Abstract
This study investigated aging changes in protective balance and startle responses to sudden drop perturbations and their effect on landing impact forces (vertical ground reaction forces, vGRF) and balance stability. Twelve healthy older (6 men; mean age = 72.5 ± 2.32 yr, mean ± SE) and 12 younger adults (7 men; mean age = 28.09 ± 1.03 yr) stood atop a moveable platform and received externally triggered drop perturbations of the support surface. Electromyographic activity was recorded bilaterally over the sternocleidomastoid (SCM), middle deltoid, biceps brachii, vastus lateralis (VL), biceps femoris (BF), medial gastrocnemius (MG), and tibialis anterior (TA). Whole body kinematics were recorded with motion analysis. Stability in the anteroposterior direction was quantified using the margin of stability (MoS). Incidence of early onset of bilateral SCM activation within 120 ms after drop onset was present during the first-trial response (FTR) for all participants. Co-contraction indexes during FTRs between VL and BF as well as TA and MG were significantly greater in the older group (VL/BF by 26%, P < 0.05; TA/MG by 37%, P < 0.05). Reduced shoulder abduction between FTR and last-trial responses, indicative of habituation, was present across both groups. Significant age-related differences in landing strategy were present between groups, because older adults had greater trunk flexion (P < 0.05) and less knee flexion (P < 0.05) that resulted in greater peak vGRFs and decreased MoS compared with younger adults. These findings suggest age-associated abnormalities of delayed, exaggerated, and poorly habituated startle/postural FTRs are linked with greater landing impact force and diminished balance stabilization.
NEW & NOTEWORTHY This study investigated the role of startle as a pathophysiological mechanism contributing to balance impairment in aging. We measured neuromotor responses as younger and older adults stood on a platform that dropped unexpectedly. Group differences in landing strategies indicated age-associated abnormalities of delayed, exaggerated, and poorly habituated startle/postural responses linked with a higher magnitude of impact force and decreased balance stabilization. The findings have implications for determining mechanisms contributing to falls and related injuries.
Keywords: aging, balance, postural control, startle
INTRODUCTION
The inherently unstable nature of human equilibrium during standing represents a major challenge to older adults with impaired balance function due to deficits in neuromuscular, sensorimotor, and musculoskeletal mechanisms (Lord and Dayhew 2001; Rubenstein et al. 1988; Sattin 1992). This inherent instability and these age-related deficits increase the risk of falls, which are a leading cause of injury and death among older people (Stevens et al. 2006). Age-related limitations in postural movements and balance-stabilizing responses to external postural perturbations are contributing factors to loss of balance and falls. For example, abnormally slower or premature postural response onset timing, reduced response magnitudes, longer response durations, increased muscle coactivation with joint stiffening, multiple corrective actions, and reduced response adaptation for stance and gait perturbations have been reported for older compared with younger adults (Allum et al. 2002; Nagai et al. 2011; Tresch et al. 2014).
Several cortical and subcortical brain regions and neural networks have been linked with the control of posture and balance and their changes with aging (Boisgontier et al. 2017; Papegaaij et al. 2014; Salat et al. 2004; Seidler et al. 2010). However, results from structural brain imaging of multiple areas, together with measures of postural performance in younger and older adults, have indicated that reduced brain stem volume with aging best accounted for poorer postural control and loss of balance than other regions of interest (Boisgontier et al. 2017). These findings are consistent with physiological studies indicating a major contribution of the brain stem structures to posture and movement, affecting both proactive and reactive balance control (Nonnekes et al. 2013a; Tresch et al. 2014). In particular, the pontomedullary reticular formation of the brain stem is fundamentally involved in the control of posture through reticulospinal pathways (Davidson et al. 2007; Drew et al. 2004; Schepens et al. 2008; Yeomans et al. 2002). Although imaging studies have identified age-related structural alterations in the brain stem (Lambert et al. 2013; Sabel and Stein 1981), neurophysiological investigations of brain stem processes for posture, balance, and movement control are limited due to methodological considerations.
In addition to postural reactions, sudden external disturbances to balance may evoke brain stem-mediated startle responses representing reflexive reactions to generally intense tactile, vestibular, or acoustic stimuli that protect the body against injury by drawing in the limbs and stiffening the body (Allum et al. 2011; Yeomans et al. 2002). Normally, excessive postural movements on the first balance perturbation trial that habituate rapidly with repetition suggest that a startle is incorporated into the balance-stabilizing first-trial response (FTR) (Allum et al. 2011; Oude Nijhuis et al. 2010). Improved postural stability with habituation during subsequent trials is likely due to attenuated muscle activity, decreased kinematic excursions, and reduced joint stiffening that lead to improved postural control (Siegmund et al. 2008). A number of studies have shown evidence of FTRs while isolating the vertical component of falls using free-fall paradigms via sudden release from an above-ground suspension to investigate the neuromuscular responses triggered by a fall (Greenwood and Hopkins 1976; Jones and Watt 1971; Sanders et al. 2015). Free-fall paradigms simulate the vertical component of falls by having the participant experience a sudden downward motion of the body due solely to the effects of gravity. On the initial exposure, the unexpected nature of these events, as well as the downward motion of the body, triggers rapid and exaggerated whole body postural responses similar to FTRs. A connection between altered startle and postural reactions with older age has been posited for recumbent free-fall perturbations of the head-torso, showing muscle onset timing delays that resembled a similar slowing found for auditory startle responses along with an increase in response frequency and amplitude (Bisdorff et al. 1995). Other reports identified reduced acoustic startle magnitude and delayed habituation and other changes with older age (Kofler et al. 2001; Ludewig et al. 2003). However, although both posture and startle responses show abnormalities with aging, their interactive effects on standing balance recovery are virtually unknown (Granacher et al. 2011; Tresch et al. 2014a). Furthermore, determining how age-related changes in FTRs and response habituation impact postural control could elucidate whether these phenomena serve as protective or disruptive mechanisms in stabilizing balance. For example, concomitant postural muscle activity and startle-evoked responses could provide a protective function by rapidly positioning the body in a defensive posture (Brown et al. 1991b; Nonnekes et al. 2013b). In contrast, excessive neuromuscular activity or coactivation of multiple muscles could alter joint stiffness, leading to less compliant or more restrictive balance recovery actions and an increased risk of injuries (Hortobágyi and DeVita 1999, 2000; Larsen et al. 2008; Macaluso et al. 2002).
Accordingly, this study investigated aging changes in protective balance and startle responses to sudden drop perturbations of the standing support surface and their effect on landing impact forces (vertical ground reaction forces, vGRF) and balance stability. We hypothesized that older adults would show delayed FTR onset timing and that habituation would be less and take longer with older age. We also expect that, compared with younger adults, older adults would demonstrate greater destabilizing ground impact recovery force/time profiles and less stable balance recovery associated with differences in prelanding startle responses.
METHODS
Participants.
Twelve healthy, community-dwelling older adults (6 men; mean age = 72.5 ± 2.32 yr, mean ± SE; range = 65–85 yr) and 12 younger adults (7 men; mean age = 28.09 ± 1.03 yr; range = 21–35 yr) volunteered to participate in the study. Younger adults were in good health with no self-reported neurological, musculoskeletal, or other medical conditions that limited their physical function. Older adult participants were screened over the telephone by the recruitment staff. Those who passed the phone screening were then evaluated by a physician geriatrician to assess their appropriateness for study participation. Exclusion criteria consisted of the following: 1) cognitive impairment [Folstein Mini Mental State Examination (MMSE) score < 22]; 2) sedative use; 3) nonambulatory; 4) presence of clinically significant osteoporosis; 5) history of prior vertebral, pelvic, or lower extremity fracture; 6) any other clinically significant functional impairment related to musculoskeletal, neurological, cardiopulmonary, or metabolic systems or other general medical problems; 7) self-reported pregnancy or possibility of pregnancy; 8) self-reported involvement in moderate to vigorous physical activity; and 9) Centers for Epidemiological Studies Depression Survey score >16. Younger adults were recruited from the local community and were excluded from the study if they met any of the following criteria: 1) any clinically significant functional impairment related to musculoskeletal, neurological, cardiopulmonary, or metabolic systems or other general medical problems, or 2) self-reported pregnancy or possibility of pregnancy.
All participants were naive of the experimental protocol before testing. Participants provided written informed consent that was approved by the research ethics committee from the Institutional Review Board of University of Maryland, Baltimore and the Baltimore Veteran’s Administration Research and Development Service before participation.
Experimental setup.
