Abstract
Changing the H reflex through operant conditioning leads to CNS multisite plasticity and can affect previously learned skills. To further understand the mechanisms of this plasticity, we operantly conditioned the initial component (M1) of the soleus stretch reflex. Unlike the H reflex, the stretch reflex is affected by fusimotor control, comprises several bursts of activity resulting from temporally dispersed afferent inputs, and may activate spinal motoneurons via several different spinal and supraspinal pathways. Neurologically normal participants completed 6 baseline sessions and 24 operant conditioning sessions in which they were encouraged to increase (M1up) or decrease (M1down) M1 size. Five of eight M1up participants significantly increased M1; the final M1 size of those five participants was 143 ± 15% (mean ± SE) of the baseline value. All eight M1down participants significantly decreased M1; their final M1 size was 62 ± 6% of baseline. Similar to the previous H-reflex conditioning studies, conditioned reflex change consisted of within-session task-dependent adaptation and across-session long-term change. Task-dependent adaptation was evident in conditioning session 1 with M1up and by session 4 with M1down. Long-term change was evident by session 10 with M1up and by session 16 with M1down. Task-dependent adaptation was greater with M1up than with the previous H-reflex upconditioning. This may reflect adaptive changes in muscle spindle sensitivity, which affects the stretch reflex but not the H reflex. Because the stretch reflex is related to motor function more directly than the H reflex, M1 conditioning may provide a valuable tool for exploring the functional impact of reflex conditioning and its potential therapeutic applications.
NEW & NOTEWORTHY Since the activity of stretch reflex pathways contributes to locomotion, changing it through training may improve locomotor rehabilitation in people with CNS disorders. Here we show for the first time that people can change the size of the soleus spinal stretch reflex through operant conditioning. Conditioned stretch reflex change is the sum of task-dependent adaptation and long-term change, consistent with H-reflex conditioning yet different from it in the composition and amount of the two components.
Keywords: humans, operant conditioning, plasticity, stretch reflex
INTRODUCTION
In animals and humans, an operant conditioning protocol can increase or decrease the size of the Hoffmann reflex (H reflex), which is produced by a wholly spinal pathway (Carp et al. 2006; Chen and Wolpaw 1995; Thompson et al. 2009; Wolpaw 1987). Acquisition of this simple skill can alter the pattern of muscle activation during walking in people and animals with spinal cord injury (Chen et al. 2011, 2014; Thompson and Wolpaw 2015). Accumulating evidence suggests induction of complex plasticity at many sites within the nervous system. Some of these changes clearly underlie the new skill of a larger or smaller H reflex, whereas others are likely to be compensatory changes that prevent the plasticity responsible for the new skill from interfering with preexisting behaviors (Wolpaw 2010; Wolpaw and Lee 1989).
The H reflex is elicited by weak electrical stimulation of the muscle nerve; it is often viewed as an electrical analog of the spinal stretch reflex, which can be increased or decreased by operant conditioning (Evatt et al. 1989; Wolpaw et al. 1983c). Stretch reflex conditioning has several advantages as a research model. First, the H reflex is elicited mainly by synchronous activation of primary afferent (group Ia and large group II) fibers; thus the afferent volley is minimally dispersed when it arrives at the spinal motoneurons (Burke et al. 1983). In contrast, the stretch reflex can be elicited by a rapid joint rotation, which produces a temporally dispersed activation of afferent fibers; the same afferent may even fire several times (Matthews 1972). The afferents are activated in a manner similar to that occurring during natural movement [e.g., the stretch reflex is less affected by presynaptic inhibition than the H reflex (Morita et al. 1998)]. Second, unlike the H reflex, the stretch reflex is affected by muscle spindle sensitivity and thus by changes in gamma drive (Arris and Henneman 1980; Matthews 1972, 1981). This provides a stretch reflex conditioning protocol an additional mechanism for changing reflex size. Third, unlike the H reflex, the stretch reflex comprises several successive peaks of excitation. The first (M1) is generated mainly by group Ia afferents. Later peaks (M2 and M3) have contributions from group Ib and II afferents (M2; af Klint et al. 2010; Dietz 1998; Dietz and Duysens 2000; Grey et al. 2001; Schieppati and Nardone 1997, 1999; Sinkjaer et al. 2000) and from transcortical pathways (M3; Capaday et al. 1991; Marsden et al. 1973, 1977; Mrachacz-Kersting et al. 2006; Palmer and Ashby 1992; Petersen et al. 1998). The pathways responsible for these later peaks contribute to normal movement. Thus the impact of M1 conditioning on these pathways should also be monitored.
The present study set out to demonstrate the feasibility of operant conditioning of the soleus (SOL) M1 stretch reflex, to characterize the time course of M1 stretch reflex changes in conditioning responders, and to compare these to the previous SOL H-reflex conditioning. It introduces a novel stretch reflex conditioning protocol for the human SOL muscle based on the H-reflex conditioning protocol of Thompson et al. (2009). Each conditioning session began with a set of control trials in which feedback was not provided. Thus, in contrast to previous stretch reflex conditioning studies in monkeys and humans (Evatt et al. 1989; Wolf and Segal 1996; Wolpaw et al. 1983b; Wolpaw and O’Keefe 1984), this study aimed to differentiate the task-dependent adaptation in reflex size that occurs within each conditioning session from the long-term change that develops over many sessions and affects pathway function outside of the conditioning paradigm. Whereas task-dependent adaptation is attributable to rapid plasticity in the cortex (Thompson et al. 2009), long-term change reflects plasticity in the spinal cord (Wolpaw 1997, 2007). This study also assessed the effects of M1 conditioning on the M2 component of the stretch reflex. The results and their differences from H-reflex conditioning provide new insights into the mechanisms and wider effects of spinal reflex conditioning.
MATERIALS AND METHODS
Participants.
Fourteen participants (8 women, 6 men; ages 19–35 yr) provided written informed consent for the study. Three participated in both up- and downconditioning protocols, with at least 6 mo between the two protocols. Since up- and downconditioning are physiologically different phenomena (Carp et al. 2001a, 2001b; Carp and Wolpaw 1994, 1995; Wolpaw and Chen 2001), the first direction of conditioning would not affect the second direction of conditioning (Thompson et al. 2009). All participants were free of any known physical or neurological disorders. Approval for the study was provided by the scientific ethics committee for Nordjylland (Reference No. N-20120044). The study was performed in accordance with the Declaration of Helsinki.
Operant conditioning study overview.
After attending a familiarization session (see Familiarization session), each participant completed 6 baseline sessions and 24 upconditioning or 24 downconditioning sessions that occurred at a pace of three times per week. Figure 1 shows the operant conditioning session schedule and the setup for stretch reflex elicitation. In each session, after preparing for the SOL and tibialis anterior (TA) electromyographic (EMG) recording (see EMG recording), the SOL H reflex/M wave recruitment curve was obtained during natural standing with a stable level of SOL and TA background EMG activity. Then, 245 trials of stretch reflexes were elicited while the sitting participant produced an ~10% maximum voluntary contraction (MVC) level of SOL EMG activity and no activation of the TA with the right lower leg fixed on the custom-made apparatus (Fig. 1B) (see Session protocol). In baseline sessions, all 245 reflexes were elicited without any feedback on reflex size. In contrast, in each conditioning session, the first 20 reflexes were elicited without any feedback on reflex size and then 225 conditioning reflexes were elicited. In these 225 conditioning trials, the participant was asked to increase (upconditioning) or decrease (downconditioning) the size of M1 reflex with the aid of visual feedback, which showed after each perturbation whether the resulting reflex was larger (for upconditioning) or smaller (for downconditioning) than a criterion value. SOL and TA background EMG levels were kept stable throughout data collection. To avoid session-to-session variability in the location of electrodes, the positions of all electrodes were mapped in relation to landmarks on the skin (e.g., moles or scars) during the familiarization session. To prevent the potential diurnal variation in reflex size from affecting the results, each participant’s sessions always occurred at the same time of day (i.e., within the same 3-h time window). A typical baseline or conditioning session took ~1.5 h.
