Abstract
Forearm rotation (supination/pronation) alters corticospinal excitability to the biceps brachii, but it is unclear whether corticospinal excitability is influenced by joint angle, muscle length, or both. Thus the purpose of this study was to separately examine elbow joint angle and muscle length on corticospinal excitability. Corticospinal excitability to the biceps and triceps brachii was measured using motor evoked potentials (MEPs) elicited via transcranial magnetic stimulation. Spinal excitability was measured using cervicomedullary motor evoked potentials (CMEPs) elicited via transmastoid electrical stimulation. Elbow angles were manipulated with a fixed biceps brachii muscle length (and vice versa) across five unique postures: 1) forearm neutral, elbow flexion 90°; 2) forearm supinated, elbow flexion 90°; 3) forearm pronated, elbow flexion 90°; 4) forearm supinated, elbow flexion 78°; and 5) forearm pronated, elbow flexion 113°. A musculoskeletal model determined biceps brachii muscle length for postures 1–3, and elbow joint angles (postures 4–5) were selected to maintain biceps length across forearm orientations. MEPs and CMEPs were elicited at rest and during an isometric contraction of 10% of maximal biceps muscle activity. At rest, MEP amplitudes to the biceps were largest during supination, which was independent of elbow joint angle. CMEP amplitudes were not different when the elbow was fixed at 90° but were largest in pronation when muscle length was controlled. During an isometric contraction, there were no significant differences across forearm postures for either MEP or CMEP amplitudes. These results highlight that elbow joint angle and biceps brachii muscle length can each independently influence spinal excitability.
NEW & NOTEWORTHY Changes in upper limb posture can influence the responsiveness of the central nervous system to artificial stimulations. We established a novel approach integrating neurophysiology techniques with biomechanical modeling. Through this approach, the effects of elbow joint angle and biceps brachii muscle length on corticospinal and spinal excitability were assessed. We demonstrate that spinal excitability is uniquely influenced by joint angle and muscle length, and this highlights the importance of accounting for muscle length in neurophysiological studies.
Keywords: biceps brachii, forearm posture, joint angle, muscle length, spinal excitability
INTRODUCTION
Limb posture significantly alters corticospinal excitability to skeletal muscles and is an integral factor in influencing both the planning and execution of motor outputs (Collins et al. 2017; Scott 2000). The processes behind this neural modulation have been credited to both cortical (Mitsuhashi et al. 2007) and spinal pathways (Nuzzo et al. 2016). Of particular interest to the present work are recent studies investigating the influence of forearm rotation on corticospinal excitability to the biceps and triceps brachii. Work by Nuzzo et al. (2016) has demonstrated that both corticospinal and spinal excitability to the biceps, but not triceps brachii, are modulated as an effect of forearm rotation during rest, with excitability largest in supination. Because of the similar responses of motor evoked potentials (MEPs) and cervicomedullary motor evoked potentials (CMEPs) across postures, spinal pathways likely drove the observed changes. Forman et al. (2016c) examined handgrip positions (forearm neutral vs. pronated) during arm cycling and isometric elbow flexion. For both tasks, biceps brachii MEP amplitudes were larger in neutral, but only in arm cycling were CMEP amplitudes greater in neutral than in pronation, suggesting a combination of supraspinal and spinal mechanisms (Forman et al. 2016c). Whereas previous forearm rotation work provides important contributions to our understanding of upper limb neural control, the biomechanical consequences of forearm rotation on biceps brachii muscle length have not been considered. The biceps brachii are shortest when the forearm is supinated and longest in pronation. Although previous work (Forman et al. 2016c; Nuzzo et al. 2016) has highlighted changes in biceps muscle length as a potential contributing factor, the influence of muscle length on corticospinal excitability was not quantified. It is therefore difficult to conclude whether changes in corticospinal excitability are modulated by posture or a consequence of changing muscle length (or both) in either study (Forman et al. 2016c; Nuzzo et al. 2016). The influence of muscle length on neurological measures is often unaccounted for in posture-related work. This may lead to erroneous conclusions and confound motor control theories. Given the number of afferent pathways that are modulated by muscle length, not accounting for such length changes across experimental designs could influence the interpretation of findings.
Alterations in afferent feedback have been proposed as the driving mechanism behind posture-induced changes in corticospinal excitability. Limb posture may result in modifications to the activity of muscle spindles, Golgi tendon organs, reciprocal inhibition (inhibition of the antagonist following activity of the agonist), and heteronymous input (excitatory or inhibitory signals from synergistic muscles acting on agonist motoneurons), all of which have synaptic connections to cortical and/or spinal pathways (Barry et al. 2008; Granit and Ström 1951; Granit and Suurosoet 1949; Laporte and Lloyd 1951; Naito 2004). Muscle length can influence all these afferents. Changes to a muscle’s length alter excitation to the agonist through the stretch reflex (and by extension, reciprocal inhibition to the antagonist) (Crone and Nielsen 1994; Nielsen et al. 1992) and modify passive tension on the muscle-tendon complex (Laporte and Lloyd 1951; Hunt 1952). Muscle length may also influence synergists through heteronymous input, if such connections exist within that muscle (Barry et al. 2008). Thus, by inadequately accounting for changes in muscle length, alterations in afferent pathways (and their subsequent effects on corticospinal and spinal excitability) can be overlooked.
Given the importance of this topic, we set out to quantify the potential influence of muscle length and how it might change interpretations of corticospinal excitability to the biceps brachii. To accomplish this objective, we utilized a novel method that used biomechanical modeling to independently control the variables of elbow joint angle and biceps brachii muscle length across three different forearm postures. Our hypotheses were as follows: 1) We hypothesized that forearm supination would increase corticospinal excitability to the biceps brachii. It was expected that this posture-induced change would occur despite any differences in afferent pathways as a result of separately controlling elbow joint angle or biceps brachii muscle length. Given previous research (Forman et al. 2016c; Nuzzo et al. 2016), it was expected that an increase in cortical excitation toward supination would override other neural inputs. 2) We hypothesized that the behavior of spinal excitability to the biceps brachii across forearm postures would differ depending on which variable (elbow joint angle or biceps brachii muscle length) was controlled. It was our expectation that spinal excitability would change across postures to a greater extent when the elbow angle was maintained because the activity of muscle afferents (specifically, length dependent) would be more greatly affected.
METHODS
Participants.
Ten male volunteers (22.9 ± 2.5 yr, 176.5 ± 7.6 cm, 83.3 ± 9.6 kg, 8 right-handed, 2 left-handed) participated in the study. Procedures were verbally explained to each volunteer before the start of the session. After all inquiries were addressed, written consent was obtained. Research was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Board at the University of Ontario Institute of Technology (REB no. 15-042). Procedures were in accordance with Tri-Council guidelines in Canada, and potential risks were fully disclosed to participants. Participants had no known neurological impairments and were screened for any contraindications to magnetic stimulation by filling out a stimulation safety checklist (Rossi et al. 2009). Participants were required to complete a Physical Activity Readiness Questionnaire to screen for any contraindications to exercise or physical activity.
Experimental setup.