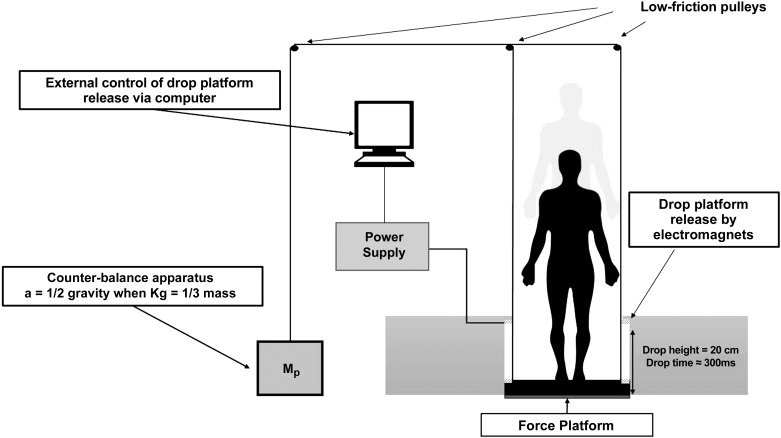
The experiment consisted of a block of 12 consecutive trials of externally triggered drop perturbations of the standing support surface spaced ~2 min apart. Participants stood atop a level, moveable support surface platform (45.6 cm wide, 50.5 cm long) secured to a fixed rigid frame using two sets of five electromagnets (12-V direct current; Magnetech) located on each side of the platform (Fig. 1). The setup enabled a 20-cm drop of the support surface. The duration of the fall was ~300 ms. For safety, all participants wore a harness that allowed unrestricted movement in response to the drop perturbation but would otherwise prevent a participant from falling. To reduce ground impact forces and the resultant risk for injury with repeated perturbations, a counterbalancing mass system (Fig. 1) was used (Greenwood and Hopkins 1976). The counterbalancing mass was attached to the support surface platform via a pulley-cable arrangement. Drop platform acceleration was standardized by changing the weight of the counter mass (M) such that acceleration = (Mp − M)g0 ÷ (Mp + M), where Mp is the participant’s mass (kg) and g0 is the acceleration due to gravity. Through further computations, a standardized acceleration of 4.91 m/s2 was chosen for each participant to reduce the peak impact force to a comfortable level of 1.5 times Mp while retaining the initial startle reaction and equaling the impact force for stair descent (Stacoff et al. 2005).
Fig. 1.
Representative experimental setup of drop perturbation. a, acceleration; Mp, participant’s mass.
Electromyography.
Electromyographic (EMG) activity was recorded bilaterally from the sternocleidomastoid (SCM), middle deltoid (DLT), biceps brachii (BIC), vastus lateralis (VL), biceps femoris (BF), medial gastrocnemius (MG), and tibialis anterior (TA) using a Noraxon TeleMyo wireless EMG system (Noraxon, Scottsdale, AZ) with a sampling frequency of 1,500 Hz. Signals were bandpass filtered (16–500 Hz), full-wave rectified, and low-pass filtered at 50 Hz using a digital 4th-order Butterworth filter. EMG data were analyzed with custom-written MATLAB interactive graphical programs (The MathWorks, Natick, MA). EMG onset latency and first-burst amplitudes for each muscle were found by calculating the mean baseline signals over a 100-ms interval before the platform drop. EMG onset latency was defined as the time when the rectified EMG value exceeded 3 SD from the mean baseline level for 30 ms and was expressed as the elapsed time from the onset of the drop. Peak EMG amplitude was calculated as the first maximum EMG value recorded from EMG onset occurring within 120 ms of drop perturbation onset to identify potential startle response contributions to the postural responses (Carlsen et al. 2007). To determine the effects of lower extremity muscle prelanding activity on vGRF during FTRs, knee and ankle co-contraction indexes (CoIs) were calculated over a period of 100 ms preceding ground contact. Muscle CoI was defined as the ratio of twice the integrated EMG activity of the less active muscle, divided by the sum of the integrated EMG activity of the two contracting muscles (Falconer and Winter 1985).
Kinematics.
Kinematic data were recorded using a 10-camera Vicon system (Vicon-USA, Denver, CO). Reflective markers were placed on the head and bilaterally on the acromion process (shoulder), lateral epicondyle of the humerus (elbow), distal end of the radius (wrist), anterior superior iliac spine (ASIS), posterior superior iliac spine (PSIS), greater trochanter (hip), lateral epicondyle of the femur (knee), lateral malleolus (ankle), first metatarsal (toe), and heel. An additional marker was placed on the drop platform to record the onset of platform movement. The onset of the platform’s movement was defined as the point in time when the vertical component of the marker trajectory reached −2 SD of the baseline reading. Marker coordinates were sampled at 120 Hz and then low-pass filtered offline at 5 Hz with a 4th-order Butterworth filter. From these data, a five-segment, two-dimensional kinematic model was developed to calculate body movements induced by the drop perturbation. The trunk segment was defined between the greater trochanter and acromion markers, the thigh was defined between the lateral femoral epicondyle and greater trochanter markers, the shank segment was defined between the lateral malleolus and lateral femoral epicondyle markers, the foot segment was defined between first metatarsal and lateral malleolus markers, and the upper arm segment was defined between the lateral humeral epicondyle and the acromion markers. Absolute two-dimensional angular displacements of the trunk, hip, knee, ankle, and upper arm were subsequently calculated offline using custom-written MATLAB programs in the sagittal (trunk, hip, knee, and ankle) and frontal (upper arm) planes.
Kinetics.
Ground reaction forces (GRFs) were collected at 600 Hz by two force platforms located underneath the drop platform (Advanced Mechanical Technology, Watertown, MA). To account for the weight of the drop platform, forces on the plates were zeroed after the platform was placed on them. The force data were time-synchronized to the kinematic data and were low-pass filtered at 10 Hz with a 4th-order Butterworth filter and used to determine the instant of drop landing and peak impact force.
Behavioral measures of anxiety.
A concomitant psychological factor associated with falls is the fear of falling, which is common among older adults (Chandler et al. 1996; Tinetti et al. 1988). Because anxiety about falling may influence startle characteristics (Carpenter et al. 2004; Painter et al. 2012), we assessed each participant’s anxiety using the self-assessment manikin (SAM), which is a nonverbal pictorial assessment that directly measures the pleasure, arousal, and dominance associated with the participant’s affective reaction to the drop perturbations (Bradley and Lang 1994). The SAM was administered at three time instants: first, immediately after participants provided informed consent and were given knowledge of the experiment before standing on the perturbation device (Base); second, while on top of the platform before perturbation initiation (Pre); and third, immediately after the last trial (Post).
Measurement of balance stability.
Stability in the anteroposterior direction was quantified using the margin of stability (MoS) (Hof et al. 2005). MoS and parameters used to compute MoS were assessed at two events, defined as follows: 1) ground contact (GC), when the GRFs exceeded 5 N after platform release, and 2) at the maximal downward displacement of the center of mass during GC (COMlow). COMlow was chosen as a surrogate marker for the completion of the landing movement strategy (Bates et al. 2013). MoS was calculated as follows: MoS = BoS − XCM, where the boundaries of the base of support (BoS) were defined by foot length. The heel marker position was the demarcation for the posterior boundary, whereas the anterior boundary was defined as the marker position at the distal end of the second metatarsal. The extrapolated center of mass (XCM) was obtained using the following equation: XCM = PCM + VCMx , where PCM is the anterior-posterior distance between the initial position of the second metatarsal marker and the vertical projection of the center of mass, VCMx is the anteroposterior velocity of the center of mass, g is the acceleration of gravity, and l is the distance between the center of mass and the ankle joint center in the sagittal plane. Postural stability is maintained in circumstances where the position of XCM is within the BoS (positive values of margin of stability), whereas stability decreases as the margin of stability decreases or becomes negative.
Statistical analyses.
All descriptive statistics are reported as means ± SE. Results were analyzed using SPSS 22.0 software. The effects of repeated drop perturbations on response parameters (EMG onsets and peak amplitude, joint kinematics, and impact force) were compared between the FTR and the average of the last two trial responses (LTR) using a two-way ANOVA [age (young vs. old) × trial (FTR and LTR)]. To examine habituation to repeated perturbations, preplanned t-tests (trial 1 vs. trials 2–11) were conducted to compare EMG, kinematic, and kinetic responses observed between FTRs and LTRs with P values corrected for multiple comparisons by the false discovery rate (FDR) correction (Benjamini and Hochberg 1995). Stepwise multiple linear regressions were used to determine predictors of MoS at the lowest vertical projection of the center of mass. Pearson product-moment correlation coefficients were reported for each dependent measure included in the regression model. The nonparametric Kruskal-Wallis H-test was used to assess any differences between the participants’ SAM scores.
