Abstract
Motor control theories propose that the same motor plans can be employed by different effectors (e.g., the hand and arm). Skills learned with one effector can therefore “transfer” to others, which has potential applications in clinical situations. However, evidence from adaptation suggests this effect is not reciprocal; learning can be generalized from proximal to distal effectors (e.g., arm to hand), but not from distal to proximal effectors (e.g., hand to arm). We propose that skill learning may not follow the same pattern, because it relies on multiple learning processes beyond error detection and correction. Participants learned a skill task involving the production of isometric forces. We assessed their ability to perform the task with the hand and arm. One group then trained to perform the task using only their hand, whereas a second group trained using only their arm. In a final assessment, we found that participants who trained with either effector improved their skill in performing the task with both their hand and arm. There was no change in a control group that did not train between assessments, indicating that gains were related to the training, not the multiple assessments. These results indicate that in contrast to adaptation, motor skills can generalize from both proximal to distal effectors and from distal to proximal effectors. We propose this is due to differences in the processes underlying skill acquisition as compared with adaptation.
NEW & NOTEWORTHY Prior research indicates that motor learning transfers from proximal to distal effectors, but not vice versa. However, this work focused on adapting existing behavior; we questioned whether different results would occur during learning of new motor skills. We found that the benefits of training on a skill task with either the hand or arm transferred across both effectors. This highlights important differences between adaptation and skill learning, and may allow therapeutic benefits for patients with impairments in specific effectors.
Keywords: motor control, motor learning, movement control, movement skill learning, skill acquisition
INTRODUCTION
Understanding how the central nervous system represents learned actions is a central question in motor control research. The theory of generalized motor programs (Schmidt 1975) proposes that learned actions can be generalized to suit the environmental requirements of the task. This suggests that actions, rather than being represented at the level of specific effectors (e.g., arm, hand, etc.), instead share a more abstract control policy that can be modified to account different contexts. A classic example of such generalization is that individuals show similar patterns when writing with their dominant hand compared with when writing with other effectors such as their nondominant hand or their feet (Schmidt and Lee 2005).
Given that action representations are effector independent, it should be possible to acquire a control policy for a task with one effector (e.g., the arm) and then generalize this policy to improve performance of the same task with an untrained effector (e.g., the hand). This feature might create therapeutic opportunities; a general movement policy developed using one effector could be used to enhance control of an impaired effector (Raghavan et al. 2010). The reverse relationship, whereby training on a task with the impaired effector leads to small but significant improvements in performance with the unimpaired effector, has recently been shown (Kitago et al. 2015). There is, however, evidence that such transfer is not reciprocal. Previous studies using visuomotor adaptation tasks indicate that actions can transfer from proximal to distal effectors, such as from the arm to the hand, but not from distal to proximal effectors (Hay and Brouchon 1972; Krakauer et al. 2006; Putterman et al. 1969). This difference has previously been attributed to the different biological constraints and history of use of these effectors (Krakauer et al. 2006); movements of the arm typically affect the position of the hand in space, but not all hand movements similarly affect the arm. Thus learning relevant actions with the arm is more likely to be transferable to the hand, whereas learning with the hand is more likely to be effector specific.
Notably, there is limited evidence that transfer is nonreciprocal beyond visuomotor adaptation paradigms. The learning process underlying adaptation requires the simple adjustment of an existing control policy, modifying executed actions with the goal of minimizing perturbation-induced error; by contrast, skill learning tasks require the development and refinement of a new control policy for actions themselves (Krakauer 2009). As such, skill learning paradigms may allow for reciprocal transfer due to the generation of a novel, centrally stored control policy. In the present study we tested this theory by training participants to perform a task with either the hand or arm, assessing performance with both effectors at different points in the learning process.
METHODS
A total of 30 healthy volunteers (21 women; mean age 23 yr) completed the study, which took place across 3 consecutive days. Participants were split evenly across three groups with no significant differences in age or sex (one-way ANOVA, P > 0.9). All participants were right handed, as determined by the Edinburgh Handedness Inventory, and had no history of neurological conditions. The procedures of the study were approved by the Johns Hopkins Institutional Review Board, and all participants gave informed written consent.
