Abstract
In aging Fischer 344 rats, phrenic motor neuron loss, neuromuscular junction abnormalities, and diaphragm muscle (DIAm) sarcopenia are present by 24 mo of age, with larger fast-twitch fatigue-intermediate (type FInt) and fast-twitch fatigable (type FF) motor units particularly vulnerable. We hypothesize that in old rats, DIAm neuromuscular transmission deficits are specific to type FInt and/or FF units. In phrenic nerve/DIAm preparations from rats at 6 and 24 mo of age, the phrenic nerve was supramaximally stimulated at 10, 40, or 75 Hz. Every 15 s, the DIAm was directly stimulated, and the difference in forces evoked by nerve and muscle stimulation was used to estimate neuromuscular transmission failure. Neuromuscular transmission failure in the DIAm was observed at each stimulation frequency. In the initial stimulus trains, the forces evoked by phrenic nerve stimulation at 40 and 75 Hz were significantly less than those evoked by direct muscle stimulation, and this difference was markedly greater in 24-mo-old rats. During repetitive nerve stimulation, neuromuscular transmission failure at 40 and 75 Hz worsened to a greater extent in 24-mo-old rats compared with younger animals. Because type IIx and/or IIb DIAm fibers (type FInt and/or FF motor units) display greater susceptibility to neuromuscular transmission failure at higher frequencies of stimulation, these data suggest that the age-related loss of larger phrenic motor neurons impacts nerve conduction to muscle at higher frequencies and may contribute to DIAm sarcopenia in old rats.
NEW & NOTEWORTHY Diaphragm muscle (DIAm) sarcopenia, phrenic motor neuron loss, and perturbations of neuromuscular junctions (NMJs) are well described in aged rodents and selectively affect FInt and FF motor units. Less attention has been paid to the motor unit-specific aspects of nerve-muscle conduction. In old rats, increased neuromuscular transmission failure occurred at stimulation frequencies where FInt and FF motor units exhibit conduction failures, along with decreased apposition of pre- and postsynaptic domains of DIAm NMJs of these units.
Keywords: aging, diaphragm muscle, neuromotor control, neuromuscular transmission, sarcopenia
INTRODUCTION
Effective neuromuscular transmission and neural control of force generation is mediated via specialized structures known as the neuromuscular junctions (NMJs). Previously, we demonstrated significant neuromuscular transmission failure (NMTF) in the rat diaphragm muscle (DIAm) during repetitive maximum stimulation of the phrenic nerve (Johnson and Sieck 1993; Kuei et al. 1990; Sieck and Prakash 1995). In the DIAm, NMTF can occur at several levels: 1) failure of axonal propagation of action potentials particularly at axonal branch points (Fournier et al. 1991; Johnson and Sieck 1993; Sieck and Prakash 1995); 2) failure of synaptic vesicle release at presynaptic terminals (Mantilla et al. 2004a; Rowley et al. 2007); 3) structural misalignment of pre- and postsynaptic components of NMJs (Prakash and Sieck 1998); and 4) postsynaptic loss or desensitization of acetylcholine receptors (Rowley et al. 2007; Sieck and Prakash 1995). In older (24 mo old) Fischer 344 (F344) rats, DIAm NMJs exhibit morphological abnormalities at larger type IIx and/or IIb muscle fibers (Prakash and Sieck 1998) that are consistent with the loss of larger phrenic motor neurons (Fogarty et al. 2018b) and subsequent denervation. These results are also consistent with DIAm sarcopenia, the age-related atrophy of type IIx and/or IIb fibers and decreased muscle specific force (Elliott et al. 2016; Gosselin et al. 1994; Greising et al. 2013; Khurram et al. 2018).
Susceptibility to NMTF depends on DIAm motor unit type such that more fatigable motor units (fast-twitch fatigue-intermediate, FInt; fast-twitch fatigable, FF) comprising type IIx and IIb muscle fibers are more vulnerable (Johnson and Sieck 1993). These more fatigable motor units are only recruited for short-duration, higher force behaviors of the DIAm (Fournier and Sieck 1988; Mantilla et al. 2010; Sieck and Fournier 1987; Sieck et al. 1989a, 1996, 2013) that are impaired with aging (Elliott et al. 2016; Gosselin et al. 1994; Greising et al. 2013; Khurram et al. 2018) and associated with increased morbidity in the elderly (Tolep et al. 1995). By contrast, slow-twitch (S) and fast-twitch fatigue-resistant (FR) DIAm motor units, which comprise smaller phrenic motor neurons that innervate type I and IIa muscle fibers, respectively, are relatively unaffected by age and ventilation is preserved (Elliott et al. 2016; Fogarty et al. 2018b; Greising et al. 2013, 2015b; Khurram et al. 2018).
During an inspiratory burst, the onset discharge rates of type S and FR motor units are lower (~8–15 Hz) with moderate peak discharge rates during inspiration (~25–40 Hz), a range that corresponds to the steep portion of the force/frequency response of these motor units (Fournier and Sieck 1988; Seven et al. 2013, 2014; Sieck et al. 1984). By comparison, peak discharge rates of type FInt and FF motor units, required for short-duration higher force maneuvers, including coughing, sneezing, and defecation (Fogarty et al. 2018a; Fogarty and Sieck 2019) can exceed 60 Hz (Fournier and Sieck 1988; Seven et al. 2013, 2014). Thus the fidelity of neuromuscular transmission appears to match the functional demands of motor unit recruitment. For example, ventilatory behaviors require only the recruitment of type S and FR motor units at lower discharge rates, where neuromuscular transmission remains effective and reliable. During higher force motor behaviors, recruitment of type FInt and FF motor units is required at higher discharge rates during which neuromuscular transmission is sustained for only short durations (Johnson and Sieck 1993; Sieck and Prakash 1995). This concept of functional tuning of neuromuscular transmission in DIAm motor units is supported by the results of a study in rats, where we showed that susceptibility to NMTF during repetitive phrenic nerve stimulation depends on stimulation frequency. NMTF is much more pronounced at 75 Hz compared with 10 Hz, with type IIx and/or IIb DIAm fibers being more susceptible than type I and IIa fibers (Johnson and Sieck 1993; Sieck and Prakash 1995).
Recently, we reported a specific loss of larger phrenic motor neurons by 24 mo of age in F344 rats (Fogarty et al. 2018b). With the loss of larger phrenic motor neurons, type IIx and/or IIb DIAm fibers are denervated, until sprouting and reinnervation can occur. Obviously, DIAm fiber denervation interrupts effective neuromuscular transmission, and the force evoked by phrenic nerve stimulation will be less than that evoked by direct muscle stimulation. Moreover, the loss of phrenic motor neurons may be triggered by retraction of presynaptic terminals at type IIx and/or IIb fibers (Prakash and Sieck 1998), thereby increasing susceptibility to neuromuscular transmission failure. This is consistent with age-related fragmentation of axon terminals and decreased apposition of the presynaptic to postsynaptic domains of NMJs at type IIx and/or IIb DIAm fibers (Prakash and Sieck 1998). Furthermore, with re-innervation of DIAm fibers, motor unit innervation ratio will expand, also increasing susceptibility to NMTF (Sieck and Prakash 1995).
In the present study, we hypothesize that in F344 rats, NMTF worsens in old age, especially at higher frequencies of stimulation. To address this hypothesis, we assessed the extent of NMTF in the DIAm at 10-, 40-, and 75-Hz stimulation in 6- and 24-mo-old F344 rats. We also hypothesize that this is concomitant with reduced overlap of the pre- and postsynaptic domains of NMJs in old rats, as assessed by fluorescent immunolabeling of DIAm NMJs.
METHODS
Animals.
Young (6 mo old, n = 12; 6 male and 6 female) and old (24 mo old, n = 12; 6 male and 6 female) F344 rats from the National Institute on Aging (NIA) colony, with ages based on survival information (100% and 50%, respectively; Miller and Nadon 2000). We combined the sexes because there was no effect of sex in other DIAm parameters in F344 rats (Fogarty et al. 2018b; Khurram et al. 2018). For NMTF experiments, n = 8 rats per age were used (4 females and 4 males of each age; data for Figs. 1–5). For the NMJ morphology, n = 4 rats per age were used (2 females and 2 males of each age; data for Fig. 6). Protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee and complied with National Institutes of Health guidelines. Animals were deeply anesthetized with ketamine (10 mg/kg) and xylazine (60 mg/kg) and were euthanized by exsanguination. The DIAm and attached phrenic nerve were excised and placed in a tissue bath containing Rees-Simpson’s buffer (pH 7.4) at 26°C and gassed with carbogen (95% O2-5% CO2).