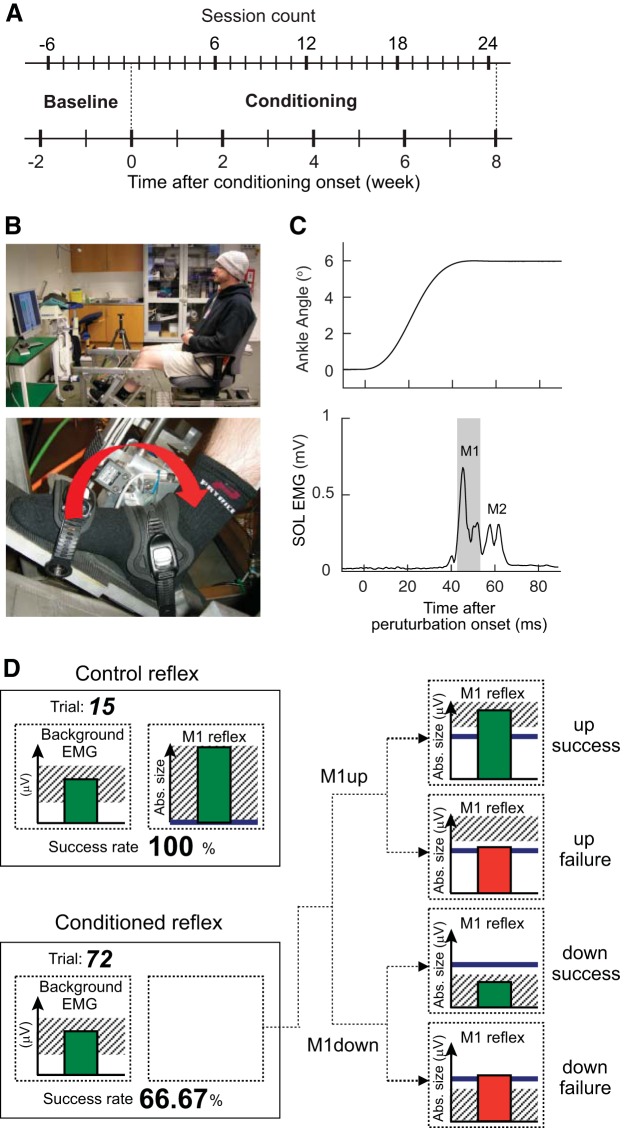
Fig. 1.
A: study session schedule: 6 baseline sessions are followed by 24 conditioning sessions, all at a pace of 3 sessions/wk. B: the stretch reflex pedal. Participants are seated comfortably with both feet on separate foot plates. C: the change in ankle angle (top) and the activity of the soleus muscle (SOL) (bottom) following a single imposed dorsiflexion perturbation. Peaks M1 and M2 are shown. Gray shaded area visualizes the time window for the M1 for which the participants received feedback during the conditioning sessions. D: visual feedback. The feedback on the screen comprises 2 parts, the background EMG and the stretch reflex size, both shown as bars. Shaded area in left panel represents the preset range for the SOL background activity, which must be maintained for at least 2 s by the participant for a stretch reflex trial to occur. Shaded area in right panel represents the targeted range for the size of the M1 component. During control trials, this shaded area is set as large as possible since the participant is not training to modify the M1 size. During M1up conditioning trials, this shaded area appears in the upper half (i.e., at criterion level and above), based on the baseline sessions. In contrast, during M1down conditioning trials, this area appears in the lower half (i.e., at criterion level and below). Immediately after a stretch reflex trial occurs (i.e., 200 ms after perturbation onset), a vertical bar reflecting M1 size is displayed. When the bar height falls within the shaded area, the participant had a successful conditioning trial and the bar is green. If the bar height falls out of the shaded area, the bar becomes red and the trial is registered as an unsuccessful trial. This provides immediate feedback on M1 size to the participant for each single trial performed. Abs., absolute; M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size.
EMG recording.
EMG activity was recorded with custom-made amplifiers and surface Ag/AgCl electrodes (Medicotest 720-01-K) placed over the belly of the right SOL and TA muscles in accordance with the recommendations of Cram and Criswell (2011) to optimize recording from these muscles and avoid contamination from other muscles. EMG activity was amplified with custom-made EMG amplifiers, filtered at 20 Hz to 2 kHz, and digitized (2 kHz) with scientific software Mr. Kick II 2.3 (Knud Larsen, Center for Sensory-Motor Interaction, Aalborg University) and stored for later off-line analysis.
Familiarization session.
All participants attended one familiarization session in which the experimental procedures were explained and implemented. This was to ensure that participants were comfortable with the electrical stimuli and perturbations that would elicit a stretch reflex (Fig. 1C). During this session, the participants were asked to perform the maximal isometric voluntary SOL contraction (MVC) while standing. The instructions were to rise up on the toes as rapidly as possible and to hold this for 1 s. This was repeated twice, and the best effort (quantified by the rectified SOL EMG amplitude) was defined as the MVC. Absolute SOL EMG amplitude range for 5–15% MVC (i.e., centering 10% MVC) level determined during the familiarization session was used for all stretch reflex measurements in the familiarization session and in all subsequent sessions. During stretch reflex trials, participants were asked to tonically activate the right SOL to produce this preset level of absolute EMG activity while sitting in a custom-made apparatus chair (Fig. 1B) with their right TA silent. EMG electrode locations were mapped in relation to permanent marks on the skin (e.g., moles and scars) so that they could be kept the same throughout the rest of the study for each participant.
Custom joint-rotation device and stretch reflex elicitation.
During all stretch reflex measurements, participants were seated in a custom-made chair that was fixed to the floor, with their knee joint flexed at ~60° (Fig. 1B). The right foot was fixed to a servo-controlled electrical actuator such that the anatomical ankle axis of rotation was closely aligned with the fulcrum of the actuator and the foot rested on a footplate. This position minimized both hip and knee movement, ensuring that the movement of the actuator was transmitted solely to the ankle joint. This knee position also minimized the possible influence of gastrocnemius activity on the SOL stretch reflex; the gastrocnemius muscle is biarticular with two heads arising from just above the femoral condyles, and act both to flex the knee joint and to plantarflex the ankle joint. The left foot was placed on a custom-made plate that extended from the actuator such that the left leg was in the same starting position as the right leg. The angular position of the actuator was monitored with an angular displacement transducer (Transtek DC ADT series 600). To elicit the stretch reflex, 6° of dorsiflexion rotation was applied at 175°/s with randomly varying intervals of 5–7 s, when the participants had maintained a background SOL contraction of 5–15% MVC for at least 2 s.
Session protocol.
In each of the baseline and conditioning sessions, first an H reflex/M wave recruitment curve was measured. Then, 245 stretch reflex trials were performed. Exact session procedures are described here.
After EMG electrode placement, an H reflex/M wave recruitment curve was obtained while the participants stood upright and provided the preset level (i.e., 5–15% of MVC level, determined during the familiarization session) of background activation in SOL. With an isolated stimulator (Noxitest IES 230), monopolar stimulation of the tibial nerve of the right leg was produced with the cathode (PALS Platinum round electrode, model 879100, 3.2 cm diameter; Axelgaard Manufacturing) in the popliteal fossa and the anode (PALS Platinum rectangular electrode, model 895340, 7.5 × 10 cm; Axelgaard Manufacturing) on the anterior aspect of the knee at the level of the patella. The cathode location was adjusted to maximize the SOL M wave. Stimuli were delivered every 5–7 s if the background level of SOL activation had been maintained at the preset level (i.e., 5–15% MVC) for at least 2 s. Stimulus intensity was increased in 5-mA increments, with three to four stimuli at each level, until an M wave (with size >50 μV) was observed; this was deemed the motor threshold (MT). Stimulus intensity continued to increase until M-wave peak-to-peak amplitude plateaued; this was defined as Mmax. Then, the scientific software Mr. Kick II 2.3 was used to control the output of the stimulator such that 10 different stimulation intensities (up to that producing Mmax) were applied randomly with three stimuli at each intensity. Stimuli were delivered every 5–7 s only if the background level of activation had been maintained at the required level for at least 2 s. The peak-to-peak values of the H reflex and M wave for each trial were extracted, and the recruitment curves were constructed. Typically, the same range of stimulus intensities was used for all baseline and conditioning sessions. The same stimulus location was maintained throughout the study for each participant.
Next, the SOL stretch reflexes were elicited in one block of 20 control trials followed by three blocks of 75 control trials (in a baseline session) or 75 conditioning trials (in a conditioning session). During control trials, no feedback was provided as to M1 size, and participants were not asked to increase or decrease it. During conditioning trials (i.e., 3 sets of 75 trials in each of 24 conditioning sessions), participants were asked to either increase (M1up) or decrease (M1down) the size of the M1 component of the stretch reflex. Immediate visual feedback was provided indicating whether the trial was a success [i.e., whether M1 size was above (M1up) or below (M1down) a size criterion; Fig. 1D].
Visual feedback.
Visual feedback provided to the participant is essentially the same as that used in the previous H-reflex conditioning studies (Makihara et al. 2014; Thompson et al. 2009, 2013), except that the feedback targeted the M1 response instead of the H reflex.
A screen ~1.5 m in front of the participant provided visual feedback on the ongoing SOL EMG activity level (left) and the size of the M1 component of the SOL stretch reflex (right), which occurred typically 39 ± 2 ms after the onset of perturbation (Fig. 1C). The background EMG panel (Fig. 1D) was the same for both control and conditioning trials. The shaded area of the background EMG panel represented the target window (i.e., corresponding to the 5–15% MVC range, determined during the familiarization session), within which the SOL EMG activity had to be maintained before reflex elicitation. The bar indicated the SOL EMG level in real time and was updated every 100 ms; it was green if the EMG level stayed within the shaded area and red if it did not.
