Abstract
Background:
With the rapidly-increased HIV epidemic among men who have sex with men worldwide, the effectiveness of voluntary medical male circumcision as the tool of HIV prevention still remains undetermined.
Purpose:
In the current study, we conducted a systematic review and meta-analysis to assess the association between voluntary medical male circumcision and HIV risk among men who have sex with men.
Methods and Conclusion:
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guideline, we conducted a comprehensive literature search through multiple databases. A total of 37 articles/abstracts were included in the analysis. We employed random-effects models and subgroup analyses based upon key study characteristics derived from empirical studies. A total of 117,293 men who have sex with men were included in the meta-analysis, and no randomized control trials have been identified. The odds of being HIV positive were 7% lower among men who have sex with men who were circumcised than among men who have sex with men who were uncircumcised (adjusted odds ratio, 0.93; 95% confidence interval, 0.88–0.99). The evidence for the potential protective effect of voluntary medical male circumcision was stronger among men who have sex with men in Asia and Africa (adjusted odds ratio, 0.62; 95% confidence interval, 0.53–0.73). Our meta-analyses may suggest a protective effect of voluntary medical male circumcision against HIV infection among men who have sex with men, especially in settings like Asia/Africa.
Keywords: Voluntary medical male circumcision, men who have sex with men, sexual positioning
Introduction
Global HIV prevalence among men who have sex with men (MSM) ranges from 3% in the Middle East and Southeast North Africa to 25% in the Caribbean countries.1,2 HIV incidence has increased in many global settings since declines were noted in the United States and western Europe in the mid-1980s, despite that behavioral (e.g. risk reduction with condom use) and biomedical (e.g. pre-exposure prophylaxis (PrEP)) prevention tools are available.3–5 Given the urgency of the global HIV epidemic in MSM, the available tools for preventing HIV acquisition among MSM may be too limited.3,5 Voluntary male medical circumcision (VMMC) is a single surgical procedure providing potential lifelong benefit.3 Both observational studies and clinical trials demonstrate that foreskin removal via VMMC reduces a man’s risk of contracting HIV through condomless heterosexual intercourses by 50%–73%.6–9 The global public health community would be thrilled to have an “HIV vaccine” with efficacy at this level.
Many scholars have examined the efficacy of VMMC for preventing HIV transmission among MSM, but the conclusions have been inconsistent.10,11 By the time of our data extraction, a few research teams conducted systematic review and meta-analyses of available observational evidence and their findings were both inconclusive.10,11 Millet et al.10 included 15 studies revealing insufficient evidence that VMMC protected against HIV infection among MSM.10 By including six more studies, Wiysonge et al.11 found the same overall conclusion, but data suggested that VMMC significantly protected MSM from HIV infection in the subgroup of men who primarily practiced insertive sex.11 Furthermore, a recent meta-analysis revealed that the odds ratio (OR) of HIV infection between circumcised and uncircumcised MSM was 0.77 (95% confidence interval (CI), 0.67–0.89) without distinguishing the effect of VMMC by different sexual positions as well as other key individual and contextual factors that may play key roles in the studied association.12
However, systematic reviews must incorporate social and contextual factors into their analyses, particularly the region of study and sexual position preferences. Compared to Western countries, the HIV epidemic in Asia differs in its later epidemic growth, comparatively low prevalence, and in the extreme social stigma faced by MSM.13 As suggested by global literature, MSM in Africa may share similar behavioral patterns and social stigmas to their Asian peers,1,14 region-specific strata can be compared to ensure that the comparatively vast literature on VMMC in MSM from the Americas and Europe does not draw out the comparatively small literature in Asia and Africa. Assessing the efficacy of VMMC among MSM by different characteristics of individuals and settings may also be revealing, for example, sex positioning, study sample size, measurement of exposure and outcome variables, and type of study design. By exploring different subgroups, we sought to better understand the efficacy of VMMC on HIV under different circumstances in the current analysis.
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (http://www.prisma-statement.org/), we sought to compare the odds of HIV infection between circumcised and uncircumcised MSM by including all available studies with appropriate study designs (e.g. randomized controlled trials (RCTs), well-designed quasi-experiments, and observational studies).
Methods
Protocol and registration
We sought to register our meta-analysis with the PROSPERO, Cochrane, and Campbell systematic review databases, but were declined as we had “progressed beyond the point of completing data extraction at the time of registration.” Nonetheless, we followed the PRISMA guidelines.
Eligibility criteria
Inclusion
Studies were included if they: (a) used an appropriate study design (e.g. RCTs, longitudinal studies, and observational studies), (b) were quantitatively evaluating effects of circumcision on HIV risk among MSM, (c) provided sufficient information to calculate effect size estimates, and (d) published (any language or year) either in peer-reviewed journals or in recognized conferences.
Exclusion criteria
The exclusion criteria are as follows: (a) descriptive studies that do not report outcomes or studies that only report qualitative outcomes, (b) studies that do not focus on MSM (e.g. focus on heterosexual men), (c) reviews, and (d) theoretical articles without original data.