RESULTS
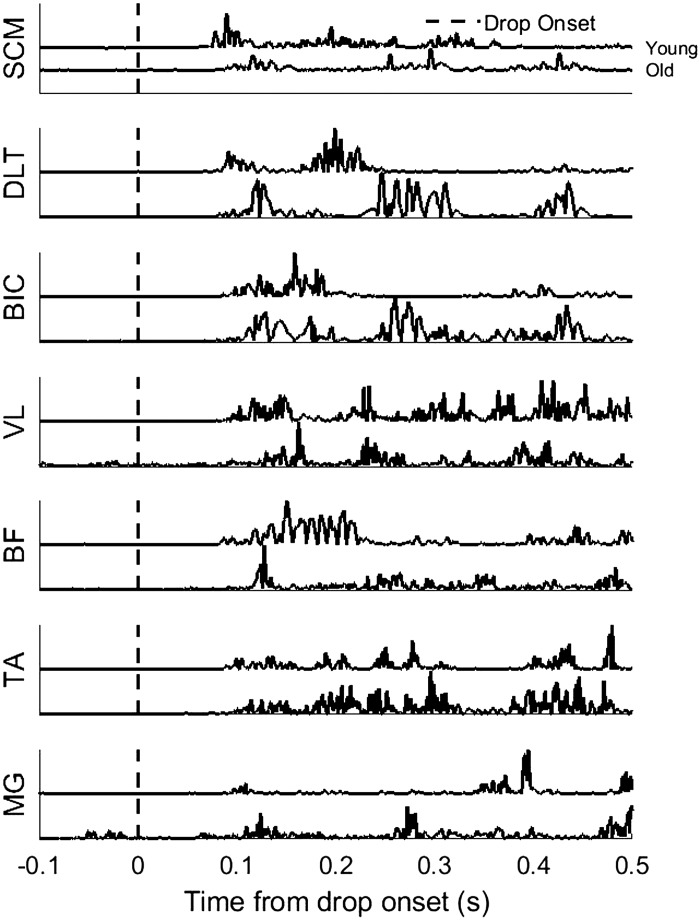
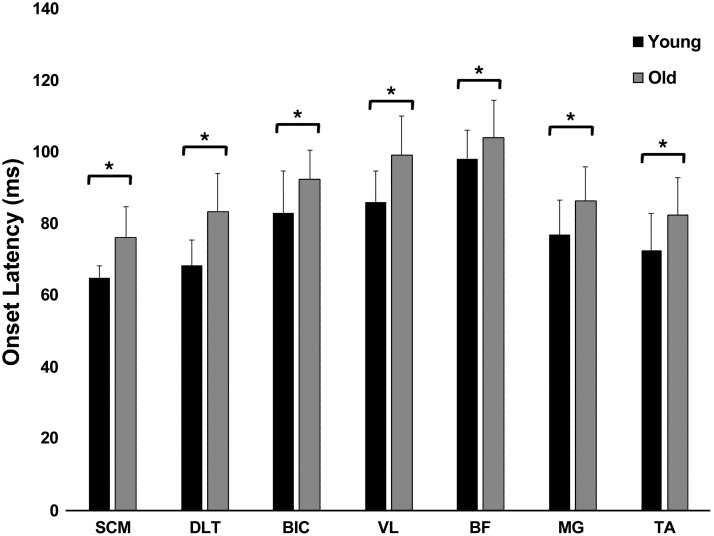
Representative muscle activation patterns during FTR for a young and older adult are shown in Fig. 2. Rapid, phasic, bilateral, and synchronous SCM activity within 120 ms after stimulus onset is a hallmark of a startle response (Carlsen et al. 2007). Incidence of early onset of bilateral SCM activation within 120 ms after drop onset was present during all FTRs for both groups. Muscle onset latencies were significantly influenced by age [F(1, 22) = 9.76, P < 0.001] during FTRs. Post hoc analysis confirmed that older adults had significantly longer EMG onset latencies across all muscle groups (Fig. 3) compared with the younger adults. Within-subject comparisons or interaction effects were not significantly different between FTRs and LTRs (P > 0.05).
Fig. 2.
Representative muscle activation patterns between younger and older adults for sternocleidomastoid (SCM), middle deltoid (DLT), biceps brachii (BIC), vastus lateralis (VL), biceps femoris (BF), medial gastrocnemius (MG), and tibialis anterior (TA) muscles. Vertical lines indicate the onset of platform release.
Fig. 3.
Mean (SE) first-trial response (FTR) onset latencies for sternocleidomastoid (SCM), middle deltoid (DLT), biceps brachii (BIC), vastus lateralis (VL), biceps femoris (BF), medial gastrocnemius (MG), and tibialis anterior (TA) muscles. *P < 0.05, young vs. older adults.
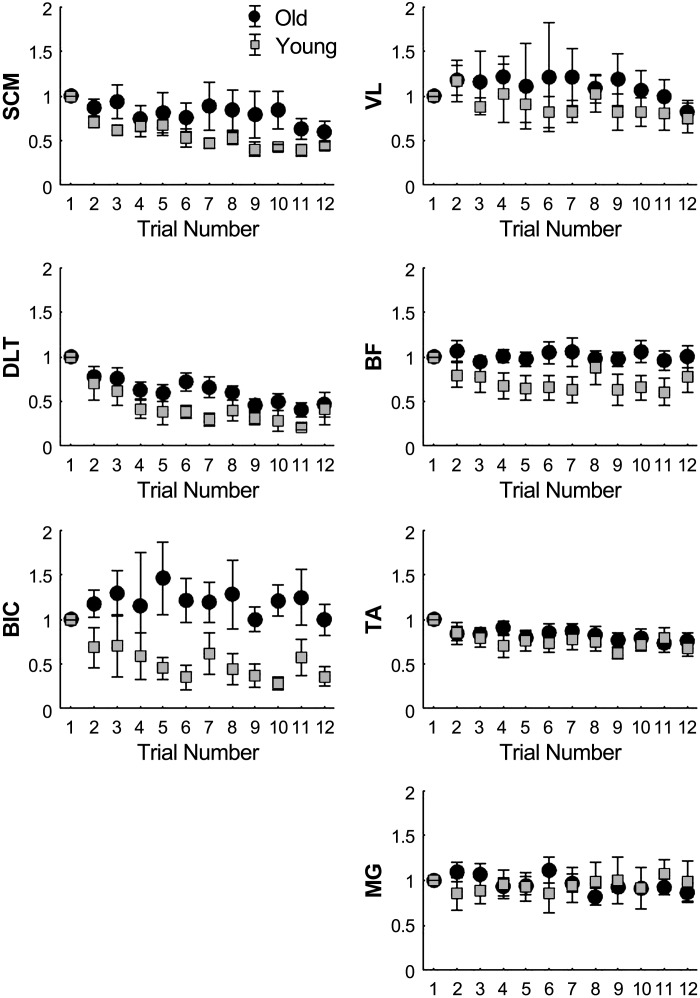
To determine whether there was a sequential effect of trials on the amplitude of EMG responses due to habituation, the FTR for each muscle was compared with that of each subsequent trial using paired t-tests (Fig. 4) with an adjusted significance level set at P < 0.004. In younger adults, SCM, DLT, and BIC EMG amplitudes were significantly reduced by trials 2, 4, and 5, respectively, whereas older adults only showed a significant reduction in SCM and DLT amplitudes after trials 11 and 4, respectively. EMG amplitudes for lower extremity muscles did not significantly decrease from trial 1 for either group (P > 0.004).
Fig. 4.
Mean (±SE) amplitude ratios relative to first-trial response (FTR) for sternocleidomastoid (SCM), middle deltoid (DLT), biceps brachii (BIC), vastus lateralis (VL), biceps femoris (BF), medial gastrocnemius (MG), and tibialis anterior (TA) muscles for younger and older adults.
