Abstract
Objective:
The present study aims to compare 24-h dietary recalls with 24-h urine collections for the estimation of sodium intake at both population and individual levels in China, Japan, the United Kingdom (UK), and the United States of America (USA), using data from the International Study of Macro- and Micro-nutrients and Blood Pressure (INTERMAP).
Methods:
Mean differences between 24-h dietary recalls and 24-h urine collections were calculated for their agreement in estimating sodium intake at the population level; relative and absolute differences as well as misclassification of salt intake groups (salt intake <6, 6–9-11.9, 12–14.9, and ≥15 g/day) were used to determine the agreement at the individual level.
Results:
The mean differences (95% CI) between dietary recalls and urine collections for China, Japan, UK, and USA were −54.0 (−59.8, −48.3), 3.9 (0.6, 7.2), 2.9 (−1.8, 7.6), and −3.5 (−5.8, −1.1) mmol/day, respectively. The proportions of individual relative differences beyond ±40% were 34.3% for China, 16.9% for Japan, 24.2% for UK, and 21.3% for USA; the proportions of individual absolute differences greater than 51.3 mmol/day (3 g salt) were 58.6% for China, 32.8% for Japan, 25.4% for UK, and 31.9% for USA. The rate for misclassification of salt intake groups at individual level for China, Japan, UK, and USA were 71.4, 60.9, 58.7, and 60.0%, respectively.
Conclusion:
The 24-h dietary recalls demonstrate greater agreement with the 24-h urine collections in estimating population sodium intake for Japan, UK, and USA, compared with China. The 24-h dietary recall has poor performance in assessing individual sodium intake in these four countries.
Keywords: 24-h dietary recall, 24-h urinary sodium, agreement, sodium intake
INTRODUCTION
High sodium (Na) intake has been widely associated with elevated blood pressure (BP) and increased risk of cardiovascular diseases [1–5]. To achieve potential cardiovascular benefits, reducing Na intake has been recommended in many guidelines on lifestyle management or dietary intake [6–9]. Accurate measurement of Na intake is essential to identify its true contribution to a relation between salt intake and health outcomes and thus evaluate the effectiveness of a population Na reduction strategy. Several approaches to measuring dietary Na intake are currently available, including urinary and dietary assessment [10]. Although multiple collections of 24-h urinary Na excretion are considered the gold standard for assessing dietary Na intake, high participant burden, analytical labor, and the difficulty in achieving complete collection of 24-h urine have limited their use in large-scale population studies. Likewise, the 24-h dietary recall has been employed in many large studies such as the China Health and Nutrition Survey [11] and the National Health and Nutrition Examination Survey (NHANES) in the United States [12]. Although self-reported dietary recalls can help to identify sources of Na and other nutrients, they can be biased by inaccuracies in recall and/or recording. Several studies have assessed the agreement between 24-h dietary recall and 24-h urine collection in estimating dietary Na intake at the population level [13–17], but most of these studies were conducted in a single country or population, hence results may not be cross-culturally applicable. Also, most previous studies failed to analyze misclassification at the individual level. Therefore, the present study aimed to address these questions using data from the population-based International Study of Macro- and Micro-nutrients and Blood Pressure (INTERMAP) to evaluate the 24-h dietary recall from all four countries [China, Japan, the United Kingdom (UK), and the United States of America (USA)] against 24-h urine collection for the estimation of Na intake at both population and individual levels.
METHODS
Study population
Participants were from the INTERMAP Study, an international epidemiologic study designed to investigate relations of dietary variables to BP, among 4680 men and women aged 40–59 years from 17 population samples in China (3 samples), Japan (4 samples), UK (2 samples), and USA (8 samples) [18]. For each participant, four 24-h dietary recalls, two timed 24-h urine specimens, eight BP measurements (two at each of four visits), and data on major possibly confounding variables were collected. Field work was conducted from 1996 to 1999, with strict quality control procedures undertaken at the international, national, and local levels throughout the field surveys. Details about the study populations and methods have been reported [18]. Written informed consent was obtained from all participants.
