom) in clipped but not unclipped kidneys of 2K1C mice.
Table 2.
Quantitative PCR analysis of kidneys from 2K1C and sham mice
| Sham |
2K1C |
|||
|---|---|---|---|---|
| Cytokine | Unclipped-Left | Unclipped-Right | Unclipped-Left | Clipped-Right |
| IL-1β | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.4 ± 0.3 | 7.3 ± 2.3*† |
| IL-2 | 1.0 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.5 | 3.7 ± 0.7*† |
| IL-5 | 1.0 ± 0.3 | 0.8 ± 0.3 | 1.1 ± 0.3 | 0.9 ± 0.1 |
| IL-6 | 1.0 ± 0.3 | 0.4 ± 0.1 | 0.6 ± 0.1 | 2.9 ± 0.7*† |
| IL-10 | 1.0 ± 0.3 | 0.4 ± 0.1 | 0.8 ± 0.1 | 3.3 ± 0.8*† |
| IL-17F | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.3 ± 0.3 | 0.8 ± 0.3 |
| IL-23 | 1.0 ± 0.1 | 1.0 ± 0.1 | 2.3 ± 0.6 | 1.2 ± 0.1 |
| TNF-α | 1.0 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 3.4 ± 1.0*† |
| MCP1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 7.9 ± 2.0*† |
| Collagen 1 | 1.0 ± 0.1 | 1.1 ± 0.2 | 1.0 ± 0.1 | 5.3 ± 1.3*† |
| Fibronectin | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 5.4 ± 1.3*† |
| TGF-β | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 2.3 ± 0.3*† |
| IFN-γ | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.4 ± 0.2 | 3.6 ± 0.6*† |
Values were normalized to β-actin and expressed as mean ± SE 2ΔΔCT in sham (n = 4) and 2-kidney,1-clip (2K1C; n = 4) mice. Statistical analysis was performed using 2-way ANOVA (group vs. kidney). MCP1, Monocyte chemoattractant protein 1; TGF-β, transforming growth factor.
P < 0.05, unclipped vs. clipped kidney in same animal.
P < 0.05, right unclipped sham vs. 2K1C-clipped kidney.
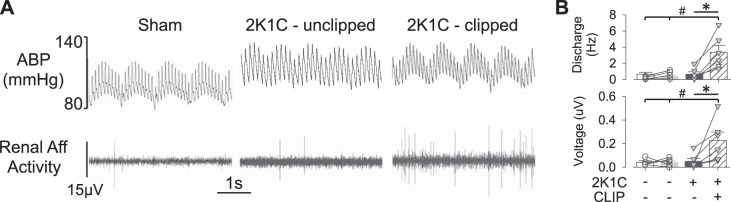
Experiment 4: Renal afferent nerve activity is elevated in 2K1C mice.
Renal afferent nerve recordings were performed to assess differences in baseline renal sensory nerve activity between 2K1C and sham mice. Baseline mean ABP was significantly elevated in this group of anesthetized 2K1C vs. sham mice (97 ± 3 vs. 86 ± 2 mmHg, respectively; P < 0.05, t-test). A qualitative assessment of the renal afferent recording suggested the presence of single units as indicated by the shape and duration of the voltage events. Quantitative analysis revealed that both renal afferent discharge and rectified/integrated voltage were significantly elevated in clipped kidneys of 2K1C mice vs. unclipped kidneys of 2K1C or sham mice (Fig. 4B). In this cohort, renal mass was significantly lower in 2K1C-clipped vs. 2K1C-unclipped or sham kidneys (2K1C-clipped: 68 ± 10 vs. 2K1C-unclipped: 181 ± 12 or sham: 182 ± 8 or 171 ± 7 mg; P < 0.05, t-tests). A linear regression revealed a significant inverse relationship between renal afferent discharge (r = 0.696, P < 0.001) or voltage (r = 0.688, P < 0.001) vs. renal mass (plots not shown).
Fig. 4.
A: raw traces of arterial blood pressure (ABP) and renal afferent (Aff) nerve activity of a sham, 2-kidney, 1-clip (2K1C)-unclipped, and 2K1C-clipped kidney. B: means ± SE and individual data points of renal afferent discharge and full-wave rectified/integrated renal afferent voltage. *P < 0.05, 2K1C-clipped vs. 2K1C-unclipped; #P < 0.05, 2K1C-clipped vs. sham (unpaired 2-tailed t-test).