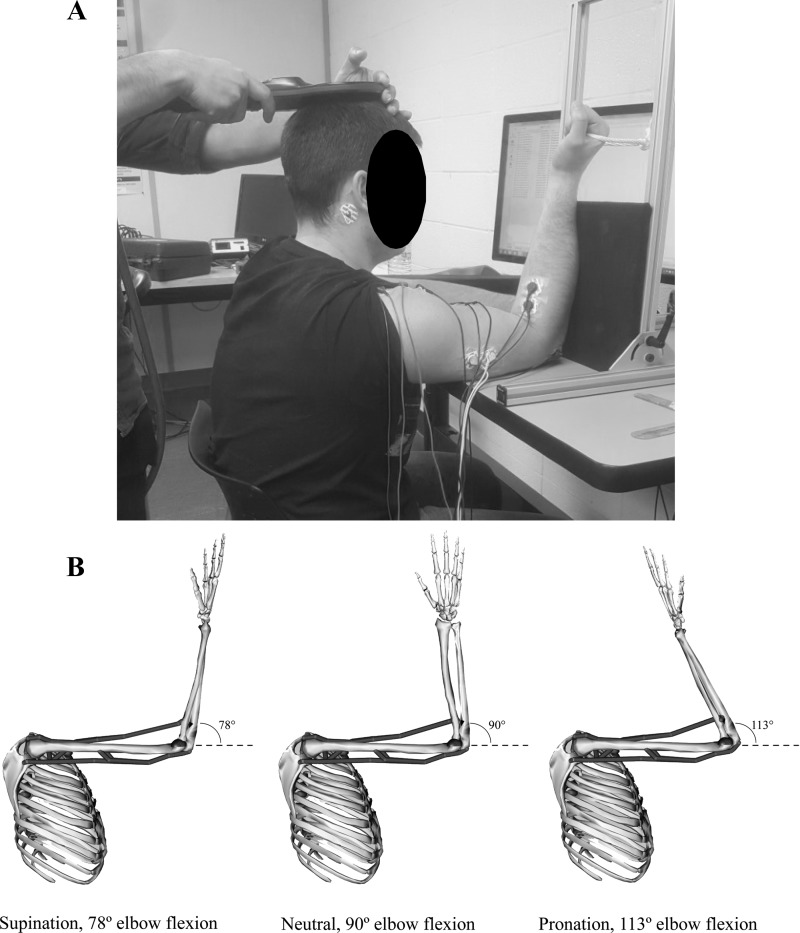
The protocol was conducted with participants seated in front of a table-mounted, custom-built apparatus (Fig. 1). The height of the table was adjusted to fully support the weight of the participant’s humerus of the dominant arm with the shoulder flexed to 90°. Throughout the experiment, participants were instructed to keep their forearm against one of two supporting boards. For resting trials, the forearm was strapped to a full-length board to immobilize the arm in the desired posture. For active/isometric trials, the forearm rested against a half-length board (Fig. 1) that allowed for a rope handle to be pulled. The apparatus was capable of moving in a range of 0–180° and could support the forearm at any elbow angle. A total of five unique postures were used in this study (see Table 1 and Fig. 1), consisting of various forearm rotations and elbow angles. Evoked responses were measured at each posture under resting and active conditions. For resting conditions, the forearm was rotated to the appropriate orientation and strapped in place. For active conditions, participants were instructed to exert an isometric elbow flexion force against the rope handle as stimulations were delivered. The rest portion of the study always preceded the active, to reduce any warm-up effect on resting measures. The order of postures was randomized within rest and active conditions.
Fig. 1.
A: experimental setup. B: musculoskeletal model and corresponding elbow angles used to produce a constant biceps brachii muscle length across forearm rotations.
Table 1.
Five upper extremity postures
| Elbow Angle Maintained | Muscle Length Maintained |
|---|---|
| Forearm supinated, elbow 90° (36.02 cm) | Forearm supinated, elbow 78° (37.05 cm) |
| Forearm neutral, elbow 90° (37.05 cm) | Forearm neutral, elbow 90° (37.05 cm) |
| Forearm pronated, elbow 90° (38.84 cm) | Forearm pronated, elbow 113° (37.05 cm) |
Forearm posture, elbow angle, and muscle length (in parentheses) are indicated in conditions where elbow angle was held at 90° of flexion with different forearm rotations or where biceps brachii muscle length was held constant despite forearm rotation.
Electromyography.
Electromyographic (EMG) activity of the biceps and triceps brachii was recorded from the dominant arm using disposable bipolar Ag-AgCl surface electrodes (Meditrace 130; Kendall, Mansfield, MA). Electrodes were positioned in line with muscle fibers, over the midline of the biceps brachii and on the lateral head of the triceps brachii. A ground electrode was placed on the lateral epicondyle of the dominant arm. Before electrode placement, hair and dead epithelial cells were removed via disposable razor, followed by sanitization with an isopropyl alcohol swab. EMG was sampled at 5 kHz using a CED 1401 interface and Signal 5 software (Cambridge Electronic Design, Cambridge, UK). Signals were amplified (gain of 300) and filtered using a three-pole Butterworth with bandpass frequencies of 10–1,000 Hz.
Stimulation conditions.
Motor responses from the biceps brachii were elicited through three separate stimulation techniques. Stimulation intensities were determined at rest with participants placed in the custom-built apparatus. The upper limb was maintained at 90° of shoulder and elbow flexion while the forearm was maintained in a supinated position. As participants were instructed to remain relaxed, a band was wrapped around the forearm/wrist and the supporting board of the apparatus to ensure that the posture was maintained.
Brachial plexus stimulation.
The maximal compound muscle action potential (Mmax) of the biceps brachii was determined by eliciting M waves through electrical stimulation of the brachial plexus at Erb’s point (DS7AH; Digitimer, Welwyn Garden City, UK). A pulse duration of 200 µs was used, and intensities ranged from 100 to 250 mA. The cathode was placed in the supraclavicular fossa and the anode on the acromion process. The initial stimulation intensity was set at 25 mA and gradually increased until the elicited M waves of the biceps brachii plateaued. The stimulation intensity that produced a plateau in M-wave amplitude was then increased by 20% to ensure maximal M waves throughout the experiment. During experimental trials, MEP and CMEP amplitudes (see below for stimulation paradigms) were normalized to the Mmax elicited under the same experimental conditions to account for changes in peripheral neuromuscular excitability (Taylor 2006).
Transcranial magnetic stimulation.
MEPs were elicited via transcranial magnetic stimulation with a Magstim 2002 (Magstim, Dyfed, UK). Stimulations were delivered over vertex via circular coil (13.5-cm outside diameter). Vertex was determined by the intersection of the midpoint between the participant’s nasion and inion and the midpoint between the participant’s tragi (Forman et al. 2016b; Philpott et al. 2015). The coil was held tangentially to the participant’s skull with the direction of the current flow preferentially activating either the left or right motor cortex (depending on hand dominance). The coil was held firmly against the participant’s head by one of the investigators to ensure consistent alignment for each trial. Stimulation intensity began at 30% of maximum stimulator output and gradually increased until the average of eight MEP amplitudes reached a target of 5–10% of the individual’s own Mmax value. This stimulation intensity was used throughout the remainder of the experiment.
Transmastoid electrical stimulation.
Transmastoid electrical stimulation was delivered using Ag-AgCl surface electrodes applied inferior to the mastoid processes. Pulse duration was fixed at 100 µs, and stimulations intensities of 135–240 mA were used (DS7AH; Digitimer). To ensure that transcranial magnetic stimulation and transmastoid electrical stimulation were eliciting evoked potentials of similar size relative to Mmax, MEP and CMEP amplitudes were matched as closely as possible. Transmastoid electrical stimulation intensity began at 25 mA and gradually increased until the average of eight CMEP amplitudes matched the average of the eight MEP amplitudes previously elicited (~5–10% of the individual’s Mmax). This stimulation intensity was used throughout the remainder of the experiment.
Musculoskeletal model of muscle length.