Dietary data collection
Each participant attended the local research center four times, two visits on consecutive days, and another such pair of visits with an interval of 3–6 weeks. Four 24-h dietary recalls (two pairs) were performed by trained certified interviewers using the in-depth multipass 24-h recall method [19]. All foods, drinks, and dietary supplements consumed in the previous 24 h were recorded. Assessment tools such as real foods, food models, measuring devices, and photographs were used in the four countries to assist in accurate recording.
In China, individual ingredients in mixed dishes were estimated by the participant, and when foods reported were prepared by someone other than the participant, the ingredients were verified with the cook. The amount of salt used in cooking was estimated using real salt, weighed and recorded. The portion of the mixture consumed was estimated by the participant. Details on country-specific procedures of dietary data collection have been reported [19].
Urine collection
Two timed 24-h urine specimens were collected, each coinciding with a pair of 24-h dietary recalls. Collections started at the local research center on the first day of each visit and were completed there the next day. The start and end times were recorded as participants began and completed the collection at the research center. Also, participants reported any missing urine on a written form. The specimen was designated ‘incomplete’ and the participant was asked to repeat the collection. Also, the collection was repeated if the collection time fell outside the range of 22–26 h, or the total volume of urine was less than 500 ml. Urine aliquots were stored frozen at −20 °C and airfreighted frozen to the Central Laboratory (Leuven, Belgium), where urinary Na, potassium (K), creatinine, and other variables were measured with rigorous internal and external quality control.
Statistical analysis
Descriptive data are presented as mean values ± standard deviation (SD) unless otherwise indicated. For analyses on the difference and agreement between the two methods, the averages of the four dietary measurements and two urinary measurements were used. Differences between urinary Na excretion measured by 24-h urine collection and dietary Na estimated by 24-h dietary recall were calculated. The relative and absolute differences between the two methods were analyzed, where the relative difference was calculated as [(24-h dietary Na – 24-h urine Na)/24-h urine Na x 100%] and the absolute difference was calculated as (24-h dietary Na – 24-h urine Na). Also, Na intake values obtained from the two methods were converted into salt intake values (17.1 mmol of Na is equivalent to 1 g of salt) and were further divided into five categories (<6, 6–9–11.9, 12–14.9, and ≥15g/day), and the percentage of participants classified into each group was calculated. Misclassification rate was defined as the proportion of individuals misclassified into other salt intake categories by dietary recall using 24-h urinary collection as the valid level. A two-tailed P value less than 0.05 was considered significant. All analyses were performed using SAS version (SAS Institute, Cary, North Carolina, USA).
RESULTS
The INTERMAP Study included 4680 participants from China (n = 839), Japan (n = 1145), UK (n = 501), and USA (n = 2195). Participants were 50.4% men, mean age 49.2 ± 5.5 years, mean daily Na excretion 181.1 ±72.4mmol. Mean of dietary Na intake estimated by 24-h dietary recall and 24-h urine collection and mean difference between the two methods are shown in Table 1. The mean 24-h urinary Na excretions for China, Japan, UK, and USA were 227.5 ± 100.3, 198.3 ± 56.2, 145.2 ± 49.1, and 162.6 ± 59.4 mmol, respectively. Mean differences (95% CI) between 24-h dietary recall and 24-h urine collection were −54.0 (−59.8, −48.3) mmol for China, 3.9 (0.6, 7.2) mmol for Japan, 2.9 (−1.8,7.6) mmol for UK, and −3.5 (−5.8, − 1.1) mmol for USA. The country Na intake was over-reported or under-reported by 2.0–2.2% for Japan, UK, and USA samples using 24-h urine collection as the reference, whereas it was under-reported by 23.7% for Chinese samples. Similar results were found for the first and repeat visit data analyzed separately.
TABLE 1.
