Significance
The organization of microtubules into a bipolar spindle is essential for chromosome segregation. Both centrosome and chromatin-dependent spindle assembly mechanisms are well studied in animals; however, the mechanism of bipolar spindle assembly in plant meiosis remains unclear. We found the key step of plant bipolar spindle assembly is the correction of multipolar spindles into bipolar spindles at metaphase I. The multipolar spindles failed to transition into bipolar spindles in OsmtopVIB. However, the absence of cohesion subunit OsREC8 resulted in bipolar spindles in the background of OsmtopVIB. Thus, OsMTOPVIB plays a crucial role in meiotic spindle assembly. Biorientation of sister kinetochores in a univalent is essential for bipolar spindle formation when homologous recombination is absent.
Keywords: rice, meiosis, spindle assembly, kinetochore
Abstract
The organization of microtubules into a bipolar spindle is essential for chromosome segregation. Both centrosome and chromatin-dependent spindle assembly mechanisms are well studied in mouse, Drosophila melanogaster, and Xenopus oocytes; however, the mechanism of bipolar spindle assembly in plant meiosis remains elusive. According to our observations of microtubule assembly in Oryza sativa, Zea mays, Arabidopsis thaliana, and Solanum lycopersicum, we propose that a key step of plant bipolar spindle assembly is the correction of the multipolar spindle into a bipolar spindle at metaphase I. The multipolar spindles failed to transition into bipolar ones in OsmtopVIB with the defect in double-strand break (DSB) formation. However, bipolar spindles were normally assembled in several other mutants lacking DSB formation, such as Osspo11-1, pair2, and crc1, indicating that bipolar spindle assembly is independent of DSB formation. We further revealed that the mono-orientation of sister kinetochores was prevalent in OsmtopVIB, whereas biorientation of sister kinetochores was frequently observed in Osspo11-1, pair2, and crc1. In addition, mutations of the cohesion subunit OsREC8 resulted in biorientation of sister kinetochores as well as bipolar spindles even in the background of OsmtopVIB. Therefore, we propose that biorientation of the kinetochore is required for bipolar spindle assembly in the absence of homologous recombination.
Meiosis is a specialized cell cycle that employs a unique “reductional” nuclear division to produce haploid gametes. At the first meiotic division, homologous chromosomes are pulled to opposite poles. At the second meiotic division, sister chromatid segregation resembles mitosis (1). To guarantee the fidelity of homologous chromosome separation, several events are needed. First, homologous pairing, synapsis, and recombination promote the formation of chiasmata between homologous chromosomes. Homologous chromosomes are physically linked so that microtubules create tensions when they pull homologous chromosomes from opposite directions. Second, in kinetochore orientation, the sister kinetochores orient side-by-side (mono-orientation) in meiosis I, whereas they face opposite directions (biorientation) at meiosis II (2). Third, the formation of a bipolar spindle is also essential for chromosome segregation.
The spindle is a complex bipolar structure consisting of microtubules and microtubule-associated proteins mediating chromosome segregation during both mitosis and meiosis. In eukaryotic cells, microtubules are major structural components composed of α-, β-tubulin heterodimers (3). Microtubule-associated proteins, located on chromosomes or at microtubule minus (or plus) ends, are important players in microtubule nucleation, polymerization, transport, organization, and dynamic behavior. Uniform polarity of microtubules (with their minus ends at the poles and their plus ends at the spindle equator) forms the bipolar spindle. Although the basic structure of the spindle is similar in all cell types of all higher eukaryotes, the routes through which the spindle assembles can be substantially different. In mitosis and male meiosis in animals, the establishment of spindle bipolarity is mediated by centrosomes (composed of a pair of centrioles) that act as microtubule organization centers (MTOCs). Duplicated centrosomes, while migrating to opposite poles of the cell, nucleate radial arrays of microtubules called “asters,” with the minus ends of microtubules at the pole and the free plus ends extending away before nuclear envelope breakdown (NEB) (4). After NEB, microtubules penetrate the nucleus and are captured by kinetochores, thereby forming a bipolar spindle via a “search-and-capture” mechanism (5).
In most species, including Drosophila melanogaster (fruit fly), Caenorhabditis elegans (nematode), Xenopus laevis (frog), Gallus gallus (chicken), Mus musculus (mice), Homo sapiens (humans), and all other mammals analyzed thus far, female meiotic cells are acentrosomal, as they lack conventional centrosomes (6). In these cell types, bipolar spindles are assembled using a chromatin-driven pathway, named “self-assembly” (7). According to this model, upon nuclear envelope breakdown, microtubules grow around chromatins. A bipolar array is progressively organized through interactions between microtubules and chromosomes in the absence of centrosomes. During female meiosis in Xenopus and Drosophila, microtubules are nucleated at dispersed sites in the vicinity of chromatin with random polarity. Microtubules of mixed polarity coalesce into bundles and are rearranged into bipolar arrays (7–9). So the transition of multipolar spindles into bipolar ones is a common process during acentrosomal meiotic spindle assembly.
