Abstract
Background
Preoperative anemia is relatively common in patients undergoing primary total knee arthroplasty (TKA), and is associated with higher medical costs due to blood transfusions.
Methods
We aimed to discuss the efficiency of tranexamic acid (TXA) administration for blood loss control in preoperatively anemic patients undergoing primary TKA. We retrospectively reviewed the clinical data regarding a consecutive series of primary unilateral TKA patients with preoperative anemia. Patients were divided into TXA group (received TXA peri-operatively) and control group (did not receive TXA). Outcome measures included drainage volume; hemoglobin, and hematocrit levels recorded preoperatively and over the first 5 days postoperatively; amount of allogeneic blood transfusion; and prevalence of thrombosis.
Results
Ninety-six patients from 996 cases were included in the study. Demographics, general health condition, and preoperative conditions were comparable between the two groups. However, significantly lower drainage volume (P < 0.001), hidden blood loss (P < 0.001), and allogeneic blood transfusion volume (χ = 4.00, P = 0.046) were noted in TXA group. The hemoglobin and hematocrit levels were significantly higher in TXA group on the first postoperative day (P = 0.006), but overall the drop in hemoglobin and hematocrit levels over the first 5 days postoperatively was similar between the groups (P = 0.763), as was the incidence of thromboembolism events (P = 0.794).
Conclusion
TXA has a positive role for patients with preoperative anemia in primary total knee arthroplasty. In patients with mild preoperative anemia, TXA decreases hidden blood loss and the need for allogeneic blood transfusion, which mainly appears effective on the first postoperative day of TKA.
Keywords: Preoperative anemia, Tranexamic acid, Total knee arthroplasty, Hemostasis
Introduction
Total knee arthroplasty (TKA) is a successful surgical intervention for treating end-stage osteoarthritis, and encouraging reports regarding long-term clinical outcomes have been published [1–3]. Approximately 350,000 TKAs are performed annually in the USA [4]; in mainland China, the number of procedures performed each year has been increasing steadily.
Approximately 24% of patients undergoing elective total joint arthroplasty have preoperative anemia [5], and, as a result, require a significant amount of allogeneic blood transfusion (ABT) [6], which is sometimes associated with prolonged hospital stay and poor clinical outcome [7, 8].
The application of the antifibrinolytic agent tranexamic acid (TXA) in TKA may significantly decrease total blood loss and the need for transfusion without increasing the incidence of venous thromboembolism for non-anemic patients [9–11]. However, a few studies have focused on the effects of TXA in patients with preoperative anemia undergoing TKA [12]. Therefore, we designed the present study to perform a preliminary evaluation of the efficiency and safety of TXA for TKA patients with preoperative anemia.
Materials and methods
Patients
We retrospectively reviewed the clinical data regarding consecutive patients who underwent primary unilateral TKA in our institution between June 2013 and December 2015. The inclusion criteria were anemia during the month before surgery, defined, according to the anemia standard put forward by the World Health Organization (WHO), as preoperative hemoglobin (Pre-op Hb) levels < 13 g/dL for males and < 12 g/dL for females; physical status level of 1 or 2, as defined by the American Society of Anesthesiologists; and Charlson comorbidity score < 6. The exclusion criteria were hemorrhagic disease; previous history of surgical infection in the affected limb; history of malignant tumor; and the patients who received TXA before 2015, because of the varied methods of drug use.
Surgical procedure and postoperative management
The patient received lumbar anesthesia, and surgery was performed with a midline skin incision and medial parapatellar approach. A pneumatic tourniquet was inflated at 300–320 mmHg, and maintained under inflated condition throughout the duration of the surgery. Total condylar prosthesis with an open design (Genesis II; Smith & Nephew, Memphis, TN, USA) was implanted in all cases. None of the patients received patella resurfacing. The whole status of equipment of the hospital, operative theater, and anesthesia medication were not different during the study period from 2013 to 2015. Drainage was performed in every patient for 24 h after surgery, and an elastic bandage was used during this period to create localized pressure. Prophylaxis for thromboembolism was performed by means of rivaroxaban (10 mg/day), and early function rehabilitation was carried out routinely in all patients. Lower extremity venous ultrasound was performed on the fifth day after surgery to identify potential deep venous thrombosis (DVT) events.
