Microbial species aren’t often found alone, but rather in complex communities containing dozens to hundreds of other species. These species affect one another in many ways. Species can harm one another by producing antibiotics, by stabbing one another and injecting toxins, or simply by consuming resources that others require (1–3). Microbes can also impact one another positively. They can, for example, degrade antibiotics and detoxify the environment, secrete iron-scavenging molecules that allow other species to take up iron, or excrete metabolic byproducts that others can consume (4–6). The ecology and evolution of these interactions are becoming more well characterized, but it’s unclear whether certain environmental conditions promote one form of interaction over another. In PNAS, Piccardi et al. (7) demonstrate that environmental stress in the form of toxic biocides causes interactions between 4 microbial species to become positive.
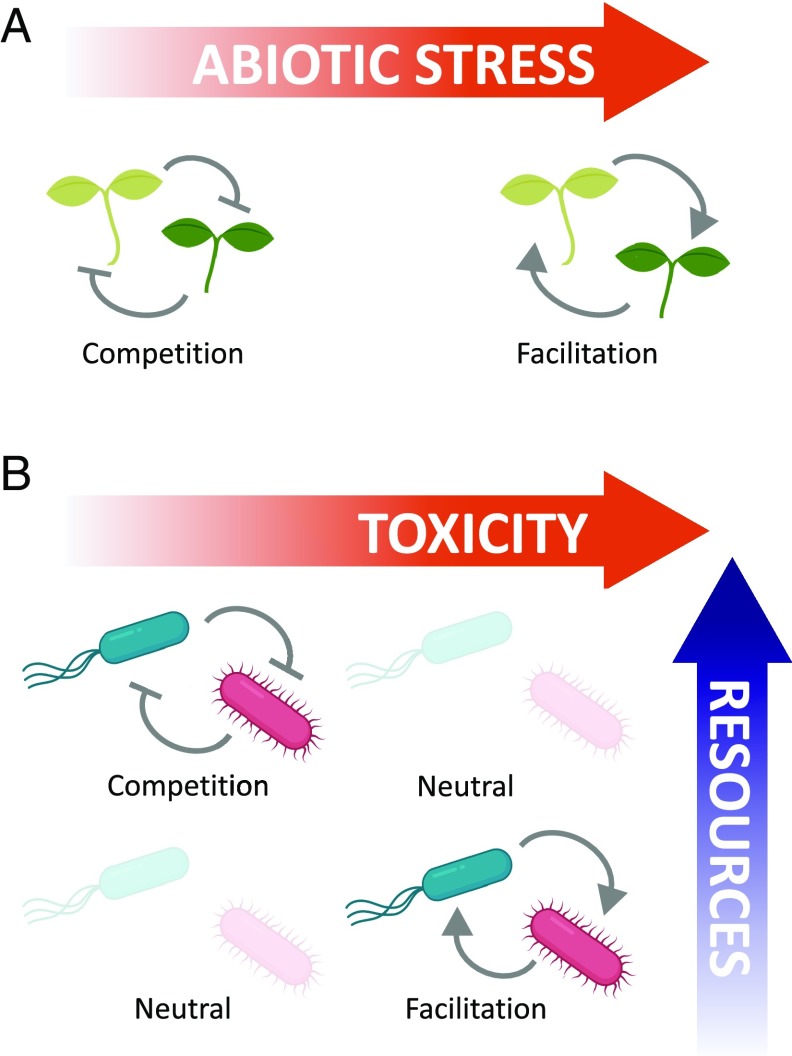
The stress gradient hypothesis (SGH) provides a framework to predict when positive or negative interactions should be observed (8). The SGH states that facilitation should be more common in stressful environments, compared with benign environments where competition should be more common (Fig. 1A). Since it was proposed, the SGH has mainly been tested in plant communities by growing species over a stress gradient (e.g., drought stress) and assessing changes in the nature and magnitude of their interactions. Metaanalyses have found that the SGH has had mixed support (9, 10). Some authors have argued that the predictive power of the SGH has been limited by a lack of specificity about which forms of stress should cause the pattern, and how other environmental gradients may influence interactions in combination with the stress gradient (11). In their study of a simple microbial community, Piccardi et al. (7) use experiments and mathematical modeling to refine the SGH by exploring the interplay between 2 abiotic factors: Environmental toxicity and resource availability.
