Significance
In the fruit fly olfactory system, 50 classes of olfactory receptor neurons (ORNs) make precise synaptic connections with 50 classes of partner projection neurons (PNs). Identification of wiring molecules here can provide general insight into how neural circuits are assembled during development. This paper reports Fish-lips (Fili), a protein spanning the cell membrane, that regulates specific connections in this circuit. We found that some ORN axons are repelled by Fili present on dendrites of nonpartner PNs, preventing them from targeting inappropriate glomeruli. Similarly, some PN dendrites are repelled by Fili expressed by nonpartner ORNs for their correct targeting. These results suggest that Fili mediates repulsion between axons and dendrites of nonsynaptic partners to ensure precise wiring patterns.
Keywords: neural connectivity, target selection, Drosophila, olfactory system, leucine-rich repeat
Abstract
Our understanding of the mechanisms of neural circuit assembly is far from complete. Identification of wiring molecules with novel mechanisms of action will provide insights into how complex and heterogeneous neural circuits assemble during development. In the Drosophila olfactory system, 50 classes of olfactory receptor neurons (ORNs) make precise synaptic connections with 50 classes of partner projection neurons (PNs). Here, we performed an RNA interference screen for cell surface molecules and identified the leucine-rich repeat–containing transmembrane protein known as Fish-lips (Fili) as a novel wiring molecule in the assembly of the Drosophila olfactory circuit. Fili contributes to the precise axon and dendrite targeting of a small subset of ORN and PN classes, respectively. Cell-type–specific expression and genetic analyses suggest that Fili sends a transsynaptic repulsive signal to neurites of nonpartner classes that prevents their targeting to inappropriate glomeruli in the antennal lobe.
The brain comprises extremely complex yet precisely wired neural circuits, which allow animals to faithfully relay and process information. To establish these specific neural connections, a coordinated sequence of developmental steps is taken: Axons and dendrites first navigate to their target zones, then identify the appropriate partners, and finally form functional synapses. Secreted and cell surface–bound factors are required for each of these steps (1–5). These factors can act as either ligands or receptors to allow a developing neurite to sample its environment and select the correct target. Although much progress has been made in both identifying wiring-related molecules and investigating cellular mechanisms underlying each of the steps just described, our understanding of neural circuit assembly is far from complete.
To gain more insight into this developmental process, we studied the Drosophila olfactory system. Here, each of the 50 classes of olfactory receptor neurons (ORNs) extend their axons that make synaptic connections with dendrites of their corresponding projection neuron (PN) class in 50 anatomically discrete glomeruli in the antennal lobe (6–8). Because of the stereotyped organization of different synaptic partner pairs, the richness of connections, and the availability of genetic tools, many wiring molecules have been identified and studied in this circuit (9, 10). Furthermore, wiring molecules discovered in this system have guided our understanding of the wiring logic of other neural circuits across different species (11–14).
Here, we designed and carried out an RNA interference (RNAi) screen to identify wiring molecules in a region of the antennal lobe that has not been extensively studied. From this screen, we identified a wiring molecule, Fish-lips (Fili), a leucine-rich repeat–containing transmembrane protein previously known only for its function in regulating dioxin receptor homolog Spineless-mediated apoptosis (15). We investigated the function of Fili in both ORN axon and PN dendrite targeting during olfactory circuit development, and found that Fili signals reciprocally between ORNs and PNs to instruct precise target selection.
Results
A Leucine-Rich Repeat Protein, Fish-Lips, Is Required for the Wiring of Olfactory Neurons.
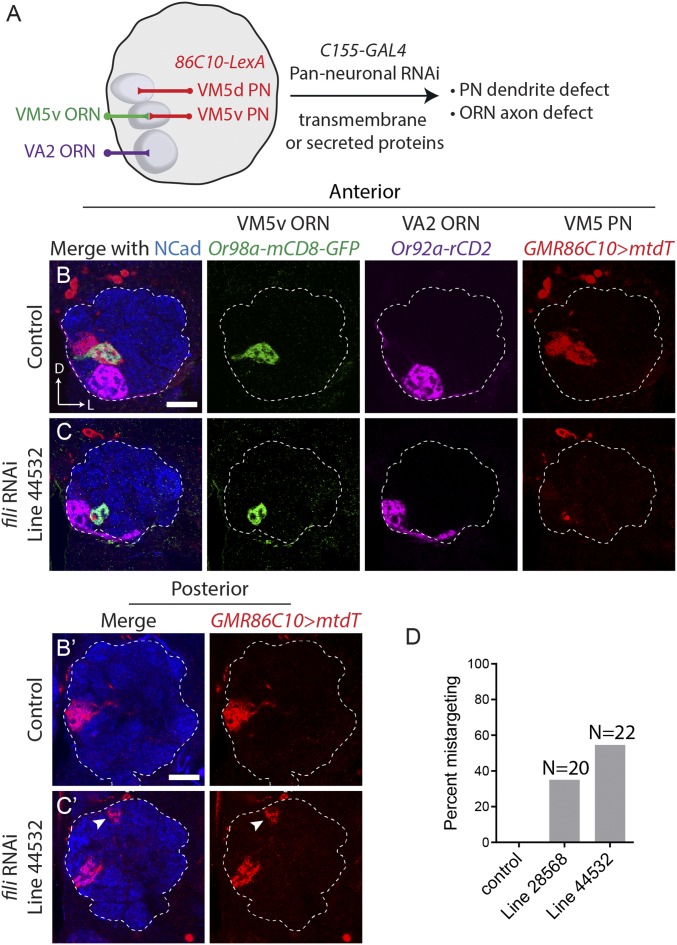
To identify molecules underlying Drosophila olfactory-circuit wiring specificity, we previously carried out genetic screens for ORN axon and PN dendrite targeting to the anterior-lateral side of the antennal lobe (11, 16). While certain molecules broadly contribute to the precise wiring of many types of olfactory neurons (17, 18), others have been found to be region- and even glomerulus-specific (9, 11, 16, 19–21). We reasoned that neurons in different locations in the antennal lobe might use a different set of wiring molecules for their circuit assembly. To identify specific PN drivers with reliable expression in other regions of the antennal lobe, we screened a collection of enhancer-GAL4 lines from the FlyLight project (22, 23). We found 5 lines that show robust, specific, and consistent labeling patterns of various PN subtypes (SI Appendix, Fig. S1). The labeled glomeruli covered distinct regions, including the dorsolateral, ventromedial, and middle regions of the antennal lobe. We also converted these enhancer-GAL4 lines into a different binary expression system, LexA/LexAop, by fusing each of the specific enhancer fragments to the LexA sequence (24). Using 1 of these lines, GMR86C10-LexA, we designed a screening scheme to identify wiring molecules that function in the ventromedial antennal lobe.