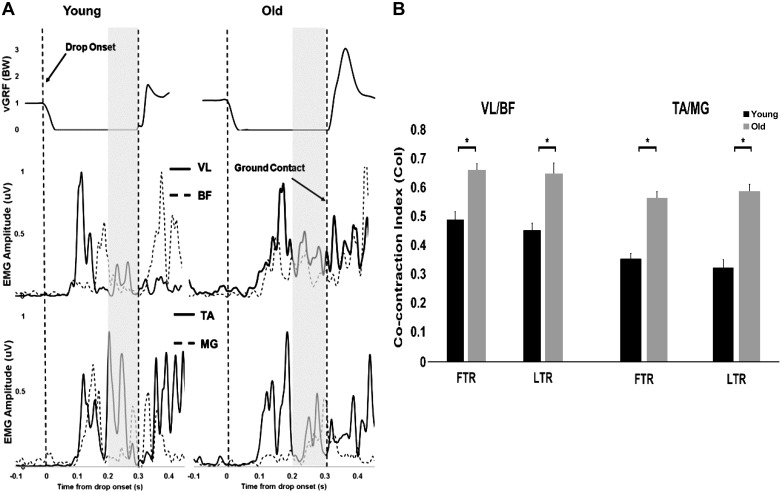
Representative trials showing prelanding muscle co-contraction patterns and accompanying vGRFs for younger and older adults are presented in Fig. 5A, with group means and SE for knee and ankle CoIs indicated in Fig. 5B. There was a significant age effect for both VL/BF CoI [F(1, 44) = 28.78, P < 0.001] and TA/MG CoI [F(1, 44) = 59.68, P < 0.001]. Post hoc analysis showed older adults landed with greater VL/BF CoI during FTR (0.66 ± 0.02 vs. 0.49 ± 0.02, P < 0.05) and LTR (0.64 ± 0.03 vs. 0.45 ± 0.04, P < 0.05). Similarly, older adults landed with greater TA/MG CoI during FTR (0.56 ±0.02 vs. 0.36 ± 0.01, P < 0.05) and LTR (0.58 ± 0.02 vs. 0.33 ± 0.03, P < 0.05). No significant differences were found between FTR and LTR CoIs (P > 0.05) for both groups.
Fig. 5.
A: representative time histories of vertical ground reaction force (vGRF; top), electromyographic (EMG) recordings of vastus lateralis (VL) and biceps femoris (BF; middle), and rectified linear envelope of the EMG of tibialis anterior (TA) and medial gastrocnemius (MG; bottom) from a younger (left) and older adult (right), during drop perturbations, with the vertical lines indicating drop onset and ground contact, respectively, and the shaded regions indicating the period of 100 ms preceding ground contact. B: mean (±SD) co-contraction indexes (CoI) for ratios VL/BF and TA/MG for first-trial (FTR) and last-trial responses (LTR). *P < 0.05, young vs. older adults.
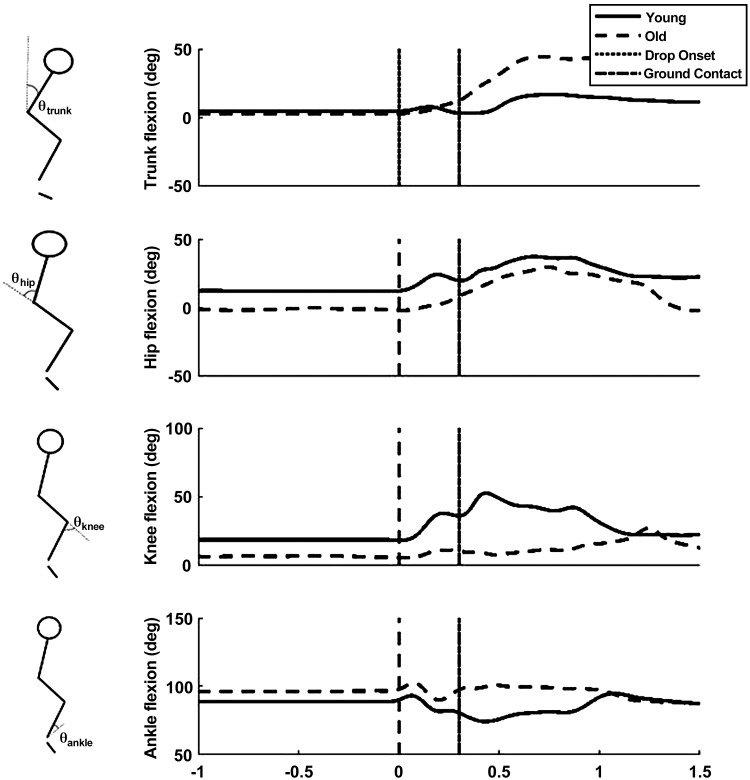
Mean trunk and lower limb joint angular position profiles during FTRs are shown in Fig. 6. Additional differences in kinematic outcomes were present between older and younger adults during the landing phase of balance recovery (i.e., from ground contact to COMlow) [F(1, 22) = 8.47, P < 0.001; Table 1]. A significant interaction of trial and age was found for trunk flexion angle [F(1, 22) = 26.05, P < 0.001] and knee flexion angle [F(1, 22) = 17.25, P < 0.001] at COMlow. Post hoc analysis confirmed that older adults had greater trunk flexion (P < 0.05) and less knee flexion (P < 0.05) during FTRs. Within-subject differences between FTR and LTR were present in the trunk (17.58 ± 1.42 vs. 7.11 ± 1.74, P < .05) and knee (23.77 ± 3.21 vs. 37.11 ± 2.96, P < 0.05) joint angles for older adults only. Peak shoulder abduction between the FTR and LTR was reduced in both older (16.57 ± 4.62 vs. 9.52 ± 2.32, P < 0.05) and younger adults (9.86 ± 3.61 vs. 6.47 ± 1.57, P < 0.05). The group data for initial peak shoulder abduction indicated that older adults had significantly larger peak shoulder abduction during FTRs than the young (P < 0.05; Table 1).
Fig. 6.
Representative kinematic profiles in order from top to bottom: trunk, knee, hip, and ankle joint angles (θ) for young and older adults.
Table 1.
Mean measures of kinematic and kinetic variables during externally triggered first- and last-trial responses
| Old |
Young |
|||
|---|---|---|---|---|
| FTR | LTR | FTR | LTR | |
| Kinematic Data | ||||
| Joint flexion angles, deg | ||||
| Shoulder (abduction) | 16.57 ± 4.62*† | 9.52 ± 2.32 | 9.86 ± 3.61† | 6.47 ± 1.57 |
| Trunk | 17.58 ± 1.24*† | 7.11 ± 1.73 | 9.48 ± 1.04 | 9.64 ± 1.14 |
| Hip | 26.86 ± 2.60 | 24.45 ± 2.97 | 30.99 ± 3.29 | 32.45 ± 3.55 |
| Knee | 23.77 ± 3.21*† | 37.11 ± 2.06 | 42.24 ± 3.08 | 47.04 ± 4.90 |
| Ankle | 86.63 ± 4.44 | 82.53 ± 1.61 | 81.67 ± 0.93 | 79.16 ± 2.05 |
| Kinetic Data | ||||
| Peak impact force, %BW | 2.65 ± 0.13* | 2.45 ± 0.19* | 1.98 ± 0.06 | 1.81 ± 0.17 |
Values are means ± SE of measures for first-trial (FTR) and last-trial responses (LTR) of older and young adults. BW, body weight.
P < 0.05, significance for corresponding measure between groups.
P < 0.05, significance for corresponding measure within group.
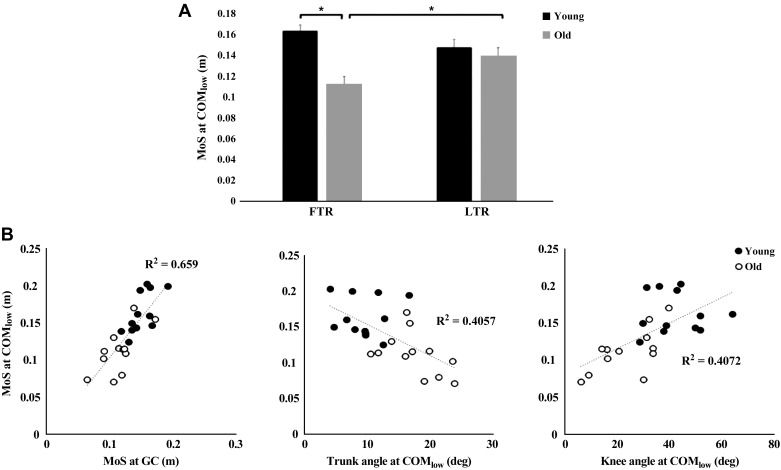
Stability in the anteroposterior direction during balance recovery was quantified using the MoS. Results indicated a significant interaction effect between trial (FTR vs. LTR) and age (old vs. young) on MoS at COMlow [F(1, 22) = 8.66, P = 0.008; Fig. 7A]. Post hoc analysis confirmed that older adults had a smaller MoS at COMlow during FTR compared with LTR (P < 0.05). The MoS was reduced at COMlow, indicating that instability was greater for older vs. young adults (P < 0.05) during FTRs. No significant difference in MoS at COMlow during LTR between young and old adults was observed. MoS at COMlow was significantly associated with MoS at GC (r = 0.812, P < 0.05), trunk flexion at COMlow (r = −0.641, P = 0.001), and knee flexion at COMlow (r = 0.639, P < 0.05; Fig. 7B). Multiple linear regression analysis indicated that MoS at GC, trunk flexion at COMlow, and knee flexion at COMlow collectively accounted for 76% of the variance in MoS at COMlow during FTRs (SE = 0.03, F(3,20) = 20.50, P < 0.001).
