Abstract
Although many studies have reported on tests of the vestibular system a valid and reliable, evidence-based screening battery for easy clinical use remains elusive. Many screening tests attempt to assess the vestibulo-ocular reflex. Therefore, head shaking, the Dix-Hallpike maneuver, the supine roll test, and head impulse tests are discussed. Other tests address the spatial orientation functions of the vestibular system, such as the Bucket Test and the Fukuda Stepping test. Still, other tests are based on the known correlates with balance skills, both static and dynamic, such as tandem walking and the modern variation of the Romberg test, the modified Clinical Test of Sensory Interaction and Balance. This review provides a critical overview of the literature on some of these tests and their value for clinical use and in epidemiological studies.
Keywords: balance, diagnostic testing, screening
INTRODUCTION
The vestibular system remains mysterious, despite more than 150 years of research. In particular, the seminal studies performed by Cohen and colleagues are the basis for much of the work on use of the vestibulo-ocular reflex (VOR) for clinical testing (Cohen and Suzuki 1963; Cohen et al. 1964a, 1964b, 1965a, 1965b, 1966, 1973, 1977, 1983, 1992; Highstein et al. 1974, 1976; Komatsuzaki et al. 1969; Matsunami and Cohen 1975; Matsuo et al. 1979; Raphan et al. 1979, 1980, 1981, 1985; Suzuki and Cohen 1964; Suzuki et al. 1964; Takemori and Cohen 1974a, 1974b; Uemura and Cohen 1973; Waespe and Cohen 1983). Although Barany et al. (1910) first described the slow phase of nystagmus as the “vestibular reflex and the quick phase as returning the eye to the normal position, Cohen and colleagues provided experimental verification (Cohen and Suzuki 1963; Cohen et al. 1964a, 1964b, 1965b; Suzuki and Cohen 1964). Further studies elucidated the neural mechanisms of the VOR. This new understanding of the VOR revolutionized clinical testing, which led to important developments in clinical screening that remain influential today, some of which are discussed below.
DEFINITION OF A SCREENING TEST
Screening tests are generalized tests, less specific than the objective diagnostic tests that comprise the accepted clinical laboratory battery. Screening tests are used to estimate the occurrence of a disorder or change in function, not to verify or quantify. Screening tests typically take only a few minutes, use minimal equipment, and can be administered by staff with minimal training and interpreted by nonspecialty physicians and other clinicians. They can be used to determine whether a patient should be referred for the more in-depth objective diagnostic test battery and for a workup with a physician who specializes in vestibular disorders. Despite the superficial nature of such tests, they must still be valid and reliable for clinical use as well as use in epidemiological or public health studies.
Screening tests have a variety of uses. Physicians may use them during the office visit to help determine whether in-depth, objective diagnostic tests are needed, such as a battery of low-frequency sinusoidal tests of the VOR in darkness, bithermal caloric tests, or vestibular-evoked myogenic potentials. Screening tests may be used when a thorough battery of objective diagnostic tests is unavailable, such as in locales with limited health care resources. They may also be used by nonphysician clinicians, such as nurses, when attempting to provide information to the primary care physician, who must determine whether a specialty care referral is needed. These tests are often used during assessments by occupational and physical therapists when patients are referred to them for vestibular rehabilitation. In these cases, the clinicians cannot make medical diagnoses, but they do need to obtain reliable data for treatment planning and for communication with physicians. Screening tests are also useful in public health studies in which resources for testing are limited (Cohen et al. 2012a, 2014a, 2014b; Li et al. 2015). See Table 1 for a summary of tests discussed in this paper.
Table 1.
Summary of screening tests mentioned
| Type of Test (Name) | Description |
|---|---|
| Tests of VOR | |
| Dix-Hallpike maneuver | Test of individual posterior semicircular canals; for diagnosis of posterior canal BPPV; best performed with infrared video-oculography for accuracy. |
| Supine roll test | Test of individual horizontal semicircular canal; for diagnosis of horizontal canal BPPV; best performed with infrared video oculography for accuracy. |
| Head impulse tests | Tests of high-velocity VOR; most useful for subjects over age 60 with >60% unilateral vestibular weakness and no cervical limitations; not useful for younger adults or older adults with reduced cervical spine ranges; may be useful for screening for bilateral vestibular impairment, but statistical data are not available; a negative response does not necessarily indicate normal vestibular function; uninstrumented test of little value; minimal research currently available on suppression head impulse tests, so clinical value remains unknown; interpret all results with caution, as results are not definitive. |
| Head shaking | Test for nystagmus elicited by head rotation; not a useful test. |
| Tests of balance | |
| Romberg on foam with eyes closed (a.k.a. CTSIB) | Tests of standing balance; best on medium density, compliant foam, performed with arms crossed; if head still condition is normal for age, test conditions with head moving in pitch or yaw at 0.3 Hz; moderately high sensitivity and good specificity; not useful for patients with lower-extremity peripheral neuropathy, however; interpret with care if patient has >1 joint replacement; no studies have tested joint replacement patients. |
| Tandem walking | Test of walking balance; eyes open condition not useful; best tested with 10 steps eyes closed after a few steps of practice, best with arms crossed; total no. of tandem steps is a more sensitive measure than number of consecutive tandem steps; problematic with patients with lower-extremity peripheral neuropathy; interpret with care if patient has >1 joint replacement; no studies have tested joint replacement patients. |
| Tests of spatial orientation | |
| Fukuda Stepping Test | Might indicate the impaired side; not useful for diagnostic or rehabilitation screening; Cohen and colleagues (Cohen et al. 2017; Dai et al. 2014, 2017) have used it successfully for pretesting patients with mal de debarquement for treatment planning. |
| Vertical and Torsional Alignment Nulling | May indicate impairments of otolith function; New test paradigm with promising data, using minimal table technology and colored lenses, but too little data so far for clinical use. |
| Bucket test | Designed as a test of subjective visual vertical; data do not support it. |
BPPV, benign paroxysmal positional vertigo of the posterior semicircular canal; CTSIB, Clinical Test of Sensory Interaction and Balance; VOR, vestibulo-ocular reflex. The order within each category indicates this author’s opinion about relative value of the test.
A few words about statistics may be helpful as background information. Typically, well-normed tests are developed by comparing a large group of healthy controls without the problem of interest to patients who have the problem of interest as defined by some gold standard. A type of statistical analysis, the Receiver Operating Characteristic (ROC), is used to determine how well a signal can be detected from background noise, i.e., as a measure of the power to discriminate a signal (Fan et al. 2006; McNeil and Hanley 1984). The ROC value ranges from 0 (no discriminatory power) to 1.0 (perfect discriminatory power). After the ROC has been calculated, to determine test norms, cut points in the range of test values must be determined. Cut points define the normal ranges, often within one to two standard deviations of the mean, but not necessarily. For example, body temperature measured orally is considered to be normal within a range ∼37°C, perhaps 36.1–37.2°C. Those upper and lower values are the cut points in the range of possible body temperatures. Cut points are determined from calculations to determine sensitivity to patients (true positives) and specificity to healthy controls (true negatives). Sensitivity and specificity range from 0.0 to 1.0. Optimally, a good test has both high sensitivity and high specificity.
SCREENING TESTS OF EYE MOVEMENTS
Dix-Hallpike Maneuver and Supine Roll Test
The most widely accepted oculomotor screening test of vestibular function is the Dix-Hallpike maneuver (see Fig. 1) (Dix and Hallpike 1952). A classical response on this test [slow phases representing the vestibulo-ocular reflex (VOR) downward and contralateral to the head movement; the more easily observable quick phases are upward and ipsilateral to the head movement] is pathognomonic for benign paroxysmal positional vertigo of the posterior semicircular canal (BPPV) (Bhattacharyya et al. 2017). With the advantage of using modern oculomotor recording technology, with infrared video-oculography (VOG), tiny beats of nystagmus that might not be observed with the naked eye or Frenzel glasses can be observed. The complex eye and head movements are now better understood, albeit still not completely elucidated. The three-dimensional spatial-temporal characteristics of the nystagmus have been described using vector analysis (Aw et al. 2005). The acceleration profiles of the semicircular canals and the head during the maneuver have also been described (Faldon and Bronstein 2008).
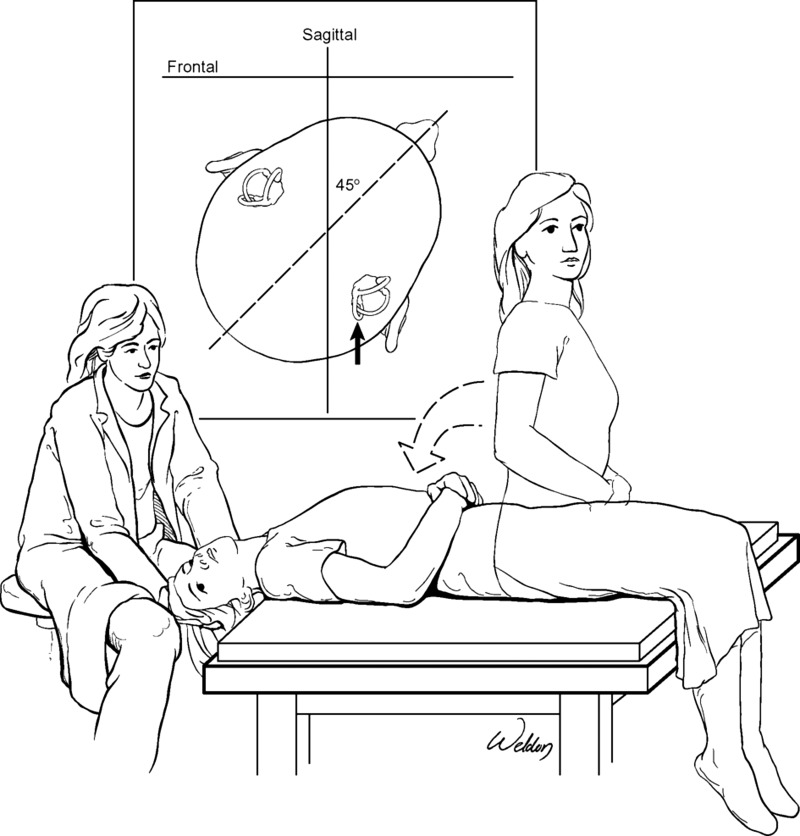
Fig. 1.
Dix-Hallpike maneuver of the right posterior semicircular canal. The patient lies supine as the head is simultaneously moved into upward pitch and yaw rotation. If positive, nystagmus commences after a brief delay. This image is shown without recording to illustrate the position of the patient’s body and head and to show a biomechanically safe position for the examiner.
The Dix-Hallpike maneuver is an essential component of a bedside screening battery (Brandt 1999; Bronstein and Lempert, 2007; Cohen 1984; Huh and Kim 2013). It is relatively easy to administer and reliable if performed correctly. Minor differences in test parameters do not seem to affect it. In our 2014 study, 69 subjects tested at two different test dates a few days apart on different treatment tables had similar response frequencies and test outcomes (Cohen et al. 2014b), indicating its reliability.