In land plants, centrosomes are typically absent (10). Plant MTOCs are diffuse and mobile entities that change in form and location during the cell and life cycles (11, 12). The cytoarchitecture of higher plant mitotic cells consists of diverse cytoskeletal arrays, such as hoop-like cortical microtubules, the preprophase band (PPB), the spindle, and the phragmoplast (10). Cortical microtubules are located beneath the plasma membrane during mitotic interphase. In early G2 phase, the cortical microtubule arrays are disassembled and the PPB appears. During the same phase, there is an explosion of microtubule assembly. Subsequently, an acentrosomal mitotic spindle that is perpendicular to the PPB assembles (13). The PPB marks the site at which the phragmoplast is formed as well as the future division plate during cytokinesis (14). In plants, analyses of mitotic microtubule arrays have been reported (15–20). The process of meiotic spindle assembly has been studied in maize and Arabidopsis thaliana (21–25). In maize, a model for meiotic spindle formation was proposed (21). Accordingly, microtubules initially associate with chromosomes at early metaphase I. Then, a bipolar spindle is constructed followed by self-organization of the microtubules. In Arabidopsis, several proteins involved in meiotic bipolar spindle assembly have been identified, including ATK1, ATK5, and PRD2/MPS1 (22, 23, 26, 27). Among them, PRD2/MPS1 is required for both DSB formation and bipolar spindle construction (23, 28).
In this study, we explored how a meiotic bipolar spindle is formed in plants, particularly the mechanism of bipolar spindle assembly upon nuclear envelope breakdown. By applying an immunofluorescence technique to monocot and dicot samples, we demonstrated that the construction of a meiotic bipolar spindle occurs via conversion of multiple poles into 2 poles. In particular, we verified that OsMTOPVIB, an initiation factor of homologous recombination conserved among various species (29, 30), is essential for bipolar spindle construction. Furthermore, we provide evidence that biorientation of sister kinetochores at the first meiotic division is essential for bipolar spindle formation when homologous recombination is aborted.
Results
From Multipolarity to Bipolarity, Spindle Assembly in Rice Meiosis I.
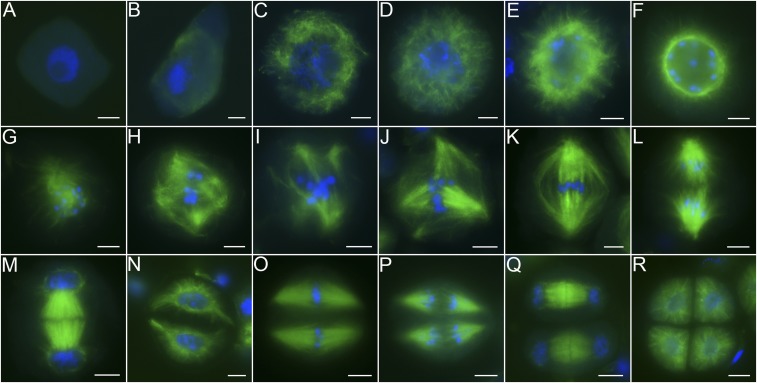
To analyze the mechanism of spindle assembly in plants, the cells of which lack centrosomes, immunofluorescence detection was carried out with rice pollen mother cells (PMCs). In wild-type PMCs, signals of α-tubulin could not be clearly detected at leptotene (Fig. 1A). Microtubules first appeared at the corner of the PMCs at early zygotene (Fig. 1B). Then, microtubules began to fill in throughout the cytoplasm from late zygotene to pachytene and a typical reticular cytoskeleton appeared at pachytene (Fig. 1 C and D). From diplotene to early diakinesis, bundles of microtubules emanating from the nuclear envelope could be clearly distinguished, as they located in 1 plane outside the nucleus with a radial cytoskeleton (Fig. 1E). After that, microtubules aggregated around the nucleus until a typical bright perinuclear ring formed around the nuclear envelope (Fig. 1F). There were no notable bipolar microtubule arrays at stages that occurred before NEB. After NEB, the perinuclear cytoskeleton structure disappeared, microtubules penetrated the nucleus, and chromosomes congregated at the middle of the cell (Fig. 1G). The phenomenon that microtubules accumulated in the vicinity of the chromosomes before chromosomes congregating at the equatorial plate might expose kinetochores to a high density of microtubules, thereby facilitating the subsequent formation of bipolar attachments. Interactions between microtubules and chromosomes resulted in multipolar intermediates at diakinesis (Fig. 1H). Redundant poles disappeared progressively, leaving tetrapolar or tripolar spindles at early metaphase I (Fig. 1 I and J). A typical bipolar spindle with bundles of microtubule fibers connecting to kinetochores appeared with chromosomes on the equator at metaphase I (Fig. 1K). At anaphase I, the spindle pulled chromosomes to opposite poles, and newly formed microtubules at the spindle equator were observed at the same time (Fig. 1L). The newly formed microtubules seem to provide a force that pulled homologous chromosomes to opposite poles. The phragmoplast, which is a cytoskeletal structure held by 2 arrays of microtubule bundles, appeared at telophase I (Fig. 1M). It centrifugally expanded and guided vesicle-mediated deposition of cell-wall materials to generate the cell plate for cytokinesis. After cytokinesis, a typical dyad formed (Fig. 1N). Chromosomes aligned on the equatorial plate by 2 sets of spindles at metaphase II (Fig. 1O). Finally, sister chromatids were separated at anaphase II, and a typical tetrad formed at telophase II (Fig. 1 P–R).