Data sources, search strategy, and study selection
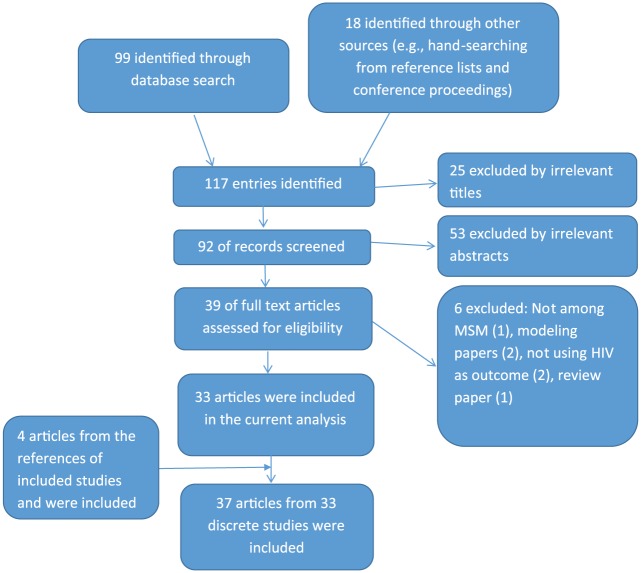
Following the PRISMA guidelines, we conducted a comprehensive literature search through multiple databases by entering different combinations of a few MeSH terms up to 4 August 2016 (Supplemental Table 1). We also searched through newspapers and conference proceedings, as well as references from article that met our inclusion criteria. The initial screening yielded 92 potentially relevant articles/abstracts from 117 entries identified in the search or through other sources (Figure 1). These 92 abstracts were reviewed, 53 abstracts were excluded, and 39 were retained for further review. Two reviewers (C.Z. and Y.L.) independently reviewed the full texts of these articles. Disagreement between reviewers was resolved by discussion, and 33 articles fulfilled the inclusion criteria and were included in the analysis. In addition, four abstracts were selected based upon this review of references and all four were deemed eligible and their corresponding papers were included in the analysis.15–18 Hence, 37 articles from 33 studies were included for the systematic review and meta-analysis.
Figure 1.
Selection procedure of included studies in the meta-analysis.
Data extraction
Two reviewers (C.Z. and Y.L.) independently extracted data from qualified articles using a standard form and developed a table containing the following information: (a) study location and time of conducting the study, (b) characteristics of participants (e.g. age and ethnicity), (c) sample size, (d) HIV assessment (e.g. either self-report or laboratory work), (e) circumcision assessment (either self-report or genital examination), (f) sex positions if applicable (e.g. receptive vs insertive), (g) number of HIV infections among circumcised and uncircumcised MSM, and (h) reported crude and/or adjusted odds ratios (aORs) of the HIV infection and VMMC. When information regarding any of the aforementioned information was unclear, we contacted the original authors of the included articles for further detailed information,19 but no response had been received. For studies with duplicate publications,20–26 we reported the study only once in the analyses, with the most complete data included.
Quality assessment
Two reviewers (C.Z. and Y.L.) independently assessed the risk of bias for each study. For our systematic review and meta-analysis, the selection bias may be unavoidable since we only considered articles with full texts among widely accessible online resources. In addition, we assessed the rigor of measuring the exposure (self-report vs genital examination) and outcome variables (self-report vs laboratory testing) in the original studies.
Statistical analysis
Measures of effects
Most studies reported strengths of association as OR with 95% CI. We preferred using aORs with 95% CIs from the given publication, available for 28 studies.14,16–45 For five studies that did not report aORs, we used raw data to calculate the crude ORs and their 95% CIs.15,46–49
Assessment of heterogeneity
To evaluate the extent to which studies’ outcomes were consistent, we employed the I2-statistics and corresponding 95% CIs to depict heterogeneity. The I2-statistics describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error, and it varies from 0% to 100% with higher percentages indicating higher heterogeneity.50 If the 95% CIs included 0, the included studies were considered to be reasonably homogeneous. P-values for I2-statistics were also reported.
Assessment of publication bias
Publication bias was assessed by the funnel plots, based on the assumption that in the absence of significant heterogeneity, study effect sizes will be normally distributed around the mean effects.50 A funnel plot is a scatterplot of study effects (i.e. x-axis) against a measure of study precision (i.e. y-axis). As a tool visually assesses publication bias, funnel plots would produce asymmetry if publication bias exists.
Data synthesis
Model selection: we employed the random-effects model, as all included studies were conducted among different populations and different settings, a feature that may influence the effects observed. The random-effects model was designed to capture the variance of effects across studies.50,51 Each study was assigned a weight directly by the calculating procedure. Forest plot of ORs from the included studies (i.e. the area of each square is proportional to study’s weight in the meta-analysis), with the summary measure (center line of diamond) and associated CIs (lateral tips of diamond), and a solid vertical line of no effect was used. STATA V12 (College Station, TX, USA), the command metan, was used for data syntheses and analyses.
Subgroup analyses were performed to examine the effect size by sex positioning (receptive vs insertive), study design (cross-sectional vs studies with follow-ups), geographical region (Asia vs non-Asia; Asia + Africa vs non-Asia/non-Africa), assessment method (self-report vs genital exam), and sample size at baseline (⩽3000 vs >3000). All stratified variables were derived from empirical literature1,14,13 as well as discussion among the research team.
