Abstract
Corticospinal tract excitability can be altered by age, physical activity (PA), and possibly sex, but whether these effects differ between upper and lower limb muscles is unknown. We determined the influence of age, PA, and sex on corticospinal excitability of an upper limb and a lower limb muscle during submaximal contractions by comparing stimulus-response curves of motor evoked potentials (MEPs). Transcranial magnetic stimulation (TMS) was used to evoke stimulus-response curves in active muscles by incrementally increasing the stimulator intensity from below the active motor threshold (AMT) until a plateau in MEP amplitudes was achieved. Stimulus-response curves were analyzed from the first dorsal interosseous (FDI) of 30 young (23.9 ± 3.8 yr) and 33 older (72.6 ± 5.6 yr) men and women and the vastus lateralis (VL) of 13 young (23.2 ± 2.2 yr) and 25 older (72.7 ± 5.5 yr) men and women. Corticospinal excitability was determined by fitting the curves with a four-parameter sigmoidal curve and calculating the maximal slope (slopemax). PA was assessed with triaxial accelerometry, and participants were dichotomized into high-PA (>10,000 steps/day, n = 15) or low-PA (<10,000 steps/day, n = 43) groups. Young adults had larger FDI MEP amplitudes (% maximum amplitude of compound muscle action potential) at higher TMS intensities (120–150% AMT) and greater slopemax than older adults (P < 0.05), with no differences between high- and low-PA groups (P > 0.05). VL MEP amplitudes and slopemax, however, were lower in the high-PA than low-PA participants, with no age or sex differences. These data suggest that aging and PA, but not sex, differentially influence the excitability of the corticospinal tracts projecting to muscles of the upper compared with the lower limb.
NEW & NOTEWORTHY Excitability of the corticospinal tract projecting to the first dorsal interosseous assessed with transcranial magnetic stimulation was reduced with age but independent of regular physical activity (steps/day) and sex of the individual. In contrast, corticospinal excitability of the vastus lateralis was not affected by age but was reduced in individuals achieving more than the physical activity recommendations of 10,000 steps/day. Aging and activity differentially affect corticospinal excitability of upper and lower limb muscles.
Keywords: aging, corticospinal excitability, physical activity, sex differences, transcranial magnetic stimulation
INTRODUCTION
Aging is accompanied by impairments in both skeletal muscle and the central nervous system leading to reductions in motor function, including reduced mechanical power and greater fatigability of limb muscles (Doherty et al. 1993; Frontera et al. 1991; Hunter et al. 2016). Age-related changes within the central nervous system that may contribute include structural degradation of cortical neurons and smaller cortical size (Salat et al. 2004), a decline in effective connectivity between motor-related cortical areas (Talelli et al. 2008), motoneuron degeneration (Hunter et al. 2016; Marner et al. 2003), and possibly altered excitability of the corticospinal tract (Clark and Taylor 2011). The integrity of the corticospinal pathway can be investigated noninvasively by stimulating the primary motor cortex with transcranial magnetic stimulation (TMS) and measuring the amplitude of the motor evoked potentials (MEPs) in the target muscle with surface electromyography (EMG). The stimulus-response curve of the MEPs represents the dose-response relationship between the increasing stimulation intensity and MEP amplitude. This curve is typically sigmoidal in shape and represents the net excitability from both the inhibitory and excitatory synaptic inputs to the cortical and spinal motoneurons (Carroll et al. 2001; Siebner and Rothwell 2003).
There are, however, disparate findings as to whether aging is associated with a change in corticospinal excitability in healthy older adults across all muscle groups and whether such findings are common to both men and women and independent of physical activity (PA). Some studies report that older adults exhibit decreased corticospinal excitability compared with young adults, because older adults had lower stimulus-response curve slopes and smaller MEP amplitudes, particularly at the lower end of the stimulus-response curve (Oliviero et al. 2006; Pitcher et al. 2005; Sale and Semmler 2005; Talelli et al. 2008). Other studies, however, observed no age-related differences in corticospinal excitability (Hassanlouei et al. 2017; Smith et al. 2009, 2011; Stevens-Lapsley et al. 2013). The different age-related findings may depend on the muscle assessed (upper vs. lower limb), whether the muscle is activated, as well as characteristics including the sex of the participant or his/her engagement in habitual PA. Pitcher et al. (2003), for example, reported that older women had smaller MEP amplitudes in the resting first dorsal interosseous (FDI) compared with young women, but no age-related differences were observed in men (Pitcher et al. 2003; Smith et al. 2011). These possible age- and sex-related differences may have been specific to the FDI muscle (Pitcher et al. 2003; Smith et al. 2011; Talelli et al. 2008), because no age- or sex-related differences have been observed in corticospinal excitability of the quadriceps muscles of the lower limb (Hassanlouei et al. 2017; Stevens-Lapsley et al. 2013). Age- and sex-related differences in corticospinal excitability could be larger for a distal upper limb muscle than a lower limb muscle because of the larger cortical representation in the motor cortex of upper limb muscles (Brouwer and Ashby 1990; Chen et al. 1998; Meier et al. 2008).
We recently demonstrated that PA influenced excitability of the corticospinal tract of a lower limb muscle (vastus lateralis, VL) independent of age (Hassanlouei et al. 2017). Young and older men and women who achieved ≥10,000 steps/day had smaller MEP amplitudes and lower stimulus-response curve slopes than young and older men and women who achieved <10,000 steps/day (Hassanlouei et al. 2017). The influence of habitual activity on corticospinal excitability may be localized to the muscles involved in performing the activity. In support of this hypothesis, short-term increases in muscle activity over a 4-wk strength training program lowered the corticospinal excitability of the trained upper limb muscles (Carroll et al. 2002; Jensen et al. 2005). Thus the increased or reduced levels of activity of a muscle group may modulate corticospinal excitability. It is unknown, however, whether the influence of PA on corticospinal excitability is specific to the corticospinal projections to the muscles that are exercised. Given that small muscles of the hand (FDI) are quite active during daily activities (Kern et al. 2001), corticospinal excitability may not differ between typically active and inactive adults when measured as steps per day.
The purpose of this study was to determine whether aging and habitual PA differentially affect the excitability of the corticospinal tract projections to upper limb muscles and lower limb muscles in men and women. All TMS measurements were obtained during low-level activation of the muscle for functional relevance and because corticospinal excitability of the FDI may be modulated with aging differently at rest than during activation (Devanne et al. 1997; Talelli et al. 2008). Data for the lower limb muscle were obtained from a recently published study (Hassanlouei et al. 2017) for a subgroup of participants who also participated in experiments of the FDI in the present study. We hypothesized that 1) older adults would have reduced corticospinal excitability compared with young adults in the upper limb muscle (FDI), as indicated by smaller peak-to-peak MEP amplitudes and lower maximal slopes of the stimulus-response curve, but not in the lower limb muscle (VL); 2) higher PA (measured as steps/day) would preferentially affect corticospinal excitability of the lower limb muscle but not the upper limb muscle; and 3) there would be no sex differences in corticospinal excitability of the upper or lower limb muscles during low-level activation.
MATERIALS AND METHODS
Participants
Sixty-three individuals participated in this study: 30 young adults (23.9 ± 3.8 yr, 15 men and 15 women) and 33 older adults (72.6 ± 5.6 yr, 18 men and 15 women) performed experiments in the FDI, and of these participants 13 young adults (23.2 ± 2.2 yr, 9 men and 4 women) and 25 older adults (72.7 ± 5.5 yr, 14 men and 11 women) completed experiments in the VL. Data for the VL were reported previously as part of a larger data set (original data set: 28 young and 50 older adults; Hassanlouei et al. 2017). All participants provided written informed consent, and all experimental procedures were approved by the Marquette University Institutional Review Board and conformed to the principles of the Declaration of Helsinki.