The M1 panel (Fig. 1D) differed between control trials, during which the participant was not asked to modify M1 size, and conditioning trials, during which the participant was asked to increase (M1up) or decrease (M1down) M1 size. During control trials, the shaded area of the M1 panel indicating the range of M1 sizes that satisfied the reward criterion was set as large as possible so that all trials with various M1 sizes would be registered as “success.” During conditioning trials, this shaded area covered only the upper (for M1up) or lower (for M1down) portion of the panel; with M1up the bottom border of the shade represented the reward criterion, and with M1down the top border represented the reward criterion. For how the reward criterion was calculated, see the next paragraph. When M1 size satisfied the criterion [i.e., when the top of the bar (i.e., M1 size) got in the shaded area], the bar was green, indicating success; when M1 size did not satisfy the criterion (i.e., the top of the bar got out of the shaded area), the bar was red, indicating failure. This feedback appeared 200 ms after the imposed ankle rotation began.
As described in previous studies (Makihara et al. 2014; Thompson et al. 2009, 2013), the reward criterion level was based on the average reflex size for the previous block of trials. Thus in each conditioning session the criterion level for the first block of 75 conditioning trials was based on the immediately preceding block of 20 control trials, and the criterion levels for the second and third blocks of conditioning trials were based on the immediately preceding block of 75 conditioning trials. The criterion level was calculated such that if M1 sizes for the new block were similar to those for the previous block, 50–60% of the trials would be successful (Chen and Wolpaw 1995; Thompson et al. 2009). The thick horizontal line represented the average M1 size for the six baseline sessions. Thus the participants also received information as to their current performance in relation to their average initial M1 size. The percentage of successful trials within the current block was displayed at the bottom of the screen and updated after each trial, and the number of completed trials was shown at the top of the screen.
Data analysis.
To calculate M1 size for each participant’s session, M1 size was defined as a root mean square (RMS) value of the rectified SOL EMG in the M1 window minus an RMS value for 100 ms of preperturbation period. For each participant, the M1 window was determined as a 10-ms window including the M1 peak (the first peak response that occurred ∼45 ms after perturbation onset) by visual inspection (e.g., Fig. 1C). Then, the average size was calculated for the 20 within-session control trials, for each block of 75 trials, and for all three blocks of 75 trials together. Values were expressed as a percentage of their average values for the six baseline sessions. The size of M2, the second set of peak responses that occur ∼60 ms after perturbation onset, was calculated in a similar way. The M2 window was typically ∼58–68 ms after perturbation onset, whereas a typical M1 window was ∼42–52 ms (see Fig. 1C).
To determine for each participant whether the conditioning (M1up or M1down) had been successful, the average M1 size for the three 75-trial blocks of conditioning trials in the last six conditioning sessions (sessions C19–C24) was compared to that for the three blocks of 75 control trials of the six baselines by a single-tailed t-test. A significant change in M1 size (P < 0.05) in the direction of conditioning (i.e., increased for M1up, decreased for M1down) defined successful conditioning.
Regardless of whether the data were from a baseline session or a conditioning session, for each participant M1 sizes from all three 75-trial blocks were averaged together and called “conditioned M1,” and M1 sizes from the 20 control trials were averaged together and called “control M1.” The final effect of the protocol on the conditioned M1 size was calculated by averaging the M1 size for the three 75-trial blocks of conditioning sessions C22–C24 and expressing the value as a percentage of the average M1 size for the three 75-trial blocks of the six baseline sessions. The final effect on the control M1 size was calculated by averaging the M1 size for the 20 control trials of conditioning sessions C22–C24 and expressing the value as a percentage of the average M1 size for the first 20 trials of the six baseline sessions. To assess the time course of changes, a repeated-measures ANOVA was used to evaluate conditioned and control M1 sizes across successive 6-session bins (i.e., baseline sessions B1–B6 and conditioning sessions C1–C6, C7–C12, C13–C18, and C19–C24). Comparable procedures were used to assess the impact of the conditioning protocol on the M2 component of the stretch reflex. This procedure was chosen over characterizing the learning via a function, based on the intersession variability of the reflexes.
To assess the session-to-session variability in EMG recording condition, the peak-to-peak Mmax and the peak-to-peak Hmax were calculated from the recruitment curve measured at the beginning of each session. To assess the stability of background EMG activity across sessions, the SOL and TA background EMG were calculated for each session, along with M1 and M2 sizes. These values were evaluated with a repeated-measures ANOVA in the same way as the time course evaluation for M1 and M2 changes.
RESULTS
Stability of Mmax, Hmax, and background EMG.
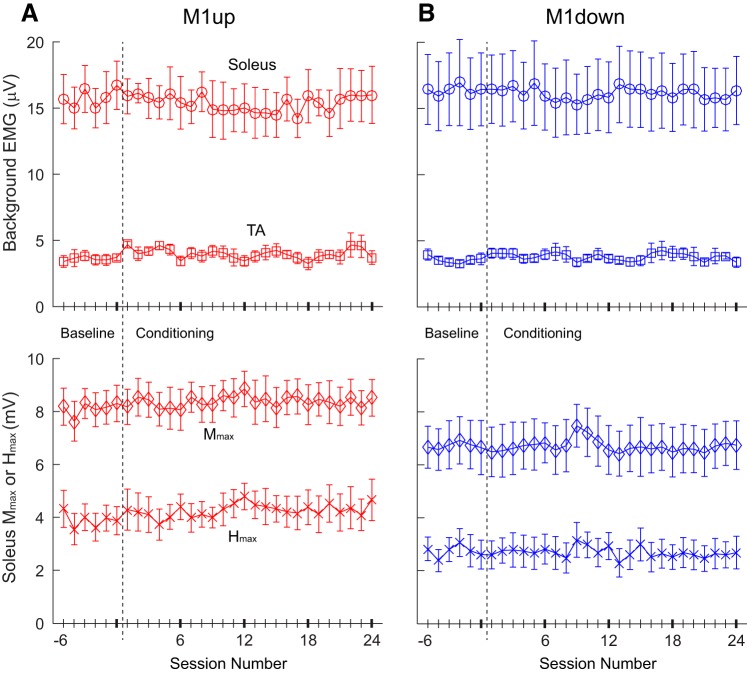
To ensure that M1 changes over sessions were not due to intersession differences in electrode placements, we measured the Mmax at the beginning of every session. Across all M1up participants, the Mmax averaged 7.7 ± 0.2 (SE) mV during the baseline sessions and 7.7 ± 0.2 mV during the conditioning sessions. One-way repeated-measures ANOVA revealed no significant difference across the sessions [F(4,28) = 0.91, P = 0.47]. Similarly, the M1down group showed no significant difference across sessions [F(4,28) = 1.01, P = 0.42; Mmax baseline sessions: 6.5 ± 0.8 mV, conditioning sessions: 6.5 ± 0.8 mV]. Hmax did not change significantly across sessions for either the M1up [F(4,28) = 0.899, P = 0.48] or the M1down [F(4,28) = 0.47, P = 0.76] group; it averaged 3.3 ± 0.2 mV for M1up and 2.8 ± 0.4 mV for M1down during the baseline sessions and 3.5 ± 0.2 mV for M1up and 2.8 ± 0.2 mV for M1down during the conditioning sessions. SOL background EMG also remained stable throughout the study [F(4,28) = 0.335, P = 0.852 for M1up group; F(4,28) = 0.338, P = 0.850 for M1down group; 1-way repeated-measures ANOVA]. TA background EMG remained at resting level (i.e., <5 μV) in both groups throughout the study. Overall, SOL Mmax and Hmax and SOL and TA background EMG values all remained within ±10% of the baseline values throughout the study. Stability of these values in the M1up and M1down groups of participants in whom conditioning was successful is displayed in Fig. 2 and summarized in Table 1.
Fig. 2.
Soleus and tibialis anterior (TA) background EMG and the soleus maximum M-wave amplitude (Mmax) and maximum H-reflex amplitude (Hmax) values for all baseline and conditioning sessions in M1up (A) and M1down (B) participants in whom conditioning was successful. Each set of a symbol and error bars represents the average (±SE) value for successfully conditioned participants. n = 5 for M1up (A), and n = 8 for M1down (B). M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size; ○, Soleus background EMG amplitude; □, TA background EMG; ◇, Mmax; ×, Hmax.
Table 1.