Sensitivity analyses were employed to examine the stability of the efficacy of circumcision by evaluating whether the overall effect size was sensitive to exclusion of any individual studies (e.g. study with highest or lowest weight and with smallest or largest sample size).
Heterogeneity was assessed by the I2-statistics and its corresponding P-value that describes the percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error, with higher percentages indicating higher heterogeneity (e.g. 30%–60% indicating moderate heterogeneity and 75%–100% indicating considerable heterogeneity) were presented to depict heterogeneity.50
Results
Overall effect size
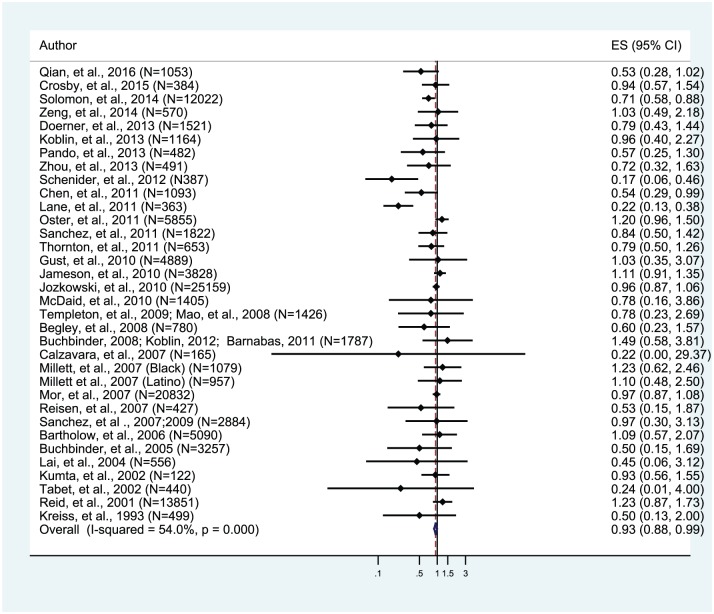
A total of 117,293 MSM participants from 33 studies contributed to examining the association between circumcision and HIV risk. Among these studies, four studies revealed that circumcision significantly reduced the odds of HIV infection among MSM,15,28,39,49 while 29 studies reported that circumcision had no statistically significant associations with HIV. The overall effect size of circumcision on HIV infection was statistically significant (aOR, 0.93; 95% CI, 0.88–0.99; Figure 2). In addition, details about each included study are presented in supplemental Table 2.
Figure 2.
Overall effect size for 33 included studies of voluntary medical male circumcision and HIV risk among men who have sex with men (N = 33).
Subgroup analyses
By sex positioning
Among included studies, 11 studies14,16,23–25,30,31,37,40,43,47 have reported circumcision data for insertive anal sex, and six studies14,23,24,37,43,47,48 reported data on receptive anal sex among MSM. We performed stratified analysis by sex positioning. The results revealed that the odds of HIV risk among circumcised MSM who primarily/exclusively practiced insertive sex did not suggest protection (aOR, 1.16; 95% CI, 0.73–1.83), nor did the odds among circumcised MSM practicing receptive anal sex (aOR, 0.97; 95% CI, 0.74–1.28). However, the insertive sex group estimate was distorted substantially by a single study (i.e. an outlier study with the aOR of HIV infection of 1.77 comparing circumcised to uncircumcised MSM among the insertive sex group, which is in the opposite direction from all other studies in this subgroup), the findings of which were highly disparate from other comparable studies.47 When this one outlier study that suggested greater risk for circumcised men who primarily/exclusively practiced insertive sex was excluded, the odds of HIV infection among circumcised MSM who primarily/exclusively practiced insertive sex (aOR, 0.51; 95% CI, 0.23–1.11) suggested a protective effect for circumcision among the insertive MSM (Table 1).
Table 1.
Subgroup analyses of included studies.