Experiments on First Dorsal Interosseous
Experimental protocol.
Participants reported to the laboratory for one experimental session to test the FDI. Testing of the FDI muscle was performed on the dominant hand as indicated by the Edinburgh Handedness Scale (Oldfield 1971). All of the participants were right handed (Table 1). The experimental session began with electrical stimulation of the median nerve at rest to determine the stimulation intensity to elicit the maximum peak-to-peak amplitude of the compound muscle action potential (Mmax). Participants then performed at least three brief (2–3 s) maximal voluntary isometric contractions (MVCs) to obtain the peak isometric force of the FDI, with at least 1-min rest between each MVC. Each participant was provided with strong verbal encouragement and visual feedback of his/her performance on a computer monitor. The highest isometric force output was recorded and used to calculate subsequent submaximal target forces.
Table 1.
Anthropometrics and stimulus-response curve characteristics of FDI for all participants
| Young |
Old |
|||
|---|---|---|---|---|
| Variable | Men (15) | Women (15) | Men (16) | Women (14) |
| Age, yr | 25.4 ± 4.2 | 22.3 ± 2.8 | 72.4 ± 4.7 | 73.0 ± 6.2† |
| Height, cm | 179 ± 7.8 | 165 ± 7.4 | 179 ± 7.6 | 158 ± 3.9†* |
| Weight, kg | 80.4 ± 13.5 | 67.5 ± 12.7 | 80.4 ± 13.1 | 63.6 ± 9.8* |
| BMI, kg/m2 | 25.0 ± 2.9 | 24.7 ± 3.7 | 26.6 ± 2.6 | 25.4 ± 3.9 |
| Body fat, % | 20.5 ± 4.6 | 33.1 ± 6.8 | 28.9 ± 5.1 | 38.1 ± 6.3†* |
| Handedness | 79.2 ± 14.7 | 80.4 ± 12.8 | 82.0 ± 21.4 | 84.3 ± 16.5 |
| MVC force, N | 64.5 ± 17.4 | 53.3 ± 27.3 | 49.6 ± 12.5 | 46.0 ± 17.3† |
| AMT, %SO | 40.1 ± 7.1 | 39.0 ± 7.0 | 44.6 ± 8.7 | 45.9 ± 8.4† |
| Mmax, mV | 13.8 ± 5.6 | 13.9 ± 3.7 | 11.7 ± 4.1 | 12.4 ± 4.5 |
| MEPmax, mV | 7.3 ± 3.7 | 7.1 ± 3.4 | 5.6 ± 2.5 | 5.3 ± 2.1† |
| MEPmax, %Mmax | 49.5 ± 13.4 | 49.5 ± 14.1 | 48.8 ± 11.3 | 41.8 ± 11.8 |
| Slopemax | 1.46 ± 1.00 | 2.05 ± 1.77 | 1.33 ± 0.77 | 0.95 ± 0.39† |
Values are means ± SD; no. of participants appear in parentheses. Handedness is expressed with Edinburgh Handedness Inventory scale. AMT, active motor threshold; BMI, body mass index; FDI, first dorsal interosseous; MEPmax, maximal motor evoked potential for a sequence of 10 stimuli; Mmax, maximal M wave in the rested state; MVC, maximal voluntary isometric contraction; slopemax, maximal slope of the stimulus-response curve; SO, stimulator output. MEPmax and slopemax were only reported for those participants with a valid 4-parameter sigmoidal stimulus-response curve.
Age difference,
sex difference (P < 0.05).
After the MVCs, the active motor threshold (AMT) and stimulus-response curves of the FDI muscle were obtained during intermittent submaximal contractions at 10% MVC. These procedures are explained in detail in Stimulus-response curve of MEPs.
Force and experimental setup.
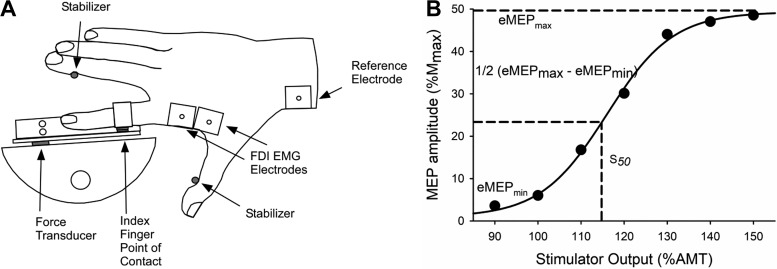
Force output of the FDI was measured with a force transducer (YB6-2KG-000-A; Sentran, Santa Ana, CA) fixed to a custom-built FDI apparatus (Fig. 1A). The experiments were conducted with each participant seated and facing a computer monitor that was positioned ~1.5 m away at eye level. The dominant arm was abducted ~40° in a comfortable resting position, and the elbow was flexed to a right angle, with the hand and forearm prone and resting on a manipulandum. The hand was placed so that the index finger was horizontal and the other three fingers were abducted and stabilized by a metal post that was positioned for comfort for each participant. The thumb was similarly stabilized in a horizontal position by a metal post that maintained the angle between the first and second metacarpals in an extended position (~80–90°). A clamp placed against the medial aspect of the wrist and the lateral aspect of the hand minimized ulnar and radial deviation of the wrist. The index finger was placed on a single-point force transducer (YB6-2KG-000-A; Sentran) that measured index finger abduction force. The index finger made contact with the force transducer with a small metal plate strapped to the proximal interphalangeal joint. The index finger abduction forces were recorded online to a computer and digitized at 1,000 Hz with a 1401 A/D converter and Spike2 software [Cambridge Electronics Design (CED), Cambridge, UK].
Fig. 1.
A: experimental setup of the first dorsal interosseous (FDI) experiments. EMG, electromyography. B: mean motor evoked potential (MEP) amplitudes of 10 stimulations at each stimulator intensity [% active motor threshold (AMT)] to obtain a stimulus-response curve for a young male participant. The stimulus-response curve was fitted with a 4-parameter sigmoidal function to generate an estimate of the minimum MEP (eMEPmin), maximum MEP (eMEPmax) and the stimulator intensity that elicited an MEP response midway between the eMEPmin and eMEPmax (S50). Mmax, maximum compound muscle action potential amplitude.
EMG recordings.
EMG signals of the FDI muscles were recorded with surface EMG electrodes (Ag/AgCl electrodes, 2.5-cm interelectrode distance; Grass Products, Natus Neurology, Warwick, RI) adhered to the skin in a bipolar arrangement over the FDI muscle belly. The reference electrode was placed over the styloid processes of the radius. Analog EMG signals were amplified (1,000×), band-pass filtered (13–1,000 Hz; Coulbourn Instruments, Allentown, PA), digitized at 2,000 Hz with a 1401 A/D converter, and stored online with Spike2 software (CED).
Electrical stimulation.
The median nerve was stimulated with a constant-current stimulator (DS7AH; Digitimer, Welwyn Garden City, UK) to obtain the Mmax of the FDI. The cathode and anode were placed on the palmar/ventral side of the wrist with a 5-cm interelectrode distance on the distal portion of the wrist near the articulation between the forearm and carpal bones (Ambu Neuroline Surface Electrodes; Ambu, Ballerup, Denmark). Single 100-µs square-wave pulses were delivered at 400 V. Stimulation intensity began at 50 mA for each participant and sequentially increased by 50 mA until a plateau in force (twitch amplitude) and the EMG response (compound muscle action potential, Mmax) occurred for the FDI. The intensity was then increased by 20% to ensure supramaximal stimulation (120–420 mA). Three stimuli were delivered with the supramaximal intensity to the resting muscle and averaged to obtain resting Mmax for the FDI muscle.
