Abstract
In the last years, endoscopic ultrasonography (EUS) has evolved from a purely diagnostic technique to a more and more complex interventional procedure, with the possibility to perform several type of therapeutic interventions. Among these, EUS-guided biliary drainage (BD) is gaining popularity as a therapeutic approach after failed endoscopic retrograde cholangiopancreatography in distal malignant biliary obstruction (MBO), due to the avoidance of external drainage, a lower rate of adverse events and re-interventions, and lower costs compared to percutaneous trans-hepatic BD. Initially, devices created for luminal procedures (e.g., luminal biliary stents) have been adapted to the new trans-luminal EUS-guided interventions, with predictable shortcomings in technical success, outcome and adverse events. More recently, new metal stents specifically designed for transluminal drainage, namely lumen-apposing metal stents (LAMS), have been made available for EUS-guided procedures. An electrocautery enhanced delivery system (EC-LAMS), which allows direct access of the delivery system to the target lumen, has subsequently simplified the classic multi-step procedure of EUS-guided drainages. EUS-BD using LAMS and EC-LAMS has been demonstrated effective and safe, and currently seems one of the most performing techniques for EUS-BD. In this Review, we summarize the evolution of the EUS-BD in distal MBO, focusing on the novelty of LAMS and analyzing the unresolved questions about the possible role of EUS as the first therapeutic option to achieve BD in this setting of patients.
Keywords: Interventional endoscopic ultrasonography, Endoscopic ultrasonography-guided biliary drainage, Endoscopic ultrasonography-guided choledocho-duodenostomy, Biliary metal stent, Lumen-apposing metal stent
Core tip: Endoscopic ultrasonography (EUS)-guided choledocho-duodenostomy represents one of the possible therapeutic options to achieve biliary drainage after failed endoscopic retrograde cholangiopancreatography. Lumen-apposing metal stent (LAMS) are fully covered metal stents specifically designed for EUS-guided transluminal interventions, such as peripancreatic fluid collection or gallbladder drainage, that have been proposed for biliary drainage in the setting of distal malignant biliary obstruction, in order to overcome the limits of non-dedicated devices. This Review focuses on the new role of LAMS in the complex scenario of EUS-guided biliary drainage.
INTRODUCTION
Management of obstructive jaundice is of paramount importance in patients with malignant biliary obstruction (MBO), as impaired biliary drainage dramatically affects the possibility of systemic therapy in unresectable disease, reduces quality of life and increases morbidity and mortality[1]. The most frequent causes of distal MBO are adenocarcinoma of the head of the pancreas, distal cholangiocarcinoma, ampullary carcinomas and adenopathy or metastasis from other cancers. It is estimated that more than half of patients with unresectable ductal adenocarcinoma of the head of the pancreas presents with obstructive jaundice, and nearly 80% of these patients will develop jaundice in absence of therapy or interventions[2,3]. Regardless of the cause, unresolved biliary obstruction increases the risk of cholangitis and liver failure; determines fat and fat-soluble vitamins malabsorption, contributing to malnutrition and cachexia; associates in up to 25% with pruritus, which poorly responds to medical therapy and dramatically compromises quality of life. It is responsible, directly or indirectly, for death of a great proportion of non-palliated patients[1,4]. For many years, palliation of obstructive jaundice has been achieved with open surgery, by performing surgical choledocho-enterostomy, cholecysto-enterostomy or hepatico-jejunostomy, with or without gastrojejunostomy in case of concomitant gastric outlet obstruction (GOO). Operative biliary bypass has shown high rate of technical success and low rate of jaundice recurrence, but at the expense of significant post-operative morbidity and mortality, which range from 27%-60% and 5.4%-23% respectively in some series[4-6]. More recent studies that compared endoscopic biliary stenting and operative biliary bypass found a higher post-operative morbidity in the operative group, while endoscopic drainage was associated with lower costs, shorter duration of hospital stay and a better quality of life[7,8]. Due to these evidences, less invasive approaches to achieve biliary drainage, namely percutaneous transhepatic biliary drainage (PTBD) and endoscopic retrograde cholangiopancreatography (ERCP) with biliary stenting, have progressively spread, with a concomitant reduction of the patients undergoing operative palliation over the years[2].
ENDOSCOPIC AND PERCUTANEOUS BILIARY DRAINAGE IN DISTAL MALIGNANT BILIARY OBSTRUCTION
Currently, ERCP with placement of plastic or self-expanding metal stent (SEMS) is widely recognized as the first strategy to achieve biliary drainage in distal MBO and, when feasible, should be preferred over PTBD and surgery[9]. Reaching the papillary region in the second portion of the duodenum and cannulating the bile duct represent the first fundamental steps to perform endoscopic operative procedures on the biliary system. The success of such steps depends on several factors related, among the others, to the patient’s anatomy and to the experience of the endoscopist. The success rate of ERCP for all indications reported in literature is high, ranging from 86%-99%. However, an underlying neoplastic process could predict a lower success rate, a higher need of advanced cannulation techniques (i.e., needle knife pre-cut, double guidewire (DGW) technique, pancreatic septotomy) with consequent higher risk of adverse events (AE)[10-12]. During ERCP, malignant biliary stricture could be very hard to pass, even for experienced endoscopists; neoplastic diseases involving the distal common bile duct (CBD) can determinate infiltration and distortion of the ampulla, thus making very difficult the identification and the subsequent attempt to cannulate the papilla (Figure 1). Moreover, advanced neoplastic disease could associate with concomitant biliary and duodenal obstruction, determining the inaccessibility of the ampullary region. In addition to the aforementioned possibilities, also common benign conditions such as intradiverticular papilla or gastroduodenal surgically altered anatomy could make ERCP difficult. For several years, a common accepted therapeutic algorithm after a failed ERCP has provided these options: in cases of an accessible papilla, a possible new attempt at the same institution or after referral to a tertiary-care hospital in 3-5 d, after the resolution of the edema of the ampulla; in case of inaccessible papilla, or after definitely failed ERCP, a PTBD performed by interventional radiologists. First described in the seventies, PTBD is performed under fluoroscopic or ultrasonographic guidance, and allows to place an external biliary catheter with subsequent drainage internalization with placement of plastic or metal stent, in a one-step or two-step procedure[13-17]. PTBD is a highly effective procedure, but is burdened of significant morbidity, with a high rate of procedure-related or drainage- related AE[18]. Most frequent AE reported are occlusion or dislocation of the catheter, cholangitis, bile leakage alongside the drain[18-21]. A retrospective study of more than 2000 PTBD procedures in 385 patients reported that 40% of patients presented at least one drainage-related AE, with malignant disease being a risk factor for drainage occlusion and cholangitis[18]. A recent retrospective study from Sarwar and co-workers on 266 PTBD procedures in 266 patients reported a 45.9% of readmission at 30 d, 63.9% of which were unplanned[22]. The high rate of AE and readmissions, in addition to the presence of the external drainage, could heavily impair the patient’s quality of life and, at the same time, significantly increases the costs. Thus far, the widespread and easy availability have confirmed PTBD as the first option to drain MBO after ERCP failure. However, alternative techniques based on interventional endoscopic ultrasonography (EUS), such as EUS-guided biliary drainage (EUS-BD) have progressively demonstrated feasibility and high effectiveness, providing useful alternatives for jaundice palliation.
Figure 1.
