Significance
Asthma is one of the leading chronic diseases among children, and there is increasing evidence that air pollution plays a role in the development of this disease. We found that had NO2 or PM2.5 concentration in 9 southern Californian communities been lower than observed in the 1990s and early 2000s, there would have been a corresponding reduction in childhood asthma incidence, with larger effect estimates for NO2. Our findings may have implications for air quality regulations, as all communities examined in this study had ambient concentrations well below the current US Environmental Protection Agency annual standard of 53 ppb for NO2.
Keywords: air pollution, nitrogen dioxide, particulate matter, asthma, child
Abstract
Childhood asthma is a major public health concern and has significant adverse impacts on the lives of the children and their families, and on society. There is an emerging link between air pollution, which is ubiquitous in our environment, particularly in urban centers, and incident childhood asthma. Here, using data from 3 successive cohorts recruited from the same 9 communities in southern California over a span of 20 y (1993 to 2014), we estimated asthma incidence using G-computation under hypothetical air pollution exposure scenarios targeting nitrogen dioxide (NO2) and particulate matter <2.5 μm (PM2.5) in separate interventions. We reported comparisons of asthma incidence under each hypothetical air pollution intervention with incidence under the observed natural course of exposure; results that may be more tangible for policymakers compared with risk ratios. Model results indicated that childhood asthma incidence rates would have been statistically significantly higher had the observed reduction in ambient NO2 in southern California not occurred in the 1990s and early 2000s, and asthma incidence rates would have been significantly lower had NO2 been lower than what it was observed to be. For example, compliance with a hypothetical standard of 20 ppb NO2 was estimated to result in 20% lower childhood asthma incidence (95% CI, −27% to −11%) compared with the exposure that actually occurred. The findings for hypothetical PM2.5 interventions, although statistically significant, were smaller in magnitude compared with results for the hypothetical NO2 interventions. Our results suggest a large potential public health benefit of air pollutant reduction in reduced incidence of childhood asthma.
The impact of pediatric asthma on children, their family members, and society is significant. Asthma is one of the leading chronic diseases among children in the United States, where an estimated 1 in 12 children has asthma (1). In addition to potential long-term health impacts, such as chronic lung disease in adulthood (2), asthma affects children’s school attendance: children with asthma missed an average of 2.2 school days per year due to asthma (3). The economic burden of pediatric asthma includes both the medical costs, an estimated $833 to $1,121 per child annually across the United States, as well as costs of absenteeism due to missed school and workdays, which in 2012 totaled $5.9 billion (3).
Childhood asthma has long been identified as a major public health concern (4, 5), and there is increasing recent evidence supporting a role for air pollution in the development of asthma. In a recent systematic review and meta-analysis of traffic-related air pollution and childhood asthma incidence, Khreis et al. (6) reported asthma risk estimates of 1.05 (95% CI, 1.02 to 1.07) per 4 μg/m3 nitrogen dioxide (NO2) and 1.03 (95% CI, 1.01 to 1.05) per 1 μg/m3 particulate matter ≤2.5 μm (PM2.5). These estimates are generally small, but given the ubiquity of ambient exposure to NO2 and PM2.5 the implications for health at the population level may be considerable. In addition, several studies have attributed substantial proportions of childhood asthma (6% to 38% in different localities) to traffic-related air pollution (7–11).
Developments in the last several decades in epidemiologic methods, along with computational advances, have provided researchers with new analytical tools to strengthen estimation of causal effects and characterization of the burden of disease (12–17). Application of causal inference methods in air pollution epidemiology is limited but has been recently increasing (17–21). With G-computation, 1 of the causal inference methods, we can estimate the disease outcomes that would have been observed had exposure been different from what was actually experienced (i.e., different counterfactual scenarios) (12–15). In a previous report from the Southern California Children’s Health Study (CHS), in which we used data from a 20-y period of air quality improvement, we observed that declining asthma incidence rates were associated with declining regional NO2 and PM2.5 concentrations (22). Using these same data, coupled with the G-computation method (12–15), a substitution estimator, we now explore what would have happened to asthma rates in this population under several hypothetical interventions (i.e., counterfactual scenarios) on these 2 air pollutants. G-computation in the current analysis was performed with the aim of computing a standardized mean outcome across the observed covariate distribution, which allows us to obtain marginal rather than conditional estimates. Comparing health outcomes under the observed natural course of exposure with those under counterfactual (i.e., possibly contrary to the fact) exposures can provide results that may be more intuitive for regulators compared with risk or rate ratios, commonly used measures of association in epidemiologic studies. For results to have a causal, rather than an associative, interpretation, several assumptions are required, which we discuss here.