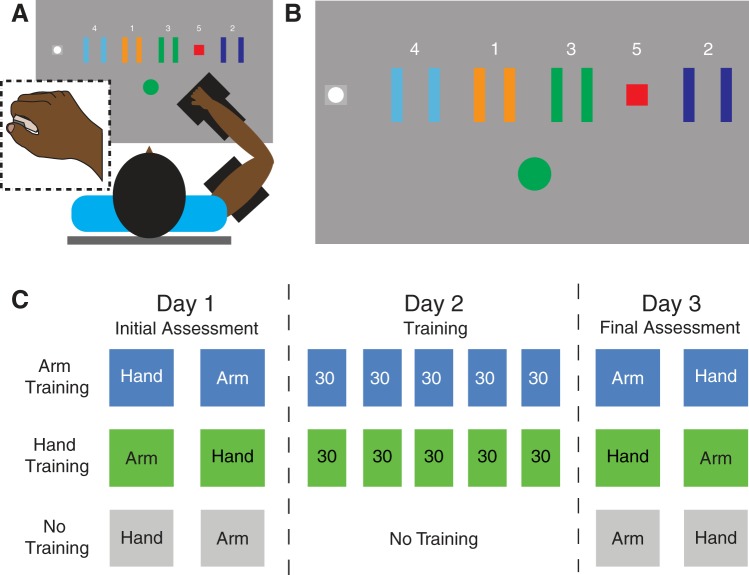
Participants sat in a KINARM robotic exoskeleton (BKIN Technologies, Kingston, ON, Canada) positioned in front of a horizontal projector and mirror system. The right arm was kept horizontal to the ground, with the shoulder fixed in 45° of transverse flexion and the elbow fixed at 90° of flexion (Fig. 1A).
Fig. 1.
Experimental setup and conditions. A: participants sat with their arm supported by a robotic exoskeleton. They produced forces with either the hand or arm to control the position of an on-screen cursor, attempting to navigate the cursor through a sequence of targets. Inset illustrates how participants interacted with the force transducer in the hand condition. B: illustration of the visual display and targets. C: illustration of the assessment and training schedule for each group of participants during the study.
Experimental task.
Participants completed a modified version of the sequential visual isometric pinch task (Hardwick et al. 2017). They controlled an on-screen cursor by performing isometric contractions, either by pinching a force transducer between the thumb and index finger or by using their elbow flexor muscles. The hand and arm were fixed at the same position/angles when performing the task with either effector. Forces at the hand were measured using a FUTEK LMD300 pinch sensor force transducer and FUTEK IMP500 signal conditioned digital display (both by FUTEK Advanced Sensor Technology, Irvine, CA) while forces at the arm were measured using an ATI Mini-45 force transducer (ATI Industrial Automation, Apex, NC).
Increasing the magnitude of the isometric force applied to the transducer moved the cursor horizontally to the right, whereas relaxing moved the cursor back toward the “Home” position. Numbered targets were arranged horizontally to the right of the Home position (Fig. 1B), and participants were instructed to move the cursor through the sequence “Home-One-Home-Two-Home-Three-Home-Four-Home-Five” to complete a single trial. The arrangement of the targets was fixed throughout the study. Force transducer input was passed through a 20-Hz low-pass Butterworth filter to dampen high-frequency noise.
As in previous studies using this paradigm, the difficulty of the task was increased by introducing a logarithmic transformation between the applied force and the displacement of the cursor. The required forces were normalized such that a contraction of 30% of the maximum for the effector being used would displace the cursor by 30 cm on the screen. This normalization procedure made the task comparable between the two effectors; at 30% of maximum voluntary contraction, neither arm flexor muscles nor intrinsic hand muscles recruit all available motor units and are therefore activated in a similar manner (Kukulka and Clamann 1981). Maximum voluntary contraction strength was calculated by taking the median of three readings recorded at the beginning of testing on the first day of the experiment.