Transcranial magnetic stimulation.
TMS was delivered to the motor cortex to elicit MEPs of the FDI. The contralateral motor cortex was stimulated by delivering a 1-ms-duration magnetic pulse with a figure-of-eight coil (70-mm diameter; Magstim, Whitland, UK) connected to a monophasic magnetic stimulator (Magstim 2002). The coil was initially positioned over the typical location to elicit MEPs in the active FDI muscle. The coil was oriented to deliver anterior-posterior-directed current into the brain, with the handle pointing 45° away from the midline of the skull. The optimal stimulator position was identified by providing suprathreshold TMS during submaximal contractions (10% MVC) along a 1-cm grid drawn on an electroencephalography (EEG) cap and was determined as the location that elicited the largest MEP amplitude in the FDI muscle. The position was marked on the EEG cap to ensure consistent placement of the TMS coil throughout the experiment.
Stimulus-response curve of MEPs.
The AMT of the FDI was determined during brief isometric contractions at 10% MVC. The target force was provided visually with a digital horizontal bar displayed on the computer monitor. To obtain the AMT and stimulus-response curve, participants were verbally cued to contract for 2–3 s once every 10 s (7- to 8-s relaxation between contractions). Single-pulse TMS was delivered after the participant had sustained the 10% MVC for 2 s. The AMT was determined as the minimum stimulator intensity to elicit MEPs in at least four of eight trials in the active FDI muscle (Hassanlouei et al. 2017; Lebon et al. 2012). After the AMT was determined, seven stimulator intensities ranging from 90% to 150% of the AMT in 10% increments were used to generate each participant’s stimulus-response curve. The seven stimulator intensities were performed in a randomized order with 10 TMS stimuli delivered at each intensity, followed by a single pulse of electrical stimulation. Each set of 11 contractions was separated by at least 60 s of rest. Peak-to-peak MEP amplitudes were measured online with custom software (Spike2), and the average MEP from the 10 TMS stimuli was calculated. During the stimulus-response curve protocol, the mean MEP amplitudes for each of the seven intensities were plotted with the stimulus intensity (% AMT) to visualize the preliminary stimulus-response curve. If there was not a clear plateau (at least 2 data points) in mean MEP amplitude after the initial seven intensities, additional TMS intensities were administered in 10% increments above the 150% AMT, until either 1) a plateau in the mean MEP amplitude was observed or 2) the maximum stimulator output was reached. The highest mean MEP amplitude recorded for a sequence of 10 stimuli was considered the maximal MEP amplitude (MEPmax).
Physical activity assessment.
PA was quantified for each participant with a triaxial accelerometer (GT3X; ActiGraph, Pensacola, FL) worn around the waist for at least 4 days with at least 8 h of wear time on each day. During assessment participants were also instructed to complete a standard PA log to provide face validity of the accuracy of all accelerometer data. Participants were given standard instructions of wear for the accelerometer, which included maintaining the accelerometer in the midline of the body, removing the accelerometer when bathing or sleeping, and ensuring a snug fit of the accelerometer around the waist with an adjustable waist strap. Data were exported at 60-s epochs, and standard analysis procedures were performed with the accelerometer software (Actilife v6; ActiGraph). Wear-time validation was performed with standard algorithms (no more than 100 counts of activity/epoch in a 60-min window was considered non-wear time) and confirmed with PA logs. Daily step count was collected, and participants were separated into two categories of either high (≥10,000 steps/day) or low (<10,000 steps/day) PA based on the upper end of the recommended walking guidelines for older populations (Tudor-Locke et al. 2011). Although PA was measured with standard scientific methodology, the measurements did not incorporate intensity of PA, and this may limit the present findings.
Five participants (2 young women, 2 older men, and 1 older woman) did not meet inclusion criteria for PA (because only 2 days were recorded) and were not included in the analyses for comparisons of high- and low-PA groups. However, these five participants were included in the analyses for comparisons of age and sex.
Experiments on Knee Extensor Muscles
A subset of 38 of the participants who completed the FDI experiments (9 young men, 4 young women, 14 older men, and 11 older women) had recently participated in a previous experiment in our laboratory to assess the stimulus-response curve of the dominant leg to test the VL muscle (Hassanlouei et al. 2017). The experimental procedures for the FDI and the VL were similar, and the experimental setup and procedures are fully explained in Hassanlouei et al. (2017). In brief, the participants were seated upright with their knee fixed at 90° flexion in a Biodex System 4 Dynamometer (Biodex Medical, Shirley, NY), and a monophasic transcranial magnetic stimulator and double-cone coil (Magstim 2002) were used (110-mm diameter) to elicit MEPs to the motor area activating the quadriceps muscles. The initial position to determine the optimal stimulation region eliciting large MEPs was 1 cm lateral to the vertex of the contralateral motor cortex. The femoral nerve was electrically stimulated (DS7AH; Digitimer) to elicit maximal M waves. The stimulus-response curves of the VL from the previous experiment and the FDI muscle from the present study were compared for these 38 participants. Data were analyzed between 90% and 140% AMT because a significant number of participants did not have MEPs at 150% AMT for the VL because 1) a clear plateau in the MEP amplitude had already occurred and/or 2) the maximum stimulator output was reached.
Data Analysis
MVC force was the highest 0.5-s average isometric force output. The percentage of the stimulator output (%SO) that elicited a MEP in at least four of eight stimulations during the 10% MVC contractions was considered the AMT. All MEP amplitudes for the FDI and VL were expressed relative to active Mmax assessed at the end of each set (in %: MEP/Mmax × 100) to reduce variability due to differences in subcutaneous fat with age and sex. The mean peak-to-peak MEP amplitudes of the 10 stimulations at each intensity (%Mmax) were plotted with stimulator intensities (% AMT) to obtain the stimulus-response curve (Fig. 1B). Each stimulus-response curve for each muscle was fitted with a four-parameter sigmoidal function (Pitcher et al. 2003; Smith et al. 2011). The four-parameter iterative procedure (SigmaPlot 12.5; Systat Software, San Jose, CA) used the Levenberg-Marquard algorithm to minimize the residuals with the following equation:
| (1) |
where MEP(s) is the MEP amplitude for a given stimulator intensity s, eMEPmax and eMEPmin are the maximum and minimum MEP amplitudes estimated by the function, respectively, S50 is the stimulator intensity eliciting a MEP response that is midway between eMEPmax and eMEPmin, b is the slope of the curve at S50, and e is the base of the natural logarithm (Hassanlouei et al. 2017). To quantify the maximal slope of the curve (slopemax), we calculated the derivative of Eq. 1 and plotted the derivative with the stimulator intensities (% AMT). To ensure that MEP amplitudes at each stimulation intensity were not influenced by differences in the level of muscle activation, MEP amplitudes were also expressed relative to the level of EMG 500 ms before the stimulation.
Data were excluded from the analysis if either a plateau in the stimulus-response curve was not obtained or the number of iterations (algorithmic attempts to fit the data) to enable a valid four-parameter sigmoidal curve fit were exceeded. For the FDI, data from two older men were removed because of an exceeded number of iterations to enable a four-parameter sigmoidal curve fit and data from one older woman because of the absence of plateau in the stimulus-response curve. Four-parameter sigmoidal curves were fitted from stimulus-response curves for 60 participants, 30 young (R2 = 0.98 ± 0.01) and 30 older (R2 = 0.97 ± 0.01) adults.
