Significance
G protein-coupled receptor (GPCR) kinases (GRKs) are responsible for negatively regulating GPCRs. During periods of cardiac stress, the binding of Ca2+ to calmodulin (CaM) promotes nuclear accumulation of GRK5 and maladaptive enlargement of the heart. Here, we use structural analysis and malbrancheamide, a natural product that we show binds to the C-terminal domain of CaM, to reveal the architecture of the CaM–GRK5 complex and the distinct regulatory roles played by each of the domains of CaM in cardiac hypertrophy and GPCR desensitization mediated by GRK5.
Keywords: G protein-coupled receptor kinase 5, calmodulin, malbrancheamide, hypertrophy
Abstract
G protein-coupled receptor (GPCR) kinases (GRKs) are responsible for initiating desensitization of activated GPCRs. GRK5 is potently inhibited by the calcium-sensing protein calmodulin (CaM), which leads to nuclear translocation of GRK5 and promotion of cardiac hypertrophy. Herein, we report the architecture of the Ca2+·CaM–GRK5 complex determined by small-angle X-ray scattering and negative-stain electron microscopy. Ca2+·CaM binds primarily to the small lobe of the kinase domain of GRK5 near elements critical for receptor interaction and membrane association, thereby inhibiting receptor phosphorylation while activating the kinase for phosphorylation of soluble substrates. To define the role of each lobe of Ca2+·CaM, we utilized the natural product malbrancheamide as a chemical probe to show that the C-terminal lobe of Ca2+·CaM regulates membrane binding while the N-terminal lobe regulates receptor phosphorylation and kinase domain activation. In cells, malbrancheamide attenuated GRK5 nuclear translocation and effectively blocked the hypertrophic response, demonstrating the utility of this natural product and its derivatives in probing Ca2+·CaM-dependent hypertrophy.
G protein-coupled receptors (GPCR) are the largest family of cell-surface receptors in humans and regulate numerous physiological processes through the activation of heterotrimeric G proteins, GPCR kinases (GRKs), and arrestins. Mechanisms to attenuate or cease downstream signaling have evolved to maintain proper physiology. The principal desensitization mechanism is initiated by GRKs, which phosphorylate the third intracellular loops or C-terminal tails of active GPCRs. This process facilitates the recruitment of arrestin which sterically occludes G protein binding and promotes receptor internalization (1, 2).
As membrane localization and recognition of activated receptors is critical for efficient receptor desensitization, several regulatory mechanisms have evolved to attenuate GRK activity and prolong receptor activation. The ubiquitous Ca2+-binding protein calmodulin (CaM) binds six of the seven GRKs and inhibits both membrane localization and receptor phosphorylation in a Ca2+-dependent manner (3–5). The interaction between Ca2+·CaM and GRK5 has been the most studied due to its high affinity (Kd = 8 nM by surface plasmon resonance) and 50-fold greater potency (IC50 = 40 to 60 nM toward rhodopsin phosphorylation) compared with other GRKs (3, 4). Coimmunoprecipitation identified N- and C-terminal helical regions of GRK5 (residues 1 to 17 and 552 to 561, respectively) and a stretch of basic residues (residues 20 to 39) near the junction between the kinase and regulator of G protein-signaling homology (RH) domains as regions involved in Ca2+·CaM binding (5, 6).
GRK5 inhibition by Ca2+·CaM is particularly significant because it plays a role in the development of maladaptive cardiac hypertrophy, which affects approximately one third of hypertensive individuals and the majority of individuals following a heart attack (7). After dissociating from membranes, the Ca2+·CaM–GRK5 complex translocates to the nucleus where GRK5 phosphorylates histone deacetylase 5 (HDAC5), leading to decreased nuclear levels of HDAC5 and derepression of genes controlled by the transcription factors myocyte enhancer factor-2 (8) and nuclear factor of activated T cells (9, 10). Therefore, blocking the translocation of Ca2+·CaM–GRK5 to the nucleus represents a potential therapeutic strategy for ameliorating heart failure (11).
Conflicting models for the Ca2+·CaM–GRK5 complex have been proposed. One depicts two separate Ca2+·CaM subunits binding to the distal N- and C-terminal elements of GRK5 in a fashion that would interfere with its membrane binding and receptor phosphorylation (5). Alternatively, recent structures of GRK5 have revealed the N- and C-terminal elements to be in close proximity to one another, thus making it possible for a single Ca2+·CaM to bind both simultaneously (12–14). To better understand the molecular mechanism of inhibition of GRK5 by Ca2+·CaM, we used multiple biophysical methods to determine the architecture of the Ca2+·CaM–GRK5 complex. Our data are consistent with a single Ca2+·CaM molecule binding to multiple elements of the kinase critical for receptor recognition, allosteric activation by phospholipids, and membrane association. We confirm that the binding of Ca2+·CaM promotes an active conformation of GRK5 resulting in increased phosphorylation of nonreceptor substrates. The Ca2+·CaM–GRK5 interaction was probed with malbrancheamide, a natural product with known activity as a Ca2+·CaM inhibitor (15–17). Cocrystallization of malbrancheamide in complex with Ca2+·CaM confirmed that the natural product binds to the C-terminal lobe, thus leaving the N-terminal lobe free to interact with other proteins. Malbrancheamide selectively inhibited GRK5 autophosphorylation without affecting substrate phosphorylation and prevented nuclear translocation of GRK5, thereby inhibiting the hypertrophic response in cardiac cells. This method of inhibition allows malbrancheamide to suppress maladaptive Ca2+·CaM-stimulated GRK5 membrane dissociation without relieving the benefit of Ca2+·CaM-mediated suppression of receptor phosphorylation.
Results
A Single Ca2+·CaM Binds the RH–Kinase Domain Junction of GRK5.