Mean difference of Na intake estimated by 24-h dietary recall and 24-h urine collection across four countries, 1996–1999
| China (n = 839) | Japan (n = 1145) | UK (n = 501) | USA (n = 2195) | |
|---|---|---|---|---|
| Mean of four visits | ||||
| Urinary Na (mmol) | 227.5 ± 100.3 | 198.3 ± 56.2 | 145.2 ± 49.1 | 162.6 ± 59.4 |
| Dietary Na (mmol) | ± 84.5 | 202.2 ± 55.6 | 148.1 ± 50.5 | 159.1 ± 58.4 |
| Mean difference (95% CI; mmol) | −54.0 (−59.8, −48.3) | 3.9 (0.6, 7.2) | 2.9 (−1.8, 7.6) | −3.5 (−5.8, −1.1) |
| Biasa (%) | −23.7 | 2.0 | 2.0 | −2.2 |
| First pair of visits | ||||
| Urinary Na (mmol) | 229.3 ± 113.9 | 198.9 ± 64.7 | 145.6 ± 57.1 | 163.0 ± 67.8 |
| Dietary Na (mmol) | ± 99.4 | 204.5 ± 66.2 | 148.3 ± 59.6 | 159.2 ± 67.3 |
| Mean difference (95% CI; mmol) | −48.9 (−56.2, −41.7) | 5.6 (1.6, 9.6) | 2.7 (−2.8, 8.3) | −3.9 (−6.8, −0.9) |
| Bias (%) | −21.3 | 2.8 | 1.9 | −2.3 |
| Repeat pair of visits | ||||
| Urinary Na (mmol) | 225.7 ± 111.8 | 197.7 ± 66.0 | 144.8 ± 58.5 | 162.1 ± 70.6 |
| Dietary Na (mmol) | 166.6 ± 89.8 | 199.9 ± 61.8 | 147.9 ± 56.1 | 159.1 ± 65.5 |
| Mean difference (95% CI; mmol) | −59.1 (−66.1, −52.2) | 2.2 (−1.9, 6.3) | 3.1 (−2.5, 8.7) | −3.1 (−6.0, −0.2) |
| Bias (%) | −26.2 | 1.1 | 2.1 | −1.9 |
Values are means± SDs unless otherwise indicated.
Bias = (Dietary Na — Urinary Na)/Urinary Na x 100%, at the population level.
For individual level analyses, distributions of relative and absolute differences between 24-h dietary recall and 24-h urine collection are shown in Figs. 1 and 2. When the 24-h urine collection was used as the reference, the proportions of relative differences within ±10% for 24-h dietary recall in China, Japan, UK, and USA were 12.8, 29.1, 24.6, and 24.2%, respectively. The proportions of relative differences beyond ±40% were 34.3% for China, 16.9% for Japan, 24.2% for UK, and 21.3% for USA. Similarly, the proportions of individual absolute differences within ±17.1 mmol/day (1 g salt) for 24-h dietary recall in the above four countries were 14.0, 26.8, 29.3, and 28.3%, respectively. In addition, the proportions of individual absolute differences greater than 51.3mmol/day (3g salt) were 58.6% for China, 32.8% for Japan, 25.4% for UK, and 31.9% for USA.
FIGURE 1.
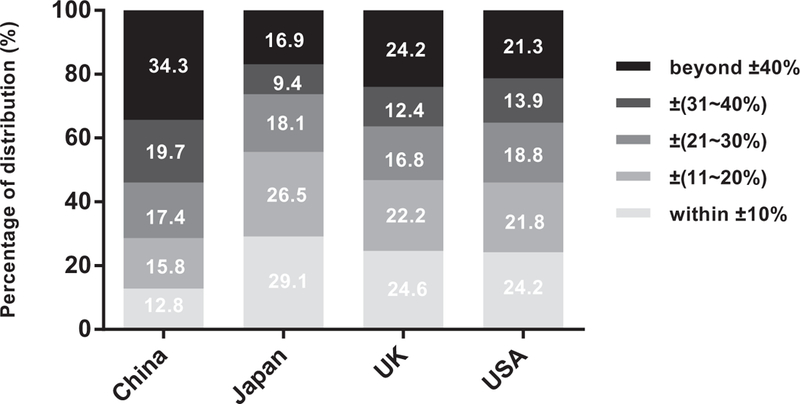
Relative difference* distributions of the 24-h dietary recall for estimating Na intake. ‘Relative difference = (24-h dietary Na — 24-h urine Na)/24-h urine Na x 100%.
FIGURE 2.