Skill assessments.
Changes in performance were examined in “skill assessments,” which empirically quantified the speed-accuracy trade-off. This allows comparison of performance across groups by controlling for the potentially confounding factors of differences in speed (Hardwick et al. 2017; Reis et al. 2009). Participants completed an initial skill assessment for both the hand and arm on day 1 of the study and a final skill assessment for both effectors on day 3 (Fig. 1C). Participants were required to complete the sequence at a pace set by an auditory metronome. The tempos used were 24, 30, 38, 45, 60, 80, 100, 110, and 120 beats/min, translating to approximate trial durations of 12.5, 10.0, 7.9, 6.7, 5.0, 3.8, 2.7, and 2.5 s, respectively. Participants completed blocks of 10 trials at each of the 9 tempos in each assessment. The order in which each block of trials at a given tempo was attempted was randomized to prevent order effects.
Training.
On day 2 of the study, the training groups practiced performing the task with either their hand or their arm. As in previous studies using this paradigm, and in contrast to the skill assessments, participants were able to perform the task at a self-selected speed (Cantarero et al. 2013a, 2013b; Hardwick et al. 2017; Reis et al. 2009; Saucedo Marquez et al. 2013; Spampinato and Celnik 2017, 2018; Statton et al. 2015; Wymbs et al. 2016). Participants were encouraged to perform the task as quickly and as accurately as they could and to attempt to improve their performance of the task throughout the session. Participants completed 5 blocks of training, each comprising 30 trials.
No-training control group.
A third group of participants acted as a control for the possibility that completing skill assessments themselves led to an increase in participant skill. This group completed an initial skill assessment on day 1 of the study but did not train before their final assessment on day 3. Thus any changes in their performance could only be attributed to acquiring skill during the assessments themselves.
Data analysis.
For skill assessments, success rates were computed as the proportion of trials within a block in which the participant hit all five targets. The mean average success rate was calculated across the nine movement tempos comprising each skill assessment. Performance in skill assessments was then examined using a mixed-design ANOVA with within-subject factors of “effector” (hand, arm) and “assessment” (initial, final) and a between-subjects factor of “group” (hand training, arm training, no training). Based on our hypothesis that skill acquisition would transfer across both effectors (but would not occur for participants in the control group), we conducted planned t-tests, comparing performance on the initial and final skill assessments for each group, split according to the effector used (Bonferroni corrected to account for the 6 comparisons being made, adjusted alpha P < 0.0083). Differences in the change in performance from the initial to the final skill assessment were examined with separate ANOVAs to assess hand skill and arm skill for the three groups, with significant differences between groups being examined using t-tests (Bonferroni corrected to account for the 3 comparisons being made, adjusted alpha P < 0.017). In cases where the magnitude of training-induced changes did not differ, further post hoc TOST (two one-sided t-tests) equivalence tests were used to determine if the change in performance for these groups should be considered equal.
For training data, online changes in performance within group were examined by conducting paired-samples t-tests on the first and final blocks of practice for each group. Separate analyses were conducted for success rates and trial durations.
RESULTS
Skill assessments.
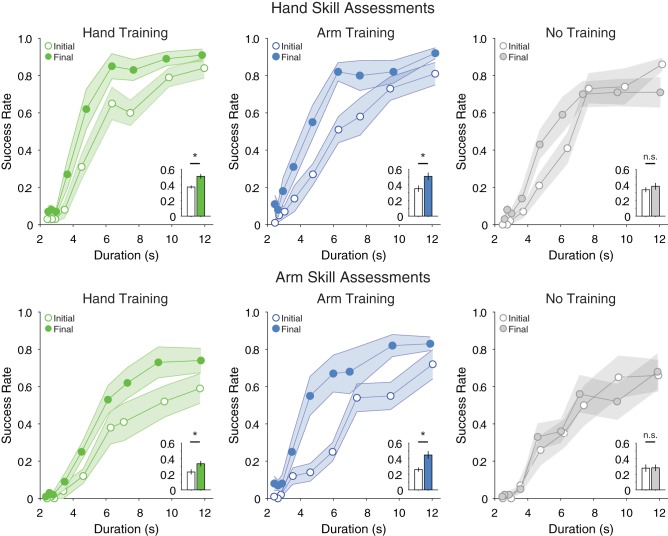
Results from the skill assessments are presented in Fig. 2. Participants performed the task with greater accuracy with their hand than their arm (main effect of effector: F1,27 = 32.32, P < 0.001). There were no differences between groups in the initial hand or arm skill assessment (one-way ANOVAs, both F < 0.5, P > 0.6).