We first determined the stoichiometry of the Ca2+·CaM–GRK5 complex via analytical size exclusion chromatography (SEC) coupled to a multiangle light scattering (MALS) detector. MALS analysis of the peak indicated a molecular weight for the complex of ∼90 kDa, indicating a 1:1 stoichiometry (Fig. 1 A and B). To determine the arrangement of Ca2+·CaM and GRK5 in complex, we performed SEC–small-angle X-ray scattering (SEC–SAXS) at multiple concentrations (SI Appendix, Fig. S1 and Table S1). The higher-concentration samples produced better signal and were used for modeling (SI Appendix, Fig. S1 and Table S2). Both samples displayed a plateau in their Porod–Debye plots at a lower qmax than their respective Kratky–Debye plots and a normal distribution in their Kratky plots, indicating that the particles do not display a high degree of flexibility (SI Appendix, Fig. S2) (18). The Porod exponents of GRK5 and the Ca2+·CaM–GRK5 complex were 3.2 and 3.8, respectively, which indicates that the complex is a more compact particle than GRK5 alone.
Fig. 1.
Biophysical characterization of the Ca2+·CaM–GRK5 complex. (A) Representative chromatograms of GRK5 ± Ca2+·CaM (3-fold molar excess) passed over an S200 size exclusion chromatography column. A shift toward a lower elution volume was observed in the presence of Ca2+·CaM. MALS determined the molecular weight of the complex to be 90 kDa (solid line inside the peak), the approximate weight of a 1:1 complex (n = 2). The molecular weights of His-tagged GRK5 and CaM are 70.7 and 19.6 kDa, respectively. (B) SDS/PAGE analysis of the chromatogram peak corresponding to the Ca2+·CaM–GRK5 complex. Both proteins coelute, consistent with complex formation. (C) Reconstructed density at 3 σ calculated via DENSS from SEC–SAXS scattering curves of the complex at 10 mg mL−1. GRK5 (PDB entry 4WNK) with terminal regions from GRK6 (PDB entry 3NYN) and Ca2+·CaM (orange cartoon, PDB entry 5J03) autodocked into the envelope. The regulator of G protein-signaling homology (RH) domain (colored beige), kinase domain (KD; colored green), active-site tether (AST; colored purple), basic phospholipid-binding patch (side-chains shown as spheres), and terminal helices (αN and αC; mauve and purple, respectively) are labeled. The model fits to the RAW SAXS data well (χ2 = 1.07) as assessed by FoXS. (D) Comparison of selected negative-stain class averages of GRK5 alone and the Ca2+·CaM–GRK5 complex. Arrows indicate density attributed to Ca2+·CaM.
Unbiased density for GRK5 alone and the Ca2+·CaM–GRK5 complex were reconstructed ab initio using the DENSS algorithm (SI Appendix, Fig. S3) (19). The crystal structures of Ca2+·CaM (PDB 5J03) (20) and GRK5 (PDB 4WNK) (14) with a modeled N-terminal helix (αN) and active site tether (AST) loop from GRK6 (PDB 3NYN) (21) were autodocked into the density (Fig. 1C), and the resulting model agrees with the RAW data (χ2 = 1.07 by FoXS) (22). The C-terminal binding site (αC) for Ca2+·CaM in GRK5, although disordered in prior crystal structures, would be in close proximity to the docked molecule of Ca2+·CaM, given the trajectory of the preceding residues in the GRK5 structure, and has been modeled as such. Compared with the density for GRK5 alone (SI Appendix, Fig. S3), extra density was observed upon the addition of Ca2+·CaM at the junction of the regulator of G protein-signaling homology (RH) and kinase domains (KD) of GRK5. This area is near the N terminus and the basic phospholipid-binding patch, which are known sites of Ca2+·CaM binding (23). The positioning of Ca2+·CaM in this configuration does not obscure the substrate or ATP-binding pockets, which is consistent with the observation that GRK5 retains catalytic activity toward soluble substrates and autophosphorylation when in complex with Ca2+·CaM (3). We next characterized GRK5 and the Ca2+·CaM–GRK5 complex by negative-stain electron microscopy and generated reference-free 2D class averages (SI Appendix, Fig. S4). Particles of the Ca2+·CaM–GRK5 complex revealed additional protein density relative to GRK5 alone in a similar position to that indicated in SAXS experiments along a surface spanning the RH domain and N lobe of the kinase domain (Fig. 1D).
Ca2+·CaM Activates GRK5 and Promotes Autophosphorylation.