A summary of the five unique postures can be found in Table 1. The humerus was supported on a raised table with the shoulder flexed to 90° (Fig. 1) for all postures. The forearm was rotated into three different orientations: maximum supination, neutral, and maximum pronation. In these three forearm rotations, elbow angle was maintained at 90°, meaning the length of the biceps brachii muscle was different at each forearm orientation (longest in pronation and shortest in supination). An upper extremity musculoskeletal model (Holzbaur et al. 2005) implemented with OpenSim V3.3 (Delp et al. 2007) predicted these musculotendon lengths to be 36.02, 37.05, and 38.84 cm for the biceps brachii in supinated, neutral, and pronated forearm postures, respectively. Next, this same model (Holzbaur et al. 2005) determined the elbow flexion/extension angle required to maintain the biceps brachii muscle at a consistent length of 37.05 cm (the length in neutral), despite the forearm rotation. In the model, muscle architecture and attachments are determined from the literature (described in detail by Holzbaur et al. 2005), and the model could estimate musculotendinous lengths and moment arms. Our desired postures were accomplished by adjusting the angle of the elbow to either shorten or lengthen the biceps brachii appropriately. First, parameters of the model were adjusted to produce 90° of shoulder and elbow flexion with the forearm in a neutral orientation. In this posture, the length of the biceps brachii muscle was determined as 37.05 cm. Next, the forearm was maximally supinated, and the elbow was gradually extended until the length of the biceps brachii matched the neutral muscle length. Finally, at neutral, the forearm was rotated into maximum pronation, and the elbow was gradually flexed until the length of the biceps brachii muscle again matched neutral. These angles corresponded to 78° and 113° of elbow flexion, respectively. In our model, full elbow extension represented 0°.
Experimental protocol.
Following EMG placement, participants performed muscle-specific maximal voluntary contractions for the biceps and triceps brachii. The dominant arm was placed against the participants’ side with the elbow flexed to 90° and the forearm supinated. Researchers provided manual resistance as participants performed a maximal elbow flexion and a maximal elbow extension contraction. From these maximal voluntary contractions, the maximal voluntary EMG (MVE) was measured for both the biceps and triceps brachii. Next, participants were secured in the custom apparatus with a neutral forearm posture, and stimulation intensities were determined (see Stimulation conditions). Once established, the resting portion of the experiment was conducted. With the dominant arm supported at 90° of shoulder flexion, participants were placed into one of the five elbow/forearm postures (see Table 1 and Fig. 1). Ten MEPs and 10 CMEPs were elicited in a randomized order throughout the configuration, with each stimulation separated by 10 s. Next, three M waves were performed under the same experimental conditions. The two configurations were repeated for each experimental posture. At all times, real-time EMG traces were monitored to ensure participants were consistently at rest. For the active portion, in the same five postures, participants were instructed to produce 10% of their biceps brachii MVE by exerting isometric elbow flexion force on the rope handle. A horizontal line representing 10% of biceps brachii MVE was placed on a computer monitor in front of each participant. The EMG signal was smoothed with a root mean square (RMS), providing participants with real-time feedback of their biceps brachii activity. Participants were instructed to contract to the target for 1 s before receiving a stimulation and were asked to relax after the stimulation. Although muscular demands were low, 2 min of rest were given between each posture to limit any effects of fatigue.
Analysis and statistics.
Data were analyzed off-line using Signal 5 software (Cambridge Electronic Design). Peak-to-peak amplitudes of MEPs, CMEPs, and M waves of the biceps and triceps brachii were measured. Peak-to-peak amplitudes for all evoked potentials were measured from the initial deflection of the voltage trace from background EMG to the return of the trace to background levels. Because changes in MEP and CMEP amplitudes can be the result of changes to the M wave, both MEPs and CMEPs were normalized to the M waves evoked during the same experimental condition. Prestimulation EMG was measured from rectified traces for both the biceps and triceps brachii and was defined as a 50-ms window of the mean rectified EMG immediately before the stimulation artifact (Forman et al. 2016b). Measurements were taken from the averaged files of all 10 MEPs or all 10 CMEPs. Separate, one-way repeated-measures ANOVAs were used to assess whether significant differences in MEP amplitudes, CMEP amplitudes, or prestimulus EMG occurred as a main effect of forearm rotation (separate tests for elbow-angle controlled and muscle-length controlled). All statistics were performed on group data with a significance level of P < 0.05 using SPSS (V24; IBM, Armonk, NY). All data are reported as means ± SD and illustrated in figures as means ± SE.
RESULTS
Corticospinal excitability during rest.
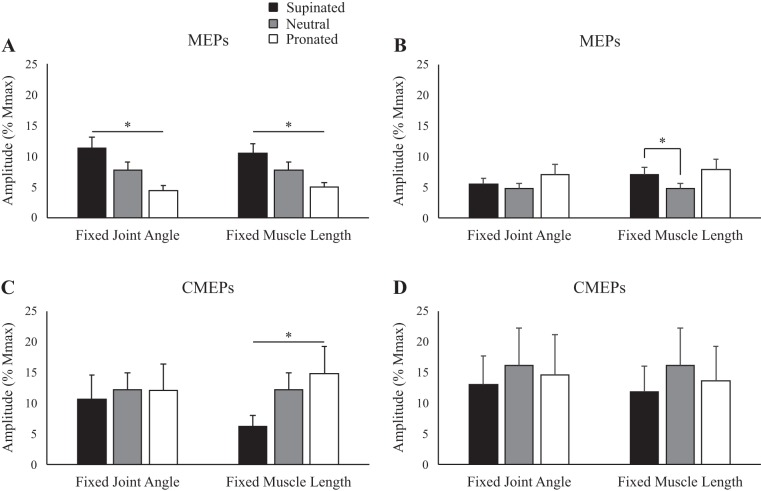
Figure 2 shows representative data of MEP amplitudes to the biceps during rest in the five postures. When elbow angle was controlled at 90°, MEPs (normalized to Mmax) were 10.6, 5.8, and 4.2% for supination, neutral, and pronation, respectively. When muscle length was maintained, MEP amplitudes were 10.6, 5.8, and 3.6% for supination, neutral, and pronation, respectively. There was a main effect of forearm rotation on MEP amplitudes for the biceps, both when elbow angle was controlled at 90° (supination: 11.3 ± 5.6% Mmax; neutral: 7.7 ± 4.3% Mmax; pronation: 4.3 ± 2.8% Mmax; P < 0.05) and when muscle length was controlled (supination: 10.4 ± 4.9% Mmax; neutral: 7.7 ± 4.3% Mmax; pronation: 5.0 ± 2.4% Mmax; P < 0.05; Fig. 3A).
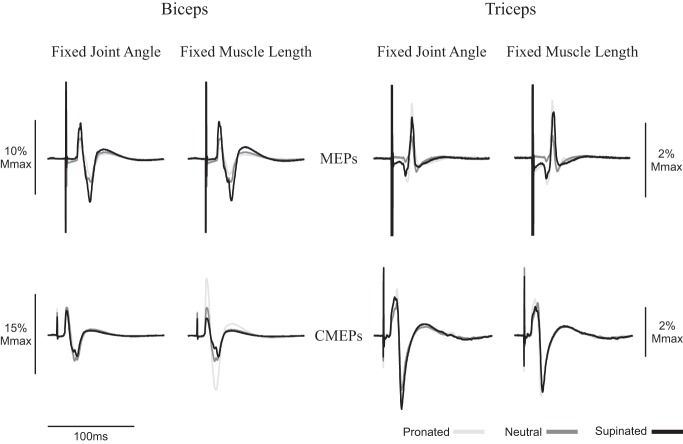
Fig. 2.
Average of 10 motor evoked potentials (MEPs) and 10 cervicomedullary motor evoked potentials (CMEPs) of the biceps and triceps brachii during rest of a representative individual. Black lines, dark gray lines, and light gray lines correspond to supinated, neutral, and pronated measurements, respectively. Traces are normalized to the average of 3 M waves elicited during the same conditions. Mmax, maximum M wave.