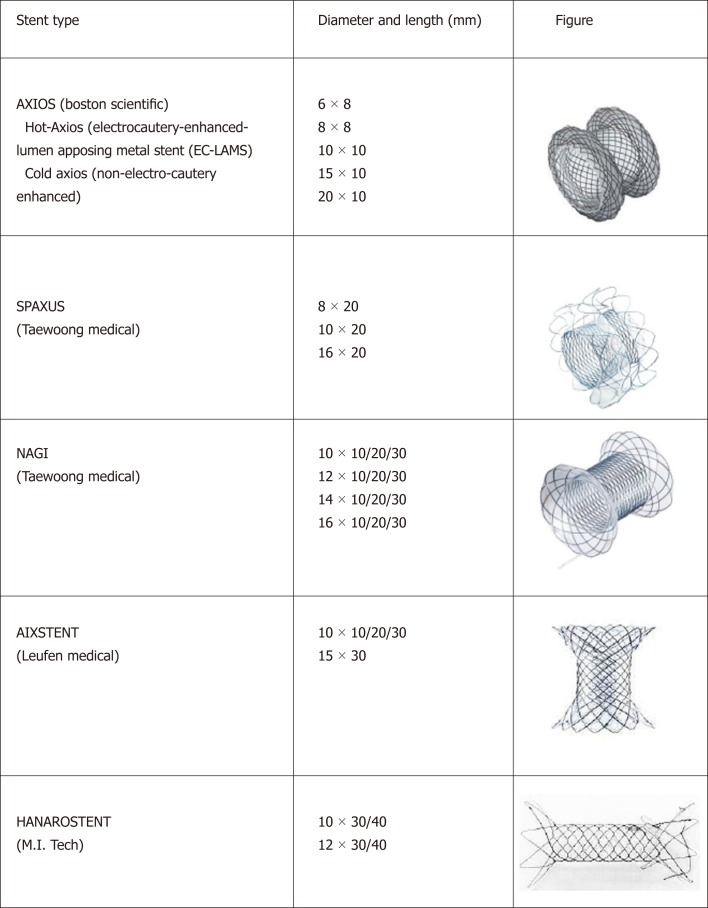
Currently lumen-apposing metal stent and fully covered self-expanding metal stent with peculiar anti-migratory shape available on the market.
EUS-GUIDED BILIARY DRAINAGE
Since the early 2000’s, the development of echoendoscope with larger operative channel allowing devices up to 10 French, opened the way for an increasingly interventional role for EUS procedures. Over the year, several types of EUS-guided procedures such as biliary and pancreatic drainage, peri-pancreatic fluid collections (PFC) drainage, gastro-enteral anastomosis, vascular interventions and ablative treatment of neoplasms have been successfully reported[23]. EUS-guided biliary access and drainage procedures are primarily performed as alternative of percutaneous or surgical drainage after failed ERCP, but the rapid widespread of interventional EUS is currently challenging the role of ERCP as primary approach for MBO[24-27]. EUS-BD can be achieved through different approaches, depending on the experience and preference of the endoscopist, the availability of specific devices, the localization of the biliary obstruction and the accessibility of the papilla: Rendez-vous (RV) technique, EUS-guided antegrade stenting; EUS-guided choledocho-duodenostomy (EUS-CD) or choledocho-gastrostomy; EUS-guided hepato-gastrostomy (EUS-HGS); EUS-guided cholecysto-gastrostomy (as a last resort). To date, which is the best technique is still a matter of debate among interventional endoscopists[28]. A recent systematic review on EUS-CD versus EUS-HGS including 10 studies with 434 patients showed a very high technical [94.1% vs 93.7%, pooled odds ratio (OR) = 0.96, 95% confidence interval (CI) = 0.39-2.33, I = 0%] and clinical success (88.5% vs 84.5%, pooled OR = 0.76, 95%CI = 0.42-1.35, I = 17%) without difference for AE (OR = 0.97, 95%CI = 0.60-1.56, I = 37%) for these procedures[29].
Regardless of the preferred approach, the first step is the access to the biliary system. Using a curved linear array echoendoscope, the bile duct is punctured with a 19 Gauge needle and the correct positioning of the needle is confirmed by aspiring bile and injecting contrast to fluoroscopically visualize the biliary tree. Then, a 0.035 inch or 0.025 inch guidewire is passed through the needle and manipulated in the desired direction. The biliary system can be accessed through a trans-hepatic route, by which the intrahepatic biliary ducts are usually punctured at the third segment with the scope positioned at the gastro-esophageal junction, or through the extra-hepatic bile duct, generally from the bulb or the stomach[23,30]. In case of RV or antegrade stenting, the guidewire is manipulated toward the papilla across the obstruction, and then coiled in the duodenum. On the contrary, the wire is directed toward the hepatic hilum in case of CD or choledocho-gastrostomy. For the RV procedure, the echoendoscope is subsequently exchanged over the guidewire and a duodenoscope is inserted in the duodenum. Then, biliary cannulation is attempted alongside the guidewire previously placed, or, after grasping the guidewire with a snare or forceps and pulling back through the operative channel of the duodendoscope, performed over the guidewire. As already mentioned, directing the guidewire toward the papilla, negotiating the obstruction and reaching the duodenum are key steps for a successful RV procedure or antegrade stenting, and are usually facilitated by keeping the scope in “short” position when puncturing the bile duct[23]. Although the high clinical success rate once these steps are achieved, they could fail in up to 25% of cases[31]. For the other EUS-BD technique, tract dilation with cystotome, needle-knife or balloon is needed before plastic stent or SEMS placement, with a sequence of over-the-wire procedural steps which are crucial for the success of the procedure as well as critical for possible AE[23]. Since the first EUS-CD has been described by Giovannini et al[32], several studies have investigated technical and clinical success of different EUS-BD. A systematic review and meta-analysis from Wang et al[33] reported the technical success rate, final success rate and AE rate in 1192 patients treated with EUS-BD, including transluminal drainages, RV procedures and antegrade stenting from 42 studies (14 prospective, 25 retrospective single-centre studies and 3 retrospective multi-centre studies). The overall technical and final success rate were 94.71% and 91.66% respectively, while technical and final success rate of EUS-guided transluminal biliary drainage procedures from 29 studies were 95.68% and 90.32%. This study was not able to compare the outcome between all types of EUS drainages, however a comparison between the trans-gastric and trans-duodenal approach did not show significant differences in success or AE. The same systematic review reported a cumulative risk of AE of 23.32% (278 patients), being bleeding (4.03%), bile leakage (4.03%), pneumoperitoneum (3.02%), stent migration (2.68%), cholangitis (2.43%), abdominal pain (1.51%), and peritonitis (1.26%) the most frequent. Strikingly, the use of metal stent vs plastic stents was associated with a lower risk of AE (17.52 vs 31.03, P = 0.013), with no significant differences in technical and clinical success rate[33]. The difference is probably due to the radial force exerted by the SEMS during the expansion, which seals the fistula between the gastrointestinal wall and the bile duct wall, reducing the risk of bile leakage and bile peritonitis. In addition, the larger calibre of metal stent compared to plastic stent probably reduces the risk of occlusion and subsequent cholangitis. Currently, SEMS instead of plastic stent should be preferred for EUS-BD[24]. The efficacy of EUS-BD questioned the primary role of PTBD after failed ERCP in MBO (Figure 2). In 2017, a systematic review and meta-analysis from Sharaiha et al[34] including 9 studies (483 patients) aimed to compare EUS-BD and PTBD outcome and safety. Despite a similar technical success, a slightly higher clinical success rate [although data from 3 randomized clinical trials (RCTs) reported no significant differences] and a lower risk of AE were found in EUS-BD compared to PTBD. Bile leak, bleeding, cholangitis, sepsis and peritonitis were the most frequent AE reported, and were all more frequent in the PTBD group. Moreover, EUS-BD was associated with less re-intervention and lower costs. A retrospective study comparing EUS-BD and PTBD in 60 patients also reported lower post-procedures pain score in EUS-BD group[35]. Analysing the issue from a different point of view, Nam et al[36] aimed to evaluate the patient’s preference in case of failed ERCP conducting a multicentre survey in 7 tertiary referral centers. Among 313 patients who responded about a simulated scenario of failed ERCP, 251 (80.2%) preferred EUS-BD, mainly for the possibility of internal drainage. Taken together, these data promoted a novel therapeutic algorithm, which favours EUS-BD, where the expertise is available, as primary approach after failed ERCP in MBO.