Experiment 5: Total or selective renal sensory nerve denervation lowers ABP in 2K1C mice.
To determine the contribution of renal efferent vs. renal afferent nerves to 2K1C hypertension, ABP telemetry recordings were performed in 2K1C mice with ipsilateral renal denervation or selective ipsilateral renal afferent denervation. Implantation of a renal clip significantly increased mean ABP from baseline levels in sham, ipsilateral renal denervation, and selective renal afferent denervation groups throughout the experimental protocol (P < 0.05 from overall ANOVAs). However, mean ABP was significantly lower in 2K1C-ipsilateral renal denervation vs. 2K1C mice at days 7–21 (Fig. 5). In addition, mean ABP was also significantly lower in 2K1C-capsaicin vs. 2K1C mice at days 1–3 and days 11–21 (Fig. 5). No significant differences were observed in mean ABP between 2K1C-ipsilateral denervation vs. 2K1C-capsaicin. Ganglionic blockade with hexamethonium did not produce a statistically greater depressor response between days 0, 14, or 21 in 2K1C-ipsilateral renal denervation (P = 0.0967 from overall 1-way ANOVA) or 2K1C-capsaicin (P = 0.06488 from overall 1-way ANOVA) groups (Fig. 5B). Renal mass was significantly lower in clipped vs. unclipped kidneys of 2K1C (105 ± 14 vs. 191 ± 5 g, respectively; P < 0.01), 2K1C-ipsilateral denervation (136 ± 16 vs. 188 ± 6 g, respectively, P < 0.01), and 2K1C-capsaicin (125 ± 11 vs. 184 ± 9 g, respectively; P < 0.01).
Fig. 5.
A: means ± SE of 24-h mean arterial blood pressure (ABP) before and after unilateral clipping of the left renal artery of mice receiving ipsilateral renal denervation or selective afferent renal denervation. Control mice are replotted from Fig. 1. *P < 0.05, sham vs. ipsilateral (2K1C-ipsi) renal denervation; #P < 0.05, control vs. capsaicin (2K1C-CAP; 2-way ANOVA followed by t-test with layered Bonferroni correction). B: means ± SE and individual data points of changes (Δ) in mean ABP in response to the ganglionic blocker hexamethonium (30 mg/kg ip). *P < 0.05 vs. day 0 (paired t-test). Ganglionic blockade with hexamethonium did not produce a greater depressor response between days 0, 14, or 21 of ipsilateral renal denervation (P = 0.0967 from overall 1-way ANOVA) or capsaicin-treated groups (P = 0.06488 from overall 1-way ANOVA). Confirmation of the denervation using immunofluorescence is presented in Fig. 6.
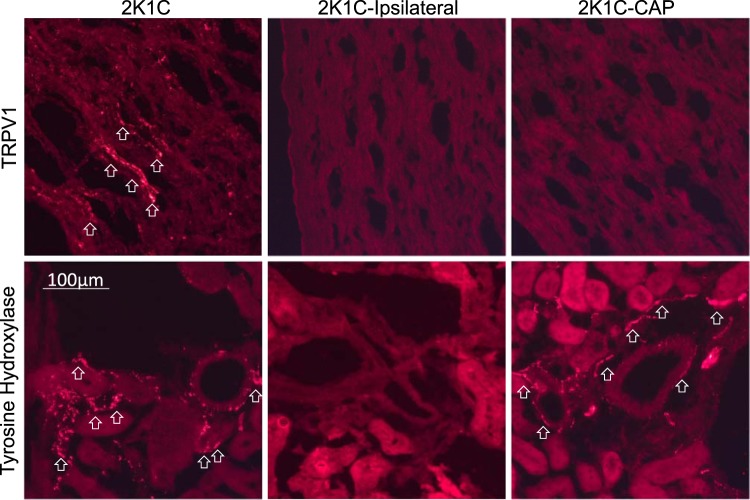
Immunofluorescence analysis confirmed the presence of TRPV1- and tyrosine hydroxylase-positive fibers in the kidney of 2K1C mice (Fig. 6). However, a qualitative analysis revealed that both markers were absent in mice with ipsilateral renal denervation. Tyrosine hydroxylase, but not TRPV1-positive fibers were present in mice with selective renal afferent denervation. It is noteworthy that three mice in the renal afferent denervation group did display TRPV1 immunofluorescence in the pelvic wall. Consequently, these mice were excluded from the blood pressure data presented in Fig. 5 but had mean ABP values on days 14 and 21 of 123 ± 5 and 125 ± 4 mmHg, respectively.