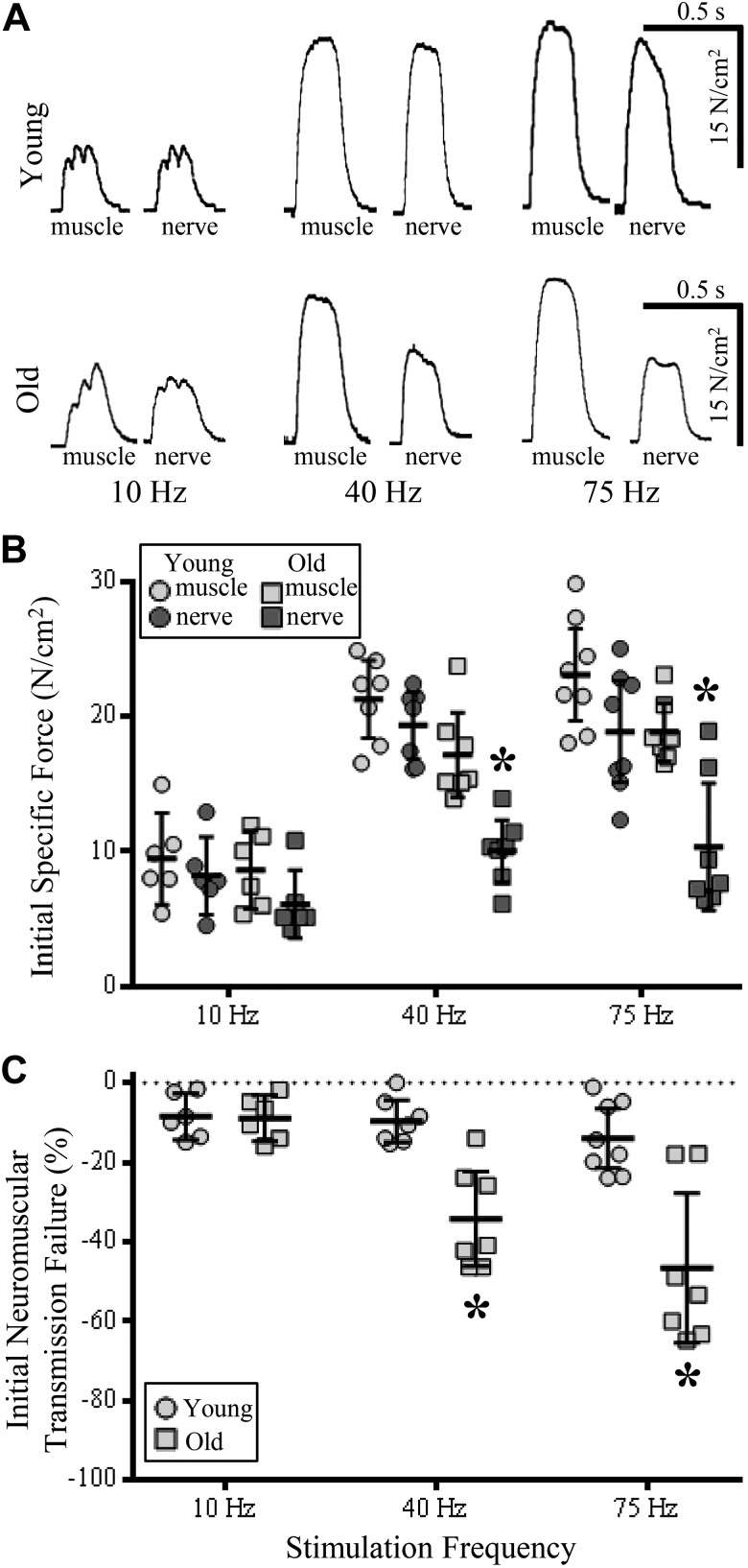
Fig. 1.
Reduced diaphragm muscle (DIAm) specific and relative force generation from initial stimulation train at 40- and 75-Hz nerve-evoked stimulations in old compared with young rats. A: pairs of muscle (left) and nerve-evoked (right) DIAm force production traces from stimulations at 10, 40, and 75 Hz in young (top row) and old rats (bottom row). B: plot [means ± 95% confidence interval (CI)] illustrating decreased force of nerve-evoked DIAm contractions at 40- and 75-Hz nerve-evoked stimulations in old rats (dark gray squares) compared with young rat muscle (light gray circles) and nerve-evoked (dark gray circles) contractions and muscle contractions of old rats (light gray squares). C: plot (means ± 95% CI) illustrating increased failure of neuromuscular transmission (% of initial direct nerve-evoked compared with initial muscle-evoked force) in old (squares) compared with young rats (circles) at 40- and 75-Hz stimulations. *P < 0.01; two-way ANOVA with Bonferroni post hoc analysis; n = 7–8 rats per age.
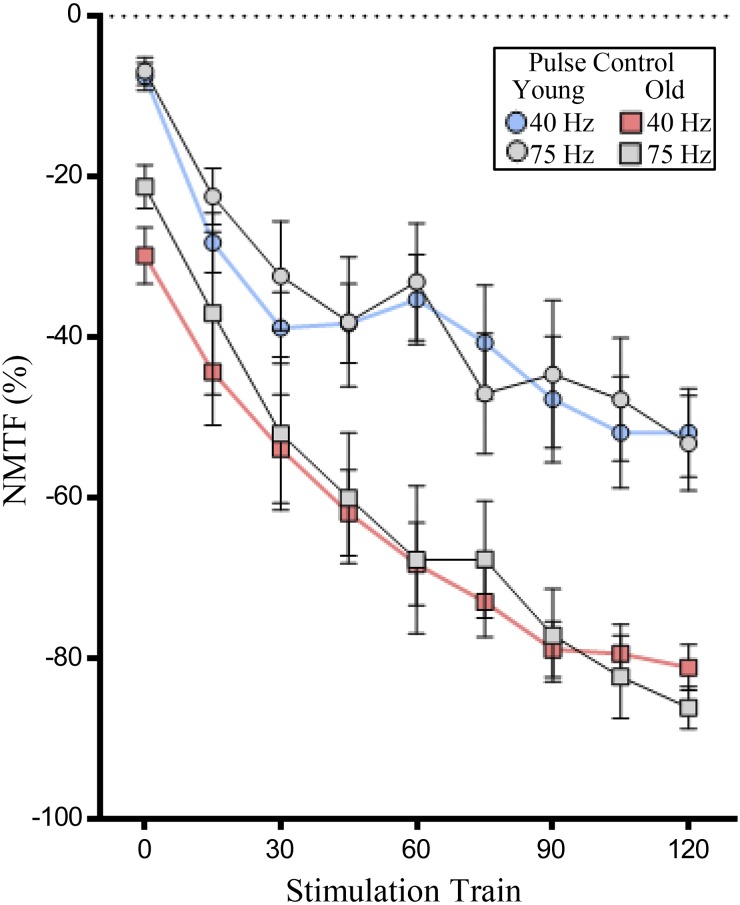
Fig. 5.
Age increases neuromuscular transmission failure (NMTF%) during 120 s of 40- and 75-Hz stimulation when pulse number per train is uniform. Plot (means ± SE) illustrates increased NMTF% in old rats during 120 s of 40 (red squares)- and 75-Hz stimulations (gray squares) compared with that in young rats during 40 (blue circles)- and 75-Hz stimulations (gray circles). In all experiments, the number of pulses per train are identical (13). *P < 0.01, two-way ANOVA with Bonferroni post hoc analysis; n = 6–8 rats per age.
Fig. 6.
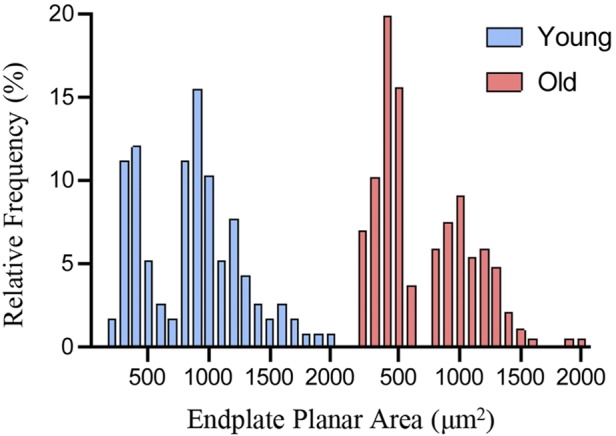
Bimodal distribution of endplate planar areas in young and old diaphragm muscle (DIAm). Frequency histogram shows two peaks in the relative distributions of neuromuscular junction endplate planar area of young and old DIAm (young: n = 116; old: n = 185).
DIAm neuromuscular transmission.
The costal portion of DIAm was positioned within the tissue bath and the central tendon attached to a force transducer (model 6350; Cambridge Technology, Cambridge, MA). Optimal DIAm length for isometric force generation and supramaximal stimulus intensities for both nerve and muscle stimulation were established in a manner identical to previous reports (Ermilov et al. 2010; Fournier et al. 1991; Greising et al. 2015a; Johnson and Sieck 1993; Kuei et al. 1990; Mantilla et al. 2004b; Sieck et al. 2012; Sieck and Prakash 1995). Neuromuscular transmission was assessed during supramaximal stimulation (model 701C; Aurora Scientific, Aurora, ON, Canada) at rates of 10, 40, and 75 Hz (separate experiments for each rate). In one set of experiments, the train duration was set to 330 ms, providing a 33% duty cycle across all stimulation frequencies. In a second set of experiments, the number of nerve stimulus pulses was fixed to 13 and the train duration (330 ms for 40-Hz stimulations, 173 ms for 75-Hz stimulations) was adjusted accordingly for each stimulus frequency (centered around the number of stimulus pulses provided in 330 ms at 40 Hz). The phrenic nerve was stimulated via a suction electrode using 0.05-ms-duration pulses in varying duration trains repeated each second for a 120-s period. Every 15 s during the 120-s period, the muscle was directly stimulated using platinum plate electrodes placed on either side of the muscle strip with 0.5-ms-duration pulses in varying duration trains depending on experimental condition (controlled duty cycle or number of pulses). At each stimulation frequency, NMTF was assessed by comparing DIAm forces evoked by phrenic nerve stimulation with that evoked by direct muscle stimulation (Ermilov et al. 2010; Fournier et al. 1991; Greising et al. 2015a; Johnson and Sieck 1993; Kuei et al. 1990; Mantilla et al. 2004b; Sieck et al. 2012; Sieck and Prakash 1995). With NMTF, muscle fibers are not activated by nerve stimulation and thus spared from muscle-derived contributions to fatigue (Rowley et al. 2005, 2007). Accordingly, increased NMTF reflects greater differences between forces evoked by nerve compared with muscle stimulation (i.e., NMTF% is the percentage of force loss that is related to neuromuscular transmission, not DIAm fatigue). Evoked DIAm forces were digitized and recorded in LabChart software (ADInstuments, Dunedin, New Zealand). As previously reported (Elliott et al. 2016; Fogarty et al. in press; Khurram et al. 2019; Lewis et al. 1986; Sieck et al. 1989b) specific force was determined by dividing absolute force by the cross-sectional area of the DIAm strip.