Fig. 2.
Results of initial and final skill assessments. Open and closed circles/bars represent data for initial and final skill assessments, respectively. Lines present the speed-accuracy trade-off data for each of the 9 movement speeds tested in each assessment. Insets show summary data (average across the 9 time points) and statistics. All error bars are SE. *P < 0.0083, initial vs. final skill assessment; n.s., not significant.
Participants in groups that trained to perform the task with either effector performed better in the final skill assessments compared with the control group (significant assessment × group interaction, F2,27 = 13.49, P < 0.001; all other interactions not significant). Participants in groups that trained were able to improve their accuracy with both their trained and untrained effectors by the final skill assessment (paired-samples t-tests on initial vs. final skill assessment were all below Bonferroni-corrected critical alpha, P < 0.0083). Participants in the no-training group did not improve their performance with either effector (paired-samples t-tests on initial vs. final skill for hand and arm skill assessments, both above the Bonferroni-corrected critical alpha, P > 0.0083).
Additional analyses examined whether the change in performance differed between groups. For hand skill assessments, both training groups had significantly greater improvements in performance than the no-training control group (ANOVA on change in hand skill, significant difference between groups, F2,29 = 5.618, P < 0.01; Bonferroni-corrected t-test comparing hand training vs. control groups, t18 = 2.69, P < 0.017, and arm training vs. control groups, t18 = 3.32, P < 0.01). Whereas a t-test analysis indicated that the amount by which performance improved did not differ between the group that trained with the hand compared with the group that trained with the arm (Bonferroni-corrected t-test above the corrected critical alpha, t18 = 0.54, P > 0.017), further analysis indicated that the change in performance for these groups was not necessarily equivalent [TOST procedure based on Student’s t-test indicated that the observed effect size (d = 0.24) was not significantly within the equivalent bounds of a change of ±0.05 scale points (i.e., a difference of ±5 successes), t18 = 0.74, P = 0.23]. Similarly, in the final arm skill assessment, both training groups improved their performance significantly more than the no-training group (ANOVA on change in arm skill, significant difference between groups, F2,29 = 10.782, P < 0.001; Bonferroni-corrected t-tests for hand training vs. control group, t18 = 3.23, P < 0.01, and arm training vs. control group, t18 = 4.40, P < 0.001). Again, whereas t-test analyses indicated there was no difference between the amount of improvement for the groups that trained with the hand or the arm (t-test above the Bonferroni-corrected alpha, t18 = 1.84, P > 0.017), further analysis indicated that the overall change between these groups was not necessarily equivalent [TOST procedure based on Student’s t-test indicated that the observed effect size (d = −0.14) was not significantly within the equivalence bounds of ±0.05 scale points (i.e., a difference of ±5 successes), t18 = −0.5, P = 0.69].
Training data.
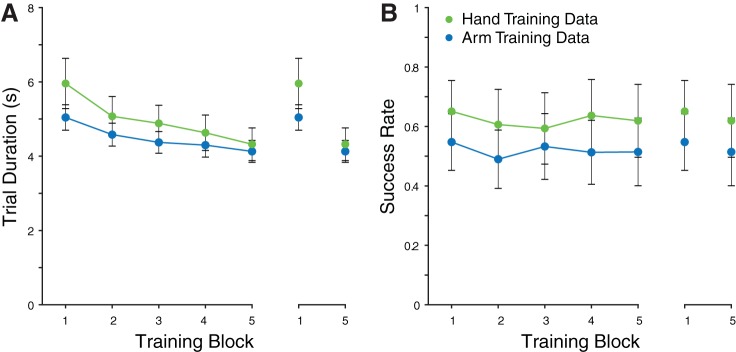
During the training session on day 2, both the hand and arm training groups improved the speed at which they completed the task (paired-samples t-tests on initial vs. final block of training, hand training group: t9 = 3.55, P < 0.01, arm training group, t9 = 3.81, P < 0.01; Fig. 3). Neither training group changed the accuracy with which they completed the task (paired-samples t-test on initial vs. final block of training, hand training group: t9 = 0.51, P > 0.05, and arm training group, t9 = 0.44, P > 0.05).