Results
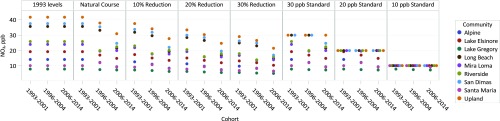
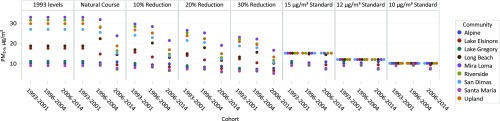
Among the 4,140 children in this study, the mean age at study entry was 9.5 y, and there was a fair balance of boys (47%) and girls (53%) overall and in each of the 3 cohorts from the CHS from 1993 to 2001, 1996 to 2004, and 2006 to 2014 (SI Appendix, Table S1). Participants were followed for an average of 5.9 y to identify 525 incident asthma cases over the study period. Over the course of the study during which these 3 cohorts were recruited, NO2 and PM2.5 concentrations declined in the 9 study communities based on community-specific central-site monitoring. In 1993, the mean NO2 concentration was 24.0 ppb (range, 8.0 to 41.7 ppb), which declined to 21.7 ppb (range, 7.6 to 38.0 ppb) in 1996, and then to 17.8 ppb (range, 7.2 to 30.9 ppb) in 2006. Similarly, the mean PM2.5 concentration declined from 20.8 μg/m3 (range, 8.9 to 32.9 μg/m3) in 1993 to 19.8 μg/m3 (range, 9.0 to 31.6 μg/m3) in 1996, and then to 13.7 μg/m3 (range, 7.1 to 23.6 μg/m3) in 2006. The distributions of NO2 and PM2.5 under the 7 hypothetical interventions for each pollutant are shown in Figs. 1 and 2, as well as SI Appendix, Table S2. The same 9 concentrations (for the 9 communities) are repeated 3 times (for the 3 cohorts) under the first hypothetical intervention, where 1993 concentrations levels are maintained. As expected, the mean air pollutant concentrations under this intervention are higher than observed mean concentrations because we did not allow levels to decline. Under hypothetical interventions in which we lowered air pollution, either by percentage reductions (−10%, −20%, and −30%) or threshold interventions (30, 20, and 10 ppb for NO2; 15, 12, and 10 μg/m3 for PM2.5), the mean and range of air pollutant concentrations were lower for these scenarios than the natural course of exposure (i.e., observed exposure). For the threshold interventions, the maximum possible air pollutant concentrations were the threshold values (i.e., hypothetical air quality standards); therefore, multiple communities had the same concentration value, as seen in Figs. 1 and 2. The numbers of participants and cohort-communities (3 cohorts each with 9 communities equates to a total of 27 cohort-communities) affected by the interventions are displayed in SI Appendix, Table S2.
Fig. 1.
Distribution of nitrogen dioxide (NO2) annual average concentrations among the 27 cohort-communities under the natural course of exposure and 7 hypothetical exposure interventions.
Fig. 2.
Distribution of particulate matter ≤2.5 μm (PM2.5) annual average concentrations among the 27 cohort-communities under the natural course of exposure and 7 hypothetical exposure interventions.
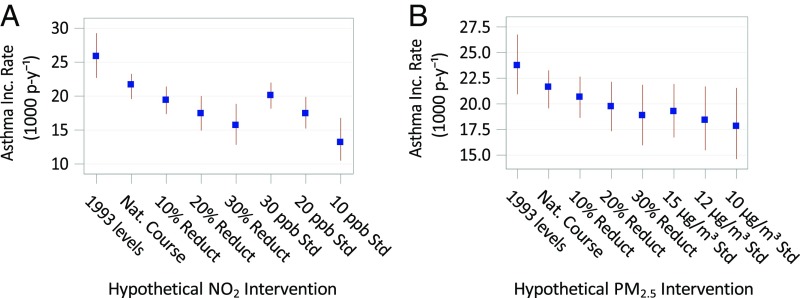
Estimates of the effect of the hypothetical NO2 and PM2.5 interventions on asthma incidence rates along with their 95% CIs are displayed in Table 1 and Fig. 3. Had NO2 concentrations remained at their 1993 levels, and the observed decline not occurred, the asthma incidence rate among our study population was estimated as 19.3% higher (95% CI, 8.9% to 31.6%) compared with the natural course. Had all communities experienced NO2 concentrations 30% lower than levels observed, we estimated the asthma incidence rate would have been 27.6% lower (95% CI, −39.3% to −14.6%) compared with the natural course. Statistically significant reductions in asthma incidence rates were observed for more moderate percentage reduction in NO2, although the effect estimates were smaller, as would be expected. The asthma incidence rate would have been 39.2% lower (95% CI, −50.4% to −23.3%) in our study population compared with the natural course, had there been complete adherence to a hypothetical air quality standard of 10 ppb NO2. For a more moderate hypothetical air quality standard of 20 ppb NO2, incidence rates would have been 19.6% lower (95% CI, −27.0% to −10.7%) compared with the natural course. Results were not markedly different in sensitivity analyses with additional covariates in the prediction model.
Table 1.