A few patients each year present with BPPV of the horizontal semicircular canal. Although no epidemiological studies have documented its frequency, it is rare. Reports from large clinical practices in tertiary care centers vary in frequency, from 1.9 to 11.8% of all BPPV patients (Cakir et al. 2006; Macias et al. 2000). Several papers have described it, all with similar descriptions of patients in whom it was elicited by having the patient slowly lie supine with the head pitched upward (in clinical language, having the neck flexed) 30° to bring the lateral canals into alignment with the vertical and then moving the head in yaw rotations leftward or rightward (Baloh et al. 1995; De la Meilleure et al. 1996; McClure 1985; Pagnini et al. 1989; White et al. 2005). If positive, the test generates horizontal nystagmus without torsional or vertical components (Casani et al. 1997). This test has come to be known as the “supine roll” test (Bhattacharyya et al. 2017; Imai et al. 2017). Unfortunately, the language used in clinical papers refers to the clinical concept of rolling, which is movement about the cephalocaudal axis, e.g., rolling onto one’s side in bed, not the scientific concept of roll, which is movement about the intra-aural axis. Therefore, reader beware, as the supine roll test has become the gold standard, although the amount of head rotation in yaw required seems to vary across clinics. Using 30° of yaw rotation is adequate (Cakir et al. 2006). It is easily performed, even in patients with limited cervical range of motion. No comparison test exists, so we have no data on sensitivity and specificity (see Fig. 2).
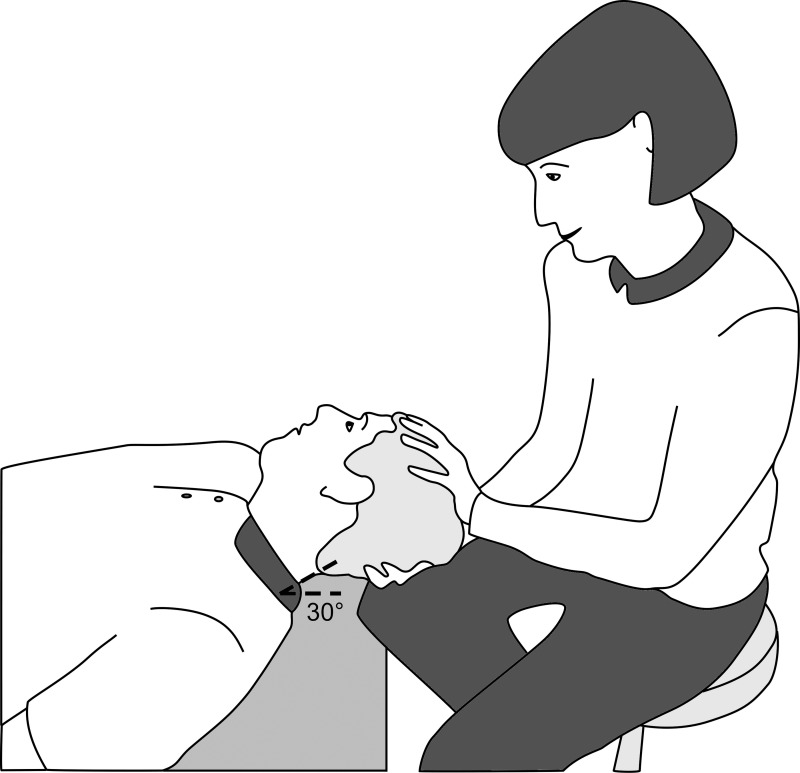
Fig. 2.
The supine roll test of the horizontal semicircular canal. The patient lies supine with the head pitched downward before the test and is then moved into yaw rotation. If positive, nystagmus commences immediately. This image is shown without recording to illustrate the position of the patient’s body and head and to show a biomechanically safe position for the examiner.
Head Shaking
Passively or actively shaking the head in yaw (i.e., left-right) has long been used as a screening test (Kamei et al. 1964), probably because the test is easy to administer and intuitively makes sense (see Fig. 3). With VOG, nystagmus may be detected in patients with Meniere’s disease and vestibular neuronitis (Asawavichiangianda et al. 1999), although it is more sensitive in patients with vestibular neuronitis than Meniere’s disease (Kim et al. 2012). Responses may vary by age as well as by diagnostic subgroups (Pérez et al. 2004). The test position may affect the result; it is usually performed in sitting, but the response may be stronger in side lying (Palla et al. 2005). In the acute phase of recovery from a vestibular insult, head shaking may be useful for bedside diagnostic assessment (Mandalà et al. 2008).
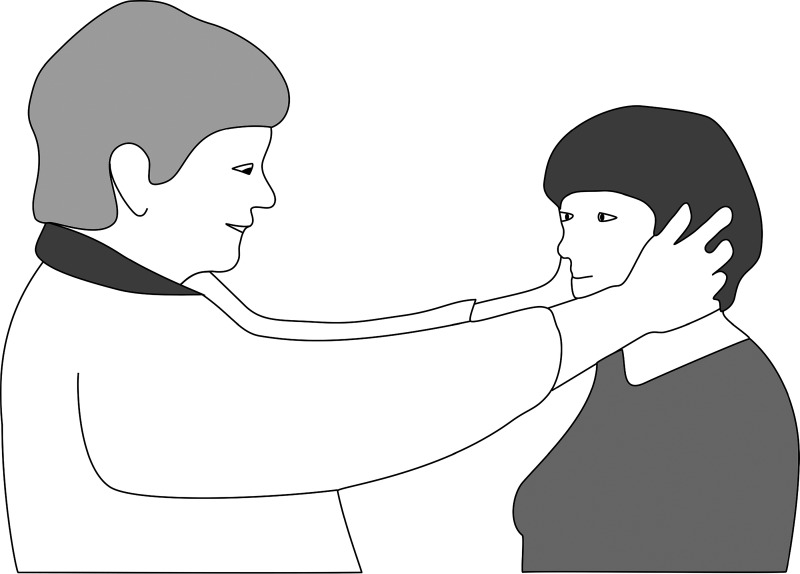
Fig. 3.
Head impulse test or head shaking test, without instrumentation.
The test is less useful for screening patients in the postacute stages of vestibular impairment. In these patients, sensitivity to patients is poor (0.27), but specificity to controls is relatively good (0.85) (Jacobson et al. 1990). We have found that even when VOG was used to record nystagmus, sensitivity is low and specificity is high: passive head shaking, subjects ≤59 yr, 0.39 sensitivity and 0.98 specificity; and similar in subjects ≥60 yr, 0.2 sensitivity, 0.97 specificity (Cohen et al. 2018b). Sensitivity and specificity are similar for active head shaking and for vertigo elicited by passive head shaking. This test illustrates the problem of widespread clinical use of a test despite a paucity of supporting evidence and even despite evidence suggesting that it is not useful for screening.
HEAD IMPULSE TESTS
Video Head Impulse Tests
The head impulse test (Halmagyi and Curthoys 1988) was developed as a quick, high-velocity test of the VOR. The initial study compared 12 patients post-unilateral vestibular neurectomy with 12 healthy controls. When subjects fixated on an earth-fixed target while the head was moved briskly, patients made compensatory refixation saccades. The test is easy to administer, except with patients who have significant cervical limitations or pain or who are otherwise unable to cooperate (see Fig. 3). Hence, rather rapidly it became widely used for screening. We have shown, however, that the ROC value for the uninstrumented head impulse test is <0.75 (Cohen et al. 2014c, 2018b) and has higher ROC only for patients >60 yr old with >60% weakness on bithermal caloric testing. After the development of head-mounted camera systems for infrared video oculography, the video head impulse test (VHIT) was adopted (see Fig. 4). VHIT has become widely used in the clinic and has been applied to epidemiological research; results are consistent with falls (Agrawal et al. 2013).
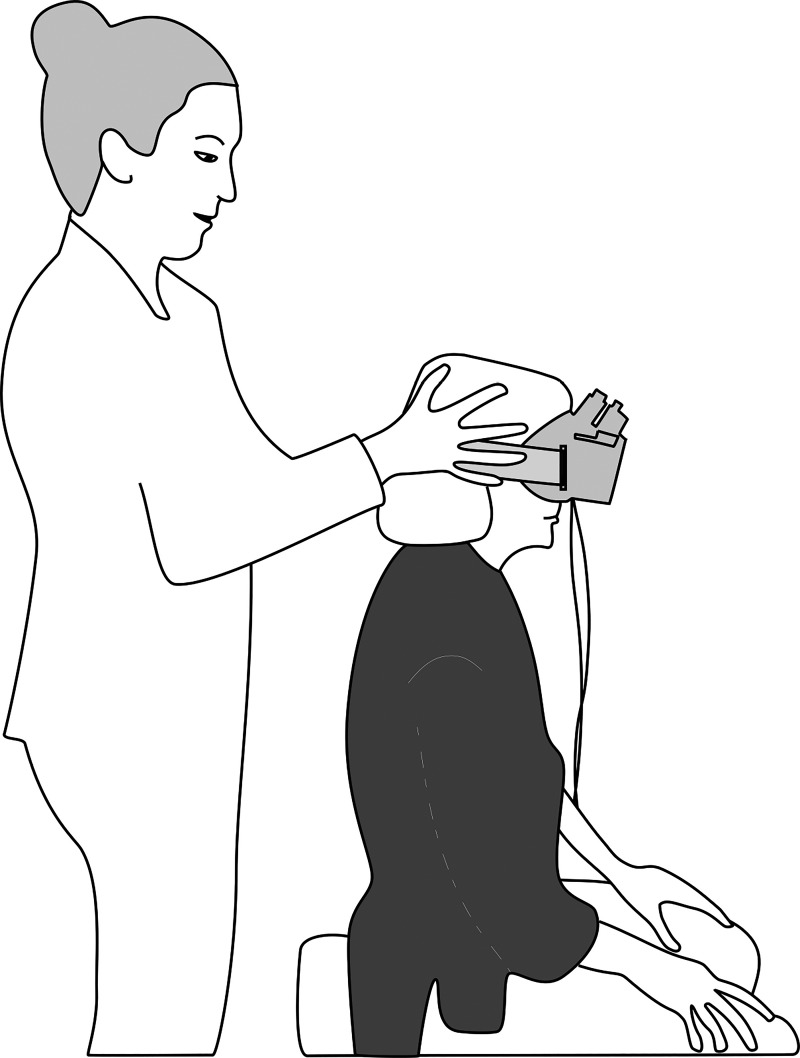
Fig. 4.
Head impulse test shown with the seated patient wearing infrared video oculography goggles. The examiner stands behind the patient, who focuses on a point straight ahead.