Soleus and tibialis anterior background EMG during stretch reflex trials and soleus Mmax and Hmax values during standing for each of successive 6-session blocks
| Group | B1–B6 | C1–C6 | C7–C12 | C13–C18 | C19–C24 | |
|---|---|---|---|---|---|---|
| Soleus EMG, μV | M1up | 15.8 ± 1.7 | 15.8 ± 1.4 | 15.2 ± 1.6 | 14.9 ± 1.7 | 15.6 ± 1.8 |
| M1down | 16.4 ± 2.8 | 16.4 ± 2.7 | 15.7 ± 2.6 | 16.3 ± 2.8 | 16.1 ± 2.5 | |
| TA EMG, μV | M1up | 3.6 ± 0.4 | 4.2 ± 0.2 | 3.9 ± 0.3 | 3.8 ± 0.4 | 4.0 ± 0.5 |
| M1down | 3.6 ± 0.3 | 3.9 ± 0.3 | 3.8 ± 0.4 | 3.8 ± 0.4 | 3.7 ± 0.4 | |
| Soleus Mmax, mV | M1up | 8.1 ± 0.6 | 8.2 ± 0.7 | 8.5 ± 0.6 | 8.4 ± 0.7 | 8.4 ± 0.7 |
| M1down | 6.7 ± 0.9 | 6.7 ± 0.9 | 6.7 ± 0.9 | 6.6 ± 0.9 | 6.7 ± 0.9 | |
| Soleus Hmax, mV | M1up | 3.9 ± 0.5 | 4.1 ± 0.6 | 4.3 ± 0.5 | 4.3 ± 0.6 | 4.3 ± 0.7 |
| M1down | 2.7 ± 0.4 | 2.7 ± 0.6 | 2.7 ± 0.6 | 2.6 ± 0.5 | 2.6 ± 0.5 |
Values (expressed as % of baseline values) are means ± SE for successful M1up or M1down participants. None of the values from conditioning sessions (C1–C24) is significantly different from the values from baseline sessions (B1–B6). Hmax, maximum H-reflex amplitude; M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size; Mmax, maximum M-wave amplitude; TA, tibialis anterior.
M1 and M2 stability in baseline sessions.
All participants completed six baseline sessions in each of which 245 control reflexes were elicited. There was no significant difference in average M1 size between the initial 20 control trials and the subsequent 225 control trials in either the M1up or M1down group (all participants included, 2-tailed paired t-test, P = 0.99 for each group). M1 size did not differ significantly among the three 75-trial blocks or across the baseline sessions [block × session interaction, P = 0.13 (M1up) and P = 0.99 (M1down)].
There was no significant difference in average M2 size between the initial 20 trials and the subsequent 225 trials in either the M1up or M1down group (2-tailed paired t-test, P = 0.99 for both types of training). M2 size did not differ significantly among the three 75-trial blocks or across the baseline sessions [block × session interaction, P = 0.41 (M1up) and P = 0.47 (M1down)].
Effect of conditioning on size of conditioned M1 reflex.
As noted above, for each session, the data for the three 75-trial blocks were combined to calculate the average conditioned M1 reflex size, which is referred to as the conditioned M1 size and is expressed as a percentage of the participant’s average M1 size for the three 75-trial blocks of the six baseline sessions. Across all participants (i.e., n = 8 for M1up and n = 8 for M1down) there was a significant change between the baseline session and the last six conditioning sessions (1-tailed paired t-test, P = 0.02 and P < 0.001 for M1up and M1down, respectively). By the analysis described in materials and methods, conditioning was successful in five of eight M1up participants and in all eight M1down participants. In the other three M1up participants, M1 size did not change significantly. Of the three participants who completed both the M1up and M1down protocols, two were successful in both and one was unsuccessful in M1up conditioning and subsequently (>6 mo later) was successful in M1down conditioning. Figure 3A shows the final conditioned M1 sizes (defined as the average conditioned M1 sizes for sessions C22–C24) for the eight M1up and eight M1down participants. Since the main aim of this study was to characterize the time course of M1 and M2 changes in the responders of M1 conditioning, the rest of this presentation focuses primarily on the data from the 13 successful participants (5 M1up and 8 M1down).
Fig. 3.
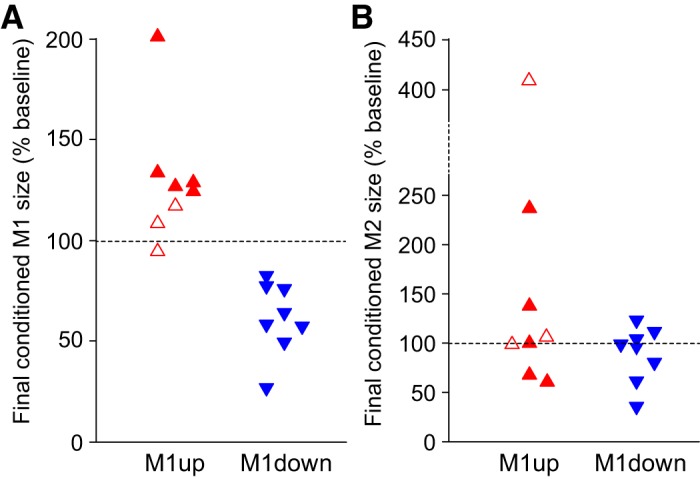
A: the final conditioned M1 size for individual participants. Filled symbols represent successful participants in whom the average conditioned M1 for conditioning sessions 19–24 was significantly increased (5 of 8 M1up participants, ▲) or decreased (8 of 8 M1down participants, ▼) compared with the average baseline M1. B: the final conditioned M2 size for individual participants. As for A, filled symbols represented the successful participants in whom the average conditioned M1 for conditioning sessions 19–24 was significantly increased (5 of 8 M1up participants, ▲) or decreased (8 of 8 M1down participants, ▼) compared with the average baseline M1. Open symbols show the 3 unsuccessful participants (i.e., 3 of 8 M1up participants). M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size.
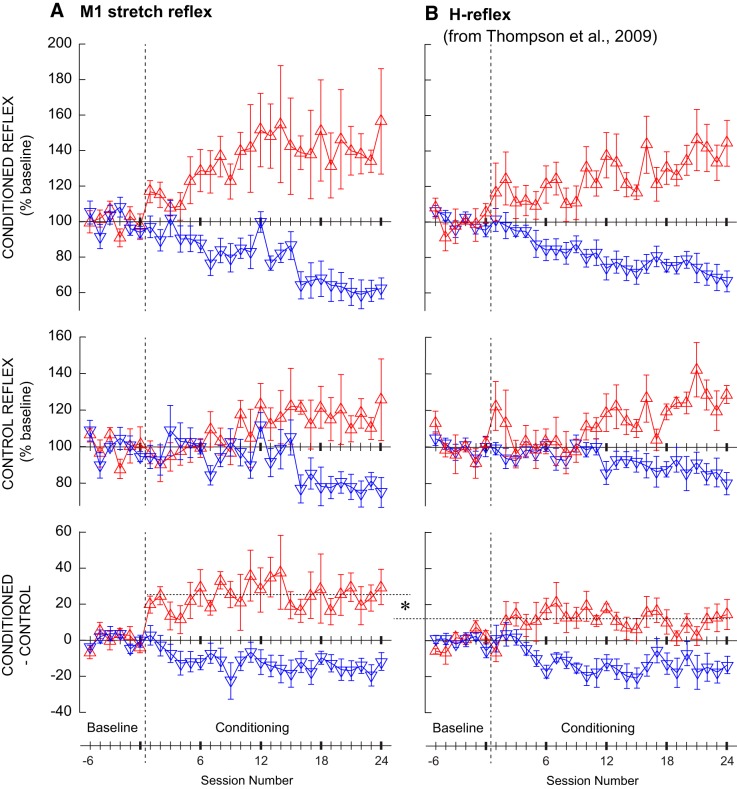
Figure 4, bottom, shows the average rectified SOL EMG data of the three 75-trial blocks for one baseline session and the last conditioning session in single participants. Figure 4, top, shows the ankle angle during these stretch reflex trials. Figure 5A summarizes the time course of M1 size changes for the successfully conditioned participants of both groups. The conditioned M1 size increased in the M1up group [F(4,16) = 4.46, P = 0.013] and decreased in the M1down group [F(4,28) = 18.79, P < 0.001] (Fig. 5A, top). The final conditioned M1 size was 143 ± 15% (SE) for the M1up group and 62 ± 6% for the M1down group. To aid in assessing the time course of M1 changes, the time course of H-reflex changes using data from participants asked to increase (HRup) or decrease (HRdown) H reflex in Thompson et al. (2009) is displayed in Fig. 5B.
Fig. 4.