Estimated effects of hypothetical air pollutant interventions on asthma incidence rates in the CHS, 1993 to 2014
| Air pollutant intervention | Asthma incidence rate (95% CI) | Absolute incidence rate difference (95% CI) | Percentage incidence rate difference (95% CI) |
| NO2 | |||
| Natural course | 21.6 (19.6 to 23.3) | Reference | Reference |
| Remain at 1993 levels | 25.8 (22.7 to 29.3) | 4.2 (1.9 to 6.9) | 19.3% (8.9% to 31.6%) |
| Percentage reduction | |||
| 10% | 19.4 (17.4 to 21.4) | −2.3 (−3.5 to −1.1) | −10.5% (−16.0% to −5.2%) |
| 20% | 17.4 (14.9 to 20.0) | −4.2 (−6.3 to −2.1) | −19.6% (−28.9% to −10.1%) |
| 30% | 15.7 (12.8 to 18.8) | −6.0 (−8.6 to −3.1) | −27.6% (−39.3% to −14.6%) |
| Hypothetical standards | |||
| 30 ppb | 20.1 (18.2 to 22.0) | −1.6 (−2.4 to −0.8) | −7.3% (−11.2% to −3.5%) |
| 20 ppb | 17.4 (15.2 to 19.9) | −4.2 (−5.9 to −2.3) | −19.6% (−27.0% to −10.7%) |
| 10 ppb | 13.2 (10.5 to 16.8) | −8.5 (−11.0 to −5.0) | −39.2% (−50.4% to −23.3%) |
| PM2.5 | |||
| Natural course | 21.6 (19.6 to 23.3) | Reference | Reference |
| Remain at 1993 levels | 23.7 (20.9 to 26.8) | 2.1 (0.2 to 4.4) | 9.8% (0.9% to 20.4%) |
| Percentage reduction | |||
| 10% | 20.6 (18.6 to 22.7) | −1.0 (−1.9 to −0.1) | −4.5% (−8.9% to −0.4%) |
| 20% | 19.7 (17.3 to 22.2) | −1.9 (−3.6 to −0.2) | −8.8% (−16.8% to −0.9%) |
| 30% | 18.9 (16.0 to 21.9) | −2.8 (−5.2 to −0.3) | −12.8% (−23.9% to −1.3%) |
| Hypothetical standards | |||
| 15 µg/m3 | 19.2 (16.7 to 21.9) | −2.4 (−4.3 to −0.2) | −11.0% (−20.1% to −1.2%) |
| 12 µg/m3 | 18.4 (15.5 to 21.7) | −3.2 (−5.7 to −0.3) | −14.9% (−26.5% to −1.6%) |
| 10 µg/m3 | 17.8 (14.6 to 21.5) | −3.8 (−6.6 to −0.4) | −17.6% (−30.7% to −1.9%) |
Estimated incidence rates (cases per 1,000 person-years) and 95% CIs based on multilevel Poisson regression models with an offset term for person-time, a random effect for cohort nested within community, and adjusted for community, age, sex, ethnicity, race, presence of gas stove in the home, participation in team sports, and baseline year mean ambient temperature.
Fig. 3.
Estimated asthma incidence rate (cases per 1,000 person-years) and 95% CIs based on G-computation for nitrogen dioxide (NO2) (A) and particulate matter ≤2.5 μm (PM2.5) (B), separately, under the natural course of exposure and 7 hypothetical pollutant interventions.
Estimates for hypothetical interventions on PM2.5, while reaching statistical significance, were smaller compared with the results for NO2. Had PM2.5 concentrations remained at their 1993 levels, and the observed decline not occurred, the asthma incidence rate among our study population would have been 9.8% higher (95% CI, 0.9% to 20.4%) compared with the natural course. Had all communities experienced PM2.5 concentrations 30% lower than levels observed, the asthma incidence rate would have been an estimated 12.8% lower (95% CI, −23.9% to −1.3%) compared with the natural course of exposure.
Parameter estimates from the main air pollution models were robust in sensitivity analyses, as shown in SI Appendix, Table S3. Restricting to subjects with longer follow-up and adjusting for additional covariates did not markedly change the point estimates. Results for time-varying air pollution exposure, based on Cox proportional hazards models with no random effect, were slightly attenuated compared with the main models. There was no evidence of heterogeneity of results by sex, ethnicity, race, exposure to smoking in utero, exposure to secondhand smoke, parental education, parental history of asthma, or high/low 1993 air pollution level, as demonstrated in SI Appendix, Table S4.
Discussion
There is mounting evidence that exposure to air pollution causes childhood asthma, but there has been limited assessment of the benefits of reducing exposure. We used G-computation techniques to estimate the effect of counterfactual scenarios of the pollutant exposure on the rate of asthma incidence in participants of the Southern California Children’s Health Study. Had regional NO2 not declined in the 1993 to 2006 period, estimates of asthma incidence rates in this study population would have been almost 20% higher than what was observed, quantifying a positive public health impact of air quality improvements in the southern California region. Furthermore, had the NO2 concentration been lower than observed, asthma incidence rates would have been markedly lower. Similar results were estimated for hypothetical interventions on PM2.5, although estimated reductions in asthma incidence were not as large as those estimated for NO2.