DIAm neuromuscular junction morphology.
In type-identified DIAm fibers, NMJs were visualized using confocal microscopy, as previously described (Gonzalez Porras et al. 2019; Mantilla et al. 2007; Sieck et al. 1999, 2012). Briefly, ex vivo DIAm strips were fixed in 4% paraformaldehyde and incubated with α-bungarotoxin conjugated to Alexa Fluor 555 (0.1 μg/ml; catalog no. B35451; Invitrogen, Carlsbad, CA) to stain the postsynaptic cholinergic receptors. Anti-synaptophysin antibody D-4 was used to stain presynaptic terminals (1 mg/ml; catalog no. sc17750; Santa Cruz Biotechnology, Santa Cruz, CA) with an Alexa 647-conjugated donkey anti-mouse IgG secondary antibody (1:200; catalog no. 715605159; Jackson ImmunoResearch Laboratories, Baltimore, PA). As an index of axon terminal complexity, the relative planar area of axon terminals occupying the motor endplate was determined. Labeled axon terminals and motor endplates were circumscribed by a rectangular region of interest in Metamorph, fluorescence intensities of α-bungarotoxin-labeled motor endplates and synaptophysin-labeled axon terminals were thresholded to generate binary images, and the extent of volumetric overlap was determined for each NMJ. Well-established morphological (size and complexity) criteria (Mantilla et al. 2004a; Sieck et al. 2012) were used to classify NMJs at type I and IIa or type IIx and/or IIb DIAm fibers. In addition, the volumetric overlap of individual NMJs was plotted against the DIAm fiber diameter in a manner identical to past reports (Sieck et al. 2012).
Statistical analysis.
The number of animals and number of NMJs per fiber type per animal were determined by power analysis based on previous reports in rats (Ermilov et al. 2010; Fournier et al. 1991; Johnson and Sieck 1993; Kuei et al. 1990; Mantilla et al. 2004b; Sieck et al. 2012). Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA) with two-way ANOVA and Bonferroni post hoc tests used to compare groups (age) and factor (stimulation frequency). All data were assessed for normality with Shapiro-Wilk tests. Within a particular data set, any data point outside 2 SD from the mean was excluded from further analysis. Pearson’s coefficients were calculated and used to compare the linearity of NMJ colocalized volumes and DIAm fiber diameter. Significance was set as P < 0.05, and all data are presented as means ± 95% confidence intervals (CI) unless otherwise stated.
RESULTS
Age-associated changes in DIAm specific force.
Specific force of the DIAm evoked by direct muscle stimulation was significantly lower in older animals at 40 and 75 Hz (Fig. 1). The maximum DIAm specific force (at 75 Hz) was 19.6 ± 2.2 N/cm2 in older rats compared with 23.8 ± 3.2 N/cm2 in younger animals (P = 0.02). These results are consistent with DIAm sarcopenia as previously reported in F344 rats (Elliott et al. 2016; Gosselin et al. 1994; Khurram et al. 2018).
Age-associated changes in NMTF during the first stimulus train are frequency dependent.
NMTF was assessed in the DIAm at different ages by comparing the specific force evoked by phrenic nerve stimulation to force evoked by direct muscle stimulation at 10, 40, and 75 Hz (Fig. 1). Even within the first stimulus train, significant NMTF was detected, which depended on age [F(1,33) = 30.3; P < 0.0001] and stimulation frequency [F(2,33) = 11.0; P = 0.0002] and displayed an age × stimulation frequency interaction [F(2,33) = 7.0; P = 0.003]. At 10 Hz, the extent of initial NMTF was not different across ages (P > 0.05; Fig. 1): 13 ± 1% in younger rats (forces: youngmuscle10 = 9.4 ± 3.4 N/cm2 vs. youngnerve10 = 8.2 ± 2.9 N/cm2) and 16.3 ± 0.9% in older animals (mean forces: oldmuscle10 = 8.6 ± 2.9 N/cm2 vs. oldnerve10 = 7.2 ± 2.6 N/cm2; P > 0.05; Fig. 1).
At 40 Hz, the extent of initial NMTF increased in older animals (P = 0.0002; Fig. 1): 41 ± 13% in older rats (mean forces: oldmuscle40 = 17.1 ± 3.1 N/cm2 vs. oldnerve40 = 10.0 ± 2.4 N/cm2) compared with 10 ± 6% in younger rats (mean forces: youngmuscle40 = 20.8 ± 3.3 N/cm2 vs. youngnerve40 = 19.1 ± 3.0 N/cm2).
Similarly, at 75-Hz stimulations, the extent of initial NMTF was much higher in older rats (P < 0.0001; Fig. 1): 47 ± 22% in older animals (mean forces: oldmuscle40 = 18.9 ± 2.2 N/cm2 vs. oldnerve75 = 10.5 ± 4.9 N/cm2) compared with 19 ± 8% in younger rats (mean forces: youngmuscle75 = 22.5 ± 4.3 N/cm2 vs. youngnerve75 = 18.0 ± 3.7 N/cm2).
Age-associated changes in NMTF during 120 s of repeated stimulation at fixed duty cycle are frequency dependent.
During 10-Hz repetitive stimulation, a reduction of DIAm specific force was detected across the 120-s period (Fig. 2A), with a significant effect of time in both muscle [F(8,88) = 22.1; P < 0.0001] and nerve-evoked stimulations [F(8,88) = 36.8; P < 0.0001; Fig. 2D]. Importantly there were no age-associated changes in specific force via muscle [F(1,11) = 0.6; P = 0.45] or nerve-evoked stimulations [F(1,11) = 0.76; P = 0.43; Fig. 2D]. Similarly, there was no effect of age × time interaction on specific force with muscle [F(8,88) = 1.3; P = 0.26] or nerve [F(8,88) = 0.4; P = 0.93] stimulations at 10 Hz (Fig. 2D). There was a progressive reduction in specific force associated with time during 10-Hz stimulation of muscle [F(8,88) = 22.1; P < 0.0001] and nerve [F(8,88) = 36.8; P < 0.0001; Fig. 2D].
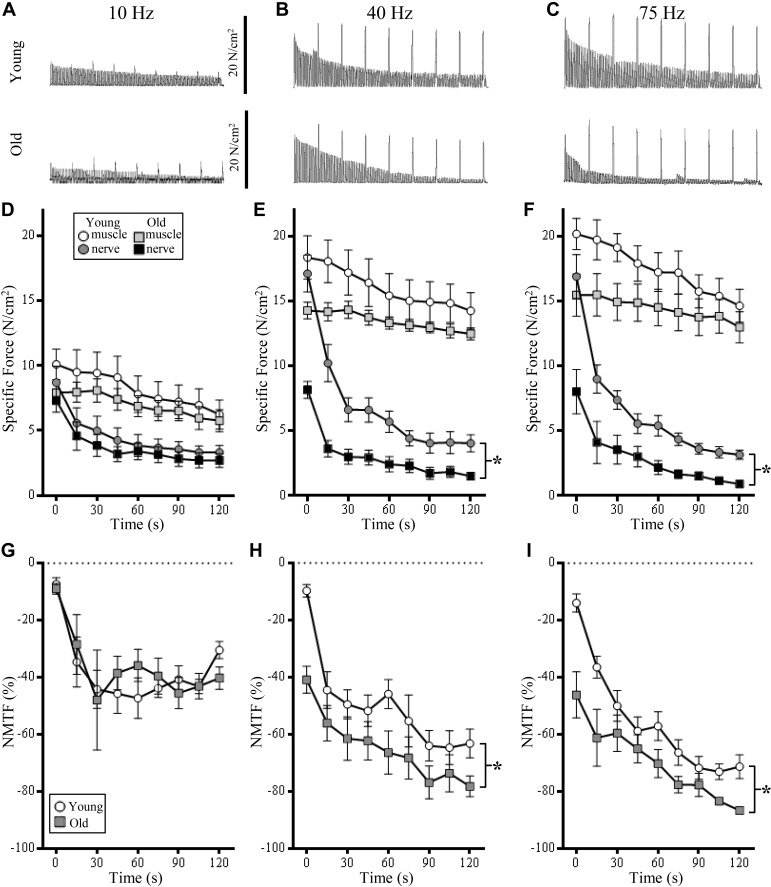
Fig. 2.
At 40- and 75-Hz stimulations, age increases the contribution of neuromuscular transmission failure (NMTF) to reduced diaphragm muscle (DIAm) force production. A–C: representative traces of continuous nerve-evoked contractions, superimposed with muscle-evoked contractions (every 15 s) at 10-, 40-, and 75-Hz stimulation frequencies, respectively, in young (top row) and old rats (bottom row). D: time course illustrating no difference in the specific force (means ± SE) generated by 10-Hz muscle stimulation (open circles, young; light gray squares, old) and nerve-evoked contraction between young (dark gray circles) and old rats (closed squares). E: time course illustrating the specific force (means ± SE) generated by 40-Hz muscle stimulation and nerve-evoked contraction between young and old rats. In old rats, the specific force generated during nerve-evoked contractions was significantly reduced compared with young rats (*P < 0.05). F: time course illustrating the specific force (means ± SE) generated by 75-Hz muscle stimulation and nerve-evoked contraction between young and old rats. In old rats, the specific force generated during nerve-evoked contractions was significantly reduced compared with young rats (*P < 0.05). G: time course illustrating no difference in the development of NMTF% (means ± SE) between young (open circles) and old rats (gray squares) at 10-Hz stimulations. H: time course illustrating increased development of NMTF% (means ± SE) in old compared with young rats at 40-Hz stimulations. I: time course illustrating increased development of NMTF% (means ± SE) in old compared with young rats at 75-Hz stimulations. Across all stimulation experiments, reduction of both muscle and nerve-evoked DIAm force occurs across the duration of this experiment (i.e., fatigue). *P < 0.05, two-way ANOVA with Bonferroni post hoc analysis; n = 7–8 rats per age.