Fig. 3.
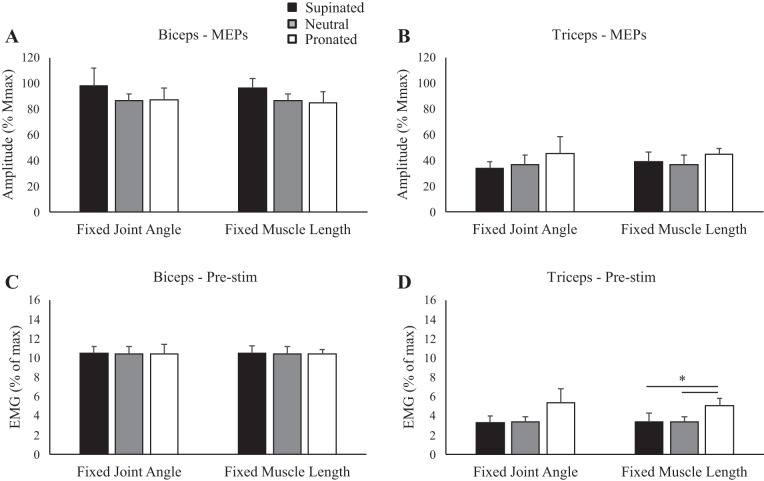
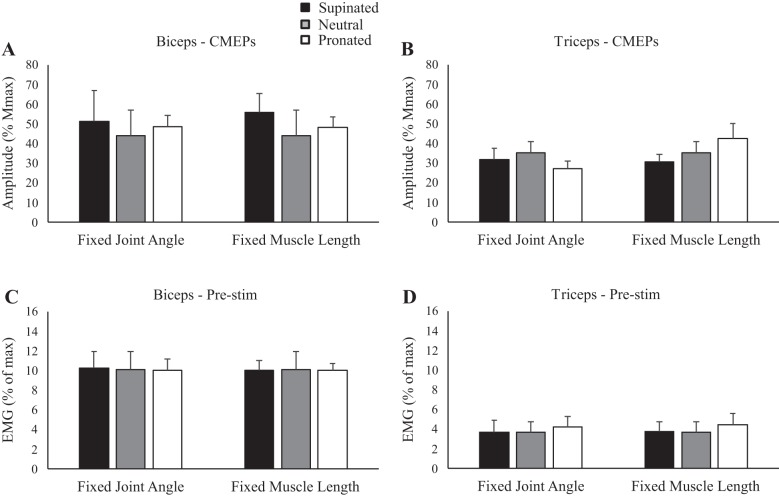
Group data (means ± SE, n = 10) during rest for motor evoked potential (MEP) amplitudes of the biceps (A) and triceps brachii (B) and group data (means ± SE, n = 5) for cervicomedullary motor evoked potential (CMEP) amplitudes of the biceps (C) and triceps brachii (D). Black, gray, and white bars correspond to supinated, neutral, and pronated forearm postures, respectively. MEP and CMEP amplitudes are shown relative to the maximum M wave (Mmax) taken during the same conditions. *P < 0.05.
Figure 2 also demonstrates MEP amplitudes in the triceps brachii during rest. At an elbow angle of 90°, MEPs were 5.1, 4.2, and 4.1% for supination, neutral, and pronation, respectively, whereas at a consistent biceps brachii muscle length, MEPs were 6.9, 4.2, and 10.9% for supination, neutral, and pronation, respectively. There were no significant effects of forearm rotation on MEP amplitudes for the triceps when elbow angle was controlled at 90° (P = 0.15). However, when biceps muscle length was controlled, MEP amplitudes were significantly larger in supination than in neutral (supination: 7.0 ± 3.8% Mmax; neutral: 4.8 ± 2.5% Mmax; P < 0.05; Fig. 3B).
Spinal excitability during rest.
Figure 2 shows an example of CMEP amplitudes to the biceps brachii during rest in the five different postures. When elbow angle was controlled at 90°, CMEPs (normalized to the Mmax) were 8.5, 10.0, and 8.4% for supination, neutral, and pronation, respectively. When muscle length was maintained, CMEP amplitudes were 7.2, 10.0, and 20.3% for supination, neutral, and pronation, respectively. For the biceps brachii, there was no significant effect of forearm rotation on CMEP amplitudes when elbow angle was controlled at 90° (P = 0.54), but CMEP amplitude was significantly influenced by forearm rotation when muscle length was controlled (supination: 6.2 ± 4.2% Mmax; neutral: 12.2 ±6.2% Mmax; pronation: 14.8 ± 9.8% Mmax; P < 0.05; Fig. 3C).
Figure 2 also shows an example of CMEP amplitudes in the triceps brachii during rest. At an elbow angle of 90°, CMEPs were 20.4, 15.2, and 20.9% for supination, neutral, and pronation, respectively, whereas at a consistent biceps brachii muscle length, CMEPs were 12.0, 15.2, and 17.4% for supination, neutral, and pronation, respectively. For the triceps brachii, there was no significant effect of forearm rotation on CMEP amplitudes, both when elbow angle was controlled at 90° (P = 0.48) and when muscle length was controlled (P = 0.11; Fig. 3D).
Corticospinal excitability during isometric elbow flexion.
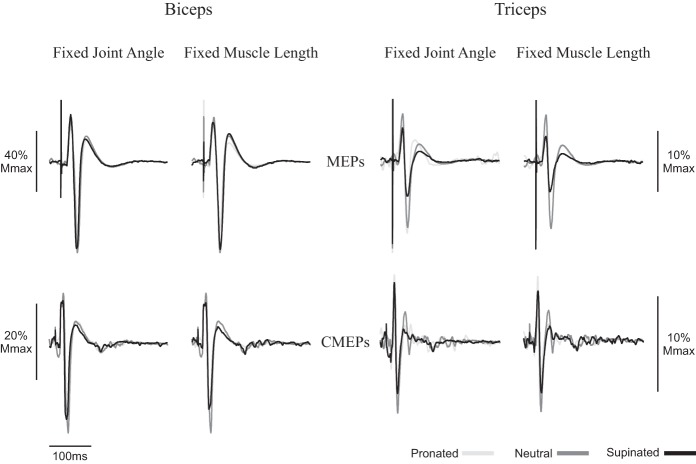
Figure 4 shows an example of MEP amplitudes to the biceps brachii during isometric elbow flexion in the five postures. When elbow angle was controlled at 90°, MEPs were 87.9, 88.7, and 76.1% for supination, neutral, and pronation, respectively. When muscle length was maintained, MEP amplitudes were 83.2, 88.7, and 69.5% for supination, neutral, and pronation, respectively. There was no influence of forearm rotation on MEP amplitudes for the biceps, either when elbow angle was controlled at 90° (P = 0.49) or when muscle length was controlled (P = 0.15; Fig. 5A). For muscle activity, prestimulus biceps brachii EMG (Fig. 5C) was not different across any posture, either when elbow angle was controlled (P = 0.99) or when muscle length was controlled (P = 0.99). Figure 4 also shows MEP amplitudes in the triceps brachii during an isometric contraction. At an elbow angle of 90°, MEPs were 38.0, 50.2, and 80.0% for supination, neutral, and pronation, respectively, whereas at a consistent biceps brachii muscle length, MEPs were 34.8, 50.2, and 48.4% for supination, neutral, and pronation, respectively. Group data of the triceps brachii (Fig. 5B) showed no effect of forearm rotation on MEP amplitudes, either when elbow angle was controlled at 90° (P = 0.36) or when muscle length was controlled (P = 0.38). For muscle activity, prestimulus triceps brachii EMG (Fig. 5D) was not different across any posture when elbow angle was controlled (P = 0.17) but was significantly higher in pronation than either supination or neutral when muscle length was controlled (supination: 3.4 ± 2.8% Mmax; neutral: 3.4 ± 1.8% Mmax; pronation: 5.0 ± 2.3% Mmax; P ≤ 0.05).