A standard protocol for administration of TXA was established in our institute at the beginning of 2015, and thereafter all patients undergoing primary unilateral TKA received TXA unless contraindicated because of the following conditions: renal insufficiency(blood creatinine levels > 133 μmol/L), hepatic insufficiency, severe respiratory disease, cardiovascular disease, coronary stent implantation during the year before TKA, coagulopathy, high risk of thrombosis (congenital/acquired thrombotic diseases), history of venous thromboembolism, or history of stroke.
The study population was divided into two groups. The TXA group was defined as containing patients who received intravenous TXA (Guangzhou Pharmaceutical Group, Guangzhou, China; batch number: H20056987; specification: 10 mL: 1.0 g), the administration of TXA was 1.0 g at 15 min before inflation of the tourniquet, and 1.0 g after tourniquet release postoperatively. The patients who did not receive TXA were included in the control group. ABT was indicated for patients with Hb < 80 g/L, hematocrit (HCT) < 20%, or other obvious anemia manifestations.
Outcome measures
The following data were recorded: baseline characteristics including demographic data (age, sex) and general information regarding the health condition (body mass index, American Society of Anesthesiologists physical status score, Charlson score); preoperative Hb, HCT, and creatinine clearance levels; tourniquet time; postoperative Hb and HCT levels over the first 5 days after surgery; postoperative drainage volume; and volume of ABT needed for each patient.
The Gross equation was used to calculate the amount of hidden blood loss (HBL). First, the total blood loss (mL) was calculated as preoperative blood volume × (preoperative HCT level − postoperative HCT level). Preoperative blood volume was calculated by the Nadler method as k1 × H3 + k2 × W+k3, where H is the height (m), W is the weight (kg), and k1, k2, and k3 are specific parameters used as follows: for males, k1 = 0.3669, k2 = 0.03219, and k3 = 0.1833; for females, k1 = 0.3561, k2 = 0.03308, and k3 = 0.1833. Finally, HBL (mL) was obtained as total blood loss + volume of autologous blood transfusion + volume of ABT − dominant blood loss.
Statistical analysis
SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) was used for data analysis. For continuous variables with a normal distribution, results were expressed as mean ± standard deviation, and the t test was used to compare the differences between the TXA group and the control group. Otherwise, results were expressed as median and 25%–75% interquartile range, and comparisons were performed using the Mann–Whitney U test. Categorical variables were expressed as numbers and percentages, and the Chi-square test was applied for comparisons. The results regarding postoperative Hb and HCT evolution were analyzed by repeated measures analysis of variance. Differences were considered statistically significant for two-sided P values < 0.05.
Results
A total of 996 consecutive patients underwent unilateral primary TKA in single institution between June 2013 and December 2015, of whom 99 patients had preoperative anemia (9.9%). Three patients with moderate or severe anemia were excluded on the basis of our exclusion criteria. A total of 96 patients (14 males and 82 females) were included in our study. The average age was 66.73 years (range, 40–85 years). All baseline characteristics including the age, gender, and preoperative anemia status were comparable (P > 0.05) between the control and TXA groups (Table 1).
Table 1.
Baseline characteristics of preoperatively anemic patients undergoing total knee arthroplasty
| TXA group | Control group | P-value | |
|---|---|---|---|
| N = 32 | N = 64 | ||
| Sex, male/female | 7/25 | 12/52 | 0.27 |
| Age, years | 67.4 ± 10.3 | 65.5 ± 7.7 | 0.96 |
| BMI, kg/m2 | 25.5 ± 3.1 | 26.2 ± 3.8 | 0.40 |
| ASA score | 1.9 ± 0.4 | 1.8 ± 0.4 | 0.42 |
| Pre-opCr, μmol/L | 65.4 ± 13.9 | 66.7 ± 9.6 | 0.58 |
| Pre-opHb, g/dL | 11.1 ± 2.6 | 11.5 ± 4.9 | 0.17 |
| Pre-opHCT | 0.338 ± 0.027 | 0.341 ± 0.019 | 0.16 |
| Tourniquet time, min | 84.7 ± 26.3 | 77.7 ± 27.7 | 0.26 |
| Charlson score | 3.00 ± 0.76 | 3.00 ± 1.49 | 1.00 |
Values given as average ± standard deviation, unless otherwise specified
TXA tranexamic acid, BMI body mass index, ASA Society of Anesthesiologists, Pre-opCr preoperative levels of creatinine, Pre-opHb preoperative levels of hemoglobin, Pre-opHCT preoperative levels of hematocrit
In the TXA group, the drainage volume and HBL were 200 (100, 320) mL and 182.6 (106.9, 301.7) mL, respectively, compared with 380 (200, 612.5) mL and 283.3 (146.7, 562.3) mL, respectively, in the control group; the difference was statistically significant. The ABT volume was dramatically lower in the TXA group (88.9 ± 230.9 mL) than in the control group (216.3 ± 293.9 mL) (P < 0.05; Table 2). Moreover, there were only 4 TXA patients who required ABT, compared to 20 in the control group (P < 0.05; Table 3). The Hb levels on the first postoperative day were significantly higher in the TXA group than in the control group (9.91 ± 0.6 g/dL vs 9.40 ± 1.1 g/dL; P = 0.006). However, no significant differences between the two groups were noted regarding Hb and HCT levels over the following 4 postoperative days (P > 0.05; Figs. 1, 2).