Specifically, we used GMR86C10-LexA > LexAop-mtdT to label dendrites of VM5v and VM5d PNs, and used Or98a-mCD8-GFP and Or92a-rCD2 to label axons of VM5v and VA2 ORNs, respectively (Fig. 1 A and B). In addition to these neuronal class–specific markers, we visualized the neuropil using an antibody against N-cadherin. Using the panneuronal C155-GAL4 driver line, we expressed RNAi against predicted transmembrane and secreted molecules. We tested more than 700 RNAi lines (Dataset S1) covering over 200 genes whose protein products contain domain types of leucine-rich repeat (LRR), Ig, cadherin, epidermal growth factor repeat, and fibronectin for their necessity in olfactory neuron–wiring accuracy.
Fig. 1.
Identification of Fish-lips as a wiring specificity molecule through an RNAi screen. (A) Schematic of RNAi screen. Panneuronal C155-GAL4 was used to drive UAS-RNAi against predicted transmembrane and secreted molecules. Dendrites of 2 PN classes, VM5v and VM5d (hereafter VM5 PNs), are labeled by GMR86C10-LexA > LexAop-mtdT. Axons of VM5v and VA2 ORNs are labeled by 2 different markers, Or98a-mCD8GFP and Or92a-rCD2, respectively. (B and C) Targeting of dendrites of VM5 PNs (red) and axons of VM5v (green) and VA2 ORNs (magenta) in the antennal lobe on the anterior side. Neuropil staining by the Ncad antibody is shown in blue. Dashed line outlines the antennal lobe neuropil. (B′ and C′) Posterior sections of the same antennal lobes as in B and C. Dendrites of VM5 PNs and VM5v/VA2 ORN axons target their corresponding glomeruli in control (B). C155-GAL4–driven UAS-fili-RNAi (VDRC 44532) shows PN dendrite–targeting defect (C). Ectopic PN targets are indicated by arrowheads on the posterior section of C′. (D) Quantification of VM5 PN mistargeting of 2 different RNAi lines against fili (Bloomington 28568 and VDRC 44532). The phenotype penetrance of 2 independent RNAi lines is 7/20 and 12/22, respectively. (Scale bars, 20 μm.) D, dorsal; L, lateral.
From the screen, we identified several candidates (Dataset S1), including a wiring specificity protein, Fish-lips (Fili). In wild-type animals, GMR86C10-LexA > LexAop-mtdT PNs extend their dendrites to only 2 glomeruli—VM5v and VM5d (hereafter VM5 PNs) (Fig. 1B). When fili was knocked down by either 1 of the 2 independent RNAi lines (i.e., against different regions of fili), VM5 PN dendrites ectopically invaded a glomerulus more dorsal and posterior to the correct target (arrowhead in Fig. 1C and SI Appendix, Fig. S2A). Penetrance of the VM5 PN dendrite–targeting phenotype is correlated with fili knockdown efficiency (Fig. 1D and SI Appendix, S2B).
fili encodes a single-pass transmembrane protein that contains 14 LRR motifs in the extracellular domain (SI Appendix, Fig. S5A). S2 cell transfection experiments validated that the extracellular domain was on the cell surface, as it could be detected by antibody staining without permeabilization (SI Appendix, Fig. S3). Fili shares the most amino acid sequence similarities with 2 other LRR proteins, Capricious (Caps) and Tartan (Trn) (15), both of which have been demonstrated to regulate PN dendrite targeting (21). The extracellular domain of Fili is 32% and 30% identical to that of Caps and Trn, respectively (SI Appendix, Fig. S4), whereas Caps and Trn are more closely related, sharing 63% identity in their extracellular domains. The intracellular domains are more divergent among these 3 proteins (SI Appendix, Fig. S4). The function of Fili in the nervous system has not been reported previously.