Because PA guidelines include a range from 8,000 to 10,000 steps/day (Tudor-Locke et al. 2011), data were analyzed with cutoffs at 10,000, 9,000, and 8,000 steps/day to differentiate high- and low-PA groups. The results did not differ for the three cutoffs, and thus the data are presented only with the 10,000 steps/day cutoff, which corresponds to the recommended PA guidelines (Tudor-Locke et al. 2011) and is similar to what we had used previously for the VL (Hassanlouei et al. 2017).
Statistical Analysis
Separate univariate analyses of variance (ANOVAs) were performed for anthropometric measures, PA metrics, and stimulus-response curve characteristics with age (young or old), sex (male or female), and/or PA level (high or low PA) as grouping variables. A two-factor repeated-measures ANOVA with age group (young or old) and sex (male or female) as between-subject factors and intensity levels for the TMS stimulus (7 levels: 90–150% AMT in 10% increments) as within-subject factors was performed to compare the FDI MEP amplitude (%Mmax) and assess the stimulus-response curve. Additional two-factor repeated-measures ANOVAs were performed to compare the stimulus-response curves between muscle groups (FDI vs. VL) and PA level (high vs. low PA), with age as between-subject factor. Significant main or interaction effects were followed up by post hoc analysis (Bonferroni’s test) when appropriate. Student’s paired t-test was used to compare the PA level of the subset of participants involved in the present study and the previous experiment on the lower limb (Hassanlouei et al. 2017). Normality and homogeneity of variance of the data were assessed with the Kolmogorov-Smirnov test and Levene’s statistics, respectively. Sphericity was verified by Mauchly’s test. If sphericity was violated, Greenhouse-Geiser correction was used. All significance levels were set at P < 0.05, and all statistics were performed with the IBM SPSS Statistics 24 for Windows statistical software package (IBM, Chicago, IL). Data are presented as means ± SD in the text and tables and means ± SE in the figures.
RESULTS
Experiments in FDI
Anthropometrics and stimulus-response curve characteristics are summarized in Table 1. Older adults had lower MVC force (48.0 ± 15.0 N) than young adults (58.9 ± 22.8 N; F[1,59] = 5.23, P < 0.05, = 0.08), with no difference between men and women (P = 0.12, = 0.04) and no interaction between age and sex (P = 0.48, < 0.01). There was no difference in MVC force between the high- and low-PA groups (50.2 ± 20.6 N vs. 55.8 ± 20.2 N; P = 0.55, < 0.01) for the young and older adults (PA × age, P = 0.82, < 0.01).
Effects of age and sex in FDI.
mmax amplitude.
The Mmax values obtained at rest did not differ between young and older men and women (age × sex, P = 0.81, < 0.01; age, P = 0.14, = 0.04; sex, P = 0.74, < 0.01). The Mmax values obtained during each 10% MVC contraction did not change throughout the experimental protocol (13.3 ± 0.1 mV; P = 0.31, = 0.02). Additionally, there were no main effects and no interactions for age or sex (all P > 0.05), such that young and older men and women showed no significant difference in Mmax values during the 10% MVC.
stimulus-response curve characteristics.
The stimulator output (%SO) that elicited the AMT was higher for the older (45.2 ± 8.6%SO) compared with young (39.5 ± 6.8%SO; F[1,56] = 7.71, P < 0.01, = 0.12) adults. There was no main effect of sex (P = 0.98, < 0.01) or interaction of age and sex (P = 0.59, < 0.01). The maximum MEP amplitude (%Mmax), however, did not differ between young and older adults (P = 0.21, = 0.03), with no age × sex interaction (P = 0.30, = 0.02; Table 1).
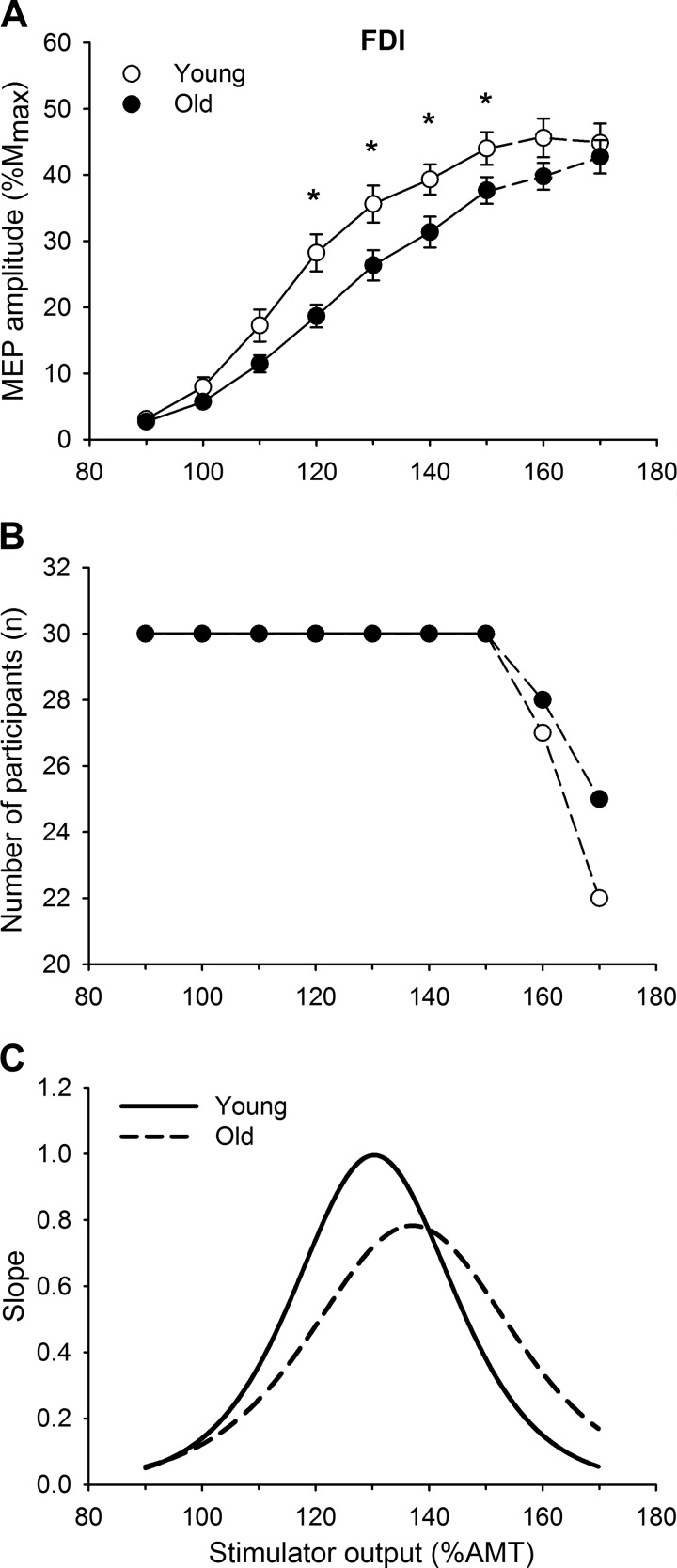
The mean stimulus-response curves for the young and older participants are shown in Fig. 2. The FDI MEP amplitude (%Mmax) increased with each increase in stimulator intensity (F[2.7,149.5] = 240.01, P < 0.001, = 0.81). There was an interaction between age and intensity in the stimulus-response curves (intensity × age, F[2.7,149.5] = 3.57, P < 0.01, =0.06), with young adults achieving larger MEP amplitudes at 120–150% AMT compared with older adults according to post hoc analysis, with no other interactions (intensity × sex, P = 0.44, ƞ2 = 0.02; intensity × age × sex, P = 0.64, < 0.01). The slopemax of the stimulus-response curve was higher for the young (1.76 ± 0.26) compared with the older (1.15 ± 0.12; F[1,56] = 4.58, P < 0.05, = 0.08; Fig. 2C) adults, with no age × sex interaction (P = 0.10, = 0.05).