Several studies have examined the burden of pediatric asthma attributable to air pollution (7–11). All have focused specifically on traffic-related air pollution, and all but 1 of these studies used a crude proxy, living within close proximity to a major roadway, as the exposure. Using a full-chain atmospheric dispersion model and a pollutant-specific meta-analytic exposure–response function, Khreis et al. estimated that 18% (95% CI, 8% to 24%) of all childhood asthma cases in Bradford, England, were attributable to NO2; this estimate increased slightly when exposure was instead based on a land-use regression model, to 24% (95% CI, 11% to 29%). The remaining studies evaluated near-roadway air pollution, which we did not evaluate in the current study. Three studies have examined asthma burden in southern California localities. In 2008, Kunzli et al. estimated 9.3% of all asthma cases in Long Beach to be attributable to living with 75 m of a busy road (11). The following year, Perez and colleagues reported a similar 9.2% (95% CI, 8.6% to 10.3%) in Long Beach and 6.1% (95% CI, 5.5% to 6.6%) in Riverside of asthma cases attributable to traffic proximity (8). A later study found that 8% (95% CI, 2% to 16%) of asthma cases in Los Angeles County was attributable to residential proximity to a major roadway, and when dispersion-modeled near-roadway NOX was used instead for exposure, this estimate increased slightly to 12% (95% CI, 2% to 20%) (7). The authors also investigated the effect of a 20% decrease in the proportion of children currently living within 75 m of a major roadway and reported a 2% reduction (95% CI, −4% to −0.3%) in asthma cases. In a study of 10 European cities, 14% (95% CI, 3% to 25%) of childhood asthma cases were attributable to near-road traffic-related pollutants. All the aforementioned studies, with the exception of Khreis et al., relied on the same odds ratio linking living <75 m of a major road to prevalent asthma (23). We cannot directly compare results from these attributable risk models to the current study’s results, since here we compare the natural course of exposure to hypothetical nonzero air pollution levels rather than no exposure. Furthermore, in contrast to these past studies, which focused exclusively on traffic-related air pollution, either based on a binary proxy or modeled exposure, the current study examined the impact of regional air pollutant concentrations. This may, however, be a reason why our results for NO2 seem generally larger compared with these previous studies.
The study has several strengths. First, we use data from a well-established, long-term prospective cohort study of cardiopulmonary outcomes in children. Second, using the rich data available in the CHS, we are evaluating the impact of air pollution on asthma incidence rather than on prevalence, which better captures the etiologic relation. Third, the exposure assessment is based on quantitative data from ambient air pollutant monitoring stations in each of the study communities that have been continuously measuring regional air pollution since the inception of the first CHS cohort (24, 25). Fourth, using a natural experiment design leveraging improvements in air quality during the study period, regional pollutant effects were identified based on changes in air pollutant levels and asthma rates in different cohorts within each community, which controls for spatial confounding. As a final strength, we utilized a causal inference method, G-computation, to estimate asthma incidence rates under several hypothetical air pollution scenarios, allowing for estimates of the answer to the intuitive question, “How would the incidence rate of asthma in our participants change if we could modify their exposure to regional NO2 (or PM2.5)?” Often in epidemiology, the choice of measure of association is dictated by the regression model employed (e.g., odds ratios from logistic regression for binary outcomes) rather than the research question of interest (26). In the current study, instead of simply presenting conditional incidence rate ratios for a 1-unit change in air pollution exposure, we chose to present a population intervention measure that estimates asthma incidence rates had exposure been, for example, no higher than 30 ppb NO2. This presentation of the results moves beyond the report of point estimates and may improve the translation of this study to policymakers (26).
This study has limitations. First is that we used baseline, rather than time-varying, community-level annual average pollutant concentration as the exposure. Although we present a sensitivity analysis using time-varying exposure fitted with Cox proportional hazards regression, this was not used as the model basis for our G-computation approach because it was not possible to obtain estimates with our multilevel modeling approach (e.g., when we included a fixed effect for community and a random effect of cohort nested within community). Point estimates from the time-varying Cox models were similar, although slightly attenuated, compared with those from the main model. This may be due to missing air pollution data across time, which were imputed and may have resulted in more exposure misclassification bias compared with the use of baseline exposure. Another consideration is the restriction to a 1-y lag on these time-varying data due to limited study central site monitoring data before 1993, which may not capture the most relevant time window of exposure. For these reasons, baseline community-level air pollution was used as the exposure metric. Second, these effect estimates are specific to this population, particularly because the interventions we employed, and their contrast with the natural course, are dependent on the observed exposure levels for the 27 cohort-communities (e.g., the estimated effect of a 10% reduction in NO2 will depend upon the starting concentrations: 50 ppb decreased to 45 ppb would have a different effect estimate compared with 20 ppb decreased to 18 ppb). Although the exposure distribution comes from southern California communities, these exposures reflect almost the full range of exposure observed across the United States. Results are also dependent on the covariate distribution in our study population and may affect the generalizability of these findings, which is a concern in any epidemiologic study. We did, however, examine potential interactions and did not find results to differ by any subgroups, lending more plausibility for the generalizability of the results. For example, there is a larger proportion of Hispanic children in the study population compared with the US child population, but there was no significant interaction between Hispanicity and NO2, indicating results are expected to be similar between Hispanics and non-Hispanic children. Last, there is a possibility of outcome misclassification bias due to a reliance on a questionnaire-based assessment of physician-diagnosed asthma in the definition of incident asthma, rather than a clinical evaluation of asthma. In validation studies of questionnaire-based asthma diagnosis in children, using similar questions to those in the present study, specificity compared with a clinical assessment as the reference standard was 87% (27), and compared with a previously validated health claims data diagnosis as the standard was 96% (28).