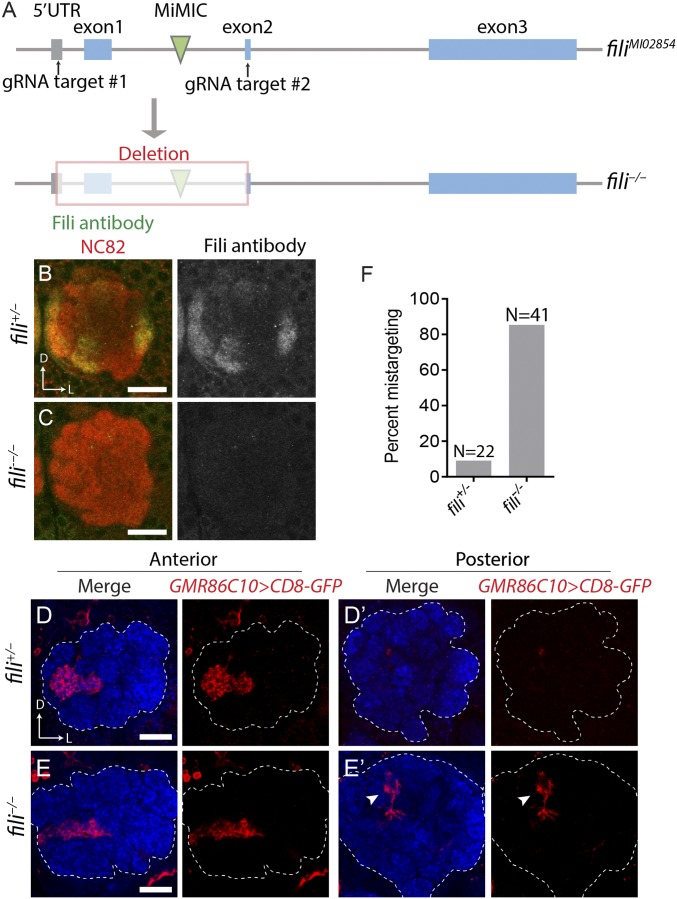
To investigate the function of Fili, we generated a null allele using CRISPR-mediated gene editing, which removed the first exon and part of the second exon of fili (Fig. 2A). According to simple modular architecture research tool (SMART) protein prediction (25), this excision should eliminate the DNA sequence that encodes the start codon, the signal peptide, and more than half of the LRR motifs. We validated this allele by staining brains of fili−/− flies with a Fili antibody we generated against an epitope on the intracellular domain of Fili (SI Appendix, Fig. S5A). Although the Fili antibody signal was clearly present in heterozygous flies, we detected no signal in fili−/− animals (Fig. 2C). Because the targeting epitope of the Fili antibody was not contained in the deleted region, our data suggested that this deletion fully disrupted production of Fili. Similar to the phenotype we observed by panneuronal RNAi knockdown of fili, fili−/− animals showed dorsoposterior ectopic targeting of VM5 PN dendrites (Fig. 2E′ arrowhead).
Fig. 2.
fili mutant recapitulates the RNAi phenotype. (A) Schematic of the generation of fili null mutant by CRISPR-mediated excision. vas-Cas9; filiMI02854 eggs were coinjected with 2 gRNAs targeting the 5′ UTR (untranslated region) and the second exon. The first coding exon and part of the second coding exon are deleted in the mutant. Blue bars, coding exons of fili. (B and C) Maximum projection of anti-Fili serum staining (green and gray) of heterozygous control (B) and fili−/− fly (C). (D and E) Targeting of VM5 PNs labeled by GMR86C10-GAL4 > UAS-mCD8GFP (red) in the antennal lobe of heterozygous fili+/− (D; mistargeting ratio: 2/22) or homozygous fili−/− animals (E; mistargeting ratio: 35/41). (F) Quantification of mistargeting ratio from D and E. (Scale bars, 20 μm.)
Fili Is Expressed in a Subset of ORNs and PNs.
The expression pattern of a wiring molecule can be informative for understanding its mechanism of action. While guidance molecules with a graded expression pattern can be important for the initial coarse targeting of developing neurites (26, 27), wiring molecules with discrete patterning may function to refine the final targeting to a specific region (21). In fili−/− animals, VM5 PN dendrites consistently mistargeted to the same glomerulus. This observation suggests that Fili could serve as a discrete signal for VM5 PN dendrites during their final target selection and refinement.
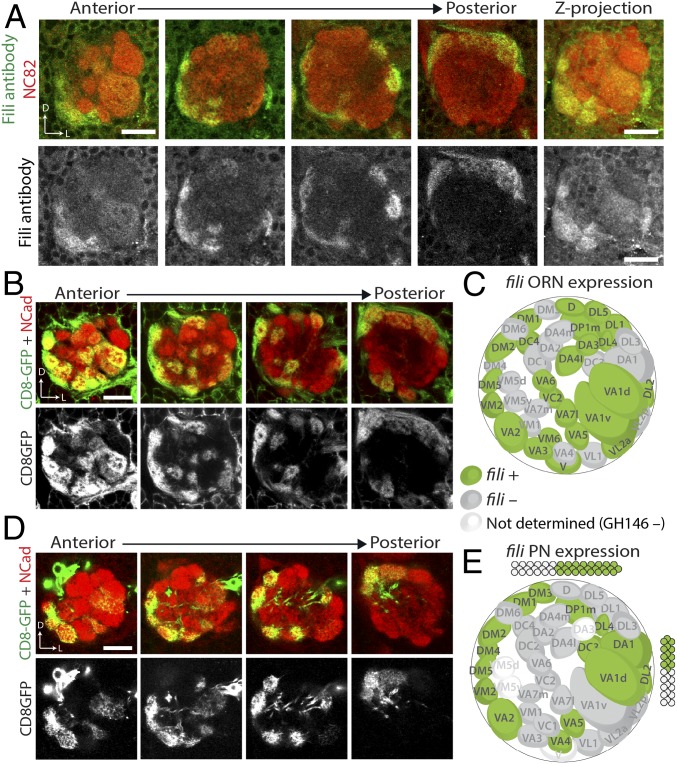
To assay the expression of Fili, we stained brains at 48 h after puparium formation (48 h APF). At this time, matching between ORN axons and PN dendrites has just been established and the expression pattern likely reflects the molecules used for the final targeting. Additionally, because discrete glomeruli have just formed at this stage, discerning the identity of neurons based on their projection pattern is possible. Immunostaining with Fili antibodies revealed the presence of Fili protein in a subset of glomeruli in the developing antennal lobe (Fig. 3A).
Fig. 3.
Fili is differentially expressed in a subset of ORNs and PNs during development. (A) anti-Fili serum staining of antennal lobe in wild-type animal (w1118) at 48 h APF (green and gray). Neuropil is stained by the NC82 antibody (red). (B and D) ey-FLP or GH146-FLP intersecting with fili-GAL4 using UAS-FRT-stop-FRT-mCD8GFP as a reporter shows fili expression pattern in ORNs (B) or PNs (D) at 48 h APF. (C and E) Schematic two-dimensional representation of the glomerular innervation pattern of fili-GAL4–expressing ORNs (C) or PNs (E). (Scale bars, 20 μm.)