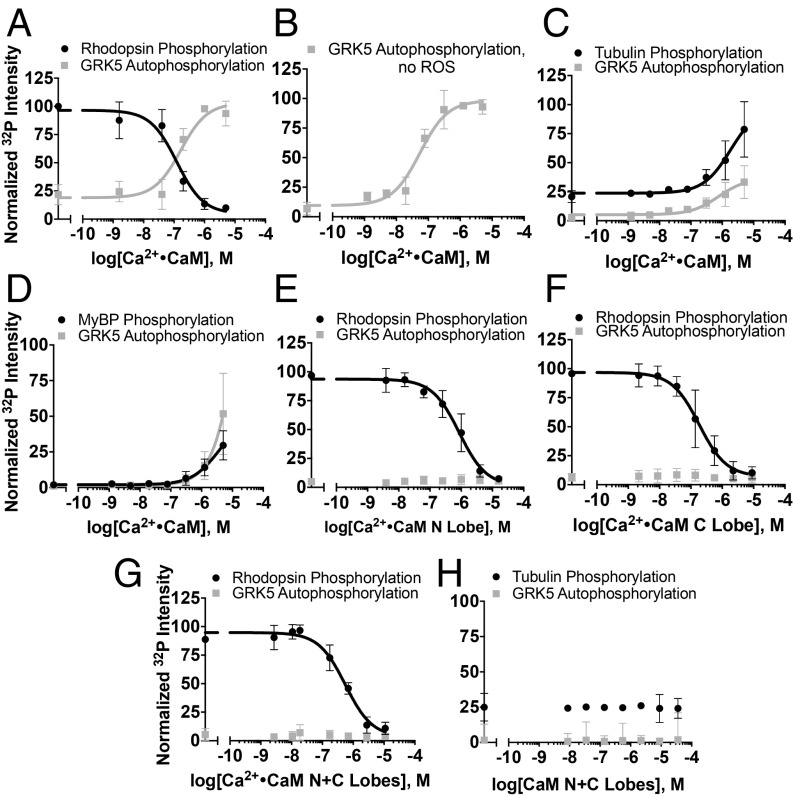
As previously reported, Ca2+·CaM inhibits GRK5-mediated receptor phosphorylation while simultaneously promoting autophosphorylation (Fig. 2A) and enhances phosphorylation of synuclein (4, 24). Our structural analyses are consistent with inhibition of receptor phosphorylation due to Ca2+·CaM interacting with regions of GRK5 critical for receptor interaction and/or membrane association. However, autophosphorylation is not simply a consequence of membrane dissociation because Ca2+·CaM stimulates autophosphorylation in the absence of membranes or other substrates (Fig. 2B). Although Ca2+·CaM inhibits receptor phosphorylation, GRK5 activity toward soluble substrates is enhanced, suggesting that Ca2+·CaM binding stabilizes a more active kinase conformation (Fig. 2 C and D). Soluble substrates attenuate autophosphorylation, likely due to competition between the soluble substrate and the C terminus of GRK5. Ca2+·CaM is even able to promote phosphorylation of myelin basic protein (MyBP), which is not phosphorylated by GRK5 in the absence of Ca2+·CaM (Fig. 2D), further demonstrating the activating effect of Ca2+·CaM. However, because Ca2+·CaM is also known to bind MyBP, we cannot exclude the possibility that Ca2+·CaM-stimulated phosphorylation of MyBP is due to the formation of a more phosphorylation-prone Ca2+·CaM–MyBP complex (25).
Fig. 2.
Modulation of substrate phosphorylation by Ca2+·CaM. (A) Rhodopsin in rod outer segments (ROS, 5 µM, light-activated) phosphorylation (IC50 = 120 ± 40 nM) and concurrent GRK5 (50 nM) autophosphorylation (EC50 = 170 ± 70 nM) in the presence of Ca2+·CaM. (B) Stimulation of GRK5 (50 nM) autophosphorylation by Ca2+·CaM (EC50 = 50 ± 20 nM) in the absence of ROS or other substrates. (C) Tubulin (10 µM) phosphorylation in the presence of Ca2+·CaM. Decreased potency of concurrent autophosphorylation is likely due to competition between the C terminus of GRK5 and tubulin. (D) Phosphorylation of myelin basic protein (MyBP, 7 µM) presence of Ca2+·CaM and concurrent autophosphorylation. Inhibition of rhodopsin phosphorylation by the (E) Ca2+·CaM N lobe (IC50 = 860 ± 230 nM), (F) C lobe (IC50 = 180 ± 60 nM), or (G) a 1:1 molar combination of N and C lobes (IC50 = 600 ± 170 nM). (H) Phosphorylation of tubulin is unaffected by the addition of both lobes. All radiometric kinase assays were performed three times and reported as mean ± SD and fit to a sigmoidal dose–response model in GraphPad Prism with the Hill slope constrained to 1.
To dissect the roles of the individual domains of Ca2+·CaM, we studied the effects of its independent N- and C-terminal lobes (residues 1 to 75 and 78 to 149, respectively), which each contain a pair of Ca2+-binding EF hand motifs. Both less potently inhibited receptor phosphorylation than full-length Ca2+·CaM, consistent with the fact that binding of even a single lobe of Ca2+·CaM along the putative membrane interacting surface would interfere with membrane association or receptor binding and diminish receptor phosphorylation (Fig. 2 E and F). Addition of both independent lobes of Ca2+·CaM was not sufficient to stimulate autophosphorylation (Fig. 2G) or increase GRK5 activity toward soluble substrates (Fig. 2H), either due to loss of avidity when the lobes are not covalently attached or, more interestingly, if the binding of intact Ca2+·CaM binding orients the C terminus of GRK5 in a more optimal way for autophosphorylation.
Malbrancheamide Binds the C Lobe of Calmodulin and Inhibits Ca2+·CaM-Stimulated GRK5 Autophosphorylation.
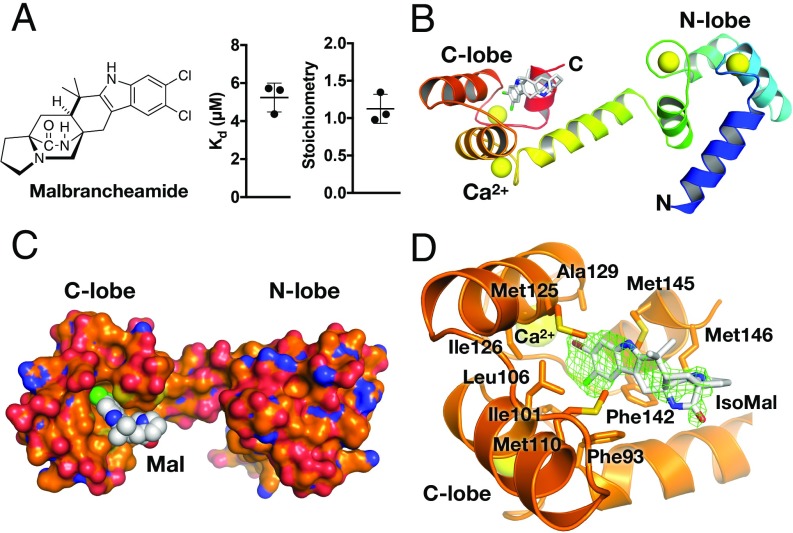
To dissect how each lobe of CaM is involved in binding the N- and C-terminal binding sites of GRK5, we turned to malbrancheamide, a halogenated fungal indole alkaloid that antagonizes Ca2+·CaM (Fig. 3A) (16) and that has been predicted to bind to the C-terminal lobe (17). We first used isothermal titration calorimetry (ITC) to confirm the binding affinity and stoichiometry of malbrancheamide and the brominated analog isomalbrancheamide D (Fig. 3A and SI Appendix, Figs. S5 and S6) (26) for Ca2+·CaM. Poor solubility hampered precise measurement of the affinities (Kd), but malbrancheamide and isomalbrancheamide D bound with affinities of 5 ± 1 and 2 ± 1 µM, respectively, consistent with prior reports (16), and bound Ca2+·CaM with a 1:1 stoichiometry (Fig. 3A and SI Appendix, Figs. S5 and S6).