Figure 2.
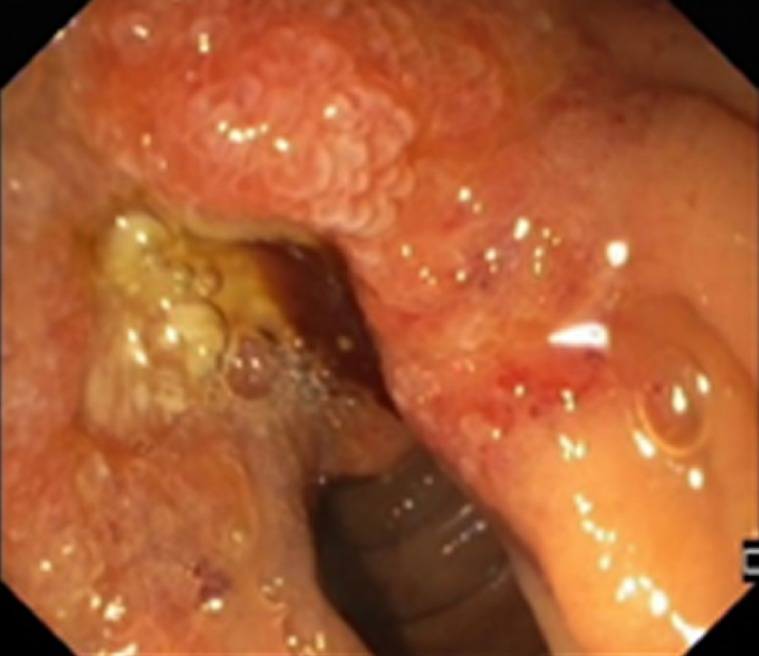
Endoscopic view of infiltration of the papilla by invasive pancreatic cancer.
LUMINAL-APPOSING METAL STENTS FOR EUS-GUIDED BILIARY DRAINAGE
Despite the exciting reports of clinical efficacy and the favourable safety data over PTBD, the AE rate for EUS-BD is not negligible and is up to 24%[33]. In the last years, interventional EUS has spread rapidly, but the devices available have remained that adapted from other interventional procedures for a long time. In fact, all the devices commonly used come from luminal indications (i.e., biliary dilation balloon, biliary stent, needle-knife), have adapted for transluminal indication and, even if the results have been motivating, it was reasonable that they could be improved. As already discussed, the use of fully covered SEMS (FCSEMS) partially resolved the issue of bile leaks and bile peritonitis due to expanding radial force of the stent that seals the transluminal fistula. However, in absence of specific anti-migratory properties, all the biliary stent designed for luminal indication present a significant risk of dislocation when used for transluminal drainage due to their tubular shape, with possible subsequent peritonitis, perforation and cholangitis. With this regard, lumen-apposing metal stent (LAMS) are fully covered “dumbbell”-shaped short stent made up of braided nitinol, specifically designed for interventional trans-luminal EUS-guided procedures, with distal anti-migratory flanges which provide the lumen-to-lumen apposition effect[37]. The device is pre-loaded in a 9 French or 10.8 French catheter with a through-the-scope delivery system compatible with therapeutic echoendoscope with a working channel of 3.7 mm or larger. Currently, two different LAMS are available on the market: Axios stent (Boston Scientific); Spaxus (Taewoong Medical). The 16 mm Spaxus stent has the largest flange (31 mm), followed by the 20 mm Axios stent (29 mm). Moreover, short FCSEMS with peculiar anti-migratory shape have been commercialized for similar indications: NAGI (Taewoong Medical); Aixstent (Leufen Medical); Hanarostent (M.I. Tech)[38] (Figure 1). LAMS have been originally designed for EUS-guided PFC drainage, as they provided large calibre to drain solid components of walled-off necrosis, low risk of leak alongside the stent and of migration, allowing trans-stent interventional procedures, such as endoscopic necrosectomy[34,39-42]. In 2011, Binmoeller and Shah first described transluminal stenting between two non-adherent lumens of the gastrointestinal tract using LAMS in an ex-vivo model[43]. Soon after, in 2012, Itoi et al[44] reported the first experience of LAMS in humans, describing the successful drainage of 15 symptomatic pancreatic pseudocyst and 5 acute cholecystitis in patients unfit for surgery. Since then, several reports have confirmed the feasibility and efficacy of LAMS in these settings, and the indication has expanded to biliary drainage, where the smaller target [i.e., the bile duct instead of PFC or gallbladder (GB)] lead to the development of smaller LAMS. In 2014, the first EUS-CD with LAMS was successfully performed by Itoi and Binmoeller[45] in a patient with unresectable pancreatic cancer and obstructive jaundice. Despite the innovative and dedicated design, the LAMS delivery system was the same of the “old” non-specific plastic stent or SEMS, and still included the same several steps: (1) Puncture of bile duct with FNA needle; (2) Guidewire introduction; (3) Tract dilation; (4) Introduction and delivery of the LAMS. As discussed above, a multi-steps procedure carries per se the risk of AE due to multiple exchanges (e.g., losing the wire and/or the scope position, bile leakage during tract dilation). To overcome these shortcomings, a LAMS delivery system has further evolved with the addition of an electrocautery tip [electrocautery-enhanced (EC)-LAMS-HOT-AXIOS, Boston Scientific Corp., Marlborough, Massachusetts, United States] which allows a single-stage technique with the access to the target lumen in one-step procedure, without the need of multiple exchanges and with reduced fluoroscopy and procedure time[46-49] (Figures 3-7). Data from the main studies on biliary LAMS are summarized in Table 1. In 2016, Kunda et al[50] reported a retrospective analysis of 57 patients who underwent EUS-CD with LAMS (27 patients) and EC-LAMS (30 patients). The overall technical and clinical success were 98.2% and 94.6% respectively. The major AE rate was 7%, with 2 duodenal perforation (one caused by the tip of the scope and not related to the delivery of the stent; the other during tract dilation for subsequent LAMS placement without cautery), 1 bleeding and 1 transient cholangitis. During the mean follow-up of 151 ± 145 d, 5 out 54 patients (9.3%) need a re-intervention (1 LAMS migration; 4 sump syndrome). A prospective study from Tsuchiya et al[51] evaluated 19 patients who underwent EUS-CD with EC-LAMS for MBO after failed ERCP. The stent was deployed using the electro-enhanced catheter over a guidewire previously placed with a 19 Gauge FNA needle puncture. The Authors reported a 100% and 95% technical and clinical success rate, with an AE rate of 36.3% (5/19), mostly with mild severity. Five patients experienced stent obstruction due to occlusion by food residue (n = 2), kinking (n = 1), tumour progression (n = 1) and spontaneous dislodgement (n = 1), and 4 patients underwent a successful re-intervention. Recently, our group reported a retrospective analysis of 46 patients with MBO treated with EC-LAMS after failed ERCP with a single-stage procedure, that is with a direct access to the bile duct with electro-enhanced catheter without a previously placed guidewire[48]. In our series, the technical and clinical success rate were 93.5% (43/46) and 97.1% (42/43), with a major AE rate of 11.6% (3 stent obstruction; 1 stent migration; 1 fatal bleeding). The only case of stent migration was a mild AE that occurred after 148 d from the procedure, and was successfully treated with a RV technique through the remaining fistula and placement of a trans-papillary biliary SEMS. Stent obstruction were also successfully managed with endoscopic interventions. Currently, no specific effective measures have been identified to avoid AE in the setting of biliary LAMS. As far as the risk of LAMS obstruction is concerned, it could be reasonable to manage concomitant duodenal obstruction in the same session, as we reported higher rate of LAMS obstruction in this sub-group of patients[48]. Technical failures with misdeployment of the first flange of the stent occurred in case of endoscope instability in the duodenal bulb, or due to a smaller CBD diameter. For such reason, we now recommend to proceed with single-stage EUS-CD in more dilated CBD (i.e., 15 mm) and to pre-load the delivery system with a guidewire in difficult cases, in order to perform an over-the-wire stent placement in case of misdeployment of the LAMS. Of note, these cases were all successfully treated during the same endoscopic session, by performing a RV technique through the fistula with subsequent transpapillary drainage or with a successful second attempt with EC-LAMS. Finally, nine patients (19.6%) with concomitant duodenal obstruction were treated in the same session with EUS-BD and subsequent duodenal stent placement, confirming the feasibility of a complete endoscopic palliation in this subgroup of patients[48,52-54]. A recent retrospective study of 52 patients treated with EC-LAMS for MBO confirmed the high rate of technical and clinical success (88.5% and 100% respectively)[55]. The Authors reported a 3.8% short-term (1 stent occlusion and 1 bleeding from pre-cut site of previous failed ERCP) and 13.5% long-term AE rate (including stent obstruction due to tumor progression or food impaction and stent migration). Various technique for EC-LAMS placement have been used in this work, and the single-stage technique, in addition to bile duct diameter > 15 mm and EC-LAMS 6 mm, was found to be significantly associated to technical success. AE related to the use of LAMS for biliary drainage in the setting of MBO are summarized in Table 2. Taken together, the cited works highlight a high efficacy and a good safety of EUS-BD with LAMS. Of note, AE reported were mostly successfully managed with endoscopic re-intervention (stent cleansing, stent-in-stent placement), and the risk of bile leakage, bile peritonitis, perforation or pneumoperitoneum compared to classic multi-steps EUS-BD was definitely lower. With an easier deployment technique, a good safety and a very short procedural time, EUS-CD with LAMS seems currently one of the most performing EUS-BD approaches[48,55].