Glomerular-specific Fili patterns could be due to Fili’s expression in ORNs, PNs, or both. To distinguish between these possibilities, we generated a transcriptional reporter, fili-GAL4 (28), to study its cell type–specific expression. An artificial exon containing a splicing acceptor, an in-frame T2A-GAL4, and a transcription terminator was inserted into a Minos mediated integration cassette (MiMIC) locus (MI02854) residing in a coding intron of fili (SI Appendix, Fig. S5B). To validate that this line faithfully represented Fili expression, we crossed it to transgenic flies expressing a membrane fluorescent reporter (UAS-mCD8-GFP) and observed a similar GFP pattern compared with the Fili protein pattern in the antennal lobe (SI Appendix, Fig. S5C). The mCD8-GFP reporter for fili-GAL4 also showed bright cell body labeling around the antennal lobe not seen in the staining with Fili antibodies. This is likely because the mCD8-GFP reporter did not reflect subcellular localization of the Fili protein.
To visualize the contribution of Fili by ORNs and PNs, we intersected fili-GAL4 with FLP recombinase lines expressed in either all ORNs (ey-FLP) or the majority of PNs (GH146-FLP). When combined with UAS-FRT-STOP-FRT-mCD8GFP, mCD8-GFP was solely produced in fili-positive ORNs or PNs. Using this strategy, we observed that Fili was expressed in ORNs and PNs in a “salt-and-pepper” pattern (Fig. 3 B and D) reminiscent of the expression of Caps and Trn (11, 21). This expression pattern suggests that Fili likely serves as a discrete determinant that constrains glomerular targeting of neurites instead of setting a gradient for trajectory selection or coarse targeting. Further analysis revealed no statistically significant correlation of fili expression between ORNs and PNs (Fisher’s exact test, P value = 0.4704). This suggests that Fili likely does not mediate homophilic attraction or repulsion between ORNs and PNs.
Fili in ORNs Signals to VM5 PNs for Proper Dendrite Targeting.
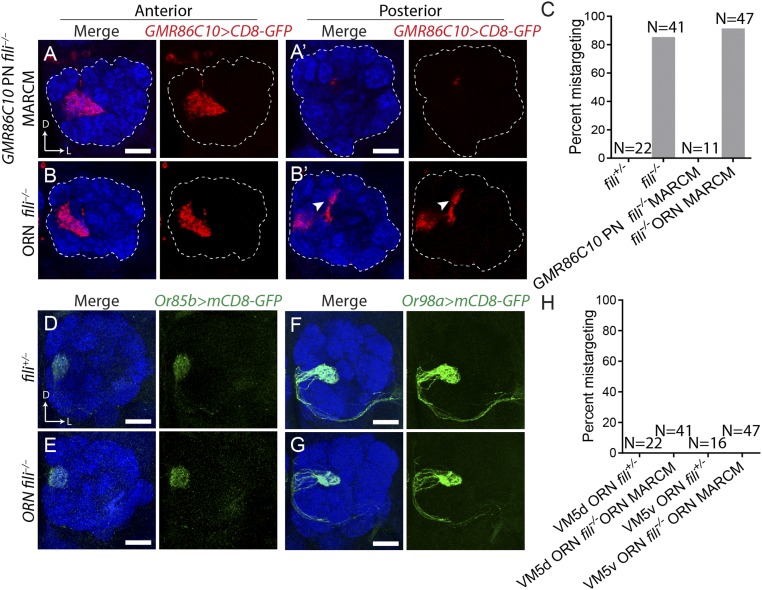
To determine which neurons require Fili functions for the proper targeting of VM5 PN dendrites, we carried out mosaic analyses with a repressible cell marker (MARCM) (29) to test a possible cell-autonomous function. Using hsFLP-based MARCM, we generated fili−/− PN neuroblast clones and observed no mistargeting of VM5 PN dendrites as visualized by GMR86C10-GAL4 (Fig. 4 A and C). This indicates that Fili is not required in VM5 PNs for their correct dendrite targeting. In contrast, deleting fili in most ORNs using ey-FLP–based MARCM combined with a cell-lethal strategy (30) recapitulated the whole-animal fili−/− phenotype (Fig. 4 B and C).
Fig. 4.
Fili in ORNs signals to VM5 PNs for the correct targeting of their dendrites. (A) Dendrite targeting of fili−/− VM5 neuroblast clone produced by hsFLP MARCM in a fili+/− background (mistargeting in 0/11 antennal lobes). (B) Dendrite targeting of fili+/− VM5 PNs in animals, with the majority of ORNs being fili−/− generated by ey-FLP MARCM combined with cell lethal strategy (mistargeting in 43/47 antennal lobes). (A′ and B′) Posterior sections of the same antennal lobe with arrowhead highlighting ectopic targeting of VM5 PN dendrites. (C) Quantification of GMR86C10+ PN mistargeting from A and B. (D and E) Targeting of VM5d ORN axons labeled by Or85b-GAL4 > UAS-mCD8GFP in fili+/− animals (mistargeting in 0/22 antennal lobes) (D) and in ORN fili−/− (mistargeting in 0/41 antennal lobes) (E). (F and G) Targeting of VM5v ORN axons labeled by Or98a-GAL4 > UAS-mCD8GFP in the antennal lobe of fili+/− animals (mistargeting in 0/16 antennal lobes) (F) and in ORN fili−/− animals (mistargeting in 0/47 antennal lobes) (G). (H) Quantification of mistargeting of VM5d and VM5v ORN axons from D–G. (Scale bars, 20 μm.)
Two mechanisms might account for the above results. First, Fili is required in VM5 ORNs for their correct axon targeting. When those ORN axons mistarget, dendrites of their partner PN classes mistarget with them (16). Second, Fili in ORNs signal to dendrites of VM5 PNs to direct their correct targeting. To test the first possibility, we removed Fili in ORNs and visualized axon targeting of VM5v or VM5d ORNs. We did not observe obvious axon mistargeting (Fig. 4 D–H). This argues against the first possibility and suggests instead that Fili is expressed by ORNs to signal to VM5 PNs for dendrite target selection. To understand where the Fili signal originates, we checked the expression pattern of Fili in ORNs. We found no Fili expression in VM5v and VM5d ORNs (SI Appendix, Fig. S6A) at 48 h APF. By contrast, the mistargeted site, DC4, has high fili expression in ORNs (SI Appendix, Fig. S6 B and C). This observation suggests that Fili expressed in the ORN class occupying the ectopic target site may repel VM5 PNs to prevent their dendrites from targeting inappropriate glomeruli.
