Significance
The prebiotic milieu was likely heterogeneous, consisting of a large number of chemicals and their associated reactions, including those not only of biological compounds, but also nonbiological compounds. Although origins of life research has focused primarily on biological molecules, the nonbiological molecules which were also present may have assisted evolving chemical systems in unforeseen ways. Thus, we synthesized and assembled membraneless polyester microdroplets from drying of pools of simple α-hydroxy acid monomers and showed that they can act as plausible prebiotic compartments. By having the capacity to undergo combinatorial rearrangement, these microdroplets could have developed versatile abilities to host early genetic and metabolic systems critical for the origins of life.
Keywords: origins of life, prebiotic chemistry, membraneless compartments, self-assembly, polyesters
Abstract
Compartmentalization was likely essential for primitive chemical systems during the emergence of life, both for preventing leakage of important components, i.e., genetic materials, and for enhancing chemical reactions. Although life as we know it uses lipid bilayer-based compartments, the diversity of prebiotic chemistry may have enabled primitive living systems to start from other types of boundary systems. Here, we demonstrate membraneless compartmentalization based on prebiotically available organic compounds, α-hydroxy acids (αHAs), which are generally coproduced along with α-amino acids in prebiotic settings. Facile polymerization of αHAs provides a model pathway for the assembly of combinatorially diverse primitive compartments on early Earth. We characterized membraneless microdroplets generated from homo- and heteropolyesters synthesized from drying solutions of αHAs endowed with various side chains. These compartments can preferentially and differentially segregate and compartmentalize fluorescent dyes and fluorescently tagged RNA, providing readily available compartments that could have facilitated chemical evolution by protecting, exchanging, and encapsulating primitive components. Protein function within and RNA function in the presence of certain droplets is also preserved, suggesting the potential relevance of such droplets to various origins of life models. As a lipid amphiphile can also assemble around certain droplets, this further shows the droplets’ potential compatibility with and scaffolding ability for nascent biomolecular systems that could have coexisted in complex chemical systems. These model compartments could have been more accessible in a “messy” prebiotic environment, enabling the localization of a variety of protometabolic and replication processes that could be subjected to further chemical evolution before the advent of the Last Universal Common Ancestor.
Compartmentalization was likely a crucial stage in the emergence of life (1). Compartments provide a boundary preventing diffusion of molecules important for evolving systems as well as a space in which chemical reactions can be enhanced due to increased concentration (2). In modern life, this is accomplished by cellularization, which also allows for both individuation and energy transduction (3). Although modern cell membranes depend on phospholipid bilayers, earlier life may have been constructed of vesicle compartments made of simpler but not necessarily easy-to-synthesize single and long-chain fatty acids (4, 5). Despite this, microscale fatty acid bilayer vesicles have often been used to model the first cell-like compartments on Earth as they are able to stably compartmentalize genetic biopolymers such as RNA even up to temperatures as high as 90 to 100 °C, while still allowing the transport of small molecules across the membrane boundary (6). Such vesicles have also been shown to be able to grow and divide easily upon incorporation of fatty acid micelles and application of shear stress (7). However, fatty acid vesicles are generally not stable to large fluctuations in pH (beyond roughly neutral) (8) or millimolar concentrations of divalent cations (at least in the absence of chelating agents like citrate) (9) such as Ca(II) or Mg(II), the latter being an essential ion that promotes the activity of primitive RNA catalysts (10). Thus, perhaps before the emergence of lipid-based cells, nonlipid bilayer-based microscale compartments may have enabled primitive biochemistry by providing the similar essential characteristics as lipid bilayer vesicles. This may have included nonvesicular compartmentalization mechanisms such as aqueous two-phase systems (ATPSs) (11), membraneless peptide coacervate droplets (12), liquid-in-liquid microdroplets made from small organics or oils (2), inorganic compartments (13), or through other liquid–liquid phase separation phenomena (14). Indeed, modern cells host a wide variety of nonlipid-based membraneless organelles and condensates. Despite having no enclosing membrane, these structures localize both RNA and protein in subcellular compartments. Some widespread examples include neuronal granules, cytoplasmic germ granules, nucleoli, promyelocytic leukemia protein bodies, Cajal bodies, and processing bodies (15).
Prebiotic chemical environments were likely much more complex than the model vesicle-based systems described above (16), and thus more investigation into the potential emergence of microscale compartments from diverse pools of simple chemicals is warranted. In this sense, the ubiquity and diversity of α-hydroxy acids (αHAs) in various primitive environments is well known, as they are synthesized in various abiotic systems such as spark discharge experiments (17) and found in carbonaceous meteorites (18, 19). Recently, Chandru et al. (20) showed that αHAs readily form combinatorial polymer libraries under evaporative conditions, which could reasonably have been synthesized on early Earth or other watery rocky planets, such as Mars (21) or even those of the TRAPPIST-1 system (22), through diurnal or seasonal oscillations in insolation (23).
We show here that polydisperse polyesters with diverse chemical functionality generated from drying αHAs at low temperatures can form gel-like phases that self-assemble into microdroplets, with diameter up to 10s of micrometers, in aqueous medium. These microdroplets are relatively stable to coalescence, and their recombination and “division” can be effected through pH and/or ionic strength fluctuations in water in conjunction with agitation. These microdroplets can differentially segregate and compartmentalize fluorescent dyes and fluorescently tagged RNAs, while still allowing biopolymer function, demonstrating their potential relevance to various origins of life models (e.g., to serve as compartments for primitive chemical systems and to host segregated reactions). These studies provide a proof-of-principle for the generation of a model primitive membraneless compartment system in the microscale and the possibility of the emergence of various “phenotypic” traits from simple monomers.