Fig. 1.
(A) The classic SGH as formulated by Bertness and Callaway (8). Negative interactions (competition) are expected to be found in benign environments, while positive interactions (facilitation) should be found in stressful environments. (B) Piccardi et al. (7) explore the combined effects of a toxicity gradient and a resource gradient. Competitive interactions dominate at low toxicity and high resource levels, while facilitation dominates at high toxicity and low resource levels.
Piccardi et al. (7) use 4 bacterial species isolated from metal working fluid (MWF), an industrial waste product that contains toxic pollutants. The authors assess pairwise interactions by growing monocultures and cocultures in sterile MWF and comparing cumulative growth of each species. For example, when species A caused species B to grow more in coculture than in monoculture, then species A affected B positively. Piccardi et al. find that facilitation is the dominant interaction, with 7 out of 12 one-way interactions being positive and the remaining interactions being neutral. In the most extreme cases of facilitation, 2 of the species were unable to survive alone (their population size decreased from 107 to zero over a 12-day growth period), but were able to increase in density when cocultured with another species, Comamonas testosteroni. The authors speculate that the facilitating species were able to detoxify the environment, which allowed the other species to grow in their presence. Overall, these findings support the SGH because positive interactions were observed in a stressful environment.
Next, Piccardi et al. (7) investigate how resource availability mediates the effects of toxicity. They first used a mathematical consumer resource model of 2 species that consume a single resource and degrade a toxin. Consistent with their experimental findings, they find that high toxicity and a low resource level generate positive interactions. Both species benefitted from the other’s toxin degradation. In contrast, when the resource level was high and toxicity was reduced, the interactions switched from positive to negative. Detoxification no longer provided much of a benefit, and resource competition caused the species to suffer in coculture (Fig. 1B). These modeling results generated a prediction about the combined effects of toxicity and resource concentrations that the authors tested in the laboratory. They first supplemented the toxic MWF medium with an amino acid mix to increase resource levels. This caused some interactions to become negative, likely due to resource competition, but some interactions became more strongly positive, possibly because facilitating species were able to provide more detoxification when they reached higher densities. However, when toxicity was removed by growing the species in a medium containing only amino acids and no toxins, competitive interactions dominated. These findings help to refine the SGH for microbial communities by teasing apart the effects of the stress gradient (toxicity) and a resource gradient. Species have the potential to facilitate other species’ growth through toxin degradation, but resource competition will dominate if the environment is not sufficiently toxic or if resource levels are high.
The SGH has been criticized for being somewhat vague about which forms of stress will produce the predicted pattern (11). Piccardi et al. (7) observe the SGH pattern with toxicity, where some of the species are able to detoxify the environment. However, if the species had not been capable of detoxification, the authors would likely not have observed any facilitation. This may be a general rule—in order for the SGH pattern to be observed for a particular stressor, at least one species in the community must be able to ameliorate the stress. This distinction can inform predictions about which stressors will cause interactions to become positive in a particular microbial community. For example, if an antibiotic is applied to a community of microbes, and the microbes are incapable of degrading the antibiotic or otherwise providing any cross-protection for sensitive species, the species will likely interact neutrally or negatively through resource competition, and the SGH predictions will not be observed. On the other hand, if at least one species is able to ameliorate the antibiotic stress (via production of a beta-lactamase that degrades a beta-lactam drug, for example), the SGH pattern is likely to be observed. However, as Piccardi et al. show, the impact of stress amelioration will also depend on the level of resource competition.
In PNAS, Piccardi et al. demonstrate that environmental stress in the form of toxic biocides causes interactions between 4 microbial species to become positive.