Fig. 3.
Malbrancheamide binds to the C lobe of Ca2+·CaM. (A) Chemical structure of malbrancheamide and ITC-binding results (average ± SD, n = 3). (B) Rainbow ribbon representation of the Ca2+·CaM·malbrancheamide structure in a trans conformation. Ca2+ are shown as yellow spheres (PDB entry 6EEB). (C) Surface representation of Ca2+·CaM (orange corresponding to carbon, blue nitrogen, and red oxygen) with malbrancheamide shown as spheres with white carbon atoms, green chlorine. (D) C-lobe hydrophobic pocket with isomalbrancheamide D (IsoMal) bound (bromine colored brown). Green cage represents simulated annealing |Fo| − |Fc| omit density contoured at 2.5 σ. Side-chains making van der Waals contacts with IsoMal are labeled and are similar to those contacting malbrancheamide (SI Appendix, Fig. S7). Color scheme is the same as in C.
We next determined crystal structures of Ca2+·CaM in complex with malbrancheamide and isomalbrancheamide D. In each complex, the ligand is observed bound with its halogenated indole buried in the hydrophobic pocket of the C lobe (Fig. 3 B–D and SI Appendix, Figs. S6 and S7 and Table S3). The halogenated indole is positioned between Leu106, Met125, and Met145, and the C-8 chlorine forms a Cl–π interaction with Phe93. Malbrancheamide buries ∼250 Å2 of surface area in the hydrophobic pocket (PISA server) (27), consistent with ligands of similar affinity (28). Isomalbrancheamide D is positioned ∼0.8 Å deeper in the Ca2+·CaM C-lobe hydrophobic pocket, which may explain its slightly higher affinity (SI Appendix, Table S3). The N-terminal lobe of Ca2+·CaM, however, is free to interact with other proteins (Fig. 3B). Due to limited quantities of isomalbrancheamide D, only malbrancheamide was examined for its ability to modulate the Ca2+·CaM–GRK5 interaction. Addition of malbrancheamide to phosphorylation reactions containing Ca2+·CaM–GRK5 and rhodopsin inhibited GRK5 autophosphorylation, but did not rescue receptor phosphorylation (Fig. 4A and SI Appendix, Fig. S8), consistent with Ca2+·CaM being simultaneously bound to malbrancheamide and GRK5. Malbrancheamide had no effect on phosphorylation of the soluble substrate tubulin (Fig. 4B).
Fig. 4.
Effects of malbrancheamide and GRK5 peptides on Ca2+·CaM modulation of GRK5 activity. (A) Rhodopsin (5 µM, light-activated) phosphorylation in the presence of 500 nM Ca2+·CaM and increasing concentrations of malbrancheamide. In these assays, Ca2+·CaM was preincubated with malbrancheamide before the addition of GRK5. (B) Tubulin (5 µM) phosphorylation in the presence of 500 nM Ca2+·CaM and varying concentrations of malbrancheamide. (C) Rhodopsin phosphorylation in the presence of 500 nM Ca2+·CaM and varying concentrations of GRK5 αN (residues 2 to 31, IC50,autophosphorylation = 540 ± 240 nM, EC50,rhodopsin = 5.6 ± 1.6 µM) or (D) αC (residues 546 to 565, IC50 = 1.1 ± 0.7 µM) peptides. (E) Tubulin phosphorylation in the presence of 500 nM Ca2+·CaM and varying concentrations of GRK5 αN (IC50 = 2.4 ± 1.7 µM) or (F) αC peptides (IC50 = 500 ± 440 nM). All assays were performed three times and reported as mean ± SD and fit to a sigmoidal dose–response model in GraphPad Prism with the Hill slope constrained to 1.
N Lobe of Ca2+·CaM Interacts with the N Terminus of GRK5 to Inhibit Receptor Phosphorylation.
To decipher which lobe of CaM is binding to the CaM-binding elements of GRK5, peptides spanning the known Ca2+·CaM binding sites on the termini of GRK5, αN (residues 2 to 31, Kd = 250 nM) (SI Appendix, Fig. S9) and αC (residues 546 to 565, Kd = 670 nM), were evaluated for their ability to disrupt regulation of GRK5 by Ca2+·CaM. In the presence of rhodopsin, both peptides inhibited autophosphorylation (Fig. 4 C and D). However, whereas the addition of the αN peptide rescued receptor phosphorylation, addition of the αC peptide did not, mimicking the effect observed upon addition of malbrancheamide (Fig. 4D). Thus, the interaction between the C terminus of GRK5 and the C lobe of Ca2+·CaM is obstructed by the binding of malbrancheamide. Neither peptide affected soluble substrate phosphorylation (Fig. 4 E and F), although similar inhibition of autophosphorylation was observed in each case. These results indicate that the C lobe of Ca2+·CaM not only binds to the C terminus of GRK5, but is also involved in dictating the autophosphorylation of the GRK5 C terminus.
Malbrancheamide Inhibits Nuclear Translocation of GRK5 and Prevents Hypertrophy.