Fili Is Required for Correct Targeting of a Small Subset of PNs and ORNs.
Because Fili is expressed in multiple ORN and PN classes, we examined its involvement in the wiring process of other neuronal classes. Identifying more classes of neurons that require Fili for their targeting allowed us to test whether Fili repels other neurites as a general mechanism of action.
We labeled different ORN and PN classes in a fili−/− background to investigate their antennal lobe–targeting fidelity. We examined dendritic targeting of 9 PN classes using 6 drivers and did not observe any obvious targeting defects (SI Appendix, Fig. S7). We also labeled 13 different ORN classes in a fili−/− background and found three classes with abnormal axon-targeting patterns (SI Appendix, Fig. S8). We observed misshapen glomerulus innervated by VA7l ORN axons in fili−/− animals (SI Appendix, Fig. S8C) but no ectopic targeting, which is likely a secondary effect caused by miswiring of other classes of neurons. For both DC1 ORNs and VA1v ORNs, we observed clear ectopic targeting of their axons (SI Appendix, Fig. S8 B and D), indicating that Fili is required for their correct axon target specificity. To better understand how Fili regulates wiring in the context of ORN axon–targeting specificity, we chose to focus on VA1v ORNs as there exists a wealth of tools available to manipulate gene expression in regions adjacent to VA1v.
Fili Is Required in VA1d/DC3 PNs to Prevent Ectopic Targeting of VA1v ORN Axons.
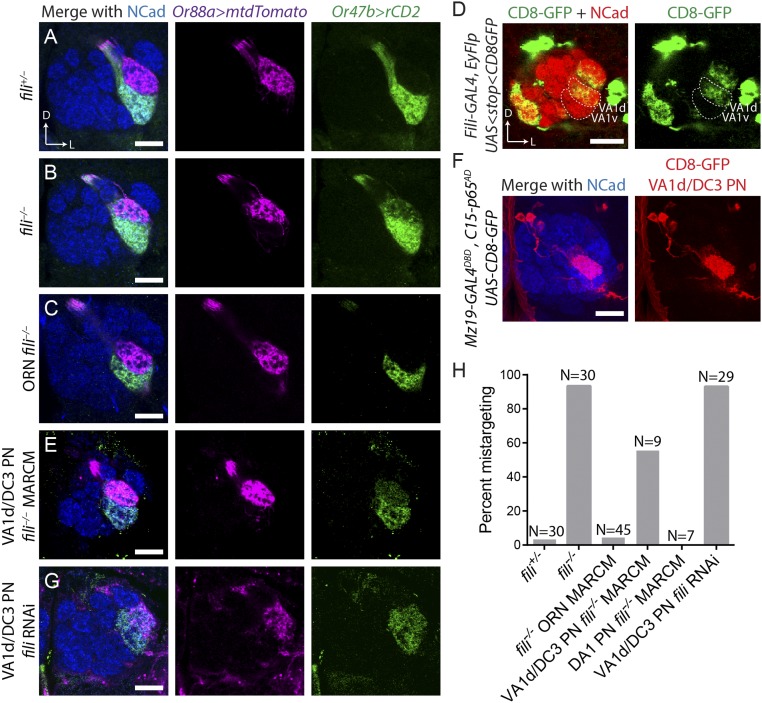
In wild-type flies, ORNs of different classes target their axons to distinct glomeruli and never intermingle (Fig. 5A). However, in fili−/− flies VA1v ORN axons invaded the VA1d glomerulus along with VA1d axons (Fig. 5 B and H). When we removed fili in most ORNs by ey-FLP–based MARCM combined with the cell lethal strategy, we did not observe any targeting defect of VA1v ORN axons (Fig. 5 C and H). Thus, Fili expression in VA1v ORNs (or other ORN classes) is not required for their axon targeting.
Fig. 5.
Fili in VA1d/DC3 PNs prevents ectopic targeting of VA1v ORN axons to VA1d glomerulus. (A) VA1d and VA1v ORNs labeled by Or88a-mTdT (magenta) and Or47b-rCD2 (green), respectively, target their axons to 2 neighboring glomeruli in a stereotyped manner in fili+/− animals (mistargeting in 2/30 antennal lobes). (B) In fili−/− animals, VA1v ORN axons invade VA1d glomerulus (mistargeting in 29/31 antennal lobes). (C) Deletion of fili in most ORNs using ey-FLP MARCM combined with cell lethal strategy does not alter VA1v ORN axon targeting (mistargeting in 2/45 antennal lobes). (D) Expression of Fili in VA1v and VA1d PNs, shown by CD8-GFP (green) using intersection between GH146-FLP and fili-GAL4. The corresponding glomeruli are outlined by dashed line. (E) Deletion of Fili in anterodorsal neuroblast clones (which include VA1d PNs) using Mz19-GAL4 hsFLP MARCM causes VA1v ORN axons to mistarget to VA1d glomerulus (mistargeting in 5/9 antennal lobes). (F) Mz19-GAL4DBD, C15-p65AD produces functional GAL4 in only VA1d/DC3 PNs to drive UAS-mCD8-GFP expression (red). Maximum z-projection is shown. (G) Mz19-GAL4DBD, C15-p65AD drives UAS-fili-RNAi (VDRC 44532), causing VA1v ORN axons to mistarget to VA1d glomerulus (mistargeting in 27/29 antennal lobes). (H) Quantification of VA1v ORN axon mistargeting from A, C, E, and G. (Scale bars, 20 μm.)