Fig. 3.
Training data. A: mean trial durations for hand and arm training groups. Inset on right shows comparison between the first and final blocks of training for each group. B: error rates for training blocks. Inset on right shows comparison between the first and final blocks of training for each group. Error bars are SE.
DISCUSSION
The present study examined transfer of motor skill within the same limb using an isometric force production task. We found that participants improved their ability to perform the task with both the trained and untrained effector. Specifically, participants who trained to perform the task using only their hand improved with both their hand and arm, and those who trained only with their arm improved with both their hand and arm. This effect was not due to learning during skill assessments, because a control group that did not train showed no changes in performance. We therefore conclude that the benefits of training on the task with one effector transferred to the untrained effector.
Notably, we found that training with either the hand or arm alone led to an improvement in skill when task was subsequently performed with either of these effectors. This non-effector-specific improvement is consistent with previous evidence for shared, generalized representations of motor skill (Schmidt 1975). In particular, previous research indicates a left hemisphere network of motor and premotor regions plays a central role in motor skill learning (Hardwick et al. 2013b, 2015; Schambra et al. 2011). Motor skill acquisition may therefore involve the development of a general control policy in left hemisphere motor regions that can be applied to perform tasks in an effector-independent manner. A further possibility is that training allowed participants to develop explicit strategies that could be applied when performing the task with either effector (Mazzoni and Krakauer 2006; Taylor et al. 2014).
In line with our central hypothesis, our results indicate that transfer occurred from both the arm to the hand and also from the hand to the arm. We also examined the secondary question of whether training with one effector led to greater improvements than training with the other. Whereas t-tests indicated no difference between the amount of improvement made between training groups, post hoc analyses of equivalence indicated that the amount of improvement for each group was not necessarily equivalent (i.e., a much larger sample size would be required to reliably identify small differences in the magnitude of improvement between groups). This question remains of interest because the ability to control these effectors differs considerably. Although we have fine control and are able to make precise movements with our hands, our arms are more suited to gross control, and the corticospinal representations of these effectors differ dramatically (Wassermann et al. 1992). Indeed, participants consistently had greater accuracy with their hand compared with their arm, indicating that controlling the cursor using the arm represented a more difficult task. Because theories propose that more challenging training leads to greater improvements in performance (Guadagnoli and Lee 2004), it may have been expected that participants who trained using the more difficult arm task may have benefitted more from it. However, evidence from a recent study of skill learning indicates no difference in performance between groups that trained on either an easy (slow) or difficult (fast) version of a skill acquisition task (Shmuelof et al. 2012). Therefore, although we have established that intralimb skill transfer can be reciprocal, further investigations are required to determine whether training with one effector leads to greater gains than training with the other.
Our results highlight the importance of considering skill acquisition as an improvement in a speed-accuracy trade-off, rather than improvements in the individual variables of speed and accuracy. During training, both the hand training and arm training groups improved their speed in performing the task while maintaining a constant level of accuracy. However, during skill assessments, in which the speed at which they were required to perform the task was fixed, both groups performed with greater levels of accuracy than at baseline. This matches the pattern of results previously found in a study that examined learning with the arm version of this task in healthy individuals and stroke patients; improvements in speed during training can be translated to improved accuracy during skill assessments (Hardwick et al. 2017). This indicates that training led to an improvement in the ability to trade-off speed and accuracy, rather than a specific improvement in the individual variables of speed and or accuracy alone. Differences in self-selected training speeds also make it difficult to directly compare performance between groups during the training conditions. For example, the hand training group was slower, but more accurate, than the arm training group; therefore, from the training data alone, it would not be possible to determine whether one group performed better overall than the other during training. Using separate skill assessments that controlled the speed at which participants performed the task allowed us to provide a like-for-like comparison between these groups, again highlighting the importance of considering the relationship between speed and accuracy when examining performance (Fitts and Peterson 1964; Hardwick et al. 2017, 2018; Reis et al. 2009; Shmuelof et al. 2012).