Fig. 4.
Average of 10 motor evoked potentials (MEPs) and 10 cervicomedullary motor evoked potentials (CMEPs) of the biceps and triceps brachii during isometric elbow flexion of a representative individual. Black lines, dark gray lines, and light gray lines correspond to supinated, neutral, and pronated measurements, respectively. Traces are normalized to the average of 3 M waves elicited during the same conditions. Mmax, maximum M wave.
Fig. 5.
Group data (means ± SE, n = 10) during isometric elbow flexion for motor evoked potential (MEP) amplitudes of the biceps (A) and triceps brachii (B) and prestimulus (Pre-stim) electromyography (EMG) before transcranial magnetic stimulation for the biceps (C) and triceps brachii (D). Black, gray, and white bars correspond to supinated, neutral, and pronated forearm postures, respectively. MEP amplitudes are shown relative to the maximum M wave (Mmax) taken during the same conditions. EMG is normalized to the maximum EMG found during muscle-specific maximal voluntary contractions. *P < 0.05 between forearm postures.
Spinal excitability during isometric elbow flexion.
Figure 4 shows an example of CMEP amplitudes to the biceps brachii during isometric elbow flexion in the five postures. When elbow angle was controlled at 90°, CMEPs were 31.2, 36.6, and 32.3% for supination, neutral and pronation, respectively. When muscle length was maintained, CMEP amplitudes were 27.9, 36.6, and 28.8% for supination, neutral, and pronation respectively. Group data to the biceps brachii (Fig. 6A) showed that there was no influence of forearm rotation on CMEP amplitudes, either when elbow angle was controlled at 90° (P = 0.86) or when muscle length was controlled (P = 0.56). For muscle activity, prestimulus biceps brachii EMG (Fig. 6C) was not different across posture, either when elbow angle was controlled (P = 0.93) or when muscle length was controlled (P = 0.99). Figure 4 also shows an example of CMEP amplitudes in the triceps brachii during the isometric contraction. At an elbow angle of 90°, CMEPs were 26.3, 28.4, and 26.0% for supination, neutral, and pronation, respectively, whereas at a consistent biceps brachii muscle length, CMEPs were 22.1, 28.4, and 30.3% for supination, neutral, and pronation, respectively. In the triceps brachii, group data (Fig. 6B) showed no effect of forearm rotation on CMEP amplitudes, either when elbow angle was controlled at 90° (P = 0.12) or when muscle length was controlled (P = 0.40). For muscle activity, prestimulus triceps brachii EMG (Fig. 6D) was not different across any posture, either when elbow angle was controlled (P = 0.764) or when muscle length was controlled (P = 0.63).
Fig. 6.
Group data (means ± SE, n = 5) during isometric elbow flexion for cervicomedullary motor evoked potential (CMEP) amplitudes of the biceps (A) and triceps brachii (B) and prestimulus (Pre-stim) electromyography (EMG) before transmastoid electrical stimulation for C) the biceps and D) triceps brachii. Black, gray, and white bars correspond to supinated, neutral, and pronated forearm postures, respectively. CMEP amplitudes are shown relative to the maximum M wave (Mmax) taken during the same conditions. EMG is normalized to the maximum EMG found during muscle-specific maximal voluntary contractions.
DISCUSSION
This study implemented a novel musculoskeletal modeling strategy to allow for an independent examination of elbow joint angle and muscle length on changes in neural excitation. Our findings are the first to demonstrate that spinal excitability across forearm postures responds differently depending on whether biceps brachii muscle length or elbow joint angle is maintained. Our findings indicate that, at rest, forearm pronation significantly increases spinal excitability to the biceps brachii compared with forearm supination, but only when the biceps brachii muscle length is held constant across forearm rotations. These results indicate that the interpretation of neurophysiology reports may change if muscle lengths are controlled or not. In contrast, corticospinal excitability to the resting biceps brachii was shown to be greatest in forearm supination and lowest in pronation, regardless of muscle length or elbow joint angle. These results suggest that muscle length and joint angle uniquely alter motor control of the upper limb, modulating both supraspinal and spinal pathways, but prove less influential during isometric contractions.
Corticospinal excitability at rest.
In the present study, corticospinal excitability to the biceps brachii was influenced by forearm rotation, with MEP amplitudes largest in supination and smallest in pronation. These findings occurred similarly both when elbow angle and muscle length were fixed, suggesting that controlling for muscle length (or not) does not influence our interpretations of corticospinal excitability data. This finding is supported by previous literature that has shown posture to be a factor in modulating corticospinal excitability (Collins et al. 2017; Forman et al. 2016a, 2016c; Mogk et al. 2014; Nuzzo et al. 2016; Perez and Rothwell 2015). Of lesser agreement is the primary source of this modulation, being driven by either cortical or spinal pathways. Our results suggest that cortical mechanisms are at least partial contributors. Whereas MEP amplitudes to the biceps brachii decreased from supination to pronation, CMEP amplitudes were either unchanged or increased across the same postures, indicating that changes in supraspinal activity were independent of spinal excitability. Support for this finding has been demonstrated in the distal upper limb, where MEPs elicited to the first dorsal interosseous were modified as an effect of forearm rotation but CMEP and F-wave (a technique using antidromic volleys elicited by peripheral stimulation to assess spinal excitability) amplitudes were unchanged (Perez and Rothwell 2015). More recent work suggests that the influence of posture on corticospinal excitability may be task dependent. Whereas both MEP and CMEP amplitudes are significantly larger during arm cycling with a neutral vs. pronated handgrip position, only MEP amplitudes are larger during tonic elbow flexion (Forman et al. 2016c). From a motor control perspective, it is intuitive that cortical excitability to the biceps brachii increases in forearm supination. Previous work has proposed that neural drive is coupled with the mechanical efficiency of a muscle (Hudson et al. 2009). As the primary supinator of the forearm and elbow flexor (Kawakami et al. 1994), the biceps brachii has the greatest mechanical advantage in a supinated forearm posture. Indeed, peak elbow flexion moment (Cnockaert et al. 1975; Jørgensen and Bankov 1971; Kohn et al. 2018) and voluntary activation (Kohn et al. 2018) are greatest in supinated and least in pronated forearm postures. It is likely that the excitability of the primary motor cortex (M1) is elevated in supinated forearm postures, facilitating activation of cortical neurons by transcranial magnetic stimulation. Greater intracortical facilitation (Dominici et al. 2005; Ginanneschi et al. 2005) and decreased intracortical inhibition (Perez and Rothwell 2015) may contribute to this finding. Alternatively, changes in proprioceptive input, from sources including cutaneous afferents, may be relevant. The firing of skin receptors reliably indicates position and movement of nearby joints (Aimonetti et al. 2007; Edin 2001). In supinated forearm postures, the stretching of the skin over the forearm and elbow might modulate activity in the dorsal column pathway, facilitating the M1 in this posture. However, recent research has demonstrated that skin stretch results in no changes to MEP or CMEP amplitudes to the biceps brachii (Dongés et al. 2019). It is therefore unclear if cutaneous inputs contributed to our findings.