Fig. 1.
The process of meiotic spindle assembly in rice. (A) Leptotene. (B) Early zygotene. (C) Late zygotene. (D) Pachytene. (E and F) Early diakinesis: radial microtubules (E) and bright “perinuclear ring” (F). (G and H) Diakinesis. (I and J) Early metaphase I, tetrapolar and tripolar spindles are shown. (K) Metaphase I showing bipolar spindles. (L) Anaphase I. (M) Telophase I. (N) Dyad. (O) Metaphase II. (P) Anaphase II. (Q) Telophase II. (R) Tetrad. Meiotic chromosomes were stained with DAPI (blue), and microtubules were immunodetected with α-tubulin antibody (green). (Scale bars: 5 μm.)
The Meiotic Spindle Assembly Process Is Conserved in Both Monocots and Dicots.
We investigated the process of spindle bipolarization in maize, Arabidopsis, and tomato (SI Appendix, Fig. S1). The perinuclear ring of cytoplasmic microtubules appeared to surround the nuclear envelope before NEB at early diakinesis (SI Appendix, Fig. S1 A, G, and M). After NEB, a chaotic stage with multipolar spindles was observed at diakinesis (SI Appendix, Fig. S1 B, H, and N). Then, tripolar spindles were frequently found at early metaphase I (SI Appendix, Fig. S1 C, I, and O). A typical bipolar spindle formed at metaphase I (SI Appendix, Fig. S1 D, J, and P). Chromosomes synchronously segregated toward opposite poles by the anaphase I spindles (SI Appendix, Fig. S1 E, K, and Q). At telophase I, an obvious dyad formed after cytokinesis (SI Appendix, Fig. S1 F, L, and R). Therefore, the similar processes of spindle assembly in rice, maize, Arabidopsis, and tomato indicated that the transition from multipolarity to bipolarity is a common occurrence in both monocots and dicots.
Transition from a Multipolar to Bipolar Spindle Requires OsMTOPVIB.
We performed a wide search of rice mutants with defects in meiotic spindle assembly. This search identified a sterile mutant in which multipolar spindles failed to transition into bipolar spindles. The target gene was mapped onto chromosome 6, a region of ∼100 kb. Within this region, sequencing identified a G-to-A transition at the sixth intron of OsMTOPVIB (SI Appendix, Fig. S2A), which introduced a new splice form resulting in the premature termination of translation. This is an allele of OsMTOPVIB that has not been previously reported. OsMTOPVIB has been proved to be essential for double-strand break (DSB) formation (29, 30). No γH2AX signals were observed in OsmtopVIB (SI Appendix, Fig. S2B), indicating that DSBs were completely hindered in OsmtopVIB. We then observed chromosomal behavior at different meiotic stages in the wild type and OsmtopVIB PMCs (SI Appendix, Fig. S2C). In the wild type, chromosomes appeared as long thin threads at leptotene, and homologous chromosome pairing and synapsis initiated at zygotene and fully synapsed at pachytene. After that, 12 highly condensed bivalents were observed at diakinesis and divided equally between the 2 poles at telophase I. In OsmtopVIB PMCs, the chromosome behavior was similar to that in the wild type at leptotene. However, obvious features of homologous chromosome pairing were not observed in the meiocytes of OsmtopVIB. Chromosomes appeared as long, thin thread-like structures at pachytene. Twenty-four chromosome univalents were present at diakinesis. Strikingly, we found that chromosomes were pulled to more than 3 groups in OsmtopVIB, in contrast to 2 sets observed in the wild type (SI Appendix, Fig. S2C).
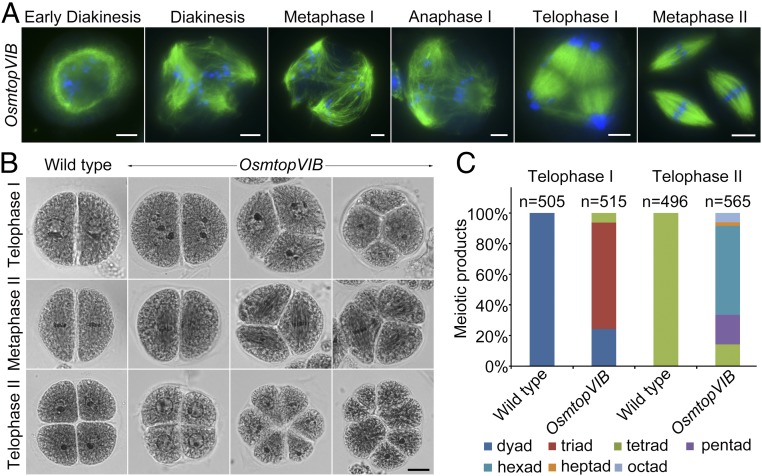
We checked spindle morphogenesis by indirect immunofluorescence staining using anti–α-tubulin antibodies on meiocytes of OsmtopVIB (Fig. 2A). In OsmtopVIB PMCs, dynamics of spindle assembly were as normal as those observed in the wild type at prophase I. Obvious cytoskeletal defects were not observed at early diakinesis. During NEB, the perinuclear cytoskeleton structure disappeared and microtubules penetrated the nucleus. Microtubules accumulated in the vicinity of the chromosomes similar to wild-type PMCs at diakinesis. In contrast to bipolar spindles in wild type at metaphase I, multipolar spindles were frequently observed in OsmtopVIB. Furthermore, most multipolar spindles could not transition into normal bipolar spindles. Chromosomes were often found unaligned, distributed along the spindle or closely positioned to the poles. They were always pulled to 3 poles at anaphase I. As a result, triads were frequently observed at telophase I. At metaphase II, 3 instead of 2 sets of spindles aligned on the equator of the cell, indicating a potential role of OsMTOPVIB in the organization and dynamics of microtubules during meiosis. Arabidopsis PRD2/MPS1 is required for both spindle orientation and DSB formation (23, 28). We found that OsMTOPVIB1-427 interacts with OsPRD2 in yeast 2-hybrid assays and bimolecular fluorescence complementation (BiFC) assays (SI Appendix, Fig. S3), implying that these proteins may function in the same pathway. Further experiments verified that OsPRD2 interacts with OsMTOPVIB359-427, but not OsMTOPVIB1-234 (SI Appendix, Fig. S3), suggesting that the transducer domain of OsMTOPVIB contributes the interaction.