| Number of studies | Number of participants | aOR (95% CI) | I2 (%) | P-value for heterogeneity chi-square | |
|---|---|---|---|---|---|
| Overall | 33 | 117,293 | 0.93 (0.88, 0.99) | 54 | <0.0001 |
| By sex positioning | |||||
| Insertive | 11 | 15,946 | 1.16 (0.73, 1.83) | 14.6 | 0.31 |
| Insertive (after deleting Zeng et al.47)a | 10 | 0.51 (0.23, 1.11) | 0.00 | 0.89 | |
| Receptive | 6 | 9244 | 0.97 (0.74, 1.28) | 28.0 | 0.23 |
| By study regions | |||||
| Asia | 8 | 17,458 | 0.69 (0.58, 0.81) | 39.3 | 0.12 |
| Non-Asia | 25 | 99,835 | 0.97 (0.91, 1.03) | 45.0 | 0.007 |
| Asia + Africa | 9 | 17,821 | 0.62 (0.53, 0.73) | 70.1 | 0.001 |
| Non-Asia/non-Africa | 24 | 99,472 | 0.99 (0.93, 1.05) | 0.00 | 0.86 |
| By sample size | |||||
| Smaller size (<3000) | 24 | 22,510 | 0.70 (0.61, 0.82) | 39.3 | 0.024 |
| Larger size (⩾3000) | 9 | 94,783 | 0.98 (0.92, 1.04) | 52.3 | 0.033 |
| By study design | |||||
| Cross-sectional | 24 | 92,937 | 0.92 (0.87, 0.98) | 61.9 | <0.0001 |
| Cohort | 9 | 24,356 | 1.01 (0.86, 1.19) | 0.0 | 0.47 |
| By sampling strategy | |||||
| Convenience sampling | 17 | 54,235 | 0.95 (0.88, 1.03) | 41.6 | 0.037 |
| Non-convenience sampling | 16 | 63,058 | 0.92 (0.85, 0.99) | 63.6 | 0.000 |
| Non-probability-based | 28 | 100,448 | 0.95 (0.68, 1.34) | 0.0 | 0.67 |
| Probability-based | 5 | 16,845 | 0.93 (0.88, 0.99) | 59.6 | <0.0001 |
| By HIV testing | |||||
| Lab test | 27 | 99,896 | 0.93 (0.88, 0.99) | 59.4 | <0.0001 |
| Self-report | 6 | 17,397 | 0.95 (0.75, 1.20) | 4.0 | 0.39 |
| By VMMC | |||||
| Genital examination | 9 | 32,715 | 0.98 (0.90, 1.07) | 0.0 | 0.61 |
| Self-report | 24 | 84,578 | 0.90 (0.84, 0.97) | 62.1 | <0.0001 |
| By exposure and outcome measurement | |||||
| Using genital examination and laboratory testing | 9 | 32,715 | 0.98 (0.90, 1.07) | 0.0 | 0.61 |
| One measured | 18 | 67,181 | 0.93 (0.88, 0.99) | 59.4 | <0.0001 |
| Neither measured | 6 | 17,397 | 0.95 (0.75, 1.20) | 4.0 | 0.39 |
aOR: adjusted odds ratio; CI: confidence interval; VMMC: voluntary medical male circumcision.
The study (Zeng et al.47) is an outlier. After deleting, the odds of HIV risk among insertive MSM were lower compared to the odds of HIV risk among MSM who primarily practice receptive or versatile sex positioning.
By study design
Among all 33 studies, 24 employed a cross-sectional study design. A protective and significant association was noted among these studies (aOR, 0.92; 95% CI, 0.87–0.98). Nine studies with a cohort design20,22,23,25,34,36,37,49,52 revealed a non-significant association (aOR, 1.01; 95% CI, 0.86–1.19; Table 1).
By region
(a) Asia versus non-Asia: eight studies14,15,17,18,27,28,47,49 were conducted in Asian countries. Among non-Asian MSM, there was no protection of circumcision against HIV infection (aOR, 0.97; 95% CI, 0.91–1.03). (b) Among studies conducted in Asian countries, the odds of being HIV infected was 31% lower compared with the odds of HIV infection among uncircumcised (aOR, 0.69; 95% CI; 0.58–0.81). (c) Only one African study was found. Examining Asian/African studies combined meant adding the African study to the five Chinese and three Indian, revealing an even lower odd of being HIV infected among circumcised MSM than Asian MSM alone (aOR, 0.62; 95% CI; 0.53–0.73). Similarly, the odds of HIV infection among circumcised MSM outside the Asian-African continents was still non-significant (aOR, 0.99; 95% CI, 0.93–1.05; Table 1).
By sample size
For studies with ⩽3000 participants (n = 24), we found that the odds of being HIV infected among circumcised MSM was significantly lower (aOR, 0.70; 95% CI, 0.61–0.82). For studies with a sample size of >3000 (n = 9),15,19,32,34,36,37,41,42,52 the OR of HIV infection was close to 1 (aOR, 0.99; 95% CI, 0.93–1.05; Table 1).
By sampling strategy
Of the 17 studies using a convenience sampling strategy, the odds of being HIV infected among circumcised MSM was 5% less compared with uncircumcised MSM (aOR, 0.95; 95% CI; 0.88–1.03). For studies employing some kind of systematic sampling, the odds of being HIV infected was significantly lower among circumcised MSM (aOR, 0.92; 95% CI, 0.85–0.99). Only five studies employed probability sampling,20,21,22,31,34,36 and the odds of being HIV infected was slightly lower among circumcised (aOR, 0.93; 95% CI, 0.88–0.99), similar to the studies that did not use probability sampling (aOR, 0.95; 95% CI; 0.68–1.34; Table 1).
By assessment method
For nine studies employing genital examinations to measure circumcision status and laboratory testing of HIV infection,14,18,20,23,24,31,37,41,44,47 the odds of being HIV infected was similar between circumcised and uncircumcised MSM (aOR, 0.98; 95% CI, 0.90–1.07). For six studies employing self-report for both variables,16,30,33,35,42,43 the odds of getting HIV was also only slightly lower among circumcised men (aOR, 0.95; 95% CI, 0.75–1.20). The rest of the 18 studies with either, but not both measures using a medical examination had a marginally significant HIV prevention benefit suggested for circumcision (aOR, 0.93; 95% CI, 0.88–0.99; Table 1).