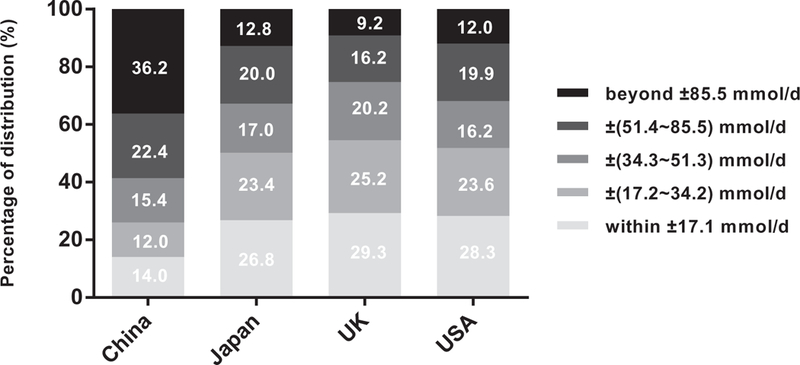
Absolute difference* distributions of the 24-h dietary recall for estimating Na intake. ‘Absolute difference = 24-h dietary Na — 24-h urine Na.
The classification of individual salt intake converted from 24-h dietary Na and 24-h urinary Na is shown in Table 2. With the 24-h urinary excretion as the valid level, the dietary value classified more than half of all individuals into wrong categories of salt intake in all four countries. The overall misclassification rates at individual level were 71.4% for China, 60.9% for Japan, 58.7% for UK, and 60.0% for USA.
TABLE 2.
Misclassification of the 24-h dietary recall for estimating individual salt intake
| Salt intake converted from 24-h urinary Naa | ||||||
|---|---|---|---|---|---|---|
| <6g | 6–8.9g | 9–11.9g | 12–14.9g | >15g | All | |
| China | ||||||
| Sample size | 80 | 133 | 168 | 157 | 301 | 839 |
| <6g | 56 (70.0) | 64 (48.1) | 34 (20.2) | 21 (13.4) | 8 (2.7) | |
| 6–8.9g | 20 (25.0) | 39 (29.3) | 70 (41.7) | 42 (26.8) | 41 (13.6) | |
| 9–11.9g | 3 (3.8) | 21 (15.8) | 34 (20.2) | 47 (29.9) | 82 (27.2) | |
| 12–14.9 g | 0 (0.0) | 6 (4.5) | 18 (10.7) | 22 (14.0) | 81 (26.9) | |
| ≥15g | 1 (1.3) | 3 (2.3) | 12 (7.1) | 25 (15.9) | 89 (29.6) | |
| Misclassificationb | 24 (30.0) | 94 (70.7) | 134 (79.8) | 135 (86.0) | 212 (70.4) | 599 (71.4) |
| Japan | ||||||
| Sample size | 18 | 241 | 423 | 291 | 172 | 1145 |
| <6g | 1 (5.6) | 7 (2.9) | 7(1.7) | 0 (0.0) | 0 (0.0) | |
| 6–8.9g | 12 (66.7) | 83 (34.4) | 72 (17.0) | 24 (8.3) | 6 (3.5) | |
| 9–11.9g | 5 (27.8) | 105 (43.6) | 188 (44.4) | 116 (39.9) | 29 (16.9) | |
| 12–14.9 g | 0 (0.0) | 37 (15.4) | 108 (25.5) | 103 (35.4) | 64 (37.2) | |
| ≥15g | 0 (0.0) | 9 (3.7) | 48(11.4) | 48 (16.5) | 73 (42.4) | |
| Misclassification | 17 (94.4) | 158 (65.6) | 235 (55.6) | 188 (64.6) | 99 (57.6) | 697 (60.9) |
| UK | ||||||
| Sample size | 87 | 231 | 121 | 49 | 13 | 501 |
| <6g | 32 (36.8) | 39 (16.9) | 10 (8.3) | 0 (0.0) | 1 (7.7) | |
| 6–8.9g | 47 (54.0) | 115 (49.8) | 48 (39.7) | 13 (26.5) | 4 (30.8) | |
| 9–11.9g | 7 (8.1) | 56 (24.2) | 45 (37.2) | 21 (42.9) | 3 (23.1) | |
| 12–14.9 g | 1 (1.2) | 16 (6.9) | 14(11.6) | 12 (24.5) | 2 (15.4) | |
| ≥15g | 0 (0.0) | 5 (2.2) | 4(3.3) | 3 (6.1) | 3 (23.1) | |
| Misclassification | 55 (63.2) | 116 (50.2) | 76 (62.8) | 37 (75.5) | 10 (76.9) | 294 (58.7) |
| USA | ||||||
| Sample size | 298 | 765 | 692 | 289 | 151 | 2195 |
| <6g | 122 (40.9) | 133 (17.4) | 65 (9.4) | 10 (3.5) | 1 (0.7) | |
| 6–8.9g | 129 (43.3) | 379 (49.5) | 231 (33.4) | 68 (23.5) | 9 (6.0) | |
| 9–11.9g | 36 (12.1) | 192 (25.1) | 257 (37.1) | 108 (37.4) | 47 (31.1) | |
| 12–14.9 g | 8 (2.7) | 49 (6.4) | 107 (15.5) | 69 (23.9) | 44 (29.1) | |
| ≥15g | 3 (1.0) | 12 (1.6) | 32 (4.6) | 34 (11.8) | 50 (33.1) | |
| Misclassification | 176 (59.1) | 386 (50.5) | 435 (62.9) | 220 (76.1) | 101 (66.9) | 1318 (60.0) |
Values are expressed as n (%).
1 g salt = 17.1 mmol Na.