Fig. 2.
OsMTOPVIB is essential for bipolar spindle assembly. (A) The process of meiotic spindle assembly in OsmtopVIB. Chromosomes were stained with DAPI (blue), and microtubules were immunostained with α-tubulin antibody (green). (Scale bars: 5 μm.) (B) Products of meiosis in wild type and OsmtopVIB. In wild-type meiocytes, dyads at telophase I and tetrads at telophase II are shown. In OsmtopVIB, chromosomes are pulled to 2, 3, or 4 groups at telophase I. Tetrads, hexads, and octads are shown in OsmtopVIB at telophase II. (Scale bars: 5 μm.) (C) Frequencies of meiotic products in wild-type and OsmtopVIB meiocytes. At telophase I, 100% dyads are in wild type, while 24.27% dyads, 70.29% triads, and 5.44% tetrads are in OsmtopVIB (n = 515). At telophase II, 100% tetrads are in wild type (n = 505), while 14.16% tetrads, 19.30% pentads, 58.23% hexads, 2.12% heptads, and 6.19% octads are in OsmtopVIB (n = 565).
Abnormal Spore Formation in OsmtopVIB.
To characterize the abnormal meiotic product formation in OsmtopVIB, the frequency of abnormal spores was quantified (Fig. 2 B and C). We found that chromosomes were divided into 2, 3, or 4 groups at anaphase I, leading to a large proportion of triads (70.29%) and tetrads (5.44%) at telophase I in OsmtopVIB. At metaphase II, the expected 2 spindles were observed in the wild-type PMCs, while 3 and 4 sets of spindles were more frequently observed in the mutant. Finally, polyads, such as pentads (19.30%), hexads (58.23%), heptads (2.12%), and octads (6.19%), were often observed in OsmtopVIB.
Meiotic Spindle Assembly Is Independent of DSB Formation.
To explore if the occurrence of multipolar spindles in OsmtopVIB meiocytes is a consequence of defects in DSB formation, we investigated the meiotic spindle assembly in 3 other mutants with defects in DSB formation, including pair2 (SI Appendix, Fig. S4), Osspo11-1, and crc1 (SI Appendix, Fig. S5). PAIR2, the rice homolog of Saccharomyces cerevisiae HOP1 and Arabidopsis ASY1, is required for homologous pairing (31). OsSPO11-1 is a homolog of subunit A of archaebacterial topoisomerase, which catalyzes DSBs and initiates homologous recombination (32). CRC1 is orthologous to S. cerevisiae Pch2 and Mus musculus TRIP13 and plays a key role in DSB formation in rice (33). We found multipolar spindles normally formed at diakinesis and transitioned to bipolar spindles at metaphase I in those mutants, suggesting bipolar spindle assembly is independent of DSB formation. We also generated a pair2 OsmtopVIB double mutant and checked its meiotic spindle assembly. Multipolar spindles were prevalently observed in the double mutant at metaphase I (SI Appendix, Fig. S4B), which is similar to those in OsmtopVIB, rather than bipolar spindles occurred in pair2. Therefore, spindle bipolarization is prevented by the mutation of OsMTOPVIB in the background of pair2.
To reveal why there is a difference between OsmtopVIB and other mutants with defects in DSB formation, we conducted fluorescence in situ hybridization (FISH) assays on metaphase I chromosomes of wild type, OsmtopVIB, pair2, crc1, Osspo11-1, and pair2 OsmtopVIB meiocytes (SI Appendix, Fig. S4C). CentO, a centromere-specific tandem repeat, was used as the probe to detect the kinetochore–microtubule attachment. In wild type, all 12 bivalents lined up on the metaphase I plate with kinetochores connecting with bundles of microtubules from opposite poles. Among them, sister kinetochores within a chromosome were attached to microtubules from 1 direction. Unexpectedly, there were always some univalents with bipolar attachments of sister kinetochores in pair2, crc1, and Osspo11-1 PMCs. However, in OsmtopVIB and pair2 OsmtopVIB meiocytes, monopolar attachments were prevalent. We speculate that the biorientation of sister kinetochores of univalents might cause bipolar spindle assembly when bivalents are absent.