During repetitive 10-Hz stimulation, a significant worsening of NMTF% was detected across the 120-s period (Fig. 2G), which depended on time alone [F(8,96) = 15.6; P < 0.0001]. Neither age [F(1,12) = 0.1; P = 0.86] nor age × time interactions [F(8,96) = 0.9; P = 0.55] had a significant effect on NMTF% at 10 Hz (Fig. 2G).
During repetitive 40-Hz stimulation, a reduction of DIAm specific force was detected across the 120-s period (Fig. 2B), with a significant effect of time in both muscle [F(8,96) = 15.8; P < 0.0001] and nerve-evoked stimulations [F(8,96) = 78.1; P < 0.0001; Fig. 2E]. Importantly, there was an age-associated reduction in DIAm specific force via nerve-evoked stimulations [F(1,12) = 20.0; P = 0.001; Fig. 2E]. The age × time interaction [F(8,96) = 10.7; P < 0.0001] showed old rats having an ~55% decrease in nerve-evoked DIAm specific force compared with young rats from 0, 30, and 60 s, a more rapid decline in nerve-evoked force (P < 0.03; Fig. 2E). There was no effect of age on DIAm specific force via muscle stimulations [F(1,12) = 2.5; P = 0.14; Fig. 2E].
During repetitive 40-Hz stimulation, a significant worsening of NMTF% was detected across the 120-s period (Fig. 2H), which depended on time [F(8,96) = 13.4; P < 0.0001] and age [F(1,12) = 14.9; P = 0.002]. Post hoc analyses showed these reductions were significant at 0, 60, 90, 105, and 120 s (P < 0.05; Fig. 2H).
During repetitive 75-Hz stimulation, a reduction of DIAm specific force was detected across the 120-s period (Fig. 2C), with a significant effect of time in both muscle [F(8,104) = 10.0; P < 0.0001] and nerve-evoked stimulations [F(8,104) = 40.7; P < 0.0001; Fig. 2F]. Importantly, there was an age-associated reduction in DIAm specific force via nerve-evoked stimulations [F(1,13) = 16.5; P = 0.001; Fig. 2F]. The age × time interaction [F(8,104) = 17.5; P < 0.0001] showed old rats having an ~50% decrease in nerve-evoked DIAm specific force compared with young rats at 0 and 30 s (P < 0.03; Fig. 2F). There was no effect of age on DIAm specific force via muscle stimulations [F(1,13) = 2.5; P = 0.14; Fig. 2F].
During repetitive 75-Hz stimulation, a significant worsening of NMTF% was detected across the 120-s period (Fig. 2I), which depended on time [F(8,104) = 31.8; P < 0.0001], age [F(1,13) = 12.2; P = 0.004], and age × time interactions [F(1,12) = 2.3; P = 0.02]. Post hoc analyses showed these reductions were significant at 0, 15, 105, and 120 s (P < 0.05; Fig. 2I).
Age-associated changes in final DIAm NMTF at a fixed duty cycle are frequency dependent.
Final NMTF was assessed in the DIAm at different ages by comparing the specific force evoked by phrenic nerve stimulation with force evoked by direct muscle stimulation at 10, 40, and 75 Hz. Following 120 s of repeated nerve and intermittent (every 15 s) muscle stimulation, a significant reduction in DIAm specific force (N/cm2) was detected, which depended on age [F(3,72) = 116.9; P < 0.0001] and, stimulation [muscle or nerve; F(2,72) = 27.0; P < 0.0001] and displayed an age × stimulation interaction [F(6,72) = 14.4; P < 0.0001; Fig. 3].
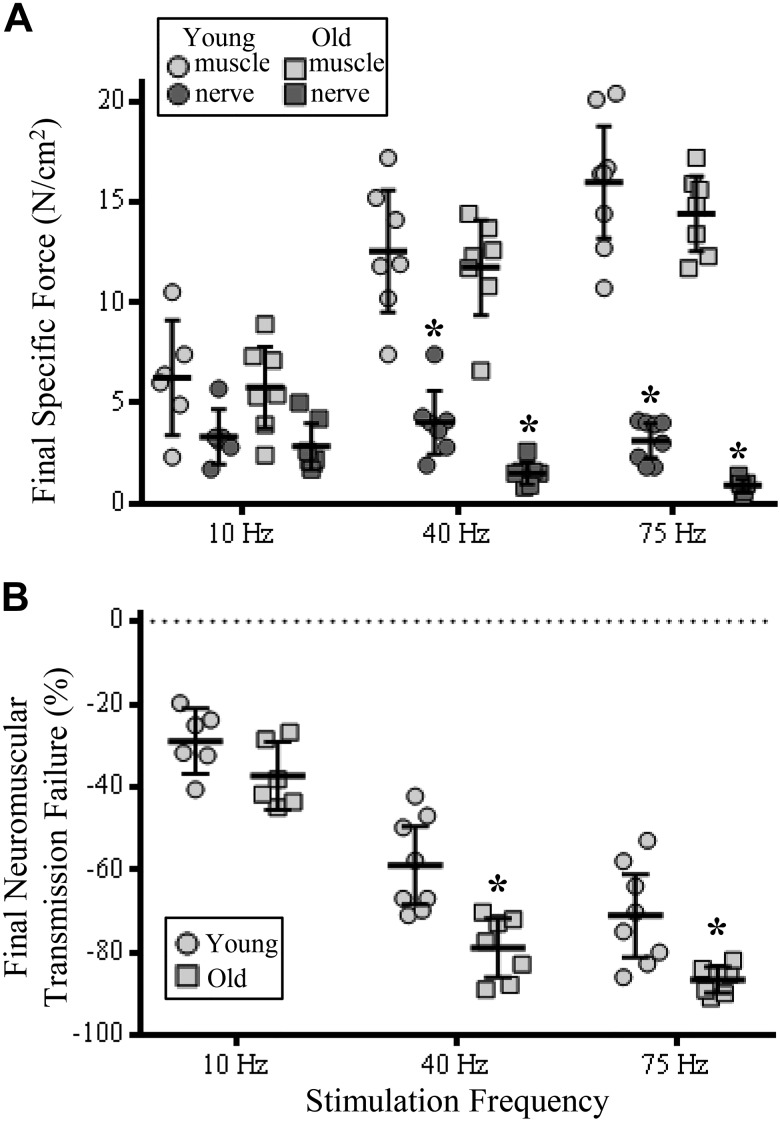
Fig. 3.
Increased contribution of neuromuscular transmission failure (NMTF) to diaphragm muscle (DIAm) reduced specific force at higher stimulation frequencies in old compared with young rats following 120 s of stimulation. A: plot [means ± 95% confidence interval (CI)] of DIAm specific force following 120 s of muscle (light gray symbols) or nerve-evoked stimulation (dark gray symbols) in old (squares) compared with young rats (circles) at 40 and 75 Hz. B: plot (means ± 95% CI) of increased NMTF% following 120 s of repeated stimulation in old (squares) compared with young rats (circles) at 40 and 75 Hz. *P < 0.05, two-way ANOVA with Bonferroni post hoc analysis; n = 7–8 rats per age.
At 10 Hz, the extent of specific force reduction in young and old rats after the 120-s stimulation was unchanged across age and stimulation frequency groups (P > 0.05; Fig. 3A). At 40 Hz, the extent of specific force reduction in young and old rats after the 120-s stimulation was significantly different. Final nerve- compared with muscle-elicited DIAm specific force was reduced by 68% in young (P < 0.0001) and 87% in old rats (P < 0.0001; Fig. 3A). Similarly, after the 120-s stimulation at 75 Hz, the extent of specific force reduction in young and old rats was significantly different. Final nerve- compared with muscle-elicited DIAm specific force was reduced by 81% in young (P < 0.0001) and 94% in old rats (P < 0.0001; Fig. 3A).
Following 120 s of nerve and muscle DIAm stimulations, age [F(1,36) = 27.3; P < 0.0001] and stimulation frequency [F(2,36) = 92.2; P < 0.0001] had an effect on final NMTF (Fig. 3B). At 10-Hz stimulations, final NMTF% in old rats (29 ± 8%) was unchanged compared with that in young rats (37 ± 8%; P = 0.34). Following 120 s of 40-Hz stimulations, final NMTF% was reduced by a further ~20% in old (79 ± 7%) compared with young rats (59 ± 8%; P = 0.0004), with a relative difference between the two groups of 33% (Fig. 3B). Following 120 s of 75-Hz stimulations, final NMTF% was reduced by a further ~15% in old (87 ± 3%) compared with young rats (71 ± 10%; P = 0.006), with a relative difference between the two groups of 22% (Fig. 3B).
Age-associated changes in NMTF during the first stimulus train and following 120 s of continuous stimulation occur at 40 and 75 Hz when pulse number is controlled for.
When stimulus was delivered in 330-ms trains to maintain a constant duty cycle, the number of pulses within each train differed. To determine if the effects of NMTF were consistent regardless of the number of pulses delivered within each train, we provided 13 pulses per train at rates of 40 and 75 Hz, with train durations and intertrain frequency set to ensure a duty cycle of 33% (see methods).