Fig. 7.
A: group means (±SE) of margin of stability (MoS) for first-trial (FTR) and last-trial responses (LTR) at the lowest vertical center of mass position (COMlow) for young and older adults. B: scatterplots showing associations between MoS at ground contact (GC) and MoS at COMlow) (left), trunk flexion angle at COMlow (middle), and knee flexion angle at COMlow (right) for young and older adults. *P < 0.05, young vs. older adults.
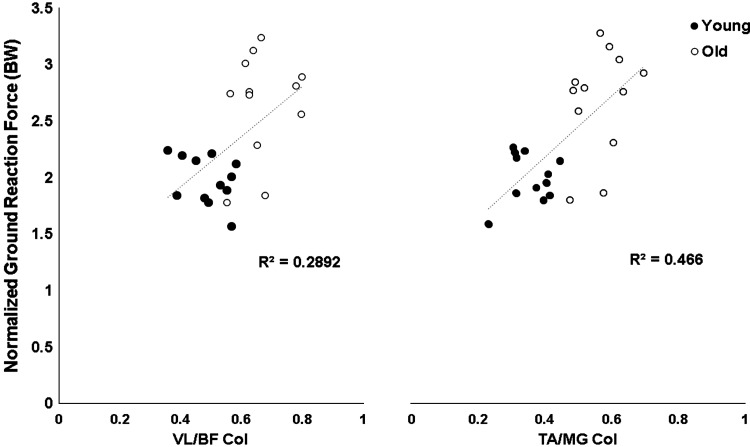
Kinetic data demonstrated a significant main effect of age on peak vGRF [F(1, 22) = 20.68, P < 0.001], with post hoc analysis confirming that older adults landed with greater peak vGRFs for FTRs (2.65 ± 0.13 vs. 1.98 ± 0.06, P < 0.05) and LTRs (2.45 ± 0.19 vs. 1.81 ± 0.07, P < 0.05; Table 1). No within-subject differences were found in both groups between FTRs and LTRs for kinetic data (P > 0.05). A significant positive correlation was present between VL/BF CoI and peak impact force (r = 0.54, P < 0.002) and TA/MG CoI and peak impact force (r = 0.68, P = . 002) during FTRs (Fig. 8).
Fig. 8.
Scatterplots of associations between normalized vertical ground reaction forces (BW, body weight) and co-contraction index (CoI) for ratios of vastus lateralis and biceps femoris (VL/BF; left) and tibialis anterior and medial gastrocnemius (TA/MG; right) for young and older adults.
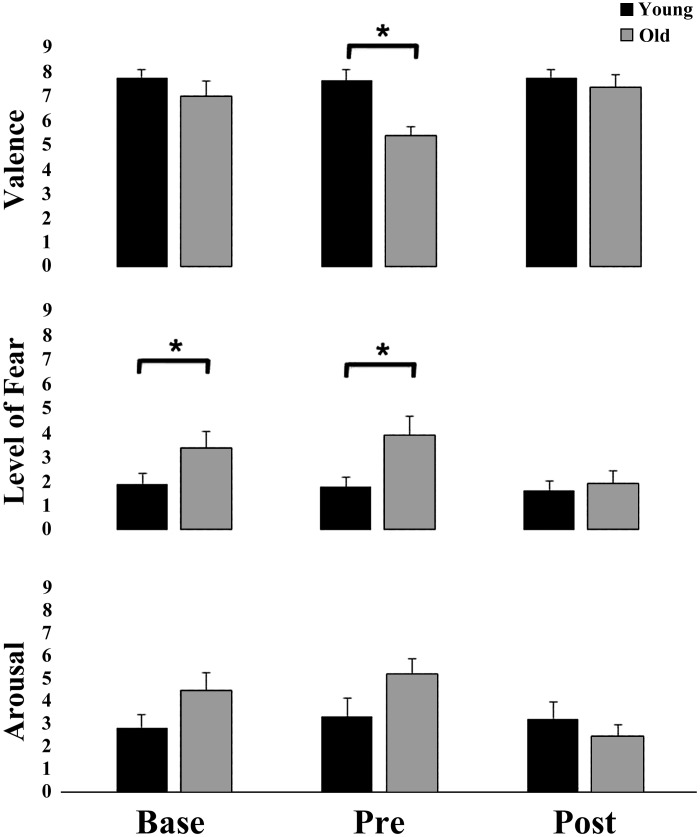
To determine the extent to which a participant’s emotional response was associated with postural/startle events, affective reactions to the drop perturbation at various time points were measured by the SAM method. A Kruskal-Wallis test showed that there was a difference in Base SAM fear score [χ2(1) =3.89, P < 0.05] and Pre-SAM valence and in fear scores between older and younger adults [χ2(1) = 7.741, P = 0.005 and χ2(2) = 3.926, P = 0.048, respectively]. Mann-Whitney U-tests revealed an age-related increase of fear during Base (U = 14.0, P < 0.05) and Pre (U = 14.0, P = 0.48) SAM assessment, with similar trends for anxiety, and a decreased valence [U = 6.50, P < 0.01] during Pre-SAM assessment (Fig. 9).
Fig. 9.
Mean (±SE) self-assessment manikin (SAM) scores for valence (top), fear (middle), and arousal (bottom) for young and older adults. *P < 0.05, young vs. older adults.
DISCUSSION
The main finding of this study was that rapid and exaggerated FTRs, and subsequent attenuated responses resembling startle-like reactions to externally imposed postural perturbations, were observed in older adults and confirmed in younger adults as previously reported (Sanders et al. 2015). These findings are consistent with other studies of postural perturbations during standing (Allum et al. 2011; Oude Nijhuis et al. 2010), walking (McIlroy and Maki 1995; Oude Nijhuis et al. 2009), and sitting (Blouin et al. 2006; Brown et al. 1991a). To our knowledge, the findings are the first to identify age-associated abnormalities of delayed, exaggerated, and poorly habituated startle/postural FTRs linked with less effective postural movements and balance stabilization through excessive muscle responses and joint stiffening with increased fall impact forces in response to sudden loss of ground support surface from a standing position. Given the generally rapid onset latencies and increased FTR magnitudes in neck and upper limb muscles, and habituation behavior during the drop perturbations, it is plausible that the age-related differences in reactive responses were, at least in part, caused by startle.
There was a high incidence of rapid (<120 ms), bilateral, and synchronous SCM neck muscle activation, which is considered a hallmark motor characteristic for identifying the presence of a startle response (Brown et al. 1991a). This response was present in all participants and supports the probability that the observed reactive responses, at least in part, included a startle component. Alternatively, it is possible that the SCM activation represented a postural response to stabilize the head/neck in preparation for ground impact. However, a prior study of younger adults using free-fall postural perturbations found consistent neck responses in all subjects and trials during externally triggered perturbations, and a marked absence of such reactions when identical perturbations were self-triggered and where startle would be less likely to occur (Sanders et al. 2015).