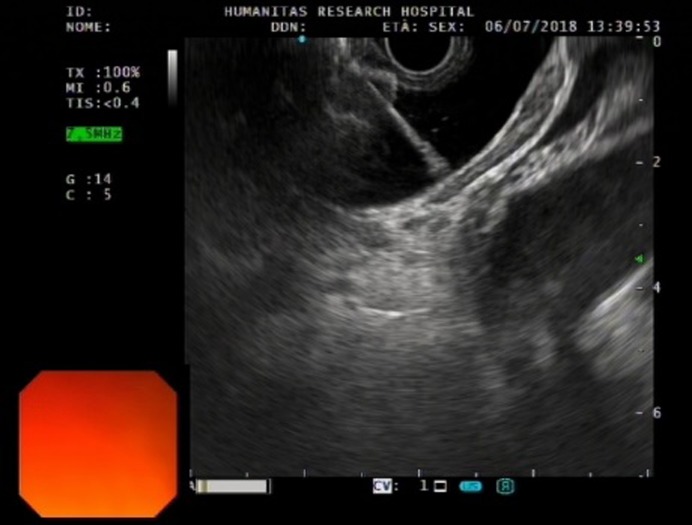
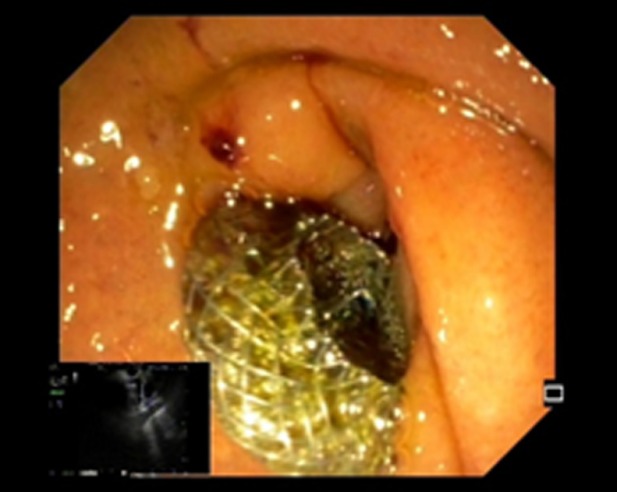
Figure 4.
Echoendoscopic view of the first flange deployment of electrocautery-enhanced lumen-apposing metal stent in a dilated common bile duct.
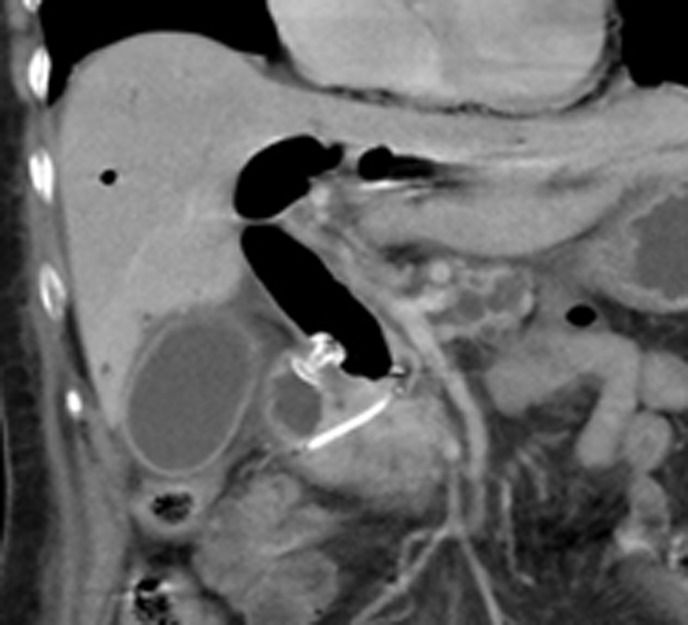
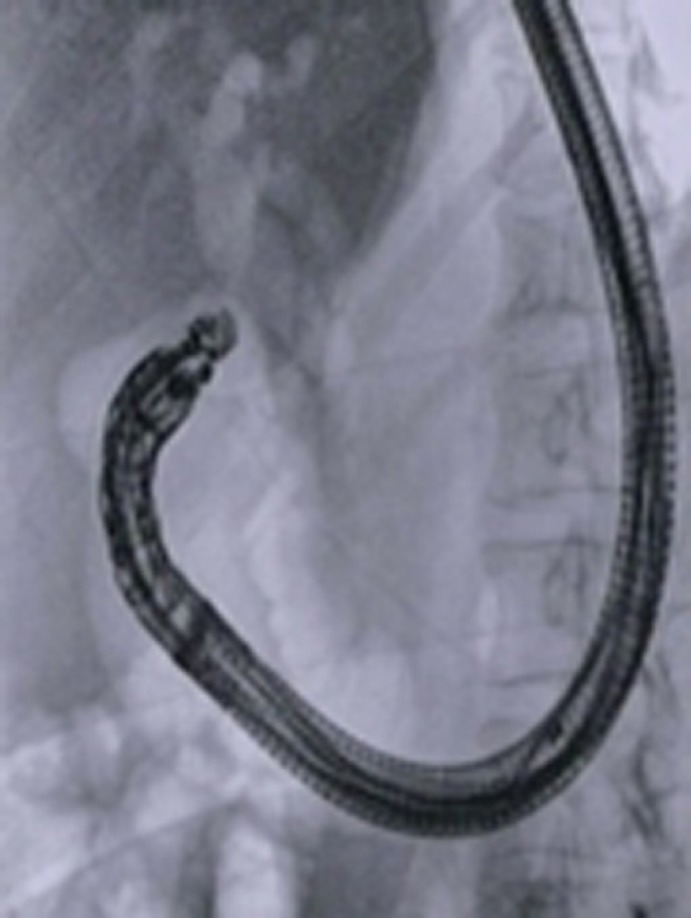
Figure 7.
Computed tomography scan appearance of electrocautery-enhanced lumen-apposing metal stent deployed across the duodenal bulb and plastic pancreatic stent previously placed during failed endoscopic retrograde cholangiopancreatography.