Several factors may affect test administration and interpretation, such as the experience and expertise of the evaluator (Jorns-Häderli et al. 2007) and the head position (Schubert et al. 2004; Seo et al. 2016). The type of test administration, whether passive or passive/assistive, may affect the result. When subjects actively participate they tend to generate small, occult saccades during the head rotation (Black et al. 2005). Patients and subjects tend to learn the test after a few trials, but current recording techniques require many repeated head impulses. Therefore, testing may become passive-assistive; i.e., the patient may actively help because relaxing completely is difficult. Placement of the examiner’s hands can affect the result (Patterson et al. 2015), probably because examiners differ in morphological characteristics such as height and hand size, which may affect force generation. Age affects the outcome in at least two ways. 1) Older subjects often have more difficulty relaxing, perhaps due to cervical arthritis, so performing the test with older subjects can be more challenging; and 2) gains in response to higher test velocities decrease with age (Matiño-Soler et al 2015; McGarvie et al. 2015). Furthermore, even in healthy control subjects, refixation saccades may occur (Yang et al. 2016). These individual variations may cause differences in the velocity profiles of the stimuli, potentially confounding data interpretation. These factors may be the reasons why the test shows a high rate of abnormalities in healthy controls (Davalos-Bichara and Agrawal 2014).
The test is insensitive to mild to moderate, clinically pathological unilateral weakness (Bartolomeo et al. 2014; Beynon et al. 1998; Cohen et al. 2014c; Hamid 2005; McCaslin et al. 2014; Perez and Rama-Lopez 2003). We found that the ROC value of VHIT is >0.8 only for subjects ≥60 yr of age, with ≥60% unilateral weakness on bithermal caloric testing (Cohen et al. 2014c). In young subjects or subjects ≥60 yr old but with milder caloric weakness, ROC values were <0.65. In other words VHIT is sensitive to patients with severe vestibular deficits, confirming the finding of Halmagyi and Curthoys (1988), but it is not sensitive to mild to moderate deficits, supporting previous findings from other laboratories. More recently, we confirmed that sensitivity is generally low, ∼0.13–0.35, and specificity is generally high, 0.85–0.90; gains for controls and patients were ∼1.0, with no differences by age or sex (Cohen et al. 2018b). If cut points are selected for high sensitivity, specificity may be poor (Perez and Rama-Lopez 2003).
These findings have important clinical implications. If the test is positive in a patient in the postacute stage of illness, i.e., refixation saccades are found and the VOR gain is decreased, then further testing may not be needed. If, however, the test is negative, the clinician still needs another way to determine whether the patient has a vestibular disorder. Also, it is not useful for estimating perception of recovery in patients with chronic vestibular impairments (Patel et al. 2016). The test may be more useful for screening patients suspected of acute vestibular neuronitis (Guan et al. 2017) or as part of a battery in the emergency room (Vanni et al. 2014). The test may also be useful for determining whether patients with Meniere’s disease have achieved the clinically desired level of unilateral vestibular destruction after gentamicin injections (Cerchiai et al. 2016).
The findings described here suggest that the test has limited use for screening and may be most valuable for patients in the acute stage of response to a naturally occurring event or for following patients with treatment-induced lesions intended to be the cause a major weakness. It is also useful in the case of suspected major bilateral loss based on history and patient complaint. Otherwise, the failure to find a positive response should be interpreted cautiously.
VHIT With Suppression Head Impulse Assessment
Refixation saccades may be hidden by so-called covert saccades during VHIT head movements, making refixation saccades difficult to find (Tjernström et al. 2012; Weber et al. 2008). Therefore, Curthoys and colleagues developed the suppression head impulse test. Rather than have the subject fixate on an earth-fixed target, as in VHIT, their subjects fixated on a head-fixed target using a head-mounted laser projecting on the wall (de Waele et al. 2017; MacDougall et al. 2016; Shen et al. 2016). They reported that healthy controls generated large refixation saccades at the end of the head impulse, and in a relatively small sample, patients with acute unilateral vestibular impairments also generated large refixation saccades when the head was turned away from the side of lesion, toward the nonlesion side. The same patients showed no refixation saccades, however, when the head was rotated toward the lesion side. They reported weak responses with the head turned toward the lesion side in a small sample of patients with subacute vestibular lesions. They found mixed responses in patients with bilateral vestibular weakness, some of whom made covert compensatory saccades, which was reminiscent of the strategy described in bilateral vestibular loss patients many years ago (Kasai and Zee 1978). Some age-related differences in responses have been reported (Rey-Martinez et al. 2018). The parameters of the saccades may also vary depending on the predictability of the target location (Rey-Martinez et al. 2017). This new test shows promise and may eventually prove to be valuable for screening. At the present time, we should consider current research findings with cautious optimism and await the results of further research.
SCREENING TESTS OF VESTIBULARLY MEDIATED SPATIAL ORIENTATION
As Cohen (1988) has shown us, the vestibular system provides a three-dimensional coordinate system for spatial orientation, which the brain can use for many functions. The vestibular system contributes to spatial orientation via path integration (Mittelstaedt and Mittelstaedt 1980), in which a moving subject must keep track of its current position with reference to a starting point and orientation with reference to the gravitational vertical. The ability to integrate this information to maintain a proper spatial reference frame probably depends on having an intact velocity storage integrator (Cohen et al. 1977; Raphan et al. 1979). Dr. Cohen and colleagues have shown that velocity storage involves neurons in the nodulus and ventral uvula (Cohen et al. 1992; Wearne et al. 1996) and subsets of neurons within the vestibular nuclei (Yakushin et al. 2017).
Path integration is severely impaired in people with bilaterally absent vestibular function (Bloomberg et al. 2000), impaired in patients with unilateral vestibular weaknesses (Roberts et al. 2011), and impaired in astronauts shortly after return from space flight (Glasauer et al. 1995). However, path integration is difficult to test clinically. Although pointing (Huebner and Bloomberg 1996) and walking tests (Cohen 2000) have been developed, these tests are not suitable for screening. Therefore, with one exception, the Fukuda Stepping Test, tests of spatial orientation have focused on orientation to the vertical.
Fukuda Stepping Test
The Fukuda Stepping Test is a screening test of path integration. Fukuda (1959) described a variation of an older test in which subjects closed their eyes and walked in place. They are not supposed to rotate or translate, but they often do. The dependent measures are the amount of rotation in either direction, the direction of rotation, and the distance walked forward or to the side. Fukuda reported that people with vestibular disorders rotate more than healthy controls. Dr. Cohen and colleagues have shown that this test is useful for treatment planning with patients who have mal de debarquement, a type of spatial disorientation syndrome that probably involves the velocity storage integrator (Dai et al. 2014, 2017) and related pathways in the cerebellar nodulus and ventral uvula (Cohen et al. 2018c).
Although the Fukuda Stepping Test has been widely used by clinicians for many years, its value for screening has not been well defined (Grommes and Conway 2011). Test results vary depending on the cognitive set and the position of the neck (Toussaint et al. 2008). The test has high variability with healthy controls (Paquet et al. 2014). We have found low sensitivity for walking and marching in place (Cohen et al. 2014c), supporting previous studies (Honaker and Shepard 2012; Honaker et al. 2009), although Honaker et al. (Honaker and Shepard 2012) did find improved sensitivity in patients with severe weaknesses on bithermal caloric tests (a standard clinical diagnostic test) of >76%. This test has demonstrated value for treatment planning with patients who have mal de debarquement, but it is not useful for initial screening patients to determine whether they have a vestibular impairment.
Subjective Visual Vertical
The vestibular system strongly influences responses to and perception of the gravitational vertical. Vestibular lesions cause impaired perception of the gravitational vertical (Anastasopoulos et al. 1997; Friedmann 1971). People with acute unilateral vestibular lesions are significantly impaired on performance of subjective visual vertical tests, but even in apparently compensated cases subjective visual vertical is impaired (Böhmer and Mast, 1999; Böhmer and Rickenmann 1995; Friedmann 1971; Grabherr et al. 2011). Therefore, not surprisingly, investigators have developed tests of perception of the vertical. Most tests such as these are not suitable for use in clinical screening, but one group did develop a screening test that has become popular.
Bucket Test
The Bucket Test is a simple bedside test of the subjective visual vertical that uses a protractor on the bottom of a small, round trash receptacle (Zwergal et al. 2009). The patient looks into the bucket, obscuring external visual information, and rotates it to make a line on the bottom vertical. The investigators reported that the test results did not differ significantly from the results of a computerized test using a hemispheric dome and a random dot pattern in the background. Norms in healthy adults were published recently (Celis-Aguilar et al. 2018). The concept is simple, and the test is easy to administer, so it quickly became popular in clinical care.
Some evidence supports the use of this test for assessing spatial orientation. Čakrt et al. (2011) reported that patients with idiopathic scoliosis, the pathophysiology of which is poorly understood, are impaired on this test compared with healthy controls. Scores are correlated with tap-evoked ocular vestibular evoked myogenic potential (oVEMP) asymmetry but not with sound-evoked cervical VEMP asymmetry (Sun et al. 2014). This finding is logical if spatial orientation depends primarily on utricular function and if oVEMP primarily measures utricular function. The current state of the science remains unclear. Because the curved surfaces of the utricle and saccule together effectively make a single surface for detecting linear acceleration in any plane in space, either or both otolith organs may be essential for spatial orientation. Further research is needed to elucidate the problem.
More recently, Čakrt et al. (2016) reported that young and older adults did not differ significantly on the estimated position and range scores. Patients with BPPV are known to have impaired spatial orientation before treatment (Nair et al. 2018). BPPV patients have been shown to improve on Bucket Test scores after successful treatments with repositioning maneuvers (Ferreira et al. 2017). One study has reported that test-retest reliability in healthy normal subjects was poor (Michelson et al. 2018), so the test should be used cautiously.
The evidence does not support using the Bucket Test for screening. When we tested the Bucket Test (Cohen and Sangi-Haghpeykar 2012), we found statistically significant differences between controls and patients with vestibular disorders, but the ROC value for males and females and normal and abnormal sides combined was only 0.75. No good cut points to differentiate patients from controls could be found. For males/females/normal side/abnormal side combined, sensitivity was 0.78 and specificity only 0.56. Despite the paucity of data, some physical therapists have uncritically recommended it for screening patients with vestibular disorders (Horn et al. 2015). The evidence does not support their recommendation. The test is clever but not sensitive or specific enough for use in screening.
SCREENING TESTS OF VESTIBULARLY MEDIATED BALANCE SKILL
Although discussing balance and vestibulo-ocular responses as separate functions is conceptually easier, we know that the vestibular system is multimodal. Otolith input affects vestibulo-ocular reflex responses in laboratory experiments with dynamic head rotations (Cohen et al. 1982; Darlot et al. 1988; Maruta et al. 2001). It also affects responses to diagnostic testing when the head is stationary (Arai et al. 1990; Coats and Smith, 1967; Cohen 2004). Recent evidence suggests that even during quiet standing on compliant foam, semicircular canal inputs affect standing balance (Anson et al. 2019). This finding supports the evidence that balance tests on unstable support surfaces are useful for screening patients.