Bipolar Spindles and Biorientation of Sister Kinetochores in Haploid Cells.
As the haploid rice contains 1 set of homologous chromosomes, only univalents are formed during meiosis. We explored the spindle assembly process in haploid rice by indirect immunofluorescence staining (SI Appendix, Fig. S6). The morphology of the microtubules was similar to that observed in wild type at prophase I (SI Appendix, Fig. S6A). The perinuclear cytoskeleton structure of microtubules was discernible at diakinesis. After NEB, microtubules penetrated the nucleus and interacted with chromosomes. Multipolar spindles were distinguishable at early metaphase I. Bipolar spindles were always assembled at metaphase I. At anaphase I, chromosomes were pulled apart unequally. Some chromosomes were toward 1 pole and the remaining chromosomes toward the opposite pole at telophase I. FISH assays using CentO as the probe were conducted on diakinesis and metaphase I chromosomes in haploid rice (SI Appendix, Fig. S6B). We found the majority of PMCs (70%) contained 1 to 3 bipolar attachments, 26% of them contained 4 to 7 bipolar attachments, and the remaining 4% PMCs contained more than 8 bipolar attachments (SI Appendix, Fig. S6 B and C).
OsREC8 Is a Suppressor of OsMTOPVIB in Multipolar Spindle Formation.
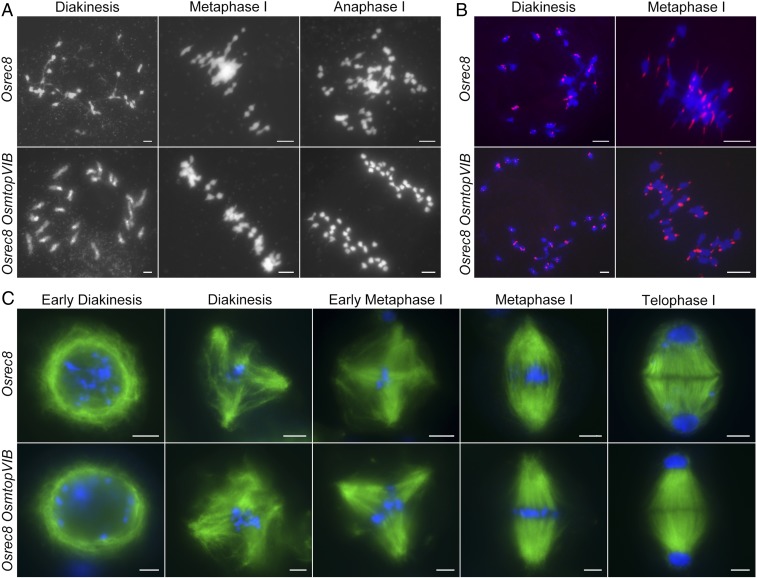
To verify that bipolar attachments of sister kinetochores can lead to bipolar spindle formation, we generated a double-mutant Osrec8 OsmtopVIB. OsREC8 is a key component of meiotic cohesion, which is required for metaphase I monopolar attachment in rice meiosis (34). In Osrec8, univalents were formed at diakinesis. Chromosomes were lined on the equatorial plate at metaphase I and segregated to the poles at anaphase I (Fig. 3A). The mutation in OsREC8 eliminated meiotic centromeric cohesion during prophase I, leading to the bipolar orientation of sister kinetochores during the first meiotic division (Fig. 3B). In Osrec8 OsmtopVIB, meiosis I was modified toward a mitotic-like division: 24 univalents aligned on the metaphase I plate, and sister chromatids segregated equally at anaphase I (Fig. 3A). FISH assays using CentO as the probe indicated that the mutation of both OsREC8 and OsMTOPVIB eliminated meiotic centromeric cohesion at diakinesis. Biorientation of sister kinetochores was universal at meiosis I (Fig. 3B). Furthermore, indirect immunofluorescence staining experiments revealed that a bipolar spindle was established in both Osrec8 and Osrec8 OsmtopVIB meiocytes at meiosis I (Fig. 3C).
Fig. 3.
The mutation of OsREC8 suppresses multipolar spindle formation in OsmtopVIB. (A) Chromosome behavior in Osrec8 and Osrec8 OsmtopVIB meiocytes. (B) Biorientation of sister kinetochores in Osrec8 and Osrec8 OsmtopVIB. Centromeres were probed with CentO (red) through fluorescence in situ hybridization. Meiotic chromosomes were stained with DAPI (blue). (C) The process of meiotic spindle assembly in Osrec8 and Osrec8 OsmtopVIB meiocytes. Chromosomes were stained with DAPI (blue), and microtubules were immunodetected with α-tubulin antibody (green). (Scale bars: 5 μm.)