Table 1.
Comparison of the main studies reporting patients treated with lumen-apposing metal stent for distal malignant biliary obstruction
| Author | n, patients | EC-LAMS, n (%) | Technical success (%) | Clinical success (%) | Adverse events (%) |
| Kunda et al[50] | 57 | 27 (47.4) | 98.2 | 96.4 | 7 |
| Tsuchiya et al[51] | 19 | 19 (100) | 100 | 94.7 | 36.8 |
| Anderloni et al[48] | 46 | 46 (100) | 93.5 | 97.7 | 11.6 |
| Jacques et al[55] | 52 | 52 (100) | 88.5 | 100 | 17.3 |
Clinical success is reported as percentage among patients with technical success. EC-LAMS: Electrocautery lumen-apposing metal stent.
Table 2.
Comparison of the adverse events reported in the main studies with lumen-apposing metal stent for distal malignant biliary obstruction
| Author | Migration | Bleeding | Obstruction | Cholangitis | Others |
| Kunda et al[50] | 0 | 1.7% (1/57) | 0 | 1.7% (1/57) | 3.5% (2/57) |
| Tsuchiya et al[51] | 0 | 0 | 26.3% (5/19) | 10.5% (2/19) | 10.5% (2/19) |
| Anderloni et al[48] | 2.2% (1/46) | 2.2% (1/46) | 6.5% (3/46) | 0 | 0 |
| Jacques et al[55] | 1.9% (1/52) | 1.9% (1/52) | 13.5% (7/52) | 11.5% (6/52) | 0 |
Others: Include perforation, pneumoperitoneum, fever. In the study from Jacques et al[55], 6 patients presented both stent obstruction and cholangitis (total adverse events: 9).
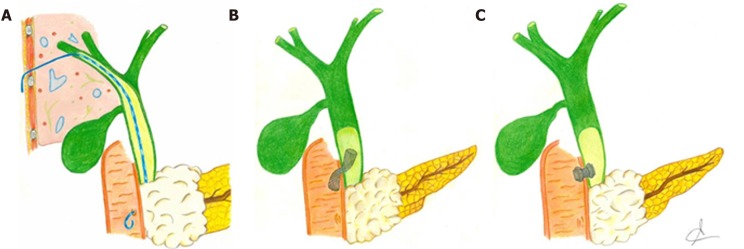
Figure 3.
Graphic representation of the main interventional techniques applied to perform biliary drainage after failed endoscopic retrograde cholangiopancreatography in malignant biliary obstruction. A: Percutaneous transhepatic biliary drainage; B: Endoscopic ultrasonography-guided choledocho-duodenostomy with placement of bilary fully covered self-expanding metal stent; C: Endoscopic ultrasonography-guided choledocho-duodenostomy with placement of lumen-apposing metal stent.
Figure 5.
Final endoscopic appearance of electrocautery-enhanced lumen-apposing metal stent deployed in the duodenal bulb.
Figure 6.
Final RX appearance of electrocautery-enhanced lumen-apposing metal stent deployed across the duodenal bulb into the common bile duct.
FUTURE PERSPECTIVES AND UNRESOLVED QUESTIONS
The impressive results of EUS-BD have recently questioned the role of interventional EUS as a mere second option after failed ERCP, advancing the hypothesis of a possible primary role in MBO alternative to ERCP. It is clear that a trans-papillary approach is difficult in case of duodenal obstruction, and EUS-BD could be the best solution, but which is actually the best drainage strategy in a patient with MBO and an accessible papilla remains debatable. ERCP for MBO can be associated with a significant morbidity, including acute pancreatitis and cholangitis, and pre-operative drainage is not indicated in patients with obstructive jaundice and surgical indication[9,10]. Moreover, some studies reported that advanced techniques of cannulation (i.e., DGW techniques, pre-cut sphincterotomy, trans-pancreatic septotomy) are associated with increased risk of AE[11,56]. These observations stressed the need of high quality evidences comparing ERCP and EUS-BD as primary approach for MBO. Recently, 3 RCT trying to address the issue have been published, and all concluded that EUS-BD has comparable outcome to ERCP in this setting[25-27]. However, it should be noted that these RCT have been powered on different outcomes: AE rate for the study from Bang et al[25]; stent patency for the study from Park et al[27]; technical success (designed as non-inferiority RCT) for the study from Paik et al[26]. Despite the good design and the importance of the data provided, these studies did not offered conclusive information about EUS-BD in primary biliary drainage. Moreover, for the study from Paik et al[26], it should also be noted that the AE rate in the ERCP group was higher than EUS-BD group, but extremely high in absolute (39.1%), probably also because of the lack of prophylactic measure to prevent post-ERCP pancreatitis[57]. However, the risk of pancreatitis in EUS-BD groups from all studies was 0%, as expected for a procedure in which the papilla is not manipulated and the pancreatic parenchyma is always spared. None of the aforementioned RCT used LAMS for EUS-BD, and a future challenge will be to address in a RCT the outcome of EUS-CD with LAMS compared to ERCP for primary biliary drainage.
Patency of biliary stents is a crucial issue in jaundice palliation, as stent occlusion determines morbidity (e.g., cholangitis) and increases the need of re-interventions, thus impacting on quality of life and costs. Biliary SEMS have demonstrated a longer patency compared to plastic stents[9,58]. However, even SEMS carries a risk of occlusion due to tumor ingrowth (for uncovered SEMS), and overgrowth (for FCSEMS)[59]. EUS-CD is performed in a CBD segment above the obstruction and the stent does not cross the neoplastic tissue. On the other hand, EUS-CD carries the risk of occlusion due to food impaction or biliary sludge deposits, and this has been reported as particularly relevant for patients with duodenal obstruction[48,50,51]. The long-term patency of LAMS for EUS-CD has yet to be evaluated, and possible technical precautions aimed to extend the patency duration needs further studies.
Another point that should be addressed is whether EUS-CD with LAMS is feasible in patients candidate for pancreatic surgery. Almost all mentioned studies included patients with unresectable malignancies, and very few information are available on performing Whipple procedures in patients with an indwelling duodenal LAMS. In the retrospective study from Jacques and colleagues[55], 2 patients underwent EUS-CD with LAMS for pre-operative drainage, and a Whipple procedure was subsequently performed without complications. Recently, a case series of patients who underwent EUS-CD with LAMS before pancreatic surgery confirmed that duodenal LAMS did not interfere with surgery[60]. Due to the small numbers, the question remain to be clarified, but data are encouraging about the possibility of LAMS placement for patient possibly candidate for surgery.
In patients with MBO and failed ERCP, an alternative to EUS-BD through the traditional approaches (i.e., EUS-CD or hepatogastrostomy) is GB drainage. EUS-guided GB drainage has emerged as an alternative treatment for acute cholecystitis in patients unfit for surgery due to relevant comorbidity[61,62]. In this setting, GB drainage with LAMS has been demonstrated as safe and effective[47,63]. A retrospective study evaluated EUS-guided GB drainage with SEMS for jaundice palliation in 12 patients with MBO[64]. The study reported a high technical and clinical success rate (100% and 91.7% respectively), with AE in 16.7% and stent dysfunction in 8.3%. Currently, further studies are needed to evaluate if LAMS could offer a better safety and efficacy in this setting. Finally, technical innovation in design and delivery system could be improve LAMS performance in the future, especially to increase the duration of patency and to reduce AE during deployment.