Fig. 6.
Digital images of immunofluorescence labeling for transient receptor potential vanilloid type 1 (TRPV1) and tyrosine hydroxylase in clipped kidneys of control, ipsilaterally denervated, and capsaicin (CAP)-treated (renal afferent denervated) mice. Note the presence of TRPV1- vs. tyrosine hydroxylase-positive fibers in the kidney of 2-kidney, 1-clip (2K1C) sham mice that were predominantly localized to the pelvis vs. cortex and medulla, respectively. However, both markers were absent in mice with ipsilateral renal denervation. Tyrosine hydroxylase-positive, but not TRPV1-positive, fibers were present in mice with selective renal afferent denervation. Arrows indicate positive-labeled terminals. Scale bar, 100 µm.
DISCUSSION
Recent clinical trials and basic research studies underscore the importance of renal afferent nerves in cardiovascular regulation and hypertension (Azizi et al. 2015; DiBona and Kopp 1997; Krum et al. 2009, 2014; Osborn and Foss 2017; Townsend et al. 2017). Although mice represent the most common biomedical research model currently employed, no information is currently available of mouse renal sensory nerves with respect to the function, anatomical location, and contribution to hypertension. The purpose of this study was to lay the foundational framework and test the contribution of mouse renal sensory nerves to a renal nerve-dependent model of hypertension. For the first time, we have documented that stimulation of these afferent nerves produces a frequency-dependent and sympathetically mediated increase in ABP. Simultaneous recordings of multiple sympathetic nerves revealed, for the first time, that activation of mouse renal sensory nerves increases splanchnic, lumbar, and renal SNA. Furthermore, the anatomical location of mouse renal sensory cell bodies are distributed in T11–L2 DRG. Next, paired renal afferent nerve recordings provide the first evidence that mouse renal sensory nerve activity is elevated in the 2K1C model of hypertension. Finally, both ipsilateral renal denervation and selective afferent denervation lowered ABP in 2K1C mice. Altogether, these findings provide multiple novel observations in the mouse to suggest that renal afferent nerves become hyperactive to elevate SNA and ABP in 2K1C hypertension.
The majority, if not all, of previous studies to understand the function of renal sensory nerves have been performed in larger animal models (i.e., cats, dogs, and rats). These studies have largely employed supraphysiological stimulation parameters (>30 Hz) and reduced preparations (i.e., barodenervated and vagotomized) to report that renal afferent nerve stimulation increases ABP and may stimulate vasopressin secretion (Caverson and Ciriello 1987, 1988; Simon et al. 1989). To date, no such data existed in the mouse. The current findings indicate renal afferent stimulation produces frequency-dependent and sympathetically mediated increases in ABP. First, stimulation frequencies of 5 or 10 Hz increased mean ABP. Although these frequencies are equivalent to the discharge rate of renal afferent nerves in the 2K1C mouse reported herein, single units were not identified, and the electrical stimulation paradigms may not reflect the patterning of renal afferent discharge. Second, blockade of ganglionic neurotransmission, but not vasopressin receptors, eliminated this pressor response. Next, to determine the extent by which renal afferent activation altered SNA, simultaneous recording of multiple sympathetic nerves revealed an increase in splanchnic, contralateral renal, and lumbar SNA. The latency (~100–200ms) suggests the involvement of supraspinal pathways and likely various neuronal populations within the hypothalamus and hindbrain (Ammons 1992; Caverson and Ciriello 1988; Xu et al. 2015). The relative contribution of sympathetic efferent nerves innervating the muscle, contralateral kidney, or mesentery to the chronic 2K1C hypertension remains unclear. However, renal denervation of the contralateral or unclipped kidney in 2K1C rats did not affect ABP (Katholi et al. 1982a). Altogether, these data provide multiple novel observations in the mouse that renal afferent neurons can elevate ABP through splanchnic, renal, and lumbar SNA.