Fig. 2.
A: first dorsal interosseous (FDI) stimulus-response curves of the motor evoked potentials (MEPs) from young and older adults. MEP [% maximum compound muscle action potential amplitude (%Mmax)] responses increased with stimulus intensity [% of active motor threshold (AMT)] for both age groups; however, the increase was greater for young than older adults. Values are means ± SE. Statistical analysis was performed (repeated-measures ANOVA) for intensities between 90% and 150% AMT (solid lines). Data at 160% and 170% AMT (dashed lines) were not included in the analysis because the number of participants declined. B: the number of participants included at each intensity decreased for intensities >150% AMT for both young and older adults because 1) a clear plateau in the MEP amplitude had already occurred and/or 2) the maximum stimulator output was reached. C: slope of the stimulus-response curve from a young male and an older male participant. The young participant had a steeper maximal slope that occurred at a lower % AMT compared with the older participant. *Age difference, P < 0.05.
The differences in the stimulus-response curves between the young and older adults were not due to EMG activity (activation) at each stimulation intensity. The EMG level 500 ms before the stimulation did not differ between all contraction sets (intensity: P = 0.43, = 0.02), young and older adults (age: P = 0.94, = 0.01), and men and women (sex: P = 0.10, = 0.05), with no interactions (intensity × age, P = 0.32, = 0.02; intensity × sex, P = 0.54, = 0.01; intensity × age × sex, P = 0.14, = 0.05).
Effect of habitual physical activity on FDI stimulus-response curve characteristics.
To determine the influence of PA on the stimulus-response curve of the FDI, only participants with a valid four-parameter sigmoidal curve and complete PA data were included in the analyses. Thirteen participants (1 young man, 4 young women, 3 older men, and 5 older women) were included in the high-PA group (≥10,000 steps/day), and 42 participants (14 young men, 9 young women, 11 older men, and 8 older women) were included in the low-PA group (<10,000 steps/day). As expected, the average steps per day were significantly greater for the high-PA group compared with the low-PA group (13,169 ± 2,981 steps/day vs. 6,804 ± 1,588 steps/day; F[1,51] = 93.54, P < 0.001, = 0.65), without an age × PA interaction (P = 0.84, < 0.01).
Stimulus-response curve characteristics.
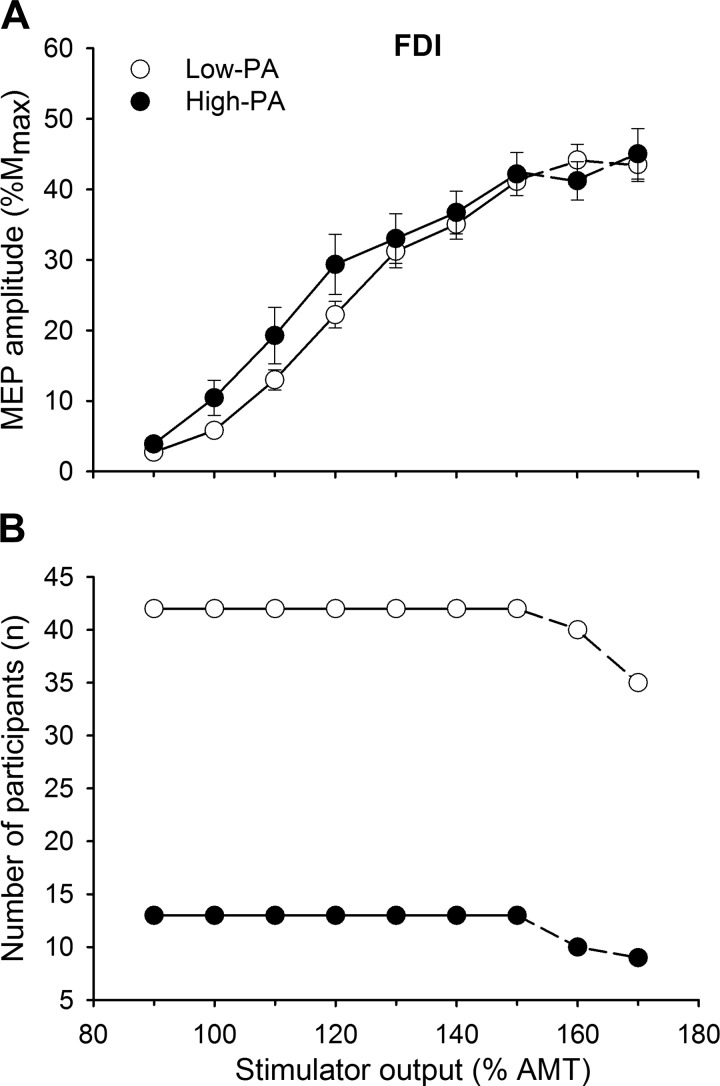
Although there was a trend toward significance, MEP amplitudes did not reach a statistical difference between the high-PA group and the low-PA group, with a low effect size (main effect of PA: F[1,51] = 3.70, P = 0.06, = 0.07). There was also no interaction between activity levels and stimulator intensity (intensity × PA: P = 0.13, = 0.04) in both young and old groups (age × PA: P = 0.24, = 0.03; Fig. 3). The slopemax of the stimulus-response curve did not reach statistical significance between the PA groups (high PA: 1.8 ± 1.6, low PA: 1.4 ± 1.0; F[1,51] = 3.00, P = 0.09, = 0.06), with no interaction between age and PA (F[1,51] = 2.94, P = 0.09, = 0.06). Furthermore, reducing the chosen cutoff did not influence the results: similar results were found for MEP amplitudes and slopemax when the cutoff between the groups was set at 9,000 steps/day (high PA, n = 17; low PA, n = 38) and 8,000 steps/day (high PA, n = 23; low PA, n = 32) (all P > 0.05).
Fig. 3.
A: stimulus-response curves of the motor evoked potentials (MEPs) from the first dorsal interosseous (FDI) in low- and high-physical activity (PA) participants. MEP [% maximum compound muscle action potential amplitude (%Mmax)] responses did not differ between low- and high-PA participants irrespective of age and sex. Values are means ± SE. Statistical analysis was performed (repeated-measures ANOVA) for intensities between 90% and 150% of active motor threshold (AMT) (solid lines). Data at 160% and 170% AMT (dashed lines) were not included in the analysis because the number of participants declined. B: the number of participants included at each intensity decreased for intensities >150% AMT for both low- and high-PA participants because 1) a clear plateau in the MEP amplitude had already occurred and/or 2) the maximum stimulator output was reached.
AMT (%SO) remained higher in older compared with young adults (young: 38.5 ± 7.9%SO, old: 45.1 ± 8.3%SO; F[1,51] = 7.81, P < 0.01, = 0.13) but was not influenced by PA (age × PA: P = 0.56, < 0.01). MEPmax (%Mmax) did not differ between the high- and low-PA groups (P = 0.55, < 0.01), with no age × PA interaction (P = 0.92, < 0.01).