Table 2.
Effect of TXA administration on blood loss and need for transfusion in preoperatively anemic patients undergoing total knee arthroplasty
| TXA group | Control group | P-value | |
|---|---|---|---|
| N = 32 | N = 64 | ||
| Drainage, mL | 200 (100, 320) | 380 (200, 612.5) | 0.00 |
| Hidden blood loss, mL | 182.6 (106.9, 301.7) | 283.3 (146.7, 562.3) | 0.04 |
| ABT volume, mL | 88.9 ± 230.9 | 216.3 ± 293.9 | 0.01 |
Values given as median (25% to 75% interquartile range) or average ± standard deviation
TXA tranexamic acid, ABT allogeneic blood transfusion
Table 3.
Chi-square test for the volume of allogeneic blood transfusion and incidence of deep venous thrombosis (DVT) events after total knee arthroplasty in preoperatively anemic patients
| TXA group | Control group | P-value | |
|---|---|---|---|
| N = 32 | N = 64 | ||
| Patients requiring ABT | 4 (12.5%) | 20 (31.3%) | 0.046 |
| Patients with DVT | 3 (9.4%) | 5 (7.8%) | 0.794 |
Data given as number (percentage)
TXA tranexamic acid, ABT allogeneic blood transfusion
Fig. 1.
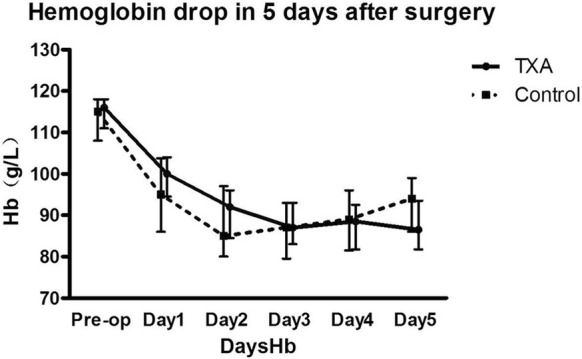
Histogram revealing the hemoglobin (Hb) drop over the first 5 days after total knee arthroplasty. Overall, no statistically significant difference (P = 0.763) was noted between the control group and the group of patients who had received tranexamic acid (TXA), although the TXA group showed significantly higher Hb levels on the first postoperative day (9.91 ± 0.6 g/dL vs 9.40 ± 1.1 g/dL; P = 0.006)
Fig. 2.
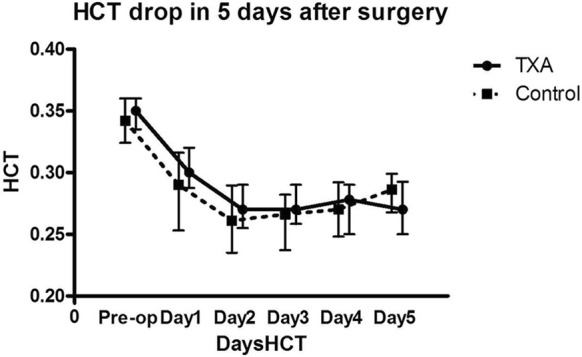
Histogram revealing the hematocrit (HCT) drop over the first 5 days after total knee arthroplasty. No statistically significant difference (P = 0.474) was noted between the control group and the group of patients who had received tranexamic acid (TXA)
The incidence of DVT events was low in both groups (3 patients in the TXA group vs 5 patients the in control group), with no statistically significant difference (P > 0.05; Table 3).
Discussion
The overall prevalence of anemia increases with age, and, according to the WHO report [13], amounts to approximately 24% in patients indicated for primary TKA. Therefore, preoperative anemia should be considered as a serious but treatable condition [14]. It was reported that ABT volume after TKA is much higher among preoperatively anemic patients than among normal patients, as are complications and medical costs [15–17]. However, few studies have investigated the effects of TXA in preoperatively anemic patients undergoing TKA. As patients with moderate or severe anemia are typically not indicated for TKA, the treatment for the primary disease should be considered as a priority; for the same reason, such patients were not included in our study.