Results
Synthesis of Polyester Condensed Phase.
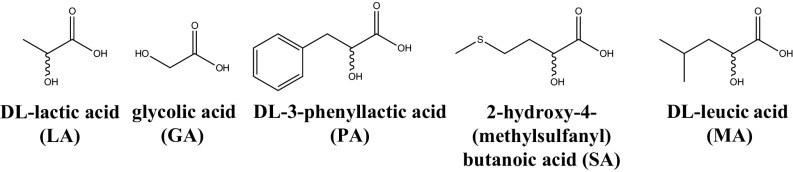
Polyesters were formed by drying 500 mM aqueous solutions (at their natural pH of 2 to 3) of each αHA [DL-lactic acid (LA), glycolic acid (GA), DL-3-phenyllactic acid (PA), 2-hydroxy-4-(methylsulfanyl)butanoic acid (SA), and DL-leucic acid (DL-2-hydroxy-4-methylpentanoic acid, MA); Fig. 1] at 80 °C for 1 wk in borosilicate glass test tubes, simulating primitive evaporative environments (SI Appendix, Figs. S1–S3). Water loss during drying drives polyester formation, and after drying, in most cases (with the exception of GA), a gel-like material formed (SI Appendix, Fig. S1, Inset photograph). Polyesters synthesized at their natural pH (2 to 3) in aqueous solution formed these phases, while reactions at pH 7 showed no detectable condensed-phase formation (except for LA), likely due to the inhibition of polymerization at pH 7 (SI Appendix, Fig. S4). The formation of the condensed phase is not glass-surface–dependent, as plastic tubes also resulted in the formation of the condensed phase (SI Appendix, Fig. S5). The resulting products were assayed by Matrix-Assisted Laser Desorption Ionization (MALDI) Time-of-Flight Mass Spectrometry and found to contain polydisperse polyesters, some up to 40 residues in length, depending on the starting material (SI Appendix, Figs. S1 and S2 and Tables S1–S5). The detected mass peaks can be assigned to discrete polymer sequences. For example, the LA spectrum (SI Appendix, Figs. S1A and S2A) shows a repetitive mass increment of Δ72.02 Da, corresponding to the (-OCH(CH3)CO-) unit. Similar results were obtained for all of the other αHA samples, which polymerized into polyesters of variable maximum length. GA and LA polymerization products were found to be longer than observed in Chandru et al. (20) which we ascribe to differences in analytical technique and synthetic protocol. To more realistically simulate heterogeneous prebiotic environments (16), we increased the complexity of the system, and all 26 possible combinations of 2 to 5 different αHAs were also prepared (i.e., all 10 combinations of 2 αHAs, 10 combinations of 3 αHAs, 5 combinations of 4 αHAs, and 1 combination of all 5 αHAs). Each of these combinations formed a condensed phase. SI Appendix, Fig. S6 shows the polyester condensed phase formed upon drying a mixture of all 5 αHAs.
Fig. 1.
The 5 αHAs studied.
Structure of Microdroplets.
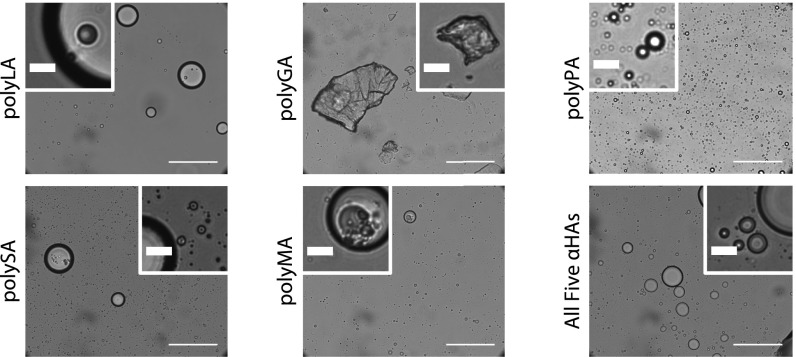
Upon addition of 4:1 (vol/vol) water/acetonitrile to the dried polyester samples and sonication and vortexing, a turbid solution formed (SI Appendix, Fig. S7) (except in the case of GA, for which the products of which remained insoluble). Acetonitrile, a potentially prebiotic solvent (24), was incorporated into the system, as a pure water solvent either did not result in formation of microdroplets at all or resulted in few microdroplets, even after several minutes of sonication (SI Appendix, Fig. S8). This turbidity suggests that the condensed phase breaks apart into smaller microscale droplets in aqueous solution, and thus the microstructure of the turbid solutions was examined using light microscopy. The formation of spherical microdroplets was observed, ranging in diameter from a few micrometers up to 10s of micrometers (Fig. 2 and SI Appendix, Fig. S9). No microdroplets formed from αHAs that were not dried, thus the droplets require polymers to form (SI Appendix, Fig. S10). Drying at room temperature also did not result in the formation of macroscopic condensed phases or microdroplets, except in the case of polyMA (SI Appendix, Fig. S11); thus, there may be a minimum temperature threshold for the formation of polyesters of sufficient length (20) to form insoluble or amphiphilic aggregates. PolyGA did not form microdroplets or a condensed phase, possibly because the GA side chain is the least hydrophobic of the αHAs studied here. Despite this, GA does not hinder the formation of condensed phases or microdroplets when reacted with other αHAs, as all of the GA-containing polyesters consisting of 2 or more αHAs form the condensed phase and microdroplets (SI Appendix, Fig. S12). This suggests even in complex prebiotic environments containing many organic chemical species (16), such microdroplets could have still assembled, as even polydisperse heteropolyesters in solution produce self-assembled droplets.