An additional consideration for why supraspinal excitability might increase in supination is to compensate for a reduction in afferent input (e.g., reduced muscle spindle activity) when a muscle is placed in shortened lengths (Lewis et al. 2001). However, this would only explain our findings when elbow angle was maintained at 90° of flexion. When muscle length was maintained across forearm postures, stretch-dependent sensory information from the biceps brachii should have theoretically remained constant. In contrast, the triceps brachii (which has no pronation/supination action) would have lengthened/shortened when the biceps brachii muscle length was held constant (due to changes in elbow angle), thereby altering the magnitude of stretch. Additionally, all other muscles crossing the elbow (e.g., brachialis, brachioradialis) would be similarly affected by changing elbow angles, potentially changing stretch-dependent heteronymous input. It is possible that reciprocal inhibition to the biceps brachii via stretch of the triceps brachii was greatest in pronation. However, this does not clarify why corticospinal excitability across forearm postures was not different between the fixed joint angle and fixed biceps brachii muscle length conditions. It could be concluded that elbow joint angle and biceps brachii muscle length do not uniquely influence corticospinal excitability during forearm rotation, but perhaps the influence of forearm rotation on corticospinal excitability overrides the contributions of joint angle and muscle length.
Spinal excitability at rest.
Unlike measures of corticospinal excitability, spinal excitability to the biceps brachii was separately modulated by elbow joint angle and biceps brachii muscle length. In the present study, spinal excitability was unchanged across forearm postures, but only when elbow angle was constant at 90° of elbow flexion. When biceps brachii muscle length was maintained, spinal excitability was greatest in pronation and lowest in supination. Importantly, our interpretations of the influence of forearm rotation change depending on whether muscle length is accounted for or not. Recently, Collins et al. (2017) found that MEP and CMEP amplitudes to the biceps brachii were larger at 90° vs. 0° of shoulder flexion during isometric elbow flexion, indicating that changes were mainly spinal in origin. Previously, Perez and Rothwell (2015) demonstrated that MEP amplitudes to intrinsic hand muscles during pinching were influenced by forearm rotation but that neither CMEPs nor F waves changed, suggesting that spinal excitability was unaffected. Conflicting results between these works suggest that the influence of posture on spinal excitability may be task dependent. Indeed, spinal excitability is elevated during arm cycling with a neutral vs. pronated forearm posture but is unchanged during tonic elbow flexion (Forman et al. 2016c).
Nuzzo et al. (2016) assessed corticospinal and spinal excitability of the biceps and triceps brachii in two upper limb positions: 1) shoulder and elbow flexed to 90° (the same posture used in our study) and 2) shoulder abducted to 75° and horizontally adducted 30°, elbow flexed to 120° (this was defined differently than our work). Measures were taken at three different forearm postures (supination, neutral, and pronation). Whereas there was no change in the triceps brachii, spinal excitability to the biceps brachii was significantly greater in supination than in either neutral or pronation. This conflicts with our findings, which showed spinal excitability to the biceps brachii to be either unchanged or greatest in pronation, depending on whether joint angle or muscle length was controlled. One reason for this discrepancy may be the different research questions between the studies. In the study by Nuzzo et al. (2016), a two-way repeated-measures ANOVA was performed to assess the influence of upper limb position and forearm rotation on evoked potentials. Supination, neutral, and pronation data were thus grouped across the two limb positions, making direct comparisons to our work (a single position) difficult.
However, where our present study differs from previous literature and provides a novel advancement is that this is the first work to demonstrate that joint angle and muscle length uniquely influence spinal excitability to the biceps brachii. It is doubtful any one pathway is responsible, but likely a separate accumulation of inhibitory and excitatory inputs from afferent receptors. For instance, muscle spindle activity in the biceps brachii (Eccles and Lundberg 1958; Granit and Ström 1951; Nielsen et al. 1992) would have changed as an effect of forearm rotation when the elbow angle was held at 90° but would likely be minimal when the biceps brachii muscle length was maintained. Heteronymous inhibition is also a possible mechanism. Group I afferents of the brachioradialis project oligosynaptic inhibition to motoneurons of the biceps brachii, an effect with a graded response that is greatest in forearm pronation (Barry et al. 2008; Naito 2004). It is currently speculative whether the length of the brachioradialis modulates its inhibitory effect on the biceps brachii.
Perhaps the most influential contributor was that of Golgi tendon organs. Golgi tendon organs typically respond to muscle tension by providing autogenic inhibition to both their source muscle and synergists (Granit and Suurosoet 1949; Hunt 1952) while producing an excitatory effect to antagonistic motoneurons (Laporte and Lloyd 1951). However, they may excite their source muscle under certain parameters by synapsing with an excitatory interneuron (Pearson and Collins 1993). It is possible in the present study, when the elbow joint was constant, that the biceps brachii received greater autogenic inhibition in the pronated posture (when the muscle length was longest and passive tendon tension would have been greatest). In contrast, when the biceps brachii muscle length was maintained, the biceps brachii may have received greater reciprocal excitation in the pronated posture, because the triceps brachii muscle length was longest in this posture. In short, it is possible that Golgi tendon organ inhibitory/excitatory input was opposite in the present study between the two controlled variables (joint angle vs. muscle length). This mechanism is somewhat speculative, considering that Golgi tendon organs are less responsive to passive than active muscle-tendon tension (Houk and Henneman 1967; Houk et al. 1971).
Corticospinal and spinal excitability during isometric elbow flexion.
In the present study, neither corticospinal nor spinal excitability to the biceps or triceps brachii were different across forearm postures during isometric elbow flexion, regardless of elbow angle or biceps brachii muscle length. This conflicts with what was found during resting trials and provides further support that the influence of posture on corticospinal excitability might be task dependent (Forman et al. 2016c). For instance, descending drive has the potential to modulate reflex pathways (Crone and Nielsen 1994; Perez et al. 2005; Roche et al. 2009). Although the absolute magnitude of afferent input may be similar across tasks, its influence on the output can change. Whereas afferent pathways may have facilitated corticospinal excitability during isometric elbow flexion in forearm supination (as they did during rest), the integration of this input was possibly modulated by descending commands. A rationale could be compensation by the central nervous system. To ensure that the biceps brachii are sufficiently excitable while producing elbow flexion torques in pronation, descending pathways may elevate corticospinal excitability to similar levels as forearm supination. Afferent inputs themselves have also been shown to change between resting and active muscle states. Discharge rate of Ia afferents increases with external loading (Burke et al. 1978), but this relationship becomes progressively less dependent on muscle length. Golgi tendon organs also respond uniquely to stimuli under resting vs. active states. At rest, passive muscle tension does activate Golgi tendon organs, but to a lesser extent than active contractile muscle tension (Fukami 1981; Houk and Henneman 1967). In the cat model, between 20 and 200 g of force are needed for Golgi tendon organ populations to reach detection threshold during passive tension, compared with just 2 to 25 g in the actively contracting muscle (Houk et al. 1971). Thus it is likely that afferent input was different between resting and isometric conditions, despite forearm posture, joint angle, and muscle lengths being the same between the two muscle states.