Misclassification: number and proportion of individuals misclassified into other salt intake categories by dietary recall using 24-h urinary collection as the valid level.
Considering that salt intake varies greatly in different regions of China, we additionally analyzed the data of three Chinese population samples (Beijing, Shanxi, and Guangxi) separately. Supplemental Tables S1-S2 and Supplemental Figures S1–S2, http://links.lww.com/HJH/B13 showed that there was great underestimation of Na intake through 24-h dietary recalls at the population level and high misclassification rate of salt intake at the individual level in all three samples. Specifically, the misclassification rates in Beijing, Shanxi, and Guangxi were 67.3, 81.7, and 64.7%.
DISCUSSION
At the population level, Na intake estimated by multiple 24- h dietary recalls was close to the Na level derived from two 24-h urine collections for Japan, UK, and USA, but not for China. The mean difference in Na intake between the two methods for the Chinese cohort was relatively large. At the individual level, the use of averaged 24-h dietary recalls performed poorly in assessing Na intake in all four countries based on both high relative and absolute differences, as well as large misclassification rate of salt intake groups.
These findings comparing methods of assessment of Na intake are consistent with data from several previous validation studies. The USDA Automated Multiple-Pass Method (AMPM) Validation Study (n = 465) compared Na intake from self-reported 24-h dietary recalls with 24-h urinary excretion and likewise found that 24-h dietary recall was accurate at the population level with less than 9% underestimation of Na compared with the urinary excretion [13]. A study conducted in a worksite sample in Ireland enrolled 50 participants aged 18–64 years and found that the mean difference between 24-h dietary recall and 24-h urine collection was only 3.8 mmol [14]. In contrast, the European Food Consumption Validation study including 365 participants from three European countries (Belgium, Norway, and the Czech Republic) found that self-reported dietary Na from 24- h recalls represented 61–75 and 59–70% of the urinary levels of the Na biomarker for men and women, respectively [15]. The Trial of Nonpharmacologic Intervention in the Elderly (TONE) included repeated standardized 24-h dietary recalls and 24-h urine collections, and reported that dietary recalls yielded estimates of Na intake that averaged 22% less than those from urine assays [17]. These studies demonstrated major underestimation of Na intake by dietary recalls at the group level. This underestimation was also found in the Chinese population in our study, and the results are in accordance with other studies conducted in Chinese populations, including studies that compared Food Frequency Questionnaire (FFQ) data with data from single 24-h urine collections in estimating dietary Na [20,21].