Fig. 2.
Spherical microdroplets formed from various polyester condensed phases in aqueous media visualized by optical microscopy. Slight differences in the size and abundance of the droplets are attributed to variations in vortexing and sonication time as well as the nonuniform distribution of the droplets within the samples. PolyGA does not form the condensed phase (Scale bars, 100 µm in the main images, 10 µm in the Insets.)
Rapid compartment coalescence or disassembly would be catastrophic to primitive evolving systems as it would result in the loss of the droplet individuality. Thus, we next examined the robustness of the polyester microdroplets under various conditions. The droplets did not rapidly disassemble upon 10-fold dilution into water (SI Appendix, Fig. S13), although some of the poly-αHA droplets decreased slightly in size over several hours upon dilution (SI Appendix, Figs. S14 and S15 and Movies S1 and S2), possibly due to the leaching out of lower molecular weight species. This suggests that they would be stable to oscillations in water level caused by environmental conditions, i.e., rain. This is in contrast with ATPSs and coacervate droplets, for which dilution could result in rapid droplet disassembly (25, 26). Additionally, while certain ATPSs and coacervates could completely coalesce on the order of minutes or hours (27) in the absence of external Pickering emulsifiers such as clay mineral particles (11) or interfacial stabilizing polymers (28), the polyester microdroplets (at their natural pH of 2 to 3) completely coalesce much more slowly, on the timescale of days (SI Appendix, Fig. S16 and Movies S3 and S4). However, when our polyester systems were brought to pH 8 in 800 mM Na-HEPES, the droplets began to coalesce much more quickly (SI Appendix, Figs. S17–S21). PolyLA microdroplets (SI Appendix, Fig. S19) coalesced rapidly (within 5 min) when brought to pH 8, while microdroplets derived from polyPA, polySA, polyMA, and the mixed sample containing all 5 αHAs all coalesced after about 1 h (Movies S5 and S6). During this time, a visible thin film separated from the aqueous phase was also present in the test tube itself. The increased propensity to coalesce may be due to an increase in ionic strength upon addition of a Na-HEPES buffer at pH 8 (the pH of which was adjusted to pH 8 by addition of NaOH), which likely results in adsorption of the ions to the surface of the hydrophobic droplets, causing a decrease in surface tension, and resulting in an increase in the aggregation and wetting propensity (to the glass coverslips) of the microdroplets (29). This is supported by evidence of the coalescence of dispersed polyPA microdroplets into a large macroscopic droplet of polyPA after only 40 min of incubation in 100 mM NaCl (SI Appendix, Fig. S22), as well as the real-time microscopic observation of coalescence into larger droplets in 100 mM NaCl and 100 mM Na-HEPES pH 8 conditions compared with the standard low-pH, no-salt conditions (SI Appendix, Fig. S23 and Movies S7–S9). However, even after 24 h of incubation in 800 mM Na-HEPES pH 8, upon vortexing and sonication, some of the samples were still able to form microdroplets (SI Appendix, Fig. S18). Although high pH and ionic strength tend to cause rapid droplet coalescence, constant agitation, such as that which might occur from wave action on shores or in fumaroles in hydrothermal fields such as those in Yellowstone (30), could have allowed such droplets to avoid complete, catastrophic coalescence in the environment and also provided a means for “division” after “recombination.” Such fumaroles are typically hot, and thus the thermal stability of these droplets at 90 °C was examined. Similar to some fatty acid vesicle systems (6), raising the ambient temperature did not result in destruction of the polyester microdroplets (SI Appendix, Fig. S24).
Segregation and Compartmentalization of Dyes.
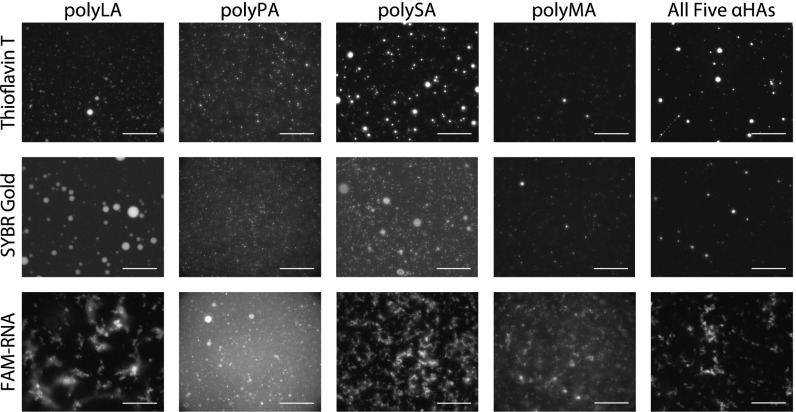
The ability of these polyester microdroplets to preferentially segregate and concentrate other molecules was then studied. Such preferential segregation would enable the microdroplets to facilitate primitive reactions which otherwise would not occur in dilute solution (31). Two fluorescent dyes—Thioflavin T (TfT) and SYBR Gold (See SI Appendix, Scheme S1 for chemical structures of these compounds)—and fluorescently labeled RNA were used for visualization. Each dye was introduced separately into each droplet system to observe phase segregation behavior. The droplets themselves are not fluorescent (SI Appendix, Fig. S25). Fig. 3 shows that TfT and SYBR Gold preferentially segregated into all of the different droplet systems, while the RNA showed differential segregation but only preferentially segregated into polyPA. The preferential segregation of TfT and SYBR Gold into all droplets suggests that the interior of the droplets are all fairly hydrophobic, as TfT preferentially binds to structures through hydrophobic interactions (32). Although the droplets appear to be fairly hydrophobic based on the preferential segregation of TfT, there may be yet still some amount water left within the dense phase even after the initial polymerization by drying. Spatial fluorescence intensity analyses of fluorescent dye-containing droplets showed the range and intensity of the dye dispersion within the droplets (SI Appendix, Figs. S26–S30). In some cases (e.g., polyLA, SI Appendix, Fig. S26), the dyes are fairly evenly dispersed within the droplets, suggesting that the polydisperse composition of the droplets is spatially evenly distributed around the entire body of the droplets. However, in