CONCLUSION
In a few years, advances in knowledge and technology radically changed the role of interventional EUS in clinical practice and, at the same time, questioned therapeutic algorithms which have been unchanged for several years. The last technological advance has been represented by LAMS, whose innovative design contributed to improve the already exciting results of EUS-BD. A consistent body of evidence highlights the advantages of EUS-BD over PTBD: lower AE; fewer re-interventions; lower costs; internal drainage with a better quality of life; lower post-procedural pain; different routes of drainage (trans-hepatic or extra-hepatic); the possibility to proceed to drainage during the same session and with the same operator after failed ERCP; concomitant jaundice and GOO palliation. With this background, LAMS contributed to make easier and faster the drainage, to reduce the shortcomings of complex multi-step procedures and probably will be responsible for further widespread of EUS-BD. RCT including LAMS to compare EUS-BD and ERCP for primary drainage are lacking, and conducting high quality studies in this field will be one of the hardest challenges in the next future.
Footnotes
Conflict-of-interest statement: Dr. Anderloni reports personal fees from Boston Scientific, during the conduct of the study; personal fees from Boston Scientific, outside the submitted work; Dr. Repici reports personal fees from Boston Scientific, personal fees from Fujifilm, during the conduct of the study; personal fees from Boston Scientific, personal fees from Fujifilm, outside the submitted work; Dr. Troncone, Dr. Fugazza, Dr. Del Vecchio Blanco, Dr. Cappello, Dr. Monteleone have nothing to disclose.
Peer-review started: March 3, 2019
First decision: April 30, 2019
Article in press: July 3, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sugimoto M S-Editor: Yan JP L-Editor: A E-Editor: Ma YJ
Contributor Information
Andrea Anderloni, Digestive Endoscopy Unit, Division of Gastroenterology, Humanitas Research Hospital, Milan 20089, Italy. andrea.anderloni@humanitas.it.
Edoardo Troncone, Department of Systems Medicine, University of Rome “Tor Vergata”, Rome 00133, Italy.
Alessandro Fugazza, Digestive Endoscopy Unit, Division of Gastroenterology, Humanitas Research Hospital, Milan 20089, Italy.
Annalisa Cappello, Digestive Endoscopy Unit, Division of Gastroenterology, Humanitas Research Hospital, Milan 20089, Italy.
Giovanna Del Vecchio Blanco, Department of Systems Medicine, University of Rome “Tor Vergata”, Rome 00133, Italy.
Giovanni Monteleone, Department of Systems Medicine, University of Rome “Tor Vergata”, Rome 00133, Italy.
Alessandro Repici, Digestive Endoscopy Unit, Division of Gastroenterology, Humanitas Research Hospital, Milan 20089, Italy; Digestive Endoscopy Unit, Division of Gastroenterology, Humanitas University, Milan 20089, Italy.
References
- 1.Stark A, Hines OJ. Endoscopic and operative palliation strategies for pancreatic ductal adenocarcinoma. Semin Oncol. 2015;42:163–176. doi: 10.1053/j.seminoncol.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Kneuertz PJ, Cunningham SC, Cameron JL, Torrez S, Tapazoglou N, Herman JM, Makary MA, Eckhauser F, Wang J, Hirose K, Edil BH, Choti MA, Schulick RD, Wolfgang CL, Pawlik TM. Palliative surgical management of patients with unresectable pancreatic adenocarcinoma: Trends and lessons learned from a large, single institution experience. J Gastrointest Surg. 2011;15:1917–1927. doi: 10.1007/s11605-011-1665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maire F, Hammel P, Ponsot P, Aubert A, O'Toole D, Hentic O, Levy P, Ruszniewski P. Long-term outcome of biliary and duodenal stents in palliative treatment of patients with unresectable adenocarcinoma of the head of pancreas. Am J Gastroenterol. 2006;101:735–742. doi: 10.1111/j.1572-0241.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 4.Singh SM, Reber HA. Surgical palliation for pancreatic cancer. Surg Clin North Am. 1989;69:599–611. doi: 10.1016/s0039-6109(16)44837-1. [DOI] [PubMed] [Google Scholar]
- 5.Huguier M, Baumel H, Manderscheid JC, Houry S, Fabre JM. Surgical palliation for unresected cancer of the exocrine pancreas. Eur J Surg Oncol. 1993;19:342–347. [PubMed] [Google Scholar]
- 6.Rosemurgy AS, Burnett CM, Wasselle JA. A comparison of choledochoenteric bypass and cholecystoenteric bypass in patients with biliary obstruction due to pancreatic cancer. Am Surg. 1989;55:55–60. [PubMed] [Google Scholar]
- 7.Artifon EL, Sakai P, Cunha JE, Dupont A, Filho FM, Hondo FY, Ishioka S, Raju GS. Surgery or endoscopy for palliation of biliary obstruction due to metastatic pancreatic cancer. Am J Gastroenterol. 2006;101:2031–2037. doi: 10.1111/j.1572-0241.2006.00764.x. [DOI] [PubMed] [Google Scholar]
- 8.Bliss LA, Eskander MF, Kent TS, Watkins AA, de Geus SW, Storino A, Ng SC, Callery MP, Moser AJ, Tseng JF. Early surgical bypass versus endoscopic stent placement in pancreatic cancer. HPB (Oxford) 2016;18:671–677. doi: 10.1016/j.hpb.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: Indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910–930. doi: 10.1055/a-0659-9864. [DOI] [PubMed] [Google Scholar]
- 10.van der Gaag NA, Rauws EA, van Eijck CH, Bruno MJ, van der Harst E, Kubben FJ, Gerritsen JJ, Greve JW, Gerhards MF, de Hingh IH, Klinkenbijl JH, Nio CY, de Castro SM, Busch OR, van Gulik TM, Bossuyt PM, Gouma DJ. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–137. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Li ZS, Liu F, Ren X, Lu NH, Fan ZN, Huang Q, Zhang X, He LP, Sun WS, Zhao Q, Shi RH, Tian ZB, Li YQ, Li W, Zhi FC. Risk factors for ERCP-related complications: A prospective multicenter study. Am J Gastroenterol. 2009;104:31–40. doi: 10.1038/ajg.2008.5. [DOI] [PubMed] [Google Scholar]
- 12.Enochsson L, Swahn F, Arnelo U, Nilsson M, Löhr M, Persson G. Nationwide, population-based data from 11,074 ERCP procedures from the Swedish Registry for Gallstone Surgery and ERCP. Gastrointest Endosc. 2010;72:1175–1184, 1184.e1-1184.e3. doi: 10.1016/j.gie.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 13.Pereiras RV, Jr, Rheingold OJ, Huston D, Mejia J, Viamonte M, Chiprut RO, Schiff ER. Relief of malignant obstructive jaundice by percutaneous insertion of a permanent prosthesis in the biliary tree. Ann Intern Med. 1978;89:589–583. doi: 10.7326/0003-4819-89-5-589. [DOI] [PubMed] [Google Scholar]
- 14.Krokidis M, Fanelli F, Orgera G, Bezzi M, Passariello R, Hatzidakis A. Percutaneous treatment of malignant jaundice due to extrahepatic cholangiocarcinoma: covered Viabil stent versus uncovered Wallstents. Cardiovasc Intervent Radiol. 2010;33:97–106. doi: 10.1007/s00270-009-9604-9. [DOI] [PubMed] [Google Scholar]
- 15.Molnar W, Stockum AE. Relief of obstructive jaundice through percutaneous transhepatic catheter--a new therapeutic method. Am J Roentgenol Radium Ther Nucl Med. 1974;122:356–367. doi: 10.2214/ajr.122.2.356. [DOI] [PubMed] [Google Scholar]