Most previous dietary validation studies using multiple 24 h recalls to report intakes of other macronutrients and micronutrients often fail to specifically discuss limitations for estimating dietary Na intake at the individual level. We used relative and absolute differences as well as misclassi- fication to determine the agreement at the individual level, in accordance with a recent validation study about spot urine in predicting 24-h Na excretion [22]. The relative differences beyond ±40% and absolute differences greater than 51.3mmol/day (equivalent to a salt intake of 3g/day) were high in all four countries, especially in China. The Prospective Urban Rural Epidemiology (PURE) Study enrolled and followed 156424 persons from 17 countries and reported that both higher and lower levels of estimated Na excretion were associated with increased cardiovascular risk, resulting in a J-shaped association curve [23]. In addition, a pooled analysis conducted by the PURE research group also reported that high Na intake was associated with an increased risk of cardiovascular events and death in hypertensive populations but not in normotensive populations, whereas the association of low Na intake with increased risk of cardiovascular events and death was observed in those with or without hypertension [24]. Although observational analysis sometimes yields a reverse causality, as was found in the Systolic Blood Pressure Intervention Trial (SPRINT) experience [25], the PURE Study was criticized mainly for using a single spot urine to estimate Na intake [26], and it was shown that spot urine produced great misclassification of salt intake groups in a recent validation study [22]. Also, a recently published analysis based on the Trials of Hypertension Prevention (TOHP) data found that spot urine estimates of Na intake resulted in a J-shaped relationship between Na and all-cause mortality whereas 24-h urine collection measured Na intake showed a linear relationship with mortality [27]. Unfortunately, over half of the INTERMAP participants’ Na intake was misclassified by diet recalls in all four countries. This finding illustrates that dietary Na intake estimated by 24-h dietary recall alone is limited for detecting relations of salt intake with health outcomes in epidemiological and clinical studies without having a urinary Na biomarker.
The present study reports that among the four countries, the use of 24-h dietary recalls had the least favorable performance in China at both the population and individual levels. Possible explanations for the difference between China and the other three countries are: first, the Na content in recipes for both processed and home-cooked foods is highly variable in the Chinese diet, and discretionary salt use is difficult to quantify in dietary surveys [28]. Our previous study has shown that dietary Na from commercially processed foods such as breads, grains, cereals, soups, sauces, and cured meats accounted for the great majority of Na consumed in Japan, UK, and USA. And discretionary Na intake, that is, salt added to foods during home preparation or at the table, was a modest contributor to overall intake in these countries. In contrast, in the three Chinese samples, discretionary Na use accounted for most Na intake [29]. Soy sauce, monosodium glutamate (MSG), and other condiments included in the home cooking process in China are difficult to accurately assess by dietary recalls, although we used scales and other tools to assist participants to estimate salt intake; second, standard recipes with amounts of condiments specified precisely, are common in the west and Japan but are hardly used in China.
Our study has several strengths. Firstly, the INTERMAP Study enrolled a large sample from different countries, with high-quality dietary data and timed 24-h urine collection, enabling assessment of the agreement between 24-h dietary recalls and 24-h urine collections for estimating dietary Na intake. Secondly, the present study not only analyzed mean differences between 24-h dietary recall and 24-h urine collection at the population level but also evaluated the agreement at the individual level by using relative and absolute differences as well as data on misclassification of salt intake groups, unlike previous studies.
Potential limitations in our study include: use of the mean of only two timed 24-h urine Na excretions in all analyses recognizing that such timed 24-h urine Na values over a short time may not reflect typical salt intake. Although multiple, nonconsecutive, 24-h urine collections are recommended to improve precision for estimating salt intake [30,31], it is hard to perform multiple 24-h urine collections in large-sample population studies. To exclude the possibility that participants with Na excretions at the extremes were particularly adherent to an unusual dietary pattern during the study, we displayed the first and repeat 24-h urinary Na excretions of participants whose mean Na excretions were in the lowest or highest 5% in Supplemental Table S3, http://links.lww.com/HJH/B20. The mean differences (95%CI) between the first and repeat 24-h urinary Na were 2.6 (−2.5, 7.8) mmol for the lowest 5% group and 1.1 (−16.3, 18.4) mmol for the highest 5% group, indicating that most of the participants with very low or high Na intake had similar intakes in the two collections in our study.