Standing Balance Studies
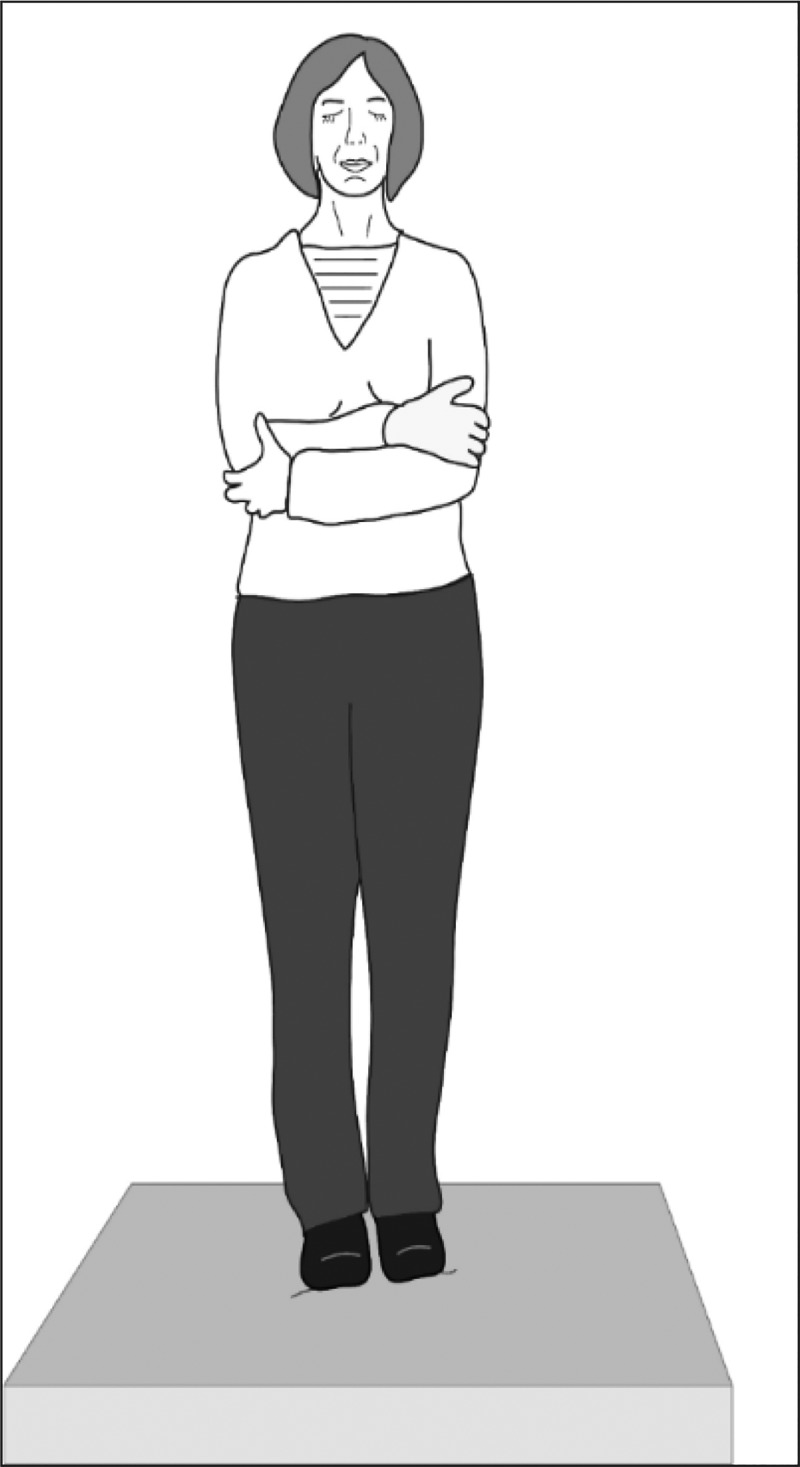
Although the gold standard for standing balance or Romberg tests (Rogers 1980) has become the computerized dynamic posturography system (Black et al. 1989), that type of system is too large, expensive, and time-consuming for screening. Shumway-Cook and Horak (1986) modernized the Romberg screening test with a condition on inexpensive, readily available, medium-density, compliant foam, renamed the Clinical Test of Sensory Interaction and Balance (CTSIB) (see Fig. 5). After initially developing norms for the CTSIB with a single set of norms for adults (Cohen et al. 1993), we later showed some changes by age (Cohen et al. 2014a), subsequently confirmed by other groups (Chaikeeree et al. 2015; Vereeck et al. 2008). We have recently published updated norms by age (Cohen et al. 2019). Sway velocity increases more in the vertical than the horizontal plane in seniors (Macedo et al. 2015). It also varies, depending on the type of shoes and socks being worn (Kim et al. 2017; Woo et al. 2018). For this reason, we have standardized testing by testing subjects without shoes but while wearing ankle-length socks. Under those conditions, we have shown that with patients who have benign paroxysmal positional vertigo, CTSIB can be more sensitive than computerized dynamic posturography (Mulavara et al. 2013). These balance responses may be influenced in particular by responses from the nodulus and ventral uvula (Cohen B, personal communication, September 2018), which are part of the circuitry of the velocity storage integrator (Cohen et al. 1992). This idea is not surprising. Balance responses have been shown to involve semicircular canal inputs (Anson et al. 2019). We know that the CTSIB and computerized Romberg tests are more challenging when sharpened with head rotations, and the sharpened tests may reveal otherwise occult balance impairments (Park et al. 2012; Cohen et al. 2014a; Wood et al. 2015).
Fig. 5.
Clinical Test of Sensory Interaction and Balance. The patient stands in stocking feet on 10-cm-thick, medium-density compliant foam, with arms crossed and eyes closed.
The test may be confounded with at least two variables. One study reported that in the head stationary condition sensitivity and specificity did not differ between tests when subjects wore their shoes or removed them (Whitney and Wrisley 2004). The influence of footwear in the more challenging conditions with the head in motion is unknown. Quite likely under conditions of less stability having more input from the feet without shoes would be useful. To avoid that potentially confounding factor studies that use this test, and tests of walking, should be standardized by having shoes removed. The type and density of foam is also a concern. Results vary depending on the type and thickness of the foam (Chaikeeree et al. 2015; Hong et al. 2015; Liu et al. 2018). We have some evidence that the density of foam also affects results (Cohen HS and Sangi-Haghpeykar H, unpublished data).
CTSIB has been used in a variety of settings. It has been used by staff with minimal expertise in balance testing in epidemiological studies (Agrawal et al. 2011; Anson et al. 2019; Cohen et al. 2012a, 2014b). It was used when visitors to a public science museum self-administered the test (Bermúdez Rey et al. 2017). Occupational and physical therapists routinely use it during clinical care in vestibular rehabilitation (Cohen et al. 2011; Horn et al. 2015).
Walking Balance: Variations on Tandem Walking
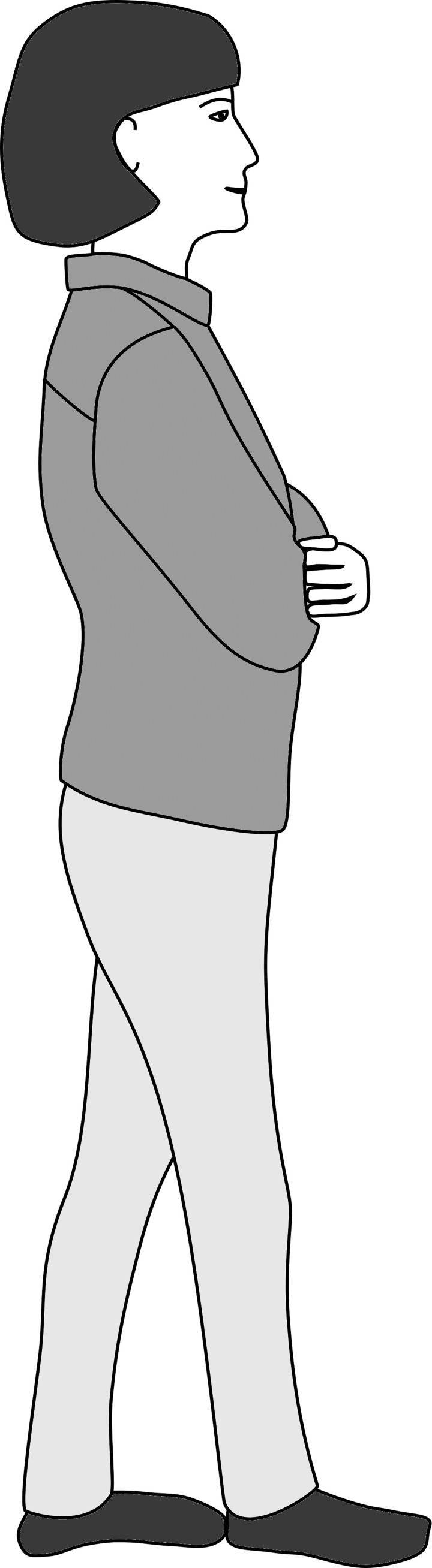
Standing and walking are different motor functions. Vestibularly mediated input to balance affects walking as well as standing. Therefore, screening tests of walking balance have been used by clinicians for many years, including tandem walking, in which the individual walks heel to toe with either eyes open or eyes closed (see Fig. 6) (Fregly and Graybiel 1970; Graybiel and Fregly 1966). In this simple test, the method for counting steps affects the outcome. Counting the number of correct consecutive steps in the eyes-closed condition is useful for screening patients for peripheral neuropathy but not for vestibular impairments (Cohen et al. 2012b, 2013). Counting the total number of correct tandem steps in the eyes closed condition is more useful for screening for vestibular disorders and results in moderately good responses: ROC = 0.8, sensitivity = 0.77, specificity = 0.72 (Cohen et al. 2018a). Less easy to measure but still, interestingly, measures of trunk sway, if equipment is available, are useful indicators of vestibular impairment (Allum et al. 2001).
Fig. 6.
Tandem walking. The patient walks heel to toe with arms crossed and eyes closed.
Test performance may be affected by the presence of peripheral neuropathy, multiple lower extremity joint replacements, deformities of the feet, or age with eyes open or closed (Cohen et al. 2018a; Longridge and Mallinson 2010; Vereeck et al. 2008). Because footwear varies, the test should be performed without shoes to eliminate that confounding variable. Performance declines with age with eyes open or closed. Repeated practice affects the outcome (Thomley et al. 1986). Nevertheless, tandem walking has been recommended as an assessment in vestibular rehabilitation (Cohen et al. 2011) and is often used by physicians for quick clinical assessments.
Test Paradigms Under Development: Vertical and Torsional Alignment Nulling
Ocular misalignment is consistent with acute vestibular impairments (Brodsky et al. 2006), most likely affecting otolith input and interpretation. It is seen in space motion sickness caused by exposure to microgravity (Diamond and Markham 1992, 1998). Based on that idea, Beaton and colleagues developed screening tests of vertical and torsional misalignment, vertical alignment nulling (VAN) and torsional alignment nulling (TAN), respectively (Beaton et al. 2017). They then developed computational models based on their preliminary data collected during parabolic flight (Beaton et al. 2015). Their data suggested that these ocular deviations represented alterations in internal state due to changes in otolith input. In a follow-up study, we compared 14 healthy controls with eight patients with vestibular disorders on a preliminary clinical version of that test (Schubert et al. 2017). We showed that patients had significantly worse scores on TAN but not on VAN, although the differences on VAN approached significance. Some military veterans have been shown to have impairments on this test, although the nature of their vestibular impairments was not clear (Schubert et al. 2018). Further technological development and testing may lead to a test battery that might be valuable for clinical screening.