An additional consideration is the manner in which our isometric elbow flexion task was executed. Rather than using isometric force as a target, participants were required to produce 10% of their biceps brachii MVE in each posture, because differences in background muscle activity can influence evoked potentials. Biceps brachii MVE was determined in supination with participants’ arms resting at their sides. The maximal biceps brachii EMG obtained in supination would not likely represent maximal EMG in other postures. Thus the 10% MVE target was made absolute to a standardized upper limb position, rather than made relative to each experimental posture. Not only is biceps brachii EMG higher during combined flexion/supination compared with flexion/pronation forces (Cnockaert et al. 1975; Jamison and Caldwell 1993), but elbow flexion maximal voluntary contraction and voluntary activation of the biceps brachii are also greater in supination (Kohn et al. 2018). The 10% MVE target was likely more difficult in the pronated forearm posture. Although the biceps brachii was still active to the same absolute magnitude, the greater exertion in pronation may have resulted in increased activity of other elbow flexors. For instance, whereas the biceps brachii are inhibited up to 50% in forearm pronation, the brachioradialis is inhibited only by 15% (Jørgensen and Bankov 1971), indicating a greater elbow flexion force contribution in this posture. This may explain why no changes in corticospinal excitability measures were observed during isometric elbow flexion in the present study. Although afferent input to the central nervous system was likely different as an effect of forearm rotation, elbow angle, and muscle length, varying muscle activity of synergists may have modulated corticospinal excitability to the biceps brachii. EMG targets normalized to each posture may provide interesting findings.
Muscle mechanics.
Biomechanical factors such as moment arms, passive properties, and force-length curves may have contributed to our findings. The moment arm of the biceps brachii significantly changes as a function of forearm rotation and flexion/extension of the elbow (Ettema et al. 1998). However, this magnitude of change is small. Our model suggests a 3-mm difference in biceps moment arm between supination and pronation with elbow flexion/extension, and this would likely have minimal influence on resting measures. Elbow flexion moment changes with elbow angle (smaller moment at both ends of the range of motion, larger moment at mid-range). However, despite this change, biceps brachii EMG is not significantly changed across these same angles (Leedham and Dowling 1995). Across postures, the relative position of the biceps on the force-length curve changes; however, it is unclear whether this influenced our measures. Last, although passive force of the biceps brachii does change throughout elbow flexion/extension, this force variation occurs mostly between 0° and 50° of elbow flexion (according to our model).This was beyond the range of the postures used in this study.
Methodological considerations.
Triceps brachii data were included, despite stimulation intensities based on biceps brachii responses. This follows publications in the field that report on multiple muscles, although intensities are typically only normalized to one (Nuzzo et al. 2016; Perez and Rothwell 2015; Spence et al. 2016). This is noteworthy for the following reason: brachial plexus stimulation intensity was set as the intensity needed to elicit a maximal M wave in the biceps, not triceps brachii, which was subsequently used to normalize transcranial magnetic stimulation and transmastoid electrical stimulation intensities. Whereas triceps brachii MEP and CMEP data are reported relative (%) to the Mmax evoked in the same experimental conditions, data were actually normalized to the largest triceps brachii M wave according to the design of our protocol (it is unclear if these M waves were truly maximal). Thus it cannot be determined which relative portion of the corticospinal and spinal pathways was being assessed in the triceps brachii. Additionally, it should be emphasized that the upper-limb postures used to maintain biceps brachii muscle length were established using predictive musculoskeletal modeling software (OpenSim). The anthropometric data from the model represent that of an adult male of average size and muscle geometry based on data from anatomical studies. Directly assessing muscle length of the biceps brachii for subject-specific modeling would be a reasonable advancement on this experimental design. However, given our sample population, with a small variability in stature, upper arm length variability was likely minimal, and thus biceps length similar. Finally, given the design and statistical approach of this study, it is difficult to precisely dissociate contributions of muscle length and elbow angle to reported results. A future investigation examining the independent influence of muscle length, elbow angle, and forearm rotation (while controlling for either muscle length or elbow angle) and a statistical design examining their interactions would address this limitation.
Conclusions.
Resting corticospinal excitability to the biceps brachii was greatest in forearm supination, regardless of elbow angle or muscle length, whereas spinal excitability was uniquely modulated by these two factors. Spinal excitability was greatest in forearm pronation when muscle length was maintained but was unchanged across forearm postures at a consistent elbow angle. This is one of the first reports to demonstrate that joint angles and muscles lengths have separate influences on the central nervous system, although these effects seem to diminish during isometric contractions. These results demonstrate that muscle length can be a contributing factor to neurological measures and should be considered in postural studies that manipulate joint angles or muscle lengths.
GRANTS
D. A. Forman is supported by a National Sciences and Engineering Research Council (NSERC) graduate scholarship. M. W. R. Holmes is supported by the Canada Research Chairs program and NSERC Discovery Grant RGPIN-2015-05765.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.A.F. and M.W.H. conceived and designed research; D.A.F., D.A.-M., C.M.B., and M.W.H. performed experiments; D.A.F., D.A.-M., and C.M.B. analyzed data; D.A.F., D.A.-M., C.M.B., and M.W.H. interpreted results of experiments; D.A.F. and M.W.H. prepared figures; D.A.F., C.M.B., and M.W.H. drafted manuscript; D.A.F., D.A.-M., C.M.B., and M.W.H. edited and revised manuscript; D.A.F., D.A.-M., C.M.B., and M.W.H. approved final version of manuscript.
REFERENCES
- Aimonetti JM, Hospod V, Roll JP, Ribot-Ciscar E. Cutaneous afferents provide a neuronal population vector that encodes the orientation of human ankle movements. J Physiol 580: 649–658, 2007. doi: 10.1113/jphysiol.2006.123075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry BK, Riley ZA, Pascoe MA, Enoka RM. A spinal pathway between synergists can modulate activity in human elbow flexor muscles. Exp Brain Res 190: 347–359, 2008. doi: 10.1007/s00221-008-1479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Löfstedt L. Muscle spindle activity in man during shortening and lengthening contractions. J Physiol 277: 131–142, 1978. doi: 10.1113/jphysiol.1978.sp012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnockaert JC, Lensel G, Pertuzon E. Relative contribution of individual muscles to the isometric contraction of a muscular group. J Biomech 8: 191–197, 1975. doi: 10.1016/0021-9290(75)90024-X. [DOI] [PubMed] [Google Scholar]
- Collins BW, Cadigan EW, Stefanelli L, Button DC. Corticospinal excitability of the biceps brachii is shoulder position dependent. J Neurophysiol 118: 3242–3251, 2017. doi: 10.1152/jn.00527.2017. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiol Scand 152: 351–363, 1994. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, Guendelman E, Thelen DG. OpenSim: open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng 54: 1940–1950, 2007. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- Dominici F, Popa T, Ginanneschi F, Mazzocchio R, Rossi A. Cortico-motoneuronal output to intrinsic hand muscles is differentially influenced by static changes in shoulder positions. Exp Brain Res 164: 500–504, 2005. doi: 10.1007/s00221-005-2270-5. [DOI] [PubMed] [Google Scholar]