In conclusion, the findings from our study indicate that multiple 24-h dietary recalls demonstrate greater agreement with the 24-h urine collections in assessing population Na intake for Japan, UK, and USA compared with China, whereas yield considerable underestimation of Na intake for Chinese populations. The 24-h dietary recall is inadequate for the accurate assessment of individual Na intake.
Supplementary Material
ACKNOWLEDGEMENTS
We thank INTERMAP participants and staff at local, national, and international centers for their invaluable efforts.
Funding source: The INTERMAP Study is supported by the National Heart, Lung, and Blood Institute, National Institutes of Health (US, grants: R01-HL50490 and R01- HL84228); by national agencies in Japan (Ministry of Education, Science, Sports, and Culture; grant 090357003), by a project grant from the West Midlands National Health Service Research and Development (UK), by the Chest, Heart, and Stroke Association (Northern Ireland, UK; grant R2019EPH). This observational study is registered at www.clinicaltrials.gov as NCT00005271.
Abbreviations:
- BP
blood pressure
- FFQ
Food Frequency Questionnaire
- INTERMAP
International Study of Macro- and Micro-nutrients and Blood Pressure
- MSG
Monosodium glutamate
- NHANES
National Health and Nutrition Examination Survey
- PURE
Prospective Urban Rural Epidemiology
- SPRINT
Systolic Blood Pressure Intervention Trial
- TOHP
Trials of Hypertension Prevention
- UK
United Kingdom
- USA
United States of America
Footnotes
The manuscript has not been submitted elsewhere for publication, except an oral presentation by Dr Xiaoxiao Wen at AHA scientific session 2015, Orlando, Florida, USA.
Conflicts of interest
There are no conflicts of interests.
REFERENCES
- 1.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ 2013; 346:f1325. [DOI] [PubMed] [Google Scholar]
- 2.Wang M, Moran AE, Liu J, Qi Y, Xie W, Tzong K, et al. A meta-analysis of effect of dietary salt restriction on blood pressure in Chinese Adults. Glob Heart 2015; 10:291.e6–299.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strazzullo P, D’Elia L, Kandala NB, Cappuccio FP. Salt intake, stroke, and cardiovascular disease: meta-analysis of prospective studies. BMJ 2009; 339:b4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerpohl JJ. Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 2013; 346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. , Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014; 371:624–634. [DOI] [PubMed] [Google Scholar]
- 6.Whelton PK, Carey RM, Aronow WS, Casey DJ, Collins KJ, Dennison HC, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/ NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2018; 71:2199–2269.29146533 [Google Scholar]
- 7.WHO. Sodium intake for adults and children. 2012. [15 January 2016]. Available at: http://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf.
- 8.Scientific Report of the 2015 Dietary Guidelines Advisory Committee. Washington, DC: US Department of Agriculture; 2015. [cited 4 January 2017]. Available at: https://health.gov/dietaryguidelines/2015-scientific-report/. [Google Scholar]
- 9.China Nutrition Society. Principle of Chinese dietary guideline 2016. Beijing, China: People’s Medical Publishing House; 2016. [Google Scholar]
- 10.McLean RM. Measuring population sodium intake: a review of methods. Nutrients 2014; 6:4651–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X, Hall J, Byles J, Shi Z. Dietary pattern is associated with obesity in older people in China: data from China Health and Nutrition Survey (CHNS). Nutrients 2015; 7:8170–8188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chmielewski J, Carmody JB. Dietary sodium, dietary potassium, and systolic blood pressure in US adolescents. J Clin Hypertens (Greenwich) 2017; 19:904–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhodes DG, Murayi T, Clemens JC, Baer DJ, Sebastian RS, Moshfegh AJ. The USDA Automated Multiple-Pass Method accurately assesses population sodium intakes. Am J Clin Nutr 2013; 97:958–964. [DOI] [PubMed] [Google Scholar]