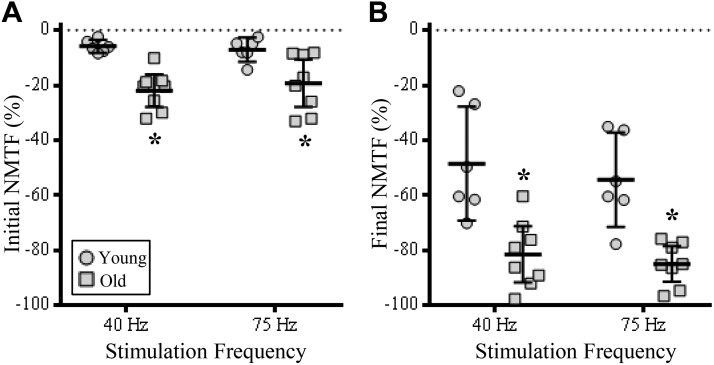
Even after the first stimulation train (containing an identical number of pulses), there were significant changes in NMTF% that were associated with age [F(1,12) = 21.7; P = 0.0006], but not stimulation frequency [F(1,12) = 0.7; P = 0.76]. Post hoc analyses showed similar increases in the initial NMTF% of older rats (~20%) compared with younger rats (~6%) at both 40 (P = 0.0007)- and 75-Hz stimulations (P = 0.009; Fig. 4A).
Fig. 4.
Increased contribution of neuromuscular transmission failure to diaphragm muscle (DIAm) specific force loss at the initial stimulation train and following 120 s of 40- and 75-Hz stimulation when pulse number per train is fixed. A: plot [means ± 95% confidence interval (CI)] illustrating increased failure of neuromuscular transmission (NMTF; % of initial direct nerve-evoked compared with initial muscle-evoked force) in old (squares) compared with young rats (circles) at 40- and 75-Hz stimulations. B: plot (means ± 95% CI) illustrating increased NMTF% in old compared with young rats following 120 s of 40- and 75-Hz stimulations. In all experiments, the number of pulses per train were fixed (13). *P < 0.01, two-way ANOVA with Bonferroni post hoc analysis; n = 6–8 rats per age.
During repetitive 40- and 75-Hz stimulations (with trains containing an identical number of pulses), a significant worsening of NMTF% was detected across the 120-s period (Fig. 5), which depended on time [F(8,104) = 31.8; P < 0.0001] and age [F(1,13) = 12.2; P = 0.004]. Post hoc analyses showed that increased NMTF% in old compared with young rats at both 40 and 75 Hz were significant at 90, 105, and 120 s (P < 0.05; Fig. 5). Importantly, within age groups, there were no significant differences in NMTF% between 40- and 75-Hz stimulations, when pulse number was controlled for (P > 0.99 in all combinations of frequency and time within age groups; Fig. 5).
Following 120 s of repeated stimulations at either 40 or 75 Hz (with trains containing an identical number of pulses), there were significant changes in final NMTF% that were associated with age [F(1,12) = 25.9; P = 0.0003], but not stimulation frequency [F(1,12) = 1.2; P = 0.29]. Post hoc analyses showed similar increases in the final NMTF% of older rats (~85%) compared with younger rats (~50%) at both 40 (P = 0.0004)- and 75-Hz stimulations (P = 0.001; Fig. 4B).
Age-associated decreases in pre- and postsynaptic apposition of NMJs innervating type IIx and/or IIb DIAm fibers.
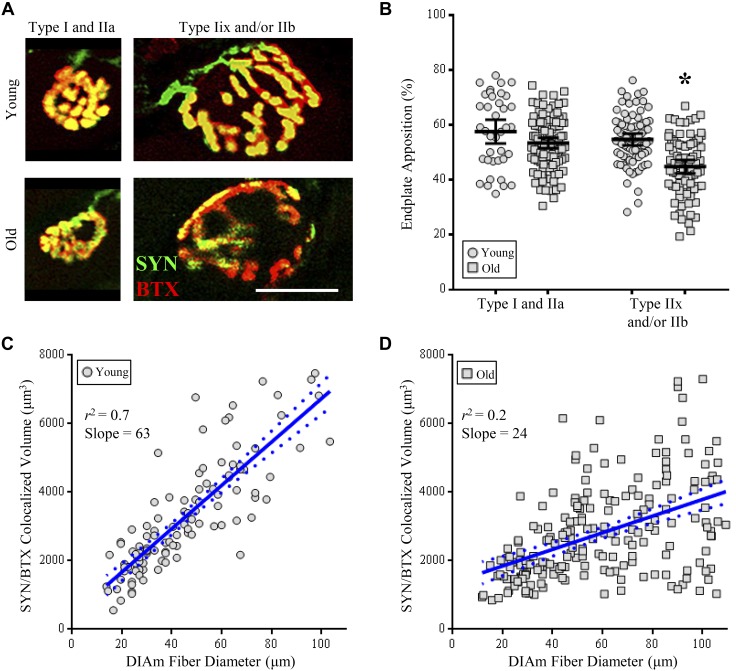
A total of 300 NMJs from 8 different animals (4 young and 4 old) were analyzed for NMJ morphology, with the distributions of the planar endplate area being bimodal in both young and old rats (Fig. 6). As previously reported in the rat DIAm (Mantilla et al. 2004a; Sieck et al. 2012; Sieck and Prakash 1997), NMJs were stratified on the basis of motor endplate areas into two groups, encompassing the smaller type I and IIa fibers or the larger type IIx and/or IIb fibers (Fig. 7). The relative volume of motor endplates occupied by axon terminals (%apposition) was dependent on age [F(1,297) = 29.7; P < 0.0001] and fiber type [F(1,297) = 19.4; P < 0.0001] and displayed an age × fiber type interaction [F(1,297) = 5.0; P < 0.03; Fig. 7B]. Post hoc analyses showed that there was no difference in %apposition of NMJs at type I and IIa fibers between young and old rats (P < 0.22; Fig. 7B). There was an 18% reduction in %apposition of NMJs at type IIx and/or IIb fibers between young and old rats (P < 0.0001; Fig. 7B). When compared between fiber types, the %apposition of old NMJs at type IIx and/or IIb fibers compared with NMJs of type I and IIa fibers was reduced by 22% compared with young (P < 0.0001) and 16% compared with old rats (P < 0.0001; Fig. 7B).
Fig. 7.
Decreased apposition of pre- and postsynaptic domains of neuromuscular junctions (NMJs) at type IIx and/or IIb diaphragm muscle (DIAm) fibers in old compared with young rats. A: representative z stack (a maximum intensity projection of eight 0.5-μm optical sections) of the axon terminal volume (synaptophysin; green) and motor endplate (α-bungarotoxin; red) in type I or IIa fibers (left column) and type IIx and/or IIb fibers (right column) of both young (top row) and old rats (bottom row). B: plot [means ± 95% confidence interval (CI)] of axon terminal fragmentation of type IIx and/or IIb fibers of old rats compared with all other groups, as measured by the relative axon terminal volume colocalized with motor endplate. *P < 0.0001, two-way ANOVA with Bonferroni post hoc analysis. C and D: relationship between the colocalized volume (axon terminal and motor endplate) and DIAm fiber diameter was linear in young (C) and old rats (D). However, there was a significant difference in the slopes between young and old groups (*P < 0.0001), and goodness of fit was reduced in old rats (young: r2 = 0.70; old: r2 = 0.23). Pearson’s coefficients, *P < 0.0001; type I and IIa (young: n = 38 NMJ endplates; old: n = 104 NMJ endplates), type IIx and/or IIb (young: n = 78 NMJ endplates; old: n = 81 NMJ endplates).
To further examine the motor unit type dependence of NMJ vulnerability to aging effects, we analyzed the volumetric colocalization of the axon terminal and motor endplate in relation to the diameter of the respective DIAm fiber, as previously reported (Sieck et al. 2012). In young rats, the relationship between colocalized volume and DIAm fiber diameter was linear (Fig. 7C), with the slope significantly non-zero [63 ± 8; F(1,110) = 258.8; P < 0.0001] and high goodness of fit (r2 = 0.70). In contrast, the linear relationship between colocalized volume and DIAm fiber diameter was significantly different in the old rats (Fig. 7D) compared with young, with the slope in old rats, though significantly non-zero [24 ± 6; F(1,207) = 60.3; P < 0.0001], being poorly predictive (r2 = 0.23) and significantly different from that in young rats [F(1,317) = 47.0; P < 0.0001; Fig. 7D].
DISCUSSION
The results of the present study demonstrate five main findings: 1) neuromuscular transmission and maximum specific force is impaired in the DIAm of older rats; 2) after 120 s of repeated stimulation, the extent of NMTF in the DIAm increases in older compared with younger rats; 3) the extent of NMTF increases at higher stimulation frequencies (40 and 75 Hz) compared with that at 10 Hz; 4) after 120 s of repeated stimulation, the frequency-dependent increase in NMTF is more pronounced in older animals; and 5) the NMJs at type IIx and/or IIb DIAm fibers are affected during aging, whereas NMJs at type I and IIa fibers are preserved. Taken together, these results support the hypothesis of increased vulnerability of neuromuscular transmission in type FInt and FF motor units during aging.
Previous work has shown that type S and type FR units are relatively less susceptible to NMTF across all frequencies (Johnson and Sieck 1993; Sieck and Prakash 1995). During stimulation scenarios where type FInt and FF motor units are more susceptible to NMTF (i.e., 40- and 75- Hz stimulations) (Johnson and Sieck 1993; Sieck and Prakash 1995), marked increases in NMTF% with age were noted. Thus our results indicate that NMTF is predominantly occurring in type FInt and FF motor units. This interpretation is consistent with the impairment of high-force expulsive/straining behaviors (Greising et al. 2015b; Khurram et al. 2018), decreased maximal DIAm contractile force (Elliott et al. 2016; Gosselin et al. 1994; Khurram et al. 2018), and the selective loss of larger phrenic motor neurons (Fogarty et al. 2018b) in old rodents. The conserved neuromuscular transmission in old rats under lower frequency stimulation (i.e., 10-Hz stimulation) is entirely consistent with the discharge rates of motor neurons during ventilation (Seven et al. 2014), the resilience of lower force ventilatory behaviors (Khurram et al. 2018), the maintenance of type I and IIa DIAm fiber cross-sectional areas (Elliott et al. 2016; Gosselin et al. 1994; Khurram et al. 2018), and the sparing of smaller phrenic motor neurons (Fogarty et al. 2018b).