These tests must be performed in a black enclosure with a small tablet computer or large-screen smart telephone, and subjects must wear red and blue lenses that match the colors of the lines on the screen. Such lenses are readily available from opticians. To date, the software is available only from Schubert or Beaton. These tests are still in the development stage and have not been normed thoroughly. They are included here as an example of evidence-based, promising screening tests with relatively minimal technology that may be useful in the future.
CONCLUSION
The literature lists many assessment tools for quick screening for vestibular disorders. Some of them, which are discussed here, are particularly common or popular, and some of those screening instruments have been shown to be valid and reliable with good sensitivity and specificity. Currently, some clinics use screening tests selectively. More research is needed before a complete, valid, and reliable evidence-based screening battery is available for clinical use.
GRANTS
This work was supported by National Institutes of Health Grant R01-DC-009031.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
H.S.C. drafted the manuscript, edited and revised the manuscript, and approved the final version of the manuscript.
ACKNOWLEDGMENTS
Deepest thanks and admiration go to my mentor, Dr. Bernard Cohen.
REFERENCES
- Agrawal Y, Carey JP, Hoffman HJ, Sklare DA, Schubert MC. The modified Romberg Balance Test: normative data in U.S. adults. Otol Neurotol 32: 1309–1311, 2011. doi: 10.1097/MAO.0b013e31822e5bee . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal Y, Davalos-Bichara M, Zuniga MG, Carey JP. Head impulse test abnormalities and influence on gait speed and falls in older individuals. Otol Neurotol 34: 1729–1735, 2013. doi: 10.1097/MAO.0b013e318295313c . [DOI] [PubMed] [Google Scholar]
- Allum JHJ, Adkin AL, Carpenter MG, Held-Ziolkowska M, Honegger F, Pierchala K. Trunk sway measures of postural stability during clinical balance tests: effects of a unilateral vestibular deficit. Gait Posture 14: 227–237, 2001. doi: 10.1016/S0966-6362(01)00132-1 . [DOI] [PubMed] [Google Scholar]
- Anastasopoulos D, Haslwanter T, Bronstein A, Fetter M, Dichgans J. Dissociation between the perception of body verticality and the visual vertical in acute peripheral vestibular disorder in humans. Neurosci Lett 233: 151–153, 1997. doi: 10.1016/S0304-3940(97)00639-3 . [DOI] [PubMed] [Google Scholar]
- Anson E, Bigelow RT, Studenski S, Deshpande N, Agrawal Y. Failure on the Foam Eyes Closed test of standing balance associated with reduced semicircular canal function in healthy older adults. Ear Hear 40: 340–344, 2019. doi: 10.1097/AUD.0000000000000619 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai Y, Suzuki J, Hess BJM, Henn V. Caloric nystagmus in three dimensions under otolithic control in rhesus monkeys. A preliminary report. ORL J Otorhinolaryngol Relat Spec 52, Spec: 218–225, 1990. doi: 10.1159/000276139 . [DOI] [PubMed] [Google Scholar]
- Asawavichiangianda S, Fujimoto M, Mai M, Desroches H, Rutka J. Significance of head-shaking nystagmus in the evaluation of the dizzy patient. Acta Otolaryngol Suppl 540: 27–33, 1999. doi: 10.1080/00016489950181152 . [DOI] [PubMed] [Google Scholar]
- Aw ST, Todd MJ, Aw GE, McGarvie LA, Halmagyi GM. Benign positional nystagmus: a study of its three-dimensional spatio-temporal characteristics. Neurology 64: 1897–1905, 2005. doi: 10.1212/01.WNL.0000163545.57134.3D . [DOI] [PubMed] [Google Scholar]
- Baloh RW, Yue Q, Jacobson KM, Honrubia V. Persistent direction-changing positional nystagmus: another variant of benign positional nystagmus? Neurology 45: 1297–1301, 1995. doi: 10.1212/WNL.45.7.1297 . [DOI] [PubMed] [Google Scholar]
- Barany R, Ibershoff AE, Copeland RS. Physiology and Pathology of the Semicircular Canals: Being an Excerpt of the Clinical Studies of Dr. Robert Barany with Notes and Addenda Gathered from the Vienna Clinics. New York: Hoeber, 1910. [Google Scholar]
- Bartolomeo M, Biboulet R, Pierre G, Mondain M, Uziel A, Venail F. Value of the video head impulse test in assessing vestibular deficits following vestibular neuritis. Eur Arch Otorhinolaryngol 271: 681–688, 2014. doi: 10.1007/s00405-013-2451-y . [DOI] [PubMed] [Google Scholar]
- Beaton KH, Huffman WC, Schubert MC. Binocular misalignments elicited by altered gravity provide evidence for nonlinear central compensation. Front Syst Neurosci 9: 81, 2015. doi: 10.3389/fnsys.2015.00081 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton KH, Schubert M, Shelhamer M. Assessment of vestibulo-ocular function without measuring eye movements. J Neurosci Methods 283: 1–6, 2017. doi: 10.1016/j.jneumeth.2017.03.012 . [DOI] [PubMed] [Google Scholar]
- Bermúdez Rey MC, Clark TK, Merfeld DM. Balance screening of vestibular function in sujects aged 4 years and older: a living laboratory experience. Front Neurol 8: 631, 2017. doi: 10.3389/fneur.2017.00631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon GJ, Jani P, Baguley DM. A clinical evaluation of head impulse testing. Clin Otolaryngol Allied Sci 23: 117–122, 1998. doi: 10.1046/j.1365-2273.1998.00112.x . [DOI] [PubMed] [Google Scholar]
- Bhattacharyya N, Gubbels SP, Schwartz SR, Edlow JA, El-Kashlan H, Fife T, Holmberg JM, Mahoney K, Hollingsworth DB, Roberts R, Seidman MD, Steiner RW, Do BT, Voelker CC, Waguespack RW, Corrigan MD. Clinical practice guideline: benign paroxysmal positional vertigo (update). Otolaryngol Head Neck Surg 156, 3_suppl: S1–S47, 2017. doi: 10.1177/0194599816689667 . [DOI] [PubMed] [Google Scholar]
- Black FO, Shupert CL, Peterka RJ, Nashner LM. Effects of unilateral loss of vestibular function on the vestibulo-ocular reflex and postural control. Ann Otol Rhinol Laryngol 98: 884–889, 1989. doi: 10.1177/000348948909801109 . [DOI] [PubMed] [Google Scholar]
- Black RA, Halmagyi GM, Thurtell MJ, Todd MJ, Curthoys IS. The active head-impulse test in unilateral peripheral vestibulopathy. Arch Neurol 62: 290–293, 2005. doi: 10.1001/archneur.62.2.290 . [DOI] [PubMed] [Google Scholar]
- Bloomberg JJ, Merkle LA, Barry SR, Huebner WP, Cohen HS, Mueller SA, Fordice J. Effects of adaptation of vestibulo-ocular reflex function on manual target localization. J Vestib Res 10: 75–86, 2000. . [PubMed] [Google Scholar]
- Böhmer A, Mast F. Assessing otolith function by the subjective visual vertical. Ann N Y Acad Sci 871: 221–231, 1999. doi: 10.1111/j.1749-6632.1999.tb09187.x . [DOI] [PubMed] [Google Scholar]
- Böhmer A, Rickenmann J. The subjective visual vertical as a clinical parameter of vestibular function in peripheral vestibular diseases. J Vestib Res 5: 35–45, 1995. doi: 10.1016/0957-4271(94)00021-S . [DOI] [PubMed] [Google Scholar]
- Brandt T. Vertigo: Its Multisensory Syndromes (2nd ed.). London: Springer, 1999. [Google Scholar]
- Brodsky MC, Donahue SP, Vaphiades M, Brandt T. Skew deviation revisited. Surv Ophthalmol 51: 105–128, 2006. doi: 10.1016/j.survophthal.2005.12.008 . [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Lempert T. Dizziness: A Practical Approach to Diagnosis and Management. New York: Cambridge, 2007. [Google Scholar]
- Cakir BO, Ercan I, Cakir ZA, Civelek S, Sayin I, Turgut S. What is the true incidence of horizontal semicircular canal benign paroxysmal positional vertigo? Otolaryngol Head Neck Surg 134: 451–454, 2006. doi: 10.1016/j.otohns.2005.07.045 . [DOI] [PubMed] [Google Scholar]
- Čakrt O, Slabý K, Kmet’ J, Kolář P, Jeřábek J. Subjective visual and haptic vertical in young and elderly. J Vestib Res 25: 195–199, 2016. doi: 10.3233/VES-150562 . [DOI] [PubMed] [Google Scholar]
- Čakrt O, Slabý K, Viktorinová L, Kolář P, Jeřábek J. Subjective visual vertical in patients with idiopatic scoliosis. J Vestib Res 21: 161–165, 2011. doi: 10.3233/VES-2011-0414 . [DOI] [PubMed] [Google Scholar]
- Casani A, Giovanni V, Bruno F, Luigi GP. Positional vertigo and ageotropic bidirectional nystagmus. Laryngoscope 107: 807–813, 1997. doi: 10.1097/00005537-199706000-00016 . [DOI] [PubMed] [Google Scholar]
- Celis-Aguilar E, Castro-Urquizo A, Mariscal-Castro J. Evaluation and interpretation of the bucket test in healthy individuals. Acta Otolaryngol 138: 458–462, 2018. doi: 10.1080/00016489.2017.1410289 . [DOI] [PubMed] [Google Scholar]
- Cerchiai N, Navari E, Dallan I, Sellari-Franceschini S, Casani AP. Assessment of vestibulo-oculomotor reflex in Meniere’s disease: defining an instrumental profile. Otol Neurotol 37: 380–384, 2016. doi: 10.1097/MAO.0000000000000983 . [DOI] [PubMed] [Google Scholar]
- Chaikeeree N, Saengsirisuwan V, Chinsongkram B, Boonsinsukh R. Interaction of age and foam types used in Clinical Test for Sensory Interaction and Balance (CTSIB). Gait Posture 41: 313–315, 2015. doi: 10.1016/j.gaitpost.2014.09.011 . [DOI] [PubMed] [Google Scholar]
- Coats AC, Smith SY. Body position and the intensity of caloric nystagmus. Acta Otolaryngol 63: 515–532, 1967. doi: 10.3109/00016486709128785 . [DOI] [PubMed] [Google Scholar]
- Cohen B. Examination of the vestibular system and the vestibulo-ocular reflex. In: Otoneurology, edited by Oosterveld WJ. New York: Wiley, 1984, p. 87–109. [Google Scholar]
- Cohen B. Representation of three-dimensional space in the vestibular, oculomotor, and visual systems. Concluding remarks. Ann NY Acad Sci 545: 239–247, 1988. doi: 10.1111/j.1749-6632.1988.tb19568.x . [DOI] [PubMed] [Google Scholar]
- Cohen B, Goto K, Shanzer S, Weiss AH. Eye movements induced by electric stimulation of the cerebellum in the alert cat. Exp Neurol 13: 145–162, 1965a. doi: 10.1016/0014-4886(65)90105-6 . [DOI] [PubMed] [Google Scholar]