Another distinguishing feature of the classical startle response is its tendency to habituate with repeated stimulus exposure, as indicated by a reduced incidence and/or magnitude of response (Allum et al. 2011; Oude Nijhuis et al. 2010; Sanders et al. 2015; Siegmund et al. 2003). This was supported by our findings of reduced EMG response amplitudes between FTR and LTR for SCM, DLT, and BIC in younger and older adults. However, older adults demonstrated a slower rate of habituation, as indicated by the greater number of trials needed to show a significant decrease from FTRs. Although this finding has not been documented previously among generally healthy older adults, reduced startle habituation has been shown in people of similar age with Parkinson’s disease (Nanhoe-Mahabier et al. 2012; Nieuwenhuijzen et al. 2006; Visser et al. 2010), suggesting the influence of age may have contributed to or exaggerated the effects already present due to Parkinson’s disease. Surprisingly, the response amplitudes for the lower extremity muscles showed little to no change between FTRs and LTRs among the older adults. This finding may reflect a need to consistently maximize joint stability on landing impact in the presence of age-associated limitations in balance ability and vulnerability to falling. Previous studies utilizing drop perturbations have suggested that the co-contraction of agonist and antagonist leg muscles before ground contact is likely to modulate the time course of joint stiffness and influence the magnitude of vGRFs, and can be altered through motor prediction (Fu and Hui-Chan 2002). During an unexpected above-ground drop perturbation, younger adults have shown increased TA/MG co-contraction but decreased co-contraction during self-initiated drop perturbations, which was associated with a reduction in peak amplitude and rate of vGRFs (Fu and Hui-Chan 2002). In the present study, older adults had increased knee and ankle co-contraction at the time of landing compared with younger adults, suggesting increased joint stiffness in the lower extremities with greater limb rigidity. Given the large external loads applied to the lower limbs at landing over a short period, older adults may not have been able to reactively respond quickly to counter these potentially damaging forces. Thus older adults may use muscle co-contraction to increase their lower limb stiffness before landing to increase dynamic stability of the lower extremities while minimizing sagittal and downward body motion to guard against collapse. However, in doing so, older adults are likely increasing the risk of injury during landing, as suggested by the increased vGRF compared with that of younger adults, who are less stiff upon landing. Previous studies have shown that modulation of lower extremity stiffness during landing can affect the magnitude of the peak vGRF (Butler et al. 2003; Devita and Skelly 1992; Zhang et al. 2000). Although stiffness was not directly measured in the present study, similar conditions appear to apply between this and previous investigations. Specifically, smaller knee flexion angles and increased vGRFs accompanying “hard landings” compared with larger knee flexion angles and decreased vGRFs for “soft landings” were found in common. Therefore, interventions aimed at modulating the landing mechanics of older adults to approach the strategy used by younger adults should be examined further as a potential means of decreasing risk of injury.
Alternatively, rather than strategic, the increased CoI and subsequent stiffness found among older adults may be startle-induced. FTRs are characterized by exaggerated postural reactions, including co-contracting muscles in multiple body segments that are thought to reflect startle-like behavior (Allum et al. 2011; Nonnekes et al. 2015). In this regard, previous studies involving drop perturbations during gait have shown that the unexpected nature of the perturbations may have elicited startle responses characterized by co-contraction of antagonist muscles as a protective attempt to “brake” the downward motion of the body (Hof and Duysens 2018; Nakazawa et al. 2004; Nieuwenhuijzen et al. 2000). Another study, though lacking SCM data, observed muscle synergy patterns characteristic of startle responses during a novel slip-induced fall, which may have disrupted balance by limiting the efficacy of the motor response through increased joint stiffness (Sawers et al. 2017). In our study, older adults did not reduce lower extremity CoI from FTR to LTR, which suggested that the effects of startle may have been prolonged in older adults. This is further supported by the delayed habituation of SCM, which is a surrogate marker for startle. This finding may serve as an important target for fall prevention and injury reduction interventions aimed at mitigating the influence of excessive startle responses on protective balance recovery mechanisms.
In both groups, there were robust shoulder abduction FTRs, as observed previously in younger adults during free-fall perturbations that habituated with repeated exposures (Sanders et al. 2015). However, shoulder abduction was greater in magnitude in older adults for both FTRs and LTRs, indicating that they were more susceptible to exaggerated upper-limb FTRs compared with young adults and that the FTRs took longer to habituate. These results were further corroborated by the arm muscle activation patterns. The observed arm responses broadly resembled the parachute reaction seen mainly in developing infants. This is thought to represent a normal protective reflex that is elicited when an infant is held in ventral suspension and tilted abruptly forward toward the floor (Zafeiriou 2004).
The increased trunk flexion during FTRs was associated with greater instability of balance among the older adults and is consistent with other studies (Oude Nijhuis et al. 2009, 2010). The greater trunk flexion at ground contact moves the COM anteriorly relative to the BoS, causing a decreased MoS. The age-related increase in trunk flexion may reflect decreased control of the trunk musculature (Hwang et al. 2008) or decreased muscle strength, or it could indicate an attempt to reduce the internal knee extensor moment during landing (Patel et al. 1997). Similarly, the reduced knee flexion at landing could reflect an age-related reduction in knee extensor control or eccentric contractile strength. Our findings indicated that trunk flexion angle, knee flexion posture, and MoS at GC are significant predictors of the MoS at COMlow. Although not statistically significant, the trend for older adults to increase their knee flexion while reducing trunk flexion during the externally triggered LTR was associated with an increased MoS that was not significantly different from that of younger participants at LTR.
Because startle reactions are known to be influenced by one’s emotional state of anxiety, arousal, or fear (Bradley et al. 1993; Carpenter et al. 2004; Lang et al. 2018), we recorded self-reported measures of emotion at different time points before and during testing. The results showed that older adults reported higher levels of valence and fear before the drop onset. The elevation in emotional state may have contributed to intensifying the level of fear-potentiated startle in anticipation of the actual drop perturbation. Moreover, the behavioral markers improved with repeated exposure to the balance perturbation in conjunction with reductions in physiological indices of startle. These findings have implications for the potential mediating role of fear of falling in contributing to aging changes in startle reactions accompanying perturbations of balance that may contribute to falls and injuries due to falls.
The observed startle-like responses were likely triggered by activation of reticulospinal motor tracts in the pontomedullary reticular formation that are known to mediate startle responses and include networks for posture and balance control (Nonnekes et al. 2015; Schepens et al. 2008; Yeomans et al. 2002). Furthermore, neurons of the pontomedullary reticular formation are not modality specific and therefore respond to different types of afferent information (Wu et al. 1988). As such, both postural and startle reactions can be triggered by somatosensory, vestibular, and visual sensory stimuli. Studies examining the incidence of startle responses during gait and various postures have shown that startle responses are modulated by afferent input. For example, startle responses are more prominent and have faster onset latencies in lower extremity muscles during standing compared with sitting (Delwaide and Schepens 1995). In addition, the occurrence of startle responses can be influenced by weight-bearing symmetry. During an asymmetrical stance with 60% of weight-bearing on the left leg, the incidence of startle responses in the TA muscle occurred more frequently in the more loaded leg compared with the less loaded leg (Nonnekes et al. 2013b). Taken together, these findings suggest that afferent loading information plays an important role in the observed incidence of startle responses. On the basis of such findings, we hypothesize that unexpected drop perturbations from a standing position mainly engage somatosensory and vestibular afferent input through the sudden unloading and loss of pressure contact on the legs and feet and/or by the abrupt motion of the head.
Among the limitations of the study, SCM activity was assumed to be a surrogate marker for startle, but the observed postural responses evoked by the drop perturbation could reflect a postural response, as well. However, there is considerable support to suggest the startle reflex was incorporated into the responses seen. The onset latency of the SCM response was under 120 ms for both young and older adults and was unchanged and consistently activated over repeated trials for each condition along with reduced response magnitude over repeated trials characteristic of behavioral habituation. These findings are consistent with other past studies of postural and startle reactions (Allum et al. 2011; Sanders et al. 2015). An activation threshold of within 120 ms of the perturbation onset was considered as the maximal time window in which a startle response may be present along with the corresponding balance stabilizing response (Carlsen et al. 2007; Tresch et al. 2014a). As such, any response onset longer than 120 ms was not considered to be consistent with the presence of a startle response. Although we acknowledge the possibility that the SCM EMG response may be attributed to the initial postural FTR in addition to startle, our findings also suggest a startle response may be present. Nonetheless, additional studies are needed to more definitively address the extent to which the observed responses are attributable to startle, and thus the findings presented in this report should be considered accordingly. Second, the participants in this study were generally healthy and moderately active older adults. Therefore, the results may not generalize to other older adult clinical populations such as individuals with mobility or balance limitations.
The present findings may have clinical implications for determining underlying mechanisms contributing to falls and increased risk of injuries due to falls among older adults by identifying the presence of startle-like responses contributing to instability and increased fall impact force. Furthermore, the observed differences in FTR between older and younger adults suggest that vertical drop perturbations demonstrate the potential for this paradigm to assess postural instability in older and young adults. Compared with younger adults, older adults showed greater co-contraction patterns, peak shoulder abduction, and decreased MoS at COMlow. Further studies are needed to fully examine the utility of drop perturbations as a diagnostic tool to assess those individuals who are more susceptible to startle-like contributions to FTRs and falls. In addition, exaggerated startle-like FTRs were reduced during subsequent trials through behavioral habituation in both young and older adults. Therefore, further work should also be directed at methods for reducing abnormal startle by exploiting the plasticity of startle reactivity through methods such as habituation training, use of motor prediction, and the influence of prestimulus modulation (Greenwood and Hopkins 1976; Kanekar and Aruin 2014; Mang et al. 2012). These findings may also help to inform about other clinical populations at risk for abnormal responses to startle and balance instability, such as individuals with Parkinson’s disease, stroke, and hyperekplexia.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants R21AG049615 and R36AG057984, Claude D Pepper – Older Americans Independence Center Grant P30AG028747, University of Maryland Advanced Neuromotor Rehabilitation Research Training Program, and NIH Post-doctoral Training Grant H133P100014.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
O.P.S. and M.W.R. conceived and designed research; O.P.S., H.Y.H., and R.A.C. performed experiments; O.P.S. and H.Y.H. analyzed data; O.P.S., D.N.S., R.A.C., and M.W.R. interpreted results of experiments; O.P.S. prepared figures; O.P.S. drafted manuscript; O.P.S., D.N.S., R.A.C., and M.W.R. edited and revised manuscript; O.P.S., H.Y.H., D.N.S., R.A.C., and M.W.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We acknowledge the Claude D. Pepper Older Americans Independence Center, University of Maryland School of Medicine, Baltimore, MD, the Geriatric Research Education and Clinical Center, and Baltimore Veterans Affairs Medical Center, as well as the contribution made by Dr. Harshvardhan Singh and the study participants for their time and effort in this experiment.