Effects of Age and Muscle Group on Stimulus-Response Curve Characteristics: FDI vs. VL
Corticospinal stimulus-response curves were assessed in the quadriceps muscles (focusing on the VL) in a subset of the participants who also participated in the FDI experiments: 13 young and 25 older adults. To determine whether age effects on the stimulus-response curve differ between upper and lower limb muscle groups, we compared the stimulus-response curves of participants who had completed experiments on both FDI and VL muscles. Habitual PA for each participant was not significantly different between the experiments on each muscle group (absolute mean difference: 1,436 ± 298 steps/day, P = 0.50). Mmax was lower in older compared with young participants in the VL only (P < 0.001; Table 2).
Table 2.
Stimulus-response curve characteristics for young adults and older adults tested on both FDI and VL
| FDI |
VL |
|||
|---|---|---|---|---|
| Variable | Young | Old | Young | Old |
| AMT, %SO | 39.5 ± 7.1 | 44.4 ± 8.4 | 39.7 ± 6.2 | 49.4 ± 7.9† |
| Mmax, mV | 15.0 ± 5.5 | 11.5 ± 4.4 | 16.0 ± 4.1 | 8.0 ± 3.3† |
| MEPmax, mV | 7.9 ± 3.8 | 5.5 ± 2.4 | 4.3 ± 2.5 | 2.5 ± 0.9†* |
| MEPmax, %Mmax | 49.2 ± 14.2 | 48.5 ± 10.6 | 26.6 ± 13.7 | 34.8 ± 15.7* |
| Slopemax | 1.5 ± 1.2 | 1.3 ± 0.8 | 0.9 ± 0.4 | 0.8 ± 0.4* |
Values are means ± SD. Stimulus-response curve characteristics are shown for the young adults (n = 13; 4 women, 9 men) and older adults (n = 25; 11 women, 14 men) tested on both the first dorsal interosseous (FDI) and vastus lateralis (VL). AMT, active motor threshold; MEPmax, maximal motor evoked potential; Mmax, maximal M wave in the rested state; slopemax, maximal slope of the stimulus-response curve; SO, stimulator output.
Age difference,
muscle difference (P < 0.05).
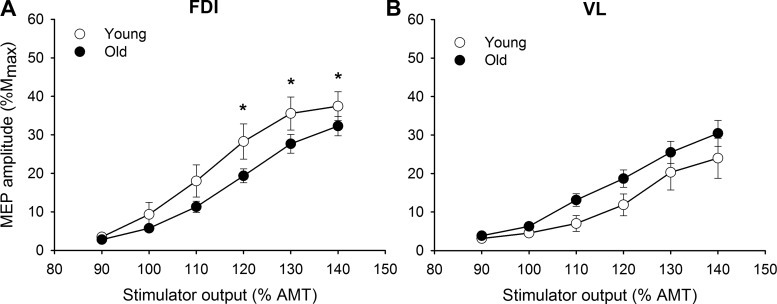
Stimulus-response curve characteristics of this subset of 38 young and older participants are summarized in Table 2. The MEP amplitudes in the stimulus-response curve were influenced by muscle and age as stimulation intensity increased (intensity × muscle × age, F[2.1,75.6] = 4.38, P < 0.05, = 0.10): the FDI muscle of the older adults had smaller MEPs at the higher intensities of stimulus output than the young adults, with no age difference for the VL muscle (Fig. 4). Furthermore, the amplitudes of the MEPs in the FDI were greater than the amplitudes in the VL for comparable intensities in young adults (intensity × muscle, F[2.6,31.3] = 7.32, P < 0.001, = 0.35) but not in older adults (intensity × muscle, P = 0.30, = 0.08). The slopemax was ~64% higher in the FDI compared with the VL (Table 2; muscle: P < 0.01, = 0.13).
Fig. 4.
Stimulus-response curves of the motor evoked potentials (MEPs) from the first dorsal interosseous (FDI; A) and vastus lateralis (VL; B) for young and older adults. MEP [% maximum compound muscle action potential amplitude (%Mmax)] responses increased with the increase in stimulator output [% of active motor threshold (AMT)] more in the young than the older adults for the FDI at high intensities (A) and similarly for the VL (B). Values are means ± SE. *Age group difference, P < 0.05.
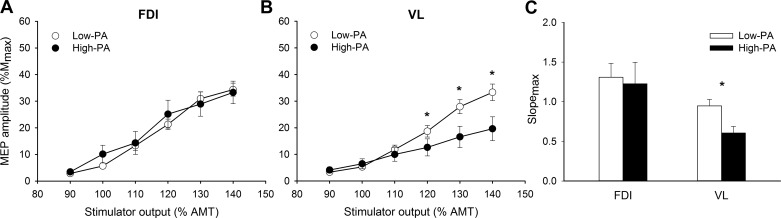
The stimulus-response curve was also differently affected by PA in the FDI and VL muscles. The stimulus-response curve of the MEP amplitudes did not differ between low- and high-PA groups for the FDI muscle (intensity × PA, P = 0.34, = 0.02), but the MEPs were larger for the low-PA compared with high-PA participants for the VL at the upper end of the curve, 120–140% (intensity × PA, F[1.5,53.7] = 7.26, P < 0.01, = 0.18) (Fig. 5).
Fig. 5.
A and B: stimulus-response curves of the motor evoked potentials (MEPs) from the first dorsal interosseous (FDI; A) and vastus lateralis (VL; B) for low- and high-physical activity (PA) participants. MEP [% maximum compound muscle action potential amplitude (%Mmax)] responses did not differ with increasing stimulus intensity [% of active motor threshold (AMT)] in low- and high-PA participants for the FDI (A) but were greater in the low- than high-PA participants for the VL at higher intensities (B). C: the low-PA participants had a steeper maximal slope (slopemax) in the stimulus-response curve for the VL compared with the high-PA participants but not for the FDI (C). Values are means ± SE. *PA group difference, P < 0.05.
Consistent with these findings, the slopemax of the stimulus-response curve of the VL was higher in the low- than the high-PA group (PA: F[1,3] = 7.18, P < 0.05, = 0.13) independent of age (age × PA: P = 0.53, < 0.01) (Fig. 5C). The slopemax of the FDI muscle, however, did not differ between all activity groups (PA: P = 0.26, = 0.02; age × PA: P = 0.16, = 0.08).
DISCUSSION
The novel findings of this study were that 1) older adults had lower corticospinal excitability of the FDI muscle (lower MEP amplitude and slopemax) compared with young adults but no differences were observed in the lower limb muscle (VL) (Hassanlouei et al. 2017); 2) PA levels influenced the corticospinal excitability of the active VL but not the FDI; and 3) there were no sex differences in corticospinal excitability of the FDI or lower limb muscles during low-level activation. Taken together, these data provide evidence that both aging and PA levels differentially influence the excitability of the corticospinal tracts projecting to a small intrinsic hand muscle compared with a larger quadriceps muscle.
Corticospinal Excitability Is Reduced with Age During Voluntary Muscle Contraction in FDI but Not VL
Compared with the young adults, the FDI of the older adults 1) required a higher stimulator intensity to elicit the AMT, 2) had smaller MEP amplitudes (%Mmax) at stimulator intensities between 120 and 150% AMT, and 3) had a lower slopemax of the stimulus-response curve. These findings confirm previous studies in the active FDI (Oliviero et al. 2006; Sale and Semmler 2005; Talelli et al. 2008) but differ from studies assessing corticospinal excitability of the resting FDI (Pitcher et al. 2003; Smith et al. 2011). For example, Pitcher et al. (2003) observed age-related differences in corticospinal excitability of the resting FDI; however, when the sexes were analyzed separately, the excitability differences with age were only evident in the women. Accordingly, Smith et al. (2011) did not observe an age-related difference in corticospinal excitability in a group of men, which suggests that the age-related changes in corticospinal excitability may differ between men and women, at least in the resting FDI muscle. Interpreting our findings together with others, the age-related differences in corticospinal excitability in the FDI muscle are likely only evident during muscle activation as we observed in our study. Additionally, any sex differences with aging in corticospinal excitability (Pitcher et al. 2003; Smith et al. 2011) appear to be minimized or not present during low levels of activation in either upper or lower limb muscles, although this study was likely underpowered to examine sex differences across muscle groups in the same participants because of a small group of young women (n = 4) who completed both experiments with upper and lower limbs. The data of young women may also be influenced by the menstrual cycle (Smith et al. 1999), which was not controlled for in this study, although conflicting results have been observed for the effects of the menstrual cycle on corticospinal excitability (Ansdell et al. 2019).