- Cohen B, Matsuo V, Raphan T. Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic after-nystagmus. J Physiol 270: 321–344, 1977. doi: 10.1113/jphysiol.1977.sp011955 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B, Suzuki J, Raphan T, Matsuo V, deJong V. Selective labyrinthine lesions and nystagmus induced by rotation about off-vertical axes. In: Functional basis of Ocular Motility Disorders, edited by Lennerstrand G. Oxford: Pergamon, 1982, p. 337–346. [Google Scholar]
- Cohen B, Suzuki JI. Eye movements induced by ampullary nerve stimulation. Am J Physiol 204: 347–351, 1963. doi: 10.1152/ajplegacy.1963.204.2.347 . [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki JI, Bender MB. Eye movements from semicircular canal nerve stimulation in the cat. Ann Otol Rhinol Laryngol 73: 153–169, 1964a. doi: 10.1177/000348946407300116 . [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki JI, Bender MB. Nystagmus induced by electric stimulation of ampullary nerves. Acta Otolaryngol 60: 422–436, 1965b. doi: 10.3109/00016486509127026. [DOI] [Google Scholar]
- Cohen B, Suzuki JI, Raphan T. Role of the otolith organs in generation of horizontal nystagmus: effects of selective labyrinthine lesions. Brain Res 276: 159–164, 1983. doi: 10.1016/0006-8993(83)90558-9 . [DOI] [PubMed] [Google Scholar]
- Cohen B, Suzuki JI, Shanzer S, Bender MB. Semicircular canal control of eye movements, In: The Oculomotor System, edited by Bender MB. New York: Harper and Row, 1964b, p. 163–172. [Google Scholar]
- Cohen B, Tokumasu K, Goto K. Semicircular canal nerve eye and head movements. The effect of changes in initial eye and head position on the plane of the induced movement. Arch Ophthalmol 76: 523–531, 1966. doi: 10.1001/archopht.1966.03850010525010 . [DOI] [PubMed] [Google Scholar]
- Cohen B, Uemura T, Takemori S. Effects of labyrinthectomy on optokinetic nystagmus (OKN) and optokinetic after-nystagmus (OKAN). Int J Equilib Res 3: 88–93, 1973. . [PubMed] [Google Scholar]
- Cohen B, Yakushin SB, Cho C. Hypothesis: the vestibular and cerebellar basis of the mal de debarquement syndrome. Front Neurol 9: 28, 2018c. doi: 10.3389/fneur.2018.00028 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Blatchly CA, Gombash LL. A study of the clinical test of sensory interaction and balance. Phys Ther 73: 346–351, 1993. doi: 10.1093/ptj/73.6.346 . [DOI] [PubMed] [Google Scholar]
- Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res 90: 526–538, 1992. doi: 10.1007/BF00230935 . [DOI] [PubMed] [Google Scholar]
- Cohen HS. Vestibular disorders and impaired path integration along a linear trajectory. J Vestib Res 10: 7–15, 2000. [PubMed] [Google Scholar]
- Cohen HS. Influence of otolith input on bithermal caloric responses: re-analyses of the data of Coats and Smith. Acta Otolaryngol 124: 223–224, 2004. doi: 10.1080/00016480310015920 . [DOI] [PubMed] [Google Scholar]
- Cohen HS, Cox C, Springer G, Hoffman HJ, Young MA, Margolick JB, Plankey MW. Prevalence of abnormalities in vestibular function and balance among HIV-seropositive and HIV-seronegative women and men. PLoS One 7: e38419, 2012a. doi: 10.1371/journal.pone.0038419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Gottshall KR, Graziano M, Malmstrom EM, Sharpe MH, Whitney SL; Barany Society Ad Hoc Committee on Vestibular Rehabilitation Therapy . International guidelines for education in vestibular rehabilitation therapy. J Vestib Res 21: 243–250, 2011. doi: 10.3233/VES-2011-0424 . [DOI] [PubMed] [Google Scholar]
- Cohen HS, Mulavara AP, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ. Tests of walking balance for screening vestibular disorders. J Vestib Res 22: 95–104, 2012b. doi: 10.3233/VES-2012-0443 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Mulavara AP, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ. Standing balance tests for screening people with vestibular impairments. Laryngoscope 124: 545–550, 2014a. doi: 10.1002/lary.24314 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Mulavara AP, Peters BT, Sangi-Haghpeykar H, Kung DH, Mosier DR, Bloomberg JJ. Sharpening the tandem walking test for screening peripheral neuropathy. South Med J 106: 565–569, 2013. doi: 10.1097/SMJ.0000000000000009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Mulavara AP, Sangi-Haghpeykar H, Peters BT, Bloomberg JJ, Pavlik VN. Screening people in the waiting room for vestibular impairments. South Med J 107: 549–553, 2014b. doi: 10.14423/SMJ.000000000000017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Mulavara AP, Stitz J, Sangi-Haghpeykar H, Williams SP, Peters BT, Bloomberg JJ. Screening for vestibular disorders using the modified Clinical Test of Sensory Interaction and Balance and Tandem Walking with eyes closed. Otol Neurotol 40: 658–665, 2019. doi: 10.1097/MAO.0000000000002173 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Sangi-Haghpeykar H. Subjective visual vertical in vestibular disorders measured with the bucket test. Acta Otolaryngol 132: 850–854, 2012. doi: 10.3109/00016489.2012.668710 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Sangi-Haghpeykar H, Ricci NA, Kampangkaew J, Williamson RA. Utility of stepping, wallking and head impulses for screening patients for vestibular impairments. Otolaryngol Head Neck Surg 151: 131–136, 2014c. doi: 10.1177/0194599814527724 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Stitz J, Sangi-Haghpeykar H, Williams SP, Mulavara AP, Peters BT, Bloomberg JJ. Tandem walking as a quick screening test for vestibular disorders. Laryngoscope 128: 1687–1691, 2018a. doi: 10.1002/lary.27022 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HS, Stitz J, Sangi-Haghpeykar H, Williams SP, Mulavara AP, Peters BT, Bloomberg JJ. Utility of quick oculomotor tests for screening the vestibular system in the subacute and chronic populations. Acta Otolaryngol 138: 382–386, 2018b. doi: 10.1080/00016489.2017.1398838 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Cohen B, Cho C, Shin S, Yakushin SB. Treatment of the mal de debarquement syndrome: a 1-year follow-up. Front Neurol 8: 175, 2017. doi: 10.3389/fneur.2017.00175 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Cohen B, Smouha E, Cho C. Readaptation of the vestibulo-ocular reflex relieves the mal de debarquement syndrome. Front Neurol 5: 124, 2014. doi: 10.3389/fneur.2014.00124 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlot C, Denise P, Droulez J, Cohen B, Berthoz A. Eye movements induced by off-vertical axis rotation (OVAR) at small angles of tilt. Exp Brain Res 73: 91–105, 1988. doi: 10.1007/BF00279664 . [DOI] [PubMed] [Google Scholar]
- Davalos-Bichara M, Agrawal Y. Normative results of healthy older adults on standard clinical vestibular tests. Otol Neurotol 35: 297–300, 2014. doi: 10.1097/MAO.0b013e3182a09ca8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Meilleure G, Dehaene I, Depondt M, Damman W, Crevits L, Vanhooren G. Benign paroxysmal positional vertigo of the horizontal canal. J Neurol Neurosurg Psychiatry 60: 68–71, 1996. doi: 10.1136/jnnp.60.1.68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waele C, Shen Q, Magnani C, Curthoys IS. A novel saccadic strategy revealed by suppression head impulse testing of patients with bilateral vestibular loss. Front Neurol 8: 419, 2017. doi: 10.3389/fneur.2017.00419 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond SG, Markham CH. Ocular torsion as a test of the asymmetry hypothesis of space motion sickness. Acta Astronaut 27: 11–17, 1992. doi: 10.1016/0094-5765(92)90168-I . [DOI] [PubMed] [Google Scholar]
- Diamond SG, Markham CH. The effect of space missions on gravity-responsive torsional eye movements. J Vestib Res 8: 217–231, 1998. doi: 10.1016/S0957-4271(97)00074-8 . [DOI] [PubMed] [Google Scholar]
- Dix MR, Hallpike CS. The pathology symptomatology and diagnosis of certain common disorders of the vestibular system. Proc R Soc Med 61: 341–354, 1952. doi: 10.1177/000348945206100403 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faldon ME, Bronstein AM. Head accelerations during particle repositioning manoeuvres. Audiol Neurootol 13: 345–356, 2008. doi: 10.1159/000136153 . [DOI] [PubMed] [Google Scholar]
- Fan J, Upadhye S, Worster A. Understanding receiver operating characteristic (ROC) curves. CJEM 8: 19–20, 2006. doi: 10.1017/S1481803500013336 . [DOI] [PubMed] [Google Scholar]
- Ferreira MM, Ganança MM, Caovilla HH. Subjective visual vertical after treatment of benign paroxysmal positional vertigo. Braz J Otorhinolaryngol 83: 659–664, 2017. doi: 10.1016/j.bjorl.2016.08.014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregly AR, Graybiel A. Labyrinthine defects as shown by ataxia and caloric tests. Acta Otolaryngol 69: 216–222, 1970. doi: 10.3109/00016487009123356 . [DOI] [PubMed] [Google Scholar]
- Friedmann G. The influence of unilateral labyrinthectomy on orientation in space. Acta Otolaryngol 71: 289–298, 1971. doi: 10.3109/00016487109125366 . [DOI] [PubMed] [Google Scholar]
- Fukuda T. The stepping test: two phases of the labyrinthine reflex. Acta Otolaryngol 50: 95–108, 1959. doi: 10.3109/00016485909129172 . [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim MA, Bloomberg JJ, Reschke MF, Peters B, Smith SL, Berthoz A. Spatial orientation during locomotion following space flight. Acta Astronaut 36: 423–431, 1995. doi: 10.1016/0094-5765(95)00127-1 . [DOI] [PubMed] [Google Scholar]
- Grabherr L, Cuffel C, Guyot JP, Mast FW. Mental transformation abilities in patients with unilateral and bilateral vestibular loss. Exp Brain Res 209: 205–214, 2011. doi: 10.1007/s00221-011-2535-0 . [DOI] [PubMed] [Google Scholar]