REFERENCES
- Allum JH, Carpenter MG, Honegger F, Adkin AL, Bloem BR. Age-dependent variations in the directional sensitivity of balance corrections and compensatory arm movements in man. J Physiol 542: 643–663, 2002. doi: 10.1113/jphysiol.2001.015644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allum JH, Tang KS, Carpenter MG, Oude Nijhuis LB, Bloem BR. Review of first trial responses in balance control: influence of vestibular loss and Parkinson’s disease. Hum Mov Sci 30: 279–295, 2011. doi: 10.1016/j.humov.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech 46: 1237–1241, 2013. doi: 10.1016/j.jbiomech.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300, 1995. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- Bisdorff AR, Bronstein AM, Gresty MA, Wolsley CJ, Davies A, Young A. EMG-responses to sudden onset free fall. Acta Otolaryngol Suppl 115: 347–349, 1995. doi: 10.3109/00016489509125267. [DOI] [PubMed] [Google Scholar]
- Blouin JS, Inglis JT, Siegmund GP. Startle responses elicited by whiplash perturbations. J Physiol 573: 857–867, 2006. doi: 10.1113/jphysiol.2006.108274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier MP, Cheval B, Chalavi S, van Ruitenbeek P, Leunissen I, Levin O, Nieuwboer A, Swinnen SP. Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiol Aging 50: 47–59, 2017. doi: 10.1016/j.neurobiolaging.2016.10.024. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatry 25: 49–59, 1994. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, Cuthbert BN. Emotion, novelty, and the startle reflex: habituation in humans. Behav Neurosci 107: 970–980, 1993. doi: 10.1037/0735-7044.107.6.970. [DOI] [PubMed] [Google Scholar]
- Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD. The effect of posture on the normal and pathological auditory startle reflex. J Neurol Neurosurg Psychiatry 54: 892–897, 1991a. doi: 10.1136/jnnp.54.10.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. The hyperekplexias and their relationship to the normal startle reflex. Brain 114: 1903–1928, 1991b. doi: 10.1093/brain/114.4.1903. [DOI] [PubMed] [Google Scholar]
- Butler RJ, Crowell HP 3rd, Davis IM. Lower extremity stiffness: implications for performance and injury. Clin Biomech (Bristol, Avon) 18: 511–517, 2003. doi: 10.1016/S0268-0033(03)00071-8. [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res 176: 199–205, 2007. doi: 10.1007/s00221-006-0610-8. [DOI] [PubMed] [Google Scholar]
- Carpenter MG, Frank JS, Adkin AL, Paton A, Allum JH. Influence of postural anxiety on postural reactions to multi-directional surface rotations. J Neurophysiol 92: 3255–3265, 2004. doi: 10.1152/jn.01139.2003. [DOI] [PubMed] [Google Scholar]
- Chandler JM, Duncan PW, Sanders L, Studenski S. The fear of falling syndrome: Relationship to falls, physical performance, and activities of daily living in frail older persons. Top Geriatr Rehabil 11: 55–63, 1996. doi: 10.1097/00013614-199603000-00007. [DOI] [Google Scholar]
- Davidson AG, Schieber MH, Buford JA. Bilateral spike-triggered average effects in arm and shoulder muscles from the monkey pontomedullary reticular formation. J Neurosci 27: 8053–8058, 2007. doi: 10.1523/JNEUROSCI.0040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwaide PJ, Schepens B. Auditory startle (audio-spinal) reaction in normal man: EMG responses and H reflex changes in antagonistic lower limb muscles. Electroencephalogr Clin Neurophysiol 97: 416–423, 1995. [DOI] [PubMed] [Google Scholar]
- Devita P, Skelly WA. Effect of landing stiffness on joint kinetics and energetics in the lower extremity. Med Sci Sports Exerc 24: 108–115, 1992. doi: 10.1249/00005768-199201000-00018. [DOI] [PubMed] [Google Scholar]
- Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 143: 251–261, 2004. doi: 10.1016/S0079-6123(03)43025-2. [DOI] [PubMed] [Google Scholar]
- Falconer K, Winter DA. Quantitative assessment of co-contraction at the ankle joint in walking. Electromyogr Clin Neurophysiol 25: 135–149, 1985. [PubMed] [Google Scholar]
- Fu SN, Hui-Chan CW. Mental set can modulate response onset in the lower limb muscles to falls in humans. Neurosci Lett 321: 77–80, 2002. doi: 10.1016/S0304-3940(02)00060-5. [DOI] [PubMed] [Google Scholar]
- Granacher U, Muehlbauer T, Zahner L, Gollhofer A, Kressig RW. Comparison of traditional and recent approaches in the promotion of balance and strength in older adults. Sports Med 41: 377–400, 2011. doi: 10.2165/11539920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Greenwood R, Hopkins A. Muscle responses during sudden falls in man. J Physiol 254: 507–518, 1976. doi: 10.1113/jphysiol.1976.sp011242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof AL, Duysens J. Responses of human ankle muscles to mediolateral balance perturbations during walking. Hum Mov Sci 57: 69–82, 2018. doi: 10.1016/j.humov.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Hof AL, Gazendam MG, Sinke WE. The condition for dynamic stability. J Biomech 38: 1–8, 2005. doi: 10.1016/j.jbiomech.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, DeVita P. Altered movement strategy increases lower extremity stiffness during stepping down in the aged. J Gerontol A Biol Sci Med Sci 54: B63–B70, 1999. doi: 10.1093/gerona/54.2.B63. [DOI] [PubMed] [Google Scholar]
- Hortobágyi T, DeVita P. Muscle pre- and coactivity during downward stepping are associated with leg stiffness in aging. J Electromyogr Kinesiol 10: 117–126, 2000. doi: 10.1016/S1050-6411(99)00026-7. [DOI] [PubMed] [Google Scholar]
- Hwang JH, Lee YT, Park DS, Kwon TK. Age affects the latency of the erector spinae response to sudden loading. Clin Biomech (Bristol, Avon) 23: 23–29, 2008. doi: 10.1016/j.clinbiomech.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Jones GM, Watt DG. Muscular control of landing from unexpected falls in man. J Physiol 219: 729–737, 1971. doi: 10.1113/jphysiol.1971.sp009685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekar N, Aruin AS. The effect of aging on anticipatory postural control. Exp Brain Res 232: 1127–1136, 2014. doi: 10.1007/s00221-014-3822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler M, Müller J, Reggiani L, Valls-Solé J. Influence of age on auditory startle responses in humans. Neurosci Lett 307: 65–68, 2001. doi: 10.1016/S0304-3940(01)01908-5. [DOI] [PubMed] [Google Scholar]
- Lambert C, Chowdhury R, Fitzgerald TH, Fleming SM, Lutti A, Hutton C, Draganski B, Frackowiak R, Ashburner J. Characterizing aging in the human brainstem using quantitative multimodal MRI analysis. Front Hum Neurosci 7: 462, 2013. doi: 10.3389/fnhum.2013.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Herring DR, Duncan C, Richter J, Sege CT, Weymar M, Limberg-Thiesen A, Hamm AO, Bradley MM. The startle-evoked potential: negative affect and severity of pathology in anxiety/mood disorders. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 626–634, 2018. doi: 10.1016/j.bpsc.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen AH, Puggaard L, Hämäläinen U, Aagaard P. Comparison of ground reaction forces and antagonist muscle coactivation during stair walking with ageing. J Electromyogr Kinesiol 18: 568–580, 2008. doi: 10.1016/j.jelekin.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Lord SR, Dayhew J. Visual risk factors for falls in older people. J Am Geriatr Soc 49: 508–515, 2001. doi: 10.1046/j.1532-5415.2001.49107.x. [DOI] [PubMed] [Google Scholar]
- Ludewig K, Ludewig S, Seitz A, Obrist M, Geyer MA, Vollenweider FX. The acoustic startle reflex and its modulation: effects of age and gender in humans. Biol Psychol 63: 311–323, 2003. doi: 10.1016/S0301-0511(03)00074-7. [DOI] [PubMed] [Google Scholar]