Sensitivity analyses
Sensitivity analyses were conducted by removing studies with highest and lowest weight, and studies with largest and smallest sample size, respectively. By comparing outcomes from sensitivity analyses with the original outcome, no difference has been found from the sensitivity analyses (not shown). In the subgroup analysis for men with an insertive sexual preference, a wide swing in aOR was noted when one study was excluded.47
Publication bias and heterogeneity assessment
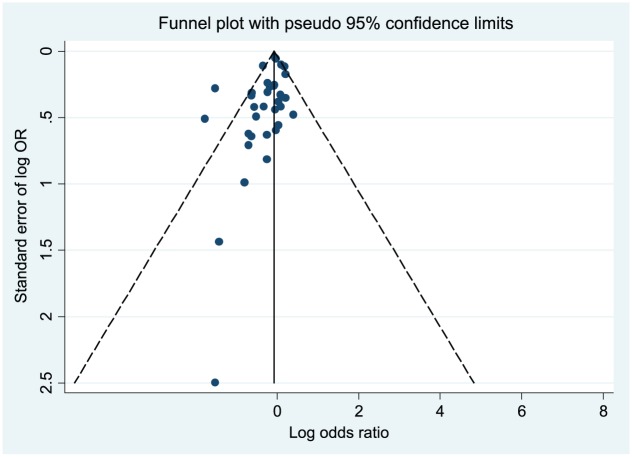
By examining the funnel plot, publication bias was present as the scatter plot shows asymmetry within the funnel, especially among studies with smaller ORs (Figure 3). The I2-statistics was 54.0% (P < 0.001), indicating moderate heterogeneity of included studies (i.e. the degree of the variation across studies due to heterogeneity rather than chance is moderate).50
Figure 3.
Funnel plot for publication bias assessment for included studies for the meta-analysis of voluntary medical male circumcision and HIV infection.
Discussion
Our meta-analytic review includes 117,293 participants from 33 studies. Our review shows that the odds of being HIV infected are lower among MSM who are circumcised than among MSM who are uncircumcised, but the effect size is modest (7% protection, 95% CI, 1%–12%). Our meta-analysis is the first to report a statistically protective effect of VMMC against HIV infection among MSM. The evidence for the protective effect of VMMC is stronger among MSM who live in Asia or Africa. Our findings suggest that VMMC may be a protective tool against HIV infection among MSM, especially for those living in Asia and Africa.53,54
In addition to the overall effect size, several key findings emerged from the subgroup analyses. First, the observation that VMMC may be an especially effective tool for HIV prevention among Asian and African MSM generates a hypothesis that circumcision may be more effective in MSM who have comparatively lower risk profiles (e.g. fewer sexual partners, less risky sexual activity) or MSM in context with stronger HIV epidemics in heterosexual transmission compared with their Western peers.3,14,49 Therefore, VMMC could be more effective among MSM with moderate risk, but that MSM with higher risk profiles may benefit less. Future studies are desired to validate this hypothesis that VMMC works more effectively among low-risk profile MSM than their high-risk peers. Second, although our findings showed increased odds of HIV infection among circumcised MSM primarily practicing insertive sex, a counterintuitive and biologically implausible finding was noted. A strong protective effect of VMMC on HIV infection among MSM exclusively or predominantly practicing insertive anal intercourse was noted after we removed an outlier from the analytic pool. Third, for studies with a sample size of ⩽3000 MSM, the OR of being HIV infected was 30% significantly lower among circumcised MSM compared to their uncircumcised peers. Based upon the funnel plot assessment, perhaps studies with fewer participants can be published only if they reported significant findings, while studies with larger sample sizes are published without bias, that is, whether the association is significant or not. Therefore, publication bias may affect the accuracy and reliability of the efficacy of VMMC, a bias away from the null hypothesis.
Our meta-analysis has several strengths. First, it included a large number of participants. Second, we stratified the data by individual and contextual characteristics to capture any potential associations in important subgroups. Third, we strictly followed the PRISMA guideline for systematic reviews and meta-analyses.