- Dongés SC, Taylor JL, Nuzzo JL. Elbow angle modulates corticospinal excitability to the resting biceps brachii at both spinal and supraspinal levels. Exp Physiol 104: 546–555, 2019. doi: 10.1113/EP087472. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Integrative pattern of Ia synaptic actions on motoneurones of hip and knee muscles. J Physiol 144: 271–298, 1958. doi: 10.1113/jphysiol.1958.sp006101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin B. Cutaneous afferents provide information about knee joint movements in humans. J Physiol 531: 289–297, 2001. doi: 10.1111/j.1469-7793.2001.0289j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettema GJ, Styles G, Kippers V. The moment arms of 23 muscle segments of the upper limb with varying elbow and forearm positions: implications for motor control. Hum Mov Sci 17: 201–220, 1998. doi: 10.1016/S0167-9457(97)00030-4. [DOI] [Google Scholar]
- Forman DA, Baarbé J, Daligadu J, Murphy B, Holmes MW. The effects of upper limb posture and a sub-maximal gripping task on corticospinal excitability to muscles of the forearm. J Electromyogr Kinesiol 27: 95–101, 2016a. doi: 10.1016/j.jelekin.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Forman DA, Philpott DT, Button DC, Power KE. Differences in corticospinal excitability to the biceps brachii between arm cycling and tonic contraction are not evident at the immediate onset of movement. Exp Brain Res 234: 2339–2349, 2016b. doi: 10.1007/s00221-016-4639-z. [DOI] [PubMed] [Google Scholar]
- Forman DA, Richards M, Forman GN, Holmes MW, Power KE. Changes in corticospinal and spinal excitability to the biceps brachii with a neutral vs. pronated handgrip position differ between arm cycling and tonic elbow flexion. Front Hum Neurosci 10: 543, 2016c. doi: 10.3389/fnhum.2016.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami Y. Responses of isolated Golgi tendon organs of the cat to muscle contraction and electrical stimulation. J Physiol 318: 429–443, 1981. doi: 10.1113/jphysiol.1981.sp013876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginanneschi F, Del Santo F, Dominici F, Gelli F, Mazzocchio R, Rossi A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp Brain Res 161: 374–382, 2005. doi: 10.1007/s00221-004-2084-x. [DOI] [PubMed] [Google Scholar]
- Granit R, Ström G. Autogenetic modulation of excitability of single ventral horn cells. J Neurophysiol 14: 113–132, 1951. doi: 10.1152/jn.1951.14.2.113. [DOI] [PubMed] [Google Scholar]
- Granit R, Suurosoet V. Self-regulation of the muscle contraction by facilitation and inhibition from its proprioceptors. Nature 164: 270–271, 1949. doi: 10.1038/164270a0. [DOI] [PubMed] [Google Scholar]
- Holzbaur KRS, Murray WM, Delp SL. A model of the upper extremity for simulating musculoskeletal surgery and analyzing neuromuscular control. Ann Biomed Eng 33: 829–840, 2005. doi: 10.1007/s10439-005-3320-7. [DOI] [PubMed] [Google Scholar]
- Houk J, Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol 30: 466–481, 1967. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Houk JC, Singer JJ, Henneman E. Adequate stimulus for tendon organs with observations on mechanics of ankle joint. J Neurophysiol 34: 1051–1065, 1971. doi: 10.1152/jn.1971.34.6.1051. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Taylor JL, Gandevia SC, Butler JE. Coupling between mechanical and neural behaviour in the human first dorsal interosseous muscle. J Physiol 587: 917–925, 2009. doi: 10.1113/jphysiol.2008.165043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC. The effect of stretch receptors from muscle on the discharge of motorneurons. J Physiol 117: 359–379, 1952. doi: 10.1113/jphysiol.1952.sp004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison JC, Caldwell GE. Muscle synergies and isometric torque production: influence of supination and pronation level on elbow flexion. J Neurophysiol 70: 947–960, 1993. doi: 10.1152/jn.1993.70.3.947. [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Bankov S. Maximum strength of elbow with flexors with pronated and supinated forearm. In: Biomechanics II. 2nd International Seminar on Biomechanics, Eindhoven, 1969. Medicine and Sport Science, edited by Vredenbregt J, Wartenweiler J. Basel: Karger, 1971, vol. 6, p. 174–180. doi: 10.1159/000392166. [DOI] [Google Scholar]
- Kawakami Y, Nakazawa K, Fujimoto T, Nozaki D, Miyashita M, Fukunaga T. Specific tension of elbow flexor and extensor muscles based on magnetic resonance imaging. Eur J Appl Physiol Occup Physiol 68: 139–147, 1994. doi: 10.1007/BF00244027. [DOI] [PubMed] [Google Scholar]
- Kohn S, Smart RR, Jakobi JM. Voluntary activation and twitch potentiation of the elbow flexors across supinated, neutral, and pronated forearm orientations. Physiol Rep 6: e13560, 2018. doi: 10.14814/phy2.13560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laporte Y, Lloyd DP. Disynaptic reflex link between muscles of a myotatic unit. Fed Proc 10: 78–79, 1951. [PubMed] [Google Scholar]
- Leedham JS, Dowling JJ. Force-length, torque-angle and EMG-joint angle relationships of the human in vivo biceps brachii. Eur J Appl Physiol Occup Physiol 70: 421–426, 1995. doi: 10.1007/BF00618493. [DOI] [PubMed] [Google Scholar]
- Lewis GN, Byblow WD, Carson RG. Phasic modulation of corticomotor excitability during passive movement of the upper limb: effects of movement frequency and muscle specificity. Brain Res 900: 282–294, 2001. doi: 10.1016/S0006-8993(01)02369-1. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi K, Seki K, Akamatsu C, Handa Y. Modulation of excitability in the cerebral cortex projecting to upper extremity muscles by rotational positioning of the forearm. Tohoku J Exp Med 212: 221–228, 2007. doi: 10.1620/tjem.212.221. [DOI] [PubMed] [Google Scholar]
- Mogk JP, Rogers LM, Murray WM, Perreault EJ, Stinear JW. Corticomotor excitability of arm muscles modulates according to static position and orientation of the upper limb. Clin Neurophysiol 125: 2046–2054, 2014. doi: 10.1016/j.clinph.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Naito A. Electrophysiological studies of muscles in the human upper limb: the biceps brachii. Anat Sci Int 79: 11–20, 2004. doi: 10.1111/j.1447-073x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y, Crone C, Hultborn H. Central facilitation of Ia inhibition during tonic ankle dorsiflexion revealed after blockade of peripheral feedback. Exp Brain Res 88: 651–656, 1992. doi: 10.1007/BF00228194. [DOI] [PubMed] [Google Scholar]
- Nuzzo JL, Trajano GS, Barry BK, Gandevia SC, Taylor JL. Arm posture-dependent changes in corticospinal excitability are largely spinal in origin. J Neurophysiol 115: 2076–2082, 2016. doi: 10.1152/jn.00885.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993. doi: 10.1152/jn.1993.70.3.1009. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BKS, Nielsen JB. Short-term adaptations in spinal cord circuits evoked by repetitive transcranial magnetic stimulation: possible underlying mechanisms. Exp Brain Res 162: 202–212, 2005. doi: 10.1007/s00221-004-2144-2. [DOI] [PubMed] [Google Scholar]
- Perez MA, Rothwell JC. Distinct influence of hand posture on cortical activity during human grasping. J Neurosci 35: 4882–4889, 2015. doi: 10.1523/JNEUROSCI.4170-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott DT, Pearcey GE, Forman D, Power KE, Button DC. Chronic resistance training enhances the spinal excitability of the biceps brachii in the non-dominant arm at moderate contraction intensities. Neurosci Lett 585: 12–16, 2015. doi: 10.1016/j.neulet.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Roche N, Lackmy A, Achache V, Bussel B, Katz R. Impact of transcranial direct current stimulation on spinal network excitability in humans. J Physiol 587: 5653–5664, 2009. doi: 10.1113/jphysiol.2009.177550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A; Safety of TMS Consensus Group . Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120: 2008–2039, 2009. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SH. Role of motor cortex in coordinating multi-joint movements: is it time for a new paradigm? Can J Physiol Pharmacol 78: 923–933, 2000. doi: 10.1139/y00-064. [DOI] [PubMed] [Google Scholar]
- Spence AJ, Alcock LR, Lockyer EJ, Button DC, Power KE. Phase- and workload-dependent changes in corticospinal excitability to the biceps and triceps brachii during arm cycling. Brain Sci 6: 60, 2016. doi: 10.3390/brainsci6040060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL. Stimulation at the cervicomedullary junction in human subjects. J Electromyogr Kinesiol 16: 215–223, 2006. doi: 10.1016/j.jelekin.2005.07.001. [DOI] [PubMed] [Google Scholar]