The results of the present study are in contrast to previous work on transfer of learning in visuomotor adaptation, which found learning generalized from the arm to the hand, but not from the hand to the arm (Hay and Brouchon 1972; Krakauer et al. 2006; Putterman et al. 1969). Krakauer et al. (2006) proposed that generalization depends on the context and prior history of action of the trained effector; all movements of the arm change the state of the hand, allowing shared learning, but not all movements of the hand affect the state of the arm, diminishing the likelihood of information learned with the hand to transfer. In comparison, the present study found transfer occurred when participants trained with either of these effectors. A primary difference between these studies is the nature of the tasks examined. As noted previously, adaptation involves modification of existing control policies, whereas motor skill learning involves development of new ones. Thus, although both skill acquisition and visuomotor adaptation examine “motor learning,” the processes and brain mechanisms underlying them differ considerably. Motor adaptation is largely attributable to cerebellum-dependent sensorimotor recalibration processes responsible for error detection/correction (Hardwick et al. 2013a) and is primarily governed by a cerebellar-prefrontal loop (Keisler and Shadmehr 2010; Liew et al. 2018). By contrast, skill learning recruits a broader network of functions and brain regions, including not only cerebellar-prefrontal error detection/correction mechanisms but also sensorimotor-striatal reinforcement learning mechanisms (Hardwick et al. 2013b; Morris et al. 2016) and sensorimotor cortical plasticity mechanisms (Mawase et al. 2017; Pascual-Leone et al. 1995). Thus, compared with motor adaptation, skill learning recruits additional physiologically and functionally separate networks, including greater reliance on memory functions (Robertson 2007; Shadmehr and Krakauer 2008; Wollenweber et al. 2014). The more extensive network of brain regions and learning mechanisms used in the present skill task may explain these differences. A second key difference between these studies is that the present study did not involve movements; participants performed isometric contractions. As such, the relationship between movements of the effectors may have been of little consequence, because no movements were performed during the experiment.
In a broader context, our finding that skill acquisition between the hand and arm is reciprocal has potential for application to rehabilitation; patients with limitations in one part of their upper extremity could benefit from training with the other effector. For example, patients with stroke who present asymmetric weakness or asymmetric synergistic muscle activations in the upper extremities could train with their less impaired effector and see benefits across both the trained and untrained segments. Similarly, musculoskeletal patients who wear a cast/orthosis experience pain at certain joints could also benefit from training with an unimpaired effector.
Conclusions.
We examined intralimb transfer in participants who learned to skillfully control the position of a cursor by producing isometric forces using either the hand or arm. Participants who trained with either effector improved their performance of the task with both their hand and arm. Participants who trained with their arm showed a trend for a larger improvement in their final skill with their arm compared with their hand, indicating some additional benefits may arise from training with effectors that have less initial fine control. These results indicate that training on a skill leads to the development of a shared control policy that can be generalized to different effectors. We propose this difference from the nonreciprocal transfer seen in visuomotor adaptation occurs because of the differences in the underlying learning processes (i.e., modification of existing actions vs. establishment of new ones). Finally, the ability to transfer the skill across effectors might be beneficial to develop training protocols for patients with motor impairment in specific effectors.