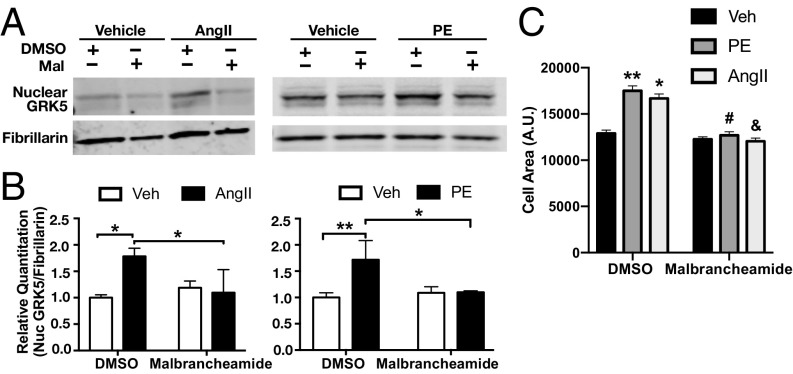
We hypothesized that malbrancheamide binding to the C lobe of Ca2+·CaM would allow the C terminus of GRK5 to bind the plasma membrane (Fig. 1C) and reduce its nuclear translocation (11). To test this, we first cultured primary neonatal rat cardiac fibroblasts and pretreated cells with 1 µM malbrancheamide or DMSO as a control. This was followed by induction of Ca2+·CaM–GRK5-dependent nuclear translocation and accumulation with the GPCR-dependent hypertrophic agonists angiotensin II (AngII) or phenylephrine (PE) (29). Malbrancheamide treatment prevented nuclear accumulation of GRK5 after addition of agonist compared with nonstimulated vehicle-treated controls, as determined by quantitative Western blotting of purified nuclear fractions of cardiac fibroblasts (Fig. 5 A and B).
Fig. 5.
Effects of malbrancheamide on GRK5 nuclear translocation and cardiomyocyte hypertrophy. (A) Representative Western blots of nuclear GRK5 following pretreatment with malbrancheamide (Mal) and stimulation with Ca2+·CaM and hypertrophic agonist angiotensin II (AngII) and phenylephrine (PE). Rat neonatal cardiac fibroblasts were pretreated with 1 μM malbrancheamide (or DMSO as control) before treatments with 5 μM AngII or 100 μM PE (or vehicle as control) for 90 min. Nuclear extracts were then prepared and GRK5 was blotted with fibrillarin as a nuclear marker and protein-loading control. (B) Quantitation of Western blot results showing significant nuclear localization of GRK5 after AngII (compared with vehicle), which was completely blocked by pretreatment with malbrancheamide (average ± SD, n = 3 biological replicates, two-way ANOVA with Tukey’s post hoc correction, *P < 0.05, **P < 0.01). (C) Quantitation of myocyte cell area was performed using F-actin stained cells and Image-J software. Three independent experiments were carried out, and 50 to 100 cells per experiment were measured for each included condition. Shown is the average ± SD (*P < 0.05 vs. DMSO vehicle, **P < 0.01 vs. DMSO vehicle, #P < 0.001 vs. DMSO PE, &P < 0.001 vs. DMSO AngII, two-way ANOVA with Tukey’s post hoc correction).
In a second set of experiments to confirm malbrancheamide inhibition of GRK5 nuclear accumulation, we examined cardiomyocyte hypertrophy in response to the same agonists (29). Pretreatment of a cardiomyocyte cell line with malbrancheamide or DMSO as a control was followed with 48 h of PE, AngII, or vehicle treatment, and myocyte hypertrophy was determined by F-actin staining and imaging quantitation of cell area (Fig. 5C and SI Appendix, Fig. S10). Malbrancheamide blocked cell hypertrophy induced by either PE or AngII. These data are consistent with a mechanism in which malbrancheamide releases the sequestered C terminus of GRK5 from CaM and thereby promotes retention of GRK5 at the plasma membrane in a state that is unable to phosphorylate GPCRs or translocate to the nucleus.
Discussion
The role of GRK5 in cardiac hypertrophy has stimulated interest in both therapeutic development targeting the kinase and the underlying molecular mechanisms by which this hypertrophy occurs (11). Recent studies have shed light on the role of GRK5 regulation by Ca2+·CaM during GRK5-mediated cardiac hypertrophy (8, 9, 11, 29), a noncanonical activity for the enzyme, but the structural basis of their interaction has not been reported. Here, we present structural and functional data that reveal a single Ca2+·CaM binds to GRK5 in a manner that interferes with both receptor recognition and membrane association while activating the kinase for phosphorylation of soluble substrates that contribute to maladaptive hypertrophy.
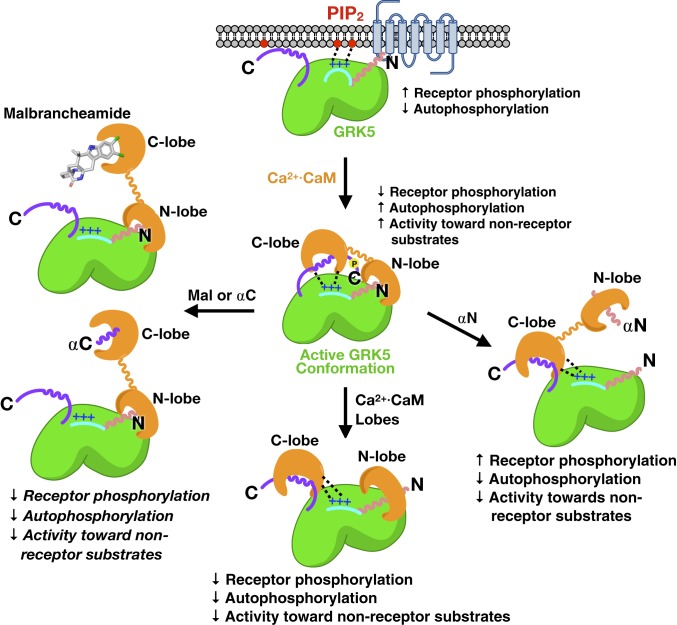
Substrates of Ca2+·CaM often contain amphipathic helices with bulky hydrophobic side-chains which anchor the hydrophobic pockets of each lobe of Ca2+·CaM (30, 31). The terminal helices of GRK5 resemble canonical Ca2+·CaM substrates, and thus our model suggests that terminal hydrophobic residues of the αN and αC helices are likely involved in binding to Ca2+·CaM with additional contribution from electrostatic interactions with the acidic helices of Ca2+·CaM with the N-terminal basic patch (Fig. 6). The αN helix has been proposed in some models to dock into the hydrophobic core of activated GPCRs and is well known to be critical for receptor recognition and phosphorylation (32, 33). It is also thought in activated receptor complexes to interact with the kinase domain and stabilize a closed, active conformation (21). Our results suggest that Ca2+·CaM binds to the small lobe of GRK5 in a manner that sterically hinders GRK5 docking with cognate receptors. A C-terminal amphipathic helix in GRK5 significantly contributes to membrane association (34, 35), and our data further suggest that the GRK5 αC helix is close enough to be sequestered by Ca2+·CaM bound via its other lobe to the GRK5 N terminus, thereby also blocking membrane association, which is important for efficient GRK5-mediated phosphorylation of active GPCRs (36).