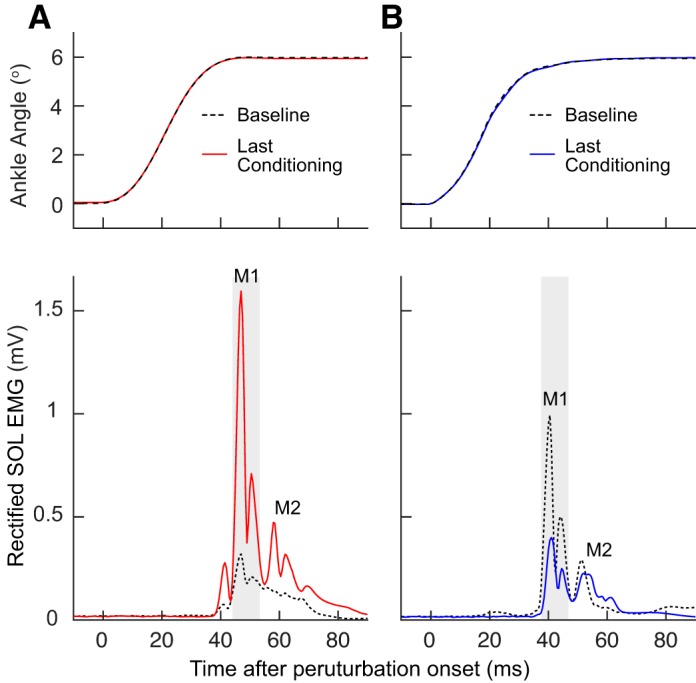
Average conditioned and control stretch reflexes for a representative participant of each of the M1up (A) and M1down (B) groups. Top: the change in ankle angle. Bottom: the average rectified soleus (SOL) EMG data of the three 75-trial blocks for 1 baseline session and the last conditioning session in single participants. Dashed black traces represent data from a single baseline session, and red (M1up) and blue (M1down) traces represent data from the last conditioning session. The gray shaded area indicates the M1 response window. M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size.
Fig. 5.
Average (±SE) M1 sizes and H-reflex sizes (from Thompson et al. 2009) for all successful M1up/HRup (red) and M1down/HRdown (blue) participants for all baseline and conditioning sessions. A, top: average conditioned M1 size. Middle: average control M1 size. Bottom: within-sessions change (average conditioned minus control M1 size). B, top: average conditioned H-reflex size. Middle: average control H-reflex size. Bottom: within-sessions change (average conditioned minus control H-reflex size). Vertical dashed line separates the baseline sessions from the conditioning sessions. M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size. *Significant difference (P < 0.05) between the two conditioning protocols in the amount of within-session reflex change.
Effect of conditioning on size of control M1 reflex.
As defined in materials and methods, the M1 reflex obtained in the first 20 control trials of the control sessions and the 20 control trials of the conditioning sessions is referred to as the “control M1.” Figure 5A, middle, shows the time course of control M1 size changes across 6 baseline and 24 conditioning sessions for the M1up and M1down participants expressed as a percentage of baseline values. The control M1 size increased in the M1up group [F(4,16) = 3.09, P = 0.046] and decreased in the M1down group [F(4,28) = 6.72, P = 0.001]. The final control M1 size was 126 ± 18% (SE) of baseline for the M1up group and 75 ± 8% of baseline for the M1down group.
It appears that the control M1 requires more time to change compared with the conditioned M1 reflex. For the M1up group, the conditioned M1 size was consistently above baseline values from conditioning session 1 on, whereas the control M1 did not exceed the baseline value until session 10. For the M1down group, the conditioned M1 was consistently below the baseline by session 4, whereas it was not until session 16 that the control M1 was consistently below baseline. Although the onset was delayed, the control M1 change was obvious toward the end. Figure 6 shows a typical example of control M1 change with M1down conditioning.
Fig. 6.
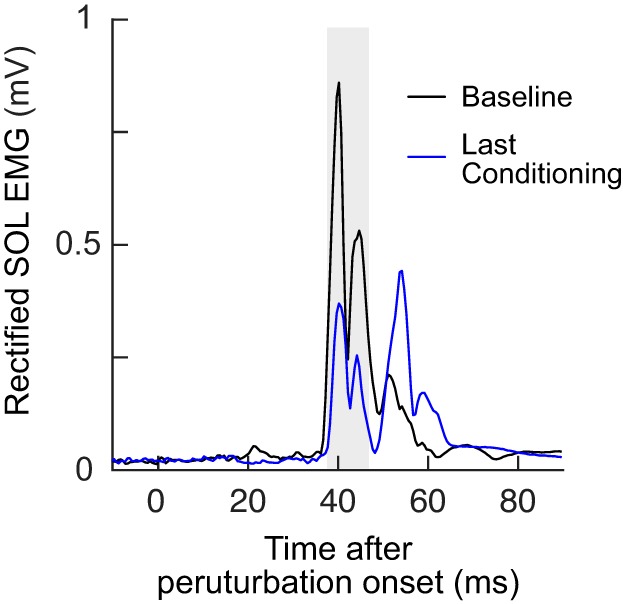
Control M1 change with M1down conditioning: control soleus (SOL) EMG during 1 baseline session and the final conditioning session for n = 1. Each trace is the average of 20 trials. The gray shaded area indicates the M1 response window. M1down, conditioning to decrease M1 size.
Within-session task-dependent adaptation in M1 reflex.
To quantify the task-dependent change in the M1 reflex from control trials to conditioning trials, for each session the control M1 size was subtracted from its conditioned M1 size (Fig. 5A, bottom). A within-session difference in the correct direction (i.e., positive for the M1up group and negative for the M1down group) is referred to as task-dependent adaptation (Thompson et al. 2009). It appears in session 1 for M1up participants and by session 4 for M1down participants, and it remains about the same over the remaining sessions. Its average final (sessions 22–24) values are 24 ± 9% (SE) and −15 ± 4% for the M1up and M1down groups, respectively. Notably, the amount of task-dependent adaptation with upconditioning was larger with M1up than with HRup (P = 0.01 by unpaired t-test for sessions 1–24). Figure 7 shows an example of within-session task-dependent adaptation in a typical M1up participant.
Fig. 7.
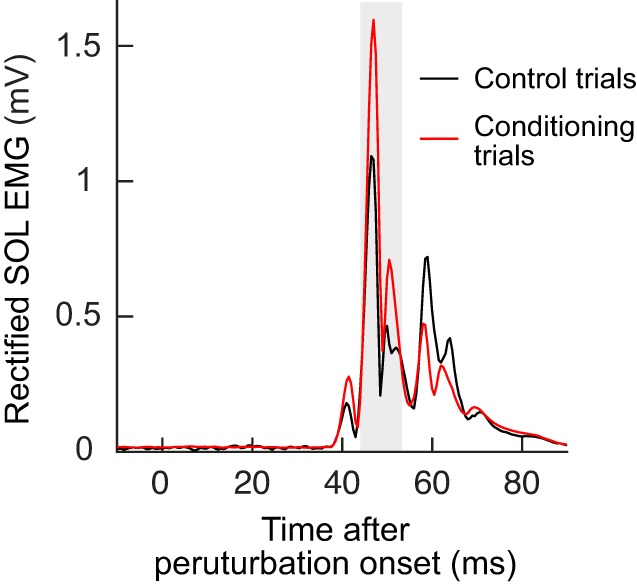
Within-session task dependent adaptation with M1up conditioning: control soleus (SOL) EMG during the control trials (the first 20 trials where no visual feedback is provided) and the conditioning trials (the 3 blocks of 75 trials where feedback is provided in relation to the size of the M1 response) for n = 1. The gray shaded area indicates the M1 response window. M1up, conditioning to increase M1 size.
Table 2 summarizes for the M1up and M1down participants the changes in control and conditioned M1 reflexes and their within-session difference (i.e., task-dependent adaptation) across the conditioning sessions grouped into four 6-session bins (C1–6, C7–12, C13–18, and C19–24). To delineate the similarities and differences between M1 conditioning and H-reflex conditioning, the data from this study are presented with the previous H-reflex conditioning data (Thompson et al. 2009); significant differences from the average of the 6 baseline sessions are indicated. Table 2 verifies the control/conditioned M1 differences in the onset of the impact of conditioning. For the M1up group the within-session difference is significant from the first bin of 6 sessions on, whereas the conditioned M1 is significantly different from the second bin of 6 sessions and the control M1 is not significantly different until the fourth (and final) bin. For the M1down group, the within-session difference is significant from the third 6-session bin, the conditioned M1 is significantly smaller from the second 6-session bin, and the control M1 is not significantly different until the fourth bin.
Table 2.