other cases, the dyes are less evenly distributed, suggesting that spatial heterogeneity of oligomer polydispersity, possibly due to the nature of their functional groups (e.g., polySA, SI Appendix, Fig. S28). We are exploring the possibility of individual droplet analysis using spatially resolved mass spectrometry. Additionally, the amphiphilic dye Rhodamine-PE (Lissamine Rhodamine B, 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine, triethylammonium salt) appears to strongly localize to the outside of polyPA droplets, confirming that the polyPA hydrophobic–hydrophilic droplet interface is amenable to and potentially even scaffolds the assembly of lipid amphiphiles around the droplets (SI Appendix, Fig. S31). Rhodamine-PE was only slightly localized at the droplet interface of PolySA droplets, perhaps due to slightly greater droplet hydrophilicity in polySA (SI Appendix, Fig. S32). Despite starting with αHAs with different chemical side chains, the resulting polyester microdroplets were all able to preferentially segregate both TfT and SYBR Gold dyes. However, the fluorescently tagged RNA only strongly segregated into polyPA droplets, while in all other cases, the RNA did not segregate into droplets and appeared to form aggregates. Although RNA is quite hydrophilic owing to its charged phosphate backbone groups, it is possible that the aromatic groups in the polyPA interact with the aromatic RNA bases or even the aromatic-rich fluorescein tag, resulting in some preferential segregation into the droplets. The exact mechanism is beyond the scope of this study.
Fig. 3.
Fluorescence microscopy images showing that fluorescent dyes and fluorescently labeled RNA preferentially segregate into polyester microdroplets. TfT and SYBR Gold both segregate into all microdroplets, while FAM-RNA (6-carboxyfluorescein-labeled RNA) only segregates into polyPA. (Scale bars, 100 µm.)
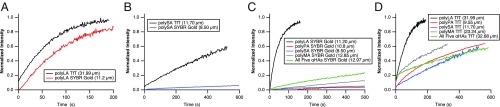
Stable compartmentalization should have been important for any early evolving system, as it prevents loss of encapsulated molecules into the surrounding environment. Thus, we next tested the ability of the microdroplets to stably compartmentalize TfT and SYBR Gold through Fluorescence Recovery After Photobleaching (FRAP), which measures the exchange rate of the encapsulated dyes from the droplet into the surrounding aqueous phase (and vice versa) (Movies S10 and S11) (33). A shorter recovery half-time t1/2 (the time for the fluorescence intensity to recover to 50% of its original value) indicates a less stably compartmentalized dye due to faster exchange, while a longer recovery half-time t1/2 indicates a more stably compartmentalized dye due to slower exchange. In some cases, such as in polyLA droplets, both dyes have similarly fast exchange rates in the same droplet type (Fig. 4A and Table 1), suggesting that neither dye is stably compartmentalized in polyLA droplets. In other cases, such as polySA, SYBR Gold has a much slower exchange rate than TfT (Fig. 4B and Table 1), suggesting that SYBR Gold is significantly more stably compartmentalized within polySA than TfT. For each dye, we also observed different exchange rates when droplets with different chemistries were compared. polyLA afforded the least stable compartmentalization for both dyes due to having the fastest exchange rate, while generally (outside of polyLA) SYBR Gold was more stably compartmentalized in all droplet types compared with TfT (Fig. 4 C and D and Table 1).
Fig. 4.
FRAP recovery curves of TfT or SYBR Gold residing in different microdroplets. A shows the recovery of both dyes in polyLA, while B shows the recovery of both dyes in polySA, highlighting the property that one droplet type may only afford stable compartmentalization to certain dyes. C shows the recovery of SYBR Gold in all droplet types, while D shows the recovery of TfT in all droplet types, highlighting the property that the same dye may be afforded stable compartmentalization only in certain droplet types. See SI Appendix, Table S6 for a list of all droplets probed with FRAP and their respective recovery kinetics, and Movies S10 and S11 for selected representative movies of the droplet FRAP experiments.
Table 1.
Representative FRAP kinetics of droplets analyzed in Fig. 4
| Dye | PolyLA | PolyPA | PolySA | PolyMA | All 5 αHAs |
| TfT | 50 s | 225 s | 705 s | 155 s | 490 s |
| SYBR Gold | 32 s | –– | –– | –– | 288 s |
FRAP curves were fit and analyzed as described (SI Appendix). t1/2, the half-time of recovery, is reported in the table. In some cases, the recovery rate was too slow to properly fit. In those cases, the table entries are labeled as “––”. See SI Appendix, Table S6 for summary of FRAP data for all droplets tested; Movies S10 and S11 show representative FRAP acquisitions.
The observed variations in exchange rate, i.e., variations in “phenotype,” are likely due primarily to the dyes having different affinities for different droplet chemistries, which have divergently arisen from relatively simple pools of similar monomers. However, droplet size and proximity to other droplets containing dyes could also contribute to the measured exchange rates, resulting in slight variations in the fluorescence recovery rate such as is observed in Fig. 4A. However, these minor variations alone cannot explain the large differences observed in Fig. 4 B–D (SI Appendix, Table S6). Thus, taken all together, these studies show that there is highly variable and composition-specific compartmentalization and exchange among these polyester droplets with respect to different solutes (SI Appendix, Table S6 and Movies S10 and S11).