Fig. 6.
Model for lobe-specific regulation of GRK5 by Ca2+·CaM. Features critical for receptor docking (αN helix shown as pink wavy line) and membrane association (purple C terminus of GRK5 and the N-terminal basic patch, shown as a blue line with + signs) are sequestered by Ca2+·CaM but also stabilize an active conformation of the kinase. Both lobes in the intact protein are required for this activation because simultaneous addition of the individual lobes does not promote an activated state of GRK5. The addition of αN peptide, αC peptide, or malbrancheamide are all able to relieve various aspects of regulation through differential binding to the two lobes of Ca2+·CaM.
Although Ca2+·CaM potently inhibits receptor phosphorylation, the activity of GRK5 toward soluble substrates is enhanced by three- to fourfold. It was previously reported that Ca2+·CaM stimulates phosphorylation of synuclein (24), and this boost in activity clearly extends to other soluble substrates, suggesting that the binding of Ca2+·CaM stabilizes an activated conformation of GRK5. Based on the crystal structure of closely related GRK6 in complex with the nucleoside inhibitor sangivamycin (21), the activated state of GRK5 is believed to be stabilized by a network of contacts between the αN helix and the two lobes of the active kinase domain. Because our data provide evidence that Ca2+·CaM binds near these elements, stabilization of the αN interaction network likely explains the increase in GRK5 activity toward its soluble substrates. Addition of the isolated lobes of Ca2+·CaM, however, did not activate kinase activity at any concentration tested, indicating that full-length Ca2+·CaM is required for stabilizing this active conformation, perhaps through improved affinity.
Ca2+·CaM-stimulated autophosphorylation of GRK5 has been mapped to noncanonical residues in the extreme C terminus (residues 579 to 590) that, similarly to phosphorylation of this region by protein kinase C (37), further reduces the ability of GRK5 to associate with membranes and thereby could represent a mechanism to prolong both inhibition of receptor phosphorylation and nuclear targeting after Ca2+·CaM has dissociated (38). Cleavage of the helix connecting the two lobes of Ca2+·CaM abolished Ca2+·CaM-stimulated autophosphorylation, suggesting that intact Ca2+·CaM positions the terminal elements of GRK5 in a specific conformation that may enable autophosphorylation in cis. Although this is the simplest model, further experiments are needed to exclude the possibility of trans-autophosphorylation or a combination of the two. A likely mechanism for this “repositioning” may simply be loss of membrane interactions of the C-terminal region of GRK5 via the binding of Ca2+·CaM, which would free this disordered region to instead enter the active site of the kinase domain. However, in the absence of membranes and Ca2+·CaM, GRK5 still does not display robust autophosphorylation (Fig. 2B). Thus, it is more likely that Ca2+·CaM both redirects the path of the C terminus into the active site while stabilizing an activated conformation of GRK5, leading to enhanced autophosphorylation.
Determination of the roles of the individual lobes of Ca2+·CaM was aided by malbrancheamide, a natural product that we show binds solely to the C lobe. This implies that addition of the compound would block the interactions of GRK5 with the C lobe. Indeed, the addition of malbrancheamide or the αC peptide inhibited kinase autophosphorylation, but did not relieve the inhibition of receptor phosphorylation mediated by Ca2+·CaM. This is consistent with the C lobe of Ca2+·CaM interacting with the C terminus of GRK5, which dissociates the kinase from membranes, whereas the N lobe interacts with the αN helix where it competitively interferes with receptor binding and receptor phosphorylation. It has been previously noted that rhodopsin phosphorylation is similarly inhibited by an antibody that recognizes epitopes in the N terminus of GRK1 without affecting soluble substrate phosphorylation (39). Thus, Ca2+·CaM acts as a GRK5 agonist and a mimic of an activated GPCR.
Extraction of GRK5 from the plasma membrane by Ca2+·CaM is thought to allow the nuclear localization sequence located in the large lobe of the kinase to promote nuclear translocation of GRK5 and pathological gene activation (34, 40). Malbrancheamide has a defined binding mode that only blocks C-lobe interactions. We show that low µM concentrations of malbrancheamide block nuclear translocation of GRK5 induced by AngII and PE, known hypertrophic agonists that are potent stimulators of nuclear GRK5 accumulation (29). Further, malbrancheamide was also shown to prevent cardiomyocyte hypertrophy induced by AngII and PE, which GRK5 has previously been shown to potentiate (29). Thus, malbrancheamide is an effective chemical probe for studying Ca2+·CaM–GRK5-dependent hypertrophic signaling in cells and may facilitate discovery of the specific roles of each lobe of CaM in other complexes. Ca2+·CaM is known to play a role in cardiomyocyte hypertrophy induced by other pathways; thus, the specific mechanisms by which malbrancheamide prevents AngII- or PE-induced cellular hypertrophy, either GRK5-dependent or otherwise, require further investigation. For example, previous work has demonstrated the biological activity of malbrancheamide as an inhibitor of Ca2+·CaM–phosphodiesterase 1 and Ca2+·CaM–myosin light-chain kinase (16, 17), inducing vasorelaxation through cGMP/NO-dependent pathways.