GRANTS
P. A. Celnik and R. M. Hardwick were supported by National Institutes of Health Grant R01HD073147. R. M. Hardwick was supported by Marie-Sklodowoska Curie Individual Fellowship NEURO-AGE 702784.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.H. and P.A.C. conceived and designed research; V.A.R. performed experiments; V.A.R. and R.M.H. analyzed data; V.A.R., R.M.H., and P.A.C. interpreted results of experiments; V.A.R. and R.M.H. prepared figures; R.M.H. drafted manuscript; V.A.R., R.M.H., and P.A.C. edited and revised manuscript; V.A.R., R.M.H., and P.A.C. approved final version of manuscript.
REFERENCES
- Cantarero G, Lloyd A, Celnik P. Reversal of long-term potentiation-like plasticity processes after motor learning disrupts skill retention. J Neurosci 33: 12862–12869, 2013a. doi: 10.1523/JNEUROSCI.1399-13.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero G, Tang B, O’Malley R, Salas R, Celnik P. Motor learning interference is proportional to occlusion of LTP-like plasticity. J Neurosci 33: 4634–4641, 2013b. doi: 10.1523/JNEUROSCI.4706-12.2013 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitts PM, Peterson JR. Information capacity of discrete motor responses. J Exp Psychol 67: 103–112, 1964. doi: 10.1037/h0045689 . [DOI] [PubMed] [Google Scholar]
- Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. J Mot Behav 36: 212–224, 2004. doi: 10.3200/JMBR.36.2.212-224 . [DOI] [PubMed] [Google Scholar]
- Hardwick RM, Dagioglou M, Miall RC. State estimation and the cerebellum. In: Handbook of the Cerebellum and Cerebellar Disorders, edited by Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N. Dordrecht, The Netherlands: Springer Netherlands, 2013a, p. 1297–1313. [Google Scholar]
- Hardwick RM, Forrence AD, Krakauer JW, Haith AM. Time-dependent competition between habitual and goal-directed response preparation. bioRxiv 201095, 2018. doi: 10.1101/201095. [DOI] [PubMed]
- Hardwick RM, Lesage E, Eickhoff CR, Clos M, Fox P, Eickhoff SB. Multimodal connectivity of motor learning-related dorsal premotor cortex. Neuroimage 123: 114–128, 2015. doi: 10.1016/j.neuroimage.2015.08.024 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rajan VA, Bastian AJ, Krakauer JW, Celnik PA. Motor learning in stroke: trained patients are not equal to untrained patients with less impairment. Neurorehabil Neural Repair 31: 178–189, 2017. doi: 10.1177/1545968316675432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage 67: 283–297, 2013b. doi: 10.1016/j.neuroimage.2012.11.020 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay L, Brouchon M. [Analysis of reorganization of visuomotor coordination in humans. Generalization of adaptation to prismatic deviation of the visual space]. Annee Psychol 72: 25–38, 1972. doi: 10.3406/psy.1972.27926 . [DOI] [PubMed] [Google Scholar]
- Keisler A, Shadmehr R. A shared resource between declarative memory and motor memory. J Neurosci 30: 14817–14823, 2010. doi: 10.1523/JNEUROSCI.4160-10.2010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitago T, Goldsmith J, Harran M, Kane L, Berard J, Huang S, Ryan SL, Mazzoni P, Krakauer JW, Huang VS. Robotic therapy for chronic stroke: general recovery of impairment or improved task-specific skill? J Neurophysiol 114: 1885–1894, 2015. doi: 10.1152/jn.00336.2015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning and consolidation: the case of visuomotor rotation. In: Progress in Motor Control, edited by Sternad D. New York: Springer, 2009, p. 405–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Mazzoni P, Ghazizadeh A, Ravindran R, Shadmehr R. Generalization of motor learning depends on the history of prior action. PLoS Biol 4: e316, 2006. doi: 10.1371/journal.pbio.0040316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res 219: 45–55, 1981. doi: 10.1016/0006-8993(81)90266-3 . [DOI] [PubMed] [Google Scholar]