As an antifibrinolytic agent, TXA has the ability to block fibrinolysin and save fibrinogen, potentially reducing postoperative blood loss and ABT volume after TKA. In a randomized control trial involving 124 patients with normal preoperative hemoglobin levels, Wong et al. [4] found that intraoperative administration of 1.5 g TXA might reduce drainage volume by one-third, and ABT volume to 12.9%. This conclusion was supported by other recent reports.
Our results suggest a similar effect for preoperatively anemic patients, as the drainage volume was lower by almost 50% in the TXA group. The volume of ABT was also significantly decreased by the application of TXA. The condition of preoperatively anemic patients could be considered as an impaired ability to store blood, which contributes to a decreased capability to cope with rapid blood loss after surgery, increasing the need for ABT. The application of TXA reduced the rate of postoperative blood loss, as suggested by the histogram in Fig. 1, which revealed that the hemoglobin levels dropped faster in the control group over the first 2 days after surgery, although the difference between the groups was statistically significant only for the first postoperative day. Therefore, administration of TXA helped the patients tolerate the postoperative hemoglobin drop, which explains the encouraging results noted in our study.
The histogram suggested higher HCT levels in the TXA group, repeated measures analysis indicated there was no significant difference between the two groups, and the Hb and Hct levels were higher at day 5 in the control group. The authors believe that the higher ABT volume in the control group should be considered as an influence factor in the analysis of the Hb and HCT levels. Therefore, further study should perform a subgroup analysis for the patients who received ABT. Additionally, it should be noted that the intravenous TXA dose of 1 g could typically be eliminated up to 90% via the kidneys within 24 h, with a half-life of 2–3 h in vivo. This might explain why only the drainage and Hb on the first postoperative day had been significantly decreased in TXA group, since the drug effect could not last during the following days.
HBL typically continues for several days after surgery, and might contribute 50% of the total blood loss [18]. In a randomized control trial study reported by Good et al. [19], HBL was reduced by TXA to 524 (330 ± 962) mL. The results obtained in the current study suggest that the improvement caused by TXA could be very significant. Considering of the PK of TXA [20], the HBL might be additionally decreased if more TXA could be administered under a safer protocol with prolonged application. Aiming to achieve this goal, a randomized control trial study is being undertaken by our research team.
Although several DVT events associated with TXA usage have been reported previously, plenty of studies have confirmed the safety of TXA application under the protocol described in the present study. In a meta-analysis of 15 randomized clinical trials, Zhang et al. [21] reported that TXA did not increase the risk of DVT, which is in agreement with many other reports. Indeed, we found that the incidence of DVT events was comparable between the TXA and control group, which suggests that TXA can be used safely to control blood loss in preoperatively anemic patients.
Considering the potential higher risk of thrombosis, nausea and vomiting, we only administrated TXA 1.0 g 15 min before inflation of the tourniquet, and 1.0 g after tourniquet release postoperatively in this study. The safety and risk of the daily and local administration of TXA need further investigation.
Conclusion
We concluded that TXA have a positive role for patients with preoperative anemia in primary total knee arthroplasty. The significance of this study is that it analyzed, for the first time, the efficiency of TXA in TKA patients with mild preoperative anemia, and noted that the application of TXA could decrease the ABT and HBD volume without increasing the DVT risk. In addition, our preliminary results suggest that TXA appears efficient mainly on the first postoperative day. Nonetheless, as the intrinsic feature of a retrospective study, the present investigation may be considered only as a preliminary assessment, and further study is warranted.
Acknowledgements
This study was funded by the Beijing Science and Technology Program (D171100003217001).
Authors’ contributions
HT: Conceived and designed the research. MWZ and XG: Collected and analyzed the data and wrote the manuscript. CW: Reviewed the research program and coordinated work. LZ: Responsible for statistical work. All authors read and approved the final manuscript.