Compatibility of Biomolecules with Droplets.
As we observed the ability for polyPA droplets to scaffold the assembly of a lipid amphiphile layer around itself (SI Appendix, Fig. S31), we further probed the effect of droplet association on the function of other biomolecules such as RNA and proteins. In vitro expressed and purified recombinant superfold green fluorescent protein (sfGFP) (34) was chosen due to its hydrophobicity and ability to be assayed for function simply via microscopy; sfGFP fluoresces when correctly folded. Within the hydrophobic polyPA microdroplets, sfGFP still fluoresced (SI Appendix, Fig. S33), and thus sfGFP remains functionally folded. This indicates that at least some proteins preserve their native structures within the droplet microenvironments, especially proteins with highly hydrophobic residues. We then performed ribozyme kinetic assays in the presence of polyPA droplets using a fluorescent self-cleaving hammerhead ribozyme (SI Appendix) (35). The reaction in the presence of polyPA proceeded (SI Appendix, Fig. S34), suggesting the compatibility between RNA and polyester microdroplets, while the rate of the ribozyme cleavage in the presence of polyPA droplets was slightly slower than in water (SI Appendix, Fig. S35). Even after 8 h of incubation with polyPA droplets in the same buffer conditions as the ribozyme self-cleavage reaction, some fluorescent RNA still segregated to the remaining droplets, which themselves appear to have decreased in number perhaps due to some hydrolysis and disassembly in these conditions (SI Appendix, Fig. S36). FRAP experiments showed that the recovery half-time of RNA within polyPA droplets was on the order of a few minutes (SI Appendix, Fig. S37 and Table S6 and Movie S12), which is far faster than the self-cleavage reaction itself (SI Appendix, Fig. S35), which occurs on the order of hours. This suggests that although RNA preferentially segregates to the droplets, it is still possible that the self-cleavage reaction occurs outside of the droplet when the RNA is exchanged into the bulk solution.
Discussion
We have shown that through simple heated wetting–drying processes, 5 types of simple αHAs (Fig. 1) alone or in combination polymerize into polydisperse polyesters after drying (SI Appendix, Fig. S1). With the exception of polyGA reacted alone, each of these polyester mixtures assembles into microdroplets with diameter up to 10s of micrometers in aqueous solution (Fig. 2). As the prebiotic Earth environment likely hosted a variety of compounds, self-assembly in the nano- or microscale arising from heterogeneous reactions may have been a common phenomenon (16). These results suggest that emergent physical behaviors relevant to chemical evolution can arise from the unguided complexification of simple monomer types even using molecular systems unrelated to the major biopolymers of modern biochemistry (14). These relatively simple heterogeneous systems also generate droplets with clear chemically distinct behaviors, namely, the differential ability to segregate and stably compartmentalize dyes and fluorescently labeled RNA.
Rehydration of the polymeric materials at pH 8 and high ionic strength conditions speeds droplet coalescence (SI Appendix, Figs. S17–S23 and Movies S5–S9). The pH and salinity of various primitive Earth surface waters, for example of the oceans or of evaporative pools, is uncertain and may have been locally variable (36). Nevertheless, significant pH (37) or salinity changes, agitation caused by water and wind movements (30), freezing and thawing (38), etc. (39) may have occurred in evaporative environments, which could have facilitated coalescence of polyester microdroplets with different encapsulated molecules or droplet chemistries, as well as dynamic polyester droplet assembly and disassembly. Modern biology uses both biologically- and environmentally controlled pH (40) and salt (41) cycling to maintain various dynamic and homeostatic systems. Oscillating pH-, ionic strength-, temperature-, and/or hydration-driven polyester microdroplet coalescence/assembly/disassembly systems with constant or periodic agitation offer a model experimental system to study primitive precellular environmentally controlled recombination and evolution.
As even this relatively simple system with only one synthesis step results in the emergence of distinct chemical traits, environmentally responsive dynamic systems may be common chemical phenomena as well as a facile way to select for microdroplets with specific attributes or functions advantageous for further chemical evolution. Since the early Earth day was much shorter than at present (∼4 h circa 4 Ga) (42), it is also possible that, depending on the ambient temperature, ebb and flow of water bodies (volume and concentration changes), ionic strength, and pH conditions, systems similar to those described could have arisen as quickly as observed here. Although this type of dynamism can be achieved in coacervate systems, for example by application of an external electrical field (43), this is not so easily accomplished in phospholipid vesicles, where fusion and division require significant external stimuli. Fatty acid vesicles, on the other hand, can easily divide from agitation or shear stress, but their fusion is not as easily achieved (7). Hence, more dynamically recombinative microscale systems, including those based on compounds such as αHAs and other simple monomers, could have played a critical role in the evolution of primitive living systems on early Earth (14).