- 16.Thornton RH, Frank BS, Covey AM, Maybody M, Solomon SB, Getrajdman GI, Brown KT. Catheter-free survival after primary percutaneous stenting of malignant bile duct obstruction. AJR Am J Roentgenol. 2011;197:W514–W518. doi: 10.2214/AJR.10.6069. [DOI] [PubMed] [Google Scholar]
- 17.Nennstiel S, Treiber M, Faber A, Haller B, von Delius S, Schmid RM, Neu B. Comparison of Ultrasound and Fluoroscopically Guided Percutaneous Transhepatic Biliary Drainage. Dig Dis. 2019;37:77–86. doi: 10.1159/000493120. [DOI] [PubMed] [Google Scholar]
- 18.Nennstiel S, Weber A, Frick G, Haller B, Meining A, Schmid RM, Neu B. Drainage-related Complications in Percutaneous Transhepatic Biliary Drainage: An Analysis Over 10 Years. J Clin Gastroenterol. 2015;49:764–770. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 19.Günther RW, Schild H, Thelen M. Percutaneous transhepatic biliary drainage: Experience with 311 procedures. Cardiovasc Intervent Radiol. 1988;11:65–71. doi: 10.1007/BF02577061. [DOI] [PubMed] [Google Scholar]
- 20.Born P, Rösch T, Triptrap A, Frimberger E, Allescher HD, Ott R, Weigert N, Lorenz R, Classen M. Long-term results of percutaneous transhepatic biliary drainage for benign and malignant bile duct strictures. Scand J Gastroenterol. 1998;33:544–549. doi: 10.1080/00365529850172142. [DOI] [PubMed] [Google Scholar]
- 21.Mueller PR, van Sonnenberg E, Ferrucci JT., Jr Percutaneous biliary drainage: Technical and catheter-related problems in 200 procedures. AJR Am J Roentgenol. 1982;138:17–23. doi: 10.2214/ajr.138.1.17. [DOI] [PubMed] [Google Scholar]
- 22.Sarwar A, Hostage CA, Jr, Weinstein JL, Kim G, Novack V, Chakrala N, Park Y, Brook OR, Ahmed M. Causes and Rates of 30-day Readmissions after Percutaneous Transhepatic Biliary Drainage Procedure. Radiology. 2019;290:722–729. doi: 10.1148/radiol.2018180279. [DOI] [PubMed] [Google Scholar]
- 23.ASGE Technology Committee. Maple JT, Pannala R, Abu Dayyeh BK, Aslanian HR, Enestvedt BK, Goodman A, Komanduri S, Manfredi M, Navaneethan U, Parsi MA, Smith ZL, Thosani N, Sullivan SA, Banerjee S. Interventional EUS (with videos) Gastrointest Endosc. 2017;85:465–481. doi: 10.1016/j.gie.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Teoh AYB, Dhir V, Kida M, Yasuda I, Jin ZD, Seo DW, Almadi M, Ang TL, Hara K, Hilmi I, Itoi T, Lakhtakia S, Matsuda K, Pausawasdi N, Puri R, Tang RS, Wang HP, Yang AM, Hawes R, Varadarajulu S, Yasuda K, Ho LKY. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209–1228. doi: 10.1136/gutjnl-2017-314341. [DOI] [PubMed] [Google Scholar]
- 25.Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: A randomized trial (with videos) Gastrointest Endosc. 2018;88:9–17. doi: 10.1016/j.gie.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Paik WH, Lee TH, Park DH, Choi JH, Kim SO, Jang S, Kim DU, Shim JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018;113:987–997. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 27.Park JK, Woo YS, Noh DH, Yang JI, Bae SY, Yun HS, Lee JK, Lee KT, Lee KH. Efficacy of EUS-guided and ERCP-guided biliary drainage for malignant biliary obstruction: Prospective randomized controlled study. Gastrointest Endosc. 2018;88:277–282. doi: 10.1016/j.gie.2018.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Guo J, Giovannini M, Sahai AV, Saftoiu A, Dietrich CF, Santo E, Fusaroli P, Siddiqui AA, Bhutani MS, Bun Teoh AY, Irisawa A, Arturo Arias BL, Achanta CR, Jenssen C, Seo DW, Adler DG, Kalaitzakis E, Artifon E, Itokawa F, Poley JW, Mishra G, Ho KY, Wang HP, Okasha HH, Lachter J, Vila JJ, Iglesias-Garcia J, Yamao K, Yasuda K, Kubota K, Palazzo L, Sabbagh LC, Sharma M, Kida M, El-Nady M, Nguyen NQ, Vilmann P, Garg PK, Rai P, Mukai S, Carrara S, Parupudi S, Sridhar S, Lakhtakia S, Rana SS, Ogura T, Baron TH, Dhir V, Sun S. A multi-institution consensus on how to perform EUS-guided biliary drainage for malignant biliary obstruction. Endosc Ultrasound. 2018;7:356–365. doi: 10.4103/eus.eus_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uemura RS, Khan MA, Otoch JP, Kahaleh M, Montero EF, Artifon ELA. EUS-guided Choledochoduodenostomy Versus Hepaticogastrostomy: A Systematic Review and Meta-analysis. J Clin Gastroenterol. 2018;52:123–130. doi: 10.1097/MCG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 30.Boulay BR, Lo SK. Endoscopic Ultrasound-Guided Biliary Drainage. Gastrointest Endosc Clin N Am. 2018;28:171–185. doi: 10.1016/j.giec.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–65. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]
- 32.Giovannini M, Moutardier V, Pesenti C, Bories E, Lelong B, Delpero JR. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 33.Wang K, Zhu J, Xing L, Wang Y, Jin Z, Li Z. Assessment of efficacy and safety of EUS-guided biliary drainage: A systematic review. Gastrointest Endosc. 2016;83:1218–1227. doi: 10.1016/j.gie.2015.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–914. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 35.Sharaiha RZ, Kumta NA, Desai AP, DeFilippis EM, Gabr M, Sarkisian AM, Salgado S, Millman J, Benvenuto A, Cohen M, Tyberg A, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500–5505. doi: 10.1007/s00464-016-4913-y. [DOI] [PubMed] [Google Scholar]
- 36.Nam K, Kim DU, Lee TH, Iwashita T, Nakai Y, Bolkhir A, Castro LA, Vazquez-Sequeiros E, de la Serna C, Perez-Miranda M, Lee JG, Lee SS, Seo DW, Lee SK, Kim MH, Park DH. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48–55. doi: 10.4103/eus.eus_100_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mussetto A, Fugazza A, Fuccio L, Triossi O, Repici A, Anderloni A. Current uses and outcomes of lumen-apposing metal stents. Ann Gastroenterol. 2018;31:535–540. doi: 10.20524/aog.2018.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stier MW, Waxman I. Lumen-Apposing Metal Stents: Which One and Why? Gastrointest Endosc Clin N Am. 2018;28:207–217. doi: 10.1016/j.giec.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Rinninella E, Kunda R, Dollhopf M, Sanchez-Yague A, Will U, Tarantino I, Gornals Soler J, Ullrich S, Meining A, Esteban JM, Enz T, Vanbiervliet G, Vleggaar F, Attili F, Larghi A. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: A large retrospective study (with video) Gastrointest Endosc. 2015;82:1039–1046. doi: 10.1016/j.gie.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Shah RJ, Shah JN, Waxman I, Kowalski TE, Sanchez-Yague A, Nieto J, Brauer BC, Gaidhane M, Kahaleh M. Safety and efficacy of endoscopic ultrasound-guided drainage of pancreatic fluid collections with lumen-apposing covered self-expanding metal stents. Clin Gastroenterol Hepatol. 2015;13:747–752. doi: 10.1016/j.cgh.2014.09.047. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui AA, Kowalski TE, Loren DE, Khalid A, Soomro A, Mazhar SM, Isby L, Kahaleh M, Karia K, Yoo J, Ofosu A, Ng B, Sharaiha RZ. Fully covered self-expanding metal stents versus lumen-apposing fully covered self-expanding metal stent versus plastic stents for endoscopic drainage of pancreatic walled-off necrosis: Clinical outcomes and success. Gastrointest Endosc. 2017;85:758–765. doi: 10.1016/j.gie.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Siddiqui AA, Adler DG, Nieto J, Shah JN, Binmoeller KF, Kane S, Yan L, Laique SN, Kowalski T, Loren DE, Taylor LJ, Munigala S, Bhat YM. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: A large retrospective, multicenter U.S. experience (with videos) Gastrointest Endosc. 2016;83:699–707. doi: 10.1016/j.gie.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 43.Binmoeller KF, Shah J. A novel lumen-apposing stent for transluminal drainage of nonadherent extraintestinal fluid collections. Endoscopy. 2011;43:337–342. doi: 10.1055/s-0030-1256127. [DOI] [PubMed] [Google Scholar]