M1 reflex values for all successful M1up and M1down participants compared with H-reflex values for all successful HRup and HRdown participants from Thompson et al. (2009)
| Group | C1–C6 | C7–C12 | C13–C18 | C19–C24 | |
|---|---|---|---|---|---|
| Conditioned reflex | M1up | 116.9 ± 6.8* | 136.8 ± 12.7* | 145.7 ± 22.4* | 141.2 ± 17.2* |
| HRup | 115.6 ± 6.2 | 122.4 ± 5.5* | 127.6 ± 7.5* | 137.3 ± 8.6* | |
| Control reflex | M1up | 96.6 ± 4.3 | 109.5 ± 5.6 | 118.4 ± 11.2* | 117.2 ± 10.9* |
| HRup | 106.4 ± 6.0 | 106.7 ± 3.7 | 116.5 ± 4.9 | 128.2 ± 5.4* | |
| Within-session change | M1up | 20.3 ± 6.2* | 27.2 ± 8.5* | 27.3 ± 12.5* | 24.0 ± 7.9* |
| HRup | 9.2 ± 5.4 | 15.6 ± 4.1* | 11.6 ± 4.2* | −12.4 ± 6.0* | |
| Conditioned reflex | M1down | 92.9 ± 5.1 | 84.9 ± 6.2* | 74.4 ± 5.9* | 62.1 ± 7.1* |
| HRdown | 93.4 ± 4.0 | 81.7 ± 4.4* | 75.1 ± 4.9* | 72.3 ± 5.3* | |
| Control reflex | M1down | 100.0 ± 5.8 | 96.8 ± 4.2 | 89.2 ± 6.5 | 77.5 ± 5.5* |
| HRdown | 97.1 ± 1.8 | 95.5 ± 2.8 | 90.5 ± 4.5 | 86.7 ± 5.9* | |
| Within-session change | M1down | −7.5 ± 3.8 | −11.9 ± 5.2 | −14.8 ± 5.0* | −15.3 ± 4.0* |
| HRdown | −3.8 ± 3.2 | −13.9 ± 3.2* | −14.4 ± 5.1* | −14.4 ± 6.3* |
Values (expressed as % of baseline values) are average ± SE M1 reflex values for all successful M1up and M1down participants and H-reflex values for all successful HRup and HRdown participants from the study by Thompson et al. (2009) for each of successive 6-session blocks (conditioning sessions C1–C24). HRdown, conditioning to decrease H-reflex size; HRup, conditioning to increase H-reflex size. M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size.
Significant differences from the 6 baseline sessions (P < 0.05, least significant difference post hoc after repeated-measures ANOVA).
Effect of M1 conditioning on size of M2 reflex.
Similar to the M1 size calculation, the M2 values from the three 75-trial blocks were combined to calculate the average M2 size for each conditioning session; this is referred to as the “conditioned M2” size and expressed as a percentage of the participant’s average M2 size across the six baseline sessions. M1 conditioning changed M2 size in the direction of M1 conditioning in four of the eight M1up participants and in five of the eight M1down participants. For the whole group, the final conditioned M2 did not change significantly in the successful M1up group (n = 5, P = 0.28, by paired t-test) or the successful M1down group (n = 8, P = 0.16) (Fig. 3B). Final conditioned M2 size averaged 120 ± 32% for the successful M1up and 89 ± 10% for the successful M1down group. Final control M2 size was 118 ± 32% for the successful M1up and 99 ± 5% for the successful M1down group. The final M2 within-session change was 2 ± 14% and −10 ± 9% for the M1up and M1down groups, respectively. Table 3 summarizes the changes in conditioned M2 and control M2, as well as M2 within-session change across the four 6-session bins of conditioning sessions (C1–6, C7–12, C13–18, and C19–24).
Table 3.
M2 stretch reflex values for all successful M1up and M1down participants for each of successive 6-session blocks
| Group | C1–C6 | C7–C12 | C13–C18 | C19–C24 | |
|---|---|---|---|---|---|
| Conditioned M2 reflex | M1up | 119.4 ± 17.5 | 126.3 ± 30.1 | 126.8 ± 28.2 | 122.7 ± 23.6 |
| M1down | 110.4 ± 7.7 | 104.8 ± 11.5 | 97.5 ± 9.3 | 89.9 ± 9.6 | |
| Control M2 reflex | M1up | 111.2 ± 14.6 | 129.1 ± 30.9 | 139 ± 40.2 | 124.4 ± 26.3 |
| M1down | 111.4 ± 3.9 | 110.5 ± 7.7 | 100.1 ± 8.3 | 97.4 ± 6.5 | |
| Within-session change | M1up | 8.2 ± 9.3 | −2.8 ± 12.4 | −12.2 ± 16.5 | −1.7 ± 11.6 |
| M1down | −1.0 ± 5.7 | −5.7 ± 4.8 | −2.6 ± 8.3 | −7.5 ± 6.8 |
Values (expressed as % of baseline values) are averages ± SE for conditioning sessions C1–C24. M1down, conditioning to decrease M1 size; M1up, conditioning to increase M1 size.
DISCUSSION
This is, to our knowledge, the first demonstration of operant conditioning of the M1 component of the human SOL stretch reflex. The reflex changed without alterations in background EMG, initial muscle length, or imposed perturbation. M1up conditioning was successful in five of eight participants and M1down conditioning in all eight participants. M1 size increased to 143 ± 15% of its initial value in the successful M1up participants and decreased to 62 ± 6% in the M1down participants. These success rates are similar to those for SOL H-reflex and biceps stretch reflex conditioning in animals (Carp et al. 2005; Chen and Wolpaw 1995; Wolpaw 1983, 1987; Wolpaw et al. 1983a) and humans (Evatt et al. 1989; Thompson et al. 2009; Wolf and Segal 1996).
The results are particularly notable in two respects. First, they confirm the two-phase acquisition of an operantly conditioned spinal reflex increase or decrease, hypothesized in 1984 (Wolpaw and O’Keefe 1984) and first documented in 2009 (Thompson et al. 2009). Second, they assess the impact of M1 conditioning on the M2 component of the stretch reflex. Because the stretch reflex is more directly related to motor function than the H reflex, both of these contributions illuminate the implications of spinal reflex conditioning for understanding normal motor function and for developing protocols that can address the reflex abnormalities associated with spinal cord injury, stroke, or other chronic disorders.
Task-dependent adaptation and long-term change in M1.
As in SOL H-reflex conditioning (Thompson et al. 2009), the data reveal a two-phase phenomenon: task-dependent adaptation within conditioning sessions (phase 1) and long-term change across conditioning sessions (phase 2). However, the time courses of these two phases differed from those found for the SOL H reflex (Thompson et al. 2009). The task-dependent adaptation for a conditioning session was defined as the conditioned M1 size (M1 size for the 225 conditioning trials) minus the control M1 size (M1 size for the 20 control trials). Thus it shows the immediate effect of asking the participant to increase (or decrease) M1 and providing immediate feedback as to whether the size criterion was met. In contrast, long-term change was indicated by the increase (or decrease) in the control M1 size over conditioning sessions. Thus it assesses the persistent effect of the conditioning sessions.
In the present results, task-dependent adaptation first appeared in conditioning session 1 (M1up) or session 4 (M1down) and remained stable over the remaining sessions. Long-term change appeared in session 10 (M1up) or session 16 (M1down) and grew gradually over the remaining sessions. The difference between the M1up and M1down groups in the onset of task-specific adaptation and long-term change [also seen for SOL H-reflex conditioning (Thompson et al. 2009)] is further evidence that upconditioning and downconditioning are not mirror images of each other but have different mechanisms (Thompson et al. 2009; Wolpaw 2007). The development of the two phases over the course of up- and downconditioning with M1 or H-reflex conditioning (Thompson et al. 2009) is summarized in Table 2. M1 upconditioning produces greater task-dependent adaptation and less long-term change (although insignificant) than H-reflex upconditioning; in contrast, M1 downconditioning produces comparable task-dependent adaptation and greater (although insignificant) long-term change compared with H-reflex downconditioning. Overall, for both task-dependent adaptation and long-term change, the time courses of development are similar for M1 and H-reflex conditioning.
How do we interpret the differences and similarities between M1 and H-reflex conditioning? The H reflex is referred to as the electrical analog of the M1 stretch reflex. A principal difference is that the H reflex bypasses the muscle spindle whereas M1 is affected by the sensitivity of the spindle and thus by γ-motoneuron activity. This may constitute an extra degree of freedom available for participants as they learn to change M1 size. Muscle spindle excitation is affected by initial muscle length (Matthews 1972) and muscle background activity (Marsden et al. 1976, 1983). Both of these variables were maintained stable throughout the study; thus it is unlikely that they contributed to the changes. It is possible that alterations in γ-motoneuron activity contributed to M1 change, as suggested for monkey biceps M1 conditioning (Wolpaw and O’Keefe 1984).
Another potential site of change is the Ia synapse, known to be modulated by presynaptic inhibition (Eccles et al. 1962; Stein 1995). This inhibition is influenced by corticospinal, reticulospinal, and vestibulospinal pathways (Baldissera et al. 1981; Iles 1996; Meunier and Pierrot-Deseilligny 1998; Pierrot-Deseilligny and Burke 2012) and is task-dependently modulated (Côté and Gossard 2003; Hultborn et al. 1987; Stein 1995; Stein and Capaday 1988). Morita et al. (1998) reported evidence that the stretch reflex is less sensitive to presynaptic inhibition than the H reflex; this is not consistent with the present finding that the conditioned M1 change is greater than the H-reflex change. In the present study, M1up produced a larger within-session M1 increase (task-dependent adaptation), for which the most plausible mechanism is a change in presynaptic inhibition (Capaday and Stein 1987). Indeed, a release of presynaptic inhibition at the segmental level has been implicated as a mechanism responsible for the increase in H-reflex and stretch reflex size during the Jendrassik maneuver (Dowman and Wolpaw 1988; Zehr and Stein 1999). However, in the present study, participants were instructed to keep the upper body (including facial muscles) relaxed throughout all the reflex trials. Thus changes in presynaptic inhibition via the Jendrassik maneuver are unlikely to have occurred here. In addition to presynaptic inhibition, animal studies, which would capture long-term physiological and/or anatomical changes, provide substantial evidence of altered motoneuron properties in GABAergic terminals on motoneurons and possibly in oligosynaptic afferent pathways (Thompson and Wolpaw 2014; Wolpaw 1997, 2010). Further studies are needed to identify the mechanisms underlying M1 and H-reflex conditioning and the extent to which they differ.