However, a number of limitations should be considered while interpreting findings from this study. First, the limited number and scope of existing studies constrained representativeness of our findings. Of the 33 studies, 22 (67%) were conducted in high-income regions (e.g. United States and Canada) with long-standing lesbian/gay/bisexual/transgender (LGBT) civil rights movements and high HIV prevalence among MSM. This geographic concentration may not reflect the full scope of sexual risk and HIV transmission dynamics worldwide.53,54 The limited number of studies (n = 13) from Asia (n = 8), Africa (n = 1), and Latin America (n = 4) limits our ability to generalize findings. Second, misclassification of circumcision status and sex positioning may lead to a bias toward the null hypothesis, minimizing the ability to detect the true magnitude of association. The limited assessment of circumcision status and sex positioning may lead to misclassification that would tend to under/overestimate VMMC benefits.11,14 When misclassification bias is eliminated by conducting direct penile examination as well as by including detailed sexual position data, promise in VMMC as a tool to reduce HIV risk among MSM than previously assumed.14 Third, the nature of cross-sectional study designs may limit the inference of the association between VMMC and HIV. In addition, our subgroup analysis among all cross-sectional studies revealed a protective association between VMMC and HIV risk, while the pooled OR among cohort studies was null. Perhaps, the significantly protective association was driven by uncontrolled/unadjusted confounding in these cross-sectional studies. Studies with more rigorous design are highly desired. Furthermore, no available information to assess potential risk compensation behaviors after circumcision among all included studies may require attention by future studies.55,56
Conclusion
Although the overall effect of VMMC on HIV prevention was marginally significant, misclassification of key exposure and confounding variables may dilute the protective effect of VMMC. In turn, publication bias may exaggerate its protective effort. Research with more rigorous study designs to objectively assess HIV infection through confirmatory serological tests and evaluation of circumcision by genital exam can significantly reduce misclassification bias. In addition, future research should collect detailed data on MSM’s sexual position preference at different time points in their lives (e.g. in the past 30 days, in the past 6 months, and lifetime), as well as the degree of their sexual risk taking. We would not be surprised if we eventually learn that circumcision is highly protective for MSM, but benefits are predominantly accrued among men practicing predominantly insertive anal sex and men without highest risk behavior patterns.57 Furthermore, two-thirds of the included studies in this meta-analysis employed convenience sampling to collect data, which may lack representativeness and constrain capacity of making casual inferences. Although an RCT could definitively determine whether VMMC reduces HIV risk among MSM, no available RCT has been conducted. An RCT, if feasible, should be conducted. An integration of behavioral and biomedical HIV prevention is highly desired.4,5,58
Supplemental Material
Supplemental material, AIM1_Supplement_Tables for Voluntary medical male circumcision and HIV infection among men who have sex with men: Implications from a systematic review by Chen Zhang, Han-Zhu Qian, Yu Liu and Sten H Vermund in SAGE Open Medicine
Acknowledgments
The authors thank Dr Bryan Shepherd for his advice on data interpretation and Ms Rachel R. Walden for her assistance on literature search.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: This is a meta-analysis, which does not need ethics approval.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from U.S. National Institutes of Health (R01AI094562 and R34AI091446). The content is solely the responsibility of the authors and does not necessarily represent the sponsor who had no role in the design or conduct of the study, the writing of this report, or its submission for publication.
Informed consent: This is a meta-analysis, which does not need informed consent.
ORCID iD: Chen Zhang  https://orcid.org/0000-0002-8771-561X
https://orcid.org/0000-0002-8771-561X
Supplemental material: Supplemental material for this article is available online.
References
- 1. Beyrer C, Baral SD, vanGriensven F, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet 2012; 380(9839): 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. vanGriensven F, deLindvanWijngaarden JW, Baral S, et al. The global epidemic of HIV infection among men who have sex with men. Curr Opin HIV AIDS 2009; 4(4): 300–307. [DOI] [PubMed] [Google Scholar]
- 3. Vermund SH, Qian HZ. Circumcision and HIV prevention among men who have sex with men: no final word. JAMA 2008; 300(14): 1698–1700. [DOI] [PubMed] [Google Scholar]
- 4. Ogbuagu O, Marshall BDL, Tiberio P, et al. Prevalence and correlates of unhealthy alcohol and drug use among men who have sex with men prescribed HIV pre-exposure prophylaxis in real-world clinical settings. AIDS Behav 2018; 23: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Paparini S, Nutland W, Rhodes T, et al. DIY HIV prevention: formative qualitative research with men who have sex with men who source PrEP outside of clinical trials. PLoS ONE 2018; 13(8): e0202830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet 2007; 369(9562): 643–656. [DOI] [PubMed] [Google Scholar]
- 7. Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007; 369(9562): 657–666. [DOI] [PubMed] [Google Scholar]