A different situation for the sister kinetochores was found between OsmtopVIB and pair2, crc1, and Osspo11-1, indicating that the divergent cohesion status could exist among those mutants. We checked the meiotic specific cohesion element OsREC8 by immunodetection using PMCs around meiosis I from those mutants. No difference in OsREC8 loading or depletion was found between OsmtopVIB and crc1, Osspo11-1, and pair2 (SI Appendix, Figs. S7 and S8). Therefore, the molecular mechanism for the involvement of OsMTOPVIB in meiotic spindle assembly remains to be revealed.
Discussion
The Acentrosomal Spindle Assembly in Plant Meiosis.
In higher eukaryotes, spindle microtubules are assembled by centrosomes or acentrosomally through chromatin-mediated pathways (5, 7, 35). However, the mechanism of bipolar spindle assembly in plant meiosis remains elusive. Here, we offer direct evidence that the bipolar spindle is established by the chromatin-dependent pathway in both monocots and dicots. During this process, microtubules fill in throughout the cytoplasm at early prophase I. Bundles of microtubules emanate from the nuclear envelope with a radial cytoskeleton at late prophase I, forming a typical perinuclear ring on the nuclear envelope at diakinesis. After NEB, the conspicuous ring-shape structure of microtubules splits into separate microtubule bundles, leading to a chaotic structure at metaphase I. Finally, multipolar spindles transition into bipolar spindles before anaphase I. We postulate that the transition from multipolar spindles into bipolar spindles is a common process in both monocots and dicots. In female meiosis of mice, Drosophila, and Xenopus, spindle assembly involves the randomly oriented growth of microtubules followed by the self-organization of microtubules into bipolar arrays (7, 36), which implies that multipolar to bipolar spindle formation appears to be a common process in acentrosomal cells.
Meiotic Spindle Bipolarity Requires OsMTOPVIB.
We revealed that a key recombination initiation factor, OsMTOPVIB, is responsible for meiotic spindle bipolarity. In OsmtopVIB PMCs, a large proportion of multipolar spindles were detected at metaphase I, leading to 85.84% polyads formed at telophase II. Mutants exhibiting a defective meiotic spindle assembly have been reported in plants. In Arabidopsis atk1, broad, unfocused, and multiaxial spindles were characterized (22). In Arabidopsis prd2/mps1, unequal bipolar or multipolar spindles were observed (23). In the present study, we found that OsMTOPVIB interacts with OsPRD2/MPS1, suggesting that they might act in the same pathway. In addition, we observed normal bipolar spindle formation in pair2, Osspo11-1, and crc1 meiocytes, indicating that bipolar spindle assembly is independent of DSB formation. There were always some univalents in pair2, Osspo11-1, and crc1, in which mono-orientation of sister kinetochores were converted into a biorientation at meiosis I. However, mono-orientation of kinetochores was prevalently observed for most of the univalents in OsmtopVIB. Therefore, we suspect that bipolar attachment of univalents in homologous recombination-depleted PMCs promotes bipolar spindle formation during meiosis I.
Centromere Cohesion May Affect Bipolar Spindle Formation in Meiosis.
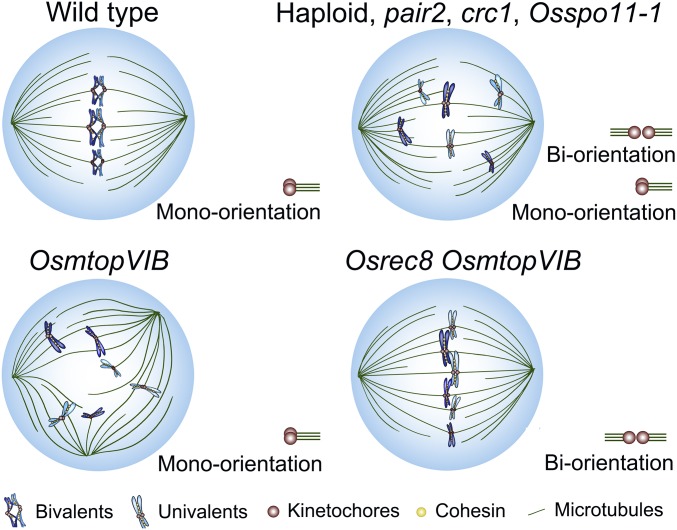
Chiasmata, the products of homologous recombination, are required for orienting bivalent chromosomes at metaphase I by creating tensions for effective interaction between kinetochores and microtubules (37). In the absence of recombination, univalents may lead to the production of either multipolar or bipolar spindles (21, 37, 38). It is proposed that univalents may disrupt the bipolar spindle due to sister kinetochores acting as a single unit and causing the monopolar attachment (39). When sister kinetochores are divided, they can attach to microtubules from both poles, leading to the bipolar attachment (21, 40). It is commonly observed that sister kinetochores in a univalent have a tendency to separate from each other when recombination is absent (41–43). We found that the spindle polarity is regulated by the orientation of sister kinetochores when recombination is absent. When the meiosis-specific cohesin subunit OsREC8 was mutated in the OsmtopVIB background, biorientation of sister kinetochores appeared and bipolar spindles were completely rescued (Fig. 4), indicating that OsMTOPVIB may control spindle assembly by regulating the centromeric cohesion. Centromere cohesion is also maintained by cohesins, similar to that on chromosome arms (2, 34, 44). However, maintaining of the centromere cohesion has a specific mechanism during meiosis. Shugoshin (SGO1) and protein phosphatase 2A (PP2A) are 2 conserved proteins in eukaryotes that protect centromeric cohesins from cleavage by separase in meiosis (45, 46). Centromere cohesion also depends on destabilizing kinetochore–microtubule attachments in Drosophila (47). Protein Phosphatase 1 regulates centromere cohesion through antagonizing Polo kinase and BubR1, 2 proteins required for stability of kinetochore–microtubule attachments. Although the mechanism of OsMTOPVIB in regulating centromeric cohesion and spindle assembly remains unclear, we suspect that OsMTOPVIB might be involved in both protection and release of cohesins in rice meiosis.