The SGH is an ecological hypothesis, but it is interesting to speculate about the effects of stress on the evolution of species interactions over longer timescales. One can imagine that adaptation could cause species to become better at tolerating or ameliorating a stress on their own, removing the potential to benefit from other species. Adaptation may also cause increased privatization of the benefits of stress amelioration, so species may no longer provide a public benefit to others. In such scenarios, evolution in a stressful environment would lead to more negative interactions over time. Consistent with this prediction, Piccardi et al. (7) passaged 2 of their species in monoculture in MWF for 10 wk and found that the evolved isolates interacted more negatively than the ancestral strains. This finding agrees with the expectation that positive interactions should be rare in natural communities, compared with competitive interactions, because natural selection favors selfish phenotypes and cooperation requires specific conditions to be maintained (12, 13). Interestingly, others have observed the opposite pattern, with evolution in a stressful environment leading to more positive interactions (14–16). For example, Lawrence et al. (16) serially passaged a small community of wild bacterial isolates in a nutrient-poor environment and found that interactions changed from mostly negative to mostly positive. They speculated that resource niche differentiation and consumption of other species’ metabolic byproducts caused species to benefit from one another. These processes could conceivably occur in Piccardi et al.’s system, and it would be interesting to perform a similar evolution experiment with all 4 species in polyculture to test how toxicity affects the evolution of their interactions. More broadly, more work is needed to investigate the ways in which abiotic factors alter the evolution of species that engage in facilitation.
In summary, Piccardi et al. (7) show that positive interactions between 4 natural bacterial isolates occur most frequently in a toxic and nutrient-poor environment. Their work lends support to the SGH, and also helps refine the hypothesis by demonstrating that high resource levels can affect species’ abilities to ameliorate the stress and can reveal underlying resource competition. It will be interesting to explore whether the same effects are seen in natural microbial communities and over evolutionary timescales.
Footnotes
The authors declare no conflict of interest.
See companion article on page 15979.
References
- 1.Cornforth D. M., Foster K. R., Antibiotics and the art of bacterial war. Proc. Natl. Acad. Sci. U.S.A. 112, 10827–10828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russell A. B., Peterson S. B., Mougous J. D., Type VI secretion system effectors: Poisons with a purpose. Nat. Rev. Microbiol. 12, 137–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hibbing M. E., Fuqua C., Parsek M. R., Peterson S. B., Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 8, 15–25 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seth E. C., Taga M. E., Nutrient cross-feeding in the microbial world. Front. Microbiol. 5, 350–355 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yurtsev E. A., Conwill A., Gore J., Oscillatory dynamics in a bacterial cross-protection mutualism. Proc. Natl. Acad. Sci. U.S.A. 113, 6236–6241 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin A. S., West S. A., Buckling A., Cooperation and competition in pathogenic bacteria. Nature 430, 1024–1027 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Piccardi P., Vessman B., Mitri S., Toxicity drives facilitation between 4 bacterial species. Proc. Natl. Acad. Sci. U.S.A. 116, 15979–15984 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertness M. D., Callaway R., Positive interactions in communities. Trends Ecol. Evol. (Amst.) 9, 191–193 (1994). [DOI] [PubMed] [Google Scholar]
- 9.Maestre F. T., Valladares F., Reynolds J. F., Is the change of plant–plant interactions with abiotic stress predictable? A meta‐analysis of field results in arid environments. J. Ecol. 93, 748–757 (2005). [Google Scholar]
- 10.He Q., Bertness M. D., Altieri A. H., Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 16, 695–706 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Maestre F. T., Callaway R. M., Valladares F., Lortie C. J., Refining the stress‐gradient hypothesis for competition and facilitation in plant communities. J. Ecol. 97, 199–205 (2009). [Google Scholar]
- 12.Mitri S., Foster K. R., The genotypic view of social interactions in microbial communities. Annu. Rev. Genet. 47, 247–273 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Ghoul M., Mitri S., The ecology and evolution of microbial competition. Trends Microbiol. 24, 833–845 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Weese D. J., Heath K. D., Dentinger B. T. M., Lau J. A., Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 69, 631–642 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Hammarlund S. P., Connelly B. D., Dickinson K. J., Kerr B., The evolution of cooperation by the Hankshaw effect. Evolution 70, 1376–1385 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Lawrence D., et al. , Species interactions alter evolutionary responses to a novel environment. PLoS Biol. 10, e1001330 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]