Funding
This study was funded by the Beijing Science and Technology Program (D171100003217001).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
The Peking University Third Hospital Medical Science Research Ethics Committee approved the study before initiation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Minwei Zhao and Xiao Geng contributed equally to the work
References
- 1.Paredes-Carnero X, Escobar J, Galdo JM, Babe JG. Total knee arthroplasty for treatment of osteoarthritis associated with extra-articular deformity. J Clin Orthop Trauma. 2018;9(2):125–132. doi: 10.1016/j.jcot.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bindawas SM. Total knee arthroplasty status and patient-reported, knee-related quality of life over a 4-year follow-up period: data from the osteoarthritis initiative. Patient Prefer Adher. 2018;12:477–482. doi: 10.2147/PPA.S155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt J, Lange T, Gunther KP, Kopkow C, Rataj E, Apfelbacher C, et al. Indication criteria for total knee arthroplasty in patients with osteoarthritis—a multi-perspective consensus study. Zeitschrift fur Orthopadie und Unfallchirurgie. 2017;155(5):539–548. doi: 10.1055/s-0043-115120. [DOI] [PubMed] [Google Scholar]
- 4.Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am Vol. 2010;92(15):2503–2513. doi: 10.2106/JBJS.I.01518. [DOI] [PubMed] [Google Scholar]
- 5.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113(2):482–495. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 6.Viola J, Gomez MM, Restrepo C, Maltenfort MG, Parvizi J. Preoperative anemia increases postoperative complications and mortality following total joint arthroplasty. The Journal of arthroplasty. 2015;30(5):846–848. doi: 10.1016/j.arth.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 7.Jans O, Jorgensen C, Kehlet H, Johansson PI, Lundbeck Foundation Centre for Fast-track H, Knee Replacement Collaborative G Role of preoperative anemia for risk of transfusion and postoperative morbidity in fast-track hip and knee arthroplasty. Transfusion. 2014;54(3):717–726. doi: 10.1111/trf.12332. [DOI] [PubMed] [Google Scholar]
- 8.Baron DM, Hochrieser H, Posch M, Metnitz B, Rhodes A, Moreno RP, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth. 2014;113(3):416–423. doi: 10.1093/bja/aeu098. [DOI] [PubMed] [Google Scholar]
- 9.Meng Y, Li Z, Gong K, An X, Dong J, Tang P. Tranexamic acid reduces intraoperative occult blood loss and tourniquet time in obese knee osteoarthritis patients undergoing total knee arthroplasty: a prospective cohort study. Ther Clin Risk Manag. 2018;14:675–683. doi: 10.2147/TCRM.S160156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed S, Ahmed A, Ahmad S, Atiquz Z, Javed S, Aziz A. Blood loss after intraarticular and intravenous tranexamic acid in total knee arthroplasty. JPMA J Pak Med Assoc. 2018;68(10):1434–1437. [PubMed] [Google Scholar]
- 11.Cao G, Xie J, Huang Z, Huang Q, Chen G, Lei Y, et al. Efficacy and safety of multiple boluses of oral versus intravenous tranexamic acid at reducing blood loss after primary total knee arthroplasty without a tourniquet: a prospective randomized clinical trial. Thromb Res. 2018;171:68–73. doi: 10.1016/j.thromres.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 12.Phan DL, Rinehart JB, Schwarzkopf R. Can tranexamic acid change preoperative anemia management during total joint arthroplasty? World J Orthop. 2015;6(7):521–527. doi: 10.5312/wjo.v6.i7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nutritional anaemias. Report of a WHO scientific group. World Health Organization technical report series. 1968;405:5-37. [PubMed]
- 14.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116(23):4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 15.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104(8):2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 16.Myers E, O’Grady P, Dolan AM. The influence of preclinical anaemia on outcome following total hip replacement. Arch Orthop Trauma Surg. 2004;124(10):699–701. doi: 10.1007/s00402-004-0754-6. [DOI] [PubMed] [Google Scholar]
- 17.Dunne JR, Malone D, Tracy JK, Gannon C, Napolitano LM. Perioperative anemia: an independent risk factor for infection, mortality, and resource utilization in surgery. J Surg Res. 2002;102(2):237–244. doi: 10.1006/jsre.2001.6330. [DOI] [PubMed] [Google Scholar]
- 18.Saleh E, McClelland DB, Hay A, Semple D, Walsh TS. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth. 2007;99(6):801–808. doi: 10.1093/bja/aem299. [DOI] [PubMed] [Google Scholar]
- 19.Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. 2003;90(5):596–599. doi: 10.1093/bja/aeg111. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson O, Kjellman H, Pilbrant A, Schannong M. Pharmacokinetics of tranexamic acid after intravenous administration to normal volunteers. Eur J Clin Pharmacol. 1974;7(5):375–380. doi: 10.1007/BF00558210. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1742–1752. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