Fig. 4.
Proposed model for OsMTOPVIB function during bipolar spindle assembly. In wild type, chiasmata together with co-orientation of sister kinetochores, enables the bipolar attachment between kinetochores and microtubules. In haploid, pair2, crc1, and Osspo11-1, the mono-orientation of sister kinetochores of some univalents is converted into a biorientation, leading to the generation of tensions and construction of bipolar spindles. However, in OsmtopVIB, only mono-orientation of sister kinetochores leads to multipolar spindles. After elimination of the cohesion subunit OsREC8 in the OsmtopVIB background, both biorientation and bipolar spindles appear.
Materials and Methods
The OsmtopVIB mutant was identified from the indica rice, Zhongxian 3037, induced by 60Co γ-ray irradiation. Details on the following are available in SI Appendix, SI Materials and Methods: plant materials, meiotic chromosome preparation, immunofluorescence, yeast 2-hybrid assays, BiFC assays, antibody production, and accession numbers. The primers used in this study are listed in SI Appendix, Table S1.
Supplementary Material
Acknowledgments
We thank Robert M. Stupar for critical reading of the manuscript. This work was supported by grants from the National Key Research and Development Program of China (2016YFD0100901) and the National Natural Science Foundation of China (31771363).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821315116/-/DCSupplemental.
References
- 1.Page S. L., Hawley R. S., Chromosome choreography: The meiotic ballet. Science 301, 785–789 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Yokobayashi S., Watanabe Y., The kinetochore protein Moa1 enables cohesion-mediated monopolar attachment at meiosis I. Cell 123, 803–817 (2005). [DOI] [PubMed] [Google Scholar]
- 3.Wittmann T., Hyman A., Desai A., The spindle: A dynamic assembly of microtubules and motors. Nat. Cell Biol. 3, E28–E34 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Compton D. A., Spindle assembly in animal cells. Annu. Rev. Biochem. 69, 95–114 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Kirschner M., Mitchison T., Beyond self-assembly: From microtubules to morphogenesis. Cell 45, 329–342 (1986). [DOI] [PubMed] [Google Scholar]
- 6.Manandhar G., Schatten H., Sutovsky P., Centrosome reduction during gametogenesis and its significance. Biol. Reprod. 72, 2–13 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Heald R., et al. , Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Petry S., Pugieux C., Nédélec F. J., Vale R. D., Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc. Natl. Acad. Sci. U.S.A. 108, 14473–14478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sköld H. N., Komma D. J., Endow S. A., Assembly pathway of the anastral Drosophila oocyte meiosis I spindle. J. Cell Sci. 118, 1745–1755 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada M., Goshima G., Mitotic spindle assembly in land plants: Molecules and mechanisms. Biology 6, E6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown R. C., Lemmon B. E., The pleiomorphic plant MTOC: An evolutionary perspective. J. Integr. Plant Biol. 49, 1142–1153 (2007). [Google Scholar]
- 12.Kosetsu K., et al. , Cytoplasmic MTOCs control spindle orientation for asymmetric cell division in plants. Proc. Natl. Acad. Sci. U.S.A. 114, E8847–E8854 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canaday J., Stoppin-Mellet V., Mutterer J., Lambert A. M., Schmit A. C., Higher plant cells: γ-Tubulin and microtubule nucleation in the absence of centrosomes. Microsc. Res. Tech. 49, 487–495 (2000). [DOI] [PubMed] [Google Scholar]
- 14.Ambrose J. C., Cyr R., Mitotic spindle organization by the preprophase band. Mol. Plant 1, 950–960 (2008). [DOI] [PubMed] [Google Scholar]
- 15.De Mey J., Lambert A. M., Bajer A. S., Moeremans M., De Brabander M., Visualization of microtubules in interphase and mitotic plant cells of Haemanthus endosperm with the immuno-gold staining method. Proc. Natl. Acad. Sci. U.S.A. 79, 1898–1902 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller S., Wright A. J., Smith L. G., Division plane control in plants: New players in the band. Trends Cell Biol. 19, 180–188 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Shimamura M., et al. , γ-Tubulin in basal land plants: Characterization, localization, and implication in the evolution of acentriolar microtubule organizing centers. Plant Cell 16, 45–59 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang G., et al. , Augmin antagonizes katanin at microtubule crossovers to control the dynamic organization of plant cortical arrays. Curr. Biol. 28, 1311–1317.e3 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Kong Z., Hotta T., Lee Y. R., Horio T., Liu B., The γ-tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana. Plant Cell 22, 191–204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaefer E., et al. , The preprophase band of microtubules controls the robustness of division orientation in plants. Science 356, 186–189 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Chan A., Cande W. Z., Maize meiotic spindles assemble around chromatin and do not require paired chromosomes. J. Cell Sci. 111, 3507–3515 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Chen C., et al. , The Arabidopsis ATK1 gene is required for spindle morphogenesis in male meiosis. Development 129, 2401–2409 (2002). [DOI] [PubMed] [Google Scholar]