Renal afferent fibers contain different fiber types, including chemo- vs. mechanosensitive or myelinated vs. unmyelinated (Kopp 2015; Recordati et al. 1978, 1980, 1981; Simon and Schramm 1984) that project to the lower thoracic and upper lumbar spinal segments (Ciriello and Calaresu 1983; Donovan et al. 1983; Kuo et al. 1983). Such data are solely based on studies performed in rats or larger animal models. The present study documents, for the first time, that injection of neuroanatomical tracers into the mouse renal pelvis labels renal sensory cell bodies primarily at the T11–L2 DRG. The renal pelvis was targeted because previous studies suggested sensory fibers primarily innervate this region of the kidney (Kopp 2015; Marfurt and Echtenkamp 1991). However, we fully acknowledge that our injections may have missed sensory fibers innervating the renal medulla or cortex and therefore underestimate the number of renal neurons. These findings also highlight that the parasympathetic nervous system does not innervate the mouse kidney. Despite the anatomical location of renal sensory neurons, little is known regarding the neurochemical phenotype and how the neurochemistry corresponds to the properties or response profiles of renal sensory fibers. For example, high concentrations of the TRPV1 agonist capsaicin are used to ablate renal sensory afferents, which assumes the majority, if not all, of renal sensory neurons express TRPV1. However, the relative expression of TRPV1 (or other common sensory phenotypes) in renal sensory neurons has not been defined for any species. Freisinger et al. (2013) reported renal sensory neurons may be distinguished by a tonic vs. phasic firing activity and the presence vs. absence of Nav1.8 or 1.9 expression. How different neurochemically distinct populations subserve different response profiles or sensory functions to impact cardiovascular function remains unexplored.
A major finding of the present study was renal afferent nerve activity of 2K1C-clipped kidneys was elevated, and denervation of these renal sensory fibers lowered ABP in 2K1C hypertensive mice. These observations extend the original reports that total renal denervation or dorsal rhizotomy lowered ABP in 2K1C or 1-kidney-1-clip rats (Katholi et al. 1982a, 1982b; Wyss et al. 1986). However, our findings do disagree with a prior study that reported no differences in baseline renal afferent nerve activity between sham and 2K1C rats (Kopp and Buckley-Bleiler 1989). Acute insertion of the clip will cause renal ischemia or a reduction in renal perfusion pressure to activate renal sensory nerves, but the mechanism(s) that chronically drive renal afferent activity remain unclear. Removal of the clip at 6 wk after insertion normalized ABP of 2K1C rats within days, thereby suggesting the renal stenosis provides a chronic stimulus (Katholi et al. 1982a). Renal afferent nerves are mechanosensitive (Kopp 2015; Niijima 1975; Recordati et al. 1978), and structural changes reflected by the reduced renal mass and renal damage may alter the mechanosensitivity and contribute to the disease. Kopp and Buckley-Bleiler (1989) reported 2K1C hypertensive rats displayed attenuated reno-renal reflexes in both kidneys rather than the stenosed kidney only. Renal afferents are also chemosensitive (Kopp 2015; Recordati et al. 1978, 1980, 1981), and the elevated activity in 2K1C-clipped kidneys may reflect changes in the local chemical environment. In this regard, qPCR indicated increased expression of numerous cytokines in the clipped but not unclipped kidneys of 2K1C mice. A subset of these cytokines is also increased in the kidneys of deoxycorticosterone-salt hypertension (Banek et al. 2016). Cytokines can activate sensory nerves originating in other end-organs (Cook et al. 2018; Dong and Dong 2018). Whether renal sensory nerves respond to local cytokines or how the renal sensory innervation of the kidney changes in such disease states remains unknown.
Unilateral renal denervation or selective sensory denervation of the clipped kidney lowered ABP in 2K1C mice. We qualitatively assessed the degree of denervation by the presence or absence of tyrosine hydroxylase or TRPV1 immunoreactivity. Although prior studies have directly assayed renal norepinephrine or CGRP content (Banek et al. 2016; Foss et al. 2015, 2016, 2018), there is not a clearly defined standard or tissue level for CGRP levels (as for norepinephrine) regarding quantification of a denervated vs. partially denervated kidney. In the present study, we were able to discriminate between these two groups by the presence or absence of TRPV1-positive fibers. Indeed, a small cohort of mice (n = 3) had residual TRPV1 immunofluorescence and displayed mean ABP values similar to 2K1C animals. Unilateral denervation was performed in the present study at the time of renal artery clipping rather than after hypertension was established. However, prior studies in rats reported that complete unilateral denervation of the clipped kidney but not the contralateral kidney at 6 wk postclipping lowered ABP in this model (Katholi et al. 1982a, 1982b). Clearly, future studies will need to evaluate the impact of selective afferent denervation after hypertension has been established. Although renal denervation lowered ABP of 2K1C mice in the present study, ABP remained elevated above baseline values (see Fig. 5), thereby suggesting additional factors contribute to the hypertension in this model. Such factors likely include renin-angiotensin-aldosterone system activation, renal dysfunction, and vascular dysfunction.