The ability of Ca2+·CaM to extract GRK5 from the plasma membrane and activate the kinase against soluble targets highlights the important role Ca2+ plays in the regulation of signaling cascades. Allosteric activation of GRKs by Ca2+·CaM thus represents an understudied area of physiology. For example, modulation of Ca2+ channels by Gβγ subunits in response to GPCR activation is well understood, particularly in the context of neurotransmission (42), but the role of Ca2+·CaM-mediated activation of GRKs in feedback mechanisms downstream of such channels has not been studied. Additionally, activation of phospholipase C β and release of Ca2+ induced by Gαq-coupled receptors will ultimately lead to formation of Ca2+·CaM, which will prolong GPCR signaling by globally inhibiting GRKs, in particular GRK5. Thus, future studies may reveal novel crosstalk between GPCRs, GRKs, and ion channels and implicate Ca2+·CaM–GRK interactions in the development of new pathologies.
Materials and Methods
Detailed methods and associated references for experiments described herein are available in the SI Appendix. The Ca2+·CaM·malbrancheamide and isomalbrancheamide D crystal structures and associated diffraction data have been deposited in the Protein Data Bank with the accession codes 6EEB and 6O5G, respectively.
Supplementary Material
Acknowledgments
We thank Elisabeth Garland-Kuntz and Monita Sieng (Purdue University) for assistance with collecting SAXS data, Srinivas Chakravarthy and Jesse Hopkins (BioCAT, Sector 18, Advanced Photon Source) for assistance with SAXS data analysis and interpretation, Stephanie Gates and Adam Yokom (Life Sciences Institute) for assistance with SEC–MALS experiments, Priya Chinnaswamy (Life Sciences Institute) for ITC assistance, and M. Claire Cato and Renee Bouley (Life Sciences Institute) for the GRK5 baculovirus. This study was supported by National Institutes of Health (NIH) Pharmacological Sciences Training Program fellowship (T32-GM007767) and US Department of Education GAANN fellowship (P200A150164) (to T.S.B.), a Rackham Predoctoral Fellowship (to A.E.F.), NIH grants HL071818, HL122416, and CA221289 (to J.J.G.T.), CA70375 (to R.M.W. and D.H.S.), R35 GM118101 (to D.H.S.), the Hans W. Vahlteich Professorship (to D.H.S.), and 9 P41 GM103622. J.J.G.T. was also supported by the Walther Cancer Foundation. This research was supported by a collaborative “LSI Cubed” grant (to T.S.B. and A.E.F.) through the University of Michigan Life Sciences Institute and used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the Pilatus 3 1M detector was provided by grant 1S10OD018090-01 from National Institute of General Medical Sciences. Use of Life Sciences-Collaborative Access Team Sector 21 was supported by the Michigan Economic Development Corporation and Michigan Technology Tri-Corridor grant 085P1000817. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Data have been deposited in the Protein Data Bank, http://www.wwpdb.org/ (PDB 6EEB, 6O5G).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1818547116/-/DCSupplemental.
References
- 1.Tesmer J. J. G., Hitchhiking on the heptahelical highway: Structure and function of 7TM receptor complexes. Nat. Rev. Mol. Cell Biol. 17, 439–450 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komolov K. E., Benovic J. L., G protein-coupled receptor kinases: Past, present and future. Cell. Signal. 41, 17–24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang T. T., Paolucci L., De Blasi A., Inhibition of G protein-coupled receptor kinase subtypes by Ca2+/calmodulin. J. Biol. Chem. 271, 28691–28696 (1996). [DOI] [PubMed] [Google Scholar]
- 4.Pronin A. N., Satpaev D. K., Slepak V. Z., Benovic J. L., Regulation of G protein-coupled receptor kinases by calmodulin and localization of the calmodulin binding domain. J. Biol. Chem. 272, 18273–18280 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Levay K., Satpaev D. K., Pronin A. N., Benovic J. L., Slepak V. Z., Localization of the sites for Ca2+-binding proteins on G protein-coupled receptor kinases. Biochemistry 37, 13650–13659 (1998). [DOI] [PubMed] [Google Scholar]
- 6.Iacovelli L., Sallese M., Mariggiò S., de Blasi A., Regulation of G-protein-coupled receptor kinase subtypes by calcium sensor proteins. FASEB J. 13, 1–8 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Cuspidi C., Sala C., Negri F., Mancia G., Morganti A.; Italian Society of Hypertension , Prevalence of left-ventricular hypertrophy in hypertension: An updated review of echocardiographic studies. J. Hum. Hypertens. 26, 343–349 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Martini J. S., et al. , Uncovering G protein-coupled receptor kinase-5 as a histone deacetylase kinase in the nucleus of cardiomyocytes. Proc. Natl. Acad. Sci. U.S.A. 105, 12457–12462 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hullmann J. E., et al. , GRK5-mediated exacerbation of pathological cardiac hypertrophy involves facilitation of nuclear NFAT activity. Circ. Res. 115, 976–985 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorriento D., et al. , The amino-terminal domain of GRK5 inhibits cardiac hypertrophy through the regulation of calcium-calmodulin dependent transcription factors. Int. J. Mol. Sci. 19, E861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato P. Y., Chuprun J. K., Schwartz M., Koch W. J., The evolving impact of g protein-coupled receptor kinases in cardiac health and disease. Physiol. Rev. 95, 377–404 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beyett T. S., Bandekar S. J., Tesmer J. J. G., “Molecular basis for targeting, inhibition, and receptor phosphorylation in the G protein-coupled receptor kinase 4 subfamily” in Methods in Pharmacology and Toxicology, Gurevich V. V., Gurevich E. V., Tesmer J. J. G., Eds. (G Protein-Coupled Receptor Kinases, Springer, New York, 2016), pp. 59–74. [Google Scholar]