- 44.Itoi T, Binmoeller KF, Shah J, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Moriyasu F. Clinical evaluation of a novel lumen-apposing metal stent for endosonography-guided pancreatic pseudocyst and gallbladder drainage (with videos) Gastrointest Endosc. 2012;75:870–876. doi: 10.1016/j.gie.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 45.Itoi T, Binmoeller KF. EUS-guided choledochoduodenostomy by using a biflanged lumen-apposing metal stent. Gastrointest Endosc. 2014;79:715. doi: 10.1016/j.gie.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 46.Walter D, Will U, Sanchez-Yague A, Brenke D, Hampe J, Wollny H, López-Jamar JM, Jechart G, Vilmann P, Gornals JB, Ullrich S, Fähndrich M, de Tejada AH, Junquera F, Gonzalez-Huix F, Siersema PD, Vleggaar FP. A novel lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: A prospective cohort study. Endoscopy. 2015;47:63–67. doi: 10.1055/s-0034-1378113. [DOI] [PubMed] [Google Scholar]
- 47.Walter D, Teoh AY, Itoi T, Pérez-Miranda M, Larghi A, Sanchez-Yague A, Siersema PD, Vleggaar FP. EUS-guided gall bladder drainage with a lumen-apposing metal stent: A prospective long-term evaluation. Gut. 2016;65:6–8. doi: 10.1136/gutjnl-2015-309925. [DOI] [PubMed] [Google Scholar]
- 48.Anderloni A, Fugazza A, Troncone E, Auriemma F, Carrara S, Semeraro R, Maselli R, Di Leo M, D'Amico F, Sethi A, Repici A. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89:69–76. doi: 10.1016/j.gie.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 49.Anderloni A, Leo MD, Carrara S, Fugazza A, Maselli R, Buda A, Amato A, Auriemma F, Repici A. Endoscopic ultrasound-guided transmural drainage by cautery-tipped lumen-apposing metal stent: Exploring the possible indications. Ann Gastroenterol. 2018;31:735–741. doi: 10.20524/aog.2018.0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunda R, Pérez-Miranda M, Will U, Ullrich S, Brenke D, Dollhopf M, Meier M, Larghi A. EUS-guided choledochoduodenostomy for malignant distal biliary obstruction using a lumen-apposing fully covered metal stent after failed ERCP. Surg Endosc. 2016;30:5002–5008. doi: 10.1007/s00464-016-4845-6. [DOI] [PubMed] [Google Scholar]
- 51.Tsuchiya T, Teoh AYB, Itoi T, Yamao K, Hara K, Nakai Y, Isayama H, Kitano M. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction: A prospective multicenter study. Gastrointest Endosc. 2018;87:1138–1146. doi: 10.1016/j.gie.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 52.Anderloni A, Buda A, Carrara S, Di Leo M, Fugazza A, Maselli R, Repici A. Single-session double-stent placement in concomitant malignant biliary and duodenal obstruction with a cautery-tipped lumen apposing metal stent. Endoscopy. 2016;48:E321–E322. doi: 10.1055/s-0042-117425. [DOI] [PubMed] [Google Scholar]
- 53.Anderloni A, Fugazza A, Auriemma F, Maia L, Maselli R, Troncone E, DʼAmico F, Repici A. Cautery-Tipped Lumen Apposing Metal Stent Placement Through the Mesh of an Indwelling Duodenal Self-Expanding Metal Stent. Am J Gastroenterol. 2018;113:644. doi: 10.1038/s41395-018-0040-9. [DOI] [PubMed] [Google Scholar]
- 54.Glessing BR, Mallery S, Freeman ML, Newcomb MD, Arain MA. EUS-guided choledochoduodenostomy with a lumen-apposing metal stent before duodenal stent placement for malignant biliary and duodenal obstruction. Gastrointest Endosc. 2015;81:1019–1020. doi: 10.1016/j.gie.2014.09.061. [DOI] [PubMed] [Google Scholar]
- 55.Jacques J, Privat J, Pinard F, Fumex F, Valats JC, Chaoui A, Cholet F, Godard B, Grandval P, Legros R, Kerever S, Napoleon B. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents: A retrospective analysis. Endoscopy. 2019;51:540–547. doi: 10.1055/a-0735-9137. [DOI] [PubMed] [Google Scholar]
- 56.Masci E, Mariani A, Curioni S, Testoni PA. Risk factors for pancreatitis following endoscopic retrograde cholangiopancreatography: A meta-analysis. Endoscopy. 2003;35:830–834. doi: 10.1055/s-2003-42614. [DOI] [PubMed] [Google Scholar]
- 57.Spadaccini M, Fugazza A, Troncone E, Craviotto V, D'Amico F, Lamonaca L, Anderloni A, Repici A. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: An Unfair Comparison. Am J Gastroenterol. 2019;114:174. doi: 10.1038/s41395-018-0371-6. [DOI] [PubMed] [Google Scholar]
- 58.Adams MA, Anderson MA, Myles JD, Khalatbari S, Scheiman JM. Self-expanding metal stents (SEMS) provide superior outcomes compared to plastic stents for pancreatic cancer patients undergoing neoadjuvant therapy. J Gastrointest Oncol. 2012;3:309–313. doi: 10.3978/j.issn.2078-6891.2011.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tringali A, Hassan C, Rota M, Rossi M, Mutignani M, Aabakken L. Covered vs. uncovered self-expandable metal stents for malignant distal biliary strictures: A systematic review and meta-analysis. Endoscopy. 2018;50:631–641. doi: 10.1055/s-0043-125062. [DOI] [PubMed] [Google Scholar]
- 60.Fabbri C, Fugazza A, Binda C, Zerbi A, Jovine E, Cennamo V, Repici A, Anderloni A. Beyond palliation: using eus-guided choledochoduodenostomy with a lumen-apposing metal stent as a bridge to surgery. J Gastrointest Liver Dis. 2019;51:e126. doi: 10.15403/jgld.2014.1121.281.eus. [DOI] [PubMed] [Google Scholar]
- 61.Law R, Baron TH. Endoscopic Ultrasound-Guided Gallbladder Drainage. Gastrointest Endosc Clin N Am. 2018;28:187–195. doi: 10.1016/j.giec.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 62.Anderloni A, Buda A, Vieceli F, Khashab MA, Hassan C, Repici A. Endoscopic ultrasound-guided transmural stenting for gallbladder drainage in high-risk patients with acute cholecystitis: A systematic review and pooled analysis. Surg Endosc. 2016;30:5200–5208. doi: 10.1007/s00464-016-4894-x. [DOI] [PubMed] [Google Scholar]
- 63.Dollhopf M, Larghi A, Will U, Rimbaş M, Anderloni A, Sanchez-Yague A, Teoh AYB, Kunda R. EUS-guided gallbladder drainage in patients with acute cholecystitis and high surgical risk using an electrocautery-enhanced lumen-apposing metal stent device. Gastrointest Endosc. 2017;86:636–643. doi: 10.1016/j.gie.2017.02.027. [DOI] [PubMed] [Google Scholar]
- 64.Imai H, Kitano M, Omoto S, Kadosaka K, Kamata K, Miyata T, Yamao K, Sakamoto H, Harwani Y, Kudo M. EUS-guided gallbladder drainage for rescue treatment of malignant distal biliary obstruction after unsuccessful ERCP. Gastrointest Endosc. 2016;84:147–151. doi: 10.1016/j.gie.2015.12.024. [DOI] [PubMed] [Google Scholar]