- 8. Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLos Med 2005; 2(11): e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a posttrial follow-up study. AIDS 2012; 26(5): 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Millett GA, Ding H, Lauby J, et al. Circumcision status and HIV infection among Black and Latino men who have sex with men in 3 US cities. J Acquir Immune Defic Syndr 2007; 46(5): 643–650. [DOI] [PubMed] [Google Scholar]
- 11. Wiysonge CS, Kongnyuy EJ, Shey M, et al. Male circumcision for prevention of homosexual acquisition of HIV in men. Cochrane Database Syst Rev 2011(6): CD007496. [DOI] [PubMed] [Google Scholar]
- 12. Yuan T, Fitzpatrick T, Ko NY, et al. Circumcision to prevent HIV and other sexually transmitted infections in men who have sex with men: a systematic review and meta-analysis of global data. Lancet Glob Health 2019; 7(4): e436–e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. AVERT. HIV and AIDS in Asia 2015, http://www.avert.org/hiv-and-aids-asia.htm
- 14. Qian HZ, Ruan Y, Liu Y, et al. Lower HIV risk among circumcised men who have sex with men in China: interaction with anal sex role in a cross-sectional study. J Acquir Immune Defic Syndr 2016; 71(4): 444–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Solomon SS, Mehta S, Srikrishnan AK, et al. (eds). Circumcision is associated with lower HIV prevalence among men who have sex with men in India. In: Proceedings of the international AIDS conference 2014, Melbourne, VIC, Australia, 20–25 July 2014. [Google Scholar]
- 16. Calzavara LM, Remis R, Myers T, et al. Circumcision and HIV/STI among MSM in the Polaris HIV seroconversion study. In: Proceedings of the 16th annual Canadian association for HIV Research 2007, Toronto, ON, Canada, 24–27 April 2007. [Google Scholar]
- 17. Lai SF, Hong CP, Lan YC, et al. Molecular epidemiology of HIV-1 in men who have sex with men from gay saunas in Taiwan from 2000 to 2003. In: Proceedings of the 15th International AIDS Conference, Bangkok, Thailand, 11–16 July 2004. [Google Scholar]
- 18. Kumta S, Setia M, Jerjani HR, et al. Men who have sex with men and male to female transgender in Mumbai: a critical emerging risk group for HIV and sexually transmitted infections in India. In: Proceedings of the 14th International AIDS Conference, Barcelona, 7–12 July 2002. [Google Scholar]
- 19. Oster AM, Wiegand RE, Sionean C, et al. Understanding disparities in HIV infection between black and white MSM in the United States. AIDS 2011; 25(8): 1103–1112. [DOI] [PubMed] [Google Scholar]
- 20. Koblin BA, Mayer KH, Noonan E, et al. Sexual risk behaviors, circumcision status, and preexisting immunity to adenovirus type 5 among men who have sex with men participating in a randomized HIV-1 vaccine efficacy trial: step study. J Acquir Immune Defic Syndr 2012; 60(4): 405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barnabas RV, Wasserheit JN, Huang Y, et al. Impact of herpes simplex virus type 2 on HIV-1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr 2011; 57(3): 238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008; 372(9653): 1881–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanchez J, Lama JR, Peinado J, et al. High HIV and ulcerative sexually transmitted infection incidence estimates among men who have sex with men in Peru: awaiting for an effective preventive intervention. J Acquir Immune Defic Syndr 2009; 51: S47-S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sanchez J. (ed.). Cutting the edge of teh HIV and epidemic among MSM. In: Proceedings of the center for HIV identification, prevention, and treatment services the future direction of male circumcision in HIV prevention working conference, Los Angeles, CA, 9 April 2007. [Google Scholar]
- 25. Templeton DJ, Jin F, Mao L, et al. Circumcision and risk of HIV infection in Australian homosexual men. AIDS 2009; 23(17): 2347–2351. [DOI] [PubMed] [Google Scholar]
- 26. Mao L, Templeton DJ, Crawford J, et al. Does circumcision make a difference to the sexual experience of gay men? Findings from the Health in Men (HIM) cohort. J Sex Med 2008; 5(11): 2557–2561. [DOI] [PubMed] [Google Scholar]
- 27. Zhou C, Raymond HF, Ding X, et al. Anal sex role, circumcision status, and HIV infection among men who have sex with men in Chongqing, China. Arch Sex Behav 2013; 42(7): 1275–1283. [DOI] [PubMed] [Google Scholar]
- 28. Schneider JA, Michaels S, Gandham SR, et al. A protective effect of circumcision among receptive male sex partners of Indian men who have sex with men. AIDS Behav 2012; 16(2): 350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koblin BA, Mayer KH, Eshleman SH, et al. Correlates of HIV acquisition in a cohort of Black men who have sex with men in the United States: HIV prevention trials network (HPTN) 061. PLoS ONE 2013; 8(7): e70413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doerner R, McKeown E, Nelson S, et al. Circumcision and HIV infection among men who have sex with men in Britain: the insertive sexual role. Arch Sex Behav 2013; 42(7): 1319–1326. [DOI] [PubMed] [Google Scholar]
- 31. Sanchez J, SalYRosas VG, Hughes JP, et al. Male circumcision and risk of HIV acquisition among MSM. AIDS 2011; 25(4): 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jozkowski K, Rosenberger JG, Schick V, et al. Relations between circumcision status, sexually transmitted infection history, and HIV serostatus among a national sample of men who have sex with men in the United States. AIDS Patient Care STDS 2010; 24(8): 465–470. [DOI] [PubMed] [Google Scholar]
- 33. Thornton AC, Lattimore S, Delpech V, et al. Circumcision among men who have sex with men in London, United Kingdom: an unlikely strategy for HIV prevention. Sex Transm Dis 2011; 38(10): 928–931. [DOI] [PubMed] [Google Scholar]