- 13.Komolov K. E., Bhardwaj A., Benovic J. L., Atomic structure of GRK5 reveals distinct structural features novel for G protein-coupled receptor kinases. J. Biol. Chem. 290, 20629–20647 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homan K. T., et al. , Crystal structure of G protein-coupled receptor kinase 5 in complex with a rationally designed inhibitor. J. Biol. Chem. 290, 20649–20659 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martínez-Luis S., Rodríguez R., Acevedo L., Malbrancheamide, a new calmodulin inhibitor from the fungus Malbranchea aurantiaca. Tetrahedron 62, 1817–1822 (2006). [Google Scholar]
- 16.Figueroa M., et al. , Fluorescence, circular dichroism, NMR, and docking studies of the interaction of the alkaloid malbrancheamide with calmodulin. J. Enzyme Inhib. Med. Chem. 26, 378–385 (2011). [DOI] [PubMed] [Google Scholar]
- 17.González-Andrade M., et al. , Insights into molecular interactions between CaM and its inhibitors from molecular dynamics simulations and experimental data. J. Biomol. Struct. Dyn. 34, 78–91 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Rambo R. P., Tainer J. A., Characterizing flexible and intrinsically unstructured biological macromolecules by SAS using the Porod-Debye law. Biopolymers 95, 559–571 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant T. D., Ab initio electron density determination directly from solution scattering data. Nat. Methods 15, 191–193 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Strulovich R., Tobelaim W. S., Attali B., Hirsch J. A., Structural insights into the M-channel proximal C-Terminus/Calmodulin complex. Biochemistry 55, 5353–5365 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Boguth C. A., Singh P., Huang C.-C., Tesmer J. J. G., Molecular basis for activation of G protein-coupled receptor kinases. EMBO J. 29, 3249–3259 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneidman-Duhovny D., Hammel M., Tainer J. A., Sali A., Accurate SAXS profile computation and its assessment by contrast variation experiments. Biophys. J. 105, 962–974 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Svergun D., Barberato C., Koch M. H., CRYSOL–a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Cryst. 28, 768–773 (1995). [Google Scholar]
- 24.Pitcher J. A., et al. , Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J. Biol. Chem. 271, 24907–24913 (1996). [DOI] [PubMed] [Google Scholar]
- 25.Pronin A. N., Morris A. J., Surguchov A., Benovic J. L., Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem. 275, 26515–26522 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Boggs J. M., Myelin basic protein: A multifunctional protein. Cell. Mol. Life Sci. 63, 1945–1961 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fraley A. E., et al. , Function and structure of MalA/MalA′, iterative halogenases for late-stage C-H functionalization of indole alkaloids. J. Am. Chem. Soc. 139, 12060–12068 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krissinel E., Henrick K., Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 372, 774–797 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Waldschmidt H. V., et al. , Structure-based design, synthesis, and biological evaluation of highly selective and potent G protein-coupled receptor kinase 2 inhibitors. J. Med. Chem. 59, 3793–3807 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gold J. I., et al. , Nuclear translocation of cardiac G protein-Coupled Receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process. PLoS One 8, e57324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kursula P., The many structural faces of calmodulin: A multitasking molecular jackknife. Amino Acids 46, 2295–2304 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya S., Bunick C. G., Chazin W. J., Target selectivity in EF-hand calcium binding proteins. Biochim. Biophys. Acta 1742, 69–79 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Huang C.-C., Tesmer J. J. G., Recognition in the face of diversity: Interactions of heterotrimeric G proteins and G protein-coupled receptor (GPCR) kinases with activated GPCRs. J. Biol. Chem. 286, 7715–7721 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pao C. S., Barker B. L., Benovic J. L., Role of the amino terminus of G protein-coupled receptor kinase 2 in receptor phosphorylation. Biochemistry 48, 7325–7333 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thiyagarajan M. M., et al. , A predicted amphipathic helix mediates plasma membrane localization of GRK5. J. Biol. Chem. 279, 17989–17995 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Pronin A. N., Carman C. V., Benovic J. L., Structure-function analysis of G protein-coupled receptor kinase-5. Role of the carboxyl terminus in kinase regulation. J. Biol. Chem. 273, 31510–31518 (1998). [DOI] [PubMed] [Google Scholar]
- 37.Yang P., Glukhova A., Tesmer J. J. G., Chen Z., Membrane orientation and binding determinants of G protein-coupled receptor kinase 5 as assessed by combined vibrational spectroscopic studies. PLoS One 8, e82072–e11 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pronin A. N., Benovic J. L., Regulation of the G protein-coupled receptor kinase GRK5 by protein kinase C. J. Biol. Chem. 272, 3806–3812 (1997). [DOI] [PubMed] [Google Scholar]
- 39.Penn R. B., Pronin A. N., Benovic J. L., Regulation of G protein-coupled receptor kinases. Trends Cardiovasc. Med. 10, 81–89 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Palczewski K., Buczyłko J., Lebioda L., Crabb J. W., Polans A. S., Identification of the N-terminal region in rhodopsin kinase involved in its interaction with rhodopsin. J. Biol. Chem. 268, 6004–6013 (1993). [PubMed] [Google Scholar]
- 41.Johnson L. R., Scott M. G. H., Pitcher J. A., G protein-coupled receptor kinase 5 contains a DNA-binding nuclear localization sequence. Mol. Cell. Biol. 24, 10169–10179 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamponi G. W., Currie K. P. M., Regulation of Ca(V)2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta 1828, 1629–1643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.