Perspectives.
This study provides further support that renal afferent nerves are hyperactive and increase SNA in certain forms of hypertension (Banek et al. 2016; Osborn and Banek 2018). The observation that renal denervation lowers ABP in 2K1C and deoxycorticosterone-salt models (Banek et al. 2016) but not the Dahl salt-sensitive rat (Foss et al. 2016), angiotensin II-salt rat (Foss et al. 2018), and angiotensin II-infused mouse (Xiao et al. 2015) indicates the therapeutic efficacy of renal denervation in hypertension may depend on the etiology or factors contributing the disease. The current findings have important clinical implications because clinical trials to evaluate the therapeutic efficacy of renal denervation exclude patients with renal stenosis. Thus this study highlights another patient population in which renal denervation may reduce ABP.
GRANTS
This study was supported by National Institutes of Health Grants HL113270 and HL128388 (to S. D. Stocker).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.O., B.J.K., A.F.S., M.D.C., and S.D.S. conceived and designed research; J.O., B.J.K., B.M.R., and S.D.S. performed experiments; J.O., B.J.K., B.M.R., and S.D.S. analyzed data; J.O., A.F.S., R.J.T., and S.D.S. interpreted results of experiments; J.O. and S.D.S. prepared figures; J.O. and S.D.S. drafted manuscript; J.O., B.J.K., A.F.S., B.M.R., R.J.T., M.D.C., and S.D.S. edited and revised manuscript; J.O., B.J.K., A.F.S., B.M.R., R.J.T., M.D.C., and S.D.S. approved final version of manuscript.
REFERENCES
- Ammons WS. Bowditch Lecture. Renal afferent inputs to ascending spinal pathways. Am J Physiol Regul Integr Comp Physiol 262: R165–R176, 1992. doi: 10.1152/ajpregu.1992.262.2.R165. [DOI] [PubMed] [Google Scholar]
- Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, Midulla M, Mounier-Véhier C, Courand PY, Lantelme P, Denolle T, Dourmap-Collas C, Trillaud H, Pereira H, Plouin PF, Chatellier G; Renal Denervation for Hypertension (DENERHTN) investigators . Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 385: 1957–1965, 2015. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68: 1415–1423, 2016. doi: 10.1161/HYPERTENSIONAHA.116.07850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 25: 878–882, 1995. doi: 10.1161/01.HYP.25.4.878. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J. Effect of stimulation of afferent renal nerves on plasma levels of vasopressin. Am J Physiol Regul Integr Comp Physiol 252: R801–R807, 1987. doi: 10.1152/ajpregu.1987.252.4.R801. [DOI] [PubMed] [Google Scholar]
- Caverson MM, Ciriello J. Contribution of paraventricular nucleus to afferent renal nerve pressor response. Am J Physiol Regul Integr Comp Physiol 254: R531–R543, 1988. doi: 10.1152/ajpregu.1988.254.3.R531. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Calaresu FR. Central projections of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst 8: 273–285, 1983. doi: 10.1016/0165-1838(83)90110-8. [DOI] [PubMed] [Google Scholar]
- Cook AD, Christensen AD, Tewari D, McMahon SB, Hamilton JA. Immune cytokines and their receptors in inflammatory pain. Trends Immunol 39: 240–255, 2018. doi: 10.1016/j.it.2017.12.003. [DOI] [PubMed] [Google Scholar]
- DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- Dong X, Dong X. Peripheral and central mechanisms of itch. Neuron 98: 482–494, 2018. doi: 10.1016/j.neuron.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan MK, Wyss JM, Winternitz SR. Localization of renal sensory neurons using the fluorescent dye technique. Brain Res 259: 119–122, 1983. doi: 10.1016/0006-8993(83)91072-7. [DOI] [PubMed] [Google Scholar]
- Ewen S, Cremers B, Meyer MR, Donazzan L, Kindermann I, Ukena C, Helfer AG, Maurer HH, Laufs U, Grassi G, Böhm M, Mahfoud F. Blood pressure changes after catheter-based renal denervation are related to reductions in total peripheral resistance. J Hypertens 33: 2519–2525, 2015. doi: 10.1097/HJH.0000000000000752. [DOI] [PubMed] [Google Scholar]