We hypothesized that, similar to our observation in GMR86C10-positive PNs (Fig. 4), Fili expressed in PNs targeting neighboring glomeruli may repel VA1v ORN axons from targeting inappropriate glomeruli. Our expression analysis using fili-GAL4 intersected with GH146-FLP was consistent with this hypothesis: While VA1v PNs did not express fili, neighboring glomeruli VA1d, DC3, and DA1 PNs did (Fig. 5D). To more directly test our hypothesis, we generated fili−/− neuroblast clones visualized by Mz19-GAL4 (expressed in DA1 PNs that belong to the lateral neuroblast lineage, and VA1d and DC3 PNs that belong to the anterodorsal neuroblast lineage). At the same time, we labeled VA1d and VA1v ORNs. When the expression of Fili in VA1d/DC3 PNs was eliminated in anterodorsal neuroblast clones, VA1v ORN axons invaded the VA1d glomerulus, similarly to the whole-animal mutant (Fig. 5 E and H). By contrast, removing fili in the lateral neuroblast including DA1 PNs did not cause VA1v ORN axon–targeting defects (Fig. 5H).
Because the MARCM strategy generates mutants stochastically, it remained possible that we generated fili−/− mutants in other cell types that were not labeled by Mz19-GAL4 to produce this phenotype. We therefore also used the RNAi strategy to knockdown fili in only VA1d and DC3 PNs. To achieve specific GAL4 expression, we utilized the split-GAL4 strategy (31, 32) to generate driver lines specific to VA1d and DC3 PNs. We inserted the hemidriver p65AD component after the last coding exons of C15, a transcription factor that is only expressed by PNs derived from the anterodorsal neuroblast (adPNs) (33), and converted Mz19-GAL4 to Mz19-GAL4DBD using the Homology Assisted CRISPR Knock-in (HACK) strategy (34, 35). When we intersected these 2 components, expression of functional GAL4 was restricted to Mz19+ adPNs (VA1d and DC3) (Fig. 5F). Using this newly developed driver line, we drove fili RNAi in only VA1d and DC3 PNs, and found that this manipulation recapitulated VA1d mistargeting for VA1v ORN axons as observed in fili−/− mutants (Fig. 5 G and H). Moreover, VA1v ORN axons did not invade the DC3 glomerulus when Fili was removed from VA1d and DC3 PNs by MARCM or RNAi. Thus, analogous to VM5 PNs, expression of Fili on the synaptic partner corresponding to the ectopic targeting site is critical for correct axon targeting of VA1v ORN axons. Together, these results suggest that Fili repels neurites of the nonmatching synaptic partner class to restrict them to the correct target.
Discussion
Here, we developed an assay for identifying wiring molecules in the ventromedial region of the Drosophila antennal lobe. Through an RNAi screen for cell surface molecules, we discovered that Fili, an LRR-containing transmembrane protein, participates in the assembly of the Drosophila olfactory circuit. Detailed expression and genetic analyses suggest that Fili acts as a repellent in ORNs for PNs, and in PNs for ORNs, to prevent invasion of neurites into inappropriate glomeruli.
Before this study, Fili had been implicated in cell–cell interaction in the detection of misspecified cells in the wing disk (15). Apoptosis induced by ectopic expression of Spineless is regulated by Fili expression. Along with Caps and Trn (36), Fili appears to participate in short-range cell interaction to support cell survival in the wing disk. In the nervous system, LRR-containing proteins have been widely implicated in neural circuit assembly (37). The unique curved structure of LRR combined with exposed β-sheets on the concave side makes LRR an effective protein-binding motif (38). Furthermore, different LRRs form distinct structures, permitting interaction with a diverse collection of proteins (39, 40). These unique binding characteristics enable secreted or membrane-associated LRR proteins to play central roles in diverse aspects of nervous system development and function.
Our results suggest that Fili sends a transsynaptic repulsive signal between ORNs and PNs. Although some LRRs are shown to have binding affinity to themselves (41–44), we note that some glomeruli are innervated by both Fili-positive ORN axons and PN dendrites (Fig. 3 C and E), arguing against a homotypic mechanism for Fili-mediated repulsion. A more probable explanation is that neurons that require Fili for their targeting specificity express a receptor for Fili and are thus repelled by regions with high Fili expression. Future identification of the Fili receptor will substantiate this hypothesis. Since VA1v ORNs express both Fili and the presumed Fili receptor, Fili does not appear to mediate ORN–ORN repulsion.
Despite Fili’s expression in many classes of ORNs and PNs, we observed that only a small fraction of these classes requires Fili for correct axon or dendrite targeting. The sparsity of neurons that manifest observable wiring defects in Fili mutants might be explained by the hypothesis that neurons use a combinatorial and redundant coding strategy to specify their connections (9). As we observed previously, a typical ORN or PN class uses multiple molecules for their axon or dendrite targeting. Our recent single-cell RNAseq data of both PNs and ORNs revealed that the cell surface landscape between different classes of PNs and ORNs is usually distinguished by multiple molecules (33, 45). Therefore, when a single wiring molecule is perturbed, the cell surface landscape may still resemble the original neuronal class more closely than other classes, thus permitting correct neurite targeting for many neurons. A more comprehensive understanding of the overarching wiring strategies of this circuit will benefit from simultaneous manipulation of multiple wiring molecules that cooperate in specifying the connectivity within individual neuronal classes.
Materials and Methods
Immunostaining.
Drosophila brain dissection and immunostaining were performed according to previously described methods (46). Primary antibodies used in this study included rat anti-Ncad [N-Ex #8; 1:40; Developmental Studies Hybridoma Bank (DSHB)], chicken anti-GFP (1:1,000; Aves Labs), rabbit anti-DsRed (1:500; Clontech), mouse anti-rCD2 (OX-34; 1:200; AbD Serotec), mouse nc82 (1:35; DSHB), anti-HRP conjugated with Cy5 (1:200; Jackson ImmunoResearch), and rat anti-Fili (1:200; custom produced by Thermo Fisher Scientific against a peptide epitope containing Fili residues 684 to 701 DDEPEHLYERFDHYEYPD). Fili antibody was preabsorbed by fili−/− larval brains to remove nonspecific binding. Secondary antibodies raised in goat or donkey against rabbit, mouse, rat, and chicken antisera were used, conjugated to Alexa 405, FITC, 568, or 647 (Jackson ImmunoResearch). Confocal images were collected with a Zeiss LSM 780 and processed with Zen software and ImageJ.