Effect on M2 component of soleus stretch reflex.
For M1up and M1down groups, the changes in M1 did not have large or consistent effects on M2 size. This differs from stretch reflex conditioning in the biceps brachii M1, which produced significant changes in M2 (Wolf et al. 1995). Since the M2 of upper limb muscles is likely generated through transcortical pathways (Capaday et al. 1991; Crago et al. 1976; Goodin et al. 1990; Marsden et al. 1977; Palmer and Ashby 1992; Rothwell et al. 1986; Thilmann et al. 1991), it might be more comparable to the M3 for lower limb muscles (Mrachacz-Kersting et al. 2006; Petersen et al. 1998). (Note that the M3 was not measurable in the present study, in which the conditioning protocol was administered in sitting participants.) The M2 of the SOL is thought to be largely mediated by group Ib and/or group II afferents (af Klint et al. 2010; Dietz 1998; Dietz and Duysens 2000; Grey et al. 2001; Sinkjaer et al. 2000; Schieppati and Nardone 1997, 1999). Thus the SOL M2 is not comparable to the M2 of the biceps brachii.
A possible explanation for why M2 did not change consistently is a potentially mixed origin of M2. Because the distinction between M1 and M2 is based on their latencies, there is a possibility that delayed Ia excitation of motoneurons may contribute to M2, in addition to the excitation from Ib and/or group II afferents. With M1 upconditioning, such delayed Ia excitation could decrease, as those motoneurons could start firing in the M1 time window. This explanation seems feasible in some but not all participants, however. Another explanation is related to the functional relevance of M2 during the sitting task in the present study. It is unlikely that the M1 or M2 in the SOL would have significant function in the sitting posture; thus changes in M2 would not necessarily reflect systematic changes [compensatory or reactive plasticity (Wolpaw 2010)]. Future studies should condition the SOL stretch reflex during more functional tasks such as standing, as has been done for the SOL H-reflex conditioning (Makihara et al. 2014; Thompson et al. 2009).
Functional implications.
Several types of afferents generate the stretch reflex: group Ia and II afferents arising from muscle spindles, group Ib afferents from Golgi tendon organs, and cutaneous afferents. Providing perturbations such as the ankle joint rotations used here is one way to probe these pathways and quantify their role during tasks such as walking. These perturbations activate the afferents in a manner similar to what occurs during normal behavior: they are dispersed in time, and the same afferents may be activated several times. Additionally, and unlike the H reflex, the stretch reflex size is affected by the sensitivity of the muscle spindle, and thus by descending gamma drive. Conditioning of the stretch reflex thereby provides a more natural paradigm for the adaptations of the underlying circuitry.
Appropriate SOL H-reflex conditioning leads to a return to a more normal gait pattern in spinal cord-injured animals and humans (Chen et al. 2006; Manella et al. 2013; Thompson et al. 2013). This favorable effect on locomotion results from the H-reflex changes triggering much wider beneficial plasticity (Thompson et al. 2013; Thompson and Wolpaw 2014). Although the H reflex depends mainly on the Ia afferent pathway, group Ib and II afferents appear to play a prominent role in generating SOL locomotor activity in humans (af Klint et al. 2010; Dietz 1998; Dietz and Duysens 2000; Grey et al. 2001; Schieppati and Nardone 1997, 1999; Sinkjaer et al. 2000). Thus operant conditioning of the SOL stretch reflex, which engages these other pathways, might prove to be a more efficient and/or effective therapeutic approach than H-reflex conditioning.
GRANTS
This work was supported in part by Villum Kann Rasmussen Foundation and Spar Nord Fond of Denmark, the National Institute of Neurological Disorders and Stroke (NS-069551 to A. K. Thompson), and the National Institute of General Medical Sciences [GM-104941, Institutional Development Award (IDeA) to Stuart Binder-MacLeod].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.M.-K., U.G.K., T.S., and A.K.T. conceived and designed research; N.M.-K., U.G.K., P.d.B.S., and Y.M. performed experiments; N.M.-K., P.d.B.S., Y.M., and A.K.T. analyzed data; N.M.-K., U.G.K., T.S., and A.K.T. interpreted results of experiments; N.M.-K. and A.K.T. prepared figures; N.M.-K., U.G.K., and A.K.T. drafted manuscript; N.M.-K., U.G.K., L.A.-N., T.S., and A.K.T. edited and revised manuscript; N.M.-K., U.G.K., P.d.B.S., Y.M., L.A.-N., T.S., and A.K.T. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Jonathan R. Wolpaw for providing invaluable advice and comments on the manuscript on behalf of the National Center for Adaptive Neurotechnologies (National Institute of Biomedical Imaging and Bioengineering, P41 EB-018783).
REFERENCES
- af Klint R, Mazzaro N, Nielsen JB, Sinkjaer T, Grey MJ. Load rather than length sensitive feedback contributes to soleus muscle activity during human treadmill walking. J Neurophysiol 103: 2747–2756, 2010. doi: 10.1152/jn.00547.2009. [DOI] [PubMed] [Google Scholar]
- Arris D, Henneman E. Feedback signals from muscle and their efferent control. In: Medical Physiology, edited by Mountcastle VB. St. Louis, MO: Mosby, 1980, p. 703–717. [Google Scholar]
- Baldissera F, Hultborn H, Illert M.. Integration in spinal neuronal systems. In: Handbook of Physiology, The Nervous System, Motor Control, edited by Brooks VB. Bethesda, MD: American Physiological Society, 1981, p. 509–595. [Google Scholar]
- Burke D, Gandevia SC, McKeon B. The afferent volleys responsible for spinal proprioceptive reflexes in man. J Physiol 339: 535–552, 1983. doi: 10.1113/jphysiol.1983.sp014732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Forget R, Fraser R, Lamarre Y. Evidence for a contribution of the motor cortex to the long-latency stretch reflex of the human thumb. J Physiol 440: 243–255, 1991. doi: 10.1113/jphysiol.1991.sp018706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaday C, Stein RB. A method for simulating the reflex output of a motoneuron pool. J Neurosci Methods 21: 91–104, 1987. doi: 10.1016/0165-0270(87)90107-5. [DOI] [PubMed] [Google Scholar]
- Carp JS, Chen XY, Sheikh H, Wolpaw JR. Operant conditioning of rat H-reflex affects motoneuron axonal conduction velocity. Exp Brain Res 136: 269–273, 2001a. doi: 10.1007/s002210000608. [DOI] [PubMed] [Google Scholar]
- Carp JS, Chen XY, Sheikh H, Wolpaw JR. Motor unit properties after operant conditioning of rat H-reflex. Exp Brain Res 140: 382–386, 2001b. doi: 10.1007/s002210100830. [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Schalk G, Wolpaw JR. Long-term spinal reflex studies in awake behaving mice. J Neurosci Methods 149: 134–143, 2005. doi: 10.1016/j.jneumeth.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Carp JS, Tennissen AM, Chen XY, Wolpaw JR. H-reflex operant conditioning in mice. J Neurophysiol 96: 1718–1727, 2006. doi: 10.1152/jn.00470.2006. [DOI] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol 72: 431–442, 1994. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron properties after operantly conditioned increase in primate H-reflex. J Neurophysiol 73: 1365–1373, 1995. doi: 10.1152/jn.1995.73.4.1365. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of H-reflex in freely moving rats. J Neurophysiol 73: 411–415, 1995. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen L, Liu R, Wang Y, Chen XY, Wolpaw JR. Locomotor impact of beneficial or nonbeneficial H-reflex conditioning after spinal cord injury. J Neurophysiol 111: 1249–1258, 2014. doi: 10.1152/jn.00756.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen L, Wang Y, Wolpaw JR, Chen XY. Operant conditioning of rat soleus H-reflex oppositely affects another H-reflex and changes locomotor kinematics. J Neurosci 31: 11370–11375, 2011. doi: 10.1523/JNEUROSCI.1526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006. doi: 10.1523/JNEUROSCI.2198-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté MP, Gossard JP. Task-dependent presynaptic inhibition. J Neurosci 23: 1886–1893, 2003. doi: 10.1523/JNEUROSCI.23-05-01886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol 39: 925–935, 1976. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Cram JR, Criswell E. Cram’s Introduction to Surface Electromyography. Sudbury, MA: Jones & Bartlett, 2011. [Google Scholar]