Past studies assessing the initial train of neuromuscular transmission have shown that failure (16%) is likely to occur only under high-frequency stimulation (75 Hz) conditions (Fournier et al. 1991, Johnson and Sieck 1993), with negligible failures at lower frequency stimulations (10 Hz). Our data are consistent with these reports (<5% failure at 10 Hz), and we interpret our current observations of conserved neuromuscular transmission effectiveness in old age during 10-Hz stimulations as being indicative of intact NMJs at type S and FR motor units. We observed an ~15% failure in initial neuromuscular transmission effectiveness of young rats across stimulation frequencies, in line with previous studies (Fournier et al. 1991; Johnson and Sieck 1993). In old rats, further reductions of initial neuromuscular transmission effectiveness occurred at 40- and 75-Hz stimulations. This impairment is likely due to axonal propagation disturbances, such as increased branch point failures, frank denervation, and/or selective degenerations of NMJs of type FInt and/or FF motor units (Greising et al. 2015a, 2017; Prakash and Sieck 1998). The possibility that anoxia of the isolated DIAm preparations during the experimental time period may differentially be affected by aging was discounted, because previous reports revealed no differences in muscle bulk and interstitial space in DIAm between 6- and 24-mo-old F344 rats (Khurram et al. 2018). Because of tissue anoxia, the magnitude of NMTF in isolated DIAm may be greater than that in the in vivo scenario, although the precise difference between in vivo phrenic neurogram and in vitro nerve stimulation remains inadequately quantified. Regardless, an effect of anoxia on only the aged DIAm tissue remains doubtful.
During sustained 10-Hz stimulations, NMTF did occur, and reductions in both relative and specific force, compared with initial force production, were ~30%, although importantly, there was no observable difference between age groups when assessed in relative or absolute terms. During sustained 40- or 75-Hz stimulations, NMTF did occur, with a reduction in relative and specific force of ~60–70% compared with initial values, similar to results in DIAm from past reports in young adult Sprague-Dawley rats (Ermilov et al. 2010; Fournier et al. 1991; Johnson and Sieck 1993; Kuei et al. 1990; Sieck et al. 2012; Sieck and Prakash 1995). Age exacerbated NMTF after repeated 120-s stimulation such that 40-Hz stimulations resulted in ~80% NMTF and 75-Hz stimulations in ~90% NMTF in DIAm of old rats.
Different factors contribute to force production from nerve-evoked and indirect muscle stimulation, and different limiting factors apply at different motor unit types (Johnson and Sieck 1993; Sieck and Prakash 1995). For example, impaired neuromuscular transmission may occur due to axonal conduction blockade, branch-point failure, synaptic vesicle release failure, or derangement in the integrity of presynaptic or postsynaptic NMJ components (Sieck and Prakash 1995). The degree of presynaptic and postsynaptic overlap is highly correlated with the efficacy of neuromuscular transmission (Prakash et al. 1996, 1999; Sieck et al. 2012). In the case of repetitive stimulations (NMTF), type FInt and FF units are more susceptible to propagation failures, particularly at higher stimulation frequencies (Johnson and Sieck 1993; Krnjević and Miledi 1958, 1959), with our mean values for initial nerve-evoked force in good agreement with past studies (Ermilov et al. 2010; Fournier et al. 1991; Greising et al. 2015a; Johnson and Sieck 1993; Kuei et al. 1990; Sieck et al. 2012; Sieck and Prakash 1995). For repeated stimulations and NMTF, postsynaptic desensitization of acetylcholine receptors also contributes to decreased force (Katz and Thesleff 1957; Sakmann et al. 1980). Additionally, repeated stimulations cause decline in the safety factor of type FInt and FF motor units, a phenomenon not observed in type S or FR units (Ermilov et al. 2007; Gertler and Robbins 1978). Differences in quantal content and nonuniformity of release at NMJs also contribute to these phenomena (Bennett and Lavidis 1989; Rowley et al. 2007), although the specific age-associated quantal characteristics across motor unit types remain ill-defined.
Our results show morphological findings consistent with our data on impaired neuromuscular transmission at higher frequency stimulations. In the current study we observed a reduced apposition of pre- and postsynaptic domains of NMJs innervating type IIx and/or IIb muscle fibers. Consistent with our findings, at low frequencies, reduced apposition is not associated with a decline in the efficacy of synaptic transmission (Willadt et al. 2016). Nevertheless, our evidence shows an increase in NMTF at higher frequencies concomitant with less apposition of pre- and postsynaptic domains of NMJs in type FInt and/or FF motor units. This indicates that age-associated NMJ remodeling is largely restricted to type FInt and/or FF motor units. Some of these properties might include increased synaptic vesicle retrieval capacity to ensure that the rate of synaptic vesicle formation is equated with the rate of release (Rowley et al. 2005). Future studies will look toward differences in functional properties of motor unit types with aging.
Similar to DIAm, NMTF in limb muscles also affects FInt and FF units to a greater extent than the generally more active type S and FR units (Hennig and Lømo 1985). Similar vulnerabilities of type FInt and FF motor units are observed in amyotrophic lateral sclerosis (ALS; Hegedus et al. 2007, 2008). In both aging and ALS, the mechanisms of motor unit dysfunction and motor neuron death may be similar. In both human pathology and animal models of ALS (Dukkipati et al. 2018; Fogarty 2018a; Kiernan and Hudson 1991) and aging (Fogarty et al. 2018b; Hashizume et al. 1988; Jacob 1998; Lexell et al. 1988; Zhang et al. 1996), the larger motor neurons of type FInt and FF motor units are selectively vulnerable, albeit over a more protracted temporal domain during aging. There has been much debate about the myogenic (die-backward) or neurogenic (die-forward) etiology of motor neuron loss in ALS and aging (Fogarty 2018b, 2019). Indeed, in multiple ALS models, perturbations are evident throughout the neuraxis (Fogarty et al. 2015, 2016a, 2016b; Jara et al. 2012; Jiang et al. 2017; Romer et al. 2017; van Zundert et al. 2008) and NMJs (Arbour et al. 2015; Chand et al. 2018; Palamiuc et al. 2015; Smittkamp et al. 2014; Tremblay et al. 2017) before detectable motor neuron death. Converging evidence suggests that neurotrophic signaling, including brain-derived neurotrophic factor (BDNF) and the high-affinity tropomyosin-related kinase receptor subtype B (TrkB), protects against motor neuron loss in ALS models (Das et al. 2016; Henderson et al. 1994; Kishino et al. 1997).
In the mouse DIAm, NMJ deficits (Greising et al. 2015a, 2015d) are observed before sarcopenia (Greising et al. 2013), although no data reporting age-associated phrenic motor neuron loss in mice are currently available. These impairments are mediated by reduced availability of TrkB receptors at NMJs during aging (Greising et al. 2017; Personius and Parker 2013). The importance of TrkB to effective neuromuscular transmission is underscored by enhancement of neuromuscular transmission by BDNF in young rats (Mantilla et al. 2014) and mice (Greising et al. 2017; Mantilla and Ermilov 2012) and the impairment of neuromuscular transmission following inhibition of effective BDNF/TrkB signaling in young mice (Greising et al. 2015a).
Throughout the life span, motor axons and target muscle fibers undergo continuous cycles of denervation/reinnervation (Larsson 1995). Usually, this process results in muscle fibers being re-innervated by the original motor axon or through collateral sprouting from a nearby axon. Age-associated impairments in the re-innervation process and persistent denervation have been shown to precede muscle fiber atrophy (Aare et al. 2016; Hepple 2018; Hepple and Rice 2016), including in studies of rodent DIAm (Greising et al. 2015a; Greising et al. 2017). Thus, during aging, the loss of motor neurons likely results in an increase in the innervation ratio of the remaining motor units (i.e., motor unit expansion) (Hepple and Rice 2016; Kanda and Hashizume 1989). In the DIAm, spared smaller motor neurons are likely to be smaller type S or FR units, accounting for age-related fiber type clustering (Bodine-Fowler et al. 1993; Fabiato 1982; Greising et al. 2015c). Muscle fibers that remain denervated for extended periods atrophy, through a process that is not entirely clear (Hepple 2018). Future studies must be designed to shed light on the specific mechanisms underlying the temporal and fiber type-specific pattern of NMJ instability during aging and the related decline in neurotrophic support.
Our results shed some light onto the possible physiological implications for impaired motor neuron-to-muscle signaling during aging. For ventilatory behaviors, motor neuron discharges at both onset and peak are of low frequency, whereas high-force expulsive behaviors usually necessitate higher onset discharge rates and greater peak discharge rates (Seven et al. 2013, 2014). For ventilatory behaviors, NMTF is not likely to have a physiological effect in the living animal, because discharge rates and durations are approximated by stimulation at 10 Hz, where NMTF was largely absent. This is underscored by the activation patterns of DIAm during ventilatory behaviors, where S and FR motor units are activated during inspiratory duty cycles of ~33% and can be performed indefinitely without notable fatigue (Belman and Sieck 1982; Seven et al. 2014; Sieck et al. 1984). By contrast, maximum activation of the DIAm, required for expulsive behaviors such as coughing and sneezing, necessitate increased motor neuron discharge rates, albeit at shorter durations (Desmedt and Godaux 1978; Seven et al. 2013, 2014). Our data show that at higher frequencies, even the initial burst of neuromuscular transmission is impaired, so it is likely that during physiological patterns of activity, NMTF is present in DIAm during these expulsive maneuvers. Although these maneuvers are unlikely to be continuous for over 120 s, a subset of patients with acute or chronic cough may exhibit uncontrolled bouts of repetitive coughing (Irwin and Madison 2000). In these cases, the physiological contribution of NMTF to ineffective airway clearance is likely, particularly because the aging population vulnerable to sarcopenia is also more at risk of respiratory infection (Polkey et al. 1997).