- 14.Kelly C, Geaney F, Fitzgerald AP, Browne GM, Perry IJ. Validation of diet and urinary excretion derived estimates of sodium excretion against 24-h urine excretion in a worksite sample. Nutr Metab Cardiovasc Dis 2015; 25:771–779. [DOI] [PubMed] [Google Scholar]
- 15.De Keyzer W, Dofkova M, Lillegaard IT, De Maeyer M, Andersen LF, Ruprich J, et al. Reporting accuracy of population dietary sodium intake using duplicate 24h dietary recalls and a salt questionnaire. Br J Nutr 2015; 113:488–497. [DOI] [PubMed] [Google Scholar]
- 16.Mercado CI, Cogswell ME, Valderrama AL, Wang CY, Loria CM, Moshfegh AJ, et al. Difference between 24-h diet recall and urine excretion for assessing population sodium and potassium intake in adults aged 18–39 y. Am J Clin Nutr 2015; 101:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espeland MA, Kumanyika S, Wilson AC, Reboussin DM, Easter L, Self M, et al. Statistical issues in analyzing 24-h dietary recall and 24-h urine collection data for sodium and potassium intakes. Am J Epidemiol 2001; 153:996–1006. [DOI] [PubMed] [Google Scholar]
- 18.Stamler J, Elliott P, Dennis B, Dyer AR, Kesteloot H, Liu K, et al. , INTERMAP Research Group. INTERMAP: background, aims, design, methods, and descriptive statistics (nondietary). J Hum Hypertens 2003; 17:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennis B, Stamler J, Buzzard M, Conway R, Elliott P, Moag-Stahlberg A, et al. , INTERMAP Research Group. INTERMAP: the dietary data- process and quality control. J Hum Hypertens 2003; 17:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu-Hage BH, Wahlqvist ML. A food frequency questionnaire for use in Chinese populations and its validation. Asia Pac J Clin Nutr 1992; 1:211–223. [PubMed] [Google Scholar]
- 21.Li J, Lu Z, Yan L, Zhang J, Tang J, Cai X, et al. Comparison of dietary survey, frequency and 24 h urinary Na methods in evaluation of salt intake in the population. Zhonghua Yu Fang Yi Xue Za Zhi 2014; 48:1093–1097. [PubMed] [Google Scholar]
- 22.Zhou L, Tian Y, Fu JJ, Jiang YY, Bai YM, Zhang ZH, et al. Validation of spot urine in predicting 24-h sodium excretion at the individual level. Am J Clin Nutr 2017; 105:1291–1296. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371:612–623. [DOI] [PubMed] [Google Scholar]
- 24.Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, et al. , PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 2016; 388:465–475. [DOI] [PubMed] [Google Scholar]
- 25.Beddhu S, Chertow GM, Cheung AK, Cushman WC, Rahman M, Greene T, et al. , SPRINT Research Group. Influence of baseline diastolic blood pressure on effects of intensive compared with standard blood pressure control. Circulation 2018; 137:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He FJ, Ivkovic V, Jelakovic B, Morris J, MacGregor GA. Estimation of sodium excretion should be made as simple as possible, but not simpler: misleading papers and editorial on spot urines. J Hypertens 2015; 33:884–886. [DOI] [PubMed] [Google Scholar]
- 27.He FJ, Campbell NRC, Ma Y, MacGregor GA, Cogswell ME, Cook NR. Errors in estimating usual sodium intake by the Kawasaki formula alter its relationship with mortality: implications for public health. Int J Epidemiol 2018; Online only, doi: 10.1093/ije/dyy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattes RD, Donnelly D. Relative contributions of dietary sodium sources. J Am Coll Nutr 1991; 10:383–393. [DOI] [PubMed] [Google Scholar]
- 29.Anderson CA, Appel LJ, Okuda N, Brown IJ, Chan Q, Zhao L, et al. Dietary sources of sodium in China, Japan, the United Kingdom, and the United States, women and men aged 40 to 59 years: the INTERMAP study. J Am Diet Assoc 2010; 110:736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olde ER, van den Hoek TC, van Noordenne ND, van den Born BH, Peters-Sengers H, Vogt L. Use of a single baseline versus multiyear 24- hour urine collection for estimation of long-term sodium intake and associated cardiovascular and renal risk. Circulation 2017; 136:917–926. [DOI] [PubMed] [Google Scholar]
- 31.Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk-measurement matters. N Engl J Med 2016; 375:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