In fatty acid vesicles, larger molecules such as nucleic acids do not leak to an appreciable degree after compartmentalization, while many smaller molecules are able to move in and out of such compartments (44). Chemically diverse polyester microdroplets are able to segregate and compartmentalize different molecules including small molecules or polymers like RNA or proteins (Figs. 3 and 4); in some droplets smaller molecules could be stably compartmentalized, e.g., small hydrophobic molecules, chemically similar to TfT, in a hydrophobic droplet interior through hydrophobic interactions. Simultaneously in these same droplets, larger molecules such as RNA or other biopolymers could be simultaneously excluded, such as in polySA, polyLA, or polyMA. In other droplets such as those formed from polyPA, larger molecules like RNA could instead preferentially cosegregate with the small hydrophobic molecules. This feature of polyester droplets could result in more diverse dynamics compared with fatty acid vesicles, and could be important for primitive chemical evolution and compartmentalized product accumulation. This could allow, for example, enhanced thermodynamic and kinetic favorability of certain reactions to occur in certain droplets, while enhancement of other reactions could occur in still other droplets (2). Certain droplets could also possibly provide stable compartmentalization of primitive biopolymers, including catalytic or genetic ones (27), providing differential fitness landscapes for different types of molecules. Although only 2 small molecule dyes, 1 fluorescently labeled amphiphile, 1 protein, and 1 fluorescently labeled RNA were studied here, such dynamics are likely also different for other prebiotic small molecules and polymers. Thus, microscale heterogeneous droplet populations could have facilitated the emergence of heterogeneous microenvironments hosting unique, localized, and selectable reaction cohorts that could be coupled into more complex reaction networks, similar to what has been proposed to occur on heterogeneous mineral surfaces (45).
The stability of individual polyester microdroplets to avoid coalescence, for extended time periods compared with other prebiotic membraneless compartment systems (11, 27) or among other polyester droplets, is an important consideration in the emergence of life using simple heterogeneous compartments in biopolymer-based origins of life models, as individuality may be required for systems to undergo Darwinian evolution (46). The observation that ribozyme catalysis is possible in association with such droplets (SI Appendix, Fig. S34) and that a protein remains functional within them (SI Appendix, Fig. S33) suggests further that these droplets could assist both RNA world-based (47) and protein/peptide-based (48) origins of life models. We could not confirm whether the ribozyme self-cleavage reaction occurred explicitly within the droplets, or whether the reaction actually occurs when the RNA molecules exchange into the bulk solution, as FRAP experiments suggested a fairly fast RNA exchange rate compared with the hammerhead ribozyme reaction itself (SI Appendix, Figs. S34 and S37). The fast exchange rate of RNA with polyPA droplets suggests fairly rapid diffusion of genetic polymers into the environment, which might hamper Darwinian evolution in polyester droplet-based systems (27). However, the observation that lipid amphiphiles can assemble into layers around these droplets (SI Appendix, Fig. S31) suggests the association of lipids with polyester droplets in primitive environments, which is plausible considering the diverse prebiotic milieu, potentially could have prevented rapid droplet coalescence and conferred greater droplet stability from hydrolysis at higher pH, while also potentially preventing rapid RNA exchange out of the droplets. Further studies combining lipids with polyester droplet systems may shed light on the ability of such droplets to serve as scaffolds for various biopolymer-based origins of life models (1, 47). Nevertheless, fast exchange of genetic polymers might actually facilitate exchange of genetic information between protocells; some models for the early evolution of life suggest this was the state of affairs before the major cell lineages became fixed (49). The droplets may also offer a model system to experimentally study the chemistry of composomes, compositional assemblies that are proposed to be able to replicate and pass on compositional information to progeny (50). Mixed heteropolyester droplet systems coupled with environmental oscillations of pH, wetting, or temperature may change in composition over time. Heteropolyester gel microdroplets could undergo repeated cycles of polymerization, depolymerization, disaggregation, and fusion, resulting in compositional evolution and selection. These droplets could even help concentrate components of simple metabolic cycles (51); the hydrophobic environments within the polyester droplets might even facilitate the emergence of other reaction networks that do not easily occur in aqueous solution.
Based on the experimental evidence described here, we suggest that these polyester-based microdroplets could have played a role in primitive compartmentalization, and offer a facile model experimental system for exploring complex chemical dynamics in populations of polymers and compartments across multiple scales and compatible with a variety of origins of life models. Prebiotic organic chemical diversity was likely higher than in our proof-of-principle study (52), and chemical characterization of the resultant chemically complex systems would likely require the development of novel analytical techniques. Nevertheless, by further increasing complexity stepwise as in this study, one can systematically track changes in a system while still observing divergent and emergent properties, such as compositional or functional changes, arising from the ensemble system. The combinatorial methods used to generate prebiotic polymers with distinct “phenotypic” traits in this work could also be used together with other compounds and chemistries possibly available on early Earth, such as formation of branched dendrimer-type polymers (53) or depsipeptides (54). While our focus is origins of life studies, application of this system toward modern biomedical applications could lead to personalized medicine delivery microvessels. These systems could even offer a simple experimental system for studying the dynamics of modern biological membraneless compartments such as those mentioned previously (15). Further development of this model system, or other similar systems, in origins of life contexts could allow closer simulation of undirected and diverse chemical systems which are more representative of complex chemistries on early Earth or other planetary bodies.
Methods
Synthesis of Polyesters.
All chemicals were purchased from Sigma-Aldrich (Chuo-ku) unless otherwise noted in SI Appendix. All experiments were conducted in open borosilicate test tubes unless otherwise noted. pH was not adjusted. Reactions were held at 80 °C for 1 wk. Starting total concentrations of all reactions were 500 mM αHA.
Microscopy.
All experiments began with dried polyester freshly hydrated in 500 µL 4:1 (vol/vol) water:acetonitrile (unless otherwise noted), followed by brief sonication and vortexing and sample slide preparation. Optical and epifluorescence microscopy images were acquired with an Olympus (Shinjuku-ku) IX73 inverted fluorescent microscope. FRAP and other confocal microscopy was performed with an Olympus IX81 confocal microscope. All images were analyzed using FIJI (Fiji is Just ImageJ, http://fiji.sc). Observations were performed in at least duplicate. See SI Appendix for sample preparation and FRAP curve-fitting details.