In summary, we provide evidence that age-related DIAm neuromuscular transmission deficits selectively afflict muscle fibers of type FInt and FF units. These data support previous observations regarding the vulnerability of larger phrenic motor neurons and type IIx and/or IIb DIAm fibers to sarcopenia.
GRANTS
This work was supported by National Institutes of Health Grants R01AG044615 (to G. C. Sieck and C. B. Mantilla) and R01AG057052 (to C. B. Mantilla and G. C. Sieck).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.J.F., C.B.M., and G.C.S. conceived and designed research; M.J.F. and M.A.G.P. performed experiments; M.J.F., M.A.G.P., and G.C.S. analyzed data; M.J.F., M.A.G.P., C.B.M., and G.C.S. interpreted results of experiments; M.J.F. and M.A.G.P. prepared figures; M.J.F. and G.C.S. drafted manuscript; M.J.F., M.A.G.P., C.B.M., and G.C.S. edited and revised manuscript; M.J.F., M.A.G.P., C.B.M., and G.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Rebecca Macken for assistance in the completion of this project.
REFERENCES
- Aare S, Spendiff S, Vuda M, Elkrief D, Perez A, Wu Q, Mayaki D, Hussain SN, Hettwer S, Hepple RT. Failed reinnervation in aging skeletal muscle. Skelet Muscle 6: 29, 2016. doi: 10.1186/s13395-016-0101-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour D, Tremblay E, Martineau É, Julien JP, Robitaille R. Early and persistent abnormal decoding by glial cells at the neuromuscular junction in an ALS model. J Neurosci 35: 688–706, 2015. doi: 10.1523/JNEUROSCI.1379-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belman MJ, Sieck GC. The ventilatory muscles. Fatigue, endurance and training. Chest 82: 761–766, 1982. doi: 10.1378/chest.82.6.761. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Lavidis NA. The probability of quantal secretion at release sites in different calcium concentrations in toad (Bufo marinus) muscle. J Physiol 418: 219–233, 1989. doi: 10.1113/jphysiol.1989.sp017836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine-Fowler SC, Unguez GA, Roy RR, Armstrong AN, Edgerton VR. Innervation patterns in the cat tibialis anterior six months after self-reinnervation. Muscle Nerve 16: 379–391, 1993. doi: 10.1002/mus.880160407. [DOI] [PubMed] [Google Scholar]
- Chand KK, Lee KM, Lee JD, Qiu H, Willis EF, Lavidis NA, Hilliard MA, Noakes PG. Defects in synaptic transmission at the neuromuscular junction precede motor deficits in a TDP-43Q331K transgenic mouse model of amyotrophic lateral sclerosis. FASEB J 32: 2676–2689, 2018. doi: 10.1096/fj.201700835R. [DOI] [PubMed] [Google Scholar]
- Das MM, Avalos P, Suezaki P, Godoy M, Garcia L, Chang CD, Vit JP, Shelley B, Gowing G, Svendsen CN. Human neural progenitors differentiate into astrocytes and protect motor neurons in aging rats. Exp Neurol 280: 41–49, 2016. doi: 10.1016/j.expneurol.2016.03.023. [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Godaux E. Ballistic contractions in fast or slow human muscles: discharge patterns of single motor units. J Physiol 285: 185–196, 1978. doi: 10.1113/jphysiol.1978.sp012566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukkipati SS, Garrett TL, Elbasiouny SM. The vulnerability of spinal motoneurons and soma size plasticity in a mouse model of amyotrophic lateral sclerosis. J Physiol 596: 1723–1745, 2018. doi: 10.1113/JP275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott JE, Omar TS, Mantilla CB, Sieck GC. Diaphragm muscle sarcopenia in Fischer 344 and Brown Norway rats. Exp Physiol 101: 883–894, 2016. doi: 10.1113/EP085703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermilov LG, Mantilla CB, Rowley KL, Sieck GC. Safety factor for neuromuscular transmission at type-identified diaphragm fibers. Muscle Nerve 35: 800–803, 2007. doi: 10.1002/mus.20751. [DOI] [PubMed] [Google Scholar]
- Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC, Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low-dose inhaled CO. Am J Physiol Regul Integr Comp Physiol 298: R784–R789, 2010. doi: 10.1152/ajpregu.00737.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium release in skinned cardiac cells: variations with species, tissues, and development. Fed Proc 41: 2238–2244, 1982. [PubMed] [Google Scholar]
- Fogarty MJ. The bigger they are the harder they fall: size-dependent vulnerability of motor neurons in amyotrophic lateral sclerosis. J Physiol 596: 2471–2472, 2018a. doi: 10.1113/JP276312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ. Driven to decay: excitability and synaptic abnormalities in amyotrophic lateral sclerosis. Brain Res Bull 140: 318–333, 2018b. [Erratum in Brain Res Bull 146: 327, 2019.] doi: 10.1016/j.brainresbull.2018.05.023. [DOI] [PubMed] [Google Scholar]
- Fogarty MJ. Amyotrophic lateral sclerosis as a synaptopathy. Neural Regen Res 14: 189–192, 2019. doi: 10.4103/1673-5374.244782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Klenowski PM, Lee JD, Drieberg-Thompson JR, Bartlett SE, Ngo ST, Hilliard MA, Bellingham MC, Noakes PG. Cortical synaptic and dendritic spine abnormalities in a presymptomatic TDP-43 model of amyotrophic lateral sclerosis. Sci Rep 6: 37968, 2016a. doi: 10.1038/srep37968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Mantilla CB, Sieck GC. Breathing: motor control of diaphragm muscle. Physiology (Bethesda) 33: 113–126, 2018a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Mantilla CB, Sieck GC. Impact of sarcopenia on diaphragm muscle fatigue. Exp Physiol. In press. doi: 10.1113/EP087558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Mu EW, Noakes PG, Lavidis NA, Bellingham MC. Marked changes in dendritic structure and spine density precede significant neuronal death in vulnerable cortical pyramidal neuron populations in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Acta Neuropathol Commun 4: 77, 2016b. doi: 10.1186/s40478-016-0347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Noakes PG, Bellingham MC. Motor cortex layer V pyramidal neurons exhibit dendritic regression, spine loss, and increased synaptic excitation in the presymptomatic hSOD1(G93A) mouse model of amyotrophic lateral sclerosis. J Neurosci 35: 643–647, 2015. doi: 10.1523/JNEUROSCI.3483-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Omar TS, Zhan WZ, Mantilla CB, Sieck GC. Phrenic motor neuron loss in aged rats. J Neurophysiol 119: 1852–1862, 2018b. doi: 10.1152/jn.00868.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Sieck GC. Evolution and functional differentiation of the diaphragm muscle of mammals. Compr Physiol 9: 715–766, 2019. doi: 10.1002/cphy.c180012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M, Alula M, Sieck GC. Neuromuscular transmission failure during postnatal development. Neurosci Lett 125: 34–36, 1991. doi: 10.1016/0304-3940(91)90124-C. [DOI] [PubMed] [Google Scholar]
- Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988. doi: 10.1152/jn.1988.59.3.1055. [DOI] [PubMed] [Google Scholar]
- Gertler RA, Robbins N. Differences in neuromuscular transmission in red and white muscles. Brain Res 142: 160–164, 1978. doi: 10.1016/0006-8993(78)90186-5. [DOI] [PubMed] [Google Scholar]
- Gonzalez Porras MA, Fogarty MJ, Gransee HM, Sieck GC, Mantilla CB. Frequency-dependent lipid raft uptake at rat diaphragm muscle axon terminals. Muscle Nerve 59: 611–618, 2019. doi: 10.1002/mus.26421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin LE, Johnson BD, Sieck GC. Age-related changes in diaphragm muscle contractile properties and myosin heavy chain isoforms. Am J Respir Crit Care Med 150: 174–178, 1994. doi: 10.1164/ajrccm.150.1.8025746. [DOI] [PubMed] [Google Scholar]
- Greising SM, Ermilov LG, Sieck GC, Mantilla CB. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J Physiol 593: 431–440, 2015a. doi: 10.1113/jphysiol.2014.282244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887, 2013. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Medina-Martínez JS, Stowe JM, Sieck GC. Functional impact of diaphragm muscle sarcopenia in both male and female mice. Am J Physiol Lung Cell Mol Physiol 309: L46–L52, 2015b. doi: 10.1152/ajplung.00064.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Medina-Martínez JS, Vasdev AK, Sieck GC, Mantilla CB. Analysis of muscle fiber clustering in the diaphragm muscle of sarcopenic mice. Muscle Nerve 52: 76–82, 2015c. doi: 10.1002/mus.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Stowe JM, Sieck GC, Mantilla CB. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp Gerontol 72: 184–191, 2015d. doi: 10.1016/j.exger.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Vasdev AK, Zhan WZ, Sieck GC, Mantilla CB. Chronic TrkB agonist treatment in old age does not mitigate diaphragm neuromuscular dysfunction. Physiol Rep 5: e13103, 2017. doi: 10.14814/phy2.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume K, Kanda K, Burke RE. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol 269: 425–430, 1988. doi: 10.1002/cne.902690309. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis 28: 154–164, 2007. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol 586: 3337–3351, 2008. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science 266: 1062–1064, 1994. [Erratum in Science 267: 777, 1995.] doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Hennig R, Lømo T. Firing patterns of motor units in normal rats. Nature 314: 164–166, 1985. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hepple RT. When motor unit expansion in ageing muscle fails, atrophy ensues. J Physiol 596: 1545–1546, 2018. doi: 10.1113/JP275981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepple RT, Rice CL. Innervation and neuromuscular control in ageing skeletal muscle. J Physiol 594: 1965–1978, 2016. doi: 10.1113/JP270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RS, Madison JM. The diagnosis and treatment of cough. N Engl J Med 343: 1715–1721, 2000. doi: 10.1056/NEJM200012073432308. [DOI] [PubMed] [Google Scholar]