- Foss JD, Fiege J, Shimizu Y, Collister JP, Mayerhofer T, Wood L, Osborn JW. Role of afferent and efferent renal nerves in the development of AngII-salt hypertension in rats. Physiol Rep 6: e13602, 2018. doi: 10.14814/phy2.13602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol 310: R262–R267, 2016. doi: 10.1152/ajpregu.00408.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308: R112–R122, 2015. doi: 10.1152/ajpregu.00427.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freisinger W, Schatz J, Ditting T, Lampert A, Heinlein S, Lale N, Schmieder R, Veelken R. Sensory renal innervation: a kidney-specific firing activity due to a unique expression pattern of voltage-gated sodium channels? Am J Physiol Renal Physiol 304: F491–F497, 2013. doi: 10.1152/ajprenal.00011.2012. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Brambilla G, Trabattoni D, Cuspidi C, Corso R, Pieruzzi F, Genovesi S, Stella A, Facchetti R, Spaziani D, Bartorelli A, Mancia G. Blood pressure responses to renal denervation precede and are independent of the sympathetic and baroreflex effects. Hypertension 65: 1209–1216, 2015. doi: 10.1161/HYPERTENSIONAHA.114.04823. [DOI] [PubMed] [Google Scholar]
- Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61: 457–464, 2013. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- Hering D, Marusic P, Walton AS, Lambert EA, Krum H, Narkiewicz K, Lambert GW, Esler MD, Schlaich MP. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 64: 118–124, 2014. doi: 10.1161/HYPERTENSIONAHA.113.03098. [DOI] [PubMed] [Google Scholar]
- Kashyap S, Boyilla R, Zaia PJ, Ghossan R, Nath KA, Textor SC, Lerman LO, Grande JP. Development of renal atrophy in murine 2 kidney 1 clip hypertension is strain independent. Res Vet Sci 107: 171–177, 2016. doi: 10.1016/j.rvsc.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension 4: 166–174, 1982a. [PubMed] [Google Scholar]
- Katholi RE, Winternitz SR, Oparil S. Decrease in peripheral sympathetic nervous system activity following renal denervation or unclipping in the one-kidney one-clip Goldblatt hypertensive rat. J Clin Invest 69: 55–62, 1982b. doi: 10.1172/JCI110441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC. Role of renal sensory nerves in physiological and pathophysiological conditions. Am J Physiol Regul Integr Comp Physiol 308: R79–R95, 2015. doi: 10.1152/ajpregu.00351.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one clip hypertensive rats. Hypertension 14: 445–452, 1989. doi: 10.1161/01.HYP.14.4.445. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275–1281, 2009. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383: 622–629, 2014. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- Kuo DC, Nadelhaft I, Hisamitsu T, de Groat WC. Segmental distribution and central projections of renal afferent fibers in the cat studied by transganglionic transport of horseradish peroxidase. J Comp Neurol 216: 162–174, 1983. doi: 10.1002/cne.902160205. [DOI] [PubMed] [Google Scholar]
- Linz D, Hohl M, Elliott AD, Lau DH, Mahfoud F, Esler MD, Sanders P, Böhm M. Modulation of renal sympathetic innervation: recent insights beyond blood pressure control. Clin Auton Res 28: 375–384, 2018. doi: 10.1007/s10286-018-0508-0. [DOI] [PubMed] [Google Scholar]
- Mahfoud F, Cremers B, Janker J, Link B, Vonend O, Ukena C, Linz D, Schmieder R, Rump LC, Kindermann I, Sobotka PA, Krum H, Scheller B, Schlaich M, Laufs U, Böhm M. Renal hemodynamics and renal function after catheter-based renal sympathetic denervation in patients with resistant hypertension. Hypertension 60: 419–424, 2012. doi: 10.1161/HYPERTENSIONAHA.112.193870. [DOI] [PubMed] [Google Scholar]