S2 Cell Staining.
The S2 cell–staining protocol was adapted from a previously described method (47). We generated pUAST-attB-Fili from cDNA and then performed 2 rounds of mutagenesis to insert V5 and FLAG tags. The final construct, UAS-SP-V5-Fili-FLAG, contained a V5 tag directly after the signal peptide of Fili and a 3XFLAG tag right before the stop codon. S2 cells were cotransfected with Actin-GAL4 and UAS-SP-V5-Fili-FLAG plasmids or only with Actin-GAL4 plasmid as a negative control. After 48 h, transfected cells were incubated with rabbit anti-V5 antibody (1:200; GeneScript) and mouse anti-FLAG M2 antibody (1:200; Sigma-Aldrich) either before (nonpermeabilized condition) or after (permeabilized condition) 4% PFA fixation and permeabilization with 0.3% Triton in PBS. Cells were then washed with PBS, incubated with secondary antibodies, and imaged.
RNAi Screening.
The RNAi screen fly was generated as follows: C155-GAL4 was recombined with UAS-dcr2 on the X chromosome. GMR86C10-LexA, LexAop-mtdT, Or98a-mCD8GFP, and Or92a-rCD2 were recombined and located on the second chromosome. UAS-RNAi males were crossed to this screening line, and the resulting flies were kept at 25 °C for 2 d after egg laying and then transferred to 29 °C to enhance the GAL4/UAS expression system.
Generation of fili Mutant.
We generated 2 gRNA constructs using the BbsI-chiRNA plasmid (48). One gRNA contained a targeting site 5′ to the start codon of fili and one 3′ to the start of the second coding exon. These 2 gRNA constructs were coinjected into Drosophila embryos with vas-cas9, a yellow gene (y) mutant allele on the X chromosome, and MI02854 containing an exogenous y gene on the second chromosome. G0 flies were crossed to balancer flies and individuals in the F1 generation were selected for loss of y. Successful events were balanced and confirmed by sequencing.
Mosaic Analysis.
The hsFLP MARCM analyses were performed as previously described (29, 49) with slight modifications. GMR86C10-GAL4 was used for labeling VM5 PNs, and Mz19-GAL4 was used for labeling VA1d, DC3, and DA1 PNs in adult-stage Drosophila. Larvae (24 to 48 h after hatching) were heat-shocked for 1 h to obtain neuroblast clones.
Transgene Generation.
To generate enhancer-LexA lines labeling different PNs, including GMR86C10-LexA, gateway vector–containing enhancer sequences (23) were recombined into the pBPnlsLexAp65Uw vector (32) through LR reaction (Invitrogen) and the resulting constructs were injected into attP2 and attP40 landing sites by integrase-mediated transgenesis. Or92a-rCD2 was made by cloning the rat CD2 coding region (50) downstream of the Or92a promoter sequence, and a transgenic animal was made through P-element transformation (51). C15-p65AD was generated by coinjecting a gRNA (cloned in pU6-BbsI-chiRNA) targeting the end of the last exon and donor sequence containing homology arms p65(AD)::Zip+ and 3XP3-RFP-SV40 (31). Mz19-Gal4DBD was generated using the HACK strategy (35). We replaced the QF2 sequence of pBPGUw-HACK-G4 > QF2 with the DNA-binding domain of GAL4 cloned from pBS-KS-attB2-SA(0)-T2A-Gal4DBD-Hsp70 polyA, and injected it into Mz19-GAL4; vas-Cas9 embryos.
Supplementary Material
Acknowledgments
We thank G. Rubin, H. Bellen, T. Lee, the Vienna Stock Center, the Bloomington Stock Center, the Drosophila Genomics Resource Center, and Addgene for reagents. We thank T. Li, C.N. McLaughlin, A. Shuster, J. Ren, J. Lui, and D. Pederick for helpful discussions and comments on the manuscript. This work was supported by NIH Grant R01 DC005982 (to L.L.). L.L. is a Howard Hughes Medical Institute investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1905832116/-/DCSupplemental.