- Jacob JM. Lumbar motor neuron size and number is affected by age in male F344 rats. Mech Ageing Dev 106: 205–216, 1998. doi: 10.1016/S0047-6374(98)00117-1. [DOI] [PubMed] [Google Scholar]
- Jara JH, Villa SR, Khan NA, Bohn MC, Ozdinler PH. AAV2 mediated retrograde transduction of corticospinal motor neurons reveals initial and selective apical dendrite degeneration in ALS. Neurobiol Dis 47: 174–183, 2012. doi: 10.1016/j.nbd.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang MC, Adimula A, Birch D, Heckman CJ. Hyperexcitability in synaptic and firing activities of spinal motoneurons in an adult mouse model of amyotrophic lateral sclerosis. Neuroscience 362: 33–46, 2017. doi: 10.1016/j.neuroscience.2017.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Sieck GC. Differential susceptibility of diaphragm muscle fibers to neuromuscular transmission failure. J Appl Physiol (1985) 75: 341–348, 1993. doi: 10.1152/jappl.1993.75.1.341. [DOI] [PubMed] [Google Scholar]
- Kanda K, Hashizume K. Changes in properties of the medial gastrocnemius motor units in aging rats. J Neurophysiol 61: 737–746, 1989. doi: 10.1152/jn.1989.61.4.737. [DOI] [PubMed] [Google Scholar]
- Katz B, Thesleff S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol 137: 267–278, 1957. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurram OU, Fogarty MJ, Rana S, Vang P, Sieck GC, Mantilla CB. Diaphragm muscle function following mid-cervical contusion injury in rats. J Appl Physiol (1985) 126: 221–230, 2019. 10.1152/japplphysiol.00481.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurram OU, Fogarty MJ, Sarrafian TL, Bhatt A, Mantilla CB, Sieck GC. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol Rep 6: e13786, 2018. 10.14814/phy2.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan JA, Hudson AJ. Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain 114: 843–853, 1991. doi: 10.1093/brain/114.2.843. [DOI] [PubMed] [Google Scholar]
- Kishino A, Ishige Y, Tatsuno T, Nakayama C, Noguchi H. BDNF prevents and reverses adult rat motor neuron degeneration and induces axonal outgrowth. Exp Neurol 144: 273–286, 1997. doi: 10.1006/exnr.1996.6367. [DOI] [PubMed] [Google Scholar]
- Krnjević K, Miledi R. Failure of neuromuscular propagation in rats. J Physiol 140: 440–461, 1958. doi: 10.1113/jphysiol.1958.sp005944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K, Miledi R. Presynaptic failure of neuromuscular propagation in rats. J Physiol 149: 1–22, 1959. doi: 10.1113/jphysiol.1959.sp006321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuei JH, Shadmehr R, Sieck GC. Relative contribution of neurotransmission failure to diaphragm fatigue. J Appl Physiol (1985) 68: 174–180, 1990. doi: 10.1152/jappl.1990.68.1.174. [DOI] [PubMed] [Google Scholar]
- Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Med Sci 50: 91–95, 1995. [DOI] [PubMed] [Google Scholar]
- Lewis MI, Sieck GC, Fournier M, Belman MJ. Effect of nutritional deprivation on diaphragm contractility and muscle fiber size. J Appl Physiol (1985) 60: 596–603, 1986. doi: 10.1152/jappl.1986.60.2.596. [DOI] [PubMed] [Google Scholar]
- Lexell J, Taylor CC, Sjöström M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Ermilov LG. The novel TrkB receptor agonist 7,8-dihydroxyflavone enhances neuromuscular transmission. Muscle Nerve 45: 274–276, 2012. doi: 10.1002/mus.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Fahim MA, Zhan WZ, Sieck GC. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve 30: 774–783, 2004a. doi: 10.1002/mus.20173. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience 146: 178–189, 2007. doi: 10.1016/j.neuroscience.2007.01.048. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Stowe JM, Sieck DC, Ermilov LG, Greising SM, Zhang C, Shokat KM, Sieck GC. TrkB kinase activity maintains synaptic function and structural integrity at adult neuromuscular junctions. J Appl Physiol (1985) 117: 910–920, 2014. doi: 10.1152/japplphysiol.01386.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ, Sieck GC. Neurotrophins improve neuromuscular transmission in the adult rat diaphragm. Muscle Nerve 29: 381–386, 2004b. doi: 10.1002/mus.10558. [DOI] [PubMed] [Google Scholar]
- Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci Med Sci 55: B117–B123, 2000. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamiuc L, Schlagowski A, Ngo ST, Vernay A, Dirrig-Grosch S, Henriques A, Boutillier AL, Zoll J, Echaniz-Laguna A, Loeffler JP, René F. A metabolic switch toward lipid use in glycolytic muscle is an early pathologic event in a mouse model of amyotrophic lateral sclerosis. EMBO Mol Med 7: 526–546, 2015. doi: 10.15252/emmm.201404433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personius KE, Parker SD. TrkB expression at the neuromuscular junction is reduced during aging. Muscle Nerve 47: 532–538, 2013. doi: 10.1002/mus.23616. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Harris ML, Hughes PD, Hamnegärd CH, Lyons D, Green M, Moxham J. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med 155: 1560–1564, 1997. doi: 10.1164/ajrccm.155.5.9154857. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Gosselin LE, Zhan WZ, Sieck GC. Alterations of diaphragm neuromuscular junctions with hypothyroidism. J Appl Physiol (1985) 81: 1240–1248, 1996. doi: 10.1152/jappl.1996.81.3.1240. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve 22: 307–319, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve 21: 887–895, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Romer SH, Seedle K, Turner SM, Li J, Baccei ML, Crone SA. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp Neurol 287: 192–204, 2017. doi: 10.1016/j.expneurol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Ermilov LG, Sieck GC. Synaptic vesicle distribution and release at rat diaphragm neuromuscular junctions. J Neurophysiol 98: 478–487, 2007. doi: 10.1152/jn.00251.2006. [DOI] [PubMed] [Google Scholar]
- Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respir Physiol Neurobiol 147: 235–251, 2005. doi: 10.1016/j.resp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Patlak J, Neher E. Single acetylcholine-activated channels show burst-kinetics in presence of desensitizing concentrations of agonist. Nature 286: 71–73, 1980. doi: 10.1038/286071a0. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Sieck GC. Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 117: 1308–1316, 2014. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol 185: 400–409, 2013. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol 180: 88–96, 2012. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Ferreira LF, Reid MB, Mantilla CB. Mechanical properties of respiratory muscles. Compr Physiol 3: 1553–1567, 2013. doi: 10.1002/cphy.c130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Contractile and fatigue properties of diaphragm motor units. In: Respiratory Muscles and Their Neuromotor Control, edited by Sieck GC, Gandevia SC, Cameron WE. New York: Liss, 1987, p. 227–237. [Google Scholar]
- Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neurosci Lett 97: 29–34, 1989a. doi: 10.1016/0304-3940(89)90134-1. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Fournier M, Prakash YS, Blanco CE. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol (1985) 80: 2179–2189, 1996. doi: 10.1152/jappl.1996.80.6.2179. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol (1985) 66: 2196–2205, 1989b. doi: 10.1152/jappl.1989.66.5.2196. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Mantilla CB, Prakash YS. Volume measurements in confocal microscopy. Methods Enzymol 307: 296–315, 1999. doi: 10.1016/S0076-6879(99)07019-6. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Fatigue at the neuromuscular junction: branch point vs. presynaptic vs. postsynaptic mechanisms. In: Neural and Neuromuscular Aspects of Muscle Fatigue, edited by Stuart DG, Gandevia S, Enoka RM, McComas AJ, Thomas CK. New York: Plenum, 1995, p. 83–100. [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Morphological adaptations of neuromuscular junctions depend on fiber type. Can J Appl Physiol 22: 197–230, 1997. doi: 10.1139/h97-014. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Trelease RB, Harper RM. Sleep influences on diaphragmatic motor unit discharge. Exp Neurol 85: 316–335, 1984. doi: 10.1016/0014-4886(84)90143-2. [DOI] [PubMed] [Google Scholar]
- Smittkamp SE, Morris JK, Bomhoff GL, Chertoff ME, Geiger PC, Stanford JA. SOD1-G93A mice exhibit muscle-fiber-type-specific decreases in glucose uptake in the absence of whole-body changes in metabolism. Neurodegener Dis 13: 29–37, 2014. doi: 10.1159/000351606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolep K, Higgins N, Muza S, Criner G, Kelsen SG. Comparison of diaphragm strength between healthy adult elderly and young men. Am J Respir Crit Care Med 152: 677–682, 1995. doi: 10.1164/ajrccm.152.2.7633725. [DOI] [PubMed] [Google Scholar]
- Tremblay E, Martineau É, Robitaille R. Opposite synaptic alterations at the neuromuscular junction in an ALS mouse model: when motor units matter. J Neurosci 37: 8901–8918, 2017. doi: 10.1523/JNEUROSCI.3090-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH Jr, Constantine-Paton M, Bellingham MC. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci 28: 10864–10874, 2008. doi: 10.1523/JNEUROSCI.1340-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadt S, Nash M, Slater CR. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci Rep 6: 24849, 2016. doi: 10.1038/srep24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Goto N, Suzuki M, Ke M. Age-related reductions in number and size of anterior horn cells at C6 level of the human spinal cord. Okajimas Folia Anat Jpn 73: 171–177, 1996. doi: 10.2535/ofaj1936.73.4_171. [DOI] [PubMed] [Google Scholar]