- Graybiel A, Fregly AR. A new quantitative ataxia test battery. Acta Otolaryngol 61: 292–312, 1966. doi: 10.3109/00016486609127066 . [DOI] [PubMed] [Google Scholar]
- Grommes C, Conway D. The stepping test: a step back in history. J Hist Neurosci 20: 29–33, 2011. doi: 10.1080/09647041003662255 . [DOI] [PubMed] [Google Scholar]
- Guan Q, Zhang L, Hong W, Yang Y, Chen Z, Lu P, Zhang D, Hu X. Video head impulse test for early diagnosis of vestibular neuritis among acute vertigo. Can J Neurol Sci 44: 556–561, 2017. doi: 10.1017/cjn.2017.202 . [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol 45: 737–739, 1988. doi: 10.1001/archneur.1988.00520310043015 . [DOI] [PubMed] [Google Scholar]
- Hamid MA. Letter to the Editor. Otol Neurotol 26: 318–319, 2005. doi: 10.1097/00129492-200503000-00042 . [DOI] [PubMed] [Google Scholar]
- Highstein SM, Cohen B, Matsunami K. Monosynaptic projections from the pontine reticular formation to the 3rd nucleus in the cat. Brain Res 75: 340–344, 1974. doi: 10.1016/0006-8993(74)90758-6 . [DOI] [PubMed] [Google Scholar]
- Highstein SM, Maekawa K, Steinacker A, Cohen B. Synaptic input from the pontine reticular nuclei to absucens motoneurons and internuclear neurons in the cat. Brain Res 112: 162–167, 1976. doi: 10.1016/0006-8993(76)90344-9 . [DOI] [PubMed] [Google Scholar]
- Honaker JA, Boismier TE, Shepard NP, Shepard NT. Fukuda stepping test: sensitivity and specificity. J Am Acad Audiol 20: 311–314, 2009. doi: 10.3766/jaaa.20.5.4 . [DOI] [PubMed] [Google Scholar]
- Honaker JA, Shepard NT. Performance of Fukuda Stepping Test as a function of the severity of caloric weakness in chronic dizzy patients. J Am Acad Audiol 23: 616–622, 2012. doi: 10.3766/jaaa.23.8.6 . [DOI] [PubMed] [Google Scholar]
- Hong SK, Park JH, Kwon SY, Kim JS, Koo JW. Clinical efficacy of the Romberg test using a foam pad to identify balance problems: a comparative study with the sensory organization test. Eur Arch Otorhinolaryngol 272: 2741–2747, 2015. doi: 10.1007/s00405-014-3273-2 . [DOI] [PubMed] [Google Scholar]
- Horn LB, Rice T, Stoskus JL, Lambert KH, Dannenbaum E, Scherer MR. Measurement characteristics and clinical utility of the Clinical Test of Sensory Interaction on Balance (CTSIB) and Modified CTSIB in individuals with vestibular dysfunction. Arch Phys Med Rehabil 96: 1747–1748, 2015. doi: 10.1016/j.apmr.2015.04.003 . [DOI] [PubMed] [Google Scholar]
- Huebner WP, Bloomberg JJ. A system for the accurate measurement of pointing responses. J Neurosci Methods 64: 233–236, 1996. doi: 10.1016/0165-0270(95)00098-4 . [DOI] [PubMed] [Google Scholar]
- Huh YE, Kim JS. Bedside evaluation of dizzy patients. J Clin Neurol 9: 203–213, 2013. doi: 10.3988/jcn.2013.9.4.203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai T, Takeda N, Ikezono T, Shigeno K, Asai M, Watanabe Y, Suzuki M; Committee for Standards in Diagnosis of Japan Society for Equilibrium Research . Classification, diagnostic criteria and management of benign paroxysmal positional vertigo. Auris Nasus Larynx 44: 1–6, 2017. doi: 10.1016/j.anl.2016.03.013 . [DOI] [PubMed] [Google Scholar]
- Jacobson GP, Newman CW, Safadi I. Sensitivity and specificity of the head-shaking test for detecting vestibular system abnormalities. Ann Otol Rhinol Laryngol 99: 539–542, 1990. doi: 10.1177/000348949009900708 . [DOI] [PubMed] [Google Scholar]
- Jorns-Häderli M, Straumann D, Palla A. Accuracy of the bedside head impulse test in detecting vestibular hypofunction. J Neurol Neurosurg Psychiatry 78: 1113–1118, 2007. doi: 10.1136/jnnp.2006.109512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei T, Kimura K, Kaneko H, Noro H. Reevaluation of the head shaking test as a method of nystagmus provocation. 1. Its nystagmus-eliciting effect. Nihon Jibiinkoka Gakkai Kaiho 67: 1530–1534, 1964. . [DOI] [PubMed] [Google Scholar]
- Kasai T, Zee DS. Eye-head coordination in labyrinthine-defective human beings. Brain Res 144: 123–141, 1978. doi: 10.1016/0006-8993(78)90439-0 . [DOI] [PubMed] [Google Scholar]
- Kim MB, Huh SH, Ban JH. Diversity of head shaking nystagmus in peripheral vestibular disease. Otol Neurotol 33: 634–639, 2012. doi: 10.1097/MAO.0b013e31824950c7 . [DOI] [PubMed] [Google Scholar]
- Kim MK, Kong BS, Yoo KT. The effect of shoe type on static and dynamic balance during treadmill walking in young healthy women. J Phys Ther Sci 29: 1653–1657, 2017. doi: 10.1589/jpts.29.1653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki A, Harris HE, Alpert J, Cohen B. Horizontal nystagmus of rhesus monkeys. Acta Otolaryngol 67: 535–551, 1969. doi: 10.3109/00016486909125481 . [DOI] [PubMed] [Google Scholar]
- Li C, Layman AJ, Carey JP, Agrawal Y. Epidemiology of vestibular evoked myogenic potentials: data from the Baltimore Longitudinal Study of Aging. Clin Neurophysiol 126: 2207–2215, 2015. doi: 10.1016/j.clinph.2015.01.008 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Leng Y, Zhou R, Liu J, Liu D, Liu J, Zhang S-L, Kong WJ. Foam pad of appropriate thickness can improve diagnostic value of foam posturography in detecting postural instability. Acta Otolaryngol 138: 351–356, 2018. doi: 10.1080/00016489.2017.1393842 . [DOI] [PubMed] [Google Scholar]
- Longridge NS, Mallinson AI. Clinical Romberg testing does not detect vestibular disease. Otol Neurotol 31: 803–806, 2010. doi: 10.1097/MAO.0b013e3181e3deb2 . [DOI] [PubMed] [Google Scholar]
- MacDougall HG, McGarvie LA, Halmagyi GM, Rogers SJ, Manzari L, Burgess AM, Curthoys IS, Weber KP. A new saccadic indicator of peripheral vestibular function based on the video head impulse test. Neurology 87: 410–418, 2016. doi: 10.1212/WNL.0000000000002827 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo C, Gazzola JM, Ricci NA, Doná F, Ganança FF. Influence of sensory information on static balance in older patients with vestibular disorder. Braz J Otorhinolaryngol 81: 50–57, 2015. doi: 10.1016/j.bjorl.2014.11.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macias JD, Lambert KM, Massingale S, Ellensohn A, Fritz JA. Variables affecting treatment in benign paroxysmal positional vertigo. Laryngoscope 110: 1921–1924, 2000. doi: 10.1097/00005537-200011000-00029 . [DOI] [PubMed] [Google Scholar]
- Mandalà M, Nuti D, Broman AT, Zee DS. Effectiveness of careful bedside examination in assessment, diagnosis, and prognosis of vestibular neuritis. Arch Otolaryngol Head Neck Surg 134: 164–169, 2008. doi: 10.1001/archoto.2007.35 . [DOI] [PubMed] [Google Scholar]
- Maruta J, Simpson JI, Raphan T, Cohen B. Orienting otolith-ocular reflexes in the rabbit during static and dynamic tilts and off-vertical axis rotation. Vision Res 41: 3255–3270, 2001. doi: 10.1016/S0042-6989(01)00091-8 . [DOI] [PubMed] [Google Scholar]
- Matiño-Soler E, Esteller-More E, Martin-Sanchez JC, Martinez-Sanchez JM, Perez-Fernandez N. Normative data on angular vestibulo-ocular responses in the yaw axis measured using the video head impulse test. Otol Neurotol 36: 466–471, 2015. doi: 10.1097/MAO.0000000000000661 . [DOI] [PubMed] [Google Scholar]
- Matsunami K, Cohen B. Afferent modulation of unit activity in globus pallidus and caudate nucleus: changes induced by vestibular nucleus and pyramidal tract stimulation. Brain Res 91: 140–146, 1975. doi: 10.1016/0006-8993(75)90473-4 . [DOI] [PubMed] [Google Scholar]
- Matsuo V, Cohen B, Raphan T, de Jong V, Henn V. Asymmetric velocity storage for upward and downward nystagmus. Brain Res 176: 159–164, 1979. doi: 10.1016/0006-8993(79)90879-5 . [DOI] [PubMed] [Google Scholar]
- McCaslin DL, Jacobson GP, Bennett ML, Gruenwald JM, Green AP. Predictive properties of the video head impulse test: measures of caloric symmetry and self-report dizziness handicap. Ear Hear 35: e185–e191, 2014. doi: 10.1097/AUD.0000000000000047 . [DOI] [PubMed] [Google Scholar]
- McClure JA. Horizontal canal BPV. J Otolaryngol 14: 30–35, 1985. [PubMed] [Google Scholar]
- McGarvie LA, MacDougall HG, Halmagyi GM, Burgess AM, Weber KP, Curthoys IS. The video head impulse test (vHIT) of semicircular canal function—age-dependent normative values of VOR gain in healthy subjects. Front Neurol 6: 154, 2015. doi: 10.3389/fneur.2015.00154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil BJ, Hanley JA. Statistical approaches to the analysis of receiver operating characteristic (ROC) curves. Med Decis Making 4: 137–150, 1984. doi: 10.1177/0272989X8400400203 . [DOI] [PubMed] [Google Scholar]
- Michelson PL, McCaslin DL, Jacobson GP, Petrak M, English L, Hatton K. Assessment of subjective visual vertical (SVV) using the “Bucket Test” and the Virtual SVV system. Am J Audiol 27: 249–259, 2018. doi: 10.1044/2018_AJA-17-0019 . [DOI] [PubMed] [Google Scholar]
- Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften 67: 566–567, 1980. doi: 10.1007/BF00450672. [DOI] [Google Scholar]
- Mulavara AP, Cohen HS, Peters BT, Sangi-Haghpeykar H, Bloomberg JJ. New analyses of the sensory organization test compared to the clinical test of sensory integration and balance in patients with benign paroxysmal positional vertigo. Laryngoscope 123: 2276–2280, 2013. doi: 10.1002/lary.24075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MA, Mulavara AP, Bloomberg JJ, Sangi-Haghpeykar H, Cohen HS. Visual dependence and spatial orientation in benign paroxysmal positional vertigo. J Vestib Res 27: 279–286, 2018. doi: 10.3233/VES-170623 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnini P, Nuti D, Vannucchi P. Benign paroxysmal vertigo of the horizontal canal. ORL J Otorhinolaryngol Relat Spec 51, Spec: 161–170, 1989. doi: 10.1159/000276052 . [DOI] [PubMed] [Google Scholar]