In contrast to upper limb muscles, studies on the lower limb consistently report similar corticospinal excitability between young and older participants when measured during both activation and rest (Hassanlouei et al. 2017; Stevens-Lapsley et al. 2013). The present study confirms these findings by directly comparing the corticospinal excitability of upper and lower limb muscles within the same participants (38 participants). We showed that the MEPmax and the MEP amplitudes at stimulator intensities between 110% and 140% AMT were larger in the FDI compared with the VL for the young participants only. The lack of age difference in the VL is consistent with other studies and adds to the evidence demonstrating differential effects of age on upper and lower limb muscles (Hassanlouei et al. 2017; Stevens-Lapsley et al. 2013). It is worth noting that the use of two different TMS coils on upper and lower limbs in this study can differentially affect the motor threshold (Dharmadasa et al. 2019), possibly influencing the FDI and VL stimulus-response curves differently. However, it is unlikely that this would change the age- or sex-related differences in stimulus-response curves reported in this study, as the same coils were used across the groups.
Corticospinal Excitability Is Influenced by PA in VL but Not FDI
A primary finding of this study was that the corticospinal excitability of the FDI muscle was not influenced by the habitual PA levels of the participants. Specifically, MEP amplitudes, slopemax of the stimulus-response curve, AMT, and MEPmax all did not differ between participants classified as high- and low-PA. The cutoff selected to differentiate between high- and low-PA participants (10,000 steps/day) also did not contribute to the lack of differences observed in corticospinal excitability, because the results were unaffected when the analyses were repeated with 9,000 and 8,000 steps/day as the activity cutoffs. This finding is in agreement with studies conducted on the upper limb muscles (Cirillo et al. 2009; Lulic et al. 2017) but differs from those conducted on a lower limb muscle (Hassanlouei et al. 2017). In the two studies investigating upper limb muscles, participants were categorized by self-identifying as highly active or sedentary with the International Physical Activity Questionnaire, and only young adults were tested (Cirillo et al. 2009; Lulic et al. 2017). Both studies found no differences in corticospinal excitability between the high- and low-PA groups in either the resting (Cirillo et al. 2009; Lulic et al. 2017) or active (Lulic et al. 2017) hand muscle. Our results in this study extend these findings by objectively measuring PA levels with triaxial accelerometry and also comparing the effects of PA on corticospinal excitability in upper and lower limbs within the same participants.
Because aging is associated with an increase in sedentary behavior (Bouchard et al. 1994; Martin et al. 2014), it can be difficult to differentiate the relative contribution of changes in corticospinal excitability that are due to aging versus inactivity. When we compared young and older adults, PA measured as steps per day did not influence FDI excitability. This finding was further supported when upper and lower limbs were compared in the same participants, where participants who demonstrated PA-related differences in corticospinal excitability in the VL did not demonstrate PA-related differences in the FDI. Specifically, the MEP amplitudes of the FDI muscle were similar between low- and high-PA groups, whereas MEP amplitudes of the VL muscles were greater for the low-PA group compared with the high-PA group irrespective of age (Fig. 5). Together these findings provide evidence that engaging in regular PA may not have a systemic effect on the excitability of the nervous system but rather is specific to the muscles involved in locomotion. One limitation of the measurement of PA in steps per day is the absence of knowledge of the PA intensity. For healthy older adults, however, walking ~7,000–10,000 steps/day is equivalent to accumulating 30 min of moderate to vigorous PA per day (Tudor-Locke et al. 2011), and steps per day are more generalizable whereas moderate to vigorous PA cutoffs have to be specific to the different populations. The alternative explanation is that, irrespective of PA measured as steps per day, the muscles of the hand were relatively active to a similar magnitude among all people. In support of this explanation, the upper limb muscles such as the FDI were more active when measured with EMG than lower limb muscles (quadriceps) during daily tasks in young (Kern et al. 2001) and older (Theou et al. 2010) adults. Further studies are needed to understand whether the level of activity specific to the upper limb muscles influences the excitability of the corticospinal tract projecting to the hand or arm muscles and also determine the possible effects of the intensity of the activity.
Consistent with our findings, increasing activity was shown to modulate corticospinal excitability locally in trained muscles. For example, after a 4-wk strength training program of the upper limb, corticospinal excitability was decreased when measured in the specific muscles involved in the training (Carroll et al. 2002; Jensen et al. 2005). In addition, prolonged periods of inactivity were also shown to alter corticospinal excitability. A number of studies have demonstrated that several weeks of cast immobilization induces an increase in corticospinal excitability when both upper limb (Clark et al. 2008; Zanette et al. 2004) and lower limb (Leukel et al. 2015; Roberts et al. 2007) muscles are immobilized. Taken together with our findings, corticospinal excitability of both upper and lower limb muscles can be modulated with prolonged activity or inactivity, although our data suggest that this excitability is probably not systemic but specific to the involved muscles.
Conclusions
This study demonstrated three important new findings. First, corticospinal excitability of the FDI was reduced in older compared with young adults, and this occurred independently of habitual PA. Second, we provided evidence that achieving the daily PA recommendation of 10,000 steps/day modulated the excitability of the corticospinal tract projecting to the involved muscles (lower limb muscles) but with no measurable differences in muscles of the upper limb in young and older adults. Third, corticospinal excitability assessed with low-level muscle activation was not different between the sexes when measured in either upper or lower limb muscles. These findings not only have important implications for characterizing the age-related changes associated with cortical control of movement but may be important for guiding future intervention/rehabilitation strategies. Given the important relationship between cortical inhibition and excitation in the effective control of movement, valuable data could be gained from studies aimed at understanding the complex relationship between corticospinal inhibition and excitation and PA with aging.
GRANTS
This work was supported by awards from the National Institutes of Health to S. K. Hunter (R21 AG-045766), a National Health and Medical Research Council-Australian Research Council Dementia Research Development Fellowship (GNT1097397) to A. E. Smith, and a National Institutes of Health Ruth L. Kirschstein predoctoral fellowship (F31 AG-052313) to C. W. Sundberg.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.R., A.E.S., and S.K.H. conceived and designed research; V.R. and C.W.S. performed experiments; V.R., J.W.S., and A.E.S. analyzed data; V.R., J.W.S., C.W.S., A.E.S., and S.K.H. interpreted results of experiments; V.R., J.W.S., and A.E.S. prepared figures; V.R., J.W.S., A.E.S., and S.K.H. drafted manuscript; V.R., J.W.S., C.W.S., A.E.S., and S.K.H. edited and revised manuscript; V.R., J.W.S., C.W.S., A.E.S., and S.K.H. approved final version of manuscript.