The method used in this study allowed for the estimation of the effect on asthma incidence of several different air pollutant intervention scenarios. For these findings to be interpreted as causal, several assumptions are required (12, 14). These assumptions are not exclusive to causal inference methods; most are common to many if not all empirical analytic approaches, but often are not expressly evaluated in the literature. By explicitly considering them here, we provide important information to the reader as to the level of interpretation of these results. We assumed exchangeability, which implies adequate control for confounders and selection bias (29, 30). Our multilevel modeling approach with an additional fixed-effect for community means we are making within-community (over time) comparisons that may help control for unmeasured community-level (i.e., spatial) confounding. Adding more individual-level covariates to the model did not markedly change effect estimates. Bias may be induced when selection of participants is informed by both the exposure and outcome of interest, thereby distorting the relation between exposure and outcome (31). Previous analysis of this cohort found that while participation rates varied by community (range, 65% to 86%), they did not correlate with either pollution concentrations or disease prevalence (25), indicating selection bias may not be of major concern.
The assumption of counterfactual consistency asserts that exposure levels correspond to a well-defined intervention (32). While exposure to a specified level of NO2 or PM2.5 is, arguably, well-defined, exactly how a community changes their air pollution levels may affect asthma incidence through pathways besides air pollution. Which and how upstream factors are targeted to reduce air pollutant levels may have ramifications for the health outcome of interest, which would violate the counterfactual consistency assumption. For example, if efforts were made to switch from vehicles to biking and walking for all short-distance trips, air quality would improve, but the population’s physical activity would also improve, which may lead to lower childhood obesity and subsequently less asthma (33). There are, however, few established modifiable risk factors for incident childhood asthma, beyond exposure to tobacco smoke and possibly obesity (33–35), meaning few other pathways through which the exposure intervention can affect the outcome. Nonetheless, we assume that the specific intervention that changes the air pollutant concentration does not matter, only that it was changed.
We also assume positivity, which means that in every confounder subgroups, all exposure values must be experienced (36). Because we include a community as a confounder in our model, there are violations of the positivity assumption because not all communities can experience all levels of the exposure, particularly very high or very low concentrations. This is why we employed dynamic interventions that imposed plausible hypothetical exposure level based on the community’s observed air pollutant level (e.g., remaining at observed 1993 levels, a percentage reduction from observed levels, etc.) and chose to contrast the estimated standardized mean outcomes under the interventions with the mean outcome under natural course of exposure (i.e., observed exposure), which is well-supported by the data. The dynamic intervention assigns exposure in response to a subject’s observed air pollution value, thereby assigning counterfactual exposures that were possible/realistic given a subject’s covariate values (37). In this manner, G-computation is less affected by violations of the positivity assumption (13). Counterfactual outcomes among some restricted range of possible values of air pollution concentration weakens the positivity assumption by requiring sufficient variability only in the assignment of treatment levels within the target range (37). It is important to note, however, that among the examined hypothetical interventions, the threshold-based (dynamic) interventions, particularly those with lower thresholds, are more susceptible to bias due to positivity violations. Our estimator, the air pollution-asthma model, allows for the G-computation estimator to extrapolate based on covariate strata with sufficient exposure levels, such as low concentrations of air pollution. This extrapolation in the G-computation depends greatly on the underlying Poisson model, and effect estimates will be biased if this model is misspecified (37).
In addition, we assumed a correctly specified model. Misspecification of the underlying Poisson model would produce biased results. Variations of the model produced similar results, and the model-based prediction of the natural course almost exactly estimates the observed incidence rate (modeled 21.649 compared with observed 21.646 per 100,000 person-years), both indicating no gross model misspecification. Nevertheless, this evidence cannot completely rule out model misspecification (38). In addition, while this model may adequately capture the exposure–response relationship at this range of air pollution concentrations, it should not be extended to concentrations beyond the support of the data. With this analytical method, we would also have to assume it is the pollutant itself (i.e., NO2 or PM2.5) that is the causal agent. We know, however, that air pollutants are neither produced nor experienced in isolation: they are complex, correlated mixtures, and a single pollutant often serves as an index of these mixtures. Therefore, we conceptualize this assumption to mean the causal agent is captured by the processes represented by the quantitative measure of the air pollutants.
Conclusion
Our study demonstrated a large potential public health benefit of air pollution reduction, both realized and hypothetical improvements, in reduced asthma incidence in children. Because regional air pollution levels are experienced by all community members, albeit with some variation in individual exposure due to differences in behavior and microenvironments’ concentrations, shifts in exposure at the population level have the potential for large benefits in reduced incidence rate of an outcome (39). These findings were observed in communities at NO2 concentrations well below the current US Environmental Protection Agency annual standard of 53 ppb (40), indicating that there may be public health benefits in reevaluating air quality standards.
Materials and Methods
The CHS is a cohort study of cardiopulmonary outcomes in children recruited from public schools starting in 1993 in 12 southern California communities. Data from 3 consecutive cohorts of the CHS, recruited in 1993, 1996, and 2002, are used in this analysis. In the parent study, follow-up for 2 of the cohorts began in fourth grade (1993 and 1996), while follow-up for the most recent cohort began in K/first grade (2002), and all cohorts were followed until graduation from high school. To have comparable data between the 3 cohorts for the current analysis, we restricted analysis to the 9 communities participating in all 3 cohorts and for the 2002 cohort set follow-up to begin in 2006, the year when most participants in this cohort were in the fourth grade. These 3 cohorts are hereafter referred to as the 1993 to 2001, 1996 to 2004, and 2006 to 2014 cohorts. Of the 6,858 participants, we excluded 892 (13.0%) with prevalent physician-diagnosis asthma at baseline and 1,348 (19.7%) with missing baseline asthma status, and an additional 478 (7.0%) had no follow-up questionnaires, leaving a sample of 4,140 children for analysis. All parents or guardians of participating children provided written informed consent. The study protocol was approved by the Institutional Review Board of the University of Southern California.