- 23.Jiang H., et al. , MULTIPOLAR SPINDLE 1 (MPS1), a novel coiled-coil protein of Arabidopsis thaliana, is required for meiotic spindle organization. Plant J. 59, 1001–1010 (2009). [DOI] [PubMed] [Google Scholar]
- 24.De Storme N., Geelen D., The Arabidopsis mutant jason produces unreduced first division restitution male gametes through a parallel/fused spindle mechanism in meiosis II. Plant Physiol. 155, 1403–1415 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.d’Erfurth I., et al. , Mutations in AtPS1 (Arabidopsis thaliana parallel spindle 1) lead to the production of diploid pollen grains. PLoS Genet. 4, e1000274 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quan L., et al. , Functional divergence of the duplicated AtKIN14a and AtKIN14b genes: Critical roles in Arabidopsis meiosis and gametophyte development. Plant J. 53, 1013–1026 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Walker J., et al. , Sexual-lineage-specific DNA methylation regulates meiosis in Arabidopsis. Nat. Genet. 50, 130–137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Muyt A., et al. , A high throughput genetic screen identifies new early meiotic recombination functions in Arabidopsis thaliana. PLoS Genet. 5, e1000654 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue Z., et al. , OsMTOPVIB promotes meiotic DNA double-strand break formation in rice. Mol. Plant 9, 1535–1538 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Fu M., et al. , The DNA topoisomerase VI-B subunit OsMTOPVIB is essential for meiotic recombination initiation in rice. Mol. Plant 9, 1539–1541 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Nonomura K., Nakano M., Eiguchi M., Suzuki T., Kurata N., PAIR2 is essential for homologous chromosome synapsis in rice meiosis I. J. Cell Sci. 119, 217–225 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Yu H., et al. , OsSPO11-1 is essential for both homologous chromosome pairing and crossover formation in rice. Chromosoma 119, 625–636 (2010). [DOI] [PubMed] [Google Scholar]
- 33.Miao C., et al. , Central region component1, a novel synaptonemal complex component, is essential for meiotic recombination initiation in rice. Plant Cell 25, 2998–3009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao T., et al. , OsREC8 is essential for chromatid cohesion and metaphase I monopolar orientation in rice meiosis. Plant Physiol. 156, 1386–1396 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steffen W., Fuge H., Dietz R., Bastmeyer M., Müller G., Aster-free spindle poles in insect spermatocytes: Evidence for chromosome-induced spindle formation? J. Cell Biol. 102, 1679–1687 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuh M., Ellenberg J., Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 130, 484–498 (2007). [DOI] [PubMed] [Google Scholar]
- 37.Dawe R. K., Meiotic chromosome organization and segregation in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 371–395 (1998). [DOI] [PubMed] [Google Scholar]
- 38.Koduru P. R. K., Rao M. K., Cytogenetics of synaptic mutants in higher plants. Theor. Appl. Genet. 59, 197–214 (1981). [DOI] [PubMed] [Google Scholar]
- 39.Dawe R. K., Cande W. Z., The role of chromosomes in maize meiotic spindle morphogenesis. J. Cell. Biochem. 21A, 438 (1995). [Google Scholar]
- 40.Khodjakov A., Cole R. W., McEwen B. F., Buttle K. F., Rieder C. L., Chromosome fragments possessing only one kinetochore can congress to the spindle equator. J. Cell Biol. 136, 229–240 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt P., LeMaire R., Embury P., Sheean L., Mroz K., Analysis of chromosome behavior in intact mammalian oocytes: Monitoring the segregation of a univalent chromosome during female meiosis. Hum. Mol. Genet. 4, 2007–2012 (1995). [DOI] [PubMed] [Google Scholar]
- 42.Rebollo E., Arana P., A comparative study of orientation at behavior of univalent in living grasshopper spermatocytes. Chromosoma 104, 56–67 (1995). [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto A., Hiraoka Y., Monopolar spindle attachment of sister chromatids is ensured by two distinct mechanisms at the first meiotic division in fission yeast. EMBO J. 22, 2284–2296 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chelysheva L., et al. , AtREC8 and AtSCC3 are essential to the monopolar orientation of the kinetochores during meiosis. J. Cell Sci. 118, 4621–4632 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Riedel C. G., et al. , Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441, 53–61 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Kitajima T. S., Kawashima S. A., Watanabe Y., The conserved kinetochore protein shugoshin protects centromeric cohesion during meiosis. Nature 427, 510–517 (2004). [DOI] [PubMed] [Google Scholar]
- 47.Wang L. I., Das A., McKim K. S., Sister centromere fusion during meiosis I depends on maintaining cohesins and destabilizing microtubule attachments. PLoS Genet. 15, e1008072 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.