REFERENCES
- Ansdell P, Brownstein CG, Škarabot J, Hicks KM, Simoes DC, Thomas K, Howatson G, Hunter SK, Goodall S. Menstrual cycle associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic females. J Appl Physiol (1985) 126: 1701–1712, 2019. doi: 10.1152/japplphysiol.01041.2018. [DOI] [PubMed] [Google Scholar]
- Bouchard C, Shephard RJ, Stephens T. Physical Activity, Fitness, and Health: International Proceedings and Consensus Statement. Champaign, IL: Human Kinetics, 1994. [Google Scholar]
- Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol 76: 509–519, 1990. doi: 10.1016/0013-4694(90)90002-2. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. Corticospinal responses to motor training revealed by transcranial magnetic stimulation. Exerc Sport Sci Rev 29: 54–59, 2001. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Riek S, Carson RG. The sites of neural adaptation induced by resistance training in humans. J Physiol 544: 641–652, 2002. doi: 10.1113/jphysiol.2002.024463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Tam A, Bütefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80: 2870–2881, 1998. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Cirillo J, Lavender AP, Ridding MC, Semmler JG. Motor cortex plasticity induced by paired associative stimulation is enhanced in physically active individuals. J Physiol 587: 5831–5842, 2009. doi: 10.1113/jphysiol.2009.181834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BC, Issac LC, Lane JL, Damron LA, Hoffman RL. Neuromuscular plasticity during and following 3 wk of human forearm cast immobilization. J Appl Physiol (1985) 105: 868–878, 2008. doi: 10.1152/japplphysiol.90530.2008. [DOI] [PubMed] [Google Scholar]
- Clark BC, Taylor JL. Age-related changes in motor cortical properties and voluntary activation of skeletal muscle. Curr Aging Sci 4: 192–199, 2011. doi: 10.2174/1874609811104030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114: 329–338, 1997. doi: 10.1007/PL00005641. [DOI] [PubMed] [Google Scholar]
- Dharmadasa T, Matamala JM, Howells J, Simon NG, Vucic S, Kiernan MC. The effect of coil type and limb dominance in the assessment of lower-limb motor cortex excitability using TMS. Neurosci Lett 699: 84–90, 2019. doi: 10.1016/j.neulet.2019.01.050. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol (1985) 74: 868–874, 1993. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol (1985) 71: 644–650, 1991. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- Hassanlouei H, Sundberg CW, Smith AE, Kuplic A, Hunter SK. Physical activity modulates corticospinal excitability of the lower limb in young and old adults. J Appl Physiol (1985) 123: 364–374, 2017. doi: 10.1152/japplphysiol.01078.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, Pereira HM, Keenan KG. The aging neuromuscular system and motor performance. J Appl Physiol (1985) 121: 982–995, 2016. doi: 10.1152/japplphysiol.00475.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JL, Marstrand PC, Nielsen JB. Motor skill training and strength training are associated with different plastic changes in the central nervous system. J Appl Physiol (1985) 99: 1558–1568, 2005. doi: 10.1152/japplphysiol.01408.2004. [DOI] [PubMed] [Google Scholar]
- Kern DS, Semmler JG, Enoka RM. Long-term activity in upper- and lower-limb muscles of humans. J Appl Physiol (1985) 91: 2224–2232, 2001. doi: 10.1152/jappl.2001.91.5.2224. [DOI] [PubMed] [Google Scholar]
- Lebon F, Byblow WD, Collet C, Guillot A, Stinear CM. The modulation of motor cortex excitability during motor imagery depends on imagery quality. Eur J Neurosci 35: 323–331, 2012. doi: 10.1111/j.1460-9568.2011.07938.x. [DOI] [PubMed] [Google Scholar]
- Leukel C, Taube W, Rittweger J, Gollhofer A, Ducos M, Weber T, Lundbye-Jensen J. Changes in corticospinal transmission following 8 weeks of ankle joint immobilization. Clin Neurophysiol 126: 131–139, 2015. doi: 10.1016/j.clinph.2014.04.002. [DOI] [PubMed] [Google Scholar]
- Lulic T, El-Sayes J, Fassett HJ, Nelson AJ. Physical activity levels determine exercise-induced changes in brain excitability. PLoS One 12: e0173672, 2017. doi: 10.1371/journal.pone.0173672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol 462: 144–152, 2003. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Martin KR, Koster A, Murphy RA, Van Domelen DR, Hung MY, Brychta RJ, Chen KY, Harris TB. Changes in daily activity patterns with age in U.S. men and women: National Health and Nutrition Examination Survey 2003–04 and 2005–06. J Am Geriatr Soc 62: 1263–1271, 2014. doi: 10.1111/jgs.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier JD, Aflalo TN, Kastner S, Graziano MS. Complex organization of human primary motor cortex: a high-resolution fMRI study. J Neurophysiol 100: 1800–1812, 2008. doi: 10.1152/jn.90531.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res 55: 74–77, 2006. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ogston KM, Miles TS. Age and sex differences in human motor cortex input-output characteristics. J Physiol 546: 605–613, 2003. doi: 10.1113/jphysiol.2002.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher JB, Robertson AL, Clover EC, Jaberzadeh S. Facilitation of cortically evoked potentials with motor imagery during post-exercise depression of corticospinal excitability. Exp Brain Res 160: 409–417, 2005. doi: 10.1007/s00221-004-2021-z. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Ricci R, Funke FW, Ramsey P, Kelley W, Carroll JS, Ramsey D, Borckardt JJ, Johnson K, George MS. Lower limb immobilization is associated with increased corticospinal excitability. Exp Brain Res 181: 213–220, 2007. doi: 10.1007/s00221-007-0920-5. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex 14: 721–730, 2004. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sale MV, Semmler JG. Age-related differences in corticospinal control during functional isometric contractions in left and right hands. J Appl Physiol (1985) 99: 1483–1493, 2005. doi: 10.1152/japplphysiol.00371.2005. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148: 1–16, 2003. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Smith AE, Ridding MC, Higgins RD, Wittert GA, Pitcher JB. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res 198: 489–500, 2009. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Smith AE, Sale MV, Higgins RD, Wittert GA, Pitcher JB. Male human motor cortex stimulus-response characteristics are not altered by aging. J Appl Physiol (1985) 110: 206–212, 2011. doi: 10.1152/japplphysiol.00403.2010. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Keel JC, Greenberg BD, Adams LF, Schmidt PJ, Rubinow DA, Wassermann EM. Menstrual cycle effects on cortical excitability. Neurology 53: 2069–2072, 1999. doi: 10.1212/WNL.53.9.2069. [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley JE, Thomas AC, Hedgecock JB, Kluger BM. Corticospinal and intracortical excitability of the quadriceps in active older and younger healthy adults. Arch Gerontol Geriatr 56: 279–284, 2013. doi: 10.1016/j.archger.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS. The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 186: 59–66, 2008. doi: 10.1007/s00221-007-1205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theou O, Jones GR, Vandervoort AA, Jakobi JM. Daily muscle activity and quiescence in non-frail, pre-frail, and frail older women. Exp Gerontol 45: 909–917, 2010. doi: 10.1016/j.exger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C, Craig CL, Aoyagi Y, Bell RC, Croteau KA, De Bourdeaudhuij I, Ewald B, Gardner AW, Hatano Y, Lutes LD, Matsudo SM, Ramirez-Marrero FA, Rogers LQ, Rowe DA, Schmidt MD, Tully MA, Blair SN. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act 8: 80, 2011. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanette G, Manganotti P, Fiaschi A, Tamburin S. Modulation of motor cortex excitability after upper limb immobilization. Clin Neurophysiol 115: 1264–1275, 2004. doi: 10.1016/j.clinph.2003.12.033. [DOI] [PubMed] [Google Scholar]