Incident asthma was defined as first reported physician-diagnosed case of asthma on an annual questionnaire during follow-up (i.e., first time answered “yes” to the question “Has a doctor ever diagnosed this child as having asthma?” when parent or guardian asked, or “Has a doctor ever said you have asthma?” when child asked). Date of diagnosis was imputed by using the midpoint between the date of the questionnaire on which asthma diagnosis was first reported and the date of the questionnaire before reporting asthma status.
Regional Air Pollutant Exposure.
Since the start of the CHS study, air pollutant monitoring stations in each study community have been continuously measuring regional air pollutants, including NO2 and PM2.5, as previously described (24, 25). We calculated community-specific annual average concentrations in the baseline year for each cohort (i.e., 1993, 1996, and 2006) based on 24-h averages for NO2 and PM2.5. Data were not available for 1993 on PM2.5 in any community; therefore, 1994 concentrations were used instead.
Covariates.
In all models we included child’s sex, age, ethnicity, race, participation in team sports, and presence of a gas stove in the home, as reported by the parent on the baseline questionnaire, as well as community-specific average temperature for the cohort-baseline year, based on data collected at monitoring stations. Additional variables collected at baseline used in sensitivity analyses include exposure to maternal smoking in utero, exposure to secondhand smoke, parental education, parental income, child’s insurance status, pests and pets in the home, carpet in the child’s bedroom and parental history of asthma.
Statistical Analysis.
We used G-computation, an imputation-based causal inference method, to estimate the potential effects of hypothetical interventions on regional NO2 and PM2.5 concentrations (separately). G-computation builds on a regression model of the outcome as a function of exposure and covariates to predict the outcome distribution under different, and possibly contrary to the fact (i.e., counterfactual), exposure scenarios (12–15). In the present analysis, this allowed us to ask, “What would have been the incidence rate of asthma if participant exposure to regional NO2 (or PM2.5) had been higher or lower?” To estimate the marginal asthma incidence rate under the natural course of exposure (no intervention) and hypothetical interventions we estimated the parameter coefficients for the observed exposure and covariate using a Poisson model with asthma incidence rates as the outcome, used the estimated coefficients to predict the asthma incidence rates in a Monte Carlo sample of 10,000 study participants under the natural course of exposure and the hypothetical exposure interventions, calculated the marginal asthma incidence rate for each exposure scenario (i.e., the natural course and interventions) by averaging the predicted outcome across participants, and contrasted the averaged outcomes to estimate the population-standardized asthma incidence rate difference. Confidence intervals were estimated by bootstrapping with replacement 10,000 times (41). We contrasted the hypothetical interventions with the natural course, using incidence rate differences.
The regression model used in step 1 above was developed as part of a prior study of regional air quality and asthma incidence in the CHS (22). This model was a multilevel Poisson regression of asthma incidence on regional air pollutant exposure and covariates with an offset term for person-time (natural log-transformed) and a fixed effect for community. Regional air pollutant exposure was defined as the community-level annual average concentration of said pollutant in the baseline year for each cohort. Using the directed acyclic graph shown in SI Appendix, Fig. S1 and directed acyclic graph theory (42), we decided a priori on a set of variables considered to be sufficient for confounding adjustment. The covariates were baseline age (continuous), sex (female, male), ethnicity (Hispanic, Non-Hispanic), race (Asian/Pacific Islander, Black, Native American Indian/other, White, mixed), presence of gas stove in the home (yes, no), physical activity defined here as team sports participation (yes, no), and community-specific mean ambient temperature for baseline year (continuous). In addition, to account for clustering effects of children by cohort and community, a random effect for cohort nested within community was included. Follow-up time was calculated as the number of days between joining the cohort (i.e., baseline questionnaire date) and either imputed date of asthma diagnosis or date of last completed questionnaire (either 12th grade or earlier if lost to follow-up), whichever came first. All hypotheses were tested assuming a 0.05 significance level and a 2-sided alternative hypothesis. All analyses were conducted using SAS software version 9.4 (SAS Institute, Cary, North Carolina).
Interventions Examined.
For each pollutant, we examined 3 sets of interventions (i.e., counterfactual scenarios): maintain air pollutant concentrations at their community-specific 1993 levels, the highest levels in the study before observed air pollution declines in the 1990s and early 2000s; an overall percentage reduction in pollutant concentration (−10%, −20%, and −30%); and a dynamic intervention using a threshold whereby only communities with a pollutant concentration above the threshold were “intervened” upon and set to the threshold value (30, 20, and 10 ppb for NO2; 15, 12, and 10 μg/m3 for PM2.5). We considered this last intervention to be dynamic because the threshold rule depends on the value of observed community-specific pollutant concentration, in contrast to a static intervention, which does not depend on the value of other variables (43). The first intervention examined what would have happened to rates of asthma incidence had air pollution not improved over the course of the study. Interventions 2 and 3 examined what would have happened to rates of asthma incidence had air pollution been lower than what was observed, with intervention 3 more closely mimicking scenarios of compliance with hypothetical air quality standards. Single-pollutant interventions were selected to be studied, as these most closely align with air quality standards, which are set for 1 pollutant at a time (e.g., US Environmental Protection Agency National Ambient Air Quality Standards).