- 34. Bartholow BN, Goli V, Ackers M, et al. Demographic and behavioral contextual risk groups among men who have sex with men participating in a phase 3 HIV vaccine efficacy trial: implications for HIV prevention and behavioral/biomedical intervention trials. J Acquir Immune Defic Syndr 2006; 43(5): 594–602. [DOI] [PubMed] [Google Scholar]
- 35. Begley EB, Jafa K, Voetsch AC, et al. Willingness of men who have sex with men (MSM) in the United States to be circumcised as adults to reduce the risk of HIV infection. PLoS ONE 2008; 3(7): e2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gust DA, Wiegand RE, Kretsinger K, et al. Circumcision status and HIV infection among MSM: reanalysis of a Phase III HIV vaccine clinical trial. AIDS 2010; 24(8): 1135–1143. [DOI] [PubMed] [Google Scholar]
- 37. Jameson DR, Celum CL, Manhart L, et al. The association between lack of circumcision and HIV, HSV-2, and other sexually transmitted infections among men who have sex with men. Sex Transm Dis 2010; 37(3): 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kreiss JK, Hopkins SG. The association between circumcision status and human immunodeficiency virus infection among homosexual men. J Infect Dis 1993; 168(6): 1404–1408. [DOI] [PubMed] [Google Scholar]
- 39. Lane T, Raymond HF, Dladla S, et al. High HIV prevalence among men who have sex with men in Soweto, South Africa: results from the Soweto men’s study. AIDS Behav 2011; 15(3): 626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McDaid LM, Weiss HA, Hart GJ. Circumcision among men who have sex with men in Scotland: limited potential for HIV prevention. Sex Transm Infect 2010; 86(5): 404–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mor Z, Kent CK, Kohn RP, et al. Declining rates in male circumcision amidst increasing evidence of its public health benefit. PLoS One 2007; 2(9): e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reid D, Weatherburn P, Hickson F, et al. Know the score: Findings from the national gay men’s sex survey 2001. London: University of Portsmouth, 2001. [Google Scholar]
- 43. Reisen CA, Zea MC, Poppen PJ, et al. Male circumcision and HIV status among Latino immigrant MSM in New York City. J LGBT Health Res 2007; 3(4): 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tabet S, Sanchez J, Lama J, et al. HIV, syphilis and heterosexual bridging among Peruvian men who have sex with men. AIDS 2002; 16(9): 1271–1277. [DOI] [PubMed] [Google Scholar]
- 45. Templeton DJ, Mao L, Prestage GP, et al. Self-report is a valid measure of circumcision status in homosexual men. Sex Transm Infect 2008; 84(3): 187–188. [DOI] [PubMed] [Google Scholar]
- 46. Crosby RA, Graham CA, Mena L, et al. Circumcision status is not associated with condom use and prevalence of sexually transmitted infections among young black MSM. AIDS Behav 2016; 20: 2538–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeng Y, Zhang L, Li T, et al. Risk Factors for HIV/syphilis infection and male circumcision practices and preferences among men who have sex with men in China. Biomed Res Int 2014; 2014: 498987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pando MA, Balan IC, Dolezal C, et al. Low frequency of male circumcision and unwillingness to be circumcised among MSM in Buenos Aires, Argentina: association with sexually transmitted infections. J Int AIDS Soc 2013; 16: 18500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen YJ, Lin YT, Chen M, et al. Risk factors for HIV-1 seroconversion among Taiwanese men visiting gay saunas who have sex with men. BMC Infect Dis 2011; 11: 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borenstein M, Hedges LV, Higgins J, et al. Introduction to meta-analysis. Hoboken, NJ: John Wiley & Sons, 2009. [Google Scholar]
- 51. Littell JH, Corcoran J, Pillai V. Systematic reviews and meta-analysis. New York: Oxford University Press, 2008. [Google Scholar]
- 52. Buchbinder SP, Vittinghoff E, Heagerty PJ, et al. Sexual risk, nitrite inhalant use, and lack of circumcision associated with HIV seroconversion in men who have sex with men in the United States. J Acquir Immune Defic Syndr 2005; 39(1): 82–89. [DOI] [PubMed] [Google Scholar]
- 53. WHO. Men who have sex with men 2016, http://www.who.int/hiv/topics/msm/about/en/
- 54. Beyrer C. Global prevention of HIV infection for neglected populations: men who have sex with men. Clin Infect Diseas 2010; 50: S108–S113. [DOI] [PubMed] [Google Scholar]
- 55. Grund JM, Chetty-Makkan CM, Ginindza S, et al. Effectiveness of an “Exclusive Intervention Strategy” to increase medical male circumcision uptake among men aged 25-49 years in South Africa. BMC Public Health 2018; 18(1): 868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kabwama SN, Ssewanyana D, Berg-Beckhoff G. The association between male circumcision and condom use behavior—a meta-analysis. Mater Sociomed 2018; 30(1): 62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang C, Penson DF, Qian HZ, et al. Modeling economic and epidemiological impact of voluntary medical male circumcision among men who have sex with men in Beijing, China. Int J STD AIDS 2019; 30(7): 630–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reed JB, Patel RR, Baggaley R. Lessons from a decade of voluntary medical male circumcision implementation and their application to HIV pre-exposure prophylaxis scale up. Int J STD AIDS. Epub ahead of print 19 August 2018. DOI: 10.1177/0956462418787896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, AIM1_Supplement_Tables for Voluntary medical male circumcision and HIV infection among men who have sex with men: Implications from a systematic review by Chen Zhang, Han-Zhu Qian, Yu Liu and Sten H Vermund in SAGE Open Medicine