Supplementary Material
Acknowledgments
We acknowledge I. Mamajanov, D. E. Smith, and C. Butch of the Earth-Life Science Institute (ELSI) at Tokyo Institute of Technology (Titech), and A. Wang (University of New South Wales). T.Z.J. is supported by Titech Seed Grant “Tane” 1798, Japan Society for the Promotion of Science (JSPS) Grant-in-Aid JP18K14354, and Japan Astrobiology Center Project Grant AB311021. K.C. is supported by the European Structural and Investment Funds Operational Programme “Research, Development and Education”-funded project “ChemJets” (CZ 02.2.69/0.0/0.0/16_027/0008351) and Research Development Fund UKM (DPP-2018-004). Pilot microscopic studies were supported by a JSPS Grant-in-Aid JP17H06459 to T.U. Crucial exploratory work by K.C. and H. J. C. was partially supported by JSPS Grant-in-Aid JP26106003 “Hadean Bioscience” and the ELSI Origins Network, which was supported by a grant from the John Templeton Foundation. The opinions expressed in this publication are those of the authors and do not necessarily reflect the views of the John Templeton Foundation. MALDI spectra were obtained with the assistance of M. Koizumi at the Titech Materials Analysis Division. FRAP experiments were performed with the assistance of Y. Sato, M. Masukawa, and M. Takinoue (Titech) and A. Baccouche and T. Fujii (University of Tokyo). We thank Y. Kuruma (The Japan Agency for Marine-Earth Science and Technology/ELSI) for providing Rhodamine-PE and for use of his epifluorescence microscope; R. Sinmyo and K. Hirose (ELSI and University of Tokyo) for assistance with focused ion beam scanning electron microscopy; P.-H. Wang from the McGlynn Group (ELSI) for technical support on expression and purification of recombinant sfGFP; and R. Kikuchi at the Titech Materials Analysis Division for assistance with transmission electron microscopy.
Footnotes
Conflict of interest statement: The authors may file a patent application within 12 months after publication of the manuscript.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902336116/-/DCSupplemental.
References
- 1.Szostak J. W., Bartel D. P., Luisi P. L., Synthesizing life. Nature 409, 387–390 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Fallah-Araghi A., et al. , Enhanced chemical synthesis at soft interfaces: A universal reaction-adsorption mechanism in microcompartments. Phys. Rev. Lett. 112, 028301 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Alberts B., Johnson A., Lewis J., Roberts K., Walter P., Eds., “The lipid bilayer” in Molecular Biology of the Cell (Garland Science, New York, 2002). https://www.ncbi.nlm.nih.gov/books/NBK26871/. Accessed 1 November 2018.
- 4.McCollom T. M., Ritter G., Simoneit B. R. T., Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 29, 153–166 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Cleaves H. J., Prebiotic chemistry: What we know, what we don’t. Evol. Educ. Outreach 5, 342–360 (2012). [Google Scholar]
- 6.Mansy S. S., Szostak J. W., Thermostability of model protocell membranes. Proc. Natl. Acad. Sci. U.S.A. 105, 13351–13355 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terasawa H., Nishimura K., Suzuki H., Matsuura T., Yomo T., Coupling of the fusion and budding of giant phospholipid vesicles containing macromolecules. Proc. Natl. Acad. Sci. U.S.A. 109, 5942–5947 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budin I., Prwyes N., Zhang N., Szostak J. W., Chain-length heterogeneity allows for the assembly of fatty acid vesicles in dilute solutions. Biophys. J. 107, 1582–1590 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamala K., Szostak J. W., Nonenzymatic template-directed RNA synthesis inside model protocells. Science 342, 1098–1100 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deamer D., The role of lipid membranes in life’s origin. Life (Basel) 7, E5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pir Cakmak F., Keating C. D., Combining catalytic microparticles with droplets formed by phase coexistence: Adsorption and activity of natural clays at the aqueous/aqueous interface. Sci. Rep. 7, 3215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann S., Systems of creation: The emergence of life from nonliving matter. Acc. Chem. Res. 45, 2131–2141 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Cooper G. J. T., et al. , Modular redox-active inorganic chemical cells: iCHELLs. Angew. Chem. Int. Ed. Engl. 50, 10373–10376 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Tena-Solsona M., Wanzke C., Riess B., Bausch A. R., Boekhoven J., Self-selection of dissipative assemblies driven by primitive chemical reaction networks. Nat. Commun. 9, 2044 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hyman A. A., Brangwynne C. P., Beyond stereospecificity: Liquids and mesoscale organization of cytoplasm. Dev. Cell 21, 14–16 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Guttenberg N., Virgo N., Chandru K., Scharf C., Mamajanov I., Bulk measurements of messy chemistries are needed for a theory of the origins of life. Philos. Trans. A Math. Phys. Eng. Sci. 375, 20160347 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker E. T., Cleaves H. J. 2nd, Bada J. L., Fernández F. M., Quantitation of α-hydroxy acids in complex prebiotic mixtures via liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 30, 2043–2051 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Cronin J. R., Cooper G. W., Pizzarello S., Characteristics and formation of amino acids and hydroxy acids of the Murchison meteorite. Adv. Space Res. 15, 91–97 (1995). [DOI] [PubMed] [Google Scholar]
- 19.Peltzer E. T., Bada J. L., α-Hydroxycarboxylic acids in the Murchison meteorite. Nature 272, 443–444 (1978). [Google Scholar]
- 20.Chandru K., et al. , Simple prebiotic synthesis of high diversity dynamic combinatorial polyester libraries. Commun. Chem. 1, 30 (2018). [Google Scholar]
- 21.Ojha L., et al. , Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat. Geosci. 8, 829–832 (2015). [Google Scholar]