Sensitivity Analysis.
To evaluate the robustness of the main models, the following sensitivity analyses were conducted: restricting to participants with longer follow-up (followed to year 5 or later, or to year 7 or later), including additional covariates to control for potential confounding, and using a time-varying air pollution exposure variable. This last sensitivity analysis was conducted using Cox proportional hazards regression, based on the same modeling approach as the main fully adjusted model, but with no random effect. No apparent violation of the underlying assumption of proportional hazards was detected based on inclusion of a time-dependent covariate for air pollution. Due to missing air pollution data for PM2.5 in all communities in 1993 and in 1 community in 2005, and no air pollution data for PM2.5 or NO2 after 2011, air pollution for these years was imputed by extending the closest years’ air pollution data (i.e., 1994 for 1993, 2006 for 2005, and 2011 for 2012 and later years). Heterogeneity of the air pollution point estimates were assessed by comparing nested models using a partial likelihood ratio test with and without interaction terms for the following variables: sex, ethnicity, race, exposure to smoking in utero, secondhand smoke exposure, parental education, parental history of asthma, and designation of high versus low air pollution community, based on whether community was above or below corresponding median annual mean concentration in 1993.
Data Availability.
Due to the limitations in the original consent forms and HIPAA requirements, the data from the CHS cannot be freely available in the manuscript, supplemental files, or in a public repository. However, we are committed to sharing the data and results acquired as part of this study. The CHS has a process in place for data sharing that involves approval of proposals by a Data Sharing Committee. Investigators who want access to data will be required to submit a research protocol, which will be reviewed by the CHS Health Data Release Committee and the USC IRB. Please send requests to access this dataset to Dr. Frank Gilliland (gillilan@usc.edu).
Supplementary Material
Acknowledgments
We thank Jon Samet for his review of the manuscript. We thank the participating students and their families, the school staff and administrators, and the members of the study field team for their efforts. This work was supported by the National Institute of Environmental Health Sciences (grants P30ES007048, P01ES009581, R01ES021801, and R01ES025786); the National Heart, Lung and Blood Institute (grant R01HL118455); the US Environmental Protection Agency (grants R826708 and RD831861); and the Hastings Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1815678116/-/DCSupplemental.
References
- 1.Zahran H. S., Bailey C. M., Damon S. A., Garbe P. L., Breysse P. N., Vital signs: Asthma in children–United States, 2001-2016. MMWR Morb. Mortal. Wkly. Rep. 67, 149–155 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeachie M. J., et al. , Patterns of growth and decline in lung function in persistent childhood asthma. N. Engl. J. Med. 374, 1842–1852 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nurmagambetov T., Khavjou O., Murphy L., Orenstein D., State-level medical and absenteeism cost of asthma in the United States. J. Asthma 54, 357–370 (2017). [DOI] [PubMed] [Google Scholar]
- 4.American Public Health Association (APHA) , “Childhood asthma a major public health problem (policy number 9101)” in Association News: APHA Policy Statements (American Journal of Public Health, 1992), vol. 82, p. 477. [Google Scholar]
- 5.Weiss K. B., Gergen P. J., Crain E. F., Inner-city asthma. The epidemiology of an emerging US public health concern. Chest 101 (suppl. 6), 362S–367S (1992). [DOI] [PubMed] [Google Scholar]
- 6.Khreis H., et al. , Exposure to traffic-related air pollution and risk of development of childhood asthma: A systematic review and meta-analysis. Environ. Int. 100, 1–31 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Perez L., et al. , Near-roadway pollution and childhood asthma: Implications for developing “win-win” compact urban development and clean vehicle strategies. Environ. Health Perspect. 120, 1619–1626 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez L., et al. , Global goods movement and the local burden of childhood asthma in southern California. Am. J. Public Health 99 (suppl. 3), S622–S628 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez L., et al. , Chronic burden of near-roadway traffic pollution in 10 European cities (APHEKOM network). Eur. Respir. J. 42, 594–605 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Khreis H., de Hoogh K., Nieuwenhuijsen M. J., Full-chain health impact assessment of traffic-related air pollution and childhood asthma. Environ. Int. 114, 365–375 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Künzli N., et al. , An attributable risk model for exposures assumed to cause both chronic disease and its exacerbations. Epidemiology 19, 179–185 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Robins J., A new approach to causal inference in mortality studies with a sustained exposure period—Application to control of the healthy worker survivor effect. Math. Model. 7, 1393–1512 (1986). [Google Scholar]
- 13.Robins J., A graphical approach to the identification and estimation of causal parameters in mortality studies with sustained exposure periods. J. Chronic Dis. 40 (suppl. 2), 139S–161S (1987). [DOI] [PubMed] [Google Scholar]