- Liew SL, Thompson T, Ramirez J, Butcher PA, Taylor JA, Celnik PA. Variable neural contributions to explicit and implicit learning during visuomotor adaptation. Front Neurosci 12: 610, 2018. doi: 10.3389/fnins.2018.00610 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawase F, Uehara S, Bastian AJ, Celnik P. Motor learning enhances use-dependent plasticity. J Neurosci 37: 2673–2685, 2017. doi: 10.1523/JNEUROSCI.3303-16.2017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Krakauer JW. An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26: 3642–3645, 2006. doi: 10.1523/JNEUROSCI.5317-05.2006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LS, Kundu P, Dowell N, Mechelmans DJ, Favre P, Irvine MA, Robbins TW, Daw N, Bullmore ET, Harrison NA, Voon V. Fronto-striatal organization: Defining functional and microstructural substrates of behavioural flexibility. Cortex 74: 118–133, 2016. doi: 10.1016/j.cortex.2015.11.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74: 1037–1045, 1995. doi: 10.1152/jn.1995.74.3.1037 . [DOI] [PubMed] [Google Scholar]
- Putterman AH, Robert AL, Bregman AS. Adaptation of the wrist to displacing prisms. Psychon Sci 16: 79–80, 1969. doi: 10.3758/BF03336628. [DOI] [Google Scholar]
- Raghavan P, Santello M, Gordon AM, Krakauer JW. Compensatory motor control after stroke: an alternative joint strategy for object-dependent shaping of hand posture. J Neurophysiol 103: 3034–3043, 2010. doi: 10.1152/jn.00936.2009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA 106: 1590–1595, 2009. doi: 10.1073/pnas.0805413106 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EM. The serial reaction time task: implicit motor skill learning? J Neurosci 27: 10073–10075, 2007. doi: 10.1523/JNEUROSCI.2747-07.2007 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo Marquez CM, Zhang X, Swinnen SP, Meesen R, Wenderoth N. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci 7: 333, 2013. doi: 10.3389/fnhum.2013.00333 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra HM, Abe M, Luckenbaugh DA, Reis J, Krakauer JW, Cohen LG. Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J Neurophysiol 106: 652–661, 2011. doi: 10.1152/jn.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RA. A schema theory of discrete motor skill learning. Psychol Rev 82: 225–260, 1975. doi: 10.1037/h0076770 14768837 [DOI] [Google Scholar]
- Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis (4th ed.). Champaign, IL: Human Kinetics, 2005. [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res 185: 359–381, 2008. doi: 10.1007/s00221-008-1280-5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol 108: 578–594, 2012. doi: 10.1152/jn.00856.2011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato D, Celnik P. Temporal dynamics of cerebellar and motor cortex physiological processes during motor skill learning. Sci Rep 7: 40715, 2017. doi: 10.1038/srep40715 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato D, Celnik P. Deconstructing skill learning and its physiological mechanisms. Cortex 104: 90–102, 2018. doi: 10.1016/j.cortex.2018.03.017 . [DOI] [PubMed] [Google Scholar]
- Statton MA, Encarnacion M, Celnik P, Bastian AJ. A single bout of moderate aerobic exercise improves motor skill acquisition. PLoS One 10: e0141393, 2015. doi: 10.1371/journal.pone.0141393 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Krakauer JW, Ivry RB. Explicit and implicit contributions to learning in a sensorimotor adaptation task. J Neurosci 34: 3023–3032, 2014. doi: 10.1523/JNEUROSCI.3619-13.2014 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol 85: 1–8, 1992. doi: 10.1016/0168-5597(92)90094-R . [DOI] [PubMed] [Google Scholar]
- Wollenweber FA, Halfter S, Brügmann E, Weinberg C, Cieslik EC, Müller VI, Hardwick RM, Eickhoff SB. Subtle cognitive deficits in severe alcohol addicts–do they show a specific profile? J Neuropsychol 8: 147–153, 2014. doi: 10.1111/jnp.12001 . [DOI] [PubMed] [Google Scholar]
- Wymbs NF, Bastian AJ, Celnik PA. Motor skills are strengthened through reconsolidation. Curr Biol 26: 338–343, 2016. doi: 10.1016/j.cub.2015.11.066 . [DOI] [PMC free article] [PubMed] [Google Scholar]