- 22.Grimm S. L., et al. , The nature of the TRAPPIST-1 exoplanets. A&A 613, A68 (2018). [Google Scholar]
- 23.Mulkidjanian A. Y., Bychkov A. Y., Dibrova D. V., Galperin M. Y., Koonin E. V., Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. U.S.A. 109, E821–E830 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adam Z. R., et al. , Estimating the capacity for production of formamide by radioactive minerals on the prebiotic Earth. Sci. Rep. 8, 265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iqbal M., et al. , Aqueous two-phase system (ATPS): An overview and advances in its applications. Biol. Proced. Online 18, 18 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priftis D., Tirrell M., Phase behaviour and complex coacervation of aqueous polypeptide solutions. Soft Matter 8, 9396–9405 (2012). [Google Scholar]
- 27.Jia T. Z., Hentrich C., Szostak J. W., Rapid RNA exchange in aqueous two-phase system and coacervate droplets. Orig. Life Evol. Biosph. 44, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason A. F., Buddingh’ B. C., Williams D. S., van Hest J. C. M., Hierarchical self-assembly of a copolymer-stabilized coacervate protocell. J. Am. Chem. Soc. 139, 17309–17312 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zangi R., Hagen M., Berne B. J., Effect of ions on the hydrophobic interaction between two plates. J. Am. Chem. Soc. 129, 4678–4686 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Hurwitz S., Harris R. N., Werner C. A., Murphy F., Heat flow in vapor dominated areas of the Yellowstone plateau volcanic field: Implications for the thermal budget of the Yellowstone Caldera. J. Geophys. Res. 117, B10207 (2012). [Google Scholar]
- 31.Deamer D., Weber A. L., Bioenergetics and life’s origins. Cold Spring Harb. Perspect. Biol. 2, a004929 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biancalana M., Koide S., Molecular mechanism of Thioflavin-T binding to amyloid fibrils. Biochim. Biophys. Acta 1804, 1405–1412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phair R. D., Gorski S. A., Misteli T., Measurement of dynamic protein binding to chromatin in vivo, using photobleaching microscopy. Methods Enzymol. 375, 393–414 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Berhanu S., Ueda T., Kuruma Y., Artificial photosynthetic cell producing energy for protein synthesis. Nat. Commun. 10, 1325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adamala K. P., Engelhart A. E., Szostak J. W., Collaboration between primitive cell membranes and soluble catalysts. Nat. Commun. 7, 11041 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland H. D., “The geologic history of seawater” in Treatise on Geochemistry, Holland H. D., Turekian K. K., Eds. (Elsevier, Amsterdam, 2007), pp. 1–46. [Google Scholar]
- 37.Shao H., Ray J. R., Jun Y.-S., Dissolution and precipitation of clay minerals under geologic CO2 sequestration conditions: CO2-brine-phlogopite interactions. Environ. Sci. Technol. 44, 5999–6005 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Takenaka N., Tanaka M., Okitsu K., Bandow H., Rise in the pH of an unfrozen solution in ice due to the presence of NaCl and promotion of decomposition of gallic acids owing to a change in the pH. J. Phys. Chem. A 110, 10628–10632 (2006). [DOI] [PubMed] [Google Scholar]
- 39.Keil L. M. R., Möller F. M., Kieß M., Kudella P. W., Mast C. B., Proton gradients and pH oscillations emerge from heat flow at the microscale. Nat. Commun. 8, 1897 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ratzke C., Gore J., Modifying and reacting to the environmental pH can drive bacterial interactions. PLoS Biol. 16, e2004248 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinn S. J., et al. , Sodium and ionic strength sensing by the calcium receptor. J. Biol. Chem. 273, 19579–19586 (1998). [DOI] [PubMed] [Google Scholar]
- 42.Meyers S. R., Malinverno A., Proterozoic Milankovitch cycles and the history of the solar system. Proc. Natl. Acad. Sci. U.S.A. 115, 6363–6368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin Y., et al. , Non-equilibrium behaviour in coacervate-based protocells under electric-field-induced excitation. Nat. Commun. 7, 10658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mansy S. S., Membrane transport in primitive cells. Cold Spring Harb. Perspect. Biol. 2, a002188 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gillams R. J., Jia T. Z., Mineral surface-templated self-assembling systems: Case studies from nanoscience and surface science towards origins of life research. Life (Basel) 8, E10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith E., Morowitz H. J., The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere (Cambridge University Press, 2016). [Google Scholar]
- 47.Gilbert W., Origin of life: The RNA world. Nature 319, 618 (1986). [Google Scholar]
- 48.Ikehara K., [GADV]-protein world hypothesis on the origin of life. Orig. Life Evol. Biosph. 44, 299–302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woese C. R., Fox G. E., The concept of cellular evolution. J. Mol. Evol. 10, 1–6 (1977). [DOI] [PubMed] [Google Scholar]
- 50.Lancet D., Zidovetzki R., Markovitch O., Systems protobiology: Origin of life in lipid catalytic networks. J. R. Soc. Interface 15, 20180159 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muchowska K. B., et al. , Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 1, 1716–1721 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt-Kopplin P., et al. , High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. U.S.A. 107, 2763–2768 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mamajanov I., Callahan M. P., Dworkin J. P., Cody G. D., Prebiotic alternatives to proteins: Structure and function of hyperbranched polyesters. Orig. Life Evol. Biosph. 45, 123–137 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Forsythe J. G., et al. , Ester-mediated amide bond formation driven by wet-dry cycles: A possible path to polypeptides on the prebiotic earth. Angew. Chem. Int. Ed. Engl. 54, 9871–9875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.