- 14.Naimi A. I., Cole S. R., Kennedy E. H., An introduction to g methods. Int. J. Epidemiol. 46, 756–762 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snowden J. M., Rose S., Mortimer K. M., Implementation of G-computation on a simulated data set: Demonstration of a causal inference technique. Am. J. Epidemiol. 173, 731–738 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zigler C. M., Dominici F., Point: Clarifying policy evidence with potential-outcomes thinking–Beyond exposure-response estimation in air pollution epidemiology. Am. J. Epidemiol. 180, 1133–1140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zigler C. M., et al. , “Causal inference methods for estimating long-term health effects of air quality regulations” (Research Report 187, Health Effects Institute, Boston, MA, 2016). [PubMed]
- 18.Moore K., et al. , Ambient ozone concentrations cause increased hospitalizations for asthma in children: An 18-year study in Southern California. Environ. Health Perspect. 116, 1063–1070 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz J., Austin E., Bind M. A., Zanobetti A., Koutrakis P., Estimating causal associations of fine particles with daily deaths in Boston. Am. J. Epidemiol. 182, 644–650 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., et al. , Estimating causal effects of long-term PM2.5 exposure on mortality in New Jersey. Environ. Health Perspect. 124, 1182–1188 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zigler C. M., Choirat C., Dominici F., Impact of national ambient air quality standards nonattainment designations on particulate pollution and health. Epidemiology 29, 165–174 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia E., et al. , Association of changes in air quality with incident asthma in children in California, 1993-2014. JAMA 321, 1906–1915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McConnell R., et al. , Traffic, susceptibility, and childhood asthma. Environ. Health Perspect. 114, 766–772 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauderman W. J., et al. , Association of improved air quality with lung development in children. N. Engl. J. Med. 372, 905–913 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters J. M., et al. , A study of twelve Southern California communities with differing levels and types of air pollution. I. Prevalence of respiratory morbidity. Am. J. Respir. Crit. Care Med. 159, 760–767 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Ahern J., Population intervention measures to connect research findings to policy. Am. J. Public Health 106, 2152–2153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hansen T. E., Evjenth B., Holt J., Validation of a questionnaire against clinical assessment in the diagnosis of asthma in school children. J. Asthma 52, 262–267 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Yang C. L., To T., Foty R. G., Stieb D. M., Dell S. D., Verifying a questionnaire diagnosis of asthma in children using health claims data. BMC Pulm. Med. 11, 52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland S., Robins J. M., Identifiability, exchangeability, and epidemiological confounding. Int. J. Epidemiol. 15, 413–419 (1986). [DOI] [PubMed] [Google Scholar]
- 30.Greenland S., Robins J. M., Identifiability, exchangeability and confounding revisited. Epidemiol. Perspect. Innov. 6, 4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole S. R., et al. , Illustrating bias due to conditioning on a collider. Int. J. Epidemiol. 39, 417–420 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole S. R., Frangakis C. E., The consistency statement in causal inference: A definition or an assumption? Epidemiology 20, 3–5 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Raj D., Kabra S. K., Lodha R., Childhood obesity and risk of allergy or asthma. Immunol. Allergy Clin. North Am. 34, 753–765 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Subbarao P., Mandhane P. J., Sears M. R., Asthma: Epidemiology, etiology and risk factors. CMAJ 181, E181–E190 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beasley R., Semprini A., Mitchell E. A., Risk factors for asthma: Is prevention possible? Lancet 386, 1075–1085 (2015). [DOI] [PubMed] [Google Scholar]
- 36.Westreich D., Cole S. R., Invited commentary: Positivity in practice. Am. J. Epidemiol. 171, 674–677 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen M. L., Porter K. E., Gruber S., Wang Y., van der Laan M. J., Diagnosing and responding to violations in the positivity assumption. Stat. Methods Med. Res. 21, 31–54 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robins J., Hernan M., Siebert U., “Effects of multiple interventions” in Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors, Ezzati M., Lopez A. D., Rodgers A., Murray C. J. L., Eds. (World Health Organization, Switzerland, 2004). [Google Scholar]
- 39.Rose G., Sick individuals and sick populations. Int. J. Epidemiol. 14, 32–38 (1985). [DOI] [PubMed] [Google Scholar]
- 40.United States Environmental Protection Agency (US EPA) , “Review of the primary national ambient air quality standards for oxides of nitrogen” (EPA-HQ-OAR-2013-0146, US EPA, Office of Air Quality Planning and Standards, Research Triangle Park, NC, 2018).
- 41.Efron B., Tibshirani R. J., An Introduction to the Bootstrap (CRC press, Boca Raton, 1993). [Google Scholar]
- 42.Greenland S., Pearl J., Robins J. M., Causal diagrams for epidemiologic research. Epidemiology 10, 37–48 (1999). [PubMed] [Google Scholar]
- 43.Hernán M., Robins J., Causal Inference (Chapman & Hall/CRC, Boca Raton, 2019), forthcoming.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the limitations in the original consent forms and HIPAA requirements, the data from the CHS cannot be freely available in the manuscript, supplemental files, or in a public repository. However, we are committed to sharing the data and results acquired as part of this study. The CHS has a process in place for data sharing that involves approval of proposals by a Data Sharing Committee. Investigators who want access to data will be required to submit a research protocol, which will be reviewed by the CHS Health Data Release Committee and the USC IRB. Please send requests to access this dataset to Dr. Frank Gilliland (gillilan@usc.edu).