References
- 1.Sanes J. R., Yamagata M., Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 25, 161–195 (2009). [DOI] [PubMed] [Google Scholar]
- 2.Yogev S., Shen K., Cellular and molecular mechanisms of synaptic specificity. Annu. Rev. Cell Dev. Biol. 30, 417–437 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Jan Y.-N., Jan L. Y., Branching out: Mechanisms of dendritic arborization. Nat. Rev. Neurosci. 11, 316–328 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Südhof T. C., Towards an understanding of synapse formation. Neuron 100, 276–293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolodkin A. L., Tessier-Lavigne M., Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb. Perspect. Biol. 3, a001727 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vosshall L. B., Stocker R. F., Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Su C.-Y., Menuz K., Carlson J. R., Olfactory perception: Receptors, cells, and circuits. Cell 139, 45–59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson R. I., Early olfactory processing in Drosophila: Mechanisms and principles. Annu. Rev. Neurosci. 36, 217–241 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong W., Luo L., Genetic control of wiring specificity in the fly olfactory system. Genetics 196, 17–29 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li H., Shuster S. A., Li J., Luo L., Linking neuronal lineage and wiring specificity. Neural Dev. 13, 5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong W., Mosca T. J., Luo L., Teneurins instruct synaptic partner matching in an olfactory map. Nature 484, 201–207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosca T. J., Hong W., Dani V. S., Favaloro V., Luo L., Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature 484, 237–241 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antinucci P., Nikolaou N., Meyer M. P., Hindges R., Teneurin-3 specifies morphological and functional connectivity of retinal ganglion cells in the vertebrate visual system. Cell Rep. 5, 582–592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berns D. S., DeNardo L. A., Pederick D. T., Luo L., Teneurin-3 controls topographic circuit assembly in the hippocampus. Nature 554, 328–333 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi-Yamada T., et al. , Wing-to-leg homeosis by Spineless causes apoptosis regulated by Fish-lips, a novel leucine-rich repeat transmembrane protein. Mol. Cell. Biol. 25, 3140–3150 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward A., Hong W., Favaloro V., Luo L., Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a Drosophila olfactory circuit. Neuron 85, 1013–1028 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H., Luo L., Diverse functions of N-cadherin in dendritic and axonal terminal arborization of olfactory projection neurons. Neuron 42, 63–75 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Zhu H., et al. , Dendritic patterning by Dscam and synaptic partner matching in the Drosophila antennal lobe. Nat. Neurosci. 9, 349–355 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Joo W. J., Sweeney L. B., Liang L., Luo L., Linking cell fate, trajectory choice, and target selection: Genetic analysis of sema-2b in olfactory axon targeting. Neuron 78, 673–686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J., et al. , Stepwise wiring of the Drosophila olfactory map requires specific Plexin B levels. eLife 7, e39088 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong W., et al. , Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat. Neurosci. 12, 1542–1550 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jenett A., et al. , A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2, 991–1001 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfeiffer B. D., et al. , Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 105, 9715–9720 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai S. L., Lee T., Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat. Neurosci. 9, 703–709 (2006). [DOI] [PubMed] [Google Scholar]
- 25.Schultz J., Milpetz F., Bork P., Ponting C. P., SMART, a simple modular architecture research tool: Identification of signaling domains. Proc. Natl. Acad. Sci. U.S.A. 95, 5857–5864 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Komiyama T., Sweeney L. B., Schuldiner O., Garcia K. C., Luo L., Graded expression of semaphorin-1a cell-autonomously directs dendritic targeting of olfactory projection neurons. Cell 128, 399–410 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Imai T., Suzuki M., Sakano H., Odorant receptor-derived cAMP signals direct axonal targeting. Science 314, 657–661 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Diao F., et al. , Plug-and-play genetic access to Drosophila cell types using exchangeable exon cassettes. Cell Rep. 10, 1410–1421 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee T., Luo L., Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron 22, 451–461 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Newsome T. P., Asling B., Dickson B. J., Analysis of Drosophila photoreceptor axon guidance in eye-specific mosaics. Development 127, 851–860 (2000). [DOI] [PubMed] [Google Scholar]
- 31.Luan H., Peabody N. C., Vinson C. R., White B. H., Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfeiffer B. D., et al. , Refinement of tools for targeted gene expression in Drosophila. Genetics 186, 735–755 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H., et al. , Classifying Drosophila olfactory projection neuron subtypes by single-cell RNA sequencing. Cell 171, 1206–1220.e22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin C.-C., Potter C. J., Editing transgenic DNA components by inducible gene replacement in Drosophila melanogaster. Genetics 203, 1613–1628 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie T., et al. , A genetic toolkit for dissecting dopamine circuit function in Drosophila. Cell Rep. 23, 652–665 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milán M., Pérez L., Cohen S. M., Short-range cell interactions and cell survival in the Drosophila wing. Dev. Cell 2, 797–805 (2002). [DOI] [PubMed] [Google Scholar]
- 37.de Wit J., Hong W., Luo L., Ghosh A., Role of leucine-rich repeat proteins in the development and function of neural circuits. Annu. Rev. Cell Dev. Biol. 27, 697–729 (2011). [DOI] [PubMed] [Google Scholar]
- 38.Kobe B., Kajava A. V., The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11, 725–732 (2001). [DOI] [PubMed] [Google Scholar]
- 39.Buchanan S. G., Gay N. J., Structural and functional diversity in the leucine-rich repeat family of proteins. Prog. Biophys. Mol. Biol. 65, 1–44 (1996). [DOI] [PubMed] [Google Scholar]
- 40.Bella J., Hindle K. L., McEwan P. A., Lovell S. C., The leucine-rich repeat structure. Cell. Mol. Life Sci. 65, 2307–2333 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nose A., Mahajan V. B., Goodman C. S., Connectin: A homophilic cell adhesion molecule expressed on a subset of muscles and the motoneurons that innervate them in Drosophila. Cell 70, 553–567 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Krantz D. E., Zipursky S. L., Drosophila chaoptin, a member of the leucine-rich repeat family, is a photoreceptor cell-specific adhesion molecule. EMBO J. 9, 1969–1977 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Howitt J. A., Clout N. J., Hohenester E., Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 23, 4406–4412 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Özkan E., et al. , An extracellular interactome of immunoglobulin and LRR proteins reveals receptor-ligand networks. Cell 154, 228–239 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li H., et al. , Coordinating receptor expression and wiring specificity in olfactory receptor neurons. bioRxiv:10.1101/594895 (31 March 2019).
- 46.Wu J. S., Luo L., A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protoc. 1, 2110–2115 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Zhang W., et al. , Ankyrin repeats convey force to gate the NOMPC mechanotransduction channel. Cell 162, 1391–1403 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gratz S. J., Wildonger J., Harrison M. M., O’Connor-Giles K. M., CRISPR/Cas9-mediated genome engineering and the promise of designer flies on demand. Fly (Austin) 7, 249–255 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J. S., Luo L., A protocol for mosaic analysis with a repressible cell marker (MARCM) in Drosophila. Nat. Protoc. 1, 2583–2589 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Dunin-Borkowski O. M., Brown N. H., Mammalian CD2 is an effective heterologous marker of the cell surface in Drosophila. Dev. Biol. 168, 689–693 (1995). [DOI] [PubMed] [Google Scholar]
- 51.Fishilevich E., Vosshall L. B., Genetic and functional subdivision of the Drosophila antennal lobe. Curr. Biol. 15, 1548–1553 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.