- Palla A, Marti S, Straumann D. Head-shaking nystagmus depends on gravity. J Assoc Res Otolaryngol 6: 1–8, 2005. doi: 10.1007/s10162-004-4052-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet N, Taillon-Hobson A, Lajoie Y. Fukuda and Babinski-Weil tests: within-subject variability and test-retest reliability in nondisabled adults. J Rehabil Res Dev 51: 1013–1022, 2014. doi: 10.1682/JRRD.2013.09.0206 . [DOI] [PubMed] [Google Scholar]
- Park MK, Lim HW, Cho JG, Choi CJ, Hwang SJ, Chae SW. A head shake sensory organization test to improve the sensitivity of the sensory organization test in the elderly. Otol Neurotol 33: 67–71, 2012. doi: 10.1097/MAO.0b013e318238f75f . [DOI] [PubMed] [Google Scholar]
- Patel M, Arshad Q, Roberts RE, Ahmad H, Bronstein AM. Chronic symptoms after vestibular neuritis and the high velocity vestibulo-ocular reflex. Otol Neurotol 37: 179–184, 2016. doi: 10.1097/MAO.0000000000000949 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JN, Bassett AM, Mollak CM, Honaker JA. Effects of hand placement technique on the video head impulse test (vHIT) in younger and older adults. Otol Neurotol 36: 1061–1068, 2015. doi: 10.1097/MAO.0000000000000749 . [DOI] [PubMed] [Google Scholar]
- Perez N, Rama-Lopez J. Head-impulse and caloric tests in patients with dizziness. Otol Neurotol 24: 913–917, 2003. doi: 10.1097/00129492-200311000-00016 . [DOI] [PubMed] [Google Scholar]
- Pérez P, Llorente JL, Gómez JR, Del Campo A, López A, Suárez C. Functional significance of peripheral head-shaking nystagmus. Laryngoscope 114: 1078–1084, 2004. doi: 10.1097/00005537-200406000-00023 . [DOI] [PubMed] [Google Scholar]
- Raphan T, Cohen B. Integration and its relation to ocular compensatory movements. Mt Sinai J Med 47: 410–417, 1980. . [PubMed] [Google Scholar]
- Raphan T, Cohen B. The role of integration in oculomotor control. In: Models of oculomotor behavior and control, edited by Zuber BL. West Palm Beach, FL: CRC, 1981. [Google Scholar]
- Raphan T, Cohen B. Velocity storage and the ocular response to multidimensional vestibular stimuli. In: Adaptive Mechanisms in Gaze Control: Facts and Theories, edited by Berthoz A, Jones GM. Amsterdam: Elsevier, 1985, p. 123–143. [PubMed] [Google Scholar]
- Raphan T, Matsuo V, Cohen B. Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248, 1979. doi: 10.1007/BF00236613 . [DOI] [PubMed] [Google Scholar]
- Rey-Martinez J, Thomas-Arrizabalaga I, Espinosa-Sanchez JM, Batuecas-Caletrio A, Trinidad-Ruiz G, Matiño-Soler E, Perez-Fernandez N. Vestibulo-ocular reflex gain values in the suppression head impulse test of healthy subjects. Laryngoscope 128: 2383–2389, 2018. doi: 10.1002/lary.27107 . [DOI] [PubMed] [Google Scholar]
- Rey-Martinez J, Yanes J, Esteban J, Sanz R, Martin-Sanz E. The role of predictbility in saccadic eye responses in the suppression head impulse test of horizontal semicircular canal function. Front Neurol 8: 536, 2017. doi: 10.3389/fneur.2017.00536 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JC, Cohen HS, Sangi-Haghpeykar H. Vestibular disorders and dual task performance: impairment when walking a straight path. J Vestib Res 21: 167–174, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JH. Romberg and his test. J Laryngol Otol 94: 1401–1404, 1980. doi: 10.1017/S002221510009023X . [DOI] [PubMed] [Google Scholar]
- Schubert MC, Gimmon Y, Millar J, Brewer KJ, Roberts D, Shelhamer M, Rohde C, Serrador JM. Veterans have greater variability in their perception of binocular alignment. PLoS One 13: e0209622, 2018. doi: 10.1371/journal.pone.0209622 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Stitz J, Cohen HS, Sangi-Haghpeykar H, Mulavara AP, Peters BT, Bloomberg JJ. Prototype tests of vertical and torsional alignment nulling for screening vestibular function. J Vestib Res 27: 173–176, 2017. doi: 10.3233/VES-170618 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert MC, Tusa RJ, Grine LE, Herdman SJ. Optimizing the sensitivity of the head thrust test for identifying vestibular hypofunction. Phys Ther 84: 151–158, 2004. . [PubMed] [Google Scholar]
- Seo YJ, Park YA, Kong TH, Bae MR, Kim SH. Head position and increased head velocity to optimize video head impulse test sensitivity. Eur Arch Otorhinolaryngol 273: 3595–3602, 2016. doi: 10.1007/s00405-016-3979-4 . [DOI] [PubMed] [Google Scholar]
- Shen Q, Magnani C, Sterkers O, Lamas G, Vidal PP, Sadoun J, Curthoys IS, de Waele C. Saccadic velocity in the new suppression head impulse test: a new indicator of horizontal vestibular canal paresis and of vestibular compensation. Front Neurol 7: 160, 2016. doi: 10.3389/fneur.2016.00160 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther 66: 1548–1550, 1986. doi: 10.1093/ptj/66.10.1548 . [DOI] [PubMed] [Google Scholar]
- Sun DQ, Zuniga MG, Davalos-Bichara M, Carey JP, Agrawal Y. Evaluation of a bedside test of utricular function - the bucket test - in older individuals. Acta Otolaryngol 134: 382–389, 2014. doi: 10.3109/00016489.2013.867456 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki JI, Cohen B. Head, eye, body and limb movements from semicircular canal nerves. Exp Neurol 10: 393–405, 1964. doi: 10.1016/0014-4886(64)90031-7 . [DOI] [PubMed] [Google Scholar]
- Suzuki JI, Cohen B, Bender MB. Compensatory eye movements induced by vertical semicircular canal stimulation. Exp Neurol 9: 137–160, 1964. doi: 10.1016/0014-4886(64)90013-5 . [DOI] [PubMed] [Google Scholar]
- Takemori S, Cohen B. Loss of visual suppression of vestibular nystagmus after flocculus lesions. Brain Res 72: 213–224, 1974a. doi: 10.1016/0006-8993(74)90860-9 . [DOI] [PubMed] [Google Scholar]
- Takemori S, Cohen B. Visual suppression of vestibular nystagmus in rhesus monkeys. Brain Res 72: 203–212, 1974b. doi: 10.1016/0006-8993(74)90859-2 . [DOI] [PubMed] [Google Scholar]
- Thomley KE, Kennedy RS, Bittner AC Jr. Development of postural equilibrium tests for examining environmental effects. Percept Mot Skills 63: 555–564, 1986. doi: 10.2466/pms.1986.63.2.555. [DOI] [Google Scholar]
- Tjernström F, Nyström A, Magnusson M. How to uncover the covert saccade during the head impulse test. Otol Neurotol 33: 1583–1585, 2012. doi: 10.1097/MAO.0b013e318268d32f . [DOI] [PubMed] [Google Scholar]
- Toussaint Y, Do MC, Fagard J. What are the factors responsible for the deviation in stepping on the spot? Neurosci Lett 435: 60–64, 2008. doi: 10.1016/j.neulet.2008.02.007 . [DOI] [PubMed] [Google Scholar]
- Uemura T, Cohen B. Effects of vestibular nuclei lesions on vestibulo-ocular reflexes and posture in monkeys. Acta Otolaryngol Suppl 315: 1–71, 1973. [DOI] [PubMed] [Google Scholar]
- Vanni S, Pecci R, Casati C, Moroni F, Risso M, Ottaviani M, Nazerian P, Grifoni S, Vannucchi P. STANDING, a four-step bedside algorithm for differential diagnosis of acute vertigo in the Emergency Department. Acta Otorhinolaryngol Ital 34: 419–426, 2014. [PMC free article] [PubMed] [Google Scholar]
- Vereeck L, Wuyts F, Truijen S, Van de Heyning P. Clinical assessment of balance: normative data, and gender and age effects. Int J Audiol 47: 67–75, 2008. doi: 10.1080/14992020701689688 . [DOI] [PubMed] [Google Scholar]
- Waespe W, Cohen B. Flocculectomy and unit activity in the vestibular nuclei during visual-vestibular interactions. Exp Brain Res 51: 23–35, 1983. doi: 10.1007/BF00236799 . [DOI] [PubMed] [Google Scholar]
- Wearne S, Raphan T, Cohen B. Nodulo-uvular control of central vestibular dynamics determines spatial orientation of the angular vestibulo-ocular reflex. Ann N Y Acad Sci 781: 364–384, 1996. doi: 10.1111/j.1749-6632.1996.tb15713.x . [DOI] [PubMed] [Google Scholar]
- Weber KP, Aw ST, Todd MJ, McGarvie LA, Curthoys IS, Halmagyi GM. Head impulse test in unilateral vestibular loss: vestibulo-ocular reflex and catch-up saccades. Neurology 70: 454–463, 2008. doi: 10.1212/01.wnl.0000299117.48935.2e . [DOI] [PubMed] [Google Scholar]
- White JA, Coale KD, Catalano PJ, Oas JG. Diagnosis and management of lateral semicircular canal benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg 133: 278–284, 2005. doi: 10.1016/j.otohns.2005.03.080 . [DOI] [PubMed] [Google Scholar]
- Whitney SL, Wrisley DM. The influence of footwear on timed balance scores of the modified clinical test of sensory interaction and balance. Arch Phys Med Rehabil 85: 439–443, 2004. doi: 10.1016/j.apmr.2003.05.005 . [DOI] [PubMed] [Google Scholar]
- Woo MT, Davids K, Liukkonen J, Chow JY, Jaakkola T. Immediate effects of wearing knee length socks differing in compression level on postural regulation in community-dwelling, healthy, elderly men and women. Gait Posture 66: 63–69, 2018. doi: 10.1016/j.gaitpost.2018.08.011 . [DOI] [PubMed] [Google Scholar]
- Wood SJ, Paloski WH, Clark JB. Assesssing sensorimotor function after long-duration spaceflight with computerized dynamic posturography. Aviat Space Environ Med 86: A45–A53, 2015. doi: 10.3357/AMHP.EC07.2015 . [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Raphan T, Cohen B. Coding of velocity storage in the vestibular nuclei. Front Neurol 8: 386, 2017. doi: 10.3389/fneur.2017.00386 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CJ, Lee JY, Kang BC, Lee HS, Yoo MH, Park HJ. Quantitative analysis of gians and catch-up saccades of video-head-impulse testing by age in normal subjects. Clin Otolaryngol Allied Sci 41: 532–538, 2016. doi: 10.1111/coa.12558. [DOI] [PubMed] [Google Scholar]
- Zwergal A, Rettinger N, Frenzel C, Dieterich M, Brandt T, Strupp M. A bucket of static vestibular function. Neurology 72: 1689–1692, 2009. doi: 10.1212/WNL.0b013e3181a55ecf . [DOI] [PubMed] [Google Scholar]