- Dietz V. Evidence for a load receptor contribution to the control of posture and locomotion. Neurosci Biobehav Rev 22: 495–499, 1998. doi: 10.1016/S0149-7634(97)00035-3. [DOI] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture 11: 102–110, 2000. doi: 10.1016/S0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Dowman R, Wolpaw JR. Jendrassik maneuver facilitates soleus H-reflex without change in average soleus motoneuron pool membrane potential. Exp Neurol 101: 288–302, 1988. doi: 10.1016/0014-4886(88)90012-X. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Schmidt RF, Willis WD. Presynaptic inhibition of the spinal monosynaptic reflex pathway. J Physiol 161: 282–297, 1962. doi: 10.1113/jphysiol.1962.sp006886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evatt ML, Wolf SL, Segal RL. Modification of human spinal stretch reflexes: preliminary studies. Neurosci Lett 105: 350–355, 1989. doi: 10.1016/0304-3940(89)90646-0. [DOI] [PubMed] [Google Scholar]
- Goodin DS, Aminoff MJ, Shih PY. Evidence that the long-latency stretch responses of the human wrist extensor muscle involve a transcerebral pathway. Brain 113: 1075–1091, 1990. doi: 10.1093/brain/113.4.1075. [DOI] [PubMed] [Google Scholar]
- Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol 534: 925–933, 2001. doi: 10.1111/j.1469-7793.2001.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol 389: 757–772, 1987. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol 491: 197–207, 1996. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makihara Y, Segal RL, Wolpaw JR, Thompson AK. Operant conditioning of the soleus H-reflex does not induce long-term changes in the gastrocnemius H-reflexes and does not disturb normal locomotion in humans. J Neurophysiol 112: 1439–1446, 2014. doi: 10.1152/jn.00225.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella KJ, Roach KE, Field-Fote EC. Operant conditioning to increase ankle control or decrease reflex excitability improves reflex modulation and walking function in chronic spinal cord injury. J Neurophysiol 109: 2666–2679, 2013. doi: 10.1152/jn.01039.2011. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Is the human stretch reflex cortical rather than spinal? Lancet 301: 759–761, 1973. doi: 10.1016/S0140-6736(73)92141-7. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB. Stretch reflex and servo action in a variety of human muscles. J Physiol 259: 531–560, 1976. doi: 10.1113/jphysiol.1976.sp011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CD, Merton PA, Morton HB, Adam J. The effect of lesions of the sensorimotor cortex and the capsular pathways on servo responses from the human long thumb flexor. Brain 100: 503–526, 1977. doi: 10.1093/brain/100.3.503. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Rothwell JC, Day BL. Long-latency automatic responses to muscle stretch in man: origin and function. Adv Neurol 39: 509–539, 1983. [PubMed] [Google Scholar]
- Matthews PB. Mammalian Muscle Receptors and Their Central Actions. London: Hodder Arnold, 1972. [Google Scholar]
- Matthews PB. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol 320: 1–30, 1981. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res 119: 415–426, 1998. doi: 10.1007/s002210050357. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Christensen LO, Sinkjaer T, Nielsen J. Sensitivity of H-reflexes and stretch reflexes to presynaptic inhibition in humans. J Neurophysiol 80: 610–620, 1998. doi: 10.1152/jn.1998.80.2.610. [DOI] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Grey MJ, Sinkjaer T. Evidence for a supraspinal contribution to the human quadriceps long-latency stretch reflex. Exp Brain Res 168: 529–540, 2006. doi: 10.1007/s00221-005-0120-0. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Evidence that a long latency stretch reflex in humans is transcortical. J Physiol 449: 429–440, 1992. doi: 10.1113/jphysiol.1992.sp019094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Christensen LO, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. J Physiol 512: 267–276, 1998. doi: 10.1111/j.1469-7793.1998.267bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E, Burke D. The Circuitry of the Human Spinal Cord. New York: Cambridge Univ. Press, 2012. [Google Scholar]
- Rothwell JC, Day BL, Berardelli A, Marsden CD. Habituation and conditioning of the human long latency stretch reflex. Exp Brain Res 63: 197–204, 1986. doi: 10.1007/BF00235664. [DOI] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Medium-latency stretch reflexes of foot and leg muscles analysed by cooling the lower limb in standing humans. J Physiol 503: 691–698, 1997. doi: 10.1111/j.1469-7793.1997.691bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieppati M, Nardone A. Group II spindle afferent fibers in humans: their possible role in the reflex control of stance. Prog Brain Res 123: 461–472, 1999. doi: 10.1016/S0079-6123(08)62882-4. [DOI] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523: 817–827, 2000. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein RB. Presynaptic inhibition in humans. Prog Neurobiol 47: 533–544, 1995. doi: 10.1016/0301-0082(95)00036-4. [DOI] [PubMed] [Google Scholar]
- Stein RB, Capaday C. The modulation of human reflexes during functional motor tasks. Trends Neurosci 11: 328–332, 1988. doi: 10.1016/0166-2236(88)90097-5. [DOI] [PubMed] [Google Scholar]
- Thilmann AF, Schwarz M, Töpper R, Fellows SJ, Noth J. Different mechanisms underlie the long-latency stretch reflex response of active human muscle at different joints. J Physiol 444: 631–643, 1991. doi: 10.1113/jphysiol.1991.sp018898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Chen XY, Wolpaw JR. Acquisition of a simple motor skill: task-dependent adaptation plus long-term change in the human soleus H-reflex. J Neurosci 29: 5784–5792, 2009. doi: 10.1523/JNEUROSCI.4326-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Pomerantz FR, Wolpaw JR. Operant conditioning of a spinal reflex can improve locomotion after spinal cord injury in humans. J Neurosci 33: 2365–2375, 2013. doi: 10.1523/JNEUROSCI.3968-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Operant conditioning of spinal reflexes: from basic science to clinical therapy. Front Integr Neurosci 8: 25, 2014. doi: 10.3389/fnint.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AK, Wolpaw JR. Restoring walking after spinal cord injury: operant conditioning of spinal reflexes can help. Neuroscientist 21: 203–215, 2015. doi: 10.1177/1073858414527541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SL, Segal RL. Reducing human biceps brachii spinal stretch reflex magnitude. J Neurophysiol 75: 1637–1646, 1996. doi: 10.1152/jn.1996.75.4.1637. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Segal RL, Heter ND, Catlin PA. Contralateral and long latency effects of human biceps brachii stretch reflex conditioning. Exp Brain Res 107: 96–102, 1995. doi: 10.1007/BF00228021. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Adaptive plasticity in the primate spinal stretch reflex: reversal and re-development. Brain Res 278: 299–304, 1983. doi: 10.1016/0006-8993(83)90259-7. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Operant conditioning of primate spinal reflexes: the H-reflex. J Neurophysiol 57: 443–459, 1987. doi: 10.1152/jn.1987.57.2.443. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The complex structure of a simple memory. Trends Neurosci 20: 588–594, 1997. doi: 10.1016/S0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. Spinal cord plasticity in acquisition and maintenance of motor skills. Acta Physiol (Oxf) 189: 155–169, 2007. doi: 10.1111/j.1748-1716.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. What can the spinal cord teach us about learning and memory? Neuroscientist 16: 532–549, 2010. doi: 10.1177/1073858410368314. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Braitman DJ, Seegal RF. Adaptive plasticity in primate spinal stretch reflex: initial development. J Neurophysiol 50: 1296–1311, 1983a. doi: 10.1152/jn.1983.50.6.1296. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Chen XY. Operant conditioning of rat H-reflex: effects on mean latency and duration. Exp Brain Res 136: 274–279, 2001. doi: 10.1007/s002210000609. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Kieffer VA, Seegal RF, Braitman DJ, Sanders MG. Adaptive plasticity in the spinal stretch reflex. Brain Res 267: 196–200, 1983b. doi: 10.1016/0006-8993(83)91059-4. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Lee CL. Memory traces in primate spinal cord produced by operant conditioning of H-reflex. J Neurophysiol 61: 563–572, 1989. doi: 10.1152/jn.1989.61.3.563. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, O’Keefe JA. Adaptive plasticity in the primate spinal stretch reflex: evidence for a two-phase process. J Neurosci 4: 2718–2724, 1984. doi: 10.1523/JNEUROSCI.04-11-02718.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Seegal RF, O’Keefe JA. Adaptive plasticity in primate spinal stretch reflex: behavior of synergist and antagonist muscles. J Neurophysiol 50: 1312–1319, 1983c. doi: 10.1152/jn.1983.50.6.1312. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Stein RB. Interaction of the Jendrássik maneuver with segmental presynaptic inhibition. Exp Brain Res 124: 474–480, 1999. doi: 10.1007/s002210050643. [DOI] [PubMed] [Google Scholar]