- Macaluso A, Nimmo MA, Foster JE, Cockburn M, McMillan NC, De Vito G. Contractile muscle volume and agonist-antagonist coactivation account for differences in torque between young and older women. Muscle Nerve 25: 858–863, 2002. doi: 10.1002/mus.10113. [DOI] [PubMed] [Google Scholar]
- Mang DW, Siegmund GP, Inglis JT, Blouin JB. The startle response during whiplash: a protective or harmful response? J Appl Physiol 113: 532–540, 2012. doi: 10.1152/japplphysiol.00100.2012. [DOI] [PubMed] [Google Scholar]
- McIlroy WE, Maki BE. Early activation of arm muscles follows external perturbation of upright stance. Neurosci Lett 184: 177–180, 1995. doi: 10.1016/0304-3940(94)11200-3. [DOI] [PubMed] [Google Scholar]
- Nagai K, Yamada M, Uemura K, Yamada Y, Ichihashi N, Tsuboyama T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch Gerontol Geriatr 53: 338–343, 2011. doi: 10.1016/j.archger.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Kawashima N, Akai M, Yano H. On the reflex coactivation of ankle flexor and extensor muscles induced by a sudden drop of support surface during walking in humans. J Appl Physiol (1985) 96: 604–611, 2004. doi: 10.1152/japplphysiol.00670.2003. [DOI] [PubMed] [Google Scholar]
- Nanhoe-Mahabier W, Allum JH, Overeem S, Borm GF, Oude Nijhuis LB, Bloem BR. First trial reactions and habituation rates over successive balance perturbations in Parkinson’s disease. Neuroscience 217: 123–129, 2012. doi: 10.1016/j.neuroscience.2012.03.064. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijzen PH, Horstink MW, Bloem BR, Duysens J. Startle responses in Parkinson patients during human gait. Exp Brain Res 171: 215–224, 2006. doi: 10.1007/s00221-005-0270-0. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuijzen PHJA, Schillings AM, Van Galen GP, Duysens J. Modulation of the startle response during human gait. J Neurophysiol 84: 65–74, 2000. doi: 10.1152/jn.2000.84.1.65. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Carpenter MG, Inglis JT, Duysens J, Weerdesteyn V. What startles tell us about control of posture and gait. Neurosci Biobehav Rev 53: 131–138, 2015. doi: 10.1016/j.neubiorev.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Scotti A, Oude Nijhuis LB, Smulders K, Queralt A, Geurts AC, Bloem BR, Weerdesteyn V. Are postural responses to backward and forward perturbations processed by different neural circuits? Neuroscience 245: 109–120, 2013a. doi: 10.1016/j.neuroscience.2013.04.036. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, van Geel K, Oude Nijhuis LB, Bloem BR, Geurts AC, Weerdesteyn V. Loading enhances the occurrence of startle responses in leg muscles. Neuroscience 240: 186–190, 2013b. doi: 10.1016/j.neuroscience.2013.02.060. [DOI] [PubMed] [Google Scholar]
- Oude Nijhuis LB, Allum JH, Borm GF, Honegger F, Overeem S, Bloem BR. Directional sensitivity of “first trial” reactions in human balance control. J Neurophysiol 101: 2802–2814, 2009. doi: 10.1152/jn.90945.2008. [DOI] [PubMed] [Google Scholar]
- Oude Nijhuis LB, Allum JH, Valls-Solé J, Overeem S, Bloem BR. First trial postural reactions to unexpected balance disturbances: a comparison with the acoustic startle reaction. J Neurophysiol 104: 2704–2712, 2010. doi: 10.1152/jn.01080.2009. [DOI] [PubMed] [Google Scholar]
- Painter JA, Allison L, Dhingra P, Daughtery J, Cogdill K, Trujillo LG. Fear of falling and its relationship with anxiety, depression, and activity engagement among community-dwelling older adults. Am J Occup Ther 66: 169–176, 2012. doi: 10.5014/ajot.2012.002535. [DOI] [PubMed] [Google Scholar]
- Papegaaij S, Taube W, Baudry S, Otten E, Hortobágyi T. Aging causes a reorganization of cortical and spinal control of posture. Front Aging Neurosci 6: 28, 2014. doi: 10.3389/fnagi.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel RR, Hurwitz DE, Andriacchi TP, Bush-Joseph CA, Bach BR Jr. Mechanisms for the “quadriceps avoidance gait” seen in ACL deficient patients. Gait Posture 5: 147, 1997. doi: 10.1016/S0966-6362(97)83364-4. [DOI] [Google Scholar]
- Rubenstein LZ, Robbins AS, Schulman BL, Rosado J, Osterweil D, Josephson KR. Falls and instability in the elderly. J Am Geriatr Soc 36: 266–278, 1988. doi: 10.1111/j.1532-5415.1988.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Sabel BA, Stein DG. Extensive loss of subcortical neurons in the aging rat brain. Exp Neurol 73: 507–516, 1981. doi: 10.1016/0014-4886(81)90284-3. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex 14: 721–730, 2004. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Sanders OP, Savin DN, Creath RA, Rogers MW. Protective balance and startle responses to sudden freefall in standing humans. Neurosci Lett 586: 8–12, 2015. doi: 10.1016/j.neulet.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattin RW. Falls among older persons: a public health perspective. Annu Rev Public Health 13: 489–508, 1992. doi: 10.1146/annurev.pu.13.050192.002421. [DOI] [PubMed] [Google Scholar]
- Sawers A, Pai YC, Bhatt T, Ting LH. Neuromuscular responses differ between slip-induced falls and recoveries in older adults. J Neurophysiol 117: 509–522, 2017. doi: 10.1152/jn.00699.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Stapley P, Drew T. Neurons in the pontomedullary reticular formation signal posture and movement both as an integrated behavior and independently. J Neurophysiol 100: 2235–2253, 2008. doi: 10.1152/jn.01381.2007. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci Biobehav Rev 34: 721–733, 2010. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund GP, Blouin JS, Inglis JT. Does startle explain the exaggerated first response to a transient perturbation? Exerc Sport Sci Rev 36: 76–82, 2008. doi: 10.1097/JES.0b013e318168f1ce. [DOI] [PubMed] [Google Scholar]
- Siegmund GP, Sanderson DJ, Myers BS, Inglis JT. Rapid neck muscle adaptation alters the head kinematics of aware and unaware subjects undergoing multiple whiplash-like perturbations. J Biomech 36: 473–482, 2003. doi: 10.1016/S0021-9290(02)00458-X. [DOI] [PubMed] [Google Scholar]
- Stacoff A, Diezi C, Luder G, Stüssi E, Kramers-de Quervain IA. Ground reaction forces on stairs: effects of stair inclination and age. Gait Posture 21: 24–38, 2005. doi: 10.1016/j.gaitpost.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev 12: 290–295, 2006. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 319: 1701–1707, 1988. doi: 10.1056/NEJM198812293192604. [DOI] [PubMed] [Google Scholar]
- Tresch UA, Perreault EJ, Honeycutt CF. Startle evoked movement is delayed in older adults: implications for brainstem processing in the elderly. Physiol Rep 2: e12025, 2014. doi: 10.14814/phy2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JE, Oude Nijhuis LB, Janssen L, Bastiaanse CM, Borm GF, Duysens J, Bloem BR. Dynamic posturography in Parkinson’s disease: diagnostic utility of the “first trial effect”. Neuroscience 168: 387–394, 2010. doi: 10.1016/j.neuroscience.2010.03.068. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev 26: 1–11, 2002. doi: 10.1016/S0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Wu MF, Suzuki SS, Siegel JM. Anatomical distribution and response patterns of reticular neurons active in relation to acoustic startle. Brain Res 457: 399–406, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafeiriou DI. Primitive reflexes and postural reactions in the neurodevelopmental examination. Pediatr Neurol 31: 1–8, 2004. doi: 10.1016/j.pediatrneurol.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Zhang SN, Bates BT, Dufek JS. Contributions of lower extremity joints to energy dissipation during landings. Med Sci Sports Exerc 32: 812–819, 2000. doi: 10.1097/00005768-200004000-00014. [DOI] [PubMed] [Google Scholar]