- Marfurt CF, Echtenkamp SF. Sensory innervation of the rat kidney and ureter as revealed by the anterograde transport of wheat germ agglutinin-horseradish peroxidase (WGA-HRP) from dorsal root ganglia. J Comp Neurol 311: 389–404, 1991. doi: 10.1002/cne.903110309. [DOI] [PubMed] [Google Scholar]
- Niijima A. Observation on the localization of mechanoreceptors in the kidney and afferent nerve fibres in the renal nerves in the rabbit. J Physiol 245: 81–90, 1975. doi: 10.1113/jphysiol.1975.sp010836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Banek CT. Catheter-based renal nerve ablation as a novel hypertension therapy: lost, and then found, in translation. Hypertension 71: 383–388, 2018. doi: 10.1161/HYPERTENSIONAHA.117.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Foss JD. Renal nerves and long-term control of arterial pressure. Compr Physiol 7: 263–320, 2017. doi: 10.1002/cphy.c150047. [DOI] [PubMed] [Google Scholar]
- Recordati G, Moss NG, Genovesi S, Rogenes P. Renal chemoreceptors. J Auton Nerv Syst 3: 237–251, 1981. doi: 10.1016/0165-1838(81)90066-7. [DOI] [PubMed] [Google Scholar]
- Recordati GM, Moss NG, Genovesi S, Rogenes PR. Renal receptors in the rat sensitive to chemical alterations of their environment. Circ Res 46: 395–405, 1980. doi: 10.1161/01.RES.46.3.395. [DOI] [PubMed] [Google Scholar]
- Recordati GM, Moss NG, Waselkov L. Renal chemoreceptors in the rat. Circ Res 43: 534–543, 1978. doi: 10.1161/01.RES.43.4.534. [DOI] [PubMed] [Google Scholar]
- Simon JK, Kasting NW, Ciriello J. Afferent renal nerve effects on plasma vasopressin and oxytocin in conscious rats. Am J Physiol Regul Integr Comp Physiol 256: R1240–R1244, 1989. doi: 10.1152/ajpregu.1989.256.6.R1240. [DOI] [PubMed] [Google Scholar]
- Simon OR, Schramm LP. The spinal course and medullary termination of myelinated renal afferents in the rat. Brain Res 290: 239–247, 1984. doi: 10.1016/0006-8993(84)90941-7. [DOI] [PubMed] [Google Scholar]
- Stella A, Golin R, Busnardo I, Zanchetti A. Effects of afferent renal nerve stimulation on renal hemodynamic and excretory functions. Am J Physiol Heart Circ Physiol 247: H576–H583, 1984. doi: 10.1152/ajpregu.1989.256.6.R1240. [DOI] [PubMed] [Google Scholar]
- Stella A, Weaver L, Golin R, Genovesi S, Zanchetti A. Cardiovascular effects of afferent renal nerve stimulation. Clin Exp Hypertens A 9, Suppl 1: 97–111, 1987. [DOI] [PubMed] [Google Scholar]
- Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M; SPYRAL HTN-OFF MED trial investigators . Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 390: 2160–2170, 2017. doi: 10.1016/S0140-6736(17)32281-X. [DOI] [PubMed] [Google Scholar]
- Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol 302: F1455–F1464, 2012. doi: 10.1152/ajprenal.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowski A, Prejbisz A, Florczak E, Kądziela J, Śliwiński P, Bieleń P, Michałowska I, Kabat M, Warchoł E, Januszewicz M, Narkiewicz K, Somers VK, Sobotka PA, Januszewicz A. Effects of renal sympathetic denervation on blood pressure, sleep apnea course, and glycemic control in patients with resistant hypertension and sleep apnea. Hypertension 58: 559–565, 2011. doi: 10.1161/HYPERTENSIONAHA.111.173799. [DOI] [PubMed] [Google Scholar]
- Wyss JM, Aboukarsh N, Oparil S. Sensory denervation of the kidney attenuates renovascular hypertension in the rat. Am J Physiol Heart Circ Physiol 250: H82–H86, 1986. doi: 10.1152/ajpheart.1986.250.1.H82. [DOI] [PubMed] [Google Scholar]
- Xiao L, Kirabo A, Wu J, Saleh MA, Zhu L, Wang F, Takahashi T, Loperena R, Foss JD, Mernaugh RL, Chen W, Roberts J 2nd, Osborn JW, Itani HA, Harrison DG. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res 117: 547–557, 2015. doi: 10.1161/CIRCRESAHA.115.306010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Zheng H, Liu X, Patel KP. Activation of afferent renal nerves modulates RVLM-projecting PVN neurons. Am J Physiol Heart Circ Physiol 308: H1103–H1111, 2015. doi: 10.1152/ajpheart.00862.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]